Open Access Article
Open Access ArticleMicelle-driven organic synthesis: an update on the synthesis of heterocycles and natural products in aqueous medium over the last decade
Mohmad Muzafar Waniab and
Bilal A. Bhat *ab
*ab
aNatural Products and Medicinal Chemistry, CSIR-Indian Institute of Integrative Medicine, Sanat Nagar, Srinagar 190005, India
bAcademy of Scientific & Innovative Research (AcSIR), Ghaziabad-201002, India. E-mail: bilal@iiim.res.in
First published on 18th July 2025
Abstract
Organic synthesis guided by micellar nanoreactors constitutes one of the fundamental fields of organic chemistry that is expected to furnish chemical synthesis in a sustainable fashion. Due to its promise, we were also attracted to using the micelles to enhance chemical reactivity and selectivity and to explore their newer applications in natural products and heterocyclic chemistry. As on date, there is no comprehensive review article that highlights the repertoire of chemical reactions developed in micelles furnishing a range heterocycles and natural products/scaffolds, barring the metal-catalyzed cross-coupling reactions. In this review, we document the last decade (2014–2024) progress of organic reactions developed in micelles to yield a range of heterocyclic and natural product-based scaffolds. Notably, we have excluded the content related to metal-catalyzed cross-coupling reactions and some other aspects of micelles due to the number of overlapping reviews written on such topics. In the current article, we briefly introduce micellar catalysis, regions of reaction sites in micelles, and various surfactants utilized in micelle chemistry. Our discussion also captures the importance of micellar catalysis compared to the conventional organic synthesis. More importantly, the focus of our review is largely on the collection of chemical transformations accomplished in the last decade in accessing heterocycles and natural products to showcase the advancement of organic synthesis through sustainable fashion. Wherever required, we have also captured the various interactions of surfactants with the substrates necessary for driving the reactions and discussed the importance of these heterocycles/natural products in intercepting the key biological pathways.
1 Introduction
Micelles represent the aggregation of surfactant molecules in a liquid, particularly in water. A typical micellar structure in water has its “hydrophilic head” in contact with the water and its “hydrophobic tail” positioned inside the assembly, resulting in the formation of dynamic nano-aggregates of various shapes and sizes.1 Often these aggregates are spherical, but shapes like ellipsoids, cylinders, and bilayers are also known. The molecular geometry of surfactant molecules, their concentration and certain environmental factors like pH, temperature and ionic strength determine the structure of micellar assemblies.2 Overall, a micellar system is a microheterogeneous two-phase colloidal system and appears homogeneous due to the colloidal size of these aggregates.3 Aggregation of surfactant molecules occur only when their concentration is at or above the critical micelle concentration (CMC) and the temperature is equal or above the Krafft temperature. During the formation of micellar assemblies, hydrophobic interactions are believed to be the driving force for their formations. These hydrophobic effects are attributed to the structural competition between the hydrogen bondings of interfacial and bulk water.4The structure of a normal micelle harbors a hydrophilic outer region-the stern layer, the inner hollow part encircled by hydrophobic tails is called the core, and the hydrophobic tail-containing area between the core and stern layer is called the palisade region (Fig. 1). The hydrophobic tails of the reverse micelle are pointing outward, and the hydrophilic heads surround the core. These different regions of the micellar nano-reactors with different environments (compartmentalization effect) act as the reaction pockets for various chemical transformations reminiscent of enzymatic catalysis in nature.5
The formation of micelles and their typical morphology are affected by the surfactant concentration, temperature, nature of surfactant and pH of the solution. Change of micellar morphology has been observed from the spherical to rod-shaped through the globular transition state on increasing the concentration of surfactant molecules.6 The CMC of micelles is also dependent on temperature (and the Krafft temperature or Krafft point is the temperature at which concentration of surfactants reaches CMC). The other factor affecting the micellization is the chain length of amphiphilic molecules which should usually be greater than ten carbon atoms. In a typical micelle, generally 50–100 surfactant molecules are present which are in thermodynamic equilibrium having an average lifetime of 10−3–10−2 seconds.7
1.1. Determination of size, structure and micellar interactions
For any organic chemistry transformations, the most crucial information required is to know the CMC, size and morphology of micelle-aggregates. Numerous approaches that are all based on the drastic change in the properties of the solution upon passing the critical micelle concentration. The most widely used techniques for calculating the CMC rely on surface tension measurements, conductometry, scattering of light, light absorbance, UV-vis or fluorescence analysis. NMR spectroscopy and several other techniques, including the recently developed smart phone based measurements, are also employed to measure the CMC.8 The average size of micellar assemblies are established by the dynamic light scattering (DLS) analysis, which gives information about the hydrodynamic radius of micellar aggregates. DLS also gives the information about the distribution of different micellar structures which are present in a given solution.9 Further, the techniques like SEM and TEM analysis help to determine the morphological features and internal structure of micelles in order to establish the actual pictures of the micellar aggregates formed by surfactants.10One of the crucial parameters in micelle-guided organic synthesis is to know the interactions between the solubilizates and micellar aggregates. Several groups have utilized NMR techniques (1D and 2D) to establish such interactions. The diffusion coefficient of micellar assemblies determined by means of pulsed field gradient diffusion NMR or DOSY compared to the value of the free solute helps in determining the interaction between the micelles and solubilizates. 2D-NMR experiments like NOESY and/or ROESY have been employed to know the site of surfactant interaction with the solute molecules.11 In an alternative method, the interactions are also established between the solute and assembled surfactants by studying the variation of the chemical shift in the various protons of surfactant by increasing the concentration of solute molecules. At higher solubilizate concentrations, sometimes the change in NMR peak shape and resolution is also observed.12
1.2. Classification of surfactants
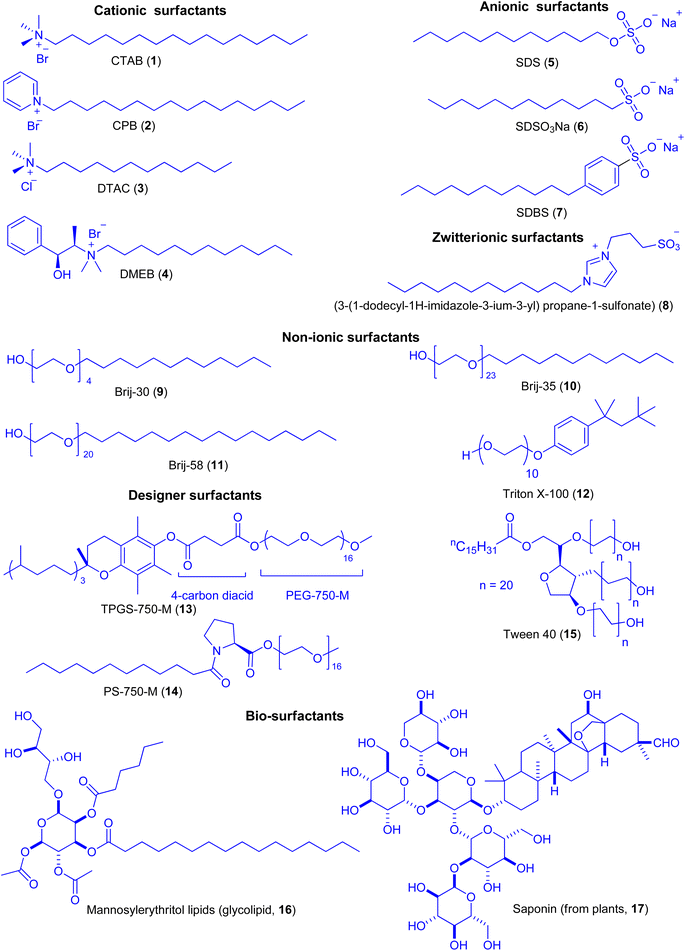
Depending upon the charge of the hydrophilic part, surfactants have been categorized into four main types.13 Cationic surfactants have positively charged hydrophilic (water-attracting) head. Cetyltrimethylammonium bromide (CTAB, 1), cetylpyridenium bromide (CPB, 2), dodecyltrimethylammonium chloride (DTAC, 3), (−)-N-dodecyl-N-methylephedrinium bromide (DMEB, 4) etc. are typical examples of cationic surfactants (Fig. 2). Anionic surfactants are distinguished by their negatively charged hydrophilic (water-attracting) head. An anionic surfactant's hydrophilic head typically contains a negatively charged group such as sulfate (SO4), sulfonate (SO3), or carboxylate (CO2−). Sodium dodecylsulfate (SDS, 5), sodium dodecylsulfonate (SDSO3Na, 6), sodium dodecylbenzenesulfate (SDBS, 7), etc. are some the typical examples of anionic surfactants. Amphiphiles containing both negative and positive charges separated by a spacer are called zwitterionic surfactants. A typical example of zwitterionic surfactant is (3-(1-dodecyl-1H-imidazole-3-ium-3-yl) propane-1-sulfonate) (8) in which the changes in the primary structure on head, tail or spacer region can improve the effectiveness of the surfactants. Nonionic surfactants function effectively as emulsifiers, foaming agents, and wetting agents because of their uncharged hydrophilic and hydrophobic groups. The hydrophilic component of nonionic surfactant is primarily composed of poly-(ethylene oxide). Brij-30, Brij-35, Brij-58, Triton X-100 and Tween 40 (9–12) are typical examples of this class of surfactants. Recently, some designer amphiphiles derived from vitamin-E and proline, such as TPGS-750-M (13) and PS-750-M (14) respectively and Tween 40 (15) also harbor poly-(ethylene oxide) as an integral part of the surfactant.14 Amphiphilic molecules exhibiting biological functions have also been found in certain groups of bio-surfactants. Among the representative examples are glycolipids (like mannosylerythritol lipids, 16), lipopeptides, phospholipids, fatty acids, neutral lipids and plant-derived saponins (17). The majority of these surfactants are either anionic or neutral, with the hydrophilic portion based on carbohydrates, amino acids, cyclic peptides, phosphates, carboxylic acids, or alcohols, and the hydrophobic part based on long-chain fatty acids, hydroxy fatty acids, or α-alkyl-β-hydroxy fatty acids. These biodegradable surfactants find interesting biological and environmental applications.152 Application of micelles in organic synthesis
During recent times, micelles have gained a considerable attention in food, medicine, beverages, cosmetics, oil refining, environment and material sciences.18–27 In chemistry, they provide an alternative way for organic chemists to perform organic reaction in nature's solvent, water, and thereby open an entry towards sustainable organic synthesis. For chemists, water was not an option, since historically, the notion that like dissolves like implies that dissolution is a pre-condition for chemical reactions to take place. Because organic substrates are usually insoluble in water, using water as a solvent has proven difficult for organic chemists. Due to the poor solubility and low reactivity of reaction partners in water, chemists did not find this option attractive and therefore embraced the organic solvents firmly. Every reaction driven by organic solvents leaves a footprint on our environment and is not a sustainable option for times to come. For efficient synthesis, selectivity- regio-, chemo- and stereoslectivity is also a major challenge for organic chemists to overcome in the future. Due to the homogeneity, reactions carried out in organic solvents attain evenly distribution of reaction partners and thereby often lead to non-selective products. Using micelles for organic transformation is like mimicking chemical reactions occurring in nature, wherein enzymatic catalysis also involve the hydrophobic and hydrophilic regions as part of the molecular structure.16 Micelles in aqueous medium are currently used not only as catalysts, but also as solvent mimics and reaction promoters in numerous chemical transformations which has been beautifully captured in a recently published review from Sachin Handa's group.17 Micelles are known to encapsulate organic substrates, improving solubility, and increasing the local concentration and reactivity of reactants as depicted in Fig. 3.18 Micelles enhance the reaction selectivity by three main effects: (a) dipoles at water/micellar lipophilic core enhance the regioselectivity; (b) formation of a specific product occurs by substrate/micellar interactions and (c) product selectivity by compartmentalization effect in various micellar regions.19 Due to these reasons, the use of micelles in synthetic organic chemistry has grown in prominence especially in the last two decades and has been employed in a number of organic transformations. F. Gallou,20 S. Handa,17 Krause,21 and, most importantly, B. H. Lipshutz22 have done ground-breaking work in this area. As a result, many researchers worldwide have reviewed this field's progress from time to time.16,17,19,23–28 In our group, we have also attempted to utilize these micellar nano-reactors to induce transformations with enhanced chemical reactivity29,30 and selectivity.31 The various organic transformations that have been implemented in aqueous micellar catalysis include C–C and C-heteroatom bond formation especially through metal-catalyzed cross-coupling coupling reactions. Further progress in this area was achieved in natural products,24 asymmetric synthesis,32 rearrangement reactions,33 oxidations/reductions18 and heterocyclic synthesis.34 In this review, we aim to chronicle the research accomplishments obtained in the last decade (2014–2024) using micellar catalysis. Our main motif is to cover the synthesis of heterocyclic compounds and natural products which have been accessed in aqueous micellar catalysis to highlight the potential of this area towards sustainable organic synthesis. The purpose is to stimulate the newer generation of chemists and to encourage them to use water as a solvent in organic synthesis for the longevity of the field of organic synthesis. An enantioselective chemoenzymatic synthesis of the antidementia drug (S)-rivastigmine using micellar catalysis by the Lipshutz group has sparked further interest among medicinal chemists in making drugs and natural products using micellar catalysis.35 Developments of newer methods in aqueous medium for the molecules of medicinal and material importance are expected to change the direction of the field of organic synthesis completely. Keeping in mind these aspects, we will discuss in detail the synthesis, micellar-solubilizate interactions and biological importance of heterocyclic compounds and natural products (wherever reported) that have been synthesized using micellar catalysis in the last decade. Due to the overlap and to keep the review focused, heterocyclic compounds and natural products synthesis involving metal-catalyzed (especially Pd-catalyzed) cross-coupling reactions have been excluded from this review and appropriate citations have been already covered (vide supra). | ||
| Fig. 3 Diagrammatic representation of reactions in aqueous micelles and in conventional organic solvents. | ||
2.1. Micellar-catalysis in heterocyclic synthesis
A large number of heterocyclic compounds have been synthesized using micellar catalysis. A repertoire of reactions involving carbon–carbon and carbon–heteroatom bond formation reactions, multicomponent reactions, oxidations/reductions, cycloaddtions and rearrangement reactions have been established to access various heterocyclic compounds. These transformations reported during the preceding decade have been discussed in the chronological order below: | ||
| Scheme 3 A three component synthesis of chromeno[2,3-b]quinolinediones in aqueous TBAB micellar medium. | ||
In its other variant, Manisha R. Bhosle et al. have developed an efficient multicomponent synthesis coumarin-fused thiazolyl chromeno[4,3-b]quinolines from the corresponding aldehydes, amines, 1,3-cycloakdiones and coumarins in CTAB micellar medium.40
 | ||
| Scheme 4 A three component synthesis of 7-aryl-benzopyrano[4,3-b]benzopyran-6,8-diones in SDS-micellar medium. | ||
A conceptually similar protocol for the synthesis of pyrano[3,2-c]chromene derivatives have been reported by Abas Ali Jafar in CTAB micellar medium by reacting 4-hydroxycoumarin, aromatic aldehydes and malononitrile.42 The reaction operates with the same mechanism and furnishes therapeutically important scaffolds in decent yields. In the same way another synthesis of 2-amino-4H-pyran derivatives was accomplished by the reaction of aldehydes, malononitrile, and cyanoketones in an aqueous CTAB micellar medium.43 Furthermore, a one-pot synthesis of fluorescent coumarin-4H-pyran conjugates has been also established from β-ketoesters, malononitrile and aldehydes in cetyltrimethylammonium chloride (CTAC) aqueous micellar medium.44 The study highlights the simplicity and effectiveness of this catalytic system and paving way for medicinal chemistry explorations to these coumarin derivatives.
 | ||
| Scheme 5 A four component synthesis of 2-amino-6-(1H-indol-3-yl)-4-arylpyridine-3,5-dicarbonitriles in aqueous CTAB micellar medium. | ||
A conceptually similar four-component reaction regime has been developed by the reaction of acetophenone, benzaldehyde, arylthiol and malanonitrile in Triton-X-100. Condensation of acetophenone with benzaldehyde leading a chalcone which undergoes subsequent reaction with malononitrile and arylthiol resulting in the formation of 8-substituted pyrido[2,3-d]pyrimidine-6-carbonitriles in excellent yields.46
Mahendra Nath established an interesting method for the synthesizing of spiro-indoline-3,2-thiazolidinones by reacting the primary amines with isatins and thioglycolic acid in micellar medium. These pharmacologically important scaffolds were synthesized through a similar set of sequential reactions in the presence of p-dodecylbenzene sulfonic acid (DBSA) as an efficient Brosnted acid surfactant combined catalyst in water.51
A conceptually similar protocol was developed by Ali Khalafi Nezhad et al. using polyethylene glycol-bonded 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU-PEG), a new surfactant-combined base catalyst for the multicomponent synthesis of 8-substituted pyrido[2,3-d]pyrimidine-6-carbonitriles (58),46 Scheme 12b. This reaction in water involving multicomponents like aldehydes, malononitrile, barbutiric acid (56) and nucleosides (57) in PEG-DBU surfactant furnishing 8-substituted pyrido[2,3-d]pyrimidine-6-carbonitrile (58) scaffolds in excellent yields.
 | ||
| Scheme 24 Reverse ZnO-nanomicelle catalyzed synthesis of 2,3-dihydroquinazolin-4(1H)-ones micellar medium. | ||
Later on, a new method for quinazolinones and spiro-quinazolionones has been accomplished through a multicomponent reaction of isatoic anhydride, amine and aldehydes/istanins.71 This reaction is catalyzed by L-proline, occurs in Triton-X-100 micellar medium and bears vast structural diversity.
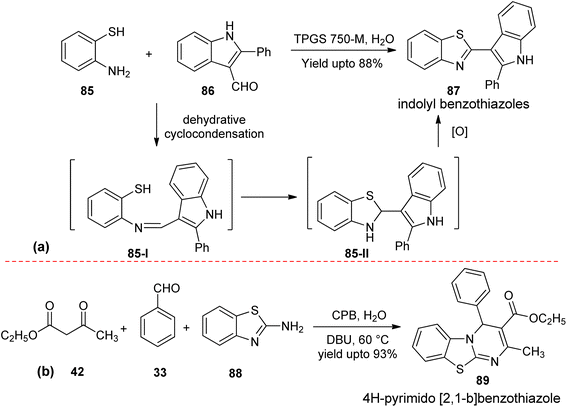 | ||
| Scheme 25 (a) Synthesis of 2-(indol-3-yl)benzothiazoles and (b) 4H-pyrimido [2,1-b]benzothiazoles in micellar medium. | ||
A conceptually related synthesis of 4H-pyrimido [2,1-b]benzothiazoles (89) have been accomplished separately from through a multicomponent cyclocondensation reaction of β-ketoester (42), aromatic aldehydes (33) and 2-aminobenzothiazole (88) reaction partners,73 Scheme 25(b). This reaction has been accomplished in CPB micellar solution using DBU as an organic base.
2.2. Micellar-catalysis in the synthesis of natural products and natural products scaffolds
After a considerable success in heterocyclic chemistry, micellar catalysis is also gaining interest in the synthesis of natural products/scaffolds through cascade reactions. Though this field is in infancy, the following examples will motivate the organic chemists to explore it in future.3 Conclusion and outlook
Over the years, the science of organic synthesis has witnessed revolutionary strides in the establishment of new methods, strategies, and routes to gain access into the complex building blocks. However, one of the key concerns has been minimizing the use of volatile organic solvents, which directly impact our day-to-day lives. Among various strategies, one of the interesting strategies developed over the past couple of decades has been the micellar catalysis, which mimics nature's machinery of enzymatic synthesis. Though this field continues to attract the traction of organic chemists in aligning their science in tune with sustainable chemistry, it has also many challenges to overcome. As a result, some particular set of reactions involving C–C/C–heteroatom bond-forming reactions, multicomponent reactions, oxidations/reductions, rearrangements, cyclo-additions, fluorination reactions etc. have been successfully reported in aqueous micellar medium. From the past several years, a large number of heterocyclic compounds have been synthesized in a robust fashion using micellar catalysis. Applying this arena on reactions furnishing natural products and natural product building blocks is largely a silent field. One of the aims of writing this article is to compile the progress of micellar catalysis in various chemical transformations and the successful attempts are expected to encourage the new generation of chemists to align their chemistry towards green and sustainable fashion. Since the natural products synthesis is a daunting task involving the synthesis of complex building blocks and controlling the stereochemistry. Micellar chemistry provides a window of opportunity to accomplish such goals with high degree of precision. Micellar catalysis not only enhances the reaction kinetics but selectivity of products also due to the compartmentalization effect. Together, these principles are expected to change the future course of organic synthesis with the discovery and development of new surfactant variants which are efficient to drive the tedious chemical transformations. Applicability of micellar catalysis is also expanding in pharmaceuticals at industrial level to chase the various drugs and their APIs in scalable quantities in water.80 A continuous progress in this arena will have a positive impact on our environment and also enhance the longevity of the field of organic synthesis.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MMW and BAB acknowledges the fellowship support and grant support of CSIR through CSIR-FIRST Scheme (FIR030302). IIIM communication No. CSIR-IIIM/IPR/00887.References
- S. Lebecque, J.-M. Crowet, M. N. Nasir, M. Deleu and L. Lins, J. Mol. Graph. Model., 2017, 72, 6–15 CrossRef CAS PubMed.
- S. Perumal, R. Atchudan and W. Lee, Polymers, 2022, 14, 2510–2529 CrossRef CAS PubMed.
- E. Bernal, M. Marchena and F. Sánchez, Molecules, 2010, 15, 4815–4874 CrossRef CAS PubMed.
- Q. Sun, Molecules, 2022, 27, 7009 CrossRef CAS PubMed.
- O. Glatter and S. Salentinig, Curr. Opin. Colloid Interface Sci., 2020, 49, 82–93 CrossRef CAS.
- C.-X. Lin, in Engineering Technology Trends, 2024, p. 2 Search PubMed.
- J. H. Clint, in Surfactant Aggregation, Springer Science & Business Media, 2012 Search PubMed.
- M. M. Mabrouk, N. A. Hamed and F. R. Mansour, Monatsh. Chem., 2022, 153, 125–138 CrossRef CAS.
- J.-L. Lemyre, S. Lamarre, A. Beaupré and A. M. Ritcey, Langmuir, 2010, 26, 10524–10531 CrossRef CAS PubMed.
- S. R. Falsafi, H. Rostamabadi, E. Assadpour and S. M. Jafari, Adv. Colloid Interface Sci., 2020, 280, 102166–102189 CrossRef CAS PubMed.
- M. M. Mabrouk, N. A. Hamed and F. R. Mansour, Appl. Spectrosc. Rev., 2023, 58, 206–234 CrossRef CAS.
- S. Rashid, U. N. Tak, M. S. Lone, O. A. Chat, P. A. Bhat, F. A. Ahanger, I. A. Bhat and A. A. Dar, Environ. Pollut., 2023, 336, 122489–122498 CrossRef CAS PubMed.
- B. Kronberg, K. Holmberg and B. Lindman, Surface Chemistry of Surfactants and Polymers, 2014, pp. 1–47 Search PubMed.
- D. Dutta, N. Gaur, P. Barman, D. Ghosh, R. Dubey and S. K. Dwivedi, Def. Life Sci. J., 2022, 7, 103–117 CrossRef.
- J. R. Kincaid, M. J. Wong, N. Akporji, F. Gallou, D. M. Fialho and B. H. Lipshutz, J. Am. Chem. Soc., 2023, 145, 4266–4278 CrossRef CAS PubMed.
- F. Fabris, M. Illner, J.-U. Repke, A. Scarso and M. Schwarze, Molecules, 2023, 28, 4809–4845 CrossRef CAS PubMed.
- J. K. Virdi, A. Dusunge and S. Handa, JACS Au, 2024, 4, 301–317 CrossRef CAS PubMed.
- B. A. Bhat and B. A. Shairgojray, Mini-Rev. Org. Chem., 2020, 17, 289–296 CrossRef CAS.
- S. Mattiello, E. Ghiglietti, A. Zucchi and L. Beverina, Curr. Opin. Colloid Interface Sci., 2023, 64, 101681–101714 CrossRef CAS.
- T. N. Ansari, F. Gallou and S. Handa, Coord. Chem. Rev., 2023, 488, 215158–215178 CrossRef CAS.
- L. Lempke, A. Ernst, F. Kahl, R. Weberskirch and N. Krause, Adv. Synth. Catal., 2016, 358, 1491–1499 CrossRef CAS.
- B. H. Lipshutz, Green Chem., 2024, 26, 739–752 RSC.
- G. La Sorella, G. Strukul and A. Scarso, Green Chem., 2015, 17, 644–683 RSC.
- V. Sorhie, B. Gogoi, B. Walling, S. A. Acharjee and P. Bharali, Sustain. Chem. Pharm., 2022, 30, 100875 CrossRef CAS.
- E. Borrego, A. Caballero and P. J. Pérez, Organometallics, 2022, 41, 3084–3098 CrossRef CAS PubMed.
- M. Yoshizawa and L. Catti, Proc. Jpn. Acad., Ser. B, 2023, 99, 29–38 CrossRef CAS PubMed.
- A. Steven, Synthesis, 2019, 51, 2632–2647 CrossRef CAS.
- A. Acharjee, A. Rakshit, S. Chowdhury and B. Saha, J. Mol. Liq., 2021, 321, 114897–114910 CrossRef CAS.
- B. A. Shairgojray, A. A. Dar and B. A. Bhat, Tetrahedron Lett., 2013, 54, 2391–2394 CrossRef CAS.
- M. M. Wani, A. A. Dar and B. A. Bhat, Org. Biomol. Chem., 2022, 20, 4888–4893 RSC.
- B. A. Shairgojray, A. A. Dar and B. A. Bhat, Catal. Commun., 2016, 83, 58–61 CrossRef CAS.
- T. Lorenzetto, D. Frigatti, F. Fabris and A. Scarso, Adv. Synth. Catal., 2022, 364, 1776–1797 CrossRef CAS.
- X. Xing, K. Zhao, Z. Li, N. Ye, N. Zhao, F. Guo, C. Qiao, G. Xu, M. Parmentier and F. Gallou, Org. Process Res. Dev., 2024, 28, 2945–2950 CrossRef CAS.
- A. Rahmati and N. Pashmforoush, J. Iran. Chem. Soc., 2015, 12, 993–1036 CrossRef CAS.
- J. Dussart-Gautheret, J. Yu, K. Ganesh, G. Rajendra, F. Gallou and B. H. Lipshutz, Green Chem., 2022, 24, 6172–6178 RSC.
- V. K. Tandon, M. K. Verma, H. K. Maurya and S. Kumar, Tetrahedron Lett., 2014, 55, 6331–6334 CrossRef CAS.
- B. Miksa, Helv. Chim. Acta, 2022, 105, e202200066 CrossRef CAS.
- A. Maity, D. Chakraborty, A. Hazra, Y. P. Bharitkar, S. Kundu, P. R. Maulik and N. B. Mondal, Tetrahedron Lett., 2014, 55, 3059–3063 CrossRef CAS.
- J. Ghosh, P. Biswas, T. Sarkar, M. G. Drew and C. Bandyopadhyay, Tetrahedron Lett., 2014, 55, 2924–2928 CrossRef CAS.
- M. R. Bhosle, S. A. Joshi and G. M. Bondle, J. Heterocycl. Chem., 2020, 57, 456–468 CrossRef CAS.
- N. C. Ganguly, S. Roy and P. Mondal, Synth. Commun., 2014, 44, 433–440 CrossRef CAS.
- A. A. Jafari and M. Ghadami, Environ. Chem. Lett., 2016, 14, 215–221 CrossRef CAS.
- Rahila, P. Rai, A. Ibad, H. Sagir and I. Siddiqui, ChemistrySelect, 2016, 1, 1300–1304 CrossRef CAS.
- A. Omar, K. Ablajan and M. Hamdulla, Chin. Chem. Lett., 2017, 28, 976–980 CrossRef CAS.
- S. Fatma, D. Singh, P. Ankit, P. Mishra, M. Singh and J. Singh, Tetrahedron Lett., 2014, 55, 2201–2207 CrossRef CAS.
- M. Shekouhy and A. Khalafi-Nezhad, Green Chem., 2015, 17, 4815–4829 RSC.
- S. R. Minkler, N. A. Isley, D. J. Lippincott, N. Krause and B. H. Lipshutz, Org. Lett., 2014, 16, 724–726 CrossRef CAS PubMed.
- M. Singh, M. Saquib, S. B. Singh, S. Singh, P. Ankit, S. Fatma and J. Singh, Tetrahedron Lett., 2014, 55, 6175–6179 CrossRef CAS.
- A. Mishra, P. Rai, Y. K. Pandey, J. Singh and J. Singh, ChemistrySelect, 2017, 2, 10979–10983 CrossRef CAS.
- J. Tiwari, S. Singh, F. Tufail, D. Jaiswal, J. Singh and J. Singh, ChemistrySelect, 2018, 3, 11634–11642 CrossRef CAS.
- A. Preetam and M. Nath, Tetrahedron Lett., 2016, 57, 1502–1506 CrossRef CAS.
- F. Tamaddon and M. Alizadeh, Tetrahedron Lett., 2014, 55, 3588–3591 CrossRef CAS.
- S. Sikandar and A. F. Zahoor, J. Heterocycl. Chem., 2021, 58, 685–705 CrossRef CAS.
- M. Singh, S. B. Singh, S. Fatma, P. Ankit and J. Singh, New J. Chem., 2014, 38, 2756–2759 RSC.
- J. Ghosh, P. Biswas, M. G. Drew and C. Bandyopadhyay, Mol. Divers., 2015, 19, 541–549 CrossRef CAS PubMed.
- G. I. Anderton, A. S. Bangerter, T. C. Davis, Z. Feng, A. J. Furtak, J. O. Larsen, T. L. Scroggin and J. M. Heemstra, Bioconjug. Chem., 2015, 26, 1687–1691 CrossRef CAS PubMed.
- I. Siddiqui, P. Rai, H. Sagir and P. Singh, RSC Adv., 2015, 5, 27603–27609 RSC.
- R. Aryan, M. Nojavan and F. Sadeghi, Phosphorus Sulfur Silicon Relat. Elem., 2015, 190, 1994–2004 CrossRef CAS.
- Y. Xu, Q. Xie, W. Li, H. Sun, Y. Wang and L. Shao, Tetrahedron, 2015, 71, 4853–4858 CrossRef CAS.
- D. He, M. Wang, S. Zhao, Y. Shu, H. Zeng, C. Xiao, C. Lu and Y. Liu, Fitoterapia, 2017, 119, 136–149 CrossRef CAS PubMed.
- T. Lohar, S. Jadhav, A. Kumbhar, A. Mane and R. Salunkhe, Res. Chem. Intermed., 2016, 42, 5329–5338 CrossRef CAS.
- M. Ravi, P. Chauhan, S. Singh, R. Kant and P. P. Yadav, RSC Adv., 2016, 6, 48774–48778 RSC.
- M. Vashishtha, M. Mishra and D. O. Shah, Green Chem., 2016, 18, 1339–1354 RSC.
- K. Rajaguru, A. Mariappan, S. Muthusubramanian and N. Bhuvanesh, Org. Chem. Front., 2017, 4, 124–129 RSC.
- P. A. More and G. S. Shankarling, New J. Chem., 2017, 41, 12380–12383 RSC.
- D.-L. Kong, G.-P. Lu, M.-S. Wu, Z.-F. Shi and Q. Lin, ACS Sustain. Chem. Eng., 2017, 5, 3465–3470 CrossRef CAS.
- T. Lohar, A. Mane, S. Kamat and R. Salunkhe, Polycycl. Aromat. Compd., 2020, 40, 1210–1222 CrossRef CAS.
- Z. T. Bhutia, D. Das, A. Chatterjee and M. Banerjee, ACS Omega, 2019, 4, 4481–4490 CrossRef CAS PubMed.
- F. Tamaddon and S. Tadayonfar, J. Mol. Liq., 2019, 280, 71–78 CrossRef CAS.
- J. Mou, N. Chen, Y. Zhao, H. Qi, S. Meng, R. Xiang and D. Pei, Front. Chem., 2020, 8, 239–250 CrossRef CAS PubMed.
- P. Kumar, N. Amber and V. D. Tripathi, Eur. J. Adv. Chem. Res., 2023, 4, 1–9 CrossRef CAS.
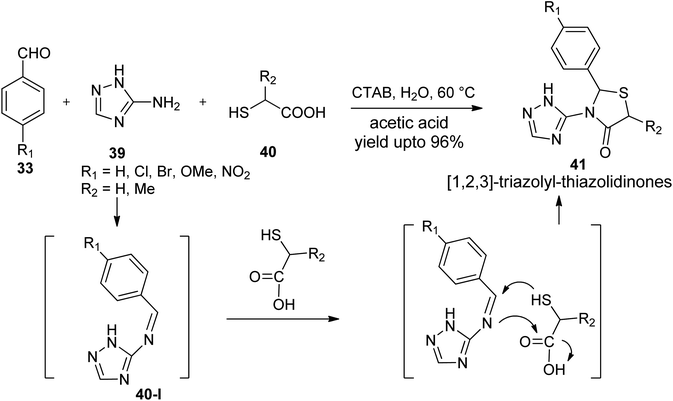
- S. Kumar, D. P. Satpute, G. N. Vaidya, M. Nagpure, S. K. Lokhande, D. Meena and D. Kumar, Tetrahedron Lett., 2020, 61, 152017–152025 CrossRef CAS.
- S. Singh and J. Lal, SN Appl. Sci., 2020, 2, 1–9 Search PubMed.
- A. Pious, R. K. Kamlekar, S. Muthusamy, A. Jothi, V. K. Praneeth, S. Ramesh and A. Veerappan, Colloids Surf., A, 2023, 664, 131129–131136 CrossRef CAS.
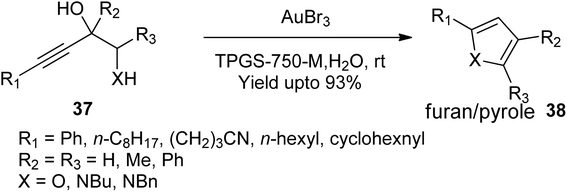
- B. Teli, M. M. Wani, S. Jan, H. R. Bhat and B. A. Bhat, Org. Biomol. Chem., 2024, 22, 6593–6604 RSC.
- J. F. Quilez del Moral, C. Ruiz Martinez, H. Perez del Pulgar, J. E. Martin Gonzalez, I. Fernández, J. L. López-Pérez, A. Fernández-Arteaga and A. F. Barrero, J. Org. Chem., 2021, 86, 3344–3355 CrossRef CAS PubMed.
- M. I. Quindt, G. F. Gola, J. A. Ramirez and S. M. Bonesi, Photochem. Photobiol. Sci., 2022, 21, 625–644 CrossRef CAS PubMed.
- S. Rashid, B. A. Bhat and G. Mehta, Eur. J. Org Chem., 2021, 2021, 6646–6651 CrossRef CAS.
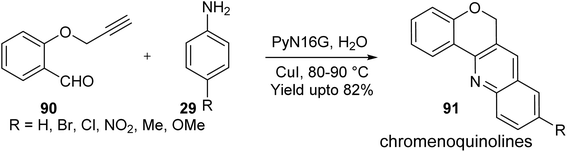
- M. M. Wani, A. Rashid and B. A. Bhat, Org. Biomol. Chem., 2023, 21, 6151–6159 RSC.
- N. Compagno, R. Profeta and A. Scarso, Curr. Opin. Green Sustain. Chem., 2023, 39, 100729 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |