 Open Access Article
Open Access ArticleStructural and luminescent properties of Dy3+-doped Ca3WO6 phosphors for white-light display applications
P. N. K. Chaitanya a,
D. Haranath
a,
D. Haranath *a,
D. Dinakar
*a,
D. Dinakar a,
M. Sree Ramanab,
K. V. R. Murthyc,
M. Rakshita
a,
M. Sree Ramanab,
K. V. R. Murthyc,
M. Rakshita a and
Govind Gupta
a and
Govind Gupta d
d
aDepartment of Physics, National Institute of Technology, Warangal-506004, India. E-mail: haranath@nitw.ac.in; praturi.chaitanya@gmail.com
bDLS, Vignayanakancha, RCI, Hyderabad-500069, India
cApplied Physics Department, Faculty of Technology and Engineering, The Maharaja Sayajirao University of Baroda, Vadodara-390001, India
dSensor Devices & Metrology, CSIR-National Physical Laboratory, Dr. K.S. Krishnan Road, New Delhi 110 012, India
First published on 11th June 2025
Abstract
This study unveils the synthesis and in-depth characterization of Dy3+-doped calcium tungstate (Ca3WO6) double perovskite phosphors, designed for advanced photoluminescence (PL) applications. These phosphors, with Dy3+ doping levels of 0.5–2.5 mol%, were synthesized using a high-temperature solid-state reaction method. Structural and morphological properties were rigorously evaluated through X-ray diffraction (XRD), scanning electron microscopy (SEM), and energy-dispersive X-ray spectroscopy (EDAX). Rietveld refinement of XRD data confirmed a monoclinic crystal structure, while SEM revealed a porous morphology attributed to high-temperature calcination, with EDAX verifying uniform elemental distribution. Excited at 278 nm, the Ca3WO6:Dy3+ phosphors emit intense white light, driven by the 4F9/2 → 6H15/2 (485 nm) and 4F9/2 → 6H13/2 (576 nm) transitions of Dy3+ ions. The effects of doping concentration on PL intensity and concentration quenching were thoroughly investigated. PL decay lifetime analysis at λex = 278 nm and λem = 576 nm elucidated the decay kinetics, affirming the phosphor's high external quantum yield (∼59%). CIE chromaticity coordinates place the emission squarely in the white light spectrum, underscoring the exceptional potential of these phosphors for advanced white light-emitting display technologies.
1. Introduction
Trivalent lanthanide ions, distinguished by their unique 4f electronic configurations, are renowned for generating sharp, vibrant emission spectra arising from intra-4f electronic transitions. These characteristics render them indispensable in the realms of advanced optics, photonics, and materials science.1,2 Among these ions, dysprosium (Dy3+) stands out for its remarkable ability to produce white light through characteristic blue (4F9/2 → 6H15/2, ∼485 nm) and yellow (4F9/2 → 6H13/2, ∼576 nm) emissions, making it a pivotal dopant for cutting-edge applications in lighting, displays, and photonic devices.3,4 The advent of phosphor-converted light-emitting diodes (pc-LEDs) has revolutionized the lighting industry, delivering unparalleled energy efficiency, exceptional brightness, and superior durability compared to conventional incandescent and fluorescent sources. Lanthanide-doped phosphors, including those activated by Dy3+, europium (Eu3+), terbium (Tb3+), and other rare-earth ions, are integral to a wide array of applications, encompassing solid-state lighting, bioimaging, laser technologies, and high-resolution display systems.5,6Double perovskite oxides, characterized by the general formula A2BB′O6, offer a highly versatile platform for luminescent materials due to their tunable structural, electronic, and optical properties.7,8 The ordered cationic frameworks of these materials provide an ideal host lattice for lanthanide ion incorporation, significantly enhancing luminescent performance for applications in LEDs, plasma display panels, and non-volatile memory devices. Recent studies on Dy3+-doped double perovskites, such as Ca2MgWO6:Dy3+ and Sr2CaWO6:Dy3+, have demonstrated exceptional luminescence properties, underscoring their potential for transformative photonic applications.9–11 Among these hosts, calcium tungstate (Ca3WO6) emerges as a particularly promising candidate for Dy3+ doping, owing to its robust monoclinic crystal structure, high thermal stability, and low phonon energy. These attributes minimize non-radiative relaxation pathways, thereby enhancing luminescence efficiency. The tungstate (WO62−) polyhedral units within Ca3WO6 create an optimal crystal field environment that facilitates efficient energy transfer to Dy3+ ions, amplifying their radiative emission. However, the substitution of divalent Ca2+ with trivalent Dy3+ introduces a charge imbalance, necessitating compensatory mechanisms such as cation vacancies, interstitial ions, or co-doping strategies. These mechanisms can profoundly influence defect formation, local site symmetry, and concentration quenching, all of which are critical factors in determining the luminescent performance of the phosphor. A comprehensive understanding of these phenomena is essential for tailoring Ca3WO6:Dy3+ phosphors to meet the stringent requirements of advanced LEDs, high-resolution displays, and scintillation detectors.
This study systematically investigates the luminescent properties of Dy3+-doped Ca3WO6, with a focus on optimizing Dy3+ doping concentration and synthesis parameters to maximize emission efficiency and color tunability. The down-conversion luminescence behavior was rigorously characterized under excitation wavelengths of 278 nm, 353 nm, and 388 nm, employing photoluminescence (PL) spectroscopy, decay kinetics analysis, and CIE chromaticity coordinate evaluation. These analyses elucidate the phosphor's suitability for high-performance display technologies and white-light-emitting devices. The novelty of this work lies in achieving unprecedented luminescence efficiency and precise chromaticity control through tailored synthesis and doping strategies, paving the way for significant advancements in white LED technology and next-generation photonic systems. By leveraging the unique structural and optical properties of Ca3WO6:Dy3+, this research establishes a robust foundation for the development of transformative optical materials, poised to redefine the landscape of solid-state lighting and advanced display technologies.
2. Materials synthesis and characterization
2.1. Synthesis of Ca3WO6:Dy3+ phosphors
A series of white-emitting Ca3WO6:Dy3+ double perovskite phosphors, doped with Dy3+ concentrations ranging from 0.5 to 2.5 mol%, was synthesized via a high-temperature solid-state reaction method. Six samples, including an undoped Ca3WO6 host, were prepared, each with a 5 gram batch size. High-purity precursors such as CaCO3 (99%), WO3 (99.9%), and Dy2O3 (99.9%) were weighed in precise stoichiometric ratios. The precursors were meticulously blended and ground for 30 minutes using an agate mortar and pestle, with acetone as a mixing medium to achieve uniform dispersion. The homogenized mixture was transferred to an alumina crucible and calcined at 1200 °C for 6 hours in a muffle furnace under an ambient air atmosphere. After calcination, the samples were slowly cooled to room temperature and finely pulverized to produce high-quality phosphor powders ready for characterization.2.2. Characterization
To comprehensively investigate the structural, morphological, and luminescent properties of the as-synthesized Ca3WO6:Dy3+ phosphors, a multifaceted characterization approach was employed. Structural properties were analyzed using X-ray diffraction (XRD) on a Rigaku SmartLab SE diffractometer with Cu Kα radiation (λ = 1.5406 Å). Diffraction patterns were collected over a 2θ range of 10–70° at a scan rate of 5° min−1, ensuring precise assessment of phase purity and crystallographic parameters. Morphological features were examined via a Carl Zeiss Supra 55 scanning electron microscope (SEM), with micrographs acquired at resolutions of 200 nm, 1 μm, and 2 μm to evaluate particle size, shape, and surface morphology. Elemental composition and distribution were confirmed using energy-dispersive X-ray spectroscopy (EDAX) integrated with the SEM system. Surface chemical states were probed through X-ray photoelectron spectroscopy (XPS) on an OMICRON Multiprobe Surface Analysis System, operating at a base pressure of 5 × 10−11 torr. Photoluminescence (PL) properties were characterized using a Shimadzu Spectrofluorophotometer equipped with a xenon lamp, capturing excitation and emission spectra at ambient temperature to elucidate the phosphors' optical performance. This rigorous, systematic characterization framework provides a robust foundation for understanding the phosphors' suitability for advanced photonic applications. The external quantum yield (QY) measurements on the samples have been carried out using Stellar Net Inc., USA made Integrating Sphere (IS6) attached to the PL instrument.3. Result and discussion
3.1. XRD phase analysis
X-ray diffraction (XRD) analysis, a pivotal tool for decoding the crystalline architecture of materials, was employed to probe the structural properties of the Ca3WO6 host matrix and its Dy3+-doped variants. Powder XRD patterns were collected using a Rigaku SmartLab SE diffractometer with Cu Kα radiation (λ = 1.5406 Å), scanning a 2θ range of 10–70° at a rate of 5° min−1. Structural refinement was performed via the Rietveld method using the FullProf Suite software, ensuring precise determination of crystal phase, lattice parameters, and unit cell characteristics.The Rietveld-refined XRD pattern of undoped Ca3WO6, presented in Fig. 1, exhibits excellent agreement between experimental and calculated profiles, validating the material's structural integrity. The analysis confirmed a monoclinic crystalline structure with the P21/c space group.12 Key crystallographic parameters—lattice constants (a, b, c), unit cell volume, reliability factors (Rp, Rwp), and goodness-of-fit (χ2)—were meticulously derived and are summarized in Table 1, offering a comprehensive structural profile. The calculated unit cell volume further reinforces the stability of the monoclinic phase.
| Formula | Ca3WO6 |
| Radiation | Cu Kα |
| 2θ range | 10–70° |
| Symmetry | Monoclinic |
| Space group | P21/c |
| a (Å) | 5.54 |
| b (Å) | 5.79 |
| c (Å) | 7.98 |
| β | 90.20° |
| Rp | 2.5 |
| Rwp | 3.5 |
| χ2 | 1.9 |
| V (Å3) | 255.97 |
Fig. 2 displays the XRD patterns of undoped and Dy3+-doped Ca3WO6 phosphors, with Dy3+ concentrations ranging from 0.5 to 2.5 mol%. The sharp diffraction peaks reflect the high crystallinity and ordered atomic structure of the materials. Notably, the XRD profiles of doped phosphors closely resemble that of the undoped host, confirming that Dy3+ incorporation preserves the monoclinic P21/c structure. Subtle peak intensity variations, attributed to the electronic effects of Dy3+ ions, were observed. The average crystallite sizes, calculated for Dy3+ concentrations of 0, 0.5, 1.0, 1.5, 2.0, and 2.5 mol%, were 22.78 nm, 21.98 nm, 22.56 nm, 22.66 nm, 22.02 nm, and 22.68 nm, respectively.
To assess Dy3+ substitution compatibility, the percentage difference in ionic radii (Dr) between Dy3+ and host cations was calculated per Pires and Davolos.13 With Dr values well below the 30% threshold, the seamless integration of Dy3+ ions into the Ca3WO6 lattice was confirmed, with no significant structural distortions. These results underscore the structural robustness and crystallographic suitability of Dy3+-doped Ca3WO6 phosphors, establishing a strong foundation for their application in advanced luminescent applications.
The following eqn (1) is used to calculate the Dr:
 | (1) |
3.2. Morphological observations
The surface morphology of phosphor materials significantly governs their luminescent efficiency and optical performance. To investigate the morphological attributes of the Ca3WO6:1.5 mol% Dy3+ phosphor, selected for its exceptional photoluminescence (PL) intensity, scanning electron microscopy (SEM) was performed using a Carl Zeiss Supra 55 instrument. Micrographs were acquired at resolutions of 200 nm, 1 μm, and 2 μm, providing a detailed depiction of the material's surface topography.As shown in Fig. 3(a–d), the phosphor exhibits a heterogeneous microstructure, characterized by micro- and nano-sized grains with irregular shapes and a pronounced porous surface. This porosity likely results from the evolution of gaseous by-products during high-temperature calcination, contributing to the observed surface irregularities. Quantitative analysis of the SEM images reveals an average grain size ranging from a few nanometers to approximately 1 μm, reflecting the intricate morphological profile formed during the solid-state synthesis process.
To substantiate the morphological findings and confirm the elemental composition, energy-dispersive X-ray spectroscopy (EDAX) was conducted. The EDAX spectrum, illustrated in Fig. 3(e–j), verifies the presence of calcium (Ca), tungsten (W), oxygen (O), and dysprosium (Dy), aligning with the stoichiometric composition of Ca3WO6:1.5 mol% Dy3+. Elemental mapped images further demonstrate the uniform distribution of these constituents across the phosphor matrix, affirming the homogeneous incorporation of Dy3+ ions despite their low doping concentration. This uniform dispersion is pivotal for ensuring consistent luminescence performance. Together, the SEM and EDAX analyses validate the structural, compositional, and morphological integrity of the Ca3WO6:1.5 mol% Dy3+ phosphor, highlighting its suitability for advanced photonic applications.
3.3. X-ray photoelectron spectroscopy analysis
To elucidate the surface chemical composition and oxidation states of the Ca3WO6:1.5 mol% Dy3+ phosphor, X-ray photoelectron spectroscopy (XPS) was conducted using an OMICRON Multiprobe Surface Analysis System under ultrahigh vacuum (base pressure: 5 × 10−11 torr). The XPS survey scan, presented in Fig. 4(a), confirms the presence of Ca, W, Dy, O, and trace carbon (C), consistent with the phosphor's stoichiometric formulation and surface characteristics. | ||
| Fig. 4 (a) XPS survey spectra of Ca3WO6:1.5 mol% Dy3+ phosphor, inset shows the core-level spectra of Dy, (b–d) shows the core-level spectra of W, Ca and O, respectively. | ||
Core-level XPS spectra of the W 4f region shown in Fig. 4(b) reveal two distinct spin–orbit split peaks, corresponding to W 4f7/2 and W 4f5/2, centered at binding energies of 35.2 eV and 37.2 eV, respectively. These peak positions, sensitive to the chemical environment of tungsten atoms, indicate that tungsten predominantly exists in the +6 oxidation state (W6+, ∼90%), with a minor contribution from the +5 oxidation state (W5+, ∼10%). This distribution underscores the stability of the WO62− tungstate group within the Ca3WO6 lattice, with the minor W5+ component likely arising from localized surface defects or partial reduction during synthesis.
The Ca 2p core-level spectra shown in Fig. 4(c) exhibit two well-resolved peaks at 346.8 eV (Ca 2p3/2) and 350.4 eV (Ca 2p1/2), confirming the presence of Ca2+ ions in the phosphor matrix. For dysprosium, the Dy 4d peak, observed at a binding energy of 167 eV, aligns with the characteristic signature of Dy2O3, verifying that Dy3+ ions are successfully incorporated into the lattice. The low intensity of the Dy peak reflects the relatively low doping concentration (1.5 mol%), yet its clear detection affirms the uniform dispersion of the dopant as shown in inset of Fig. 4(a).
The O 1s core-level spectrum shown in Fig. 4(d) displays a broad peak centered at 531.2 eV, accompanied by shoulder features, indicative of multiple oxygen bonding environments. Deconvolution of the O 1s spectrum reveals four distinct contributions at 529.0 eV, 530.4 eV, 531.8 eV, and 533.2 eV, corresponding to lattice O2−, W–O bonds, surface hydroxyl groups (OH−), and adsorbed oxygen species, respectively. The dominance of the W–O peak highlights the structural integrity of the tungstate groups, while the presence of OH− and adsorbed oxygen suggests minor surface interactions with the ambient environment.
This comprehensive XPS analysis not only validates the elemental composition and chemical states of the Ca3WO6:1.5 mol% Dy3+ phosphor but also provides critical insights into its surface chemistry. The findings confirm the successful integration of Dy3+ ions and the stability of the host lattice, reinforcing the phosphor's potential for high-performance luminescent applications in advanced photonic devices.
3.4. Photoluminescence analysis
To thoroughly investigate the luminescent properties of Dy3+-activated Ca3WO6 phosphors, a comprehensive photoluminescence (PL) study was conducted across Dy3+ doping concentrations of 0.5–2.5 mol%.Photoluminescence excitation (PLE) spectra, recorded at room temperature with an emission wavelength of 576 nm, are depicted in Fig. 5. These spectra reveal a complex array of optical transitions within the 220–500 nm range, characterized by broad charge transfer bands and sharp Dy3+ intra-4f transitions. Two prominent charge transfer bands dominate the 220–350 nm region, centered at 278 nm and ∼300 nm. The 278 nm peak arises from electron transfer between the O2− 2p and Dy3+ 4f orbitals, while the ∼300 nm band is attributed to ligand-to-metal charge transfer involving O2− and W6+ ions within the Ca3WO6 host lattice.15 Additionally, distinct excitation peaks at 353 nm, 368 nm, and 388 nm correspond to Dy3+ transitions from the 6H15/2 ground state to the 6P7/2, 6P5/2, and 4I13/2 excited states, respectively, highlighting the versatility of Dy3+ as a dopant.16,17
Emission spectra of the Ca3WO6:D3+ phosphors, excited at 278 nm, 353 nm, and 388 nm, are presented in Fig. 6(a–c).18,19 Regardless of excitation wavelength, the emission profiles consistently feature two intense peaks at 485 nm and 576 nm, corresponding to the characteristic Dy3+ transitions of 4F9/2 → 6H13/2 (blue) and 4F9/2 → 6H13/2 (yellow), respectively.20–22 These radiative transitions, critical for white light emission, underscore the phosphors' potential in display and lighting applications. An energy level diagram of Dy3+ ions, shown in Fig. 7, elucidates the luminescence mechanisms, emphasizing the 4F9/2 level as the primary emissive state driving efficient visible emissions through radiative and non-radiative pathways.23,24
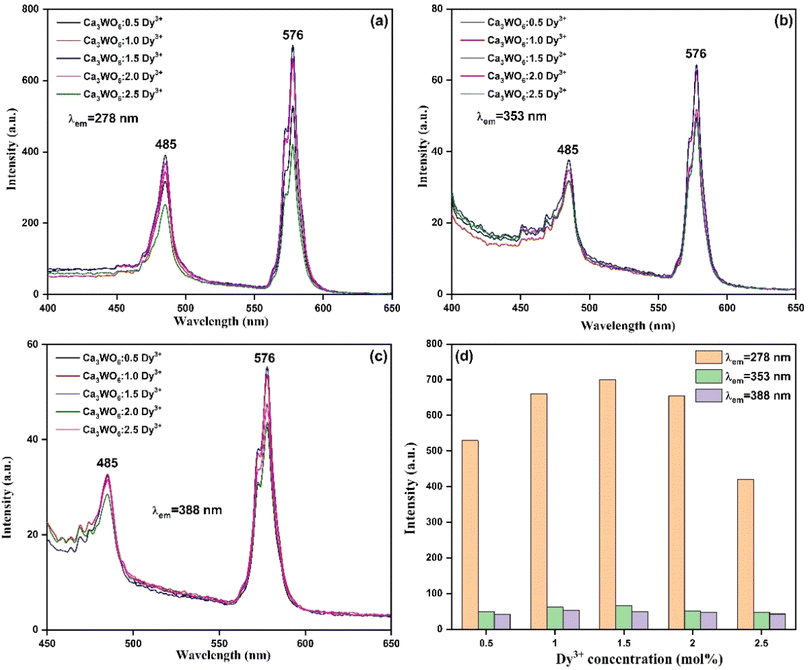 | ||
| Fig. 6 PL emission spectra of Ca3WO6:xDy3+ (x = 0.5–2.5 mol%) phosphors recorded at (a) 278 nm, (b) 353 nm, and (c) 388 nm; and (d) plot of PL intensity as a function of Dy3+ concentration. | ||
The effect of Dy3+ doping concentration on luminescence intensity was systematically explored, as illustrated in Fig. 5 and 6(a–c). Emission intensity increases with Dy3+ content, peaking at an optimal concentration of 1.5 mol%, before declining due to concentration quenching, as shown in Fig. 6(d). This quenching, pronounced at 2.0 mol% Dy3+, results from non-radiative energy transfer among closely spaced Dy3+ ions, facilitated by multipolar interactions or exchange mechanisms, which dissipate excitation energy and reduce luminescence efficiency.25 These findings highlight the critical need for precise dopant optimization to maximize the luminescent performance of Ca3WO6:Dy3+ phosphors. To identify the exact mechanism of intensity quenching, the concept of critical distance is utilized, for which the following eqn (2) was used:26,27
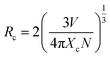 | (2) |
The interplay between the Ca3WO6 host and Dy3+ ions significantly influences the phosphors' luminescent properties. The host lattice provides an optimal crystal field environment, enabling efficient energy transfer from the WO62− tungstate groups to Dy3+ ions, which subsequently emit characteristic blue and yellow luminescence. The local symmetry and crystal field strength around Dy3+ ions, modulated by the host, further shape the emission spectra, color purity, and overall optical performance, reinforcing the phosphor's suitability for advanced photonic applications.
QY measurements reveal variations in luminescent efficiency with excitation wavelength. At 278 nm, the QY reaches 12%, indicating moderate efficiency, while at 353 nm and 388 nm, it decreases to 0.49% and 1.18%, respectively, reflecting lower efficiency at these wavelengths. In comparison, the commercial CREE phosphor achieves a QY of 37%, outperforming Ca3WO6:Dy3+. These results underscore areas for further optimization to enhance the phosphors' efficiency, positioning Ca3WO6:Dy3+ as a promising candidate for next-generation display and lighting technologies.
3.5. PL decay analysis
To unravel the relaxation dynamics and energy transfer mechanisms underpinning the luminescent behavior of Dy3+-doped Ca3WO6 phosphors, photoluminescence (PL) decay lifetime measurements were meticulously performed across samples with Dy3+ concentrations of 0.5–2.5 mol%. Decay kinetics offer profound insights into the temporal evolution of excited states, elucidating the balance between radiative and non-radiative processes within the phosphor system. Decay curves were acquired at room temperature under 278 nm excitation, with emission monitored at 576 nm, corresponding to the 4F9/2 → 6H13/2 transition of Dy3+ ions. These curves, depicted in Fig. 8 for Ca3WO6:xDy3+ (x = 0.5–2.5 mol%), were analyzed using sophisticated fitting techniques to derive precise lifetime parameters, capturing the intricate luminescence dynamics influenced by Dy3+ ion interactions within the Ca3WO6 host lattice.30,31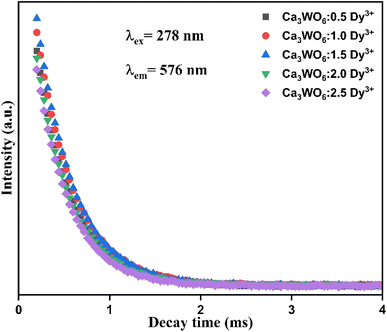 | ||
| Fig. 8 PL decay curves of Ca3WO6:xDy3+ (x = 0.5–2.5 mol%) phosphors recorded with λex = 278 nm and λem = 576 nm. | ||
The decay profiles were best described by a bi-exponential decay model, indicative of heterogeneous relaxation pathways, and fitted using the following equation:32
I(t) = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e(−t/τ1) + A2 e(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e(−t/τ2) e(−t/τ2)
| (3) |
 | (4) |
The computed average lifetimes for the Ca3WO6:Dy3+ phosphors were calculated as 0.409 ms (0.5 mol%), 0.403 ms (1.0 mol%), 0.394 ms (1.5 mol%), 0.391 ms (2.0 mol%), and 0.373 ms (2.5 mol%). A discernible trend emerges: the lifetime progressively shortens with increasing Dy3+ concentration. This reduction is primarily driven by enhanced non-radiative energy transfer among neighboring Dy3+ ions, facilitated by their reduced inter-ionic distances at higher doping levels.32 Such interactions, likely mediated by multipolar or exchange mechanisms, accelerate the depopulation of the 4F9/2 excited state, resulting in shorter lifetimes. These findings illuminate the complex decay kinetics of the Ca3WO6:Dy3+ system, highlighting the pivotal role of dopant concentration in optimizing luminescent efficiency for advanced photonic applications, such as solid-state lighting and high-resolution displays.30
3.6. Temperature-dependent photoluminescence studies
To evaluate the thermal stability and color emission sensitivity of Ca3WO6:Dy3+ phosphors for solid-state lighting and high-power energy applications, temperature-dependent PL (TDPL) studies were conducted over a temperature range of 25–200 °C. The results, presented in Fig. 9(a), reveal that the emission peak intensity at 576 nm (4F9/2 → 6H13/2) decreases steadily with rising temperature, while the peak position remains largely unchanged. Quantitative analysis indicates that the integrated emission intensity at 200 °C retains 59% of its room-temperature value, underscoring the phosphor's robust thermal stability. This intensity reduction is attributed to the diversion of excitation energy from the luminescent core toward non-radiative thermal emission pathways via lattice relaxation. These non-radiative transitions, associated with a Stokes shift, diminish PL emission due to thermal quenching effects.34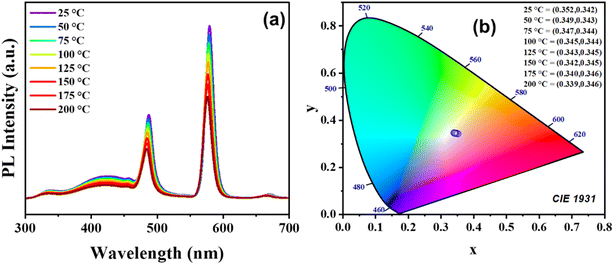 | ||
| Fig. 9 (a) Temperature dependent PL emission spectra and (b) CIE chromaticity diagram of Ca3WO6:Dy3+ phosphor. | ||
The CIE chromaticity coordinates, plotted in Fig. 9(b), exhibit a temperature-dependent shift, with the phosphor's emission approaching the ideal white light coordinates (0.33, 0.33) as temperature increases. This trend suggests that elevated temperatures enhance the chromaticity alignment with optimal white light, offering potential for tailoring the phosphor's color properties in specific applications, such as white LEDs and displays. Comparative data from the literature, summarized in Table 2, further contextualize the thermal performance of Ca3WO6:Dy3+, affirming its competitive standing among similar phosphor systems. These TDPL results highlight the phosphor's thermal resilience and tunable optical properties, positioning it as a promising candidate for high-performance luminescent applications under varying thermal conditions.
3.7. Photometric analysis
To assess the chromatic performance and applicability of Dy3+-doped Ca3WO6 phosphors in advanced lighting and display technologies, a meticulous photometric analysis was conducted by determining their Commission Internationale de l'Éclairage (CIE) chromaticity coordinates. These coordinates provide a standardized framework for evaluating color quality, enabling precise quantification of the phosphors' emitted light within the visible spectrum.38,39 Using photoluminescence (PL) emission data, CIE color coordinates were calculated for Ca3WO6:Dy3+ phosphors with Dy3+ concentrations of 0.5–2.5 mol%, under excitation wavelengths of 278 nm and 388 nm, to elucidate their chromatic behavior across varying conditions.The results, vividly depicted in Fig. 10(a and b), showcase the CIE chromaticity coordinates for the phosphors under both excitation wavelengths. Under 278 nm excitation, the Ca3WO6:1.5 mol% Dy3+ phosphor yields coordinates of (0.319, 0.343), closely aligning with the ideal white light point of (0.333, 0.333) per CIE standards.40,41 This near-ideal chromaticity highlights the phosphor's exceptional ability to produce high-quality white light, a critical attribute for next-generation display and lighting applications. Across all doping concentrations under 278 nm excitation, the CIE coordinates consistently fall within the white light region of the CIE diagram, affirming the robust white-emitting capability of the Ca3WO6:Dy3+ series. In contrast, under 388 nm excitation, the coordinates shift toward the bluish-white region, reflecting excitation-dependent variations in Dy3+ transition dynamics that subtly alter the emission type.42
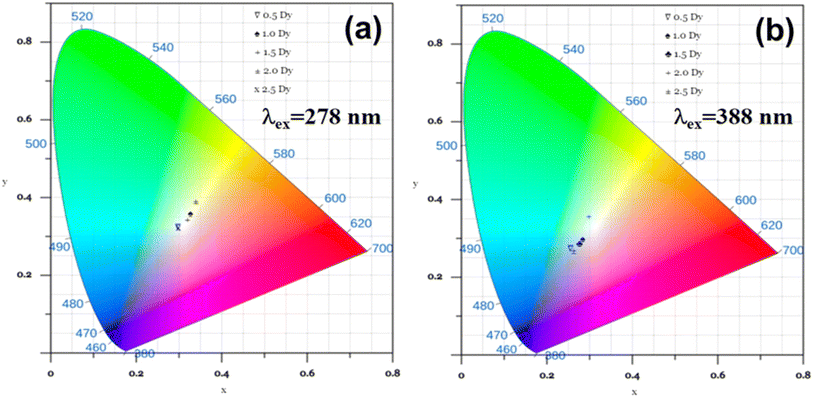 | ||
| Fig. 10 CIE color coordinates of Ca3WO6:xDy3+ (x = 0.5–2.5 mol%) phosphors estimated for (a) λex = 278 nm and (b) λex = 388 nm. | ||
The superior photometric performance under 278 nm excitation underscores the phosphors' potential as versatile candidates for optoelectronic applications. Their ability to deliver stable, tunable white light, as evidenced by their precise placement in the CIE chromaticity space, addresses the stringent requirements of modern display technologies. However, the QY of Ca3WO6:Dy3+ phosphors, particularly at longer excitation wavelengths (e.g., 0.49% at 353 nm and 1.18% at 388 nm), is relatively low compared to the 12% QY at 278 nm and the 37% QY of commercial CREE phosphors. While the moderate QY at 278 nm supports niche applications, the lower QY at longer wavelengths may limit suitability for high-efficiency LED systems, necessitating further optimization to enhance luminescent efficiency. These findings position Ca3WO6:Dy3+ phosphors as promising materials with significant potential for tailored photonic applications, provided QY improvements are achieved.
4. Conclusions
This study comprehensively validates the synthesis, structural robustness, and superior luminescent properties of Dy3+-doped Ca3WO6 phosphors, optimized across 0.5–2.5 mol% Dy3+ concentrations, establishing their potential as high-efficiency materials for advanced white light-emitting displays. X-ray diffraction with Rietveld refinement confirmed a stable monoclinic P21/c crystal structure, while SEM revealed a porous, agglomerated microstructure in the optimal 1.5 mol% Dy3+ phosphor, with particle sizes ranging from nanometers to micrometers, corroborated by EDAX's demonstration of uniform elemental distribution. XPS analysis verified Dy3+ incorporation, with the Dy 4d peak at 167 eV indicating Dy2O3 formation. Photoluminescence studies, monitored at 576 nm, displayed robust charge transfer bands at 278 nm and ∼300 nm, alongside Dy3+ intra-4f transitions, with emission spectra under 278 nm, 353 nm, and 388 nm excitations showcasing intense blue (485 nm) and yellow (576 nm) emissions, peaking at 1.5 mol% Dy3+ before concentration quenching due to multipolar interactions. Decay kinetics showed lifetimes decreasing from 0.409 ms to 0.373 ms with rising Dy3+ content, reflecting enhanced non-radiative energy transfer. CIE chromaticity coordinates under 278 nm excitation (0.319, 0.343) closely approached the ideal white point (0.333, 0.333), affirming the phosphors' exceptional suitability for advanced white light-emitting display technologies.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
P. N. K. Chaitanya – data curation, investigation, writing original-draft, D. Haranath – formal analysis, methodology, writing – review & editing, D. Dinakar – formal analysis, writing – review & editing, M. Sree Ramana – supervision, formal analysis, K. V. R. Murthy – conceptualization, supervision, M. Rakshita – formal analysis, writing – review & editing, Govind Gupta – formal analysis, writing – review & editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The author, DH, expresses profound gratitude to the Department of Science and Technology (DST), Government of India, for their generous financial support through project #CRG/2021/007142, which was instrumental in advancing this research. MR is grateful to the Council of Scientific & Industrial Research (CSIR), Government of India, for providing financial support under various project viz CSIR – SRF #09/0922(11518)/2021-EMR-I.References
- J. Xue, Y. Guo, B. K. Moon, S. H. Park, J. H. Jeong, J. H. Kim and L. Wang, Improvement of photoluminescence properties of Eu 3+ doped SrNb 2 O 6 phosphor by charge compensation, Opt. Mater., 2017, 66, 220–229, DOI:10.1016/j.optmat.2017.02.002.
- T. Grzyb, A. Szczeszak, J. Rozowska, J. Legendziewicz and S. Lis, Tunable Luminescence of Sr 2 CeO 4 :M 2+ (M = Ca, Mg, Ba, Zn) and Sr 2 CeO 4 :Ln 3+ (Ln = Eu, Dy, Tm) Nanophosphors, J. Phys. Chem. C., 2012, 116, 3219–3226, DOI:10.1021/jp208015z.
- M. H. Im and Y. J. Kim, Energy transfer and multiple photoluminescence of LuNbO4 co-doped with Eu3+ and Tb3+, Mater. Res. Bull., 2019, 112, 399–405, DOI:10.1016/j.materresbull.2019.01.009.
- R. G. Kunghatkar, V. L. Barai and S. J. Dhoble, Synthesis route dependent characterizations of CaMgP2O7: Gd3+ phosphor, Results Phys., 2019, 13, 102295, DOI:10.1016/j.rinp.2019.102295.
- V. M. Krishna, S. Mahamuda, R. A. Talewar, K. Swapna, M. Venkateswarlu and A. S. Rao, Dy3+ ions doped oxy-fluoro boro tellurite glasses for the prospective optoelectronic device applications, J. Alloys Compd., 2018, 762, 814–826, DOI:10.1016/j.jallcom.2018.05.191.
- C. Wei, D. Xu, Z. Yang, Y. Jia, X. Li and J. Sun, Luminescence and energy transfer of Tm3+ and Dy3+ co-doped Na3 ScSi2 O7 phosphors, RSC Adv., 2019, 9, 27817–27824, 10.1039/C9RA04727A.
- Q. Sun, S. Wang, L. Sun, J. Liang, B. Devakumar and X. Huang, Achieving full-visible-spectrum LED lighting via employing an efficient Ce3+-activated cyan phosphor, Mater. Today Energy, 2020, 17, 100448, DOI:10.1016/j.mtener.2020.100448.
- C. Wei, D. Xu, J. Li, A. Geng, X. Li and J. Sun, Synthesis and luminescence properties of Eu3+-doped a novel double perovskite Sr2YTaO6 phosphor, J. Mater. Sci.: Mater. Electron., 2019, 30, 2864–2871, DOI:10.1007/s10854-018-0563-2.
- K. Li and R. Van Deun, Obtaining Efficiently Tunable Red Emission in Ca3−δ Ln δ WO6 :Mn4+ (Ln = La, Gd, Y, Lu, δ = 0.1) Phosphors Derived from Nearly Nonluminescent Ca3 WO6 :Mn4+ via Ionic Substitution Engineering for Solid-State Lighting, ACS Sustain. Chem. Eng., 2020, 8, 7256–7261, DOI:10.1021/acssuschemeng.0c02444.
- X. Zhao, J. Wang, L. Fan, Y. Ding, Z. Li, T. Yu and Z. Zou, Efficient red phosphor double-perovskite Ca3WO6 with A-site substitution of Eu3+, Dalton Trans., 2013, 42, 13502, 10.1039/c3dt51029h.
- T. H. Q. Vu, D. Stefańska and P. J. Dereń, Effect of A-Cation Radius on the Structure, Luminescence, and Temperature Sensing of Double Perovskites A2MgWO6 Doped with Dy3+ (A = Ca, Sr, Ba), Inorg. Chem., 2023, 62, 20020–20029, DOI:10.1021/acs.inorgchem.3c02798.
- N. Degda, N. Patel, V. Verma, K. V. R. Murthy and M. Srinivas, Luminescence and dosimetry approach in terbium(III)-activated tungstate double perovskite, Luminescence, 2024, 39, e4622, DOI:10.1002/bio.4622.
- G. Hu, S. Yi, Z. Fang, Z. Hu and W. Zhao, Luminescence properties of high thermal stability Sr2LaNbO6:xLn3+(Ln3+=Eu3+/Sm3+) phosphors with double-perovskite structures, Opt. Mater., 2019, 98, 109428, DOI:10.1016/j.optmat.2019.109428.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A, 1976, 32, 751–767, DOI:10.1107/S0567739476001551.
- N. Prabhu, S. Agilan, N. Muthukumarasamy and T. S. Senthil, Enhanced photovoltaic performance of WO3 nanoparticles added dye sensitized solar cells, J. Mater. Sci.: Mater. Electron., 2014, 25, 5288–5295, DOI:10.1007/s10854-014-2303-6.
- Z. Yang, Y. Liu, C. Liu, F. Yang, Q. Yu, X. Li and F. Lu, Multiwavelength excited white-emitting Dy3+ doped Sr3Bi(PO4)3 phosphor, Ceram. Int., 2013, 39, 7279–7283, DOI:10.1016/j.ceramint.2013.02.044.
- L. Fu, X. Yang, Z. Fu, Z. Wu and J. H. Jeong, Hydrothermal synthesis and tunable luminescence of CaSiO3:RE3+(RE3+=Eu3+, Sm3+, Tb3+, Dy3+) nanocrystals, Mater. Res. Bull., 2015, 65, 315–319, DOI:10.1016/j.materresbull.2015.01.060.
- T. Sh. Atabaev, H. H. T. Vu, H.-K. Kim and Y.-H. Hwang, Synthesis and optical properties of Dy3+-doped Y2O3 nanoparticles, J. Korean Phys. Soc., 2012, 60, 244–248, DOI:10.3938/jkps.60.244.
- A. K. Vishwakarma, K. Jha, M. Jayasimhadri, B. Sivaiah, B. Gahtori and D. Haranath, Emerging cool white light emission from Dy3+ doped single phase alkaline earth niobate phosphors for indoor lighting applications, Dalton Trans., 2015, 44, 17166–17174, 10.1039/C5DT02436F.
- P. Muralimanohar, G. Srilatha, K. Sathyamoorthy, P. Vinothkumar, M. Mohapatra and P. Murugasen, Preparation and luminescence properties of Dy3+ doped BaAlBO3F2 glass ceramic phosphor for solid state white LEDs, Optik, 2021, 225, 165807, DOI:10.1016/j.ijleo.2020.165807.
- H. Wu, Z. Sun, S. Gan and L. Li, Luminescence properties of Dy3+ or/and Sm3+ doped LiLa(WO4)2 phosphors and energy transfer from Dy3+ to Sm3+, Solid State Sci., 2018, 85, 48–53, DOI:10.1016/j.solidstatesciences.2018.09.013.
- Y. Lian, Y. Wang, J. Li, Z. Zhu, Z. You, C. Tu, Y. Xu and W. Jie, Structural and optical properties of Dy3+:YAlO3 phosphors for yellow light-emitting diode applications, J. Rare Earths, 2021, 39, 889–896, DOI:10.1016/j.jre.2020.06.012.
- Q. Xu, J. Sun, D. Cui, Q. Di and J. Zeng, Synthesis and luminescence properties of novel Sr3Gd(PO4)3:Dy3+ phosphor, J. Lumin., 2015, 158, 301–305, DOI:10.1016/j.jlumin.2014.10.034.
- M. Gao, K. Li, Y. Yan, S. Xin, H. Dai, G. Zhu and C. Wang, Novel thermally robust warm white light emitting phosphor Ca18Li3Y(PO4)14:Dy3+: Synthesis, crystal structure and luminescence property investigation, J. Mol. Struct., 2021, 1228, 129471, DOI:10.1016/j.molstruc.2020.129471.
- J. Zhao, D. Zhao, Z. Ma, M.-J. Ma, B.-Z. Liu, W.-J. Guo and G.-Y. Wang, Synthesis and photoluminescent properties of orange-emitting Sm3+-activated KPb4(PO4)3 phosphor for LEDs, Displays, 2019, 59, 16–20, DOI:10.1016/j.displa.2019.05.003.
- N. Jain, B. P. Singh, R. K. Singh, J. Singh and R. A. Singh, Enhanced photoluminescence behaviour of Eu3+ activated ZnMoO 4 nanophosphors via Tb3+ co-doping for light emitting diode, J. Lumin., 2017, 188, 504–513, DOI:10.1016/j.jlumin.2017.05.007.
- N. P. Patel, N. Degda, V. Verma, K. V. R. Murthy and M. Srinivas, Thermally stable photoluminescence of Eu3+ doped strontium pyrophosphate: red emitting phosphor with high color purity, New J. Chem., 2024, 48, 5399–5411, 10.1039/D3NJ03994C.
- Y. Zhou, Y. Li, H. Wu, X. Li and M. Gou, High temperature persistent luminescence in Tb3+ doped CaSr2Al2O6 phosphor, Optik, 2021, 242, 167103, DOI:10.1016/j.ijleo.2021.167103.
- H. Ait ahsaine, M. Ezahri, A. Benlhachemi, B. Bakiz, S. Villain, J.-C. Valmalette, F. Guinneton, M. Arab and J.-R. Gavarri, Structural, vibrational study and UV photoluminescence properties of the system Bi(2−x)Lu(x)WO6(0.1 ≤ x ≤ 1), RSC Adv., 2015, 5, 96242–96252, 10.1039/C5RA19424E.
- C. Kumari, R. Gopal, H. Yadav and J. Manam, SrNb2O6: Dy3+: a single phase warm white light emitting phosphor for solid-state lighting, J. Mater. Sci.: Mater. Electron., 2024, 35, 638, DOI:10.1007/s10854-024-12396-9.
- R. Gopal, A. Kumar and J. Manam, Enhanced photoluminescence and abnormal temperature dependent photoluminescence property of SrWO4:Dy3+ phosphor by the incorporation of Li+ ion, Mater. Chem. Phys., 2021, 272, 124960, DOI:10.1016/j.matchemphys.2021.124960.
- J. Dalal, M. Dalal, S. Devi, A. Hooda, A. Khatkar, V. B. Taxak and S. P. Khatkar, Radiative and non-radiative characteristics of Ca9Bi(PO4)7:Eu3+ nano-phosphor for solid state lighting devices, J. Lumin., 2019, 216, 116697, DOI:10.1016/j.jlumin.2019.116697.
- S. Liu, J. He, Z. Wu, J. H. Jeong, B. Deng and R. Yu, Preparation and study on the spectral properties of garnet-type Li3Gd3Te2O12:Dy3+ single-phase full-color phosphor, J. Lumin., 2018, 200, 164–168, DOI:10.1016/j.jlumin.2018.03.089.
- M. Rakshita, A. A. Sharma, P. P. Pradhan, K. A. K. Durga Prasad, K. Jayanthi and D. Haranath, Highly efficient and self-activating Zn3V2O8 phosphor for the fabrication of cool-white light emitting devices, Ceram. Int., 2023, 49, 16775–16785, DOI:10.1016/j.ceramint.2023.02.038.
- K. Poria, R. Lohan, S. Bhatia, A. Kumar, R. Singh, N. Deopa, R. Punia, J. S. Shahi and A. S. Rao, Lumino-structural properties of Dy3+ activated Na3Ba2LaNb10O30 phosphors with enhanced internal quantum yield for w-LEDs, RSC Adv., 2023, 13, 11557–11568, 10.1039/d3ra01260c.
- C. Y. Chang, T. H. Hsu and C. L. Huang, Novel and thermostable double-perovskite La2ZnTiO6: Sm3+, Dy3+ phosphors with high quantum efficiency, Opt. Mater., 2023, 135, 113361, DOI:10.1016/J.OPTMAT.2022.113361.
- S. Yang, Y. Dai, Y. Shen, C. Duan, Q. Rao, H. Peng, F. Yang, Y. Shan and Q. Zhao, Blue emission from Sr0.98Ga2B2O7: 0.01Bi3+, 0.01Dy3+ phosphor with high quantum yield, J. Alloys Compd., 2019, 810, 151849, DOI:10.1016/J.JALLCOM.2019.151849.
- K. Mariselvam and J. Liu, Judd-Ofelt analysis and visible luminescence of Sm3+: MCZBP glass for reddish-orange laser and multi-colour display applications, Solid State Sci., 2021, 115, 106606, DOI:10.1016/j.solidstatesciences.2021.106606.
- B. Fan, J. Liu, W. Zhou and L. Han, Luminescence properties of new red-emitting phosphor Li2Al2Si3O10:Eu3+ for near UV-based white LED, Opt. Mater., 2019, 98, 109499, DOI:10.1016/j.optmat.2019.109499.
- B. C. Jamalaiah, M. Jo, J. Zehan, J. J. Shim, S. Il Kim, W.-Y. Chung and H. J. Seo, Luminescence, energy transfer and color perception studies of Na3Gd(PO4)2:Dy3+:Tm3+ phosphors, Opt. Mater., 2014, 36, 1688–1693, DOI:10.1016/j.optmat.2014.01.016.
- D. Zhu, M. Liao, Z. Mu and F. Wu, Preparation and Luminescence Properties of Ca9NaZn(PO4)7:Dy3+ Single-Phase White Light-Emitting Phosphor, J. Electron. Mater., 2018, 47, 4840–4844, DOI:10.1007/s11664-018-6380-9.
- G. Zhang, L. Zhao, F. Fan, Y. Bai, B. Ouyang, W. Chen, Y. Li and L. Huang, Near UV-pumped yellow-emitting Ca3TeO6:Dy3+ phosphor for white light-emitting diodes, Spectrochim. Acta, Part A, 2019, 223, 117343, DOI:10.1016/j.saa.2019.117343.
| This journal is © The Royal Society of Chemistry 2025 |





