DOI:
10.1039/D5RA02615F
(Paper)
RSC Adv., 2025,
15, 18577-18592
Synthesis and anti-SARS-CoV-2 potential of novel coumarin hybrids: a combined wet/dry lab approach targeting MPro, Nsp15 and spike protein†
Received
14th April 2025
, Accepted 23rd May 2025
First published on 3rd June 2025
Abstract
This study focuses on the synthesis of novel hybrids with a coumarin scaffold as potential SARS-CoV-2 inhibitors. All the novel coumarin-1,2,4-triazole hybrids 14(a–h) and phenylacetamide linked coumarin derivatives 17(a–h) were synthesized by following a standard procedure in good to excellent yields i.e., 51–75% for 14(a–h) and 62–82% for 17(a–h). The synthesized derivatives were subjected to in silico modelling to evaluate their anti-SARS-CoV-2 potential, targeting Mpro (main protease), Nsp15 (nonstructural protein) and spike protein. Among all, compounds 14b and 14c expressed excellent potency against their respective targets with corresponding binding affinities of −9.5 kcal mol−1 (6VWW), −9.2 kcal mol−1 (6Y84), and −8.6 (6WPT) kcal mol−1, even better than all standards i.e., chloroquine, lopinavir, remdesivir, favipiravir, and nirmatrelvir. The stability of the potent compounds (14b and 14c) was further supported by a 100 ns MD simulation, emphasizing their potent and stable interactions with the main protease, endoribonuclease, and spike protein. The current study highlights the coumarin-based conjugates 14(a–h) and 17(a–h) as attractive and promising candidates for future pharmacological interventions against SARS-CoV-2.
1 Introduction
Right from the outset of 2020, a testing situation emerged due to the SARS-CoV-2 (coronavirus) pandemic. This deadly disease affected 774.47 million people around the globe leading to more than 7.03 million deaths.1 The global healthcare and socio-economic structure have been disrupted significantly due to COVID-19.2 The healthcare facilities turned out to be overburdened by the immense responsibility of treating affected people, resulting in a divergence of resources and attention from other important medical needs.3–9 According to phylogenic research, the main contributory agent of coronavirus is from the sarbecovirus subgenus (genus β-coronavirus).10,11 It was concluded from different research that the transmission of this deadly pathogenic virus is controlled by ACE2 (host receptor) and RBD (spike protein).12–20 SARS-CoV-2 had the ability to undergo persistent mutations that spread around the world during COVID-19.21–25 The new subvariants of COVID (Omicron, delta, beta, and alpha) attenuate the proficiency of known antibody treatment together with antibody-mediated immunity, established via infection and vaccination. Therefore, increasing number of these variants emphasize the need for development of new coronavirus inhibitors.26 The coronavirus life cycle commences with attachment of spike protein (S protein) with host ACE2 (angiotensin-converting enzyme 2) receptors. The viral genome causes the translation of Nsps (16 non-structural proteins) by confining the host ribosomes and cause the proteolytic cleavage by PLpro and 3Clpro. The sub genome (synthesized from transcription of +ssRNA) translates the structural protein i.e., nucleocapsid (N), membrane protein (M), spike protein (S), and envelop (E). The mature protein and +ssRNA assemble to form new virion after successful transcription and replication of genome. Therefore, main protease, Nsps and spike protein make substantial contribution in transmission and replication. Hence, blocking the activities of these proteins may provide an intriguing therapeutic agent for the treatment of SARS-CoV-2.27–29 Few traditional and FDA-approved drugs (chloroquine 1,30–32 lopinavir 2,33–35 remdesivir 3,36–38 favipiravir 4,39–41 and nirmatrelvir 5 (ref. 42–44)) (Fig. 1) have been known to provide some relief to combat coronavirus, however, there is no particular drug synthesized specifically to combat COVID-19.
 |
| | Fig. 1 Structures of important drugs used to combat SARS-CoV-2. | |
The in silico modelling analysis brought about a seismic shift in rational drug design by providing a time-effective and economical solution to conventional experimental techniques. By exploiting computational tools, researchers can quickly screen extensive libraries of compounds, detect lead compounds and can enhance their pharmacological features.45 These strategies facilitate the prediction of toxicity, binding affinities, and pharmacokinetic profiles.46,47 Additionally, in silico modelling approach assists the machine learning algorithms that further enhances the scope of this methodology in drug discovery process.48
The heterocyclic compounds49–51 are important naturally occurring structural motifs52 found in biologically and pharmaceutically important compounds.53 Literature survey revealed that these derivatives can act as neuroprotective agents,54 pesticide agents,55 anti-Alzheimer agents,56 anti-fungal agents,57 anti-oxidative agents,58 anti-tyrosinase agents,59,60 anti-mycobacterium tuberculosis agents,61 and anti-cancer agents.62 The heterocyclic coumarin scaffolds have also been exploited for designing and synthesizing potential anti-SARS-CoV-2 inhibitors.63–65 Considering the biological and pharmaceutical potential of coumarin derivatives, we have synthesized novel coumarin hybrids in good to excellent yields. In silico computer-aided molecular-docking strategy enables swift-identification of inhibitory potential of targeted molecules. Therefore, all the synthesized coumarin-based conjugates were analyzed using molecular docking and MD simulation. The complete set of these novel-hybrids were tested against different targets such as 6Y84 (main protease (Mpro)), 6VWW (Nsp15), and 6WPT (spike protein). These findings may lead to in-future pre-clinical investigations owing to the anti-viral potential of these conjugates against SARS-CoV-2.
2 Result and discussion
2.1. General protocol for construction of thio-linked triazole coumarin hybrids (14a–h)
Initially, resorcinol 6 (1 equivalent) was made to react and stir with ethyl acetoacetate (1 equivalent) in sulfuric acid (few drops) to afford substrate 7 in 73% yield. In the next step, 7-hydroxy-4-methylcoumarin 7 (1 equivalent) and bromoacetylbromide (1 equivalent) were allowed to stir in chloroform (15 mL) and pyridine (1 equivalent) for 16 h to yield bromoacetyl derivative of coumarin 8 (84% yield). By a reaction between methyl alcohol (1 equivalent) and various substituted carboxylic acid 9(a–h) (1 equivalent), different esters 10(a–h) were afforded in 72–77% yields. Esters 10(a–h) were further converted into hydrazides 11(a–h) (85–89% yield) by reacting them with hydrazine hydrate in the presence of ethanol. Hydrazides 11(a–h) were reacted with phenylisothiocyanate in DCM to afford intermediate 12 followed by refluxing it with aqueous sodium hydroxide to yield differently substituted triazoles 13(a–h) in 75–80% yields.51 In the last step, triazoles 13(a–h) were reacted with bromoacetyl derivative of coumarin 8 in the presence of DCM to afford targeted triazole–coumarin hybrids 14(a–h). After the reaction completion, n-hexane and water were added to get products' precipitates. These precipitates were recrystallized with ethanol to yield pure targeted coumarin–triazoles hybrids 14(a–h) in good yields (51–75%) (Scheme 1, Fig. 2).
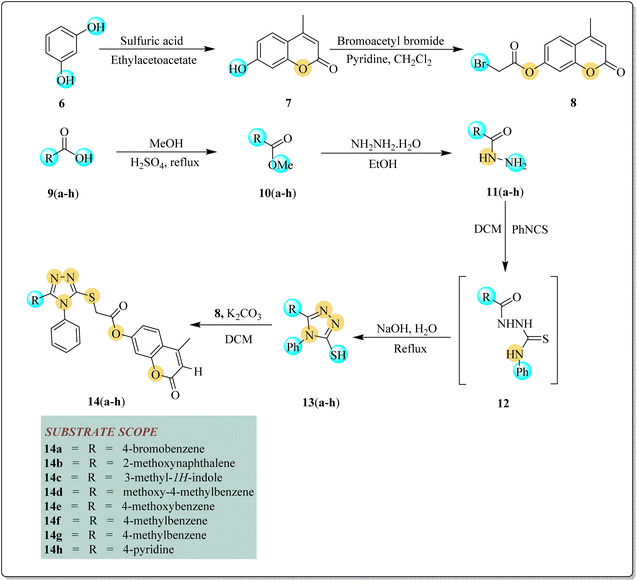 |
| | Scheme 1 Synthesis of thio-linked triazole coumarin hybrids 14(a–h). | |
 |
| | Fig. 2 Substrate scope of coumarin–triazole hybrids 14(a–h). | |
2.2. Synthesis of phenylacetamide linked coumarin derivatives 17(a–h)
A two-step synthetic approach was applied to afford unreported phenylacetamide linked coumarin derivatives 17(a–h). In the first step, a series of variously substituted 2-bromo-N-phenylacetamides 16(a–h) were yielded by the reaction of bromo acetylbromide and different anilines 15(a–h) in DCM and pyridine. The stirring of 7-hydroxy-4-methyl coumarin 7 with the substituted 2-bromo-N-phenylacetamide 16(a–h) in the presence of K2CO3 and DMF (dimethylformamide) yielded the corresponding phenyl acetamide linked coumarin derivatives 17(a–h) in good yields (62–82%), as outlined in Scheme 2 (Fig. 3).
 |
| | Scheme 2 Reaction protocol of phenylacetamide linked coumarin derivatives 17(a–h). Reagents and conditions: (a) 2-bromoacetyl bromide, pyridine, DCM, stirring at 30 °C (b) K2CO3, dimethylformamide (DMF). | |
 |
| | Fig. 3 Substrate scope of phenylacetamide linked coumarin derivatives 17(a–h). | |
2.3. Ligand–protein interactions analysis of 6Y84 MPro (main protease)
SARS-CoV-2 depends on main protease for effective gene expression and replication.66 The suppression of main protease (Mpro) is considered as an efficient route and strategic approach for the treatment of corona virus.67 The inhibitory potential of all newly synthesized derivatives 14(a–h) & 17(a–h) was assessed through molecular docking.68 The poses with lowest binding scores were selected for interactions study and their binding affinities were compared with already known inhibitors (chloroquine,69 lopinavir,70 remdesivir,71 favipiravir,72 and nirmatrelvir73) potent against SARS-CoV-2. From the results (Table 1), 14c (−9.2 Kcal mol−1) displayed exceptional binding with Mpro even better than standards i.e., chloroquine (−5.8 Kcal mol−1), lopinavir −7.1 Kcal mol−1), remdesivir (−7.6 Kcal mol−1), favipiravir (−5.0 Kcal mol−1) and nirmatrelvir (−8.8 Kcal mol−1).
Table 1 Docking scores of compounds (14(a–h) & 17(a–h)) with binding sites of 6Y84
| Sr. no. |
Compounds |
Docking score (Kcal mol−1) |
| 1 |
14a |
−7.8 |
| 2 |
14b |
−8.7 |
| 3 |
14c |
−9.2 |
| 4 |
14d |
−8.2 |
| 5 |
14e |
−7.9 |
| 6 |
14f |
−8.0 |
| 7 |
14g |
−7.2 |
| 8 |
14h |
−7.4 |
| 9 |
17a |
−7.9 |
| 10 |
17b |
−7.4 |
| 11 |
17c |
−7.5 |
| 12 |
17d |
−7.9 |
| 13 |
17e |
−7.8 |
| 14 |
17f |
−7.7 |
| 15 |
17g |
−7.3 |
| 16 |
17h |
−7.4 |
| 17 |
Chloroquine |
−5.8 |
| 18 |
Lopinavir |
−7.1 |
| 19 |
Remdesivir |
−7.6 |
| 20 |
Favipiravir |
−5.0 |
| 21 |
Nirmatrelvir |
−8.8 |
The comprehensive evaluation of compound 14c with active site of 6Y84 (ref. 74) revealed that the carbonyl group of 14c interacted with LYS100 (1.73 Å) and TYR101 (2.63 Å) via carbon–hydrogen as well as conventional H-bonding interactions, respectively. The aromatic rings of the synthesized hybrid 14c were revealed to contribute in pi–alkyl and pi–sigma associations with VAL157 and LYS97 having bond distances of 5.22 Å and 2.21 Å, which play a significant role towards the inhibition of SARS-CoV-2.75–80 (Fig. 4 & 5). In comparison, standards i.e., chloroquine, lopinavir, remdesivir, favipiravir and nirmatrelvir displayed strong hydrogen bonding interactions with ARG298, THR111, THR292, GLN110, ASN151, SER158, ARG4, SER284, TRP207, and ASP289. These standards also established alkyl, π–alkyl, π–π sigma, and pi–pi stacked hydrophobic interactions with PHE294, VAL104, VAL297, VAL303, LYS5, PHE291 and LEU286 (Table 2) (S49, ESI†).
 |
| | Fig. 4 Interactions of 14c with binding pocket of 6Y84. | |
 |
| | Fig. 5 (A–C) 3D binding interactions of 14c with 6Y84 receptor. (D) 2D representation of binding analysis of 14c with 6Y84 receptor. | |
Table 2 Ligand–protein interactions study of 14(a–h) & 17(a–h) with binding sites of 6Y84
| Ligand–protein interactions (6Y84) |
| Sr. no. |
Compounds |
Hydrophobic interactions |
Hydrogen bonding interactions |
| Interacted residues |
Interactions |
Distance (Å) |
Interacted residues |
Interactions |
Distance (Å) |
| 1 |
14c |
VAL157 |
π–Alkyl |
5.22 |
LYS100 |
C–H bonding |
1.73 |
| |
|
LYS97 |
π–Sigma, π–alkyl |
2.21, 4.24 |
TYR101 |
Conventional H-bonding |
2.63 |
| 2 |
Chloroquine |
PHE294 |
π–π stacked |
4.30 |
ARG298 |
Conventional H-bonding |
2.83 |
| |
|
VAL104 |
Alkyl |
4.66 |
THR111 |
C–H bonding |
3.40 |
| |
|
|
|
|
THR292 |
C–H bonding |
3.69 |
| |
|
|
|
|
GLN110 |
Conventional H-bonding |
2.38 |
| 3 |
Lopinavir |
PHE294 |
π–Alkyl |
5.21 |
ARG298 |
Conventional H-bonding |
2.41 |
| |
|
VAL297 |
π–Alkyl |
4.74 |
|
|
|
| |
|
VAL104 |
Alkyl |
4.59 |
|
|
|
| |
|
VAL303 |
π–Sigma |
5.11 |
|
|
|
| 4 |
Remdesivir |
PHE294 |
π–π stacked |
3.91 |
THR111 |
C–H bonding |
2.59 |
| |
|
VAL297 |
π–Alkyl |
4.45 |
ASN151 |
Conventional H-bonding |
2.73 |
| |
|
|
|
|
SER158 |
C–H bonding |
3.43 |
| 5 |
Favipiravir |
|
|
|
ARG298 |
Conventional H-bonding |
2.79 |
| |
|
|
|
|
GLN110 |
Conventional H–Bonding |
2.77 |
| 6 |
Nirmatrelvir |
LYS5 |
Alkyl |
4.02 |
ARG4 |
C–H bonding |
3.46 |
| |
|
PHE291 |
Alkyl |
4.68 |
SER284 |
C–H bonding |
3.34 |
| |
|
LEU286 |
Alkyl |
4.37 |
TRP207 |
Conventional H-bonding |
2.28 |
| |
|
|
|
|
ASP289 |
Conventional H-bonding |
1.98 |
2.4. Ligands–protein interactions analysis of 6VWW endoribonuclease (Nsp15)
The 6VWW endoribonuclease (Nsp15) is accountable for the lysis of viral RNA especially in the case of COVID-19 and assumes a crucial function in eluding innate response of host.81 The synthesized compounds 14(a–h) & 17(a–h) were subjected to molecular docking with 6VWW.82 As revealed by docking analysis (Table 3), it was found that compound 14b displayed exceptional binding affinity (−9.5 Kcal mol−1) with the active site of endoribonuclease in comparison with the reference ligands i.e., chloroquine,69 lopinavir,70 remdesivir,71 favipiravir,72 and nirmatrelvir,73 whose docking scores were observed to be ranging from −6.1 to −9.2 Kcal mol−1.
Table 3 Docking scores of compounds (14(a–h) & 17(a–h)) with binding sites of 6VWW
| Sr. no. |
Compounds |
Docking score (Kcal mol−1) |
| 1 |
14a |
−8.4 |
| 2 |
14b |
−9.5 |
| 3 |
14c |
−9.1 |
| 4 |
14d |
−8.9 |
| 5 |
14e |
−7.2 |
| 6 |
14f |
−8.3 |
| 7 |
14g |
−8.0 |
| 8 |
14h |
−8.2 |
| 9 |
17a |
−7.7 |
| 10 |
17b |
−7.6 |
| 11 |
17c |
−7.6 |
| 12 |
17d |
−8.0 |
| 13 |
17e |
−8.1 |
| 14 |
17f |
−7.6 |
| 15 |
17g |
−6.9 |
| 16 |
17h |
−7.2 |
| 17 |
Chloroquine |
−6.1 |
| 18 |
Lopinavir |
−7.6 |
| 19 |
Remdesivir |
−8.9 |
| 20 |
Favipiravir |
−6.1 |
| 21 |
Nirmatrelvir |
−9.2 |
The ligand–protein interactions disclosed that the ASN200 interacted with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of 14b via conventional hydrogen bonding at the corresponding distance of 2.75 Å. In addition, coumarin core of 14b established conventional hydrogen bonding as well as C–H interactions with LYS71 (2.62 Å) and SER198 (3.22 Å), respectively. The naphthyl and coumarin core of 14b were found to be engaged with VAL295 (4.82 Å), LEU252 (3.63 Å), LYS277 (4.38 Å), LEU266 (5.25 Å), LEU201 (4.47 Å) and PHE259 (5.86 Å) via pi–alkyl, alkyl and pi–pi T-shaped hydrophobic interactions (Fig. 6 & 7).83–85 In comparison, standards i.e., chloroquine, lopinavir, remdesivir, favipiravir, and nirmatrelvir established C–H and conventional hydrogen bonding interactions with LYS71, GLY165, LYS90, ARG199, LYS277, ASP268, SER198, ASN200, SER274, LEU201 and GLN202 (Table 4). Moreover, all of them also interacted with LYS277, LEU252, VAL295, LYS90, TYR279, ARG91, LEU266, SER198, LEU201 and LYS277 via hydrophobic interactions (Fig. S50, ESI†).
O group of 14b via conventional hydrogen bonding at the corresponding distance of 2.75 Å. In addition, coumarin core of 14b established conventional hydrogen bonding as well as C–H interactions with LYS71 (2.62 Å) and SER198 (3.22 Å), respectively. The naphthyl and coumarin core of 14b were found to be engaged with VAL295 (4.82 Å), LEU252 (3.63 Å), LYS277 (4.38 Å), LEU266 (5.25 Å), LEU201 (4.47 Å) and PHE259 (5.86 Å) via pi–alkyl, alkyl and pi–pi T-shaped hydrophobic interactions (Fig. 6 & 7).83–85 In comparison, standards i.e., chloroquine, lopinavir, remdesivir, favipiravir, and nirmatrelvir established C–H and conventional hydrogen bonding interactions with LYS71, GLY165, LYS90, ARG199, LYS277, ASP268, SER198, ASN200, SER274, LEU201 and GLN202 (Table 4). Moreover, all of them also interacted with LYS277, LEU252, VAL295, LYS90, TYR279, ARG91, LEU266, SER198, LEU201 and LYS277 via hydrophobic interactions (Fig. S50, ESI†).
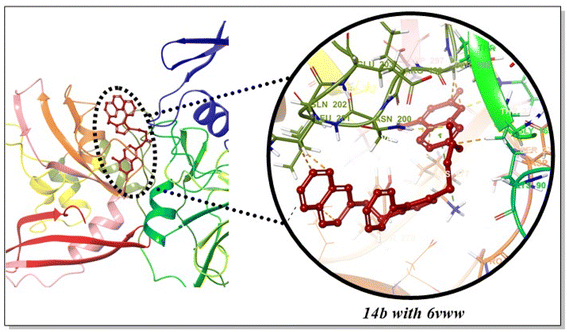 |
| | Fig. 6 Interactions of 14b with 6VWW binding pocket. | |
 |
| | Fig. 7 (A–C) 3D binding interactions of 14b with active site of 6VWW. (D) 2D representation of binding analysis of 14b with 6VWW receptor. | |
Table 4 Ligand–protein interactions study of (14(a–h) & 17(a–h)) with binding sites of 6VWW (Nsp15)
| Ligand–protein interactions (6VWW) |
| Sr. no. |
Compounds |
Hydrophobic interactions |
Hydrogen bonding interactions |
| Interacted residues |
Interactions |
Distance (Å) |
Interacted residues |
Interactions |
Distance (Å) |
| 1 |
14b |
VAL295 |
Alkyl |
4.82 |
ASN200 |
Conventional H-bonding |
2.75 |
| |
|
LEU252 |
Alkyl |
3.63 |
LYS71 |
Conventional H-bonding |
2.62 |
| |
|
LYS277 |
Pi–Alkyl |
4.38 |
SER198 |
C–H bonding |
3.22 |
| |
|
LEU266 |
Pi–Alkyl |
5.25 |
|
|
|
| |
|
LEU201 |
Pi–Alkyl |
4.47 |
|
|
|
| |
|
PHE259 |
Pi–Pi T-shaped |
5.86 |
|
|
|
| 2 |
Chloroquine |
LYS277 |
Pi–Alkyl |
3.73 |
LYS71 |
Conventional H-bonding |
2.80 |
| |
|
LEU252 |
Pi–Alkyl |
3.46 |
GLY165 |
C–H bonding |
3.74 |
| |
|
VAL295 |
Alkyl |
4.53 |
|
|
|
| |
|
LYS90 |
Alkyl |
4.04 |
|
|
|
| 3 |
Lopinavir |
TYR279 |
Alkyl |
5.04 |
LYS90 |
Conventional H-bonding |
2.42 |
| |
|
ARG91 |
Pi–Alkyl |
3.68 |
ARG199 |
Van der Waals |
|
| |
|
LEU266 |
Pi–Sigma |
3.52 & 4.92 |
LYS277 |
C–H bonding |
3.10 |
| |
|
SER198 |
Amide Pi–alkyl |
4.53 |
|
|
|
| |
|
LEU201 |
Alkyl |
3.81 |
|
|
|
| |
|
LYS90 |
Pi–Alkyl |
5.83 |
|
|
|
| 4 |
Remdesivir |
ARG91 |
Alkyl |
3.36 |
ASP268 |
Conventional hydrogen bond |
3.22 |
| |
|
LEU266 |
Alkyl |
5.20 |
LYS90 |
Conventional hydrogen bond |
2.48 |
| |
|
LEU201 |
Alkyl |
4.32 |
SER198 |
Conventional hydrogen bond |
3.34 |
| |
|
LYS277 |
π–Alkyl |
3.73 |
ASN200 |
Conventional hydrogen bond |
2.59 |
| 5 |
Favipiravir |
LYS90 |
π–Alkyl |
5.01 |
LYS71 |
Conventional hydrogen bond |
3.54 |
| |
|
|
|
|
SER274 |
Conventional hydrogen bond |
2.30 |
| |
|
|
|
|
ARG199 |
Conventional hydrogen bond |
2.34 |
| 6 |
Nirmatrelvir |
LYS90 |
Alkyl |
4.23 |
LYS90 |
Conventional hydrogen bond |
2.53 |
| |
|
|
|
|
SER274 |
Conventional hydrogen bond |
2.12 |
| |
|
|
|
|
LYS71 |
Conventional hydrogen bond |
2.64 |
| |
|
|
|
|
SER198 |
C–H bonding |
3.39 |
| |
|
|
|
|
LEU201 |
Conventional hydrogen bond |
2.95 |
| |
|
|
|
|
GLN202 |
Conventional hydrogen bond |
2.24 |
2.5. Ligands–protein interactions analysis of 6WPT (spike protein)
The association of ligand with target receptor 6WPT was also explored using molecular docking. The same docking protocols were used to dock all ligands (14(a–h) & 17(a–h)) with binding pocket of receptor 6WPT. The ligands (14(a–h) & 17(a–h)) exhibited docking scores between −6.2 to −8.6 Kcal mol−1. Among them, 14c displayed the highest potency with binding affinity of −8.6 Kcal mol−1. However, chloroquine,69 lopinavir,70 remdesivir,71 favipiravir,72 and nirmatrelvir73 interacted with target receptors with binding scores of −5.3, −7.1, −7.0, −6.0, and −8.0 Kcal mol−1 respectively (Table 5).
Table 5 Docking scores of compounds (14(a–h) & 17(a–h)) with binding sites of 6WPT
| Sr. no. |
Compounds |
Docking score (Kcal mol−1) |
| 1 |
14a |
−7.5 |
| 2 |
14b |
−7.9 |
| 3 |
14c |
−8.6 |
| 4 |
14d |
−7.6 |
| 5 |
14e |
−7.5 |
| 6 |
14f |
−7.4 |
| 7 |
14g |
−7.9 |
| 8 |
14h |
−7.4 |
| 9 |
17a |
−7.9 |
| 10 |
17b |
−7.9 |
| 11 |
17c |
−6.2 |
| 12 |
17d |
−7.5 |
| 13 |
17e |
−7.4 |
| 14 |
17f |
−6.9 |
| 15 |
17g |
−7.9 |
| 16 |
17h |
−6.5 |
| 17 |
Chloroquine |
−5.3 |
| 18 |
Lopinavir |
−7.1 |
| 19 |
Remdesivir |
−7.0 |
| 20 |
Favipiravir |
−6.0 |
| 21 |
Nirmatrelvir |
−8.0 |
The protein–ligand interaction study revealed that 14c interacted with coumarin and triazole core by making conventional and C–H bonding interactions with LYS1038 (2.42 Å), SER1037 (3.35 Å), GLY1035 (3.43 Å), and GLN1036 (2.53 Å). The compound 14c also exhibited strong hydrophobic associations with GLU1031, and TRP886 having bond distances of 4.07 and 4.72 Å (Fig. 8 & 9).86–88 Additionally, standards i.e., chloroquine, lopinavir, remdesivir, favipiravir, and nirmatrelvir established both hydrophobic interactions (with ALA890, LEU1034, TRP886, LYS1038, LYS90, PRO863, ILE870, PRO862, PHE823 and VAL860) and hydrogen bonding interactions (with TRP886, ARG905, LEU1034, GLY1035, GLN1036, HIS1058, ASP867, THR827, PHE82![[3 with combining cedilla]](https://www.rsc.org/images/entities/char_0033_0327.gif) THR778, ASP867 and THR732) (Fig. S51, ESI†) (Table 6).
THR778, ASP867 and THR732) (Fig. S51, ESI†) (Table 6).
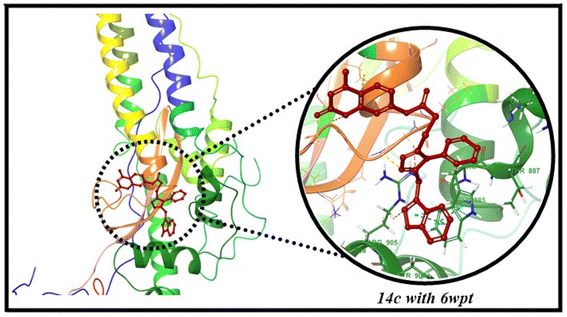 |
| | Fig. 8 Interactions of 14c with active site of 6WPT receptor. | |
 |
| | Fig. 9 (A–C) 3D interactions of potent compound 14c with spike protein. (D) 2D representation of binding analysis of 14c with 6WPT receptor. | |
Table 6 Ligand–protein interactions study of (14(a–h) & 17(a–h)) with binding sites of 6WPT
| Ligand–protein interactions (6WPT) |
| Sr. no. |
Compounds |
Hydrophobic interactions |
Hydrogen bonding interactions |
| Interacted residues |
Interactions |
Distance (Å) |
Interacted residues |
Interactions |
Distance (Å) |
| 1 |
14c |
GLU1031 |
Pi–sigma, Pi–Pi T-shaped |
4.07 |
GLN1036 |
Conventional H-bonding |
2.53 |
| |
|
TRP886 |
Pi–sigma, Pi–Pi stacked |
4.72 |
LYS1038 |
Conventional H-bonding |
2.42 |
| |
|
|
|
|
GLY1035 |
C–H bonding |
3.43 |
| |
|
|
|
|
SER1037 |
C–H bonding |
3.35 |
| 2 |
Chloroquine |
ALA890 |
Alkyl |
4.21 |
ARG905 |
C–H bonding |
3.41 |
| |
|
LEU1034 |
Pi-alkyl |
4.85 |
TRP886 |
C–H bonding |
4.48 |
| |
|
|
|
|
LEU1034 |
Conventional H-bonding |
2.72 |
| 3 |
Lopinavir |
LEU1034 |
Alkyl |
4.19 |
GLY1035 |
C–H bonding |
3.35 |
| |
|
ALA890 |
Alkyl |
4.13 |
GLN1036 |
Conventional H-bonding |
2.06 |
| |
|
TRP886 |
π–π stacked |
3.87 |
|
|
|
| |
|
LYS1038 |
Alkyl |
4.17 |
|
|
|
| |
|
LYS90 |
Pi-alkyl |
5.83 |
|
|
|
| 4 |
Remdesivir |
PRO863 |
Alkyl |
4.96 |
HIS1058 |
C–H bonding |
2.64 |
| |
|
ILE870 |
Alkyl |
4.82 |
ASP867 |
Conventional hydrogen bond |
3.35 |
| |
|
|
|
|
THR827 |
Conventional hydrogen bond |
2.09 |
| |
|
|
|
|
PHE823 |
Conventional H-bonding |
2.50 |
| 5 |
Favipiravir |
PRO863 |
π–Alkyl |
4.64 |
THR778 |
Conventional H-bonding |
2.59 |
| 6 |
Nirmatrelvir |
PRO862 |
Pi–alkyl |
4.95 |
ASP867 |
Conventional H-bonding |
1.79 |
| |
|
PHE823 |
Alkyl |
5.09 |
THR732 |
Conventional H-bonding |
2.49 |
| |
|
VAL860 |
Pi–alkyl |
5.30 |
HIS1058 |
Conventional H-bonding |
1.91 |
| |
|
PRO863 |
Pi–alkyl |
4.74 |
|
|
|
2.6. Dynamic simulation studies
Molecular dynamic (MD) simulations present dynamic validation of docking results, facilitating a clearer perception about the structural stability of 14b and 14c complexes. Multiple benchmarks such as RMSF, RMSD of C-alpha atoms and protein–ligand interactions have been extracted from the MD trajectories.
2.6.1 RMSD analysis of 14b and 14c with target receptors. MD simulation trajectories are employed to estimate deviation in complexes via protein Cα RMSD. The dynamic stability of potent compounds 14b and 14c with respective receptors (6VWW, 6Y84 and 6WPT) were assessed through 100 ns MD simulations via RMSD. These RMSD calculations of protein–ligand complexes were calculated with reference to the original state, that served as an indicator for determining ligand–protein complex stability. Compound 14b displayed conformational changes in RMSD during the initial 40 ns, after which moderate stability was attained from 40–100 ns with the 6VWW receptor. Similarly, compound 14c, when complexed with 6WPT, showed stability in RMSD from 35–100 ns after an initial conformational change period. Additionally, 14c exhibited slight deviations with the binding pocket during the initial 0–35 ns period, after which higher stability was attained between 35–100 ns with main protease (6Y84) (Fig. 10).
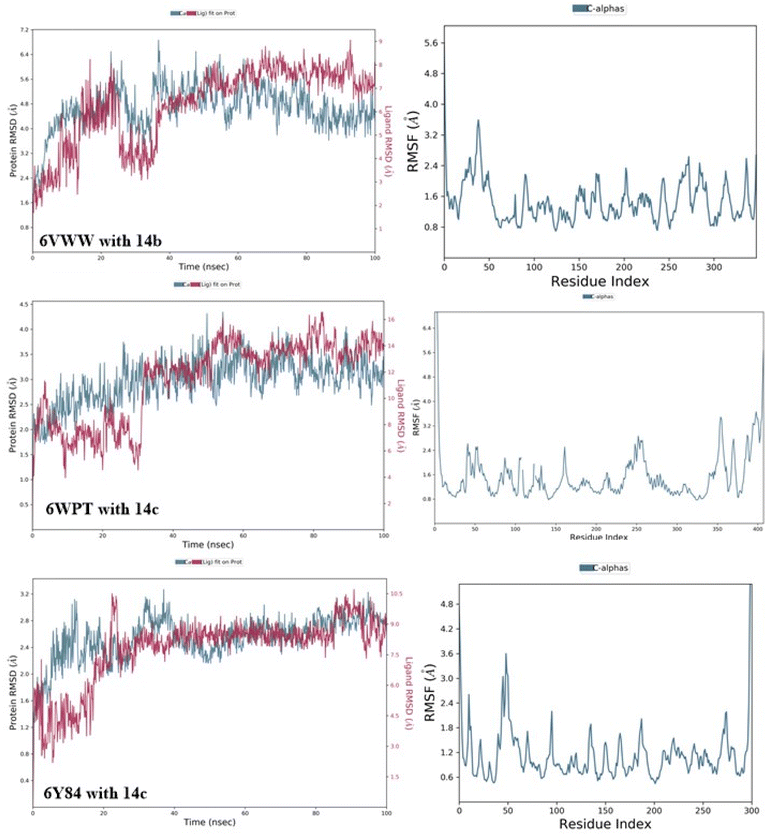 |
| | Fig. 10 RMSD and RMSF plots of 14b and 14c with 6VWW, 6WPT and 6Y84. | |
2.6.2 RMSF analysis of potent 14c and 14b complexes. During MD simulation period, average deviation of each protein residue from its initial position is calculated via RMSF. Moreover, stability of each complex is determined by RMSF of corresponding amino acid. The analysis of MD simulation via RMSF unearthed new observations about the flexibility of protein–ligand complexes with individual residues. During MD simulation period, complexes with relatively higher RMSF values tend to be more flexible as compared to those having low RMSF values. Compound 14b exhibited minimal fluctuations, ranging from 0.8 to 2.4 Å, at residues 55–250 of 6VWW receptor. Similarly, compound 14c exhibited a similar fluctuation profile with the spike protein, achieving stability between 0.8–2.9 Å at residues 25–325, after which higher fluctuations were observed between residues 350–400. Additionally, compound 14c displayed slightly higher fluctuations due to conformational changes between residues 25–75, after which minimal fluctuations were observed within a range of 0.6–2 Å. The corresponding RMSF analysis images have been presented in Fig. 10.
2.6.3 Protein–ligand contacts 14b and 14c with target receptors. To interpret the suppressive potential of compound 14b and 14c against SARS-CoV-2, a comprehensive analysis of the different interactions formed between the active site and the potent compound was conducted during a 100 ns simulation time. Within the simulation trajectory, ligand–residue interactions greater than 30% are referred as protein–ligand contacts. The compound 14b formed prominent hydrophobic interactions with LYS277 (40%) of 6VWW receptor (Fig. 11). Moreover, 14c displayed moderate hydrophobic bonding interactions with TRP886 and TYR904 of spike protein (Fig. S52, ESI†). Additionally, 14c formed strong hydrophobic as well as hydrogen bonding interactions with LYS97 (53%) and PHE150 (66%) (Fig. 12).
 |
| | Fig. 11 Protein–ligand contacts plot of 14b with 6VWW receptor. | |
 |
| | Fig. 12 Protein–ligand contacts plot of 14c with 6Y84 receptor. | |
The stable protein–ligand complexes are formed as a result of these significant interactions duration the MD simulation.
3 Materials and methods
3.1. Materials
The analytical-grade solvents, chemicals, and reagents (purity ≥ 99%) used throughout this investigation were sourced from Sigma-Aldrich. The NMR signals are denoted as singlet (s), doublet (d), triplet (t) and multiplet (m). The value of J (coupling constant) is given in hertz (Hz). For thin layer chromatography, aluminum plates precoated with silica gel 60F-254 (Merck) were employed, and the resulting chromatograms were visualized under UV illumination at 254 nm to 365 nm. Melting point determinations were performed using a Gallenkamp apparatus. An AVANCE AV 400 MHz spectrometer was used to record NMRs of all synthesized compounds. MS spectra were tracked in Agilent 6400 series (triple quadrupole) instrument. CE-440 Elemental analyzer was used to determine (CHN) elemental analysis.
3.2. Synthesis of substituted 4-methyl-2-oxo-2H-chromen-7-yl-2-((4-phenyl-4H-1,2,4-triazol-3-yl)thio) acetate 14(a–h)
A mixture of 4-methyl-2-oxo-2H-chromen-7-yl-2-bromoacetate (8, 0.22 mmol) in DCM was combined with various 4-phenyl-4H-1,2,4-triazole-3-thiols (13a–h, 0.21 mmol) and K2CO3 (0.23 mmol). The reaction mixture was agitated at room temperature for 20 hours. The reaction progression was tracked using thin-layer chromatography (TLC). Upon reaching completion, the target compounds (14a–h) were isolated by adding distilled water and n-hexane to the reaction mixture, inducing precipitation. The resulting solids were collected through filtration, dried, and subsequently recrystallized from ethanol.
3.3. Synthesis of substituted 2-((4-methyl-2-oxo-2H-chromen-7-yl)oxy)-N-(p-tolyl)acetamide 17(a–h)
The reaction was performed by dissolving 7-hydroxy-4-methylcoumarin (7, 0.0045 mol) and various 2-bromo-N-phenylacetamides (16a–h, 0.0045 mol) in DMF (6 mL) in the presence of K2CO3 (0.0049 mol) as a base. The reaction mixture was stirred at ambient temperature for 22 hours, with periodic assessment of reaction progress via TLC. After the reaction was complete, the target compounds (17a–h) were isolated as precipitates by adding water to the reaction mixture. The precipitates were washed with water, dried, and then crystallized from ethanol to access the pure compounds.
3.4. In silico studies
The synthesized coumarin scaffolds underwent computational molecular docking analysis to assess their binding affinity and anti-SARS-CoV-2 potential. Docking studies were performed with Autodock Vina 1.1.2.89 The 3D crystallized protein structures (6Y84, 6VWW, and 6WPT) were downloaded from PDB (Protein Data Bank). The standards (chloroquine lopinavir, remdesivir, favipiravir, nirmatrelvir) and targeted compound structures were drawn via ChemDraw software. The AutoDock Tools package was utilized to find the binding pocket by using grid box. The search grids for 6Y84, 6VWW, and 6WPT were determined as follows: 6Y84 (x = 11.869, y = 0.552, z = 4.89), 6VWW (x = −71.795, y = 21.929, z = −24.02), and 6WPT (x = 211.008, y = 222.549, z = −172.693), all with 40 xyz dimensions. The exhaustiveness was set to 8 for all targeted receptors.90–92 The poses with the lowest binding scores were chosen for further ligand–protein interaction study using Discovery Studio v24.1.0.23298. MD (molecular dynamics) simulations (100 ns) were run to determine stability of all the protein–ligand complexes. All the complexes were prepared using Maestro (academic version) from Schrödinger LLC, which corrected structural irregularities. MD simulations were performed by using Desmond module in order to examine the conformational changes and dynamic properties of complexes. The Desmond93 system builder module was utilized to launch orthorhombic cubic box and solvent model (TIP3P).94 Counterions (Na+ and Cl−) were incorporated to attain charge neutrality, and 0.15 M NaCl was added to simulate isotonic conditions.95 The system underwent energy minimization using the OPLS2005 force field,96 followed by a 100 ns molecular dynamics simulation at 1 atm pressure and 300 K temperature, generating 1000 trajectories, which were then analyzed using the Simulation Interaction Diagram (SID) tool to compute RMSD, RMSF, and protein–ligand contacts.
4 Conclusion
To conclude, two series of coumarin derivatives 14(a–h) & 17(a–h) have been synthesized in good to excellent yields ranging from 51–75% for 14(a–h) and 62–82% for 17(a–h). All of these hybrids 14(a–h) & 17(a–h) were docked against three targets known as 6WPT (spike protein), 6Y84 (main-protease) and 6VWW (Nsp15). Remarkable potency was exhibited by 14b and 14c against the selected targets, even better than all chosen standards (chloroquine, lopinavir, remdesivir, favipiravir, and nirmatrelvir). Compounds 14b and 14c interacted with their respective targets, 6VWW and 6Y84/6WPT, by forming hydrogen bonds and hydrophobic interactions. Molecular dynamic simulations were performed to assess the conformational stability and binding efficacy of potent inhibitors, as determined by RMSD, RMSF and ligand–protein contact analysis. The results of this computational study predict that the compounds 14b and 14c merit further investigations through in vitro and in vivo assays to evaluate their potential as therapeutic agents against SARS-CoV-2.
Data availability
All data is contained in the manuscript and ESI.†
Conflicts of interest
Authors have no conflict of interest for this research work.
References
- World Health Organization, WHO Coronavirus Disease (COVID-19) Dashboard, https://covid19.who.int/ Search PubMed.
- O. Delardas, K. Kechagias, P. Pontikos and P. Giannos, Sustainability, 2022, 14, 9699 CrossRef CAS
 .
. - F. He, Y. Deng and W. Li, J. Med. Virol., 2020, 92, 719–725 CrossRef CAS PubMed
 .
. - M. M. Khan, M. R. Amin, A. Al Mamun and A. A. Sajib, J. Software Eng. Appl., 2021, 14, 26 CrossRef
 .
. - J. Liu and S. Liu, J. Med. Virol., 2020, 92, 1484–1490 CrossRef CAS PubMed
 .
. - W.-j. Guan, Z.-y. Ni, Y. Hu, W.-h. Liang, C.-q. Ou, J.-x. He, L. Liu, H. Shan, C.-l. Lei and D. S. Hui, N. Engl. J. Med., 2020, 382, 1708–1720 CrossRef CAS PubMed
 .
. - S. Chavez, B. Long, A. Koyfman and S. Y. Liang, Am. J. Emerg. Med., 2021, 44, 220–229 CrossRef PubMed
 .
. - H. Ouassou, L. Kharchoufa, M. Bouhrim, N. E. Daoudi, H. Imtara, N. Bencheikh, A. ELbouzidi and M. Bnouham, J. Immunol. Res., 2020, 2020, 1357983 Search PubMed
 .
. - V. A. Verma, J. Mol. Struct., 2024, 1309, 138268 CrossRef CAS
 .
. - N. Zhu, D. Zhang, W. Wang, X. Li, B. Yang, J. Song, X. Zhao, B. Huang, W. Shi and R. Lu, N. Engl. J. Med., 2020, 382, 727–733 CrossRef CAS PubMed
 .
. - D. Wu, T. Wu, Q. Liu and Z. Yang, Int. J. Infect. Dis., 2020, 94, 44–48 CrossRef CAS PubMed
 .
. - F. Li, Annu. Rev. Virol., 2016, 3, 237–261 CrossRef CAS PubMed
 .
. - R. J. Hulswit, C. A. de Haan and B.-J. Bosch, Adv. Virus Res., 2016, 96, 29–57 CAS
 .
. - R. N. Kirchdoerfer, C. A. Cottrell, N. Wang, J. Pallesen, H. M. Yassine, H. L. Turner, K. S. Corbett, B. S. Graham, J. S. McLellan and A. B. Ward, Nature, 2016, 531, 118–121 CrossRef CAS PubMed
 .
. - I. Glowacka, S. Bertram, P. Herzog, S. Pfefferle, I. Steffen, M. O. Muench, G. Simmons, H. Hofmann, T. Kuri and F. Weber, J. Virol., 2010, 84, 1198–1205 CrossRef CAS PubMed
 .
. - Z. Liu, H. Zheng, H. Lin, M. Li, R. Yuan, J. Peng, Q. Xiong, J. Sun, B. Li, J. Wu, L. Yi, X. Peng, H. Zhang, W. Zhang, R. J. G. Hulswit, N. Loman, A. Rambaut, C. Ke, T. A. Bowden, O. G. Pybus and J. Lu, J. Virol., 2020, 94, e00790 CAS
 .
. - S. Matsuyama and F. Taguchi, J. Virol., 2002, 76, 11819–11826 CrossRef CAS PubMed
 .
. - I. Glowacka, S. Bertram, M. A. Müller, P. Allen, E. Soilleux, S. Pfefferle, I. Steffen, T. S. Tsegaye, Y. He and K. Gnirss, J. Virol., 2011, 85, 4122–4134 CrossRef PubMed
 .
. - E. Lontok, E. Corse and C. E. Machamer, J. Virol., 2004, 78, 5913–5922 CrossRef CAS PubMed
 .
. - X. Tian, C. Li, A. Huang, S. Xia, S. Lu, Z. Shi, L. Lu, S. Jiang, Z. Yang and Y. Wu, Emerging Microbes Infect., 2020, 9, 382–385 CrossRef CAS PubMed
 .
. - B. Cosar, Z. Y. Karagulleoglu, S. Unal, A. T. Ince, D. B. Uncuoglu, G. Tuncer, B. R. Kilinc, Y. E. Ozkan, H. C. Ozkoc and I. N. Demir, Cytokine Growth Factor Rev., 2022, 63, 10–22 CrossRef CAS PubMed
 .
. - W. T. Harvey, A. M. Carabelli, B. Jackson, R. K. Gupta, E. C. Thomson, E. M. Harrison, C. Ludden, R. Reeve, A. Rambaut and C.-G. U. Consortium, Nat. Rev. Microbiol., 2021, 19, 409–424 CrossRef CAS PubMed
 .
. - L. Van Dorp, M. Acman, D. Richard, L. P. Shaw, C. E. Ford, L. Ormond, C. J. Owen, J. Pang, C. C. Tan and F. A. Boshier, Infect., Genet. Evol., 2020, 83, 104351 CrossRef CAS PubMed
 .
. - S. Thakur, S. Sasi, S. G. Pillai, A. Nag, D. Shukla, R. Singhal, S. Phalke and G. Velu, Front. Biomed., 2022, 9, 815389 Search PubMed
 .
. - Y. Kaku, M. S. Yo, J. E. Tolentino, K. Uriu, K. Okumura, J. Ito and K. Sato, Lancet Infect. Dis., 2024, 24, e482–e483 CrossRef CAS PubMed
 .
. - Y. Cao, F. Jian, J. Wang, Y. Yu, W. Song, A. Yisimayi, J. Wang, R. An, X. Chen and N. Zhang, Nature, 2023, 614, 521–529 CAS
 .
. - A. Sethi, S. Sanam, S. Munagalasetty, S. Jayanthi and M. Alvala, RSC Adv., 2020, 10, 29873–29884 RSC
 .
. - V. Mody, J. Ho, S. Wills, A. Mawri, L. Lawson, M. C. Ebert, G. M. Fortin, S. Rayalam and S. Taval, Commun. Biol., 2021, 4, 93 CrossRef CAS PubMed
 .
. - J. Köppke, L.-E. Keller, M. Stuck, N. D. Arnow, N. Bannert, J. Doellinger and O. Cingöz, Nat. Commun., 2024, 15, 299 CrossRef PubMed
 .
. - P. Colson, J.-M. Rolain, J.-C. Lagier, P. Brouqui and D. Raoult, Int. J. Antimicrob. Agents, 2020, 55, 105932 CrossRef CAS PubMed
 .
. - C. A. Devaux, J.-M. Rolain, P. Colson and D. Raoult, Int. J. Antimicrob. Agents, 2020, 55, 105938 CrossRef CAS PubMed
 .
. - M. Huang, T. Tang, P. Pang, M. Li, R. Ma, J. Lu, J. Shu, Y. You, B. Chen and J. Liang, J. Mol. Cell Biol., 2020, 12, 322–325 CrossRef CAS PubMed
 .
. - S. Meini, A. Pagotto, B. Longo, I. Vendramin, D. Pecori and C. Tascini, J. Clin. Med., 2020, 9, 2050 CrossRef CAS PubMed
 .
. - B. Cao, Y. Wang, D. Wen, W. Liu, J. Wang, G. Fan, L. Ruan, B. Song, Y. Cai and M. Wei, N. Engl. J. Med., 2020, 382, 1787–1799 CrossRef PubMed
 .
. - S. Saghir and Z. Xiao, Bioresour. Technol., 2024, 391, 129916 CrossRef CAS PubMed
 .
. - J. H. Beigel, K. M. Tomashek, L. E. Dodd, A. K. Mehta, B. S. Zingman, A. C. Kalil, E. Hohmann, H. Y. Chu, A. Luetkemeyer and S. Kline, N. Engl. J. Med., 2020, 383, 1813–1826 CrossRef CAS PubMed
 .
. - J. J. Malin, I. Suárez, V. Priesner, G. Fätkenheuer and J. Rybniker, Clin. Microbiol. Rev., 2020, 34, e00162 CrossRef PubMed
 .
. - A. Rezagholizadeh, S. Khiali, P. Sarbakhsh and T. Entezari-Maleki, Eur. J. Pharmacol., 2021, 897, 173926 CrossRef CAS PubMed
 .
. - S. Joshi, J. Parkar, A. Ansari, A. Vora, D. Talwar, M. Tiwaskar, S. Patil and H. Barkate, Int. J. Infect. Dis., 2021, 102, 501–508 CrossRef CAS PubMed
 .
. - B. Dadonaite, J. Brown, T. E. McMahon, A. G. Farrell, M. D. Figgins, D. Asarnow, C. Stewart, J. Lee, J. Logue and T. Bedford, Nature, 2024, 631, 617–626 CrossRef CAS PubMed
 .
. - S. Hassanipour, M. Arab-Zozani, B. Amani, F. Heidarzad, M. Fathalipour and R. Martinez-de-Hoyo, Sci. Rep., 2021, 11, 11022 CrossRef CAS PubMed
 .
. - R. Arbel, Y. Wolff Sagy, M. Hoshen, E. Battat, G. Lavie, R. Sergienko, M. Friger, J. G. Waxman, N. Dagan and R. Balicer, N. Engl. J. Med., 2022, 387, 790–798 CrossRef CAS PubMed
 .
. - J. Hammond, H. Leister-Tebbe, A. Gardner, P. Abreu, W. Bao, W. Wisemandle, M. Baniecki, V. M. Hendrick, B. Damle and A. Simón-Campos, N. Engl. J. Med., 2022, 386, 1397–1408 CrossRef CAS PubMed
 .
. - C. Marzolini, D. R. Kuritzkes, F. Marra, A. Boyle, S. Gibbons, C. Flexner, A. Pozniak, M. Boffito, L. Waters and D. Burger, Clin. Pharmacol. Ther., 2022, 112, 1191–1200 CrossRef CAS PubMed
 .
. - W. L. Jorgensen, Science, 2004, 303, 1813–1818 CrossRef CAS PubMed
 .
. - M. P. Gleeson, J. Med. Chem., 2008, 51, 817–834 CrossRef CAS PubMed
 .
. - J. D. Durrant and J. A. McCammon, BMC Biol., 2011, 9, 1–9 Search PubMed
 .
. - J. Vamathevan, D. Clark, P. Czodrowski, I. Dunham, E. Ferran, G. Lee, B. Li, A. Madabhushi, P. Shah and M. Spitzer, Nat. Rev. Drug Discovery, 2019, 18, 463–477 CrossRef CAS PubMed
 .
. - Z. Sajid, M. Ahmad, S. Aslam, U. A. Ashfaq, A. F. Zahoor, F. A. Saddique, M. Parvez, A. Hameed, S. Sultan and H. Zgou, Pharm. Chem. J., 2016, 50, 172–180 CrossRef CAS
 .
. - S. Faiz and A. F. Zahoor, Mol. Diversity, 2016, 20, 969–987 CrossRef CAS PubMed
 .
. - S. Ahmad, A. F. Zahoor, S. A. R. Naqvi and M. Akash, Mol. Diversity, 2018, 22, 191–205 CrossRef CAS PubMed
 .
. - I. Rasool, M. Ahmad, Z. A. Khan, A. Mansha, T. Maqbool, A. F. Zahoor and S. Aslam, Trop. J. Pharm. Res., 2017, 16, 723–733 CrossRef CAS
 .
. - A. Kanwal, F. A. Saddique, S. Aslam, M. Ahmad, A. F. Zahoor and N.-u.-A. Mohsin, Pharm. Chem. J., 2018, 51, 1068–1077 CrossRef CAS
 .
. - P. S. Mishra, A. Kumar, K. Kaur and V. Jaitak, Curr. Med. Chem., 2024, 31, 5702–5738 CrossRef CAS PubMed
 .
. - H. Ma, K. Wang, B. Wang, Z. Wang, Y. Liu and Q. Wang, J. Agric. Food Chem., 2024, 72, 4658–4668 CrossRef CAS PubMed
 .
. - N. N. Kamel, H. F. Aly, G. I. Fouad, S. S. Abd El-Karim, M. M. Anwar, Y. M. Syam, S. A. Elseginy, K. A. Ahmed, H. F. Booles and M. B. Shalaby, RSC Adv., 2023, 13, 18496–18510 RSC
 .
. - P. P. Song, J. Zhao, Z. L. Liu, Y. B. Duan, Y. P. Hou, C. Q. Zhao, M. Wu, M. Wei, N. H. Wang and Y. Lv, Pest Manage. Sci., 2017, 73, 94–101 CrossRef CAS PubMed
 .
. - A. Sahar, Z. A. Khan, M. Ahmad, A. F. Zahoor, A. Mansha and A. Iqbal, Trop. J. Pharm. Res., 2017, 16, 203–210 CrossRef CAS
 .
. - R. Kausar, A. F. Zahoor, H. Tabassum, S. Kamal and M. Ahmad Bhat, Pharmaceuticals, 2024, 17, 532 CrossRef CAS PubMed
 .
. - S. Saeed, M. J. Saif, A. F. Zahoor, H. Tabassum, S. Kamal, S. Faisal, R. Ashraf, S. G. Khan, U. Nazeer and A. Irfan, RSC Adv., 2024, 14, 15419–15430 RSC
 .
. - R. Z. Batran, A. Sabt, J. Dziadek and A. F. Kassem, RSC Adv., 2024, 14, 21763–21777 RSC
 .
. - A. Irfan, S. Faiz, A. Rasul, R. Zafar, A. F. Zahoor, K. Kotwica-Mojzych and M. Mojzych, Molecules, 2022, 27, 1023 CrossRef CAS PubMed
 .
. - A. Wu, K. Shi, J. Wang, R. Zhang and Y. Wang, Eur. J. Med. Chem., 2024, 263, 115923 CrossRef CAS PubMed
 .
. - K. Sharma, M. Singh, P. Sharma, S. C. Sharma, S. Mujwar, M. Kapoor, K. K. Mishra and T. A. Wani, Molecules, 2024, 29, 1406 CrossRef CAS PubMed
 .
. - S. Chidambaram, M. A. El-Sheikh, A. H. Alfarhan, S. Radhakrishnan and I. Akbar, Saudi J. Biol. Sci., 2021, 28, 1100–1108 CrossRef CAS PubMed
 .
. - M. S. Bekheit, S. S. Panda, B. M. Kariuki, S. H. Mahmoud, A. Mostafa and A. S. Girgis, Eur. J. Med. Chem., 2023, 258, 115563 CrossRef CAS PubMed
 .
. - K. B. Lokhande, S. Doiphode, R. Vyas and K. V. Swamy, J. Biomol. Struct. Dyn., 2021, 39, 7294–7305 CrossRef CAS PubMed
 .
. - K. Shinohara, T. Kobayakawa, K. Tsuji, Y. Takamatsu, H. Mitsuya and H. Tamamura, Eur. J. Med. Chem., 2024, 280, 116963 CrossRef CAS PubMed
 .
. - S. Saeedi, A. Rahmati and Z. Chavoshpour-Natanzi, RSC Adv., 2022, 12, 19579–19589 RSC
 .
. - S. De Vita, M. G. Chini, G. Lauro and G. Bifulco, RSC Adv., 2020, 10, 40867–40875 RSC
 .
. - S. Koulgi, V. Jani, M. V. Uppuladinne, U. Sonavane and R. Joshi, RSC Adv., 2020, 10, 26792–26803 RSC
 .
. - L. C. Assis, A. A. de Castro, J. P. A. de Jesus, E. F. F. da Cunha, E. Nepovimova, O. Krejcar, K. Kuca, T. C. Ramalho and F. d. A. La Porta, RSC Adv., 2021, 11, 35228–35244 RSC
 .
. - H. S. Elbordiny, N. Z. Alzoman, H. M. Maher and S. I. Aboras, RSC Adv., 2023, 13, 26719–26731 RSC
 .
. - O. M. Ogunyemi, G. A. Gyebi, I. M. Ibrahim, C. O. Olaiya, J. O. Ocheje, M. M. Fabusiwa and J. O. Adebayo, RSC Adv., 2021, 11, 33380–33398 RSC
 .
. - S. Sonadevi, D. Rajaraman, M. Saritha, P. Solo and L. Athishu Anthony, Mol. Phys., 2025, 123, e2353331 CrossRef
 .
. - D. Douche, Y. Sert, S. A. Brandán, A. A. Kawther, B. Bilmez, N. Dege, A. El Louzi, K. Bougrin, K. Karrouchi and B. Himmi, J. Mol. Struct., 2021, 1232, 130005 CrossRef CAS PubMed
 .
. - A. Rani, G. Singh, A. Singh, U. Maqbool, G. Kaur and J. Singh, RSC Adv., 2020, 10, 5610–5635 RSC
 .
. - M. M. Abdelshaheed, H. I. El Subbagh, M. A. Tantawy, R. T. Attia, K. M. Youssef and I. M. Fawzy, RSC Adv., 2023, 13, 15689–15703 RSC
 .
. - F. Shiri, S. Shahraki, S. Baneshi, M. Nejati-Yazdinejad and M. H. Majd, RSC Adv., 2016, 6, 106516–106526 RSC
 .
. - S. Jalil, Z. Hussain, S. M. A. Abid, A. Hameed and J. Iqbal, RSC Adv., 2024, 14, 8905–8920 RSC
 .
. - M. Maddah, R. Bahramsoltani, N. H. Yekta, R. Rahimi, R. Aliabadi and M. Pourfath, New J. Chem., 2021, 45, 15977–15995 RSC
 .
. - S. Kumar, P. Kashyap, S. Chowdhury, S. Kumar, A. Panwar and A. Kumar, Phytomedicine, 2021, 85, 153317 CrossRef CAS PubMed
 .
. - M. Saeed, A. Saeed, M. J. Alam and M. Alreshidi, Molecules, 2020, 25, 5657 CrossRef CAS PubMed
 .
. - B. Gopi and V. Vijayakumar, RSC Adv., 2024, 14, 13218–13226 RSC
 .
. - D. P. Tran, Y. Taira, T. Ogawa, R. Misu, Y. Miyazawa and A. Kitao, Sci. Rep., 2022, 12, 3860 CrossRef CAS PubMed
 .
. - S. Skariyachan, D. Gopal, S. Chakrabarti, P. Kempanna, A. Uttarkar, A. G. Muddebihalkar and V. Niranjan, Comput. Biol. Med., 2020, 126, 104054 CrossRef CAS PubMed
 .
. - S. Skariyachan, D. Gopal, A. G. Muddebihalkar, A. Uttarkar and V. Niranjan, Comput. Biol. Med., 2021, 132, 104325 CrossRef CAS PubMed
 .
. - B. N. Marak, J. Dowarah, L. Khiangte and V. P. Singh, Drug Dev. Res., 2021, 82, 374–392 CrossRef CAS PubMed
 .
. - T. Gaillard, J. Chem. Inf. Model., 2018, 58, 1697–1706 CrossRef CAS PubMed
 .
. - G. Wang, Z. Peng, J. Wang, X. Li and J. Li, Eur. J. Med. Chem., 2017, 125, 423–429 CrossRef CAS PubMed
 .
. - T. N. H. Pham, T. H. Nguyen, N. M. Tam, T. Y. Vu, N. T. Pham, N. T. Huy, B. K. Mai, N. T. Tung, M. Q. Pham and V. V. Vu, J. Comput. Chem., 2022, 43, 160–169 CrossRef CAS PubMed
 .
. - J. Ding, S. Tang, Z. Mei, L. Wang, Q. Huang, H. Hu, M. Ling and J. Wu, J. Chem. Inf. Model., 2023, 63, 1982–1998 CrossRef CAS PubMed
 .
. - Y. Ali, A. A. Khan, A. M. Alanazi, S. A. Abdikakharovich, J. A. Shah, Z.-G. Ren and S. Khattak, Mol. Diversity, 2024, 1–14 Search PubMed
 .
. - Y. Moukhliss, Y. Koubi, M. Alaqarbeh, N. Alsakhen, S. Hamzeh, H. Maghat, A. Sbai, M. Bouachrine and T. Lakhlifi, New J. Chem., 2022, 46, 10154–10161 RSC
 .
. - B. Jójárt, R. Kiss, B. Viskolcz, I. Kolossváry and G. r. M. Keserű, J. Phys. Chem. Lett., 2010, 1, 1008–1013 CrossRef
 .
. - Y. Hua, X. Tan, J. Zhang, N. Xu, R. Chen, S. Zhou, S. Liu, K. Li, W. Chen and Q. Luo, Sci. Rep., 2024, 14, 29873 CrossRef CAS PubMed
 .
.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article * and
Muhammad Haroon
* and
Muhammad Haroon


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of 14b via conventional hydrogen bonding at the corresponding distance of 2.75 Å. In addition, coumarin core of 14b established conventional hydrogen bonding as well as C–H interactions with LYS71 (2.62 Å) and SER198 (3.22 Å), respectively. The naphthyl and coumarin core of 14b were found to be engaged with VAL295 (4.82 Å), LEU252 (3.63 Å), LYS277 (4.38 Å), LEU266 (5.25 Å), LEU201 (4.47 Å) and PHE259 (5.86 Å) via pi–alkyl, alkyl and pi–pi T-shaped hydrophobic interactions (Fig. 6 & 7).83–85 In comparison, standards i.e., chloroquine, lopinavir, remdesivir, favipiravir, and nirmatrelvir established C–H and conventional hydrogen bonding interactions with LYS71, GLY165, LYS90, ARG199, LYS277, ASP268, SER198, ASN200, SER274, LEU201 and GLN202 (Table 4). Moreover, all of them also interacted with LYS277, LEU252, VAL295, LYS90, TYR279, ARG91, LEU266, SER198, LEU201 and LYS277 via hydrophobic interactions (Fig. S50, ESI†).
O group of 14b via conventional hydrogen bonding at the corresponding distance of 2.75 Å. In addition, coumarin core of 14b established conventional hydrogen bonding as well as C–H interactions with LYS71 (2.62 Å) and SER198 (3.22 Å), respectively. The naphthyl and coumarin core of 14b were found to be engaged with VAL295 (4.82 Å), LEU252 (3.63 Å), LYS277 (4.38 Å), LEU266 (5.25 Å), LEU201 (4.47 Å) and PHE259 (5.86 Å) via pi–alkyl, alkyl and pi–pi T-shaped hydrophobic interactions (Fig. 6 & 7).83–85 In comparison, standards i.e., chloroquine, lopinavir, remdesivir, favipiravir, and nirmatrelvir established C–H and conventional hydrogen bonding interactions with LYS71, GLY165, LYS90, ARG199, LYS277, ASP268, SER198, ASN200, SER274, LEU201 and GLN202 (Table 4). Moreover, all of them also interacted with LYS277, LEU252, VAL295, LYS90, TYR279, ARG91, LEU266, SER198, LEU201 and LYS277 via hydrophobic interactions (Fig. S50, ESI†).

![[3 with combining cedilla]](https://www.rsc.org/images/entities/char_0033_0327.gif) THR778, ASP867 and THR732) (Fig. S51, ESI†) (Table 6).
THR778, ASP867 and THR732) (Fig. S51, ESI†) (Table 6).



.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.
.






