 Open Access Article
Open Access ArticleDirect determination of rare earth elements in atmospheric precipitation using a membrane desolvation ICP-MS/MS with N2O as the reaction gas
Jiang-yi Zhangac,
Wen-jing Liu *bc,
Di Liud,
Guang-liang Wuac and
Zhi-fang Xubc
*bc,
Di Liud,
Guang-liang Wuac and
Zhi-fang Xubc
aInstitutional Center for Shared Technologies and Facilities, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, 100029, China. E-mail: liuwenjing@mail.iggcas.ac.cn
bState Key Laboratory of Lithospheric and Environmental Coevolution, Institute of Geology and Geophysics, Chinese Academy of Sciences, Beijing, 100029, China
cCollege of Earth and Planetary Sciences, University of Chinese Academy of Sciences, Beijing, 100049, China
dState Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry, Institute of Atmospheric Physics, Chinese Academy of Sciences, Beijing, 100029, China
First published on 15th July 2025
Abstract
Analyzing rare earth elements (REEs) in atmospheric precipitation can reveal their sources and migration patterns. However, their direct determination in atmospheric precipitation using ICP-MS/MS remains challenging owing to low (sub-ng L−1) levels and mass spectral interference. This study established a reliable ICP-MS/MS method using a membrane desolvation system to enhance the sensitivity of REEs detections and employing N2O as the reaction gas to eliminate spectral interference for the direct measurement of REEs in atmospheric precipitation. The production rates of REE monoxides were significantly enhanced when using N2O as the reaction gas instead of O2. In particular, the yield of EuO+ increased to 64.9%, while that of YbO+ increased to 39.5%. A regular 10% signal suppression of REE+ was observed in the presence of matrix using membrane desolvation and employing 185Re as an internal standard effectively improved the results by >98%. The instrumental detection limits of the proposed method ranged from 0.001 ng L−1 for Lu to 0.022 ng L−1 for Nd. The results for REEs in the certified reference material (SLRS-6) at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution was consistent with the values reported in the literature. Thus, the proposed method was employed to analyze atmospheric precipitation samples. The accuracy of the results demonstrated that this method has the potential for routinely measuring sub ng L−1 levels of REEs in freshwater samples, offering advantages regarding sample throughput and reduced handling.
10 dilution was consistent with the values reported in the literature. Thus, the proposed method was employed to analyze atmospheric precipitation samples. The accuracy of the results demonstrated that this method has the potential for routinely measuring sub ng L−1 levels of REEs in freshwater samples, offering advantages regarding sample throughput and reduced handling.
1. Introduction
Rare earth elements (REEs) are crucial for tracing natural and human-induced processes in water owing to their unique chemical and physical properties.1–4 Research has indicated that the atmospheric inputs of particles and rainwater significantly contribute to the presence of REEs in the oceans and often surpass river inputs.5–7 However, the distribution of REEs in natural systems and their migration pathways remain insufficiently studied, particularly concerning atmospheric precipitation.8 Accurately determining the presence of REEs in atmospheric precipitation is essential to understand their sources and migration patterns. However, currently employed methods to measure REE concentrations in atmospheric precipitation require 30-fold9 to 200-fold10,11 preconcentration, rendering the direct determination of REEs challenging.Currently, quadrupole inductively coupled plasma mass spectrometry (Q-ICP-MS) is an excellent method for REE quantification in waters owing to its high sensitivity and multi-elemental measurement. However, directly measuring REEs in natural waters remains challenging for two main reasons. First, the extremely low concentration of REEs (sub-ng L−1) in natural waters, is often below the detection limit of the Q-ICP-MS system.3,12 Second, oxide and hydroxide interference are generated by Ba (such as BaO+ on Eu+ and BaH+ on La+) and light REEs oxides and hydroxides on heavy REEs.13 Therefore, the separation and pre-concentration of REEs from matrix elements are typically required before obtaining Q-ICP-MS.13–17 These sample pretreatment processes are time-consuming, labor-intensive, and require large sample volumes, and the risk of sample contamination increases. Developing a convenient, rapid, and cost-effective method for REEs determination would significantly enhance the quantification analysis of these elements in water samples.
The direct Q-ICP-MS analysis of natural water enables analysts to minimize sample manipulation and reduce the potential for blank contamination. For example, Lawrence et al.18 proposed a method for directly determining REEs in natural water using Q-ICP-MS. However, this method was only suitable for analyzing water samples with high REE concentrations. Specialized sample introduction systems, such as ultrasonic nebulization and microflow nebulization/desolvation, can be used in conjunction with sector field (SF)-ICP-MS to enhance the signal response and reduce polyatomic spectral interference, enabling the direct analysis of REEs in water samples.19–25 Despite its high sensitivity, there is an increased risk of interference. For example, barium hydroxide and lanthanum oxide can affect the 155Gd+ signal by 20%, with 13% and 7% contributed by 138Ba16O1H+ and 139La16O+, respectively.19 After connecting to the membrane desolvation device, Q-ICP-MS still has a poor detection limit (1–3 ng L−1).20 SF-ICP-MS with a membrane desolvation is the most sensitive method for ultra-trace REEs analysis, however, its high cost (over US $600,000) limits its use.19,25
Recently, inductively coupled plasma tandem mass spectrometry (ICP-MS/MS) has attracted attention owing to its efficient and direct approach for ultra-trace level analysis. Moreover, it can effectively reduce spectrochemical and isobaric interferences.26–30 Several studies have reported the determination of REEs using ICP-MS/MS.31–35 These studies utilized a mass-shift mode that enabled each REE isotope to react with oxygen gas (O2) or nitrous oxide gas (N2O) to form monoxide ions. This process effectively removes spectral interference, enabling the accurate determination of REEs without requiring a correction for interferences. Zhu et al.36 developed a method to directly measure REEs in natural water using O2 as the reaction gas. However, the sensitivities for Eu and Yb were lower due to the conversion efficiency of REE+ to REEO+, which was lower (14% to 20%) for Eu and Yb.34,36 Notably, the formation of REEO+ was enhanced using N2O instead of O2 as a reaction gas, resulting in improved sensitivity and limits of detection, and the significant enhancement of Eu and Yb oxides sensitivity.32,37–39
This study aims to develop a reliable method for the accurate and direct determination of sub-ng L−1 REEs in atmospheric precipitation using ICP-MS/MS. This method employed N2O as an oxidizing reaction gas to selectively eliminate possible spectral interferences of the analytes. Additionally, it used a membrane desolvation sample introduction system to enhance the sensitivity of the measurements. The validity of the proposed method was confirmed by the direct analysis of REEs in the river water certified reference material SLRS-6 at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution. Determination of REEs was also carried out for atmospheric precipitation samples. Finally, REE concentrations in real atmospheric precipitation samples collected from Xizang and Yunnan provinces were determined, and the distribution patterns of the REEs were compared.
10 dilution. Determination of REEs was also carried out for atmospheric precipitation samples. Finally, REE concentrations in real atmospheric precipitation samples collected from Xizang and Yunnan provinces were determined, and the distribution patterns of the REEs were compared.
2. Experimental
2.1 Instrumentation
In this study, a NexION 5000 Multi-Quadrupole ICP-MS Instrument (PerkinElmer Inc., Shelton, CT, USA) was used, equipped with platinum cones and a hyper skimmer, as previously described.40 A CETAC Aridus 3 membrane desolvation system (Omaha, NF, USA) was used to enhance the sensitivity of REEs, incorporating a 100 μL min−1 microconcentric nebulizer. The conditions for ICP-MS/MS (Table 1) ensured optimal performance in measuring REEs with high sensitivity and low limit of quantification (LOQ). The quantification process utilized external calibration with 185Re as the internal standard to correct for signal drift.| Instrument parameters | Value |
|---|---|
| RF power (W) | 1600 |
| Plasma gas flow (L min−1) | 16 |
| Nebuliser gas flow (L min−1) | 1.10 |
| Auxiliary gas flow (L min−1) | 1.4 |
| QID fixed voltage (V) | −12 |
| Hyper skimmer park voltage (V) | 13 |
| Omniring park voltage (V) | −210 |
| Inner target lens voltage (V) | 4 |
| Outer target lens voltage (V) | −1 |
| Deflector exit voltage (V) | −8 |
| Differential aperture voltage (V) | −3.5 |
| Cell gas | N2O |
| Reaction gas flow rate (mL min−1) | 1.2 |
| Analytical mode | Mass-shift |
| Internal standard | 185Re |
| PFA spray chamber (°C) | 110 |
| Membrane (°C) | 140 |
| Membrane sweep gas (L min−1) | 3.5 |
| Nitrogen addition gas (mL min−1) | 5.0 |
2.2 Reagents and standards
Ultrapure water (18.2 MΩ cm) was obtained from a Milli-Q Element water purification system (Millipore, France) and used throughout the experiments. High-purity nitric acid (HNO3) was purchased from Sinopharm Chemical Reagent Co., Ltd (Beijing, China) and purified twice using a sub-boiling distillation system (DST-1000, Savillex) prior to use. High-purity oxygen (O2, 99.999%) and nitrous oxide (N2O, 99.999%) gases were sourced from Beijing Hongba Gas Technology Co., Ltd and used as cell gases. Single-element standard solutions (1000 mg L−1) of REEs, Ba, K, Na, Ca, and Mg, were obtained from the National Institute of Metrology, China. The REE stock standard solution (10 mg L−1) was obtained from PerkinElmer, Inc. (Shelton, CT, USA). To confirm the accuracy of the proposed method, certified reference materials (CRMs, SLRS-6) for river water was purchased from the National Research Council of Canada and analyzed at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dilution.
10 dilution.
2.3 Analytical measurement
To eliminate interferences in REE+ determination, the mass-shift method was employed, which involved oxidizing REE+ to REEO+ using N2O or O2, resulting in a 16 amu shift for accurate measurement. The signal intensities of REEO+ were measured over the range of 0–2 mL min−1 gas flows of N2O or O2, and the maximum signal intensity of REEO+ was observed at 1.2 mL min−1 for N2O or O2. Before each measurement, the cell was flushed for about 10 min with the respective reaction gas (O2 or N2O).2.4 Sample preparation
Six atmospheric precipitation samples were collected from Yunnan and Xizang Province, respectively. To avoid the influence of dry precipitation, rainwater samples were collected half an hour after the start of each rain event using a polyethylene bucket with a polyethylene lid. The collector and container were pre-cleaned with 5–7 N HNO3, rinsed with ultrapure water, and dried. To prevent contamination from dry deposition, the lid was immediately removed before the rain began. The water samples were filtered using 0.45 μm membrane filters immediately after sampling and acidified to 2% (v/v) HNO3 for storage.3. Results and discussion
3.1 Comparison of REE oxidation yields using O2 and N2O
High yields of REE oxides are crucial to improve the sensitivity of REE measurements using the ICP-MS/MS mass-shift method. Thus, this study compared the REE oxidation yields obtained from the ICP-MS/MS mass-shift method using O2 and N2O, as shown in Fig. 1. The REE oxidation yields were <60% when using O2 as the cell gas. These values were lower than those reported by Yang et al.33 and Ding et al.35 (>80%), except for Eu and Yb. The yields of EuO+ and YbO+ were 15.3% and 8.6%, respectively, which were significantly lower than those (21% for Eu and 36% for Yb, respectively) reported by Yang et al..33 However, the O-binding energy of N2O is lower than that of O2. As a result, the reaction enthalpies for REE+ with N2O can be reduced compared to those with O2, which can improve the formation rates of REE+ and enhance sensitivity.38,41 In this study, the yields of EuO+ and YbO+ increased to 64.9% and 39.5%, respectively, when using N2O compared to that for O2. For the other REEs, the oxidation yields were exceeding 60% when using N2O as the cell gas than O2, except for La and Ce, which were comparable to those reported by Sugiyama.42 Therefore, N2O was used as the reaction cell gas in this study.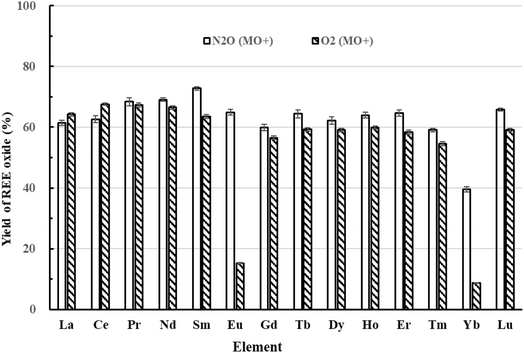 | ||
| Fig. 1 Yields of REE oxides using the ICP-MS/MS mass-shift method with O2 or N2O as the cell gas. The flow rate of O2 or N2O was 1.2 mL min−1, and the concentration of REE was 1 μg L−1. | ||
3.2 Matrix effect on REE sensitivities
Although ICP-MS/MS offer high sensitivity, the mass-shift mode (REE+ REEO+) was insufficient to directly measure all REEs at low concentrations in atmospheric precipitation samples. Therefore, a membrane desolvation sample introduction system (MD-ICP-MS/MS) was used to further enhance the REEs' sensitivity. This technique can effectively introduce samples by using a polytetrafluoroethylene (PTFE) membrane to separate solvent vapors from the sample aerosol, improving transport efficiency, increasing ion signal intensity, and minimizing the formation of oxides and hydroxides. Because of these advantages, membrane desolvation has been successfully employed in numerous trace and ultra-trace element studies.44,45 To quantify the possible matrix effects to determine REEs in water, three solutions containing REE + 2% HNO3, REE + matrix + 2% HNO3, and REE + matrix + 185Re + 2% HNO3 were applied. The matrix induced effects were expressed as the ratio of the signals for REE + matrix + 2% HNO3 to those obtained for REE + 2% HNO3, as shown in Fig. 2. The introduced sample matrix had a signal suppression effect on all REE+ species,18,20 and a regular 10% signal suppression for all REE+ species was observed, consistent with the 15% suppression reported by Chung et al.21 but lower than the 30–60% reported by Gabrielli et al..19 This signal reduction could be effectively compensated using 185Re as the internal standard (Fig. 2), achieving an improvement of >98%. Therefore, the precision and accuracy of REE determination were improved using MD-ICP-MS/MS method.3.3 Performance of the proposed method
Under optimized conditions, the blank equivalent concentration (BEC) and instrument detection limit (IDL) values were obtained by measuring 2% HNO3, while sensitivity was determined by measuring 1 μg L−1 REE in the same solution using MD-ICP-MS/MS (Table 2). Using our proposed method, the BEC and IDL values of REEs were lower than those obtained by Zhu et al.36 using ICP-MS/MS directly. Moreover, the sensitivity for each REE was improved by 2.6–9.7 times, with Eu and Yb showing improvements of 9.7 and 6.2 times, respectively. Therefore, the proposed method can be used to directly and effectively determine low concentrations of REEs.| Element | Present work | Ref. 36 | ||||
|---|---|---|---|---|---|---|
| BEC ng L−1 | IDL ng L−1 | Sensitivity CPS mL ng−1 | BEC ng L−1 | IDL ng L−1 | Sensitivity CPS mL ng−1 | |
| La | 0.005 | 0.006 | 966![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.24 | 0.15 | 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Ce | 0.012 | 0.009 | 965![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 816 816 |
0.14 | 0.13 | 268![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Pr | 0.004 | 0.007 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 221 221![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 966 966 |
0.03 | 0.08 | 312![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Nd | 0.012 | 0.022 | 461![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 511 511 |
0.11 | 0.26 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Sm | 0.013 | 0.011 | 347![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535 535 |
0.03 | 0.01 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Eu | 0.009 | 0.002 | 622![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 913 913 |
0.023 | 0.007 | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Gd | 0.008 | 0.004 | 255![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 608 |
0.03 | 0.009 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Tb | 0.001 | 0.001 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 085 085![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 937 937 |
0.009 | 0.06 | 343![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Dy | 0.014 | 0.005 | 286![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 438 438 |
0.017 | 0.005 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Ho | 0.001 | 0.002 | 977![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 734 734 |
0.017 | 0.07 | 356![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Er | 0.005 | 0.002 | 323![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 943 943 |
0.014 | 0.004 | 114![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Tm | 0.0008 | 0.001 | 868![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 418 418 |
0.009 | 0.06 | 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Yb | 0.001 | 0.003 | 184![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 686 686 |
0.05 | 0.014 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Lu | 0.0008 | 0.001 | 893![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204 204 |
0.009 | 0.06 | 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.4 Measurement results of REEs in SLRS-6 and atmospheric precipitation samples
To confirm the accuracy of the proposed method, the river water CRM SLRS-6 was used following dilution 10 times. As shown in Table 3, REE concentrations ranged from 1.70 to 288.5 ng L−1, which were consistent with the literature-reported values of 1.79–292.7 ng L−1.43 The proposed method was also applied to determine REEs in atmospheric precipitation samples collected from the Yunnan and Xizang provinces, and the results are presented in Table 3. The concentrations of REEs in atmospheric precipitation significantly varied, with the MT region in the Xizang province exhibiting the lowest levels, ranging from 0.003 to 0.24 ng L−1 for Lu to Ce. These values were higher than the BEC and IDL values presented in Table 2. Therefore, the proposed method could successfully determine the low concentrations of REEs in these samples.| SLRS-6 reported41 | SLRS-6 this study | GGQ-S | GGQ-W | LZ-S | LZ-W | MT-S | MT-W | |
|---|---|---|---|---|---|---|---|---|
| La | 248.3 ± 12.1 | 244.3 ± 10.2 | 1.32 ± 0.12 | 1.13 ± 0.11 | 0.75 ± 0.08 | 0.55 ± 0.08 | 0.30 ± 0.09 | 0.22 ± 0.06 |
| Ce | 292.7 ± 15.1 | 288.5 ± 12.3 | 2.50 ± 0.20 | 2.10 ± 0.12 | 1.43 ± 0.21 | 0.83 ± 0.14 | 0.43 ± 0.10 | 0.24 ± 0.04 |
| Pr | 59.1 ± 1.9 | 58.3 ± 1.1 | 0.31 ± 0.06 | 0.29 ± 0.04 | 0.19 ± 0.04 | 0.12 ± 0.02 | 0.068 ± 0.008 | 0.043 ± 0.009 |
| Nd | 227.8 ± 9.4 | 225.3 ± 5.6 | 1.29 ± 0.10 | 1.17 ± 0.15 | 0.82 ± 0.08 | 0.52 ± 0.10 | 0.27 ± 0.06 | 0.19 ± 0.03 |
| Sm | 39.5 ± 1.7 | 38.7 ± 1.1 | 0.23 ± 0.05 | 0.21 ± 0.06 | 0.16 ± 0.05 | 0.10 ± 0.02 | 0.056 ± 0.010 | 0.037 ± 0.009 |
| Eu | 7.26 ± 0.35 | 7.20 ± 0.21 | 0.054 ± 0.010 | 0.050 ± 0.011 | 0.037 ± 0.008 | 0.026 ± 0.003 | 0.017 ± 0.004 | 0.013 ± 0.004 |
| Gd | 31.6 ± 2.5 | 30.1 ± 2.2 | 0.21 ± 0.04 | 0.20 ± 0.05 | 0.15 ± 0.03 | 0.093 ± 0.010 | 0.061 ± 0.009 | 0.042 ± 0.07 |
| Tb | 4.07 ± 0.27 | 3.89 ± 0.22 | 0.033 ± 0.011 | 0.031 ± 0.008 | 0.023 ± 0.005 | 0.015 ± 0.005 | 0.010 ± 0.003 | 0.007 ± 0.001 |
| Dy | 21.9 ± 1.1 | 21.1 ± 0.9 | 0.22 ± 0.05 | 0.21 ± 0.06 | 0.16 ± 0.02 | 0.10 ± 0.02 | 0.051 ± 0.006 | 0.039 ± 0.004 |
| Ho | 4.3 ± 0.3 | 4.15 ± 0.2 | 0.04 ± 0.007 | 0.04 ± 0.004 | 0.03 ± 0.004 | 0.017 ± 0.003 | 0.009 ± 0.002 | 0.007 ± 0.002 |
| Er | 12.4 ± 0.7 | 11.9 ± 0.7 | 0.12 ± 0.03 | 0.12 ± 0.03 | 0.091 ± 0.006 | 0.057 ± 0.005 | 0.029 ± 0.003 | 0.019 ± 0.003 |
| Tm | 1.79 ± 0.18 | 1.70 ± 0.13 | 0.018 ± 0.005 | 0.017 ± 0.004 | 0.013 ± 0.003 | 0.008 ± 0.002 | 0.004 ± 0.001 | 0.003 ± 0.001 |
| Yb | 11.2 ± 0.7 | 11.7 ± 0.4 | 0.12 ± 0.03 | 0.11 ± 0.03 | 0.086 ± 0.008 | 0.056 ± 0.006 | 0.034 ± 0.003 | 0.021 ± 0.003 |
| Lu | 1.91 ± 0.23 | 1.86 ± 0.21 | 0.018 ± 0.005 | 0.017 ± 0.004 | 0.013 ± 0.002 | 0.008 ± 0.001 | 0.005 ± 0.001 | 0.003 ± 0.001 |
Shale-normalized REE patterns can assist in identifying the sources of atmospheric precipitation. Four primary types of shale-normalized patterns emerged from the analysis of dissolved REEs: (1) middle rare earth elements (MREE)-enriched (Sm to Dy); (2) Flat REE patterns; (3) heavy rare earth elements (HREE)-enriched (Ho to Lu); and (4) light rare earth elements (LREE)-enriched (La to Nd). These patterns provided insights into the origins of REEs in atmospheric precipitation. The shale-normalized REE distribution patterns for these samples are plotted in Fig. 3, and were enriched in MREE. MREE enrichment can result from the interaction of water and airborne particles which occurs through the dissolution or leaching of these particles when they interact with water.46
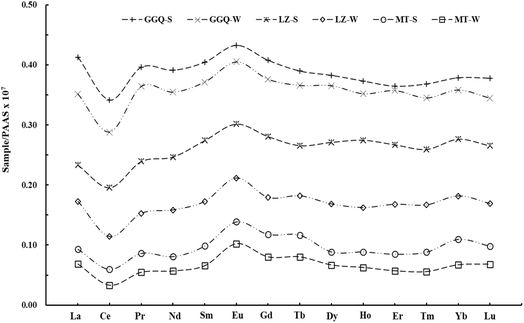 | ||
| Fig. 3 Patterns of shale-normalized REEs in atmospheric precipitation from the Yunnan and Xizang provinces. | ||
4. Conclusion
This study presents an alternative method for the direct measurement of REEs in atmospheric precipitation samples using MD-ICP-MS/MS. Spectral interferences were eliminated using ICP-MS/MS in the mass-shift mode, employing N2O as the reaction cell gas. This approach shifted the mass-to-charge ratio (m/z) of the target analyte by 16 amu.38 The use of membrane desolvation was beneficial for directly measuring ultra-trace REEs in atmospheric precipitation samples, eliminating the need for tedious, contamination-prone preconcentration and matrix separation procedures. The analytical results for REEs using SLRS-6 were consistent with those of previous studies, confirming the reliability of the proposed method for measuring REEs in atmospheric precipitation samples. This method shows promise for routinely measuring sub ng L−1 levels of REEs in freshwater samples, offering advantages in terms of multi-element capability, sample throughput, and reduced handling.Data availability
Data will be made available upon request.Author contributions
Jiang-yi Zhang: writing – original draft, review & editing, methodology, data curation; Wen-jing Liu: review & editing, methodology, data curation, resources; Di Liu: methodology, data curation; Guang-liang Wu: investigation, data curation; Zhi-fang Xu: review & editing, methodology, resources, supervision, project administration.Conflicts of interest
The authors have no conflicts of interest to declare.Acknowledgements
This work was supported by the National Key Research and Development Program of China (No. 2023YFC3710600, 2020YFA0607700), the National Natural Science Foundation of China (No. 42422303), and the Key Research Program of the Institute of Geology & Geophysics, CAS (No. IGGCAS-202204), Wenjing Liu extends gratitude for the support from the Youth Innovation Promotion Association CAS (Y2023014).References
- S. Kulaksiz and M. Bau, Anthropogenic dissolved and colloid/nanoparticle-bound samarium, lanthanum and gadolinium in the Rhine River and the impending destruction of the natural rare earth element distribution in rivers, Earth Planet. Sci. Lett., 2013, 362, 43–50 CrossRef CAS.
- V. Hatje, K. W. Bruland and A. R. Flegal, Increases in anthropogenic gadolinium anomalies and rare earth element concentrations in San Francisco Bay over a 20 year record, Environ. Sci. Technol., 2016, 50, 4159–4168 CrossRef CAS PubMed.
- K. C. Crocket, E. Hill, R. E. Abell, C. Johnson, S. F. Gary, T. Brand and E. C. Hathorne, Rare earth element distribution in the NE Atlantic: evidence for benthic sources, longevity of the seawater signal, and biogeochemical cycling, Front. Mar. Sci., 2018, 5, 1–22 CrossRef.
- C. W. Noack, D. A. Dzombak and A. K. Karamalidis, Rare earth element distributions and trends in natural waters with a focus on groundwater, Environ. Sci. Technol., 2014, 48, 4317–4326 CrossRef CAS PubMed.
- E. R. Sholknovitz, T. M. Church and R. Arimoto, Rare earth element composition of precipitation, precipitation particles, and aerosols, J. Geophys. Res., 1993, 98, 20587–20599 CrossRef.
- M. J. Greaves, P. J. Stanthanm and H. Elderfield, Rare earth element mobilization from marine atmospheric dust into seawater, Mar. Chem., 1994, 46, 255–260 CrossRef CAS.
- L. J. Spokes, T. D. Jickells and K. Jarvis, Atmospheric inputs of trace metals to the northeast Atlantic Ocean: the importance of southeasterly flow, Mar. Chem., 2001, 76, 319–330 CrossRef CAS.
- V. I. Radomskaya, D. V. Yusupov and L. M. Pavlova, Rare-Earth Elements in the Atmospheric Precipitation of the City of Blagoveshchensk, Geochem. Int., 2018, 56, 189–198 CrossRef CAS.
- M. Iwashita, A. Saito, M. Arai, Y. Furusho and T. Shimamura, Determination of rare earth elements in rainwater collected in suburban Tokyo, Geochem. J., 2011, 45, 187–197 CrossRef CAS.
- J. Zhang and C. Q. Liu, Major and rare earth elements in rainwaters from Japan and East China Sea: Natural and anthropogenic sources, Chem. Geol., 2004, 209, 315–326 CrossRef CAS.
- Z. Z. Zhu, C. Q. Liu, Z. L. Wang, X. L. Liu and J. Li, Rare earth elements concentrations and speciation in rainwater from Guiyang, an acid rain impacted zone of Southwest China, Chem. Geol., 2016, 442, 23–34 CrossRef CAS.
- Y. Li, W. Guo, Z. Wu, L. Jin, Y. Ke, Q. Guo and S. Hu, Determination of ultra-trace rare earth elements in high-salt groundwater using aerosol dilution inductively coupled plasma-mass spectrometry (ICP-MS) after iron hydroxide co-precipitation, Microchem. J., 2016, 126, 194–199 CrossRef CAS.
- I. Wysocka, Determination of rare earth elements concentrations in natural waters - A review of ICP-MS measurement approaches, Talanta, 2021, 221, 121636 CrossRef CAS PubMed.
- A. Fisher and D. Kara, Determination of rare earth elements in natural water samples- A review of sample separation, preconcentration and direct methodologies, Anal. Chim. Acta, 2016, 935, 1–29 CrossRef CAS PubMed.
- T. Watanabe, Y. Saito-Kokubu, H. Murakami and T. Iwatsuki, Onsite chelate resin solid-phase extraction of rare earth elements in natural water samples: its implication for studying past redox changes by inorganic geochemistry, Limnology, 2018, 19, 21–30 CrossRef CAS.
- Z. Arslan, T. Oymak and J. White, Triethylamine-assisted Mg(OH)2 coprecipitation/preconcentration for determination of trace metals and rare earth elements in seawater by inductively coupled plasma mass spectrometry (ICP-MS), Anal. Chim. Acta, 2018, 1008, 18e28 CrossRef PubMed.
- H. Li, R. Tong, W. Guo, Q. Xu, D. Tao, Y. Lai, L. Jin and S. Hu, Development of a fully automatic separation system coupled with online ICP-MS for measuring rare earth elements in seawater, RSC Adv., 2022, 12, 24003 RSC.
- M. G. Lawrence, A. Greig, K. D. Collerson and B. S. Kamber, Direct quantification of rare earth element concentrations in natural waters by ICP-MS, Appl. Geochem., 2006, 21, 839–848 CrossRef CAS.
- P. Gabrielli, C. Barbante, C. Turetta, A. Marteel, C. Boutron, G. Cozzi, W. Cairns, C. Ferrari and P. Cescon, Direct determination of rare earth elements at the subpicogram per gram level in Antarctic Ice by ICP-SFMS using a desolvation system, Anal. Chem., 2006, 78, 1883–1889 CrossRef CAS PubMed.
- D. Dick, A. Wegner, P. Gabrielli, U. Ruth, C. Barbante and M. Kriews, Rare earth elements determined in Antarctic ice by inductively coupled plasma-Time of flight, quadrupole and sector field-mass spectrometry: An inter-comparison study, Anal. Chim. Acta, 2008, 621, 140–147 CrossRef CAS PubMed.
- C. H. Chung, Isaac Brenner and Chen-Feng You, Comparison of microconcentric and membrane-desolvation sample introduction systems for determination of low rare earth element concentrations in surface and subsurface waters using sector field inductively coupled plasma mass spectrometry, Spectrochim. Acta, Part B, 2009, 64, 849–856 CrossRef.
- L. Halicz, I. Segal and O. Yoffe, Direct REE determination in fresh waters using ultrasonic nebulization ICP-MS, J. Anal. At. Spectrom., 1999, 14, 1579–1581 RSC.
- M. P. Aliaga-Campuzano, J. P. Bernal, S. B. BricenoPrieto, O. Perez-Arvizu and E. Lounejeva, Direct analysis of lanthanides by ICPMS in calcium-rich water samples using a modular high-efficiency sample introduction system–membrane desolvator, J. Anal. At. Spectrom., 2013, 28, 1102 RSC.
- X. Y. Zheng, J. Yang and G. M. Henderson, A Robust Procedure for High-Precision Determination of Rare Earth Element Concentrations in Seawater, Geostand. Geoanal. Res., 2014, 39, 277–292 CrossRef.
- J. H. Zhu, F. Y. Chu, F. Liang, X. Y. Luo, Q. Liu, Q. H. Xu, W. Yu, Y. C. Li, J. G. Lu, Y. X. Li, Y. H. Dong, H. M. Li, J. Zhao and C. Zhang, Accurate determination of ultra-trace REEs in seawater using a membrane desolvation Q-ICP-MS coupled with an online automatic separation system, J. Anal. At. Spectrom., 2024, 39, 2870 RSC.
- D. Yan, W. Guo, Q. Guo, L. Jin and S. Hu, Determination of Ultra-trace Hg in Geothermal Water Using ICP-MS/MS, Atom. Spectrosc., 2024, 45, 525–531 CrossRef CAS.
- D. Wang, H. Li, L. Guo, H. Guo, X. Li, S. Li and Z. Zhang, Determination and Evaluation of Ultra-trace Levels of Titanium in Bearing Steel by Inductively Coupled Plasma Tandem Mass Spectrometry, Atom. Spectrosc., 2024, 45, 473–480 CrossRef CAS.
- J. Zhang, W. Liu, Z. Xu and G. Wu, Determination of Sulfur Isotope Ratios (34S/32S) in Atmospheric Precipitation Samples by Triple Quadrupole ICP-MS, Atom. Spectrosc., 2024, 45, 438–444 CrossRef CAS.
- L. Balcaen, E. Bolea-Fernandez, M. Resano and F. Vanhaecke, Inductively coupled plasma – Tandem mass spectrometry (ICP-MS/MS): A powerful and universal tool for the interference-free determination of (ultra)trace elements–A tutorial review, Anal. Chim. Acta, 2015, 894, 7–19 CrossRef CAS PubMed.
- E. Bolea-Fernandez, L. Balcaen, M. Resano and F. Vanhaecke, Overcoming spectral overlap via inductively coupled plasma-tandem mass spectrometry (ICP-MS/MS). A tutorial review, J. Anal. At. Spectrom., 2017, 32, 1660–1679 RSC.
- Y. Zhu, Determination of rare earth elements in seawater samples by inductively coupled plasma tandem quadrupole mass spectrometry after coprecipitation with magnesium hydroxide, Talanta, 2020, 209, 120536 CrossRef CAS PubMed.
- O. Klein, T. Zimmermann and D. Pröfrock, Improved determination of technologically critical elements in sediment digests by ICP-MS/MS using N2O as a reaction gas, J. Anal. At. Spectrom., 2021, 36, 1524–1532 RSC.
- M. Yang, W. Wei, Y. H. Yang, R. L. Romer, S. T. Wu, T. Wu and L. F. Zhong, Accurate determination of ultra-trace rare earth elements by LA-ICP-MS/MS and its application to cassiterite for effective elimination of Gd and Tb false positive anomalies, J. Anal. At. Spectrom., 2024, 39, 2992–2999 RSC.
- M. A. Amr, N. D. A. Dawood, A. I. Helal and B. Russell, Rare earth elements and 143Nd/144Nd isotope ratio measurements using tandem ICP-CRC-MS/MS: characterization of date palm (Phoenix dactylifera L.), J. Anal. At. Spectrom., 2017, 32, 1554–1565 RSC.
- X. T. Ding, W. T. Bu, Y. Y. Ni, X. P. Shao, K. Xiong, C. T. Yang and S. Hu, Determination of trace rare earth elements in uranium ore samples by triple quadrupole inductively coupled plasma mass spectrometry, J. Anal. At. Spectrom., 2021, 36, 2144–2152 RSC.
- Y. B. Zhu, K. Nakano, Y. Shikamori and A. Itoh, Direct determination of rare earth elements in natural water samples by inductively coupled plasma tandem quadrupole mass spectrometry with oxygen as the reaction gas for separating spectral interferences, Spectrochim. Acta, Part B, 2021, 179, 106100 CrossRef CAS.
- V. K. Zepeda, B. S. Kamber and O. Y. A. Ghidan, Direct accurate Eu anomaly analysis in very high Ba/Eu silicate samples by triple-quadrupole ICP-MS in MS/MS mass shift mode, Chem. Geol., 2024, 647, 121827 CrossRef CAS.
- S. T. Lancaster, T. Prohaska and J. Irrgeher, Characterisation of gas cell reactions for 70+ elements using N2O for ICP tandem mass spectrometry measurements, J. Anal. At. Spectrom., 2023, 38, 1135 RSC.
- Y. Zhu, Determination of rare earth elements by inductively coupled plasma–tandem quadrupole mass spectrometry with nitrous oxide as the reaction gas, Front. Chem., 2022, 10, 912938 CrossRef CAS PubMed.
- J. Y. Zhang, W. J. Liu, D. Liu, G. L. Wu and Z. F. Xu, Rapid determination of the 87Sr/86Sr ratio in water samples using ICP-MS/MS without concentration matching and matrix matching, Spectrochim. Acta, Part B, 2024, 221, 107046 CrossRef CAS.
- G. K. Koyanagi and D. K. Bohme, Oxidation Reactions of Lanthanide Cations with N2O and O2: Periodicities in Reactivity, J. Phys. Chem. A, 2001, 105, 8964–8968 CrossRef CAS.
- N. Sugiyama, Direct Analysis of Ultratrace Rare Earth Elements in Environmental Waters by ICP-QQQ, Agilent Application Note, 2020, pp. 1–5 Search PubMed.
- D. Yeghicheyan, D. Aubert, M. B. Coz, J. Chmeleff, S. Delpoux, I. Djouraev, G. Granier, F. Lacan, J. Piro, T. Rousseau, C. Cloquet, A. Marquet, C. Menniti, C. Pradoux, R. Freydier, E. V. da Silva-Filho and K. Suchorski, A New Interlaboratory Characterisation of Silicon, Rare Earth Elements and Twenty-Two Other Trace Element Concentrations in the Natural River Water Certified Reference Material SLRS-6 (NRC-CNRC), Geostand. Geoanal. Res., 2019, 43, 475–496 CrossRef CAS.
- B. C. Russell, I. W. Croudace, P. E. Warwick and J. A. Milton, Determination of precise 135Cs/137Cs ratio in environmental samples using sector field inductively coupled plasma mass spectrometry, Anal. Chem., 2014, 86, 8719–8726 CrossRef CAS PubMed.
- D. He, Z. Zhu, X. Miao, H. Zheng, X. Li, N. S. Belshaw and S. Hu, Determination of trace cadmium in geological samples by membrane desolvation inductively coupled plasma mass spectrometry, Microchem. J., 2019, 148, 561–567 CrossRef CAS.
- R. E. Hannigan and E. R. Sholkovitz, The development of middle rare earth element enrichments in freshwaters: weathering of phosphate minerals, Chem. Geol., 2001, 175, 495–508 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |

