Open Access Article
Open Access ArticleFacile synthesis of a Ni–Cu composite reinforced with a para-phenylenediamine layer for enhanced hydrogen evolution reaction†
Soufiane Skakri a,
Anas El Attarab,
Saad Benhaiba
a,
Anas El Attarab,
Saad Benhaiba a,
Badr Bouljoihel‡
a,
Abdellah Aaddanea,
Anas Mouakkara,
Adiba Raisa and
Mama El Rhazi
a,
Badr Bouljoihel‡
a,
Abdellah Aaddanea,
Anas Mouakkara,
Adiba Raisa and
Mama El Rhazi *a
*a
aLaboratory of Materials, Membranes, and Environment, Faculty of Sciences and Technologies Mohammedia, University Hassan II of Casablanca, Morocco. E-mail: Mama.elrhazi@fstm.ac.ma
bCentrale Lille, UMET–Unité Matériaux et Transformation, UMR 8207, F-59000 Lille, France
First published on 10th July 2025
Abstract
In the race to develop new catalysts for water splitting, researchers are increasingly focusing on the design of cost-effective materials, particularly first-row transition metals. Nickel and copper catalysts are promising candidates due to their low cost and excellent compatibility. Herein, for the first time, the electrochemical synthesis of Ni–Cu-based nanomaterials reinforced with a para-phenylenediamine (pPD) layer for the HER is reported. A very simple method involving the electrodeposition of a pPD layer on a carbon paste electrode (CPE), followed by the electrodeposition of Ni–Cu particles at a constant current to form Ni4Cu1/pPD/CPE, was employed. The morphological, structural and electrochemical properties of the catalyst were thoroughly characterized using several techniques such as field-emission scanning electron microscopy (FE-SEM), X-ray diffraction (XRD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The catalytic performance and stability of the catalyst were evaluated in a 1 M KOH solution using linear sweep voltammetry and chronoamperometry, respectively. The prepared Ni4Cu1/pPD/CPE electrocatalyst exhibited high activity toward HER in an alkaline medium, achieving a very low overpotential of −70 mV vs. RHE at 10 mA cm−2 and a value of 87 mV dec−1 for Tafel slope. The results indicate that the prepared Ni4Cu1/pPD/CPE electrocatalyst is a highly promising catalytic material for effective green hydrogen production.
Introduction
With the tremendous increase in the global population and the continuous advancement of various technologies, the worldwide demand for energy consumption has escalated, leading to a critical challenge in meeting human energy needs.1 Currently, fossil fuels are the primary source of energy; however, their usage releases numerous pollutants into the environment and contributes to future challenges such as greenhouse gas emissions, CO2 emissions, and global warming.2 The development of clean energy technology, such as water splitting, has emerged as a promising strategy for producing green hydrogen.3,4 The HER generally requires three stages in alkaline solutions.5 The first is the Volmer reaction, also known as electrochemical hydrogen adsorption: H2O + M + e− → MHads + OH−. In this step, a water molecule dissociates, producing a hydroxide ion and hydrogen adsorbed on the catalyst surface. The second step is electrochemical desorption, or the Heyrovsky reaction, in which the hydrogen adsorbed during the Volmer step is discharged with an electron at the electrode–solution interface, leading to the formation of hydrogen gas (H2). Finally, the third step is chemical desorption, known as the Tafel reaction, where two adsorbed hydrogen atoms formed during the Volmer step combine to produce hydrogen gas (H2).6Platinum is the most effective catalyst for the HER. However, its high cost and limited earth abundance nature affect the cost-effectiveness of HER technology.7,8 To overcome this issue, many strategies have been investigated using first-row transition metals such as nickel, copper and cobalt.9–11 Nickel-based catalysts have garnered significant interest and have been thoroughly investigated. However, the activity and durability of Ni-based catalysts remain unsatisfactory. Recent research on synthetic Ni-based alloys has demonstrated that the nickel content in the alloy has a significant impact on both the alloy's morphological characteristics and electrochemical properties.12,13 On the other hand, copper is an inexpensive, nonprecious transition metal with a face-centered cubic structure and lattice characteristics comparable to those of nickel. It has been reported that alloying nickel with copper exhibits favorable synergistic effects and good electrocatalytic properties toward HER with an overpotential of 140 mV at 10 mA cm−2; meanwhile, the corresponding Tafel slope was 79 mV dec−1.14 However, the synthesis method requires a significant amount of time. Hüner et al. succeeded in depositing Ni–Cu on 3D printed electrodes, enhancing their kinetic activity. The combination of Pd nanoparticles with nickel–copper foam leads to a special fractal structure with a high surface area and a very low overpotential towards HER.15,16
One of the most advantageous approaches to improving the catalytic performance of catalysts is to combine them with conducting polymers, as reported recently by many authors.17–20 This combination provides a large specific surface area, strong electrical conductivity, and excellent stability under alkaline conditions. This approach has already been used in many studies for the oxidation of alcohol (ethanol oxidation reaction EOR and methanol oxidation reaction MOR) using different conducting polymers, such as polyaniline,21 polypyrrole,22 poly-para-phenylenediamine,23 and poly-ortho-phenylenediamine, as support of bimetallic nanoparticles.24 The presence of polyaniline as a supporting matrix ensures a homogeneous distribution of the deposited palladium–silver nanoparticles, leading to excellent electrocatalytic activity and higher resistance to intermediate catalyst species.25 Furthermore, Zhanzhao Li and coworkers elaborated a MoS2/rGO/PPD/O-MWCNT catalyst via a hydrothermal process at 220 °C for 24 h, and they investigated the effect of para-phenylenediamine on the HER of the MoS2/rGO/PPD/O-MWCNT catalyst. The presence of pPD ensures good catalytic activity with 47.6 mA cm−2 as current density at an overpotential of 200 mV. This is mainly due to the fast electron transfer during the electrocatalytic reaction, coupled with a large surface area providing numerous active sites.26 Park et al. synthesized a catalyst via a solvothermal process at 120 °C for 72 h and studied the effect of amine-based COF on the HER performance of the catalyst. It was concluded that the introduction of p-phenylenediamine and 2-nitro-p-phenylenediamine enhances the performance of the catalysts due to the modification of the electrocatalytic properties by increasing the proton transfer at the electrode–solution interface.27 Ji and coworkers prepared different catalysts with metal cobalt nanoparticles and different phenylenediamines (o-phenylenediamine, m-phenylenediamine, p-phenylenediamine) via pyrolysis at 900 °C for 2 h in N2 at 5 °C min−1 to investigate HER and OER. The catalyst Co@OPDBS exhibited a remarkable performance for both cathodic HER and anodic OER reactions, which was assigned to the highest charge-transfer kinetics, conversion efficiency and exposed more active sites with an overpotential η10 of 172 mV and 289 mV, respectively.28 However, these studies suffer from serious drawbacks, such as the time-consuming synthesis process and high temperature. These aspects increase the cost of catalyst preparation and limit its widespread use in large-scale applications.29,30
To the best of our knowledge, this is the first report on the combination of para-phenylenediamine as a support matrix and Ni–Cu-based composite using an electrochemical synthesis approach to enhance the electrocatalytic performance of HER. Herein, a detailed investigation of the electrodeposition of para-phenylenediamine, copper and nickel particles was conducted using two simple pot synthesis methods. In order to achieve this purpose, our electrocatalysts were electro-synthesized in two steps using a single batch of potassium chloride solution containing copper and nickel chloride and another batch containing an appropriate concentration of para-phenylenediamine in sulfuric acid. The electrodeposition mode of para-phenylenediamine was investigated using cyclic voltammetry, chronoamperometry and chronopotentiometry. Galvanostatic and potentiostatic modes were applied to allow the deposition of nickel–copper particles. We carefully examined parameters that could affect the deposition process, including the salt concentration, deposition time, and deposition mode. The electrochemical behavior and stability of our catalyst towards HER were investigated using linear sweep voltammetry (LSV), electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV) and chronopotentiometry (CP) methods. The acquired results show that our electrocatalysts exhibit good durability, long stability, and good catalytic activity under alkaline conditions.
Experimental
Compounds and chemicals
Graphite (powder, <20 μm, synthetic, 100%) (MW = 12), H2SO4 (36%) (MW = 98), (para-phenylenediamine) (pPD) (99%) (MW = 108.14), mineral oil (heavy) (99%), CuCl2, 2H2O (99%) (MW = 170.48), NiCl2, 6H2O (97%) (MW = 237.69), potassium ferri/ferrocyanide K3Fe(CN)6/K4Fe(CN)6 3H2O (ACS reagent >99%), potassium chloride (KCl, extra pure, >99%) (MW = 74.55), and potassium hydroxide (KOH, 85%) (MW = 56.11) were provided by Sigma-Aldrich. All other chemicals were of analytical reagent grade and were used as received.Preparation of the modified electrodes
A carbon paste electrode was prepared by mixing graphite powder (1 g) and paraffin oil (300 μl) in a mortar until a homogeneous paste was formed, which was then packed into the Teflon tube electrode (3 mm) cavity (CPE).31–33 The electrochemical deposition of para-phenylenediamine was performed in a solution containing the monomer (5 mM of pPD in 1 M of H2SO4), followed by the galvanostatic electrodeposition of Ni–Cu within a solution containing NiCl2 and CuCl2 in 0.5 M of KCl with different salt concentrations. The electrode was named Ni4Cu1/pPD/CPE.Synthesis of modified carbon paste electrode using para-phenylenediamine
The synthesis of poly para-phenylenediamine (pPD) was carried out via cyclic voltammetry using a three-electrode system. A carbon paste electrode, a platinum wire, and an Ag/AgCl electrode was used as working, auxiliary, and reference electrodes, respectively. First, the pPD monomer (5.10−3 M) was dissolved in 1 M H2SO4 solution, followed by electrodeposition on the CPE for 15 cycles in a potential range between −0.3 and 0.9 V vs. Ag/AgCl at a scan rate of 0.05 V s−1. Two other approaches were used to synthesize the (pPD), which comprises galvanostatic and potentiostatic modes. The as-prepared pPD/CPE electrodes were used for electrodeposition of nickel–copper particles.Fabrication of nickel–copper particles
It is important to indicate that the deposition mechanism is a significant factor in the determination of the shape of the bimetallic particles on the electrode.34,35 The as-prepared pPD/CPE electrodes were carefully washed with bi-distilled water and placed in another aqueous solution containing 0.2 M NiCl2–0.05 M CuCl2 in 0.5 M KCl (the ratio 4/1 was already optimized in our previous work35). Two approaches have been investigated to prepare the Ni4Cu1 particles: the galvanostatic mode (applying a constant current density) and the potentiostatic mode (the applied potential of the prepared electrode was taken from the galvanostatic curve). Fig. 1 summarizes the fabrication procedures of the Ni4Cu1/pPD/CPE electrocatalyst.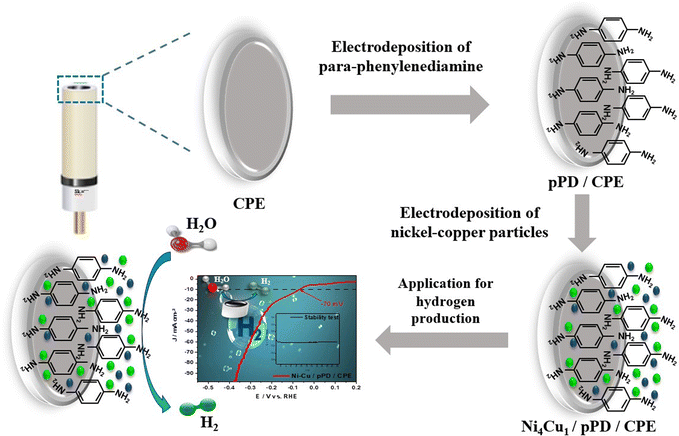 | ||
| Fig. 1 Schematic of the fabrication steps of the Ni4Cu1/pPD/CPE electrocatalyst and its application for the HER in alkaline media. | ||
Characterization and electrochemical measurements
SEM imaging was performed using a field-emission scanning electron microscope (HITACHI SU8220) at an accelerating voltage of 10 kV. The morphologies and compositions were further investigated using an energy-dispersive X-ray (EDX) detector coupled to FEG-SEM with an EDX detector (Oxford) and Transmission Electron Microscope (TEM) (acceleration voltage 200 kV, source: field emission gun, resolution 0.12 nm in imaging mode). XRD patterns were obtained by a Bruker D8-Advance X-ray diffractometer with Cu Kα irradiation (λ = 1.541874 A).All electrochemical measurements were controlled with the Versa-Studio software and conducted using the VersaSTAT 4 system in a typical three-electrode configuration with the modified electrode as the working electrode, a silver chloride electrode Ag/AgCl as the reference electrode, and a platinum disc as the auxiliary electrode. All recorded potentials were adjusted to the reversible hydrogen electrode according to the following equation: ERHE = EAg/AgCL + 0.197V + 0.059 pH.36 The catalytic performance of our modified electrode (electrocatalyst) was evaluated using linear sweep voltammetry from 0.1 to −0.6 (V vs. RHE) at a scan rate of 0.005 V s−1.37 Tafel plots were extracted from LSV curves using the equation: η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(j) + a (η: overpotential, j: current density, b: Tafel slope, a: Tafel constant).38 The electrochemical active surface area (ECSA) was calculated by the double layer capacitance method, and the charge transfer electron was determined by electrochemical impedance spectroscopy (EIS).
log(j) + a (η: overpotential, j: current density, b: Tafel slope, a: Tafel constant).38 The electrochemical active surface area (ECSA) was calculated by the double layer capacitance method, and the charge transfer electron was determined by electrochemical impedance spectroscopy (EIS).
Results and discussion
Effect of electrodeposition modes on pPD
The effect of the electrodeposition mode was investigated using three different electrodeposition modes on pPD on the CPE electrode. The first experiment was conducted using cyclic voltammetry (CV) from −0.3 to 0.9 at a scan rate of 0.05 V s−1 using 15 cycles (Fig. S1a†). Notably, the monomer oxidation occurs at 0.6 V vs. Ag/AgCl during the first cycle, while we can observe that in the backward scan, two reduction peaks appear at 0.27 and 0.45 V vs. Ag/AgCl, in line with the previous findings obtained by Halim et al.39 It's worth mentioning that the current of the cathodic and anodic peaks of the polymer increased with the consecutive potential cycling, indicating the formation of an electroactive layer of the polymer.40 The second electrode was prepared using the galvanostatic mode (CP) at a current of 0.1 mA for 40 s. As shown in Fig. S1b,† the potential increased instantly during the first seconds and then stabilized at 1.45 V, demonstrating that the pPD film was successfully synthesized.41 The last experiment was conducted using the chronoamperometry (CA) method at 0.7 V for 40 s (Fig. S1c†). According to the potentiostatic curve, the electrodeposition process comprises two steps. During the first step, the current increases (t < 3 s), indicating the formation of a radical cation. The current then decreased from 110 μA to 30 μA for 40 s, which was due to the deposition of the poly-pPD film.42After the modification with poly-pPD, the electrode was placed in a solution containing 200 mM NiCl2 and 50 mM CuCl2 prepared in 0.5 M KCl and a current of −3 mA was applied for 150 s. Fig. 2a shows the galvanostatic deposition curves of the as-prepared electrodes. During the first second, the potential decreases rapidly for the three electrocatalysts until a value of −0.95 V/vs. Ag/AgCl, indicating the reduction of nickel–copper cations on the electrode surface. Then, between 20 and 150 s, the potential gradually increased with time, attributed to the formation of hydrogen bubbles on the Ni–Cu particles.43
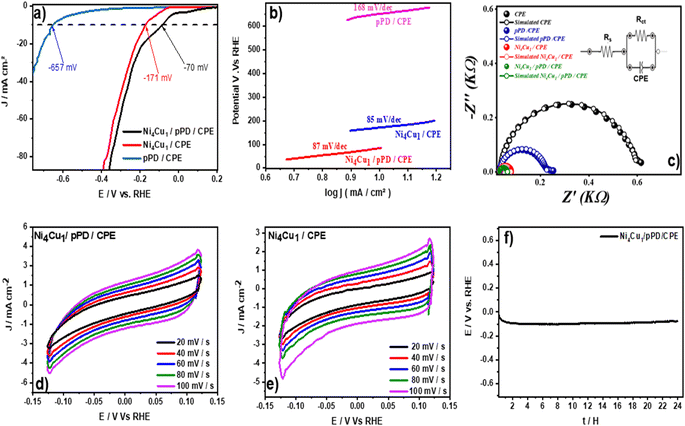
To evaluate the catalytic efficiency of the as-prepared electrodes, the electrocatalytic response in 1 M KOH was investigated by LSV (Fig. 2b). The LSV curves reveal that the deposition of pPD with cyclic voltammetry (CPE/pPD)CV exhibits poor HER activity compared to the galvanostatic mode (CPE/pPD)CP and the potentiostatic mode (CPE/pPD)CA, with an overpotential η10 of −503 mV, which might be as a result of a polymer layer formation on the surface of the electrode, which limits its conductivity and consequently reduces its active sites and performance. In contrast, the use of the galvanostatic mode (CPE/pPD)CP and the potentiostatic mode (CPE/pPD)CA demonstrates better electrocatalytic activity with an overpotential η10 of −206 mV and −245 mV, respectively. It should be noted that with the enhancement in overpotential (−275 mV>), the (CPE/pPD)CA exhibits higher electrocatalytic performance toward HER than (CPE/pPD)CP. For (CPE/pPD)CA, the higher activity toward HER is clearly due to the good conductivity of pPD deposited by the potentiostatic mode, as demonstrated in previous reported experiments.26,27 SEM images of Ni4Cu1 particles deposited on pPD by CV methods (CPE/pPD)CV, and by potentiostatic method (CPE/pPD)CA are shown in Fig. S3.† The electrocatalyst prepared with pPD by potentiostatic deposition (Fig. S3a and b†) reveals the presence of a smooth surface with a slice-shaped which provides a significant surface area. While the electrocatalyst prepared with pPD by the CV method (Fig. S3c and d†) shows a good distribution of Ni–Cu particles on the electrode, it may be noted that the particles are flower-shaped. Since reducing the overpotential is the primary challenge in HER,44 the effect of the deposition potential of pPD was investigated in order to determine the most suitable deposition potential to generate a higher amount of hydrogen. The electrocatalytic response of the different electrodes is shown in Fig. 2c; the lowest overpotential η10 (−109 mV) was obtained at an applied potential of 0 V. vs. Ag/AgCl, while the recording overpotential for the applied potentials −0.2, −0.1, 0.1, and 0.7 V were −356, −124, −176, and −245 mV, respectively. The higher catalytic efficiency of the prepared electrocatalyst at 0 V vs. Ag/AgCl toward HER may be related to the fact that the deposition of the pPD on the surface of the electrode by a simple adsorption of the monomer onto the electrode surface, which provides a large number of active sites for the nickel–copper deposition. It is important to highlight that the surface state plays an important role before the deposition of the bimetallic particle, as mentioned by many authors.34
Effect of the electrodeposition mode of nickel–copper particles
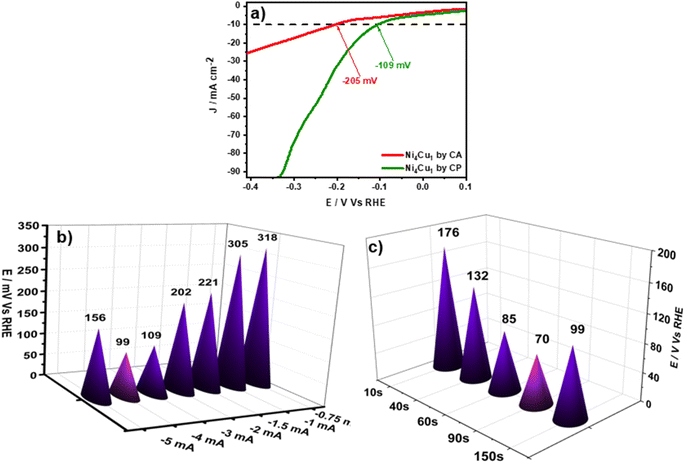
Once the electrodeposition mode of the para-phenylenediamine has been optimized, and to incorporate nickel–copper on the surface of the as-prepared electrode (CPE/pPD)CA, the next step is to find the appropriate deposition mode of the Ni4Cu1 particles. To this end, the behavior of our catalyst will be investigated using two different electrodeposition techniques, galvanostatic mode and potentiostatic mode. Fig. S4a† shows a chronopotentiometry curve of the deposition of Ni–Cu at −3 mA for 150 s. The potential decreases rapidly to a more negative value from −0.4 V to −0.9 V vs. Ag/AgCl, and represents the reduction of nickel–copper cations on the electrode surface. The potential stabilizes after 10 s at −0.9 (V vs. Ag/AgCl), reaching a plateau, probably due to the evolution of hydrogen on the crystallites of the Ni–Cu particles, as reported by Chemchoub et al.45 The second approach involved the potentiostatic deposition of Ni–Cu particles by applying a constant potential of −0.9 V vs. Ag/AgCl for 150 s (Fig. S4b†). The chronoamperometry curve shows that the potential increases and stabilizes after 25 s, indicating the nucleation of the nickel–copper particles. The same behavior for nickel cobalt particles was reported by indicating 3D nucleation.46 The growth of nickel–copper particles and their activity toward HER were examined in 1 M KOH. Fig. 3a shows the results obtained using the two approaches explained above. It can be noticed that a low overpotential η10 of −109 mV was recorded using galvanostatic mode, while an overpotential of −205 mV was observed using potentiostatic mode. A potential shift of −96 mV was observed between the two methods. Subsequent investigations will be conducted under galvanostatic conditions.The mass of nickel–copper particles deposited on the electrode ((CPE/pPD)CA) related to different electrodeposition currents was studied (the current was varied from −0.75 mA to −5 mA during 150 s). The Benchmark response of different prepared electrodes toward HER in 1 M KOH was compared (Fig. 3b). We can notice that the overpotential values decrease with the increase of electrodeposited nickel–copper particles until −4 mA (−99 mV at η10). The additional mass growth of nickel–copper leads to an elevation of the overpotential, indicating that an excess of nickel–copper amount can negatively affect the electrocatalyst by decreasing the number of its active sites.47
The effect of the electrodeposition time of Ni4Cu1 particles was investigated. For this purpose, we performed LSV tests in 1 M KOH at −4 mA, varying the durations from 10 to 150 s. Increasing the deposition duration decreased the overpotential, reaching an optimal value of −70 mV at 10 mA cm−2 for 90 s (Fig. 3c). This is probably due to the enhancement of the active surface area and the presence of coordination sites available for HER at the interface Ni–Cu, as mentioned in previous reports.48 However, higher time did not produce any additional gains. As mentioned above, an excessive amount of nickel–copper particles can negatively affect the electrocatalyst by decreasing the number of its active sites.47,49 As far as we are aware, this value was recorded for the first time using a transition metal reinforced by a layer of para-phenylenediamine. The free amine group present in the pPD plays an important role in the catalyst's performance enhancement, especially by facilitating the electrode/electrolyte interaction. Moreover, amines can influence the selectivity of reactions by favoring specific reaction pathways, which decreases the activation energy required for HER. Integrating the amine groups into the structure of the catalysts improves not only the activity but also the stability and durability, as recently reported when using multiwalled carbon nanotubes doped with nitrogen as a metal-free electrocatalyst. It has also been suggested that amine-based COF combined with Pt could facilitate proton transfer and hydrogen molecule formation.27,50 Fig. S5a† shows the electrodeposition of pPD on the CPE electrode at a constant potential of 0 V vs. Ag/AgCl for 40 s. As shown, the current increased during the first few seconds, then it stabilized at a value of −5 μA, indicating that the pPD monomer was well deposited on the carbon paste electrode. Once the pPD was formed, and in order to incorporate nickel–copper on the surface of the as-prepared electrode pPD/CPE, the electrode was promptly and carefully washed with bi-distilled water and placed in the appropriate solution (0.2 M NiCl2–0.05 M CuCl2 in 0.5 M KCl). Fig. S5b† shows the chronopotentiometry curve of Ni–Cu particle deposition (−4 mA for 90 s). The first plot decreases rapidly to a more negative value from −0.3 to −1.2 V vs. Ag/AgCl due to the reduction of nickel–copper cations. The potential stabilizes after 10 s at −1.2 (V vs. Ag/AgCl), reaching a plateau, which is in agreement with the results of previous studies.35,51 The prepared electrocatalyst was named Ni4Cu1/pPD/CPE.
Characterization of the Ni4Cu1/pPD/CPE electrocatalyst
An energy-dispersive X-ray (EDX) detector coupled to FEG-SEM and elemental mapping analysis was employed to identify the composition and distribution of element species in the prepared catalyst (Fig. 5d and e). The EDX analysis of the prepared electrocatalyst Ni4Cu1/pPD/CPE (Fig. 5e) revealed the presence of N, Ni and Cu at the surface of the electrocatalyst. The elemental atom percentages of the sample are illustrated as an inset table in Fig. 5e, which indicates that more nickel is deposited at the electrode surface compared to copper and nitrogen. This elemental mapping image (Fig. 5d) demonstrates the homogenous distribution of the deposited species.
TEM images of the optimized electrode Ni4Cu1/pPD/CPE are shown in Fig. 6. In agreement with XRD, the images (Fig. 6a and b) show a cluster of overlapping nanostructures with a relatively high contrast, suggesting denser or thicker materials. Some elongated, rod- or needle-like features are visible. The dark, elongated particles correspond to metallic nickel or copper nanoparticles. The high-aspect-ratio features are likely nanorods or nanoneedles, which could be Cu2O crystals due to their crystalline growth habits. The images (Fig. 6c and d) show well-defined rod- or platelet-like structures clustered in a roughly parallel fashion. These smaller features may correspond to Cu2O nanocrystals, which tend to form such elongated crystalline domains. The surrounding matrix might be a thin film or support of CPE/pPD, given the faint background.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log(j) + a (η, b and j are the overpotential, Tafel slope and the current density, respectively).60,61 A Tafel slope inferior to 40 mV dec−1 suggests facile water dissociation and hydrogen production via the Volmer–Tafel mechanism, whereas a Tafel slope between 40 and 120 mV dec−1 suggests fast reaction kinetics, indicating the Volmer–Heyrovsky mechanism. A Tafel slope superior to 120 mV dec−1 indicates slow reaction kinetics and slow water dissociation.62 The Tafel slopes for Ni4Cu1/pPD/CPE, and Ni4Cu1/CPE were 87, and 85 mV dec−1, respectively, indicating faster reaction kinetics compared to pPD/CPE (168 mV dec−1) (Fig. 7b), the higher Tafel slope for Ni4Cu1/pPD/CPE compared to Ni4Cu1/CPE (2 mV dec−1) might be related to higher activation energy and the adsorption characteristic of the intermediates. These smaller Tafel slopes suggest the Volmer–Heyrovsky mechanism, as the slopes are inferior to 120 mV dec−1, indicating fast adsorbed hydrogen formation followed by the reduction of hydrogen cations on the electrode followed by the discharge of the Hads with an electron and water molecule (another hydrogen source) present on the electrode surface, allowing the formation of the hydrogen gas (H2).6 The values of the Tafel slope and recorded overpotential for the previously reported electrocatalysts for HER are compared with the values obtained by the Ni4Cu1/pPD/CPE electrocatalyst Table 1. The electrocatalytic performance of our catalyst was higher than many reported catalysts, such as NiTe2 nanoflakes/Ni foam and NiSe/Ni foam.
log(j) + a (η, b and j are the overpotential, Tafel slope and the current density, respectively).60,61 A Tafel slope inferior to 40 mV dec−1 suggests facile water dissociation and hydrogen production via the Volmer–Tafel mechanism, whereas a Tafel slope between 40 and 120 mV dec−1 suggests fast reaction kinetics, indicating the Volmer–Heyrovsky mechanism. A Tafel slope superior to 120 mV dec−1 indicates slow reaction kinetics and slow water dissociation.62 The Tafel slopes for Ni4Cu1/pPD/CPE, and Ni4Cu1/CPE were 87, and 85 mV dec−1, respectively, indicating faster reaction kinetics compared to pPD/CPE (168 mV dec−1) (Fig. 7b), the higher Tafel slope for Ni4Cu1/pPD/CPE compared to Ni4Cu1/CPE (2 mV dec−1) might be related to higher activation energy and the adsorption characteristic of the intermediates. These smaller Tafel slopes suggest the Volmer–Heyrovsky mechanism, as the slopes are inferior to 120 mV dec−1, indicating fast adsorbed hydrogen formation followed by the reduction of hydrogen cations on the electrode followed by the discharge of the Hads with an electron and water molecule (another hydrogen source) present on the electrode surface, allowing the formation of the hydrogen gas (H2).6 The values of the Tafel slope and recorded overpotential for the previously reported electrocatalysts for HER are compared with the values obtained by the Ni4Cu1/pPD/CPE electrocatalyst Table 1. The electrocatalytic performance of our catalyst was higher than many reported catalysts, such as NiTe2 nanoflakes/Ni foam and NiSe/Ni foam.
Electrochemical impedance measurements were performed on the prepared electrocatalysts (Ni4Cu1/pPD/CPE, Ni4Cu1/CPE, pPD/CPE, CPE) to examine the electrochemical behavior at the electrode–solution interface. The measurements were performed in 1 M KOH at a frequency range between 100 kHz–0.1 Hz, a potential amplitude of 0.01 V and an applied potential of −0.15 V vs. RHE to calculate the charge transfer resistance (Rct), which is a crucial parameter to evaluate the catalytic reaction's kinetics.68,69 Fig. 7c displays the findings obtained using the prepared electrocatalysts Ni4Cu1/pPD/CPE, Ni4Cu1/CPE, pPD/CPE, and CPE, where the semicircle in the low-frequency area represents the kinetics of HER. The smaller the radius of the semicircle, the higher the electrocatalyst conductivity, and the higher the electron transfer.70,71 According to Fig. 7c, the prepared electrocatalyst Ni4Cu1/pPD/CPE shows a smaller radius compared to other prepared electrocatalysts (Ni4Cu1/CPE, pPD/CPE, CPE), indicating a rapid hydrogen evolution reaction kinetics. The improvement in the electron transfer rate is attributed to a good synergetic impact between Ni4Cu1 particles and the pPD, which provides a high surface area and good electrical properties, facilitating electron transport.72–74 The fitting circuit is shown in the inset of Fig. 7c, where Rs represents the electrolyte resistance, Rct represents the charge transfer resistance, and CPE is used as a constant phase element to replace the double-layer capacitor of the charge transfer process. The Rct reached the lowest value with the Ni4Cu1/pPD/CPE electrocatalyst (33 and 11 Ω), while Rct for Ni4Cu1/CPE, pPD/CPE, and CPE were 45, 91, 197, 570, and 5 Ω, respectively. The EIS response confirms the HER results obtained from polarization tests (LSV).
In addition, the double layer capacitance Cdl method was used to determine the electrochemically active surface area ECSA of the prepared electrocatalysts. The CV curves at different scan rates of (d) Ni4Cu1/pPD/CPE electrocatalyst and (e) Ni4Cu1/CPE electrocatalyst are shown in Fig. 7d and e. The non-faradic potential range was used to examine CV curves, as well as the cathodic current density Jc and the anodic current density Ja to calculate the double-layer capacitance using the following expressions:75
The value of Cdl is 14.23 mF cm−2 and 12.15 mF cm−2 for the Ni4Cu1/pPD/CPE and Ni4Cu1/CPE electrocatalysts, respectively (Fig. S6†). The ECSA was estimated using the following formula:76
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 706 cm2), and 20 μF cm−2 is the ideal double-layer capacitance value.77 The calculated ECSA values for the prepared electrocatalysts Ni4Cu1/pPD/CPE and Ni4Cu1/CPE are 50.23 cm2 and 42.89 cm2, respectively. Consequently, the prepared electrocatalyst has a large effective surface area and high overall conductivity, leading to an enhancement of catalytic performance.78,79
706 cm2), and 20 μF cm−2 is the ideal double-layer capacitance value.77 The calculated ECSA values for the prepared electrocatalysts Ni4Cu1/pPD/CPE and Ni4Cu1/CPE are 50.23 cm2 and 42.89 cm2, respectively. Consequently, the prepared electrocatalyst has a large effective surface area and high overall conductivity, leading to an enhancement of catalytic performance.78,79
Durability and stability are important parameters for assessing the quality of the prepared electrocatalysts.80 To this end, we used chronopotentiometry and linear voltammetry to investigate the stability of the prepared electrocatalyst Ni4Cu1/pPD/CPE at −10 mA cm−2 in alkaline media, 1 M KOH. As shown in Fig. 7f, the continuous chronopotentiometry curve did not show any movement in the negative direction during the continuous electrolysis, and the overpotential shifted slightly towards the positive direction within 24 h. This confirms that the Ni4Cu1/pPD/CPE electrocatalyst is highly stable during long-term electrolysis and maintains its hydrogen evolution catalytic activity under stable current density conditions. The long-term stability of the Ni4Cu1/pPD/CPE electrocatalyst was also tested in 1 M KOH by cyclic voltammetry. Fig. S7† shows a comparison of the polarization curve after 1000 cycles by cyclic voltammetry (CV) in a potential region of the linear sweep polarization region. The polarization curve after the CV test shifted slightly to a negative value, reaching a value of −105 mV at η10. We can notice that for higher current density (100 mA cm−2), only a difference of 10 mV was observed. These comparisons highlight the high performance of the optimized Ni4Cu1/pPD/CPE electrocatalyst composition.
Conclusion
A novel and highly efficient hybrid electrocatalyst based on Ni–Cu reinforced with a layer of pPD was successfully synthesized using a straightforward and rapid electrodeposition method for monitoring the HER in alkaline media. Three electrodepositions modes of pPD have been investigated: galvanostatic mode, potentiostatic mode and cyclic voltammetry. The electrodeposition of nickel–copper particles was performed using the galvanostatic mode. The estimated time for catalyst fabrication was 2 to 3 minutes, given a rapid and efficient pathway to the synthesis of electrocatalysts. The best response in terms of overpotential was obtained by pPD deposition at 0 V vs. Ag/AgCl for 40 s, followed by deposition of Ni–Cu particles at −4 mA for 90 s. The presence of amino groups at the electrode-solution interface with negative charge at 0 V appears to promote uniform dispersion of Ni–Cu particles, preventing their agglomeration. The ESCA calculation and the EIS show that the prepared electrocatalyst has a high surface area and low charge transfer resistance compared to other catalysts, Ni4Cu1/CPE and pPD/CPE. Interestingly, the Ni4Cu1/pPD/CPE electrocatalyst demonstrated an excellent catalytic performance for HER, exhibiting a very low overpotential η10 of −70 mV. Moreover, it maintained high stability and durability for 24 h in alkaline media. These findings highlight the superior electrocatalytic properties of the Ni4Cu1/pPD/CPE electrocatalyst for efficient HER activity in water splitting, indicating its promising potential for sustainable H2 production.Abbreviations
| HER | Hydrogen Evolution Reaction |
| OER | Oxygen Evolution Reaction |
| Ni–Cu particles | Nickel–Copper particles |
| Ni | Nickel |
| Cu | Copper |
| CPE | Carbon Paste Electrode |
| pPD | para-Phenylenediamine |
| PpPD | Poly-para-Phenylenediamine |
| Ni4Cu1/pPD/CPE | The prepared electrocatalyst by Ni–Cu particles supported by pPD |
| FE-SEM | Field Emission coupled with Scanning Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| EDX | Energy-dispersive X-ray |
| XRD | X-ray Diffraction |
| LSV | Linear Sweep Voltammetry |
| CV | Cyclic Voltammetry |
| CA | Chronoamperometry |
| CP | Chronopotentiometry |
| η10 | The overpotential required for an electrocatalyst to reach 10 mA cm−2 |
| RES | Renewable Energy Sources |
| DC | Direct Current |
| RHE | Reversible Hydrogen Electrode |
| Ag/AgCl | Silver Chloride Electrode |
| WE | Working Electrode |
| RE | Reference Electrode |
| CE | Counter Electrode or auxiliary electrode |
Data availability
Data ESI† of this study can be found within the article.Author contributions
Skakri Soufiane: writing – review and editing, writing – original draft, methodology, investigation, data curation, and conceptualization. El Attar Anas: review and editing and conceptualization. Benhaiba Saad: conceptualization. Bouljoihel Badr: data curation. Mouakkar Anas: investigation. Aaddane Abdellah: co-supervision. Adiba Rais: data curation. El Rhazi Mama: writing – review and editing, methodology, data curation, conceptualization, supervision, and funding acquisition.Conflicts of interest
The authors declare that they have no conflicts of interest.Acknowledgements
The authors thank the Centre Nationale pour la Recherche Scientifique et Technique (CNRST), especially Mr El Bouji Mohamed, Mr Nejmi Youssef, and Mr Bouchaqour Zakaria for the SEM and TEM facilities.References
- F. Dawood, M. Anda and G. M. Shafiullah, Hydrogen production for energy: An overview, Int. J. Hydrogen Energy, 2020, 45, 3847–3869 CrossRef CAS.
- S. P. Filippov and A. B. Yaroslavtsev, Hydrogen energy: development prospects and materials, Russ. Chem. Rev., 2021, 90, 627–643 CrossRef CAS.
- M. Nemiwal, V. Gosu, T. C. Zhang and D. Kumar, Metal organic frameworks as electrocatalysts: Hydrogen evolution reactions and overall water splitting, Int. J. Hydrogen Energy, 2021, 46, 10216–10238 CrossRef CAS.
- M. Cai, L. Xu, J. Guo, X. Yang, X. He and P. Hu, Recent advances in metal-free electrocatalysts for the hydrogen evolution reaction, J. Mater. Chem. A, 2024, 12, 592–612 RSC.
- A. Lasia, in Handbook of Fuel Cells, ed. W. Vielstich, A. Lamm, H. A. Gasteiger and H. Yokokawa, Wiley, 1st edn, 2010 Search PubMed.
- F. Bao, E. Kemppainen, I. Dorbandt, R. Bors, F. Xi, R. Schlatmann, R. Van De Krol and S. Calnan, Understanding the Hydrogen Evolution Reaction Kinetics of Electrodeposited Nickel-Molybdenum in Acidic, Near-Neutral, and Alkaline Conditions, Chemelectrochem, 2021, 8, 195–208 CrossRef CAS.
- X. Zou and Y. Zhang, Noble Metal-Free Hydrogen Evolution Catalysts for Water Splitting, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- M. Schalenbach, O. Kasian, M. Ledendecker, F. D. Speck, A. M. Mingers, K. J. J. Mayrhofer and S. Cherevko, The Electrochemical Dissolution of Noble Metals in Alkaline Media, Electrocatalysis, 2018, 9, 153–161 CrossRef CAS.
- S. Muthu, M. B. Gandhi, S. Saravanan, S. Sekar, S. Arun Kumar, P. Ilanchezhiyan, S. Lee and M. B. Sridharan, Hierarchical nanocomposite of cobalt sulfide nanoflakes-decorated reduced graphene oxide for enhancing the electrocatalytic hydrogen evolution reaction, Int. J. Hydrogen Energy, 2024, 52, 1405–1414 CrossRef CAS.
- R. Manikandan, S. Sadhasivam, S. Lee, S.-C. Chang, K. Ashok Kumar, C. Bathula, V. Gopalan Sree, D. Young Kim and S. Sekar, Deep eutectic solvents assisted synthesis of AC-decorated NiO nanocomposites for hydrogen evolution reaction, J. Mol. Liq., 2023, 375, 121338 CrossRef CAS.
- H. Zhou, D. Zhang, W. Dong, C. Zhou, T. Jiang, Y. Liu, C. Tian, Y. Liu, Y. Liu and B. Mao, Stainless steel mesh-based CoSe/Ni3Se4 heterostructure for efficient electrocatalytic overall water splitting, Int. J. Hydrogen Energy, 2023, 48, 14554–14564 CrossRef CAS.
- R. Zahra, E. Pervaiz, M. Yang, O. Rabi, Z. Saleem, M. Ali and S. Farrukh, A review on nickel cobalt sulphide and their hybrids: Earth abundant, pH stable electro-catalyst for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2020, 45, 24518–24543 CrossRef CAS.
- M. Zhang, D. Hu, Z. Xu, B. Liu, M. Boubeche, Z. Chen, Y. Wang, H. Luo and K. Yan, Facile synthesis of Ni-, Co-, Cu-metal organic frameworks electrocatalyst boosting for hydrogen evolution reaction, J. Mater. Sci. Technol., 2021, 72, 172–179 CrossRef CAS.
- Z. Li, C. Yu, Y. Wen, Y. Gao, X. Xing, Z. Wei, H. Sun, Y.-W. Zhang and W. Song, Mesoporous Hollow Cu–Ni Alloy Nanocage from Core–Shell Cu@Ni Nanocube for Efficient Hydrogen Evolution Reaction, ACS Catal., 2019, 9, 5084–5095 CrossRef CAS.
- B. Hüner, N. Demir and M. F. Kaya, Electrodeposition of NiCu bimetal on 3D printed electrodes for hydrogen evolution reactions in alkaline media, Int. J. Hydrogen Energy, 2022, 47, 12136–12146 CrossRef.
- S. Mojabi and S. Sanjabi, Decorated fractal Ni-Cu foam with Pd nanoparticles as a high-performance electrocatalyst toward hydrogen evolution reaction, Thin Solid Films, 2022, 758, 139415 CrossRef CAS.
- H. Ashassi-Sorkhabi, A. Kazempour, S. Moradi-Alavian, E. Asghari and J. J. Lamb, 3D nanostructured nickel film supported to a conducting polymer as an electrocatalyst with exceptional properties for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2023, 48, 29865–29876 CrossRef CAS.
- S. Cogal, G. Celik Cogal, M. Mičušík, M. Kotlár and M. Omastová, Cobalt-doped WSe2@conducting polymer nanostructures as bifunctional electrocatalysts for overall water splitting, Int. J. Hydrogen Energy, 2024, 49, 689–700 CrossRef CAS.
- K. E. Ramohlola, K. D. Modibane, M. M. Ndipingwi and E. I. Iwuoha, Polyaniline-based electrocatalysts for electrochemical hydrogen evolution reaction, Eur. Polym. J., 2024, 213, 113125 CrossRef CAS.
- R. Zahra, N. Drissi and A. Kumar, Enhanced HER activity through FeAlO3 perovskite oxide incorporated with conducting polymer PANI, Inorg. Chem. Commun., 2024, 113609 Search PubMed.
- N. M. Ivanova, E. A. Soboleva and Y. A. Visurkhanova, Bimetallic Co–Cu polyaniline composites: Structure and electrocatalytic activity, Russ. J. Appl. Chem., 2016, 89, 1072–1081 CrossRef CAS.
- P. Pattanayak, N. Pramanik, P. Kumar and P. P. Kundu, Fabrication of cost-effective non-noble metal supported on conducting polymer composite such as copper/polypyrrole graphene oxide (Cu 2 O/PPy–GO) as an anode catalyst for methanol oxidation in DMFC, Int. J. Hydrogen Energy, 2018, 43, 11505–11519 CrossRef CAS.
- S. Chemchoub, M. Elbasri, El. M. Halim and M. El Rhazi, The electrocatalytic oxidation of methanol on a carbon paste electrode modified by poly(para-phenylenediamine) and Nickel particles, Mater. Today: Proc., 2019, 13, 720–729 CAS.
- P. Gajendran and R. Saraswathi, Electrocatalytic performance of poly(o-phenylenediamine)-Pt–Ru nanocomposite for methanol oxidation, J. Solid State Electrochem., 2013, 17, 2741–2747 CrossRef CAS.
- Z. Nodehi, A. A. Rafati and A. Ghaffarinejad, Palladium-silver polyaniline composite as an efficient catalyst for ethanol oxidation, Appl. Catal., A, 2018, 554, 24–34 CrossRef CAS.
- Z. Li, X. Dai, K. Du, Y. Ma, M. Liu, H. Sun, X. Ma and X. Zhang, Reduced Graphene Oxide/O-MWCNT Hybrids Functionalized with p-Phenylenediamine as High-Performance MoS2 Electrocatalyst Support for Hydrogen Evolution Reaction, J. Phys. Chem. C, 2016, 120, 1478–1487 CrossRef CAS.
- J. H. Park, C. H. Lee, S. Yu, P. Kharel, R. Choi, C. Zhang, P. Y. Huang, J. S.-I. Kwon and H. Yang, Effects of amine-based covalent organic framework on platinum electrocatalyst performance towards hydrogen evolution reaction, Nano Energy, 2024, 128, 109947 CrossRef CAS.
- X. Ji, L. Bo, Y. Zhang, W. Shi, X. Guan, L. Xia, Y. Shen, Y. Wang and J. Tong, Simply constructed highly dispersed cobalt nanoparticles in diverse N-doped graphitic carbon with remarkable performances for water electrolysis, Int. J. Hydrogen Energy, 2022, 47, 25511–25521 CrossRef CAS.
- K. Sun, Z. Li, Y. Cao, F. Wang, M. A. Qyyum and N. Han, Recent advancements in perovskite electrocatalysts for clean energy-related applications: Hydrogen production, oxygen electrocatalysis, and nitrogen reduction, Int. J. Hydrogen Energy, 2024, 52, 1104–1126 CrossRef CAS.
- H. N. Nguyen and D. A. Khuong, in Engineered Biochar, ed. S. Ramola, D. Mohan, O. Masek, A. Méndez and T. Tsubota, Springer Nature Singapore, Singapore, 2022, pp. 105–126 Search PubMed.
- F. E. Salih, L. Oularbi, E. Halim, M. Elbasri, A. Ouarzane and M. El Rhazi, Conducting Polymer/Ionic Liquid Composite Modified Carbon Paste Electrode for the Determination of Carbaryl in Real Samples, Electroanalysis, 2018, 30, 1855–1864 CrossRef.
- L. Oularbi, M. Turmine, F. E. Salih and M. El Rhazi, Ionic liquid/carbon nanofibers/bismuth particles novel hybrid nanocomposite for voltammetric sensing of heavy metals, J. Environ. Chem. Eng., 2020, 8, 103774 CrossRef CAS.
- A. El Attar, L. Oularbi, S. Chemchoub and M. El Rhazi, Preparation and characterization of copper oxide particles/polypyrrole (Cu2O/PPy) via electrochemical method: Application in direct ethanol fuel cell, Int. J. Hydrogen Energy, 2020, 45, 8887–8898 CrossRef CAS.
- Z. Zhang, Y. Wu and D. Zhang, Potentiostatic electrodeposition of cost-effective and efficient Ni–Fe electrocatalysts on Ni foam for the alkaline hydrogen evolution reaction, Int. J. Hydrogen Energy, 2022, 47, 1425–1434 CrossRef CAS.
- S. Chemchoub, A. El Attar, L. Oularbi, S. A. Younssi, F. Bentiss, C. Jama and M. El Rhazi, Electrosynthesis of eco-friendly electrocatalyst based nickel-copper bimetallic nanoparticles supported on poly-phenylenediamine with highest current density and early ethanol oxidation onset potential, Int. J. Hydrogen Energy, 2022, 47, 39081–39096 CrossRef CAS.
- Y. Li, K. Dastafkan, Q. Sun, Y. Ma, X. Wang, X. Yang, Z. Wang and C. Zhao, Ni-based 3D hierarchical heterostructures achieved by selective electrodeposition as a bifunctional electrocatalyst for overall water splitting, Electrochim. Acta, 2021, 379, 138042 CrossRef CAS.
- S. Dongre S, A. Iqbal, R. Thapa, P. M, S. Ramu and R. G. Balakrishna, Synergistic Catalyst Design for Enhanced Electrochemical Hydrogen Evolution: Fe 2 O 3/MoS 2/Ti 3 C 2 T x MXene Ternary Composite, ACS Appl. Eng. Mater., 2024, 2, 943–954 CrossRef CAS.
- A. Kumar and S. Bhattacharyya, Porous NiFe-Oxide Nanocubes as Bifunctional Electrocatalysts for Efficient Water-Splitting, ACS Appl. Mater. Interfaces, 2017, 9, 41906–41915 CrossRef CAS PubMed.
- E. M. Halim, M. Elbasri, H. Perrot, O. Sel, K. Lafdi and M. El Rhazi, Synthesis of carbon nanofibers/poly(para-phenylenediamine)/nickel particles nanocomposite for enhanced methanol electrooxidation, Int. J. Hydrogen Energy, 2019, 44, 24534–24545 CrossRef CAS.
- A. K. D. Diaw, D. Gningue-Sall, A. Yassar and J. J. Aaron, New poly(p-substituted-N-phenylpyrrole)s. Electrosynthesis, electrochemical properties and characterization, Synth. Met., 2013, 179, 74–85 CrossRef CAS.
- L. M. Samyn, R. S. Babu, M. Devendiran and A. L. F. De Barros, Electropolymerization of p-phenylenediamine films on carbon fiber fabrics electrode for flexible supercapacitors: surface and electrochemical characterizations, Ionics, 2020, 26, 3041–3050 CrossRef CAS.
- E. M. Halim, H. Perrot, O. Sel, C. Debiemme-Chouvy, K. Lafdi and M. El Rhazi, Electrosynthesis of hierarchical Cu2O–Cu(OH)2 nanodendrites supported on carbon nanofibers/poly(para-phenylenediamine) nanocomposite as high-efficiency catalysts for methanol electrooxidation, Int. J. Hydrogen Energy, 2021, 46, 19926–19938 CrossRef CAS.
- X. Qiao, H. Li, W. Zhao and D. Li, Effects of deposition temperature on electrodeposition of zinc–nickel alloy coatings, Electrochim. Acta, 2013, 89, 771–777 CrossRef CAS.
- Y. Li, X. Xie, Y. Wang, X. Yuan and F. Li, Nickel-based alloy nanoparticles anchored on nickel mesh towards excellent and sustainable water electrolysis, Int. J. Electrochem. Sci., 2023, 18, 100216 CrossRef.
- S. Chemchoub, A. El Attar, L. Oularbi, S. A. Younssi, F. Bentiss, C. Jama and M. El Rhazi, Electrosynthesis of eco-friendly electrocatalyst based nickel-copper bimetallic nanoparticles supported on poly-phenylenediamine with highest current density and early ethanol oxidation onset potential, Int. J. Hydrogen Energy, 2022, 47, 39081–39096 CrossRef CAS.
- W. Li, J. Hao, S. Mu and W. Liu, Electrochemical behavior and electrodeposition of Ni-Co alloy from choline chloride-ethylene glycol deep eutectic solvent, Appl. Surf. Sci., 2020, 507, 144889 CrossRef CAS.
- H. Zhu, J. Wang, X. Liu and X. Zhu, Three-dimensional porous graphene supported Ni nanoparticles with enhanced catalytic performance for Methanol electrooxidation, Int. J. Hydrogen Energy, 2017, 42, 11206–11214 CrossRef CAS.
- A. Irshad and N. Munichandraiah, Electrodeposited Nickel–Cobalt–Sulfide Catalyst for the Hydrogen Evolution Reaction, ACS Appl. Mater. Interfaces, 2017, 9, 19746–19755 CrossRef CAS PubMed.
- W. Tan, X. Liu, W. Liu, H. He and Y. Yang, Galvanostatic electrodeposition of a self-supported Ni–Se–Lu/NF electrocatalyst for efficient alkaline hydrogen evolution reaction, Int. J. Hydrogen Energy, 2024, 80, 270–279 CrossRef CAS.
- R. P. Dighole, A. V. Munde, B. B. Mulik, S. C. Dhawale and B. R. Sathe, Facile synthesis of nitrogen-containing multiwalled carbon nanotubes as a worth metal-free electrocatalyst for hydrogen evolution reaction and semicarbazide oxidation, Int. J. Hydrogen Energy, 2024, 91, 285–293 CrossRef CAS.
- N. A. M. Barakat, M. A. Yassin, F. S. Al-Mubaddel and M. T. Amen, New electrooxidation characteristic for Ni-based electrodes for wide application in methanol fuel cells, Appl. Catal., A, 2018, 555, 148–154 CrossRef CAS.
- F. M. Souza, P. Böhnstedt, V. S. Pinheiro, L. A. Oliveira, B. L. Batista, L. S. Parreira, R. A. Antunes and M. C. Santos, Niobium increasing the electrocatalytic activity of palladium for alkaline direct ethanol fuel cell, J. Electroanal. Chem., 2020, 858, 113824 CrossRef CAS.
- X. Chen, R. Chen, X. Zhu, Q. Liao, Y. Zhang, D. Ye, B. Zhang, Y. Yu and J. Li, A solar responsive cubic nanosized CuS/Cu2O/Cu photocathode with enhanced photoelectrochemical activity, J. Catal., 2019, 372, 182–192 CrossRef CAS.
- A. El Attar, L. Oularbi, S. Chemchoub and M. El Rhazi, Preparation and characterization of copper oxide particles/polypyrrole (Cu2O/PPy) via electrochemical method: Application in direct ethanol fuel cell, Int. J. Hydrogen Energy, 2020, 45, 8887–8898 CrossRef CAS.
- C. Cui, Y. Liu, S. Mehdi, H. Wen, B. Zhou, J. Li and B. Li, Enhancing effect of Fe-doping on the activity of nano Ni catalyst towards hydrogen evolution from NH3BH3, Appl. Catal., B, 2020, 265, 118612 CrossRef CAS.
- X. Wang, S. Yang, Y. Yu, M. Dou, Z. Zhang and F. Wang, Low-loading Pt nanoparticles embedded on Ni, N-doped carbon as superior electrocatalysts for oxygen reduction, Catal. Sci. Technol., 2020, 10, 65–69 RSC.
- S. A. Al-Thabaiti, Z. Khan and M. A. Malik, Bimetallic Ag-Ni nanoparticles as an effective catalyst for hydrogen generation from hydrolysis of sodium borohydride, Int. J. Hydrogen Energy, 2019, 44, 16452–16466 CrossRef CAS.
- M. Negem, H. Nady and C. W. Dunnill, Nanocrystalline Ni–Cu Electroplated Alloys Cathodes for Hydrogen Generation in Phosphate-Buffered Neutral Electrolytes, J. Bio Tribo Corros., 2020, 6, 116 CrossRef.
- M. Negem and H. Nady, Electroplated Ni-Cu nanocrystalline alloys and their electrocatalytic activity for hydrogen generation using alkaline solutions, Int. J. Hydrogen Energy, 2017, 42, 28386–28396 CrossRef CAS.
- Y. Zhu, T. Liu, L. Li, S. Song and R. Ding, Nickel-based electrodes as catalysts for hydrogen evolution reaction in alkaline media, Ionics, 2018, 24, 1121–1127 CrossRef CAS.
- J. Ping, Y. Wang, Q. Lu, B. Chen, J. Chen, Y. Huang, Q. Ma, C. Tan, J. Yang, X. Cao, Z. Wang, J. Wu, Y. Ying and H. Zhang, Self-Assembly of Single-Layer CoAl-Layered Double Hydroxide Nanosheets on 3D Graphene Network Used as Highly Efficient Electrocatalyst for Oxygen Evolution Reaction, Adv. Mater., 2016, 28, 7640–7645 CrossRef CAS PubMed.
- H. Sun, C. Tian, Y. Li, J. Wu, Q. Wang, Z. Yan, C. Li, F. Cheng and M. Du, Coupling NiCo Alloy and CeO2 to Enhance Electrocatalytic Hydrogen Evolution in Alkaline Solution, Adv. Sustainable Syst., 2020, 4, 2000122 CrossRef CAS.
- S. Anantharaj, K. Karthick and S. Kundu, NiTe2 Nanowire Outperforms Pt/C in High-Rate Hydrogen Evolution at Extreme pH Conditions, Inorg. Chem., 2018, 57, 3082–3096 CrossRef CAS PubMed.
- L. Yang, H. Xu, H. Liu, D. Cheng and D. Cao, Active Site Identification and Evaluation Criteria of In Situ Grown CoTe and NiTe Nanoarrays for Hydrogen Evolution and Oxygen Evolution Reactions, Small Methods, 2019, 3, 1900113 CrossRef.
- J.-X. Feng, J.-Q. Wu, Y.-X. Tong and G.-R. Li, Efficient Hydrogen Evolution on Cu Nanodots-Decorated Ni3S2 Nanotubes by Optimizing Atomic Hydrogen Adsorption and Desorption, J. Am. Chem. Soc., 2018, 140, 610–617 CrossRef CAS PubMed.
- N. Jiang, Q. Tang, M. Sheng, B. You, D. Jiang and Y. Sun, Nickel sulfides for electrocatalytic hydrogen evolution under alkaline conditions: a case study of crystalline NiS, NiS2, and Ni3S2 nanoparticles, Catal. Sci. Technol., 2016, 6, 1077–1084 RSC.
- H. Ren, Z.-H. Huang, Z. Yang, S. Tang, F. Kang and R. Lv, Facile synthesis of free-standing nickel chalcogenide electrodes for overall water splitting, J. Energy Chem., 2017, 26, 1217–1222 CrossRef.
- N. Hallemans, W. D. Widanage, X. Zhu, S. Moharana, M. Rashid, A. Hubin and J. Lataire, Operando electrochemical impedance spectroscopy and its application to commercial Li-ion batteries, J. Power Sources, 2022, 547, 232005 CrossRef CAS.
- L. Oularbi, M. Turmine and M. El Rhazi, Preparation of novel nanocomposite consisting of bismuth particles, polypyrrole and multi-walled carbon nanotubes for simultaneous voltammetric determination of cadmium(II) and lead(II), Synth. Met., 2019, 253, 1–8 CrossRef CAS.
- C. Hitz and A. Lasia, Experimental study and modeling of impedance of the her on porous Ni electrodes, J. Electroanal. Chem., 2001, 500, 213–222 CrossRef CAS.
- B. Fei, Z. Chen, J. Liu, H. Xu, X. Yan, H. Qing, M. Chen and R. Wu, Ultrathinning Nickel Sulfide with Modulated Electron Density for Efficient Water Splitting, Adv. Energy Mater., 2020, 10, 2001963 CrossRef CAS.
- C. R. Rawool, N. S. Punde, A. S. Rajpurohit, S. P. Karna and A. K. Srivastava, High energy density supercapacitive material based on a ternary hybrid nanocomposite of cobalt hexacyanoferrate/carbon nanofibers/polypyrrole, Electrochim. Acta, 2018, 268, 411–423 CrossRef CAS.
- Z. Kang, M. Zhao, Y. Wu, T. Xia, J.-P. Cao, W. Cai and L. Chen, Facial fabrication of yolk-shell Pd-Ni-P alloy with mesoporous structure as an advanced catalyst for methanol electro-oxidation, Appl. Surf. Sci., 2019, 484, 441–445 CrossRef CAS.
- L. Oularbi, M. Turmine and M. El Rhazi, Electrochemical determination of traces lead ions using a new nanocomposite of polypyrrole/carbon nanofibers, J. Solid State Electrochem., 2017, 21, 3289–3300 CrossRef CAS.
- H. Dong, T. Lei, Y. He, N. Xu, B. Huang and C. T. Liu, Electrochemical performance of porous Ni3Al electrodes for hydrogen evolution reaction, Int. J. Hydrogen Energy, 2011, 36, 12112–12120 CrossRef CAS.
- A. Kellenberger, N. Vaszilcsin, W. Brandl and N. Duteanu, Kinetics of hydrogen evolution reaction on skeleton nickel and nickel–titanium electrodes obtained by thermal arc spraying technique, Int. J. Hydrogen Energy, 2007, 32, 3258–3265 CrossRef CAS.
- N. Krstajic, V. Jovic, L. Gajickrstajic, B. Jovic, A. Antozzi and G. Martelli, Electrodeposition of Ni–Mo alloy coatings and their characterization as cathodes for hydrogen evolution in sodium hydroxide solution, Int. J. Hydrogen Energy, 2008, 33, 3676–3687 CrossRef CAS.
- M. A. Lukowski, A. S. Daniel, F. Meng, A. Forticaux, L. Li and S. Jin, Enhanced Hydrogen Evolution Catalysis from Chemically Exfoliated Metallic MoS 2 Nanosheets, J. Am. Chem. Soc., 2013, 135, 10274–10277 CrossRef CAS PubMed.
- M. Y. Gao, C. Yang, Q. B. Zhang, Y. W. Yu, Y. X. Hua, Y. Li and P. Dong, Electrochemical fabrication of porous Ni-Cu alloy nanosheets with high catalytic activity for hydrogen evolution, Electrochim. Acta, 2016, 215, 609–616 CrossRef CAS.
- Y. Wei, R. A. Soomro, X. Xie and B. Xu, Design of efficient electrocatalysts for hydrogen evolution reaction based on 2D MXenes, J. Energy Chem., 2021, 55, 244–255 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02533h |
| ‡ Dr Badr Bouljoihel, who made significant contributions to the development of this work, sadly passed away before the submission of this manuscript. He was a brilliant scientist, and the authors gratefully acknowledge his insight, dedication, and lasting impact on this research. |
| This journal is © The Royal Society of Chemistry 2025 |