Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Biopolymer-based electrospun nanofiber membranes for smart food packaging applications: a review
Maria Mathew
,
Sagitha Paroly
and
Sujith Athiyanathil
 *
*
Material Research Laboratory, Department of Chemistry, National Institute of Technology, Calicut, Kerala 673601, India. E-mail: sujith@nitc.ac.in; athiyanathil.sujith@gmail.com
First published on 25th June 2025
Abstract
Food safety is an important concern impacting public health and the well-being of society. Novel food packaging systems are adopted by the market owing to the increased consumer preference for healthy food products. To minimize food spoilage, it is essential to monitor the food quality throughout the food supply chain in real time. The development of food packaging systems such as active and intelligent packaging has been greatly accelerated to meet these requirements. It relies on the development of new materials and technologies to monitor the quality, shelf life, and usability of food products for its end-users. Polymeric nanofibers, which can be easily functionalized, are good candidates for these applications. This review describes the role of electrospun nanofibers in fabricating various active and intelligent food packaging systems. Electrospun nanofibers encapsulated with bioactive ingredients can serve as a promising and environmentally sustainable alternative to conventional plastic systems for widespread use. Moreover, nanofiber packaging materials have many functional advantages such as controlled release of active agents, gas permeability, and flexibility. This review will be a valuable source of information for a wide group of researchers working in the area of electrospinning and biopolymer-based active and intelligent food packaging systems.
1. Introduction
Food packaging is considered a crucial step in the food production process as it has a significant impact on the quality, safety, and freshness of food items. It acts as a barrier to various contaminants, including air, light, moisture, microbes, and many physical and chemical pollutants.1 Traditional food packaging is meant to only provide physical support and protection against external environmental stimuli such as light, temperature, moisture, oxidation, and various microbial contaminations.2 This packaging ensures the primary purpose of protecting a product during the processes of transportation, distribution, and storage.3 Materials that have traditionally been used for food packaging include glass, metals (aluminium foils and laminates, tin plates, and steel), paper, and plastics. However, food deterioration during distribution, transport, and storage results in food loss and food-related health issues. Proper food packaging can reduce these issues to a great extent. Considering the increased demand for improving the shelf life of food products and real-time quality monitoring, traditional food packaging is being replaced by smart packaging technologies. Smart food packaging is an innovative packaging solution that can detect, record, and sense the quality of food products and convey efficiently to the consumers. Smart food packaging utilizes sensors or indicators that can detect and analyse various factors such as freshness, pathogens, pH, CO2, oxygen, or temperature.4Active food packaging and intelligent food packaging are the two broad classifications of smart “interactive” food packaging. Active food packaging involves the incorporation of certain active components into the packaging material that can release antioxidants, antibacterial agents, CO2 emitters, etc. Additionally, they can absorb moisture, ethylene gas, and oxygen from the packaging itself and its surrounding environment.5 Thus, an active packaging system modifies the packaging environment during the preservation period without compromising the quality and safety of packaged foods. The next category of smart food packaging system is intelligent packaging. The goal of intelligent food packaging is to convey information regarding food freshness via various signals, i.e. using sensors, indicators, and data carriers.6 Recently, such smart food packaging has attracted great attention due to its ability for the real-time monitoring of food freshness and spoilage. It also enables the tracking and tracing of a product throughout its lifecycle, and this information can be utilized by the manufacturers, retailers, or consumers to understand the real-time condition of the products.7
To fulfill the criteria for smart food packaging, the active agents integrated within the packaging should exhibit exceptional stability, enabling their controlled and gradual release without any loss or degradation. Among the various techniques employed in food packaging, membrane-based processes are particularly favored due to their adaptability, cost-effectiveness, low energy requirements, high reliability, ease of scale-up, and environmentally sustainable nature.8 Several methods such as solution casting, 3D printing, sol–gel method, extrusion, and electrospinning are available for the fabrication of smart food packaging.9 However, since most active agents evaporate easily due to their high volatility, it is not feasible to incorporate them directly through conventional polymer processing methods. To overcome these problems, electrospinning technique can be employed due to its simplicity and cost-effectiveness in producing nanofibers with large surface area, porosity, flexibility, and other tunable features. Nanofibers are typically defined as fibers having diameters less than 1000 nm.10 Due to the high encapsulation efficiency, electrospun nanofibers can act as excellent carriers of various active agents for smart food packaging applications.11,12 Electrospinning offers several advantages over the conventional encapsulation and film-forming methods. It enables the production of nanofiber membranes with a high surface area-to-volume ratio and tunable porosity, enhancing barrier properties and functional activities. The process allows for the efficient encapsulation of bioactive agents (antioxidants, antibacterial agents, enzymes, and prebiotics) under mild conditions. The introduction of active agents into electrospun membranes can be achieved through two main approaches. The first method involves the incorporation of agents directly into the electrospinning solution, via dissolving or dispersing the agents within the polymer matrix.13 The second approach relies on post-treatment methods such as grafting, dip coating, and surface adsorption.14 Incorporation during electrospinning ensures uniform distribution and better encapsulation, while post-treatment allows for surface functionalization without altering the fiber structure. This is particularly advantageous for preserving the structural integrity and functionality of heat-sensitive compounds such as essential oils, antioxidants, and antimicrobials. In contrast, conventional encapsulation techniques require elevated temperatures or harsh chemical conditions, which can badly affect the functional properties of bioactive compounds. Moreover, electrospinning is a relatively simple, portable, and low-cost technique that requires minimal manpower, making it suitable for both laboratory and industrial settings. Its versatility in polymer selection and precise control over fiber morphology, mechanical, and transport properties further support its application in developing advanced, sustainable food packaging materials. Additionally, over the years, electrospinning has been developed with diverse structures and morphologies to fulfill specific requirements such as uniaxial, core–shell, hollow, and porous structures.10,15
A wide range of polymers can be processed through electrospinning to produce fibrous membranes. The use of biopolymers to develop active and intelligent packaging materials can provide excellent biodegradability, biocompatibility, and low toxicity.16 To reduce environmental impacts and dependence on fossil fuels, many efforts have been made to substitute synthetic polymers with biodegradable polymers, especially those derived from natural sources. Biopolymers for food packaging can be either extracted directly from biomass or produced by microorganisms or synthesized from bioderived monomers.17,18
The existing research studies have independently explored various aspects of electrospinning and smart food packaging.19,20 Our review presents a comprehensive and integrative analysis of biopolymeric electrospun membranes within the context of smart food packaging, setting it apart from the existing literature. We investigate the principles of both active and intelligent packaging systems, elucidating their functionalities, and the synergistic role of electrospun biopolymeric membranes in enhancing these systems. A detailed examination of the electrospinning process, including critical factors influencing the fiber morphology and functionality, which is essential for tailoring packaging materials to specific food preservation applications is also provided in this review. We emphasize the use of sustainable and biodegradable biopolymers in electrospinning, aligning with environmental concerns for developing eco-friendly packaging solutions. Furthermore, we highlight the capacity of electrospun membranes to encapsulate bioactive agents to facilitate their sustained release, a feature pivotal for extending the shelf life and ensuring food safety. By critically assessing recent studies, identifying gaps, and proposing future research outlook, our review serves as a consolidated resource that not only summarizes current advancements but also provides strategic insights for future research and development in smart food packaging technologies.
2. Active packaging
Active food packaging is meant to improve the properties of packaged food products by maintaining their quality and extending their shelf life. According to European regulation No. (EC) 450/2009, active food packaging systems are designed to “deliberately incorporate components that would release or absorb substances into or from the packaged food or the environment surrounding the food”.21 The active components are generally incorporated into the bulk of the food. However, for the majority of fresh and processed foods, food degradation and microbial growth occur on the surfaces of food. Moreover, certain active components when added directly to food will undergo unwanted chemical interactions with the food components causing the inhibition or reduction of their activity. Therefore, adding active components to the packaging system is the most efficient way compared to adding them directly to the food. The active packaging systems are classified into two types: active scavenging systems and active releasing systems, as shown in Fig. 1. The active scavenging systems are also known as ‘absorbers’, which can absorb components such as ethylene, oxygen, and moisture. This category includes ethylene scavengers, moisture scavengers, and oxygen scavengers. In contrast, the active releasing systems are known as ‘emitters’, which can emit active components such as antioxidants, antimicrobials and carbon dioxide. Hence, the active releasing systems are classified as antioxidant emitters, antimicrobial emitters, and carbon dioxide emitters.222.1. Ethylene scavengers
Ethylene (C2H4) is one of the most important plant hormones released from fruits and vegetables, which controls physiological mechanisms, growth, ripening, and senescence. Ethylene also stimulates chlorophyll loss and enhances excessive softening of climacteric fruits such as apple, tomato, avocado, banana, pear, mango, and kiwi.23 Mainly, there are two types of ethylene responsible for the various physiological changes in fruits. It includes endogenous ethylene, produced biologically by plants, and exogenous ethylene generated by external sources such as neighbouring crops, automotive exhaust, and tobacco.24 Exogenous ethylene is responsible for the browning and peel color transformation in postharvest fruits. Since most fruits and vegetables are ethylene sensitive, prolonged exposure to ethylene often leads to over-ripening, spoilage, change in taste, color, and odor. Therefore, ethylene scavengers are used in food packages to limit ethylene accumulation for retaining the quality and thereby extending the shelf life of fresh produce.25 Ethylene scavengers will absorb ethylene gas produced during storage either via physical or via chemical adsorption processes. The ethylene scavengers include inhibitors, adsorbents, and catalytic oxidants. Specific examples of each category are summarized in Table 1.| Type of ethylene scavengers | Examples |
|---|---|
| Inhibitors | 1-Methylcyclopropene (1-MCP), silver (Ag) ions, 2-aminoethoxyvinyl glycine (AVG) |
| Adsorbents | Natural clays like montmorillonite, halloysite nanotubes, activated charcoal, aluminosilicates, silica gel, palladium-based systems |
| Catalytic oxidants | Potassium permanganate (KMnO4), ozone (O3), titanium dioxide (TiO2) |
Potassium permanganate (KMnO4)-based ethylene scavengers are the most studied systems. KMnO4 functions as a potent oxidizing agent, facilitating the removal of ethylene (C2H4) via an oxidation reaction. At the molecular level, KMnO4 oxidizes ethylene by cleaving its double bond, subsequently converting it into carbon dioxide (CO2) and water (H2O), while KMnO4 itself undergoes reduction to form manganese dioxide (MnO2). The simplified reaction is represented in eqn (1):26
| 3C2H4 + 12KMnO4 + 18H2O → 12MnO2 + 12KOH + 6CO2 | (1) |
This redox reaction is characterized by a visible color transition from purple (KMnO4) to brown (MnO2), which serves as an indicator of the scavenger's saturation and subsequent loss of activity. The oxidation process occurs on the surface of the support material, typically initiated by the physisorption of ethylene, followed by chemisorption and reaction with KMnO4.
However, due to the high sensitivity of KMnO4, it can be deactivated during membrane or film processing. Consequently, Tirgar et al.27 proposed an innovative method for incorporating potassium permanganate (PPM) into electrospun nanofiber membranes to delay banana ripening. This study employs two distinct approaches for fabricating PPM-loaded nanofibers. The first approach utilizes carbon nanofibers (CNFs) loaded with alumina nanoparticles (ANPs) as carriers for KMnO4 (Fig. 2a), as carbonized fibers are stable and non-reactive with KMnO4. The second approach involves alumina nanofibers (ANFs) loaded with ANPs as a support for PPM, offering high surface area and stability (Fig. 2b). When comparing the membrane absorption capacity with various forms of ethylene scavengers such as beads, films, and liners, the alumina nanofiber membrane, with its exceptionally high surface area, demonstrated a superior ethylene absorption capacity over an extended period. However, the main disadvantage of KMnO4-based systems is that they cannot be used in those packages that make direct contact with food because of their toxicity. Hence, generally safe and non-toxic adsorbents incorporated in polymeric films are always receiving popularity. The main non-toxic adsorbents used for ethylene removal include zeolites, clay, alumina, silica, and activated carbon. The high specific surface area of these porous materials makes them capable of absorbing ethylene released from fruits and vegetables effectively. These materials exhibit distinct adsorption isotherms based on their surface chemistry, pore structure, and interaction mechanisms with ethylene. Silica typically exhibits physical adsorption, due to its relatively low surface energy and mesoporous structure.28 Zeolites, being microporous and highly crystalline with well-defined pore sizes and surface polarities, often follow Langmuir-type isotherms, indicating monolayer chemisorption or selective adsorption based on molecular sieving.29 In contrast, activated carbon, with its high surface area and heterogeneous pore structure, supports both physisorption and chemisorption, depending on surface functional groups and activation conditions. Due to this variation in isotherm behavior, it is important to select appropriate scavengers based on the desired adsorption mechanism and application.
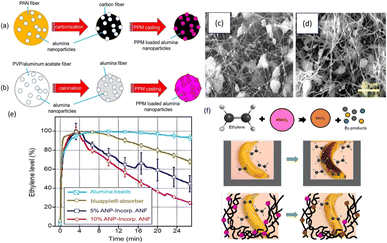 | ||
| Fig. 2 Production of ANP-incorporated (a) CNFs and (b) ANFs followed by PPM casting. SEM images of ANP-incorporated (c) carbon nanofibers and (d) alumina nanofibers. (e) Ethylene absorption capacity for various absorbers. (f) Electrospun nanofiber membranes as ethylene scavengers.27. [Copyright © 2018, American Chemical Society]. | ||
The chitosan/zeolite packaging film developed by Sousa et al.,30 the corn starch-gum acacia film impregnated with sepiolite clay reported by Upadhyay et al.,31 the dendritic mesoporous silica-supported platinum catalyst as an ethylene scavenger developed by Wei et al.32 and the palladium-modified activated carbon-based ethylene adsorbent for delaying the ripening of banana33 are few examples of recent research works on ethylene scavengers. Recently, a group of researchers have reported that brick ash, the industrial byproduct of brick kilns can exhibit excellent ethylene adsorption capacity at room temperature. Suraj et al.34 activated the brick ash at a higher temperature to study its adsorption properties, and it was observed that the material showed higher ethylene adsorption capacity as the activation temperature varied from 500 °C to 1000 °C. The practical applicability of this newly developed material was evaluated through shelf-life studies on raw bananas at 22–24 °C temperature and 65% relative humidity (RH) conditions. The results indicated that the new material could provide an increased shelf life of up to 20 days, compared to the control (12 days).
Photocatalytic oxidation is another effective way of ethylene removal for the preservation of postharvest fruits and vegetables. This technique involves the use of photocatalysts such as TiO2, which get activated upon irradiation with UV light and facilitate the oxidation of ethylene to CO2 and H2O.35,36 Fig. 3a shows the schematic diagram representing the mechanism of photocatalytic degradation of ethylene and the antimicrobial activity of the chitosan-TiO2 film developed by Siripatrawan et al.37 Reactive oxygen species (ROS) such as hydroxyl radicals (OH) and superoxide ions (O22−) generated upon UV irradiation react with ethylene and polyunsaturated phospholipids in the cell membrane of microorganisms. Even though this technique has been extensively studied for air and water purification, only limited attention has been paid to the postharvest preservation of fruits and vegetables.
 | ||
| Fig. 3 Schematics: (a) mechanism of photocatalytic degradation of ethylene and antimicrobial activity of the chitosan-TiO2 film [reprinted with permission from ref. 37 Copyright Elsevier 2018]. (b) Moisture condensation mechanism in packaging trays containing fresh produces. (c) Oxidation mechanism (i) without and (ii) with antioxidants in the packaging material [reproduced with permission from ref. 38 Springer Nature]. | ||
2.2. Moisture scavengers
Moisture content and elevated relative humidity (RH) are critical factors influencing the quality, safety, and shelf life of food products. Excess moisture accelerates microbial spoilage, promotes lipid oxidation, and induces undesirable changes in texture and appearance, thereby significantly reducing product stability and consumer acceptability. Fish, meat, poultry, and other minimally processed fruits and vegetables are examples of highly moisture-sensitive food items. For these types of water-sensitive products, controlled moisture levels are essential for keeping them safe from microbial growth to enhance their shelf life and sensory properties. Moisture accumulation within packaged food products can occur as a result of excessive transpiration and condensation (Fig. 3b). Both transpiration and condensation are key factors that can negatively impact postharvest quality by causing defects in external appearance and promoting the growth of spoilage microorganisms.39,40 Therefore, achieving a stable temperature-humidity equilibrium within the packaging system is essential for extending the shelf life of fresh produce and maintaining product integrity.41 Transpiration is a physiological process through which moisture moves from the internal tissues of the product to its surface and eventually into the surrounding air. This movement is driven by differences in moisture levels between the product and its environment, specifically differences in water activity, moisture concentration gradient, and water vapor pressure. However, under saturated storage conditions (100% relative humidity and constant temperature), transpiration can still occur, primarily due to the internal heat generated by the respiration process. When the water vapor transmission rate (WVTR) of the packaging material is lower than the product's transpiration rate, the moisture released by the product cannot escape efficiently. Consequently, water vapor accumulates inside the package, causing an increase in internal water vapor pressure, eventually leading to condensation on the inner surface. Similarly, once the interior surface temperature of the packaging falls below the dew point of the enclosed air, condensation occurs. This results in the formation of liquid droplets on the internal surfaces. This process is driven by vapor pressure differentials between the food product, the package headspace, and the external environment. Temperature fluctuations during storage and transportation further accelerate these differentials, promoting internal moisture migration and droplet formation.Similarly, the action of metabolizing fats, carbohydrates, and other biological compounds can also generate water. As a result, food with a higher moisture content tends to exhibit elevated vapor pressure, and the degradation of these organic molecules results in the accumulation of moisture within the headspace of food packages. An effective way to control this excess moisture is to use an appropriate moisture absorber/scavenger.42 Moisture absorbers or scavengers are usually incorporated inside sachets, pads, and films to maintain relative humidity (RH) within packages. Desiccants are examples of materials used to remove moisture in packaging headspace. Desiccants work by absorbing or adsorbing water molecules, thereby reducing humidity and moisture-activated microbial growth and food spoilage.43 Conventional drying methods require high-temperature conditions.44,45 However, desiccants are effective for food packaging, as they produce dry air by adsorbing moisture, enabling low-temperature, low-humidity drying ideal for preserving color, texture, and nutrients in heat-sensitive foods. The process relies on vapor pressure differences between the air and the desiccant, and dry desiccants absorb moisture until equilibrium is reached. Common examples of desiccants include silica gel, activated charcoal, activated clay, molecular sieves, and humectant salts (CaCl2, MgCl2, and CaSO4). Generally, desiccants are considered as renewable. To regenerate the desiccant, it must be heated, so its surface vapor pressure exceeds that of the surrounding air. This can be achieved using either renewable (e.g., solar, waste heat) or non-renewable energy sources. New formulations of desiccants allow regeneration at lower temperatures, enhancing the energy efficiency and sustainability. Renewable desiccants, often derived from natural materials such as clay or plant-based substances, are generally more biodegradable and environmentally friendly, but may have lower moisture absorption capacity than synthetic ones. Synthetic desiccants manufactured from chemical processes typically offer higher efficiency, but may have a larger environmental footprint. End-of-life management options for both types include reusability via regeneration processes.41 Synthetic desiccants (molecular sieves, activated alumina, and phosphorous pentoxide) typically offer more regeneration cycles and can be thermally regenerated multiple times. Biodegradability also varies, with renewable desiccants typically degrading more readily. Certain desiccants can be either recycled or thermally regenerated; however, the associated energy consumption must be carefully evaluated in sustainability assessments. Kenyo et al.46 studied the effect of desiccant characteristics on the properties of polystyrene/zeolite-active packaging materials using five different categories of zeolites as adsorbents. The results proved that the moisture adsorption capacity is determined by the zeolite content and not its type. In addition to this, super absorbent polymers (SAP) are used as desiccants.
Superabsorbent polymers (SAPs) are highly hydrophilic materials characterized by a cross-linked network structure, typically in the form of microbeads capable of absorbing and retaining large quantities of water, 1000 times as much as their own weight, significantly exceeding the capacity of conventional hydrogels. Their remarkable absorption capacity is attributed to the presence of strongly hydrophilic functional groups (e.g. –OH, –COOH/–COO−M+, –CONH2, and –SO3H/–SO3−M+). These groups will undergo strong interactions with water via hydrogen bonding and ion-dipole interactions. However, to prevent dissolution in water, a stable three-dimensional cross-linked structure is required, ensuring that the SAPs undergo extensive swelling without dissolving.47 Superabsorbent hydrogel films are widely used in active food packaging applications to extend the shelf life of perishable food products.48 Superabsorbent polymers (SAPs) can be categorized into natural and synthetic types based on their origin.49 Natural polymer-based SAPs include materials such as starch, cellulose, and chitin. These natural SAPs are highly biodegradable, but have a limited capacity for water absorption. In contrast, SAPs derived from synthetic polymers such as polyacrylic acid (PAA), polyacrylamide (PAM), and polyvinyl alcohol (PVA) generally exhibit water absorption and retention capabilities superior to their natural counterparts. However, the degradation of synthetic SAPs in soil takes many years, leading to significant soil pollution. Jiao et al.50 developed an antibacterial smart adsorbent pad with a Janus structure for preserving meat using the electrospinning technique (Fig. 4a). This pad is composed of two layers, each with distinct wettability. The top layer is made of hydrophobic polyurethane (PU) nanofibers infused with a cationic antimicrobial agent, while the bottom layer is a superabsorbent hydrophilic PVA containing anthocyanins. This dual-layer membrane with varying wettability facilitates the movement of meat juice from the hydrophobic to the hydrophilic layer, with the hydrophobic top layer remaining dry to inhibit bacterial growth. Water conductivity tests demonstrated that liquid moves more swiftly from the hydrophobic side to the hydrophilic side than in the reverse direction. When the hydrophobic force (HF) is less than the hydrophilic pressure (HP), liquid droplets penetrate the hydrophobic layer to reach the hydrophilic layer. The droplets are then subjected to capillary force (CF) from the hydrophilic PVA nanofibers, and quickly spread within the absorbent layer (Fig. 4b). This multifunctional antimicrobial water-absorbing indicator pad offers new insights and methods for meat preservation and freshness indication.
 | ||
| Fig. 4 (a) Schematic representation showing the fabrication of antibacterial smart adsorbent pad. (b) Principle behind water conductivity. [Reprinted with permission from ref. 50 Copyright Elsevier 2023]. | ||
Other approaches for moisture regulation include modified atmospheric packaging (MAP). This kind of packaging can be achieved by exchanging the humid air within the headspace with a dry modified atmosphere gas, usually nitrogen and carbon dioxide. Alternatively, vacuum packaging can be used, where humid air is removed from the headspace.51 MAP is particularly effective for perishable foods such as meat, dairy, and fresh produce, as it helps retain freshness, color, texture, and nutritional value without the need for chemical preservatives.
Recently, Kumar et al.52 have conducted experimental studies on the moisture barrier properties of four different polymeric films including ethylene vinyl alcohol (EVOH), polypropylene (PP), low-density polyethylene (LDPE) and high-density polyethylene (HDPE). The analysis was performed by measuring the water vapor transmission rates under accelerated conditions according to the ASTM standard E96, at 80% relative humidity and 40 °C temperature conditions. The moisture absorption increases linearly with time and becomes constant beyond a specific threshold. Notably, the polypropylene film displayed superior moisture barrier characteristics coupled with robust mechanical and structural stability, making it a cost-effective choice.
2.3. Oxygen scavengers
Oxygen scavengers (antioxidants) are additives capable of retarding oxidation reactions that cause rancidity.53 Lipid peroxidation is a biochemical process wherein oxidants such as free radicals or nonradical species target lipids, particularly polyunsaturated fatty acids (PUFAs). This involves hydrogen abstraction from a carbon, forming lipid peroxy radicals and hydroperoxides via oxygen insertion.54 The PUFA oxidation products such as lipid hydroperoxides and secondary reactive aldehydes (malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE)) can disturb cellular homeostasis. These reactive species can create adducts with proteins, lipids, and DNA, resulting in structural damage and functional degradation. For example, 4-HNE functions as a molecular signal that activates genes associated with inflammation, hence contributing to chronic inflammatory states and potentially to diseases such as cancer, atherosclerosis, or neurological disorders.55,56 Additionally, lipid peroxides can impair membrane integrity and mitochondrial function, hence intensifying cellular stress.Another issue related to PUFA-rich food is the off-flavour due to aldehydes, ketones, and volatile secondary oxidation products. For example, 2-pentyl furan and its isomers are responsible for the undesirable “reversion flavor” of soybean oil produced by light-induced singlet oxygen oxidation of linoleic acid.57 Thus, in antioxidant packages, oxygen scavengers or oxygen emitters are one of the main components used for preventing uncontrolled radical generation and propagation. Both natural and synthetic antioxidants have been widely used in food packaging to prevent lipid oxidation.58 Fig. 3c represents the antioxidant mechanism of packaging material.38 Natural antioxidants such as tocopherols, flavonoids, and essential oils derived from plant or animal sources are generally regarded as safe. They are attractive due to their biodegradability, lower toxicity, and alignment with consumer trends toward natural ingredients. However, their efficacy may vary based on the source, stability under processing conditions, and extraction costs. Additionally, natural antioxidants often have a lower activity than that of their synthetic counterparts and may impart unwanted flavors or colors to food products. Synthetic antioxidants including butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA), and tert-butylhydroquinone (TBHQ) are chemically synthesized compounds that offer high stability, consistent performance, and cost-effectiveness. They are widely used in the food industry due to their potent antioxidant activity and well-established regulatory frameworks. However, some synthetic antioxidants have raised safety concerns, leading to restrictions or bans in certain countries, and increasing consumer resistance to their use. Therefore, while natural antioxidants are preferable for sustainable and consumer-friendly packaging solutions, synthetic antioxidants continue to play a role where the performance, cost, and regulatory approvals align with application requirements. A hybrid approach or the use of encapsulated natural antioxidants might offer an optimal balance in active food packaging systems.
Vidal et al.59 developed an antioxidant packaging film by incorporating green coffee oil by-products into a carboxymethyl cellulose film to prevent fish oil oxidation. The capacity of the active film was monitored by analysing peroxides and thiobarbituric acid reactive species (TBARS), primary and secondary products of oxidation, etc. Almonds are susceptible to lipid oxidation due to their high content of PUFA, mainly linoleic acid. Almond skin is a potent natural antioxidant that is being incorporated into poly(ε-caprolactone) (PCL) by Garcia et al.60 to develop an efficient antioxidant food packaging film. Even though no significant differences were observed for the oxygen barrier properties, an increase in water absorption and water vapor permeability was observed for active films compared to the PCL control. This indicates the suitability of this novel packaging for extending the shelf life of fatty foods.
2.4. Antimicrobial food packaging systems
Antimicrobial food packaging systems are meant to inhibit the growth of undesired microorganisms responsible for the spoilage of packaged food products. It is an efficient packaging technology, where antimicrobial agents are incorporated into polymeric films that suppress the activities of food-borne pathogens or other targeted microorganisms dangerous to consumer health.61,62 Antimicrobial agents can be either directly integrated into food or incorporated into packaging materials, where it is slowly released to kill the pathogens and, thus, maintain the food quality and extend the shelf life. The selection of antimicrobial agents depends on the characteristics of microorganisms. The most commonly used antimicrobial agents for food packaging include essential oils, natural extracts, enzymes, bacteriocins, organic acids, and other derivatives. Close or direct contact with the microorganisms is the most essential requirement for the action of antimicrobial agents. Antimicrobial agents can prevent microbial growth by several mechanisms such as63 (i) modifying or denaturing the protein composition within microorganisms, influencing their functionality; (ii) disrupting cell membrane proteins or membrane lipids; (iii) inhibiting the synthesis of cell wall constituents, thus obstructing a stable cell wall structure; and (iv) preventing the reproduction, transcription, and translation of nucleic acids within microorganisms, hindering their ability to replicate. Table 2 summarizes some of the recent research works on antimicrobial food packaging systems.| Antimicrobial agents | Target microorganism | Food | Packaging material | References | |
|---|---|---|---|---|---|
| a EO: essential oil; PLA: polylactic acid; PBAT: poly(butylene adipate-co-terephthalate); LDPE: low-density polyethylene; PEG: polyethylene glycol; E. coli: Escherichia coli; S. aureus: Staphylococcus aureus; C. parapsilosis: Candida parapsilosis; A. brasil. | |||||
| Essential oils | Thyme–clove EO | S. aureus | — | PLA-PBAT | 64 |
| E. coli | |||||
| Basil EO | Gram-positive bacteria | Cooked ham | Chitosan | 65 | |
| Clove EO | E. coli | — | Pullulan/gelatin | 66 | |
| S. aureus | |||||
| Thyme–cinnamon–oregano EO | E. coli | Strawberry | Zein/chitosan | 67 | |
| S. aureus | |||||
| C. parapsilosis | |||||
| A. brasiliensis | |||||
| Chrysanthemum EO | L. monocytogenes | Beef | Chitosan nanofiber | 68 | |
| Enzymes | Lysozyme | Gram-positive bacteria | Cheese | _— | 69 |
| Lactoperoxidase | Gram-positive bacteria | Mango | Chitosan | 70 | |
| Gram-negative bacteria | |||||
| Bacteriocins | Nisin | M. luteus ATCC 10240 bacterial flora | Tryptone soya broth (TSB) milk | LDPE | 71 |
| Nisin and EDTA | E. coli | Fish fillet | Chitosan-polylactate | 72 | |
| S. aureus | |||||
| Natural extracts | Grape pomace | E. coli | — | Polypropylene | 73 |
| B. subtilis | |||||
| Date palm fruit | S. aureus | Strawberry | Chitosan-PEG | 74 | |
| Curcumin | S. aureus | Meat | Cellulose laurate | 75 | |
| Organic acids | Benzoic acid | E. coli | Meat | Chitosan | 76 |
| S. aureus | |||||
| Sorbic acid | B. cereus | ||||
| P. fluorescens | |||||
| Acetic acid | Salmonella | Meat | — | 77 | |
| Citric acid | Poultry products | ||||
| Lactic acid | |||||
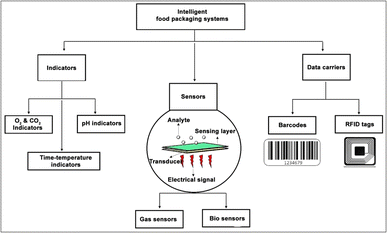
3. Intelligent food packaging
Even though food quality tests are regularly performed during the production and packing stage, no quality assessments are usually done aftermarket delivery. Therefore, food spoilage during this period could result in significant food loss and consumer health issues. Intelligent food packaging is a smart solution for this scenario. Intelligent packaging is an emerging food packaging technology that is capable of monitoring the condition of packed foods.78 It helps to sense and share the quality status of the packed foods with the stake holders of the food supply chain. This technology also helps to monitor the presence of specific chemicals, growth of microbial metabolites, humidity, pH, and gaseous (O2 and CO2) levels inside the packaging. In general, intelligent food packaging systems rely on three main technologies: indicators, sensors and data carriers (Fig. 5).79In smart food packaging, both indicators and sensors are employed to monitor the quality and safety of food products, yet they differ in their mechanism of action and outputs. Indicators are typically presented in the form of labels or tags and are designed to detect changes in environmental parameters such as pH, temperature, or gas composition. These changes are then transformed into visual responses, most commonly through color changes.80 Unlike sensors, indicators do not generate electronic signals, rather, they react directly with target analytes such as volatile amines, CO2 or other spoilage-related compounds.78 The response of an indicator can be either cumulative or threshold-based: cumulative if the indicator gradually changes over time as the spoilage condition progresses and threshold if becoming activated only when a critical limit is exceeded. For example, as microorganisms proliferate in food, they produce metabolic byproducts that can alter the pH of the environment. A pH-sensitive dye will change its color only when the acidity or alkalinity reaches a threshold value, thereby serving as a visual indicator of microbial spoilage. However, sensors are more advanced and active systems that are capable of detecting specific chemicals or biological analytes and converting them into measurable signals. Most sensors are made up of two basic components: a receptor and a transducer. The function of the receptor or sensing material is to interact with the target analyte and this interaction induces some physicochemical changes. The transducer then converts these changes into readable signals, typically an electrical, chemical, thermal, or optical signal.
3.1. Indicators
Indicators are devices incorporated within the food packaging that can provide information about the quality status of the food through some visual indications. Most indicators are based on colorimetric dyes, which produce different colors depending upon the presence or absence of target chemicals, the extent of reactions between two or more substances or the difference in the concentration of chemicals inside or outside the packaged food. Oxygen and carbon dioxide indicators, pH indicators, and time–temperature indicators are the most commonly used indicators in intelligent packaging.81An example of a commercially available redox-based oxygen indicator is AGELESS EYE™. The color pattern of the indicator is given in Fig. 6a. Normally the color of the AGELESS EYE™ indicator will be pink. If the oxygen in the respective package gets depleted, the color of the indicator will change from pink to blue or purple. Once the oxygen in the package reduces, the indicator retains its original color. Won et al.84 developed a novel oxygen indicator based on natural components such as laccase, guaiacol and cysteine with in-pack activation. After physically separating these natural components into two compartments, the barrier separating them was breached. Upon rupture, the oxygen indicator was activated, undergoing color changes in response to the presence of oxygen. However, carbon dioxide (CO2) is generally a beneficial gas in food preservation, as it can inhibit microbial growth and extend the shelf life. In addition, CO2 is an inert flushing gas used during MAP for inhibiting microbial metabolism. Hence, any variation in CO2 concentration would result in food spoilage due to microbial/mold growth. Controlling CO2 levels is, therefore, crucial in maintaining the shelf life of food products. Thus, colorimetric oxygen and carbon dioxide indicators are key constituents in intelligent packaging, which is widely used to detect the amount of gas involved in food spoilage by means of a color change.
 | ||
| Fig. 6 (a) Color pattern of the AGELESS EYE™ oxygen indicator [reproduced from ref. 85 with permission from Royal Society of Chemistry]. (b) Structure of anthocyanin at different pH values.86 (c) Classification of time–temperature indicators (TTIs) based on the working principle [reproduced with permission from ref. 87 Copyright 2023 John Wiley and Sons]. | ||
| Indicator | Analyte | Food | Polymer matrix | References |
|---|---|---|---|---|
| Lysine/ε-polylysine/anthocyanin | CO2 | Poultry meat | PET | 96 |
| Anthocyanin/limonene | Bacillus subtilis, Aspergillus niger, and Staphylococcus aureus | Pasteurized milk | Starch/PVA | 97 |
| Anthocyanin–ovalbumin-CMC nanocomplex | CO2 | Mushroom | Corn starch/PVA | 98 |
| Curcumin/anthocyanin | Volatile amines | Fish | Starch/PVA | 99 |
| Red radish anthocyanin | Volatile amines | Fish | Gelatin/gellan gum | 100 |
| Organic acids | Milk | |||
| Anthocyanin/propolis extract | Escherichia coli and methicillin-resistant Staphylococcus aureus | Pasteurized milk | PVA/starch | 101 |
| Black rice bran anthocyanin/oregano essential oil | Ammonia | Pork | Chitosan | 102 |
| Alizarin/grapefruit seed extract | Volatile gas | CMC/agar | 103 | |
| Anthocyanin | Ammonia | Pork | Starch/carbon dots | 104 |
| Microbes |
3.2. Sensors
Sensors are devices that detect, locate, or quantify a problem and then send signals to measure its physical or chemical characteristics. Sensors can provide continuous output signals in response to changes in the surrounding environment. Most sensors are made up of two basic components: receptor and transducer. The function of the receptor or sensing material is to interact with the target analyte and to convert the resulting physical or chemical information into the form of energy. This energy will then be converted by the transducer into a readable signal, typically an electrical, chemical, thermal, or optical signal.105 Depending upon the response stimuli, the sensors are of two different types: gas sensors and bio-sensors.The microbial decomposition of nutrients in food products can lead to the emission of various volatile organic compounds (VOCs) including amines (such as ammonia, dimethylamine, and trimethylamine), hydrogen sulfide (H2S), carbon dioxide (CO2), oxygen (O2), ethylene (C2H4), and aldehydes and ketones. These compounds serve as indicators of spoilage and alter the food quality. The gas sensors can detect and quantify the concentrations of these VOCs, thus enabling the real-time monitoring of food freshness.106 Thus, incorporating gas sensors to food packaging can provide consumers and suppliers with immediate information regarding the condition of the food, thereby enhancing food safety and reducing waste. Fig. 7 illustrates the gas sensing mechanism in food quality monitoring.
As already mentioned in Section 3.1.1., CO2 is generally accepted as an active packaging gas that can minimize the oxygen content inside the food package to inhibit the growth of aerobic microbes, thereby extending the shelf life of food products. Therefore, food deterioration can be directly determined from the CO2 concentration. For example, conventional CO2 sensors can be broadly classified into two types, based on the type of transducer: optical and electrochemical CO2 sensors. Optical sensors can be further classified into colorimetric CO2 sensors and fluorescent CO2 sensors. Both the fluorescent and colorimetric CO2 sensors detect carbon dioxide via chemical interactions between carbon dioxide and the sensing materials, but they differ significantly in their detection mechanisms and practical applications. Fluorescent CO2 sensors utilize fluorescent dyes or probes that respond to CO2 by exhibiting changes in their fluorescence properties such as intensity, wavelength, or lifetime.107 Typically, CO2 reacts with water in the sensor matrix to form carbonic acid (H2CO3), which alters the pH and consequently affects the fluorescence of pH-sensitive dyes. In some systems, CO2 directly interacts with amine groups on the fluorescent molecules, forming carbamate species that cause fluorescence quenching or spectral shifts. These changes are detected by exciting the sensor with UV or visible light and measuring the emitted fluorescence, enabling highly sensitive and quantitative detection of CO2 at low concentrations.108 However, colorimetric CO2 sensors operate through a visible color change caused by similar chemical interactions, where CO2 dissolves in water to produce carbonic acid, lowering the pH and triggering a color transition in pH-sensitive dyes such as bromothymol blue or phenol red.109 Unlike fluorescent sensors, colorimetric sensors provide a simple, low-cost, and power-free way to monitor CO2 levels that can often be interpreted by the naked eye, but with lower sensitivity and less precise quantification. Fluorescent sensors require instrumentation for excitation and emission detection but offer faster response times, higher sensitivity, and better suitability for real-time and quantitative applications.
Bigiani et al.110 have developed a MnO2-based ethylene gas sensor for monitoring fruit ripening. The fabrication was carried out by a two-step plasma-assisted process, where the first step is the chemical vapor deposition of MnO2 on the polycrystalline Al2O3 substrate followed by functionalization with Ag and Au nanoparticles by a sputtering process. This composite has exhibited high sensitivity towards ethylene gas even at a very low ppm range.
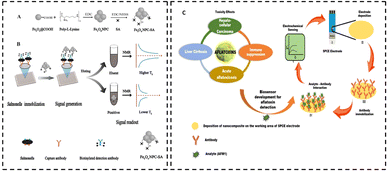 | ||
| Fig. 8 (A) Preparation of Fe3O4 NPC and Fe3O4 NPC-SA. (B) Outline of the NMR biosensor for Salmonella detection in milk [reprinted with permission from ref. 112 Copyright Elsevier 2019]. (C) Electrochemical sensor based on the MoS2/UiO-66-NH2 composite for AFM1 detection [Copyright © 2022, American Chemical Society]. | ||
| Target analyte | Company | Country |
|---|---|---|
| Ethanol, methanol, glucose, lactate, glycerol | Analox Instruments | UK and USA |
| E. coli in lettuce | Massachusetts Institute of Technology | USA |
| Proteins, toxins, viruses, bacteria, spores and fungi | Research International | USA |
| Atrazine | Universitat Autonoma de Barcelona in collaboration with CSIC | USA |
| Allergens, vitamins, microorganisms | Biotech-lgG | Sweden |
| Amines | Oriental electric | China |
One of the drawbacks associated with these sensors is their selectivity. Real food systems are highly complex, and hence, potential interference from various components in the food matrix such as other volatile organic compounds (VOCs), moisture, and fat can affect the accuracy and reliability of indicator responses. For example, if a sensor is particularly designed to detect a specific spoilage analyte like hydrogen sulfide or ammonia, they may exhibit cross-reactivity with other volatiles naturally present in the food matrix. This can lead to a false-negative response. Quartz crystal microbalance (QCM) biosensors are effective for the detection of pathogens and other quality indicators. However, their sensitivity can be influenced by food components such as fats and proteins. These substances may compete with the target analyte binding on the sensor surface, thus minimizing the accuracy.115
3.3. Data carriers
Data carriers are automatic recognition devices incorporated within the food packages that store and transmit information in the food supply chain.116 Data carriers are not intended to provide any information regarding the food quality status; instead, they are utilized for traceability, automatization, theft prevention, or counterfeit protection. They are mostly placed onto tertiary packaging systems such as large cardboard boxes, pallets, and multi-box containers. Barcode labels and radio frequency identification devices (RFID) are the most common data carriers used in the food packaging industry.1174. Electrospun membranes for smart food packaging applications
Electrospinning is a simple and highly versatile technique used for developing smooth and thin fibrous membranes with high surface area and porosity. Due to its unique surface characteristics, electrospun membranes are widely used in membrane-based filtration, sensing, water treatment, energy storage, and biomedical applications.124,125 In the food industry, electrospun membranes are extensively used as novel platforms for smart food packaging. Since these membranes possess tunable surface characteristics, they can be easily functionalized by incorporating active components. These encapsulated active components can ensure sustained and controlled release from the membranes due to the inherent porosity and fibrous nature associated with the electrospun membranes.Electrospun membrane porosity plays a crucial role in controlling the respiration rates and moisture levels in perishable food packaging, especially in the case of fresh fruits and vegetables (FFV).126 As mentioned in Section 2.2, FFV's are easily susceptible to postharvest loses mainly due to transpiration and condensation. The interconnected porous structure of electrospun membranes allows for selective gas permeability, which can be tailored to match the respiration rates of specific fruits and vegetables. By optimizing the pore size and distribution, these membranes can regulate gas exchange to effectively slow down the ripening process and extend the shelf life. Additionally, the high surface area-to-volume ratio facilitates interfacial interactions with gases, providing selective barrier properties against water vapor, oxygen, and other environmental gases by creating a long and tortuous diffusion path, which enhances gas and moisture resistance compared to conventional packaging materials. This selective permeability not only prevents excessive moisture accumulation that could foster microbial growth, but also minimizes excessive moisture loss that can cause food dehydration. Thus, tuning the membrane porosity via customized packaging solutions can be achieved that meets specific requirements of perishable foods.
Recently, electrospun-blended nanofibers composed of zein and gelatin, enriched with natural polyphenols, were used as active packaging materials for extending the shelf life of fresh cherries (Fig. 9a).127 These biopolymer-based nanofiber mats demonstrated high hydrophobicity, which effectively minimized moisture loss from the fruit during storage. This moisture retention was evidenced by reduced firmness loss, lower respiratory activity, and decreased ethylene production in the cherries when stored at 20 °C for up to 12 days. In dairy product packaging, the high porosity enables the incorporation of antimicrobial agents and antioxidants that help extend the shelf life. Zein-based nanofibers embedded with an inclusion complex of citral and hydroxypropyl-β-cyclodextrin (HP-β-CD) demonstrated favourable characteristics for active food packaging, especially in cheese preservation (Fig. 9b).128 The addition of zein improved the tensile strength, thermal stability, and hydrophobicity of the nanofibers, making them suitable for moisture-sensitive applications. Moreover, the porous structure of the nanofiber matrix enabled a prolonged release of citral, a bioactive agent known for its antibacterial properties. This controlled release facilitated the preservation of cheese by preventing the growth of Listeria monocytogenes and prolonging its shelf life under the storage conditions of 4 °C and 12 °C. An effective antibacterial hydrogel was developed using electrospun nanofibers composed of gelatin, chitosan, and 3-phenyllactic acid, for extending the shelf life of chilled chicken meat (Fig. 9c).129 The distinctive structural characteristics of electrospun nanofibers, including their uniform and dense network, high surface area, effective entrapment of pathogens, and controlled release of active compounds render them highly suitable for food preservation applications. Upon absorbing moisture, the nanofibers rapidly transformed into a hydrogel, which demonstrated significant antimicrobial activity against foodborne pathogens and successfully prolonged the shelf life of chilled chicken by up to four days.
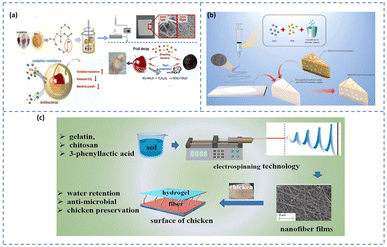 | ||
| Fig. 9 (a) Schematic showing the fabrication and application of electrospun zein/gelatin films with polyphenols for cherry preservation [reprinted with permission from ref. 127 Copyright Elsevier 2023]. (b) Zein nanofiber membrane encapsulated with an HP-β-CD inclusion complex for cheese preservation [reprinted with permission from ref. 128 Copyright Elsevier 2022]. (c) Antibacterial gelatin/chitosan/3-phenyllactic acid for extending the shelf life of chilled chicken meat [reprinted with permission from ref. 129 Copyright Elsevier 2022]. | ||
In order to demonstrate the superiority of electrospun nanofiber membranes over solventcast films, Turanli et al.130 conducted a comparative study. In this study, polyvinyl alcohol (PVA/chitosan (CS) blend materials were fabricated via electrospinning and solvent casting. This comparative analysis demonstrated enhanced performance of electrospun fibers in terms of thermal stability, antioxidant activity, and adsorption capacity, attributed to their higher surface area, porosity, and fiber alignment. Despite their improved thermal stability, electrospun samples showed lower glass transition (Tg) and melting temperatures (Tm), probably due to enhanced chain mobility from their porous architecture. A comparative summary of the obtained values is presented in Table 5.
| Sample | Water contact angle (°) | Tg (°C) | Tm (°C) | Radical scavenging activity (%) | |
|---|---|---|---|---|---|
| DPPH | ABTS | ||||
| a CF = solvent cast, NF = nanofiber. | |||||
| CF-PVA-CS | 43 ± 1 | 80 | 225 | 28.8 | 22.8 |
| NF-PVA-CS | 40 ± 1 | 74 | 223 | 38.9 | 34.6 |
In electrospinning, the production of continuous nanofibers from the polymer solution or melt is done with the application of high voltage.131 This is achieved with the help of a high-voltage supply system, a syringe pump system, and a grounded collector. The schematic of a basic electrospinning unit is shown in Fig. 10a. The first step of the process involves polymer solution preparation. The prepared solution can be then taken in a suitable syringe system equipped with a metallic needle. The next step involves proper fixing of the solution-filled syringe in the syringe pump setup. Once all these initial processes are done, a high voltage is applied between the tip of the metallic spinneret and the collector. The application of electric field results in the charging of the polymer solution. As the process progresses, there occurs charge repulsion, and hence, the solution gets extruded through the metallic spinneret as a liquid drop. Once the electric field intensity is sufficient enough to overcome the surface tension, the liquid droplet deforms into a conical shape known as the “Taylor cone”. When the electric field is increased further, the electrostatic repulsive force overcomes the surface tension, and the liquid jet is ejected out. The ejected liquid jet is then directed towards the metallic collector. During this time, the liquid jet initially extends in a straight line. However, due to bending instabilities and lengthening, the straight jet undergoes vigorous whipping motion. This whipping motion allows the polymer chains within the solution to stretch and slide past each other, resulting in the formation of fine fibers with a diameter in the nanoscale range. Finally, the excess solvent in the formed fibers get evaporated, and solid fibers are deposited onto the collector surface.132
 | ||
| Fig. 10 (a) Schematic of a basic electrospinning setup with syringe pump, high voltage supply and collector systems. (b) Outline of various electrospinning parameters. | ||
4.1. Electrospray-electrospinning
Electrospray-electrospinning is an emerging technology that combines two complementary techniques, namely electrospraying and electrospinning, to fabricate advanced functional materials. Electrospraying is a variation of the electrospinning technique that creates micro/nanoparticles through the hydrodynamic atomization of a polymer solution when exposed to a strong electric field.133,134 This method has received significant attention in food packaging applications for the development of films or bioactive coatings that impart distinct properties to the final product. Although bioactive compounds offer significant benefits in food packaging, they often lose their functionality when exposed to organic solvents in polymer solutions for extended periods. Electrospraying provides an effective strategy to overcome this limitation by enclosing sensitive compounds within polymeric nanoparticles. This protective coating preserves their biological activity and shields them from environmental stresses such as fluctuations in pH, temperature, humidity, and light. Schmatz et al.135 developed an innovative nanocomposite material consisting of polycaprolactone (PCL) or poly-L-lactic acid (PLLA) nanofibers coated with phycocyanin-incorporated polyvinyl alcohol (PVA) (PC-PVA) nanoparticles, using electrospinning and electrospraying techniques. Fig. 11a demonstrates the electrospray–electrospinning process along with the SEM images of PCL nanofibers (Fig. 11b) and PLLA nanofibers (Fig. 11c) coated with PC-PVA nanoparticles, respectively. The resulting nanomaterial exhibited favorable mechanical strength (31 ± 7 to 228 ± 26 kPa) and thermal stability (285–366 °C), effectively protecting phycocyanin from mechanical stress and thermal degradation. The direct deposition of nanoparticles onto the nanofiber surface mitigates challenges related to nanoparticle recovery from the collector. A significant advantage of this method is the immobilization of phycocyanin within the interstitial spaces of the polymeric matrix, facilitating a more controlled and sustained release of the bioactive compound upon contact with the target food. This prolonged release enhances antioxidant activity and aids in preserving the sensory qualities of the packaged food. | ||
| Fig. 11 (a) Schematic showing electrospraying of nanoparticles over static collectors containing nanofibers. (b) and (c) SEM images of PCL and PLLA nanofibers coated with PC-PVA nanoparticles [reprinted with permission from ref. 135 Copyright Elsevier 2019]. | ||
4.2. Parameters affecting fiber characteristics
Adjusting electrospinning parameters is essential for customizing nanofiber characteristics to satisfy the specific requirements of food packaging applications, especially in the context of smart and active packaging systems. Adjusting parameters including polymer concentration, applied voltage, solution flow rate, tip-to-collector distance, and solvent composition allow for the precise control over fiber morphology, which influences the diameter, porosity, surface roughness, and alignment. The structural features directly impact functional performance. Smaller fiber diameters and increased porosity contribute to an enhanced surface area, facilitating improved interaction with embedded sensors or bioactive compounds, which is crucial for responsive packaging. Optimizing surface roughness enhances the adhesion and visibility of colorimetric indicators. Additionally, modulating hydrophilicity influences moisture sensitivity and barrier properties. Strategic tuning of electrospinning parameters ensures uniform and stable fiber formation, enabling the design of packaging membranes with tailored functionalities for real-time monitoring, extended shelf life, and improved food safety.There are three main parameters that influence the overall fiber characteristics including fiber diameter, fiber morphology, and fiber distribution.136 These include the processing, solution, and environmental parameters (Fig. 10b).
The next process parameter is flow rate. Flow rate is defined as the amount of polymer solution flowing out of the needle tip to the Taylor cone per unit time. The flow rate can influence the droplet size, stability of the Taylor cone, trajectory of the jet, fiber diameter, and fiber distribution.138 Every polymer solution possesses a critical flow rate at which uniform beadless nanofibers are generated. At lower flow rates, the solvent will get enough time for evaporation, generating fibers of smaller diameter. However, increasing the flow rate above a critical value will result in beaded and thicker fibers with larger diameters. This is because of the insufficient drying time for the evaporation of the solvent and the low stretching force of the solution during the time of flight between the needle and the collector. A study conducted by Zargham et al.139 on electrospun nylon 6 nanofibers showed that the Taylor cone becomes more stable at a low flow rate of 0.5 mL h−1, and at this low flow rate, fibers with the narrowest fiber distribution were obtained. However, at higher flow rates, some defects such as fiber splitting, branching, and formation of web-like structures were observed due to insufficient solvent evaporation.
The distance between the needle tip and the collector is also considered as an important processing parameter. If the distance is too short, the fibers will not get enough time for solvent evaporation and proper stretching. This results in the formation of beaded fibers with higher diameters. Similarly, if the distance is too long, very thin fibers with small diameters are observed. Therefore, an optimum distance must be maintained for getting fibers with appropriate fiber characteristics. Recently, Mariola et al.140 have studied the influence of processing parameters such as distance, voltage, and flow rate on the morphology and diameter of electrospun polyvinyl alcohol (PVA) fibers. The fiber diameter was analyzed by varying the tip-to-collector distance: 10 cm; 836 ± 301 nm, 15 cm; 814 ± 309 nm, 20 cm; 727 ± 218 nm. A minimum fiber diameter was observed at a distance of 20 cm, which can be explained by two effects that occurred simultaneously causing opposing tendencies. That is, if the tip-to-collector distance is increased, the intensity of the electric field will decrease, forming a less stretched polymer solution with thicker fibers. At the same time, long distances will provide enough time for solvent evaporation forming thinner fibers.
The molecular weight of the polymer is another important factor, as it influences other factors such as viscosity, surface tension, conductivity, and dielectric strength of the polymer solution.144 Generally, in electrospinning, high molecular weight polymers generate smooth fibers with uniform morphology than low-molecular-weight polymers.
Solution viscosity is another important factor in the electrospinning process.145 At very low viscosity, continuous and smooth fibers cannot be produced and at very high viscosity, the polymer jet cannot be easily discharged from the needle tip. The polymer concentration and molecular weight are closely related to the respective solution viscosity. Hence, by adjusting these two parameters, a solution with proper viscosity that can produce optimum fiber morphology can be made. Nezarati et al.146 investigated the effect of solution viscosity on the fiber morphology by mixing polycarbonate urethane (PCU) in N,N-dimethylacetamide (DMAc) at different concentrations. Generally, as the solution concentration increases, the solution viscosity also increases. It was observed that beaded fibers were formed at a low viscosity (7.2 ± 1.7 Pa s), uniform fibers at an intermediate viscosity (10.1 ± 0.5 Pa s), and fibers with a large diameter at a higher viscosity (22.5 ± 1.4 Pa s).
Another important solution parameter influencing the fiber morphology is the surface tension. For the initiation of the electrospinning process, the charged solution needs to overcome surface tension. It has been reported that a lower surface tension can produce beadless fibers with smaller diameters, whereas a higher surface tension leads to instabilities in the polymer jet and thus produces fibers with non-uniform morphology. Surface tension is strongly dependent on the nature of the solvent used for making the polymer solution. Yang et al.147 have investigated the effect of solvents with different surface tensions (DMF, ethanol, and DCM) on the morphology of polyvinyl pyrrolidone (PVP) electrospun fibers. A single solvent system with 4 wt% PVP concentration in ethanol displayed a smooth fibrous texture, in contrast to those produced using DMF and DCM, which showed a beaded morphology. Within mixed-solvent configurations, the combination of ethanol and DMF at a 50/50 mass ratio provided fine PVP fibers with a very small diameter of 20 nm. Therefore, solvent selection is an important criterion in the electrospinning process.148
The electrospinning process is mainly dependent on the Coulomb forces between charges on the polymer jet surface and electrostatic force due to the external electric field.149 The repulsion between these surface charges is responsible for the stretching and formation of nanofibers. Surface charge interactions can thus be studied by altering the solution's conductivity. The solution conductivity not only affects the formation of the Taylor cone, but also influences the fiber diameter. A significant drop in nanofiber diameter has been observed with the increase in electrical conductivity due to the accumulation of more charges on the jet.150 Conductivity can be altered by adding salts, ions or conducting polymers to the electrospinning solution.151 Angammana et al.149 conducted a detailed study on the polyethylene oxide (PEO)/water system to investigate the effect of solution conductivity on the entire electrospinning process and fiber morphology by the addition of a NaCl salt. The group initially observed an increase in average jet current with the increase in conductivity and then it gradually declined. However, the average fiber diameter decreased with an increase in conductivity. They also found out that polymer solutions with low conductivity cannot undergo an electrospinning process, as there is no charge on the liquid surface to form the Taylor cone.
Similarly, variations in humidity can also influence the fiber morphology in terms of pore size, its number, and fiber diameter. Ramakrishnan et al.155 studied the effect of humidity on electrospun polycaprolactone (PCL) nanofibers for drug delivery applications. When the humidity factor was increased from 40% to 60% RH, an increase in fiber diameter from 100 nm to 145 nm was observed. The fibers produced were also more uniform at 60% relative humidity. It was also reported that, at high humidity, small circular pores can be formed at the fiber surface.156 If the humidity is too low, the solvent evaporation will be faster causing the solution near the needle tip to be clogged, hence preventing the proper electrospinning process.
4.3. Biopolymers suitable for electrospinning and their applications in smart food packaging
The smart food packaging materials can offer many additional benefits over the traditional packaging systems. The highly porous structure and large surface area-to-volume ratio of electrospun fibers make them an ideal platform for fabricating multifunctional nanostructured films for food packaging applications. In the food industry, electrospun nanofibers can be used in many ways such as reinforcing fillers and structural components in food matrixes, and for the encapsulation of bioactive substances such as antimicrobials, antioxidants, enzymes and vitamins.157 These nanofibers can protect the food from oxidation, moisture, and light. Additionally, it facilitates the release of bioactive compounds and improves the viability of probiotics. By combining proteins and polysaccharides, these fibers provide an environmentally friendly, biodegradable, and cost-efficient packaging solution using materials such as starch, zein, gelatin, PHB, PHBV, and PLA.158 For food-related applications, the material used has to be safe and biocompatible. In this regard, natural polymers are safer than synthetic ones due to their compatibility, availability, easy processing, and tunable properties. A schematic outline of biopolymers for food packaging is given in Fig. 12.4.3.1.1 Proteins. Proteins are highly biocompatible and biodegradable components possessing multiple binding sites for the encapsulation of bioactive components.159 Even though protein-based electrospun nanofibers have several advantages, the electrospinning of proteins themselves is quite challenging due to their complex secondary and tertiary structure.160 Depending on the protein type, there are many other ways to make proteins favorable for electrospinning such as blending with other biopolymers, denaturation of protein structure, and use of appropriate solvent systems.161
Whey protein isolate (WPI) is one of the main biopolymers used in food packaging materials due to its film-forming and gas barrier properties.163,164 WPI is an excellent carrier of both bioactive and pharmaceutical compounds, hence it has wide-ranging applications in the food industry and biomedical field. It is isolated from milk by-products and is added to improve the textural and nutritional values in food formulations due to its superior gelling and emulsifying properties. Since the electrospinning of pure whey protein is challenging, Mohammadi et al.162 evaluated the spinnability of whey protein isolate (WPI) at various concentrations with the addition of guar gum (GG), demonstrating that fiber formation is significantly influenced by the WPI-to-GG ratio. At a concentration of 7% WPI with 0.9% GG, fibers containing beads were observed, whereas no fibers formed at lower WPI concentrations (5%), irrespective of the GG content. This indicates that adequate chain entanglement, essential for electrospinning, occurs only at specific WPI/GG ratios. Higher WPI concentrations (9% and 12%) further enhanced spinnability, resulting in uniform, bead-free nanofibers with diameters of approximately 466 ± 87 nm and 510 ± 77 nm, respectively (Fig. 13). Panahi et al.165 fabricated PVA/WPI fibers encapsulated with probiotics as a novel platform for active food packaging to improve the food safety and to extend the shelf life. Bifidobacterium bifidum was successfully encapsulated within the optimized nanofiber system and these systems demonstrated effective antimicrobial properties against L. monocytogenes and E. coli. A color indicator film based on whey protein isolate nanofibers incorporated with anthocyanin as an indicator was developed by Han et al.166 This pH indicator film was used for sensing the pH of salmon, which changes with deterioration. Anthocyanin displayed a color shift from dark pink to grey with the increase in pH. Additionally, glycerol and pullulan were also incorporated with the film to improve its plasticizing and mechanical properties.
 | ||
| Fig. 13 SEM images of WPI–GG electrospun nanofibers at varying whey protein isolate (WPI) and guar gum (GG) concentrations. Images (a–c), (d–f), (g–i), and (j–l) correspond to increasing WPI concentrations of 5%, 7%, 9%, and 12% (w/w), respectively, arranged as rows, each column represents different GG concentration (0.7%, 0.8%, and 0.9% w/w) at a resolution of 5 μm [reprinted with permission from ref. 162 Copyright 2019 Elsevier]. | ||
Zein is another important protein known for its biodegradability, biocompatibility, hydrophobicity and film-forming properties. It is a prolamine protein obtained from maize corn.168 Zein-based systems are exclusively studied as food packaging and edible coating materials. Recently, bioactive compounds are incorporated into zein nanofibers for smart food packaging applications due to their resistance to moisture, oxidation, and heat.169 In a study conducted by Deng et al., gelatin/zein electrospun membranes and solvent-cast films were fabricated to find out which one has the greater potential for food packaging applications.167 Fig. 14 shows the morphology of electrospun and cast gelatin/zein films immersed in water and ethanol to study the solvent resistance. Gelatin/zein nanofibers showed good solvent resistance to water and ethanol, while cast films exhibited poor solvent resistance. Interestingly, the electrospun film had a hydrophobic surface with a water contact angle of 118° and the cast film had a hydrophilic surface with a water contact angle of 53°. The high water contact angle observed for the electrospun membranes is due to the formation of hydrogen bonding between zein and gelatin structures. Thus, the results confirmed the suitability of electrospun gelatin/zein nanofibers rather than the corresponding solvent-cast films for food packaging applications. Similarly, electrospun PVA/gelatin/zein membrane-based active food packaging developed by Ullah et al.170 and tetradecane-encapsulated zein nanofibers for sausage packaging171 are some other examples of zein-based nanofibers for smart food packaging applications.
 | ||
| Fig. 14 Morphology of the electropsun and cast zein/gelatin films after immersion in water and ethanol for studying solvent resistance capability and their water contact angle. [Reprinted with permission from ref. 167 Copyright Elsevier 2018]. | ||
Gelatin is one of the versatile biopolymers used for food packaging and it can be formed by the hydrolysis of collagen, the most abundant protein in animals.172 Gelatin is rarely used alone due to its limiting properties like brittleness, low bioactivities, and highly hydrophilic nature. Thus, they need to be modified by crosslinking, grafting, and blending to overcome its limitations in food packaging applications.173 Electrospinning of gelatin can be achieved in mild solvents such as a mixture of acetic acid/water and ethanol/formic acid/water. Yavari et al.174 fabricated a novel electrospun gelatin nanofiber reinforced using oxidized xanthan gum (OXG) as a crosslinking agent. The presence of OXG reduced water vapor permeability, water solubility, and moisture content, while their thermal and mechanical properties were changed.
Recently, a pH-responsive membrane based on gelatin and red anthocyanin extract was developed by Chayavanich et al.175 for the real-time monitoring of meat spoilage. The developed membrane exhibited a distinct colour change with the change in pH as a result of the volatile basic compounds released from spoiled meat. Similarly, Duan et al.176 fabricated a multifunctional food packaging system based on gelatin/chitosan nanofibers loaded with curcumin as a novel active and intelligent packaging material. The incorporation of curcumin has improved its antioxidant and antibacterial activities. Moreover, the developed electrospun membrane acts as a colorimetric indicator in ammonia sensing for monitoring the freshness of meat and seafood.
Huang et al.177 developed an innovative electrospun gelatin nanofiber membrane incorporating purple potato anthocyanin (PPA) and syringic acid (SA) for the purpose of preserving and monitoring the freshness of pork (Fig. 15). Syringic acid, a plant-derived phenolic compound, is known for its significant antibacterial and antioxidant properties. These biological activities are primarily attributed to the presence of hydroxy (–OH) and methoxy (–OCH3) functional groups within the aromatic rings. The study thoroughly examined the impact of SA on mechanical strength, thermal stability, and antioxidant and antibacterial activities. An optimized SA loading was found to enhance the thermal stability and mechanical strength via intermolecular hydrogen bond interactions. Furthermore, GA/PPA/SA nanofibers demonstrated over 80% antioxidant activity and exhibited strong antibacterial efficacy against both E. coli and S. aureus. The inhibitory mechanism involves damage to the cell membrane, increased membrane permeability, and elevated intracellular pH. In pork spoilage tests, the ammonia sensitivity response resulted in a noticeable color change from purple to green. These findings indicate the suitability of the GA/PPA/SA nanofiber membrane for multifunctional food packaging applications.
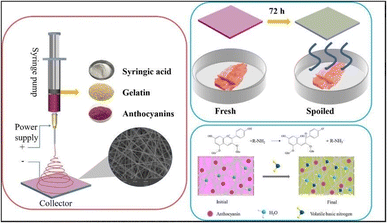 | ||
| Fig. 15 Schematic representation showing the fabrication and application of the GA/PPA/SA nanofiber membrane for pork preservation and freshness monitoring.177 | ||
Casein is a thermally stable phosphoprotein present in milk and cheese. Casein is responsible for the white color of milk. Due to their high nutritional value, water solubility, emulsification, non-toxicity, microbiological stability, and biodegradability, the milk proteins casein and whey alone or in combination can be utilized for making edible films.178 Compared to polysaccharide-based edible films/coatings, the three-dimensional complexed structure of milk proteins generates films with greater stability, excellent barrier properties, and longer durability.179 Even though casein films have great potential to be used in food packaging, the presence of hydrophilic residues and the moisture-sensitive nature lower their mechanical strength, thereby limiting their commercial acceptance. Crosslinkers and plasticizers are generally used to improve the protein film functionality.180 Sodium or calcium caseinates are the most commonly used caseinates for food packaging.
Pure sodium caseinate is not suitable for electrospinning due to the extensive network of inter- and intra-molecular hydrogen bonding. Therefore, suitable carrier polymers are required for the production of electrospun casein membranes. For example, polyethylene oxide (PEO) can be used as a suitable carrier polymer to make casein/PEO composites.181 The carrier polymer makes casein electrospinning feasible through the enhancement of solution viscosity and lowering of surface tension or electrical conductivity. Tomasula et al.182 have conducted a similar study to determine the feasibility of creating nanofibers from the food proteins calcium caseinate (CaCAS) and sodium caseinate (NaCAS) from an aqueous solution with and without a food-grade carrier polysaccharide, pullulan. They performed an extensive study to determine the effect of the solution and process parameters on fiber morphology. Similarly, Dai et al.183 fabricated a novel electrospun casein/PEO nanofiber membrane loaded with a thymol/β-cyclodextrin inclusion complex for beef preservation. Antibacterial studies revealed that casein nanofibers could facilitate the controlled release of thymol via the interaction of casein with bacterial proteases.
Soy protein isolate (SPI) is a plant-based protein originating from soybeans, and it is widely used in the food industry due to its biocompatibility, good film-forming nature, low cost, and high availability.184 The films made up of SPI will be transparent, smooth, and highly flexible. However, the main challenges associated with SPI films are their high water solubility and low mechanical stability. Additionally, electrospinning of pure SPI is also a challenge due to its low solubility in organic solvents and poor mechanical properties. These shortcomings can be rectified using suitable carrier polymers and functional components. PVA is an excellent carrier polymer that can provide required strength, and thus, it helps in the formation of nanofibers.185 Bruni et. al.186 developed an electrospun β-carotene-loaded SPI/PVA fiber mat as a bioactive coating for food packaging applications. Due to the lipophilic nature of β-carotene, emulsion electrospinning was used to encapsulate the carotenoid to a hydrophilic SPI/PVA hybrid system. Since proteins are excellent carriers for emulsion electrospinning, here SPI has been proposed as the carrier matrix for β-carotene. Therefore, SPI has been blended with spinnable biopolymer polyvinyl alcohol (PVA). A stable emulsion of SPI/PVA was prepared using soybean oil as the carrier oil for hydrophobic carotenoids. This emulsion was directly spun over a polyhydroxyalkanoate (PHA) film to form a continuous coating over it. They also studied the effect of annealing treatment on the controlled release of bioactive compounds from the electrospun membranes. The annealing process favored the adhesion of coating onto a PHA film and contributed to the sustained release of β-carotene. Similarly, Wang et al.187 fabricated functionalized electrospun SPI fiber mats using anthocyanin-rich raspberry extract as the active component. The film exhibited excellent antibacterial activity against Staphylococcus epidermidis, and hence, it could be used as an active packaging film. A novel bioactive electrospun nanofiber based on ethyl cellulose/SPI containing bitter orange peel extract was developed by Rashidi et al.188 Bitter orange peel, a rich source of phenolic compounds, makes this nanofiber membrane an excellent antioxidant and antibacterial active food packaging film.
4.3.1.2 Polysaccharides. Polysaccharides are polymeric carbohydrates composed of long chains of monosaccharide units joined by glycosidic bonds with the general formula (C6H10O5)n, 40 ≤ n ≤ 3000.189 Polysaccharide-based packaging materials are promising solutions for reducing the dependence on petroleum-based plastics. Among biopolymers, polysaccharides are the most widely used raw materials since they originate from plant or marine organism's biomass. Due to their inherent characteristics such as biocompatibility, biodegradability and non-toxicity, polysaccharides are suitable for edible films and coatings in smart food packaging applications.190,191 Many polysaccharides (e.g. starch, cellulose, chitosan, alginate, carrageenan, dextran, and hyaluronic acid) have been used to obtain electrospun nanofibers for various intelligent applications. Protein-based nanofibers will undergo denaturation at elevated temperatures.192 Compared to protein-based nanofibers, polysaccharide-based nanofibers are most often used for high-temperature applications (nanoencapsulation) due to their excellent thermal stability. However, one of the major challenges with polysaccharide-based electrospinning is their low spinnability, which can be resolved by using various carrier polymers, co-solvents, and functional additives while preparing the electrospinning solution.193
Starch is a biodegradable natural polymer originating from rice, wheat, corn and potatoes. Starch mainly consists of two microstructures: linear structure known as amylose and branched structure known as amylopectin, whose properties and ratios vary depending on the source.194 Starch has great potential to form electrospun nanofibers due to its good biocompatibility, also it can be electrospun with other polymers such as polylactic acid (PLA), polyvinyl alcohol (PVA), polycaprolactone (PCL), polyepoxyethane and poly(ethylene-co-vinyl alcohol) to achieve combined features of these polymers.195 Starch electrospinning is quite challenging because of its semicrystalline nature, higher molecular weight, and extensive hydrogen bonding interactions.196 Heat annealing and cross-linking are some of the ways to enhance the crystallinity and wet stability of fibers. To improve the moisture sensitivity and mechanical properties of starch-based films, Zhu et al.197 developed crosslinked starch nanofiber (SNF) films using glutaraldehyde as the crosslinking agent. Electrospun SNF possessed higher flexibility and enhanced water sensitivity compared to solution-cast films. However, the presence of hydrophilic hydroxyl groups on the fiber surface and the disrupted crystalline region in starch contribute to the hydrophobic nature and poor mechanical strength to SNFs. A multifunctional soluble potato starch-carvacrol nanofiber was developed by Fonseca et al.198 with potential antimicrobial and antioxidant activities. Carvacrol is an excellent antioxidant and antimicrobial agent. It can accumulate on the cell membrane of bacteria, causing a 90% increase in the cellular permeability of microorganisms. This inhibits its growth and induces dehydration in bacterial cells. Liu et al.199 fabricated starch/PVA nanofiber composite films (SPVANFs) crosslinked with glutaraldehyde (GTA) and modified with silver sodium zirconium phosphate (Ag–ZrP) to improve their hydrophobic and antibacterial properties. The addition of glutaraldehyde improved the hydrophobicity of starch/PVA nanofiber films, and Ag–ZrP provided outstanding antibacterial properties against both Gram-positive and Gram-negative bacteria (Fig. 16).
 | ||
| Fig. 16 Schematic of (a) fabrication of SPVANFs, (b) crosslinking of SPVANFs with GTA vapors, and (c) preparation of SPVA-silver nanofibers (SPVA-AgNFs). [Reprinted with permission from ref. 199 Copyright Elsevier 2022]. | ||
Cellulose is one of the most abundant polysaccharides with unique properties. All natural fibers such as wood fibers, fruit, seed, and fruit fibers are examples of cellulosic fibers.200 Cellulose is resistant to strong alkalis but easily hydrolyzed by acids to soluble sugars. Due to the strong intra- and intermolecular hydrogen bonds and van der Waals interactions, it is insoluble in common organic solvents. The highly reactive and regularly arranged hydroxyl groups along the cellulose chains can be utilized to make a wide variety of cellulose derivatives via simple reactions between the functional substituents and active hydroxyl groups. Cellulose derivatives include various esters and ethers, such as cellulose acetate (CA), methylcellulose (MC), carboxymethyl cellulose (CMC), hydroxyethyl cellulose (HEC), and hydroxyl propyl methylcellulose (HPMC).
Since cellulose is not soluble in most common solvents, electrospinning is the best-known method to prepare cellulose nanofibers with versatile applications.201 Nowadays, cellulose nanofibers form the basis of functionalized materials and fiber-reinforced composites. Cellulose electrospinning is quite challenging due to its poor solubility; however, many research works have been published on the electrospinning of cellulose derivatives.202,203 In food packaging, cellulose and its derivatives are incorporated with active substances for spinning and the spun films act as carriers for active packaging. Baikzadeh et al.204 fabricated electrospun ethyl cellulose/polycaprolactone/gelatin (ECL/PCL/GEL) nanofibers incorporated with Zataria multiflora essential oil (ZEO) and zinc oxide (ZnO) nanoparticles for active food packaging applications. The ECL/PCL/GEL nanofiber with a weight ratio of 20/70/10, incorporated with 30% ZEO and 3% ZnO exhibited the highest biocompatibility, antioxidant, and antifungal properties. A biopolymeric pH indicator film based on cellulose nanofiber/chitosan dyed with methyl red (CCM) and coated with PLA was developed by Sobhan et al.205 and used for the real-time monitoring of beef and fish spoilage. The PLA/CCM films effectively biodegraded within 5 weeks and in response to pH variation and ammonia sensitivity test, the film showed noticeable color changes. In the study conducted by Pittarate et al., an electrospun nanofiber film of cellulose acetate and polyethylene oxide (CA/PEO) blend was developed to study the effects of PEO on the morphology, moisture adsorption, and tensile properties of the film.206 The fibrous film fabricated by the electrospinning technique gives smooth, continuously long, ultrathin fibers with an average diameter of 800–900 nm. The addition of PEO significantly enhanced its mechanical properties and the incorporation of ZnO nanoparticles provided potential antibacterial properties to the film.
A multifunctional active and intelligent film based on cellulose nanofibers (CNFs) integrated with Brassica oleracea (BO) anthocyanin and BO-biowaste-derived carbon dots was fabricated by Wagh et al.207 The BO-carbon dot/CNF film is characterized by high UV-blocking capacity, and antibacterial and antioxidant properties compared to the neat CNF. BO anthocyanin enables the real-time freshness monitoring of pork, fish, and shrimp stored at 25 °C with a distinct color change from red to yellow. Corn straw core/CNF-based biodegradable nanocomposite film with bacteria blocking, UV, and water vapor barrier properties developed by Li et al.208 and CNF-based ethylene scavenging films incorporated with different types of TiO2 nanoparticles by Riahi et al.209 are examples of some recent works on cellulose nanofiber-based food packaging systems.
Chitosan is an amino polysaccharide obtained from the de-acetylation of chitin, the second most common biopolymer after cellulose.210 It is a copolymer of (1 → 4)-2-amino-2-deoxy-β-D-glucan and (1→4)-2-acetamido-2-deoxy-β-D-glycan. Chitosan is characterized by its low toxicity, high biocompatibility, biodegradability and good physical, chemical and biological (antibacterial) properties that promote their applications in food packaging, agriculture, cosmetics and biomedical field. Because of the highly hydrogen-bonded and semi-crystalline structure, it is difficult to dissolve both chitin and chitosan in most organic solvents. However, the solubility of chitosan can be enhanced through the functionalization of hydroxyl and amino-functional groups. Since chitosan is soluble in dilute acids, electrospinning solutions can be easily made in dilute acids such as acetic acid, trifluoroacetic acid (TFA), acrylic acid, and lactic acid.211 Recently, there have been many research works on electrospun chitosan nanofibers for antibacterial applications with the encapsulation of bioactive compounds such as essential oils, antioxidants, antimicrobial agents, and enzymes to develop functional materials for active food packaging.212 In a study conducted by Zou et al.213 electrospun chitosan/polycaprolactone nanofibers containing chlorogenic acid-loaded halloysite (CGA@HNT) were developed as active food packaging films. The CGA@HNT-loaded nanofiber mat exhibited long-term sustained release of bioactive components with enhanced antibacterial and antioxidant properties. Imino-chitosan/quaternized chitosan-based nanofibers with vanillin as antioxidant and antibacterial films for fruit preservation were developed by Andreica et al.214 The results revealed that the valuable combination of vanillin and quaternized chitosan into nanofibers proved to be effective in establishing a physical barrier against pathogens while maintaining the optimal humidity level. In the preservation of raspberries, the nanofiber membrane extended the shelf life to 7 days under normal atmospheric conditions (Fig. 17).
 | ||
| Fig. 17 Quaternized chitosan/vanillin-based nanofiber membrane as an active food packaging material [reprinted with permission from ref. 214 Copyright 2023 Elsevier]. | ||
Similarly, several research studies have revealed the promising applications of chitosan-based electrospun nanofibers as active/intelligent food packaging to extend the shelf life and sensory properties of food products.215–218
Alginates are natural anionic polysaccharides found in the cell walls of certain brown seaweeds. The chemical structure of alginate consists of long chains of repeating units of two different monosaccharides, α-L glucuronic acid and β-D mannuronic acid.219 Alginates are extensively used for food and biomedical applications due to their biocompatibility, low toxicity, cost-effectiveness, and mild gelation by the addition of divalent cations such as Ca2+ and Ba2+.220 In food packaging applications, alginate-based films/coatings exhibit excellent film-forming properties, flexibility, water solubility, low water vapor permeability, and good tensile strength. When combined with various additives such as essential oils, plant extracts, metal nanoparticles, enzymes, and chelating agents, they offer a multitude of benefits. These include moisture retention, antioxidant, and antibacterial properties, preventing color and texture degradation, enhancing mechanical and barrier properties, and improving sensor acceptability.
Alginate being a naturally occurring polysaccharide tends to have a relatively high viscosity, which hinders the formation of smooth and continuous nanofibers during the electrospinning process. The electrospinnability of alginates can be improved through various approaches such as blending with synthetic polymers, ionic crosslinking, solution modification (viscosity, concentration), and fine-tuning the electrospinning parameters.221 Kyziol et al.222 demonstrated that the addition of a carrier polymer (PEO) and surfactant (Pluronic F-127) can generate uniform alginate nanofibers with a cylindrical shape. For the successful electrospinning of sodium alginate (SA) from aqueous solutions, Nie et al.223 introduced a strong polar co-solvent, namely glycerol. The results indicated that glycerol has improved the flexibility and entanglement of SA chains by disrupting the strong inter- and intramolecular hydrogen bonding among SA chains.
A novel electrospun nanofiber membrane based on zein/sodium alginate incorporated with TiO2 nanoparticles and betanin as an active food packaging system was developed by Amjadi et al.224 The combination of zein and sodium alginate helps in overcoming the drawbacks of pure zein (weak mechanical properties and rapid dissolution rate) and sodium alginate (high conductivity, surface tension, and low chain entanglements)-based nanofibers. Photocatalytic TiO2 nanoparticles with high antibacterial activity and betanin, rich in phenolic and cyclic amino groups, with high antioxidant properties loaded to zein/sodium alginate nanofibers are used as highly potential active food packaging systems for meat products, cheese, and cereals.
Pullulan is a natural polysaccharide consisting of repeating units of maltotriose residues linked by α-1,4-glycosidic bonds, with occasional branching through α-1,6-glycosidic bonds. It is produced from starch by the fungus Aureobasidium pullulans.225 Due to its unique properties such as excellent water solubility, biodegradability, biocompatibility, thermal stability, and non-toxicity pullulan finds applications in various industries including food, beverages, cosmetics, and packaging. Pullulan possesses exceptional characteristics such as remarkable flexibility and amorphous nature, enabling it to create a wide array of versatile products including thin layers, electrospun nanofibers, nanoparticles, flexible coatings and polymer films. These features make pullulan highly comparable to synthetic petroleum-derived polymers such as PVA and nylon 6, 6.226 Sun et al.227 fabricated pure pullulan nanofibers with a diameter of ∼100 to 700 nm using redistilled water as a solvent. They also studied the influence of various spinning parameters on the morphology and diameter of nanofibers. The optimized parameters were 22 wt% solution concentration, 31 kV voltage, 20 cm capillary-screen distance, and 0.5 mL h−1 flow rate.
Recently, pullulan/polyvinyl alcohol nanofibers incorporated with a thymol-loaded porphyrin metal–organic framework have been developed by Min et al.228 as antibacterial food packaging materials. The developed nanofiber matrix system offered good flexibility, biocompatibility, and biodegradability. As an antibacterial packaging material, the nanofiber exhibited synergistic antibacterial activity against E. coli (∼99%) and S. aureus (∼98%) under light irradiation. Crosslinking is another way to enhance the properties of pullulan nanofibers because it reduces the hydrophilicity of nanofibers making them less susceptible to water absorption and it also reinforces the intermolecular interactions between pullulan chains, resulting in improved mechanical properties. Glutaraldehyde is the most commonly used crosslinking agent in biopolymer film making; however, some natural crosslinking agents are used these days to substitute the toxic agents. One such green crosslinking strategy was used by Quin et al.229 to fabricate electrospun chitosan/pullulan composite nanofiber films. In this study, cinnamaldehyde, the major component of cinnamon essential oil, was used as the green crosslinking agent. The crosslinked film showed superior water resistance, displaying hydrophobic characteristics along with enhanced mechanical and thermal properties. Electrospun pullulan/chitin nanofibers (PCN) loaded with curcumin and anthocyanin were developed by Duan et al.230 as an active-intelligent food packaging material. PCN containing both anthocyanin and curcumin (PCN/ATH/CR) had better antioxidant and antibacterial activities than nanofibers containing curcumin (PCN/CR) or anthocyanin (PCN/ATH) alone. In response to pH, PCN/CR did not show any significant color change, but PCN/ATH and PCN/ATH/CR fibers exhibited significant color changes (Fig. 18). An antibacterial food packaging system based on core–shell nanofibers with pullulan as the core layer and carboxymethyl chitosan/PEO as the shell layer loaded with nisin nanogels was developed by Duan et al.231 Nisin is a promising natural antibacterial agent against Gram-positive bacteria. Due to its high sensitivity to temperature and pH, coaxial electrospinning was chosen for the stabilization of nisin in food packaging materials. A tea polyphenol-loaded pullulan/carboxymethyl cellulose nanofiber membrane for fruit preservation developed by Shao et al.232 and an antioxidant electrospun nanofiber membrane based on pullulan/amaranth protein isolates loaded with curcumin by Blanco et al.233 are few examples for smart food packaging applications based on pullulan nanofibers.
 | ||
| Fig. 18 (a) Color changes in PCN/CR, PCN/ATH, and PCN/CR/ATH nanofiber membranes immersed in different buffer solutions (pH 2–11). (b) Mechanism behind the color change [reprinted with permission from ref. 230 Copyright 2021 Elsevier]. | ||
Dextran is a bacterial polysaccharide synthesized by lactic acid bacteria or their enzymes in the presence of sucrose.234 Dextran is composed of an α-1,6-glycosidic linkage in which D-glucose units are linked by α-(1 → 2) or α-(1 → 3) or α-(1 → 4) bonds. The properties of dextran vary with their molecular weight and branching. The solubility of dextran in both water and organic solvents makes it a versatile biopolymer for preparing nanofiber membranes. They can be blended with water-soluble bioactive agents or hydrophobic biodegradable polymers to tailor the properties for food packaging applications. Luo et al.235 fabricated a curcumin-loaded dextran/zein hybrid nanofiber membrane as an antioxidant packaging film. A curcumin-encapsulated dextran/zein fiber membrane made from a solution comprising 50% dextran and 30% zein exhibited desired functional and mechanical properties. While there is limited research on electrospun dextran-based membranes for food packaging applications, film-based systems are more prevalent. Davidovic et al.236 developed a dextran-based edible film for the preservation of blueberries. The film was plasticized with different concentrations of polyglycerol. The results from the studies conducted on both coated and non-coated samples suggested the effectiveness of dextran/polyglycerol coatings to extend the shelf life of blueberries. A biodegradable antibacterial and antioxidant nanocomposite film based on Dextran/PVA/TiO2 developed by Islamipour et al. and an antioxidant trilayer film of gelatin/dextran-propyl gallate/gelatin by Wang et al.237 are examples of dextran-based systems for active food packaging.
Polyhydroxybutyrate (PHB), polyhydroxyvalerate (PHV), and polyhydroxyhexanoate (PHH) are some of the most commonly used PHAs in biomedical and food packaging fields. Even though PHAs are biodegradable and biocompatible, their low mechanical strength, thermal properties, high production cost, and poor functionalities limit their applications in various fields. To overcome these limitations, they have to be modified to ensure better performance for specific applications. Blending with natural biopolymers such as starch, cellulose, and lignin is one way to improve their properties, and chemical modification via block and graft copolymerization is another way. However, electrospinning is gaining much popularity over other methods due to its remarkable versatility.239
Among various PHAs, the highly crystalline poly-3-hydroxybutyrate (P (3HB)) and its copolymers are the most extensively studied ones. Electrospinning helps to improve their mechanical properties and decrease the crystallinity. Aligned fibers offer better mechanical properties than randomly oriented fibers. Blended PHA fibers also render enhanced properties. Monolayered and multilayered electrospun PHA nanofiber membranes that can operate at room temperature can be synthesized via electrospinning by applying high electric voltages and annealing treatments.
Lopez et al.240 in their research study developed eugenol-incorporated ultrathin fibers of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) and annealed them to obtain antimicrobial monolayers. It was found that 15 wt% eugenol was the optimal concentration in PHBV monolayers since it exhibited high electrospinnability and thermal stability as well as the most effective antibacterial reduction against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). Several studies have reported on the application of bio-waste-derived electrospun PHBs incorporated with various essential oils and natural extracts as antioxidant and antimicrobial food package materials.241,242 Electrospun PHBV biopapers loaded with cerium oxide nanoparticles (CeO2 NPs) as oxygen-scavenging active food packaging films were developed by Lopez et al.243 The electrospun fibers were transformed into biopapers via an annealing process. Hexadecyltrimethylammonium bromide (CTAB) was used as the surfactant for loading CeO2 NPs into a PHBV solution. The best results for antibacterial, antioxidant, and oxygen scavenging performance were obtained for the PHBV film with 1.5 wt% CeO2 NPs and 5 wt% CTAB.
Polylactic acid (PLA) is a biodegradable, thermoplastic, renewable biopolymer obtained through the polymerization of lactic acid monomers. PLA can be synthesized by the bacterial fermentation of plant starch such as corn, cassava, potato, and sugar beet pulp. PLA possesses excellent characteristics such as high mechanical strength, biodegradability, biocompatibility, transparency, easy processability, and non-toxicity.244 It has been accepted as ‘Generally Recognized as Safe’ (GRAS) by the Food and Drug Administration (FDA) and hence suitable for direct food contact applications. Thus, PLA has been given special significance in food packaging applications as packaging films or coatings. Various processing techniques such as thermoforming and extrusion can be utilized to develop various PLA products such as cups, wrapping films, and food and beverage containers. However, electrospinning is one of the facile technologies for making PLA-based nanomaterials with enhanced gas barrier and thermal and mechanical properties. The nanofibers so obtained will have high porosity and large surface area-to-volume ratio, providing high encapsulation efficiency and controlled release of bioactive ingredients for active and intelligent food packaging applications.245
Wu et al.246 reviewed the recent advances and potential applications of PLA-based electrospun nanofibers for food packaging. By incorporating specific active agents or indicator substances, PLA fibers can act as antioxidant, antibacterial, or pH indicating fibrous mats that can provide information about food spoilage/contamination to the consumers. A novel antimicrobial electrospun nanofiber based on polylactic acid/hydroxypropyl methylcellulose (PLA/HPMC) containing pomegranate peel extract was developed by Bodbodak et. al.247 After optimizing various electrospinning parameters, they concluded that PLA/HPMC electrospun nanofibers in a concentration ratio of 80/20 with 10% pomegranate extract showed good fiber morphology with excellent antioxidant and antibacterial properties. Very recently, Liu et al.248 have developed a multifunctional PLA/butterfly pea flower extract (BPA)/cinnamaldehyde (CIN) (PLA/BPA/CIN) nanofiber film for monitoring the beef freshness. The nanofiber film exhibited remarkable antioxidant, antibacterial, hydrophobic, and colorimetric properties, which make it a potential intelligent packaging film for beef spoilage detection. Liao et al.249 modified electrospun PLA fibers with silver oxide-deposited hemp fibers as antibacterial fruit preservation films. Hemp fibers are natural fibers that can reinforce composite materials, and silver oxide has strong antimicrobial properties. Nanofiber membranes of the PLA/PEG blend incorporated with peppermint essential oil were produced via solution-blow-spinning (SBS). Peppermint essential oil (PO) is characterized by excellent antibacterial property due to the presence of L-menthol, menthone, methyl acetate, and limonene. SBS technique is a cost-effective technique, where a gas is used to accelerate the polymer solution into fiber. Thus, the PLA/PEG/PO film effectively extended the shelf life of strawberries.
As a novel packaging solution, Min et al.250 fabricated an antimicrobial film based on porous PLA nanofibers with a hydrophilic PVA/PEG coating for the controlled release of thyme essential oil (TEO). A highly volatile solvent and a high relative humidity are the main factors that influence the formation of pores via phase separation mechanism as shown in Fig. 19a. TEO was successfully encapsulated within the pores of PLA nanofibers providing high loading capacity. However, it is still very difficult to achieve controlled release of antimicrobials from packaging films. Therefore, in this study, hydrophilic polymer molecules having swelling behavior that enhances their gas permeability when exposed to water vapor were used to develop a controlled release system. For that, PVA/PEG blend was coated onto a PLA/TEO fiber surface (PLA/TEO/PVA/PEG) and the in vitro release of TEO was controlled by adjusting the humidity (20% to 80% RH). The reduction in water contact angle from 128° to 0° confirms the hydrophilic nature of fibers with PVA/PEG coating (Fig. 19b). The release of TEO from the PLA/TEO/PVA/PEG composite film was very slow compared to PLA/TEO fibers. The cumulative release of TEO at different RH values is also given in Fig. 19c. The results indicated the increase in diffusion rate with the increase in relative humidity. This is because of the increased gas permeability facilitated by the swelling of polymer wall upon exposure to moisture. In addition to that, the composite exhibited excellent antibacterial action against E. coli and S. aureus.
 | ||
| Fig. 19 (a) Schematic showing the fabrication of PLA/TEO/PVA/PEG composite films using an electrospinning technique. (b) SEM images of (i) pure PLA nanofibers, (ii) pore size distribution, water contact angle of (iii) pure PLA nanofibers, and (iv) PVA/PEG coated PLA/TEO. (c) Cumulative release of TEO from the PLA/TEO/PVA/PEG composite film at different RH values. [Reprinted with permission from ref. 250 Copyright Elsevier 2020]. | ||
5. Natural polyphenols as bioactive agents for smart food packaging applications
Polyphenols are plant-based naturally occurring bioactive compounds with excellent antioxidant and antibacterial properties. Based on the chemical structure, polyphenols can be classified into four main groups such as, phenolic acids, flavonoids, stilbenes, and lignans.251 Due to their inherent antioxidant and antibacterial properties, they can be used as functional components in active food packaging systems. The characteristic phenolic OH groups present in polyphenols help them to chelate with highly redox-active metal ions, which enhance their ability to protect against oxidative damage.252 Some commercial applications of polyphenols include functional food ingredients, antioxidants for preservation, and cosmetic ingredients. Raw fruits and vegetables are the main sources of polyphenols. Wheat, rice, maize, oats, coffee, tea, herbs, spices, and essential oils are the rich sources of phenolic compounds.253 Natural polyphenolic compounds such as curcumin, anthocyanins, tea polyphenols and gallic acid are widely used in biopolymer-based active food packaging films due to their diverse physiological, biological and beneficial health properties. Table 6 summarizes some recent research works related to natural polyphenols.| Type of polyphenol | Source | Fiber matrix | Properties obtained | Ref. |
|---|---|---|---|---|
| Curcumin | Curcuma longa L. (turmeric) | Polycaprolactone-carboxymethyl chitosan (PCL-CMCH) | Encapsulation of curcumin enhanced thermal stability, tensile strength and hydrophobicity of PCL-CMCH nanofiber film | 254 |
| Good biocompatibility, low toxicity and better inhibition of S. aureus under blue light irradiation | ||||
| Pectin–gelatin | Composite film exhibited good antioxidant activity, mechanical properties and antibacterial activity against E. coli and S. aureus | 255 | ||
| Excellent water vapor barrier capability | ||||
| pH-responsive color change from yellow to dark red with increase in pH. Hence, suitable for monitoring the freshness of shrimp | ||||
| Anthocyanins | Papaver rhoeas extract | Guar gum–pectin | Color changes from white (fresh) to dark violet (spoiled) in the preservation of lamb meat | 256 |
| Black carrot extract | PVA | Change in the morphology and structure of PVA-black carrot extract/SnO2 nanofiber film on interaction with released volatiles (confirmed by SEM, XRD, and FTIR) is due to the formation of complexes through new bonds | 257 | |
| Turnip peel extract | Potato starch | Turnip peel extract improved the thermal stability of potato starch nanofibers | 258 | |
| In the preservation of lamb meat, the nanofiber mat showed visible color change from white to purplish red in response to the microbial growth and chemical changes | ||||
| Tea polyphenols (TP) | Tea leaf extract | Starch | Tea polyphenol endowed antioxidant activity to the starch nanofiber film | 259 |
| Hydrophobicity enhanced with addition of TP | ||||
| High amylose corn starch and PVA | Addition of 15% TP enhanced the mechanical and water vapor barrier properties | 260 | ||
| Effective antimicrobial activity against S. aureus by destroying the bacterial cell wall and degrading DNA fragments | ||||
| Pullulan–carboxymethyl cellulose (CMC) | Decreased weight loss, maintained the firmness, and prolonged the shelf life of strawberries | 261 | ||
| PVA-ethyl cellulose (EC) | Superior thermal stability of PVA-EC/-TP composite film compared to pure PVA nanofiber | 262 | ||
| Significant increase in water contact angle from 22° to 109° | ||||
| Reduced water vapor permeability | ||||
| Good antioxidant and antibacterial properties | ||||
| Gallic acid | Pea flour-PEO | Thermal degradation of gallic acid decreased due to its successful encapsulation of gallic acid in the nanofiber matrix | 263 | |
| Total phenolic content of the film increased with gallic acid concentration |
Curcumin is one of the important polyphenolic natural bioactive compounds isolated from Curcuma longa L. (turmeric) belonging to the Zingiberaceae family.264 Among the three curcuminoid derivatives present in turmeric, curcumin (70%) is the principal curcuminoid. Curcumin has a yellowish-orange color with powerful antioxidant and antibacterial properties, which are attributed to the presence of ortho-methoxy and hydroxyl derivatives. Curcumin has many more biological functions such as anticancer, anti-inflammatory, and antimutagenic activities.265 Despite its reported benefits, one of the major problems that limits its bioavailability is poor water solubility, thermal sensitivity, and pH instability.266 Therefore, many studies have been conducted to overcome these limitations. One of the promising technologies is the encapsulation of bioactive agents in various delivery systems such as nanoparticles,267 nanoemulsions,268 nanofibers,269 β-cyclodextrin complexes,270 and biopolymer matrixes.271
In food packaging, curcumin-based systems are of great interest due to their good biocompatibility, low toxicity with food, UV protection, and free-radical scavenging properties.272 In intelligent food packaging, curcumin is used as a pH indicator with distinct pH-responsive color changes for the real-time freshness monitoring of food items.273,274 Recently, Du et al.275 have developed a green and efficient method for the delivery of active components to food by loading curcumin to a starch/pullulan nanofiber matrix. An inclusion complex of hydroxypropyl-β-cyclodextrin/curcumin was used to overcome the poor solubility of curcumin. The developed nanofiber membrane exhibited good antioxidant properties and fast dissolving since it was made in a 100% aqueous medium. A dynamically crosslinked chitosan/cellulose nanofiber integrated with a γ-cyclodextrin/curcumin inclusion complex was fabricated by Zhou et al.276 as a multifunctional packaging material for the preservation of perishable fruits. In this study, they used curcumin as a photodynamic antibacterial agent. Since curcumin is a natural photosensitizer, it generates reactive oxygen species (ROS) when exposed to blue light, resulting in bacterial cell death. In addition, the antioxidant property of curcumin helps to reduce the respiration of fruits and helps in prolonging their shelf life. A highly porous colorimetric indicator based on curcumin-loaded (1 wt%) polycaprolactone nanofibers (PCLCU1) was developed by a non-solvent-induced phase separation technique.277 These porous membranes interact with amine vapors, causing reversible color changes that help in the real-time monitoring of food spoilage. A comparative study was also conducted with non-porous PCL-curcumin (PCLCU-NP) mats. Compared to the non-porous fibers, the developed system showed an immediate response to amine vapors with distinct color change even at very low vapor concentrations (2.33 ppm). Fig. 20 shows the reversibility of color of mats upon exposure to dimethylamine (DMA) vapors of specific concentrations.
 | ||
| Fig. 20 (A) SEM image of the porous PCLCU1 electrospun mat. (B) Kinetic curves showing CIELAB color space analysis, used to study the color responsiveness of the fibers upon exposure to dimethylamine (DMA) vapors: (a) response of PCLCU1 mat (b) response of nanoparticle-loaded PCLCU1 (PCLCU1-NP) mat (c) time taken by PCLCU1 and PCLCU1-NP mats to reach their maximum fluorescence intensity (∼640 nm) after DMA vapor exposure. (d) Photograph of the PCLCU1 mat before exposure to DMA vapors. (e) PCLCU1 and (f) PCLCU1-NP mats during exposure to DMA vapors, showing visible color change.277 | ||
Among polyphenols, anthocyanins are an interesting class of water-soluble flavonoid pigments found in fruits, vegetables, flowers, and leaves.91 Anthocyanins are responsible for the red-to-blue color of flowers and fruits. Besides, they have excellent antioxidant properties. Chemically, anthocyanins are glycosylated forms of anthocyanidins, sugar-free pigments in plants. The rich sources of anthocyanins include blueberries, grapes, red cabbage, radishes, currants, pomegranates, and black carrots.278 They possess a wide variety of biological and functional properties such as antioxidant, anticancer, antimicrobial, antiobesity, detoxification and antidiabetic effects.279
As a natural colorant, anthocyanins can be used as pH-responsive color indicators in smart food packaging applications like monitoring food quality and extending the shelf life of food products.280 Biopolymers such as polysaccharides and proteins are mainly used as solid supports for anthocyanins, since they are biodegradable, biocompatible, less-toxic, and eco-friendly. Recently, a starch-based electrospun nanofiber mat incorporated with roselle anthocyanin has been developed by Lv et al.281 for the real-time freshness monitoring of pork and shrimp. In this study, octenyl succinic anhydride starch (OSA) was used as the film forming material and PVA was added to enhance the electrospinnability of starch. During the spoilage of protein-based food items, the release of volatile base nitrogen increases, which causes an increase in the environmental pH level. Since roselle anthocyanin is a pH-sensitive dye, the fresh-to-spoilage transformation could be easily visualized as the color changed from red to dark green. Thus, this novel starch nanofiber mat-based smart food label can provide the best consumption period and spoilage information to the consumers. Jiang et al. fabricated282 electrospun pullulan/PVA nanofiber membranes with bayberry pomace anthocyanin extract (BAE) to monitor the freshness of aquatic products (Fig. 21). SEM, FTIR, XRD, and TGA results confirmed the successful loading of BAE to electrospun polymer matrixes. The pH value for fresh Penaeus vannamei is 6.92, which increases with deterioration and reaches a maximum value of 7.7. If the pH value exceeds 7.7 it is not good to consume. In this study, the shrimp freshness was monitored by dividing it into three states according to their TVB-N content: (i) ‘fresh’ state - TVB-N content <10 mg/100 g of shrimp, (ii) ‘sub-fresh’ state - 10 mg/100 g < TVB-N content < 30 mg/100 g, (iii) ‘spoiled’ state - TVB-N content ≥ 30 mg/100 g. The film efficiently differentiated the three freshness states (fresh, sub-fresh, and spoiled) of Penaeus vannamei during storage at 4 °C by exhibiting varying colors, from pink to grayish-purple to green.
 | ||
| Fig. 21 Pullulan/PVA nanofiber membranes incorporated with bayberry pomace anthocyanin extract for freshness monitoring. [Reprinted with permission from ref. 282 Copyright Elsevier 2024]. | ||
Tea polyphenols (TPs) are the predominant bioactive agents present in tea leaf extract.283 TPs belong to a subclass of flavonoids, known as flavanols. Due to their effective antioxidant, antibacterial, anti-inflammatory, and preservative properties, TPs are widely used in various fields such as active food packaging, healthcare, and medicine.284 In active food packaging, TPs can act as excellent antioxidant and antibacterial agents. Moreover, through various interactive forces with the polymer matrix, they can improve the physical and chemical properties of the active film.285 Catechins, chemically flavan-3-ols, are the major components of TPs, which are primarily responsible for the antioxidant properties of green tea.286 The antioxidant efficacy of TPs is attributed to the presence of phenolic hydroxyl groups within polyphenol rings. The antioxidant mechanism of TPs involves its reaction with reactive oxygen species (ROS) to produce relatively stable phenolic oxygen radicals to scavenge free radicals.287 The antibacterial activity involves the attachment of TPs to the surface of the bacterial cell membrane through hydrogen bonding to produce hydrogen peroxide and damages the bacterial cell membrane by denaturing the proteins and disrupting the function of DNA and mRNA.
Liu et al.288 fabricated an antimicrobial PLA/TP nanofiber membrane for food packaging applications. The investigation on the release of TP from the PLA/TP composite fiber on food simulants such as 50% ethanol and 95% ethanol revealed the easy release of TP due to its excellent hydrophilic nature. For the initial 20 hours, there was a burst release followed by a slow and sustained release. This can be attributed to the fact that TP was adsorbed on the surface of PLA and randomly distributed over the PLA matrix by non-covalent interactions. The composite fiber exhibited the highest 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical-scavenging capacity (95.07 ± 10.55%) and good antibacterial activity against E. coli and S. aureus (92.26% ± 5.93% and 94.58% ± 6.53%, respectively). A multifunctional active food packaging film based on gelatin nanofibers was developed by Lan et al.289 In this study, the antioxidant agent TP and the antibacterial agent ε-poly(L-lysine) were incorporated into a gelatin nanofiber membrane. The highest radical scavenging activity (>97%) and antibacterial activity (>93%) were obtained for 0.5% TP and 0.5% ε-poly(L-lysine) contents, respectively.
Gallic acid (GA) is a well-known natural antioxidant present in fruits, vegetables, and herbal medicines. They are secondary polyphenolic metabolites with the chemical formula C7H6O5 (3,4,5-trihydroxybenzoic acid).290 GA and its derivatives such as lauryl gallate, propyl gallate, octyl gallate, tetradecyl gallate and hexadecyl gallate have been found to possess antioxidant properties that inhibit oxidation by scavenging free radicals.291 Hence, they are useful additives in food preservation. In addition to the antioxidant effect, GA has many more biological functions such as antimicrobial, anticancer, and anti-inflammatory activities. Since GA is highly sensitive to temperature, pH, and light, it is usually encapsulated to conserve its bioactivity. Electrospinning is a versatile technique to encapsulate antioxidants susceptible to fast deterioration.
An effective antioxidant active food packaging material based on hydroxypropyl methylcellulose (HPMC) nanofibers was fabricated by incorporating GA to delay the oxidation of walnuts.292 Due to high amounts of polyunsaturated fatty acids, walnuts are highly prone to oxidation. HPMC nanofibers with 10% GA delayed the oxidation of walnut when compared to control. Moreover, the fiber diameter decreased with the increase in GA concentration. Chuysinuan et al.293 fabricated an electrospun PVA-based hydrogel composite with GA and studied their antioxidant activities by monitoring DPPH and ABTS radical scavenging assays and ferric reducing power. The highest activity was obtained for 7.5% GA/PVA electrospun fiber.
6. Sustainability and biodegradability of electrospun membranes
The sustainability of electrospun membranes largely depends on the choice of polymer. Biodegradable and bio-based polymers such as polylactic acid (PLA), polyvinyl alcohol (PVA), chitosan, gelatin, and cellulose derivatives degrade naturally in composting or normal soil conditions, compared to conventional petroleum-based plastics. These materials reduce both plastic waste and greenhouse gas emissions effectively.294 Replacing synthetic polymers with biodegradable polymers helps lower the carbon footprint while preserving the freshness of products. For example, PLA is fully biodegradable and industrially compostable biopolymer widely used for active food packaging applications. It is derived from renewable resources such as corn starch or sugarcane, making it an environmentally friendly alternative.295 Biodegradable electrospun membranes contribute significantly to sustainability by decomposing into harmless substances, thereby reducing long-term ecological effects. Recent advancements in the membrane technology field focus on green fabrication methods such as avoiding hazardous organic solvents, using renewable biopolymers, and facilitating membrane reuse or recovery.296 These advances align with sustainable packaging goals, offering eco-friendly alternatives to plastic materials.While the recyclability of nanofiber membranes remains limited due to their complex morphology and contamination after use, their single-use environmental impact can be minimized by utilizing renewable materials and incorporating bioactive components. As regulatory and consumer demands are increasing progressively, the inclusion of green synthesis methods and biopolymer composites presents a viable approach towards sustainable, functional food packaging systems that align with circular economy goals.158 Even though electrospun membranes are biodegradable, their degradation rate is prolonged from six months to two years in a natural environment, highlighting the need for further research to accelerate biodegradation without compromising functional performance.
7. Conclusion and future outlooks
Advanced food packaging technology plays a pivotal role in ensuring food safety by preserving and extending the shelf life of food products without compromising their quality. Unlike traditional food packaging, active and intelligent packaging systems can convey the current quality status of food to consumers through visual color changes, written texts, and various other indicators. Although there are multiple methods to prevent microbial damage and contamination in food, such as incorporating preservatives directly into the food, adding toxic additives to extend shelf life, and employing UV/visible light irradiation, these approaches have certain limitations. The direct interaction of these substances with food can adversely affect its nutritional value and safety. Therefore, instead of using toxic preservatives, various bioactive agents are incorporated into packaging films, as they offer several advantages over direct addition to food. Biopolymer-based electrospun nanofibers have garnered significant attention as food packaging materials due to the growing environmental and health concerns associated with petroleum-based plastics. In comparison to films produced through alternative fabrication methods, electrospun nanofibers exhibit a high surface area-to-volume ratio, attributed to their small diameter and porous structure. These characteristics along with their compatibility with a diverse range of polymers, both natural and synthetic, facilitate the development of composite nanofibers with enhanced gas barrier, antimicrobial, and antioxidant properties.Despite the numerous advantages discussed, the electrospinning technique also presents certain limitations. One of the major limitations of conventional electrospinning is its low productivity, hindering its large-scale industrial applications. Scaling up the process, while ensuring uniform quality and fiber characteristics, can be difficult. Safety concerns associated with electrospinning primarily come from the toxicity of the solvents employed in the process, as most biopolymers are water insoluble and hence require the use of organic solvents to dissolve them. The utilization of toxic organic solvents poses risks to human health and the environment, thereby reducing the environmental friendliness of this technology. Additionally, the incorporation of bioactive compounds to electrospinning solution poses further challenges, as many of these compounds are volatile and sensitive to external conditions. Exposure to high voltages during electrospinning may result in their degradation or inactivation, thereby compromising their functional performance. However, numerous electrospinning techniques have been developed to address these challenges. Coaxial electrospinning is a new technology that facilitates the encapsulation of sensitive compounds within a core–shell structure, thereby enabling controlled release and the utilization of un-spinnable materials. Multifluid electrospinning further enhances fiber complexity by incorporating multiple components. For large-scale production, multi-needle electrospinning increases output by enabling simultaneous spinning from multiple nozzles. Additionally, techniques such as emulsion electrospinning, blend electrospinning, needleless electrospinning, and near-field electrospinning offer improved control over the fiber structure and production efficiency, thereby advancing the applicability of electrospun materials in smart food packaging. Thus, by exploring innovative approaches, researchers and industries can develop advanced packaging materials that address consumers' demand and key challenges in food preservation, safety and sustainability.
Data availability
No primary research results, software or code has been included and no new data were generated or analysed as part of this review.Author contributions
Maria Mathew: conceptualization and writing the original draft. Sagitha Paroly: review and editing. Sujith Athiyanathil: supervision, review, and editing.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Institute of Technology Calicut (NITC), India, for this research work.References
- M. Aman Mohammadi, S. Dakhili, A. Mirza Alizadeh, S. Kooki, H. Hassanzadazar, M. Alizadeh-Sani and D. J. McClements, New Perspectives on Electrospun Nanofiber Applications in Smart and Active Food Packaging Materials, Crit. Rev. Food Sci. Nutr., 2024, 64(9), 2601–2617 CrossRef CAS PubMed.
- H. Xu, L. Chen, D. Julian McClements, Y. Hu, H. Cheng, C. Qiu, H. Ji, C. Sun, Y. Tian, M. Miao and Z. Jin, Progress in the Development of Photoactivated Materials for Smart and Active Food Packaging: Photoluminescence and Photocatalysis Approaches, Chem. Eng. J., 2022, 432, 134301 CrossRef CAS.
- K. Lloyd, M. Mirosa and J. Birch, Active and Intelligent Packaging, Encycl. Food Chem., 2019, 177–182 CAS.
- P. R. Salgado, L. Di Giorgio, Y. S. Musso and A. N. Mauri, Recent Developments in Smart Food Packaging Focused on Biobased and Biodegradable Polymers, Front. Sustain. Food Syst., 2021, 5, 1–30 Search PubMed.
- J. R. Westlake, M. W. Tran, Y. Jiang, X. Zhang, A. D. Burrows and M. Xie, Biodegradable Biopolymers for Active Packaging: Demand, Development and Directions, Sustainable Food Technol., 2023, 1(1), 50–72 RSC.
- B. Modi, H. Timilsina, S. Bhandari, A. Achhami, S. Pakka, P. Shrestha, D. Kandel, D. B. GC, S. Khatri, P. M. Chhetri and N. Parajuli, Current Trends of Food Analysis, Safety, and Packaging, Int. J. Food Sci., 2021, 2021(1), 9924667 Search PubMed.
- A. U. Alam, P. Rathi, H. Beshai, G. K. Sarabha and M. J. Deen, Fruit Quality Monitoring with Smart Packaging, Sensors, 2021, 21(4), 1509 CrossRef PubMed.
- P. Bora, C. Bhuyan, P. Rajguru and S. Hazarika, A Gemini Basic Ionic Liquid and Functionalized Cellulose Nanocrystal-Based Mixed Matrix Membrane for CO2/N2 Separation, Chem. Commun., 2023, 59(86), 12887–12890 RSC.
- S. Forghani, H. Almasi and M. Moradi, Electrospun Nanofibers as Food Freshness and Time-Temperature Indicators: A New Approach in Food Intelligent Packaging, Innovative Food Sci. Emerging Technol., 2021, 73, 102804 CrossRef CAS.
- M. Mathew, R. R. Paul, A. R. Abraham and A. K. Haghi, Introduction to Electrospinning of Nanofibers, in Electrospun Porous Nanofibers: an Introduction, Springer Nature Switzerland, Cham, 2025, pp. 1–18 Search PubMed.
- H. Chen, J. Wang, Y. Cheng, C. Wang, H. Liu, H. Bian, Y. Pan, J. Sun and W. Han, Application of Protein-Based Films and Coatings for Food Packaging: A Review, Polymers, 2019, 11(12), 1–32 Search PubMed.
- M. Mathew, R. R. Paul, A. R. Abraham and A. K. Haghi, Eletcrospun Nanofiber Membranes, in Electrospun Porous Nanofibers: An Introduction, Springer Nature Switzerland, Cham, 2025, pp. 35–49 Search PubMed.
- H. Rostamabadi, E. Assadpour, H. S. Tabarestani, S. R. Falsafi and S. M. Jafari, Electrospinning Approach for Nanoencapsulation of Bioactive Compounds; Recent Advances and Innovations, Trends Food Sci. Technol., 2020, 100, 190–209 CrossRef CAS.
- P. Sagitha, C. R. Reshmi, S. P. Sundaran and A. Sujith, Recent Advances in Post-Modification Strategies of Polymeric Electrospun Membranes, Eur. Polym. J., 2018, 105, 227–249 CrossRef CAS.
- M. Mathew, S. Paroly and S. Athiyanathil, Fabrication and Optimization of Highly Porous Electrospun PLA Fibers: A Comparative Study with Curcumin-Loaded Systems, J. Polym. Res., 2025, 32(4), 1–15 Search PubMed.
- R. Priyadarshi, P. Ezati and J.-W. Rhim, Recent Advances in Intelligent Food Packaging Applications Using Natural Food Colorants, ACS Food Sci. Technol., 2021, 1(2), 124–138 CrossRef CAS.
- S. Rahman, J. Gogoi, S. Dubey and D. Chowdhury, Animal Derived Biopolymers for Food Packaging Applications: A Review, Int. J. Biol. Macromol., 2024, 255, 128197 CrossRef CAS PubMed.
- Y. Yin and M. W. Woo, Transitioning of Petroleum-Based Plastic Food Packaging to Sustainable Bio-Based Alternatives, Sustainable Food Technol., 2024, 2(3), 548–566 RSC.
- T. Wang and E. Su, Electrospinning Meets Food Packaging: A Promising Pathway towards Novel Opportunities in Food Preservation, Food Packag. Shelf Life, 2024, 41, 101234 CrossRef CAS.
- F. Topuz and T. Uyar, Electrospinning of Sustainable Polymers from Biomass for Active Food Packaging, Sustainable Food Technol., 2024, 1266–1296 RSC.
- S. Yildirim, B. Röcker, M. K. Pettersen, J. Nilsen-Nygaard, Z. Ayhan, R. Rutkaite, T. Radusin, P. Suminska, B. Marcos and V. Coma, Active Packaging Applications for Food, Compr. Rev. Food Sci. Food Saf., 2018, 17(1), 165–199 CrossRef PubMed.
- R. K. Deshmukh, L. Hakim and K. K. Gaikwad, Active Packaging Materials, Curr. Food Sci. Technol. Reports, 2023, 1(2), 123–132 CrossRef.
- K. K. Gaikwad, S. Singh and Y. S. Negi, Ethylene Scavengers for Active Packaging of Fresh Food Produce, Environ. Chem. Lett., 2020, 18(2), 269–284 CrossRef CAS.
- M. A. A. Mariah, J. M. Vonnie, K. H. Erna, N. M. Nur’aqilah, N. Huda, R. A. Wahab and K. Rovina, The Emergence and Impact of Ethylene Scavengers Techniques in Delaying the Ripening of Fruits and Vegetables, Membranes, 2022, 12(2), 1–19 CrossRef PubMed.
- H. Wei, F. Seidi, T. Zhang, Y. Jin and H. Xiao, Ethylene Scavengers for the Preservation of Fruits and Vegetables: A Review, Food Chem., 2021, 337, 127750 CrossRef CAS PubMed.
- D. U. Shin, B. J. Park, H. W. Cho, S. W. Kim, E. S. Kim, Y. W. Jung, D. H. Kim and S. J. Lee, Potassium Permanganate-Based Ethylene Gas Indicator of Kiwifruit Ripeness, Postharvest Biol. Technol., 2023, 200, 112330 CrossRef CAS.
- A. Tirgar, D. Han and A. J. Steckl, Absorption of Ethylene on Membranes Containing Potassium Permanganate Loaded into Alumina-Nanoparticle-Incorporated Alumina/Carbon Nanofibers, J. Agric. Food Chem., 2018, 66(22), 5635–5643 CrossRef CAS PubMed.
- N. Jiang, M. Erdős, O. A. Moultos, R. Shang, T. J. H. Vlugt, S. G. J. Heijman and L. C. Rietveld, The Adsorption Mechanisms of Organic Micropollutants on High-Silica Zeolites Causing S-Shaped Adsorption Isotherms: An Experimental and Monte Carlo Simulation Study, Chem. Eng. J., 2020, 389, 123968 CrossRef CAS.
- E. Pérez-Botella, S. Valencia and F. Rey, Zeolites in Adsorption Processes: State of the Art and Future Prospects, Chem. Rev., 2022, 122(24), 17647–17695 CrossRef PubMed.
- S. D. do Nascimento Sousa, R. G. Santiago, D. A. Soares Maia, E. de Oliveira Silva, R. S. Vieira and M. Bastos-Neto, Ethylene Adsorption on Chitosan/Zeolite Composite Films for Packaging Applications, Food Packag. Shelf Life, 2020, 26, 100584 CrossRef.
- A. Upadhyay, P. Kumar, S. K. Kardam and K. K. Gaikwad, Ethylene Scavenging Film Based on Corn Starch-Gum Acacia Impregnated with Sepiolite Clay and Its Effect on Quality of Fresh Broccoli Florets, Food Biosci., 2022, 46, 101556 CrossRef CAS.
- H. Wei, L. Li, T. Zhang, F. Seidi and H. Xiao, Platinum-Loaded Dendritic Mesoporous Silica as Novel Ethylene Scavenger to Extend Shelf Life of Banana (Musa Nana), Food Chem., 2023, 424, 136415 CrossRef CAS PubMed.
- P. Tepamatr, Efficacy of a Palladium-Modified Activated Carbon in Improving Ethylene Removal to Delay the Ripening of Gros Michel Banana, J. Agric. Food Res., 2023, 12, 100561 CAS.
- P. Suraj, M. Shantanu, S. P. Patil, S. R. Chaudhari and R. S. Matche, Ethylene Scavenger from Brick Ash: A Sustainable Alternative, ACS Sustain. Chem. Eng., 2023, 11(24), 8764–8773 CrossRef.
- R. Asrey, S. Sharma, K. Barman, U. Prajapati, N. Negi and N. K. Meena, Biological and Postharvest Interventions to Manage the Ethylene in Fruit: A Review, Sustainable Food Technol., 2023, 1(6), 803–826 RSC.
- J. de Matos Fonseca, S. M. Miranda, J. P. Monteiro, R. de Fátima Peralta Muniz Moreira, G. A. Valencia, A. R. Monteiro and V. J. P. Vilar, Ethylene Photocatalytic Degradation Using TiO2 Immobilized in a Net Mix Mili-Photoreactor, J. Environ. Chem. Eng., 2023, 11(5), 110976 CrossRef.
- U. Siripatrawan and P. Kaewklin, Fabrication and Characterization of Chitosan-Titanium Dioxide Nanocomposite Film as Ethylene Scavenging and Antimicrobial Active Food Packaging, Food Hydrocoll., 2018, 84, 125–134 CrossRef CAS.
- R. K. Deshmukh and K. K. Gaikwad, Natural Antimicrobial and Antioxidant Compounds for Active Food Packaging Applications, Biomass Convers. Biorefinery, 2024, 14(4), 4419–4440 CrossRef CAS.
- S. Volpe, P. V. Mahajan, G. Rux, S. Cavella and E. Torrieri, Condensation and Moisture Regulation in Packaged Fresh-Cut Iceberg Lettuce, J. Food Eng., 2018, 216, 132–137 CrossRef.
- P. V. Mahajan and D. S. Lee, Modified Atmosphere and Moisture Condensation in Packaged Fresh Produce: Scientific Efforts and Commercial Success, Postharvest Biol. Technol., 2023, 198, 112235 CrossRef CAS.
- Z. A. Nur Hanani, A. D. Sonawane and P. V. Mahajan, Impact of Humidity, Temperature and Condensation on O2 and CO2 Transmission Rate of Modified Atmosphere Packages, Food Packag. Shelf Life, 2023, 39, 101132 CrossRef CAS.
- K. K. Gaikwad, S. Singh and A. Ajji, Moisture Absorbers for Food Packaging Applications, Environ. Chem. Lett., 2019, 17(2), 609–628 CrossRef CAS.
- L. Anokye-Bempah, J. Han, K. Kornbluth, W. Ristenpart and I. R. Donis-González, The Use of Desiccants for Proper Moisture Preservation in Green Coffee during Storage and Transportation, J. Agric. Food Res., 2023, 11, 100478 CAS.
- S. Misha, S. Mat, M. H. Ruslan and K. Sopian, Review of Solid/Liquid Desiccant in the Drying Applications and Its Regeneration Methods, Renew. Sustain. Energy Rev., 2012, 16(7), 4686–4707 CrossRef CAS.
- R. Srivastava, M. Chandrashekara and A. Yadav, The Regeneration of Various Solid Desiccants Using Novel Heated Surface Plate Based on Heat Pipe Vacuum Tube Collector: An Experimental Investigation, Energy Sources, Part A, 2023, 45(3), 9341–9358 CrossRef CAS.
- C. Kenyó, K. Renner, J. Móczó, E. Fekete, C. Kröhnke and B. Pukánszky, Effect of Desiccant Characteristics on the Properties of PS/Zeolite Functional Packaging Materials, Polym. Compos., 2014, 35(11), 2112–2120 CrossRef.
- J. Chen, J. Wu, P. Raffa, F. Picchioni and C. E. Koning, Superabsorbent Polymers: From Long-Established, Microplastics Generating Systems, to Sustainable, Biodegradable and Future Proof Alternatives, Prog. Polym. Sci., 2022, 125, 101475 CrossRef CAS.
- M. A. Qureshi, N. Nishat, S. Jadoun and M. Z. Ansari, Polysaccharide Based Superabsorbent Hydrogels and Their Methods of Synthesis: A Review, Carbohydr. Polym. Technol. Appl., 2020, 1, 100014 Search PubMed.
- L. Chang, L. Xu, Y. Liu and D. Qiu, Superabsorbent Polymers Used for Agricultural Water Retention, Polym. Test., 2021, 94, 107021 CrossRef CAS.
- X. Jiao, J. Xie, H. Du, X. Bian, C. Wang, L. Zhou and Y. Wen, Antibacterial Smart Absorbent Pad with Janus Structure for Meat Preservation, Food Packag. Shelf Life, 2023, 37, 101066 CrossRef CAS.
- Y. Yan, Y. Zhang, Z. Fang, Z.-C. Wang, Y. Nan, H. Shi, H. Zhang, W. Song and H. Gu, Modified Atmosphere Packaging and Plant Extracts Synergistically Enhance the Preservation of Meat: A Review, Food Control, 2024, 164, 110622 CrossRef CAS.
- V. Kumar, A. Kumar Vinayak and S. Venkatachalam, Moisture Barrier Behavior of Polymer Films on Food Packaging Plastic Materials, Int. J. Technol. Res. Eng., 2022, 9(5), 6–12 Search PubMed.
- W. F. Lai, Design of Polymeric Films for Antioxidant Active Food Packaging, Int. J. Mol. Sci., 2022, 23(1), 1–12 Search PubMed.
- H. T. Endale, W. Tesfaye and T. A. Mengstie, ROS Induced Lipid Peroxidation and Their Role in Ferroptosis, Front. Cell Dev. Biol., 2023, 11, 1–7 Search PubMed.
- Y. Zheng, J. Sun, Z. Luo, Y. Li and Y. Huang, Emerging Mechanisms of Lipid Peroxidation in Regulated Cell Death and Its Physiological Implications, Cell Death Dis., 2024, 15(11), 859 CrossRef PubMed.
- S. Atalay Ekiner, A. Gęgotek and E. Skrzydlewska, Inflammasome Activity Regulation by PUFA Metabolites, Front. Immunol., 2024, 15, 1–23 Search PubMed.
- D. B. Min, A. L. Callison and H. O. Lee, Singlet Oxygen Oxidation for 2-Pentylfuran and 2-Pentenyfuran Formation in Soybean Oil, J. Food Sci., 2003, 68(4), 1175–1178 CrossRef CAS.
- T. A. Uzombah, The Implications of Replacing Synthetic Antioxidants with Natural Ones in the Food Systems, in Natural Food Additives, 2022 Search PubMed.
- O. L. Vidal, M. C. Barros Santos, A. P. Batista, F. F. Andrigo, B. Baréa, J. Lecomte, M. C. Figueroa-Espinoza, N. Gontard, P. Villeneuve, V. Guillard, C. M. Rezende, C. Bourlieu-Lacanal and M. S. Larraz Ferreira, Active Packaging Films Containing Antioxidant Extracts from Green Coffee Oil By-Products to Prevent Lipid Oxidation, J. Food Eng., 2022, 312, 110744 CrossRef CAS.
- A. V. García, N. J. Serrano, A. B. Sanahuja and M. C. Garrigós, Novel Antioxidant Packaging Films Based on Poly(ε-Caprolactone) and Almond Skin Extract: Development and Effect on the Oxidative Stability of Fried Almonds, Antioxidants, 2020, 9(7), 1–18 Search PubMed.
- R. Chawla, S. Sivakumar and H. Kaur, Antimicrobial Edible Films in Food Packaging: Current Scenario and Recent Nanotechnological Advancements- A Review, Carbohydr. Polym. Technol. Appl., 2021, 2, 100024 CAS.
- T. Fadiji, M. Rashvand, M. O. Daramola and S. A. Iwarere, A Review on Antimicrobial Packaging for Extending the Shelf Life of Food, Processes, 2023, 11(2), 1–30 CrossRef.
- M. A. Hudson and S. W. Lockless, Elucidating the Mechanisms of Action of Antimicrobial Agents, MBio, 2022, 13(3), 1–9 CrossRef CAS PubMed.
- S. Sharma, S. Barkauskaite, B. Duffy, A. K. Jaiswal and S. Jaiswal, Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application, Foods, 2020, 9, 1117 CrossRef CAS PubMed.
- G. Amor, M. Sabbah and L. Caputo, et al., Basil Essential Oil: Composition, Antimicrobial Properties, and Microencapsulation to Produce Active Chitosan Films for Food Packaging, Foods, 2021, 10(1), 121 CrossRef CAS PubMed.
- Y. Shen, Z.-J. Ni, K. Thakur, J.-G. Zhang, F. Hu and Z.-J. Wei, Preparation and Characterization of Clove Essential Oil Loaded Nanoemulsion and Pickering Emulsion Activated Pullulan-Gelatin Based Edible Film, Int. J. Biol. Macromol., 2021, 181, 528–539 CrossRef CAS PubMed.
- L. Pavlátková, J. Sedlaříková, P. Pleva, P. Peer, I. Uysal-Unalan and M. Janalíková, Bioactive zein/chitosan systems loaded with essential oils for food-packaging applications, J. Sci. Food Agric., 2023, 103, 1097–1104 CrossRef PubMed.
- L. Lin, X. Mao, Y. Sun, G. Rajivgandhi and H. Cui, Antibacterial Properties of Nanofibers Containing Chrysanthemum Essential Oil and Their Application as Beef Packaging, Int. J. Food Microbiol., 2019, 292, 21–30 CrossRef CAS PubMed.
- N. Khorshidian, E. Khanniri, M. R. Koushki and S. Sohrabvandi, An Overview of Antimicrobial Activity of Lysozyme and Its Functionality in Cheese, Front. Nutr., 2022, 9, 833618 CrossRef PubMed.
- M. Cissé, J. Polidori, D. Montet, G. Loiseau and M. N. Ducamp-Collin, Preservation of Mango Quality by Using Functional Chitosan-Lactoperoxidase Systems Coatings, Postharvest Biol. Technol., 2015, 101, 10–14 CrossRef.
- G. Mauriello, E. De Luca, A. La Storia, F. Villani and D. Ercolini, Antimicrobial Activity of a Nisin-Activated Plastic Film for Food Packaging, Lett. Appl. Microbiol., 2005, 41(6), 464–469 CrossRef CAS PubMed.
- S. Chang, Y. Chen, H. Tseng, H. Hsiao, H. Chai, K. Shang, C. Pan and G. Tsai, Applications of Nisin and EDTA in Food Packaging for Improving Fabricated Chitosan-Polylactate Plastic Film Performance and Fish Fillet Preservation, Membranes, 2021, 11(11), 852 CrossRef CAS PubMed.
- D. da Silva, M. de Oliveira, S. Wang, D. Carastan and D. Rosa, Designing Antimicrobial Polypropylene Films with Grape Pomace Extract for Food Packaging, Food Packag. Shelf Life, 2022, 34, 100929 CrossRef CAS.
- Z. N.S, M. Albalawi, A. Alalawy, M. Al Duais, S. Alzahrani and M. Kasem, Modification of Edible Chitosan/Polyethylene Glycol Films Fortified with Palm Date Fruit Waste Extract as Promising Antimicrobial Food Packaging Materials for Fresh Strawberry Conservation, Eur. Polym. J., 2023, 194, 112171 CrossRef.
- T. Ma, Y. Chen, X. Zhi and B. Du, Cellulose Laurate Films Containing Curcumin as Photoinduced Antibacterial Agent for Meat Preservation, Int. J. Biol. Macromol., 2021, 193, 1986–1995 CrossRef CAS PubMed.
- M. C. Cruz-Romero, T. Murphy, M. Morris, E. Cummins and J. P. Kerry, Antimicrobial Activity of Chitosan, Organic Acids and Nano-Sized Solubilisates for Potential Use in Smart Antimicrobially-Active Packaging for Potential Food Applications, Food Control, 2013, 34(2), 393–397 CrossRef CAS.
- E. Mani-López, H. S. García and A. López-Malo, Organic Acids as Antimicrobials to Control Salmonella in Meat and Poultry Products, Food Res. Int., 2012, 45(2), 713–721 CrossRef.
- Y. Palanisamy, V. Kadirvel and N. D. Ganesan, Recent Technological Advances in Food Packaging: Sensors, Automation, and Application, Sustainable Food Technol., 2024, 161–180 Search PubMed.
- P. Shao, L. Liu, J. Yu, Y. Lin, H. Gao, H. Chen and P. Sun, An Overview of Intelligent Freshness Indicator Packaging for Food Quality and Safety Monitoring, Trends Food Sci. Technol., 2021, 118, 285–296 CrossRef CAS.
- W. Heo and S. Lim, A Review on Gas Indicators and Sensors for Smart Food Packaging, Foods, 2024, 13(19), 3047 CrossRef CAS PubMed.
- S. Kalpana, S. R. Priyadarshini, M. M. Leena, J. A. Moses and C. Anandharamakrishnan, Trends in Food Science & Technology Intelligent Packaging : Trends and Applications in Food Systems, Trends Food Sci. Technol., 2019, 9, 145–157 CrossRef.
- K. W. McMillin, Modified Atmosphere Packaging, in Food Safety Engineering, ed. Demirci, A., Feng, H. and Krishnamurthy, K., Springer International Publishing, Cham, 2020, pp. 693–718 Search PubMed.
- A. Tilley, M. P. McHenry, J. A. McHenry, V. Solah and K. Bayliss, Enzymatic Browning: The Role of Substrates in Polyphenol Oxidase Mediated Browning, Curr. Res. Food Sci., 2023, 7, 100623 CrossRef CAS PubMed.
- K. Won, N. Y. Jang and J. Jeon, A Natural Component-Based Oxygen Indicator with In-Pack Activation for Intelligent Food Packaging A Natural Component-Based Oxygen Indicator with In-Pack Activation for Intelligent Food Packaging, J. Agric. Food Chem., 2016, 64(51), 9675–9679 CrossRef CAS PubMed.
- X. Wang and O. S. Wolfbeis, Optical Methods for Sensing and Imaging Oxygen: Materials{,} Spectroscopies and Applications, Chem. Soc. Rev., 2014, 43(10), 3666–3761 RSC.
- A. Ananga, V. Georgiev, J. Ochieng, B. Phills and V. Tsolova, Production of Anthocyanins in Grape Cell Cultures: A Potential Source of Raw Material for Pharmaceutical, Food, and Cosmetic Industries, ed. B. Sladonja, 2013, pp. 247–287 Search PubMed.
- Y. Liu, L. Li, Z. Yu, C. Ye, L. Pan and Y. Song, Principle, Development and Application of Time–Temperature Indicators for Packaging, Packag. Technol. Sci., 2023, 36(10), 833–853 CrossRef.
- J. Zhang, S. Liu, C. Xie, C. Wang, Y. Zhong and K. Fan, Recent Advances in PH-Sensitive Indicator Films Based on Natural Colorants for Smart Monitoring of Food Freshness: A Review, Crit. Rev. Food Sci. Nutr., 2023, 64(33), 12800–12819 CrossRef PubMed.
- L. Zheng, L. Liu, J. Yu and P. Shao, Novel Trends and Applications of Natural PH-Responsive Indicator Film in Food Packaging for Improved Quality Monitoring, Food Control, 2022, 134, 108769 CrossRef CAS.
- D. Liu, C. Zhang, Y. Pu, S. Chen, L. Liu, Z. Cui and Y. Zhong, Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness, Foods, 2022, 11(13), 1884 CrossRef CAS PubMed.
- R. Mattioli, A. Francioso, L. Mosca and P. Silva, Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases, Molecules, 2020, 25(17), 3809 CrossRef CAS PubMed.
- K. Halake, M. Birajdar and J. Lee, Structural Implications of Polyphenolic Antioxidants, J. Ind. Eng. Chem., 2016, 35, 1–7 CrossRef CAS.
- A. N. Mafe, G. I. Edo, R. S. Makia, O. A. Joshua, P. O. Akpoghelie, T. S. Gaaz, A. N. Jikah, E. Yousif, E. F. Isoje, U. A. Igbuku, D. S. Ahmed, A. E. A. Essaghah and H. Umar, A Review on Food Spoilage Mechanisms, Food Borne Diseases and Commercial Aspects of Food Preservation and Processing, Food Chem. Adv., 2024, 5, 100852 CrossRef.
- T. Gao, Y. Tian, Z. Zhu and D.-W. Sun, Modelling, Responses and Applications of Time-Temperature Indicators (TTIs) in Monitoring Fresh Food Quality, Trends Food Sci. Technol., 2020, 99, 311–322 CrossRef CAS.
- A. Pavelková, Time Temperature Indicators as Devices Intelligent Packaging, Acta Univ. Agric. Silvic. Mendelianae Brun., 2013, 61(1), 245–251 CrossRef.
- F. Saliu and R. Della Pergola, Carbon Dioxide Colorimetric Indicators for Food Packaging Application: Applicability of Anthocyanin and Poly-Lysine Mixtures, Sens. Actuators, B, 2018, 258, 1117–1124 CrossRef CAS.
- B. Liu, H. Xu, H. Zhao, W. Liu, L. Zhao and Y. Li, Preparation and Characterization of Intelligent Starch/PVA Films for Simultaneous Colorimetric Indication and Antimicrobial Activity for Food Packaging Applications, Carbohydr. Polym., 2017, 157, 842–849 CrossRef CAS PubMed.
- L. Liu, W. Wu, L. Zheng, J. Yu, P. Sun and P. Shao, Intelligent Packaging Films Incorporated with Anthocyanins-Loaded Ovalbumin-Carboxymethyl Cellulose Nanocomplexes for Food Freshness Monitoring, Food Chem., 2022, 387, 132908 CrossRef CAS PubMed.
- H. Chen, M. Zhang, B. Bhandari and C. Yang, Novel PH-Sensitive Films Containing Curcumin and Anthocyanins to Monitor Fish Freshness, Food Hydrocoll., 2020, 100, 105438 CrossRef CAS.
- X. Zhai, Z. Li, J. Zhang, J. Shi, X. Zou, X. Huang, D. Zhang, Y. Sun, Z. Yang, M. Holmes, Y. Gong and M. Povey, Natural Biomaterial-Based Edible and PH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging, J. Agric. Food Chem., 2018, 66(48), 12836–12846 CrossRef CAS PubMed.
- P. Mustafa, M. B. K. Niazi, Z. Jahan, G. Samin, A. Hussain, T. Ahmed and S. R. Naqvi, PVA/Starch/Propolis/Anthocyanins Rosemary Extract Composite Films as Active and Intelligent Food Packaging Materials, J. Food Saf., 2020, 40(1), e12725 CrossRef.
- Y. Hao, J. Kang, X. Guo, M. Sun, H. Li, H. Bai, H. Cui and L. P. H.- Shi, Responsive Chitosan-Based Film Containing Oregano Essential Oil and Black Rice Bran Anthocyanin for Preserving Pork and Monitoring Freshness, Food Chem., 2023, 403, 134393 CrossRef CAS PubMed.
- S. Roy and J.-W. Rhim, Fabrication of Carboxymethyl Cellulose/Agar-Based Functional Films Hybridized with Alizarin and Grapefruit Seed Extract, ACS Appl. Bio Mater., 2021, 4(5), 4470–4478 CrossRef CAS PubMed.
- R. R. Koshy, J. T. Koshy, S. K. Mary, S. Sadanandan, S. Jisha and L. A. Pothan, Preparation of PH Sensitive Film Based on Starch/Carbon Nano Dots Incorporating Anthocyanin for Monitoring Spoilage of Pork, Food Control, 2021, 126, 108039 CrossRef CAS.
- A. M. Alizadeh, M. Masoomian, M. Shakooie, M. Z. Khajavi and M. Farhoodi, Trends and Applications of Intelligent Packaging in Dairy Products: A Review, Crit. Rev. Food Sci. Nutr., 2022, 62(2), 383–397 CrossRef PubMed.
- M. Nami, M. Taheri, I. A. Deen, M. Packirisamy and M. J. Deen, Nanomaterials in Chemiresistive and Potentiometric Gas Sensors for Intelligent Food Packaging, TrAC Trends Anal. Chem., 2024, 174, 117664 CrossRef CAS.
- P. Li, J. Li, S. Song, J. Chen, N. Zhong, Q. Xie, Y. Liu, B. Wan, Y. He and H. Karimi-Maleh, Recent Advances in Optical Gas Sensors for Carbon Dioxide Detection, Measurement, 2025, 239, 115445 CrossRef.
- H. Wang, S. I. Vagin, B. Rieger and A. Meldrum, An Ultrasensitive Fluorescent Paper-Based CO2 Sensor, ACS Appl. Mater. Interfaces, 2020, 12(18), 20507–20513 CrossRef CAS PubMed.
- A. Mills, L. McDonnell and D. Yusufu, Colorimetric CO2 Indicators, Accounts Mater. Res., 2023, 4(7), 570–579 CrossRef CAS PubMed.
- L. Bigiani, D. Zappa, E. Comini, C. Maccato, A. Gasparotto and D. Barreca, Manganese Oxide Nanoarchitectures as Chemoresistive Gas Sensors to Monitor Fruit Ripening, J. Nanosci. Nanotechnol., 2020, 20(5), 3025–3030 CrossRef CAS PubMed.
- V. Naresh and N. Lee, A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors, Sensors, 2021, 21(4), 1–35 CrossRef PubMed.
- D. Zou, L. Jin, B. Wu, L. Hu, X. Chen, G. Huang and J. Zhang, Rapid Detection of Salmonella in Milk by Biofunctionalised Magnetic Nanoparticle Cluster Sensor Based on Nuclear Magnetic Resonance, Int. Dairy J., 2019, 91, 82–88 CrossRef CAS.
- G. Kaur, S. Sharma, S. Singh, N. Bhardwaj and A. Deep, Selective and Sensitive Electrochemical Sensor for Aflatoxin M1 with a Molybdenum Disulfide Quantum Dot/Metal–Organic Framework Nanocomposite, ACS Omega, 2022, 7(21), 17600–17608 CrossRef CAS PubMed.
- S. Neethirajan, V. Ragavan, X. Weng and R. Chand, Biosensors for Sustainable Food Engineering: Challenges and Perspectives, Biosensors, 2018, 8(1), 23 CrossRef PubMed.
- I. Nastasijevic, I. Kundacina, S. Jaric, Z. Pavlovic, M. Radovic and V. Radonic, Recent Advances in Biosensor Technologies for Meat Production Chain, Foods, 2025, 14(5), 744 CrossRef CAS PubMed.
- A. M. Alizadeh, M. Masoomian, M. Shakooie, Z. Khajavi and M. Farhoodi, Trends and Applications of Intelligent Packaging in Dairy Products : A Review, Crit. Rev. Food Sci. Nutr., 2020, 1–15 Search PubMed.
- M. Ghaani, C. A. Cozzolino, G. Castelli and S. Farris, An Overview of the Intelligent Packaging Technologies in the Food Sector, Trends Food Sci. Technol., 2016, 51, 1–11 CrossRef CAS.
- P. Li, J. Yang, A. M. Jiménez-Carvelo and S. W. Erasmus, Applications of Food Packaging Quick Response Codes in Information Transmission toward Food Supply Chain Integrity, Trends Food Sci. Technol., 2024, 146, 104384 CrossRef CAS.
- J. Z. Gao, L. Prakash and R. Jagatesan, Understanding 2D-BarCode Technology and Applications in M-Commerce - Design and Implementation of A 2D Barcode Processing Solution, IEEE Xplore, 2021, 49–56 CAS.
- J. Zuo, J. Feng, M. G. Gameiro, Y. Tian, J. Liang, Y. Wang, J. Ding and Q. He, RFID-Based Sensing in Smart Packaging for Food Applications: A Review, Future Foods, 2022, 6, 100198 CrossRef CAS PubMed.
- T. Athauda, R. Bhattacharyya, N. Karmakar and S. Sarma, Electromagnetic Characterization of a Food Safe, Organic Smart Material for Customizable Temperature Threshold Sensing in Cold Chain Applications, in 2019 IEEE International Conference on RFID, RFID, 2019, pp. 1–6 Search PubMed.
- A. Vena, L. Sydänheimo, M. M. Tentzeris and L. Ukkonen, A Novel Inkjet Printed Carbon Nanotube-Based Chipless RFID Sensor for Gas Detection, in 2013 European Microwave Conference, 2013, pp. 9–12 Search PubMed.
- J. Jun, J. Oh, D. H. Shin, S. G. Kim, J. S. Lee, W. Kim and J. W. Jang, Room Temperature Volatile Organic Compound Sensor Based on Polypyrrole Nanoparticle Immobilized Ultrahigh Frequency Radio Frequency Identification Tag, ACS Appl. Mater. Interfaces, 2016, 8(48), 33139–33147 CrossRef CAS PubMed.
- M. Mathew, R. R. Paul, A. R. Abraham and A. K. Haghi Applications of Electrospun Nanofibers, in Electrospun Porous Nanofibers: an Introduction, Springer Nature Switzerland, Cham, 2025, pp. 19–34 Search PubMed.
- A. Nadaf, A. Gupta, N. Hasan, N. Fauziya, S. Ahmad, P. Kesharwani and F. J. Ahmad, Recent Update on Electrospinning and Electrospun Nanofibers: Current Trends and Their Applications, RSC Adv., 2022, 12(37), 23808–23828 RSC.
- C. Shi, D. Fang, C. Huang, L. Lyu, W. Wu and W. Li, Electrospun Biopolymer Material for Antimicrobial Function of Fresh Fruit and Vegetables: Application Perspective and Challenges, LWT, 2023, 174, 114374 CrossRef CAS.
- Y. Zhao, G. Guo, B. Xu, H. Liu, H. Tian, J. Li, Y. Ouyang, A. Xiang and R. Kumar, Electrospun Natural Polypeptides Based Nanofabrics Enriched with Antioxidant Polyphenols for Active Food Preservation, Food Chem., 2023, 405, 134991 CrossRef CAS PubMed.
- C. Shen, W. Chen, C. Li, Y. Ye, H. Cui and L. Lin, Preparation and Physicochemical Effects of Zein Nanofiber Membrane Encapsulated with Citral/HP-β-CD Inclusion Complex and Its Application on Cheese, Food Biosci., 2022, 50, 101990 CrossRef CAS.
- Y. Liu, R. Wang, D. Wang, Z. Sun, F. Liu, D. Zhang and D. Wang, Development of a Food Packaging Antibacterial Hydrogel Based on Gelatin, Chitosan, and 3-Phenyllactic Acid for the Shelf-Life Extension of Chilled Chicken, Food Hydrocoll., 2022, 127, 107546 CrossRef CAS.
- A. Turanli, C. Altinkok, E. C. Kacakgil, C. Dizman and G. Acik, A Comprehensive Study on the Comparison between Casted Films and Electrospun Nanofibers of Poly(Vinyl Alcohol)/Chitosan Blends: Wettability, Thermal, Antioxidant, and Adsorbent Properties, Int. J. Biol. Macromol., 2025, 315, 144467 CrossRef CAS PubMed.
- L. Zhao, G. Duan, G. Zhang, H. Yang and S. He, Electrospun Functional Materials toward Food Packaging Applications : A Review, Nanomaterials, 2020, 10(1), 150 CrossRef CAS PubMed.
- R. Rubert, M. Mathew and R. Rajan Paul, Electrospinning Technique and Magnetic Nanofibers, inModern Magnetic Materials, 2023, pp. 247–290 Search PubMed.
- Q. F. Lim, R. C. C. Yap, C. P. Teng, J. C. C. Yeo, M. Y. Tan, J. P. W. Toh, Q. Zhu, W. Thitsartarn, C. He, S. Liu and J. Kong, Electrospray-on-Electrospun Breathable, Biodegradable, and Robust Nanofibrous Membranes with Photocatalytic Bactericidal Activity, ACS Appl. Nano Mater., 2023, 6(3), 1828–1838 CrossRef CAS.
- S. Soheili, B. Dolatyar, M. R. Adabi, D. Lotfollahi, M. Shahrousvand, P. Zahedi, E. Seyedjafari and J. Mohammadi-Rovshandeh, Fabrication of Fiber-Particle Structures by Electrospinning/Electrospray Combination as an Intrinsic Antioxidant and Oxygen-Releasing Wound Dressing, J. Mater. Chem. B, 2024, 9074–9097 RSC.
- D. A. Schmatz, J. A. V. Costa and M. G. de Morais, A Novel Nanocomposite for Food Packaging Developed by Electrospinning and Electrospraying, Food Packag. Shelf Life, 2019, 20, 100314 CrossRef.
- R. Abdulhussain, A. Adebisi, B. R. Conway and K. Asare-Addo, Electrospun Nanofibers: Exploring Process Parameters, Polymer Selection, and Recent Applications in Pharmaceuticals and Drug Delivery, J. Drug Deliv. Sci. Technol., 2023, 90, 105156 CrossRef CAS.
- S. S. Syed Bakar, K. M. Foong, N. Abdul Halif and S. Yahud, Effect of Solution Concentration and Applied Voltage on Electrospun Polyacrylonitrile Fibers, IOP Conf. Ser. Mater. Sci. Eng., 2019, 701(1), 12018 CrossRef.
- M. F. Abdullah, A. Andriyana, F. Muhamad and B. C. Ang, Effect of Core-to-Shell Flowrate Ratio on Morphology, Crystallinity, Mechanical Properties and Wettability of Poly(Lactic Acid) Fibers Prepared via Modified Coaxial Electrospinning, Polymer, 2021, 237, 124378 CrossRef CAS.
- S. Zargham, S. Bazgir, A. Tavakoli, A. S. Rashidi and R. Damerchely, The Effect of Flow Rate on Morphology and Deposition Area of Electrospun Nylon 6 Nanofiber, J. Eng. Fibers Fabr., 2012, 7(4), 42–49 CAS.
- F.-R. Mariola, Á. A. Beltrán-Osuna, J. A. Gómez-Tejedor and L. Gómez, Electrospun PVA/Bentonite Nanocomposites Mats for Drug Delivery, Materials, 2017, 10(12), 1448 CrossRef PubMed.
- A. M. Al-Dhahebi, M. S. M. Saheed and M. Mustapha, Effects of Solution Concentration on the Synthesis of Polyvinylidene Fluoride (PVDF) Electrospun Nanofibers, Mater. Today: Proc., 2023, 80, 2119–2124 CAS.
- V. Pillay, C. Dott, Y. E. Choonara, C. Tyagi, L. Tomar, P. Kumar and L. C. Toit, Ndesendo A Review of the Effect of Processing Variables on the Fabrication of Electrospun Nanofibers for Drug Delivery Applications, J. Nanomater., 2013, 2013, 1–22 Search PubMed.
- J. Tao and S. Shivkumar, Molecular Weight Dependent Structural Regimes during the Electrospinning of PVA, Mater. Lett., 2007, 61, 2325–2328 CrossRef CAS.
- F. T. Zahra, Y. Zhang, A. O. Ajayi, Q. Quick and R. Mu, Optimization of Electrospinning Parameters for Lower Molecular Weight Polymers: A Case Study on Polyvinylpyrrolidone, Polymers, 2024, 16(9), 1217 CrossRef CAS PubMed.
- E. Ewaldz, J. Randrup and B. Brettmann, Solvent Effects on the Elasticity of Electrospinnable Polymer Solutions, ACS Polym. Au, 2022, 2(2), 108–117 CrossRef CAS PubMed.
- R. M. Nezarati, M. B. Eifert and E. Cosgriff-hernandez, Effects of Humidity and Solution Viscosity on Electrospun Fiber Morphology, Tissue Eng., Part C, 2013, 19(10), 810–819 CrossRef CAS PubMed.
- Q. Yang, Z. Li, Y. Hong, Y. Zhao, S. Qiu, C. E. Wang and Y. E. N. Wei Influence of Solvents on the Formation of Ultrathin Uniform Poly(Vinyl Pyrrolidone) Nanofibers With. 2004, pp. 7–11 Search PubMed.
- A. K. Haghi and M. Akbari, Trends in Electrospinning of Natural Nanofibers, Phys. Status Solidi A, 2007, 1834(6), 1830–1834 CrossRef.
- C. J. Angammana, S. Member and S. H. Jayaram, Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology, IEEE Trans. Ind. Appl., 2011, 47(3), 1109–1117 CAS.
- B. Sun, Y. Z. Long, H. D. Zhang, M. M. Li, J. L. Duvail, X. Y. Jiang and H. L. Yin, Advances in Three-Dimensional Nanofibrous Macrostructures via Electrospinning, Prog. Polym. Sci., 2014, 39, 862–890 CrossRef CAS.
- A. Haider, S. Haider and I. Kang, REVIEW A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology, Arab. J. Chem., 2018, 11(8), 1165–1188 CrossRef CAS.
- G. Z. Yang, H. P. Li, J. H. Yang, J. Wan and D. G. Yu, Influence of Working Temperature on The Formation of Electrospun Polymer Nanofibers, Nanoscale Res. Lett., 2017, 12(1), 55 CrossRef PubMed.
- M. Wildy, W. Wei, K. Xu, J. Schossig, X. Hu, D. C. Hyun, W. Chen, C. Zhang and P. Lu, Heat's Role in Solution Electrospinning: A Novel Approach to Nanofiber Structure Optimization, Langmuir, 2024, 40(15), 7982–7991 CrossRef CAS PubMed.
- S. Mahdi, S. Shahabadi, A. Kheradmand, V. Montazeri and H. Ziaee, Effects of Process and Ambient Parameters on Diameter and Morphology of Electrospun Polyacrylonitrile Nanofibers, Polym. Sci., Ser. A, 2015, 57(2), 155–167 CrossRef.
- R. Ramakrishnan, P. Ramakrishnan, B. Ranganathan, C. Tan, T. M. Sridhar and J. Gimbun, ScienceDirect Effect of Humidity on Formation of Electrospun Polycaprolactone Nanofiber Embedded with Curcumin Using Needdleless Electrospinning, Mater. Today: Proc., 2019, 19, 1241–1246 CAS.
- D. Mailley, A. Hébraud and G. Schlatter, A Review on the Impact of Humidity during Electrospinning: From the Nanofiber Structure Engineering to the Applications, Macromol. Mater. Eng., 2021, 306(7), 2100115 CrossRef CAS.
- T. S. Muthu, K. S. Kumar, N. Rajini, S. Siengchin, N. Ayrilmis and A. V. Rajulu, A Comprehensive Review of Electrospun Nanofibers : Food and Packaging Perspective, Composites, Part B, 2019, 175, 107074 CrossRef.
- A. R. Borah, P. Hazarika, R. Duarah, R. Goswami and S. Hazarika, Biodegradable Electrospun Membranes for Sustainable Industrial Applications, ACS Omega, 2024, 9(10), 11129–11147 CrossRef CAS PubMed.
- X. Zhang, L. Li, J. Ouyang, L. Zhang, J. Xue, H. Zhang and W. Tao, Electroactive Electrospun Nanofibers for Tissue Engineering, Nano Today, 2021, 39, 101196 CrossRef CAS.
- Y. Wang, M. A. Khan, K. Chen, L. Zhang and X. Chen, Electrospinning of Natural Biopolymers for Innovative Food Applications : A Review, Food Bioprocess Technol., 2023, 704–725 CrossRef.
- S. R. Falsafi, F. Topuz, Z. Esfandiari, A. Can Karaca, S. M. Jafari and H. Rostamabadi, Recent Trends in the Application of Protein Electrospun Fibers for Loading Food Bioactive Compounds, Food Chem.:X, 2023, 20, 100922 CAS.
- M. Aman mohammadi, S. Ramazani, M. Rostami, M. Raeisi, M. Tabibiazar and M. Ghorbani, Fabrication of Food-Grade Nanofibers of Whey Protein Isolate–Guar Gum Using the Electrospinning Method, Food Hydrocoll., 2019, 90, 99–104 CrossRef CAS.
- H. Song, I. Choi, J.-S. Lee, Y. Chang, C. S. Yoon and J. Han, Whey Protein Isolate Coating Material for High Oxygen Barrier Properties: A Scale-up Study from Laboratory to Industrial Scale and Its Application to Food Packaging, Food Packag. Shelf Life, 2022, 31, 100765 CrossRef CAS.
- A. Etxabide, M. Arregi, S. Cabezudo, P. Guerrero and K. de la Caba, Whey Protein Films for Sustainable Food Packaging: Effect of Incorporated Ascorbic Acid and Environmental Assessment, Polymers, 2023, 15(2), 387 CrossRef CAS PubMed.
- Z. Panahi, M. Mohsenzadeh and M. Hashemi, Fabrication and Characterization of PVA/WPI Nanofibers Containing Probiotics Using Electrospinning Technique, Nanomed. J., 2023, 10(3), 216–226 CAS.
- B. Han, P. Chen, J. Guo, H. Yu, S. Zhong, D. Li, C. Liu, Z. Feng and B. Jiang, A Novel Intelligent Indicator Film: Preparation, Characterization, and Application, Molecules, 2023, 28(8), 3384 CrossRef CAS PubMed.
- L. Deng, X. Kang, Y. Liu, F. Feng and H. Zhang, Characterization of Gelatin/Zein Films Fabricated by Electrospinning vs Solvent Casting, Food Hydrocoll., 2018, 74, 324–332 CrossRef CAS.
- X. Lan, X. Zhang, L. Wang, H. Wang, Z. Hu, X. Ju and Y. Yuan, A Review of Food Preservation Based on Zein: The Perspective from Application Types of Coating and Film, Food Chem., 2023, 424, 136403 CrossRef CAS PubMed.
- J. Kong, X. Ge, Y. Sun, M. Mao, H. Yu, R. Chu and Y. Wang, Multi-Functional PH-Sensitive Active and Intelligent Packaging Based on Highly Cross-Linked Zein for the Monitoring of Pork Freshness, Food Chem., 2023, 404, 134754 CrossRef CAS PubMed.
- S. Ullah, M. Hashmi, J. Shi and I. S. Kim, Fabrication of Electrospun PVA/Zein/Gelatin Based Active Packaging for Quality Maintenance of Different Food Items, Polymers, 2023, 15(11), 2538 CrossRef CAS PubMed.
- M. Karim, M. Fathi, S. Soleimanian-Zad and G. Spigno, Development of Sausage Packaging with Zein Nanofibers Containing Tetradecane Produced via Needle-Less Electrospinning Method, Food Packag. Shelf Life, 2022, 33, 100911 CrossRef CAS.
- N. S. Said, N. K. Howell and N. M. Sarbon, A Review on Potential Use of Gelatin-Based Film as Active and Smart Biodegradable Films for Food Packaging Application, Food Rev. Int., 2023, 39(2), 1063–1085 CrossRef CAS.
- F. M. Fakhouri, D. Costa, F. Yamashita, S. M. Martelli, R. C. Jesus, K. Alganer, F. P. Collares-Queiroz and L. H. Innocentini-Mei, Comparative Study of Processing Methods for Starch/Gelatin Films, Carbohydr. Polym., 2013, 95(2), 681–689 CrossRef CAS PubMed.
- L. Yavari Maroufi, R. Norouzi, S. Ramezani and M. Ghorbani, Novel Electrospun Nanofibers Based on Gelatin/Oxidized Xanthan Gum Containing Propolis Reinforced by Schiff Base Cross-Linking for Food Packaging, Food Chem., 2023, 416, 135806 CrossRef CAS PubMed.
- K. Chayavanich, R. Kaneshige, P. Thiraphibundet, T. Furuike, H. Tamura and A. P. H.- Imyim, Responsive Nanofibrous Membrane Fabricated from Gelatin and Red Radish Anthocyanins for Meat Spoilage Monitoring, Dyes Pigm., 2023, 216, 111331 CrossRef CAS.
- M. Duan, J. Sun, Y. Huang, H. Jiang, Y. Hu, J. Pang and C. Wu, Electrospun Gelatin/Chitosan Nanofibers Containing Curcumin for Multifunctional Food Packaging, Food Sci. Hum. Wellness, 2023, 12(2), 614–621 CrossRef CAS.
- C. Huang, J. Tang, X. Chen, X. Zeng, W. Zhong, J. Pang and C. Wu, Novel Electrospun Gelatin Nanofibers Loaded with Purple Potato Anthocyanin and Syringic Acid for Multifunctional Food Packaging, Foods, 2024, 13(16), 2538 CrossRef CAS PubMed.
- V. Chaudhary, P. Kajla, P. Kumari, S. P. Bangar, A. Rusu, M. Trif and J. M. Lorenzo, Milk Protein-Based Active Edible Packaging for Food Applications: An Eco-Friendly Approach, Front. Nutr., 2022, 9, 942524 CrossRef PubMed.
- A. Shendurse, Milk Protein Based Edible Films and Coatings–Preparation, Properties and Food Applications, J. Nutr. Heal. Food Eng., 2018, 8(2), 219–226 Search PubMed.
- H. M. C. Azeredo and K. W. Waldron, Crosslinking in Polysaccharide and Protein Films and Coatings for Food Contact – A Review, Trends Food Sci. Technol., 2016, 52, 109–122 CrossRef CAS.
- J. Xie and Y. Lo. Hsieh, Ultra-High Surface Fibrous Membranes from Electrospinning of Natural Proteins: Casein and Lipase Enzyme, J. Mater. Sci., 2003, 38(10), 2125–2133 CrossRef CAS.
- P. M. Tomasula, A. M. M. Sousa, S. C. Liou, R. Li, L. M. Bonnaillie and L. S. Liu, Short Communication: Electrospinning of Casein/Pullulan Blends for Food-Grade Applications, J. Dairy Sci., 2016, 99(3), 1837–1845 CrossRef CAS PubMed.
- J. Dai, W. Hu, H. Yang, C. Li, H. Cui, X. Li and L. Lin, Controlled Release and Antibacterial Properties of PEO/Casein Nanofibers Loaded with Thymol/β-Cyclodextrin Inclusion Complexes in Beef Preservation, Food Chem., 2022, 382, 132369 CrossRef CAS PubMed.
- L. Chen, Y. Ramezan, H. Pourramezan, A. Najafi, A. Kamkari, G. Goksen, Z. Huang and W. Zhang, Soy Protein Isolate (SPI)-Based Films/Coatings for Food Packaging: Research Progress on Properties and Applications, Compr. Rev. Food Sci. Food Saf., 2025, 24(3), e70181 CrossRef PubMed.
- D. Cho, A. N. Netravali and Y. L. Joo, Mechanical Properties and Biodegradability of Electrospun Soy Protein Isolate/PVA Hybrid Nanofibers, Polym. Degrad. Stab., 2012, 97(5), 747–754 CrossRef CAS.
- G. Pinheiro Bruni, J. P. de Oliveira, L. G. Gómez-Mascaraque, M. J. Fabra, V. Guimarães Martins, E. da R. Zavareze and A. López-Rubio, Electrospun β-Carotene–Loaded SPI:PVA Fiber Mats Produced by Emulsion-Electrospinning as Bioactive Coatings for Food Packaging, Food Packag. Shelf Life, 2020, 23, 100426 CrossRef.
- S. Wang, M. F. Marcone, S. Barbut and L. T. Lim, Electrospun Soy Protein Isolate-Based Fiber Fortified with Anthocyanin-Rich Red Raspberry (Rubus Strigosus) Extracts, Food Res. Int., 2013, 52(2), 467–472 CrossRef CAS.
- M. Rashidi, S. Seyyedi Mansour, P. Mostashari, S. Ramezani, M. Mohammadi and M. Ghorbani, Electrospun Nanofiber Based on Ethyl Cellulose/Soy Protein Isolated Integrated with Bitter Orange Peel Extract for Antimicrobial and Antioxidant Active Food Packaging, Int. J. Biol. Macromol., 2021, 193, 1313–1323 CrossRef CAS PubMed.
- Y. Xu, Hierarchical Materials, Mod. Inorg. Synth. Chem., 2017, 545–574 CAS.
- J. Deng, E. Q. Zhu, G. F. Xu, N. Naik, V. Murugadoss, M. G. Ma, Z. Guo and Z. J. Shi, Overview of Renewable Polysaccharide-Based Composites for Biodegradable Food Packaging Applications, Green Chem., 2022, 24(2), 480–492 RSC.
- M. Ureña, T. T.-T. Phùng, M. Gerometta, L. de Siqueira Oliveira, J. Chanut, S. Domenek, P. Dole, G. Roudaut, A. Lagorce and T. Karbowiak, Potential of Polysaccharides for Food Packaging Applications. Part 1/2 : An Experimental Review of the Functional Properties of Polysaccharide Coatings, Food Hydrocoll., 2023, 144, 108955 CrossRef.
- M. Fathi, Á. Martín and D. J. McClements, Nanoencapsulation of Food Ingredients Using Carbohydrate Based Delivery Systems, Elsevier Ltd, 2014, p. 39 Search PubMed.
- D. N. Poshina, I. V. Tyshkunova, V. A. Petrova and Y. A. Skorik, Electrospinning of Polysaccharides for Tissue Engineering Applications, Rev. Adv. Chem., 2021, 11(1–2), 112–133 CrossRef CAS.
- H. Chen, F. Xie, L. Chen and B. Zheng, Effect of Rheological Properties of Potato, Rice and Corn Starches on Their Hot-Extrusion 3D Printing Behaviors, J. Food Eng., 2019, 244, 150–158 CrossRef CAS.
- M. Mondragón, O. López-Villegas, S. Sánchez-Valdés and F. J. Rodríguez-González, Effect of Thermoplastic Starch and Photocrosslinking on the Properties and Morphology of Electrospun Poly(Ethylene-Co-Vinyl Alcohol) Mats, Polym. Eng. Sci., 2020, 60(3), 474–480 CrossRef.
- G. Liu, Z. Gu, Y. Hong, L. Cheng and C. Li, Electrospun Starch Nanofibers: Recent Advances, Challenges, and Strategies for Potential Pharmaceutical Applications, J. Control. Release, 2017, 252, 95–107 CrossRef CAS PubMed.
- W. Zhu, D. Zhang, X. Liu, T. Ma, J. He, Q. Dong, Z. ud Din, J. Zhou, L. Chen, Z. Hu and J. Cai, Improving the Hydrophobicity and Mechanical Properties of Starch Nanofibrous Films by Electrospinning and Cross-Linking for Food Packaging Applications, LWT, 2022, 169, 114005 CrossRef CAS.
- L. M. Fonseca, C. E. Cruxen, S. dos, G. P. Bruni, Â. M. Fiorentini, E. da R. Zavareze, L. T. Lim and A. R. G. Dias, Development of Antimicrobial and Antioxidant Electrospun Soluble Potato Starch Nanofibers Loaded with Carvacrol, Int. J. Biol. Macromol., 2019, 139, 1182–1190 CrossRef CAS PubMed.
- X. Liu, L. Chen and Q. Dong, et al., Emerging starch composite nanofibrous films for food packaging: Facile construction, hydrophobic property, and antibacterial activity enhancement, Int. J. Biol. Macromol., 2022, 222(Pt A), 868–879 CrossRef CAS PubMed.
- K. El Bourakadi, F.-Z. Semlali, M. Hammi and M. El Achaby, A Review on Natural Cellulose Fiber Applications: Empowering Industry with Sustainable Solutions, Int. J. Biol. Macromol., 2024, 281, 135773 CrossRef CAS PubMed.
- K. Peranidze, T. V. Safronova and N. R. Kildeeva, Electrospun Nanomaterials Based on Cellulose and Its Derivatives for Cell Cultures: Recent Developments and Challenges, Polymers, 2023, 15(5), 1174 CrossRef CAS PubMed.
- Y. Zhang, C. Zhang and Y. Wang, Recent Progress in Cellulose-Based Electrospun Nanofibers as Multifunctional Materials, Nanoscale Adv., 2021, 3(21), 6040–6047 RSC.
- N. Qosim, H. Majd, J. Ahmed, G. Williams and M. Edirisinghe, Making Fibers from Cellulose Derivatives by Pressurized Gyration and Electrospinning, Cellulose, 2024, 31(5), 2815–2832 CrossRef CAS.
- S. Beikzadeh, S. M. Hosseini, V. Mofid, S. Ramezani, M. Ghorbani, A. Ehsani and A. M. Mortazavian, Electrospun Ethyl Cellulose/Poly Caprolactone/Gelatin Nanofibers: The Investigation of Mechanical, Antioxidant, and Antifungal Properties for Food Packaging, Int. J. Biol. Macromol., 2021, 191, 457–464 CrossRef CAS PubMed.
- A. Sobhan, K. Muthukumarappan and L. Wei, A Biopolymer-Based PH Indicator Film for Visually Monitoring Beef and Fish Spoilage, Food Biosci., 2022, 46, 101523 CrossRef CAS.
- C. Pittarate, T. Yoovidhya, W. Srichumpuang, N. Intasanta and S. Wongsasulak, Effects of Poly(Ethylene Oxide) and ZnO Nanoparticles on the Morphology, Tensile and Thermal Properties of Cellulose Acetate Nanocomposite Fibrous Film, Polym. J., 2011, 43(12), 978–986 CrossRef CAS.
- R. V. Wagh, A. Khan, R. Priyadarshi, P. Ezati and J.-W. Rhim, Cellulose Nanofiber-Based Multifunctional Films Integrated with Carbon Dots and Anthocyanins from Brassica Oleracea for Active and Intelligent Food Packaging Applications, Int. J. Biol. Macromol., 2023, 233, 123567 CrossRef CAS PubMed.
- Z. Li, J. Guan, C. Yan, N. Chen, C. Wang, T. Liu, F. Cheng, Q. Guo, X. Zhang, X. Ye, Y. Liu and Z. Shao, Corn Straw Core/Cellulose Nanofibers Composite for Food Packaging: Improved Mechanical, Bacteria Blocking, Ultraviolet and Water Vapor Barrier Properties, Food Hydrocoll., 2023, 143, 108884 CrossRef CAS.
- Z. Riahi, P. Ezati, J. W. Rhim, R. Bagheri and G. Pircheraghi, Cellulose Nanofiber-Based Ethylene Scavenging Antimicrobial Films Incorporated with Various Types of Titanium Dioxide Nanoparticles to Extend the Shelf Life of Fruits, ACS Appl. Polym. Mater., 2022, 4(7), 4765–4773 CrossRef CAS.
- F. Hisham, M. H. M. Akmal, F. Ahmad, K. Ahmad and N. Samat, Biopolymer Chitosan : Potential Sources , Extraction Methods , and Emerging Applications, Ain Shams Eng. J., 2024, 15(2), 102424 CrossRef.
- C. Salas, Z. Thompson and N. Bhattarai, Electrospun Chitosan Fibers, Elsevier Ltd, 2017 Search PubMed.
- C. Zhang, Y. Li, P. Wang and H. Zhang, Electrospinning of Nanofibers: Potentials and Perspectives for Active Food Packaging, Compr. Rev. Food Sci. Food Saf., 2020, 19(2), 479–502 CrossRef CAS PubMed.
- Y. Zou, C. Zhang, P. Wang, Y. Zhang and H. Zhang, Electrospun Chitosan/Polycaprolactone Nanofibers Containing Chlorogenic Acid-Loaded Halloysite Nanotube for Active Food Packaging, Carbohydr. Polym., 2020, 247, 116711 CrossRef CAS PubMed.
- B. I. Andreica, A. Anisiei, I. Rosca and L. Marin, Quaternized Chitosan-Based Nanofibers with Strong Antibacterial and Antioxidant Activity Designed as Ecological Active Food Packaging, Food Packag. Shelf Life, 2023, 39, 101157 CrossRef CAS.
- E. Yildiz, G. Sumnu and L. N. Kahyaoglu, Monitoring Freshness of Chicken Breast by Using Natural Halochromic Curcumin Loaded Chitosan/PEO Nanofibers as an Intelligent Package, Int. J. Biol. Macromol., 2021, 170, 437–446 CrossRef CAS PubMed.
- L. Lin, L. Xue, S. Duraiarasan and C. Haiying, Preparation of ε-Polylysine/Chitosan Nanofibers for Food Packaging against Salmonella on Chicken, Food Packag. Shelf Life, 2018, 17, 134–141 CrossRef.
- S. F. Hosseini, Z. Nahvi and M. Zandi, Antioxidant Peptide-Loaded Electrospun Chitosan/Poly(Vinyl Alcohol) Nanofibrous Mat Intended for Food Biopackaging Purposes, Food Hydrocoll., 2019, 89, 637–648 CrossRef CAS.
- C. Chancharoonpong, R. Tepsorn, J. Somsap, K. Kanjanapongkul and S. Supapvanich, Antimicrobial Activity of Edible Electrospun Chitosan/Cellulose Acetate/Gelatin Hybrid Nanofiber Mats Incorporating Eugenol, Curr. Appl. Sci. Technol., 2019, 19(3), 235–247 Search PubMed.
- W. R. Gombotz and S. Fong Wee, Protein Release from Alginate Matrices, Adv Drug Deliv Rev, 1998, 31(3), 267–285 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Alginate: Properties and Biomedical Applications, Prog. Polym. Sci., 2012, 106–126 CrossRef PubMed.
- C. D. Saquing, C. Tang, B. Monian, C. A. Bonino, J. L. Manasco, E. Alsberg and S. A. Khan, Alginate-Polyethylene Oxide Blend Nanofibers and the Role of the Carrier Polymer in Electrospinning, Ind. Eng. Chem. Res., 2013, 52(26), 8692–8704 CrossRef CAS.
- A. Kyzioł, J. Michna, I. Moreno, E. Gamez and S. Irusta, Preparation and Characterization of Electrospun Alginate Nanofibers Loaded with Ciprofloxacin Hydrochloride, Eur. Polym. J., 2017, 96, 350–360 CrossRef.
- H. Nie, A. He, J. Zheng, S. Xu, J. Li and C. C. Han, Effects of Chain Conformation and Entanglement on the Electrospinning of Pure Alginate, Biomacromolecules, 2008, 9(5), 1362–1365 CrossRef CAS PubMed.
- S. Amjadi, H. Almasi, M. Ghorbani and S. Ramazani, Preparation and Characterization of TiO2NPs and Betanin Loaded Zein/Sodium Alginate Nanofibers, Food Packag. Shelf Life, 2020, 24, 100504 CrossRef.
- N. Aquinas, C. H. Chithra and M. R. Bhat, Progress in Bioproduction, Characterization and Applications of Pullulan: A Review, Springer, Berlin Heidelberg, 2024, vol. 81 Search PubMed.
- S. Farris, I. U. Unalan, L. Introzzi, J. M. Fuentes-Alventosa and C. A. Cozzolino, Pullulan-Based Films and Coatings for Food Packaging: Present Applications, Emerging Opportunities, and Future Challenges, J. Appl. Polym. Sci., 2014, 131(13), 40539 CrossRef.
- X. Sun, D. Jia, W. Kang, B. Cheng and Y. Li, Research on Electrospinning Process of Pullulan Nanofibers, Appl. Mech. Mater., 2013, 268, 198–201 Search PubMed.
- T. Min, X. Sun, L. Zhou, H. Du, Z. Zhu and Y. Wen, Electrospun Pullulan/PVA Nanofibers Integrated with Thymol-Loaded Porphyrin Metal−organic Framework for Antibacterial Food Packaging, Carbohydr. Polym., 2021, 270, 118391 CrossRef CAS PubMed.
- Z. Qin, X. Jia, Q. Liu, B. Kong and H. Wang, Enhancing Physical Properties of Chitosan/Pullulan Electrospinning Nanofibers via Green Crosslinking Strategies, Carbohydr. Polym., 2020, 247, 116734 CrossRef CAS PubMed.
- M. Duan, S. Yu, J. Sun, H. Jiang, J. Zhao, C. Tong, Y. Hu, J. Pang and C. Wu, Development and Characterization of Electrospun Nanofibers Based on Pullulan/Chitin Nanofibers Containing Curcumin and Anthocyanins for Active-Intelligent Food Packaging, Int. J. Biol. Macromol., 2021, 187, 332–340 CrossRef CAS PubMed.
- M. Duan, J. Sun, S. Yu, Z. Zhi, J. Pang and C. Wu, Insights into Electrospun Pullulan-Carboxymethyl Chitosan/PEO Core-Shell Nanofibers Loaded with Nanogels for Food Antibacterial Packaging, Int. J. Biol. Macromol., 2023, 233, 123433 CrossRef CAS PubMed.
- P. Shao, B. Niu, H. Chen and P. Sun, Fabrication and Characterization of Tea Polyphenols Loaded Pullulan-CMC Electrospun Nanofiber for Fruit Preservation, Int. J. Biol. Macromol., 2018, 107, 1908–1914 CrossRef CAS PubMed.
- A. Blanco-Padilla, A. López-Rubio, G. Loarca-Piña, L. G. Gómez-Mascaraque and S. Mendoza, Characterization, Release and Antioxidant Activity of Curcumin-Loaded Amaranth-Pullulan Electrospun Fibers, Lwt, 2015, 63(2), 1137–1144 CrossRef CAS.
- E. Díaz-Montes, Dextran: Sources, Structures, and Properties, Polysaccharides, 2021, 2(3), 554–565 CrossRef.
- S. Luo, A. Saadi, K. Fu, M. Taxipalati and L. Deng, Fabrication and Characterization of Dextran/Zein Hybrid Electrospun Fibers with Tailored Properties for Controlled Release of Curcumin, J. Sci. Food Agric., 2021, 101(15), 6355–6367 CrossRef CAS PubMed.
- S. Davidović, M. Miljković, M. Gordic, G. Cabrera-Barjas, A. Nesic and S. Dimitrijević-Branković, Dextran-Based Edible Coatings to Prolong the Shelf Life of Blueberries, Polymers, 2021, 13(23), 4252 CrossRef PubMed.
- P. Wang, Y. Li, C. Zhang, F. Que, J. Weiss and H. Zhang, Characterization and Antioxidant Activity of Trilayer Gelatin/Dextran-Propyl Gallate/Gelatin Films: Electrospinning versus Solvent Casting, Lwt, 2020, 128, 109536 CrossRef CAS.
- A. Z. Naser, I. Deiab and B. M. Darras, Poly(Lactic Acid) (PLA) and Polyhydroxyalkanoates (PHAs), Green Alternatives to Petroleum-Based Plastics: A Review, RSC Adv., 2021, 11(28), 17151–17196 RSC.
- C. Sanhueza, F. Acevedo, S. Rocha, P. Villegas, M. Seeger and R. Navia, Polyhydroxyalkanoates as Biomaterial for Electrospun Scaffolds, Int. J. Biol. Macromol., 2019, 124, 102–110 CrossRef CAS PubMed.
- K. J. Figueroa-Lopez, L. Cabedo, J. M. Lagaron and S. Torres-Giner, Development of Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Monolayers Containing Eugenol and Their Application in Multilayer Antimicrobial Food Packaging, Front. Nutr., 2020, 7, 1–16 CrossRef PubMed.
- K. J. Figueroa-Lopez, A. A. Vicente, M. A. M. Reis, S. Torres-Giner and J. M. Lagaron, Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers, Nanomaterials, 2019, 9(2), 1–22 CrossRef PubMed.
- J. L. Castro Mayorga, M. J. Fabra Rovira, L. Cabedo Mas, G. Sánchez Moragas and J. M. Lagarón Cabello, Antimicrobial Nanocomposites and Electrospun Coatings Based on Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Copper Oxide Nanoparticles for Active Packaging and Coating Applications, J. Appl. Polym. Sci., 2018, 135(2), 1–11 CrossRef.
- K. J. Figueroa-Lopez, C. Prieto, M. Pardo-Figuerez, L. Cabedo and J. M. Lagaron, Development and Characterization of Electrospun Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Biopapers Containing Cerium Oxide Nanoparticles for Active Food Packaging Applications, Nanomaterials, 2023, 13(5), 823 CrossRef CAS PubMed.
- T. A. Swetha, A. Bora, K. Mohanrasu, P. Balaji, R. Raja, K. Ponnuchamy, G. Muthusamy and A. Arun, A Comprehensive Review on Polylactic Acid (PLA) – Synthesis, Processing and Application in Food Packaging, Int. J. Biol. Macromol., 2023, 234, 123715 CrossRef CAS PubMed.
- J. Wu, T. Hu, H. Wang, M. Zong, H. Wu and P. Wen, Electrospinning of PLA Nanofibers: Recent Advances and Its Potential Application for Food Packaging, J. Agric. Food Chem., 2022, 70(27), 8207–8221 CrossRef CAS PubMed.
- J. Wu, T. Hu, H. Wang, M. Zong, H. Wu and P. Wen, Electrospinning of PLA Nano Fi Bers: Recent Advances and Its Potential Application for Food Packaging, J. Agric. Food Chem., 2022, 70(27), 8207–8221 CrossRef CAS PubMed.
- S. Bodbodak, N. Shahabi, M. Mohammadi, M. Ghorbani and A. Pezeshki, Development of a Novel Antimicrobial Electrospun Nanofiber Based on Polylactic Acid/Hydroxypropyl Methylcellulose Containing Pomegranate Peel Extract for Active Food Packaging, Food Bioprocess Technol., 2021, 14(12), 2260–2272 CrossRef CAS.
- X. Liu, X. Song, D. Gou, H. Li, L. Jiang, M. Yuan and M. Yuan, A Polylactide Based Multifunctional Hydrophobic Film for Tracking Evaluation and Maintaining Beef Freshness by an Electrospinning Technique, Food Chem., 2023, 428, 136784 CrossRef CAS PubMed.
- M. Liao, Y. Pan, X. Fu, S. Wu, S. Gan, Z. Wu, H. Zhao, W. Zheng, Y. Cao, W. Zhou and X. Dong, Electrospun Polylactic Acid Nanofiber Film Modified by Silver Oxide Deposited on Hemp Fibers for Antibacterial Fruit Packaging, Int. J. Biol. Macromol., 2023, 253, 126569 CrossRef CAS PubMed.
- T. Min, X. Sun, Z. Yuan, L. Zhou, X. Jiao, J. Zha, Z. Zhu and Y. Wen, Novel Antimicrobial Packaging Film Based on Porous Poly(Lactic Acid) Nanofiber and Polymeric Coating for Humidity-Controlled Release of Thyme Essential Oil, LWT, 2021, 135, 110034 CrossRef CAS.
- A. N. Li, S. Li, Y. J. Zhang, X. R. Xu, Y. M. Chen and H. B. Li. Resources and Biological Activities of Natural Polyphenols, Nutrients, MDPI AG, 2014, pp. 6020–6047 Search PubMed.
- A. K. Singh, J. Y. Kim and Y. S. Lee, Phenolic Compounds in Active Packaging and Edible Films/Coatings: Natural Bioactive Molecules and Novel Packaging Ingredients, Molecules, 2022, 27(21), 7513 CrossRef CAS PubMed.
- U. Tylewicz, M. Nowacka, B. Martín-García, A. Wiktor and A. M. Gómez Caravaca, Target Sources of Polyphenols in Different Food Products and Their Processing By-Products, Elsevier, 2018 Search PubMed.
- C. Shen, J. Zhong, F. Guo, C. Zhang, C. Zhu, D. Wu and K. Chen, Curcumin-Loaded Polycaprolactone/Carboxymethyl Chitosan Nanofibers with Photodynamic Inactivation of Staphylococcus Aureus Developed by Solution Blow Spinning, LWT, 2023, 186, 115261 CrossRef CAS.
- S. Li, N. Wei, J. Wei, C. Fang, T. Feng, F. Liu, X. Liu and B. Wu, Curcumin and Silver Nanoparticles Loaded Antibacterial Multifunctional Pectin/Gelatin Films for Food Packaging Applications, Int. J. Biol. Macromol., 2024, 266, 131248 CrossRef CAS PubMed.
- P. Rashidi Karambasti and N. Shavisi, Development of Guar Gum-Pectin Nanofiber Mats Containing Papaver Rhoeas Petal Anthocyanins and Cellulose Nanocrystals for Real-Time Visual Detection of Lamb Meat Freshness, LWT, 2024, 194, 115786 CrossRef CAS.
- T. Cetinkaya, Evaluation of Intelligent Packaging Functions of Black Carrot Extract-Infused Polyvinyl Alcohol Nanofibers, Appl. Surf. Sci. Adv., 2024, 19, 100571 CrossRef.
- Y. Khaledian, H. Moshtaghi and Y. Shahbazi, Development and Characterization of Smart Double-Layer Nanofiber Mats Based on Potato Starch-Turnip Peel Anthocyanins and Guar Gum-Cinnamaldehyde, Food Chem., 2024, 434, 137462 CrossRef CAS PubMed.
- D. Zhang, L. Chen, J. Cai, Q. Dong, Z. Din, Z.-Z. Hu, G.-Z. Wang, W.-P. Ding, J.-R. He and S.-Y. Cheng, Starch/Tea Polyphenols Nanofibrous Films for Food Packaging Application: From Facile Construction to Enhance Mechanical, Antioxidant and Hydrophobic Properties, Food Chem., 2021, 360, 129922 CrossRef CAS PubMed.
- L. Chen, F. Wu, M. Xiang, W. Zhang, Q. Wu, Y. Lu, J. Fu, M. Chen, S. Li, Y. Chen and X. Du, Encapsulation of Tea Polyphenols into High Amylose Corn Starch Composite Nanofibrous Film for Active Antimicrobial Packaging, Int. J. Biol. Macromol., 2023, 245, 125245 CrossRef CAS PubMed.
- P. Shao, B. Niu, H. Chen and P. Sun, Fabrication and Characterization of Tea Polyphenols Loaded Pullulan-CMC Electrospun Nanofiber for Fruit Preservation, Int. J. Biol. Macromol., 2018, 107, 1908–1914 CrossRef CAS PubMed.
- Y. Yang, Y. Shi, X. Cao, Q. Liu, H. Wang and B. Kong, Preparation and Functional Properties of Poly(Vinyl Alcohol)/Ethyl Cellulose/Tea Polyphenol Electrospun Nanofibrous Films for Active Packaging Material, Food Control, 2021, 130, 108331 CrossRef CAS.
- A. Aydogdu Emir, E. Yildiz, Y. Aydogdu, G. Sumnu and S. Sahin, Gallic Acid Encapsulated Pea Flour-Based Nanofibers Produced by Electrospinning as a Potential Active Food Packaging Material, Legum. Sci., 2021, 3(2), 1–10 Search PubMed.
- S. Roy, R. Priyadarshi, P. Ezati and J. W. Rhim, Curcumin and Its Uses in Active and Smart Food Packaging Applications - A Comprehensive Review, Food Chem., 2022, 375(2021), 131885 CrossRef CAS PubMed.
- S. Yadav, G. K. Mehrotra, P. Bhartiya, A. Singh and P. K. Dutta, Preparation, Physicochemical and Biological Evaluation of Quercetin Based Chitosan-Gelatin Film for Food Packaging, Carbohydr. Polym., 2020, 227, 115348 CrossRef CAS PubMed.
- S. Sabet, A. Rashidinejad, L. D. Melton and D. J. McGillivray, Recent Advances to Improve Curcumin Oral Bioavailability, Trends Food Sci. Technol., 2021, 110, 253–266 CrossRef CAS.
- Y. Jin, S. Yang, F. Li, Y. Zhang, T. Zhao, S. Cheng and X. Mei, Fabrication of Hordein/Chitosan Nanoparticles for Encapsulation of Curcumin, J. Food Eng., 2024, 371(17), 111994 CrossRef CAS.
- Y. J. Kim, H. I. Yong, Y. G. Chun, B. K. Kim and M. H. Lee, Physicochemical Characterization and Environmental Stability of a Curcumin-Loaded Pickering Nanoemulsion Using a Pea Protein Isolate-Dextran Conjugate via the Maillard Reaction, Food Chem., 2024, 436, 137639 CrossRef CAS PubMed.
- B. S. Munteanu and C. Vasile, Encapsulation of Natural Bioactive Compounds by Electrospinning—Applications in Food Storage and Safety, Polymers, 2021, 13(21), 1–28 CrossRef PubMed.
- Y. Liu, C. Yuan, B. Cui, M. Zhao, B. Yu, L. Guo, P. Liu and Y. Fang, Encapsulation of Apigenin into β -Cyclodextrin Metal-Organic Frameworks with High Embedment Efficiency and Stability, Food Chem., 2024, 443, 138543 CrossRef CAS PubMed.
- X. Lan, Y. Liu, L. Wang, H. Wang, Z. Hu, H. Dong, Z. Yu and Y. Yuan, A Review of Curcumin in Food Preservation: Delivery System and Photosensitization, Food Chem., 2023, 424, 136464 CrossRef CAS PubMed.
- M. Seidi Damyeh, R. Mereddy, M. E. Netzel and Y. Sultanbawa, An Insight into Curcumin-Based Photosensitization as a Promising and Green Food Preservation Technology, Compr. Rev. Food Sci. Food Saf., 2020, 19(4), 1727–1759 CrossRef CAS PubMed.
- A. Etxabide, P. A. Kilmartin and J. I. Maté, Color Stability and PH-Indicator Ability of Curcumin, Anthocyanin and Betanin Containing Colorants under Different Storage Conditions for Intelligent Packaging Development, Food Control, 2021, 121, 107645 CrossRef CAS.
- S. Hazer and A. Aytac, Monitoring Food Quality - Effect of Curcumin in the Development of Polyethylene/Thermoplastic Starch Based Smart Packaging, J. Vinyl Addit. Technol., 2023, 29(5), 826–839 CrossRef CAS.
- Z. Du, H. Lv, C. Wang, D. He, E. Xu, Z. Jin, C. Yuan, L. Guo, Z. Wu, P. Liu and B. Cui, Organic Solvent-Free Starch-Based Green Electrospun Nanofiber Mats for Curcumin Encapsulation and Delivery, Int. J. Biol. Macromol., 2023, 232, 123497 CrossRef CAS PubMed.
- Y. Zhou, R. Liu, C. Zhou, Z. Gao, Y. Gu, S. Chen, Q. Yang and B. Yan, Dynamically Crosslinked Chitosan/Cellulose Nanofiber-Based Films Integrated with γ-Cyclodextrin/Curcumin Inclusion Complex as Multifunctional Packaging Materials for Perishable Fruit, Food Hydrocoll., 2023, 144, 108996 CrossRef CAS.
- D. Kossyvaki, A. Barbetta, M. Contardi, M. Bustreo, K. Dziza, S. Lauciello, A. Athanassiou and D. Fragouli, Highly Porous Curcumin-Loaded Polymer Mats for Rapid Detection of Volatile Amines, ACS Appl. Polym. Mater., 2022, 4(6), 4464–4475 CrossRef CAS.
- H. E. Khoo, A. Azlan, S. T. Tang and S. M. Lim, Anthocyanidins and Anthocyanins: Colored Pigments as Food, Pharmaceutical Ingredients, and the Potential Health Benefits, Food Nutr. Res., 2017, 61(1), 1361779 CrossRef PubMed.
- M. Hu, J. Du, L. Du, Q. Luo and J. Xiong, Anti-Fatigue Activity of Purified Anthocyanins Prepared from Purple Passion Fruit (P. Edulis Sim) Epicarp in Mice, J. Funct. Foods, 2020, 65, 104365 Search PubMed.
- S. Roy and J. W. Rhim, Anthocyanin Food Colorant and Its Application in PH-Responsive Color Change Indicator Films, Crit. Rev. Food Sci. Nutr., 2021, 2297–2325 CrossRef CAS PubMed.
- H. Lv, C. Wang, D. He, H. Zhao, M. Zhao, E. Xu, Z. Jin, C. Yuan, L. Guo, Z. Wu, P. Liu and B. Cui, Intelligent Food Tag: A Starch-Anthocyanin-Based PH-Sensitive Electrospun Nanofiber Mat for Real-Time Food Freshness Monitoring, Int. J. Biol. Macromol., 2024, 256, 128384 CrossRef CAS PubMed.
- L. Jiang, C. Wang, F. Zhao, S. Li, D. Sun, Q. Ma, Z. Yu, B. Zhang, Y. Liu and W. Jiang, Development of Electrospun Nanofiber Films Based on Pullulan/Polyvinyl Alcohol Incorporating Bayberry Pomace Anthocyanin Extract for Aquatic Products Freshness Monitoring, Food Biosci., 2024, 58, 103717 CrossRef CAS.
- J. Dai, D. E. Sameen, Y. Zeng, S. Li, W. Qin and Y. Liu, An Overview of Tea Polyphenols as Bioactive Agents for Food Packaging Applications, Lwt, 2022, 167, 113845 CrossRef CAS.
- W. Zhang, H. Jiang, J. W. Rhim, J. Cao and W. Jiang, Tea Polyphenols (TP): A Promising Natural Additive for the Manufacture of Multifunctional Active Food Packaging Films, Crit. Rev. Food Sci. Nutr., 2022, 288–301 Search PubMed.
- L. H. Zhang and Q. Shen, Fully Green Poly(Vinyl Alcohol)/Tea Polyphenol Composites and Super Anti-Ultraviolet and -Bacterial Properties, Macromol. Mater. Eng., 2020, 305(3), 1900669 CrossRef CAS.
- C. Musial, A. Kuban-Jankowska and M. Gorska-Ponikowska, Beneficial Properties of Green Tea Catechins, Int. J. Mol. Sci., 2020, 21(5), 1744 CrossRef CAS PubMed.
- Z. Miao, Y. Zhang and P. Lu, Novel Active Starch Films Incorporating Tea Polyphenols-Loaded Porous Starch as Food Packaging Materials, Int. J. Biol. Macromol., 2021, 192, 1123–1133 CrossRef CAS PubMed.
- Y. Liu, X. Liang, S. Wang, W. Qin and Q. Zhang, Electrospun Antimicrobial Polylactic Acid/Tea Polyphenol Nanofibers for Food-Packaging Applications, Polymers, 2018, 10(5), 561 CrossRef PubMed.
- X. Lan, T. Luo, Z. Zhong, D. Huang, C. Liang, Y. Liu, H. Wang and Y. Tang, Green Cross-Linking of Gelatin/Tea Polyphenol/ε-Poly (L-Lysine) Electrospun Nanofibrous Membrane for Edible and Bioactive Food Packaging, Food Packag. Shelf Life, 2022, 34, 100970 CrossRef CAS.
- N. Kahkeshani, F. Farzaei, M. Fotouhi, S. S. Alavi, R. Bahramsoltani, R. Naseri, S. Momtaz, Z. Abbasabadi, R. Rahimi, M. H. Farzaei and A. Bishayee, Pharmacological Effects of Gallic Acid in Health and Disease: A Mechanistic Review, Iran. J. Basic Med. Sci., 2019, 22(3), 225–237 Search PubMed.
- S. Choubey, L. R. ache. Varughese, V. Kumar and V. Beniwal, Medicinal Importance of Gallic Acid and Its Ester Derivatives: A Patent Review, Pharm. Pat. Anal., 2015, 305–315 CrossRef CAS PubMed.
- A. Aydogdu, G. Sumnu and S. Sahin, Fabrication of Gallic Acid Loaded Hydroxypropyl Methylcellulose Nanofibers by Electrospinning Technique as Active Packaging Material, Carbohydr. Polym., 2019, 208, 241–250 CrossRef CAS PubMed.
- P. Chuysinuan, T. Thanyacharoen, S. Techasakul and S. Ummartyotin, Electrospun Characteristics of Gallic Acid-Loaded Poly Vinyl Alcohol Fibers: Release Characteristics and Antioxidant Properties, J. Sci. Adv. Mater. Devices, 2018, 3(2), 175–180 CrossRef.
- K. Babaremu, O. P. Oladijo and E. Akinlabi, Biopolymers: A Suitable Replacement for Plastics in Product Packaging, Adv. Ind. Eng. Polym. Res., 2023, 6(4), 333–340 CAS.
- A. K. Trivedi, M. K. Gupta and H. Singh, PLA Based Biocomposites for Sustainable Products: A Review, Adv. Ind. Eng. Polym. Res., 2023, 6(4), 382–395 CAS.
- J. Avossa, G. Herwig, C. Toncelli, F. Itel and R. M. Rossi, Electrospinning Based on Benign Solvents: Current Definitions, Implications and Strategies, Green Chem., 2022, 24(6), 2347–2375 RSC.
| This journal is © The Royal Society of Chemistry 2025 |