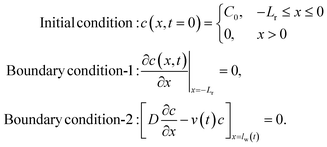Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Experiment and modeling of concentration-dependent diffusion in a solution with dual transport-phase flow for paper-based sensing†
Md. Saykat Hassan Sajib ,
Md. Sakif Rafid
,
Md. Sakif Rafid and
M. Ryyan Khan
and
M. Ryyan Khan *
*
Department of Electrical and Electronic Engineering, East West University, Dhaka, Bangladesh. E-mail: ryyan@ewubd.edu
First published on 4th June 2025
Abstract
Paper-based sensors are promising as low-cost, passive devices for point-of-care clinical diagnostics, food quality assessment, and environmental monitoring. The diffusion process in paper-based sensors, and component detection through multistage transport or multiphase solution flow (e.g., in chromatography) have been studied in literature. However, observation or analysis of concentration dependent analyte flow in such systems has not been considered. In this work, we performed a set of experiments on concentration dependent liquid-flow in paper strips, developed a video processing technique for automatic fluid-flow-distance detection, and explained the observation through a mathematical model. We use KMnO4 solutions – such solutes which have weak bonds with water (solvent) can have decoupled transport-phases: (i) water flow, followed by (ii) analyte diffusion. We have presented a video processing algorithm to automatically extract the time-series analyte and water flow distance measurements by analyzing the pixel values frame-by-frame of the recorded experiment videos. This ensures consistency in distance measurements among the experiments. We finally explain the physical process of the flow using a corresponding mathematical model to describe the concentration effects in paper-like materials. The model includes how analyte undergoes drift force (due to water flow velocity) along with diffusion. We found that the numerical solution of the model agrees with the trends seen in the experimental results. This can help us better understand the liquid-wicking behavior in different concentrations through a mathematical model and provide guidance in the design and optimization of paper-based sensors.
1. Introduction
Over the last few decades, paper-based sensor technologies have shown great promise due to its lower cost and simpler implementation. There have been significant progress in relevant fabrication, fluid control techniques for sample processing and analysis. Paper-based devices are widely used for medical diagnosis such as simple blood sugar level and protein testing, urine testing to measure pregnancy hormone or glucose contents,1 and DNA detection.2 Paper-based devices can also be used to monitor food quality and preservation,3 and for environmental monitoring of chemicals and pollution in air4 and water.5 The fundamental physical process of these applications is mass transfer within the porous structure of paper-like materials, where liquid-wicking behavior by capillary action plays a significant role. It is crucial to clearly understand the fluid flow behavior and fluid control mechanisms to establish a framework for designing paper-based diagnostics or sensing devices.61.1. Fluid control (device design)
Various fluid control methods have been proposed to improve the sensitivity of paper-based devices, such as changing geometrical structures, making hydrophobic patterns, adding moderator reagents, and so on. In geometric control strategies, different wicking behavior is achieved by changing the dimension of the paper strips. The simplest geometric control is a distance-based delay. For instance, Fu et al. investigated liquid flow in multiple connected straight paper strips with varying widths to control wicking times to achieve multi-step reactions in one paper strip.7 Apilux et al. created a serpentine channel by printing baffles on the paper to increase the wicking length that delayed flow.8 These studies showed how to improve the sensitivity by controlling fluid speed by chaining the geometrical structure of paper strips. Besides changing paper structure, the porous structure of papers can also be modified with hydrophobic material (e.g., paraffin wax) or dissolvable barriers (e.g., sugar and trehalose) to slow down fluid speed directly. Toley et al.9 and Shin et al.10 pass the fluid through a dissolvable barrier (known as a shunt) to obtain a flow delay, which is added onto paper strips to reduce the available wicking area. Lutz et al. deposited different concentrations of sugar into paper strips to create flow delays for an automated multi-step assay device.11 Noh et al. demonstrated a 3-D vertical flow assay, where various amounts of paraffin wax were deposited into paper strips to create delay. They found that paraffin wax could generate a delay of several minutes.12 Additionally, Camplisson et al.; Channon et al. accelerated flow by creating two-ply channels.13,14 They created a hollow region in the center of the channel by sandwiching two paper strips between hollow layers of double-sided tape, which has much lower fluid resistance compared to the paper around it. Renault et al.; Shin et al. created ‘macro-capillaries’ for the liquid to flow through by removing regions of the paper to create faster wicking channels.15,16 Other groups Giokas et al. and Liu et al. etched grooves into paper using a craft plotter, and CO2 laser cutter respectively to speed up flow.17,18 The different fluid control strategies on paper-devices discussed above are mostly developed experimentally through trial and error. The simplistic theoretical imbibition models are however unsuitable for complex device design and application conditions.1.2. Concentration detection
Several works have experimentally shown concentration dependent variation in solution flow in paper based sensors. For example, Mirzaei et al. proposed a distance-based method for the detection of blood lactate concentration using a paper strip coated with gold nanoparticles (AuNPs).19 As the lactate flows through the paper strip, it interacts with the nanoparticles and changes color from red to purple. The lactate concentration was detected by distance analysis. The researchers found a linear relationship between the color length and lactate concentration in the range of 1.0 to 30.0 mM. A similar method was used by Phoonsawat et al. to determine chloride ions (Cl−) in water by functionalization of silver nanoparticles (AgNPs) on the paper channel.20 Soda et al. introduced an ion-selective capillary sensor, which can determine the potassium ion (K+) concentration.21 They demonstrated that ion-selective membrane films cast on the inner wall of the capillary channel can respond sensitively to a narrow range of K+ concentrations (1.0–6.0 mM), and can be used in a sensing mode. Moreover, Patari et al. detected the presence of added water in milk by measuring changes in capillary height resulting from varying water concentrations. They added 25%, 50%, and 75% water to pure milk and measured changes in capillary height. As the water content increased, the capillary height increased as well.22 Xu et al. modified paper cellulose by depositing water-insoluble oxidants onto the paper channel to create a distance-based analytical device for detecting reducing substances.23 Using different concentrations of ascorbic acid and captopril solutions, they established a calibration curve and observed a linear relationship between the concentration and the length of the discolored band. These works, however, do not provide theoretical or mathematical explanation of the concentration dependent fluid flow.1.3. Fluid flow physics
Besides experimental studies, the mathematical model also plays an important role in predicting liquid-wicking behavior for better fluid control in the paper. Lucas–Washburn (L–W) model has been widely used in the study of one-dimensional liquid-wicking behavior in porous materials.24 They defined a relationship between liquid wicking distances (h) with time (t) as , which showed good agreement with experimental results. However, this model ignored some key factors such as characteristics of materials, evaporation, gravitational force, channel boundary effects, and the environmental temperature and humidity which are significant in real applications. Consequently, this model cannot predict the experimental results of the liquid-wicking phenomenon in porous materials over long time intervals. Therefore, various modified models were developed considering different factors to give better descriptions. Ponomarenko et al. developed a general relationship between liquid front and time considering the gravitational force because it is an important factor for long-term liquid-wicking behavior.25 The relationship showed that liquid imbibition height has a power-law relationship with time as
, which showed good agreement with experimental results. However, this model ignored some key factors such as characteristics of materials, evaporation, gravitational force, channel boundary effects, and the environmental temperature and humidity which are significant in real applications. Consequently, this model cannot predict the experimental results of the liquid-wicking phenomenon in porous materials over long time intervals. Therefore, various modified models were developed considering different factors to give better descriptions. Ponomarenko et al. developed a general relationship between liquid front and time considering the gravitational force because it is an important factor for long-term liquid-wicking behavior.25 The relationship showed that liquid imbibition height has a power-law relationship with time as 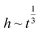 . Li et al. proposed the criteria for using the L–W model in the case of spontaneous imbibition of a liquid in porous media.26 They also described the criteria for including the effect of gravity on wicking using the CGR number (capillary pressure-to-gravity force ratio). Besides, Fries et al. modified the L–W model to account for the evaporation effect in the wicking of different liquids in porous media.27 They showed that capillary rise is inversely proportional to the evaporation rate. Liu et al. also developed an evaporation model in the case of paper matrix to predict liquid wicking height and mass by varying the width of paper strips.28 Hong and Kim proposed an empirical model to investigate the wicking behavior in hydrophobic channels with various boundaries.29 They illustrated a relationship between hydrophobic channels and the dynamic wicking behavior in channels. This relationship showed a novel strategy to decrease the liquid velocity by adjusting the contact angle with the wax boundaries. These models are focused towards studying wicking behavior in porous materials with 2D or 3D complex geometric structures and the effects of micro structural parameters of fibrous materials such as porosity, fiber diameter, etc.
. Li et al. proposed the criteria for using the L–W model in the case of spontaneous imbibition of a liquid in porous media.26 They also described the criteria for including the effect of gravity on wicking using the CGR number (capillary pressure-to-gravity force ratio). Besides, Fries et al. modified the L–W model to account for the evaporation effect in the wicking of different liquids in porous media.27 They showed that capillary rise is inversely proportional to the evaporation rate. Liu et al. also developed an evaporation model in the case of paper matrix to predict liquid wicking height and mass by varying the width of paper strips.28 Hong and Kim proposed an empirical model to investigate the wicking behavior in hydrophobic channels with various boundaries.29 They illustrated a relationship between hydrophobic channels and the dynamic wicking behavior in channels. This relationship showed a novel strategy to decrease the liquid velocity by adjusting the contact angle with the wax boundaries. These models are focused towards studying wicking behavior in porous materials with 2D or 3D complex geometric structures and the effects of micro structural parameters of fibrous materials such as porosity, fiber diameter, etc.
Considering properties of the media such as porosity and permeability, Darcy first described fluid flow in porous materials.30 He found that the flow rate in porous media is proportional to the pressure drop through the porous medium. This relationship is known as Darcy's law. The electrical circuit analogy (ECA) model has also attracted significant interest because it is a simple approach for modeling the fluidic network by electric circuits. Fu et al. calculated the transport time for flow through a paper strip with different widths using Darcy's law by analogy to Ohm's law.7 Furthermore, the ECA approach has been used to model different paper networks that have utilized various materials such as a sponge shunt integrated on lateral flow assay (LFAs) with varying contact angles and coated an absorbent shunt on a strip.9,31 However, these models did not consider the effect of concentration on the wicking process in the paper matrix. Piquemal et al. modeled species movement in porous media using a dispersion–convection equation for the mobile fluid and a diffusion equation for the stagnant fluid (two-stage transport). Their study focused on a cylindrical tube with stagnant regions along its walls and also explored the behavior in a layered permeable medium.32
Such two-transport-phase flow in porous medium is regularly utilized in chromatography. The concept of chromatography has been appended with multiple techniques for applications in different applications. For example, Masanobu Motooka et al. improved gas sensing using chromatography paper for breath analysis, with potential diagnostic applications such as diabetes screening.33 Patrick Ryan et al. developed a graphite electrode-based electrochemical probe integrated to chromatography paper using ethylene glycol-choline chloride electrolyte for sensitive TNT detection in soil.34 Xiao-Lei Huo et al. integrated electrochemiluminescence (ECL) with paper chromatography for simultaneous separation and detection of (bisphenol A, BPA, and dibutyl phthalate, DBP) for multi-target environmental analysis.35 The underlying mechanism of multi-stage transport and multi-phase fluid dynamics and its applications have been discussed in the literature.36 Fei He et al. numerically investigated the transient two-phase fluid flow with liquid phase transitions through micro- and nanoscale porous medium.37
There are prior works on paper-based or chromatography-like systems with well established models38 explaining the physics of the underlying diffusion process. However, there is no analysis of concentration dependency and its potential detection in the multi-stage-transport or multi-phase flow systems on nonfunctionalized paper channels.
1.4. This work
In this paper, (i) we develop an automatic fluid-flow-distance detection method by video processing, (ii) through experiments and a mathematical model, we explain how the wicking distance of a two-transport-phase flow shows concentration dependency, and (iii) study how the behavior may change for different source conditions and device orientations. To elaborate: we present a detailed time-series experiment of solution flow through paper strips for various concentrations. To measure the experimental observations of fluid flow, we implement a video processing algorithm for robust and consistent extraction of time-series analyte flow distance through the paper channel. We choose KMnO4 solutions for the experiment—this shows a 2-stage transport where water flows faster than the purple MnO4− ions. The detailed experimental measurements (time and distance) for concentration dependent solution flow yields in estimation of solvent (water) velocity and analyte flow distance. We finally present a convection–diffusion equation-based, physically relevant mathematical model representing this system. Our mathematical model and numerical analysis agree with the trends observed in the experiments. Such mathematical models to represent the concentration effect are vital for the design and performance analysis of paper-based sensor or diagnostic devices.2. Concept/problem statement
Most paper-based sensors are explained using some form of L–W model. According to L–W model,24 we do not expect variations in fluid or solution flow distance through paper-channels for different concentrations. This is consistent with several experiments in literature. For some solutions, however, a concentration dependent solution flow distance have been observed.22,23 It is unclear how or why such dependencies occur. In case of solute with weak bonds with the solvent, there can be a two-stage transport. For such cases, the solvent (e.g., water) flows through the paper channel first, and then the solute (e.g., KMnO4) shows a secondary diffusion (two transport-phases). Although two transport-stage flow models have been discussed in the literature,32,36 concentration dependency has not been explored. We hypothesize that a velocity assisted diffusion can have concentration dependencies. We will test this hypothesis, and explain it using a general mathematical model describing this process.3. Experimental setup
3.1. Paper sensor structure
In most of the research studies, Whatman filter paper is used because it is cheap, thin, easily available, lightweight, easy to store and transport, disposable, and biodegradable.39 We used Whatman no. 1 filter papers because of its homogeneous and hydrophilic nature. A common problem in paper-based device is the reduction in wicking speed due to evaporation. Therefore, we sandwiched (laminate) the paper between polymer films to prevent liquid leakage and evaporation during flow. A thin strip of laminated paper works as the fluid flow channel for the paper based sensor.3.2. Device preparation
The polymer film was attached to the filter paper on both sides using a laminator (JL330T, PFEC) at 130 °C with a feeding speed of 1 cm s−1. The laminated filter paper was cut to make the channels of the devices using a laser cutting machine (R500 Laser cutter, Rayjet) according to the design of the paper-sensor (device). The paper strip has two parts (see Fig. 1(c)): a reservoir (10 mm × 10 mm) where the liquid was deposited and a straight channel to flow the liquid with a width of 3 mm and a length of channel 80 mm. A double-sided tape was used to attach the prepared device to a flat surface. Additionally, a scale bar was placed alongside the paper strips to calibrate and measure the length of the liquid front during video and image processing. We will use different concentrations of aqueous solutions of KMnO4 as the working fluids in various tests. This purple solution will facilitate the development of distance-based detection on paper-based microfluidics device aiming to quantify analyte concentration based on color length.3.3. Experimental process
The experiments were conducted in different ways depending on the positioning of the paper and solution source (i.e., solution reservoir) to examine the effects of concentration on liquid-wicking behavior in paper-like materials: (i) infinite source: vertical strip; (ii) infinite source: horizontal strip; (iii) finite source: horizontal strip.Every experiment was run multiple times for each specific concentration to average out variability – see Sec. S1 in the ESI† document for relevant details. Fluid-flow into the paper channel was video-recorded for 20 minutes after introducing the solution at the paper channel. The position of the fluid flow wavefront was obtained by video and image analysis methods. See Table 1 for a summary of the experiments.
| Items | Details |
|---|---|
| Device material | Whatman no. 1 |
| Channel size | 3 mm × 80 mm |
| Reservoir size | 10 mm × 10 mm |
| Imaging system | Camera (smartphone), fixture or mount, post-processing algorithm |
| Solution | KMnO4 in water |
| Concentrations | 10, 20, 30, 40, and 50 mM |
| Experiments | Type | Source volume | Channel orientation |
|---|---|---|---|
| IVS | Infinite | Vertical | |
| IHS | Infinite | Horizontal | |
| FHS | Finite | Horizontal |
3.4. Data extraction
We developed an algorithm to extract data from the recorded experimental video. The video was imported into the computer, and data was extracted from each frame to determine the distance flowed by the analyte front. Use of an algorithm eliminates human error and ensures consistency among the information extracted from all the experiments. The calculation steps followed in the algorithm is summarized in Fig. 2.Step-1: each of the experiments were video-recorded. The camera was fixtured vertically away from the paper-device plane. As a reference for physical length, a scale bar was placed alongside the paper-devices within the video capture frame. The video is then loaded into a computer program for frame-by-frame analysis. The pixel dimension of the video was converted to millimeters by calibrating to the scale in the video frame. This would need to be done in the first frame of each video. The calibrated value can vary for different experiments (i.e., different videos). For our various sets of experiments and repetitions, we had found values ranging between 6 and 13 pixelsper mm. All our video captures were at 30 fps (which can also be extracted from the metadata of a video file).
Step-2: the analysis region of interest is the paper channel where the analyte will flow. The video frame is therefore appropriately rotated and cropped to this region (starting beyond the reservoir). This is only needed to be done in the first frame – the selected region will automatically remain the same, given that the camera fixture was sturdy. The part of the channel where the analyte (i.e., KMnO4) has diffused is dark, and represented in the video frame as a low pixel value.
Step-3: the channel image is extracted for each frame of the video (see sample images shown in step-3 in Fig. 2). Each of the frames are converted to gray-scale for further processing. The rightmost part of the channel is never wicked and always has the highest pixel value (see step-2 in the figure). Each of the video frames are normalized to this high pixel value to correct for possible temporal fluctuations in ambient lighting.
Step-4: at each frame, the pixel values are averaged along the width (i.e., along y-axis, also see Fig. 1) to get a 1D intensity matrix along the channel length. For example, such plots for 5-different time frames are shown in step-4 of Fig. 2. The pixel values are low (i.e., dark wetted part) along the channel, followed by a clear transition to brighter pixels (i.e., unwetted part). The transition obviously marks the flow front of the analyte. For consistency among all experiments, we set a threshold pixel value of 0.75 (marked by dashed line in the figure) to mark the analyte flow distance at a given time frame. The water flow front was detected by taking a spatial derivative of the pixel value vs. distance curve and following the corresponding transition peak – see Sec. S2 in the ESI† document for full details.
Step-5: the flow distance estimated for each time-frame found in the previous step is collated to form the flow-distance versus time relationship.
The sample time-series analyte and water flow distance extraction videos provided as additional ESI† demonstrate the algorithm. In Sec. S5 of the ESI† document, we have also illustrated how the 5-step process works on the video frames.
The post-processing of the captured video (i.e., steps 1–4) is such that the data extraction process works consistently regardless of the imaging system or camera (any DSLR or smartphone camera would be fine). The only requirement is that the video capture should have a resolution high enough to detect the flow front at each time step.
4. Results: data analysis
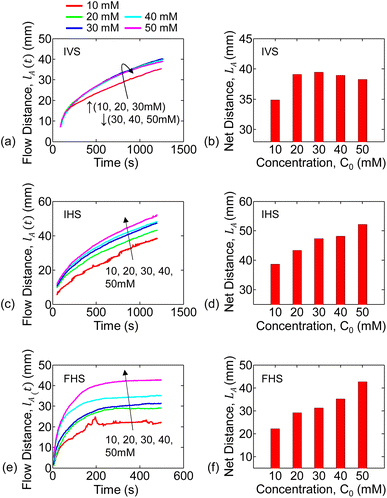
Aqueous solutions of KMnO4 (analyte) were prepared with concentrations of 10, 20, 30, 40, and 50 mM. The experiment steps were repeated for each concentration, for each of the experimental setups (discussed in the previous section). Multiple paper strips were used in each experiment setup and concentration combination. For a given setup, the capillary flow distance versus time data is averaged over the multiple runs of each concentration.For the infinite source with vertical strip (IVS) setup, the analyte (i.e., KMnO4) flow distance versus time for each concentration is shown in Fig. 3(a). The flow distance after 1200 s for various concentrations are shown in Fig. 3(b). It is observed that the analyte distance increased with concentration from 10 mM to 30 mM and then decreased for 40 mM to 50 mM. This can be due to the combined effect of gravitational force and inconsistent flow velocity of the liquid solvent (i.e., water). We only observed the inconsistent water flow velocity in the vertical strip setups. This is further discussed in Section 5.2 and in Sec. S3 of the ESI† document.
The temporal change in flow distance and the final distance (at 1200 s) for various concentrations for the infinite source with horizontal strip (IHS) is shown in Fig. 3(c and d). The corresponding results (at 500 s) for finite source with horizontal strip (FHS) is in Fig. 3(e and f). Clearly, as seen in Fig. 3(d and f), the flow distance increases with concentration. However, there is a distinctive characteristic difference in analyte imbibition with time for IHS and FHS setups as observed from Fig. 3(c and e). For IHS, the analyte flow distance continuously increases with time. On the other hand, the analyte imbibition distance saturates after some time for FHS. We hypothesize that in FHS setup, the imbibition distance is limited because the solution volume and hence the analyte is finite. In the following section, we will explain the trends of the experimental data of the analyte flow using our mathematical and numerical model.
5. Model and interpretation
5.1. Numerical model for two transport-phase flow
Mass transport by capillary action through porous media exhibits diffusive behavior. When a KMnO4 crystal is placed into the water, it dissolves into K+ and MnO4− ions. The MnO4− ion's purple color spreads throughout the water. When the KMnO4 solution contacts with the paper strip, we observe that water flows through the paper channel first, followed by a secondary flow of purple analyte (MnO4− ions). Such a solution flow with two transport-phases can happen for any weakly soluble chemical or biochemical compounds.23 The fluid velocity and molecular diffusion are the two main factors that govern the mass transport of the moving fluid through the porous media in this wicking process. Fick's second law describes how the spatial distribution of concentration changes over time due to diffusion. For the capillary-driven flow, this law can be reformulated as follows:
 | (1) |
The second term on the right hand side of eqn (1) represents the drift force on the analyte due to the water flow velocity. The overall process can be interpreted as follows: the carrier fluid flows along the channel (transport phase-1) and its velocity enhances the net diffused analyte flow distance (transport phase-2). The dual transport-phase flow is consistent with our experimental observation. The flow and velocity of the carrier fluid (i.e., water in our experiment) along the paper strip can be estimated from mathematical and numerical modeling.22 This will however vary with the experimental setups. For our analysis, the water flow velocity was determined by image processing and curve fitting methods described as follows. For a given setup (IVS, IHS, or FHS), it is expected that the water will travel the same distance for all concentrations. We estimated the water flow distance (lw) versus time following a technique similar to that discussed in Sec. 3.4. We found the trends to be of the following form:
| lw(t) = Lw∞(1 − exp(−t/τ)). | (2) |
 | (3) |
 | (4) |
| Parameter | Value |
|---|---|
| D (mm2 s−1) | 0.07 |
| Parameter | IVS | IHS | FHS |
|---|---|---|---|
| a There is no gravitational effect on the horizontal setups (IHS, FHS). IVS also modeled in this case for comparison.b With gravity, for IVS: α = 0.53, and β = 2.1 × 10−4 mm s−1 mM−1. | |||
| Lw∞ (mm) | 49.95 | 64.15 | 45.94 |
| τ (s) | 383.53 | 428.89 | 83.18 |
| No gravity:a,b (α, β) | (0.48, 0) | (0.48, 0) | (0.48, 0) |
We assume our system to be represented by a 1D channel (no analyte flow vertical to the channel) of length L. The analyte will flow along x – direction (channel) with the source at x = 0 and the channel terminating at x = L. Eqn (4) is solved to find concentration distribution along the channel at each time step (i.e., c(x, t)). This same equation will appropriately give results for IVS, IHS and FHS based on the boundary conditions. There are two conditions based on the analyte source: (i) infinite source, and (ii) finite source (mass conservation). We will discuss these in the following.
Here, C0 is the concentration of the solution being tested. These conditions are the same for the IVS and IHS methods; only the carrier fluid (water) velocities are different. Note that, the boundary condition-2 at x = lw(t) indicates that the solute cannot flow out of the water at any time. As a negligible amount of the solute reaches x = lw(t) (solute is much slower than the water flow front), the boundary condition-2 can be approximated to c(x = L, t) = 0 (with only 0.3% difference in the answers).
For the finite source model, we assume a 1D system (with −Lr ≤ x ≤ L) where the reservoir is −Lr ≤ x < 0, and the channel is 0 ≤ x ≤ L. While the water velocity is vw(t) in the channel, we assume it to be zero within the reservoir. The conditions to solve eqn (4) in this case are:
 .
.
In summary, we extracted the water flow dynamics from the experiments and then used the convection–diffusion equation to model the overall analyte flow. We do not explicitly model the wicking of water. The flow of water or an aqueous solution in paper predominantly relies on capillary action, and hence could be modeled using Darcy's law. A more detailed approach would be a 2D transient flow model through a porous medium.41–43 However, this is beyond the scope and target of this work and is left for future studies.
5.2. Results and interpretation
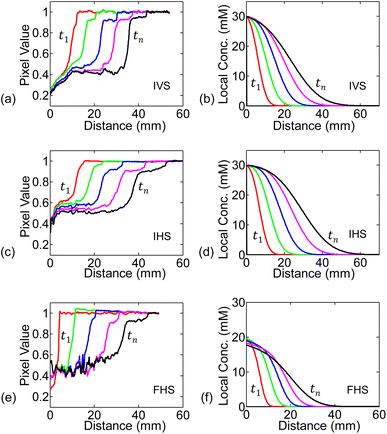
We numerically solve eqn (4) following the conditions discussed in the previous sub-section. The spatial and time steps have been set small enough to ensure stability in the solutions. The numerically found spatial distribution of concentration at different time steps (c(x, t)) for 30 mM solution are shown in Fig. 5(b, d and f), alongside the experimental measurements in Fig. 5(a, c and e).As seen in Fig. 5(b, d and f), for any of the three systems: IVS, IHS, or FHS, we observe that at time t1, concentration is high near the source reservoir (i.e., near x = 0) and then falls off as we move along the channel (i.e., at higher x). Higher concentration of KMnO4 will be darker (corresponding low pixel value in experiments) and vice versa. The transition from dark to bright region marks the flow distance of KMnO4. On the other hand, in the simulations, the transition in the local concentration marks the flow distance of KMnO4. There is no one to one mapping of the KMnO4 local concentration to the darkness (or intensity) measured in the experiments. Regardless, the numerically found concentration profile is conceptually consistent with the experimentally measured analyte distribution (pixel values). At later time tn, the analyte spreads further along the channel.
For the infinite source systems, we expect that the concentration of the analyte (hence its color) at the source (x = 0) to remain unchanged. On the other hand, the finite source gets depleted with time and the analyte concentration decreases (color becomes less dark, i.e., higher pixel value). These characteristics are, of course, expected from the simulated results due to the boundary conditions. We observe the trends in experimental measurements as well (especially in the initial time steps) – see Fig. 5.
The spatial profile of concentration observed at time t1 will further move along the channel over time (from t1 to tn). For the numerical simulations, we assume the analyte flow distance to be the position at which the concentration profile reaches the threshold Cth = 2 mM. The Cth has been chosen here to obtain a closer match in the net flow distance (LA) between simulations and experiments. The flow distance for each time step is combined to find the simulated analyte net flow-distance versus time. This is repeated for all the concentrations under study – see Fig. 6(a, c and e). Here, the results for β = 0 have been illustrated for comparative discussions. As observed in the simulation (Fig. 6(a and c)) and experimental (Fig. 3(a and c)) results, the flow-distance versus time characteristics for infinite sources (IVS, IHS) does not saturate as early as in the case of finite source (FHS). This is dominated by the time-series behaviour of water flow and velocity. Due to the finite volume, water velocity quickly decays towards zero and consequently slows down the analyte flow in case of FHS. Due to the same reason, the analyte in FHS flows slower and to a shorter distance than that in IVS or IHS. See water flow distance lw vs. time for the three configurations in the ESI document.
The total flow-distance found after 1200 s (IVS and IHS) or 500 s (FHS) for the different analyte concentrations are summarized in the bar plots in Fig. 6. The blue and red bar plots represents results from the numerical simulation and the experiments, respectively. The numerically predicted results are consistent with the experiments for IHS and FHS. Trends of both the time-series flow-distance, and the net flow for different concentration are consistently represented by the numerical results – this indicates that our hypothesis of dual transport-phase flow for this solution system is adequate. Overall, the characteristics observed determines the relationship between liquid sample concentration and flow distance. This relation can be used for other chemicals (with dual transport or two-phase flow) to identify the concentration.
In general, for a given setup (IVS, IHS or FHS), the water flow velocity (vw(t)) is expected to be consistent for repeated experiments. The vw(t) should be independent of the concentration used (due to the dilute solutions), as previously mentioned in Sec. 5.1. We do observe this consistency for IHS and FHS experiments (see Sec. S2 in the ESI† document). Therefore, using the fixed set of parameter values as listed in Table 2 give practically relatable numerical results for all concentrations for IHS and FHS. The net flow distance of analyte (LA) for IVS predicted by the numerical simulation (based on parameters in Table 2) does not follow the experiments – see blue and red bar plots in Fig. 6, respectively. This can be due to the combined effects of: (i) gravitational force on analyte, and (ii) the fact that the water flow velocity (vw(t)) for IVS varied among experiments. The effect of gravitational force can be modeled with non-zero βin eqn (4), as discussed earlier. We extracted the vw(t) profiles separately for each of the IVS experiments (Sec. S2†) and then did corresponding numerical analysis including graviational force on analyte – see Sections S2 and S3 in the ESI† document for full details. The resulting net flow distances for the various analyte concentrations are shown by the green bar plots (labeled as Simulation*) in Fig. 6(b). These results are more consistent with the experimental observations (red bar plots). It is unclear why there was observable inconsistencies among water velocities among the IVS experiments. Further study is needed to explain the effects of gravity in the IVS systems.
5.3. Flow distance vs. diffusion length
Conventionally, we do not expect concentration dependency of diffusion distance. It is therefore important to discuss the difference between the ‘diffusion distance’ and the ‘flow distance’ (lA). In the following, we will explain why the ‘flow distance’ shows concentration dependency, even though ‘diffusion distance’ does not.The flow distance at any time (lA(t)) is the distance as perceived by the intensity threshold in the experiment (i.e., edge where the dark analyte front ends), or equivalently by a concentration threshold as explained in the numerical simulations results in Sec. 5.2. For example, in the IHS setup, the concentration profiles after t = 1200 s for 50 mM and 10 mM solutions are shown in Fig. 7(a). As per definition, the flow distance (lA) is the distance at which the local concentration goes below the threshold Cth, which equivalently represents the intensity threshold. Clearly, the 50 mM solution travels more than the 10 mM source solution. Our results are, in fact, also consistent with the conventional ‘diffusion length’ concept. Within the diffusion length, c/C0 decreases by a fixed amount. For example, if we plot our results as ‘c/C0 vs. distance’, as shown in Fig. 7(b), the curves for the 10 mM and 50 mM sources overlap. This indicates that the diffusion distance for different concentrations would be the same – however, the flow distance (lA) as seen by the observer would be concentration dependent.
6. Conclusion
In this work, we have experimentally and numerically analyzed the spatio-temporal wicking behavior of KMnO4 solutions through paper strips for different concentrations. In the solution chosen (KMnO4 in water), the analyte (i.e., KMnO4) forms a weak bond with the solvent (water). As a result, its wicking through the paper shows two transport stages: a capillary-driven water flow, followed by a convection–diffusion mode analyte transport. We have conducted experiments and numerical modeling focusing on time-series progression of water and analyte flow through the paper-strip for different setup conditions (vertical and horizontal paper channel) and concentrations.All the experiments were video recorded for time-series flow data extraction. We determined the water and analyte flow distance using our proposed video processing algorithm to ensure measurement consistency among experiments. We also explained the physics of the analyte flow through the paper-channels through experimental results and simulations. We have explained how the convection–diffusion equation with appropriate boundary conditions can model this diffusion-based analyte transport process. The water flow (concentration independent) contributes to a drift force on the analyte particles which accentuates the concentration dependent flow distance. We have explained through our model why the observed flow distance is concentration dependent, in contrary to concentration independent diffusion distance.
For horizontal paper channel setups (IHS and FHS), the average end-of-experiment final flow distance of the analyte (LA) showed monotonically larger values with increasing concentration. This is a key characteristic behavior that is specific to such two-transport phase flow (water assisted analyte flow) solution that can be useful for paper-based sensing devices. Concentration dependency would not be observed for fully dissolved solution with single transport phase flow which follows Lucas–Washburn like behavior. For the vertical setup (i.e., IVS), on the other hand, the end-of-experiment final flow distance of the analyte (LA) increases for 10–30 mM concentrations, and then decreases for 40 mM and 50 mM solutions. We theorize that the gravitational forces on the solution in the vertical flow system causes this behavior. Indeed, our corresponding numerical results including gravitational effects consolidates this observation – this model, however, requires further development. Overall, the experimental and simulated data show good characteristic agreement. However, more studies are needed to improve the model to achieve a better quantitative match between simulations and experiments.
Paper-based detection has great potential for low-cost and point-of-care devices. Our work can be generalized to test and analyze other solute–solvent systems with two transport-stage flows. The numerical analysis can be further refined with more detailed 2D, transient models and by including environmental factors (e.g., humidity, temperature, and evaporation). Deeper understanding of spatio-temporal behavior of analyte flow, such as that presented in this paper, will aid in developing and designing more complex and practically relevant paper-based sensors.
Data availability
The extracted data (analyte flow distance with time) for all experiments are available at Zenodo at https://doi.org/10.5281/zenodo.14000186.Author contributions
Md. Saykat Hassan Sajib: data curation, validation, methodology, investigation, formal analysis, software, visualization, writing – original draft, writing – review & editing. Md. Sakif Rafid: data curation, validation, methodology, investigation, software, writing – review & editing. M. Ryyan Khan: conceptualization, project administration, funding acquisition, supervision, methodology, investigation, formal analysis, validation, software, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We greatly thank Prof. Muhammad A. Alam (Purdue University, USA) for detailed discussions. This work was partly funded by UIU-LCT project. AI-tools (Grammarly and QuillBot) were used only to help improve the language – the final text has been rechecked, and represents the authors' views.References
- A. W. Martinez, S. T. Phillips, M. J. Butte and G. M. Whitesides, Patterned paper as a platform for inexpensive, low-volume, portable bioassays, Angew. Chem., 2007, 119(8), 1340–1342 CrossRef.
- Y. Wang, S. Wang, S. Ge, S. Wang, M. Yan and D. Zang, et al., Ultrasensitive chemiluminescence detection of DNA on a microfluidic paper-based analytical device, Monatsh. Chem., 2014, 145, 129–135 CrossRef.
- S. Z. Hossain, R. E. Luckham, M. J. McFadden and J. D. Brennan, Reagentless bidirectional lateral flow bioactive paper sensors for detection of pesticides in beverage and food samples, Anal. Chem., 2009, 81(21), 9055–9064 CrossRef PubMed.
- Z. Nie, C. A. Nijhuis, J. Gong, X. Chen, A. Kumachev and A. W. Martinez, et al., Electrochemical sensing in paper-based microfluidic devices, Lab Chip, 2010, 10(4), 477–483 RSC.
- L. Wang, W. Chen, D. Xu, B. S. Shim, Y. Zhu and F. Sun, et al., Simple, rapid, sensitive, and versatile SWNT- paper sensor for environmental toxin detection competitive with ELISA, Nano Lett., 2009, 9(12), 4147–4152 CrossRef PubMed.
- Z. Liu, X. He, J. Han, X. Zhang, F. Li and A. Li, et al., Liquid wicking behavior in paper-like materials: mathematical models and their emerging biomedical applications, Microfluid. Nanofluid., 2018, 22, 1–20 CrossRef.
- E. Fu, S. A. Ramsey, P. Kauffman, B. Lutz and P. Yager, Transport in two-dimensional paper networks, Microfluid. Nanofluid., 2011, 10, 29–35 CrossRef.
- A. Apilux, Y. Ukita, M. Chikae, O. Chailapakul and Y. Takamura, Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing, Lab Chip, 2013, 13(1), 126–135 RSC.
- B. J. Toley, B. McKenzie, T. Liang, J. R. Buser, P. Yager and E. Fu, Tunable-delay shunts for paper microfluidic devices, Anal. Chem., 2013, 85(23), 11545–11552 CrossRef.
- J. H. Shin, J. Park, S. H. Kim and J. K. Park, Programmed sample delivery on a pressurized paper, Biomicrofluidics, 2014, 8(5), 054121 CrossRef.
- B. Lutz, T. Liang, E. Fu, S. Ramachandran, P. Kauffman and P. Yager, Dissolvable fluidic time delays for programming multi-step assays in instrument-free paper diagnostics, Lab Chip, 2013, 13(14), 2840–2847 RSC.
- H. Noh and S. T. Phillips, Fluidic timers for time-dependent, point-of-care assays on paper, Anal. Chem., 2010, 82(19), 8071–8078 CrossRef.
- C. K. Camplisson, K. M. Schilling, W. L. Pedrotti, H. A. Stone and A. W. Martinez, Two-ply channels for faster wicking in paper-based microfluidic devices, Lab Chip, 2015, 15(23), 4461–4466 RSC.
- R. B. Channon, M. P. Nguyen, A. G. Scorzelli, E. M. Henry, J. Volckens and D. S. Dandy, et al., Rapid flow in multilayer microfluidic paper-based analytical devices, Lab Chip, 2018, 18(5), 793–802 RSC.
- C. Renault, X. Li, S. E. Fosdick and R. M. Crooks, Hollow-channel paper analytical devices, Anal. Chem., 2013, 85(16), 7976–7979 CrossRef CAS.
- J. H. Shin, G. J. Lee, W. Kim and S. Choi, A stand-alone pressure-driven 3D microfluidic chemical sensing analytic device, Sens. Actuators, B, 2016, 230, 380–387 CrossRef CAS.
- D. L. Giokas, G. Z. Tsogas and A. G. Vlessidis, Programming fluid transport in paper-based microfluidic devices using razor-crafted open channels, Anal. Chem., 2014, 86(13), 6202–6207 CrossRef CAS.
- Q. Liu, C. Xu and H. Liang, Laser carved micro-crack channels in paper-based dilution devices, Talanta, 2017, 175, 289–296 CrossRef CAS PubMed.
- Y. Mirzaei, A. Gholami and M. M. Bordbar, A distance-based paper sensor for rapid detection of blood lactate concentration using gold nanoparticles synthesized by Satureja hortensis, Sens. Actuators, B, 2021, 345, 130445 CrossRef CAS.
- K. Phoonsawat, N. Ratnarathorn, C. S. Henry and W. Dungchai, A distance-based paper sensor for the determination of chloride ions using silver nanoparticles, Analyst, 2018, 143(16), 3867–3873 RSC.
- Y. Soda, D. Citterio and E. Bakker, Equipment-free detection of K+ on microfluidic paper-based analytical devices based on exhaustive replacement with ionic dye in ion-selective capillary sensors, ACS Sens., 2019, 4(3), 670–677 CrossRef CAS PubMed.
- S. Patari and P. S. Mahapatra, Liquid wicking in a paper strip: An experimental and numerical study, ACS Omega, 2020, 5(36), 22931–22939 CrossRef CAS.
- C. Xu, G. Zhou, H. Cai, Y. Chen, L. Huang and L. Cai, et al., Modification of microfluidic paper-based devices with an oxidant layer for distance readout of reducing substances, ACS Omega, 2022, 7(23), 20383–20389 CrossRef CAS PubMed.
- E. W. Washburn, The dynamics of capillary flow, Phys. Rev., 1921, 17(3), 273 CrossRef.
- A. Ponomarenko, D. Quéré and C. Clanet, A universal law for capillary rise in corners, J. Fluid Mech., 2011, 666, 146–154 CrossRef.
- K. Li, D. Zhang, H. Bian, C. Meng and Y. Yang, Criteria for applying the Lucas-Washburn law, Sci. Rep., 2015, 5(1), 14085 CrossRef CAS PubMed.
- N. Fries, K. Odic, M. Conrath and M. Dreyer, The effect of evaporation on the wicking of liquids into a metallic weave, J. Colloid Interface Sci., 2008, 321(1), 118–129 CrossRef CAS PubMed.
- Z. Liu, J. Hu, Y. Zhao, Z. Qu and F. Xu, Experimental and numerical studies on liquid wicking into filter papers for paper-based diagnostics, Appl. Therm. Eng., 2015, 88, 280–287 CrossRef.
- S. Hong and W. Kim, Dynamics of water imbibition through paper channels with wax boundaries, Microfluid. Nanofluid., 2015, 19, 845–853 CrossRef.
- H. Darcy, Les fontaines publiques de Dijon, 1856 Search PubMed.
- R. Tang, H. Yang, Y. Gong, Z. Liu, X. Li and T. Wen, et al., Improved analytical sensitivity of lateral flow assay using sponge for HBV nucleic acid detection, Sci. Rep., 2017, 7(1), 1360 CrossRef.
- J. Piquemal, On the modelling conditions of mass transfer in porous media presenting capacitance effects by a dispersion-convection equation for the mobile fluid and a diffusion equation for the stagnant fluid, Transport Porous Media, 1993, 10, 271–283 CrossRef.
- M. Motooka and S. Uno, Improvement in limit of detection of enzymatic biogas sensor utilizing chromatography paper for breath analysis, Sensors, 2018, 18(2), 440 CrossRef PubMed.
- P. Ryan, D. Zabetakis, D. A. Stenger and S. A. Trammell, Integrating paper chromatography with electrochemical detection for the trace analysis of tnt in soil, Sensors, 2015, 15(7), 17048–17056 CrossRef PubMed.
- X. L. Huo, Y. Chen, N. Bao and C. G. Shi, Electrochemiluminescence integrated with paper chromatography for separation and detection of environmental hormones, Sens. Actuators, B, 2021, 334, 129662 CrossRef.
- G. Yan, Z. Li, S. A. Galindo Torres, A. Scheuermann and L. Li, Transient two-phase flow in porous media: a literature review and engineering application in geotechnics, Geotechnics, 2022, 2(1), 32–90 CrossRef.
- F. He, W. Dong and J. Wang, Modeling and numerical investigation of transient two-phase flow with liquid phase change in porous media, Nanomaterials, 2021, 11(1), 183 CrossRef PubMed.
- D. DeVault, The theory of chromatography, J. Am. Chem. Soc., 1943, 65(4), 532–540 CrossRef.
- M. Z. Hua, S. Li, S. Wang and X. Lu, Detecting chemical hazards in foods using microfluidic paper-based analytical devices (μPADs): the real-world application, Micromachines, 2018, 9(1), 32 CrossRef.
- A. Atangana, Fractional Operators with Constant and Variable Order with Application to Geo-Hydrology, Academic Press, 2017 Search PubMed.
- G. Yan, Z. Li, T. Bore, S. A. G. Torres, A. Scheuermann and L. Li, A lattice Boltzmann exploration of two-phase displacement in 2D porous media under various pressure boundary conditions, J. Rock Mech. Geotech. Eng., 2022, 14(6), 1782–1798 CrossRef.
- G. Yan, Z. Li, T. Bore, S. A. G. Torres, A. Scheuermann and L. Li, Discovery of dynamic two-phase flow in porous media using two-dimensional multiphase lattice Boltzmann simulation, Energies, 2021, 14(13), 4044 CrossRef.
- R. Lenormand, E. Touboul and C. Zarcone, Numerical models and experiments on immiscible displacements in porous media, J. Fluid Mech., 1988, 189, 165–187 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: The ESI document includes additional discussions on the experiments and results, analysis details, and data extraction process. Sample time-series analyte and water flow distance extraction videos are provided as additional ESI materials. See DOI: https://doi.org/10.1039/d5ra02347e |
| This journal is © The Royal Society of Chemistry 2025 |