Open Access Article
Open Access ArticleHeterogeneous silicotungstic acid constructed via defect and morphology engineering for boosting the oxidative desulfurization of fuel oil†
Xiyang Zhou ab,
Li Yub,
Xu Lib,
Weizhou Jiaob and
Ruixin Wang*b
ab,
Li Yub,
Xu Lib,
Weizhou Jiaob and
Ruixin Wang*b
aDepartment of Chemical and Material Engineering, Lyuliang University, Lv Liang, Shanxi 033001, China. E-mail: 20111013@llu.edu.cn; Fax: +86-351-3921497; Tel: +86-18234838896
bSchool of Chemistry and Chemical Engineering, North University of China, Taiyuan, Shanxi 030051, China. E-mail: zbdxwrx@nuc.edu.cn; zbdxjwz@nuc.edu.cn
First published on 30th May 2025
Abstract
It is well-known that defect engineering and morphology engineering are key strategies for giving an impetus to the catalytic activity of catalysts. This study reports the successful regulation of oxygen vacancy (OV) defects and the morphology of a Keggin-type silicotungstic acid (SiW12)-loaded N-doped carbon (NC) matrix, which is obtained by calcining a copolymer of acryloyloxyethyl trimethyl ammonium chloride (DAC) and acrylamide (AM) as precursors in air. It was unveiled that adjusting the proportion of AM in the copolymer could be simultaneously conducive to the targeted regulation of OV defects of catalysts and their morphological evolution from bowls to spheres. The as-prepared OV-SiW12/SNC sphere catalyst showed an excellent oxidative desulfurization (ODS) performance with H2O2 as a green oxidant. Complete removal of DBT (2000 ppm) was achieved within 12 minutes at 60 °C with a large rate constant of 0.4055 min−1. The outstanding ODS performance was critically ascribed to the highly active W sites promoted by the abundant OV defects, and the easy diffusion and contact between the substrate and W sites were led by the spherical structure. Moreover, the as-prepared catalyst exhibited prominent reusability. This ODS reaction was found to follow a HO˙ radical mechanism.
1. Introduction
Despite the continuous development of the new energy industry, crude oil remains the most commonly used energy source in most industries in the modern world.1–3 However, fuel reserves continue to dwindle, leading to the use of low-quality fossil fuels containing a large amount of refractory sulfides, such as thiophene (Th), dibenzothiophene (DBT), benzothiophene (BT), 4,6-dimethyldibenzothiophenes (4,6-DMDBT), and other derivatives. The combustion of these sulfides could result in the release of sulfur oxides into air so as to the acid and smog, therefore posing serious threats to human health and ecosystems.4–6 Moreover, thiophene compounds can poison catalysts used for automobile exhaust treatment and corrode refining equipment.7 Currently, the limit for sulfur content in liquid fuels are becoming increasingly strict in various countries (even less than 60 ppb for organic sulfur compounds).8 Therefore, it is urgent to develop highly efficient and feasible desulfurization techniques.Although traditional hydrodesulfurization (HDS) has high efficiency for removing mercaptan, sulfide and disulfide from fuel feedstocks, it finds difficulty in removing refractory sulfides (such as DBT and its derivatives), and it requires harsh conditions (such as high temperature, high pressure, and substantial hydrogen consumption).4 Recently, some non-hydrodesulfurization methods, including extractive desulfurization (EDS), adsorptive desulfurization (ADS), biological desulfurization (BDS), and oxidative desulfurization (ODS), have been developed to supplement HDS for the removal of refractory sulfides.4,9,10 In particular, ODS is an attractive method with strong elimination ability for refractory sulfides under mild operating conditions, and hence, it is expected to produce ultra-clean fuels.9,11 During ODS process, aromatic sulfur compounds are usually oxidized into sulfones, which could be then easily separated and recovered through adsorption, crystallization, rectification, extraction, and so on.9,11–13
A cost-effective and highly active catalyst is always a key factor for boosting the commercial viability of ODS technology.1 To date, various catalysts, including polyoxometalates (POMs),14,15 metal–organic frameworks (MOFs),16,17 transition metal oxides (VOx, WOx, and MoOx) or nitrides (W2N and Mo2N),1,18–21 transition metal carbides (Mo2C and W2C),22,23 and transition metals (Co),24 have been widely explored for ODS of fuel oil. Among them, POMs, as a class of metal–oxygen clusters consisting of early transition metals (W, V, Mo, etc.) in their high oxidation states, have received significant attention as effective catalysts owing to their appealing properties, such as their particular structure, excellent redox activity, good stability, and mild reaction conditions.25,26 Notably, the heterogeneous POMs with SiO2, porous carbon materials, and MOFs as supports, are atttracting increasingly widespread interest owing to more exposed active sites triggered by the good distribution of active components.27,28 Morphology engineering is always a good strategy for improving the catalytic activity of catalysts. Generally, the spherical shape has the advantages of good fluidity and high specific surface area, which makes it easier for reactant molecules to come into contact with the active center, thus exhibiting an excellent catalytic effect.27,29 Furthermore, defect engineering has been found to be promising for greatly improving the performance of catalysts.5,30–33 Typically, the oxygen vacancy (OV) defect can generate favorable effects for regulating the surface-electron state and boosting the redox reaction (such as the oxidative desulfurization reaction).5 Ma et al.34 synthesized an OV-defective catalyst, H4PVMo4W7O40@rht-MOF-1-De, using a one-pot method for fuel oil desulfurization. It exhibited high catalytic activity and recyclability, achieving 99% DBT and 96% 4,6-DMDBT removal under optimized conditions. In the context of oxidative desulfurization (ODS) reactions, Jiao et al.1 discovered that the presence of oxygen vacancies significantly promoted the generation of active intermediates, thereby enhancing the overall rate of the ODS reaction. This observation underscores the crucial role of oxygen vacancies in facilitating efficient sulfur removal.
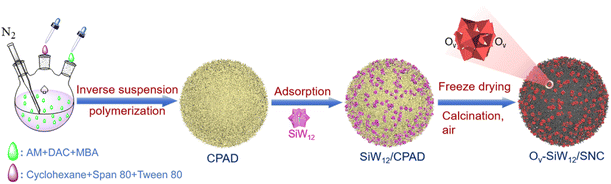
Herein, a novel strategy for effectively modulating oxygen vacancy (OV) defects and the morphology of a Keggin-type silicotungstic acid (SiW12)-loaded N-doped carbon (NC) matrix is developed by adjusting the content of acrylamide (AM) in a copolymer of acryloyloxyethyl trimethyl ammonium chloride (DAC) and acrylamide (AM) (abbreviated as CPAD) as precursors. Finally, by in situ low-temperature calcination of CPAD in air, a spherical catalyst with abundant OV defects (OV-SiW12/SNC) was successfully prepared and applied to the deep ODS of fuel oil. The as-prepared OV-SiW12/SNC catalyst shows an excellent oxidative desulfurization (ODS) performance, wonderful adaptability to fuel oils with a wide range of sulfur concentrations, and remarkable reusability. In addition, a series of radical scavenging studies were performed to elucidate the ODS mechanism of OV-SiW12/SNC.
2. Experimental
2.1. Materials
Acrylamide (AM, AR, C3H5NO, Tianjin Dengfeng Chemical Reagent Factory); acryloyloxyethyl trimethyl ammonium chloride (DAC, 80 wt% in H2O, C8H16ClNO2, Shanghai Maclean Biochemical Technology Co., Ltd); silicotungstic acid (H4SiW12O40·xH2O, abbreviated as SiW12, AR, Shanghai Maclean Biochemical Technology Co., Ltd); dibenzothiophene (DBT, ≥99%, C12H8S, Aladdin); 4,6-dimethyldibenzothiophene (4,6-DMDBT) (97%, C14H12S, Aladdin); thiophene (BT, 99%, C4H4S); n-octane (AR, Tianjin Damao Chemical Reagent Factory); H2O2 aqueous solution (AR, 30 wt%, Tianjin Damao Chemical Reagent Factory); and acetonitrile (AR, CH3CN, Tianjin Concord Technology Co., Ltd). All the other chemicals and reagents are commercially available and analytically pure (see Section 1 in the (ESI)† for more details).2.2. Preparation of catalysts
First, copolymerized crosslinked microspheres (CPAD) of acrylamide (AM) and acryloyloxyethyl trimethyl ammonium chloride (DAC) were prepared by applying the reverse suspension polymerization method (see Section 2 of the (ESI)† for more details). Then, a certain amount of silicotungstic acid (SiW12) can be combined with CPAD through the electrostatic interaction to produce the spherical precursor SiW12/CPAD (Fig. S1†). Finally, SiW12/CPAD is calcined at different temperatures to obtain spherical catalysts, i.e. SiW12/CPAD-300, SiW12/CPAD-400, SiW12/CPAD-500, and SiW12/CPAD-700, where the numbers represent calcination temperatures. Importantly, during the synthesis process, the feed ratio of DAC and AM is explored in the range of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–4
0–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to regulate the morphology of the final calcination products. Unless otherwise specified, the DAC/AM feed ratio is determined to be 4
3 to regulate the morphology of the final calcination products. Unless otherwise specified, the DAC/AM feed ratio is determined to be 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, and the corresponding precursor and spherical SiW12/CPAD-400 product are denoted as SiW12/CPAD and OV-SiW12/SNC, respectively. Besides, to highlight OV-SiW12/SNC, the obtained calcinating products of pure SiW12 and the SiW12/CPAD precursor with a feed molar ratio of DAC to AM of 4
2, and the corresponding precursor and spherical SiW12/CPAD-400 product are denoted as SiW12/CPAD and OV-SiW12/SNC, respectively. Besides, to highlight OV-SiW12/SNC, the obtained calcinating products of pure SiW12 and the SiW12/CPAD precursor with a feed molar ratio of DAC to AM of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 at 400 °C are marked as SiW12-400 and SiW12/NC, respectively. The preparation process of OV-SiW12/SNC is schematically shown in Fig. 1, and the molecular structures of DAC, AM, MBA, and CPAD are depicted in Fig. S2.†
0 at 400 °C are marked as SiW12-400 and SiW12/NC, respectively. The preparation process of OV-SiW12/SNC is schematically shown in Fig. 1, and the molecular structures of DAC, AM, MBA, and CPAD are depicted in Fig. S2.†
2.3. Characterizations
Fourier Infrared Spectrometer (FT-IR, KBr method, L1600300 Spectrum Two Lita Model, Liantrisant, UK); UV-Visible Spectrophotometer (UV-Vis, UV-2802, Unico, China); High Performance Liquid Chromatograph (HPLC, Model Classical 3100, Dalian Yi lite Analytical Instrument Co., Ltd, Dalian, China); element analyser (EA, PE2400 Series II CHNS/O type, Qingdao Huaqing Group Co., Ltd China); and a Powder X-ray diffractometer (XRD, Empyrean, PANalytical B.V., Holland, Cu K(α) radiation (λ = 1.5418 Å, 40 kV and 40 mA) over a range of 2θ = 5–90°). Electron paramagnetic resonance spectroscopy (EPR) was performed using a MiniScope MS 5000 spectrometer (Magnettech, Germany) in the dark at room temperature. Scanning electron microscopy (SEM, Zeiss Merlin Compact, Germany); X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha+, USA); Synchronous thermal analysis (TG-DSC, LF-1100, Mettler Toledo Instruments Co., Ltd, Shanghai, China, air atmosphere, a heating rate of 5 °C min−1); and High-performance liquid chromatography (HPLC, EClassical 3100, Dalian Yilite Analytical Instrument Co., Ltd, Dalian, China). Inductively coupled plasma optical emission spectrometry (ICP-OES, Agilent 5110, USA) was used to determine the W content.2.4. ODS experiments
First, the model oil with a sulfur content of 500–4000 ppm (parts per million by weight) was produced by dissolving DBT (or BT, 4,6-DMDBT) into n-octane. The extraction-catalyzed ODS reaction was carried out in a two-phase system consisting of model oil and acetonitrile. Specifically, 20 mL of the model oil and 20 mL of acetonitrile were mixed and stirred at the reaction temperature for 20 min to achieve extraction equilibrium. Subsequently, an ODS reaction occurred, followed by the addition of OV-SiW12/SNC and 30 wt% of H2O2 aqueous solution with a certain H2O2/sulfur (O/S) molar ratio into the above two-phase system. During the reaction, trace samples were periodically removed from the two phases and then diluted with n-octane and acetonitrile to detect the residual sulfur concentration by employing the HPLC external standard method (see Section 3 of the ESI† for details). At the end of the reaction, the catalyst was collected, washed with acetonitrile and ethanol, dried at 50 °C, and then recovered for reuse.3. Results and discussion
3.1. Characteristics of catalysts
The morphology of the calcination products with the feed ratios of DAC and AM in the range of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–4
0–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 at 400 °C was observed by SEM. As displayed in Fig. 2(a–c) and S3,† with the feed DAC/AM ratio in the range of 4
3 at 400 °C was observed by SEM. As displayed in Fig. 2(a–c) and S3,† with the feed DAC/AM ratio in the range of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–4
0–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 during the preparation of precursors, the morphology of the calcination products varies from bowl shape with thin walls to the bowl with increased wall thickness and then to spherical structure, which may be related to the thermal decomposition of CPAD microspheres. Herein, the feed DAC/AM ratio is determined to be 4
3 during the preparation of precursors, the morphology of the calcination products varies from bowl shape with thin walls to the bowl with increased wall thickness and then to spherical structure, which may be related to the thermal decomposition of CPAD microspheres. Herein, the feed DAC/AM ratio is determined to be 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to guarantee the spherical OV-SiW12/SNC catalyst. Moreover, the EDX element mapping in Fig. 2(d) shows that the C, N, O, Si, and W elements are evenly distributed in the OV-SiW12/SNC microspheres, indicating the even distribution of OV-SiW12 in the spherical NC matrix. Furthermore, the SiW12/CPAD precursors with different DAC/AM feed molar ratios of 4
2 to guarantee the spherical OV-SiW12/SNC catalyst. Moreover, the EDX element mapping in Fig. 2(d) shows that the C, N, O, Si, and W elements are evenly distributed in the OV-SiW12/SNC microspheres, indicating the even distribution of OV-SiW12 in the spherical NC matrix. Furthermore, the SiW12/CPAD precursors with different DAC/AM feed molar ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 4
2 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 present similar TG and DSC curves (Fig. 3), in which the precursor with a DAC/AM feed molar ratio of 4
0 present similar TG and DSC curves (Fig. 3), in which the precursor with a DAC/AM feed molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 is named SiW12/CPAD-0. The TG curves of SiW12/CPAD show four distinct weight loss stages. The weight loss below 280 °C is owing to the removal of physically and chemically adsorbed water and decomposition of MBA in CPAD; the weight loss at 280–400 °C with an exothermic peak at 365 °C is ascribed to the damage of the quaternary ammonium group35,36 and the loss of crystal water in H4SiW12O40·xH2O;27 the weight loss at 400–505 °C along with an exothermic peak at 441 °C is attributed to the combustion of residual carbon skeleton and the deprotonation of H4SiW12O40 into SiW12O38,27,35,37 which can be further demonstrated by the color of products at 400 and 500 °C (Fig. S4a†); the weight loss at 506–600 °C along with two exothermic peaks at 555 °C and 572 °C is accredited to the transformation of SiW12 to silicon- and tungsten-oxides.38,39 Compared to the TG and DSC curves of the SiW12/CPAD-0 precursor, it is depicted that the thermo-decomposed temperatures of the SiW12/CPAD precursor in each pyrolysis stage increase owing to the addition of AM, which could also be unmasked by comparing their colors at 300–500 °C (Fig. S4†). Therefore, when the temperature increases to 400 °C, the residual weight of SiW12/CPAD (87.9%) is greater than that of SiW12/CPAD-0 (80.8%) (Fig. 3a), which favors better preservation of the spherical carbon skeleton after calcining SiW12/CPAD.
0 is named SiW12/CPAD-0. The TG curves of SiW12/CPAD show four distinct weight loss stages. The weight loss below 280 °C is owing to the removal of physically and chemically adsorbed water and decomposition of MBA in CPAD; the weight loss at 280–400 °C with an exothermic peak at 365 °C is ascribed to the damage of the quaternary ammonium group35,36 and the loss of crystal water in H4SiW12O40·xH2O;27 the weight loss at 400–505 °C along with an exothermic peak at 441 °C is attributed to the combustion of residual carbon skeleton and the deprotonation of H4SiW12O40 into SiW12O38,27,35,37 which can be further demonstrated by the color of products at 400 and 500 °C (Fig. S4a†); the weight loss at 506–600 °C along with two exothermic peaks at 555 °C and 572 °C is accredited to the transformation of SiW12 to silicon- and tungsten-oxides.38,39 Compared to the TG and DSC curves of the SiW12/CPAD-0 precursor, it is depicted that the thermo-decomposed temperatures of the SiW12/CPAD precursor in each pyrolysis stage increase owing to the addition of AM, which could also be unmasked by comparing their colors at 300–500 °C (Fig. S4†). Therefore, when the temperature increases to 400 °C, the residual weight of SiW12/CPAD (87.9%) is greater than that of SiW12/CPAD-0 (80.8%) (Fig. 3a), which favors better preservation of the spherical carbon skeleton after calcining SiW12/CPAD.
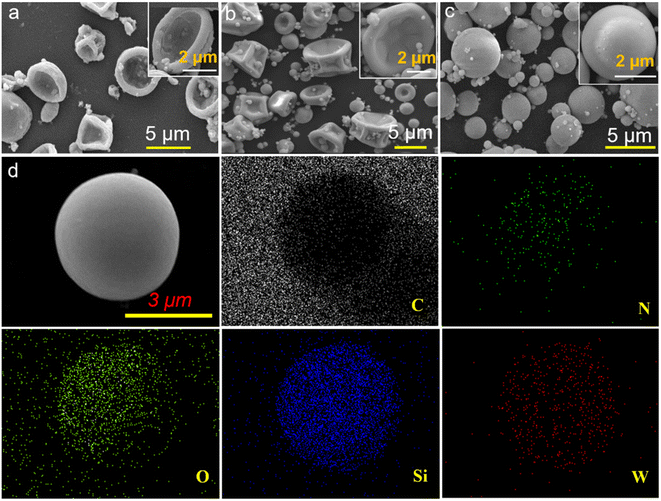 | ||
Fig. 2 SEM images of OV-SiW12/SNC derived from different DAC/AM feed molar ratios of (a) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, (b) 4 0, (b) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, (c) 4 1, (c) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and (d) HADDF and EDX elemental mapping images of OV-SiW12/SNC. 2 and (d) HADDF and EDX elemental mapping images of OV-SiW12/SNC. | ||
 | ||
Fig. 3 (a) TG and (b) DSC curves of precursors SiW12/CPAD with feed DAC/AM molar ratios of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 and 4 0 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (in air). 2 (in air). | ||
The FT-IR spectrum of the CPAD (Fig. S5†) shows that not only the characteristic absorption peaks of DAC (at 1164 cm−1, 1479 cm−1 and 1740 cm−1 corresponding to the stretching vibration of C–O, –CH2–N+ and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively)40 but also those of AM (at 1667 cm−1 corresponding to the stretching vibration of –NH2) appear,41 confirming the formation of the copolymerized crosslinked microspheres CPAD. In the FT-IR spectrum of SiW12/CPAD, in addition to peaks of CPAD, the characteristic absorption peaks of SiW12 are found at 1017, 973, 920, 881 and 796 cm−1 ascribed to the stretching vibration of Si–Oa, W–Od, W–Ob–W, O–O and W–Oc–W bonds, respectively,42,43 verifying the successful combination of SiW12 onto CPAD.
O, respectively)40 but also those of AM (at 1667 cm−1 corresponding to the stretching vibration of –NH2) appear,41 confirming the formation of the copolymerized crosslinked microspheres CPAD. In the FT-IR spectrum of SiW12/CPAD, in addition to peaks of CPAD, the characteristic absorption peaks of SiW12 are found at 1017, 973, 920, 881 and 796 cm−1 ascribed to the stretching vibration of Si–Oa, W–Od, W–Ob–W, O–O and W–Oc–W bonds, respectively,42,43 verifying the successful combination of SiW12 onto CPAD.
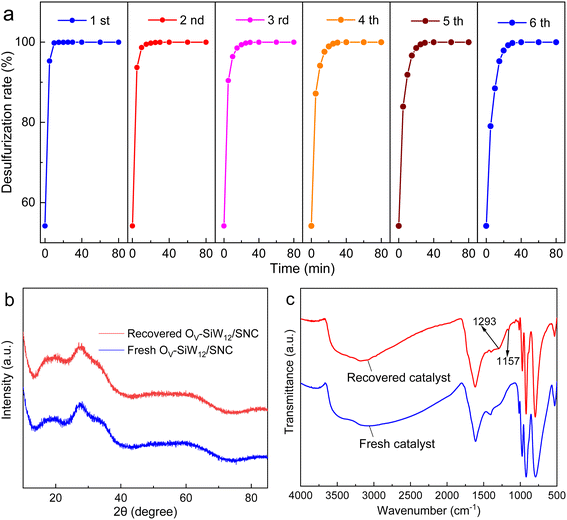
Fig. 4a illustrates the FT-IR spectra of the calcination products of SiW12/CPAD at different temperatures. When calcinating at 300 °C, the peaks of SiW12 remain basically unchanged, but the peak of AM in CPAD at 1667 cm−1 is greatly reduced, suggesting that the structure of CPAD has changed. After calcinating SiW12/CPAD at 400 °C, the peaks assigned to AM and DAC in CPAD have almost gone, and the peaks of SiW12 at 1017, 973, 920, and 796 cm−1 remain basically the same except for the peak at 880 cm−1 corresponding to the O–O bond that almost disappears, indicating that not only the structure of CPAD is destroyed owing to carbonization (see the blackened product shown in Fig. S4†), but also that of SiW12 is damaged to some extent owing to the formation of oxygen vacancies, which is further supported by the presented symmetric peak at g = 2.003 in the electron paramagnetic resonance (EPR) spectrum compared with the SiW12/CPAD precursor and its calcined product at 300 °C (Fig. S6†). As the calcination temperature exceeds 500 °C, the peaks of both CPAD and SiW12 disappear completely, and only a large, wide peak appears at about 790 cm−1 corresponding to the W–O–W bond.27 This significant structural change is also disclosed by XRD patterns. As shown in Fig. 4b, the calcination products at 400 and 300 °C exhibit XRD patterns similar to that of the SiW12/CPAD precursor, with almost no diffraction peaks, indicating the uniform dispersion of SiW12 in the CPAD matrix or its derived carbon materials39,44,45 and the preservation of the basic SiW12 structure even after calcination at 300 or 400 °C in air, which agrees with the literature.5,27 However, as the calcination temperature exceeds 500 °C, the XRD patterns of the calcination products are distinctly different, and some clear diffraction peaks appear, corresponding to the monochromatic WO3 phase (PDF # 71-0131), which comes from the oxidation and decomposition of SiW12. The XRD results are in good agreement with those of the FT-IR spectra. To sum up, the calcination temperature of 400 °C is indispensable for ensuring the basic structure of SiW12 while creating partial oxygen vacancy defects.
Furthermore, X-ray photoelectron spectroscopy (XPS) was performed to evaluate the chemical environment and composition of OV-SiW12/SNC, as shown in Fig. 4c and S7.† The XPS survey spectrum (Fig. S7a†) shows the presence of C, O, N, W, and Si elements in accordance with the EDX element mapping results. In the high resolution W 4f spectrum of OV-SiW12/SNC (Fig. 4c), two split peaks at 36.10 and 38.21 eV respectively correspond to W6+ of SiW12,5 and are redshifted compared to SiW12/NC, suggesting that the electronic structure of W elements changes. In addition, it is clearly found in the N 1s spectra of OV-SiW12/SNC and SiW12/NC (Fig. 4d) that two peaks at 400.1 and 401.8 eV are ascribed to pyrrolic N and graphitic N, respectively,46 and the ratio of pyrrolic N to graphitic N for OV-SiW12/SNC is notably greater than that for SiW12/NC. The coordination of pyrrolic N with metal (W) atoms can regulate its electronic structure and thus promote its catalytic activity.47 Hence, a higher ratio of pyrrolic N to graphitic N for OV-SiW12/SNC is propitious to facilitate its catalytic activities. The O 1s spectrum (Fig. 4e) can be fitted into three peaks at 530.9 eV, 531.8 eV and 532.8 eV, corresponding to the W–O, Ov, and Si–O bonds, respectively.48,49 Importantly, it can be found from Fig. 4e and f that the OV contents of various specimens decrease in the order OV-SiW12/SNC > SiW12/NC > SiW12-400, which is also verified by changes in the intensity of signal peaks of OV defects at g = 2.003 observed in the EPR spectrum depicted in Fig. 4g. Therefore, the addition of AM to the precursor facilitates the formation of more OV defects, which could modulate the electronic structure of the active W sites in SiW12, thus accelerating the decomposition of H2O2 and improving the ODS performance of OV-SiW12/SNC.
3.2. ODS performance evaluation
Furthermore, it is surprising to find that with the feed DAC/AM ratio in the range of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–4
0–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 during the preparation of precursors, not only the morphology of the corresponding calcined products varies from bowl shape to spherical structure (as shown in Fig. S3†), but also the OV defects of calcined products increase until the precursor with the feed DAC/AM ratio of 4
3 during the preparation of precursors, not only the morphology of the corresponding calcined products varies from bowl shape to spherical structure (as shown in Fig. S3†), but also the OV defects of calcined products increase until the precursor with the feed DAC/AM ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 produces the almost same number of OV defects as that with 4
2 produces the almost same number of OV defects as that with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fig. 5b). Accordingly, with the addition of AM, fuel oil achieves ultra-deep desulfurization in a short time with a lower O/S ratio (3
3 (Fig. 5b). Accordingly, with the addition of AM, fuel oil achieves ultra-deep desulfurization in a short time with a lower O/S ratio (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Moreover, with the increased feed amount of AM, the feed DAC/AM ratio was stretched up in the range of 4
1). Moreover, with the increased feed amount of AM, the feed DAC/AM ratio was stretched up in the range of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0–4
0–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and the ODS performance of the corresponding calcined products becomes better and better owing to the fast mass transfer process supported by the spherical morphology, more OV defects induced by the introduction of AM, and the modulated electronic structure of W active sites by OV defects (Fig. 4c). In view of almost the same desulfurization ratio of the sample with a feed DAC/AM ratio of 4
3, and the ODS performance of the corresponding calcined products becomes better and better owing to the fast mass transfer process supported by the spherical morphology, more OV defects induced by the introduction of AM, and the modulated electronic structure of W active sites by OV defects (Fig. 4c). In view of almost the same desulfurization ratio of the sample with a feed DAC/AM ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 as that with 4
2 as that with 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the feed DAC/AM ratio is optimized as 4
3, the feed DAC/AM ratio is optimized as 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
In addition, the ODS performances of the calcinated products of the precursor SiW12/CPAD with a DAC/AM feed molar ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 at different temperatures are explored to uncover the particularity of the calcination temperature of 400 °C, as shown in Fig. 5d. It is clear that too high or too low a calcination temperature is not conducive to the ODS performance of the calcinated products. Combined with FT-IR, XRD, and EPR results (Fig. 4a, b, and S6†), the structure of SiW12 in the calcined product at 300 °C is the same as that in the precursor, with no OV defects, thus demonstrating a lower ODS activity. Although the Keggin structure of SiW12 in the calcined product at 400 °C is maintained, more OV defects appear (Fig. S6†), resulting in excellent ODS activity. As the calcination temperature increases beyond 400 °C, the desulfurization rate decreases instead, which is related to the structural transformation of SiW12 into WO3 (Fig. 4b). The reduction of exposed active sites led to the aggregation of WO3 species owing to the loss of carbon layers as a barrier during high temperature calcination (as shown in the TG result in Fig. 3a).
2 at different temperatures are explored to uncover the particularity of the calcination temperature of 400 °C, as shown in Fig. 5d. It is clear that too high or too low a calcination temperature is not conducive to the ODS performance of the calcinated products. Combined with FT-IR, XRD, and EPR results (Fig. 4a, b, and S6†), the structure of SiW12 in the calcined product at 300 °C is the same as that in the precursor, with no OV defects, thus demonstrating a lower ODS activity. Although the Keggin structure of SiW12 in the calcined product at 400 °C is maintained, more OV defects appear (Fig. S6†), resulting in excellent ODS activity. As the calcination temperature increases beyond 400 °C, the desulfurization rate decreases instead, which is related to the structural transformation of SiW12 into WO3 (Fig. 4b). The reduction of exposed active sites led to the aggregation of WO3 species owing to the loss of carbon layers as a barrier during high temperature calcination (as shown in the TG result in Fig. 3a).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively.
1, respectively.Generally, the reaction temperature plays a dominant role in improving ODS efficiency. As illustrated in Fig. 6a, the desulfurization efficiency of OV-SiW12/SNC is substantially improved with an increased temperature, which is ascribed to the facilitated probability of collision between the reactants, oxidants, and catalysts, and hence the promoted reaction rate. Optimally, it reaches 100% of DBT removal from the 2000 ppm of model fuel within 12 minutes at 60 °C, better than most reports (Table 1). Surprisingly, even at lower temperatures, the DBT removal rate was still up to 100% within 40 min (at 40 °C) and 99.7% within 80 min (at 30 °C), indicating the outstanding ODS activity of OV-SiW12/SNC. Furthermore, the kinetic plots for ODS reactions over the catalyst are performed, as shown in Fig. 6b (see Section 4 of ESI† for the fitting method). These results demonstrate that all of them follow the pseudo-first-order kinetic model, as confirmed by the high correlation factor (R2 > 0.99). When the reaction temperature is 60 °C, the rate constant is as high as 0.4055 min−1.
 | ||
Fig. 6 (a) ODS efficiencies at different reaction temperatures and (b) pseudo-first-order kinetic curves (DBT content: 2000 ppm; catalysts: 0.2 g; n(H2O2)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(S) = 4 n(S) = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). | ||
| Catalyst | Reaction conditions | Time | Desulfurization rate | Ref. |
|---|---|---|---|---|
| HPW@UiO-66 | 800 ppm, 0.08 g catalysts, 20 mL mode oil, O/S = 7, T = 60 °C | 90 min | 100% | 50 |
| WOx@mSnO2 | 500 ppm, 0.1 g catalysts, 20 mL mode oil, O/S = 5, T = 60 °C | 120 min | 100% | 51 |
| [mim(CH2)3COO]3PW@UiO-66 | 1000 ppm, 0.16 g catalysts, 20 mL mode oil, O/S = 5, T = 70 °C | 60 min | 100% | 44 |
| 30HPW/ZrO2 | 500 ppm, 0.1 g catalysts, 20 mL mode oil, O/S = 4, T = 60 °C | 120 min | 100% | 52 |
| HPW-vim | 500 ppm, 0.20 g catalysts, 20 mL mode oil, O/S = 5, T = 50 °C | 30 min | 100% | 53 |
| CeO2-WOx-TiO2 | 1154 ppm, 0.20 g catalysts, 100 mL mode oil, T = 60 °C | 30 min | 98% | 54 |
| OV-SiW12/SNC | 2000 ppm, 0.2 g catalysts, 20 mL mode oil, O/S = 4, T = 60 °C | 12 min | 100% | This work |
Additionally, the OV-SiW12/SNC catalyst achieves a turnover frequency (TOF) value of 10.65 h−1 at 60 °C by utilizing the W atom as the active site based on a W content of 2.93 mmol g−1 calculated by the ICP-OES test according to Formula (S2) in the ESI.† Notably, the TOF value of the spherical OV-SiW12/SNC catalyst with more OV defects is about 10 times that (1.39 h−1) of the bowl SiW12/NC catalyst with less OV defects obtained by the corresponding precursor with no addition of AM,5 verifying the great advantage of introducing AM components into the precursor once again.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2:2
2:2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, confirming the presence of the HO˙ radical and thus a free radical reaction mechanism for the ODS reaction, which is similar to the results in the literature.5,29
1, confirming the presence of the HO˙ radical and thus a free radical reaction mechanism for the ODS reaction, which is similar to the results in the literature.5,29
Furthermore, Fig. S11† shows HPLC chromatograms of the n-octane and acetonitrile phases during the ODS reaction. As the ODS reaction proceeds, DBT in the acetonitrile phase is initially oxidized into DBTO and eventually into DBTO2. Combined with the aforementioned results, the possible reaction mechanism (Fig. 8c) is inferred as follows. DBT is first transferred from the n-octane phase to the acetonitrile phase. Simultaneously, in the acetonitrile phase, H2O2 is activated by OV-SiW12/SNC to generate HO˙; then, DBT is oxidized by HO˙ into DBTO and further into DBTO2. During the key process, not only the W active sites with high electron density triggered by OV defects boost the cleavage of O-O bonds of H2O2 and thus the generation of a substantial quantity of electrophilic HO˙ species,58 but also the spherical structure of OV-SiW12/SNC is conducive to the exposure of W active sites and the diffusion of DBT and H2O2, thereby providing opportunities for excellent interaction with active species and reactants, which thus facilitates the ODS reaction. With the transformation of DBT in the acetonitrile phase into DBTO and DBTO2, the extraction equilibrium is disturbed, and the residual DBT in the n-octane phase is further extracted into the acetonitrile phase for the subsequent oxidation reaction. In this way, extraction and oxidation are carried out alternately until all DBTs are completely transferred from the n-octane phase into the acetonitrile phase and converted into DBTO2 in the acetonitrile phase, thereby obtaining the final complete removal of DBT from the n-octane phase.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of DBTO2.5,58
O bond of DBTO2.5,58
4. Conclusion
In conclusion, the spherical OV-SiW12/SNC catalyst with abundant OV defects was successfully synthesized through in situ low-temperature calcination of the copolymer of AM and DAC as a precursor in air for deep ODS of fuel oil. It is depicted that during the preparation, the introduction of AM in the precursor could facilitate the formation of more OV defects and the evolution of the morphology from bowl to sphere. The as-prepared OV-SiW12/SNC catalyst exhibits an excellent ODS activity and outstanding reusability. It could effectively eliminate DBT from fuel oil with a sulfur content of 2000 ppm within 12 minutes at 60 °C and maintain a desulfurization rate of 100% after 6 cycles despite a slightly extended time. Notably, it can still efficiently remove DBT derivatives (such as BT and 4,6-DMDBT) in the same way that DBT is removed from fuel oil. This may be attributed to the W active sites with high electron density induced by abundant OV defects, the good dispersion and high exposure of the W sites, and the easy diffusion and contact between the substrate and active sites led by the spherical structure. The ODS reaction follows the HO˙ radical mechanism. Overall, this research presents a controllable method for constructing heterogeneous SiW12 with abundant OV defects, which could potentially expand its application in the deep ODS of fuel oils.Data availability
Data for this article, including raw graphical data and ESI files, are available at Science Date Bank at https://doi.org/10.57760/sciencedb.22886.Conflicts of interest
The authors report no declarations of interest.Acknowledgements
This research was supported by the Research Project Supported by Shanxi Scholarship Council of China (2021-121, 2023-128), the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (20240013), and the Scientific Research Start-up Funds for Introduced Doctoral Talents of Lyuliang University (UP-2024-0304442).References
- J. Jiao, X. Zhou, S. Zhao, W. Jiao and R. Wang, In situ highly dispersed loading of molybdenum dioxide with oxygen vacancies on N-doped graphene for enhanced oxidative desulfurization of fuel oil, J. Environ. Chem. Eng., 2023, 11(2), 109402 CrossRef CAS.
- J. Zou, Y. Lin and C. Yang, Covalency triggers high catalytic activity of amorphous molybdenum oxides for oxidative desulfurization, Sci. China: Chem., 2023, 66(4), 1211–1220 CrossRef CAS.
- M. Yuan, H. Yuan, S. Xun, R. Le, Y. Huang, M. He, L. Zhu, W. Zhu and H. Li, VO2 uniformly supported by 3D g-C3N4: A highly effective catalyst for deep oxidative desulfurization, Fuel, 2022, 319, 123792 CrossRef CAS.
- J. Guo, L. Chu, H. Yang, Z. Huang, M. Yang and G. Wang, Amphiphilic halloysite nanotube enclosing molybdenum oxide as nanoreactor for efficient desulfurization of model fuels, Chem. Eng. J., 2023, 451, 138595 CrossRef CAS.
- X. Zhou, J. Jiao, W. Jiao and R. Wang, Oxidative desulfurization of model oil over the bowl-shaped N-doped carbon material loaded by the defective silicotungstic acid, Sep. Purif. Technol., 2023, 310, 123180 CrossRef CAS.
- G. Ye, Y. Gu, W. Zhou, W. Xu and Y. Sun, Synthesis of Defect-Rich Titanium Terephthalate with the Assistance of Acetic Acid for Room-Temperature Oxidative Desulfurization of Fuel Oil, ACS Catal., 2020, 10(3), 2384–2394 CrossRef CAS.
- M. O. Azeez and S. A. Ganiyu, Review of biomass derived-activated carbon for production of clean fuels by adsorptive desulfurization: Insights into processes, modifications, properties, and performances, Arabian J. Chem., 2023, 16(10), 105182 CrossRef CAS.
- J. Li, X. Jiang, M. Zhu and B. Dai, Cu(I) anchoring in MOF-808 as a stable catalyst in ultra-deep oxidation desulfurization, Fuel, 2023, 341, 127674 CrossRef CAS.
- J. Wang, B. Yang, X. Peng, Y. Ding, S. Yu, F. Zhang, L. Zhang, H. Wu and J. Guo, Design and preparation of polyoxometalate-based catalyst [MIMPs]3PMo6W6O40 and its application in deep oxidative desulfurization with excellent recycle performance and low molar O/S ratio, Chem. Eng. J., 2022, 429, 132446 CrossRef CAS.
- L. Hao, L. Sun, T. Su, D. Hao, W. Liao, C. Deng, W. Ren, Y. Zhang and H. Lü, Polyoxometalate-based ionic liquid catalyst with unprecedented activity and selectivity for oxidative desulfurization of diesel in [Omim]BF4, Chem. Eng. J., 2019, 358, 419–426 CrossRef CAS.
- G. Zhang, F. Yang, W. Yang and Y. Li, N-doped carbon nanotube encapsulated nZVI as a high-performance bifunctional catalyst for oxidative desulfurization, Particuology, 2023, 81, 109–118 CrossRef CAS.
- G. Ye, Y. Sun, D. Zhang, W. Zhou, C. Lancelot, A. Rives, C. Lamonier and W. Xu, Hierarchical porous titanium terephthalate based material with highly active sites for deep oxidative desulfurization, Microporous Mesoporous Mater., 2018, 270, 241–247 CrossRef CAS.
- R. Ghubayra, C. Nuttall, S. Hodgkiss, M. Craven, E. F. Kozhevnikova and I. V. Kozhevnikov, Oxidative desulfurization of model diesel fuel catalyzed by carbon-supported heteropoly acids, Appl. Catal., B, 2019, 253, 309–316 CrossRef CAS.
- S. O. Ribeiro, D. Julião, L. Cunha-Silva, V. F. Domingues, R. Valença, J. C. Ribeiro, B. de Castro and S. S. Balula, Catalytic oxidative/extractive desulfurization of model and untreated diesel using hybrid based zinc-substituted polyoxometalates, Fuel, 2016, 166, 268–275 CrossRef CAS.
- R. Wang, G. Zhang and H. Zhao, Polyoxometalate as effective catalyst for the deep desulfurization of diesel oil, Catal. Today, 2010, 149(1–2), 117–121 CrossRef CAS.
- J. Kim, N. D. McNamara, T. H. Her and J. C. Hicks, Carbothermal reduction of Ti-modified IRMOF-3: an adaptable synthetic method to support catalytic nanoparticles on carbon, ACS Appl. Mater. Interfaces, 2013, 5(21), 11479–11487 CrossRef CAS PubMed.
- X. Li, L. Zhang and Y. Sun, Titanium-modified MIL-101(Cr) derived titanium-chromium-oxide as highly efficient oxidative desulfurization catalyst, Catalysts, 2020, 10(9), 1091 CrossRef CAS.
- L. Rivoira, M. L. Martínez, O. Anunziata and A. Beltramone, Vanadium oxide supported on mesoporous SBA-15 modified with Al and Ga as a highly active catalyst in the ODS of DBT, Microporous Mesoporous Mater., 2017, 254, 96–113 CrossRef CAS.
- X. Li, S. Huang, Q. Xu and Y. Yang, Preparation of WO3-SBA-15 mesoporous molecular sieve and its performance as an oxidative desulfurization catalyst, Transition Met. Chem., 2009, 34(8), 943–947 CrossRef CAS.
- H. Yan, Y. Xie, Y. Jiao, A. Wu, C. Tian, X. Zhang, L. Wang and H. Fu, Holey Reduced Graphene Oxide Coupled with an Mo2N–Mo2C Heterojunction for Efficient Hydrogen Evolution, Adv. Mater., 2017, 30(2), 1704156 CrossRef PubMed.
- N. A. Khan, B. N. Bhadra, S. W. Park, Y. Han and S. H. Jhung, Tungsten nitride, well-dispersed on porous carbon: remarkable catalyst, produced without addition of ammonia, for the oxidative desulfurization of liquid fuel, Small, 2020, 16(12), 1901564 CrossRef CAS PubMed.
- F. Wang, Y. Liu, Y. Lv, J. Ren, R. Wang and W. Jiao, Oxidative desulfurization of liquid fuels catalyzed by W2C@C derived from metallophthalocyanine/phosphotungstic acid composites, Sep. Purif. Technol., 2022, 281, 119953 CrossRef CAS.
- P. Zuo, Y. Liu, J. Jiao, J. Ren, R. Wang and W. Jiao, Ultrafine W2C well-dispersed on N-doped graphene: extraordinary catalyst for ultrafast oxidative desulfurization of high sulfur liquid fuels, Appl. Catal., A, 2022, 643, 118791 CrossRef CAS.
- B. N. Bhadra, M. M. H. Mondol and S. H. Jhung, Metallic cobalt-anchored carbon with non-metallic heteroatom decoration: Remarkably effective oxidative desulfurization catalyst, Sep. Purif. Technol., 2024, 330, 125425 CrossRef CAS.
- Y. Liu, X. Yin, C. Li, Z. Xie, F. Zhao, J. Li, J. Hei, Y. Han, N. Wang and P. Zuo, Defective silicotungstic acid-loaded magnetic floral N-doped carbon microspheres for ultra-fast oxidative desulfurization of high sulfur liquid fuels, Dalton Trans., 2023, 52(46), 17524–17537 RSC.
- Y. Liu, P. Zuo, R. Wang, Y. Liu and W. Jiao, Covalent immobilization of Dawson polyoxometalates on hairy particles and its catalytic properties for the oxidation desulfurization of tetrahydrothiophene, J. Cleaner Prod., 2020, 274, 122774 CrossRef CAS.
- Y. Lv, J. Jiao, R. Wang and W. Jiao, Silicotungstic acid-supported C@SiO2 nanospheres as an efficient oxidative desulfurization catalyst, Chem. Eng. Sci., 2022, 248, 117225 CrossRef CAS.
- E. A. Eseva, M. O. Lukashov, L. A. Kulikov, O. Y. Grafov, A. F. Bikbaeva and A. V. Akopyan, Transition to Carbon Materials: The Effect of Support Nature on the Catalytic Properties of Anderson-Type Polyoxometalate in Aerobic Oxidation of Sulfur-Containing Compounds, Energy Fuels, 2023, 37(22), 17461–17472 CrossRef CAS.
- Y. Zhang and R. Wang, Synthesis of silica@C-dots/phosphotungstates core-shell microsphere for effective oxidative-adsorptive desulfurization of dibenzothiophene with less oxidant, Appl. Catal., B, 2018, 234, 247–259 CrossRef CAS.
- J. Y. Qiu, F. H. Qin, B. Y. Xiao, M. T. Zhang, T. Wan, J. P. Liu, J. H. Chen and Z. Y. Huang, Surface-electronic-state-modulated, single-crystalline (001) alpha-Fe2O3 nanosheets with dual reaction sites for efficient fenton-like catalysis, Chin. J. Inorg. Chem., 2019, 35(9), 1665–1677 CAS.
- C. Mao, H. Cheng, H. Tian, H. Li, W.-J. Xiao, H. Xu, J. Zhao and L. Zhang, Visible light driven selective oxidation of amines to imines with BiOCl: does oxygen vacancy concentration matter?, Appl. Catal., B, 2018, 228, 87–96 CrossRef CAS.
- J. Zou, Y. Lin, S. Wu, Y. Zhong and C. Yang, Molybdenum dioxide nanoparticles anchored on nitrogen-doped carbon nanotubes as oxidative desulfurization catalysts: role of electron transfer in activity and reusability, Adv. Funct. Mater., 2021, 31(22), 2100442 CrossRef CAS.
- X. Chang, X. F. Yang, Y. Qiao, S. Wang, M. H. Zhang, J. Xu, D. H. Wang and X. H. Bu, Confined Heteropoly Blues in Defected Zr-MOF (Bottle Around Ship) for High-Efficiency Oxidative Desulfurization, Small, 2020, 16(14), 1906432 CrossRef CAS PubMed.
- L. Ma, P. Wei, J. Li, X. Lu and G. Li, Preparation and catalysis of defected PVMo4W7@rht-MOF-1 for oxidation desulfurization under air, Mol. Catal., 2024, 553, 113784 CrossRef CAS.
- J. Sun, X. Ma, X. Li, J. Fan, Q. Chen, X. Liu and J. Pan, Synthesis of a cationic polyacrylamide under UV initiation and its flocculation in estrone removal, Int. J. Polym. Sci., 2018, 2018, 1–11 Search PubMed.
- Y. Sun, M. Ren, C. Zhu, Y. Xu, H. Zheng, X. Xiao, H. Wu, T. Xia and Z. You, UV-initiated graft copolymerization of cationic chitosan-based flocculants for treatment of zinc phosphate-contaminated wastewater, Ind. Eng. Chem. Res., 2016, 55(38), 10025–10035 CrossRef CAS.
- J. Ma, J. Shi, H. Ding, G. Zhu, K. Fu and X. Fu, Synthesis of cationic polyacrylamide by low-pressure UV initiation for turbidity water flocculation, Chem. Eng. J., 2017, 312, 20–29 CrossRef CAS.
- Y. Yu, D. Sun, S. Wang, M. Xiao, L. Sun and Y. Meng, Heteropolyacid salt catalysts for methanol conversion to hydrocarbons and dimethyl ether: effect of reaction temperature, Catalysts, 2019, 9(4), 320 CrossRef.
- Y. Chen, H.-y. Song, Y.-z. Lu, H. Meng, C.-x. Li, Z.-g. Lei and B.-h. Chen, Unified catalytic oxidation–adsorption desulfurization process using cumene hydroperoxide as oxidant and vanadate based polyionic liquid as catalyst and sorbent, Ind. Eng. Chem. Res., 2016, 55(39), 10394–10403 CrossRef CAS.
- J. Men, R. Wang, H. Li, X. Li, S. Yang, H. Liu and B. Gao, Preparation of crosslinked poly (acryloyloxyethyltrimethyl ammonium chloride) microsphere and its adsorption and mechanism towards shikimic acid, Mater. Sci. Eng., C, 2017, 71, 167–175 CrossRef CAS PubMed.
- K. E. Lee, B. T. Poh, N. Morad and T. T. Teng, Synthesis and characterization of hydrophobically modified cationic acrylamide copolymer, Int. J. Polym. Anal. Charact., 2008, 13(2), 95–107 CrossRef CAS.
- H.-F. Pang, X. Xiang, Z.-J. Li, Y.-Q. Fu and X.-T. Zu, Hydrothermal synthesis and optical properties of hexagonal tungsten oxide nanocrystals assisted by ammonium tartrate, Phys. Status Solidi A, 2012, 209(3), 537–544 CrossRef CAS.
- Y.-L. Zou, H.-Y. Li, W. Zhou, X.-G. Cui, G.-H. Zou and G.-Z. Shen, Introduction of the antibacterial drugs norfloxacin and Ciprofloxacin into a polyoxometalate structure: Synthesis, characterization, and antibacterial activity, J. Mol. Struct., 2020, 1205, 127584 CrossRef CAS.
- Z. Qi, Z. Huang, H. Wang, L. Li, C. Ye and T. Qiu, In situ bridging encapsulation of a carboxyl-functionalized phosphotungstic acid ionic liquid in UiO-66: a remarkable catalyst for oxidative desulfurization, Chem. Eng. Sci., 2020, 225, 115818 CrossRef CAS.
- S. Wang, X. Chen, Y. Tan, Z. Zhao, W. Yang, C. Huang, X. Wang, M. Chen, Z. Du and Y. Ding, Highly efficient synthesis of isoprene from methyl tert-butyl ether and formaldehyde over activated carbon supported silicotungstic acid catalysts, Mol. Catal., 2020, 485, 110840 CrossRef CAS.
- P. Wu, J. Yi, L. Feng, X. Li, Y. Chen, Z. Liu, S. Tian, S. Li, S. Khan and Y. Sun, Microwave assisted preparation and characterization of a chitosan based flocculant for the application and evaluation of sludge flocculation and dewatering, Int. J. Biol. Macromol., 2020, 155, 708–720 CrossRef CAS PubMed.
- N. Zhang, T. Zhou, M. Chen, H. Feng, R. Yuan, C. . a. Zhong, W. Yan, Y. Tian, X. Wu, W. Chu, C. Wu and Y. Xie, High-purity pyrrole-type FeN4 sites as a superior oxygen reduction electrocatalyst, Energy Environ. Sci., 2020, 13(1), 111–118 RSC.
- T. Wang, X. Tang, S. Zhang, J. Zheng, H. Zheng and L. Fang, Roles of functional microbial flocculant in dyeing wastewater treatment: Bridging and adsorption, J. Hazard. Mater., 2020, 384, 121506 CrossRef CAS PubMed.
- K. Ye, K. Li, Y. Lu, Z. Guo, N. Ni, H. Liu, Y. Huang, H. Ji and P. Wang, An overview of advanced methods for the characterization of oxygen vacancies in materials, TrAC, Trends Anal. Chem., 2019, 116, 102–108 CrossRef CAS.
- Y. Ma, A. Li, C. Wang and X. Ge, Preparation of HPW@UiO-66 catalyst with defects and its application in oxidative desulfurization, Chem. Eng. J., 2021, 404, 127062 CrossRef CAS.
- A. Rajendran, H.-X. Fan, T.-Y. Cui, J. Feng and W.-Y. Li, Enrichment of polymeric WOx species in WOx@SnO2 catalysts for ultra-deep oxidative desulfurization of liquid fuels, Fuel, 2021, 290, 120036 CrossRef CAS.
- Y. Du, L. Zhou, Z. Liu and L. Yang, Polyoxometalate-based 3DOM ZrO2 material for deep oxidative desulfurization of DBT with ultrahigh stability, J. Porous Mater., 2020, 28(1), 109–116 CrossRef.
- Z. Ren, Q. Yuan, C. Dai and L. Zhu, Experimental and theoretical density functional theory approaches for desulfurization of dibenzothiophene from diesel fuel with imidazole-based heteropolyacid catalysts, ACS Omega, 2023, 8(6), 5593–5606 CrossRef CAS PubMed.
- C. A. Virtudazo-Ligaray, M. D. G. de Luna, A. E. S. Choi and M. C. Lu, Novel cerium–tungsten–titanium-based catalyst for an efficient oxidative desulfurization material in fuel oil under a laboratory-scale setup, Clean Technol. Environ. Policy, 2023, 26(4), 1135–1148 CrossRef.
- M. Craven, D. Xiao, C. Kunstmann-Olsen, E. F. Kozhevnikova, F. Blanc, A. Steiner and I. V. Kozhevnikov, Oxidative desulfurization of diesel fuel catalyzed by polyoxometalate immobilized on phosphazene-functionalized silica, Appl. Catal., B, 2018, 231, 82–91 CrossRef CAS.
- I. Shafiq, S. Shafique, P. Akhter, M. Ishaq, W. Yang and M. Hussain, Recent breakthroughs in deep aerobic oxidative desulfurization of petroleum refinery products, J. Cleaner Prod., 2021, 294, 125731 CrossRef CAS.
- W. Jiang, H. Jia, H. Li, L. Zhu, R. Tao, W. Zhu, H. Li and S. Dai, Boric acid-based ternary deep eutectic solvent for extraction and oxidative desulfurization of diesel fuel, Green Chem., 2019, 21(11), 3074–3080 RSC.
- M. Y. Masoomi, M. Bagheri and A. Morsali, Application of two cobalt-based metal-organic frameworks as oxidative desulfurization catalysts, Inorg. Chem., 2015, 54(23), 11269–11275 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02085a |
| This journal is © The Royal Society of Chemistry 2025 |