Open Access Article
Open Access ArticlePhoto-induced decarboxylative radical cascade cyclization of unactivated alkenes: access to CF- and CF2-substituted ring-fused imidazoles†
Huinan Wang‡
a,
Shengbao Lin‡a,
Hui Honga,
Zhangjie Hua,
Yawen Huanga,
Xiaolan Zhanga,
Sheng-Nan Lin *a and
Bin-Miao Yang*b
*a and
Bin-Miao Yang*b
aCollege of Chemistry and Environment Science, Shangrao Normal University, Shangrao 334001, China. E-mail: 307192@sru.edu.cn
bThe International Joint Institute of Tianjin University, Fuzhou, Tianjin University, Tianjin 300072, China. E-mail: bmyang@tjufz.org.cn
First published on 22nd April 2025
Abstract
A mild and effective visible-light-induced decarboxylative radical cascade reaction of olefin-containing imidazoles with α-fluorinated carboxylic acids as building blocks containing CF or ArCF2 moieties, has been developed to afford a series of monofluoromethylated or aryldifluoromethylated polycyclic imidazoles in medium to excellent yields with features of simple operation, available raw materials, and wide substrate scopes. In addition, the mechanistic experiments indicated that the methodology involved a radical pathway.
Introduction
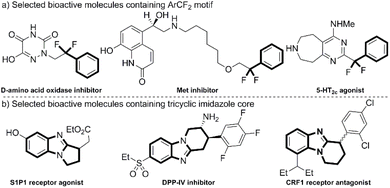
Fluorine-containing moieties, owing to the existence of the most electronegative element, could significantly alter the physicochemical properties and biological activities of parent molecules.1 Synthetic methods for fluorinated compounds are consistently in high demand in the fields of pharmaceutical and agricultural chemistry.2 Among various fluorine-containing groups, the difluoromethylene moiety (CF2), which can serve as a bioisoster of ethereal oxygen or carbonyl groups,3 has been regarded as a valuable candidate substituent group in the process of drug discovery.4 Consequently, the methods for synthesizing CF2-substituted compounds have been well developed.5 Notably, the attractive benzylic difluoromethylene group (ArCF2) has been widely identified in bioactive molecules (Fig. 1a).6 Alongside the development of attractive methods for direct construction of the ArCF2 moiety, such as the direct deoxygenative fluorination of aldehydes or ketones,7 transition-metal-catalyzed difluoroalkylation of arenes,8 visible-light-promoted difluoroalkylation of arenes,9 and direct fluorination of benzylic C–H bonds,10 a number of effective protocols for direct incorporation of external ArCF2 groups into parent skeletons have also been established in recent years.11 Nowadays, α,α-difluoroarylacetic acids have been recognized as general and effective aryldifluoromethyl radical precursors due to their beneficial features, including stability, the generation of CO2 as a byproduct, and easy accessibility.12 To incorporate ArCF2 moieties into diverse bioactive frameworks, the development of decarboxylative radical aryldifluoromethylation reactions, which often involve heat-promoted oxidation13 or a photo-induced process,14 has become one of the hotspots in the field of fluorine chemistry in the last decade. In addition, significant progress has been made in the construction of C–CF bonds in recent years.15 Furthermore, methods for synthesizing CF-substituted compounds via decarboxylative radical monofluoromethylation using α-monofluorinated carboxylic acids have also been reported.16Nitrogen-containing heterocyclic moieties exist in numerous bioactive molecules.17 Among them, the benzimidazole-fused polycyclic scaffolds are frequently encountered.18 In particular, the tricyclic benzimidazole skeletons have attracted extensive attention in the fields of synthetic and pharmaceutical chemistry (Fig. 1b).19 Therefore, various methods for constructing polycyclic benzimidazole skeletons have been well-developed.20 Among these strategies, the direct cyclization of substituted benzimidazoles with alkenes, which simultaneously incorporates functional groups into molecules and constructs complex heterocyclic skeletons in a single-step reaction, has been considered as a convenient and atom-economical approach to access ring-fused benzimidazole derivatives with promising potential.21 Despite huge efforts devoted to synthesizing functionalized polycyclic benzimidazoles through radical tandem reactions of N-alkenoxyl 2-aryl benzimidazoles,22 studies on the intramolecular cyclization of substituted imidazoles with olefins at the C-2 position to afford ring-fused imidazoles have continued to attract more and more interest in recent years, including transition-metal-catalyzed cross-coupling,23 and cascade radical cyclization.24 In 2021, Li and co-workers described a direct radical cyclization of imidazoles with olefins to afford difluoroalkylated polycyclic imidazoles using BrCF2COR as a radical source (Scheme 1a).25 Subsequently, Chen,26 Li,27 and Jin28 independently disclosed a series of interesting works on radical cascade cyclization of imidazoles with olefins to synthesize CF3/HCF2-substituted tricyclic benzimidazoles using CF3/HCF2SO2Na (Scheme 1b-d). Compared to the preparation of CF3-substituted polycyclic imidazoles,26–29 the introduction of ArCF2 groups into ring-fused imidazoles remains largely unexplored. Given the biological activities of benzimidazole core and aryldifluoromethyl group, it makes sense to incorporate ArCF2 motif into polycyclic benzimidazole skeletons. In 2025, Li's group reported a protocol for introducing ArCF2 groups to synthesize aryldifluoromethylated polycyclic imidazoles (Scheme 1e).30 Nevertheless, Li's work exhibited several limitations, including limited substrates, unexplored scope of aryldifluoroacetic acids, low yields, excessive amounts of fluorine sources, and ambiguities in the proposed photochemical mechanistic pathway. During the same period, motivated by ongoing interest in photo-induced synthesis of fluorinated heterocyclic compounds,31 we attempted to develop the protocol to synthesize CF/ArCF2-substituted polycyclic imidazoles through visible-light-promoted decarboxylative radical cascade cyclization of olefin-containing imidazoles with corresponding ɑ-fluorinated carboxylatic acids, simultaneously constructing C(sp3)–CF or C(sp3)–CF2Ar bonds (Scheme 1f).
Results and discussion
To evaluate the reaction conditions, 1-(pent-4-en-1-yl)-1H-benzo[d]imidazole (1a) and 2,2-difluoro-2-phenylacetic acid (2b) were chosen as model substrates (Table 1, details in the ESI†). Initially, PhI(OAc)2 was employed as an oxidant, and the reaction was conducted in THF with the irradiation of 405 nm LEDs (10w) at ambient temperature under a nitrogen atmosphere, generating the expected product 3aa in 56% isolated yield (Table 1, entry 1). Then, other oxidants were investigated, such as PhI(OCOCF3)2 and (NH4)2S2O8, but no reaction was observed (Table 1, entries 2 and 3). Subsequently, we screened several common solvents, including DMSO, MeCN, DCM, and so on, but unsatisfactory results were demonstrated (Table 1, entries 4–9). Interestingly, a mixed solvent systerm of THF and H2O proved equally suitable for the template reaction (Table 1, entry 10). Employing KHCO3 as a base failed to enhance the reaction efficiency (Table 1, entry 11). Fortunately, when we decreased the concentration of 1a, the isolated yield of 3aa increased to 80% (Table 1, entry 12), and a 12 hours irradiation period was adequate to achieve complete conversion of the substrate (Table 1, entry 13). However, the addition of FeCl2 did not further improve the efficiency of reaction (Table 1, entry 14). Additionally, the reaction failed to ignited in the absence of either PhI(OAc)2 or light irradiation, highlighting the indispensable roles of both PhI(OAc)2 and light irradiation (Table 1, entries 15 and 16). Therefore, the optimized reaction conditions were determined as follows: the reaction mixture of 1a (0.2 mmol), 2a (0.4 mmol), and PhI(OAc)2 (0.6 mmol) in THF (2 mL) was exposed to 405 nm blue LEDs (10 W) at room temperature for 12 h.| Entry | Oxidant | Solvent | Yieldb (%) |
|---|---|---|---|
a Reaction conditions: 1a (0.2 mmol), 2a (0.6 mmol) and oxidant (0.6 mmol) in solvent (2 mL) irradiated with 405 nm 10 W blue LEDs at room temperature for 12 h under a N2 atmosphere n.r. no reaction.b Isolated yields.c In THF-H2O (2 mL, v/v, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).d KHCO3 (0.6 mmol) as additive.e 2a (0.4 mmol), 19F NMR yield are given in parentheses with (trifluoromethoxy)benzene as the internal standard.f 2a (0.4 mmol) 16 h.g 2a (0.4 mmol) and FeCl2 (0.02 mmol) as additive.h No irradiation. 1).d KHCO3 (0.6 mmol) as additive.e 2a (0.4 mmol), 19F NMR yield are given in parentheses with (trifluoromethoxy)benzene as the internal standard.f 2a (0.4 mmol) 16 h.g 2a (0.4 mmol) and FeCl2 (0.02 mmol) as additive.h No irradiation. |
|||
| 1 | PhI(OAc)2 | THF | 5.3 |
| 2 | PIFA | THF | n.r. |
| 3 | (NH4)2S2O8 | THF | n.r. |
| 4 | PhI(OAc)2 | DMSO | Trace |
| 5 | PhI(OAc)2 | DMAc | 11 |
| 6 | PhI(OAc)2 | CH3CN | n.r. |
| 7 | PhI(OAc)2 | Et2O | 9 |
| 8 | PhI(OAc)2 | DCM | n.r. |
| 9 | PhI(OAc)2 | Toluene | 20 |
| 10c | PhI(OAc)2 | THF-H2O | 48 |
| 11d | PhI(OAc)2 | THF | 54 |
| 12e | PhI(OAc)2 | THF | 80(89) |
| 13f | PhI(OAc)2 | THF | 78 |
| 14g | PhI(OAc)2 | THF | 79 |
| 15h | PhI(OAc)2 | THF | n.r. |
| 16 | — | THF | n.r. |
Having established the optimal reaction conditions, we then explored the scope of this oxidative decarboxylative aryldifluoromethylation/cyclization reaction with respect to substituted N-alkenyl benzimidazoles. As shown in Table 2, a variety of substituted N-alkenyl benzimidazoles with electron-withdrawing groups (F, Cl, Br, CF3, COCH3 and COOMe) or electron-donating groups (OMe and Me) at different positions of phenyl ring successfully underwent the decarboxylative cascade cyclization reaction, yielding the corresponding products 3aa–oa in 67–86% yields. Pleasingly, the substrates with halogen atoms (F, Cl and Br) at different positions of aryl group proved to be well-tolerated under the standard conditions, smoothly affording the desired products in moderate yields (3ba–ga, 71–79%), which enable following modifications. It is worth mentioning that the substrate with electron-withdrawing groups (COCH3) exhibited higher reactivity than the model substrate under the optimized conditions, generating the target product 3ia in up to 86% yield. The sterically hindered substrate with a methyl group at the 4- position of benzene ring underwent the cascade cyclization reaction smoothly, affording the corresponding product 3la with a yield of 72%, indicating that steric hindrance has a negligible effect on this transformation. Subsequently, the 5,6-disubstituted substrates with dimethyl or dihalogen demonstrated good compatibility, and were transformed into the expected products in moderate yields (3pa–ra, 70–85%).
When it comes to the substrates containing 4- or 7-azobenzimidazole core (1s and 1t), the desired products (3sa and 3ta) were generated in 66% and 91% yields, respectively. We then turned our attention to the modification of olefin motif. To our delight, N-but-3-en-1-yl benzimidazole 1u successfully performed the tandem reaction, forming the five-membered fused product 3ua in a considerable yield of 62%. Moreover, we successfully constructed seven-membered cyclized benzimidazole 3va with an acceptable yield of 57%. Considering the effect of radical stability, we installed methyl group into olefin motif. However, the relative product 3wa was obtained with a slightly decreased yield of 78%. Furthermore, when N-alkenoxyl substrate 1x was employed, the corresponding product 3xa was obtained in reasonable yield of 71%. Then, we focused on imidazole-derived substrates, and found that the desired bicyclic aryldifluoromethylated imidazoles could be obtained in moderate to good yields (3ya, 85%; 3za 62%; 3a1, 70%; 3a2, 79%; 3a3, 67%). These results suggested that substrates with electron-donating groups on imidazole ring were generally more reactive than those with electron-withdrawing groups (3ya, 3a2 vs. 3za, 3a1). Finally, to our disappointment, the anticipated product 3a4 could not be obtained, indicating that the N-alkenyl indole 1a4 was unsuitable for this transformation. To our disappointment, the anticipated product 3a4 could not be obtained, indicating that the N-alkenyl indole 1a4 was unsuitable for this transformation. Finally, when 3-methyl substituted N-alkenyl indole 1a5 was employed for this transformation, the reaction could not proceed smoothly under the present conditions, leading to obvious raw material remaining, and indicating that the indole core was probably incompatible with this protocol.
Afterwards, we investigated the scope of this reaction with respect to substituted aryldifluoroacetic acids. As revealed in Table 3, a broad range of aryldifluoroacetic acids 2b–m, containing electron-donating (Ph, Me, OMe) or electron-withdrawing groups (F, Br, CN) at different positions of the phenyl ring, were well tolerated under the standard conditions, affording the corresponding products 3ab–am in yields of 71–84%. Comparing substituents at the same positions of the aromatic ring, we found that the reactivities of substrates with electron-donating groups (Ph, Me, OMe) were higher than those with electron-withdrawing groups (F, Br, CN) in reaction (3ab, 3ac, 3ad vs. 3ae, 3af, 3ag; 3ah, 3ai vs. 3aj; 3ak, 3al vs. 3am). Moreover, this protocol exhibited good tolerance to halogens (F and Br), and the corresponding products were obtained in moderate yields, offering potential for further modifications (3ae, 75%; 3af, 71%; 3aj, 74%; 3am, 72%). To our delight, when α,α-difluoropropanoic acid (MeCF2COOH, 2n) was used as a representative example of aliphatic difluoroacetic acids, the preparation of product 3an proceeded smoothly under the standard conditions with a satisfactory yield of 72%. Remarkably, the difluoroacetic acid (HCF2COOH, 2o) also exhibited good compatibility with this cascade cyclization reaction, generating the product 3ao in 66% yield. To further extend the application of this photo-induced decarboxylative cascade cyclization reaction to α-fluorinated carboxylatic acids, we found that aliphatic α-monofluoroacetic acids, such as Me2CFCO2H (2p), MeCHFCO2H (2q), and 1-(tert-butoxycarbonyl)-4-fluoropiperidine-4-carboxylic acid (2r), were effectively transformed into the corresponding products 3ap, 3aq, and 3ar, which were obtained in 63%, 58%, and 41% yields, respectively. Regretfully, when we investigated PhCHFCOOH (2s), the desired reaction did not proceed with unreacted starting materials, indicating that α-fluorobenzeneacetic acid was incompatible with this protocol, possibly because the corresponding stable radical intermediate could not be continuously generated under the standard experimental conditions. Moreover, when we investigated CF3COOH (2t), which has high oxidation potential, the reaction could not proceed smoothly under the standard conditions, likely due to difficulty in forming the stable radical intermediate.
To broaden the application of this strategy, we explored the construction of PhCF2-substituted benzimidazole-dihydroisoquinoline skeleton via a photo-induced decarboxylative radical cascade reaction. Encouragingly, the ring-fused tetracyclic product (5) was successfully prepared from N-alkenyl 2-phenyl benzimidazole (4) under the optimized conditions with a synthetically useful yield of 78% (Scheme 2). Additionally, to explore the potential synthetic utility of this protocol, a gram-scale experiment was carried out, demonstrating an isolated yield of 77% (1.44 g, details in the ESI†), and confirming the appropriate suitability for large-scale preparation of PhCF2-substituted ring-fused imidazoles.
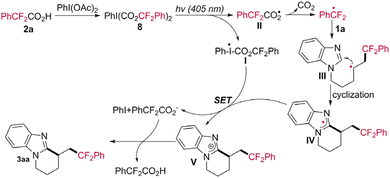
To investigate the mechanism of this protocol, several control experiments were conducted under the standard conditions. Initially, the template reaction was remarkably suppressed by the addition of 2,2,6,6-tetramethyl-piperidin-1-oxyl (TEMPO, 3.0 equiv.) as a radical scavenger. Only a trace amount of 3aa was detected, and radical-trapping adduct TEMPO-CF2Ar (6) was identified by high-resolution mass spectrometry (HRMS) (Scheme 3a), indicating significant inhibition of the transformation into 3aa. Moreover, the conversion from 1a to 3aa was completely inhibited upon the addition of 1,1-diphenylethylene as a radical scavenger (3.0 equiv.), resulting in the absence of 3aa and the detection of compound 7 by HRMS (Scheme 3b). The results of radical-trapping experiments showed that the cascade cyclizaition reaction might be relative to a free radical process. Furthermore, the reaction of 1a with phenyl-λ3-iodanediyl bis(2,2-difluoro-2-phenylacetate) (8) proceeded smoothly to deliver 3aa in an isolated yield of 71% under the standard conditions, indicating that PhI(OCOCF2Ph)2 (8) is probably the key intermediate in this conversion process (Scheme 3c). To display the role of visible-light irradiation in this transformation, an On/Off light-illumination experiment was performed. The results showed that continuous illumination is essential for the reaction to proceed (Scheme 3d).
Based on the above mechanistic studies as well as previous related literature,14 a plausible reaction pathway is proposed in Scheme 4 (taking the reaction of 1a with 2a as an example). Initially, the intermediate PhI(OCOCF2Ph)2 (8) is formed by ligand exchange between PhI(OAc)2 and PhCF2COOH (2a), simultaneously genenerating an oxygen-centred radical intermediate II and an idoine-centred radical intermediate I through homolysis of the C–I bond under the irradiation of 405 nm visible light. The radical intermediate II undergoes a decarboxylative process to generate a PhCF2 radical, which then attacks the terminal alkenyl moiety of substrate 1a to afford carbon-centred radical intermediate III. Subsequently, intramolecular radical cyclization of intermediate III occurs to form intermediate IV, which is oxidized by intermediate I through a single-electron transfer (SET) process, leading to the cationic intermediate V. Finally, deprotonation and rearomation of intermediate V take place, yielding the target product 3aa.
Conclusions
In summary, we developed an efficient and simple protocol for photo-induced decarboxylative radical coupling of unactivated olefin-containing imidazoles with α-fluorinated carboxylic acids, affording CF- or ArCF2-substituted ring-fused imidazoles in moderate to good yields. It was noteworthy that this practical methodology has several beneficial merits, including simple operation, readily available starting materials, and good functional group compatibility. Moreover, mechanistic experiments confirmed that this photo-induced decarboxylative radical cascade reaction proceeds via a radical pathway.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
H. Wang and S. Lin performed the experiments, compound characterization and data analysis. H. Hong, Z. Hu, Y. Huang and X. Zhang synthesized the raw materials. S.-N. Lin and B.-M. Yang finalized the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of China (22201205).References
- For selected reviews: (a) P. Jeschke, Pest Manag. Sci., 2024, 80, 3065–3087 CrossRef CAS PubMed; (b) G. Chandra, D. V. Singh, G. K. Mahato and S. Patel, Chem. Pap., 2023, 77, 4085–4106 CrossRef CAS PubMed; (c) C. Zhang, ACS Omega, 2022, 7, 18206–18212 CrossRef CAS PubMed; (d) O. Kamble, R. Dandela and S. Shinde, Curr. Org. Chem., 2021, 25, 2650–2665 CrossRef CAS.
- For selected reviews: (a) X. Yin, X. wang, L. Song, J. Zhang and X. Wang, Molecules, 2024, 29, 3677 CrossRef CAS PubMed; (b) D. Lin, M. Coe, V. Krishnamurti, X. Ispizua-Rodriguez and G. K. Surya Prakash, Chem. Rec., 2023, 23, e202300104 CrossRef CAS PubMed; (c) Purushotam, A. Bera and D. Banerjee, Org. Biomol. Chem., 2023, 21, 9298–9315 RSC; (d) P. L. Kalar, S. Agrawal, S. Kushwaha, S. Gayen and K. Das, Curr. Org. Chem., 2023, 27, 190–205 CrossRef CAS; (e) T. T. Bui and H.-K. Kim, Chem.–Asian J., 2021, 16, 2155–2167 CrossRef CAS PubMed.
- M. Chen, Y. Cui, X. Chen, R. Shang and X. Zhang, Nat. Commun., 2024, 15, 419–428 CrossRef CAS PubMed.
- For selected reviews: (a) C. Lu, Y. Song, L. Gao and Y. Wang, Org. Biomol. Chem., 2024, 22, 8700–8713 RSC; (b) S. Ali and J. Zhou, Eur. J. Med. Chem., 2023, 256, 115476–115488 CrossRef CAS PubMed; (c) C. Rao, T. Zhang and H. Huang, Nat. Commun., 2024, 15, 7924 CrossRef CAS PubMed.
- For selected review: (a) L.-J. Tang, Y. Wen, H.-X. Jin, W. Luo, L. Long, J. Shen, H. Luo and D. Yu, Org. Chem. Front., 2025, 12, 2052–2075 RSC; (b) L. Shi, D. An and G.-J. Mei, Org. Chem. Front., 2022, 9, 4192–4208 RSC; (c) J. Feng, X. Jia, S. Zhang, K. Lu and D. Cahard, Org. Chem. Front., 2022, 9, 3598–3623 RSC; (d) D.-Q. Dong, H. Yang, J.-L. Shi, W.-J. Si, Z.-L. Wang and X.-M. Xu, Org. Chem. Front., 2020, 7, 2538–2575 RSC; (e) E. Oftadeh, M. J. Wong, J. Yu, X. Li, Y. Cao, F. Gallou, L. Heinz and B. H. Lipshutz, J. Org. Chem., 2024, 89, 17331–17337 CrossRef CAS PubMed; (f) E. Miller, S. Kim, K. Gibson, J. S. Derrick and F. D. Toste, J. Am. Chem. Soc., 2020, 142, 8946–8952 CrossRef CAS PubMed; (g) C. Xu, Z.-F. Yang, L. An and X. Zhang, ACS Catal., 2019, 9, 8224–8229 CrossRef CAS.
- (a) M. Nambo, J. C.-H. Yim, L. B. O. Freitas, Y. Tahara, Z. T. Ariki, Y. Maekawa, D. Yokogawa and C. M. Crudden, Nat. Commun., 2019, 10, 4528 CrossRef PubMed; (b) N. Hin, B. Duvall, D. Ferraris, J. Alt, A. G. Thomas, R. Rais, C. Rojas, Y. Wu, K. M. Wozniak, B. S. Slusher and T. Tsukamoto, J. Med. Chem., 2015, 58, 7258–7272 CrossRef CAS PubMed; (c) P. Montuschi and G. Ciabattoni, J. Med. Chem., 2015, 58, 4131–4164 CrossRef CAS PubMed.
- (a) P. Bhattarai, S. Koley, K. Goel and R. A. Altman, Chem. Commun., 2025, 61, 2524 RSC; (b) E. Mao, C. N. Prieto Kullmer, H. A. Sakai and D. W. C. MacMillan, J. Am. Chem. Soc., 2024, 146, 5067–5073 CrossRef CAS PubMed; (c) Y. Li, K. A. Wheeler and R. Dembinski, Org. Biomol. Chem., 2012, 10, 2395–2408 RSC; (d) A. Khalaf, D. Grée, H. Abdaliah, N. Jaber, A. Hachem and R. Grée, Tetrahedron Lett., 2010, 51, 2413–2415 CrossRef.
- For selected review: (a) Z. Feng, Y.-L. Xiao and X. Zhang, Acc. Chem. Res., 2018, 51, 2264–2278 CrossRef CAS PubMed; (b) Y.-C. Luo, F.-F. Tong, Y. Zhang, C.-Y. He and X. Zhang, J. Am. Chem. Soc., 2021, 143, 13971–13979 CrossRef CAS PubMed; (c) Y.-L. Xiao, Q.-Q. Min, C. Xu, R.-W. Wang and X. Zhang, Angew. Chem., Int. Ed., 2016, 55, 5837–5841 CrossRef CAS PubMed; (d) Q.-Q. Min, Z. Yin, Z. Feng, W.-H. Guo and X. Zhang, J. Am. Chem. Soc., 2014, 136, 1230–1233 CrossRef CAS PubMed.
- (a) X.-L. Zhu, H. Wang, C.-K. Zhai and W. He, Org. Biomol. Chem., 2024, 22, 720–724 RSC; (b) Y. Cheng, X. Zhang, G. An, G. Li and Z. Yang, Chin. Chem. Lett., 2023, 34, 107625 CrossRef CAS.
- (a) F. Rasheed, J. Shi, T. Zeng, Y. Krishna, D. Fishlock and A. Orellana, Org. Lett., 2023, 25, 8628–8633 CrossRef CAS PubMed; (b) J.-B. Xia, C. Zhu and C. Chen, J. Am. Chem. Soc., 2013, 135, 17494–17500 CrossRef CAS PubMed.
- (a) J. Huang, L. Fu, Z. Tang, X. Ma, X. Zhao and D. Zhao, Chin. Chem. Lett., 2024, 110505 CrossRef; (b) J. Wang, Q. Zhou, L. Zhou and Z. Zhang, ACS Catal., 2024, 14, 18499–18506 CrossRef CAS; (c) C. Guo, X. Han, X. Li, Z. Diao, X. Li and Y. Dong, Asian J. Org. Chem., 2022, 11, e202100663 CrossRef CAS.
- For selected reviews: (a) Y. Zhu, T. Xiao, D. Xia and W. Yang, Chin. J. Org. Chem., 2022, 42, 4067–4077 CrossRef CAS; (b) W. Mei, Y. Kong and G. Yan, Org. Chem. Front., 2021, 8, 5516–5530 RSC.
- (a) Q. Sun, H. Li, X. Chen, J. Hao, H. Deng and H. Jiang, Molecules, 2023, 28, 3578–3591 CrossRef CAS PubMed; (b) J.-W. Yuan, M.-Y. Zhang, Y. Liu, W.-Y. Hu, L.-R. Yang, Y.-M. Xiao, X.-Q. Diao, S.-R. Zhang and J. Mao, Org. Biomol. Chem., 2022, 20, 9722–9733 RSC; (c) F. Zhao, S. Guo, Y. Zhang, T. Sun, B. Yang, Y. Ye and K. Sun, Org. Chem. Front., 2021, 8, 6895–6900 RSC; (d) Z. Chen, X. Bai, J. Sun and Y. Xu, J. Org. Chem., 2020, 85, 7674–7682 CrossRef CAS PubMed.
- (a) Z. Song, L. Guo, C. Yang and W. Xia, Org. Chem. Front., 2024, 11, 4436–4441 RSC; (b) A. Gutiérrez-Bonet and W. Liu, Org. Lett., 2023, 25, 483–487 CrossRef PubMed; (c) J. Yuan, L. Shen, N. Guo, Y. Yin, P. Yang, L. Yang, Y. Xiao and S. Zhang, J. Org. Chem., 2023, 88, 16598–16608 CrossRef CAS PubMed; (d) C. Guo, X. Han, Y. Feng, Z. Liu, Y. Li, H. Liu, L. Zhang, Y. Dong and X. Li, J. Org. Chem., 2022, 87, 9232–9241 CrossRef CAS PubMed; (e) Q.-W. Gui, F. Teng, H. Yang, C. Xun, W.-J. Huang, Z.-Q. Lu, M.-X. Zhu, W.-T. Ouyang and W.-M. He, Chem. - Asian J., 2022, 17, e202101139 CrossRef CAS PubMed.
- For selected reviews: (a) B.-J. Li, Y.-L. Ruan, L. Zhu, J. Zhou and J.-S. Yu, Chem. Commun., 2024, 60, 12302–12314 RSC; (b) M. Y. Moskalik, Int. J. Mol. Sci., 2023, 24, 17593 CrossRef CAS PubMed; (c) T. W. Butcher, W. M. Amberg and J. F. Hartwig, Angew. Chem., Int. Ed., 2022, 61, e202112251 CrossRef CAS PubMed; (d) C. Sun, Q. Zhou, C.-Y. Li, Z.-W. Hou and L. Wang, Org. Lett., 2024, 26, 883–888 CrossRef CAS PubMed; (e) T. Zeng, C. Huang, Y. Zhang, Y. Luo and D. Niu, Green Chem., 2024, 26, 8701–8705 RSC; (f) R. Wang, J. Xu, J.-X. Li, B.-B. Wu, R.-X. Jin, Y.-X. Bi and X.-S. Wang, Chin. Chem. Lett., 2023, 34, 108490 CrossRef CAS; (g) J.-D. Hamel and J.-F. Paquin, Chem. Commun., 2018, 54, 10224–10239 RSC.
- (a) F. Pasca, Y. Gelato, M. Andresini, D. Serbetci, P. Natho, G. Romanazzi, L. Degennaro, M. Colella and R. Luisi, JACS Au, 2025, 5, 684–692 CrossRef CAS PubMed; (b) H. Wang, C.-F. Liu, Z. Song, M. Yuan, Y. A. Ho, O. Gutierrez and M. J. Koh, ACS Catal., 2020, 10, 4451–4459 CrossRef CAS.
- For selected reviews: (a) G. Wu, W. Wang, F. Li, C. Xu, Y. Zhou, Z. Li, B. Liu, L. Shao, D. Chen, S. Bai and Z. Wang, Molecules, 2024, 29, 5155 CrossRef CAS PubMed; (b) S. Majee, Shilpa, M. Sarav, B. K. Banik and D. Ray, Pharmaceuticals, 2023, 16, 873 CrossRef CAS PubMed.
- For selected reviews: (a) S. Venugopal, B. Kaur, A. Verma, P. Wadhwa, M. Magan, S. Hudda and V. Kakoty, Chem. Biol. Drug Des., 2023, 102, 357–376 CrossRef CAS PubMed; (b) N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS PubMed.
- (a) T. Kojima, M. Mochizuki, T. Takai, Y. Hoashi, S. Morimoto, M. Seto, M. Nakamura, K. Kobayashi, Y. Sako, M. Tanaka, N. Kanzaki, Y. Kosugi, T. Yano and K. Aso, Bioorg. Med. Chem., 2018, 26, 2229–2250 CrossRef CAS PubMed; (b) I. Beltran-Hortelano, V. Alcolea, M. Font and S. Pérez-Silanes, Eur. J. Med. Chem., 2020, 206, 112692–112704 CrossRef CAS PubMed; (c) S. D. Edmondson, A. Mastracchio, J. M. Cox, G. J. Eiermann, H. He, K. A. Lyons, R. A. Patel, S. B. Patel, A. Petrov, G. Scapin, J. K. Wu, S. Xu, B. Zhu, N. A. Thornberry, R. S. Roy and A. E. Weber, Bioorg. Med. Chem. Lett., 2009, 19, 4097–4101 CrossRef CAS PubMed.
- For selected reviews: (a) A. E. Alami, H. Sdassi and S. Bouzikri, Synth. Commun., 2024, 54, 613–635 CrossRef; (b) N. T. Chung, V. C. Dung and D. X. Duc, RSC Adv., 2023, 13, 32734–32771 RSC; (c) D. J. Twardy, K. A. Wheeler, B. Török and R. Dembinski, Molecules, 2022, 27, 2062–2078 CrossRef CAS PubMed; (d) M. Sweeney, D. Conboy, S. I. Mirallai and F. Aldabbagh, Molecules, 2021, 26, 2684–2712 CrossRef CAS PubMed.
- For selected reviews: (a) P. V. Kumar, K. Aravindraj, G. Madhumitha and S. M. Roopan, Curr. Org. Chem., 2023, 27, 997–1009 CrossRef CAS; (b) H. Liu, L. Wang and J.-T. Yu, Asian J. Org. Chem., 2023, 12, e202300101 CrossRef CAS.
- (a) Y. Xu, C.-J. Wang, C. Lv, J. Wang, Q. Zhang, J. Wang, R.-P. Shen, B. Sun and C. Jin, New J. Chem., 2024, 48, 14684–14689 RSC; (b) K. Sui, Y. Leng and Y. Wu, ACS Omega, 2023, 8, 7517–7528 CrossRef CAS PubMed; (c) P. Meher, R. K. Samanta, S. Manna and S. Murarka, Chem. Commun., 2023, 59, 6092–6095 RSC; (d) D. Chen, X. Yang, D. Wang, Y. Li, L. Shi and D. Liang, Org. Chem. Front., 2023, 10, 2482–2490 RSC; (e) G. C. Upreti, T. Singh, K. Khanna and A. Singh, J. Org. Chem., 2023, 88, 4422–4433 CrossRef CAS PubMed; (f) J.-Z. Li, L. Mei, X.-E. Cai, C.-C. Zhang, T.-T. Cao, X.-J. Huang, Y.-L. Liu and W.-T. Wei, Adv. Synth. Catal., 2022, 364, 2080–2085 CrossRef CAS; (g) H.-C. Li, K. Sun, X. Li, S.-Y. Wang, X.-L. Chen, S.-Q. He, L.-B. Qu and B. Yu, J. Org. Chem., 2021, 86, 9055–9066 CrossRef CAS PubMed; (h) L. Liu, D.-Y. Yang, Y.-H. He and Z. Guan, J. Org. Chem., 2020, 85, 11892–11901 CrossRef CAS PubMed; (i) Y. Yuan, Y. Zheng, B. Xu, J. Liao, F. Bu, S. Wang, J.-G. Hu and A. Lei, ACS Catal., 2020, 10, 6676–6681 CrossRef CAS.
- (a) M. Lu, W. Xu and M. Ye, Molecules, 2023, 28, 736–746 CrossRef CAS PubMed; (b) Z.-J. Liu, J.-F. Li, F.-P. Zhang, X.-T. Xu and M. Ye, Org. Lett., 2023, 25, 353–357 CrossRef CAS PubMed; (c) S.-J. Lou, Z. Mo, M. Nishiura and Z. Hou, J. Am. Chem. Soc., 2020, 142, 1200–1205 CrossRef CAS PubMed; (d) X. Wu, G. Xiao, Y. Ding, Y. Zhan, Y. Zhao, R. Chen and T.-P. Loh, ACS Catal., 2020, 10, 14107–14116 CrossRef CAS; (e) Y.-T. He, Y.-J. Mao, H.-Y. Hao, Z.-Y. Xu, S.-J. Lou and D.-Q. Xu, Org. Lett., 2020, 22, 8250–8255 CrossRef CAS PubMed.
- (a) H.-X. Jin, Y. Zhu, J. Zhang, Y.-P. Zhang, L. Zhu, L. Long, Z. Chen, H. Luo and D. Yu, Org. Chem. Front., 2024, 11, 5762–5768 RSC; (b) Y. Liu, Z. Li, L. Yang, S. Li and Z. Chen, Org. Biomol. Chem., 2023, 21, 8690–8694 RSC; (c) Y. Wu, H. Liu, L. Liu and J.-T. Yu, Org. Biomol. Chem., 2023, 21, 7079–7084 RSC; (d) B. Sun, H.-X. Tian, Z.-G. Ni, P.-Y. Huang, H. Ding, B.-Q. Li, C. Jin, C.-L. Wu and R.-P. Shen, Org. Chem. Front., 2022, 9, 3669–3676 RSC; (e) Z. Guo, X. Chen, Y. Xue, J. Li, D. Zou, Y. Wu and Y. Wu, Adv. Synth. Catal., 2022, 364, 4231–4236 CrossRef CAS.
- S.-N. Lin, Y. Chen, X.-D. Luo and Y. Li, Eur. J. Org Chem., 2021, 31, 4485–4489 CrossRef.
- Z. Chen, Z. Li, S. Li, G. Qian and Y. Sun, New J. Chem., 2023, 47, 11465–11470 RSC.
- Q.-H. Zhou, J.-Y. Dai, W.-J. Zhao, X.-Y. Zhong, C.-Y. Liu, W.-W. Luo, Z.-W. Li, J.-S. Li and W.-D. Liu, Org. Biomol. Chem., 2023, 21, 3317–3322 RSC.
- P. Huang, C. Lv, H. Song, C. Wang, J. Du, J. Li, B. Sun and C. Jin, Green Chem., 2024, 26, 7198–7205 RSC.
- (a) R. Ma, Y. Ren, Z. Deng, K.-H. Wang, J. Wang, D. Huang, Y. Hu and X. Lv, Molecules, 2022, 27, 8389–8397 CrossRef CAS PubMed; (b) S. Lin, J. Cui, Y. Chen and Y. Li, J. Org. Chem., 2021, 86, 15768–15776 CrossRef CAS PubMed.
- Y. Pang, J. Yan, N. Al-Maharik, Q. Zhang, Z. Fang and D. Li, Beilstein J. Org. Chem., 2025, 21, 234–241 CrossRef CAS PubMed.
- S.-N. Lin, Y. Deng, H. Zhong, L.-L. Mao, C.-B. Ji, X.-H. Zhu, X. Zhang and B.-M. Yang, ACS Omega, 2024, 9, 28129–28143 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02023a |
| ‡ Both authors contributed to this work equally. |
| This journal is © The Royal Society of Chemistry 2025 |