Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Organic fibrous nanostructures via the droplet-assisted growth and shaping (DAGS) mechanism
Rabab Azizi and
Stefan Seeger *
*
Department of Chemistry, University of Zurich, Winterthurerstrasse 190, 8057 Zurich, Switzerland. E-mail: s.seeger@uzh.ch
First published on 4th July 2025
Abstract
The Droplet-Assisted Growth and Shaping mechanism (DAGS) represents a bottom-up approach for the fabrication of versatile one-dimensional polymeric (1D) nanomaterials and involves the polymerisation of a highly reactive monomer with water nanodroplets formed on a substrate surface. The unidimensional growth of the resulting polymer is sustained with its water insolubility. To date, all 1D polymeric nanostructures grown via the DAGS mechanism were either based on silicone, alumina, or germanium oxide but not on a carbonic backbone. In this paper, we demonstrate for the first time that the DAGS mechanism can also be used for the growth of organic 1D polymeric nanostructures using ethyl-2-cyanoacrylate (ECA) as a monomer. The polymerisation is carried out in n-hexane/toluene mixtures with different water contents (WCs). The obtained poly(ethyl-2-cyanoacrylate) (PECA) fibrous nanostructures (PECA-FNS), which were coated on glass, manifested as nanofibers and nanoribbons with an aspect ratio ranging from 4.9 to 18.3. Attenuated total reflectance infrared (ATR-IR) spectroscopy revealed the presence of the carbonyl bond on the coated glass substrates, confirming the presence of the PECA-FNS. The topography and the root mean square roughness (Sq) of the PECA-FNS were examined via atomic force microscopy (AFM). Both static contact angle measurements and UV-Vis spectrophotometry showed that the PECA-FNS coatings displayed a high transparency and moderate hydrophobicity.
Introduction
One-dimensional (1D) polymeric nanostructures such as nanofibers, nanotubes, and nanowires, are nanomaterials with a typical diameter ranging from 1 to 100 nm.1,2 Compared to their bulk polymeric analogues, the miniaturisation into the nanoscale usually results in novel chemical, thermal, and mechanical properties,3 for example, the high surface-to-volume ratio and optical anisotropy exhibited by nanofibers.4 Among the various methods for the fabrication of 1D polymeric nanostructures, techniques such as electrospinning, self-assembly, and templating are widely employed.2,5,6 However, electrospinning and self-assembly processes, while very useful for creating 1D nanostructures, often face challenges in scalability and precise control of the morphology.7,8A few decades ago, Artus et al. introduced the droplet-assisted growth and shaping (DAGS) mechanism, a bottom-up approach for manufacturing 1D polymeric nanostructures. This method uses water nanodroplets formed on a substrate (e.g. glass) as confined reaction volumes. The water droplets act as initiation centres of reactive inorganic monomers, such as silanes, and the unidimensional growth of the resulting polymer is ensured by its water-insoluble nature.9 The advantages of the DAGS mechanism include mild reaction conditions, i.e. processing at room temperature and atmospheric pressure, as well as the ability to achieve one-dimensional growth of the polymers in both gas and/or liquid phase.9,10 For instance, the straightforward implementation of the DAGS mechanism makes it suitable for scaling up the production of superhydrophobic coatings at the pilot plant level.11 Moreover, it has been successfully applied to synthesise further various inorganic 1D polymers, including silicone nanofilaments, nanorods, helices, germanium oxide nanofilaments, and alumina necklaces.9,12,13 Our present strategic vision entails not only the growth of diverse inorganic but also organic and eventually hybrid 1D polymeric nanostructures, which are particularly well-known for their tuneable and synergistic properties.14 Therefore, the main aim of this paper is to demonstrate the universality of the DAGS mechanism and to validate its applicability to organic monomers. For this purpose, we have chosen ethyl-2-cyanoacrylate (ECA), an organic monomer, mostly commercialised as an instant adhesive.15 ECA undergoes a rapid anionic polymerisation in the presence of trace amounts of water16,17 and was previously used to obtain nanofibers in the gas phase at high relative humidity values.18 Although these PECA nanofibers were obtained only by the mean of specific initiators such as alcohols or salts such as NaCl, KCl, CH3COOK,19–21 while our unique method relies solely on the initiation of water nanodroplets present on the substrate surface. Namely, the ECA anionic polymerisation in our work was carried out in n-hexane/toluene mixtures with water contents ranging from 30 to 120 ppm using pre-treated glass slides as substrates. Scanning electron microscope (SEM) images unveiled versatile PECA fibrous nanostructures (PECA-FNS) including nanofibers and nanoribbons, with an aspect ratio up to ∼18.3. This breakthrough represents a new generation of 1D nanostructures, demonstrating the unprecedented successful transition of the DAGS mechanism from inorganic to organic 1D nanomaterials.
Experimental section
Materials
Glass slides (25 mm × 60 mm × 1 mm) from Epredia-Menzel were used as a substrate. ECA n-hexane and toluene were purchased from Merck, Switzerland. Hydranal Coulomat AG used as a Karl Fischer agent was purchased from Fischer Scientific, Switzerland. Deconex 11 Universal was obtained from Borer Chemie, Switzerland. All solvents and chemicals are of analytical grade and were used as received unless otherwise specified. Milli-Q water was generated from the Simplicity UV system.Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VTol were 4
VTol were 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, respectively. The water content in each mixture was 30, 50, 90, and 120 ppm with a permissible variation of ±7 ppm. The previously cleaned glass substrates were put vertically in a cylindrical reactor with an inner cavity lined with Teflon to ensure inertness towards organic solvents and avoid the adhesion of monomer on the reactor walls. A total volume of 100 mL of n-hexane or n-hexane/toluene mixture was introduced into the reactor, which was then tightly sealed. The water content in the organic solvent was adjusted under constant stirring (300 rpm) at a temperature of 21 ± 1 °C using a stream of either dry or wet nitrogen gas. The latter was generated by passing dry nitrogen through a bottle filled with Milli Q-water. The water content was determined using a Mettler Toledo C20 Karl-Fischer coulometer equipped with a platinum reference electrode and a diaphragmaless generator electrode. Once the desired water content is reached, at least two further values are measured for verification. Subsequently, 0.85 mmol of ECA was injected into the reactor and allowed to polymerise for 18 to 22 hour under continuous stirring (120 rpm). The PECA-coated substrates are finally taken out and dried at ambient conditions using a nitrogen gun and preserved in glass slide mailers made from polypropylene until later analysis. The entire experimental procedure for the fabrication of PECA nanostructures is depicted in Fig. 1.
4, respectively. The water content in each mixture was 30, 50, 90, and 120 ppm with a permissible variation of ±7 ppm. The previously cleaned glass substrates were put vertically in a cylindrical reactor with an inner cavity lined with Teflon to ensure inertness towards organic solvents and avoid the adhesion of monomer on the reactor walls. A total volume of 100 mL of n-hexane or n-hexane/toluene mixture was introduced into the reactor, which was then tightly sealed. The water content in the organic solvent was adjusted under constant stirring (300 rpm) at a temperature of 21 ± 1 °C using a stream of either dry or wet nitrogen gas. The latter was generated by passing dry nitrogen through a bottle filled with Milli Q-water. The water content was determined using a Mettler Toledo C20 Karl-Fischer coulometer equipped with a platinum reference electrode and a diaphragmaless generator electrode. Once the desired water content is reached, at least two further values are measured for verification. Subsequently, 0.85 mmol of ECA was injected into the reactor and allowed to polymerise for 18 to 22 hour under continuous stirring (120 rpm). The PECA-coated substrates are finally taken out and dried at ambient conditions using a nitrogen gun and preserved in glass slide mailers made from polypropylene until later analysis. The entire experimental procedure for the fabrication of PECA nanostructures is depicted in Fig. 1.
Results and discussion
ECA anionic polymerisation in n-hexane and its toluene mixtures
The polymerisation of ECA in n-hexane and its mixtures with toluene proceeds via an anionic mechanism, initiated by trace amounts of water. We hypothesised that the rapid reactivity of ECA with even minimal water content would make it a suitable and a promising candidate for extending the DAGS mechanism from inorganic to organic 1D nanostructures structures. The water molecules are both involved in the initiation and termination steps. The initiation step occurs when a water molecule attacks the ECA β-carbon, leading to the formation of a carbanion, which will react with a further ECA molecule. The propagation of the macromolecular chain will continue as long as the monomer is available or as long as there is no chain termination, for example via a proton transfer.22 The detailed anionic polymerisation mechanism of ECA is shown in Scheme 1. | ||
| Scheme 1 Anionic polymerisation of ECA in the presence of a nucleophile, for example a water molecule. | ||
We initially investigated the growth of PECA-based coatings on glass, using water contents (WCs) in the range of 30, 50, 90, and 120 ppm in n-hexane and n-hexane/toluene mixtures with a volumetric ratio VHex![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VTol = 4
VTol = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 2
2, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 4
3, and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. A water content of 120 ppm could not be reached in pure n-hexane. Indeed, the maximum reachable water content in pure n-hexane was reported to be 90 ppm at 25 °C.23 The water content was systematically adjusted to find the optimal amount of initiator (water) for the formation of PECA 1D nanostructures. The nomenclature of the PECA-coated samples follows a systematic approach based on solvent composition and water content. For samples polymerised in only n-hexane, the designation takes the form (x)H(z)p, where x represents the percentage of n-hexane (abbreviated as H), and z indicates the water content (WC) in ppm. For coatings performed in n-hexane/toluene mixtures, the designation expands to (x)H(y)T(z)p, where x and y represent the percentages of n-hexane and toluene (abbreviated as H and T, respectively), followed by z denoting the WC range. In both cases, the letter ‘p’ is appended to signify ppm of the water in the solvent system. For example, the sample synthesised in pure n-hexane at the lowest water content (30 ppm) will be denoted as 100H30p. Subsequent designation of a sample fabricated within the same WC range but with a VHex
1. A water content of 120 ppm could not be reached in pure n-hexane. Indeed, the maximum reachable water content in pure n-hexane was reported to be 90 ppm at 25 °C.23 The water content was systematically adjusted to find the optimal amount of initiator (water) for the formation of PECA 1D nanostructures. The nomenclature of the PECA-coated samples follows a systematic approach based on solvent composition and water content. For samples polymerised in only n-hexane, the designation takes the form (x)H(z)p, where x represents the percentage of n-hexane (abbreviated as H), and z indicates the water content (WC) in ppm. For coatings performed in n-hexane/toluene mixtures, the designation expands to (x)H(y)T(z)p, where x and y represent the percentages of n-hexane and toluene (abbreviated as H and T, respectively), followed by z denoting the WC range. In both cases, the letter ‘p’ is appended to signify ppm of the water in the solvent system. For example, the sample synthesised in pure n-hexane at the lowest water content (30 ppm) will be denoted as 100H30p. Subsequent designation of a sample fabricated within the same WC range but with a VHex![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VTol = 4
VTol = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 will be designated as 80H20T30p etc. Finally, the sample with the highest water content (120 ppm) and the highest toluene proportion (VHex
1 will be designated as 80H20T30p etc. Finally, the sample with the highest water content (120 ppm) and the highest toluene proportion (VHex![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VTol = 1
VTol = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
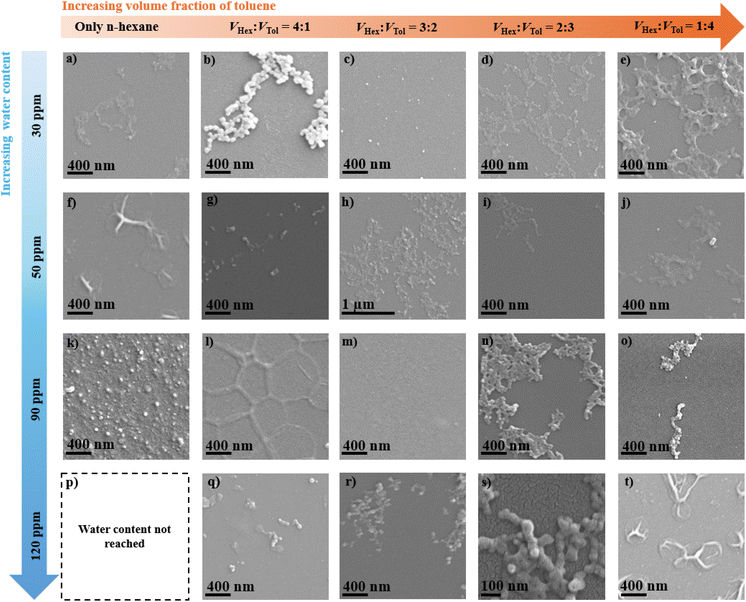
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) is denoted as 20H80T120p. SEM top-view images revealed that the majority of the obtained PECA nanostructures (PECA-NS) are relatively similar to the alumina necklace-like nanostructures previously reported to be obtained via the DAGS mechanism in the gaseous phase.12,13 These PECA necklace-like nanostructures manifested in various forms, as prominent necklace-like nanostructures in form of either ‘continuous islands’ (Fig. 2b, n, r, and s) or ‘disrupted islands’ (Fig. 2g, o, and q). In Fig. 2a, d, e, h, i, and j the PECA necklace nanostructures still form in continuous islands but with a flatter surface profile. On the other hand, a few PECA-NS display punctual patterns on the surface (Fig. 2c and k), while others seem to build up a polymer film (Fig. 2m) with some sub-circular patterns as seen in Fig. 2f, l and t. SEM top-view images clearly indicate that minor modifications in the polymerisation parameters (solvent system and WC) can result in significant morphological variations within the PECA-NS. However, initial observations did not indicate a straightforward correlation between the PECA-NS morphology and the increase of either toluene fraction in n-hexane or WC values. This suggests that additional factors, including diffusion, adsorption of water molecules, and their stochastic distribution on the substrate, may interact to affect the development of PECA-NS via the DAGS mechanism in the solvent phase.
4) is denoted as 20H80T120p. SEM top-view images revealed that the majority of the obtained PECA nanostructures (PECA-NS) are relatively similar to the alumina necklace-like nanostructures previously reported to be obtained via the DAGS mechanism in the gaseous phase.12,13 These PECA necklace-like nanostructures manifested in various forms, as prominent necklace-like nanostructures in form of either ‘continuous islands’ (Fig. 2b, n, r, and s) or ‘disrupted islands’ (Fig. 2g, o, and q). In Fig. 2a, d, e, h, i, and j the PECA necklace nanostructures still form in continuous islands but with a flatter surface profile. On the other hand, a few PECA-NS display punctual patterns on the surface (Fig. 2c and k), while others seem to build up a polymer film (Fig. 2m) with some sub-circular patterns as seen in Fig. 2f, l and t. SEM top-view images clearly indicate that minor modifications in the polymerisation parameters (solvent system and WC) can result in significant morphological variations within the PECA-NS. However, initial observations did not indicate a straightforward correlation between the PECA-NS morphology and the increase of either toluene fraction in n-hexane or WC values. This suggests that additional factors, including diffusion, adsorption of water molecules, and their stochastic distribution on the substrate, may interact to affect the development of PECA-NS via the DAGS mechanism in the solvent phase.
Characterisation of PECA fibrous nanostructures (PECA-FNS)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dav) are presented below in Table 1.
dav) are presented below in Table 1.
| Sample | Lav![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) dav dav |
Classification of the PECA-FNS |
|---|---|---|
| 60H40T30p | 6.4 | Nanofibers (NF) |
| 40H60T30p | 18.3 | Nanofibers (NF) |
| 20H80T50p | 4.9 | Nanoribbons (NR) |
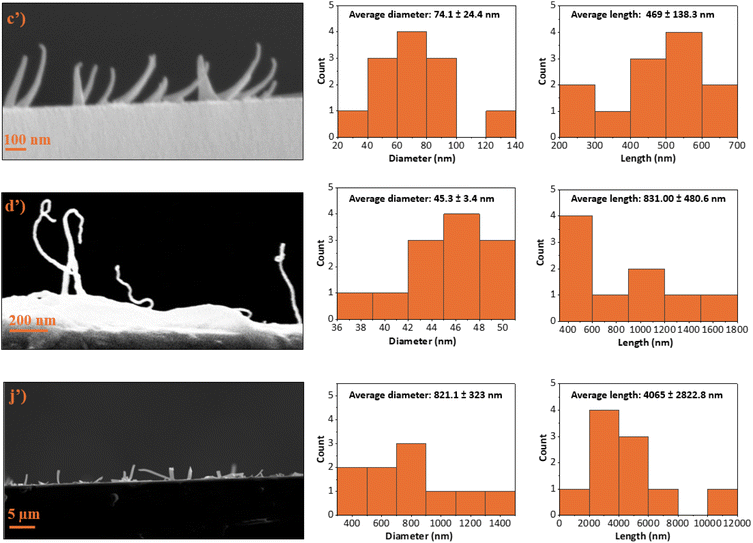
The observed aspect ratios of the obtained PECA-FNS are lower compared to those of previously fabricated silicone-based 1D nanostructures via the DAGS mechanism. For instance, silicone nanotubes with an aspect ratio up to 800 were produced in toluene with a water content of 155 ± 5 ppm,24 whereas in our case, the maximum water content used to polymerise ECA was 120 ppm. A plausible explanation for the dimensional differences between silicone-based and PECA-FNS may be attributed to the distinct polymerisation mechanisms of ECA and silanes. ECA undergoes polyaddition, characterised by the sequential addition of monomer molecules without the release of by-products. In this case, water acts as an initiator, and trace amounts are already sufficient to induce a spontaneous anionic polymerisation.25 Conversely, silanes polymerise via hydrolysis with a subsequent polycondensation. Polycondensation is a step-growth polymerisation that involves the elimination of small molecules such as HCl. In silane polymerisation, water functions more as a reactant, and the molar water-to-silane ratio is crucial in controlling the overall polymerisation, alongside other factors such as pH, temperature or catalysts.26 The contrasting role of water in each process – as an initiator in ECA polyaddition and as a reactant in silane polycondensation is likely to also contribute to the distinct dimensional variations in the corresponding polymeric 1D nanostructures obtained by means of the DAGS mechanism. While the precise conditions and factors contributing to the formation of PECA-FNS require further investigation, the morphological similarities of the PECA-FNS to the previously obtained 1D inorganic nanostructures via the DAGS mechanism still provides evidence for a shared underlying growth process as shown in Fig. 4. In addition to the hydroxyl (OH) groups present on the pre-treated glass substrate, we believe that water nanodroplets formed on its surface also serve as initiation sites for the ECA anionic polymerisation (Fig. 4a). Once initiated, the reaction continues propagating as long as ECA molecules are available to sustain chain growth of the macro-carbanion (Fig. 4b). The resulting PECA is then deposited due to its water insolubility,27 ensuring a one-dimensional growth of the PECA-FNS (Fig. 4c). This process continues until the full consumption of ECA and/or until the active PECA chain interacts with a nearby water molecule, which will terminate the polymerisation and lead to the formation of the PECA-FNS (Fig. 4d). Moreover, the solvent is thought to have a significant influence on the growth mechanism of the PECA-FNS, since the nature of the solvent determines the interactions polymer–solvent, which are known to impact the macromolecular conformation of the polymer chains.28 The interaction of a solute-which are here ECA, water, and the later formed PECA with a certain solvent, can be predicted via the Hansen solubility parameters (HSP). This approach is based on the principle ‘like dissolves like’ and divides cohesive energy into three components: dispersion interactions, polar interactions, and hydrogen bonding,29–32 which are represented by their respective energy densities δd, δp, and δH (MPa1/2 in the SI system).33 In general, if the HSP components (δd, δp, and δH) of a solvent and a solute are close enough, the two substances are likely to be ‘compatible’.30 We aim to clarify the formation of the PECA-FNS in a simplified manner by utilising HSP, with a primary focus on the interactions between the solvent and water. We also assert that the interactions involving ECA with water, as well as those between ECA and the solvent system and the glass surface, likely play a significant role in the one-dimensional growth via the DAGS mechanism. The morphological differences observed in the PECA-FNS between samples 60H40T30p and 40H60T30p can be attributed to variations in solvent compositions, given that both samples were polymerised with the same amount of water. This modification suggests a change in how the solvent system interacts with water nanodroplets on the glass substrate. For example, in sample 60H40T30p, where n-hexane predominates, the substantial difference in HSP values between water and n-hexane (see Table 2) compels the formation of larger water nanodroplet ‘islands’ on the substrate, likely due to a greater affinity for the hydrophilic glass surface. This phenomenon probably leads to rapid initial consumption of the monomer, resulting in wider but shorter nanofibers due to the limited availability of ECA molecules necessary for supporting macromolecular chain propagation. In contrast, in sample 40H60T30p, where toluene is the predominant solvent, the favourable interaction between water and toluene, characterised by a minimal difference in HSP values, as evidenced in Table 2, facilitates the formation of smaller water islands on the glass surface. This distribution of smaller initiation sites contributes to the development of thinner nanofibers. Furthermore, the higher proportion of toluene in sample 40H60T30p likely retains more unreacted ECA molecules in solution, which can further support macromolecular growth after initiation, ultimately resulting in longer PECA nanofibers. On the other hand, the PECA-FNS observed in the sample 20H80T50p exhibit both a larger dav and Lav, likely due to wider and elongated water islands formed on the substrate surface, which could potentially explain their ribbon like appearance. Additionally, the solvent mixture in this sample, primarily composed of toluene, might have facilitated the interactions among all solutes (ECA, water and the growing PECA), promoting the propagation of significantly longer polymeric chains or even causing the arrangement of PECA macromolecules, resulting in a longer Lav.
| δd | δp | δH | |
|---|---|---|---|
| ECA | 15.2 | 10.3 | 9 |
| n-Hexane | 14.9 | 0 | 0 |
| Toluene | 18 | 1.4 | 2 |
| Water | 15.5 | 16 | 42.3 |
It is noticeable that the signals from glass are very prominent in the PECA-coated substrates as shown in Fig. 5a.
This might be due to the low concentration of ECA used during polymerisation. The peak at 783 cm−1 is attributed to the symmetric stretching of Si–O–Si,35 while the sharp band at 956 cm−1 corresponds to the stretching of the Si–O–B band.36 The blue arrow around 1250 cm−1 shows the absorption of the C–O–C bond coming from the crude PECA.37 In contrast, this absorption band was blended by signals from the glass substrate in all the PECA-FNS samples. The broad band at 1395 cm−1 represents the asymmetric stretching relaxation of the B–O bond originating from the trigonal BO3 units of glass.38 Finally, the IR signals at ∼1738–1753 cm−1 (Fig. 5b) can be ascribed to the carbonyl groups found in PECA. The black arrows indicate a shift in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of the PECA-FNS samples compared to the C
O bond of the PECA-FNS samples compared to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O absorption band observed in the sample with crude PECA.
O absorption band observed in the sample with crude PECA.
The background of sample 60H40T30p shows a rough surface with a root mean square roughness (RMS roughness Sq) of 22.73 nm, and a bimodal height distribution characterised by two distinct ρ peaks. The primary peak occurred at ∼53 μm−1, corresponding to a height of approximately 81 nm, while the secondary peak reached a minimum of ∼18 μm−1 at a height of circa 97 nm. This bimodal distribution suggests a hierarchical surface structure with two predominant PECA-NFs populations with distinct different heights. In contrast, sample 40H60T30p displayed a smoother surface (Sq = 15.16 nm) with a multimodal height distribution, indicative of a heterogeneous surface topography. The maximum peak was observed at ∼38 μm−1, corresponding to a height of approximately 47 nm, while the minimal peak appears at ∼10 μm−1 for a height of ca. 10 nm. This complex distribution implies a highly varied surface topography, potentially comprising both small-scale rough and other larger PECA-NFs. The surface of sample 20H80T50p was the smoothest (Sq = 8.85 nm). Its height distribution was relatively narrow and symmetric with a ρ value of 0.19 μm−1, corresponding to a maximum height of ca. 15 nm. This uniform height distribution suggests a homogeneous surface topography with consistent PECA-NRs morphologies.
 | ||
| Fig. 7 Transmittance spectra measured from 300–800 nm: crude PECA (green), sample 60H40T30p (yellow), sample 40H60T30p (blue), 80H20T50p (red), and a pristine pre-treated glass slide (purple). | ||
The differences in transmittance noted within the PECA-FNS samples can be attributed to variations in their topology or coating thickness. These discrepancies can lead to light scattering, resulting in reduced light transmission. For example, sample 40H60T30p exhibits the lowest transmittance value among all the PECA-FNS samples, likely due to optical losses associated with its heterogeneous surface, as indicated by previous AFM topological analysis. However, the overall high transparency of the PECA-FNS can be well suited for coating applications, in which preserving the original appearance of the substrate (i.e., colour) is required. When compared to the previously fabricated silicone nanofilaments, the transmittance values of the PECA-FNS in the visible range remain slightly lower.42
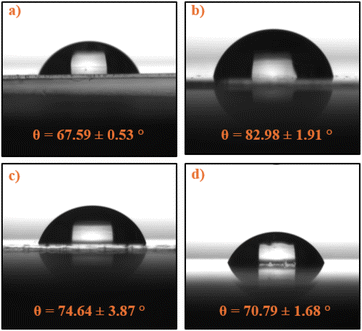 | ||
| Fig. 8 Static contact angle measurements on PECA-coated glass substrates: (a) crude PECA on glass, (b) sample 60H40T, (c) sample 40H60T30p, and (d) sample 20H80T50p. | ||
Contact angles varied from 67.59° for crude PECA on glass (Fig. 8a) to 82.98° for sample 60H40T30p (Fig. 8b), with samples 40H60T30p and 20H80T50p exhibiting intermediate values of 74.64° (Fig. 8c) and 70.79°(Fig. 8d), respectively. These results indicate a slight enhancement in hydrophobicity compared to the crude PECA coating, although the surfaces can not be described as hydrophobic, as the contact angle does not exceed 90°.43 The observed moderate hydrophilicity of PECA-FNS aligns with expectations based on its molecular structure, which contains polar CN and COOEt groups. Previous studies have estimated the surface tension of PECA at 33 mN m−1,44 and our findings for the contact angle of the crude PECA are consistent with reported values in the literature.45,46 Despite the complex relationship between surface roughness and wettability,47 a correlation between surface roughness (Sq) and contact angle was observed, in our case: the contact angle increased with increasing Sq, suggesting that topographical features of the PECA-FNS have a role in modulating surface wettability. Interestingly, sample 60H40T30p exhibited the highest hydrophobicity among all the measured samples, which may be attributed to its high surface roughness (Sq = 22.73 nm). It is also noteworthy that PECA-FNS samples displayed markedly different wetting behaviour compared to previously reported PECA nanofibers (CA = 158°).46 However, the moderately hydrophilic nature of the PECA-FNS could be advantageous for example in wound healing applications where a balance between hydrophobicity and hydrophilicity is desired.48
Conclusions
In this study, we successfully demonstrated the extension of the DAGS mechanism to organic monomers using ECA. The anionic polymerisation was carried out in n-hexane and n-hexane/toluene mixtures with water contents ranging from 30 to 120 ppm, resulting in PECA fibrous nanostructures (PECA-FNS) appearing as either nanofibers (PECA-NFs) and or nanoribbons (PECA-NRs). The aspect ratio of the PECA-FNS ranges from 4.9 to 18.3. These 1D nanostructures bear resemblance to the well-known silicon nanofilaments (SNF) formed via the DAGS mechanism, providing evidence that a similar mechanism underpins the growth of these organic nanostructures. This similarity further validates the versatility of the DAGS mechanism and highlights its potential for producing a wide range of one-dimensional nanomaterials. While the exact factors contributing to the formation of the PECA-FNS remain partially unrevealed, the applicability of the DAGS mechanism to organic monomers represents a significant advancement, as it enables the fabrication of organic 1D nanostructures while retaining the advantages of DAGS, including operation under mild conditions at room temperature and atmospheric pressure. This extension not only broadens the scope of the mechanism but also opens new opportunities for developing hybrid materials with tuneable and synergistic properties, significantly expanding the application potential of 1D polymeric nanostructures. Notably, PECA-FNS exhibit relatively high transparency and moderate hydrophilicity, positioning them as a promising alternative to nanocoatings prepared via the DAGS mechanism, particularly when both tailored surface wettability and excellent transparency are desired.Data availability
All the raw data used in this article can be accessed from OSF repository at OSF|PECA-FNS via the DAGS mechanism (https://osf.io/mkd7p/).Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to express our sincere gratitude to Dr Thomas Möhl for conducting the AFM measurements and providing valuable insights during the discussion of the obtained data. We are deeply thankful to Dr Clamor for offering constructive feedback on the draft of this manuscript. Furthermore, we also acknowledge the Center of Microscopy of the University of Zurich (ZMB) for using their facilities.Notes and references
- Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim and H. Yan, Adv. Mater., 2003, 15, 353–389 CrossRef CAS.
- M. Ikegame, K. Tajima and T. Aida, Angew. Chem., 2003, 42, 2154–2157 CrossRef CAS.
- M. Arruebo, M. Valladares and Á. González-Fernández, J. Nanomater., 2009, 2009, 439389 CrossRef.
- R. Mishra and J. Militky, Nanotechnology in Textiles. Theory and Application, Woodhead Publishing, Duxford England, Cambridge MA, 2019 Search PubMed.
- Y. Zhang, H. Ouyang, C. T. Lim, S. Ramakrishna and Z.-M. Huang, J. Biomed. Mater. Res., Part B, 2005, 72, 156–165 CrossRef.
- J. D. Hartgerink, E. Beniash and S. I. Stupp, Science, 2001, 294, 1684–1688 CrossRef CAS.
- F. E. Ahmed, B. S. Lalia and R. Hashaikeh, Desalination, 2015, 356, 15–30 CrossRef CAS.
- J. Li, X. Liu, J. Xi, L. Deng, Y. Yang, X. Li and H. Sun, Polymers, 2023, 15, 3616 CrossRef CAS.
- G. R. J. Artus, S. Olveira, D. Patra and S. Seeger, Macromol. Rapid Commun., 2017, 38, 1600558 CrossRef.
- J. Zhang, L. Li, B. Li and S. Seeger, RSC Adv., 2014, 4, 33424–33430 RSC.
- G. R. J. Artus and S. Seeger, Ind. Eng. Chem. Res., 2012, 51, 2631–2636 CrossRef CAS.
- N.-U.-H. Saddiqi, D. Patra and S. Seeger, ChemPhysChem, 2019, 20, 538–544 CrossRef CAS.
- N.-U.-H. Saddiqi and S. Seeger, J. Colloid Interface Sci., 2020, 560, 77–84 CrossRef CAS.
- J. Yuan and A. H. Müller, Polymer, 2010, 51, 4015–4036 CrossRef CAS.
- Handbook of Adhesives, ed., I. Skeist, Springer US, Boston, MA, 1990 Search PubMed.
- G. L. Schneberger, Adhesives in Manufacturing, Routledge, 2018, pp. 51–66 Search PubMed.
- P. R. Raja, Rev. Adhes. Adhes., 2016, 4, 398–416 CrossRef CAS.
- P. J. Mankidy, R. Rajagopalan, C. G. Pantano and H. C. Foley, Chem. Mater., 2009, 21, 831–842 CrossRef CAS.
- P. J. Mankidy, R. Rajagopalan and H. C. Foley, Chem. Commun., 2006, 1139–1141 RSC.
- N. Marinov and M. Simeonova, Bulg. Chem. Commun., 2015, 47, 87–92 Search PubMed.
- V. G. Georgieva, M. Y. Simeonova, S. C. Turmanova and N. M. Marinov, Polym. Int., 2022, 71, 715–723 CrossRef CAS.
- D. A. Dillard, Advances in Structural Adhesive Bonding, CRC Press, Boca Raton, FL, Oxford, 2010 Search PubMed.
- J. Polak and B. C.-Y. Lu, Can. J. Chem., 1973, 51, 4018–4023 CrossRef CAS.
- A. Stojanovic, S. Olveira, M. Fischer and S. Seeger, Chem. Mater., 2013, 25, 2787–2792 CrossRef CAS.
- E. F. Donnelly, D. S. Johnston, D. C. Pepper and D. J. Dunn, J. Polym. Sci., Part B, 1977, 15, 399–405 CAS.
- I. B, R. Gunasekhar, P. Sathiyanathan and A. P. Arun, ECS Trans., 2022, 107, 14539–14546 CrossRef CAS.
- N. Behan, C. Birkinshaw and N. Clarke, Biomaterials, 2001, 22, 1335–1344 CrossRef CAS.
- M. Mishra, Encyclopedia of Polymer Applications, CRC Press, Boca Raton, 2018 Search PubMed.
- O. Segarceanu and M. Leca, Prog. Org. Coat., 1997, 31, 307–310 CrossRef CAS.
- C. M. Hansen, Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient, Importance in Surface Coating Formulation, Doctoral dissertation, University of Copenhagen, 1967 Search PubMed.
- C. M. Hansen and A. Beerbower,Kirk-Othmer Encyclopedia of Chemical Technology, Supplement Volume, ed., A. Standen, Interscience, New York, 2nd edn, 1971, pp. 889–910 Search PubMed.
- C. M. Hansen, J. Paint Technol., 1967, 39, 505–510 CAS.
- E. Stefanis and C. Panayiotou, Int. J. Thermophys., 2008, 29, 568–585 CrossRef CAS.
- C. M. Hansen, Hansen Solubility Parameters. A User's Handbook, CRC Press, Boca Raton, Florida, 2000 Search PubMed.
- G. Shao, X. Wu, Y. Kong, S. Cui, X. Shen, C. Jiao and J. Jiao, Surf. Coat. Technol., 2015, 270, 154–163 CrossRef CAS.
- T. Rao, A. Rupesh Kumar, K. Neeraja, N. Veeraiah and M. Rami Reddy, J. Alloys Compd., 2013, 557, 209–217 CrossRef CAS.
- D. Li, H. Zhou, X. Hui, X. He, H. Huang, J. Zhang, X. Mu, C. Lee and Y. Yang, Adv. Sci., 2021, 8, e2101879 CrossRef.
- V. G. Chekhovskii, Glass Phys. Chem., 1985, 11, 1 Search PubMed.
- A. J. Christy and S. T. Phillips, Sci. Adv., 2023, 9, eadg2295 CrossRef CAS.
- P. D. Rufe, Fundamentals of Manufacturing, Society of Manufacturing Engineers, Dearborn, MI, 3rd edn, 2013 Search PubMed.
- J. H. Townsend, Adhesives, Coatings and Consolidants, Routledge, Abingdon Oxon, New York NY, 2025 Search PubMed.
- J. Zimmermann, G. R. J. Artus and S. Seeger, J. Adhes. Sci. Technol., 2008, 22, 251–263 CrossRef CAS.
- K.-Y. Law and H. Zhao, Surface Wetting, Springer International Publishing, Cham, 1st edn, 2016 Search PubMed.
- E. Mele, J. A. Heredia-Guerrero, I. S. Bayer, G. Ciofani, G. G. Genchi, L. Ceseracciu, A. Davis, E. L. Papadopoulou, M. J. Barthel, L. Marini, R. Ruffilli and A. Athanassiou, Sci. Rep., 2015, 5, 14019 CrossRef.
- J. Kreuter, Int. J. Pharm., 1983, 14, 43–58 CrossRef CAS.
- L. Zhang, N. Zhao, X. Li, Y. Long, X. Zhang and J. Xu, Soft Matter, 2011, 7, 4050 RSC.
- F. Leroy and F. Müller-Plathe, Langmuir, 2011, 27, 637–645 CrossRef CAS.
- S. M. Oliveira, N. M. Alves and J. F. Mano, J. Adhes. Sci. Technol., 2014, 28, 843–863 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |