Open Access Article
Open Access ArticleRecent advances in zirconium-based catalysis and its applications in organic synthesis: a review
Saima Bibia,
Muhammad Zubair *a,
Rehana Riaza,
Aqsa Kanwala and
Syed Adnan Ali Shahbc
*a,
Rehana Riaza,
Aqsa Kanwala and
Syed Adnan Ali Shahbc
aDepartment of Chemistry, Government College University, Faisalabad, Pakistan. E-mail: zubairmkn@gcuf.edu.pk
bFaculty of Pharmacy, Universiti Teknologi MARA Cawangan Selangor Kampus Puncak Alam, Bandar Puncak Alam 42300, Selangor D. E., Malaysia
cAtta-ur-Rahman Institute for Natural Product Discovery (AuRIns), Universiti Teknologi MARA CawanganSelangor Kampus Puncak Alam, Bandar Puncak Alam 42300, Selangor D. E., Malaysia
First published on 9th May 2025
Abstract
In recent years, transition metal-catalysed organic synthesis has received great importance. Zirconium, a second-row transition metal, has gained prominence owing to its luster and abundance, but it is more expensive than other transition metals because it is difficult to refine and process. In particular, active zirconia-based catalysts have fascinated researchers owing to their low toxicity, affordability, flexibility and excellent dispersion. This review focuses on the latest zirconium catalysts used in the manufacturing of medicinal compounds, bioactive molecules and pertinent synthesis mechanisms reported since 2020. In this review, the synthesis of various heterocycles such as imidazoles, pyrazole, pyrimidinones, quinolines, quinazolinones, pyridines, pyrroles, benzopyrans, substituted amides and triazolidine-based bioactive molecules is discussed in detail. Future research in this area is based on further understanding the scope of zirconium catalysed sustainable and approachable synthesis of biologically active compounds.
1 Introduction
The second-row transition metal zirconium (Zr) is very lustrous and abundant and may be used in dental implants, prosthetic limbs, knee and hip replacements, and nuclear medicine. It is also suitable for use in these devices as a biomaterial.1–3 In the areas of chemical reactions, electrical fields, catalysis, electrochemical sensors and proton conduction, it exhibits encouraging outcomes.4–7 Zr has been the focus of a great deal of research since the middle of the 20th century because it is an extremely reactive metallic compound. Because zirconium is just as biocompatible as titanium, it can be used in orthopaedic and dental applications. It has low ionic cytotoxicity and is highly osteocompatible.8 Zr-containing sorbents have been applied to hemofiltration, haemodialysis, peritoneal dialysis and wearable kidney development in the field of nephrology.9–13 Zr is not considered a material that is harmful to human health.14A tris(hydroxymethyl)aminomethane-zirconium complex (SBA-15@n-Pr-THMAM-ZrO) (1) supported on modified SBA-15 is a novel mesoporous catalyst, effectively catalysing the multicomponent synthesis of industrially significant six-membered N-containing heterocyclic compounds.15 The hexagonal mesoporous silica-based catalyst HMS/Pr-Rh-Zr (2) features a large surface area, large pore volume, and wormhole pores, making it an ideal support for heterogeneous catalyst synthesis. The HMS/Pr-Rh-Zr catalyst, prepared by immobilizing a Zr-rhodanine complex on functionalized hexagonal mesoporous silica (HMS), efficiently catalyzes the synthesis of tetrahydrobenzo[b]pyran derivatives and is reusable without significant loss of activity.16 The synthesis of 3,4-dihydropyrimidine-2(1H)-ones via the Biginelli reaction was used to test the catalytic activity of Zr(IV) porphyrin graphene oxide, a cross-linked catalyst that can catalyze reactions in a short time with good to excellent yields. The catalyst was created via the nucleophilic reaction of Zr(IV) coordinated 5,10,15,20-tetrakis(aminophenyl)porphyrin (ZrPPh) with carboxyl groups of the edges of GO (GO-ZrPPh) (Fig. 5).17 Fe3O4@MCM-41@ZrCl2 (3) is a core–shell magnetic mesoporous nanocomposite that was investigated for synthesizing naphthols via a multicomponent reaction. The favorable efficiency of the targeted nanocomposite in the synthesis was induced by the combination of special qualities of MCM-41 as a unique mesoporous compound, the pleasing magnetic nature of Fe3O4 magnetic nanoparticles, and significant catalytical applications of zirconium.18 The catalytic activity of SPVAZP, a hybrid catalyst composed of sulfonated polyvinyl alcohol dispersed in Al-pillared α-zirconium phosphate, was investigated for one-pot synthesis of multicomponent 4,6-diarylpyrimidin-2(1H)-ones. ZP and AZP materials served as the host lattice for sulfonated polyvinyl alcohol dispersion.19 The α-zirconium phosphate-based nanocatalysts BSA@α-ZrP 4 and α-ZrP/uracil/Cu2+ demonstrated promising catalytic activity in synthesizing 2-substituted benzimidazoles. α-ZrP is employed as a stable support for green organic molecules such as butane sulfonic acid (BSA) and uracil as well as to tune the metal interactions.20 The mesoporous catalyst FeCl4@[Zr-UiO-66-PDC-SO3H] 5 is used for synthesizing biologically significant dihydrobenzo[g]pyrimido[4,5-b]quinoline derivatives incorporating uracil and henna moieties.21 With ZrO2 supported on a magnetic charcoal material, CoFe2O4/BC-ZrO2 is an effective catalyst that can be retrieved by an external magnetic field and recycled at least five times without noticeably losing its activity. For the one-pot synthesis of spiro[furo[3,4-b]quinoline], it exhibits high catalytic activity.22 The primary benefit of nano-ZrO2 is that it demonstrated moderate recyclability and could be recovered using a decantation approach or basic filtration, in addition to its selectivity. The primary benefits of nano-ZrO2 are its high reproducibility, ease of handling, recyclable nature, low mole percentage, and lack of leaching effect during organic conversions. Excellent recyclability of up to five cycles in dry ethanol was demonstrated by the nano-ZrO2 catalyst. Catalytic activity in the synthesis of benzimidazoles,23 dihydropyrimidinones,24 imidazoles,25 and pyranopyrazoles26 is being studied. A cobalt-incorporated sulphated zirconia, (ZrO2/SO42−/Co), is an effective heterogeneous recyclable multifunctional nanocatalyst. The catalyst's heterogeneous nature with nearly minimal metal leaching was demonstrated by a hot filtration test. With minimal efficiency loss, the catalyst was recycled for at least eight runs in a row. The catalyst's ability to catalyze the production of 1,8-dioxo-octahydroxanthene was investigated.27 Zr-KIT-6 is an inexpensive, environmentally friendly Lewis acid catalyst, investigated for synthesizing N-aryl pyrroles. Since adding Zr to the KIT-6 framework improved its dispersion, it exhibits better activity than ZrO2. KIT-6 was chosen because of its high surface area and distinctive Ia3d cubical porous network, which improve mass transport for reactants and large product molecules (Fig. 1).28
Zirconium catalyst is used in the synthesis of organic compounds that are important to biology in a variety of forms. Two naturally occurring pyranone derivatives with medical significance were synthesized quickly and efficiently using chiral zirconium complexes and the asymmetric hetero-Diels–Alder reaction: (+)-prelactone C 6 (ref. 29–32) and (+)-9-deoxygoniopypyrone 7.30,33 Pyridopyrimidine 8 derivatives are very desirable because of their many biological applications,34 including antiaggressive,35 antihypertensive,36 antiasthmatic,37 tuberculostatic,38 antimicrobial,39 calcium channel antagonists,40 antileishmanial,41 anticonvulsants,42 antibacterial,43 diuretic and potassium-sparing,44 anti-inflammatory and analgesic45 as well as antiallergic and antifolate46 properties. Furthermore, pyridines are known to function as herbicides, insecticidal agents, antagonists and inhibitors.47–50 The synthesis of β-amino carbonyl derivatives 10 and benzylamino coumarin derivatives 11 is facilitated by ZrO2, a magnetic heterogeneous nanocatalyst.51 The Mannich reaction yields β-amino carbonyl compounds, which are crucial synthetic intermediates found in numerous natural and pharmaceutical products. Benzylamino coumarins are significant members of the heterocyclic chemical family with antioxidant, anti-HIV and anticoagulant properties.52–54 Coumarins catalysed by nano-crystalline sulfated zirconia (benzo-2-pyrone derivatives)55 are significant coumarin compounds with a range of bioactivities and other uses.56 β-Methylumbelliferone, or 7-hydroxy 4-methyl coumarin 9, is a useful laser dye and fluorescent brightener that can be used as a raw material to make insecticides and furano coumarins.57–59 Similarly, the main applications for 7-amino 4-methyl coumarin are as an intermediate in the synthesis of bioactive compounds and as a laser dye.60
One of the primary nanomaterials used in the manufacturing of ceramics, foundry sands and refractories is zirconia (ZrO2) NPs. They are helpful in the biomedical domains of implants, cancer treatment, dentistry and biosensors, among others, because of their robust mechanical qualities. It works well as a catalyst for chemical processes like oxidation, hydrogenation, dehydration and elimination because of its unique chemical properties. Zirconium catalyst is used to combine heterocyclic spiroindoles, quinoxaline, quinoline, and furan-2,4[2H,5H]-dione to create a variety of unique compounds with biological and structural activity. Derivatives of tetrahydropyridines acted as a favourable scaffold for a variety of naturally occurring and artificially produced bioactive compounds, including ciprofloxacin 15, arecoline 14, awaine 13, homoclausenamide 12 and Lapadin B.61,62 N-Aryl pyrroles are a significant part of certain drugs such as pyrrolylimide 16 (anti-mycobacterial), lamellarin 17 (antiviral), pyrvinium 18 (anthelmintic), and BM212 19 (anti-microbial) (Fig. 2).
A vast variety of heterocyclic compounds are synthesized using zirconium catalyst. Zirconium in various forms such as ZrO2, Zr(IV), zirconium silicates, zirconium inorganic salts, organozirconium complexes, zirconium bimetal oxides and zirconium based MOFs are employed in synthesis of bioactive organic compounds. This review covers the catalytic applications of zirconium-mediated multicomponent reactions, C–H functionalization (Fig. 3), hydroaminoalkylation, etc.
Considering the synthetic utility of zirconium catalysis, significant research groups have summarized its remarkable applications. However very few reports have been published to emphasize the use of zirconium catalysis towards organic synthesis. In 2022, Peng's research group63 summarized the zirconium catalysis in organic synthesis. This work emphasizes the application of inorganic zirconium catalysts—both those incorporating cyclopentadienyl (Cp) ligands and those without—in facilitating diverse organic transformations. In 2024, Chattopadhyay provided a comprehensive review of zirconium-based metal–organic frameworks (MOFs), detailing their synthesis and highlighting their potential in biomedical applications.64 In 2011, Zhang reviewed the applications of zirconium catalysts in organic synthesis that cover the zirconium mediated organic named reactions.65 An overview of zirconia based solid acids for organic synthesis, which is restricted to their usage, was provided by the K. Patil research group in 2011.66 Zirconium mediated synthesis of heterocyclic bioactive organic compounds and their intricate reaction mechanisms is the main topic of our review. Since 2022, there have been no comprehensive reviews published on zirconium-mediated organic synthesis. This represents a significant research gap that our review aims to address.
2 Literature review
2.1. Synthesis of substituted imidazoles
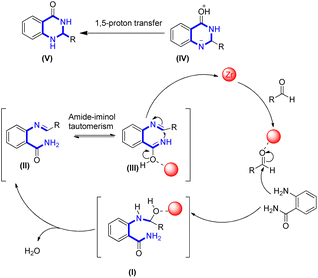
Imidazole is a 1,3-diazole that is a member of the alkaloid class. Heterocycles in the imidazole class have different substitutions but an analogous ring structure. Important cellular architectures like histidine 24 and histamine 25 hormone have this ring system. The imidazole ring is also present in antifungal medications and nitroimidazole 26.67–70 Imidazole derivatives 23 were created by Singh and colleagues in a one pot at 110 °C without the use of solvents, wherein isatin derivatives 20 reacted with ammonium acetate 21 and substituted benzaldehydes 22 (Scheme 1).25Scheme 2 provides the efficient mechanism for the synthesis of substituted imidazoles. Diamine intermediate I speeds up the process. Condensing diamine with derivatives of isatin, dehydrating it, and then rearranging it through imine intermediate II produced the desired result.
Benzimidazoles are essential synthetic intermediates in drug discovery. Many biological activities including antitumor, antibacterial, antifungal, anti-inflammatory, antiviral and analgesic effects are exhibited by benzimidazoles and their derivatives.71 Rao and colleagues produced novel benzimidazole derivatives with antifungal and antibacterial properties using a simple, highly productive and environmentally safe catalyst. Using dry ethanol as a solvent, O-phenylenediamine (o-PDA) 27 and aromatic aldehydes 28 react with ZrO2 at 60 °C to produce 2-arylbenzimidazoles 29. The highest active potency of benzimidazole derivatives 29a–c is against Escherichia coli bacterium. These derivatives are all extremely effective substances with antifungal properties (Scheme 3).23
Benzimidazole derivatives are a specific category of heterocyclic compounds in chemical72–78 and pharmaceutical settings.79–83 Using benzamidine 30, HOAc, and ZrP/uracil/Cu2+ in CH3CN refluxing at 80 °C under direct O2 flow, Hajipour and colleagues synthesized benzimidazoles 31.
A proposed mechanism for the synthesis of benzimidazole is shown in Scheme 4 along with background information from earlier literature.20,84,85 This mechanism suggests that the reaction of benzamidine 30 with Cu(II) in α-ZrP/Uracil/Cu2+ results in I, where HOAc aids in the coordination of nitrogen and copper. The transfer of the ring bond generates a positive charge on the aromatic ring, which is promptly offset by the bond shift, and a metal cycle with the copper centre is formed II. The processes of aromatization and reductive elimination lead to the formation of the benzimidazole product III. Both the catalyst and the HOAc were eventually recovered. Hajipour and colleagues made benzimidazoles 34 by dissolving 1,2-phenylenediamine 33 (1 mmol) in 3 mL of ethanol. BSA@α-ZrP (15 mg) and aromatic aldehyde 32 (1.1 mmol) were added to the solution that was previously prepared (Scheme 5).
A feasible process for the synthesis of benzimidazoles is shown in Scheme 6. BSA@α-ZrP first activates the aromatic aldehyde. An aldehyde containing the amino group of 1,2-phenylenediamine condenses to form imimine I. Intermediate II is created when a nucleophile targets the leftover NH2 in the presence of a catalyst. In the end, intermediate II was oxidatively aromatized at room temperature to create the benzimidazole product III. To ensure that air and oxygen played a critical role in the final step, the reaction was conducted in an argon atmosphere; no product was synthesized.20
2.2. Synthesis of substituted pyrazole derivatives
The two nitrogen atoms in pyrazole and its derivatives make them heterocyclic compounds with a variety of biological activities. These properties include antioxidant, antidiabetic, anticancer, and antimicrobial effects.86–91 Zirconium magnetic nanocomposite is a green catalyst employed in the production of derivatives of pyrazole. It can be reused without losing any of its effectiveness. Because of its large specific surface area, the zirconium magnetic nanocomposite (Fig. 4) exhibited effective biological activity against Gram-positive and Gram-negative fungal species. Asiri and colleagues produced pyrazole derivatives by a one-pot component reaction involving aromatic aldehyde derivatives, malononitrile, phenyl hydrazine and ethyl acetoacetate using a zirconium magnetic nanocomposite as a catalyst.92Aldehyde 35, malononitrile 36, ethyl 3-oxobutanoate 37, phenylhydrazine 38 and zirconium magnetic nanocomposite (Fig. 4) are combined in a one pot reaction to synthesize 1,4-dihydropyrano pyrazole 39 in excellent yield (Scheme 7).
 | ||
| Scheme 7 Zr-catalysed one pot synthesis of (R)-6-amino-3-methyl-1,4-diphenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile. | ||
Using a zirconium magnetic nanocomposite, Scheme 8 provides a believable reaction mechanism for the synthesis of (R)-6-amino-3-methyl-1,4-diphenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile 34.
 | ||
| Scheme 8 Proposed mechanism for synthesizing (R)-6-amino-3-methyl-1,4-diphenyl-1,4-dihydropyrano[2,3-c]carbonitrile pyrazole-5. | ||
Using multi-component reactions (MCRs), Basappa and colleagues synthesized environmentally friendly nano-ZrO2 catalysed pyranopyrazoles 44. Compared to well-known synthetic methods, this method produced a good yield of products with fewer by-products while reducing the cost, time and energy required for the synthesis. The five-component reaction involving benzaldehyde 40, substituted hydrazine 41, malononitrile 42, ethyl 3-oxobutanoate 43 and nano-ZrO2 20 mol% in an H2O–EtOH mixture produced a noteworthy yield of 75% in 50 minutes. MCF-7 cell viability is lost by pyrazopyrazole derivatives 44a and 44c, with IC50 values of 17.83 and 23.79 μM, respectively. Research on the in vitro and in silico mechanisms of action of pyranopyrazoles has revealed that 44b and 44c derivatives have strong IC50 values and target the cyclin-dependent kinase (Cdk1) protein on human breast cancer cells (Scheme 9).
Scheme 10 shows a presumable mechanism for the synthesis of pyranopyrazoles, in which nano-ZrO2 functions as both a base and a Lewis acid. Initially, ethyl-3-oxobutanoate and substituted hydrazine hydrate were condensed to create pyrazolone I derivatives. By absorbing an electron pair from the carbonyl oxygen, ZrO2 functions as a Lewis acid, facilitating the reaction between hydrazine hydrate and ethyl acetoacetate. ZrO2's base site can be activated by malononitrile II to produce an active methylene group. It initiates the benzaldehyde and malononitrile Knoevenagel condensation reaction, which yields arylidene malononitrile. After arylidene malononitrile and pyrazolone undergo the Michael addition reaction, cyclization and tautomerization take place to produce pyranopyrazoles 44.26
Aldehyde 47, hydrazine hydrate 48, ethyl acetate 49 and malononitrile 50 were mixed and heated to 35 °C. EtOH acts as a catalyst for (R)-6-amino-3-methyl-4-phenyl-1,4-dihydropyrano[2,3-c] when combined with HMS/Pr-Rh-Zr (0.01 g) to synthesize 5-carbonitrile pyrazole 51 (Scheme 11).
 | ||
| Scheme 11 Scheme for the synthesis of (R)-6-amino-3-methyl-4-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile. | ||
A tenable process for 1,4-dihydropyrano pyrazole-5-carbonitrile 51 synthesis is illustrated in Scheme 12. With the use of a catalyst, malononitrile and activated aldehyde undergo Knoevenagel condensation, generating intermediate I, an arylidene malononitrile intermediate. Pyrazone (intermediate II) was formed from the condensation reaction that occurred among hydrazine and activated ethyl acetoacetate. Ultimately, a Michael addition reaction was observed between the enolized pyrazolone and the arylidene malononitrile. The intermediate is then tautomerized to yield 1,4-dihydropyrano pyrazole-5-carbonitrile.93
 | ||
| Scheme 12 Mechanism for the synthesis of (R)-6-amino-3-methyl-4-phenyl-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile. | ||
2.3. Synthesis of substituted pyrimidinones
Deveshegowda and colleagues synthesised dihydropyrimidinones 55 using polarized surface nano-ZrO2. Peroxisome proliferator-activated receptor (PPAR)-γ 56 was discovered to be the target of the DHPs; these ligands were observed to be toxic against MCF-7 cell proliferation, and compounds 55a and 55b inhibit proliferation, with IC50 values of 11.8 and 15.8 μM, respectively. By using nano-ZrO2 as a catalyst, organic substrates and reagents can selectively form products due to the increased surface area of the nano-constituents. The Biginelli reaction was carried out by refluxing urea 52, benzaldehyde 53, ethyl acetoacetate 54 and 20 mol% nano-ZrO2 catalyst in EtOH for 60 minutes, yielding 90% products (Scheme 13).The Biginelli reaction mechanism (Scheme 14) begins with a condensation reaction between urea and benzaldehyde, which is then catalysed by a second urea molecule to add ethyl acetoacetate. It is carried out using a catalyst system decorated with sulfonic acid and imidazolium in a solvent-free environment.24
Recently, synthetic organic chemistry has been very interested in the pharmacological and therapeutic properties of 3,4-dihydropyrimidin-2(1H)-ones, which has led to their synthesis.94 Fig. 5 shows the synthesis of GO-ZrPPh 57 catalyst that catalyse 3,4-dihydropyrimidin-2(1H)-one synthesis.
Ghadamyari and colleagues added GO-ZrPPh 57 catalyst to a solution of aromatic aldehydes 58, ethyl acetoacetate 59 and urea 60 in a solvent-free environment at 70 °C for 35–60 minutes, yielding substituted (R)-ethyl 6-methyl-2-oxo-4-phenyl-1,2,3,4-tetrahydropyrimidine-5-carboxylate 61. The catalyst was sucked out of the reaction mixture and heated ethanol was added when the reaction was completed, with excellent yields of 80–93% observed for the various derivatives 61a–d (Scheme 15).
Scheme 16 presents a plausible process for zirconium-coordinated porphyrin bonding to graphene oxide (GO), yielding 3,4-dihydropyrimidin-2(1H)-ones 61. To produce the final product, urea must be attacked with a nucleophile, condensed with ethyl acetoacetate, water must be removed, and Zr IV Lewis acid must react to activate the carbonyl group of aromatic aldehydes.17
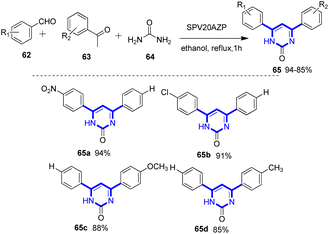
The pyrimidine moiety of organic compounds has attracted a lot of interest in synthetic chemistry due to its biological significance, potential as a therapeutic agent and frequent presence in a wide range of natural products.95,96 The pyrimidine ring is a fundamental structural component of numerous synthetic and natural drug molecules, vitamins, chemotherapeutic agents, herbicides, and dyes. In recent decades, an enormous research has been conducted on the pharmacological actions of the structural analogues of pyrimidine, specifically 3,4-dihydropyrimidin-2(1H)-ones and 4,6-diarylpyrimidin-2(1H)-ones 60.95–97 The biological activities of these nitrogen-containing heterocycles are diverse and include antibacterial, anti-inflammatory, anti-hypertensive, antiviral, and anti-tumour properties.95–98 Majhi and colleagues synthesized 4,6-diarylpyrimidin-2(1H)-ones 65 shown in Scheme 17. For the one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones, the catalytic activities of the SPVxZP and SPVxAZP materials have been assessed. This was achieved by condensation of aryl aldehydes 62, ketones 63, and urea 64 in an ethanolic medium under reflux conditions.19
2.4. Synthesis of substituted quinolines and quinazolinone
Quinolines possess various pharmacological properties such as antimalarial,99 antitubercular,100 antifungal,101 anthelmintic, cardiotonic, anticonvulsant, analgesic,102 anti-inflammatory, antibacterial and anticancer103 activities. Tasqeeruddin and colleagues synthesized hexahydroquinoline derivatives 70 via one pot reaction of benzaldehyde 66, 5,5-dimethylcyclohexane-1,3-dione 67, pentane-2,4-dione 68, ammonium acetate 69 and 10 mol% ZrCl2·8H2O conducted in a variety of solvents, including EtOH, DMF, CH2Cl2, and MeCN (Scheme 18).104Fig. 6 illustrates potential biological and pharmacological uses for 1,4-dihydropyridine structures containing uracil and henna (2-hydroxynapthalene-1,4-dione).105,106 Frameworks containing uracil moieties have antitumor,107 cardiotonic,108 heptaprotactive,109 anticoagulants,110 antibronchitic111 and antifungal activity.112
 | ||
| Fig. 6 Biological substances with moieties of dihydropyridine, henna, and uracil in their structure. | ||
Substituted hexahydroquinoline carboxamide shows a variety of bioactivities such as antitubercular113 and anticancer114 and is also used for the blockage of calcium channels. Sandeep and colleagues synthesized 1,4-dihydropyridine 75 using aromatic aldehyde 71, 1,3-cyclohexadione 72, acetoacetanilide 73 and ammonium acetate 74 (Scheme 19).
The catalyst's Lewis acidic patches will help the reaction proceed more smoothly overall. Lewis acidic sites and carbonyl oxygen have to coordinate to form a Knoevenagel condensation product. As a result, an intermediate I carbonium ion formed. Then, there was an interaction between the intermediate I and the active methylene group of the acetoacetanilide. After the intermediate separates from the catalyst surface, it becomes intermediate II by absorbing a proton from the EtOH. The crucial intermediate I′ is then produced by intramolecular dehydration, and the target molecules are obtained by condensation of intermediate II′ (Scheme 20).
Metal–organic frameworks, or MOFs, are coordinating networks with an open structure made up of organic linkers and inorganic nodes that may have voids.115–117 Zr-based MOFs are interesting materials for biological applications like drug administration and bio imaging because of their low toxicity, adaptable surface properties, and structural stability under physiological conditions.118 For instance, it has been shown that Zr-based MOFs may effectively encapsulate azo chemicals with anticancer properties that are soluble in water, especially in the hypoxic environments linked to pancreatic cancer.119 Since zirconium has low toxicity,120 zirconium-based metal–organic frameworks (MOFs) are probably one of the most studied classes of materials in MOF chemistry because they are easily obtainable and reasonably priced. This stability is consistent with the hard and soft acids and bases (HSAB) principle and results from the robust coordination link between the strongly Lewis acidic Zr4+ ions and the strongly Lewis basic oxygen anions from the ligand molecules.121 MOFs with large surface areas and stability are typically favoured for potential uses such as catalysis, gas storage, heat transformation, and medical programs.122,123 Initially, the MOFs [Zr-UiO-66-PDC] were made by a previously established method.124 A mixture of ClSO3H 77 and [Zr-UiO-66-PDC] 76 in dry CH2Cl2 at 0 °C was stirred for two hours in a 50 mL round-bottom flask. Following that, a white precipitate developed and was vacuum-dried after being separated by centrifugation. Next, a mixture of [Zr-UiO-66-PDC-SO3H]Cl 78 and FeCl3 79 was stirred in a mortar for two hours at 50 °C using the anion exchange method. The reaction mixture was allowed to cool to room temperature once the reaction was finished. Lastly, [Zr-UiO-66-PDC-SO3H]FeCl4 80 was triturated in acetone to purify it (Fig. 7).
Combining [Zr-UiO-66-PDC-SO3H]FeCl4 80 with aldehyde 81, 2-hydroxynaphthalene-1,4-dione 82, and 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione 83, Jalili and colleagues synthesized the compound (S)1,3-dimethylbenzo[g]-5-(4-methoxyphenyl)2,4,6,11 pyrimido[4,5-b]quinoline (1H,3H,5H,12H)tetraone 84 (Scheme 21).
 | ||
| Scheme 21 Synthetic procedure for (S) derivatives of 1,3-dimethylbenzo[g]-5-(4-methoxyphenyl)pyrimidine[4,5-b]2,4,6,11(1H,3H,5H,12H)-quinoline-tetraone. | ||
According to the suggested mechanism, the aldehyde's carbonyl functional group is activated by the [Zr-UiO-66-PDC-SO3H]FeCl4 80 catalyst. 4-Methoxy benzaldehyde 81 was reacted with [Zr-UiO-66-PDC-SO3H]FeCl4 80 at room temperature to examine the activation of the aldehyde. One H2O molecule is then extracted from the aldehyde by the henna (2-hydroxynaphthalen-1,4-dione) 82 moiety when it combines with the carbonyl to form intermediate I. The next step is to react intermediate I with 6-amino-1,3-dimethylpyrimidine-2,4(1H,3H)-dione 83 to create intermediate II. After intramolecular cyclization and the loss of an additional H2O molecule, intermediate II produces the desired product in two steps (Scheme 22).21
 | ||
| Scheme 22 Proposed mechanism for the synthesis of (S)1,3-dimethylbenzo[g]-5-(4-methoxyphenyl)pyrimidine[4,5-b]2,4,6,11(1H,3H,5H,12H) quinolinetetraone. | ||
Polyhydroquinolines, which are heterocyclic compounds, are mostly present in a variety of natural products and are crucial for the creation of novel medications and synthetic organic chemistry.125–131 Arash and colleagues utilized the tris(hydroxymethyl)aminomethane-ZrO complex to fill the SBA-15's pores and produce a new type of heterogeneous mesoporous catalyst. It was shown that the mesopore material may be used to combine several components to create a broad range of industrially important six-membered N-containing heterocyclic compounds, such as (S)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one and polyhydroquinolines (Scheme 23).
Arash and colleagues synthesized polyhydroquinolines 90 by dissolving a mixture of dimedone 86, aryl aldehyde 87, ammonium acetate 88, ethyl acetate 89 and ZrO catalyst in ethanol and stirring it under reflux conditions for the necessary amount of time. The reaction mechanism for the synthesis of polyhydroquinolines 90 is shown in Scheme 24.
Arash and colleagues prepared (S)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one 93 by filling the flask with ZrO catalyst, swirling the mixture during reflux and dissolving the combination of aromatic aldehyde 91 and anthranilamide 92 in three millilitres of ethanol (Scheme 25).15 Scheme 26 presents the plausible reaction mechanism for the synthesis of (S)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one 93.
Because of its biological and pharmaceutical properties, quinazolinone and its derivatives are a significant class of heterocycles that contain nitrogen.132–135 These substances are also useful starting points for the synthesis of several pharmaceuticals that are sold commercially.136 Quinazolinone 96 are synthesized by the reaction of 2-aminobenzamide 94 and ketone 95 in the presence of acetic acid and UiO-66-MW Zr complex (Scheme 27). The possible reaction mechanism for quinazolinone synthesis is shown in (Scheme 28).
Polyheterocyclic compounds are extensively used in food, medicine, building materials and fine chemicals.137 Many natural alkaloids and pharmaceuticals contain heterocyclic spiroindoles, which are also used as building blocks in synthetic organic chemistry.138 Quinoxaline derivatives have a variety of pharmacological characteristics, such as antihyperglycemic, anticonvulsant, analgesic, anti-HIV, antidepressant and anticancer effects.22,139 Furan-2,4[3H,5H]-dione, a heterocyclic tetronic acid, exhibits antibacterial, fungicidal, and insecticidal properties.140,141 Biochar (BC) is a biomass carbon based material that is obtained from agricultural waste, pyrolysis of sediments and biomass in a hypoxic environment. BC qualities include a large surface area, high permeability, potent adsorption capability, good stability, and modifiable functional groups.142,143 Conversely, ZrO2 is a commonly utilized catalyst in organic reactions.63 Zhang and colleagues designed an effective magnetic recyclable catalyst (CoFe2O4/BC-ZrO2) supported on magnetic BC for the synthesis of bioactive organic compounds. By using benzene-1,2-diamine 97, tetronic acid 98 and indoline-2,3-dione 99 as three separate components in a three-component reaction, spiro[furo[3,4-b]quinolones] 100 were synthesized. Yields are adjusted by varying the reaction parameters and the neutral, electron-donating or electron-withdrawing R groups (Scheme 29).
 | ||
| Scheme 29 Zr-catalysed synthesis of 1H,1′H-spiro[furo[3,4-b]quinoline-9,2′-quinoxaline]-1,3′(3H,4H,4′H)-diones. | ||
A possible mechanism (Scheme 30) for this reaction is that enormous number of hydroxyl groups present on catalyst surface form hydrogen bonding with the indoline-2,3-dione carbonyl group to produce intermediate I. Intermediate II is synthesized when the amino groups of benzene-1,2-diamine undergoes an aldoamine condensation reaction with intermediate I. Attack of amino groups of intermediate II on the activated carbonyl group generates intermediate III. The catalyst activates the tetronic acid carbonyl position by forming a hydrogen bond with the OH group, resulting in the production of intermediate IV. With the help of the catalyst, intermediate IV is formed into σ–π. Intermediate V is then produced by intramolecular cyclization, and its tautomerization yields the final products 100.22
 | ||
| Scheme 30 Mechanism for the formation of 7-chloro-1-H-spiro[furo[3,4-b]quiloxaline-9,2′-quinoline]-1,3′(3H,4H,4′H)-dione. | ||
2.5. Synthesis of substituted pyridines
Tetrahydropyridine derivatives are favourable scaffolds for a variety of naturally occurring and artificially produced bioactive compounds, including ciprofloxacin, arecoline, homoclausenamide 105, awaine 106, and lapadin B.61,62 Tetrahydropyridines are interesting target molecules for synthesis due to their varied pharmacological activities such as analgesic,144 hyperglycemic,145 anticonvulsant,146 antitumor,147 vasodilators,148 antioxidant149 and HIV protease inhibitor.150 Yelwande and colleagues used a polyaniline zirconium oxide catalyst to synthesize tetrahydropyridines 104 in a one-pot manner by condensation of aldehydes 101, anilines 102, and β-keto esters 103 (Scheme 31).Scheme 32 illustrates a reasonable process for the synthesis of tetrahydropyridines 104. ZrO2 functions as a Lewis acid catalyst when aniline and methyl acetoacetate or benzaldehyde react, resulting in the production of β-enaminone or imine. The steps involved in forming a product are intermolecular Mannich reaction, condensation, tautomerism, intramolecular Mannich reaction and tautomerism at the last step.151
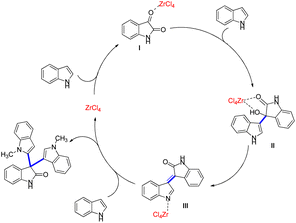
Indole derivatives are substances with biological and pharmacological activity that are used as intermediate products in the synthesis of organic compounds.152,153 The pharmacological activities of indole derivatives [2,3':3′,3′′-terindolin]-2′-one include antiproliferative,154 antibacterial,155 anti-inflammatory,156 laxative157 and anticonvulsant qualities.158 Hojati and colleagues synthesized indole derivatives 109, when isatin 107 and indole 108 in EtOH were reacted in the presence of ZrCl4 at 50 °C, resulting in a 95% yield (Scheme 33).
Scheme 34 provides a feasible mechanism for indole derivative synthesis. It is plausible that ZrCl4 will coordinate with isatins non-amidic carbonyl group and activate intermediate I. Then, (S)-3-hydroxy-3-(1H-indol-3-yl)indolin-2-one II is produced as a critical intermediate by nucleophilic attack of indole at position I, and this is converted into III as a result of H2O loss. N-activated III is combined with the second indole to create the analogous product, and ZrCl4 proceeds to the subsequent catalytic cycle.159
The Flavivirus genus includes a family of encased RNA arthropod-borne viruses, which cause a number of serious diseases in humans and animals, such as the Kyasanur Forest disease virus (KFDV), (JEV), (WNV), Dengue virus (DENV), Zika virus (ZIKV), and Yellow fever virus. 2-Benzylidene-1H-indene-1,3(2H)-dione derivatives exhibit flavivirus protease inhibitory activity.160 2-Benzylidene-1H-indene-1,3(2H)-dione 112 was designed using a simple and efficient Knoevenagel method. ZrOCl2.8H2O was used as a catalyst in the reaction between indan-1,3-dione 110 and various aromatic aldehydes 111 to create these moieties, with water acting as a solvent. The generated compounds were evaluated for their capacity to inhibit the NS2B-NS3 protease of the West Nile virus (WNV) (Scheme 35).161
2.6. Synthesis of substituted pyrrole derivatives
Pyrroles rings have gained considerable devotion because of their advantages in biological and pharmaceutical products, such as cytomegalovirus protease,162 CD45 protein tyrosinphoshate,163 anticancer,164 thiomarinol A3 antibiotic,165 alkaloids,166 UCS1025A,167 and oteromycin.168 These rings show herbicidal activities169 and are a component of HIV integrase.170Using a mixture of amine 113 and dialkyl acetylenedicarboxylate 114, which was stirred in MeOH for 15 minutes, Mohamadpour synthesized substituted dihydro-2-oxopyrroles 117 by adding ZrOCl2·8H2O (15 mol%), amine 115 and formaldehyde 116, and stirring the reaction for the appropriate amount of time (Scheme 36).
Scheme 37 depicts the suggested procedure for producing highly substituted dihydro-2-oxopyrroles 117 when ZrOCl2·8H2O is present. To produce intermediate I, dialkyl acetylenedicarboxylate 114 and an amine 113 first react. Second, imine is produced by the condensation of amine and formaldehyde 116 in the presence of ZrOCl2·8H2O. Because of its enamine nature, intermediate I can easily react with imine II in ZrOCl2·8H2O to produce intermediate III. The final step involves tautomerizing intermediate IV to the corresponding highly substituted dihydro-2-oxopyrroles V after intermediate IV is cyclized.171
Pyrroles are needed to make agrochemicals, drugs, dyes, and molecular materials for electronics and transmission applications.172–174 N-Aryl pyrroles exhibit noteworthy biological properties such as anticancer,175 antitumor,176 antifungal,177 anti-tubular,178 anti-mycobacterial,179 anti-HIV,180 and anti-inflammatory.181 Currently, the pharmaceutical, polymer, and other chemical-based industries require more than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of pyrroles per year to meet their needs. A low-cost, easily scalable Lewis acid catalyst based on zirconium is called Zr-KIT-6. Using Zr-KIT-6 catalyst, condensation of 2,5 DMF 118, and aniline 119 under ideal reaction conditions, Manal and Srivastava produced N-aryl pyrrole 120 (Schemes 38 and 39).28
000 tons of pyrroles per year to meet their needs. A low-cost, easily scalable Lewis acid catalyst based on zirconium is called Zr-KIT-6. Using Zr-KIT-6 catalyst, condensation of 2,5 DMF 118, and aniline 119 under ideal reaction conditions, Manal and Srivastava produced N-aryl pyrrole 120 (Schemes 38 and 39).28
2.7. Synthesis of substituted benzopyran
Zirconium silicates are abundant in nature and have received an abundance of intrigue, mostly involving the resolution of general geophysical and mineralogical issues, due to their creation under hydrothermal circumstances (between ≈300 and ≈550 °C).182 Approximately one-third of the known zirconium silicates, both natural and manufactured, have crystal structures that have been determined. Maurice conducted some of the earliest hydrothermal synthesis of zirconium silicates in 1949.183,184 Derivatives of tetrahydro-1-benzopyran show various effects on biological processes, such as anti-anaphylactic, anti-cancer, diuretic, spasmolytic and anticoagulant properties.185 They are also used to treat AIDS, amyotrophic lateral sclerosis, neurodegenerative diseases and cognitive enhancers.186 Mishra and colleagues used multicomponent condensation of different aldehydes 123 with malononitrile 124 and dimedone 125 to create bioactive tetrahydro-1-benzopyran derivatives 126. Many benefits are provided by this protocol, including high yields, an easy-to-follow experimental work-up process, a quick reaction time, absence of by products, affordability, simple reliability of catalysts and detoxification (Scheme 40).Scheme 41 provides a tenable mechanism for tetrahydro-1-benzopyran synthesis 126. ZrRHSi activates aldehyde 123 in the reaction, which is then attacked by dicyanomethanide to form Knoevenagel adduct I. This adduct then combines with the enol form of dimedone 125 to produce intermediate II. Later, it is cyclized to produce tetrahydro-1-benzopyrans.187
Benzopyran and pyrano[2,3-c] pyrazole are extensively used in pharmaceutical chemistry and biosciences. They are used as antibacterial,188,189 anticancer,190 anti-inflammatory191,192 and antioxidants.193,194 Abdolahi and colleagues synthesized tetrahydrobenzo[b]pyran 130 using HMS/Pr-Rh-Zr added to a mixture of aldehyde 127, malononitrile 129 and dimedone 128 in polyethylene glycol (PEG) at 80 °C (Scheme 42).
 | ||
| Scheme 42 Synthesis of (S)-2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile derivatives. | ||
Tetrahydrobenzo[b]pyran formation in the presence of HMS/Pr-Rh-Zr is shown to be a likely reaction mechanism in Scheme 43. When activated aldehyde 127 and malononitrile 129 react, arylidene malononitrile intermediate I is produced, marking the start of the reaction. Arylidene malononitrile intermediate I produced in the preceding step reacts with enolized dimedone 128 II in the subsequent step. Ultimately, tetrahydrobenzo[b]pyran 130 is created because of rearranging and intramolecular cyclization.16
 | ||
| Scheme 43 Plausible mechanism for the synthesis of (S)-2-amino-7,7-dimethyl-5-oxo-4-phenyl-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile. | ||
The core xanthene structure can be efficiently synthesized using ZrO2/SO42−/Co catalyst. Further functionalization of this core structure lead to the formation of various artificial dyes, such as rhodamine, fluoresceins and eosins, which have diverse applications (Fig. 8).
Nasseri and colleagues195 synthesized 1,8-dioxo-octahydroxanthene 135 using aldehyde 133 (1 equiv.), 1,3-cyclohexadione 134 (2.1 equiv.) and water as solvent. After adding the catalyst (0.005 g), the mixture was agitated for the required time at 25 °C (Scheme 44).
 | ||
| Scheme 44 Scheme for the synthesis of 3,3,6,6,9-pentamethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione derivatives. | ||
A plausible mechanism was proposed in Scheme 45.196–199 At first, the catalyst's Co and Zr Lewis acid sites activated aldehyde and cyclohexadione derivatives. A cyclohexadione is attacked nucleophilically to initiate the reaction, which is then followed by an aldehyde capturing a proton with the catalyst's Brønsted acid (Scheme 45 I and II). Once more, a water molecule is eliminated by Brønsted acid to create a Knoevenagel product III (Scheme 45). The cyclic intermediate IV is produced by the reaction continuing with another nucleophilic assault of cyclohexadione to III. Lewis and Brønsted acid mediators are used in the catalyst to facilitate the cyclization process V. Ultimately, the process of dehydrating VI yields the required 1,8-dioxo-octahydroxanthene product and regenerates the ZrO2/SO42−/Co catalyst for the subsequent cycle (Scheme 45).27
 | ||
| Scheme 45 Suggested process for synthesizing derivatives of 3,3,6,6,9-pentamethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-dione. | ||
2.8. Synthesis of substituted amides
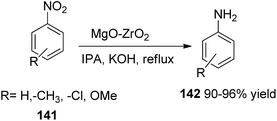
In synthetic organic and coordination chemistry, cyanamides are a prominent class of reactive organic molecules with a wide variety of uses.200–203 It is known that compounds based on cyanamide have a range of intriguing biological properties, including antiviral and anticancer properties.204,205 The suggested method for using zirconium silicate to N-benzylate arylcyanamides is depicted in Scheme 46. The Lewis acidity of the zirconium silicate nanocomposite material in the N-benzyl-N-phenylcyanamide is probably going to play a significant part in this. It was proposed that using a zirconium silicate nanocomposite to activate the C–Br bond of benzyl bromide would enable the synthesis of N-benzyl-N-phenylcyanamide via an SN2-type mechanism in an aprotic solvent of MeCN with nucleophile attack of the phenylcyanamides (Scheme 47).206Aromatic nitro compounds 141 reduction: The catalytic transfer hydrogenation of aromatic nitro compounds 141 to the corresponding amines 142 is an important step in the commercial synthesis of colours, physiologically active compounds, medications, rubber chemicals, photography, and agricultural chemicals.207 Numerous techniques have been created for this aim (Scheme 48).208
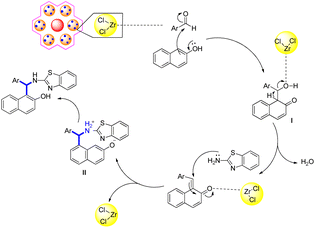
Creating derivatives of (S)-N-(1-(2-hydroxynaphthalen-1-yl)ethyl)acetamide is an interesting use for these MCRs. A lot of attention has focused on derivatives of 1-amidoalkyl-2-naphthols with 1,3-amino functional groups that are oxygenated because they are essential parts of many potent drugs, synthetic pharmaceuticals and bioactive natural products, including HIV protease inhibitors and several nucleoside antibiotics.209 Dipake and colleagues synthesized 1-aminoalkyl naphthols 146 by combining benzamide 145, aldehyde 144 and naphthol 143 at 110 °C with zirconium silicate catalyst without the use of a solvent (Scheme 49).
Scheme 50 illustrates the suggested reaction mechanism for the ZS-1 catalyst-mediated synthesis of (S)-N-(1-(2-hydroxynaphthalen-1-yl)ethyl)acetamide.210,211 Because of the characteristics of the ZS-1 catalyst, the carbonyl group of aldehydes has a lower electron density. B-naphthol's nucleophilic attack on the carbonyl group of activated aldehydes forms intermediate II. Next, the water molecule condensed to form the ortho-quinone methide III intermediate. After that, the ZS-1 catalyst activates the ortho-quinone methide III intermediate, which then allows the Michael addition of amide to yield (S)-N-(1-(2-hydroxynaphthalen-1-yl)ethyl)acetamide IV as expected.212
It is certain that the 2-aminobenzothiazole core plays a wide and important role in industrial, biological, and pharmaceutical chemistry as a preferred scaffold.213–221 Among these kinds of compounds, benzo[d]thiazol-2-amine and a Betti base are the two biologically active components of ((benzo[d]thiazol-2-ylamino)methyl)naphthalen-2-ol. The molecule known as 1-(amino(phenyl)methyl)naphthalen-2-ol, which has two active amino and hydroxyl groups, is called a Betti base and is essential in synthetic chemistry because it can create C–C bonds in mild laboratory settings.221 The targeted nanocomposite exhibited favourable efficiency in the multicomponent condensation reaction of aromatic aldehydes 147, naphthalen-2-ol 148 and benzo[d]thiazol-2-amine 149 in the absence of solvent, owing to the presence of zirconium. These reactions have excellent yields (70–90%) of ((benzo[d]thiazol-2-ylamino)methyl)naphthalen-2-ol 150 (Scheme 51).
The Lewis acidic catalyst Fe3O4@MCM-41@ZrCl2 is said to initiate the reaction by activating the aldehyde's carbonyl group, according to the proposed mechanism. Next, 2-naphthol attacks the activated aldehyde nucleophilically, forming intermediate I. The next stage produces intermediate II and finally the main product by reacting this intermediate with 2-aminobenzothiazole (Scheme 52).18
 | ||
| Scheme 52 Proposed mechanism for the synthesis of ((benzo[d]thiazol-2-ylamino)methyl)naphthalen-2-ol. | ||
α-Arylated amines are used as construction blocks to make medications like L-733-060 154, an antidepressant/anxiolytic and repaglinide 155, an antidiabetic. A novel and cost-effective catalytic technique for creating Csp3–Csp3 bonds α-to nitrogen in primary and secondary amine substrates is hydroaminoalkylation.222–226 The synthesis of (R)-1-phenyl-3-(trimethylsilyl)propan-1-amine 153 through zirconium catalysis was reported by Ana and colleagues. This involved using N-silylated benzyl amines 151 to hydroaminoalkylate activated and unactivated alkenes 152. An easy-to-find commercially accessible Zr(NMe2)4 was discovered to catalyse the formation of Csp3–Csp3 bonds by activating the carbon–hydrogen bond α to nitrogen, resulting in the wanted products after aqueous workup (Scheme 53).
Scheme 54 presents a plausible mechanism for hydroaminoalkylation catalysed by transition metals. After complex I formation, amido groups are transmuted with N-silylamine to form complex II, and the catalytically active zirconaaziridine III with its phenyl substituent is produced upon hydrogen removal. Complex III then undergoes alkene insertion into the more reactive carbon–carbon bond to form zirconapyrrolidine intermediates IV and V, where the steric bulk of the alkene affects the reaction's regioselectivity. Diastereomers IV and IV′ may arise from the formation of intermediate IV, and the major diastereomer is obtained by trans-orienting the metallacyclic intermediates.227,228
Three VANOL 156 ligands encircle zirconium 157 (Fig. 9) and charge is balanced by two protonated N-methylimidazoles. Scheme 56 presents the liberation of α-amino phosphonates.
2.9. Synthesis of substituted triazolidine
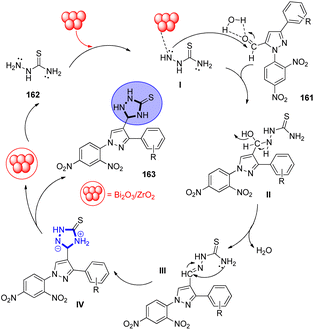
N-heterocycles are essential components of many natural product analogues with a variety of biological functions and are basic components in the discipline of heterocyclic chemistry. Numerous triazoles exhibit antibacterial, anticonvulsant,238 anti-leishmanial,239 anti-tubercular,240 and anticancer241 properties. Among them, triazoles with pyrazole groups stand out due to their potent PDE4 inhibitory properties.242 Kerru and colleagues243 used thiosemicarbazide 165 and water, an environmentally friendly solvent, to create 1,2,4-triazolidine-3-thiones 166 by heating 1-(2,4-dinitrophenyl)-3-phenyl-1H-pyrazole-4-carbaldehyde 164 at 80 °C for 30 to 45 minutes (Scheme 57).Active intermediate I is produced in the first reaction, which is the probable adsorption of thiosemicarbazide 165 onto the Bi2O3/ZrO2 catalyst's active site. The reaction is made possible by the multiple Lewis acid catalytic centres that allow the active sites of bismuth and zirconia to cooperate. The two oxides' active sites working together synergistically results in the improved production of the product. Water may then form a hydrogen bond with the oxygen atom of the carbonyl group and the water molecule, increasing the electrophilicity of the second precursor, pyrazole-4-carbaldehyde 164 carbonyl carbon. Thus, hemiminal intermediate II was created.244,245 The water molecule (H2O) was eliminated to form thiosemicarbazone III, and the ring closed as a result of the free –NH2 group III's intramolecular cyclization and tautomerization. The intended target compound was 5-(1-(2,4-dinitrophenyl)-3-phenyl-1H-pyrazol-4-yl)-1,2,4-triazolidine-3-thione 166 (Scheme 58).
3 Conclusion
The exploration of zirconium metal-based catalysis in synthesizing bioactive molecules has yielded significant potential for advancing pharmaceutical development and creating biologically active compounds. This research showcases the latest breakthroughs in zirconium catalysis, underscoring its effectiveness and versatility. Zirconium catalysis emerges as a sustainable, efficient and adaptable tool for the synthesis of various bioactive heterocyclic molecules such as imidazoles, pyrazole, pyrimidinones, quinolines, quinazolinones, pyridines, pyrroles, benzopyrans, substituted amides and triazolidine. This makes it easier to synthesize intricate chemical structures with excellent efficiency and encourages the formulation of novel proposals for modifying pharmacological compounds. Zirconium catalysis is a powerful synthetic tool, enabling complex reactions under mild conditions while promoting sustainability. Due to its cost-effectiveness, stability and recyclability, zirconium catalysts offers promising advantages for future applications. It is significant to use green and stable zirconium catalysts in pharmaceutical production instead of other metal catalysts. Developments of greener ligands, chiral zirconium catalysts and Zr-based MOFs as heterogeneous catalysts enhances future research. Since there is no comprehensive review on zirconium-mediated organic synthesis published after 2022, there is a significant research gap in this area. Our review aims to address and potentially fill this gap.Data availability
No new data have been generated, and all data are present in the manuscript.Author contributions
S. Bibi: wrote the manuscript. M. Zubair: supervised the project. R. Riaz: conducted literature survey and data analysis. A. Kanwal & S. A. A. Shah: review and editing. All the authors reviewed and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We strongly acknowledge the Organic Material Synthesis Lab of Government College University, Faisalabad.References
- X. Hu, et al., Removal of Zr (IV) from aqueous solution using hydrated manganese oxide derived from the modified Hummers method, Chem. Phys. Lett., 2020, 752, 137585 CrossRef CAS.
- L. Xia, et al., High temperature nano-indentation on the mechanical properties of Zr and Zr–Fe alloys: experimental and theoretical analysis, Mech. Mater., 2021, 162, 104053 CrossRef.
- N. A. Patil and B. Kandasubramanian, Biological and mechanical enhancement of zirconium dioxide for medical applications, Ceram. Int., 2020, 46(4), 4041–4057 CrossRef CAS.
- Y. Cheng, et al., Minimalistic synthesis of α-zirconium diammonium phosphate and zirconia for applications in ion exchange and catalysis, ACS Sustain. Chem. Eng., 2018, 7(1), 895–904 CrossRef.
- G.-Y. Zhang, et al., Magnetic zirconium hexacyanoferrate (II) nanoparticle as tracing tag for electrochemical DNA assay, Anal. Chem., 2015, 87(17), 9093–9100 CrossRef CAS.
- E.-X. Chen, G. Xu and Q. Lin, Robust porphyrin-spaced zirconium pyrogallate frameworks with high proton conduction, Inorg. Chem., 2019, 58(6), 3569–3573 CrossRef CAS PubMed.
- G. Singh, et al., Synthesis of Schiff base functionalized organosilatranes for the detection of Zirconium (IV) ion: Their cytotoxicity evaluation and anti-inflammatory activity against cyclooxygenase-2 via computational approach, Appl. Organomet. Chem., 2024, 38(1), e7297 CrossRef CAS.
- T. Vompe, et al., Sintering of additively manufactured zirconium by MoldJet technology, Powder Technol., 2024, 436, 119494 CrossRef CAS.
- A. Murisasco, et al., Continuous arterio-venous hemofiltration in a wearable device to treat end-stage renal disease, ASAIO J., 1986, 32(1), 567–571 CAS.
- A. Davenport, et al., A wearable haemodialysis device for patients with end-stage renal failure: a pilot study, Lancet, 2007, 370(9604), 2005–2010 CrossRef.
- D. B. Lee and M. Roberts, A peritoneal-based automated wearable artificial kidney, Clin. Exp. Nephrol., 2008, 12, 171–180 CrossRef CAS.
- D. B. Lee, et al., Zirconium: biomedical and nephrological applications, ASAIO J., 2010, 56(6), 550–556 CrossRef CAS.
- N. Ofsthun and A. Stennett, An integrated membrane/sorbent PD approach to a wearable artificial kidney, in World Congress on Medical Physics and Biomedical Engineering, September 7-12, 2009, Munich, Germany: Vol. 25/7 Diagnostic and Therapeutic Instrumentation, Clinical Engineering, Springer, 2009 Search PubMed.
- H. A. Schroeder and A. P. Nason, Trace-element analysis in clinical chemistry, Clin. Chem., 1971, 17(6), 461–474 CrossRef CAS.
- A. Ghorbani-Choghamarani, H. Aghavandi and M. Mohammadi, Mesoporous SBA-15@ n-Pr-THAM-ZrO organic–inorganic hybrid: as a highly efficient reusable nanocatalyst for the synthesis of polyhydroquinolines and 2, 3-dihydroquinazolin-4 (1h)-ones, J. Porous Mater., 2021, 28, 1167–1186 CrossRef CAS.
- S. Abdolahi, M. Hajjami and F. Gholamian, An approach to the synthesis and characterization of HMS/Pr-Rh-Zr as efficient catalyst for synthesis of tetrahydrobenzo [b] pyran and 1, 4-dihydropyrano [2, 3-c] pyrazole derivatives, Res. Chem. Intermed., 2021, 47, 1883–1904 CrossRef CAS.
- Z. Ghadamyari, et al., Zirconium (IV) porphyrin graphene oxide: a new and efficient catalyst for the synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones, Appl. Organomet. Chem., 2019, 33(9), e5091 CrossRef.
- R. Pourhasan Kisomi, F. Shirini and M. Golshekan, Fe3O4@ MCM-41@ ZrCl2: A novel magnetic mesoporous nanocomposite catalyst including zirconium nanoparticles for the synthesis of 1-(benzothiazolylamino) phenylmethyl-2-naphthols, Appl. Organomet. Chem., 2021, 35(6), e6212 CrossRef CAS.
- D. Majhi, et al., Preparation and catalytic application of sulfonated polyvinyl alcohol-Al-pillared α-zirconium phosphate (SPV-AZP) hybrid material towards synthesis of 4, 6-diarylpyrimidin-2 (1H)-ones, J. Porous Mater., 2020, 27, 355–368 CrossRef CAS.
- A. R. Hajipour, S. Zakery and Z. Khorsandi, Synthesis of benzimidazoles by two methods (C–H functionalization and condensation reaction) catalyzed by α-zirconium hydrogen phosphate-based nanocatalyst, J. Iran. Chem. Soc., 2020, 17, 1919–1931 CrossRef CAS.
- F. Jalili, et al., Application of novel metal–organic framework [Zr-UiO-66-PDC-SO 3 H] FeCl 4 in the synthesis of dihydrobenzo [g] pyrimido [4, 5-b] quinoline derivatives, RSC Adv., 2022, 12(15), 9058–9068 RSC.
- S. Z. Zhang, et al., Magnetic biochar-supported ZrO2 as an efficient catalyst for one-pot synthesis of 1′, 4′-dihydro-3 H, 3′ H-spiro [furo [3, 4-b] quinoline-9, 2′-quinoxaline]-1, 3′(4 H)-diones, Appl. Organomet. Chem., 2023, 37(2), e6949 CrossRef CAS.
- T. N. Rao, et al., Reusable nano-zirconia-catalyzed synthesis of benzimidazoles and their antibacterial and antifungal activities, Molecules, 2021, 26(14), 4219 CrossRef CAS.
- S. N. Deveshegowda, et al., Nano-ZrO2-Catalyzed Biginelli Reaction and the Synthesis of Bioactive Dihydropyrimidinones That Targets PPAR-γ in Human Breast Cancer Cells, Catalysts, 2023, 13(2), 228 CrossRef CAS.
- S. Singh and S. Bajpai, Eco-Friendly and Facile Synthesis of Substituted Imidazoles via Nano Zirconia Catalyzed One-Pot Multicomponent Reaction of Isatin Derivatives with Ammonium Acetate and Substituted Aromatic Aldehydes under Solvent Free Conditions, in Nanocatalysts, IntechOpen, 2019 Search PubMed.
- B. Basappa, et al., Nano-Zirconium Dioxide Catalyzed Multicomponent Synthesis of Bioactive Pyranopyrazoles That Target Cyclin Dependent Kinase 1 in Human Breast Cancer Cells, Biomedicines, 2023, 11(1), 172 CrossRef CAS.
- A. N. Dadhania, V. K. Patel and D. K. Raval, Ionic liquid promoted facile and green synthesis of 1, 8-dioxo-octahydroxanthene derivatives under microwave irradiation, J. Saudi Chem. Soc., 2017, 21, S163–S169 CrossRef CAS.
- A. K. Manal and R. Srivastava, Zr-KIT-6 catalyzed renewable synthesis of N-aryl pyrroles for producing bioactive synthetic compounds, Appl. Catal., A, 2023, 119018 CrossRef CAS.
- K. U. Bindseil and A. Zeeck, Metabolic products of microorganisms. Part 265. Prelactones C and B, oligoketides from Streptomyces producing concanamycins and bafilomycins, Helv. Chim. Acta, 1993, 76(1), 150–157 CrossRef CAS.
- Y. Yamashita, et al., Chiral Hetero Diels− Alder Products by Enantioselective and Diastereoselective Zirconium Catalysis. Scope, Limitation, Mechanism, and Application to the Concise Synthesis of (+)-Prelactone C and (+)-9-Deoxygoniopypyrone, J. Am. Chem. Soc., 2003, 125(13), 3793–3798 CrossRef CAS.
- T. Esumi, et al., Enantioselective preparation of 1-benzyloxy-3-methyl-6-heptene-2, 4-diols: Total synthesis of (+)-prelactone C, Tetrahedron Lett., 1997, 38(27), 4823–4826 CrossRef CAS.
- T. K. Chakraborty and S. Tapadar, Diastereoselective opening of trisubstituted epoxy alcohols: application in the synthesis of (+)-prelactone C, Tetrahedron Lett., 2001, 42(7), 1375–1377 CrossRef CAS.
- C. Mukai, S. Hirai and M. Hanaoka, Stereoselective Syntheses of (+)-Goniotriol,(+)-8-Acetylgoniotriol,(+)-Goniodiol,(+)-9-Deoxygoniopypyrone,(+)-Altholactone, and (−)-Goniofupyrone, J. Org. Chem., 1997, 62(19), 6619–6626 CrossRef CAS.
- S. Abdolmohammadi and S. Balalaie, A clean procedure for synthesis of pyrido [d] pyrimidine derivatives under solvent-free conditions catalyzed by ZrO2 nanoparticles, Comb. Chem. High Throughput Screening, 2012, 15(5), 395–399 CrossRef CAS PubMed.
- H. Sladowska, A. Bartoszko-Malik and T. Zawisza, Synthesis and properties of new derivatives of ethyl 7-methyl-2, 4-dioxo-1, 2, 3, 4-tetrahydropyrido [2, 3-d] pyrimidine-5-carboxylate, Farmaco, 1990, 45(1), 101–110 CAS.
- J. W. Ellingboe, Substituted Pyridopyrimidines and Antihypertensives, 1995 Search PubMed.
- A. Rosowsky, C. E. Mota and S. F. Queener, Synthesis and antifolate activity of 2, 4-diamino-5, 6, 7, 8-tetrahydropyrido [4, 3-d] pyrimidine analogues of trimetrexate and piritrexim, J. Heterocycl. Chem., 1995, 32(1), 335–340 CrossRef CAS.
- I. Bystryakova, et al., Synthesis and biological-activity of some pyrido [2, 3-d] pyrimidine derivatives, Khim.-Farm. Zh., 1991, 25(12), 31–33 CAS.
- I. O. Donkor, et al., Synthesis and antimicrobial activity of 6, 7-annulated pyrido [2, 3-d] pyrimidines, J. Pharm. Sci., 1995, 84(5), 661–664 CrossRef CAS PubMed.
- A. Pastor, et al., Synthesis and structure of new pyrido [2, 3-d] pyrimidine derivatives with calcium channel antagonist activity, Tetrahedron, 1994, 50(27), 8085–8098 CrossRef CAS.
- N. Satti, et al., Synthesis and Antileishmanial Activity of Some Pyrido (1, 2-a) pyrimidines and Phenanthrolines, Cheminf., 1993, 24(51), 978–980 Search PubMed.
- A. Deyanov, et al., Amides, Nitriles of 2-Arylamino-5-Carboxy (Carbethoxy)-6-Methylnicotinic Acids And 1-Aryl-6-Carbethoxy-7-Methyl-4-Oxo-1, 4-Dihydropyrido [2, 3-D] Pyrimidines-Synthesis And Biological-Activity, Khim.-Farm. Zh., 1991, 25(4), 26–28 CAS.
- K. S. Babu, et al., Discovery of substituted imidazo [1, 2-c] pyrido [3, 2-e] pyrimidine based derivatives as novel anti-microbial agents, World J. Pharm. Res., 2016, 1339–1362 CAS.
- A. Monge, et al., 2-Arylamino-4-oxo-3, 4-dihydropyrido [2, 3-d] pyrimidines: synthesis and diuretic activity, Eur. J. Med. Chem., 1989, 24(3), 209–216 CrossRef CAS.
- V. Kolla, et al., Investigation of the anti-inflammatory and analgesic activity of 2-substituted 1-aryl-6-carboxy (carbethoxy)-7-methyl-4-oxo-1, 4-dihydropyrido [2, 3-d] pyrimidines, Pharm. Chem. J., 1993, 27, 635–636 CrossRef.
- A. M. Thompson, et al., Tyrosine kinase inhibitors. 7. 7-Amino-4-(phenylamino)-and 7-amino-4-[(phenylmethyl) amino] pyrido [4, 3-d] pyrimidines: a new class of inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor, J. Med. Chem., 1995, 38(19), 3780–3788 CrossRef CAS.
- S. Shabalala, et al., A facile, efficacious and reusable Sm2O3/ZrO2 catalyst for the novel synthesis of functionalized 1, 4-dihydropyridine derivatives, Catal. Commun., 2016, 79, 21–25 CrossRef CAS.
- J. El Bakali, et al., 4-Oxo-1, 4-dihydropyridines as selective CB2 cannabinoid receptor ligands part 2: discovery of new agonists endowed with protective effect against experimental colitis, J. Med. Chem., 2012, 55(20), 8948–8952 CrossRef CAS.
- M. Iman, et al., Design and synthesis of new 1, 4-dihydropyridines containing 4 (5)-chloro-5 (4)-imidazolyl substituent as a novel calcium channel blocker, Arch. Pharmacal Res., 2011, 34, 1417–1426 CrossRef CAS.
- L. G. Yue, et al., Synthesis and herbicidal activity of novel 3-aminocarbonyl-2-oxazolidinethione derivatives containing a substituted pyridine ring, J. Agric. Food Chem., 2006, 54(1), 125–129 CrossRef PubMed.
- H. Ghafuri, et al., Fe3O4@ ZrO2/SO42-: A recyclable magnetic heterogeneous nanocatalyst for synthesis of β-amino carbonyl derivatives and synthesis of benzylamino coumarin derivatives through Mannich reaction, Appl. Organomet. Chem., 2018, 32(3), e4147 CrossRef.
- S. Hesse and G. Kirsch, A rapid access to coumarin derivatives (using Vilsmeier–Haack and Suzuki cross-coupling reactions), Tetrahedron Lett., 2002, 43(7), 1213–1215 CrossRef CAS.
- J.-C. Jung, Y.-J. Jung and O.-S. Park, A convenient one-pot synthesis of 4-hydroxycoumarin, 4-hydroxythiocoumarin, and 4-hydroxyquinolin-2 (1 H)-one, Synth. Commun., 2001, 31(8), 1195–1200 CrossRef CAS.
- A. D. Patil, et al., The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn, J. Med. Chem., 1993, 36(26), 4131–4138 CrossRef CAS PubMed.
- B. Tyagi, M. K. Mishra and R. V. Jasra, Synthesis of 7-substituted 4-methyl coumarins by Pechmann reaction using nano-crystalline sulfated-zirconia, J. Mol. Catal. A: Chem., 2007, 276(1–2), 47–56 CrossRef CAS.
- A. Estévez-Braun and A. G. González, Coumarins, Nat. Prod. Rep., 1997, 14(5), 465–475 RSC.
- N. A. Kuznetsova and O. L. Kaliya, The photochemistry of coumarins, Usp. Khim., 1992, 61(7), 1243–1267 CAS.
- E. Musgrove, C. Rugg and D. Hedley, Flow cytometric measurement of cytoplasmic pH: a critical evaluation of available fluorochromes, Cytometry, 1986, 7(4), 347–355 CrossRef CAS PubMed.
- R. D. H. Murray, J. Méndez, and S. A. Brown, The Natural Coumarins, 1982 Search PubMed.
- M. Nowakowska, M. Smoluch and D. Sendor, The effect of cyclodextrins on the photochemical stability of 7-amino-4-methylcoumarin in aqueous solution, J. Inclusion Phenom. Macrocyclic Chem., 2001, 40, 213–219 CrossRef CAS.
- A. Ravindernath and M. S. Reddy, Synthesis and evaluation of anti-inflammatory, antioxidant and antimicrobial activities of densely functionalized novel benzo [d] imidazolyl tetrahydropyridine carboxylates, Arabian J. Chem., 2017, 10, S1172–S1179 CrossRef CAS.
- S. T. Harini, et al., Synthesis, antioxidant and antimicrobial activity of novel vanillin derived piperidin-4-one oxime esters: Preponderant role of the phenyl ester substituents on the piperidin-4-one oxime core, Arabian J. Chem., 2012, 22(24), 7588–7592 CAS.
- L. Peng, et al., Zirconium-Based Catalysts in Organic Synthesis, Top. Curr. Chem., 2022, 380(5), 41 CrossRef CAS PubMed.
- K. Chattopadhyay, M. Mandal and D. K. Maiti, A review on zirconium-based metal–organic frameworks: synthetic approaches and biomedical applications, Mater. Adv., 2024, 5(1), 51–67 RSC.
- L.-P. Mo and Z.-H. Zhang, Recent applications of zirconium compounds as catalysts or reagents in organic synthesis, Curr. Org. Chem., 2011, 15(22), 3800–3823 CrossRef CAS.
- M. K. Patil, A. N. Prasad and B. M. Reddy, Zirconia-based solid acids: green and heterogeneous catalysts for organic synthesis, Curr. Org. Chem., 2011, 15(23), 3961–3985 CrossRef CAS.
- C. W. Bird and A. R. Katritzky, Comprehensive Heterocyclic Chemistry: the Structure, Reactions, Synthesis and Uses of Heterocyclic Compounds;[in 8 Volumes]. 4, Pergamon Press, 1984 Search PubMed.
- M. R. Grimmett, Imidazole and Benzimidazole Synthesis, Academic press, 1997 Search PubMed.
- A. Pozharskii, A. Soldatenkov, and A. Katritzky, Heterocycles and health, Heterocycles in Life and Society, 1997, pp. 135–164 Search PubMed.
- E. G. Brown, Ring Nitrogen and Key Biomolecules: the Biochemistry of N-Heterocycles, Springer Science & Business Media, 2012 Search PubMed.
- A. Arena, et al., Nanostructured zirconia-based ceramics and composites in dentistry: A state-of-the-art review, Nanomaterials, 2019, 9(10), 1393 CrossRef CAS.
- V. O. Rodionov, et al., Benzimidazole and related ligands for Cu-catalyzed azide–alkyne cycloaddition, J. Am. Chem. Soc., 2007, 129(42), 12696–12704 CrossRef CAS.
- P. Dubey, P. Prasada Reddy and K. Srinivas, A facile tandem synthesis of α-benzyl benzimidazole acetonitriles, Arkivoc, 2007, 15, 192–198 Search PubMed.
- H. Wu, et al., Synthesis and characterization of the ligand based on benzimidazole and its copper complex: DNA binding and antioxidant activity, Bioinorg. Chem. Appl., 2011, 2011, 105431 Search PubMed.
- R. Sharma, M. Abdullaha and S. B. Bharate, Metal-Free Ionic-Liquid-Mediated Synthesis of Benzimidazoles and Quinazolin-4 (3H)-ones from Benzylamines, Asian J. Org. Chem., 2017, 6(10), 1370–1374 CrossRef CAS.
- J. Lu and H. Fu, Copper-catalyzed cascade synthesis of alkyl 6-aminobenzimidazo [2, 1-a] isoquinoline-5-carboxylates, J. Org. Chem., 2011, 76(11), 4600–4605 CrossRef CAS PubMed.
- R. T. Stibrany, et al., A geometrically constraining bis (benzimidazole) ligand and its nearly tetrahedral complexes with Fe (II) and Mn (II), Inorg. Chem., 2004, 43(4), 1472–1480 CrossRef CAS.
- K. Niknam and A. Fatehi-Raviz, Synthesis of 2-substituted benzimidazoles and bis-benzimidazoles by microwave in the presence of alumina-methanesulfonic acid, J. Iran. Chem. Soc., 2007, 4, 438–443 CrossRef CAS.
- H. Zarrinmayeh, et al., Synthesis and evaluation of a series of novel 2-[(4-chlorophenoxy) methyl]-benzimidazoles as selective neuropeptide Y Y1 receptor antagonists, J. Med. Chem., 1998, 41(15), 2709–2719 CrossRef CAS PubMed.
- P. Martins, et al., Heterocyclic anticancer compounds: recent advances and the paradigm shift towards the use of nanomedicine's tool box, Molecules, 2015, 20(9), 16852–16891 CrossRef CAS PubMed.
- E. I. Elnima, M. U. Zubair and A. A. Al-Badr, Antibacterial and antifungal activities of benzimidazole and benzoxazole derivatives, Antimicrob. Agents Chemother., 1981, 19(1), 29–32 CrossRef CAS.
- V. G. Atyam, et al., Synthesis, characterization, and biological evaluation of benzimidazole derivatives as potential anxiolytics, J. Young Pharm., 2010, 2(3), 273–279 CrossRef.
- I. Mohammadpoor-Baltork, et al., Silica sulfuric acid catalyzed synthesis of benzoxazoles, benzimidazoles and oxazolo [4, 5-b] pyridines under heterogeneous and solvent-free conditions, J. Iran. Chem. Soc., 2008, 5, S65–S70 CrossRef CAS.
- K. Yamada, et al., A mild copper-mediated intramolecular amination of aryl halides, Synlett, 2002, 2002(02), 0231–0234 CrossRef.
- H. Wang, et al., A direct intramolecular C–H amination reaction cocatalyzed by copper (II) and iron (III) as part of an efficient route for the synthesis of pyrido [1, 2-a] benzimidazoles from N-aryl-2-aminopyridines, J. Am. Chem. Soc., 2010, 132(38), 13217–13219 CrossRef CAS PubMed.
- H. Kumar, et al., Pyrazole scaffold: a remarkable tool in the development of anticancer agents, Eur. J. Med. Chem., 2013, 70, 248–258 CrossRef CAS.
- T. S. Reddy, et al., Design, synthesis and biological evaluation of 1, 3-diphenyl-1H-pyrazole derivatives containing benzimidazole skeleton as potential anticancer and apoptosis inducing agents, Eur. J. Med. Chem., 2015, 101, 790–805 CrossRef CAS PubMed.
- K. R. Abdellatif, et al., Design, synthesis, modeling studies and biological evaluation of thiazolidine derivatives containing pyrazole core as potential anti-diabetic PPAR-γ agonists and anti-inflammatory COX-2 selective inhibitors, Bioorg. Chem., 2019, 82, 86–99 CrossRef CAS PubMed.
- N. Bakthavatchala Reddy, et al., Design and synthesis of some new benzimidazole containing pyrazoles and pyrazolyl thiazoles as potential antimicrobial agents, J. Heterocycl. Chem., 2019, 56(2), 589–596 CrossRef CAS.
- R. Verma, et al., Pyrazole-based analogs as potential antibacterial agents against methicillin-resistance staphylococcus aureus (MRSA) and its SAR elucidation, Eur. J. Med. Chem., 2021, 212, 113134 CrossRef CAS PubMed.
- S. Kumari, S. K. Paliwal and R. Chauhan, An improved protocol for the synthesis of chalcones containing pyrazole with potential antimicrobial and antioxidant activity, Curr. Bioact. Compd., 2018, 14(1), 39–47 CrossRef CAS.
- M. Asiri, et al., Synthesis of New Zirconium Magnetic Nanocomposite as a Bioactive Agent and Green Catalyst in the Four-Component Synthesis of a Novel Multi-Ring Compound Containing Pyrazole Derivatives, Nanomaterials, 2022, 12(24), 4468 CrossRef CAS PubMed.
- M. Dadaei and H. Naeimi, An environment-friendly method for green synthesis of pyranopyrazole derivatives catalyzed by CoCuFe2O4 magnetic nanocrystals under solvent-free conditions, Polycyclic Aromat. Compd., 2021, 42(1), 204–217 CrossRef.
- H. G. Alvim, et al., Facts, presumptions, and myths on the solvent-free and catalyst-free Biginelli reaction. What is catalysis for?, J. Org. Chem., 2014, 79(8), 3383–3397 CrossRef CAS.
- J. Safari and S. Gandomi-Ravandi, A novel protocol for solvent-free synthesis of 4, 6-diaryl-3, 4-dihydropyrimidine-2 (1H)-ones catalyzed by metal oxide–MWCNTs nanocomposites, J. Mol. Struct., 2014, 1074, 71–78 CrossRef CAS.
- G. Sabitha, et al., Iodotrimethylsilane-Accelerated One-Pot Synthesis of 5-Unsubstituted 3, 4-Dihydropyrimidin-2 (1H)-ones: A Novel Procedure for the Biginelli-Like Cyclocondensation Reaction at Room Temperature, Helv. Chim. Acta, 2005, 88(11), 2996–2999 CrossRef CAS.
- B. G. Mishra, D. Kumar and V. Rao, H3PW12O40 catalyzed expeditious synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones under solvent-free conditions, Catal. Commun., 2006, 7(7), 457–459 CrossRef CAS.
- Z.-T. Wang, et al., Novel Biginelli-like three-component cyclocondensation reaction: efficient synthesis of 5-unsubstituted 3, 4-dihydropyrimidin-2 (1H)-ones, Tetrahedron Lett., 2004, 45(42), 7951–7953 CrossRef CAS.
- A. J. Bridges, et al., N6-(2, 2-diphenylethyl) adenosine, a novel adenosine receptor agonist with antipsychotic-like activity, J. Med. Chem., 1987, 30(10), 1709–1711 CrossRef CAS.
- I. Jarak, et al., Novel cyano-and amidino-substituted derivatives of thieno [2, 3-b]-and thieno [3, 2-b] thiophene-2-carboxanilides and thieno [3′, 2′: 4, 5] thieno-and thieno [2′, 3′: 4, 5] thieno [2, 3-c] quinolones: Synthesis, photochemical synthesis, DNA binding, and antitumor evaluation, Bioorg. Med. Chem., 2006, 14(8), 2859–2868 CrossRef CAS.
- S. Tasqeeruddin, Y. Asiri and J. A. Alsherhri, An efficient and green microwave-assisted synthesis of quinoline derivatives via Knoevengal Condensation, Lett. Org. Chem., 2020, 17(2), 157–163 CrossRef CAS.
- S. Tasqeeruddin and Y. I. Asiri, An environmentally benign, green, and efficient ionic liquid catalyzed synthesis of Quinoline derivatives via Knoevenagel condensation, J. Heterocycl. Chem., 2020, 57(1), 132–139 CrossRef CAS.
- E. A. Fehnel, Friedländer syntheses with o-Aminoaryl ketones. I. Acid-catalyzed condensations of o-Aminobenzophenone with ketones1, J. Org. Chem., 1966, 31(9), 2899–2902 CrossRef CAS.
- S. Tasqeeruddin, Y. Asiri and S. Shaheen, Zirconium (IV) Oxychloride Octahydrate (ZrOCl2· 8H2O): An Efficient Catalyst for the One-Pot Multicomponent Synthesis of Hexahydroquinoline Derivatives under Conventional Heating and Microwave Irradiation, Russ. J. Org. Chem., 2022, 58(7), 1008–1014 CrossRef CAS.
- W. Yu and C. Li, Regioselective one-pot C–N coupling of substituted naphthoquinones: selective intramolecular ring fusion of sulfonamides, Tetrahedron, 2014, 70(2), 459–464 CrossRef CAS.
- J. Tyleckova, et al., Cancer cell response to anthracyclines effects: mysteries of the hidden proteins associated with these drugs, Int. J. Mol. Sci., 2012, 13(12), 15536–15564 CrossRef CAS.
- S. ElKalyoubi and E. Fayed, Synthesis and evaluation of antitumour activities of novel fused tri-and tetracyclic uracil derivatives, J. Chem. Res., 2016, 40(12), 771–777 CrossRef.
- H. R. Lamazian, V. T. Pidchenko, and V. M. Minarchenko, Standardization of Citrullus colocynthis (L.) Shrad. Fruits Dry Extract for Futher Study of its Antidiabetic Activity, 2019 Search PubMed.
- T. Mirsaev, Experimental study of hepatoprotective activity of hydroxymethyluracil, Bull. Exp. Biol. Med., 2007, 143(5), 575 CrossRef CAS PubMed.
- M. Eltze, Investigations on the mode of action of a new antihypertensive drug, urapidil, in the isolated rat vas deferens, Eur. J. Pharmacol., 1979, 59(1–2), 1–9 CrossRef CAS PubMed.
- J. I. Bardagi and R. A. Rossi, Advances in the synthesis of 5-and 6-substituted uracil derivatives, Org. Prep. Proced. Int., 2009, 41(6), 479–514 CrossRef CAS.
- A. Mai, et al., Discovery of uracil-based histone deacetylase inhibitors able to reduce acquired antifungal resistance and trailing growth in Candida albicans, Bioorg. Med. Chem. Lett., 2007, 17(5), 1221–1225 CrossRef CAS PubMed.
- N. C. Desai, et al., Design, synthesis, and biological evaluation of 1, 4-dihydropyridine derivatives as potent antitubercular agents, Chem. Biol. Drug Des., 2015, 86(3), 370–377 CrossRef CAS.
- R. N. Goto, et al., Anti-cancer activity of a new dihydropyridine derivative, VdiE-2N, in head and neck squamous cell carcinoma, Eur. J. Pharmacol., 2018, 819, 198–206 CrossRef CAS PubMed.
- S. R. Batten, et al., Coordination polymers, metal–organic frameworks and the need for terminology guidelines, CrystEngComm, 2012, 14(9), 3001–3004 RSC.
- D. B. Trushina, et al., Doxorubicin-loaded core–shell UiO-66@ SiO2 metal–organic frameworks for targeted cellular uptake and cancer treatment, Pharmaceutics, 2022, 14(7), 1325 CrossRef CAS PubMed.
- O. M. Yaghi and H. Li, Hydrothermal synthesis of a metal-organic framework containing large rectangular channels, J. Am. Chem. Soc., 1995, 117(41), 10401–10402 CrossRef CAS.
- J. B. DeCoste, et al., Stability and degradation mechanisms of metal–organic frameworks containing the Zr 6 O 4 (OH) 4 secondary building unit, J. Mater. Chem. A, 2013, 1(18), 5642–5650 RSC.
- C. Zhang, et al., Metal–Organic Framework (MOF)-Based Ultrasound-Responsive Dual-Sonosensitizer Nanoplatform for Hypoxic Cancer Therapy, Adv. Healthcare Mater., 2022, 11(2), 2101946 CrossRef CAS.
- P. Couture, et al., Zirconium toxicity assessment using bacteria, algae and fish assays, Water, Air, Soil Pollut., 1989, 47, 87–100 CrossRef CAS.
- M. Taddei, When defects turn into virtues: The curious case of zirconium-based metal-organic frameworks, Coord. Chem. Rev., 2017, 343, 1–24 CrossRef CAS.
- M. Bosch, M. Zhang and H.-C. Zhou, Increasing the stability of metal-organic frameworks, Adv. Chem., 2014, 2014(182327.10), 1155 Search PubMed.
- C. Pettinari, et al., Application of metal− organic frameworks, Polym. Int., 2017, 66(6), 731–744 CrossRef CAS.
- S. Waitschat, et al., Synthesis of M-UiO-66 (M= Zr, Ce or Hf) employing 2, 5-pyridinedicarboxylic acid as a linker: defect chemistry, framework hydrophilisation and sorption properties, Dalton Trans., 2018, 47(4), 1062–1070 RSC.
- M. Dabiri, et al., Silica sulfuric acid: An efficient reusable heterogeneous catalyst for the synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones in water and under solvent-free conditions, Catal. Commun., 2008, 9(5), 785–788 CrossRef CAS.
- T. Tamoradi, S. M. Mousavi and M. Mohammadi, Praseodymium (iii) anchored on CoFe 2 O 4 MNPs: an efficient heterogeneous magnetic nanocatalyst for one-pot, multi-component domino synthesis of polyhydroquinoline and 2, 3-dihydroquinazolin-4 (1 H)-one derivatives, New J. Chem., 2020, 44(7), 3012–3020 RSC.
- P. Salehi, et al., Silica sulfuric acid and silica chloride as efficient reagents for organic reactions, Curr. Org. Chem., 2006, 10(17), 2171–2189 CrossRef CAS.
- B. D. Rupnar, et al., Green and efficient synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones in aqueous medium using ZnFe 2 O 4 catalyst under microwave irradiation, J. Iran. Chem. Soc., 2017, 14, 1853–1858 CrossRef CAS.
- P. V. G. Reddy, et al., A review on multicomponent reactions catalysed by zero-dimensional/one-dimensional titanium dioxide (TiO2) nanomaterials: Promising green methodologies in organic chemistry, J. Environ. Manage., 2021, 279, 111603 CrossRef.
- M. A. Zolfigol, et al., Nanometasilica disulfuric acid (NMSDSA) and nanometasilica monosulfuric acid sodium salt (NMSMSA) as two novel nanostructured catalysts: applications in the synthesis of Biginelli-type, polyhydroquinoline and 2, 3-dihydroquinazolin-4 (1 H)-one derivatives, J. Iran. Chem. Soc., 2017, 14, 121–134 CrossRef CAS.
- P. Salehi, et al., A novel method for the one-pot three-component synthesis of 2, 3-dihydroquinazolin-4 (1H)-ones, Synlett, 2005, 2005(07), 1155–1157 CrossRef.
- A. Kamal, et al., Synthesis and biological evaluation of 3, 5-diaryl isoxazoline/isoxazole linked 2, 3-dihydroquinazolinone hybrids as anticancer agents, Eur. J. Med. Chem., 2011, 46(2), 691–703 CrossRef CAS.
- G.-H. Zhang, et al., Phthalazino [1, 2-b] quinazolinones as p53 Activators: Cell Cycle Arrest, Apoptotic Response and Bak–Bcl-xl Complex Reorganization in Bladder Cancer Cells, J. Med. Chem., 2017, 60(16), 6853–6866 CrossRef CAS.
- T. Abe, et al., An Ullmann N-arylation/2-amidation cascade by self-relay copper catalysis: one-pot synthesis of indolo [1, 2-a] quinazolinones, Org. Chem. Front., 2017, 4(11), 2124–2127 RSC.
- D. N. Garad and S. B. Mhaske, Diversification of quinazolinones by Pd-catalyzed C (sp3)-acetoxylation, J. Org. Chem., 2017, 82(19), 10470–10478 CrossRef CAS.
- L. He, H. Li, J. Chen and X.-F. Wu, RSC Adv., 2014, 4, 12065–12077 RSC.
- O. Ghashghaei, et al., Extended multicomponent reactions with indole aldehydes: access to unprecedented polyheterocyclic scaffolds, ligands of the aryl hydrocarbon receptor, Angew. Chem., 2021, 133(5), 2635–2640 CrossRef.
- J. Bariwal, L. G. Voskressensky and E. V. Van der Eycken, Recent advances in spirocyclization of indole derivatives, Chem. Soc. Rev., 2018, 47(11), 3831–3848 RSC.
- L. Fabian, et al., Evaluation of quinoxaline compounds as ligands of a site adjacent to S2 (AS2) of cruzain, Bioorg. Med. Chem. Lett., 2019, 29(16), 2197–2202 CrossRef CAS.
- R. Schobert and A. Schlenk, Tetramic and tetronic acids: an update on new derivatives and biological aspects, Bioorg. Med. Chem., 2008, 16(8), 4203–4221 CrossRef CAS.
- D. Matiadis, et al., Synthesis, biological evaluation and structure-activity relationships of 5-arylidene tetramic acids with antibacterial activity against methicillin-resistant Staphylococcus aureus, Bioorg. Med. Chem. Lett., 2020, 30(10), 127107 CrossRef CAS.
- W.-J. Liu, H. Jiang and H.-Q. Yu, Development of biochar-based functional materials: toward a sustainable platform carbon material, Chem. Rev., 2015, 115(22), 12251–12285 CrossRef CAS PubMed.
- T. Chhabra, P. Dwivedi and V. Krishnan, Acid functionalized hydrochar as heterogeneous catalysts for solventless synthesis of biofuel precursors, Green Chem., 2022, 24(2), 898–910 RSC.
- M. A. Ghaffari, et al., Synthesis of N-Substituted Carbonylamino-1, 2, 3, 6-Tetrahydropyridines as Potential Anti-Inflammatory Agents, Synth. Commun., 2011, 41(17), 2615–2623 CrossRef CAS PubMed.
- J. M. Yeung, L. A. Corleto and E. E. Knaus, Synthesis of N-[[(substituted-phenyl) carbonyl] amino]-1, 2, 3, 6-tetrahydropyridines with analgesic and hyperglycemic activity, J. Med. Chem., 1982, 25(6), 720–723 CrossRef CAS.
- B. Ho, A. M. Crider and J. P. Stables, Synthesis and structure–activity relationships of potential anticonvulsants based on 2-piperidinecarboxylic acid and related pharmacophores, Eur. J. Med. Chem., 2001, 36(3), 265–286 CrossRef CAS.
- R. Aeluri, et al., Synthesis and Antiproliferative Activity of Polysubstituted Tetrahydropyridine and Piperidin-4-one-3-carboxylate Derivatives, Asian J. Org. Chem., 2012, 1(1), 71–79 CrossRef CAS.
- C. Kappe, Synthesis of octahydroquinazolinone derivatives using silica sulfuric acid as an efficient catalyst, Eur. J. Med. Chem., 2000, 35, 1043–1052 CrossRef CAS PubMed.
- N. Leeantha, et al., Antimicrobial and antioxidant activities of piperidine derivatives, Afr. J. Pharm. Pharmacol., 2015, 9(31), 783–792 CrossRef.
- A. A. Mohammadi, et al., Diastereoselective synthesis and molecular docking studies of novel fused tetrahydropyridine derivatives as new inhibitors of HIV protease, J. Mol. Struct., 2017, 1139, 166–174 CrossRef CAS.
- A. A. Yelwande, et al., One-pot multicomponent synthesis approach for tetrahydropyridines using polyaniline-zirconium oxide composites, Synth. Commun., 2022, 52(7), 1039–1049 CrossRef CAS.
- R. J. Sundberg, Indoles, Elsevier, 1996 Search PubMed.
- S. J. Garden, et al., A modified Sandmeyer methodology and the synthesis of (±)-convolutamydine A, Tetrahedron Lett., 1997, 38(9), 1501–1504 CrossRef CAS.
- K. Joshi, V. Pathak and S. Jain, Studies of potential organo-fluorine antibacterial agents. Part 5: Synthesis and antibacterial activity of some new fluorine-containing indole-2, 3-dione derivatives, Pharmazie, 1980, 35(11), 677–679 CAS.
- V. Bolotov, et al., Synthesis and biological activity of 1-aminomethyl-3, 3-diaryl-2-oxoindolines, Pharm. Chem. J., 1982, 16(1), 48–51 CrossRef.
- H. Pajouhesh, R. Parson and F. D. Popp, Potential anticonvulsants VI: Condensation of isatins with cyclohexanone and other cyclic ketones, J. Pharm. Sci., 1983, 72(3), 318–321 CrossRef CAS PubMed.
- F. Garrido, J. Ibanez, E. Gonalons and A. Giraldez, Eur. J. Med. Chem., 1975, 143 CAS.
- C. Praveen, A. Ayyanar and P. T. Perumal, Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di (indolyl) indolin-2-ones, Arabian J. Chem., 2011, 21(13), 4072–4077 CAS.
- S. F. Hojati and A. S. Kaheh, New Mothod for Synthesis of Indole Derivatives via Zirconium (IV) Chloride Catalyst, Jordan J. Chem., 2019, 14(1), 17–27 CAS.
- M. A. Martin-Acebes, A. Vazquez-Calvo and J.-C. Saiz, Lipids and flaviviruses, present and future perspectives for the control of dengue, Zika, and West Nile viruses, Prog. Lipid Res., 2016, 64, 123–137 CrossRef CAS PubMed.
- A. F. C. d. S. Oliveira, et al., Zirconium catalyzed synthesis of 2-arylidene Indan-1, 3-diones and evaluation of their inhibitory activity against NS2B-NS3 WNV protease, Eur. J. Med. Chem., 2018, 149, 98–109 CrossRef CAS.
- A. D. Borthwick, et al., Design and synthesis of pyrrolidine-5, 5-trans-lactams (5-oxohexahydropyrrolo [3, 2-b] pyrroles) as novel mechanism-based inhibitors of human cytomegalovirus protease. 2. Potency and chirality, J. Med. Chem., 2002, 45(1), 1–18 CrossRef CAS.
- W.-R. Li, et al., Efficient total synthesis of pulchellalactam, a CD45 protein tyrosine phosphatase inhibitor, J. Org. Chem., 2002, 67(14), 4702–4706 CrossRef CAS PubMed.
- J. W. Lampe, et al., (Imidazolylphenyl) pyrrol-2-one inhibitors of cardiac cAMP phosphodiesterase, J. Med. Chem., 1993, 36(8), 1041–1047 CrossRef CAS.
- H. Shiozawa and S. Takahashi, Configurational studies on thiomarinol, J. Antibiot., 1994, 47(7), 851–853 CrossRef CAS.
- Y. Chen, et al., Study on photochromism of diarylethenes with a 2, 5-dihydropyrrole bridging unit: a convenient preparation of 3, 4-diarylpyrroles from 3, 4-diaryl-2, 5-dihydropyrroles, J. Org. Chem., 2005, 70(13), 5001–5005 CrossRef CAS PubMed.
- C. Grunwald, et al., Synthesis, pharmacology, and structure− activity relationships of novel Imidazolones and Pyrrolones as modulators of GABAA receptors, J. Med. Chem., 2006, 49(6), 1855–1866 CrossRef CAS.
- S. B. Singh, et al., Oteromycin: a novel antagonist of endothelin receptor, J. Org. Chem., 1995, 60(21), 7040–7042 CrossRef CAS.
- L. Zhang, et al., Design, syntheses and 3D-QSAR studies of novel N-phenyl pyrrolidin-2-ones and N-phenyl-1H-pyrrol-2-ones as protoporphyrinogen oxidase inhibitors, Bioorg. Med. Chem., 2010, 18(22), 7948–7956 CrossRef CAS.
- T. Kawasuji, et al., 3-Hydroxy-1, 5-dihydro-pyrrol-2-one derivatives as advanced inhibitors of HIV integrase, Bioorg. Med. Chem., 2007, 15(16), 5487–5492 CrossRef CAS.
- F. Mohamadpour, Efficient and Mild Four-component Process for the Synthesis of Highly Substituted Dihydro-2-oxopyrroles using ZrOCl2 8H2O as an Environmental-ly Friendly Catalyst, Makara J. Sci., 2020, 24(4), 2 Search PubMed.
- H. Li, et al., Cycloamination strategies for renewable N-heterocycles, Green Chem., 2020, 22(3), 582–611 RSC.
- X. Chen, et al., Expanding the boundary of biorefinery: organonitrogen chemicals from biomass, Acc. Chem. Res., 2021, 54(7), 1711–1722 CrossRef CAS PubMed.
- F. W. Lichtenthaler, Unsaturated O-and N-heterocycles from carbohydrate feedstocks, Acc. Chem. Res., 2002, 35(9), 728–737 CrossRef CAS PubMed.
- C. Avendaño and J. C. Menéndez, DNA Intercalators and topoisomerase inhibitors, Med. Chem. Anticancer Drug, 2008, 199–228 Search PubMed.
- S. D. Joshi, et al., Synthesis, characterization, biological activity, and 3D-QSAR studies on some novel class of pyrrole derivatives as antitubercular agents, Med. Chem. Res., 2014, 23, 1123–1147 CrossRef CAS.
- J. T. Gupton, Pyrrole natural products with antitumor properties, Heterocyclic Antitumor Antibiotics, 2006, pp. 53–92 Search PubMed.
- S. Bhakta, et al., Design and synthesis of 1-((1, 5-Bis (4-chlorophenyl)-2-methyl-1 H-pyrrol-3-yl) methyl)-4-methylpiperazine (BM212) and N-Adamantan-2-yl-N′-((E)-3, 7-dimethylocta-2, 6-dienyl) ethane-1, 2-diamine (SQ109) pyrrole hybrid derivatives: Discovery of potent antitubercular agents effective against multidrug-resistant mycobacteria, J. Med. Chem., 2016, 59(6), 2780–2793 CrossRef CAS PubMed.
- S. Zhang, et al., Design, synthesis, and antifungal evaluation of novel coumarin-pyrrole hybrids, J. Heterocycl. Chem., 2021, 58(2), 450–458 CrossRef CAS.
- M. Pei, et al., Gold-Catalyzed Cyclization of Ynones Involving cis-Hydrofunctionalizations: Rapid Assembly of C-, O-, or S-Functionalized Pyrroles by a Single Methodology, Org. Lett., 2022, 24(7), 1541–1545 CrossRef CAS.
- T. Fukuda, F. Ishibashi and M. Iwao, Synthesis and biological activity of lamellarin alkaloids: an overview, Heterocycles, 2011, 83(3), 491 CrossRef CAS.
- A. I. Bortun, L. N. Bortun and A. Clearfield, Hydrothermal synthesis of sodium zirconium silicates and characterization of their properties, Chem. Mater., 1997, 9(8), 1854–1864 CrossRef CAS.
- P. Ferreira, et al., Synthesis and structural characterization of zirconium silicates, Chem. Mater., 2001, 13(2), 355–363 CrossRef CAS.
- O. D. Maurice, Transport and deposition of the non-sulphide vein Minerals;[Part] 5, Zirconium minerals, Econ. Geol., 1949, 44(8), 721–731 CrossRef.
- L. Bonsignore, et al., Synthesis and pharmacological activity of 2-oxo-(2H) 1-benzopyran-3-carboxamide derivatives, Eur. J. Med. Chem., 1993, 28(6), 517–520 CrossRef CAS.
- P. Salvi, et al., An efficient protocol for synthesis of tetrahydrobenzo [b] pyrans using amino functionalized ionic liquid, C. R. Chim., 2011, 14(10), 878–882 CrossRef CAS.
- M. Mishra, et al., Zirconia Supported on Rice Husk Silica from Biowaste: A Novel, Efficient, and Recoverable Nanocatalyst for the Green Synthesis of Tetrahydro-1-benzopyrans, Russ. J. Org. Chem., 2020, 56, 1784–1789 CrossRef CAS.
- P. T. Mistry, et al., Synthesis, characterization, and in vitro biological studies of some novel pyran fused pyrimidone derivatives, J. Heterocycl. Chem., 2012, 49(2), 349–357 CrossRef CAS.
- P. M. Ronad, et al., Synthesis and antimicrobial activity of 7-(2-substituted phenylthiazolidinyl)-benzopyran-2-one derivatives, Eur. J. Med. Chem., 2010, 45(1), 85–89 CrossRef CAS.
- W. JL, D. Liu, Z. J. Zhang, S. Shan, X. Han, S. M. Srinivasula, C. M. Croce, E. S. Alnemri and Z. Huang, Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells, Proc. Natl. Acad. Sci., 2000, 97, 7124–7129 CrossRef.
- S. Singh, et al., Synthesis of 3, 5-dihydroxy-7, 8-dimethoxy-2-(4-methoxyphenyl) benzopyran-4-one derivatives as anticancer agents, Arabian J. Chem., 2016, 26(21), 5322–5327 CAS.
- M. E. Zaki, et al., Pyrazolopyranopyrimidines as a class of anti-inflammatory agents, Z. Naturforsch., C: J. Biosci., 2006, 61(1–2), 1–5 CAS.
- S. Hasan, et al., Synthesis of 6-aminomethyl derivatives of benzopyran-4-one with dual biological properties: anti-inflammatory-analgesic and antimicrobial, Eur. J. Med. Chem., 2009, 44(12), 4896–4903 CrossRef CAS.
- R. S. Aliabadi and N. O. Mahmoodi, Green and efficient synthesis of pyranopyrazoles using [bmim][OH−] as an ionic liquid catalyst in water under microwave irradiation and investigation of their antioxidant activity, RSC Adv., 2016, 6(89), 85877–85884 RSC.
- M. A. Nasseri, et al., Efficient preparation of 1, 8-dioxo-octahydroxanthene derivatives by recyclable cobalt-incorporated sulfated zirconia (ZrO 2/SO 4 2−/Co) nanoparticles, J. Nanopart. Res., 2019, 21, 214 CrossRef.
- M. Sayyafi, et al., One-pot, three-component route to 2H-indazolo [2, 1-b] phthalazine-triones, Tetrahedron, 2008, 64(10), 2375–2378 CrossRef CAS.
- M. Khazaei, et al., Novel electronic and magnetic properties of two-dimensional transition metal carbides and nitrides, Adv. Funct. Mater., 2013, 23(17), 2185–2192 CrossRef CAS.
- H. Naeimi and Z. S. Nazifi, Convenient Synthesis of 14-Aryl-14-H-dibenzo [a, j] xanthenes Catalyzed by Acyclic Brønsted Acidic Ionic Liquid [H—NMP]+[HSO4]− under Microwave Irradiation, J. Chin. Chem. Soc., 2013, 60(9), 1113–1117 CrossRef CAS.
- F. Shirini, et al., One-pot synthesis of 4, 4-(arylmethylene)-bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols) catalyzed by Brönsted acidic ionic liquid supported on nanoporous Na+-montmorillonite, J. Mol. Liq., 2015, 208, 291–297 CrossRef CAS.
- A. Servais, et al., Radical Cyclization of N-Acylcyanamides: Total Synthesis of Luotonin A, Angew. Chem., Int. Ed., 2007, 46(4), 576–579 CrossRef CAS.
- D. Nekrasov, Synthesis and chemical transformations of mono-and disubstituted cyanamides, Russ. J. Org. Chem., 2004, 40, 1387–1402 CrossRef CAS.
- R. L. Giles, J. D. Sullivan, A. M. Steiner and R. E. Looper, Angew. Chem., Int. Ed., 2009, 48(17), 3116–3120 CrossRef CAS PubMed.
- L.-C. Kang, et al., Hydrogencyanamido bridged multinuclear copper (II) complexes: from strong antiferromagnetic couplings to weak ferromagnetic couplings, Dalton Trans., 2011, 40(19), 5200–5209 RSC.
- Y. Lu, et al., Design, synthesis, and SAR studies of 4-substituted methoxylbenzoyl-aryl-thiazoles analogues as potent and orally bioavailable anticancer agents, J. Med. Chem., 2011, 54(13), 4678–4693 CrossRef CAS.
- R. Kumar, et al., Synthesis and antiviral activity of novel 5-(1-cyanamido-2-haloethyl) and 5-(1-hydroxy (or methoxy)-2-azidoethyl) analogues of uracil nucleosides, J. Med. Chem., 2001, 44(21), 3531–3538 CrossRef CAS.
- M. Nasrollahzadeh, M. Sajjadi and S. M. Sajadi, Green synthesis of Cu/zirconium silicate nanocomposite by using rubia tinctorum leaf extract and its application in the preparation of N-benzyl-N-arylcyanamides, Appl. Organomet. Chem., 2019, 33(2), e4705 CrossRef.
- B. M. Trost and I. Fleming, Comprehensive Organic Synthesis: Selectivity, Strategy, and Efficiency in Modern Organic Chemistry, Elsevier, 1991, vol. 8 Search PubMed.
- R. C. Larock, Comprehensive Organic Transformations, Wiley Online Library, 1989 Search PubMed.
- R. Tayebee, et al., Preparation and characterization of a novel Wells–Dawson heteropolyacid-based magnetic inorganic–organic nanohybrid catalyst H 6 P 2 W 18 O 62/pyridino-Fe 3 O 4 for the efficient synthesis of 1-amidoalkyl-2-naphthols under solvent-free conditions, Dalton Trans., 2014, 43(4), 1550–1563 RSC.
- N. M. Ghohe, R. Tayebee and M. Amini, Synthesis and characterization of mesoporous NbZr/KIT-6 as a productive catalyst for the synthesis of benzylpyrazolyl coumarins, Mater. Chem. Phys., 2019, 223, 268–276 CrossRef.
- F. Narenji-Sani, R. Tayebee and M. Chahkandi, New task-specific and reusable ZIF-like grafted H6P2W18O62 catalyst for the effective esterification of free fatty acids, ACS Omega, 2020, 5(17), 9999–10010 CrossRef CAS.
- S. S. Dipake, et al., ZS-1 Zeolite as a Highly Efficient and Reusable Catalyst for Facile Synthesis of 1-amidoalkyl-2-naphthols Under Solvent-Free Conditions, Catal. Lett., 2022, 152(3), 755–770 CrossRef CAS.
- Y. Oba, et al., Biosynthesis of Firefly Luciferin in Adult Lantern: Decarboxylation of L-Cysteine is a Key Step for Benzothiazole Ring Formation in Firefly Luciferin Synthesis, PLoS One, 2013, 8(12), e84023 CrossRef PubMed.
- M. C. Van Zandt, et al., Discovery of 3-[(4, 5, 7-trifluorobenzothiazol-2-yl) methyl] indole-N-acetic acid (lidorestat) and congeners as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications, J. Med. Chem., 2005, 48(9), 3141–3152 CrossRef CAS.
- E. R. Atkinson and F. E. Granchelli, Antimalarials V: aminobenzothiazoles, J. Pharm. Sci., 1976, 65(4), 618–620 CrossRef CAS.
- S. Manjula, et al., Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents, Eur. J. Med. Chem., 2009, 44(7), 2923–2929 CrossRef CAS.
- C. G. Mortimer, et al., Antitumor benzothiazoles. 26. 2-(3, 4-Dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzothiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines, J. Med. Chem., 2006, 49(1), 179–185 CrossRef CAS.
- C. Rodríguez-Rodríguez, et al., Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer's disease, J. Am. Chem. Soc., 2009, 131(4), 1436–1451 CrossRef.
- C. Desai and K. Desai, Synthesis of bifunctional reactive dyes using various benzthiozole and their dyeing properties, Orient. J. Chem., 2000, 16(2), 311–314 CAS.
- D. Fajkusova, et al., Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles, Bioorg. Med. Chem., 2012, 20(24), 7059–7068 CrossRef CAS.
- M. T. Maghsoodlou, et al., A green protocol for one-pot three-component synthesis of 1-(benzothiazolylamino) methyl-2-naphthol catalyzed by oxalic acid, J. Iran. Chem. Soc., 2017, 14, 329–335 CrossRef CAS.
- P. W. Roesky, Catalytic hydroaminoalkylation, Angew. Chem., Int. Ed., 2009, 48(27), 4892–4894 CrossRef CAS.
- E. Chong, P. Garcia and L. L. Schafer, Hydroaminoalkylation: early-transition-metal-catalyzed α-alkylation of amines, Synthesis, 2014, 46(21), 2884–2896 CrossRef.
- P. Edwards and L. Schafer, Early transition metal-catalyzed C–H alkylation: hydroaminoalkylation for C sp3–C sp3 bond formation in the synthesis of selectively substituted amines, Chem. Commun., 2018, 54(89), 12543–12560 RSC.
- M. Holmes, L. A. Schwartz and M. J. Krische, Intermolecular metal-catalyzed reductive coupling of dienes, allenes, and enynes with carbonyl compounds and imines, Chem. Rev., 2018, 118(12), 6026–6052 CrossRef CAS.
- L. Gonnard, A. Guérinot and J. Cossy, Transition metal-catalyzed α-alkylation of amines by C (sp3)–H bond activation, Tetrahedron, 2019, 75(2), 145–163 CrossRef CAS.
- P. R. Payne, et al., Tantalum Catalyzed Hydroaminoalkylation for the Synthesis of α-and β-Substituted N-Heterocycles, Org. Lett., 2013, 15(9), 2182–2185 CrossRef CAS.
- A. Koperniku, et al., Zirconium hydroaminoalkylation. an alternative disconnection for the catalytic synthesis of α-arylated primary amines, J. Am. Chem. Soc., 2019, 141(48), 18944–18948 CrossRef CAS.
- B. Lejczak and P. Kafarski, Biological activity of aminophosphonic acids and their short peptides, Phosphorous Heterocycles I, 2009, pp. 31–63 Search PubMed.
- A. Mucha, P. Kafarski and Ł. Berlicki, Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry, J. Med. Chem., 2011, 54(17), 5955–5980 CrossRef CAS.
- J. Hiratake and J. i. Oda, Aminophosphonic and aminoboronic acids as key elements of a transition state analogue inhibitor of enzymes, Biosci., Biotechnol., Biochem., 1997, 61(2), 211–218 CrossRef CAS.
- F. R. Atherton, C. H. Hassall and R. W. Lambert, Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl) phosphonic acid and (aminomethyl) phosphonic acid, J. Med. Chem., 1986, 29(1), 29–40 CrossRef CAS.
- V. A. Solodenko and V. P. Kukhar, Stereoselective papain-catalyzed synthesis of alafosfalin, Tetrahedron Lett., 1989, 30(49), 6917–6918 CrossRef CAS.
- P. P. Giannousis and P. A. Bartlett, Phosphorus amino acid analogs as inhibitors of leucine aminopeptidase, J. Med. Chem., 1987, 30(9), 1603–1609 CrossRef CAS PubMed.
- C. Stamper, et al., Inhibition of the aminopeptidase from Aeromonas proteolytica by L-leucinephosphonic acid. Spectroscopic and crystallographic characterization of the transition state of peptide hydrolysis, Biochemistry, 2001, 40(24), 7035–7046 CrossRef CAS.
- P. A. Bartlett, J. E. Hanson and P. P. Giannousis, Potent inhibition of pepsin and penicillopepsin by phosphorus-containing peptide analogs, J. Org. Chem., 1990, 55(26), 6268–6274 CrossRef CAS.
- Y. Dai, et al., Zirconium-catalyzed asymmetric Kabachnik–Fields reactions of aromatic and aliphatic aldehydes, Chem. Sci., 2021, 12(37), 12333–12345 RSC.
- M. M. Kamel and N. Y. M. Abdo, Synthesis of novel 1, 2, 4-triazoles, triazolothiadiazines and triazolothiadiazoles as potential anticancer agents, Eur. J. Med. Chem., 2014, 86, 75–80 CrossRef CAS.
- N. Süleymanoğlu, et al., 1, 2, 4-triazole derivative with Schiff base; thiol-thione tautomerism, DFT study and antileishmanial activity, J. Mol. Struct., 2017, 1150, 82–87 CrossRef.
- N. Nayak, et al., Synthesis of new pyrazole-triazole hybrids by click reaction using a green solvent and evaluation of their antitubercular and antibacterial activity, Res. Chem. Intermed., 2016, 42, 3721–3741 CrossRef CAS.
- A. Almasirad, et al., Synthesis and anticonvulsant activity of new 2-substituted-5-[2-(2-fluorophenoxy) phenyl]-1, 3, 4-oxadiazoles and 1, 2, 4-triazoles, Arabian J. Chem., 2004, 14(24), 6057–6059 CAS.
- Y.-S. Li, et al., Synthesis and bioactivity of pyrazole and triazole derivatives as potential PDE4 inhibitors, Arabian J. Chem., 2016, 26(15), 3632–3635 CAS.
- N. Kerru, et al., Efficient synthesis of novel pyrazole-linked 1, 2, 4-triazolidine-3-thiones using bismuth on zirconium oxide as a recyclable catalyst in aqueous medium, Mol. Diversity, 2020, 24, 345–354 CrossRef CAS.
- R. Ramesh and A. Lalitha, Facile and Green Chemistry Access to 5-aryl-1, 2, 4-Triazolidine-3-thiones in Aqueous Medium, ChemistrySelect, 2016, 1(9), 2085–2089 CrossRef CAS.
- S. Nagaraju, et al., Synthesis of functionalized isoxazole–oxindole hybrids via on water, catalyst free vinylogous Henry and 1, 6-Michael addition reactions, RSC Adv., 2015, 5(100), 81768–81773 RSC.
| This journal is © The Royal Society of Chemistry 2025 |