Open Access Article
Open Access ArticleUpcycling waste zirconia block dental powders: towards a facile and highly selective on-off optical probe for sensing Zn2+ and Hg2+ in aqueous media†
Amin Moghaddasfar a,
Ghodsi Mohammadi Ziarani
a,
Ghodsi Mohammadi Ziarani b and
Alireza Badiei
b and
Alireza Badiei *a
*a
aSchool of Chemistry, College of Sciences, University of Tehran, Iran. E-mail: abadiei@ut.ac.ir
bDepartment of Organic Chemistry, Faculty of Chemistry, Alzahra University, Iran
First published on 15th May 2025
Abstract
Upcycling waste materials to produce high-value-added substances can pave the way for sustainable development. Waste block dental powders (WBDPs), a valuable source of zirconia, represent a significant portion of dentistry wastage and are valuable candidates for upcycling. Herein, a highly selective and facile optical probe based on upcycled WBDPs with surface interaction of 8-hydroxyquinoline-5-sulfonate (8-HQS) was developed to produce a powerful solid-state optical chemo-probe for sensing Zn2+ and Hg2+ in aqueous media. ZrO2-8-HQS provided high selectivity for sensing Zn2+ over a wide range of cations and anions, with a remarkable fluorescence intensity enhancement (λem = 517 nm) over a wide pH range (4–10). The as-prepared optical probe had a remarkable sensitivity, with a limit of detection (LoD) of 5.2 μM for Zn2+. The fluorescence of the Zn2+ probe complex was quenched in the presence of aqueous solutions of Hg2+, allowing the as-prepared chemo-probe to sense Hg2+ in aqueous media (LoD of 0.8 μM for Hg2+). The Stern–Volmer equation revealed static and dynamic mechanisms in the quenching process, and the (KS × KD) and (KS + KD) values were 0.0012 and 0.0076, respectively.
1. Introduction
Upcycling of waste materials is a promising method to convert them into high-value-added substances in their second life.1,2 In the past few decades, with the development of industries, a wide range of high-value inorganic solids have been produced for use in various sectors. However, significant amounts of these materials have been wasted and have not been recycled or upcycled. The development of a recycling or upcycling system for inorganic solid wastes is one of the important issues that help to continue environmentally sustainable development in the future.3 For instance, Badiei's group4 recycled e-waste tantalum and PET waste to synthesize an upcycled metal–organic framework as an optical chemo-sensor for the detection of chloroacetaldehyde.One of the inorganic solids is yttria tetragonal zirconia (YTZ), which has attracted attention as a promising material for dentistry. It is mainly used for building prosthodontics due to its biocompatibility, mechanical strength, and excellent esthetic properties.5 The utilization of computer-aided design/computer-aided manufacturing (CAD/CAM) technology allows dental laboratories and clinicians to manufacture dental restorations with precision and efficiency. However, the CAD/CAM milling process results in up to 80% of waste from the original disc's or block's bulk due to indirect milling of restoration. During this process, 30% of the waste becomes powder, while up to 50% remains unused, leading to noticeable environmental and economic losses.6 Nowadays, residual dental YTZ has been recycled by some companies. Unfortunately, the recycled YTZ has an irregular shape and a larger particle size, which can negatively impact its molding and sintering kinetics.7
In the last decade, human civilization development has rapidly increased water pollution.8,9 Conventional detection techniques such as GC, LC, and HPLC are limited by some shortcomings. The drawbacks of traditional methods are that some cannot detect low levels of harmful pollutants in water, especially free metal ions, and are costly, inaccessible, and complicated.10,11 Recently, fluorescence chemo-probes have been developed as a powerful method for sensing a wide range of metal ions, due to their high sensitivity, simplicity, selectivity, and on-site detection. Among the chemo-probes, some normally have a receptor that selectively interacts with the specific contaminant and a fluorophore that translates the molecular recognition into a fluorescence signal.12–14 Selective detection of transition metal ions has attracted the attention of researchers due to their fundamental effects on environmental, medical, and biological processes. Among metal ions, zinc ion (as a d10 metal ion) cannot effectively be detected by conventional methods such as absorption spectroscopy.15,16 Behind iron, zinc (Zn2+), the second most essential and abundant transition element in the human body, plays a crucial role in biological processes, including gene transcription, brain function, signal transmission, and mammalian reproduction.17,18 A wide range of Zn2+ fluorescence sensors have been developed based on various mechanisms including internal charge transfer (ICT),19 excimer/exciplex formation and extinction,20 photoinduced electron transfer (PET),21 and fluorescence resonance energy transfer (FRET).22 However, some of them also respond to other metal ions such as Pb2+, Cd2+, Ni2+, and Co2+. Therefore, researchers still desire to develop novel optical chemo-probes with selective high affinity for Zn2+ over other relevant metal ions. Mercury (Hg2+) is also a hazardous heavy metal ion that is discharged into water because of human industrial activity developments, leading to environmental pollution that can adversely affect human health.23–25 Therefore, the development of a facile and selective optical probe for sensing Zn2+ and Hg2+ in aqueous media is very essential for the development of human civilization.26,27
One of the most promising compounds for this purpose is the use of 8-hydroxyquinoline-5-sulfonate (8-HQS) and its derivatives due to their affinity and chelating interaction towards a wide range of metal ions and the high luminescence efficiency of the resulting metal complexes.28,29 As a ligand, it exhibits weak fluorescence activity primarily due to an intramolecular proton (H+) transfer from oxygen to nitrogen in the excited state, leading to a non-radiative relaxation pathway. The interaction of 8-HQS with a wide range of metal ions can result in hydroxyl H+ replacement with metal ions and consequently suppress the intramolecular H+ transfer.30 Therefore, the chelation of metal ions to 8-HQS produces a solid-state probe, bestowing it with significant enhancement in the emission intensity. According to research, zirconia (ZrO2) presumably interacts with the sulfonic acid head of 8-HQS, for which two scenarios are possible: tripodal and chelating configuration.31 Furthermore, Zn2+ presumably bonds to 8-HQS through the nitrogen and oxygen donor atoms.32–34
This study, to the best of our knowledge, is the first attempt to upcycle WBDPs with 8-HQS as a powerful solid-state optical chemo-probe. The result revealed that ZrO2 nanoparticles (NPs) could successfully enhance the PL properties of 8-HQS for the sensing of Zn2+ ions. The PL properties, stability in a wide pH range, particle size distribution, and zeta potential of the as-prepared solid-state optical probe were investigated. The quenching of emission intensity in the presence of Hg2+ allows the Zn2+ optical probe complex to sense Hg2+ in aqueous media. The quenching mechanism was evaluated by the Stern–Volmer equation and illustrated static and dynamic mechanisms in the quenching process.
2. Experimental section
2.1. Chemicals
The chemicals used in this study are waste zirconia block, 8-HQS (Merck), sodium hydroxide (NaOH, Sigma), hydrochloric acid (HCl, Sigma), nitrate salts of the metal cations (K+, Ag+, Hg+, Ni2+, Mn2+, Cd2+, Ca2+, Pb2+, Fe2+, Mg2+, Cu2+, Zn2+, Hg2+, Cr3+, Fe3+, and Al3+) and sodium salts of anions (I−, Br−, Cl−, NO3−, NO2−, CH3COO−, MoO42−, CO32−, Cr2O72−, SO42−, S2O32− and SCN−). All the above-mentioned compounds were of HPLC-reagent grade and used without any further purification.2.2. Characterization techniques
For Fourier-transform infrared (FTIR) spectroscopy measurement of ZrO2 NPs, Rayleigh WQF-510A (China) was used. A tablet mixture of ZrO2 NPs and potassium bromide (KBr) was scanned in the range of 4000 cm−1 to 400 cm−1 with 9 scans. The X-ray diffraction (XRD) pattern to determine the crystallinity and phase of the ZrO2 NPs was recorded using a Rigaku Ultima IV (Belgium) instrument (the characterization was done at ambient temperature (Kα X-ray of Cu was used)). Scanning electron microscopy (SEM) was performed using a MIRA3-Tescan for morphology investigation. The Raman spectrum was studied using a Teksan N1-541 instrument (Nd:YAG laser source, λ = 785 nm/Iran). To find the optical properties of the as-prepared chemo probe, a UV-vis spectrophotometer (Raleigh UV-1600/China) and a PL spectrometer (Agilent-G980A/USA) were used. Horiba SZ-100 was used for dynamic light scattering (DLS) and zeta potential to estimate the particle size of ZrO2-8-HQS and ZrO2 and to confirm the interaction between HQS and ZrO2 in the liquid phase, respectively.2.3. Suspension preparation and photoluminescence property examination
The WBDP ball milling was used to achieve a fine ZrO2 NPs powder. To activate the ZrO2 NP surface, 0.03 g of the obtained ZrO2 NPs were dispersed in 50 mL of distilled water and sonicated in an ultrasonic bath for 20 min. Then, 0.01 g of 8-HQS was dissolved in 50 mL of water and poured into the ZrO2-activated suspension. The obtained suspension was sonicated for 20 min and then stirred for 24 hours (Scheme 1). For PL studies, 2 mL of prepared suspension was poured into a cuvette. Subsequently, 5 μL of different cations and anions (10−2 M) was added to examine the chemo-sensitivity of the as-prepared probe. The pH of the solution was adjusted by utilizing the desired volumes of HCl and NaOH solutions. | ||
| Scheme 1 Schematic of the preparation of ZrO2-8-HQS as an on-off optical chemo-probe for sensing Zn2+ and Hg2+. | ||
3. Results and discussion
3.1. Characterization of ZrO2 NPs and ZrO2-8-HQS
The XRD analysis obtained from ZrO2 NPs revealed that the particles are highly pure and crystalline (Fig. 1a). The deflection peaks revealed sharp and high intensity. The XRD obtained result confirms the dominance of the tetragonal phase of ZrO2 NPs over the monoclinic phase (JCPDS cards no. 17-0923 and 37-1484). The positions of the deflections corresponding to the monoclinic phase were (110), (−111), (111), (002), (−012), (−211), (−202), (022), (221), (−231), and (−041), while the positions corresponding to the tetragonal phase were (011), (110), (112), (021), (121), and (022). Furthermore, the crystallite size of the ZrO2 NPs was estimated from the XRD data using the Scherrer equation given in eqn (1):
 | (1) |
The SEM characterization of the as-prepared ZrO2 NPs is illustrated in Fig. 1b. The SEM result reveals the aggregation of NPs. Furthermore, it confirmed a uniform morphology of the as-prepared NPs, and the particle size was estimated using the Digimizer software and obtained as about 14 nm. The Fourier transform infrared (FTIR) spectra recorded in the range of 4000–400 cm−1 are presented in Fig. 2. In the fingerprint region at 900–500 cm−1, the transmittance peak at 733 cm−1 corresponds to the Zr–O–Zr vibration bond. The peak at 523 cm−1 exhibits a Zr–O peak. To distinguish the structure of ZrO2, the Raman spectra of ZrO2 are displayed in Fig. 3. The vibration bonds of m-ZrO2 appeared at 170, 366, and 544 cm−1. Meanwhile, the vibration bonds of t-ZrO2 appeared at 135, 247, 316, 461, and 625 cm−1.35
The DLS results of ZrO2-8-HQS are shown in Fig. S1.† According to these results, some NPs successfully interacted with 8-HQS, forming complexes of 23.1 nm, while others remain dispersed as ZrO2 NPs in water, measuring 16.1 nm. This behavior can be clarified by the formation of coordination complexes between the ZrO2 NPs and 8-HQS. It is pertinent to mention that the PL properties obtained from DLS analysis confirm this interaction. As indicated in Table 1, the PL properties of ZrO2 NPs interacting with 8-HQS show a remarkable increase in emission intensity compared to ZrO2 NPs alone. Additionally, the DLS peak of ZrO2 NPs is sharp, whereas the DLS peak of ZrO2-8-HQS is broader. This phenomenon is attributed to the uniform hydrodynamic radius around ZrO2 NPs. However, the interaction between 8-HQS and the ZrO2 network is not uniform, resulting in a non-uniform hydrodynamic radius and a broad non-uniform DLS peak for ZrO2-8-HQS.
| Sample | Hydrodynamic radius (nm) | PL |
|---|---|---|
| ZrO2 | 16.1 | 0.076 |
| ZrO2-8-HQS | 23.1 | 0.230 |
The zeta potential of ZrO2-8-HQS, as shown in Fig. S2,† revealed a negative charge of −0.3 mV, indicating the successful interaction of 8-HQS with ZrO2 NPs.
3.2. Photoluminescence examination
 | ||
| Fig. 4 Fluorescence emission comparison of 8-HQS (dark blue line) and ZrO2-8-HQS (red line) (λex = 270 nm). | ||
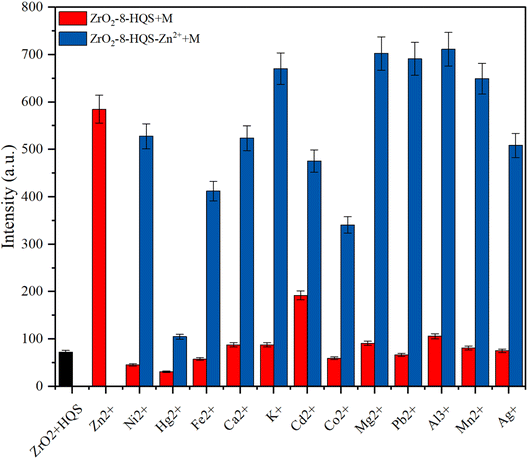
To evaluate the selectivity of the as-prepared chemo-probe for sensing ions in water, 2 mL of ZrO2-8-HQS was poured into the cuvette, followed by the addition of 5 μL of various metal ions and anions (the concentration of all metal ions and anions adjusted at 10−2 M). Fig. 5 reveals the response of the chemo-probe to different metal ions and anions. Compared to other metal ions, the emission intensity was enhanced for Al3+, Cd2+, and Ag+, while other ions caused quenching. Upon the addition of Zn2+, the emission intensity was remarkably enhanced and showed a red shift from 487.01 nm to 517.951 nm. Jianbo and co-workers34 reported that Zn2+ interacts with oxygen and nitrogen groups of 8-HQ and produces 8-HQ zinc complexes that suppress the intramolecular H+ transfer (Scheme 2). The absorption spectroscopy results (Fig. S3†) clearly indicated a blue shift after the interaction of Zn2+ with the as-prepared solid-state optical probe, leading to an ICT mechanism.37 According to Fig. S4,† the LoD of sensing Zn2+ was calculated as 5.2 μM. The response time of the as-prepared chemo probe interacting with Zn2+ was evaluated, and is shown in Fig. S5.† The results indicated that the interaction of ZrO2-8-HQS occurred rapidly within the first 10 seconds, and no noticeable change in emission intensity was observed afterward, even when the duration was extended to 300 seconds. Table 2 demonstrates the compression of various studies to detect Zn2+ with this work.
| Sensor | LoD (M) | Measured signal | Reference |
|---|---|---|---|
| 4-Methyl-2,6-bis((E)-(2-(phthalazin-1-yl)hydrazono)methyl)phenol | 2.3 × 10−6 | Fluorescence | 38 |
| (E)-1-((2-(9-(Naphthalen-1-yl)-8-(thiophen-2-yl)-9H-purin-6-yl)hydrazono)methyl)naphthalen 2-ol | 6.1 × 10−8 | Fluorescence | 39 |
| 2-(Pyridin-2-yl)-4,7-di(thiophen-2-yl)-3H-benzo[d]imidazole | 1.6 × 10−8 | Fluorescence | 40 |
| 3-[1-(4 Dimethylamino)phenylimino]ethyl 4-hydroxy-2H-chromen-2-one | 6.5 × 10−5 | Fluorescence | 41 |
| 7-(2′,4′-Dihydroxy benzylidene amino)-4-methylcoumarin | 3.8 × 10−6 | Fluorescence | 42 |
| Dipicolinohydrazonamide | 24 × 10−6 | Fluorescence | 43 |
| 4,4′-(Propane-2,2-diyl)bis(2-(((2morpholinoethyl)imino)methyl)phenol) | 7.05 × 10−8 | Fluorescence | 44 |
| (E)-2-((2-(2,4-Dinitrophenyl)hydrazineylidene) methyl)phenol | 1.1 × 10−8 | Fluorescence | 45 |
| (E)-3,5-Di-tert-butyl-2-hydroxy-N′-((1-hydroxynaphthalen-2-yl)methylene)benzohydrazide | 2.2 × 10−9 | Fluorescence | 46 |
| 2-(Benzo[d]thiazol-2-yl)-6-(1-(pyridin-2-yl)imidazo[1,5-a] pyridin-3-yl) phenol | 2.36 × 10−8 | Fluorescence | 47 |
| ZrO2-8-HQS | 5.2 × 10−6 | Fluorescence | This study |
 | (2) |
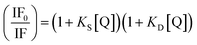 | (3) |
 | ||
| Fig. 7 (a) Titration plot of ZrO2-8-HQS-Zn2+ through the addition of Hg2+. (b) Non-linear modified Stern–Volmer quenching plot. | ||
To further evaluate the quenching mechanism of the optical probe, absorption spectroscopy was performed. The results indicated an isosbestic point at a wavelength of 333 nm following titration with Hg2+ (Fig. 7). Therefore, the quenching process allows the ZrO2-8-HQS + Zn2+ complex optical probe to detect Hg2+ selectively in aqueous solutions (LoD = 0.8 μM). The interaction of Hg2+ with ZrO2-8-HQS + Zn2+ occurred rapidly within the first 10 seconds and showed no significant change over 300 seconds, as depicted in Fig. S7.† Table 3 demonstrates the comparison of various sensors that detect Hg2+ reported in previous studies with that demonstrated in this work.
| Sensor | LoD (M) | Measured signal | Reference |
|---|---|---|---|
| rGO@MoS2 | 2.3 × 10−9 | Fluorescence | 49 |
| S,N-GQDs | 9.1 × 10−6 | Fluorescence | 50 |
| Tetra(p-dimethylaminophenyl)porphyrin | 8.0 × 10−9 | Fluorescence | 51 |
| CNPs-RhB nanohybrid | 4.2 × 10−8 | Fluorescence | 52 |
| PET-CHEF-FRET | 1.5 × 10−10 | Fluorescence | 53 |
| Rhodamine pyrene conjugate | 1.9 × 10−5 | Fluorescence | 54 |
| 2-(Rhodamine-b-hydrazido)-N-(quinolin-8-yl)acetamide | 4.5 × 10−7 | Fluorescence | 55 |
| NETBZ | 1.4 × 10−8 | Fluorescence | 56 |
| ZrO2-8-HQS-Zn | 8.04 × 10−7 | Fluorescence | This study |
3.3. pH effect
To investigate the pH effects on the as-prepared solid-state optical chemo-probe, a wide range of pH values from 2 to 10 were studied. Fig. S8† illustrates the result of optical chemo-probe performance in the sensing of Zn2+ ion in a wide pH range. According to the result, the as-prepared probe had good performance in various pH values from 4 to 10. Under harsh acidic conditions, the intensity of the chemo-probe was completely quenched (close to zero). At pH from 2 to 3, the intensity of the probe is approximately the same as fresh ZrO2-8-HQS. Therefore, this optical probe is unable to work under harsh acidic conditions. At pH = 4, the intensity enhanced to about 688, but above this pH, the intensity of the chemo-probe had not changed significantly.4. Conclusion
In summary, upcycling valuable waste materials can pave the way for preventing the loss of important resources by converting them into high-value-added materials for use in specific applications. In this study, a facile and highly selective optical probe for Zn2+ in an aqueous environment was developed by upcycling WBDPs with 8-HQS. The results clearly confirm that the prepared optical probe could selectively detect Zn2+ with a significant enhancement at λem = 517 nm. Furthermore, the as-prepared optical probe could operate within a wide range of pH with high sensitivity. Through the competitive test with various ions, it was observed that Hg2+ caused quenching effects. It allows the Zn2+ optical complex to detect Hg2+ in aqueous media. Therefore, upcycling waste materials to create high-value-added substrates, such as solid-state optical chemo-probes, can contribute to sustainable development.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- D. Sajwan, A. Sharma, M. Sharma and V. Krishnan, Upcycling of Plastic Waste Using Photo-, Electro-, and Photoelectrocatalytic Approaches: A Way toward Circular Economy, ACS Catal., 2024, 14, 4865–4926 CrossRef CAS.
- X. Zhao, B. Boruah, K. F. Chin, M. Đokić, J. M. Modak and H. S. Soo, Upcycling to Sustainably Reuse Plastics, Adv. Mater., 2022, 34, 2100843 CrossRef CAS.
- L. Ou, R. Li, H. Zhu, H. Zhao and R. Chen, Upcycling waste phosphogypsum as an alternative filler for asphalt pavement, J. Cleaner Prod., 2023, 420, 138332 CrossRef CAS.
- R. Yousefi, S. Ahmadi, G. Mohammadi Ziarani and A. Badiei, Synthesis of [Ta6O19] @MOF composite as a chloroacetaldehyde optical sensor in wastewater obtained from two waste-resourced precursors: Tantalum from capacitors and terephthalic acid from PET bottle, Mater. Today Sustain., 2023, 24, 100604 Search PubMed.
- T. M. B. Campos, C. dos Santos, L. M. M. Alves, E. B. Benalcazar-Jalkh, H. B. Strazzi-Sahyon, E. T. P. Bergamo, S. M. Tebcherani, L. Witek, P. G. Coelho, S. Yamaguchi, G. P. Thim and E. A. Bonfante, Minimally processed recycled yttria-stabilized tetragonal zirconia for dental applications: Effect of sintering temperature on glass infiltration, J. Mech. Behav. Biomed. Mater., 2024, 150, 106311 CrossRef CAS.
- C. Siligardi, S. Barbi, R. Casini, L. Tagliaferri and V. Remigio, Recycling of yttria-stabilized zirconia waste powders in glazes suitable for ceramic tiles, Int. J. Appl. Ceram. Technol., 2017, 14, 1236–1247 CrossRef CAS.
- Y. Yang, J. Jiang, G. Shen and R. Yu, An optical sensor for mercury ion based on the fluorescence quenching of tetra(p-dimethylaminophenyl)porphyrin, Anal. Chim. Acta, 2009, 636, 83–88 CrossRef CAS PubMed.
- X. Wang, Q. Lin, S. Ramachandran, G. Pembouong, R. B. Pansu, I. Leray, B. Lebental and G. Zucchi, Optical chemosensors for metal ions in aqueous medium with polyfluorene derivatives: Sensitivity, selectivity and regeneration, Sens. Actuators, B, 2019, 286, 521–532 CrossRef CAS.
- S. Chakraborty, V. Ravindran, P. V. Nidheesh and S. Rayalu, Optical Sensing of Copper and Its Removal by Different Environmental Technologies, ChemistrySelect, 2020, 5, 10432–10474 CrossRef CAS.
- Q. Li, T. Wang, Y. Jin, C. Wierzbicka, F. Wang, J. Li and B. Sellergren, Synthesis of highly selective molecularly imprinted nanoparticles by a solid-phase imprinting strategy for fluorescence turn-on recognition of phospholipid, Sensor. Actuator. B Chem., 2022, 368, 132193 CrossRef CAS.
- J. Krämer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Molecular Probes, Chemosensors, and Nanosensors for Optical Detection of Biorelevant Molecules and Ions in Aqueous Media and Biofluids, Chem. Rev., 2022, 122, 3459–3636 CrossRef.
- G. Gigi and A. M. Mohan, Probe-impregnated monolithic polymer as a robust solid-state colorimetric chemosensor for selective sensing of Hg2+ in environmental water and cigarette samples, Environ. Res., 2023, 220, 115210 CrossRef CAS PubMed.
- J. Khan, Optical Chemosensors Synthesis and Appplication for Trace Level Metal Ions Detection in Aqueous Media: A Review, J. Fluoresc., 2024, 1–22 CAS.
- V. K. Gupta, A. K. Singh, L. K. Kumawat and N. Mergu, An easily accessible switch-on optical chemosensor for the detection of noxious metal ions Ni(II), Zn(II), Fe(III) and UO2(II), Sens. Actuators, B, 2016, 222, 468–482 CrossRef CAS.
- J.-H. Hu, J.-B. Li, J. Qi and Y. Sun, Acylhydrazone based fluorescent chemosensor for zinc in aqueous solution with high selectivity and sensitivity, Sens. Actuators, B, 2015, 208, 581–587 CrossRef CAS.
- K. H. Alharbi, A Review on Organic Colorimetric and Fluorescent Chemosensors for the Detection of Zn(II) Ions, Crit. Rev. Anal. Chem., 2023, 53, 1472–1488 CrossRef CAS PubMed.
- S. Das, M. Das, A. Bag, S. Laha, B. Chandra Samanta, I. Choudhury, N. Bhattacharya and T. Maity, Selective recognition of Zn(II) by a novel Schiff base chemosensor with the formation of an AIE active Zn(II) complex having picric acid detection ability: Application in live cell imaging study, J. Photochem. Photobiol. Chem., 2024, 447, 115214 CrossRef CAS.
- M. Budri, G. Naik, S. Patil, P. Kadolkar, K. Gudasi and S. Inamdar, A novel switch on and reversible optical sensor as an efficient, selective receptor for Zn(II) ion and its biological application, Spectrochim. Acta Mol. Biomol. Spectrosc., 2020, 224, 117462 CrossRef CAS.
- S. H. Mashraqui, R. Betkar, S. Ghorpade, S. Tripathi and S. Britto, A new internal charge transfer probe for the highly selective detection of Zn(II) by means of dual colorimetric and fluorescent turn-on responses, Sens. Actuators, B, 2012, 174, 299–305 CrossRef CAS.
- M. Sohrabi, M. Amirnasr, S. Meghdadi, M. Lutz, M. Bikhof Torbati and H. Farrokhpour, A highly selective fluorescence turn-on chemosensor for Zn2+, and its application in live cell imaging, and as a colorimetric sensor for Co2+: experimental and TD-DFT calculations, New J. Chem., 2018, 42, 12595–12606 RSC.
- J. Mishra, R. Kaur, A. K. Ganguli and N. Kaur, Urea/thiourea based dipodal nanoreceptors: Aqueous medium fluorescent chemosensor for Zn(II) and Hg(II) ions by photoinduced electron transfer, Microchem. J., 2024, 206, 111501 CrossRef CAS.
- V. Venkatesan, S. A. Kumar and S. K. Sahoo, Highly selective turn-on fluorogenic chemosensor for Zn2+ based on chelation enhanced fluorescence, Inorg. Chem. Commun., 2019, 102, 171–179 CrossRef CAS.
- D. Paderni, L. Giorgi, M. Voccia, M. Formica, L. Caporaso, E. Macedi and V. Fusi, A New Benzoxazole-Based Fluorescent Macrocyclic Chemosensor for Optical Detection of Zn2+ and Cd2+, Chemosensors, 2022, 10, 188 CrossRef CAS.
- V. Kumar, D. Singh, P. Kumar, G. Chaudhary, A. P. Singh and R. Gupta, Turn-on fluorescent detection of nickel and zinc ions by two related chemosensors containing naphthalimide ring(s), J. Mol. Struct., 2022, 1261, 132901 CrossRef CAS.
- A. Kolbus, A. Danel, P. Moskwa, K. Szary and T. Uchacz, Pyrazoloquinoline-based fluorescent sensor for the detection of Pb2+, Zn2+ and the realization of an OR-type optical logic gate, Dyes Pigm., 2024, 223, 111956 CrossRef CAS.
- P. Srinivasan, S. P. Sivaraman, A. M. Mohan, D. K. Madhu, P. K. Chinaraga, C. B. Rao, S. Nagarajan and P. Deivasigamani, Chromoionophoric molecular probe infused bimodal porous polymer rostrum as solid-state ocular sensor for the selective and expeditious optical sensing of ultra-trace toxic mercury ions, J. Hazard. Mater., 2024, 478, 135483 CrossRef CAS PubMed.
- Z. Ruan, X. Dong, T. Long, S. Liu, Y. Chen and J. Lin, A novel fluorescent chemosensor enables dual-channel selective “turn-on” detection of Hg2+ and Ag+ via distinct thiophilic effects, essential mechanisms, and excellent sensing performance for mercury(ii) in aggregated states, J. Mater. Chem. C, 2024, 12, 7572–7579 RSC.
- C. Bissani Gasparin and D. A. Pilger, 8-Hydroxyquinoline, Derivatives and Metal-Complexes: A Review of Antileukemia Activities, ChemistrySelect, 2023, 8, e202204219 CrossRef CAS.
- K. Soroka, R. S. Vithanage, D. A. Phillips, B. Walker and P. K. Dasgupta, Fluorescence properties of metal complexes of 8-hydroxyquinoline-5-sulfonic acid and chromatographic applications, Anal. Chem., 1987, 59, 629–636 CrossRef CAS.
- Rohini, K. Paul and V. Luxami, 8-Hydroxyquinoline Fluorophore for Sensing of Metal Ions and Anions, Chem. Rec., 2020, 20, 1430–1473 CrossRef CAS.
- A. I. Rabee, G. A. Mekhemer, A. Osatiashtiani, M. A. Isaacs, A. F. Lee, K. Wilson and M. I. Zaki, Acidity-Reactivity Relationships in Catalytic Esterification over Ammonium Sulfate-Derived Sulfated Zirconia, Catalysts, 2017, 7, 204 CrossRef.
- H. Seo, M. K. Jackl, M. Kalaj and S. M. Cohen, Developing Metal-Binding Isosteres of 8-Hydroxyquinoline as Metalloenzyme Inhibitor Scaffolds, Inorg. Chem., 2022, 61, 7631–7641 CrossRef CAS PubMed.
- Z. H. Syed, M. R. Mian, R. Patel, H. Xie, Z. Pengmei, Z. Chen, F. A. Son, T. A. Goetjen, A. Chapovetsky, K. M. Fahy, F. Sha, X. Wang, S. Alayoglu, D. M. Kaphan, K. W. Chapman, M. Neurock, L. Gagliardi, M. Delferro and O. K. Farha, Sulfated Zirconium Metal–Organic Frameworks as Well-Defined Supports for Enhancing Organometallic Catalysis, J. Am. Chem. Soc., 2022, 144, 16883–16897 CrossRef CAS.
- H. Jianbo, Z. Tingting, C. Yongjing, Z. Yuanyuan, Y. Weiqing and M. Menglin, Study on Relationship Between Fluorescence Properties and Structure of Substituted 8-Hydroxyquinoline Zinc Complexes, J. Fluoresc., 2018, 28, 1121–1126 CrossRef CAS.
- M. H. Zare and A. Mehrabani-Zeinabad, Photocatalytic activity of ZrO2/TiO2/Fe3O4 ternary nanocomposite for the degradation of naproxen: characterization and optimization using response surface methodology, Sci. Rep., 2022, 12, 10388 CrossRef CAS PubMed.
- M. Tao, S. Ishikawa, T. Ikeda, S. Yasumura, K. Shimoda, R. Osuga, Y. Jing, T. Toyao, K.-i. Shimizu, H. Matsuhashi and W. Ueda, Acid Catalysis over Crystalline Zr3SO9: Role of the Local Structure in Generating Acidity, ACS Catal., 2023, 13, 4517–4532 CrossRef CAS.
- L. Wu, A. C. Sedgwick, X. He and T. D. James, Fluorescent Chemosensors, Royal Society of Chemistry, 2023 Search PubMed.
- M. Khatun, P. Ghorai, J. Mandal, S. Ghosh Chowdhury, P. Karmakar and A. Saha, Design and synthesis of a hydrazinopthalazine derived chemosensor to detect metal ions Zn2+, Al3+ via CHEF effect with biological study and theoretical calculation, J. Photochem. Photobiol. Chem., 2024, 446, 115145 CrossRef CAS.
- H. Xu, W. Chen, W. Zhang, L. Ju and H. Lu, A selective purine-based fluorescent chemosensor for the “naked-eye” detection of zinc ions (Zn2+): applications in live cell imaging and test strips, New J. Chem., 2020, 44, 15195–15201 RSC.
- N. Honnappa, A. G. Anil, S. Shekar, S. K. Behera and P. C. Ramamurthy, Design of a Highly Selective Benzimidazole-Based Derivative for Optical and Solid-State Detection of Zinc Ion, Inorg. Chem., 2022, 61, 15085–15097 CrossRef CAS PubMed.
- D. Sarkar, A. K. Pramanik and T. K. Mondal, Coumarin based dual switching fluorescent ‘turn-on’ chemosensor for selective detection of Zn2+ and HSO4−: an experimental and theoretical study, RSC Adv., 2014, 4, 25341–25347 RSC.
- J.-c. Qin, L. Fan, B.-d. Wang, Z.-y. Yang and T.-r. Li, The design of a simple fluorescent chemosensor for Al3+/Zn2+via two different approaches, Anal. Methods, 2015, 7, 716–722 RSC.
- K. Kumarasamy, Z.-W. Wu, W.-J. Chien, M.-C. Lin and S. K. Ramasamy, A ratiometric turn-on chemosensor for highly selective and sensitive visual detection of Zn2+ and its applications to environmental samples, J. Environ. Chem. Eng., 2024, 12, 114615 CrossRef CAS.
- S. Bari, D. Mridha, T. Roychowdhury and P. Roy, Detection of Zn2+ and its imaging in plant roots by a bisphenol A-Based fluorescent chemosensor, Inorg. Chim. Acta, 2024, 566, 122011 CrossRef CAS.
- R. Behura, P. P. Dash, P. Mohanty, S. Behera, M. Mohanty, R. Dinda, S. K. Behera, A. K. Barick and B. R. Jali, A Schiff base luminescent chemosensor for selective detection of Zn2+ in aqueous medium, J. Mol. Struct., 2022, 1264, 133310 CrossRef CAS.
- M. Budri, G. Naik, S. Patil, P. Kadolkar, K. Gudasi and S. Inamdar, An ESIPT blocked highly ICT based molecular probe to sense Zn (II) ion through turn on optical response: Experimental and theoretical studies, J. Photochem. Photobiol., A., 2020, 390, 112298 CrossRef CAS.
- S. Enbanathan, S. Munusamy, D. Jothi, S. Manojkumar, S. Manickam and S. K. Iyer, Zinc ion detection using a benzothiazole-based highly selective fluorescence “turn-on” chemosensor and its real-time application, RSC Adv., 2022, 12, 27839–27845 RSC.
- A. S. Tanwar, R. Parui, R. Garai, M. A. Chanu and P. K. Iyer, Dual “Static and Dynamic” Fluorescence Quenching Mechanisms Based Detection of TNT via a Cationic Conjugated Polymer, ACS Meas. Sci. Au, 2022, 2, 23–30 CrossRef CAS PubMed.
- A. Kumar, N. Ahmad, Y. Jadeja, S. Ganesan, J. Abd Hamid, P. Singh, K. Kaur and L. Hassen jaseem, Fluorescence sensor for mercury ions in aqueous mediums based on reduced graphene oxide linked with molybdenum disulfide, J. Phys. Chem. Solids, 2025, 196, 112305 CrossRef CAS.
- Z. Liu, Z. Mo, X. Niu, X. Yang, Y. Jiang, P. Zhao, N. Liu and R. Guo, Highly sensitive fluorescence sensor for mercury(II) based on boron- and nitrogen-co-doped graphene quantum dots, J. Colloid Interface Sci., 2020, 566, 357–368 CrossRef CAS PubMed.
- H. Yang, L. Sun, H. Yu, A. P. Nugraha, J. R. V. Sáenz and G. Hong, Current prospect of dental zirconia recycling: A scoping review, J Prosthodont Res., 2024, 68, 522–531 CrossRef.
- M. Lan, J. Zhang, Y.-S. Chui, P. Wang, X. Chen, C.-S. Lee, H.-L. Kwong and W. Zhang, Carbon Nanoparticle-based Ratiometric Fluorescent Sensor for Detecting Mercury Ions in Aqueous Media and Living Cells, ACS Appl. Mater. Interfaces, 2014, 6, 21270–21278 CrossRef CAS.
- M. Banerjee, M. Ghosh, S. Ta, J. Das and D. Das, A smart optical probe for detection and discrimination of Zn2+, Cd2+ and Hg2+ at nano-molar level in real samples, J. Photochem. Photobiol. Chem., 2019, 377, 286–297 CrossRef CAS.
- K.-H. Chu, Y. Zhou, Y. Fang, L.-H. Wang, J.-Y. Li and C. Yao, Rhodamine–pyrene conjugated chemosensors for ratiometric detection of Hg2+ ions: Different sensing behavior between a spirolactone and a spirothiolactone, Dyes Pigm., 2013, 98, 339–346 CrossRef CAS.
- B. Sen, M. Mukherjee, S. Pal, K. Dhara, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, A water soluble FRET-based ratiometric chemosensor for Hg(ii) and S2− applicable in living cell staining, RSC Adv., 2014, 4, 14919–14927 RSC.
- D. Aydin and I. Yilmaz, Simple synthesis and sensing applications of a new and low cost fluorescent chemosensor for selective recognition and facile removal of Hg2+, J. Photochem. Photobiol. Chem., 2021, 414, 113280 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01728a |
| This journal is © The Royal Society of Chemistry 2025 |