Open Access Article
Open Access ArticleFacile access to quinazolin-4(3H)-ones by tandem Cu-catalyzed annulation of 2-nitrobenzonitrile and alcohols under air†
Xuan Lam Vua,
Ban Van Phucb,
Minh Hoang Nguyena,
Thuy Anh T. Nguyena,
Thuy Linh Nguyena,
Hien Nguyenc,
Quoc Anh Ngobd,
Nguyen Quang Anb,
Tran Quang Hung *bd and
Tuan Thanh Dang
*bd and
Tuan Thanh Dang *a
*a
aFaculty of Chemistry, VNU-Hanoi University of Science, 19 Le Thanh Tong, Hanoi, Vietnam. E-mail: dangthanhtuan@hus.edu.vn
bInstitute of Chemistry, Vietnamese Academy of Science and Technology (VAST), 18 Hoang Quoc Viet, Hanoi, Vietnam. E-mail: tqhung@ich.vast.vn
cFaculty of Chemistry, Hanoi National University of Education (HNUE), Vietnam
dGraduate University of Science and Technology, Vietnam Academy of Science and Technology, Vietnam
First published on 21st July 2025
Abstract
A convenient Cu-catalyzed synthesis of 2-arylquinazolin-4(3H)-one derivatives has been developed from simple starting materials such as 2-nitrobenzonitriles and alcohols under mild conditions. This procedure showed a broad substrate scope and tolerance of functional groups, high yields, and no need to use oxidants or reductants, making synthesis simple, convenient, and sustainable. Our methodology may be utilized for the one-pot synthesis of glycosminine alkaloid.
Fused N-heterocyclic compounds have been recognized as critical targets in designing of new bioactive molecules, agrochemicals, medicines, and advanced materials.1,2 Among these, 2-arylquinazolin-4(3H)-ones have been studied and found to have interesting bioactivities, for example, anti-allergy, antidepressant, antihypertensive, anti-malarial, antimicrobial, anticonvulsant, anti-inflammatory, anti-cancer, and others.3 Notably, 2-arylquinazolin-4(3H)-ones have been found in the structures of several alkaloids.4 In fact, over 75 quinazolinone-based natural products with intriguing biological activities have been identified (Fig. 1).3,4 Interestingly, a number of major quinazolinone-based pharmaceutics have been utilized clinically to treat a variety of disorders, including albaconazole, afloqualone, balaglitazone, halofuginone, cloroqualone, metolazone, febrifugine, quinethazone A, luotonin, raltitrexed, and others.3,4
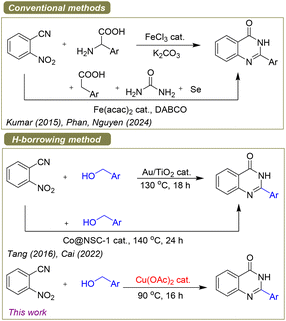
Numerous synthetic methods to prepare quinazolinone structures have been described because 2-arylquinazolin-4(3H)-ones are crucial to the advancement of pharmaceutical studies and drug discovery.3,4 Quinazolinones can generally be made using the following methods (Scheme 1): (i) the classical cyclocondensations of 2-aminobenzamides with aldehydes and carboxylic acids,5 (ii) the annulation reactions of 2-2-nitrobenzonitriles, aminobenzonitriles with carbonyl compounds including aldehydes, ketones as well as carboxylic acids,6 (iii) the cyclization reactions of 2-halobenzamide derivatives with aldehydes, benzylamines, α-amino acids, nitriles, amidines and other related transformations with 2-halobenzoic acids,7 (iv) the autohydrogen-transfer and/or dehydrogenation tandem annulations of 2-aminobenzonitriles, 2-nitrobenzamides or 2-aminobenzamides with alcohols.8
These previous methods have some advantages; however, they frequently have several drawbacks such as narrow substrate scope, hard conditions, and require the employment of an excessive number of nonrenewable oxidants. In recent years, the advancement of more convenient and greener synthetic approaches based on cost-effective and non-hazardous starting materials has tremendously benefited sustainable operations in future.9 In comparison to classical condensation methods, acceptorless dehydrogenation and hydrogen-auto transfer pathways in the employment of less toxic and low-cost chemicals such as amines, alcohols, nitro compounds and nitriles, which produce only hydrogen gas and/or water as byproducts, could be a more sustainable solution to preparing N-heterocyclic compounds.10 Interestingly, 2-arylquinazolin-4(3H)-ones could be prepared using readily low-cost starting materials, for example, 2-nitrobenzonitriles and alcohols. Up to now, two catalytic methods have been developed so far for generating the 2-arylquinazolin-4(3H)-ones from 2-nitrobenzonitrile derivatives.11 In 2016, Tang et al. disclosed the first preparation of 2-arylquinazolin-4(3H)-ones by hydrogen-transfer process employing Au nanoparticles/TiO2 as heterogeneous catalyst.11a Cai and coworkers recently reported a unique Co nanoparticles/N,S co-doped carbon as a cheap and active heterogeneous catalyst for the production of 2-arylquinazolin-4(3H)-one derivatives from 2-nitrobenzonitriles and benzyl alcohols.11b In fact, these approaches using heterogeneous catalysts demonstrated advantages, their catalysts needed huge effort to synthesize and characterize the nanostructure catalysts, which may limit potential uses in organic synthesis and medicinal chemistry research. Additionally, their processes had to be carried out at high temperatures (over 130 °C) in an inert gas atmosphere. To address these limitations, in early 2025, we reported an efficient method for the 2-arylquinazolin-4(3H)-ones synthesis from 2-nitrobenzonitriles and alcohols using [Ru(COD)Cl2]n catalyst.11c However, Ru metal has been known as a precious and quite toxic transition metal which limits its possible application in pharmaceutical and fine chemical industries. In continuous progress, herein, we present the first homogeneous copper catalyst for producing high yields of various quinazolin-4(3H)-ones, therefore aiding in the advancement of hydrogen-transfer methods using cheap transition metal catalysts. This tandem procedure involved the first reduction of the nitro group by copper hydride to generate an amine group, followed by the second cyclization step of this amine intermediate with in situ-formed aldehyde under mild conditions.
To develop a straightforward approach for synthesizing 2-phenylquinazolin-4(3H)-one product 3a, we choose the reaction of 2-nitrobenzonitrile 1a with benzyl alcohol 2a in the employment of LiOtBu base to optimize copper catalysts. Table 1 lists the key factors that may influence the reaction, such as ligands, Cu precursors, and bases, at 120 °C. First, we used bidentate phosphines as ligands when combined with Cu(OAc)2 precursor using well-established methods. Under these conditions, the BINAP ligand in conjunction with Cu(OAc)2 appears to be the best choice for this transformation, resulting in 75% yield of the product 3a (Table 1, entry 2). Especially, when we carried out this reaction using only Cu(OAc)2 catalyst in the absence of any ligands, product 3a was received with 77% yield (Table 1, entry 6). After that, a number of common bases were also looked into. Notably, using KOH as the base and solvent, respectively, allowed us to achieve a yield of up to 84% of the target product 3a (entry 10). Product 3a's synthesis at 80 °C produced a comparable yield when the reaction temperature was lowered (entries 14). Notably, when this transformation was carried out in an air atmosphere, the Cu(OAc)2 catalyst was discovered to be stable and extremely active, allowing for the preparation of product 3a in an 86% yield (Entry 15). It means that oxygen (in air) did not play a critical role in this transformation. Then, two control reactions were conducted in which either the base or the Cu(OAc)2 catalyst was absent in order to obtain useful insights of the catalyst's actual function. Product 3a was only observed in trace amounts (entries 17 and 18).
| Entry | Catalyst | Ligand | Base | Temp. (°C) | Yieldb (%) |
|---|---|---|---|---|---|
| a Condition: 1a (0.34 mmol), 2a (5 equiv.), base (2.0 equiv.), Cu(OAc)2 catalyst (10 mol%), ligand (5 mol%), 16 h.b Yield of isolated products are reported.c Reaction was conducted under air. | |||||
| 1 | CuI | BINAP | LiOtBu | 120 | 67 |
| 2 | Cu(OAc)2 | BINAP | LiOtBu | 120 | 75 |
| 3 | Cu(OAc)2 | dppe | LiOtBu | 120 | 50 |
| 4 | Cu(OAc)2 | 1,10-Phenanthroline | LiOtBu | 120 | 70 |
| 5 | Cu(OAc)2 | L-Proline | LiOtBu | 120 | 60 |
| 6 | Cu(OAc)2 | — | LiOtBu | 120 | 77 |
| 7 | Cu(OAc)2 | — | LiOtBu | 120 | 63 |
| 8 | Cu(OAc)2 | — | KOtBu | 120 | 80 |
| 9 | Cu(OAc)2 | — | NaOEt | 120 | 78 |
| 10 | Cu(OAc)2 | — | KOH | 120 | 84 |
| 11 | Cu(OAc)2 | — | NaOH | 120 | 80 |
| 12 | Cu(OAc)2 | — | K2CO3 | 120 | 20 |
| 13 | Cu(OAc)2 | — | KOH | 100 | 85 |
| 14 | Cu(OAc)2 | — | KOH | 80 | 84 |
| 15 | Cu(OAc)2 | — | KOH | 80 | 86c |
| 16 | Cu(OAc)2 | — | KOH | 70 | 60 |
| 17 | — | — | KOH | 80 | n.d. |
| 18 | Cu(OAc)2 | — | — | 80 | n.d. |
Using optimized conditions, we began to study the possibility of the cyclization reaction of compound 1a with several alcohols 2a–r, as shown in Table 2. The intended quinazolinone compounds were successfully produced, with isolated yields reaching up to 90%. Typically, the cyclization of compound 1a using benzylic alcohol derivatives produced the desired products 3a–p in high yield, with the tolerance of several functional groups reaching up to 90%. Interestingly, furan-2-ylmethanol may be efficiently used as an alcohol substrate in this reaction, yielding the matching quinazolinone 3p in 83% isolated yield. Notably, the cyclization of starting material 1a with challenging aliphatic alcohols could be achieved when these reactions were conducted under harsh conditions (160 °C, 48 h). Especially, product 3q which was known as a bioactive alkaloid (glycosminine) isolated from Indian medicinal plant (Glycosmis arborea).12
Finally, we intended to investigate the scope of this reaction by using 2-nitrobenzonitrile derivatives 1b–g as starting materials in a one-pot annulation process with benzyl alcohols 2a–e and Cu(OAc)2 catalyst under our optimal conditions. Notably, the 2-arylquinazolin-4(3H)-one products 4a–k were achieved in up to 89% isolated yield (Table 3). The preparation of quinazolinones utilizing earlier heterogeneous catalysts necessitated well-designed catalysts operating at high temperatures (over 130 °C), which may be cumbersome for convenient applications in medicinal chemistry and organic synthesis. Our method significantly improved the preparation of 2-arylquinazolin-4(3H)-one derivatives under milder conditions, utilizing a low-cost and air-stable Cu(OAc)2 catalyst.
| a Condition: 1b–g (0.34 mmol), 2a–e (5 equiv.), KOH (2.0 equiv.), Cu(OAc)2 catalyst (10 mol%). Yields of isolated products are given. Reaction were performed under air atmosphere. |
|---|
 |
Specifically, the Cu-catalyzed annulation reactions of starting materials containing bromine and fluorine substituents (1h,i) with benzyl alcohol 2a did not produce the predicted products (4l,m). Instead of obtaining desired products (4l,m), 2-phenylquinazolin-4(3H)-one products (4l′,m′) were isolated in 43% and 60%, respectively (Table 4). In fact, SNAr (Nucleophilic Aromatic Substitution) reaction of substrates (1h,i) with the in situ-formed benzylic alkoxide occurred faster than the hydrogen-transfer and annulation reactions. Finally, the coupling reaction of 2-nitro-4-(trifluoromethyl)benzonitrile substrate 1j with benzyl alcohol 1a resulted in the formation of uncyclized amide 4n′ as a side productin 78% isolated yield. It means that the in situ-formed amide should be the key intermediate in this transformation.
| a Condition: 1h–j (0.34 mmol), 2a (5 equiv.), KOH (2.0 equiv.),Cu(OAc)2 catalyst (10 mol%). Yields of isolated products are given. Reactions were performed under air atmosphere. |
|---|
 |
To better understand the reaction process and the true role of the Cu(OAc)2 catalyst in this reaction, we conducted some control experiments, as shown in Scheme 2. First, to investigate the hydrogen-transfer reaction in the presence of Cu(OAc)2 catalyst, the reaction of 2-nitrobenzonitrile 1a with benzaldehyde 2a′ was conducted under optimum conditions (Scheme 2, reaction (1)). In fact, we did not observe even a trace amount of quinazolinone 3a. In this Cu-catalyzed reaction, we hypothesize that 2-aminobenzonitrile 4a and 2-aminobenzamide 5a would be in situ-formed intermediates. Then, 2-aminobenzonitrile 4a and 2-aminobenzamide 5a were used in the annulation processes with benzyl alcohol 1a. Indeed, quinazolinone 3a was obtained in 85% and 91% of cases (Scheme 2, reaction (2) and (3)). Finally, Cu-catalyzed annulation of 2-aminobenzamide 5a with benzaldehyde 2a′ produced the dehydrogenative product 3a in 90% isolated yield (Scheme 2, reaction (4)). Based on these achievements, we can realize that benzyl alcohol 2a and nitro group in the compound 1a contributed as hydrogen donors and acceptors in the Cu(OAc)2-catalyzed preparation of quinazolinone products. In order to explain the negative effect of any ligands in this transformation (Table 1), the reaction of nitrobenzene with benzyl alcohol 1a was performed under optimized conditions (reaction (5)). As we predicted, this reaction did not work due to the lack of any directing group as the primary amide in the intermediate B which is proposed in Scheme 3. Indeed, ligands may well hinder the formation of Cu–H complex as well as the hydrogen transfer process in the intermediate B.
Based on the achieved results in control experiments, a plausible mechanism for the Cu(OAc)2-catalyzed preparation of quinazolinones is described (Scheme 3). Firstly, benzyl alcohol 2a was transformed into benzaldehyde 2a′ in the employment of Cu(OAc)2 catalyst and KOH base. This step produced the catalytic active species (AcO)Cu–H (intermediate A) reducing the intro group of 1a′ to give an amino group of the benzamide intermediate 4 via Cu–H complex intermediate B. Indeed, the in situ-formed amide group played a key role as the directing group for the success of this transformation. In the next step, the benzamide intermediate 4 reacted with benzaldehyde 2a′ to form the dihydroquinazolinone intermediate 5, which was further transformed into the quinazolinone product 3a. In fact, a second Cu-catalyzed hydrogen-transfer process happens with the intermediate 5 yielding quinazolinone product 3a in the presence of Cu(OAc)2 catalyst, and catalytic (AcO)Cu–H species A could be again in situ-generated, which are required for the reduction of the nitro group in the structure of starting material 1a in order to attend the next catalytic cycle.
Conclusions
In conclusion, we disclosed a feasible and practical approach for synthesizing a series of 2-arylquinazolin-4(3H)-one products in high yields from readily available compounds such as 2-nitrobenzonitriles and alcohols under mild conditions. Relying on the results of the control reactions, a viable mechanism for this transformation involving a Cu(OAc)2 catalyst and a hydrogen-borrowing route was proposed. Our approach, which employed a practical, air-stable, and inexpensive Cu(OAc)2 catalyst, outperformed earlier methods that used heterogeneous catalysts and required multiple steps to prepare. The findings given herein may be useful to explore promising applications in organic synthesis and medicinal chemistry.Data availability
The data supporting this research are available in the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Vietnam Academy of Science and Technology under Grant Number: NCXS 01.02/24-26.Notes and references
- (a) R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed; (b) J. A. Joule and K. Mills, Heterocyclic Chemistry, Wiley, Chichester, UK, 5th edn, 2010 Search PubMed; (c) R. Vardanyan and V. Hruby, Synthesis of Best-Seller Drugs, Elsevier Science, 2016 Search PubMed.
- (a) M. L. Fascio, M. I. Errea and N. B. D'Accorso, Eur. J. Med. Chem., 2015, 90, 666 CrossRef CAS PubMed; (b) L. Kielesinski, M. Tasior and D. T. Gryko, Org. Chem. Front., 2015, 2, 21 RSC; (c) T. N. Ngo, P. Ehlers, T. T. Dang and P. Langer, Org. Biomol. Chem., 2015, 13, 3321 RSC; (d) T. N. Ngoc, O. A. Akrawi, T. T. Dang, A. Villinger and P. Langer, Tetrahedron Lett., 2015, 56, 86 CrossRef; (e) B. V. Phuc, N. T. Nguyen, N. T. H. Van, T. L. Nguyen, V. H. Nguyen, C. M. Tran, H. Nguyen, M. T. Nguyen, T. Q. Hung and T. T. Dang, Chem. Commun., 2023, 59, 1947 RSC; (f) T. Q. Hung, N. N. Thang, D. H. Hoang, T. T. Dang, A. Villinger and P. Langer, Org. Biomol. Chem., 2014, 12, 2596 RSC; (g) T. Q. Hung, N. N. Thang, D. H. Hoang, T. T. Dang, A. Villinger, K. Ayub, S. Lochbrunner, G. Flechsig and P. Langer, Eur. J. Org Chem., 2015, 5, 1007 CrossRef; (h) T. T. Dang, U. Albrecht, K. Gerwien, M. Siebert and P. Langer, J. Org. Chem., 2006, 71, 2293 CrossRef CAS PubMed; (i) N.-K. Nguyen, M.-T. Ha, H. Y. Bui, Q. T. Trinh, B. N. Tran, T. Q. Hung, T. T. Dang and X. H. Vu, Catal. Commun., 2021, 149, 106240 CrossRef CAS; (j) N.-K. Nguyen, D. H. Nam, B. V. Phuc, V. H. Nguyen, Q. T. Trịnh, T. Q. Hung and T. T. Dang, Mol. Catal., 2021, 505, 111462 CAS.
- P. S. Auti, G. George and A. T. Paul, RSC Adv., 2020, 10, 41353 RSC.
- S. B. Mhaske and N. P. Argade, Tetrahedron, 2006, 62, 9787 CrossRef CAS.
- (a) N. Y. Kim and C. H. Cheon, Tetrahedron Lett., 2014, 55, 2340 CrossRef CAS; (b) Z. Bie, G. Li, L. Wang, Y. Lv, J. Niu and S. Gao, Tetrahedron Lett., 2016, 57, 4935 CrossRef CAS; (c) R. Cheng, L. L. Tang, T. J. Guo, D. Zhang-Negrerie, Y. F. Du and K. Zhao, RSC Adv., 2014, 4, 26434 RSC; (d) X. Chen, T. Chen, F. Ji, Y. Zhou and S.-F. Yin, Catal. Sci. Technol., 2015, 5, 2197 RSC.
- (a) M. Kumar, Richa, S. Sharma, V. Bhatt and N. Kumar, Adv. Synth. Catal., 2015, 357, 2862 CrossRef CAS; (b) T. B. Nguyen, J. L. Bescont, L. Ermolenko and A. Al-Mourabit, Org. Lett., 2013, 15, 6218 CrossRef CAS PubMed; (c) T. T. Nguyen, D. T. M. Chung, K. X. Nguyen, V. P. T. Nguyen, N. T. S. Phan and T. T. Nguyen, ChemistrySelect, 2024, 9, e202303762 CrossRef CAS.
- (a) W. Xu, Y. Jin, H. Liu, Y. Jiang and H. Fu, Org. Lett., 2011, 13, 1274 CrossRef CAS PubMed; (b) T. Kotipalli, V. Kavala, D. Janreddy, V. Bandi, C. W. Kuo and C. F. Yao, Eur. J. Org Chem., 2016, 1182 CrossRef CAS; (c) W. Xu and H. Fu, J. Org. Chem., 2011, 76, 3846 CrossRef CAS PubMed; (d) X. Yu, L. Gao, L. Jia, Y. Yamamoto and M. Bao, J. Org. Chem., 2018, 83, 10352 CrossRef CAS PubMed; (e) T. Songsichan, J. Promsuk, V. Rukachaisirikul and J. Kaeobamrung, Org. Biomol. Chem., 2014, 12, 4571 RSC; (f) L. Xu, Y. Jiang and D. Ma, Org. Lett., 2012, 14, 1150 CrossRef CAS PubMed; (g) W. Xu and H. Fu, J. Org. Chem., 2011, 76, 3846 CrossRef CAS PubMed.
- (a) H. Wang, X. Cao, F. Xiao, S. Liu and G. J. Deng, Org. Lett., 2013, 15, 4900 CrossRef CAS PubMed; (b) S. Mondal, S. Chakraborty, S. Khanra, S. Chakraborty, S. Pal, P. Brandao and N. D. Paul, J. Org. Chem., 2024, 89, 5250 CrossRef CAS PubMed; (c) J. Zhou and J. Fang, J. Org. Chem., 2011, 76, 7730 CrossRef CAS PubMed; (d) A. J. A. Watson, A. C. Maxwell and J. M. J. Williams, Org. Biomol. Chem., 2012, 10, 240 RSC; (e) Z. Bie, G. Li, L. Wang, Y. Lv, J. Niu and S. Gao, Tetrahedron Lett., 2016, 57, 4935 CrossRef CAS; (f) S. Parua, S. Das, R. Sikari, S. Sinha and N. D. Paul, J. Org. Chem., 2017, 82, 7165 CrossRef CAS PubMed; (g) W. Zhang, C. Meng, Y. Liu, Y. Tang and F. Li, Adv. Synth. Catal., 2018, 360, 3751 CrossRef CAS; (h) Y. Hu, S. Li, H. Li, Y. Li, J. Li, C. Duanmu and B. Li, Org. Chem. Front., 2019, 6, 2744 RSC; (i) Sk. A. Samim, B. C. Roy, S. Nayak and S. Kundu, J. Org. Chem., 2020, 85(17), 11359 CrossRef CAS PubMed; (j) S. M. A. H. Siddiki, K. Kon, A. S. Touchy and K. I. Shimizu, Catal. Sci. Technol., 2014, 4, 1716 RSC; (k) J. Qiao, H. Jiang, X. Liu, C. Xu, Z. Sun and W. Chu, Eur. J. Org Chem., 2019, 13, 2428 CrossRef; (l) M. Vageesh, P. Omkar, P. Hima and D. Raju, Synlett, 2024, 35, 2496–2502 CrossRef.
- C.-J. Li and B. Trost, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 13197 CrossRef CAS PubMed.
- (a) N. Hofmann and K. C. Hultzsch, Eur. J. Org Chem., 2021, 46, 6206 CrossRef; (b) B. Paul, M. Maji, K. Chakrabarti and S. Kundu, Org. Biomol. Chem., 2020, 18, 2193 RSC.
- (a) L. Tang, X. Zhao, G. Zou, Y. Zhou and X. Yang, Asian J. Org. Chem., 2016, 5, 335 CrossRef CAS; (b) T. Su, K. Sun, G. Lu and C. Cai, ACS Sustain. Chem. Eng., 2022, 10, 3872 CrossRef CAS; (c) B. V. Phuc, T. D. Nguyen, N. T. M. Tuan, P. A. Dao, P. T. T. Vy, N. T. Nguyen, Q. A. Ngo, T. Q. Hung and T. T. Dang, Org. Biomol. Chem., 2025, 23, 603 RSC.
- S. C. Pakrashi, J. Bhattacharyya, L. F. Johnson and H. Budzikiewicz, Tetrahedron, 1963, 19, 1011 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra01550b |
| This journal is © The Royal Society of Chemistry 2025 |