Open Access Article
Open Access ArticleMethyl chlorothioformate as a convenient reagent for thionoester synthesis†
Mykhailo O. Pashkoab,
Kiril V. Pashkovac,
Dmitriy S. Granatac,
Yurii L. Yagupolskiic,
Serhiy V. Ryabukhin *abc and
Dmytro M. Volochnyuk*abc
*abc and
Dmytro M. Volochnyuk*abc
aEnamine Ltd, 78 Winston Churchill str., 02094 Kyiv, Ukraine. E-mail: s.v.ryabukhin@gmail.com; d.volochnyuk@gmail.com
bInstitute of Organic Chemistry, National Academy of Sciences of Ukraine, 5 Akademik Kuhar str., 02660 Kyiv, Ukraine
cTaras Shevchenko National University of Kyiv, 60 Volodymyrska str., 01601 Kyiv, Ukraine
First published on 8th May 2025
Abstract
A promising reagent for introducing the methyl thionoester function into organic molecules through a straightforward synthetic sequence based on common magnesium organics is proposed. A scalable procedure for its production is elaborated. The potential of the reaction, along with its advantages/simplicity, convenience, and effectiveness, is demonstrated and discussed.
Introduction
Thionoesters are the thiocarbonyl analogues of esters and possess unique reactivity. The difference in the reactivity of thionoesters and classical esters is explained by the difference in C = X double bond energy (the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond is almost 50 kcal mol−1 more robust than the C
O bond is almost 50 kcal mol−1 more robust than the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond),1 higher polarisability of sulfur compared with oxygen and the significant influence of the C
S bond),1 higher polarisability of sulfur compared with oxygen and the significant influence of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S function on the reactivity of the leaving group at the Csp2 carbon atom.2 This difference allows for unique functional group transformations, such as the reduction of thionoesters to ethers,3 the addition of organolithium with a subsequent reduction to modified branched ethers,4 and dethiofluorination to difluoroalkyl ethers,5 including the possibility of radiotracer production,6 and, in some cases, usage in Newman–Kwart-like rearrangement, leading to rare thiols.7 Additionally, thionoesters are valuable and irreplaceable C1-synthons8 and dipolarophiles9 for the synthesis of sulfur-containing heterocycles (Fig. 1). However, the synthetic potential of these compounds has not yet been fully revealed owing to their limited availability, especially in large quantities. Generally, synthetic methodologies can be divided into four categories: A–D. Category A is based on the sulfo-hydrolysis of imino esters with H2S in pyridine,10 which has a drawback of forming thioamides as side products. Next, category B is based on the thionation of esters by P4S10 with different modifications.11 However, such procedures still require harsh reaction conditions, which limit the diversity of substrates and the scale of synthesis. Category C, which is the largest category, uses alcohols for thioacylation,12 including Pd-catalysed.13 These processes require multiple non-trivial additional synthetic steps to prepare activated precursors. Among such protocols, the Newton base-catalyzed transesterification of thionoesters seems the most promising owing to the possibility of keeping on stock only corresponding methyl thionoesters as “surrogates” of all other esters.14 Category D, which is rare, is based on C–C bond creation using C-nucleophiles and electrophilic counterparts with a thionoester function. Among recent protocols, the Ni-catalyzed cross-coupling of organozinc reagents and thiocarbonyl-containing starting materials could be highlighted.15 Inspired by Newton's transesterification and the possibility of C–C bond creation, we decided to develop a convenient and scalable method for methyl thionoesters from readily available starting materials. Methyl chlorothioformate was selected as the electrophilic counterpart. Except for the “neglected” Delepine report from 1911 without experimental details,16 only Pd-catalyzed thioacylation of mono-substituted alkynes with methyl chlorothioformate was described for this type of process.17 Moreover, only phenyl chlorothioformate was used for thionoester synthesis in the reaction with PhMgBr18 and the AlCl3-catalyzed acylation of arenes.19 We report preliminary and essential results for the TM-free synthesis of thionoesters from methyl chlorothioformate and commonly used Li, Mg and Zn organometallics. Such a protocol has considerable potential to significantly increase the diversity and availability of this rare class of compounds on a 10–100 gram scale, with a possibility for further scale-up.
S function on the reactivity of the leaving group at the Csp2 carbon atom.2 This difference allows for unique functional group transformations, such as the reduction of thionoesters to ethers,3 the addition of organolithium with a subsequent reduction to modified branched ethers,4 and dethiofluorination to difluoroalkyl ethers,5 including the possibility of radiotracer production,6 and, in some cases, usage in Newman–Kwart-like rearrangement, leading to rare thiols.7 Additionally, thionoesters are valuable and irreplaceable C1-synthons8 and dipolarophiles9 for the synthesis of sulfur-containing heterocycles (Fig. 1). However, the synthetic potential of these compounds has not yet been fully revealed owing to their limited availability, especially in large quantities. Generally, synthetic methodologies can be divided into four categories: A–D. Category A is based on the sulfo-hydrolysis of imino esters with H2S in pyridine,10 which has a drawback of forming thioamides as side products. Next, category B is based on the thionation of esters by P4S10 with different modifications.11 However, such procedures still require harsh reaction conditions, which limit the diversity of substrates and the scale of synthesis. Category C, which is the largest category, uses alcohols for thioacylation,12 including Pd-catalysed.13 These processes require multiple non-trivial additional synthetic steps to prepare activated precursors. Among such protocols, the Newton base-catalyzed transesterification of thionoesters seems the most promising owing to the possibility of keeping on stock only corresponding methyl thionoesters as “surrogates” of all other esters.14 Category D, which is rare, is based on C–C bond creation using C-nucleophiles and electrophilic counterparts with a thionoester function. Among recent protocols, the Ni-catalyzed cross-coupling of organozinc reagents and thiocarbonyl-containing starting materials could be highlighted.15 Inspired by Newton's transesterification and the possibility of C–C bond creation, we decided to develop a convenient and scalable method for methyl thionoesters from readily available starting materials. Methyl chlorothioformate was selected as the electrophilic counterpart. Except for the “neglected” Delepine report from 1911 without experimental details,16 only Pd-catalyzed thioacylation of mono-substituted alkynes with methyl chlorothioformate was described for this type of process.17 Moreover, only phenyl chlorothioformate was used for thionoester synthesis in the reaction with PhMgBr18 and the AlCl3-catalyzed acylation of arenes.19 We report preliminary and essential results for the TM-free synthesis of thionoesters from methyl chlorothioformate and commonly used Li, Mg and Zn organometallics. Such a protocol has considerable potential to significantly increase the diversity and availability of this rare class of compounds on a 10–100 gram scale, with a possibility for further scale-up.
Results and discussion
Since methyl chlorothioformate is commercially unavailable in multi-gram quantities at a reasonable price, we decided to develop a convenient protocol for its synthesis from CSCl2 and MeOH. The optimal conditions (for the optimization protocol see ESI†) are the mixing of CSCl2 and MeOH in Et2O at 0 °C, leading to methyl chlorothioformate 2, which is isolated and purified by distillation at 0.5 bar in 62% yield on a 100 g scale from one synthetic run (Scheme 1A). It is crucial that while producing 2, we avoid expensive and uncomfortable synthetic, separation and purification techniques, which make the protocol very attractive for further optimization process.Then, we checked the reaction of compound 2 with commercially available PhMgBr 3a, PhLi 4 and PhZnCl 5 prepared by transmetallation of PhLi with ZnCl2 in THF.20 The reaction of 2 with PhMgBr was successful at −78 °C, giving the target thionoester 6a in 83% preparative yield. This procedure is easily scaled up to 25 g of the final product from one synthetic run. However, using corresponding Li or Zn derivatives does not lead to the formation of the desired product. In the case of Zn derivatives, thioacylation does not proceed. Meanwhile, in the case of Li derivatives, a complex mixture of products was formed (Scheme 1B). These data are in accordance with Nicolaou's results regarding the reaction ability of cyclic thionoester towards organometallics. Here, it was shown that the addition of organomagnesium reagents (except allyl magnesium bromide) resulted in excellent yields. In contrast, reagent organolithium reacts poorly.4 Therefore, organomagnesium C-nucleophiles appeared to be the optimal substrates for the synthesis of thionoesters from chlorothioformates. Assuming a lack of commercial availability of diverse aryl magnesium compounds, we also checked the synthesis of the desired thionoester, starting with bromides. We obtained a comparable result on the model synthesis of thionoester 6a by generating it from phenyl bromide 7a or using commercial PhMgBr (Scheme 1C).
The next step of the investigation was mini-screening aryl bromides, which can form the corresponding thionoesters in the above-mentioned protocol. The “functional group free” aryl bromide 7a–l was subjected to a developed protocol and possessed good preparative results, affording the desired thionoester 6a–l in 32–77% yields. In most cases, the procedure was easily scaled up to 20 g (Scheme 2). The moderate yields in our situation are not critical. Owing to easy separation and purification steps without the usage of expansive techniques, there is a broad potential for further scale-up of the protocol and elaboration of industrial regulations.
Unexpectedly, the fluorine-based aryl bromides 6h and i afforded extremely low yields, and in the case of 1-bromo-2-fluorobenzene 6j, the product was undetected. To solve this problem, we checked the milder bromine/magnesium exchange using turbo-Grignard reagent iPrMgCl·LiCl at lower temperatures.21 Such changes in methodology for generating organomagnesium species significantly improved the preparative yields of products 6h–j, achieving up to 62%. This procedure is also helpful for scaling up to multi-gram quantities.
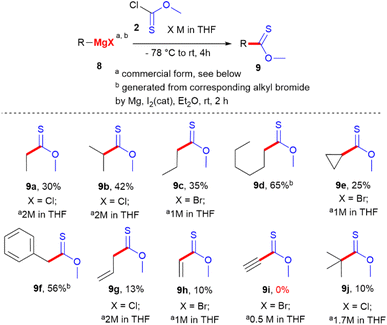
Unfortunately, our preliminary attempts to use bromides bearing masked protected functional groups as substrates still failed. We attempted to use the Grignard reagent obtained from 4-bromo-N,N-dimethylaniline 7m in the reaction, but the desired product 6m was not observed. This could probably be explained by the incompatibility of chlorothioformates with tertiary amines owing to the formal dealkylation reaction.22 To avoid such a side process, we attempted mono- and di-Boc derivatives 7n and o in the reaction to generate both variants of organomagnesium intermediates, but all attempts failed. Using nitro derivative 7p via turbo-Grignard-mediated bromine/magnesium exchange also did not lead to the target products. Unexpectedly and still unclear were the unsuccessful attempts to use t-Bu-protected benzoic acid derivative 7q in both protocols (Scheme 2), we also tested aliphatic organomagnesium derivatives in the reaction. We selected a set of commercially available alkyl-based Grignard reagent 8a–c and Grignard reagent 8e. The desired products were formed in the reaction from low to moderate preparative yields in all cases. The worst result was observed for allyl magnesium chloride 8g (commercial, 2 M in THF), which afforded the desired compound with only 13% after isolation (Scheme 3). Nevertheless, the efficient separation/purification steps and use of common, non-expensive starting materials make these results reasonable and promising.
These results also agree with Nicolaou's results regarding the reaction ability of cyclic thionoester towards allyl magnesium species.4 Despite low yield, the method remains attractive for the synthesis of alkyl thionoesters owing to the simplicity of the procedures and easy accessibility of starting materials. Besides commercially available Grignard reagents, we tested the protocol by adding the in situ generation of organomagnesium species in Et2O from alkyl bromides. In the case of n-butyl (8d) and benzyl bromide (8f), the approach gives better results (65% for 9d and 56% for 9f) than with commercial organometallics. Notably, in the reaction, our attempts to use the commercial Norman reagent 8h, Iotsich reagent 8i, or Grignard reagent 8j failed. In the case of compounds 8h and 8j, the desired products 9h and 9j were detected at only 10% of the mixture after isolation; meanwhile, in the case of compound 8i, product 9i was not detected at all.
Conclusions
In summary, we translated methyl chlorothioformate from a rare to a promising common reagent by developing a preparative, scalable procedure for its production and understanding of the stability and storage conditions. We significantly improved the transition-metal-free crosscoupling of organomagnesium reagents and methyl chlorothioformate to access an array of methyl thionoesters. The synthesis of starting methyl chlorothioformate was optimized and scaled up to 100 g from 1 synthetic run. The coupling protocols were scaled up to 25 g from 1 synthetic run. It was shown that organomagnesium reagents are the optimal C-nucleophiles for this transformation. The lithium-based substrates appeared over-reactive towards methyl chlorothioformate, leading to complex reaction mixtures. Meanwhile, zinc-based reagents appeared insufficiently reactive. The preliminary scope of the reaction was investigated. Broad examples of commercial and on-site prepared aryl and alkyl Grignard reagents were used. Among aryl reagents, the reagents bearing dialkylamino- or protected carboxyl functions are out of scope, as well as Norman and Iotsich reagents.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Mykhailo O. Pashko: methodology, validation, formal analysis, investigation Kiril V. Pashkov: validation, formal analysis, resources, investigation Dmitriy S. Granat: methodology, formal analysis, investigation, resources, data curation, supervision Yurii L. Yagupolskii: conceptualization, methodology, formal analysis, supervision Serhiy V. Ryabukhin: conceptualization, funding acquisition, methodology, supervision, writing–review & editing, visualization Dmytro M. Volochnyuk: conceptualization, methodology, writing–original draft, visualization, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was funded by internal Enamine grant and National Research Foundation of Ukraine (grant number 0124U003838). The authors thank Enamine Ltd for access to the building blocks' stock, Prof. Andrey A. Tolmachev for his encouragement and support and Dr Halyna Buvailo for her help with manuscript preparation.Notes and references
- K. B. Wiberg and Y. Wang, A Comparison of Some Properties of C=O and C=S Bonds, ARKIVOC, 2010, 2011(5), 45–56 Search PubMed.
- K. B. Wiberg, Y. Wang, S. J. Miller, A. L. A. Puchlopek, W. F. Bailey and J. D. Fair, Disparate Behavior of Carbonyl and Thiocarbonyl Compounds: Acyl Chlorides vs. Thiocarbonyl Chlorides and Isocyanates vs. Isothiocyanates, J. Org. Chem., 2009, 74(10), 3659–3664 CrossRef CAS PubMed.
- S. L. Baxter and J. S. Bradshaw, A New Conversion of Esters to Ethers and Its Application to the Preparation of Furano-18-Crown-6, J. Org. Chem., 1981, 46(4), 831–832 CrossRef CAS.
- K. C. Nicolaou, D. G. McGarry, P. K. Somers, B. H. Kim, W. W. Ogilvie, G. Yiannikouros, C. V. C. Prasad, C. A. Veale and R. R. Hark, Synthesis of Medium-Sized Ring Ethers from Thionolactones. Applications to Polyether Synthesis, J. Am. Chem. Soc., 1990, 112(17), 6263–6276 CrossRef CAS.
- W. H. Bunnelle, B. R. McKinnis and B. A. Narayanan, Difluorination of Esters. Preparation of .Alpha.,.Alpha.-Difluoro Ethers, J. Org. Chem., 1990, 55(2), 768–770 CrossRef CAS.
- J. Newton, D. Driedger, M. B. Nodwell, P. Schaffer, R. E. Martin, R. Britton and C. M. Friesen, A Convenient Synthesis of Difluoroalkyl Ethers from Thionoesters Using Silver(I) Fluoride, Chem.–Eur. J., 2019, 25(70), 15993–15997 CrossRef CAS.
- J. J. Monteith, J. W. Pearson and S. A. L. Rousseaux, Photocatalytic O- to S-Rearrangement of Tertiary Cyclopropanols, Angew. Chem., Int. Ed., 2024, 63(17), e202402912 CrossRef CAS.
- (a) G. D. Hartman and L. M. Weinstock, A Novel 1,3-Thiazole Synthesis via α-Metallated Isocyanides and Thiono Esters, Synthesis, 1986, 10, 681–682 Search PubMed; (b) T. Aoyama, Y. Iwamoto and T. Shioiri, New Methods and Reagents in Organic Synthesis. 59. Lithium Trimethylsilydiazomethane: A New Synthon for the Preparation of 5-Substituted 1,2,3-Thiadiazoles, Heterocycles, 1986, 24(3), 589–592 CrossRef CAS; (c) A. Couture and P. Grandclaudon, One-Pot Synthesis of 2-Arylthiazolo[5,4-b]Pyridines, Synthesis, 1985, 5, 533–535 CrossRef; (d) M. Yokoyama, Y. Menjo, M. Watanabe and H. Togo, Synthesis of Oxazoles and Thiazoles Using Thioimidates, Synthesis, 1994, 12, 1467–1470 CrossRef.
- (a) B. Junge, Eine Neue Synthese von 3-Amino-5-Alkyl-1,2,4-Thiadiazolen, Adv. Cycloaddit., 1975, 1975(11), 1961–1966 Search PubMed; (b) T. Miura, Y. Funakoshi, Y. Fujimoto, J. Nakahashi and M. Murakami, Facile Synthesis of 2,5-Disubstituted Thiazoles from Terminal Alkynes, Sulfonyl Azides, and Thionoesters, Org. Lett., 2015, 17(10), 2454–2457 CrossRef CAS PubMed; (c) Y. Matsumoto, D. Nakatake, R. Yazaki and T. Ohshima, An Expeditious Route to Trans-Configured Tetrahydrothiophenes Enabled by Fe(OTf)3-Catalyzed [3+2] Cycloaddition of Donor–Acceptor Cyclopropanes with Thionoesters, Chem.–Eur. J., 2018, 24(23), 6062–6066 CrossRef CAS PubMed.
- D. H. R. Barton, C. Chavis, M. K. Kaloustian, P. D. Magnus, G. A. Poulton and P. J. West, Photochemical Transformations. Part XXXI. Photolysis of Thiobenzoic Acid O-Esters. Part II. General Methods for the Preparation of Thiobenzoic Acid O-Esters, J. Chem. Soc., Perkin Trans. 1, 1973, 1, 1571–1574 RSC.
- (a) J. W. Scheeren, P. H. J. Ooms and R. J. F. Nivard, A General Procedure for the Conversion of a Carbonyl Group into a Thione Group with Tetraphosphorus Decasulfide, Synthesis, 1973, 03, 149–151 CrossRef; (b) B. S. Pedersen, S. Scheibye, K. Clausen and S.-O. Lawesson, Studies on Organophosphorus Compounds XXII The Dimer of P-Methoxyphenylthionophosphine Sulfide as Thiation Reagent. a New Route to O-Substituted Thioesters and Dithioesters, Bull. Soc. Chim. Belg., 1978, 87(4), 293–297 CrossRef CAS; (c) T. J. Curphey, Thionation with the Reagent Combination of Phosphorus Pentasulfide and Hexamethyldisiloxane, J. Org. Chem., 2002, 67(18), 6461–6473 CrossRef CAS PubMed; (d) D. Cho, J. Ahn, K. A. De Castro, H. Ahn and H. Rhee, P4S10/Dimethicone Tandem: Efficient Reagent for Thionation of Various Aromatic Amides and Esters, Tetrahedron, 2010, 66(30), 5583–5588 CrossRef CAS.
- K. A. Latif and M. Y. Ali, Reaction of Thiobenzoyldisulphides with Bases Synthesis of Thion-Esters, Tetrahedron, 1970, 26(18), 4247–4249 CrossRef.
- Y. Liu, X. Mo, I. Majeed, M. Zhang, H. Wang and Z. Zeng, An Efficient and Straightforward Approach for Accessing Thionoesters via Palladium-Catalyzed C–N Cleavage of Thioamides, Org. Biomol. Chem., 2022, 20(7), 1532–1537 RSC.
- J. J. Newton, R. Britton and C. M. Friesen, Base-Catalyzed Transesterification of Thionoesters, J. Org. Chem., 2018, 83(20), 12784–12792 CrossRef CAS PubMed.
- J. J. Monteith, K. Scotchburn, L. R. Mills and S. A. L. Rousseaux, Ni-Catalyzed Synthesis of Thiocarboxylic Acid Derivatives, Org. Lett., 2022, 24(2), 619–624 CrossRef CAS PubMed.
- M. Delépine, Sur Les Sulfo-Éthers-Sels Ou Éthers Thioniques R.CS.OR, C. R. Hebd. Seances Acad. Sci., 1911, 153, 279–282 Search PubMed.
- K. Hartke, H.-D. Gerber and U. Roesrath, α,β-Acetylenic Dithio and Thiono Esters, Tetrahedron Lett., 1989, 30(9), 1073–1076 CrossRef CAS.
- M. M. Cerda, Y. Zhao and M. D. Pluth, Thionoesters: A Native Chemical Ligation-Inspired Approach to Cysteine-Triggered H2S Donors, J. Am. Chem. Soc., 2018, 140(39), 12574–12579 CrossRef CAS PubMed.
- H. Viola, S. Scheithauer and R. Mayer, Organische Schwefelverbindungen,97. Friedel-Crafts-Reaktionen Mit Thiosäurechloriden, Chem. Ber., 1968, 101(10), 3517–3529 CrossRef CAS.
- R. M. Keenan and L. I. Kruse, A Convenient Synthesis of 5-Aryltropones, Synth. Commun., 1989, 19(5–6), 793–798 CrossRef CAS.
- A. Kremsmair, J. H. Harenberg, K. Schwärzer, A. Hess and P. Knochel, Preparation and Reactions of Polyfunctional Magnesium and Zinc Organometallics in Organic Synthesis, Chem. Sci., 2021, 12(17), 6011–6019 RSC.
- (a) D. S. Millan, D. S. Millan, R. H. Prager and R. H. Prager, Phenyl Chloro(Thionoformate): A New Dealkylating Agent of Tertiary Amines, Aust. J. Chem., 1999, 52(9), 841–850 CrossRef CAS; (b) M. A. Shalaby and H. A. Rapoport, General and Efficient Route to Thionoesters via Thionoacyl Nitrobenzotriazoles, J. Org. Chem., 1999, 64(3), 1065–1070 CrossRef CAS PubMed; (c) C. Yao, J. Yang, X. Lu, S. Zhang and J. Zhao, Ynamide-Mediated Thionoester and Dithioester Syntheses, Org. Lett., 2020, 22(16), 6628–6631 CrossRef CAS PubMed; (d) X. Zhu, K. Wan, J. Zhang, H. Zhao, Y. He, Y. Ma, X. Yang and M. Szostak, Esterification of Thioamides via Selective N–C(S) Cleavage under Mild Conditions, Org. Lett., 2023, 25(33), 6149–6154 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experiments and synthesis; spectral and analytical data for the synthetized compounds; copies of NMR spectra. See DOI: https://doi.org/10.1039/d5ra01538c |
| This journal is © The Royal Society of Chemistry 2025 |