 Open Access Article
Open Access ArticleRecent exploration of inorganic sonosensitizers for sonodynamic therapy of tumors
Conghao Liu†
ab,
Xueyu Liu†ab,
Ling Zha†c,
Yulong Zhang†ab,
Ruizhuo Ouyang *ab,
Dong Sun*d and
Yuqing Miao
*ab,
Dong Sun*d and
Yuqing Miao *ab
*ab
aInstitute of Bismuth and Rhenium Science, University of Shanghai for Science and Technology, Shanghai 200093, China. E-mail: ouyangrz@usst.edu.cn; yqmiao@usst.edu.cn
bUSST-UH International Joint Laboratory for Tumor Diagnosis and Energy Treatment, University of Shanghai for Science and Technology, Shanghai 200093, China
cDepartment of Laboratory Diagnosis, Changhai Hospital, Naval Medical University, Shanghai 20043, P. R. China
dHenan Normal University, Xinxiang 453007, China. E-mail: sundong2004@126.com
First published on 11th June 2025
Abstract
With the rapid development of nanomedicine and nanobiotechnology, various therapeutic methods have been applied with good efficacy and biological safety. As a non-invasive treatment method, sonodynamic therapy (SDT) can effectively treat deep tumors with less damage to the surrounding tissue and high adaptability. The ultrasound sensitizer is an indispensable and important part of the SDT process, and its structure and properties directly determine the therapeutic effect of SDT. Compared with conventional organic sonosensitizers, inorganic sonosensitizers including noble metal-based, transition metal-based, silicon-based and carbon-based sonosensitizers have high stability, controllable morphology and long circulation time in the human body, which has greatly expanded their research applications in SDT. In this review, the possible mechanisms of SDT, i.e. the cavitation effect and the generation of reactive oxygen species, are briefly discussed. Subsequently, recent research progress of inorganic sonosensitizers is systematically summarized in terms of their formulations and antitumor effects with a focus on strategies to optimize therapeutic efficacy. The current challenges and future are presented to provide insights into strengthening the interdisciplinary collaborations so as to promote the innovation and development of SDT technology in clinical application.
1 Introduction
Currently, cancer remains one of the most important diseases threatening human life and health.1 It is known that cancer develops through a multi-step pathological process involving changes in many cellular physiological systems, such as cell differentiation and apoptosis.2 Cancers initially start as localized diseases, but their tendency to spread to distant parts of the body makes them difficult to cure.3 Conventional cancer treatments include surgical removal, chemotherapy (CT) and radiotherapy (RT).4 While all of these treatments have achieved good clinical results, they all cause varying side effects after treatment. For example, surgical resection is the most direct and effective treatment for most solid tumors.5 However, resection can cause acute pain at the incision site, and the tumor tissue or cells remaining after surgery usually re-develop into solid tumors locally or distantly, leading to tumor recurrence or metastasis;6 chemotherapy is a form of adjuvant tumor treatment that mainly inhibits further growth and division of cancer cells.7 Due to the poor solubility and specificity of chemotherapeutic agents, they cannot be targeted directly against cancer cells, which leads to severe toxic effects.With the development and application of novel nanotechnology platforms, researchers have developed a variety of cancer treatments based on the unique benefits of nanomaterials.8 For example, researchers have developed various types of nano-photothermal sensitizers for photothermal therapy (PTT) of tumors that can be heated to kill tumor cells under specific light irradiation.9 Similarly, small molecule photosensitizers for photodynamic therapy (PDT) can induce apoptosis and necrosis of tumor cells when the photosensitizer is activated to generate reactive oxygen species (ROS) under irradiation with light of specific wavelengths.10 Although PDT reduces the side effects of conventional cancer therapies on the human body to a certain extent, its effectiveness in treating deep-seated tumors is limited due to the low penetration depth of PDT, and its therapeutic spectrum may not include deep-seated tumors.11
Sonodynamic therapy (SDT) is a non-invasive treatment method that triggers cell death through the generation of ROS by sonosensitizers under the influence of ultrasound (US). In 1989, Yumita and Umemura12 discovered that hematoporphyrin (HP) can be activated by US to kill tumor cells. Since then, SDT has been considered the most promising treatment method for tumors.13 US is often used as a mechanical wave in biomedical fields.14 With SDT, the ultrasound can penetrate deep into the tissue and focus precisely on the tumor cells, which in turn stimulates the acoustic sensitizer to generate ROS that kill the tumor cells. SDT offers two key advantages: (1) deeper tissue penetration with spatial precision, and (2) minimized damage to surrounding healthy tissues.15
In the course of the development of nanotechnology, sonosensitizers are frequently used to control SDT15 due to their strong imaging capability. Sonosensitizers play a crucial role in the process of SDT. sonosensitizers can be enriched and generate cytotoxic ROS in the tumor region under the influence of US, which in turn triggers apoptosis and necrosis of tumor cells and achieves the killing effect on tumor cells.16 The currently known sonosensitizers are mainly organic sonosensitizers and inorganic sonosensitizers.17 Initially, photosensitizers such as Hp, hematoporphyrin monomethyl ether,18 Ga-porphyrin,19 photoporphyrin IX,20 and other porphyrin derivatives used in PDT were used directly as sonosensitizers for SDT.21 Subsequently, xanthones,22 organic compounds with specific chemical structures, have also shown some acoustic sensitization. In addition, several antitumor drugs, including doxorubicin (DOX),23 curcumin,24 and artemisinin,25 have been investigated as sonosensitizers for SDT to enhance the killing effect on tumor cells after US activation.26
Organic sonosensitizers have only a limited therapeutic effect of SDT due to their hydrophobicity, non-specificity, and short residence time in the body, which leads to insufficient accumulation at the tumor site. In contrast to organic sonosensitizers, recently developed inorganic sonosensitizers, such as titanium dioxide (TiO2) nanoparticles,27 manganese dioxide (MnO2) nanoparticles,28 and ultrasmall quantum dots (QDs), have been well developed in terms of improved chemical stability, and phototoxicity, and offer a broad perspective for the application of SDT. as shown below. (1) Due to their unique semiconducting properties, inorganic sonosensitizers are able to trigger the production of electrons (e−), and holes (h+) under certain conditions, leading to the generation of ROS.29 (2) Inorganic sonosensitizers exhibit excellent stability in physiological environments, and can circulate in the body for long periods without being rapidly degraded.30 (3) They are relatively easy to control in terms of size, and morphology, which facilitates further biomedical applications such as tumor targeting or drug delivery.31 Despite the above advantages of inorganic sonosensitizers, the SDT efficiency of most modern inorganic sonosensitizers is still unsatisfactory. To improve the SDT efficiency of inorganic sonosensitizers, the development of suitable sonosensitizers has become an important topic of current research.
Here we provide an overview of the latest developments in inorganic sonosensitizers. First, the potential therapeutic mechanisms of sonodynamic therapy are discussed in detail. Then, the current design, synthesis, and biological effects of inorganic sonosensitizers (based on noble metals, transition metals, carbon (C), and silicon (Si) (Fig. 1)) are discussed and some constructive ideas to improve their therapeutic efficiency for SDT are presented. Finally, challenges and key issues in this field for the future development of SDT are discussed.
2 Mechanisms of acoustic energy therapy
The mechanism of SDT has been much discussed in recent years, but due to the complexity of the process, its exact mode of operation remains unclear. (1) US-induced cavitation effect, and (2) ROS generation is the possible mechanisms of SDT. The most widely accepted mechanism is the generation of ROS induced by pyrolysis or acoustic luminescence (Fig. 2).2.1 Cavitation effects
The cavitation effect, also known as ultrasonic cavitation, refers to the vibration of microscopic gaseous cavitation bubbles (also known as cavitation nuclei) in a liquid in the presence of sound waves. When the sound pressure reaches a certain level, these cavitation bubbles undergo a kinetic process of growth and decay. During this process, the cavitation bubbles undergo a series of changes such as oscillation, expansion, contraction, and bursting32 releasing enormous energy. There are two types of cavitation effects: inertial cavitation and stable cavitation.33 In inertial cavitation, the cavitation bubbles expand and contract rapidly under the action of high-intensity ultrasonic waves, which eventually leads to the bursting of the bubbles. During this process, enormous amounts of energy are released, generating shock waves, acoustic luminescence, and localized high-temperature and high-pressure environments. These extreme conditions can activate sonosensitizers and promote the production of ROS,34 ultimately leading to the lysis of cancer cells. In contrast, stable cavitation is a process in which the cavitation bubbles are continuously vibrated in a small area by low-intensity ultrasound, and this vibration increases the rate of diffusion of the nuclear gases, facilitating the translocation of charge carriers into the cell. Stabilized cavitation is primarily used in SDT in combination with sonosensitizers to achieve therapeutic effects targeting the focal point, including targeted release of carrier drugs and enhancement of drug uptake, penetration, and alteration of the immune microenvironment.35 The cavitation effect has a broad application perspective in sound power therapy. On the one hand, tumor cells can be killed directly and indirectly to improve the therapeutic effect; on the other hand, the permeability of cell membranes can be altered and the targeting accuracy and permeability of sonosensitizers15 can be improved. With the continuous development of molecular imaging and molecular biology, as well as the constant advances in ultrasound technology and nanotechnology, the application of the cavitation effect in sound therapy will become more extensive and profound. Ultrasonic microbubbles as optimal cavitation nuclei can significantly enhance and precisely regulate the cavitation effect. In the future, the application of the cavitation effect in sound power therapy will become even more promising with the continuous progress of the corresponding technologies.2.2 ROS generation
Sonosensitizers are stimulated by the US to transfer energy to molecular oxygen (O2), which in turn generates different types of ROS, e.g. singlet oxygen (1O2), superoxide anions (O2−), and hydroxyl radicals (·OH).36 These reactive oxygen species can cause cell damage and trigger apoptosis in cancer cells. ROS production37 is one of the most important steps in SDT. Currently, cavitation is considered the main process of reactive oxygen species production by sonosensitizers under US exposure. The most widely accepted mechanism is pyrolysis or acoustic luminescence-induced ROS production.38 Specifically, pyrolysis occurs when microbubbles burst during the cavitation effect, releasing large amounts of energy and interacting with the surrounding liquid; this interaction leads to pyrolysis of the liquid, resulting in the formation of ROS.35 Acoustic luminescence occurs in the inertial cavitation process, the sharp collapse of the bubble will produce acoustic luminescence phenomenon. Acoustic luminescence is a phenomenon in which light radiation is excited by ultrasound, causing the acoustic sensitizer to transition from the ground state to an excited state. When the activated acoustic sensitizer returns to the ground state, it releases energy that is transferred to the surrounding oxygen, resulting in the formation of large amounts of reactive oxygen species.39 In both mechanisms, the high temperature and pressure generated when the bubbles burst lead to sonochemical effects that generate ROS and damage mitochondrial membranes by promoting lipid peroxidation, resulting in a decrease in mitochondrial membrane potential and an increase in membrane permeability.37 In addition, cytochrome c released from the damaged mitochondrial membrane activates the apoptotic signaling pathway, which ultimately leads to the apoptosis of cancer cells. Although experimental evidence supports both pathways of ROS generation, it is not clear which pathway is the main mechanism of ROS generation in SDT. This remains an important area of research, and further experimental and theoretical studies are needed to uncover the exact mechanism of ROS generation in SDT. A more comprehensive understanding of ROS generation pathways will help to develop and apply sonosensitizers more effectively, thereby improving the therapeutic efficacy and safety of SDT. In summary, ROS generation in SDT is a complex and interesting process involving multiple physical and chemical mechanisms. Future studies will focus on uncovering the intrinsic relationships and interactions between these mechanisms, providing a more solid theoretical basis for the clinical application of SDT.Notably, the tumor microenvironment (TME) critically modulates SDT efficacy. Hypoxia, a hallmark of solid tumors, may limit O2 availability for ROS generation, particularly for sonosensitizers relying on type II photodynamic pathways. Recent studies suggest that MnO2-based sonosensitizers can alleviate hypoxia by catalyzing H2O2 decomposition to O2.40 Additionally, the acidic TME (pH 6.5–7.0) could influence charge states of transition metal-based sonosensitizers, altering their catalytic activity. Redox homeostasis, maintained by elevated glutathione (GSH) levels in tumors, also necessitates sonosensitizers with GSH-depleting capabilities.
3 Inorganic nanomaterials in sound power therapy
SDT is a new non-invasive tumor treatment in which US is used to stimulate the acoustic sensitizer to generate ROS that induce tumor cell death to achieve the therapeutic goal. Sonosensitizers play a crucial role in SDT. In recent years, inorganic nanostructures such as noble metals, transition metal oxides, carbon-based and silicon-based materials have been used as sonosensitizers with remarkable results due to their high stability and special physicochemical properties. The recent advances in these inorganic sonosensitizers are reviewed below in terms of design principles to improve their therapeutic efficacy:3.1 Noble metal-based sonosensitizers
The unique physicochemical properties of metal-based sonosensitizers as a key component in SDT, in particular their high stability, good electron transfer capability and catalytic activity, enable these materials to efficiently generate ROS under ultrasound irradiation, which in turn induce apoptosis or necrosis of tumor cells. Metal-based sonosensitizers usually have high stability and special physicochemical properties, such as good electron transfer capability and catalytic activity, which make them potentially useful for SDT. Various noble metal nanoparticles such as gold, silver, and platinum nanoparticles and their modified forms are considered excellent sonosensitizers due to their high stability, good water solubility, non-dermal photosensitization and inherent acoustic cavitation properties. | ||
| Fig. 3 (a) Schematic representation of the acoustic catalytic effect of Au NRsALG on cancer cells. Ultrasound-activatable Au NRsALG catalyzes the production of ROS through the mitochondrial cell death pathway, leading to severe DNA damage and triggering apoptosis in cancer cells; Copyright 2023, Elsevier.43 (b) Schematic illustration of the synthesis process of self-assembled SM nanoparticles and SMAH heterostructures; shows the mechanism of photocatalytic, acoustic catalytic and anti-tumor effects of SMAH heterostructures; (c) infrared thermal images of SMAH (80 μM) aqueous solution and water using 1064 nm light irradiation; (d) consumption of GSH by SMAH with DTNB as scavenger at different reaction times; (e) XPS spectra of Mn 2p peaks in SMAH before and after incubation with GSH; (f) cell viability of 4T1 cells after different treatments. Copyright 2024, American Chemical Society.40 | ||
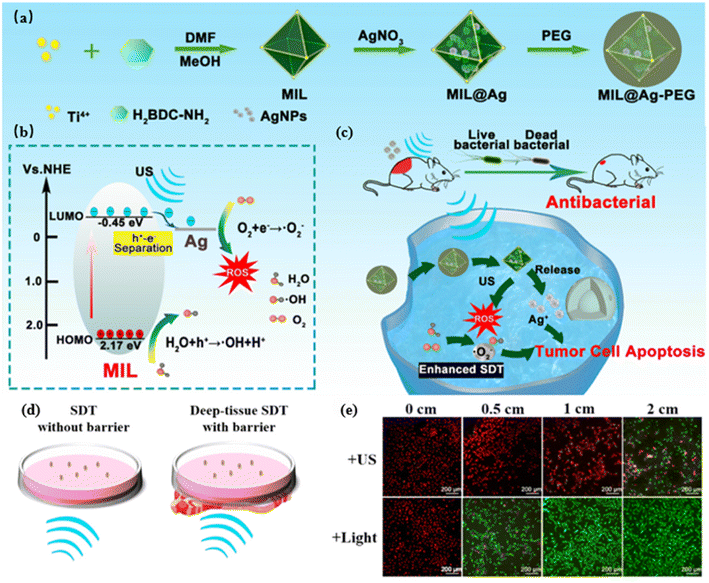 | ||
| Fig. 4 (a) Schematic preparation process of MIL@Ag-PEG; (b) schematic mechanism of MIL@Ag-PEG for enhancing the mechanism of action of SDT schematic of the mechanism of action of SDT; (c) schematic of the mechanism of MIL@Ag-PEG enhancing the mechanism of SDT for cancer treatment and rapid wound healing; (d) schematic of the MIL@Ag-PEG used to kill the deep cancer cells. Lean meat was used to simulate the barrier to show the SDT and deep tissue SDT settings in vitro; (e) CLSM images of A549 cells irradiated with US (2.0 W cm−2) or 635 nm laser light exposed to MIL@Ag-PEG at different thicknesses of the tissue barrier. Copyright 2023, American Chemical Society.55 | ||
 | ||
| Fig. 5 (a) Schematic representation of Pt-MOCs prepared by coordination crystallization; (b) schematic representation of Pt-MOCs with acoustic sensitization by SDT; (c) cell viability of 4T1 cells incubated with Pt-MOCs under different treatments; Copyright 2023, The Royal Society of Chemistry.59 (d) Schematic synthesis pathways of CDP@HP-T; and (e) combination of chemotherapy and SDT for CDP@HP-T. Copyright 2020, Elsevier.62 | ||
The use of precious metal nanoparticles in SDT has seen advancements in research, but their high cost and limited effectiveness in eliminating tumors have hindered their wide use. However, combining precious metal nanoparticles with other materials, such as PTT or CDT, has proven to be an effective strategy for enhancing the therapeutic efficiency of SDT under ultrasound irradiation. This combination leads to a synergistic effect that significantly improves the anti-tumor efficacy of SDT. The text discusses the impact of combining noble metal nanoparticles with other materials in influencing the properties of the tumor microenvironment, such as pH and redox state. This combination can facilitate the formation and accumulation of ROS, which can be beneficial in cancer treatment. Additionally, when combined with tumor microenvironment-responsive materials, noble metal nanoparticles can more easily penetrate tumor tissue, enhancing the therapeutic effect. However, the high cost and limited availability of noble metal nanoparticles limit their widespread use. As a result, researchers are now focusing on developing high-performance sonosensitizers using non-precious metals as a solution to this challenge.
3.2 Transition metal-based sonosensitizers
Sonosensitizers based on transition metals have great potential for SDT due to their unique electronic structure and chemical properties. They usually have good biocompatibility and stability can be stabilized in living organisms and have therapeutic effects. In addition, due to their energy band structure, transition metal-based sonosensitizers can be easily activated by ultrasound to generate large amounts of ROS, which can have a killing effect on tumor cells. Sonosensitizers based on transition metals have a wide range of applications in the medical field. They can be used not only for the treatment of bacterial infections and viral diseases, but also for the treatment and diagnosis of tumors.67 By adapting the structure and properties of the sonosensitizers, the generation and release of ROS can be precisely controlled, thereby improving the therapeutic effect and reducing side effects. | ||
| Fig. 6 (a) The synthetic process of CaCO3@Pt–TiO2 NP; (b) schematic illustration of CaCO3@Pt–TiO2 NPs for sonodynamic-cooperated immunotherapy; (c) band structures of TiO2 NPs and Pt–TiO2 NPs; (d) cell viability of 4T1 cells incubation for 24 h after different treatments; (e) mean tumor volume ((1) control, (2) US, (3) Pt–TiO2 (PT), (4) PT + US, (5) CaPT, (6) CaPT + US) in each group of loaded nude mice after 14 days of treatment (n = 5). Copyright 2023, Elsevier.73 | ||
The combination of SDT and PTT is commonly used for tumor treatment. PTT can generate ROS, while SDT can overcome the limited penetration depth of PTT and target deeper tissue. SDT–PTT combination therapy has shown promise in improving tumor oxygenation, increasing ROS production, and enhancing the thermal sensitivity of cancer cells, resulting in effective tumor treatment. Titanium-based nanomaterials, specifically titanium nitride (TiN) nanodots,80 have been widely used as sonosensitizers for SDT due to their unique nanostructures and multiple valence states. TiN nanoparticles have demonstrated excellent physicochemical properties, such as good biocompatibility and photothermal characteristics. It is worth noting that TiN nanoparticles have vacancies in their crystal lattice structure. In conclusion, the combination of SDT and PTT, using TiN nanodots as sonosensitizers, holds promise for effective tumor treatment due to its ability to enhance ROS production and penetrate deeper tissues. The electron cloud is unevenly distributed around the vacancies, resulting in a lack of electric dipoles, which enhances microwave-excited thermoacoustic therapy. Wang et al.81 successfully synthesized ultrasmall TiN nanodots82,83 for PTT-enhanced synergistic SDT against cancer.84 First, they synthesized ultra-small TiN nanodots with an average size of 1.5 nm using the liquid stripping method85 (Fig. 7a). The fabricated TiN nanodots have secondary heat-absorbing properties in the near-infrared region and are oxidized to TiO2 (ref. 86) due to their special low-oxygen structure and surface fraction. Therefore, the TiN nanodots can be used not only for photoacoustic imaging and photothermal therapy of tumors, but also as efficient sonosensitizers to enhance the separation of e− and h+ from the energy band structure under US irradiation, leading to the good SDT performance of TiN nanodots (Fig. 7b). TiN nanorods induce mild photothermal heating of the tumor under near-infrared laser irradiation, which in turn promotes tumor blood flow and improves tumor oxygenation, leading to a stronger effect of the combined PTT and SDT treatment. The ultra-small size of TiN nanodots, most of which can be rapidly degraded in mice, also reduces concerns about the long-term toxicity of nanomaterials (Fig. 7d). They first designed a treatment protocol for PTT–SDT with TiN nanodots in a 4T1 tumor model (Fig. 7c). They then evaluated the killing performance of PVP-TiN nanodots on 4T1 cells under NIR or US irradiation and combined NIR and US irradiation. For the in vitro cell therapy experiments, the following eight groups were formed: (1) control; (2) US; (3) NIR; (4) US + NIR; (5) PVP-TiN; (6) PVP-TiN + NIR (PTT group); (7) PVP-TiN + US (SDT group); (8) PVP-TiN + NIR + US (PTT–SDT group). It can be seen that the relative cell survival rate in groups (1)–(5) is more than 90%, indicating that US and/or NIR laser irradiation alone does not cause damage to 4T1 cells. However, in the presence of PVP-TiN nanodots in combination with NIR laser irradiation, US irradiation and NIR laser irradiation + US irradiation, the relative cell survival rates in the corresponding groups (6)–(8) were 68.46%, 41.03% and 7.78%, respectively (Fig. 7e). These results show that the therapeutic effect of PVP-TiN is significantly enhanced in the PTT–SDT combination group. This indicates that the therapeutic effect of PVP-TiN nanodots is mainly due to the SDT properties and the combined PTT–SDT treatment can produce a significant synergistic effect. In the in vivo experiments, all mice were randomly divided into the following six groups: (1) control; (2) NIR + US; (3) PVP-TiN nanorods; (4) PVP-TiN + NIR (PTT group); (5) PVP-TiN nanorods + US (SDT group); (6) PVP-TiN + NIR + US (PTT + SDT group). Tumors were treated with 1064 nm laser irradiation followed by 2 hours of US irradiation 8 hours after intravenous injection of PVP-TiN nanodots. Tumor growth in mice was monitored after treatment. In the control group, tumors grew rapidly, and neither injection of PVP-TiN nanodots alone nor NIR/US treatment in mice injected with saline showed any inhibitory effect on tumors compared to the control group. Both the PTT group (PVP-TiN + NIR) and the SDT group (TiN + US) showed some inhibition of tumors in the mice, suggesting that the PVP-TiN nanodots have excellent photothermal and acoustic properties. In the PTT + SDT group (PVP-TiN + NIR + US), the tumor growth of the mice was significantly inhibited and the therapeutic performance was much better than that of the SDT group (TiN + US) and the PTT group (PVP-TiN + NIR) (Fig. 7f). Both in vitro and in vivo evaluations showed high synergistic performance of the PTT–SDT combination treatment, which significantly inhibited tumor growth. This study highlights that TiN nanodots can be used as a novel acoustic sensitizer for improved acoustic dynamics in cancer treatment and expand the application of metal nitrides in cancer imaging and therapy.
 | ||
| Fig. 7 (a) Schematic preparation and modification of TiN nanodots; (b) TiN nanodots prepared by liquid stripping method for photothermal enhancement of SDT in cancer; (c) TiN nanodot-mediated PTT–SDT in 4T1 tumor model; (d) schematic representation of PVP-TiN nanodots used for mild PTT-enhancement of PTT in cancer cells; (e) 4T1 cells treated with different treatments (control, NIR, US, US + NIR, PVP-TiN, PVP-TiN + NIR, PVP-TiN + US, and PVP-TiN + NIR + US); (f) relative cell survival after various treatments (control, NIR + US, PVP-TiN, PVP-TiN + NIR, PVP-TiN + US, and PVP TiN + NIR + US) after the average tumor growth curves of mice. Copyright 2021, Elsevier.81 | ||
 | ||
| Fig. 8 (a) Schematic preparation of cMn-MOF@CM; (b) schematic representation of cMn-MOF@CM-triggered SDT and nanovaccines for improving anti-PD-1 potency; (c) PBS, Mn-MOF, cMn-MOF, Mn-MOF@CM, and cMn-MOF@CM at Zr concentrations of 10 and 20 μg mL−1 together with 5 μM GSH GSH content after 40 min of incubation; (d) ROS generation by PBS, Mn-MOF, cMn-MOF, Mn-MOF@CM and cMn-MOF@CM at a Zr concentration of 10 μg mL−1 after US irradiation under low oxygen conditions with or without treatment for different time intervals (1 MHz, 0.9 W cm−2, 30% duty cycle). Copyright 2021, Elsevier.92 | ||
Zn-based nanomaterials, such as ZnO, have unique physicochemical properties and good biocompatibility. In SDT, Zn-based nanomaterials can generate ROS through surface effects, quantum size effects, etc., and produce killing effects on tumor cells. In addition, Zn-based nanomaterials can synergistically enhance the therapeutic effect of SDT by modulating the tumor microenvironment and enhancing the immune response. For example, Hu et al.96 designed a zinc oxide nano-acoustic sensitizer co-doped with Fe and Mn to improve the anti-tumor efficiency of SDT by inducing multiple iron apoptosis in tumor cells. Doping the Fe/Mn component into the nanostructure of the ZnO nanosensitizer97 (Fig. 9a) not only catalyzed the Fenton reaction to generate ROS in the tumor microenvironment with hydrogen peroxide overexpression, but also decreased intracellular glutathione to inhibit ROS degradation.88 To verify whether D-ZnO-PEG was able to undergo the Fenton reaction, 3,3,5,5-tetramethylbenzidine (TMB) was used as a probe for ·OH. In brief, TMB and 0.1 mM H2O2 were mixed with different solution groups (control, US, D-ZnO-PEG, D-ZnO-PEG + US) and then the characteristic peaks were recorded at 650 nm. The results showed that the D-ZnO-PEG groups exhibited a significant increase in intensity, indicating that the doped Fe/Mn underwent a Fenton reaction in the ZnO NPs (Fig. 9b). To investigate the potential mechanism by which iron oxidation enhances the SDT effect, GSH loss was detected using UV spectroscopy. It is shown that the D-ZnO-PEG + US group has a higher GSH consumption than the ZnO-PEG + US group due to the Fe/Mn reference. When irradiated with US, GSH was further oxidized by US-triggered ROS during SDT. It was clearly observed that the D-ZnO-PEG + US group caused a higher degree of GSH degradation98 than the D-ZnO-PEG group (Fig. 9c). The therapeutic effect of SDT on tumors is enhanced by the development of a unique Fe/Mn-doped ZnO nanosensitizer that simultaneously induces multiple apoptotic effects of iron in a specific tumor microenvironment. This study provides a unique paradigm for improving the SDT performance of nanoscale sensitizers by doping with transition metals and provides important information for further exploration of other efficient nanotherapeutic tumor modalities based on iron death99 and SDT to achieve highly potent therapeutic effects on tumors.
 | ||
| Fig. 9 (a) Schematic preparation of D-ZnO-PEG NPs; (b) characteristic peaks of TMB oxidation of control, US, D-ZnO-PEG and D-ZnO-PEG + US groups over time; (c) comparison of GSH depletion in control, US, D-ZnO-PEG and D-ZnO-PEG + US groups; Copyright 2022, Elsevier.96 (d) Mechanism of ROS generation by ultrasound-catalyzed ABO nanocatalysts; (e) schematic diagram process of ICD induction in tumor cells by ultrasound-catalyzed ABO nanocatalysts; (f) relative viability of 4T1 cells after different treatments; (g) weights of primary and (h) distal tumors and photos of the corresponding tumors after 14 days of treatment (scale bar = 1 cm). Copyright 2024, Elsevier.100 | ||
Bismuth oxide is a non-toxic and chemically stable material that forms the basis for its use in biomedical applications. Although there are relatively few direct studies on the use of bismuth oxide as an acoustic sensitizer for SDT, bismuth oxide has certain similarities with TiO2 in terms of chemical structure, and therefore it is speculated that bismuth oxide can also be used as an acoustic sensitizer for SDT to treat tumors. For example, the integration of noble metal nanoparticles or the introduction of oxygen defects could further improve the efficacy of bismuth oxide in SDT. Chen et al.100 designed a polymer-modified metal–semiconductor Schottky heterostructure nanocomposite (ABO) as a therapeutic nano platform for inducing ICD (Fig. 9d). In this catalytic system, electrons generated by ultrasonic excitation could be transported from the surface of Bi2O3 (BO) semiconductors to Au nanorods (Au NRs) via Schottky junctions, and the energy bands effectively prevented the electron backflow.101 Meanwhile, the synergistic effect of Bi2O3 semiconductor and GSH exacerbated the oxidative stress of TME. In addition, the Au NRs exhibited significant photothermal conversion efficiency under 808 nm laser irradiation. Thus, under ultrasound and near-infrared (NIR) light irradiation, ABO nanocomposites activated the ICD in tumor cells, which then synergistically disrupted the tumor's oxidative stress defense system by GSH depletion, reversed the immunosuppressed TME, and inhibited tumor progression (Fig. 9e). In the in vitro study, the therapeutic effect of the synergistic group was approximately 7 times that of the additive group at an ABO concentration of 100 μg mL−1. This result indicated that ultrasound and laser irradiation greatly enhanced the therapeutic effect of ABO, and its synergistic effect was far superior to the purely additive effect, suggesting that the tumor therapeutic effect could be greatly enhanced under US and NIR irradiation (Fig. 9f). In the in vivo treatment, the tumor-bearing mice were randomly divided into seven groups: control, ABO, NIR, US, ABO + NIR, ABO + US, and Synergy. Subsequently, the mice in the seven groups received the corresponding treatments. Compared with the control group, the ABO, NIR, and US groups had little effect on tumor growth. In contrast, primary and distal tumors were significantly inhibited in the ABO + NIR, ABO + US, and synergy groups (Fig. 9g and f). In in vitro and in vivo studies, the nano-acoustic catalytic system exhibited strong reactive oxygen species generation and photothermal properties and synergistically triggered the ICD of tumor cells to inhibit tumor progression, which effectively improved the effectiveness of tumor therapy.
Fe3O4 has received increasing attention in the field of SDT due to its superparamagnetism, Fenton-like reactivity and peroxidase-like activity.102 In the biomedical field, Fe3O4 can be used as a magnetic carrier for drug delivery, cell imaging and biosensing, etc. Fe3O4 has Fenton-like reactivity. It can catalyze the decomposition of H2O2 to generate ROS with strong oxidative properties, such as ·OH, under certain conditions. In tumor immunotherapy, Fe3O4 nanoparticles are often designed to generate ROS via the Fenton reaction in response to the specific characteristics of the tumor microenvironment (e.g. low pH, high H2O2, etc.), thereby eliminating cancer cells. This Fenton-like reactivity provides a broad prospect for the application of Fe3O4 in fields such as cancer therapy and environmental protection. In addition to the Fenton-like reactivity, Fe3O4 also has the activity of mimicking catalase. Hydrogen peroxidase is an enzyme that catalyzes the decomposition of H2O2 to produce O2 and H2O, and Fe3O4 nano-enzymes can mimic the activity of this enzyme to catalyze the decomposition of H2O2 to produce O2. This mimicry of peroxidase activity makes Fe3O4 potentially valuable for biomedical applications. For example, Chen et al.103 reported a tumor-targeted biomimetic acoustic sensitizer-conjugated Fe3O4 nanocatalyst combined with CDT and SDT for the treatment of colorectal cancer. They synthesized bovine serum albumin (BSA)-modified Fe3O4 nanoparticles by alkaline co-precipitation and coupled them with chlorine e6 (Ce6) as an acoustic sensitizer, and then surface camouflaged CT26 cancer cell membranes to construct tumor-targeting mimetic biomimetic nanocatalysts (MBFCs) for homologous targeting of cancer cells (Fig. 10a). The obtained MBFC nanocatalysts possessed strong catalytic ability and efficient acoustic kinetic properties.104 Under US irradiation, MBFC could generate a large amount of ROS in the tumor microenvironment (Fig. 10b). The high cellular uptake efficiency of MBFC was confirmed by cell internalization experiments due to the cell membrane-mediated homologous targeting mechanism. The iron staining in MBFC-treated cells was much more pronounced than that in BFC-treated cells, which was mainly due to the fact that iron staining in MBFC-treated cells was much more pronounced than that in BFC-treated cells, and the intracellular uptake ability of the nanoparticles was quantitatively investigated through the measurement of iron content. The results showed that the total iron uptake of MBFC-treated cells was 1.03 μg, while the total iron uptake of BFC-treated cells was 0.25 μg, which was 4.1-fold lower than that of MBFC (Fig. 10c). MBFC nanocatalysts were able to achieve the combined effect of CDT and SDT and significantly induced apoptosis in CT26 cells in vitro, and the cell viability in the presence of H2O2 was lower than that of the group without added H2O2 for both the BFC and MBFC treated cells, the cell viability in the presence of H2O2 was lower than that of the group without added H2O2 (Fig. 10d), demonstrating that CDT has the effect of killing cancer cells. Under US treatment, the cell viability of BFC and MBFC treated cells was significantly reduced compared with that of control cells (Fig. 10e), which was due to the SDT effect. In US treatment, both BFC and MBFC treatment significantly inhibited tumor growth, suggesting that the combined effect of CDT and SDT could improve the anti-tumor effect. According to the experimental results, it is obvious that the relative tumor volume in the MBFC + US group is much smaller than that in the BFC + US group, further confirming that MBFC is superior to BFC in terms of therapeutic effect due to its targeting ability. The relative weight of the tumor gradually decreased (Fig. 10f), which strongly inhibited the growth of CT26 tumors in living mice. The synergistic effect of CDT and SDT achieved by MBFC can kill cancer cells in vitro and inhibit the growth of intestinal tumors in vivo, providing a tumor-targeted bionic platform for effective tumor therapy.
 | ||
| Fig. 10 (a) Schematic illustration of the synthesis of MBFC nanoparticles; (b) schematic illustration of MBFC-mediated targeted combinational CDT/SDT of colon tumors in living mice; (c) quantitative analysis of the cellular uptake of BFC or MBFC nanoparticles by CT26 cells; (d) cell viability of CT26 cells after incubation with BFC or MBFC nanoparticles at the Fe concentration of 50 mg mL−1 without or with the addition of H2O2 (100 μM); (e) cell viability of CT26 cells after incubation with BFC or MBFC nanoparticles at the Fe concentration of 50 mg mL−1 with or without the addition of H2O2 (100 μM) and US treatment (1.0 MHz, 1.0 W cm−2). (f) Relative tumor weight in different treatment groups. Copyright 2022, The Royal Society of Chemistry.103 | ||
Cerium oxide (CeO2) NPs have nano-enzymatic activity, and their excellent photocatalytic properties, antioxidant properties, high stability, and good biocompatibility have led to their widespread use in tumor therapy. CeO2 NPs can be used as sonosensitizers or in combination with other sonosensitizers to generate free radicals under the action of ultrasound, which in turn kills tumor cells.105 The presence of hypoxia in tumor microenvironments (TEMs) results in limited therapeutic efficacy of SDT and PDT. Studies have shown that nanomaterials with catalase-like activity can convert H2O2 to O2, which can alleviate hypoxia in TEMs.106 The enzyme-like activities of CeO2 NPs mainly include catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD). CeO2 can improve the tumor microenvironment and enhance the yield and killing effect of ROS through its catalytic properties. Cao et al.107 designed a direct catalytic synthesis of ultrasmall Cu2O-liganded carbon nitride for multimodal antitumor therapy. They synthesized Cu2O@CeO2 nanocomposites108 on a biocompatible CeO2 substrate by a self-assisted catalytic growth strategy. Cu2O can catalyze the pyrolysis of dicyandiamide (DCD) to generate carbon nitride, and ultimately ultra-small Cu2O-liganded carbon nitride (CuO2-CNx@CeO2) was created on a CeO2 substrate (Fig. 11a). The peroxidase (POD)-like biocatalytic activity of ROS generation was investigated according to the colorimetric method of 3,3,5,5,-TMB. In the presence of H2O2, Cu2O-CNx@CeO2 could catalyze the oxidation process of TMB to produce blue oxidized TMB (oxTMB) with a characteristic absorbance of 652 nm (Fig. 11b). The experimental results showed that Cu2O-CNx@CeO2 possessed the most excellent peroxidase activity. Cu2O-CNx@CeO2 due to its optimal Cu2O-CNx coordination structure and the catalytic activity of CeO2 as a substrate. Cu2O-CNx@CeO2 not only exhibited highly efficient POD-like activity to generate –O2 (Fig. 11c). and can promote abundant ·OH and 1O2 generation under US irradiation (Fig. 11d). Photoexcitation and ultrasonic radiation have been shown to promote the generation of reactive oxygen species. In an in vitro experimental study, the intracellular ROS levels of Cu2O-CNx@CeO2 on B16F10 cells under different treatments were recorded using 2,7-dichlorofluorescein diacetate (DCFH-DA). DCFH-DA reacts with ROS to generate 2,7-dichlorofluorescein (DCF) with green fluorescence. The highest intensity of green fluorescence and the strongest level of ROS generation could be observed in the Cu2O-CNx@CeO2 + US + L group (Fig. 11e). A further process was carried out to examine the biocatalytic killing ability of Cu2O-CNx@CeO2 on B16F10 cells, and the cytotoxicity was detected by CCK8. It could be observed that cell death was most significant in the Cu2O-CNx@CeO2 + US + L group (Fig. 11f). In the in vivo experimental study, B16F10 tumor xenografts of Balb/c male mice were used to detect their in vivo anti-tumor efficiency. It could be observed that the Cu2O-CNx@CeO2 + US/L group inhibited tumor growth compared with the control group. The Cu2O-CNx@CeO2 + US + L group significantly inhibited tumors with the highest inhibition rate of 78.8% (Fig. 11g). The in vitro and in vivo experiments demonstrated that Cu2O-CNx@CeO2 could effectively inhibit the growth of malignant melanoma through the US/light multimodal antitumor ability. This work provides novel biocatalysts with dual catalytic activities for the generation of ROS and O2, and offers a new way to engineer multimodal nanoreagents to achieve synergistic inhibition of malignant tumors.
 | ||
| Fig. 11 (a) Illustrated formation procedures for the Cu2O-CNx@CeO2 biocatalyst; (b) UV-vis absorption spectra via the TMB method with Cu2O@CeO2 and Cu2O-CNx@CeO2; (c) schematic illustration of the CAT-like and ultrasound-enhanced POD-like activity (with H2O2); (d) the type I and type II PDT mechanism of Cu2O-CNx@CeO2 (without H2O2); (e) fluorescence images of DCFH-DA stained B16F10 cells; (f) quantitative analysis of cell death rate after different treatments; (g) tumor growth inhibition in B16F10 tumors. Copyright 2023, The Royal Society of Chemistry.107 | ||
In addition to the Zn, Bi, Fe, and Ce-based materials mentioned above, there are other transition metal oxide-based nanomaterials that may be used as sonosensitizers in SDT. The selection and design of these materials usually depend on factors such as their physicochemical properties, biocompatibility, stability, and ability to generate ROS. The performance of these sonosensitizers can be further optimized and the therapeutic efficacy of SDT can be improved by rational material design and preparation processes. In summary, transition metal oxide-based nanomaterials have a promising application as inorganic sonosensitizers in SDT. However, further studies and explorations on the specific mechanism of action, biosafety, and therapeutic efficacy of these materials are still needed. With the continuous development of nanotechnology and biomedicine, it is believed that more novel and efficient transition metal oxide-based nanomaterials will be developed in the future to provide richer options for SDT.
3.3 Non-metallic acoustic sensitizer
Inorganic non-metallic sonosensitizers are chemical agents consisting of inorganic non-metallic elements that are capable of producing an acoustic dynamic effect in the presence of ultrasound. These elements typically include non-metallic elements such as silicon and carbon, as well as their compounds or nanomaterials. These sonosensitizers usually exhibit superior chemical stability, and low phototoxicity, and are easy to be chemically modified and surface functionalized for modification, thus showing unique advantages in SDT. Under US irradiation, inorganic non-metallic sonosensitizers are able to absorb acoustic energy and undergo separation of electron–hole pairs. These separated electrons and holes can react with surrounding oxygen or water molecules to generate cytotoxic ROS. These ROS are capable of damaging biomolecules such as cell membranes, DNA and proteins of tumor cells, thus leading to the death of tumor cells.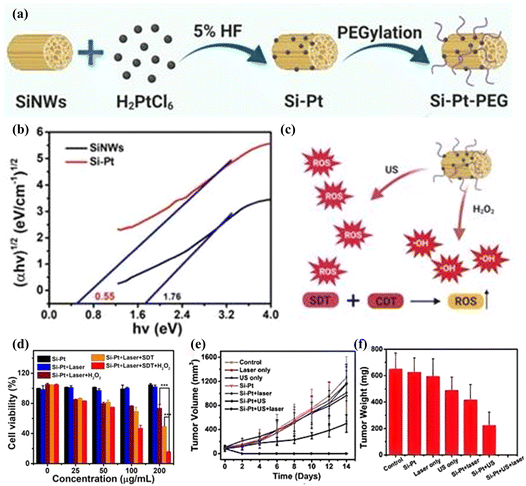 | ||
| Fig. 12 (a) Schematic illustration of Si–Pt nanocomposites (NCs) with unique photo and ultrasonic properties for photothermal enhanced sonodynamic therapy; (b) the bandgap of SiNWs and Si–Pt NCs; (c) the schematic of sonodynamic and chemodynamic capability of Si–Pt NCs; (d) Si–Pt NCs, 50 μM H2O2 and US irradiation in the presence or absence of 1064 nm laser; (e) biodistribution of Si–Pt NCs in mice at different days post-intratumor injection of 4T1 tumor growth curve and (f) tumor weight. Copyright 2021, Ivyspring International.111 | ||
 | ||
| Fig. 13 (a) Synthesis process of ZnO and C–ZnO; (b) optical band gaps of ZnO and C–ZnO; (c) N2 adsorption–desorption isotherms and pore size distribution of C–ZnO. Copyright 2024, Wiley-VCH.115 | ||
Specific elements in the fullerene molecule (e.g., Gd) can generate hydrophilic groups to achieve good contrast effects. It has been shown that fullerenes containing Gd perform well in MRI contrast, and their contrast effect is far superior to that of conventional contrast agents. This property makes fullerenes have a broad application prospect in the field of bio-imaging. The molecular structure of fullerenes enables them to carry large amounts of therapeutic drugs and has good biocompatibility.117 Studies have shown that fullerenes can be used as drug delivery carriers to deliver drugs to tumor sites, showing promise in the diagnosis and treatment of tumors. Iwase et al.118 investigated the acoustic kinetics-induced antitumor effects of pyrrolidinetriacontanic acid fullerenes (PTFs). PTFs showed significant ultrasound-induced antitumor effects as well as a significant enhancement of ultrasound-induced cellular damage in vitro. PTF increased the rate of ultrasound-induced cell injury in isolated sarcoma 180 cells by approximately 5-fold. Tumor tissue destruction was observed in the combination of PTF and US treatment, whereas neither PTF alone nor ultrasound alone induced necrosis. The above results demonstrated that PTF showed excellent US-induced antitumor effects.
CDs are an emerging class of zero-dimensional fluorescent carbon nanomaterials with small highly carbonized cores and polymer surface groups. Their unique structure endows CDs with excellent optical and electronic properties, making them a preferred material in the field of bioimaging. The imaging function of CDs stems from their unique optical features or functional reagents bound to their cores or surfaces, which enable imaging at the cellular or even single-molecule level. CDs are characterized by low toxicity, high chemical stability, excellent water-solubility, and good biocompatibility.119 These properties make CDs promising for drug delivery applications. The tunable functional properties of CDs make them ideal nanocapsules and nanocarriers120 for loading and delivering drugs and genes to specific targets in the body. Recently, iron-doped multivalent manganese oxide nanoparticles (FDMNs) studied by Sun et al.121 have been well applied as sonosensitizers in SDT for tumor therapy. Due to the presence of oxygen vacancies, a large number of oxygen molecules adsorbed on the surface of FDMNs can enter the tumor microenvironment, effectively preventing ultrasound-triggered electron–hole pair recombination and generating a large number of ROS for SDT tumor therapy. Based on the study of FDMNs for SDT tumor therapy, Cheng et al.122 designed a novel acoustic sensitizer synthesized with Cu–CDs (Fig. 14a) for the acoustic kinetic therapy of glioblastoma multiforme (GBM) (Fig. 14b). The Cu–CDs have unique p–n junctions and abnormally narrow bandgaps, and Cu referencing converts the CDs into a 1.58 eV bandgap p–n semiconductors, resulting in improved separation efficiency of electrons and holes and improved ROS generation. The p–n-CD acoustic sensitizer acts as a US transducer, which can generate 1O2 through the energy transfer and SDT mechanisms. Briefly, due to the small band gap, when p–n-CDs undergo rapid separation of surface charges and holes under US irradiation, the separated electrons absorb energy from the ground state (S0) to jump to a single-line excited state (S1). A portion of this absorbed energy is released through the interlinear crossover to form a triplet excited state (T1). p–n-CD triplet state's relatively long half-life (10.7 μs) permits efficient energy transfer to nearby oxygen molecules, which results in the production of 1O2 via the type II pathway (Fig. 14c). Consequently, this process destroys cells in the surrounding region, ultimately leading to cell death. This is the mechanism by which ROS generation by carbon-based sonosensitizers induces tumor cell death. By using 2,2,6,6-tetramethylpiperidine (TEMP) as a trapping agent for 1O2, electron spin resonance (ESR) spectra were recorded (Fig. 14d). The results showed that “Cu–CDs + US” produced significantly stronger ESR signals, indicating enhanced 1O2 production. In addition, the introduction of copper doping induced copper body deposition, and Cu–CDs induced a biological reaction leading to cell death called copper death. Specifically, Cu–CDs efficiently bound to lipoylated mitochondrial enzymes and induced the aggregation of lipoylated dihydrosulfonyltransacetylase (DLAT), leading to copper conversion. The copper death mechanism further amplified the effects of SDT, leading to more potent therapeutic outcomes compared to SDT alone. In cellular in vitro assays, to determine the in vitro SDT performance of Cu–CDs on U87 cells, 5 min of US irradiation at 1.0 w cm−2 was performed. Under the same concentration (50 μg ml−1) and US irradiation conditions, the cell survival of different treatment groups (US, CDs, Cu–CDs, CDs + US and Cu–CDs + US) were compared relative to the control group for 24 h. The SDT effect was greatest in the Cu–CD + US group (Fig. 14f). Flow cytometry analysis supported the WST-1 results and live/dead cell analysis, and Cu–CDs showed that US enhanced ROS generation and tumor death (Fig. 14e). The proportion of late apoptotic cells was higher in the US-enhanced Cu–CDs group (75.9%). Cu–CDs effectively inhibited the growth of glioblastoma tumors and prolonged the survival of mice with these tumors. This study provides support for the application of carbon-based nanomaterials as ultrasound sensitizers in tumor therapy.
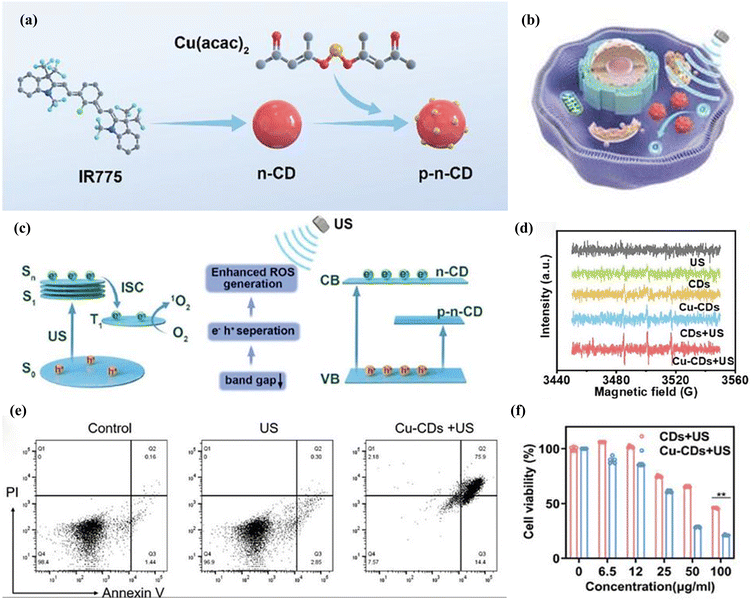 | ||
| Fig. 14 (a) Design and mechanism of Cu–CDs for sonodynamic cancer therapy; (b) mechanisms of Cu–CDs for acoustic power cancer therapy; (c) schematic diagram of 1O2 generation mechanisms of Cu–CDs; (d) ESR spectra of US-triggered 1O2 generation using TEMP as the trapping agent for 1O2; (e) flow cytometry analysis of U87 cells after different treatments stained with annexin V-FITC/PI; (f) relative cell viability of U87 cells incubated with CDs and Cu–CDs + US (1.0 w cm−2). Copyright 2024, Wiley-VCH.122 | ||
3.4 Stability, biocompatibility, and metabolic pathways of inorganic sonosensitizers
To systematically evaluate the performance of inorganic sonosensitizers in sonodynamic therapy (SDT), we conducted a comparative analysis of three primary material classes: noble metals (Au, Ag, Pt), transition metal oxides (e.g., MnO2), and carbon-based nanomaterials. This assessment focuses on critical parameters including stability, biocompatibility, reactive oxygen species (ROS) yield, and practical limitations (Table 1). Noble metal-based sonosensitizers exhibit high structural stability and excellent biocompatibility, yet their moderate ROS generation efficiency is constrained by high production costs and limited tissue penetration depth. Transition metal oxides, represented by MnO2, demonstrate pH-dependent stability and superior ROS yields via Fenton-like reactions; however, their efficacy is compromised by glutathione (GSH) scavenging in the tumor microenvironment. Carbon-based materials, while achieving ultra-high stability, suffer from inconsistent biocompatibility (dependent on surface functionalization) and low ROS productivity, alongside poor tumor-targeting specificity. This comparative framework underscores the necessity of balancing material properties with clinical requirements, guiding the rational selection and optimization of sonosensitizers for SDT applications.| Material class | Stability | Biocompatibility | ROS yield | Key limitations |
|---|---|---|---|---|
| Noble metals | High | Excellent | Moderate | High cost, limited depth penetration |
| Transition metals | pH-dependent | Good | High | GSH sensitivity |
| Non-metallic | Ultra-high | Variable | Low | Poor tumor targeting |
The clinical translation of inorganic sonosensitizers necessitates rigorous evaluation of their biosafety. Noble metal-based nanoparticles (e.g., Au, Ag) exhibit excellent biocompatibility but face challenges in long-term accumulation due to slow renal clearance. Recent studies suggest that sub-5 nm Au nanoparticles can enhance urinary excretion. Transition metal oxides (e.g., MnO2) are degradable in acidic TME via Mn2+/Mn4+ redox cycles, yet excessive Mn2+ may induce neurotoxicity. Silicon-based materials, while biodegradable into orthosilicic acid (Si(OH)4), require precise size control (<10 nm) to avoid pulmonary inflammation. Carbon-based sonosensitizers (e.g., graphene quantum dots) show pH-dependent degradation but raise concerns about prolonged retention in reticuloendothelial systems. Future studies should prioritize real-time tracking of sonosensitizer metabolism using isotopic labeling (e.g., 64Cu-labeled probes).
4 Conclusion and outlook
With the booming of nanomedicine technology, the research on sonosensitizers at the core of SDT, an emerging cancer treatment, is experiencing an unprecedented boom in exploration. In this in-depth review, our attention is focused on the key area of inorganic sonosensitizers, especially precious metals (e.g., Au, Ag, Pt): these metals are used in SDT mainly due to their unique photovoltaic properties and stability. However, they are costly and may trigger an immune response in some cases. Therefore, future research should focus on the development of more cost-effective and biocompatible precious metal nanostructures; transition metals (e.g., Ti, Mn, etc.): the tunable redox activity of these metals offers new possibilities for SDT, while their biosafety and long-term effects still need to be further evaluated. By precisely controlling the size, shape and surface chemistry of the nanoparticles, their biodistribution and clearance mechanisms can be optimized; other metals (e.g., Zn, Bi, Fe, and Ce, etc.): these metals have attracted much attention due to their good catalytic activity. However, their biocompatibility and stability still need to be further verified. Their SDT performance can be improved by composite or surface modification with other materials; silicon-based materials are ideal candidates for SDT due to their good biocompatibility and redox activity, but their mechanical strength and long-term stability still need to be improved. Their SDT effect can be enhanced by introducing functionalized groups or constructing composite structures; carbon-based materials, such as graphene, fullerenes and carbon dots, are popular for their excellent conductivity and biocompatibility. Whereas, their biodistribution and clearance mechanisms still need to be thoroughly investigated. Their SDT performance can be improved by precisely controlling the size and shape of the materials and introducing targeting groups.We not only comprehensively review the basic mechanism of action of SDT, the direct killing of cancer cells by ROS or physical effects generated by the interaction of ultrasound and sonosensitizers – but also analyze in depth a variety of cutting-edge strategies aimed at enhancing the efficacy of SDT. These strategies cover: (1) material design and cavitation effect: by designing nanoparticles with specific shapes and structures, the cavitation effect can be enhanced, thereby improving the efficiency of ultrasound energy delivery and utilization in tumor tissues. For example, the use of nanoparticles with a porous structure can increase the scattering and absorption of ultrasound; (2) metal coupling and multifunctional nanoplatforms: through metal coupling technology, multifunctional nanoplatforms can be constructed to enhance the stability of the sonosensitizers, as well as to facilitate the subsequent biomodification and targeted delivery. This strategy can significantly improve the specificity and therapeutic efficacy of SDT; (3) defect engineering and energy band structure modulation: through the implementation of defect engineering, it is possible to modulate the energy band structure of the sonosensitizers and optimize their response performance to ultrasound. This strategy can significantly improve the efficiency and sensitivity of SDT; (4) material modification and conductivity enhancement: increasing conductivity through material modification can promote the electron transfer process and accelerate the generation of ROS. This strategy can significantly improve the killing effect of SDT; (5) targeting strategy and tumor microenvironment remodeling: the use of advanced targeting strategies can improve the specific recognition of tumor cells by sonosensitizers. Meanwhile, exploring new ways to remodel the tumor microenvironment, such as improving the oxygen supply and blood circulation inside the tumor, can enhance the therapeutic effect of SDT emerging strategies to enhance SDT include surface engineering of sonosensitizers with tumor-targeting ligands (e.g., anti-EGFR antibodies) and stimuli-responsive coatings.123 For instance, pH-sensitive polymers could enable site-specific drug release in acidic TME. Combinatorial approaches integrating SDT with immune checkpoint inhibitors (e.g., anti-PD-1) have shown synergistic abscopal effects in recent preclinical trials. Additionally, nanoparticle-mediated sonodynamic-chemo therapy hybrids (e.g., DOX-loaded TiO2) demonstrate enhanced tumor penetration through ultrasound-triggered cavitation). Multimodal synergistic treatment can be achieved by researching combined treatment modes, combining chemotherapy, radiotherapy and photodynamic therapy; as well as using image-guided technology to achieve precise positioning and real-time monitoring of the SDT process to ensure the safety and effectiveness of the treatment.
However, despite the remarkable progress made by researchers in developing efficient, safe, and biocompatible sonosensitizers, unfortunately, no acoustic sensitizer has yet to successfully cross the laboratory-to-clinical divide and achieve true clinical application. This is mainly due to the fact that there are still many challenges regarding the stability, biodistribution, clearance mechanism, and long-term safety of sonosensitizers in vivo.
Future of SDT requires interdisciplinary convergence: (1) material scientists and clinicians should co-design sonosensitizers with real-time imaging capabilities (e.g., US-guided); (2) pharmacologists need to establish standardized protocols for sonosensitizer dosing and US parameter optimization; (3) regulatory frameworks must adapt to assess nanotherapeutic safety in SDT combination regimens. With global initiatives like the International SDT Consortium (2024), clinical trials may soon validate these laboratory breakthroughs.
Data availability
All data have been included in the main text.Conflicts of interest
There is no conflict to declare.Acknowledgements
This work was financially supported by the Science and Technology Project of China Minmetals Corporation (2022ZXA02), Shanghai Collaborative Innovation Center of Energy Therapy for Tumors, the Medical-Engineering Interdisciplinary Project of University of Shanghai for Science and Technology (1022310503), Open Research Fund of School of Chemistry and Chemical Engineering, Henan Normal University (2024Y12), the Technical Innovation Team of Henan Normal University (2022TD03). The authors greatly appreciated these supports.References
- C. Dong, H. Hu, L. Sun and Y. Chen, Biomed. Mater., 2021, 16, 032006 CrossRef CAS.
- U. Anand, A. Dey, A. K. S. Chandel, R. Sanyal, A. Mishra, D. K. Pandey, V. De Falco, A. Upadhyay, R. Kandimalla, A. Chaudhary, J. K. Dhanjal, S. Dewanjee, J. Vallamkondu and J. M. Pérez de la Lastra, Gene Dis., 2023, 10, 1367–1401 CrossRef CAS.
- L. Sun, P. Wang, J. Zhang, Y. Sun, S. Sun, M. Xu, L. Zhang, S. Wang, X. Liang and L. Cui, Biomater. Sci., 2021, 9, 1945–1960 RSC.
- K. D. Miller, L. Nogueira, T. Devasia, A. B. Mariotto, K. R. Yabroff, A. Jemal, J. Kramer and R. L. Siegel, Ca-Cancer J. Clin., 2022, 72, 409–436 Search PubMed.
- A. R. Lima, L. Santos, M. Correia, P. Soares, M. Sobrinho-Simões, M. Melo and V. Máximo, Genes, 2018, 9, 115 CrossRef PubMed.
- M. Cedzyński and A. S. Świerzko, Cancers, 2024, 16, 3116 Search PubMed.
- L.-L. Bu, J. Yan, Z. Wang, H. Ruan, Q. Chen, V. Gunadhi, R. B. Bell and Z. Gu, Biomaterials, 2019, 219, 119182 Search PubMed.
- F. Peng, J. Liu, J. Chen, W. Wu, Y. Zhang, G. Zhao, Y. Kang, D. Gong, L. He, J. Wang, W. Zhang and F. Qiu, ACS Nano, 2023, 17, 20135–20152 Search PubMed.
- X. Shi and X. Sun, Cancer Chemother. Pharmacol., 2017, 80, 909–917 Search PubMed.
- M. L. S. Silva, Int. J. Pharm., 2024, 665, 124685 Search PubMed.
- L. Han, X.-Y. Zhang, Y.-L. Wang, X. Li, X.-H. Yang, M. Huang, K. Hu, L.-H. Li and Y. Wei, J. Controlled Release, 2017, 259, 40–52 Search PubMed.
- N. Yumita, R. Nishigaki, K. Umemura and S.-I. Umemura, Jpn. J. Cancer Res., 1989, 80, 219–222 Search PubMed.
- J. H. Correia, J. A. Rodrigues, S. Pimenta, T. Dong and Z. Yang, Pharmaceutics, 2021, 13, 1332 CrossRef CAS PubMed.
- X. Song, F. Li, F. Tian, L. Ren, Q. Wang, C. Jiang, T. Yan and S. Zhang, Acta Biomater., 2023, 157, 538–550 CrossRef CAS PubMed.
- Z. Dai and A. A. Exner, Bioconjugate Chem., 2022, 33, 991–992 CrossRef CAS PubMed.
- J. Guo, X. Pan, C. Wang and H. Liu, Bioconjugate Chem., 2022, 33, 993–1010 Search PubMed.
- K. Bian, W. Yang, Y. Xu, W. Zeng, H. Wang, H. Liang, T. Cui, Z. Wang and B. Zhang, Small, 2022, 18, 2202921 Search PubMed.
- A. Radivoievych, S. Prylutska, O. Zolk, U. Ritter, M. Frohme and A. Grebinyk, Pharmaceutics, 2023, 15, 2616 Search PubMed.
- L. Wang, M. Niu, C. Zheng, H. Zhao, X. Niu, L. Li, Y. Hu, Y. Zhang, J. Shi and Z. Zhang, Adv. Healthcare Mater., 2018, 7, 1800819 CrossRef PubMed.
- X. Wang, Y. Wang, P. Wang, X. Cheng and Q. Liu, Ultrasonics, 2011, 51, 539–546 CrossRef PubMed.
- J. Chen, Q. Zhou and W. Cao, Adv. Funct. Mater., 2024, 34, 2405844 CrossRef CAS.
- X. Feng, Y. Shi, L. Xie, K. Zhang, X. Wang, Q. Liu and P. Wang, J. Med. Chem., 2018, 61, 7189–7201 Search PubMed.
- A. Luiz-Ferreira, T. Pacifico, Á. C. Cruz, F. Laudisi, G. Monteleone and C. Stolfi, Int. J. Mol. Sci., 2023, 24, 16596 CrossRef CAS PubMed.
- S. Liang, X. Deng, G. Xu, X. Xiao, M. Wang, X. Guo, P. a. Ma, Z. Cheng, D. Zhang and J. Lin, Adv. Funct. Mater., 2020, 30, 1908598 CrossRef CAS.
- T. Kasai, K. Miyauchi, N. Yanagisawa, K. Kajimoto, N. Kubota, M. Ogita, S. Tsuboi, A. Amano and H. Daida, Heart, 2013, 99, 22–29 Search PubMed.
- L. Wang, Y. Hu, Y. Hao, L. Li, C. Zheng, H. Zhao, M. Niu, Y. Yin, Z. Zhang and Y. Zhang, J. Controlled Release, 2018, 286, 74–84 CrossRef CAS PubMed.
- J. Bogdan, J. Pławińska-Czarnak and J. Zarzyńska, Nanoscale Res. Lett., 2017, 12, 225 Search PubMed.
- X. Feng, C. Wu, W. Yang, J. Wu and P. Wang, Int. J. Mol. Sci., 2022, 23, 7981 Search PubMed.
- S. Yamamoto, M. Ono, E. Yuba and A. Harada, Nanomaterials, 2017, 7, 268 Search PubMed.
- H.-Y. Xia, B.-Y. Li, Y. Zhao, Y.-H. Han, S.-B. Wang, A.-Z. Chen and R. K. Kankala, Coord. Chem. Rev., 2022, 464, 214540 CrossRef CAS.
- T. Xu, S. Zhao, C. Lin, X. Zheng and M. Lan, Nano Res., 2020, 13, 2898–2908 Search PubMed.
- G. Wang, W. Wu, J.-J. Zhu and D. Peng, Ultrason. Sonochem., 2021, 79, 105781 Search PubMed.
- N. H. Ince, G. Tezcanli, R. K. Belen and I. G. Apikyan, Appl. Catal., B, 2001, 29, 167–176 Search PubMed.
- P. Yan, L.-H. Liu and P. Wang, ACS Appl. Bio Mater., 2020, 3, 3456–3475 Search PubMed.
- Y. Xin, Z. Guo, A. Ma, E. Shi, Z. Li, Z. Liang, Z. Qian, L. Yang, Y. Wang, M. Cao and X. Yang, Chem. Eng. J., 2023, 451, 138782 CrossRef CAS.
- J. An, H. Hong, M. Won, H. Rha, Q. Ding, N. Kang, H. Kang and J. S. Kim, Chem. Soc. Rev., 2023, 52, 30–46 RSC.
- R. G. Thomas, U. S. Jonnalagadda and J. J. Kwan, Langmuir, 2019, 35, 10106–10115 CrossRef CAS PubMed.
- D. Huang, C. Zhao, B. Wen, X. Fu, L. Shang, W. Kong and Y. Zhao, Chem. Eng. J., 2022, 435, 134871 CrossRef CAS.
- X. Wang, X. Zhong, F. Gong, Y. Chao and L. Cheng, Mater. Horiz., 2020, 7, 2028–2046 RSC.
- P. Xu, C. Wen, C. Gao, H. Liu, Y. Li, X. Guo, X.-C. Shen and H. Liang, ACS Nano, 2024, 18, 713–727 CrossRef CAS PubMed.
- A. Sazgarnia, A. Shanei, A. R. Taheri, N. T. Meibodi, H. Eshghi, N. Attaran and M. M. Shanei, J. Ultrasound Med., 2013, 32, 475–483 CrossRef PubMed.
- N. S. Abadeer and C. J. Murphy, J. Phys. Chem. C, 2016, 120, 4691–4716 CrossRef CAS.
- Y. L. Loke, A. Beishenaliev, P.-W. Wang, C.-Y. Lin, C.-Y. Chang, Y. Y. Foo, F. N. Faruqu, B. F. Leo, M. Misran, L. Y. Chung, D.-B. Shieh, L. V. Kiew, C.-C. Chang and Y. Y. Teo, Ultrason. Sonochem., 2023, 96, 106437 CrossRef CAS PubMed.
- Y. Zhang, Y. Chen, Y. Zhang, H. Cong, B. Fu, S. Wen and S. Ruan, J. Nanopart. Res., 2013, 15, 2014 CrossRef.
- H. Hu, J. Zhao, K. Ma, J. Wang, X. Wang, T. Mao, C. Xiang, H. Luo, Y. Cheng, M. Yu, Y. Qin, K. Yang, Q. Li, Y. Sun and S. Wang, J. Controlled Release, 2023, 359, 188–205 CrossRef CAS PubMed.
- B. Geng, S. Zhang, X. Yang, W. Shi, P. Li, D. Pan and L. Shen, Chem. Eng. J., 2022, 435, 134777 CrossRef CAS.
- K. Li, C. Lin, M. Li, K. Xu, Y. He, Y. Mao, L. Lu, W. Geng, X. Li, Z. Luo and K. Cai, ACS Nano, 2022, 16, 2381–2398 CrossRef CAS PubMed.
- B. Geng, S. Xu, L. Shen, F. Fang, W. Shi and D. Pan, Carbon, 2021, 179, 493–504 CrossRef CAS.
- C. Shuai, W. Guo, P. Wu, W. Yang, S. Hu, Y. Xia and P. Feng, Chem. Eng. J., 2018, 347, 322–333 Search PubMed.
- V. Bernard, V. Mornstein, J. Jaroš, M. Sedláčková and J. Škorpíková, J. Appl. Biomed., 2014, 12, 137–145 CrossRef.
- C. Hu, B. Hou and S. Xie, RSC Adv., 2022, 12, 22722–22747 RSC.
- A. Mysara, M. Morsy, A. O. Ahmed, F. A. Ibrahim and A. Elzwawy, J. Mater. Sci., 2024, 59, 20964–20981 CrossRef CAS.
- Y. Yu, J. Geng, E. Y. X. Ong, V. Chellappan and Y. N. Tan, Adv. Healthcare Mater., 2016, 5, 2528–2535 CrossRef CAS.
- P. Liang, Q. Guo, T. Zhao, C.-Y. Wen, Z. Tian, Y. Shang, J. Xing, Y. Jiang and J. Zeng, Anal. Chem., 2022, 94, 8466–8473 CrossRef CAS PubMed.
- X. Meng, S. Sun, C. Gong, J. Yang, Z. Yang, X. Zhang and H. Dong, ACS Nano, 2023, 17, 1174–1186 CrossRef CAS.
- R. M. Abdelhameed, M. M. Q. Simões, A. M. S. Silva and J. Rocha, Chem.–Eur. J., 2015, 21, 11072–11081 CrossRef CAS PubMed.
- X. Zhong, X. Wang, L. Cheng, Y. a. Tang, G. Zhan, F. Gong, R. Zhang, J. Hu, Z. Liu and X. Yang, Adv. Funct. Mater., 2020, 30, 1907954 CrossRef CAS.
- L.-H. Fu, Y. Wan, C. Qi, J. He, C. Li, C. Yang, H. Xu, J. Lin and P. Huang, Adv. Mater., 2021, 33, 2006892 CrossRef CAS PubMed.
- T. Sun, R. Wang, W. Lu, X. Shi, F. Gao, T. Wu, G. Wang, X. Su and Z. Teng, J. Mater. Chem. B, 2023, 11, 11280–11289 RSC.
- K. Cheng, X.-Q. Yang, X.-S. Zhang, J. Chen, J. An, Y.-Y. Song, C. Li, Y. Xuan, R.-Y. Zhang, C.-H. Yang, X.-L. Song, Y.-D. Zhao and B. Liu, Adv. Funct. Mater., 2018, 28, 1803118 Search PubMed.
- G. Yang, L. Xu, J. Xu, R. Zhang, G. Song, Y. Chao, L. Feng, F. Han, Z. Dong, B. Li and Z. Liu, Nano Lett., 2018, 18, 2475–2484 CrossRef CAS PubMed.
- J. An, Y.-G. Hu, K. Cheng, C. Li, X.-L. Hou, G.-L. Wang, X.-S. Zhang, B. Liu, Y.-D. Zhao and M.-Z. Zhang, Biomaterials, 2020, 234, 119761 Search PubMed.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 Search PubMed.
- X.-C. Liu, G.-C. Wang, R.-P. Liang, L. Shi and J.-D. Qiu, J. Mater. Chem. A, 2013, 1, 3945–3953 Search PubMed.
- Q. Ren, N. Yu, L. Wang, M. Wen, P. Geng, Q. Jiang, M. Li and Z. Chen, J. Colloid Interface Sci., 2022, 614, 147–159 Search PubMed.
- Z. Dong, L. Feng, Y. Hao, M. Chen, M. Gao, Y. Chao, H. Zhao, W. Zhu, J. Liu, C. Liang, Q. Zhang and Z. Liu, J. Am. Chem. Soc., 2018, 140, 2165–2178 CrossRef CAS PubMed.
- L. Ding, X. Zhu, Y. Wang, B. Shi, X. Ling, H. Chen, W. Nan, A. Barrett, Z. Guo, W. Tao, J. Wu and X. Shi, Nano Lett., 2017, 17, 6790–6801 CrossRef CAS PubMed.
- A. León, P. Reuquen, C. Garín, R. Segura, P. Vargas, P. Zapata and P. A. Orihuela, Appl. Sci., 2017, 7, 49 Search PubMed.
- A. Sosnik, I. Zlotver and H. Potthuri, Prog. Mater. Sci., 2025, 148, 101384 Search PubMed.
- Y. Harada, K. Ogawa, Y. Irie, H. Endo, L. B. Feril, T. Uemura and K. Tachibana, J. Contr. Release, 2011, 149(2), 190–195 CrossRef CAS PubMed.
- Z. Qin, L. Chen, R. Ma, R. Tomovska, X. Luo, X. Xie, T. Su and H. Ji, J. Alloys Compd., 2020, 836, 155428 CrossRef CAS.
- S. Liang, X. Xiao, L. Bai, B. Liu, M. Yuan, P. a. Ma, M. Pang, Z. Cheng and J. Lin, Adv. Mater., 2021, 33, 2100333 CrossRef CAS PubMed.
- C. Tang, H. Li, M. Sha, J. Song, X. Bai, K. Liu, Y. Liu, B. Yuan, J. Yan, J. Chang and J. Kang, Chem. Eng. J., 2023, 475, 146054 CrossRef CAS.
- M. Scrocco, Chem. Phys. Lett., 1979, 61, 453–456 CrossRef CAS.
- V. Abdelsayed, S. Moussa, H. M. Hassan, H. S. Aluri, M. M. Collinson and M. S. El-Shall, J. Phys. Chem. Lett., 2010, 1, 2804–2809 CrossRef CAS.
- O. Elbanna, M. Fujitsuka, S. Kim and T. Majima, J. Phys. Chem. C, 2018, 122, 15163–15170 CrossRef CAS.
- Q. Li, Y. Xia, C. Yang, K. Lv, M. Lei and M. Li, Chem. Eng. J., 2018, 349, 287–296 CrossRef CAS.
- G. K. Naik, S. M. Majhi, K.-U. Jeong, I.-H. Lee and Y. T. Yu, J. Alloys Compd., 2019, 771, 505–512 CrossRef CAS.
- M. Wu, D. Xu, B. Luo, H. Shen, C. Wang and W. Shi, Mater. Lett., 2015, 161, 45–48 CrossRef CAS.
- J. Zhang, J. Xi and Z. Ji, J. Mater. Chem., 2012, 22, 17700–17708 RSC.
- X. Wang, X. Wang, Q. Yue, H. Xu, X. Zhong, L. Sun, G. Li, Y. Gong, N. Yang, Z. Wang, Z. Liu and L. Cheng, Nano Today, 2021, 39, 101170 CrossRef CAS.
- J. Shao, J. Zhang, C. Jiang, J. Lin and P. Huang, Chem. Eng. J., 2020, 400, 126009 CrossRef CAS.
- W. He, K. Ai, C. Jiang, Y. Li, X. Song and L. Lu, Biomaterials, 2017, 132, 37–47 CrossRef CAS PubMed.
- M. Xu, L. Zhou, L. Zheng, Q. Zhou, K. Liu, Y. Mao and S. Song, Cancer Lett., 2021, 497, 229–242 CrossRef CAS PubMed.
- C. Wang, C. Dai, Z. Hu, H. Li, L. Yu, H. Lin, J. Bai and Y. Chen, Nanoscale Horiz., 2019, 4, 415–425 RSC.
- B. Hu, A. Huang, X. Zhang, Z. Chen, R. Tu, W. Zhu, Z. Zhuang, C. Chen, Q. Peng and Y. Li, Nano Res., 2021, 14, 3482–3488 CrossRef CAS.
- J. Ruan and H. Qian, Adv. Therapeut., 2021, 4, 2100018 CrossRef CAS.
- B. Ding, P. Zheng, P. a. Ma and J. Lin, Adv. Mater., 2020, 32, 1905823 CrossRef CAS PubMed.
- H. Zhang, X. Pan, Q. Wu, J. Guo, C. Wang and H. Liu, Exploration, 2021, 1, 20210010 CrossRef PubMed.
- M. R. Smith, J. Fernandes, Y.-M. Go and D. P. Jones, Biochem. Biophys. Res. Commun., 2017, 482, 388–398 CrossRef CAS PubMed.
- W. Yue, L. Chen, L. Yu, B. Zhou, H. Yin, W. Ren, C. Liu, L. Guo, Y. Zhang, L. Sun, K. Zhang, H. Xu and Y. Chen, Nat. Commun., 2019, 10, 2025 Search PubMed.
- G. Zhan, Q. Xu, Z. Zhang, Z. Wei, T. Yong, N. Bie, X. Zhang, X. Li, J. Li, L. Gan and X. Yang, Nano Today, 2021, 38, 101195 Search PubMed.
- Q. Xu, G. Zhan, Z. Zhang, T. Yong, X. Yang and L. Gan, Theranostics, 2021, 11, 1937–1952 CrossRef CAS PubMed.
- H. Sun, J. Su, Q. Meng, Q. Yin, L. Chen, W. Gu, P. Zhang, Z. Zhang, H. Yu, S. Wang and Y. Li, Adv. Mater., 2016, 28, 9581–9588 Search PubMed.
- R. Yang, J. Xu, L. Xu, X. Sun, Q. Chen, Y. Zhao, R. Peng and Z. Liu, ACS Nano, 2018, 12, 5121–5129 CrossRef CAS PubMed.
- Z. Hu, X. Song, L. Ding, Y. Cai, L. Yu, L. Zhang, Y. Zhou and Y. Chen, Mater. Today Bio, 2022, 16, 100452 Search PubMed.
- S. Bai, N. Yang, X. Wang, F. Gong, Z. Dong, Y. Gong, Z. Liu and L. Cheng, ACS Nano, 2020, 14, 15119–15130 Search PubMed.
- L. Wang, Y. Chen, J. Zhao, D. Luo and W. Tian, Transl. Cancer Res., 2022, 11, 1970–1976 Search PubMed.
- J. Li, H. Fang, L. Fan, J. Yang, T. Ji and Q. Chen, Waste Manage., 2022, 139, 96–104 Search PubMed.
- G. Chen, J. Du, L. Gu, Q. Wang, Q. Qi, X. Li, R. Zhang, H. Yang, Y. Miao and Y. Li, Chem. Eng. J., 2024, 482, 148953 CrossRef CAS.
- M. Wu, Z. Zhang, Z. Liu, J. Zhang, Y. Zhang, Y. Ding, T. Huang, D. Xiang, Z. Wang, Y. Dai, X. Wan, S. Wang, H. Qian, Q. Sun and L. Li, Nano Today, 2021, 37, 101104 CrossRef CAS.
- Y. Zhang, Y. Xu, D. Sun, Z. Meng, W. Ying, W. Gao, R. Hou, Y. Zheng, X. Cai, B. Hu and X. Lin, Chem. Eng. J., 2020, 390, 124521 CrossRef CAS.
- X. Chen, D. Cheng, M. Ding, N. Yu, J. Liu, J. Li and L. Lin, J. Mater. Chem. B, 2022, 10, 4595–4604 Search PubMed.
- C. He, X. Zhang, C. Chen, X. Liu, Y. Chen, R. Yan, T. Fan, Y. Gai, R. J. Lee, X. Ma, J. Luo, Y. Lu, T. Yang and G. Xiang, Acta Biomater., 2021, 122, 354–364 CrossRef CAS PubMed.
- R. W. Tarnuzzer, J. Colon, S. Patil and S. Seal, Nano Lett., 2005, 5, 2573–2577 CrossRef CAS PubMed.
- R. W. Tarnuzzer, J. Colon, S. Patil and S. Seal, Nano Lett., 2005, 5, 2573–2577 CrossRef CAS PubMed.
- L. Cao, Z. Feng, R. Guo, Q. Tian, W. Wang, X. Rong, M. Zhou, C. Cheng, T. Ma and D. Deng, Mater. Horiz., 2023, 10, 1342–1353 RSC.
- Y. Qu, Z. Li, W. Chen, Y. Lin, T. Yuan, Z. Yang, C. Zhao, J. Wang, C. Zhao, X. Wang, F. Zhou, Z. Zhuang, Y. Wu and Y. Li, Nat. Catal., 2018, 1, 781–786 CrossRef CAS.
- F. Tang, L. Li and D. Chen, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- F. Erogbogbo, K.-T. Yong, R. Hu, W.-C. Law, H. Ding, C.-W. Chang, P. N. Prasad and M. T. Swihart, ACS Nano, 2010, 4, 5131–5138 CrossRef CAS PubMed.
- L. Sun, X. Wang, F. Gong, K. Yin, W. Zhu, N. Yang, S. Bai, F. Liao, M. Shao and L. Cheng, Theranostics, 2021, 11, 9234–9242 CrossRef CAS PubMed.
- R. Que, M. Shao, S. Wang, D. D. Ma and S. T. Lee, Nano Lett., 2011, 11, 4870–4873 Search PubMed.
- G. Gollavelli and Y.-C. Ling, Biomaterials, 2012, 33, 2532–2545 CrossRef CAS PubMed.
- H. Zhao, J. Duan, Y. Xiao, G. Tang, C. Wu, Y. Zhang, Z. Liu and W. Xue, Chem. Mater., 2018, 30, 3438–3453 CrossRef CAS.
- X. Pan, Z. Huang, J. Guo, Q. Wu, C. Wang, H. Zhang, J. Zhang and H. Liu, Adv. Mater., 2024, 36, 2400142 Search PubMed.
- A. Yildirim, R. Chattaraj, N. T. Blum, G. M. Goldscheitter and A. P. Goodwin, Adv. Healthcare Mater., 2016, 5, 1290–1298 Search PubMed.
- A. N. Sukumar, P. D. Duraisamy, P. M. S. Paul, P. Gopalan and A. Angamuthu, J. Biomol. Struct. Dyn., 2024, 1–14 CrossRef PubMed.
- Y. Iwase, K. Nishi, J. Fujimori, T. Fukai, N. Yumita, T. Ikeda, F.-S. Chen, Y. Momose and S.-I. Umemura, Jpn. J. Appl. Phys., 2016, 55, 07KF02 CrossRef.
- W. Ren, H. Wang, Q. Chang, N. Li, J. Yang and S. Hu, Carbon, 2021, 184, 102–108 Search PubMed.
- S. Yang, X. Wang, P. He, A. Xu, G. Wang, J. Duan, Y. Shi and G. Ding, Small, 2021, 17, 2004867 CrossRef CAS PubMed.
- L. Sun, Y. Cao, Z. Lu, P. Ding, Z. Wang, F. Ma, Z. Wang and R. Pei, Nano Today, 2022, 43, 101434 CrossRef CAS.
- M. Cheng, Y. Liu, Q. You, Z. Lei, J. Ji, F. Zhang, W.-F. Dong and L. Li, Adv. Sci., 2024, 11, 2404230 CrossRef CAS PubMed.
- Q. Zhang, J. Liang, S. L. J. Yun, K. Liang, D. Yang and Z. Gu, Biomater. Sci., 2020, 8, 4129–4146 RSC.
Footnote |
| † C. Liu, X. Liu, L. Zha and Y. Zhang contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |


