Open Access Article
Open Access ArticleBrønsted acid-catalyzed two-component tandem condensation and cycloisomerization to 6(2H)-isoquinolinones†
Fang Yang‡
,
Lin-Lin Wang‡,
Yu-Hua Liu,
Ke-Xin Wu,
Zi-Sheng Chen * and
Kegong Ji
* and
Kegong Ji *
*
College of Chemistry and Pharmacy, Northwest A&F University, Shaanxi Key Laboratory of Natural Products & Chemical Biology, 3 Taicheng Road, Yangling, 712100, Shaanxi, P. R. China. E-mail: jikegong@nwsuaf.edu.cn
First published on 24th March 2025
Abstract
An attractive Brønsted acid-catalyzed two-component reaction of 2-alkynyl-4-hydroxybenzaldehydes 1 and primary amines 2 to various 6(2H)-isoquinolinones 3 has been developed. This catalytic system realized an efficient tandem condensation and cycloisomerization reaction to 6(2H)-isoquinolinones 3 in good to excellent yields via a one-pot synthesis, in which two different kinds of C–N bonds were constructed in a straightforward manner. Remarkably, the reaction tolerated various aliphatic, aryl-substituted amines, including chiral amino alcohols and amino acids. The practicality of this approach rendered it a viable alternative for the construction of various 6(2H)-isoquinolinones.
Introduction
Isoquinolinones and their derivatives,1 like 6(2H)-isoquinolinones and natural nitrogenated azaphilone products, bearing a highly nitrogenated pyridinoquinone bicyclic core, have continuously aroused considerable attention owing to their structural diversity and significant biological activities (Fig. 1).2,3 As a class of alkaloids, isoquinolinone derivatives have antiplasmodial, anticancer, anti-MRSA, antimicrobial, antiviral, and cytotoxicity activities and other functional properties.2a,b,4–8As a privileged core structure, the traditional strategies for the synthesis of 6(2H)-isoquinolinones are achieved through the following two pathways, as shown in Scheme 1. (a) The conversion strategies based on 6-hydroxyisochromenylium A as the precursor. Normally, the transformation of 6-hydroxyisochromenylium is initiated by the addition of primary amines under basic conditions and followed by isomerizations to 6(2H)-isoquinolinones. This strategy was applied by the Thamyongkit group in the synthesis of alkaloid Cassiarin B from barakol in four steps with a 16.6% total yield (Scheme 1a).2c Alternatively, Cassiarin B could also be synthesized from 8-chromenone B. In 2008, Yao and co-workers reported a silver-catalyzed 6-exo-dig cycloisomerization to 8-chromenone B in 75% yield under acidic and harsh conditions, which was directly converted to Cassiarin B in a moderate yield, as shown in Scheme 1b.2a (b) The other conversion strategy was based on 6-isoquinolinol as the nucleophilic reagent. The transformation of 6-isoquinolinol was accessed via the selective activation of a nitrogen atom by highly active electrophilic reagents to realize the C–N bond formation and isomerization to 6(2H)-isoquinolinone, as shown in Scheme 1c. You and co-workers have reported an Ir-catalyzed enantiodivergent synthesis of chiral 6(2H)-isoquinolinones 3aa from 6-isoquinolinol.2d However, these strategies also need more steps, harsh conditions or noble metals, which demands more efforts in exploring more efficient strategies in the synthesis of 6(2H)-isoquinolinones.
2-Alkynylbenzaldehyde, as a useful precursor, was successfully applied in the synthesis of many nature products via the isochromenylium salt intermediate.3a,9 For a more useful application of 2-alkynylbenzaldehyde and the development of an efficient approach to 6(2H)-isoquinolinones, as shown in Scheme 1d, our design anticipated that with 2-alkynyl-4-hydroxybenzaldehydes 1 and primary amines 2 as substrates, a novel 6-hydroxyisoquinolin-2-ium salt intermediate could be selectively generated under acidic conditions,10 which was subsequently isomerized to 6(2H)-isoquinolinones 3. As part of our ongoing work in the field of alkyne chemistry,11 we reported herein a Brønsted acid-catalyzed two-component tandem condensation and cycloisomerization to 6(2H)-isoquinolinones in one-pot.
Results and discussion
At the outset, we employed 2-alkynyl-4-hydroxybenzaldehyde (1a) and aniline (2a) as the template substrates for condition optimization as shown in Table 1. Initially, CF3COOH (20 mol%) was chosen as the catalyst, the reaction was carried out in 1,2-dichloroethane (DCE) with MgSO4 (3.4 equiv.) as an additive at 80 °C for 24 h. To our delight, the desired product 3a was indeed formed in 76% isolated yield (Table 1, entry 1). The structure of product 3a was unambiguously assigned by X-ray crystallography (Fig. 2. CCDC 2081746). An attempt to optimize this reaction in the absence of the catalyst was fruitless (see the ESI†). Solvent effect was also considered, toluene was found to be the most effective solvent and afford 3a in 82% yield (entries 2–6). Other catalysts such as CH3SO3H, HCl·Et2O, HOTf and CH3COOH were also investigated, but no superior results were obtained even after a longer time (entries 7–10, respectively). The catalyst loading was also studied, it was found that 10 mol% of CF3COOH gave a better yield of 3a (entry 11). Furthermore, the equivalent of aniline 2a was also investigated, it was found that aniline 2a (1.1 equivalent) gave the optimal yield of 3a in 92% (entry 13). Other additive, like 5 Å molecular sieve, was also considered, but no better yields were observed (entry 15, for more details, see the ESI†).| Entry | 2a (equiv.) | Catalyst (mol%) | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: The reaction was carried out in a tube in the presence of 1a (0.2 mmol) and 2a with 3.4 equivalent of MgSO4 at 80 °C for 24 h; [1a] = 0.1 M.b Isolated yields.c Instead of MgSO4, 5 Å molecular sieve (100 mg) as additive was added. | ||||
| 1 | 1.0 | CF3COOH (20) | DCE | 76 |
| 2 | 1.0 | CF3COOH (20) | 1,4-Dioxane | 65 |
| 3 | 1.0 | CF3COOH (20) | DMSO | 66 |
| 4 | 1.0 | CF3COOH (20) | DMF | 73 |
| 5 | 1.0 | CF3COOH (20) | Toluene | 82 |
| 6 | 1.0 | CF3COOH (20) | PhCl | 79 |
| 7 | 1.0 | CH3SO3H (20) | Toluene | 78 |
| 8 | 1.0 | HCl·Et2O (20) | Toluene | 71 |
| 9 | 1.0 | HOTf (20) | Toluene | 64 |
| 10 | 1.0 | CH3COOH (20) | Toluene | 72 |
| 11 | 1.0 | CF3COOH (10) | Toluene | 83 |
| 12 | 1.0 | CF3COOH (5) | Toluene | 79 |
| 13 | 1.1 | CF3COOH (10) | Toluene | 92 |
| 14 | 1.3 | CF3COOH (10) | Toluene | 90 |
| 15c | 1.1 | CF3COOH (10) | Toluene | 55 |
With the optimal reaction conditions in hand (Table 1, entry 13), the reaction scope was investigated. As shown in Table 2, a broad range of primary amines 2b–2s could be utilized in this tandem condensation and cycloisomerization to 6(2H)-isoquinolinones 3b–3s with 2-alkynyl-4-hydroxybenzaldehyde 1a. Aryl-substituted primary amines, like 2b–2h, upon installation both electron-withdrawing groups (2b–2d) and electron-donating groups (2e–2h) on aryl ring, worked as expected to give 3b–3h in good to excellent yields. The aryl amines with more steric hindrance, like 2, 6-dimethylaniline 2g, proceeded smoothly to provide corresponding product 3g in 94% yield. Furthermore, the reaction was also tolerated different aliphatic-substituted primary amines. Substrates 2i–2o possessing different aliphatic substituted R groups, such as benzyl, β-substituted ethyl, isopropyl and cyclopropyl groups, proceeded smoothly to give 3i–3o in 57–83% yields. Interestingly, primary amines, like chiral amino alcohols 2p–2q with two nucleophilic sites, also worked as expected to give the desired products 3p–3q in 38% and 41% yields, respectively. However, chiral amino acids, like 2s–2r, almost gave no desired products under the standard conditions, which implied that the relative low solubility of substrates in toluene and multi-nucleophilic sites of amino acids may affect the overall yields of the transformation. To our delight, chiral amino acids, like 2s–2r, proceeded smoothly to give desired products 3s–3r in 71% and 51% yields, respectively, by employing acetic acid as the solvent.
Moreover, for more synthetically useful transformations, various 2-alkynyl-4-hydroxybenzaldehydes, like 1t–1ab, possessing different R1 substitutions were also investigated under the standard conditions and gave the desired 3t–3ab, respectively, in good to excellent yields, as shown in Table 3. The reaction was tolerant of various aliphatic and aromatic R1 groups. Substrates 1u–1w, by installing different substituents at the end of the alkyl chain, such as Cl, OH and OBn group, were readily tolerated and afforded the corresponding products 3u–3w in moderate to good yields (61–87%). Substrate 1x, with more steric hindrance R1 group, proceeded smoothly to afford the desired product 3x in a moderate yield. Moreover, 2-alkynyl-4-hydroxybenzaldehydes 1y–1ab, by installing different electron-withdrawing and electron-donating aryl R1 groups, worked smoothly to provide corresponding products 3y–3ab in excellent yields (up to 96% yield).
To demonstrate the synthetic utility of this method, 2-alkynyl-4-hydroxybenzaldehyde 1a was reacted at a gram scale to afford the desired product 3a in 91% yield (Scheme 2). Furthermore, this approach was also successfully applied in the synthesis more useful 8-hydroxyl-6(2H)-isoquinolinones 3ac–3ae in good to excellent yields, which can be easily modified to construct natural azaphilone scaffolds12 (Scheme 2).
In order to gather additional experimental evidence for the mechanism, the control experiments were conducted, as shown in Scheme 3. 2-Alkynyl-4-hydroxybenzaldehyde 1a was carried out under the standard conditions without primary amines 2, no desired 3-phenyl-6H-isochromen-6-one 4 was observed. However, when the substrate 3-(phenylethynyl)-4-((phenylimino)methyl)phenol 1a′ was investigated under the standard conditions, the desired 6(2H)-isoquinolinone 3a was obtained in 80% yield, which indicated that the 6-hydroxyisoquinolin-2-ium might be the real intermediate of this transformations.
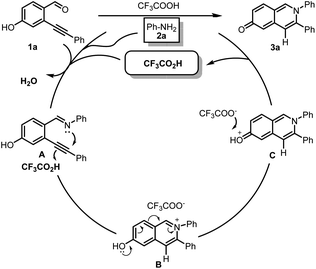
On the basis of the above observations and literature reported,1c we proposed the following plausible mechanism for this transformation (Scheme 4). Firstly, the substrates 2-alkynyl-4-hydroxybenzaldehyde 1a and aniline 2a are condensed and dehydrated to form imine A in acidic condition. Secondly, the proton (H+) generated from CF3COOH selectively coordinates with the C–C triple bonds of imine A. Subsequently, the nitrogen of imine attacks the activated C–C triple bonds via a 6-endo-dig cyclization1c to afford 6-hydroxyisoquinolinium intermediate B, which is isomerized to (2,3-diphenylisoquinolin-6(2H)-ylidene)oxonium C. Finally, the proton on oxonium intermediate C is trapped by trifluoroacetate to give final product 3a and releases CF3COOH for next catalytic cycle.
Conclusions
In summary, we have developed a novel one-pot strategy to construct 6(2H)-isoquinolinones 3 from 2-alkynyl-4-hydroxybenzaldehydes 1 and primary amines 2 via a Brønsted acid-catalyzed tandem condensation and cycloisomerization reaction. This approach provides a facile access to various 6(2H)-isoquinolinones 3 in good to excellent yields, including chiral 6(2H)-isoquinolinones. This protocol tolerates various commercially available materials, such as aliphatic, aryl-substituted amines, including chiral amino alcohols and amino acids. The mild metal-free conditions, atom economy and gram scale application of the reaction render the present method attractive for future applications.Data availability
Data available within the article or its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for the financial support from Natural Science Foundation of Shaanxi province (2023-JC-QN-0139) and the National Natural Science Foundation of China (NSF-22171228).Notes and references
- (a) P. Pigeon and B. Decroix, A New Access to Isoindolo[2,1-b] [2,4]benzodiazepines through an N-Acyliminium Ion-Amide Cyclization, Tetrahedron Lett., 1997, 38, 2985–2988 CrossRef CAS; (b) S. Dhanasekaran, A. Suneja, V. Bisai and V. K. Singh, Approach to Isoindolinones, Isoquinolinones, and THIQs via Lewis Acid-Catalyzed Domino Strecker-Lactamization/Alkylations, Org. Lett., 2016, 18, 634–637 CrossRef CAS PubMed; (c) P. Fernández, F. J. Fañanás and F. Rodríguez, Nitrogenated Azaphilone Derivatives through a Silver-Catalysed Reaction of Imines from ortho-Alkynylbenzaldehydes, Chem.–Eur. J., 2017, 23, 3002–3006 CrossRef PubMed; (d) W. Chen, R. Chen, Q. Liu, Y. He, K. He, X. Ding, L. Kang, X. Guo, N. Xie, Y. Zhou, Y. Lu, R.-J. Cox, I. Molnár, M. Li, Y. Shao and F. Chen, Orange, red, yellow: biosynthesis of azaphilone pigments in Monascus fungi, Chem. Sci., 2017, 8, 4917–4925 RSC.
- (a) Y.-S. Yao and Z.-J. Yao, Biomimetic Total Syntheses of Cassiarins A and B, J. Org. Chem., 2008, 73, 5221–5225 CrossRef CAS PubMed; (b) E. Wenkert, J. S. Pyrek, S. Uesato and Y. D. Vankar, A Synthesis of (±)-Yohimbine, J. Am. Chem. Soc., 1982, 104, 2244–2246 CrossRef CAS; (c) S. Kanputhorn, A. Petsom and P. Thamyongkit, Transformation of barakol into cassiarins A, B, and their derivatives, Tetrahedron, 2010, 66, 7539–7543 CrossRef CAS; (d) H.-F. Tu, P. Yang, Z.-H. Lin, C. Zheng and S.-L. You, Time-dependent enantio divergent synthesis via sequential kinetic resolution, Nat. Chem., 2020, 12, 838–844 Search PubMed.
- (a) J. M. Gao, S. X. Yang and J. C. Qin, Azaphilones: Chemistry and Biology, Chem. Rev., 2013, 113, 4755–4811 Search PubMed; (b) C. Chen, H. Tao, W. Chen, B. Yang, X. Zhou, X. Luo and Y. Liu, Recent advances in the chemistry and biology of structurally diverse azaphilones from 2012 to 2019, RSC Adv., 2020, 10, 10197–10220 CAS; (c) C. Pavesi, V. Flon, S. Mann, S. Leleu, S. Prado and X. Franck, Biosynthesis of azaphilones: a review, Nat. Prod. Rep., 2021, 38, 1058–1071 Search PubMed; (d) N. Osmanova, W. Schultze and N. Ayoub, Azaphilones: a class of fungal metabolites with diverse biological activities, Phytochem. Rev., 2010, 9, 315–342 CAS.
- (a) Y. Zheng, Y. Xin, X. Shi and Y. Guo, Cytotoxicity of Monascus Pigments and Their Derivatives to Human Cancer Cells, J. Agric. Food Chem., 2010, 58, 9523–9528 Search PubMed; (b) W. Wang, J. Yang, Y.-Y. Liao, G. Cheng, J. Chen, X.-D. Cheng, J.-J. Qin and Z. Shao, Cytotoxic Nitrogenated Azaphilones from the Deep-Sea-Derived Fungus Chaetomium globosum MP4-S01-7, J. Nat. Prod., 2020, 83, 1157–1166 Search PubMed.
- M. Chen, N. X. Shen, Z. Q. Chen, F. M. Zhang and Y. Chen, Penicilones A–D, Anti-MRSA Azaphilones from the Marine-Derived Fungus Penicillium janthinellum HK1-6, J. Nat. Prod., 2017, 80, 1081–1086 CrossRef CAS PubMed.
- (a) S. A. S. Mapari, U. Thrane and A. S. Meyer, Trends Biotechnol., 2010, 28, 300–307 Search PubMed; (b) N. Osmanova, W. Schultze and N. Ayoub, Phytochem. Rev., 2010, 9, 315–342 CrossRef CAS; (c) H. Hussain, A. M. Al-Sadi, B. Schulz, M. Steinert, A. Khan, I. R. Green and I. Ahmed, Future Med. Chem., 2017, 9, 1631–1648 CrossRef CAS PubMed.
- (a) J.-L. Tang, Z.-Y. Zhou, T. Yang, C. Yao, L.-W. Wu and G.-Y. Li, Azaphilone Alkaloids with Anti-inflammatory Activity from Fungus Penicillium sclerotiorum cib-411, J. Agric. Food Chem., 2019, 67, 2175–2182 CrossRef CAS PubMed; (b) K. Becker, S. Pfütze, E. Kuhnert, R. J. Cox, M. Stadler and F. Surup, Hybridorubrins A–D: Azaphilone Heterodimers from Stromata of Hypoxylon fragiforme and Insights into the Biosynthetic Machinery for Azaphilone Diversification, Chem.–Eur. J., 2021, 27, 1438–1450 CrossRef CAS.
- S. A. S. Mapari, U. Thrane and A. S. Meyer, Fungal polyketide azaphilone pigments as future natural food colorants?, Trends Biotechnol., 2010, 28, 300–307 CrossRef CAS PubMed.
- For selected references on isochromenylium chemistry: (a) B. Yu, Gold(I)-Catalyzed Glycosylation with Glycosyl o-Alkynylbenzoates as Donors, Acc. Chem. Res., 2018, 51, 507–516 CrossRef CAS PubMed; (b) Z.-L. Hu, W.-J. Qian, S. Wang, S. Wang and Z.-J. Yao, Construction of Multiring Frameworks by Metal-Free Cascade Reactions of Stable Isochromenylium Tetrafluoroborate, J. Org. Chem., 2009, 74, 8787–8793 CrossRef CAS PubMed; (c) T. Miao, Z.-Y. Tian, Y.-M. He, F. Chen, Y. Chen, Z.-X. Yu and Q.-H. Fan, Asymmetric Hydrogenation of In Situ Generated Isochromenylium Intermediates by Copper/Ruthenium Tandem Catalysis, Angew. Chem., Int. Ed., 2017, 56, 4135–4139 CrossRef CAS PubMed; (d) J. Zhu, A. R. Germain and J. A. Porco, Synthesis of Azaphilones and Related Molecules by Employing Cycloisomerization of o-Alkynylbenzaldehydes, Angew. Chem., Int. Ed., 2004, 43, 1239–1243 CrossRef CAS PubMed.
- T. Wang, Y. Wang, D. Feng, M. Wang, X. Yang and Z.-J. Yao, Isochromenylium/Isoquinolinium-Mediated One-Pot Annulation to Hexahydropyrazinoisoquinolines. Synthesis of Quinocarcinol, Org. Lett., 2023, 25, 8803–8808 CrossRef CAS PubMed.
- For selected work: (a) W.-S. Chen, F. Yang, T. Wang, G.-Q. Zhang, Y. Wei, M.-H. Wang, Z.-S. Chen and K. Ji, Chemoselective Transformations of Amides: An Approach to Quinolones from β-Amido Ynones, Org. Lett., 2023, 25, 5762–5767 CrossRef CAS; (b) G.-Q. Zhang, F. Yang, W.-S. Chen, X. Zhao, T. Wang, Z.-S. Chen and K. Ji, A practical and economic route for regioselective cyclization of β-phenoxyl ynones to flavonoid derivatives, Green Chem., 2024, 26, 8711–8717 RSC.
- (a) R. C. Clark, S. Y. Lee and D. L. Boger, Total Synthesis of Chlorofusin, Its Seven Chromophore Diastereomers, and Key Partial Structures, J. Am. Chem. Soc., 2008, 130, 12355–12369 CrossRef CAS PubMed; (b) J. Zhu, N. P. Grigoriadis, J. P. Lee and J. A. Porco, Synthesis of the Azaphilones Using Copper-Mediated Enantioselective Oxidative Dearomatization, J. Am. Chem. Soc., 2005, 127, 9342–9343 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2081746. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra01267h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |