Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photo-induced-photo-catalytic SERS with silver-deposited TiO2 nanorods for ultrasensitive and sustainable detection of low Raman cross-section molecules
Quan-Doan Mai *a,
Dang Thi Hanh Tranga,
Ngo Thi Loana,
Nhu Hoa Tran Thi
*a,
Dang Thi Hanh Tranga,
Ngo Thi Loana,
Nhu Hoa Tran Thi b,
Ong Van Hoangc,
Ta Ngoc Bachd,
Nguyen Quang Hoae,
Anh-Tuan Phamaf and
Anh-Tuan Le
b,
Ong Van Hoangc,
Ta Ngoc Bachd,
Nguyen Quang Hoae,
Anh-Tuan Phamaf and
Anh-Tuan Le *a
*a
aPhenikaa University Nano Institute (PHENA), Phenikaa University, Hanoi 12116, Vietnam. E-mail: doan.maiquan@phenikaa-uni.edu.vn; tuan.leanh@phenikaa-uni.edu.vn
bFaculty of Materials Science and Technology, University of Science, Ho Chi Minh City, Vietnam
cUniversity of Transport Technology, Trieu Khuc, Thanh Xuan District, Hanoi, Vietnam
dInstitute of Materials Science (IMS), Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Hanoi 10000, Vietnam
eFaculty of Physics, VNU University of Science, Vietnam National University, Hanoi, Thanh Xuan, Hanoi, Vietnam
fFaculty of Biotechnology, Chemistry and Environmental Engineering, Hanoi 12116, Vietnam
First published on 24th April 2025
Abstract
Surface-enhanced Raman spectroscopy (SERS) offers significant advantages, including label-free, non-invasive analysis and ultrasensitivity down to the single-molecule level, making it widely applicable in analytical chemistry and biology. However, its effectiveness is limited when detecting molecules with inherently low Raman scattering cross-sections, restricting its broader applications. In this study, we apply the photo-induced-photo-catalytic SERS (PI-PC SERS) technique, utilizing an Ag-deposited TiO2 nanorod (Ag/TiO2 NR) substrate to overcome this limitation. The PI-PC SERS technique combines two optoelectronic effects: photo-induced enhanced Raman scattering (PIERS) and the photocatalytic activity of the metal/semiconductor substrate. PIERS amplifies Raman signals beyond normal SERS, while the photocatalytic effect facilitates the removal of residual analytes. The PI-PC SERS process follows three sequential irradiation steps: (i) pre-irradiation with 365 nm UV light to activate PIERS, (ii) laser excitation at 785 nm to capture the enhanced Raman signal, and (iii) post-irradiation with 365 nm UV light to trigger photocatalytic degradation. Two low Raman cross-section molecules, 4-nitrophenol (a widely used pesticide) and urea (an important biomarker), were selected to evaluate the performance of the PI-PC SERS technique on the Ag/TiO2 NR substrate. The results demonstrated that PI-PC SERS not only enhanced detection sensitivity tenfold compared to normal SERS but also enabled self-cleaning by efficiently removing residual analytes after measurement, ensuring substrate reusability. These findings pave the way for advancing SERS-based techniques for detecting low Raman cross-section molecules while broadening their potential applications in chemical and biological sensing fields.
1. Introduction
Surface-enhanced Raman spectroscopy (SERS) sensors, known for their unique advantages such as ultrasensitivity down to the single-molecule level, label-free, and non-invasive detection, have attracted significant attention and found widespread applications in analytical chemistry (e.g., food safety, water quality) and bioanalysis (e.g., early diagnostics, pharmaceutical quality).1–5 The recent advances in nanoscience have further propelled SERS research, as purposefully designed nanostructures effectively exploit their unique properties to achieve significantly enhanced SERS signals while maintaining reliable performance.4,6–8 These nanostructures, ranging from simple colloidal and self-assembled metallic nanoparticles to more complex systems like nanocomposites, and even novel techniques based on the optical, electronic, and thermal properties of nanomaterials, have made notable strides in advancing SERS applications.9–17 Despite its remarkable sensitivity and widespread use, SERS faces an inherent limitation when detecting molecules with low Raman cross-sections. These molecules, due to their intrinsic low scattering efficiency, produce weak SERS signals, making detection challenging.18,19 The Raman cross-section, typically expressed in cm2 sr−1 per molecule, defines the probability of a photon being scattered inelastically by a given molecule.20 High-scattering analytes such as rhodamine 6G possess high Raman cross-sections on the order of 10−25 cm2 sr−1, yielding strong SERS signals that facilitate easy detection.21,22 Conversely, small or weakly polar molecules such as gases, urea, or 4-nitrophenol exhibit much lower Raman cross-sections (∼10−30 to 10−27 cm2 sr−1), often resulting in poor signal enhancement even under optimized SERS conditions.23,24 As a consequence, detection limits for these targets generally remain in the micromolar range (10−3 to 10−4 M), limiting their practical utility. Nonetheless, many low Raman cross-section molecules hold substantial value across various domains such as food safety and healthcare diagnostics. Thus, developing strategies to enhance SERS detection for these molecules could significantly expand the potential applications and impact of this powerful sensing platform.Among the nanomaterials developed for SERS, metal/semiconductor structures have received significant attention due to the synergistic integration of two key enhancement mechanisms: electromagnetic (EM) and chemical (CM), resulting in significantly enhanced overall SERS performance.25–27 In addition, several intriguing physical and chemical effects in these structures have been leveraged to improve sensor efficacy, offering advantages such as enhanced stability and cost-effectiveness.16,27,28 In 2016, Ben-Jaber et al. discovered the photo-induced enhanced Raman scattering (PIERS) effect on SERS substrates based on metal/semiconductor structures by pre-irradiating SERS substrates composed of gold (Au) nanoparticles on a TiO2 surface, leading to a remarkable increase in sensing performance compared to conventional SERS for a wide range of molecular targets.16 Since then, this technique has been explored and applied to various metal/semiconductor-based SERS substrates and diverse target molecules, resulting in even further optimization of sensing performance.29–31 The enhancement effect of PIERS, as established in prior studies, may have the potential to improve the SERS signal of low Raman cross-section molecules – where normal SERS techniques often fall short. In 2022, by implementing PIERS on Ag/TiO2 nanoparticle substrates (with TiO2 in particle shape and anatase phase), we achieved significantly improved detection of urea and 4-nitrophenol as representative low Raman cross-section molecules.32 The system reached a detection limit as low as 10−6 M, representing an enhancement of two to three orders of magnitude over normal SERS. Beyond that, in light of growing real-world demands, there is increasing interest in the development of SERS substrates that not only provide high sensitivity but also offer additional features such as reusability, aiming to reduce operational costs and improve sustainability. Most recently, we introduced the photo-induced-photo-catalytic (PI-PC SERS) technique, which combines the PIERS effect with photocatalysis in metal/semiconductor nanostructures to achieve superior sensing performance and reusability compared to normal SERS.33 This technique has been successfully applied to Raman-sensitive molecules, such as methylene blue (a dye) and thiram (a pesticide). Applying the PI-PC SERS technique to low Raman cross-section molecules may offer not only the advantage of enhancing their weak SERS signals to improve sensitivity, but also the potential to meet other practical demands such as reducing operational costs and improving sustainability. Moreover, since the semiconductor component plays a central role in both PIERS and photocatalysis, variations in its properties – such as morphology and crystal phase – can significantly influence the overall performance of the PI-PC SERS technique.
In this study, we developed Ag-deposited TiO2 nanorods (Ag/TiO2 NRs – with TiO2 in rod shape and rutile phase) as metal (Ag)/semiconductor (TiO2) SERS substrates and applied the PI-PC SERS technique to improve the sensing performance for low Raman cross-section molecules, namely 4-nitrophenol (4-NP, a high-priority toxic pollutant and widely used pesticide, important in food safety) and urea (a biomarker for early diagnosis, relevant to kidney and cardiovascular diseases), compared to normal SERS. The results showed that the PI-PC SERS technique significantly outperforms normal SERS on the Ag/TiO2 NR substrate for both 4-nitrophenol and urea. Specifically, for 4-nitrophenol, PI-PC SERS achieved a detection limit of 6.8 × 10−7 M, approximately 10 times better than the case of normal SERS with a detection limit of 8.1 × 10−6 M. A similar result was observed for urea, with PI-PC SERS reaching a detection limit of 6.9 × 10−7 M, compared to only 8.9 × 10−6 M for normal SERS. Furthermore, the photocatalysis of the Ag/TiO2 NR substrate in PI-PC SERS was demonstrated, showing that after the sensing process, residual 4-NP and urea molecules on surface of the Ag/TiO2 NRs were efficiently removed via photocatalytic degradation, providing self-cleaning and reusability, thus ensuring cost-efficiency and sustainability. The enhanced sensing capability for low Raman cross-section molecules makes the PI-PC SERS technique a promising approach for applications in food safety, early diagnosis, and other fields involving low Raman cross-section molecules.
2. Materials and methods
2.1. Materials
The precursors, including silver nitrate (AgNO3, ≥99.0%), sodium borohydride (NaBH4, 99%), ethanol (C2H5OH, 98%), and cetyltrimethylammonium bromide (C19H42NBr, 99.9%), were procured from Shanghai Chemical Reagent and utilized without any additional purification. The low Raman cross-section molecules, namely 4-nitrophenol (C6H5NO3, ≥99%) and urea (CO(NH2)2, 99%), were obtained from Sigma-Aldrich. Titanium foils (99.99% purity) with dimensions of 100 mm × 20 mm × 1 mm were used in the experiments. All procedures were carried out using double-distilled water.2.2. Synthesis of Ag-deposited TiO2 nanorods material and their characterizations
Ag-deposited TiO2 nanorods (Ag/TiO2 NRs) were fabricated using a two-step process. Initially, TiO2 nanorods were formed by an electrochemical method, followed by the deposition of Ag nanoparticles onto the TiO2 substrate through chemical reduction. The electrochemical fabrication process of TiO2 nanorods is described in detail in our previous study, with slight modifications in the applied voltage, reaction time, and calcination temperature, which may lead to differences in morphology and crystal phase composition.34 The reaction system consisted of three key components: two parallel titanium electrodes spaced 3 cm apart, a direct current (DC) power supply, and an electrolyte solution in a 200 mL beaker. The electrolyte solution consisted of 0.1 M cetyltrimethylammonium bromide (CTAB) dispersed in 200 mL of distilled water. The TiO2 nanorod formation reaction occurred over 5 hours under a 25 V DC current applied across the titanium electrodes. The resulting bright white solution containing the TiO2 nanorods was dried at 80 °C for 6 hours to obtain TiO2 powder. Subsequently, the TiO2 was annealed at 750 °C for 4 hours to achieve a crystalline structure. After the thermal treatment, the resulting crystalline TiO2 powder appeared bright white. The Ag/TiO2 NR material was formed through the reduction of AgNO3 in the presence of TiO2. First, 100 mg of crystalline TiO2 nanorods were then dispersed in 100 mL of distilled water under ultrasonic agitation for 15 minutes and then stirred uniformly. A calculated amount of 158 mg of AgNO3, dispersed in 20 mL of distilled water, was added and stirred for 1 hour to ensure optimal interaction between the Ag+ ions and TiO2. Afterward, a 10 mL solution containing 35 mg of NaBH4 was gradually introduced to reduce the Ag+ ions to Ag nanoparticles. During the addition of NaBH4, the solution color changed from bright white to brown, indicating the reduction of Ag+ ions into Ag nanomaterial. After the addition of NaBH4, the reaction was allowed to proceed for an additional hour to ensure complete reduction. The final resulting solution containing Ag/TiO2 NRs was used directly for subsequent experiments. The Ag to TiO2 ratio was selected as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as this ratio has been shown to provide optimal SERS enhancement in previous studies.32 The morphology and structure of the Ag/TiO2 NRs were analyzed using a field emission scanning electron microscopy (FE-SEM, Hitachi S-4800). The samples were prepared by drop-casting the Ag/TiO2 NR suspension onto a clean silicon wafer and drying under ambient conditions. Imaging was performed under high vacuum at an accelerating voltage of 5 kV, providing high-resolution surface morphology observations. Crystalline properties of Ag/TiO2 NRs were investigated by X-ray diffraction (XRD) analysis was performed using a Bruker D5005 diffractometer equipped with a Cu Kα radiation source (λ = 1.5406 Å). The measurements were conducted at an operating voltage of 40 kV and a current of 30 mA. Diffraction patterns were recorded over a 2θ range of 20° to 80°, with a step size of 0.05° and a counting time of 1 second per step. Prior to analysis, the sample were prepared by drop-casting the Ag/TiO2 NR suspension onto a clean silicon wafer, followed by drying under ambient conditions. The chemical properties and surface interactions of the Ag/TiO2 NRs were examined using Raman spectroscopy with a MacroRaman™ Raman spectrometer (Horiba) using a 785 nm laser for excitation. The spectra were collected in the range of 200 cm−1 to 2000 cm−1 with a spectral resolution of 1 cm−1. The laser power was set to 10 mW, and the exposure time was set to 30 seconds for signal acquisition.
1, as this ratio has been shown to provide optimal SERS enhancement in previous studies.32 The morphology and structure of the Ag/TiO2 NRs were analyzed using a field emission scanning electron microscopy (FE-SEM, Hitachi S-4800). The samples were prepared by drop-casting the Ag/TiO2 NR suspension onto a clean silicon wafer and drying under ambient conditions. Imaging was performed under high vacuum at an accelerating voltage of 5 kV, providing high-resolution surface morphology observations. Crystalline properties of Ag/TiO2 NRs were investigated by X-ray diffraction (XRD) analysis was performed using a Bruker D5005 diffractometer equipped with a Cu Kα radiation source (λ = 1.5406 Å). The measurements were conducted at an operating voltage of 40 kV and a current of 30 mA. Diffraction patterns were recorded over a 2θ range of 20° to 80°, with a step size of 0.05° and a counting time of 1 second per step. Prior to analysis, the sample were prepared by drop-casting the Ag/TiO2 NR suspension onto a clean silicon wafer, followed by drying under ambient conditions. The chemical properties and surface interactions of the Ag/TiO2 NRs were examined using Raman spectroscopy with a MacroRaman™ Raman spectrometer (Horiba) using a 785 nm laser for excitation. The spectra were collected in the range of 200 cm−1 to 2000 cm−1 with a spectral resolution of 1 cm−1. The laser power was set to 10 mW, and the exposure time was set to 30 seconds for signal acquisition.
2.3. Substrate preparation, SERS and PI-PC SERS measurements
The SERS substrate was prepared by depositing the material onto an aluminum (Al) base with dimensions of 1 cm × 1 cm × 0.1 cm, featuring a circular hole of 0.2 cm in diameter on the surface where the material was deposited. The Al base was first cleaned with ethanol and then allowed to dry naturally at room temperature (RT). A suspension of Ag/TiO2 NRs was drop-cast onto the active surface area and allowed to dry at room temperature. Aqueous solutions of 4-NP (ranging from 10−3 M to 5 × 10−7 M) and urea (10−3 M to 5 × 10−7 M) were prepared at different concentrations (the pH of the mixture was 7, as distilled water was used as the solvent and no pH-adjusting agents were added). For each SERS measurement, 5 μL of the analyte solution was deposited onto the prepared substrate and left to dry naturally at room temperature. Raman spectra were collected using a MacroRamamTM spectrometer (Horiba) with a 785 nm laser excitation source. Measurements were performed with a 100× objective lens (numerical aperture: 0.90), while the laser power was maintained at 45 mW at a 45° contact angle. The laser spot had a diffraction-limited diameter of 1.1 μm (calculated as 1.22λ/NA) with a focal depth of 115 nm. Each spectrum was obtained with an exposure time of 10 s and three accumulations, followed by baseline correction to ensure data accuracy.The PI-PC SERS technique was performed as described in detail in our previous study.33 The PIERS effect of the Ag/TiO2 NR SERS substrate was activated by pre-irradiating the substrate with light at a wavelength of 365 nm for 30 minutes. The sensing signal was then collected immediately to ensure optimal retention of the PIERS effect. The self-cleaning capability of the Ag/TiO2 NR SERS substrate was demonstrated by activating the photocatalytic effect of the Ag/TiO2 NRs through post-irradiation of the SERS substrate containing residual analyte, using light at a wavelength of 365 nm. The degradation of the residual analyte molecules on the SERS substrate was monitored in real-time by observing the SERS spectrum and the intensity of their characteristic peaks. Finally, the SERS substrate was deemed reusable when the characteristic peaks of the residual analyte were no longer visible in the SERS spectrum.
2.4. Calculation of limit of detection (LOD)
The LOD value was calculated based on the linear equation established for each technique (SERS, PI-PC SERS) and the Raman signal of the corresponding analyte in its powder form. The LOD is calculated using the following equation:35| LOD = 10[(Yaverage+3SD)/Yaverage−A]/B | (1) |
SD is calculated via the well-known formula:
 | (2) |
3. Results and discussion
3.1. Characterizations of Ag-deposited TiO2 nanorod materials
The morphology of the Ag/TiO2 NR material was investigated using FE-SEM, as shown in Fig. 1a and b. Two distinct shapes were clearly observed: a large rod-like form and smaller spherical particles, which are attributed to the presence of TiO2 and Ag. The rod-shaped structures observed in the FE-SEM images are TiO2, fabricated using an electrochemical method, as demonstrated in our previous work.34 Notably, Ag nanoparticles were uniformly distributed across the TiO2 rods, with no observable aggregation or separation from TiO2 materials. Fig. 1c presents the XRD pattern, confirming the diffraction peaks of TiO2 in the rutile phase at 2θ positions of 28.7, 36.8, 39.3, 41.5, 44.1, and 54.3, corresponding to the (110), (101), (200), (111), (210), and (211) crystal planes (JCPDS-PDF card no. 21-1276). Additionally, diffraction peaks at 2θ values of 32.4, 33.5, 46.9, and 64.5, characteristic of Ag crystal planes (122), (111), (231), and (220) (JCPDS-PDF card no. 04-0783), confirm the formation and presence of Ag nanoparticles in the Ag/TiO2 NR structure. Fig. 1d shows the Raman spectra of the Ag/TiO2 NRs, where two characteristic peaks at 455 cm−1 and 618 cm−1 confirm the presence of rutile TiO2 with Eg and A1g vibrational modes in its crystal structure.36 Moreover, a relatively strong scattering peak at 246 cm−1 is observed, which is attributed to the Ag–O vibration, likely due to the interaction between Ag and the TiO2 surface.32 This suggests a close interaction between Ag and TiO2, which is also supported by the FE-SEM images, where Ag nanoparticles are dispersed across the surface of the TiO2 nanorods. Furthermore, no additional scattering peaks were detected in the Raman spectrum, indicating that the Ag/TiO2 NR material has high purity. Based on these analyses, the Ag/TiO2 NR material was successfully synthesized with Ag nanoparticles decorating the surface of rutile TiO2 nanorods, demonstrating a close interaction between Ag and TiO2.3.2. Application of the PI-PC SERS technique for low Raman cross-section molecules – 4-nitrophenol – a pesticide
4-NP is a commonly used pesticide in agriculture, applied to control a wide range of pests and diseases in crops. As mentioned, 4-NP is a small organic molecule with relatively low polarity compared to highly Raman-active compounds such as rhodamine 6G, which exhibit both strong dipole moments and high Raman cross-sections (∼10−25 cm2 sr−1). In contrast, small molecules like 4-NP typically exhibit Raman cross-sections in the range of 10−30 to 10−27 cm2 sr−1, and are thus generally classified as low Raman cross-section analytes.23,24 While it is true that 4-NP can exhibit enhanced Raman activity under specific resonance conditions – as demonstrated by Jahncke et al., where only the conjugate base of 4-NP in combination with a 405 nm laser produced a resonance-enhanced Raman signal – such conditions are not commonly applicable in real-world sensing scenarios.37 In practical applications, 4-NP is usually present under near-neutral pH conditions, where its conjugate base is not dominant. Moreover, 405 nm laser sources are not commonly used in standard Raman instrumentation due to their high energy, which can cause photodegradation of samples or generate strong fluorescence backgrounds. Therefore, our experiment was designed to closely reflect real-world conditions, utilizing a neutral pH and a low-energy laser source in the near-infrared range (785 nm). Under these conditions, 4-NP remains in a non-resonant state and exhibits a low Raman cross-section. This inherent property can result in a weak scattering probability, leading to low sensing efficiency when using traditional Raman spectroscopy techniques. However, its residue in the environment, particularly in food products, poses significant health risks. Prolonged exposure to 4-NP has been linked to various harmful effects, including potential toxicity to the liver, kidneys, and central nervous system, as well as potential carcinogenic properties.38 Beyond its use as a pesticide, 4-NP also serves as an important intermediate in the production of dyes, pharmaceuticals, and petrochemicals. Its discharge into the environment can lead to serious contamination of water and soil, posing substantial risks to both human health and ecological systems. Therefore, monitoring its presence in food, water, and soil is critical to ensure food safety, environmental monitoring and risk prevention. However, the weak SERS signal of 4-nitrophenol presents a challenge, highlighting the need for improved detection sensitivity.First, the normal SERS experiment was conducted to detect low Raman cross-section molecules for comparing the sensing performance with the PI-PC SERS technique. Fig. 2a briefly illustrates the SERS signal collection from the Ag/TiO2 NR SERS substrate in the normal SERS setup. After depositing the Ag/TiO2 NRs onto the Al substrate, a 4-NP solution at various concentrations was applied to the substrate surface and allowed to air dry at RT. The SERS signal of 4-NP was then recorded and displayed in Fig. 2b. At a high concentration of 10−3 M, distinct scattering peaks at 876, 1120, 1343, and 1598 cm−1, characteristic of the 4-NP molecular structure, were observed. First, the observed peaks were compared with the Raman spectrum of pure 4-NP in powdered form (Fig. 2c). A strong match was observed between the characteristic peaks of the pure powder and those in the SERS spectrum of 4-NP at a concentration of 10−3 M on the Ag/TiO2 NR substrate, confirming the accurate identification of the analyte. These peaks correspond to the bending vibration of the –NO2 group, C–H bending vibration, symmetric stretching of the –NO2 group, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration in the 4-NP molecule.39,40 These characteristic peaks gradually decreased as the concentration of 4-NP was reduced. At 10−5 M (still considered a high concentration for sensitive platforms like SERS), the characteristic peaks remained visible but with significantly reduced intensity, and completely disappeared at 5 × 10−6 M. As expected, the normal SERS technique struggled to detect 4-NP due to its low Raman cross-section. The relationship between 4-NP concentration and the obtained SERS intensity of the characteristic peaks, plotted on a logarithmic scale, is shown in Fig. 3. The 1343 cm−1 peak displayed the best linear correlation (R2 = 0.96), while the peaks at 876, 1120, and 1598 cm−1 yielded R2 values of 0.90, 0.87, and 0.88, respectively. Based on the 1343 cm−1 peak, the linear equation relating concentration and intensity was derived as y = (6.29 ± 0.15) + (0.97 ± 0.03)x, where x and y represent the logarithms of the 4-NP concentration and SERS intensity, respectively. From this equation, the limit of detection (LOD) was calculated to be 8.1 × 10−6 M (detailed LOD determination methods are provided in Section 2.4). With a highly sensitive sensor platform capable of single-molecule detection like SERS, achieving a detection limit of only 10−6 M falls short of expectations. This can be improved by applying new techniques and effectively leveraging other effects of the SERS substrate.
C stretching vibration in the 4-NP molecule.39,40 These characteristic peaks gradually decreased as the concentration of 4-NP was reduced. At 10−5 M (still considered a high concentration for sensitive platforms like SERS), the characteristic peaks remained visible but with significantly reduced intensity, and completely disappeared at 5 × 10−6 M. As expected, the normal SERS technique struggled to detect 4-NP due to its low Raman cross-section. The relationship between 4-NP concentration and the obtained SERS intensity of the characteristic peaks, plotted on a logarithmic scale, is shown in Fig. 3. The 1343 cm−1 peak displayed the best linear correlation (R2 = 0.96), while the peaks at 876, 1120, and 1598 cm−1 yielded R2 values of 0.90, 0.87, and 0.88, respectively. Based on the 1343 cm−1 peak, the linear equation relating concentration and intensity was derived as y = (6.29 ± 0.15) + (0.97 ± 0.03)x, where x and y represent the logarithms of the 4-NP concentration and SERS intensity, respectively. From this equation, the limit of detection (LOD) was calculated to be 8.1 × 10−6 M (detailed LOD determination methods are provided in Section 2.4). With a highly sensitive sensor platform capable of single-molecule detection like SERS, achieving a detection limit of only 10−6 M falls short of expectations. This can be improved by applying new techniques and effectively leveraging other effects of the SERS substrate.
To enhance the detection capability of 4-NP, the PI-PC SERS technique was applied to Ag/TiO2 NRs, leveraging the PIERS effect of this nanostructure. Fig. 4a provides a brief overview of the SERS signal collection process for 4-NP using PI-PC SERS, with a pre-irradiation step of the Ag/TiO2 NR substrate using UV light (365 nm) for 30 minutes. After irradiation is ceased, solutions of 4-NP at different concentrations are applied to the substrate, and the SERS signals are immediately collected. The resulting sensing data is shown in Fig. 4b. At higher concentrations, 10−3 M and 10−4 M, the characteristic peaks for 4-NP are clearly visible with high intensity. Notably, at a concentration of 5 × 10−6 M, where normal SERS would fail to detect 4-NP, the characteristic peaks are still clearly distinguishable. These peaks remain detectable at a concentration of 10−6 M and disappear only at 5 × 10−7 M. This demonstrates that the PI-PC SERS technique applied to Ag/TiO2 NRs, thanks to the PIERS (PI) effect, significantly enhances the detection capability compared to normal SERS. The linear relationship between 4-NP concentration and the PI-PC SERS signal is quantitatively calculated and displayed in Fig. 5. The peak at 1343 cm−1 shows the best linearity, with an R2 value of 0.99, and a wide linear range from 10−4 to 5 × 10−7 M. The corresponding linear equation is y = (7.03 ± 0.17) + (0.96 ± 0.03)x. The limit of detection (LOD) for PI-PC SERS is calculated to be 6.8 × 10−7 M, which is 10 times lower than the normal SERS LOD of 8.1 × 10−6 M. Table 1 compares the detection performance of 4-NP on various SERS substrates under both conventional SERS and the PI-PC SERS technique. It can be observed that, although highly sophisticated SERS substrates have been developed – such as Au-coated silicon pillars or reduced graphene oxide nanocomposites functionalized with cysteamine and Ag nanoparticles – normal SERS still faces challenges in detecting 4-NP due to its small molecular size and low Raman cross-section, with detection limits typically reaching only around 10−5 to 10−6 M. Our 2022 study, which applied the PIERS technique on a SERS substrate based on Ag/TiO2 (with TiO2 in nanoparticle form and anatase phase), achieved significantly enhanced sensing performance compared to normal SERS, reaching a detection limit as low as 1.4 × 10−6 M, despite the relatively simple fabrication process of the Ag/TiO2 nanoparticles. In this study, by leveraging the PIERS effect through the PI-PC SERS technique on Ag/TiO2 NR substrate – with rutile-phase TiO2 nanorods – we achieved a further improvement in sensing performance, reaching a detection limit as low as 6.8 × 10−7 M. This performance surpasses that of normal SERS using sophisticated substrates and even exceeds the previous PIERS results obtained from Ag/TiO2 nanoparticle substrate with anatase-phase TiO2 nanoparticles. Therefore, by simply pre-irradiating the Ag/TiO2 NR substrate, the detection efficiency for the low Raman cross-section molecule, 4-NP, is enhanced by approximately 10 times and reaches down to the level of 10−7 M. This achievement not only improves the detection of low Raman cross-section molecules but also highlights the intriguing enhancement mechanisms of the PI-PC SERS in Ag/TiO2 NR substrate.
 | ||
| Fig. 5 The linear relationship between the concentration of 4-NP and the PI-PC SERS intensity obtained as a logarithmic function at peaks 876 cm−1 (a), 1120 cm−1 (b), 1343 cm−1 (c) and 1598 cm−1 (d). | ||
| Substrate | Technique | LOD | Linear range | Reusability | Ref. |
|---|---|---|---|---|---|
| Au-coated silicon pillars | SERS | 1 × 10−5 M | — | — | 39 |
| Ag-PDMS | SERS | 1 × 10−6 M | — | — | 41 |
| rGOSHAg | SERS | 1 × 10−6 M | — | — | 39 |
| Ag/TiO2 nanoparticles | PIERS | 1.4 × 10−6 M | 1 × 10−3–1 × 10−6 M | — | 32 |
| Ag/TiO2 NRs | PI-PC SERS | 6.8 × 10−7 M | 1 × 10−4–5 × 10−7 M | Yes | This work |
Fig. 6a and b compare the SERS signals obtained for 4-NP at concentrations of 10−3 M and 5 × 10−6 M in both normal SERS and PI-PC SERS. Based on the signal intensity of 4-NP at the 1343 cm−1 peak – selected for its strong enhancement and its association with the characteristic –NO2 group of 4-NP – the PI-PC SERS technique demonstrated signal enhancements of approximately 2.4-fold and 8.9-fold at concentrations of 10−3 M and 5 × 10−6 M, respectively, compared to normal SERS. The SERS signal enhancement driven by the PIERS effect has also been demonstrated in other nanostructures and analytes. Ben-Jaber et al. observed significant PIERS-induced enhancement using Au/TiO2 nanoparticle substrates across a wide range of molecular targets, including organic dyes, biomolecules, and explosives.16 Similarly, Barbillon et al. reported up to a 7.52-fold enhancement for thiophenol compared to conventional SERS using Au/ZnO nanoparticle structures.42 The enhanced SERS signal in PI-PC SERS, attributed to the PIERS effect, arises from photoelectric interaction and the energy level transitions between Ag and TiO2 in the Ag/TiO2 NR structure, where the presence of TiO2 facilitates more efficient charge transfer between Ag and 4-NP.29,30 During the measurement, the localized surface plasmon resonance (LSPR) of the Ag nanoparticles excites free charges that oscillate around the nanoparticle surfaces.43 These excited charges can transfer to nearby analyte molecules, and the recovery of these charges within the analyte molecules generates characteristic Raman signals corresponding to molecular vibrations.44 The transferred charge from Ag to the analyte tends to reach the lowest unoccupied molecular orbital (LUMO) energy level (in this case, for the 4-NP molecule), where the charges then relax to the highest occupied molecular orbital (HOMO) of 4-NP, contributing to the overall SERS signal (Fig. 6c).45,46 The TiO2 component in the SERS substrate enhances the charge transfer efficiency between Ag and 4-NP. Upon applying the PI-PC SERS technique to the Ag/TiO2 NR structure by pre-irradiating the substrate with UV light at a wavelength of 365 nm (to activate PIERS effect), this illumination causes oxygen atoms on the TiO2 surface to be ejected, forming oxygen vacancy sites – a phenomenon previously documented in other studies.16,30 Mezhenny et al. demonstrated that UV irradiation induces the formation of oxygen vacancies on the surface of TiO2, which can remain stable for up to 48 hours after the irradiation ceases.47 These oxygen vacancies create a temporary intermediate energy level between the valence band and the conduction band of TiO2, providing a site where electrons can be accepted.48 This temporary energy level was demonstrated by Henrich et al. to lie approximately 0.7 eV below the conduction band of TiO2.48 This intermediate energy level in TiO2 facilitates more efficient charge transfer from the LSPR of Ag to TiO2, thus increasing the probability and number of charges transferred to the 4-NP molecule.49,50 As a result, the SERS signal is significantly enhanced in the PI-PC SERS technique compared to normal SERS (Fig. 6d).
The optoelectronic phenomenon of the Ag/TiO2 NR structure not only proves highly effective in enhancing the detection of 4-NP through the PIERS effect but also offers the potential for self-cleaning and reuse of the SERS substrate. The Ag/TiO2 structure is widely known for its superior photocatalytic efficiency.51,52 Leveraging this property, the Ag/TiO2 NR SERS substrate, which contains residual 4-NP molecules after the sensing process, can be treated to self-clean its surface and be reused for subsequent analyses. This approach can reduce both the effort and cost involved in fabricating new SERS substrates while also minimizing waste generated from discarded substrates after each analysis. After the sensing process, the contaminated Ag/TiO2 NR substrate, which has 4-NP molecules on its surface, was dropped with a sufficient of water and then subjected to UV irradiation at a wavelength of 365 nm to activate the photocatalytic decomposition property of the Ag/TiO2 structure (Fig. 7a). The effectiveness of removing residual 4-NP molecules is monitored through the SERS signal obtained from the Ag/TiO2 substrate. The results are depicted in Fig. 7b. At time point 0 minutes, the SERS signal of 4-NP at a concentration of 5 × 10−4 M is measured before the photocatalytic process begins. Subsequently, time intervals of 5, 10, 20, 30… minutes of the degradation reaction are monitored using SERS spectra to assess the reduction in 4-NP concentration on the contaminated SERS substrate. As time progresses, the intensity of the characteristic peaks of 4-NP diminishes, indicating the gradual breakdown of molecular bonds within the 4-NP structure. This demonstrates that the photocatalytic activity of the Ag/TiO2 NR substrate is effectively decomposing the 4-NP molecules present on its surface. After 70 minutes of irradiation and reaction, the SERS spectrum of the contaminated Ag/TiO2 NR substrate no longer shows characteristic peaks of 4-NP, implying that the residual 4-NP molecules on the surface of the SERS substrate have been sufficiently decomposed, and their signal no longer influences the overall SERS response.
Fig. 7c succinctly outlines the mechanism behind the photocatalytic decomposition of 4-NP by the Ag/TiO2 NR substrate. Upon UV irradiation at a wavelength of 365 nm, the electrons in the valence band of the TiO2 nanorods with the rutile crystal phase are excited, jumping to the conduction band and creating electron–hole pairs.53 These electron–hole pairs then react with water and oxygen in the environment to produce hydroxyl radicals (·OH), which directly interact with the 4-NP molecules, leading to the formation of CO2, water, and other by-products.53,54 The presence of Ag nanoparticles further enhances the photocatalytic decomposition activity of the Ag/TiO2 NR structure by reducing the recombination rate of the electron–hole pairs on TiO2, thereby increasing the number of electron–hole pairs available to participate in the reaction.51,52 Ultimately, after the self-cleaning process, the Ag/TiO2 NR SERS substrate is heated at 60 °C to remove water and by-products, resulting in a renewable and reusable SERS substrate.
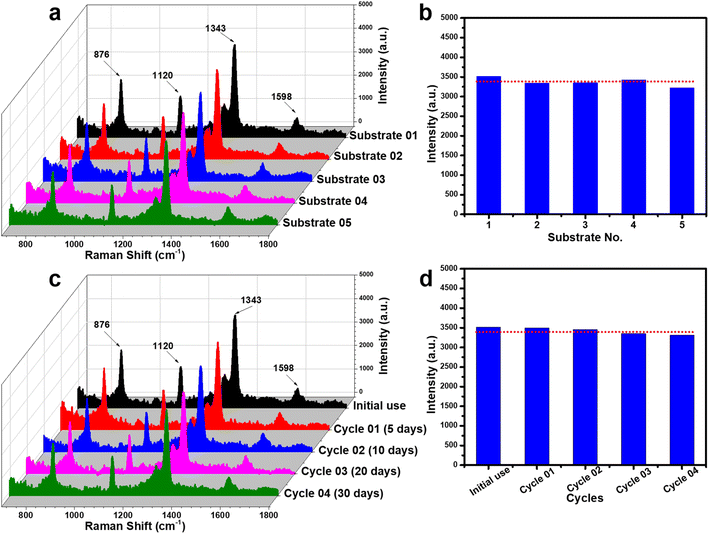
To assess the reliability of the PI-PC SERS technique using Ag/TiO2 NR substrates, two key parameters were examined: reproducibility and reusability. Reproducibility was evaluated by collecting SERS signals of 4-NP (5 × 10−4 M) from five independently prepared Ag/TiO2 NR substrates, each subjected to the same PI-PC SERS protocols. Reusability was assessed by reapplying one substrate across multiple cycles at various storage intervals. The results are presented in Fig. 8. For reproducibility, five independent experiments were conducted, encompassing substrate fabrication, pre-irradiation, and signal collection. As shown in Fig. 8a, the SERS spectra from all five substrates display consistent characteristic peaks of 4-NP. The intensity of the 1343 cm−1 peak – selected for its strong linearity – was used to calculate the relative standard deviation (RSD), which was 8.22% (Fig. 8b). This low RSD value indicates excellent reproducibility of the PI-PC SERS technique with Ag/TiO2 NR substrate. The reusability of the substrate, enabled by its photocatalytic self-cleaning property, was also investigated. After each use, the substrate was cleaned and reused to detect 4-NP. Fig. 8c shows that the characteristic SERS peaks of 4-NP remain clearly visible across reuse cycles, with negligible intensity variation. As depicted in Fig. 8d, while the 1343 cm−1 peak shows a slight decline in intensity with successive uses, the signal remains strong, demonstrating robust reusability. Additionally, the long-term stability of the substrate was examined by storing the Ag/TiO2 NRs in sealed containers protected from light and conducting reuse tests after 5, 10, 20, and 30 days. The SERS signals obtained at these intervals (Fig. 8c and d) remained stable, confirming the substrate's good stability. From these results, the Ag/TiO2 NR substrate, when coupled with the PI-PC SERS strategy, exhibits good reproducibility, reusability, and storage stability, highlighting its strong reliability.
3.3. Application of the PI-PC SERS technique for low Raman cross-section molecules – urea – a biomarker
Urea, a diamide derivative of carbonic acid, is a vital nitrogen-containing compound and a crucial biomarker present in human blood and urine. It plays a critical role in clinical diagnoses related to kidney, liver, and heart diseases.55,56 Beyond clinical applications, urea detection is important in agriculture, environmental monitoring, and food safety. Elevated urea levels in soil and water can indicate nutrient imbalances or contamination from agricultural runoff, which affects crop health and water quality.57,58 In food safety, urea contamination in food products can signal improper handling or excessive fertilizer use, posing potential health risks.59 Despite its significance, urea has a low Raman scattering cross-section, which limits the effectiveness of the SERS technique for detecting this biomarker due to weak SERS signals. Enhancing the detection sensitivity for urea could offer valuable applications in early diagnosis, healthcare, food safety and environmental protection. The PI-PC SERS technique, applied to Ag/TiO2 NR SERS substrates, was further explored to improve the detection of this important molecule. The Ag/TiO2 NR SERS substrates, cleaned after the 4-NP experiments, were reused in this urea experiment to assess their reusability.Fig. 9a shows the results obtained from normal SERS experiments for urea. As a small organic molecule consisting of a carbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and two amino groups (–NH2), urea adsorbed on SERS substrates produced a straightforward SERS spectrum, characterized by a distinct peak at 1010 cm−1 corresponding to the C–N stretching vibration.60 Compared to the Raman spectrum of pure urea in powdered state, the SERS spectrum obtained from the urea solution shows a slight peak shift, with the powder spectrum displaying a characteristic peak at 1020 cm−1 (Fig. 9b). This characteristic peak appeared clearly with relatively high intensity at a concentration of 10−3 M and gradually decreased at lower concentrations. At a concentration of 10−5 M (Fig. 9a), the characteristic peak was still observable but very weak, and it completely disappeared at a concentration of 5 × 10−6 M. Additionally, the SERS spectrum of urea used on the reused substrate from the 4-NP experiment showed no unusual scattering peaks and did not affect the ability to detect urea. This demonstrates the good reusability of this SERS substrate through the PI-PC SERS technique. The calculated LOD value in this normal SERS case was 8.9 × 10−6 M. Fig. 9c shows the detection results for urea when applying the PI-PC SERS technique on the Ag/TiO2 NR SERS substrate over the concentration range of 10−3–5 × 10−7 M. When comparing the SERS signal intensity at a concentration of 10−3 M, PI-PC SERS clearly shows the superior detection performance compared to normal SERS (Fig. 9d). The 1010 cm−1 peak in the PI-PC SERS case appears more clearly and with significantly higher intensity than in the normal SERS. At a concentration of 5 × 10−6 M, normal SERS could not detect urea, while the characteristic peak of urea remained clearly visible when applying PI-PC SERS (Fig. 9e). The LOD value in the PI-PC SERS was 6.9 × 10−7 M, enhanced by over 10 times compared to normal SERS. Table 2 presents a comparison of urea sensing performance across various SERS substrates using both normal SERS and the PI-PC SERS technique. Although these substrates – such as Ag dendritic structures, highly ordered Ag/Cu nanostructure arrays, and Au@Ag nanoparticles – were meticulously engineered, their detection capability for urea, a small molecule with a low Raman scattering cross-section, remains limited to the range of 10−3–10−4 M under normal SERS. By employing the PIERS technique on Ag/TiO2 substrates containing anatase-phase TiO2 nanoparticles, the detection limit is significantly enhanced, reaching 10−6 M. Remarkably, the Ag/TiO2 NRs used in this study, featuring rutile-phase TiO2 in rod morphology, enable an even lower detection limit of 10−7 M when using the PI-PC SERS technique. It should be noted that PI-PC SERS synergistically integrates PIERS and photocatalysis, with the observed signal enhancement attributed to the PIERS effect. This substantial improvement in urea detection underscores the excellent SERS enhancement capability of the Ag/TiO2 NR substrate when applying the PI-PC SERS approach. The Ag/TiO2 NR SERS substrate contaminated with urea was also cleaned through the photocatalytic effect (Fig. 9f), and after 60 minutes of post-irradiation, the urea signal was completely removed.
O) and two amino groups (–NH2), urea adsorbed on SERS substrates produced a straightforward SERS spectrum, characterized by a distinct peak at 1010 cm−1 corresponding to the C–N stretching vibration.60 Compared to the Raman spectrum of pure urea in powdered state, the SERS spectrum obtained from the urea solution shows a slight peak shift, with the powder spectrum displaying a characteristic peak at 1020 cm−1 (Fig. 9b). This characteristic peak appeared clearly with relatively high intensity at a concentration of 10−3 M and gradually decreased at lower concentrations. At a concentration of 10−5 M (Fig. 9a), the characteristic peak was still observable but very weak, and it completely disappeared at a concentration of 5 × 10−6 M. Additionally, the SERS spectrum of urea used on the reused substrate from the 4-NP experiment showed no unusual scattering peaks and did not affect the ability to detect urea. This demonstrates the good reusability of this SERS substrate through the PI-PC SERS technique. The calculated LOD value in this normal SERS case was 8.9 × 10−6 M. Fig. 9c shows the detection results for urea when applying the PI-PC SERS technique on the Ag/TiO2 NR SERS substrate over the concentration range of 10−3–5 × 10−7 M. When comparing the SERS signal intensity at a concentration of 10−3 M, PI-PC SERS clearly shows the superior detection performance compared to normal SERS (Fig. 9d). The 1010 cm−1 peak in the PI-PC SERS case appears more clearly and with significantly higher intensity than in the normal SERS. At a concentration of 5 × 10−6 M, normal SERS could not detect urea, while the characteristic peak of urea remained clearly visible when applying PI-PC SERS (Fig. 9e). The LOD value in the PI-PC SERS was 6.9 × 10−7 M, enhanced by over 10 times compared to normal SERS. Table 2 presents a comparison of urea sensing performance across various SERS substrates using both normal SERS and the PI-PC SERS technique. Although these substrates – such as Ag dendritic structures, highly ordered Ag/Cu nanostructure arrays, and Au@Ag nanoparticles – were meticulously engineered, their detection capability for urea, a small molecule with a low Raman scattering cross-section, remains limited to the range of 10−3–10−4 M under normal SERS. By employing the PIERS technique on Ag/TiO2 substrates containing anatase-phase TiO2 nanoparticles, the detection limit is significantly enhanced, reaching 10−6 M. Remarkably, the Ag/TiO2 NRs used in this study, featuring rutile-phase TiO2 in rod morphology, enable an even lower detection limit of 10−7 M when using the PI-PC SERS technique. It should be noted that PI-PC SERS synergistically integrates PIERS and photocatalysis, with the observed signal enhancement attributed to the PIERS effect. This substantial improvement in urea detection underscores the excellent SERS enhancement capability of the Ag/TiO2 NR substrate when applying the PI-PC SERS approach. The Ag/TiO2 NR SERS substrate contaminated with urea was also cleaned through the photocatalytic effect (Fig. 9f), and after 60 minutes of post-irradiation, the urea signal was completely removed.
| Substrate | Technique | LOD | Linear range | Reusability | Ref. |
|---|---|---|---|---|---|
| Ag dendrites | SERS | 0.2 mg mL−1 (∼3.33 × 10−3 M) | 0.17–3.33 × 10−3 M | — | 61 |
| Au/Cu nanostructure arrays | SERS | 1 × 10−3 M | 0.3–1 × 10−3 M | — | 19 |
| Ag–Au compound | SERS | 1 × 10−3 M | 2 × 10−2–1 × 10−3 M | — | 62 |
| Au@Ag NPs | SERS | 5 mg dL−1 (∼8.33 × 10−4 M) | 1.3 × 10−3–8.3 × 10−4 M | — | 63 |
| Ag/TiO2 nanoparticles | PIERS | 4.6 × 10−6 M | 1 × 10−3–1 × 10−6 M | — | 32 |
| Ag/TiO2 NRs | PI-PC SERS | 6.9 × 10−7 M | 1 × 10−4–5 × 10−7 M | Yes | This work |
Similar to the case of 4-NP, the reliability of the PI-PC SERS technique using Ag/TiO2 NR substrates was also further evaluated for urea at a concentration of 5 × 10−4 M by assessing reproducibility and reusability. The experimental procedure was conducted in the same manner as with 4-NP to ensure repeatability. The results shown in Fig. 10a and b confirm the high reproducibility of the PI-PC SERS technique for urea, with a RSD value of 6.34%. Furthermore, Fig. 10c and d demonstrate the excellent reusability and stability of the Ag/TiO2 NR substrate across multiple reuse cycles and various storage time points. These results, together with the findings obtained for 4-NP, further highlight the high reliability of combining Ag/TiO2 NR substrates with the PI-PC SERS technique, which not only offers sensitive sensing performance, good reproducibility, reusability, and stability, but also demonstrates applicability to various low Raman cross-section molecules.
4. Conclusions
This study successfully demonstrates the application of the photo-induced photo-catalytic (PI-PC SERS) technique using Ag-deposited TiO2 nanorod-based SERS substrates to enhance the detection of low Raman cross-section molecules, specifically the pesticide 4-NP and the biomarker urea. Normal SERS struggles with detecting such molecules, with detection limits restricted to 10−6 M. By leveraging the unique PIERS effect of Ag/TiO2 NRs, PI-PC SERS significantly improves detection sensitivity, achieving a tenfold enhancement and lowering the detection limit to 10−7 M. Beyond its enhanced detection capability, PI-PC SERS harnesses the high photocatalytic activity of Ag/TiO2 NRs to degrade residual analyte molecules post-sensing, enabling an efficient self-cleaning and reusable SERS platform. These advancements not only improve detection efficiency but also enhance the cost-effectiveness and sustainability of SERS-based analytical techniques. The findings of this study highlight the transformative potential of PI-PC SERS in overcoming the challenge of detecting low Raman cross-section molecules, paving the way for future research in ultrasensitive sensing while expanding its applications in food safety, biomedical diagnostics, and many related areas. However, some limitations remain. This study tested on two model low Raman cross-section analytes (4-NP and urea); further investigations are needed to confirm PI-PC SERS performance across a broader range of such molecules. Additionally, its practical applicability should be further validated using complex real-world samples to ensure reliability and reproducibility under practical conditions.Data availability
All experimental data, including the characterization of the Ag-deposited TiO2 nanorod substrate and the detection results for 4-nitrophenol and urea molecules, are included in the manuscript.Author contributions
Q. D. Mai: conceptualization, methodology, investigation, formal analysis, data curation, supervision, writing – original draft; D. T. H. Trang: formal analysis, investigation, validation; N. T. Loan: validation, investigation; N. H. T. Thi: validation, formal analysis; O. V. Hoang: validation, investigation; T. N. Bach: validation, investigation; N. Q. Hoa: validation, investigation; A. T. Pham: methodology, supervision; A. T. Le: conceptualization, methodology, supervision, project administration, writing – review & editing.Conflicts of interest
The authors confirm that no financial interests or personal relationships exist that could have influenced the findings presented in this paper.Acknowledgements
This study was supported by Phenikaa University under grant number PU2023-2-A-03 and A&A Green Phoenix Group JSC through Financial Support for the Key Research Group (NEB Lab). The authors express their gratitude to NEB Lab (Phenikaa University) for assistance with Raman measurements and to IMS-VAST for providing FE-SEM measurement support.References
- X. X. Han, R. S. Rodriguez, C. L. Haynes, Y. Ozaki and B. Zhao, Surface-enhanced Raman spectroscopy, Nat. Rev. Methods Primers, 2021, 1(1), 87 CrossRef CAS.
- S. Zeng, D. Baillargeat, H.-P. Ho and K.-T. Yong, Nanomaterials enhanced surface plasmon resonance for biological and chemical sensing applications, Chem. Soc. Rev., 2014, 43(10), 3426–3452 RSC.
- S. Nie and S. R. Emory, Probing single molecules and single nanoparticles by surface-enhanced Raman scattering, Science, 1997, 275(5303), 1102–1106 CrossRef CAS PubMed.
- S.-Y. Ding, J. Yi, J.-F. Li, B. Ren, D.-Y. Wu and R. Panneerselvam, et al., Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials, Nat. Rev. Mater., 2016, 1(6), 1–16 Search PubMed.
- J. Langer, D. Jimenez de Aberasturi, J. Aizpurua, R. A. Alvarez-Puebla, B. Auguié and J. J. Baumberg, et al., Present and future of surface-enhanced Raman scattering, ACS Nano, 2019, 14(1), 28–117 CrossRef PubMed.
- J. Wang, K. M. Koo, Y. Wang and M. Trau, Engineering state-of-the-art plasmonic nanomaterials for SERS-based clinical liquid biopsy applications, Adv. Sci., 2019, 6(23), 1900730 CrossRef CAS PubMed.
- H.-L. Liu, K. Zhan, K. Wang and X.-H. Xia, Recent advances in nanotechnologies combining surface-enhanced Raman scattering and nanopore, TrAC, Trends Anal. Chem., 2023, 159, 116939 CrossRef CAS.
- Z. Huang, A. Zhang, Q. Zhang and D. Cui, Nanomaterial-based SERS sensing technology for biomedical application, J. Mater. Chem. B, 2019, 7(24), 3755–3774 RSC.
- S. E. Bell and M. R. McCourt, SERS enhancement by aggregated Au colloids: effect of particle size, Phys. Chem. Chem. Phys., 2009, 11(34), 7455–7462 RSC.
- Q. D. Mai, H. A. Nguyen, T. L. H. Phung, N. Xuan Dinh, Q. H. Tran and T. Q. Doan, et al., Silver nanoparticles-based SERS platform towards detecting chloramphenicol and amoxicillin: an experimental insight into the role of HOMO–LUMO energy levels of the analyte in the SERS signal and charge transfer process, J. Phys. Chem. C, 2022, 126(17), 7778–7790 CrossRef CAS.
- R. G. Freeman, K. C. Grabar, K. J. Allison, R. M. Bright, J. A. Davis and A. P. Guthrie, et al., Self-assembled metal colloid monolayers: an approach to SERS substrates, Science, 1995, 267(5204), 1629–1632 CrossRef CAS PubMed.
- M. J. Banholzer, J. E. Millstone, L. Qin and C. A. Mirkin, Rationally designed nanostructures for surface-enhanced Raman spectroscopy, Chem. Soc. Rev., 2008, 37(5), 885–897 RSC.
- S. Fateixa, H. I. Nogueira and T. Trindade, Hybrid nanostructures for SERS: materials development and chemical detection, Phys. Chem. Chem. Phys., 2015, 17(33), 21046–21071 RSC.
- J. F. Li, Y. F. Huang, Y. Ding, Z. L. Yang, S. B. Li and X. S. Zhou, et al., Shell-isolated nanoparticle-enhanced Raman spectroscopy, Nature, 2010, 464(7287), 392–395 CrossRef CAS PubMed.
- S. Almohammed, F. Zhang, B. J. Rodriguez and J. H. Rice, Electric field-induced chemical surface-enhanced Raman spectroscopy enhancement from aligned peptide nanotube–graphene oxide templates for universal trace detection of biomolecules, J. Phys. Chem. Lett., 2019, 10(8), 1878–1887 CrossRef CAS PubMed.
- S. Ben-Jaber, W. J. Peveler, R. Quesada-Cabrera, E. Cortés, C. Sotelo-Vazquez and N. Abdul-Karim, et al., Photo-induced enhanced Raman spectroscopy for universal ultra-trace detection of explosives, pollutants and biomolecules, Nat. Commun., 2016, 7(1), 12189 CrossRef CAS PubMed.
- Q.-D. Mai, D. C. Thanh, N. T. Anh, T. Van Manh, T. N. Bach and H.-A. Nguyen, et al., Smart 3D Ag-decorated TiO2 Nanostructure: An Advanced Synergistic SERS Substrate for Trace Detection of Analytes with Diverse Natures, Sens. Actuators, B, 2024, 135651 CrossRef CAS.
- R. A. Alvarez-Puebla and L. M. Liz-Marzán, SERS detection of small inorganic molecules and ions, Angew. Chem., Int. Ed., 2012, 51(45), 11214–11223 CrossRef CAS PubMed.
- K. Chen, X. Zhang and D. R. MacFarlane, Ultrasensitive surface-enhanced Raman scattering detection of urea by highly ordered Au/Cu hybrid nanostructure arrays, Chem. Commun., 2017, 53(56), 7949–7952 RSC.
- C. Penney, L. Goldman and M. Lapp, Raman scattering cross sections, Nat. Phys. Sci., 1972, 235(58), 110–112 CrossRef CAS.
- S. Shim, C. M. Stuart and R. A. Mathies, Resonance Raman cross-sections and vibronic analysis of Rhodamine 6G from broadband stimulated Raman spectroscopy, ChemPhysChem, 2008, 9(5), 697–699 CrossRef CAS PubMed.
- C. D. Tschannen, G. Gordeev, S. Reich, L. Shi, T. Pichler and M. Frimmer, et al., Raman scattering cross section of confined carbyne, Nano Lett., 2020, 20(9), 6750–6755 CrossRef CAS PubMed.
- R. Aggarwal, L. Farrar, S. Di Cecca and T. Jeys, Raman spectra and cross sections of ammonia, chlorine, hydrogen sulfide, phosgene, and sulfur dioxide toxic gases in the fingerprint region 400-1400 cm− 1, AIP Adv., 2016, 6(2), 025310 CrossRef.
- D. Fouche and R. Chang, Relative Raman cross section for O3, CH4, C3H8, NO, N2O, and H2, Appl. Phys. Lett., 1972, 20(7), 256–257 CrossRef CAS.
- Y. Liu, H. Ma, X. X. Han and B. Zhao, Metal–semiconductor heterostructures for surface-enhanced Raman scattering: synergistic contribution of plasmons and charge transfer, Mater. Horiz., 2021, 8(2), 370–382 RSC.
- M. Procházka, D. Novák, E. Kočišová, K. Oe, W. Ji and Y. Ozaki, New insights into SERS mechanism of semiconductor–metal heterostructure: a case study on vanadium pentoxide nanoparticles decorated with gold, J. Phys. Chem. C, 2024, 128(28), 11732–11740 CrossRef.
- Q.-D. Mai, T. H. T. Dang, T. T. Nguyen, T. T. T. Nguyen, T. Ngoc Bach and A. S. Nguyen, et al., Activating SERS Signals of Inactive Analytes: Creating an Energy Bridge between Metal/Molecule Energy Alignment via Metal/Semiconductor Transitions, Anal. Chem., 2024, 97(1), 994–1002 CrossRef PubMed.
- H. Pu, T. Fang, Z. Wu and D.-W. Sun, Advancements in recyclable photocatalytic semiconductor substrates for SERS detection in food safety applications, Trends Food Sci. Technol., 2023, 697–707 CrossRef CAS.
- J. Zhao, Z. Wang, J. Lan, I. Khan, X. Ye and J. Wan, et al., Recent advances and perspectives in photo-induced enhanced Raman spectroscopy, Nanoscale, 2021, 13(19), 8707–8721 RSC.
- J. Ye, R. Arul, M. K. Nieuwoudt, J. Dong, T. Zhang and L. Dai, et al., Understanding the chemical mechanism behind photoinduced enhanced Raman spectroscopy, J. Phys. Chem. Lett., 2023, 14(19), 4607–4616 CrossRef CAS PubMed.
- A. Brognara, B. R. Bricchi, L. William, O. Brinza, M. Konstantakopoulou and A. L. Bassi, et al., New mechanism for long photo-induced enhanced Raman spectroscopy in Au nanoparticles embedded in TiO2, Small, 2022, 18(25), 2201088 CrossRef CAS PubMed.
- Q. D. Mai, H. A. Nguyen, T. L. H. Phung, N. Xuan Dinh, Q. H. Tran and T. Q. Doan, et al., Photoinduced Enhanced Raman Spectroscopy for the Ultrasensitive Detection of a Low-Cross-Section Chemical, Urea, Using Silver–Titanium Dioxide Nanostructures, ACS Appl. Nano Mater., 2022, 5(10), 15518–15530 CrossRef CAS.
- Q.-D. Mai, D. T. H. Trang, T. N. Bach, V. T. Le Na, A.-T. Pham and A.-T. Le, Synergizing PIERS and photocatalysis effects in a photo-responsive Ag/TiO 2 nanostructure for an ultrasensitive and renewable PI-PC SERS technique, RSC Adv., 2025, 15(6), 4149–4162 RSC.
- Q. D. Mai, H. A. Nguyen, N. N. Huyen, P. C. Thanh, D. Q. Thuc and N. A. Son, et al., Large-Scale Green Electrochemical Synthesis of Smart Titanium Dioxide Nanomaterials: Controlled Morphology and Rotatable Surface Ligands via Tuning Electrolyte Structures, J. Electron. Mater., 2023, 1–17 Search PubMed.
- R. Chen, H. Shi, X. Meng, Y. Su, H. Wang and Y. He, Dual-amplification strategy-based SERS chip for sensitive and reproducible detection of DNA methyltransferase activity in human serum, Anal. Chem., 2019, 91(5), 3597–3603 CrossRef CAS PubMed.
- T. Mazza, E. Barborini, P. Piseri, P. Milani, D. Cattaneo and A. Li Bassi, et al., Raman spectroscopy characterization of Ti O 2 rutile nanocrystals, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75(4), 045416 CrossRef.
- C. L. Jahncke, W. Zhang, B. M. DeMuynck and A. D. Hill, Exploring resonance Raman scattering with 4-nitrophenol, J. Chem. Educ., 2022, 99(9), 3233–3241 CrossRef CAS.
- V. Uberoi and S. K. Bhattacharya, Toxicity and degradability of nitrophenols in anaerobic systems, Water Environ. Res., 1997, 69(2), 146–156 CrossRef CAS.
- L. Hostert, C. Blanc, A. J. Zarbin, E. Anglaret and E. S. Orth, SERS detection and comprehensive study of p-nitrophenol: towards pesticide sensing, New J. Chem., 2021, 45(8), 3886–3891 RSC.
- M. Muniz-Miranda, SERS monitoring of the catalytic reduction of 4-nitrophenol on Ag-doped titania nanoparticles, Appl. Catal., B, 2014, 146, 147–150 CrossRef CAS.
- M. Wang, B. De Vivo, W. Lu and M. Muniz-Miranda, Sensitive surface-enhanced Raman scattering (SERS) detection of nitroaromatic pollutants in water, Appl. Spectrosc., 2014, 68(7), 784–788 CrossRef CAS PubMed.
- G. Barbillon, T. Noblet and C. Humbert, Highly crystalline ZnO film decorated with gold nanospheres for PIERS chemical sensing, Phys. Chem. Chem. Phys., 2020, 22(37), 21000–21004 RSC.
- M. R. Philpott, Effect of surface plasmons on transitions in molecules, J. Chem. Phys., 1975, 62(5), 1812–1817 CrossRef CAS.
- M. Moskovits, Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals, J. Chem. Phys., 1978, 69(9), 4159–4161 CrossRef CAS.
- L. Jensen, C. M. Aikens and G. C. Schatz, Electronic structure methods for studying surface-enhanced Raman scattering, Chem. Soc. Rev., 2008, 37(5), 1061–1073 RSC.
- S. M. Morton, D. W. Silverstein and L. Jensen, Theoretical studies of plasmonics using electronic structure methods, Chem. Rev., 2011, 111(6), 3962–3994 CrossRef CAS PubMed.
- S. Mezhenny, P. Maksymovych, T. Thompson, O. Diwald, D. Stahl and S. Walck, et al., STM studies of defect production on the TiO2 (110)-(1× 1) and TiO2 (110)-(1× 2) surfaces induced by UV irradiation, Chem. Phys. Lett., 2003, 369(1–2), 152–158 CrossRef CAS.
- V. E. Henrich, G. Dresselhaus and H. Zeiger, Observation of Two-Dimensional Phases Associated with Defect States on the Surface of Ti O 2, Phys. Rev. Lett., 1976, 36(22), 1335 CrossRef CAS.
- K. Iida and M. Noda, Electron Transfer Governed by Light− Matter Interaction at Metal− Semiconductor Interface, npj Comput. Mater., 2020, 5 CrossRef CAS.
- Z. Mao, W. Song, L. Chen, W. Ji, X. Xue and W. Ruan, et al., Metal–semiconductor contacts induce the charge-transfer mechanism of surface-enhanced Raman scattering, J. Phys. Chem. C, 2011, 115(37), 18378–18383 CrossRef CAS.
- C. Zarzzeka, J. Goldoni, G. G. Lenzi, M. D. Bagatini and L. M. S. Colpini, Photocatalytic action of Ag/TiO2 nanoparticles to emerging pollutants degradation: a comprehensive review, Sustainable Chem. Environ., 2024, 100177 CrossRef.
- D. Kanakaraju, F. D. anak Kutiang, Y. C. Lim and P. S. Goh, Recent progress of Ag/TiO2 photocatalyst for wastewater treatment: Doping, co-doping, and green materials functionalization, Appl. Mater. Today, 2022, 27, 101500 CrossRef.
- Y. Nam, J. H. Lim, K. C. Ko and J. Y. Lee, Photocatalytic activity of TiO 2 nanoparticles: a theoretical aspect, J. Mater. Chem. A, 2019, 7(23), 13833–13859 RSC.
- C. B. Anucha, I. Altin, E. Bacaksiz and V. N. Stathopoulos, Titanium dioxide (TiO2)-based photocatalyst materials activity enhancement for contaminants of emerging concern (CECs) degradation: In the light of modification strategies, Chem. Eng. J. Adv., 2022, 10, 100262 CrossRef CAS.
- S. N. Botewad, D. K. Gaikwad, N. B. Girhe, H. N. Thorat and P. P. Pawar, Urea biosensors: A comprehensive review, Biotechnol. Appl. Biochem., 2023, 70(2), 485–501 CrossRef CAS PubMed.
- C. Bai, H. Wang, D. Dong, T. Li, Z. Yu and J. Guo, et al., Urea as a by-product of ammonia metabolism can Be a potential serum biomarker of hepatocellular carcinoma, Front. Cell Dev. Biol., 2021, 9, 650748 CrossRef PubMed.
- M. Gomes, T. J. Ralph, M. S. Humphries, B. P. Graves, T. Kobayashi and D. B. Gore, Waterborne contaminants in high intensity agriculture and plant production: A review of on-site and downstream impacts, Sci. Total Environ., 2025, 958, 178084 Search PubMed.
- D. F. Lambert, J. E. Sherwood and P. S. Francis, The determination of urea in soil extracts and related samples—a review, Soil Res., 2004, 42(7), 709–717 CrossRef CAS.
- F. Shalileh, H. Sabahi, M. Dadmehr and M. Hosseini, Sensing approaches toward detection of urea adulteration in milk, Microchem. J., 2023, 193, 108990 CrossRef CAS.
- A. Culka and J. Jehlička, Raman microspectrometric investigationof urea in calcite and gypsum powder matrices, J. Raman Spectrosc., 2010, 41(12), 1743–1747 CrossRef.
- Dendritic silver microstructures as highly sensitive SERS platform for the detection of trace urea, IOP Conference Series: Materials Science and Engineering, ed. J. Wen, F. Song, Y. Du, W. Yu and R. Qiang, IOP Publishing, 2019 Search PubMed.
- Y. Li, Q. Li, C. Sun, S. Jin, Y. Park and T. Zhou, et al., Fabrication of novel compound SERS substrates composed of silver nanoparticles and porous gold nanoclusters: A study on enrichment detection of urea, Appl. Surf. Sci., 2018, 427, 328–333 CrossRef CAS.
- A. Hussain, D.-W. Sun and H. Pu, SERS detection of urea and ammonium sulfate adulterants in milk with coffee ring effect, Food Addit. Contam., Part A, 2019, 36(6), 851–862 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |