Open Access Article
Open Access ArticleRecovery of cellulose nanocrystal from mixed office wastepaper and the development of bio-based coating matrixes with enhanced water, gas, oil, and grease resistances for packaging
Anik Baral†
a,
Niloy Roy Kerjee†a,
Nazia Afrin Jashibc,
Md. Ismail Hossen Emona,
Munmun Basakc,
Md. Mostafizur Rahmand,
A. F. M. Mustafizur Rahmana,
Mohammed Mizanur Rahmana,
Lokendra Pal c,
Mohammad Shahruzzaman
c,
Mohammad Shahruzzaman a and
Khandoker Samaher Salem
a and
Khandoker Samaher Salem *a
*a
aApplied Chemistry and Chemical Engineering, University of Dhaka, Bangladesh. E-mail: samaher.salem@du.ac.bd
bInstitute of Leather Engineering and Technology, University of Dhaka, Bangladesh
cDepartment of Forest Biomaterials, North Carolina State University, 431 Dan Allen Dr., Raleigh, NC 27695-8005, USA
dPulp and Paper Research Division, Bangladesh Council of Scientific and Industrial Research, Dhaka, Bangladesh
First published on 25th April 2025
Abstract
This study on sustainable nanocellulose modifications for various packaging applications is driven by the global social awareness and growing demand for bioproducts to reduce the use of single-use plastics. Mixed office waste (MOW) was used as the raw material to extract cellulose nanocrystals (CNCs) via the acid hydrolysis method. The average length and diameter of the CNCs were approximately 104.08 ± 0.1 nm and 9.49 ± 0.3 nm, respectively. The crystallinity index was 87%, as confirmed using transmission electron microscopy and X-ray diffraction analysis. Coating solutions of varying concentrations were prepared by mixing CNCs with collagen hydrolysate and glycerin, and the coatings were applied to the surface of uncoated paper via a rod coating process. FTIR spectra confirmed the presence of CNCs in the coated paper. High-resolution FE-SEM images provided detailed information on the surface morphology of the coated papers. The barrier and mechanical performances of the coated paper were evaluated using the oil and grease resistance KIT, hot oil, water vapor permeability, air permeability, water contact angle, tensile index, burst index, percentage of elongation, and fold tests. All the coated paper samples passed the hot oil test and exhibited the highest KIT rating. The water vapor and air resistance values of the coated paper samples increased 14 times and 250 times, respectively, compared with the uncoated paper samples. The water contact angle of the coated paper samples increased to 99.40° from 60.13°, and the surface roughness decreased from 2.37 μm to 0.85 μm. The presence of coating also increased the tensile and burst indices by 5.28 times and 1.79 times, respectively, compared with the uncoated paper samples. No cytotoxic effects were found in the optimized coated paper, and all the samples were fully degraded within 49 days, as confirmed using the soil biodegradability test. Therefore, the coated paper can be a potential alternative to existing single-use plastics for packaging materials.
1 Introduction
Packaging plays a critical role in the field of product supply chain management. The primary function of packaging is to protect the products from external factors, such as dust, moisture, chemical pollution, microbial contamination, and volatile substances, while preventing physical damage during transportation in all logistic phases.1–3 In the past few decades, plastic packaging has replaced traditional packaging materials, such as cardboard, metal, and paper, owing to its lightness, cost-effectiveness, compatibility, and ease of production.4,5 Most plastics are produced from petroleum, and their long-term degradation leaves detrimental effects on the environment.6 Nondegradable plastics accumulate in the environment, generate secondary microplastics and nano-plastics, and release hazardous chemicals while manufacturing. The extensive use of plastic and its improper disposal and combustion lead to environmental pollution.7–10 Poor plastic waste management can cause drain clogging in cities and surrounding areas.11 Approximately 10–12% of plastic residues from municipal solid waste is combusted, releasing greenhouse gases into the environment. As a result, air pollution increases, contributing to the greenhouse effect.5,11 At the turn of the 21st century, owing to the depletion of petroleum resources and the emergence of environmental problems, biodegradable and bio-based packaging gained increased attention as a replacement for plastic packaging. Hence, the world is currently moving towards naturally derived bio-based packaging materials to replace plastic packaging products.12,13Bio-based packaging materials are derived from renewable sources, which results in a lower carbon footprint, less environmental impact,14 waste management,15,16 more customer acceptability, preservation of the packed good's barrier qualities and shelf life, and sustainable end-of-life management.17 Many companies, including PepsiCo, Kraft Heinz, and McDonald's, have recently announced plans to shift toward completely biodegradable packaging with 100% renewable and recyclable materials by 2025.18,19 In 2022, Eurostat data show that Europeans generated 83.4 million tons of packaging waste, equivalent to 186 kg per inhabitant. The share of plastic packaging waste, paper and cardboard, glass, wood, and metal was 19.4%, 40.8%, 18.8%, 16%, and 4.9%, respectively. Wastepaper, being a valuable resource for extracting cellulose, can be chemically modified to produce economically significant cellulose derivatives, as well as a variety of environmentally friendly and useful specialty end products.20,21 Wastepaper from office waste is an economic resource to produce CNCs. Mixed office waste (MOW) includes phone books, old mail, paperboard, and daily office paper waste that are recycled to produce products such as tissues, chipboard, wallboard, and cellulose insulation through the pulping and deinking process.22,23 However, this production is less economically feasible than virgin pulp production. The biggest challenge in the recycling processes of MOW is reducing the cellulose fiber length, which results in weaker and lower-quality paper products than those made from virgin pulp.24
Wastepaper recycling results in shorter fiber lengths of cellulose, which leads to poor-quality paper. Since the highest percentage of paper-to-paper recycling is 65%,25,26 enormous amounts of byproducts are produced, which must be disposed of eventually. With the rising expense of generating paper from recycled paper and the disposal of waste fibers that are unfit for use, the development of new techniques to recycle wastepaper is essential. Due to its cellulosic composition, wastepaper can be used as a raw material in the manufacturing of CNCs. Manufacturing CNCs from wastepaper would give an alternative to paper recycling while potentially addressing the issue of byproduct generation from paper-to-paper recycling.1,27 In the past decade, cellulose nanomaterials have garnered significant interest due to their lightweight nature, low cost, ease of recycling, and environmental friendliness.28,29 The ecotoxicity of nanocellulose is very low.30,31 CNCs are used in packaging as a filler32,33 and reinforcing agents in polymeric matrices due to their strength, high aspect ratio, surface area, and high tensile modulus.34 CNCs can form hydrogen bonds that create a dense and strong network within the packaging material, preventing the permeation of various molecules through the packaging layers and thereby increasing the barrier properties.35,36
The main motive of the present work was to upcycle MOW by extracting CNCs from it through an acid hydrolysis process. A coating recipe was developed using CNCs from recycled sources, collagen hydrolysate, and glycerin, and was applied as a coating on paper to evaluate its barrier and mechanical properties. The work emphasizes upcycling waste paper while contributing to environmental sustainability. The findings explore the potential of recycled, biodegradable materials as promising alternatives to conventional non-biodegradable single-use plastics.
2 Experimental sections
2.1 Materials
Mixed office waste (MOW) and brown uncoated base paper (100 g m−2) were collected from the local market. Collagen hydrolysate was extracted from shaving dust collected from the local tannery industry, a procedure established in our laboratory.37 Sodium sulfate (Na2SO4, lab grade, >99% purity), sodium carbonate (Na2CO3, lab grade, >99.5% purity), sodium hydroxide (NaOH, lab grade, 1 M), glacial acetic acid (CH3COOH, lab grade, 99% purity), concentrated sulfuric acid (H2SO4, lab grade, 98%) and glycerin were purchased from Merck, Germany. Hydrogen peroxide (H2O2, technical grade, 70% purity) was purchased from Research-Lab Fine Chemical Industries, India.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 minutes. Finally, the sample was transferred into a cyclone separator and kept for 24 h to settle down. Residue was collected from the bottom, and the suspension from the upper section containing nanocellulose was further centrifuged to remove water content. The final product contained CNCs in the form of suspension. Fig. 1 represents the extraction process of CNCs from mixed office waste (MOW).
000 rpm for 30 minutes. Finally, the sample was transferred into a cyclone separator and kept for 24 h to settle down. Residue was collected from the bottom, and the suspension from the upper section containing nanocellulose was further centrifuged to remove water content. The final product contained CNCs in the form of suspension. Fig. 1 represents the extraction process of CNCs from mixed office waste (MOW).
2.2 Formulation of the coating recipe
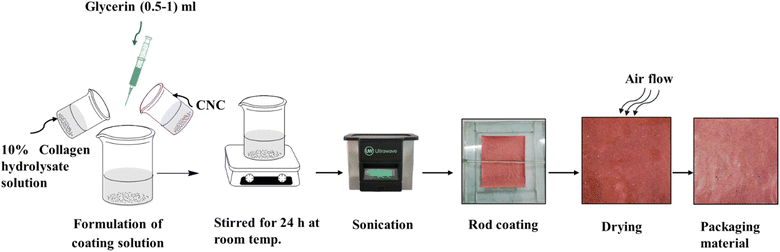
A 10% collagen hydrolysate solution was prepared by dissolving collagen hydrolysate powder in 1% acetic acid solution and 0.5–1 mL of glycerin. Then, 1, 3, 5, 7, and 10% of CNCs were taken in the weight ratio of the collagen hydrolysate powder. The formulations were prepared following Table 1 by stirring for 24 h at room temperature and sonicated for 20 min to remove the bubbles. Fig. 2 represents the application steps of CNC-based coating on the packaging material.| Serial no. | Coating recipes | Sample ID |
|---|---|---|
| 1 | Uncoated base paper | UP |
| 2 | Paper coated with 1% CNCs, 10% collagen hydrolysate, and (0.5–1) mL of glycerin | CP-1% |
| 3 | Paper coated with 3% CNCs, 10% collagen hydrolysate, and (0.5–1) mL of glycerin | CP-3% |
| 4 | Paper coated with 5% CNCs, 10% collagen hydrolysate, and (0.5–1) mL of glycerin | CP-5% |
| 5 | Paper coated with 7% CNCs, 10% collagen hydrolysate, and (0.5–1) mL of glycerin | CP-7% |
| 6 | Paper coated with 10% CNCs, 10% collagen hydrolysate, and (0.5–1) mL of glycerin | CP-10% |
2.3 Characterization
| Crystallinity index (CI) = 100 × (I200 − Iam)/I200 | (1) |
According to the equation, the Segal technique39 was used to determine the crystallinity index (CI) from the heights of the (200) peak (I200, 2θ = 22.6°) and the minimum intensity between the (200) and (110) peaks (Iam, 2θ = 18°). Iam stands for the amorphous material, while I200 denotes both crystalline and amorphous materials.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 2% gentamicin, and 10% fetal bovine serum (FBS). Cells (3.0 × 104/200 μL) were seeded into 48-well plates and incubated at 37 °C with 5% CO2. The following day, 50 μL (autoclaved) samples were added to each well, and after 48 hours of incubation, the cytotoxicity was investigated using an inverted light microscope.
1), 2% gentamicin, and 10% fetal bovine serum (FBS). Cells (3.0 × 104/200 μL) were seeded into 48-well plates and incubated at 37 °C with 5% CO2. The following day, 50 μL (autoclaved) samples were added to each well, and after 48 hours of incubation, the cytotoxicity was investigated using an inverted light microscope.3 Results and discussion
Fig. 3A represents the XRD data, where the crystallinity index of CNCs was determined through the Segal peak height method.39 The crystallinity index of CNCs obtained from mixed office waste (MOW) was 87%. The increase in the crystallinity index (CI) for CNCs was due to the effective removal of non-cellulosic components from the fibers, specifically the dissolution of amorphous regions, through a controlled acid hydrolysis process, which accounts for the increased crystalline nature of nanocellulose.40The TEM image shown in Fig. 3B exhibits the morphology of CNC samples. The treated CNC suspension consisted of long, rod-like particles, comprising individual filaments and some agglomerated bundles. The acid hydrolysis process seems to have removed amorphous cellulose and hemicellulose. The average length, diameter, and aspect ratio of CNCs were, respectively, 104.08 ± 0.1 nm, 9.49 ± 0.3 nm, and 10.97 ± 0.2 nm.
The coated and uncoated papers were examined using FTIR, as shown in Fig. 4A. Compared with uncoated paper, new absorption peaks were found for CP-3% at 2955 cm−1 for amide, 1659 cm−1 for amide I (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), 1560 cm−1 for amide II (N–H bending), 1268 cm−1 for amide III (C–N stretching), 1471 cm−1 for –CH2 bending vibration, and 1350 cm−1 for C
O stretching), 1560 cm−1 for amide II (N–H bending), 1268 cm−1 for amide III (C–N stretching), 1471 cm−1 for –CH2 bending vibration, and 1350 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration due to collagen hydrolysate. Compared with the neat collagen hydrolysate and uncoated paper, the peaks at 1125 cm−1 corresponding to collagen and 1029 cm−1 corresponding to uncoated paper shifted and formed a new peak at 1096 cm−1 because of hydrogen bonding between the –OH group of cellulose and –NH2 and –COOH groups of collagen, ensuring the coating effect of collagen to the uncoated paper.
O stretching vibration due to collagen hydrolysate. Compared with the neat collagen hydrolysate and uncoated paper, the peaks at 1125 cm−1 corresponding to collagen and 1029 cm−1 corresponding to uncoated paper shifted and formed a new peak at 1096 cm−1 because of hydrogen bonding between the –OH group of cellulose and –NH2 and –COOH groups of collagen, ensuring the coating effect of collagen to the uncoated paper.
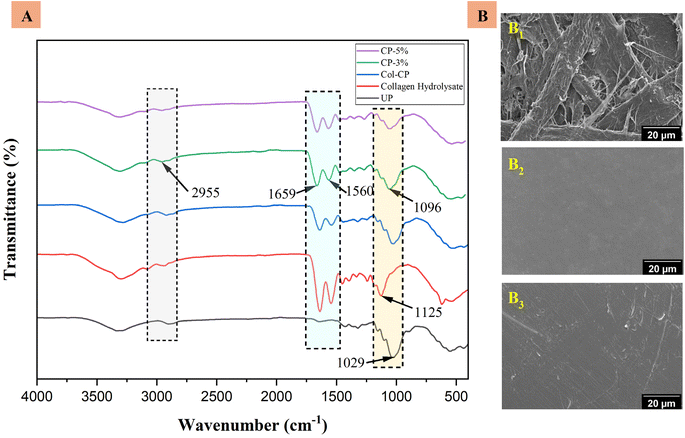 | ||
| Fig. 4 (A) FTIR spectra of collagen hydrolysate, uncoated base paper and coated paper. (B) FE-SEM image of UP (B1), CP-3% (B2), and CP-5% (B3). | ||
The FE-SEM images provide detailed information about the morphology of coated and uncoated paper, as shown in Fig. 4B. A smooth surface was observed when the uncoated paper was coated with CP-3%. However, as the concentration of CNCs in the CP-5% coated paper increased, agglomeration occurred between the CNC particles, which reduced the uniformity of the coating layer on the paper.
Grease resistance KIT tests were also conducted on coated and uncoated paper samples using 12 different oil and grease concentrations, as specified in the TAPPI standard. In Fig. 5A, the uncoated base paper (UP) failed to pass KIT rating 1, whereas the coated paper samples (CP-1%, CP-3%, CP-5%, CP-7%, and CP-10%) passed the highest KIT rating 12, indicating better oil and grease resistance. Oil and grease are nonpolar substances, whereas CNCs and glycerol have polar hydroxyl groups that do not interact with nonpolar oil and grease. As a result, all the coated papers have oil and grease-repellent properties. Fig. 5B represents the hot oil test, which was performed to evaluate the oil barrier properties of both coated and uncoated papers, and the results are shown in Fig. 5B. The uncoated base paper (UP) failed the hot oil test. Still, all the coated papers passed the hot oil barrier test. Red stains were seen on the surface of UP and the tissue paper under it. No stains were observed on the surface of the coated paper or under its tissue papers because of the uniform coating and oil repellency of the CNC-based coating.
In water vapor transmission and air resistance tests, the coated paper exhibited higher barrier properties than the uncoated paper due to its smooth coating surface, on which CNCs were dispersed homogeneously. Here, the CNC-based coating acted as a reinforcement filler, decreasing the air permeability of the paper and forming a dense, compact structure through the penetration of cellulose crystals into the cellulose matrix of the paper, which resulted in strong hydrogen bonding. The decrease in water vapor permeability of the coated paper was attributed to the blocking of fiber gaps by CNCs and the hydrophobic properties of the collagen hydrolysate. However, as the percentage of CNCs increased, the value of water vapor resistance did not change much. Almost similar values for CP-5%, CP-7%, and CP-10% are shown in Fig. 6A. The air permeability values of coated paper samples incorporating different concentrations of CNCs are illustrated in Fig. 6B. The coated paper showed higher air barrier properties than uncoated paper. The higher porosity and air permeability of uncoated base paper were due to the presence of large pores, as shown in Fig. 4B1. Coated paper formed a dense, compact structure with CNCs, collagen hydrolysate, and glycerin on the surface of the uncoated-based paper, which provided a more tortuous pathway for the diffusion of air molecules and enhanced the air barrier performance.
The mechanical properties of packaging materials were evaluated to determine their stability and strength during handling, transportation, and storage of products in packaging. The tensile index and burst index of samples were measured, as shown in Fig. 7A and B. The coated paper CP-3% exhibited the highest tensile and burst index values due to the addition of CNCs, which reinforced the uncoated paper by making the polymers with a cellulose matrix more rigid and compact. The enhanced mechanical properties are due to increased interaction between the OH groups of CNCs and the available groups (COOH, NH2, C(NH2)2, and CONH2) of collagen hydrolysate with the OH group of cellulose of uncoated paper. For the CP-3% coating sample, the ultimate tensile index was 74.43 ± 2.895 N m g−1, which was 5.28 times higher than that of the UP sample (14.10 ± 0.315 N m g−1). The burst index for the CP-3% coating sample was 2.10 ± 0.09 kPa m2 g−1, which was 1.79 times higher than that of the UP sample (1.17 ± 0.15). The tensile and burst indexes decreased slightly for coated paper samples (CP-5%, CP-7%, and CP-10%) due to the agglomeration of CNCs at increased concentrations, resulting from non-uniform coating. Fig. 7C shows that the percentage of elongation decreased in CP-1% and then slightly increased due to the presence of CNC content in the coating. The presence of CNCs and collagen on the paper did not significantly impact the elongation. Fig. 7D depicts the bending properties of the coated paper, where no crease was observed due to folding or stretching, as the presence of coating added flexibility to the coated paper.
TGA and DTGA were used to depict the thermal behavior of uncoated and coated papers over a temperature range of 50–620 °C. The incorporation of CNCs and collagen hydrolysate into uncoated paper resulted in a delay in the mass loss of the coated papers within the temperature range (Fig. 7E). The DTGA curves for coated papers (Fig. 7F) showed less degradation of the CP-3%, CP-5%, CP-7%, and CP-10% samples than the uncoated papers. This could be related to the strong interactions between the cellulosic matrix of the paper and the coating solution, as identified by FTIR spectroscopy (Fig. 4A).
The hydrophilic properties of the uncoated base paper (UP) and coated paper samples were measured using the water contact angle (WCA), as shown in Fig. 8A. The WCA of UP was found to be 60.13° due to the porosity of the paper and higher interaction of the cellulosic matrices and water hydroxyls. In Fig. 8B, the WCA of CP-1% and CP-3% gradually increased from 69.25° to 99.40° because of the hydrophobic properties of collagen hydrolysate, as it covers the porous structure of the cellulosic matrix throughout the uniform coating of the paper, which significantly increased water repellency. However, the WCA of CP-5%, CP-7% and CP-10% gradually decreased to 97.71°, 92.59° and 83.51°. As the concentration of CNCs increased, the presence of more surface hydroxyl groups reduced the contact angle. Fig. 8D illustrates how surface roughness was reduced with a uniform coating of CNCs and collagen hydrolysate nanoparticles due to the uniform distribution of coating layers, which filled the gaps in the cellulose fiber matrix. As the WCA values gradually decreased in the case of CP-5%, CP-7%, and CP-10%, only CP-3% and CP-5% were considered for surface roughness tests. The non-uniform coating of CP-5% due to the agglomeration of CNCs at increased concentrations resulted in an uneven distribution of cellulose nanoparticles, thereby enhancing the surface roughness. Uniform coating resulted in a reduction of both average surface roughness and average roughness depth. Among them, CP-3% showed the best results, as shown in Fig. 8D.
A cytotoxicity assessment of the UP (control) and paper-coated CP-3% samples was conducted. The tested papers were found to be non-toxic when the test was conducted using a Vero cell line for DMEM (Dulbecco's modified Eagle's medium) in Fig. 9B. Direct contact with the UP and CP-3% resulted in cell viability of more than 95% after 48 h of incubation. No toxic effect was found in the coated papers. Cells were found to be alive, indicating no cytotoxic effect. Therefore, the paper could be used as a packaging material for food.
 | ||
| Fig. 9 Cytotoxic effect assessment of (A) uncoated base paper and (B) coated paper sample (CP-3%) using water as the solvent. | ||
The biodegradability of plastics (polyethylene and polystyrene), uncoated base paper (UP), and coated papers was assessed using a soil burial test.5,37 The results of the soil degradation test are depicted in Fig. 10. The UP sample and coated paper samples (CP-3%, CP-5%, and CP-7%) took almost 7 weeks to degrade fully. The coating did not delay the biodegradability of the paper samples by enhancing the barrier and physical properties. However, plastic samples were not changed after 11 weeks because microorganisms cannot quickly degrade synthetic polymers.
4 Conclusions
The application of CNCs, extracted from MOW, collagen hydrolysate, and glycerin on a paper substrate, introduced a new pathway for the development of bio-based coating applications. The crystallinity index of CNCs was around 87%, and the average length and diameter were approximately 104.08 ± 0.1 nm and 9.49 ± 0.3 nm, respectively. The modification was confirmed by the presence of FTIR peaks at 1096 cm−1 and 1020 cm−1, which ensured the hydrogen bonding between the –OH group of cellulose and the –NH2 and –COOH groups of collagen hydrolysate. FE-SEM images were acquired to investigate the surface morphology of the uncoated and coated samples, revealing a uniform coating on the surface of CP-3%. The barrier tests were conducted, and all coated paper samples passed; however, the uncoated paper failed in both the KIT and hot oil barrier tests. The water vapor resistance and air resistance of CP-3% increased by 14 times and 250 times, respectively, compared to the uncoated base paper, due to the uniform coating. The tensile index and burst index of CP-3% increased by 5.28 times and 1.79 times, respectively, compared to the uncoated paper. The water contact angle of the sample CP-3% increased by 39%, and the surface roughness decreased by 64.02%. No cytotoxic effect was observed in the CP-3% sample, with more than 95% of cells surviving. All the uncoated and coated paper samples took almost 77 days to fully degrade, whereas plastics such as polyethylene and polystyrene did not degrade. All the results proved that the CP-3% sample was highly suitable for packaging applications.Ethical statement
The cytotoxic study was conducted in strict accordance with the Guidelines for the Care and Use of Laboratory Animals of the Centre for Advanced Research in Sciences at the University of Dhaka, utilizing their commercial services. The experimental procedures were approved by the Committee on the Ethics of Animal Experiments of the Centre for Advanced Research in Sciences at the University of Dhaka. In brief, Vero (a kidney cell line from an African green monkey) was kindly provided by Professor H. Hoshino (Gunma University Graduate School of Medicine, Gunma, Japan) and maintained in DMEM (Dulbecco's modified Eagle's medium) containing 1% penicillin–streptomycin (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), 0.2% gentamycin and 10% fetal bovine serum (FBS).
1), 0.2% gentamycin and 10% fetal bovine serum (FBS).
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.Author contributions
K. S. S. suggested the idea, designed the project, and secured the funding. A. B. and N. R. K. performed experiments and data analysis, prepared the figures, and wrote the manuscript. N. A. J. and M. I. H. E. performed the experiments, and M. B., M. M. R., A. F. M. M. R., M. R., L. P., and M. S. reviewed the manuscript. K. S. S. directed and monitored the research and reviewed and edited the manuscript. All authors significantly contributed to the writing of the manuscript.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This research was conducted in the Soft Materials Research Laboratory. Applied Chemistry and Chemical Engineering, University of Dhaka. The study of mechanical properties and water vapor permeability, and TGA were conducted at the Institute of Leather Engineering and Technology, University of Dhaka. The air permeability test was conducted at the Pulp and Paper Research Division (PPRD) of BCSIR, Dhaka. TEM, SEM, contact angle, and surface roughness tests were conducted in the Department of Forest Biomaterials at North Carolina State University. The authors acknowledge the funding support from the Grants for Advanced Research in Education (Project ID: ET-20222100), BANBEIS, Ministry of Education, Government of Bangladesh.References
- W. H. Danial, Z. Abdul Majid, M. N. Mohd Muhid, S. Triwahyono, M. B. Bakar and Z. Ramli, Carbohydr. Polym., 2015, 118, 165–169 CrossRef CAS PubMed.
- S. J. Risch, J. Agric. Food Chem., 2009, 57, 8089–8092 CrossRef CAS PubMed.
- I. Rahmawati, A. W. Pratama, S. A. Pratama, M. N. Khozin, A. Firmanda, F. H. Irawan, Asranudin, A. N. M. Ansori and T. H. Sucipto, Case Stud. Chem. Environ. Eng., 2024, 10, 100776, DOI:10.1016/j.cscee.2024.100776.
- L. K. Ncube, A. U. Ude, E. N. Ogunmuyiwa, R. Zulkifli and I. N. Beas, Materials, 2020, 13, 4994 CrossRef CAS PubMed.
- K. S. Salem, M. Debnath, S. Agate, K. M. Y. Arafat, H. Jameel, L. Lucia and L. Pal, Carbohydr. Polym. Technol. Appl., 2024, 7, 100421 CAS.
- M. M. A. Allouzi, D. Y. Y. Tang, K. W. Chew, J. Rinklebe, N. Bolan, S. M. A. Allouzi and P. L. Show, Sci. Total Environ., 2021, 788, 147815 CrossRef CAS PubMed.
- J. R. Jambeck, R. Geyer, C. Wilcox, T. R. Siegler, M. Perryman, A. Andrady, R. Narayan and K. L. Law, Science, 2015, 347, 768–771 CrossRef CAS PubMed.
- M. Revel, A. Châtel and C. Mouneyrac, Curr. Opin. Environ. Sci. Hlth., 2018, 17–23 CrossRef.
- D. Biryol, C. I. Nicolas, J. Wambaugh, K. Phillips and K. Isaacs, Environ. Int., 2017, 108, 185–194 CrossRef CAS.
- J. N. Hahladakis, C. A. Velis, R. Weber, E. Iacovidou and P. Purnell, J. Hazard. Mater., 2018, 179–199 CrossRef CAS.
- N. Evode, S. A. Qamar, M. Bilal, D. Barceló and H. M. N. Iqbal, Case Stud. Chem. Environ. Eng., 2021, 4, 100142 CrossRef CAS.
- D. Mahapatra, J. Food Process Eng., 2024, 165–187 Search PubMed.
- P. Ezati, A. Khan, R. Priyadarshi, T. Bhattacharya, S. K. Tammina and J. W. Rhim, Food Hydrocolloids, 2023, 108771 CrossRef CAS.
- N. M. Stark and L. M. Matuana, Mater. Today Sustain., 2021, 15, 100084 Search PubMed.
- C. Andersson, Packag. Technol. Sci., 2008, 339–373 CrossRef CAS.
- B. G. Hermann, L. Debeer, B. De Wilde, K. Blok and M. K. Patel, Polym. Degrad. Stab., 2011, 96, 1159–1171 CrossRef CAS.
- C. L. Reichert, E. Bugnicourt, M. B. Coltelli, P. Cinelli, A. Lazzeri, I. Canesi, F. Braca, B. M. Martínez, R. Alonso, L. Agostinis, S. Verstichel, L. Six, S. De Mets, E. C. Gómez, C. Ißbrücker, R. Geerinck, D. F. Nettleton, I. Campos, E. Sauter, P. Pieczyk and M. Schmid, Polymers, 2020, 12, 1558 CrossRef CAS PubMed.
- C. Slaggert, Kraft Heinz Online, https://news.kraftheinzcompany.com/press-releases-details/2023/Kraft-Heinz-Announces-Goal-to-Reduce-the-Use-of-Virgin-Plastic-Globally-by-20-Percent--or-more-than-100-Million-Pounds--by-2030/default.aspx Search PubMed.
- McDonald’s Corporation, https://corporate.mcdonalds.com/corpmcd/our-stories/article/renewable_packaging.html.
- V. Kumar, P. Pathak and N. K. Bhardwaj, Waste Manag., 2021, 102, 281–303, DOI:10.1016/j.wasman.2019.10.041.
- M. Debnath, K. S. Salem, V. Naithani, N. Starrett, E. Musten, M. A. Hubbe and L. Pal, in TAPPICon 2023 – ‘Rock the Roll: Unleashing the Harmonies of the Paper Industry’, TAPPI Press, 2023 Search PubMed.
- P. Bajpai, in Recycling and Deinking of Recovered Paper, Elsevier, 2024, pp. 57–87 Search PubMed.
- A. Singh, L. M. Varghese, R. Dutt Yadav and R. Mahajan, Environ. Sci. Pollut. Res., 2020, 45814–45823 CrossRef CAS PubMed.
- W. Wolska and E. Małachowska, Forestry and Wood Technology, 2023, 66–75 CrossRef.
- M. Zubair, N. D. Mu'azu, M. Nasir, M. S. Manzar, M. A. Aziz, M. Saleem and M. A. Al-Harthi, Arabian J. Sci. Eng., 2022, 47, 5377–5393 CrossRef CAS.
- Y. Ikeda, E. Y. Park and N. Okuda, Bioresour. Technol., 2006, 97, 1030–1035 CrossRef CAS PubMed.
- H. Abushammala, M. A. Masood, S. T. Ghulam and J. Mao, Sustainability, 2023, 15, 6915 CrossRef CAS.
- N. M. Stark, J. Renewable Mater., 2016, 14, 313–326 CrossRef.
- A. J. Sayyed, D. V. Pinjari, S. H. Sonawane, B. A. Bhanvase, J. Sheikh and M. Sillanpää, J. Environ. Chem. Eng., 2021, 9, 106626 CrossRef CAS.
- T. Rusconi, L. Riva, C. Punta, M. Solé and I. Corsi, Environ. Sci.: Nano, 2024, 11, 61–77 RSC.
- T. Kovacs, V. Naish, B. O'Connor, C. Blaise, F. Gagné, L. Hall, V. Trudeau and P. Martel, Nanotoxicology, 2010, 4, 255–270 CrossRef CAS PubMed.
- V. Dharini, A. Biswal, P. S. Sellamuthu, J. Jayaramudu and E. R. Sadiku, Int. J. Food Sci. Technol., 2024, 59, 318–332 CrossRef.
- A. Ferrer, L. Pal and M. Hubbe, Ind. Crops Prod., 2017, 95, 574–582 CrossRef CAS.
- C. Amara, A. El Mahdi, R. Medimagh and K. Khwaldia, Curr. Opin. Green Sustainable Chem., 2021, 31, 100512 CrossRef CAS.
- S. S. Ahankari, A. R. Subhedar, S. S. Bhadauria and A. Dufresne, Carbohydr. Polym., 2021, 255, 117479 CrossRef CAS.
- S. S. Nair, J. Zhu, Y. Deng and A. J. Ragauskas, Sustainable Chem. Processes, 2014, 2, 23 CrossRef.
- N. A. Jashi, M. Debnath, L. Lucia, L. Pal, M. M. Rahman and K. S. Salem, J. Polym. Environ., 2025, 33, 1814–1828 CrossRef CAS.
- K. S. Salem, N. K. Kasera, M. A. Rahman, H. Jameel, Y. Habibi, S. J. Eichhorn, A. D. French, L. Pal and L. A. Lucia, Chem. Soc. Rev., 2023, 52, 6417–6446 RSC.
- L. Segal, J. J. Creely, A. E. Martin and C. M. Conrad, Text. Res. J., 1952, 29, 43 Search PubMed.
- B. Deepa, E. Abraham, N. Cordeiro, M. Mozetic, A. P. Mathew, K. Oksman, M. Faria, S. Thomas and L. A. Pothan, Cellulose, 2015, 22, 1075–1090 CrossRef CAS.
- K. S. Salem, H. R. Starkey, L. Pal, L. Lucia and H. Jameel, ACS Sustain. Chem. Eng., 2020, 8, 1471–1478 CrossRef CAS.
- P. Tyagi, L. A. Lucia, M. A. Hubbe and L. Pal, Carbohydr. Polym., 2019, 206, 281–288 CrossRef CAS PubMed.
- V. Muralidharan, M. S. Arokianathan, M. Balaraman and S. Palanivel, Polym. Test., 2020, 81, 106250 CrossRef CAS.
Footnote |
| † Co first author. |
| This journal is © The Royal Society of Chemistry 2025 |