Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Advances in mercury ion sensing using BODIPY-based compounds: a sexennial update
Supriya Routray
,
Subhadeep Acharya
 ,
Laxmipriya Nayak
,
Simran Pattnaik
and
Rashmirekha Satapathy
,
Laxmipriya Nayak
,
Simran Pattnaik
and
Rashmirekha Satapathy
 *
*
Department of Chemistry, Ravenshaw University, Cuttack-753003, Odisha, India. E-mail: rashmi@ravenshawuniversity.ac.in
First published on 1st April 2025
Abstract
Pollution from mercury ions (Hg2+) continues to pose a significant threat to the environment and public health because of its extreme toxicity and bioaccumulative nature. BODIPY-based compounds are emerging as strong candidates for creating selective and sensitive chemosensors for mercury ion detection. Their structural tunability facilitates the introduction of various functional groups, improving their binding affinity and specificity toward mercury ions. This review elucidates various sensing mechanisms and provides comprehensive insights into the performance of these sensors, particularly with regard to selectivity, sensitivity, and detection limits. The synthetic routes for synthesizing the chemosensors are mentioned in detail. Given their reliability and flexibility, BODIPY-based sensors are poised to make significant contributions in the fields of both sensors and analytical chemistry.
1. Introduction
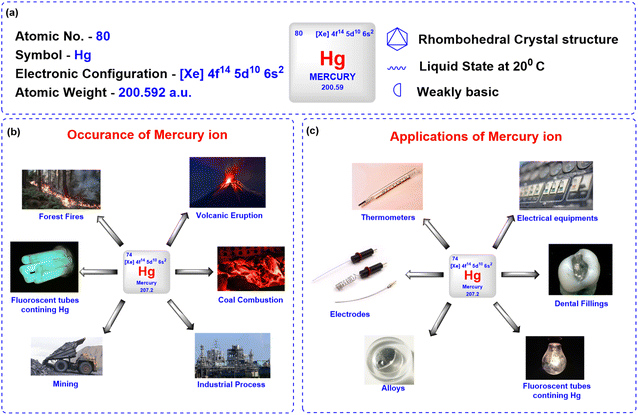
Mercury ions pose a significant threat to environmental safety and human health due to their toxicity and tendency to bioaccumulate in both natural and artificial environments.1 Naturally occurring in the Earth's crust, mercury exists in various forms such as elemental form (liquid metal), inorganic compound form (e.g., mercuric chloride, mercuric sulfide), and organic compound form like methylmercury, which is a potent neurotoxin that accumulates in food chains. Additionally, mercury can appear as ions, mainly mercurous (Hg22+) and mercuric (Hg2+).2 Natural sources, such as volcanic eruptions and wildfires, contribute to mercury emissions; however, human activities, including mining, coal combustion, industrial processes, and waste incineration, are the primary contributors to mercury pollution (Fig. 1).3Mercury has been widely utilized in various applications due to its distinct chemical and physical properties, including its liquid state at room temperature and high density. In laboratories, mercury is commonly used in the manufacturing of thermometers, barometers, and diffusion pumps, as well as in sphygmomanometers for medical purposes (Fig. 1).3 Its excellent electrical conductivity makes it valuable in the production of electrical switches, relays, and other electronic devices. Mercury serves as a liquid electrode in electrochemical processes, such as the industrial synthesis of chlorine and sodium hydroxide.4 In the chemical industry, mercury acts as a catalyst in various reactions, while its ability to form amalgams with metals like gold and silver has historically been exploited in dental fillings and gold extraction processes. Additionally, mercurous chloride, a mercury compound, has been used in medicine as a purgative and as a reference electrode (calomel) in electrochemical measurements. However, due to its toxicity, the use of mercury in many of these applications is now regulated or being phased out in favour of safer alternatives.
Despite its utility in various sectors, mercury's toxic effects are profound. The World Health Organization (WHO) and the US Environmental Protection Agency (EPA) set strict allowable limits for mercury in drinking water, highlighting the serious risks associated with exposure. The World Health Organization (WHO) has established a permissible limit of mercury ions in drinking water at 0.5 μg L−1. In contrast, the US Environmental Protection Agency (EPA) has determined the acceptable limit for inorganic mercury in drinking water to be 0.002 mg L−1 (2 ppb).
Exposure to mercury has profound and far-reaching consequences on human health and the environment. Mercury can severely damage the central nervous system, kidneys, and immune system, leading to cognitive impairments, neuromuscular disorders, and kidney dysfunction.5 Methylmercury, a highly toxic organic form, is particularly harmful to developing fetuses and infants, as it can cross the placental barrier and disrupt normal brain development, causing long-term neurological damage.6–9 Acute mercury exposure can result in symptoms such as headaches, tremors, insomnia, and respiratory irritation, while chronic exposure may lead to Minamata disease, characterized by severe motor and sensory impairments (Fig. 2).10–13 Mercury also disrupts proteins and enzymes, causing oxidative stress, endocrine disturbances, and altered gut flora.14 Additionally, it acts as an epigenetic toxicant, potentially influencing gene expression in future generations. Environmental contamination from mercury persists throughout the food chain, with bioaccumulation in fish posing a significant risk to human populations that rely on seafood as a dietary staple.15,16 The severity of these adverse effects underscores the need for stringent regulatory measures and innovative detection methods to mitigate mercury contamination.
Fluorescent sensors have emerged as a compelling alternative17 to conventional methods such as reduced graphene oxide field-effect transistor (rGO FET), cold vapor integrated quartz crystal microbalance (CV-QCM), electrochemical analysis, atomic absorption spectrometry, gas chromatography-triple quadrupole mass spectrometry (GC-MS/MS), mercury analyzers, and mass spectrometry, which often require expensive equipment and are too cumbersome for on-site or live-cell detection.18–24 These sensors are particularly advantageous for metal ion detection because of their simplicity, quick response, and low detection limits. Unlike traditional approaches, fluorescent sensors enable real-time monitoring without complex sample preparation, making them ideal for dynamic imaging in living cells and a wide range of biomedical and environmental applications.25–31 Several mechanisms facilitate analyte detection, including ET (energy transfer), PET (photo-induced electron transfer),32–34 CHEF (chelation-enhanced fluorescence),35–37 FRET (Förster resonance energy transfer)38–41 and ESIPT (excited state intramolecular proton transfer).42,43 By leveraging carefully designed fluorophore–ligand systems, these sensors achieve extreme sensitivity and outstanding selectivity, reducing interference from different metal ions and ensuring accurate detection of low mercury concentrations in aqueous environments (Fig. 3). Furthermore, their ability to integrate into portable devices or test strips enhances their practicality and cost-effectiveness for on-site metal analysis, underscoring their versatility in real-world scenarios.44,45 A notable challenge in the realm of metal ion sensing is the targeted identification of mercury ions. Consequently, the development of differential sensing methodologies for Hg2+ has emerged as a significant area of research. To this end, diverse rational design strategies utilizing fluorophore scaffolds such as cyanine, rhodamine, fluorescein, coumarin, anthracene, and BODIPY-based derivatives have been instrumental in creating novel functional fluorescent probes capable of sensitively and selectively detecting mercury (Hg2+) ions.46–51
4,4-Difluoro-4-bora-3a,4a-diaza-s-indacene (BODIPY) is a highly versatile fluorophore renowned for its exceptional photonic and electronic characteristics (Fig. 4).52,53 Since its discovery in 1968 by Treibs and Kreuzer,54 BODIPY-based compounds have garnered extensive attention across diverse fields, including biochemistry, materials science, physics, and electronics.55 Its unique features, such as excellent absorption and emission in the UV-vis region, high fluorescence quantum yields, and molar extinction coefficients with remarkable photochemical and thermal stability, have established BODIPY as a pivotal building block in the development of advanced fluorescent materials.56,57 Furthermore, its highly conjugated core and tunable electronic properties make it an ideal candidate for functionalization and structural modification.58,59 Moreover, anchoring BODIPY compounds onto a solid support offers several practical benefits. This approach not only enhances sensor performance by increasing operational stability but also minimizes issues such as aggregation and leaching while enabling more straightforward integration into portable devices and sensor platforms. An analysis of the literature on BODIPY-based fluorescent sensors showed that 1183 research papers were published over the past decade (2015–2025). The analysis reveals an upward trend in the number of publications and citations over time, indicating a substantial increase in interest in this rapidly growing field (Fig. 5).
 | ||
| Fig. 4 (a) Structure and different substitution positions of BODIPY; (b) key characteristics of BODIPY. | ||
 | ||
| Fig. 5 Analysis of publications and citations of BODIPY-based fluorescent sensors from 2015–2025 (Web of Science). | ||
The versatile characteristics of BODIPY have been harnessed to design BODIPY-based fluorescent sensors capable of detecting a broad spectrum of analytes, including anions,60 cations,61,62 phosgene,63 and nerve gases.64,65 BODIPY scaffolds stand out as particularly promising for mercury ion detection as these sensors exhibit fluorescence changes, manifested by turn-on or turn-off or colorimetric responses, providing a simple and visually observable detection mechanism. Incorporating specific receptors or functional groups into the BODIPY scaffold enables the creation of chemosensors that exhibit excellent selectivity and sensitivity towards mercury ions.
While a number of comprehensive reviews in the last decade exist detailing the broader development of BODIPY-based sensors, a dedicated analysis focusing specifically on the synthesis and mechanisms of BODIPY-based mercury ion sensors remains absent from the literature.56,66–69 This review addresses this gap by examining recent advancements (2019–2024) in BODIPY-based chemosensors designed for mercury ion detection. To begin, we present and outline the general principles of designing effective fluorescent imaging probes, as well as their various sensing methods, to better understand rational design approaches and discuss how these fluorescent probes interact with analytes. Then, we comprehensively analyze their colorimetric and fluorogenic properties, utilizing data obtained from different in situ and ex situ spectroscopic techniques like fluorescence, UV-vis, and NMR spectroscopic techniques across diverse solvent systems. The central objective is to provide an in-depth understanding of these sensors, with a particular emphasis on synthetic strategies, binding mechanisms, complex stoichiometry, and the thermodynamics governing host–guest interactions. The review is structured thematically, with subsections dedicated to different types of substitution on the BODIPY core. Ultimately, we wrap up with a discussion of the realities and future challenges in this field (Fig. 6).
2. Photophysical sensing mechanism of fluorescent sensors
The design of fluorescent sensors prioritizes key attributes such as selectivity, stability, sensitivity, fluorescence brightness, signal modulation, pH independence, spectral compatibility, and binding affinity to ensure accurate and dependable analyte detection (Fig. 7).70 These indicators operate by exhibiting changes in fluorescence properties, such as intensity or emission wavelength, upon interaction with a target analyte. Central to their functionality are photophysical sensing mechanisms, which enable the conversion of chemical interactions into measurable optical signals, such as fluorescence or absorption. These mechanisms offer high sensitivity, selectivity, and the capability to monitor analytes in real time. Commonly utilized mechanisms include PET (photo-induced electron transfer), CHEF (chelation-enhanced fluorescence), FRET (Förster resonance energy transfer), ICT (intramolecular charge transfer), AIE (aggregation-induced emission), and ESIPT (excited state intramolecular proton transfer), each offering distinct advantages tailored to specific sensing applications.71 Below is a concise overview of various fluorescence-sensing mechanisms.2.1 Photoinduced electron transfer (PET)
Photoinduced Electron Transfer (PET) is a fundamental mechanism in fluorescence sensing, characterized by the transfer of an electron between a fluorophore and a recognition unit, which modulates the fluorescence signal. Typically, PET-based systems are designed with a fluorophore linked to a recognition site via a spacer, allowing for selective interactions with target analytes (Fig. 8). The process is governed by the alignment of molecular orbital energy levels, where the highest occupied molecular orbital (HOMO) of the recognition unit lies between the HOMO and lowest unoccupied molecular orbital (LUMO) of the fluorophore. In the absence of analyte binding, PET leads to fluorescence quenching through nonradiative electron transfer. However, upon analyte binding, the energy levels are altered, inhibiting PET and restoring fluorescence. This mechanism allows PET sensors to function as “on-off” or “off-on” switches, making them highly sensitive tools for detecting ions, biomolecules, and environmental changes.722.2 The intramolecular charge transfer (ICT)
The Intramolecular Charge Transfer (ICT) mechanism, first introduced by Valeur, serves as a foundational strategy for designing fluorescent chemosensors. This approach relies on a conjugated π-system that integrates an electron-donating unit (D) and an electron-accepting unit (A), thereby forming a “push–pull” electronic configuration. Upon excitation, charge redistribution occurs, with the HOMO localized near the donor and the LUMO near the acceptor, generating an intense dipole moment (Fig. 9).73 Analyte binding modulates this electronic system: interactions at the donor site reduce its electron-donating capacity, weakening the ICT and inducing a blue shift in absorption/emission spectra. Conversely, interactions at the acceptor site enhance ICT, producing a red shift. These spectral shifts, coupled with changes in fluorescence quantum yields and lifetimes, enable ratiometric sensing—a method resistant to environmental interference. The direct conjugation of the receptor and fluorophore (without spacers) ensures efficient electronic communication, allowing precise modulation of optical properties. ICT-based probes are widely employed for detecting cations, protons, and polar analytes, leveraging their sensitivity to solvent polarity and analyte-induced electronic perturbations.2.3 Fluorescence resonance energy transfer (FRET)
Fluorescence Resonance Energy Transfer (FRET) is a photophysical phenomenon involving non-radiative energy transfer between two chromophores, an acceptor and a donor mediated by dipole–dipole coupling (Fig. 10).38 Energy transfer happens when the donor's emission spectrum coincides with the acceptor's absorption spectrum, allowing for transfer over distances of 1–10 nm. FRET efficiency (E) exhibits an inverse sixth-power dependence on the donor–acceptor separation distance (E ∝ r−6), rendering it highly sensitive to nanoscale molecular proximity and conformational changes. This distance-dependent mechanism facilitates real-time monitoring of biomolecular interactions, such as protein binding and nucleic acid dynamics, with applications spanning biological imaging and ratiometric sensing of analytes like Hg2+ and Zn2+. The absence of photon emission during energy transfer distinguishes FRET from conventional fluorescence methods, allowing precise spatial resolution in dynamic molecular systems.2.4 Excited-state intramolecular proton transfer (ESIPT)
Excited-State Intramolecular Proton Transfer (ESIPT) is a photophysical process characterized by the ultrafast relocation of a proton between adjacent atoms within a molecule following photoexcitation, leading to tautomerization between enol (E) and keto (K) forms (Fig. 11).74–76 This mechanism is mediated by a pre-existing intramolecular hydrogen bond (H-bond) between a proton donor group, such as hydroxyl or amino, and an acceptor site, typically carbonyl oxygen or imine nitrogen. Upon excitation, the strengthened H-bond and charge redistribution in the excited state lower the energy barrier for proton transfer, enabling spontaneous conversion from the enol (E*) to keto (K*) tautomer in the S1 state. The process follows a four-level photocycle (E → E* → K* → K), where emission occurs from the keto form, resulting in significant Stokes shifts (>200 nm) due to significant structural and electronic differences between the tautomers. ESIPT-based systems exhibit enhanced photostability and reduced excited-state reactivity, making them ideal for designing fluorescent probes with environmental sensitivity. Derivatives like 2-(2-hydroxyphenyl)benzothiazole (HBT) demonstrate substituent-dependent dynamics, where electron-donating groups optimize H-bond strength and modulate energy barriers (∼2–3 kcal mol−1), as validated by time-dependent density functional theory (TDDFT) and experimental spectroscopic analyses. These properties enable applications in ratiometric sensing, bioimaging, and optoelectronic devices.3. Recent advances in BODIPY-based mercury ion sensors
3.1 Pillararene-BODIPY-based mercury ion sensor
Pillararenes represent a class of macrocyclic compounds characterized by a framework composed of hydroquinone or dialkoxybenzene units, typically ranging in number from five to ten. These units are interconnected at the para positions via methylene bridges. The inherent synthetic accessibility of pillararenes facilitates the attachment of diverse functional groups to their periphery. This modification strategically provides tailored interaction sites for specific applications such as cation recognition.77In 2023, Yemisci and colleagues created a new macrocyclic fluorescent probe 9 by integrating pillar[5]arene with BODIPY components for the specific detection of Hg2+ in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) acetonitrile and water solution.78 Spectroscopic investigations revealed a photoinduced electron transfer (PET) mechanism between the doner atoms of probe 9 and Hg2+ ions, supported by fluorescence quenching at 515 nm (1130 units to 110 units) and decrease in the UV-vis absorption spectra at 500 nm, which is attributed to complex formation between probe 9 and Hg2+ ion (Fig. 12). The sensor, through fluorometric analysis, demonstrated a detection limit of 0.2 μM, and Job plot analysis confirmed a 1
1 (v/v) acetonitrile and water solution.78 Spectroscopic investigations revealed a photoinduced electron transfer (PET) mechanism between the doner atoms of probe 9 and Hg2+ ions, supported by fluorescence quenching at 515 nm (1130 units to 110 units) and decrease in the UV-vis absorption spectra at 500 nm, which is attributed to complex formation between probe 9 and Hg2+ ion (Fig. 12). The sensor, through fluorometric analysis, demonstrated a detection limit of 0.2 μM, and Job plot analysis confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry. It has been found that probe 9 captures the Hg2+ ion at two separate sites: the amino groups of BODIPY units and the oxygen atoms in the pillar[5]arene framework. The heightened sensitivity of probe 9 to Hg2+ is attributed to the soft acid–soft base interactions (Scheme 1).
2 binding stoichiometry. It has been found that probe 9 captures the Hg2+ ion at two separate sites: the amino groups of BODIPY units and the oxygen atoms in the pillar[5]arene framework. The heightened sensitivity of probe 9 to Hg2+ is attributed to the soft acid–soft base interactions (Scheme 1).
 | ||
Fig. 12 (a) Fluorescence spectra of probe 9 in H2O/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (b) Absorption spectra of probe 9 in H2O/CH3CN (1 1). (b) Absorption spectra of probe 9 in H2O/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). (c) The interaction between probe 9 and Hg2+ in H2O/CH3CN (1 1). (c) The interaction between probe 9 and Hg2+ in H2O/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Reproduced with permission from ref. 78. Copyright 2023, Elsevier. 1). Reproduced with permission from ref. 78. Copyright 2023, Elsevier. | ||
Kursunlu and colleagues developed a novel dual-channel macrocyclic sensor (probe 14) by combining BODIPY fluorophores with pillar[5]arene for the selective detection of Hg2+ and Sn2+ in aqueous-acetonitrile media (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) (Scheme 2).62 In the fluorescence spectra, probe 14 displayed quenching upon interaction with Hg2+ due to photoinduced electron transfer (PET). Conversely, it increased fluorescence when interacting with Sn2+, attributed to chelation-enhanced fluorescence (CHEF) (Fig. 13). The sensor demonstrated a limit of detection (LOD) of 1.09 μM for Hg2+ and 0.31 μM for Sn2+ using fluorometric analysis. The analysis of the Job's plot established a 1
1 v/v) (Scheme 2).62 In the fluorescence spectra, probe 14 displayed quenching upon interaction with Hg2+ due to photoinduced electron transfer (PET). Conversely, it increased fluorescence when interacting with Sn2+, attributed to chelation-enhanced fluorescence (CHEF) (Fig. 13). The sensor demonstrated a limit of detection (LOD) of 1.09 μM for Hg2+ and 0.31 μM for Sn2+ using fluorometric analysis. The analysis of the Job's plot established a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding ratio between probe 14 and the Hg2+/Sn2+ ion. The sensor exhibited exceptional selectivity among competing metal ions and maintained functionality across a broad pH range (3–9).
2 binding ratio between probe 14 and the Hg2+/Sn2+ ion. The sensor exhibited exceptional selectivity among competing metal ions and maintained functionality across a broad pH range (3–9).
 | ||
Fig. 13 (a) Emission spectra of probe 14 in H2O/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (1 μM) in presence of various metal ions. (b) The absorption spectra of probe 14 in H2O/CH3CN (1 1) (1 μM) in presence of various metal ions. (b) The absorption spectra of probe 14 in H2O/CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (1 μM) in presence of various metal ions. Reproduced with permission from ref. 62. Copyright 2022, Elsevier. 1) (1 μM) in presence of various metal ions. Reproduced with permission from ref. 62. Copyright 2022, Elsevier. | ||
3.2 Rhodamine-BODIPY-based mercury ion sensor
Rhodamine is a family of xanthene-based dyes known for their vibrant fluorescence and excellent photophysical properties. Due to their strong absorption and emission characteristics, these dyes are widely utilized in diverse fields, including water tracing, biotechnology, and materials science. In the realm of sensing, rhodamine-based compounds have gained prominence as chemosensors for detecting ions (e.g., Hg2+, Cu2+), anions (e.g., CN−), and biomolecules (e.g., ATP).79,80 These sensors rely on rhodamine's unique “turn-on” fluorescence mechanism triggered by specific interactions with target analytes.In 2019, Shi et al. developed two BODIPY-based fluorescent featuring bis(1,2,3-triazole)amino receptors (probe 19) and its rhodamine-substituted analogous (probe 20) for selective Hg2+ detection in acetonitrile-aqueous media (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) (Scheme 3).81 Probe 19 displayed enhanced fluorescence intensity with an 80-fold increase in fluorescence quantum yield upon interaction with 60 equivalent Hg2+ ions. Meanwhile, other evaluated metal ions such as Cd2+, Zn2+, Pb2+, Cu2+, Ba2+, Ni2+, Co2+, Ag+, Mg2+, Ca2+, K+, and Na+ did not change the emission spectra significantly. The fluorescence enhancement of probe 19 could be attributed to the inhibition of PET quenching following interaction with Hg2+. The rhodamine-substituted analogous (probe 20) displayed similar fluorescence enhancement ascribed to FRET arising from rhodamine moieties (Fig. 14). Job's plot analysis revealed a 1
1 v/v) (Scheme 3).81 Probe 19 displayed enhanced fluorescence intensity with an 80-fold increase in fluorescence quantum yield upon interaction with 60 equivalent Hg2+ ions. Meanwhile, other evaluated metal ions such as Cd2+, Zn2+, Pb2+, Cu2+, Ba2+, Ni2+, Co2+, Ag+, Mg2+, Ca2+, K+, and Na+ did not change the emission spectra significantly. The fluorescence enhancement of probe 19 could be attributed to the inhibition of PET quenching following interaction with Hg2+. The rhodamine-substituted analogous (probe 20) displayed similar fluorescence enhancement ascribed to FRET arising from rhodamine moieties (Fig. 14). Job's plot analysis revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry in CH3CN for the interactions between the probes and Hg2+ ions, with binding constants determined to be 1.2 ± 0.1 × 1022 M−2 and 1.3 ± 0.3 × 1010 M−2 for probes 19 and 20 respectively. The LOD was found to be 0.01 μM for 19 and 0.44 μM for 20. The higher LOD of probe 19 can be attributed to the bulky rhodamine moiety, which may impede efficient analyte binding. Proton NMR titration displayed broadened and downfield shifted triazole proton singlet in probes 19 and 20 due to binding with Hg2+ ions.
2 binding stoichiometry in CH3CN for the interactions between the probes and Hg2+ ions, with binding constants determined to be 1.2 ± 0.1 × 1022 M−2 and 1.3 ± 0.3 × 1010 M−2 for probes 19 and 20 respectively. The LOD was found to be 0.01 μM for 19 and 0.44 μM for 20. The higher LOD of probe 19 can be attributed to the bulky rhodamine moiety, which may impede efficient analyte binding. Proton NMR titration displayed broadened and downfield shifted triazole proton singlet in probes 19 and 20 due to binding with Hg2+ ions.
 | ||
Fig. 14 (a) Emission spectra of 19 (2 μm) in CH3CN/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) upon interaction with different metal ions and the color shifts when exposed to a UV lamp at 365 nm. (b) Emission spectra of 20 (2 μm) in CH3CN/H2O (9 1, v/v) upon interaction with different metal ions and the color shifts when exposed to a UV lamp at 365 nm. (b) Emission spectra of 20 (2 μm) in CH3CN/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) upon interaction with different metal ions and the color shifts when exposed to a UV lamp at 365 nm. Reproduced with permission from ref. 81. Copyright 2019, Wiley-VCH. 1, v/v) upon interaction with different metal ions and the color shifts when exposed to a UV lamp at 365 nm. Reproduced with permission from ref. 81. Copyright 2019, Wiley-VCH. | ||
Wen et al. developed an innovative BODIPY-Rhodamine-based probe 27 for the selective detection of Hg2+ ions, employing a fluorescence resonance energy transfer (FRET) mechanism, with BODIPY serving as the energy donor and Rhodamine functioning as the energy acceptor (Scheme 4).82 Spectroscopic measurements were conducted in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) mixture of methanol and water at pH 6.0. By the addition of Hg2+ ions, a distinct increase in absorbance at approximately 554 nm (characteristic of the Rhodamine moiety) was observed in the UV-vis spectra. This spectral change was accompanied by a visible colorimetric response, with the probe solution changing from yellow to pink under ambient light and from green to orange under UV irradiation. Experimental evidence indicates that Hg2+ ions facilitate the conversion of the rhodamine moiety from its spirolactam form to an open-ring structure. Fluorescence studies demonstrated a significant enhancement in emission intensity with the gradual addition of Hg2+ ions (Fig. 15). Compared to the blank solution, the fluorescence spectrum of the Hg2+-treated probe exhibited a new emission band at 586 nm, while the intensity of the original emission band at 513 nm decreased. This “turn-on” fluorescence response is attributed to the efficient energy transfer from the excited BODIPY donor to the Rhodamine acceptor. The LOD for Hg2+ ions using probe 27 was determined to be 0.3 μM. The colorimetric and fluorometric responses showed remarkable selectivity for Hg2+ ions, displaying no significant changes with other metal ions present.
3 (v/v) mixture of methanol and water at pH 6.0. By the addition of Hg2+ ions, a distinct increase in absorbance at approximately 554 nm (characteristic of the Rhodamine moiety) was observed in the UV-vis spectra. This spectral change was accompanied by a visible colorimetric response, with the probe solution changing from yellow to pink under ambient light and from green to orange under UV irradiation. Experimental evidence indicates that Hg2+ ions facilitate the conversion of the rhodamine moiety from its spirolactam form to an open-ring structure. Fluorescence studies demonstrated a significant enhancement in emission intensity with the gradual addition of Hg2+ ions (Fig. 15). Compared to the blank solution, the fluorescence spectrum of the Hg2+-treated probe exhibited a new emission band at 586 nm, while the intensity of the original emission band at 513 nm decreased. This “turn-on” fluorescence response is attributed to the efficient energy transfer from the excited BODIPY donor to the Rhodamine acceptor. The LOD for Hg2+ ions using probe 27 was determined to be 0.3 μM. The colorimetric and fluorometric responses showed remarkable selectivity for Hg2+ ions, displaying no significant changes with other metal ions present.
 | ||
Fig. 15 (a) Fluorescence emission of probe 27 (10 μM) with and without various metal ions (100 μM); (b) color change of probe 27 (10 μM) with different concentrations of Hg2+ (0–16 μM) in MeOH/20 mM HEPES buffer (V/V = 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, pH = 6.0) under the daylight. Reproduced with permission from ref. 82. Copyright 2021, Elsevier. 3, pH = 6.0) under the daylight. Reproduced with permission from ref. 82. Copyright 2021, Elsevier. | ||
3.3 Thiosemicarbazide-BODIPY-based mercury ion sensor
Thiosemicarbazide, a versatile organosulfur compound, is widely recognized for its strong ability to coordinate with metal ions thanks to its electron-donating nitrogen and sulfur atoms. This property makes it a valuable reagent in analytical chemistry, particularly for detecting transition and heavy metal ions. Its chemical structure enables it to form stable complexes with metals, facilitating its use in various sensing platforms.83 In sensing mercury ions (Hg2+), thiosemicarbazide-based compounds have proven highly effective because of their high affinity towards mercury ions and capacity to induce distinct optical or fluorescence changes upon binding.In 2019, Haldar and Lee developed a water-soluble polymeric chemosensor (probe 32) integrating BODIPY fluorophores and thiosemicarbazone receptors for selective Hg2+ detection and removal in pure aqueous media (Scheme 5).84 The copolymer exhibited a turn-on fluorescence response at 545 nm (ΦF = 0.17) upon Hg2+ coordination, achieving a detection limit of 0.37 μM in HEPES buffer (pH 7.4). Spectroscopic analysis showed that the binding of Hg2+ inhibited isomerization at the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the thiosemicarbazone group, thereby restoring the inherent fluorescence of BODIPY. Job plot analysis confirmed a 2
N bond of the thiosemicarbazone group, thereby restoring the inherent fluorescence of BODIPY. Job plot analysis confirmed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between probe 32 and Hg2+, with 1H NMR indicating upfield shifts of imino and methyne protons upon complexation. The sensor demonstrated exceptional selectivity among 20 competing metal ions and maintained functionality in aqueous solution, addressing hydrolysis issues observed in its small-molecule analog (probe 30). Notably, probe 32 enabled 86% Hg2+ removal from contaminated water via precipitation of coordination complexes, validated by ICP-OES analysis.
1 binding stoichiometry between probe 32 and Hg2+, with 1H NMR indicating upfield shifts of imino and methyne protons upon complexation. The sensor demonstrated exceptional selectivity among 20 competing metal ions and maintained functionality in aqueous solution, addressing hydrolysis issues observed in its small-molecule analog (probe 30). Notably, probe 32 enabled 86% Hg2+ removal from contaminated water via precipitation of coordination complexes, validated by ICP-OES analysis.
Chen et al. reported the development of a novel fluorescence sensor (probe 37) for selective detection of Hg2+ ions based on a thiosemicarbazide-functionalized boron dipyrromethene (BODIPY) derivative (Scheme 6).44 Spectroscopic investigations were conducted in a binary solvent mixture of water and dimethylformamide (DMF) (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v). After adding four equivalents of Hg2+, probe 37's green emission was significantly quenched, unlike other metal ions (Fig. 16). The fluorescence titration demonstrated a reduction in the intensity of the emission band at 517 nm. The limit of detection was determined to be 0.49 μM, suggesting a 1
2, v/v). After adding four equivalents of Hg2+, probe 37's green emission was significantly quenched, unlike other metal ions (Fig. 16). The fluorescence titration demonstrated a reduction in the intensity of the emission band at 517 nm. The limit of detection was determined to be 0.49 μM, suggesting a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between probe 37 and Hg2+ ions based on the analysis conducted via Job's plot analysis. The association constant for the interaction between probe 37 and Hg2+ was determined to be 2.98 × 104 M−1. 1H NMR titration indicated broadening of the singlet at 11.52 ppm, attributed to N–H, upon the addition of Hg2+ ions. To demonstrate practical applicability, a successful paper strip test for Hg2+ ion detection was implemented using the synthesized probe.
1 binding stoichiometry between probe 37 and Hg2+ ions based on the analysis conducted via Job's plot analysis. The association constant for the interaction between probe 37 and Hg2+ was determined to be 2.98 × 104 M−1. 1H NMR titration indicated broadening of the singlet at 11.52 ppm, attributed to N–H, upon the addition of Hg2+ ions. To demonstrate practical applicability, a successful paper strip test for Hg2+ ion detection was implemented using the synthesized probe.
 | ||
Fig. 16 (a) Emission spectra of probe 37 in H2O/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) upon the addition of 4 equivalents of various metal ions; (b) fluorescence intensity at 517 nm of probe 37 in H2O/DMF (8 2) upon the addition of 4 equivalents of various metal ions; (b) fluorescence intensity at 517 nm of probe 37 in H2O/DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) with various metal ions (5 equivalents). (c) Test strips exposed to various metal ions when illuminated at 365 nm. (d) Images of the test strips treated with different concentrations of mercury ions. Reproduced with permission from ref. 44. Copyright 2022, Elsevier. 2) with various metal ions (5 equivalents). (c) Test strips exposed to various metal ions when illuminated at 365 nm. (d) Images of the test strips treated with different concentrations of mercury ions. Reproduced with permission from ref. 44. Copyright 2022, Elsevier. | ||
3.4 Styryl-BODIPY-based mercury ion sensor
The styryl group is a conjugated system that plays a crucial role in medicinal chemistry, materials science, and sensing applications.85–88 Its extended π-conjugation imparts unique optical and electronic properties, including solvatochromism, fluorescence, and high photostability.89 Additionally, the styryl group can be functionalized to enhance its interaction with specific targets, allowing for its integration into various chemical and biological systems. In sensing technologies, styryl-based compounds are widely used as chemosensors to identify metal ions, anions, and biomolecules through resonance energy transfer (RET) or intramolecular charge transfer (ICT).90In 2019, Xue et al. developed a distyryl BODIPY-based chemosensor (probe 41) for dual-mode detection of Hg2+, demonstrating both chromogenic and fluorogenic responses in an aqueous-THF medium (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v; HEPES buffer, pH 7.2) (Scheme 7).91 The probe demonstrated a distinct color transition from green to light yellow (absorption shift: 653 nm → 515 nm) and fluorescence quenching at 681 nm (quantum yield: 0.89 → 0.03) upon Hg2+ coordination, enabling naked-eye detection (Fig. 17). Spectroscopic analyses revealed a Hg2+-induced blocking of the intramolecular charge transfer (ICT) process from hydroxyl-substituted distyryl groups to the BODIPY core observed, with a detection limit of 0.7 μM calculated via fluorometric titration. Job plot and 1H NMR studies confirmed a 1
1 v/v; HEPES buffer, pH 7.2) (Scheme 7).91 The probe demonstrated a distinct color transition from green to light yellow (absorption shift: 653 nm → 515 nm) and fluorescence quenching at 681 nm (quantum yield: 0.89 → 0.03) upon Hg2+ coordination, enabling naked-eye detection (Fig. 17). Spectroscopic analyses revealed a Hg2+-induced blocking of the intramolecular charge transfer (ICT) process from hydroxyl-substituted distyryl groups to the BODIPY core observed, with a detection limit of 0.7 μM calculated via fluorometric titration. Job plot and 1H NMR studies confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry (probe 41: Hg2+), where Hg2+ initially oxidizes hydroxyl groups to quinone intermediates before coordinating with the phenolic oxygen atoms. The sensor exhibited exceptional selectivity among 10 competing metal ions, including Pb2+ and Cu2+, with minimal interference. Unique among Hg2+ probes, probe 41 utilized hard donor hydroxyl receptors rather than conventional soft sulfur-based ligands, achieving a binding constant of 4.3 × 103 M−1.
2 binding stoichiometry (probe 41: Hg2+), where Hg2+ initially oxidizes hydroxyl groups to quinone intermediates before coordinating with the phenolic oxygen atoms. The sensor exhibited exceptional selectivity among 10 competing metal ions, including Pb2+ and Cu2+, with minimal interference. Unique among Hg2+ probes, probe 41 utilized hard donor hydroxyl receptors rather than conventional soft sulfur-based ligands, achieving a binding constant of 4.3 × 103 M−1.
 | ||
Fig. 17 (a) UV-vis spectra of probe 41 upon addition of 5 equivalent of different cations; (b) absorption ratio of (A515/A653) of probe 41 containing 50 equivalent of different metals; (c) color change of probe 41 in the presence of different metal ions; (d) fluorescence spectra of probe 41 upon addition of 50 equivalent of different cations; (e) the fluorescence responses at 681 nm (I681) of probe 41 containing 50 eq. of Hg2+ ions introduced to chosen metal ions (50 eq.) in a THF–H2O mixture (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; HEPES 10 mM, pH = 7.2) solution; (f) color of solution with different metal ions under UV irradiation at 360 nm. Reproduced with permission from ref. 91. Copyright 2019, Elsevier. 1; HEPES 10 mM, pH = 7.2) solution; (f) color of solution with different metal ions under UV irradiation at 360 nm. Reproduced with permission from ref. 91. Copyright 2019, Elsevier. | ||
In 2019, Huang et al. developed a near-infrared distyryl BODIPY-based chemosensor (probe 47) functionalized with a bis(1,2,3-triazole)amino receptor for quick and selective detection of Hg2+ and Cu2+ in CH3CN/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) (Scheme 8).92 The probe 47 demonstrated notable spectral responses upon interaction with Hg2+ and Cu2+ but did not display any changes upon interaction with other metals such as Ni2+, Mn2+, Zn2+, Fe3+, Co2+, Cd2+, Pb2+, Ba2+, Ag+, K+, Mg2+, Na+, Pd2+, and Ca2+. Hg2+ induced a blue shift of 22 nm in absorption (from 663 nm to 641 nm) and exhibited ratiometric fluorescence enhancement at 673 nm (detection limit: 0.09 μM). In contrast, Cu2+ resulted in absorption broadening (from 663 nm to 597 nm) and fluorescence quenching at 730 nm (detection limit: 1.02 μM) (Fig. 18). Binding studies revealed a 1
1 v/v) (Scheme 8).92 The probe 47 demonstrated notable spectral responses upon interaction with Hg2+ and Cu2+ but did not display any changes upon interaction with other metals such as Ni2+, Mn2+, Zn2+, Fe3+, Co2+, Cd2+, Pb2+, Ba2+, Ag+, K+, Mg2+, Na+, Pd2+, and Ca2+. Hg2+ induced a blue shift of 22 nm in absorption (from 663 nm to 641 nm) and exhibited ratiometric fluorescence enhancement at 673 nm (detection limit: 0.09 μM). In contrast, Cu2+ resulted in absorption broadening (from 663 nm to 597 nm) and fluorescence quenching at 730 nm (detection limit: 1.02 μM) (Fig. 18). Binding studies revealed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry (probe 47: Hg2+/Cu2+) for both ions through Job plot analysis, with binding constants of 1.0 × 109 M−2 (Hg2+) and 2.0 × 1010 M−2 (Cu2+), indicating stronger coordination with Cu2+. The response to Hg2+ was attributed to intramolecular charge transfer (ICT) inhibition, whereas Cu2+ prompted the quenching of photoinduced electron transfer (PET). The study demonstrated exceptional selectivity among 20 competing metal ions, including Pb2+ and Fe3+, with rapid response kinetics of less than five minutes. Cellular imaging conducted in HeLa cells confirmed dual-channel discrimination, indicating a 14-fold increase in the I640–700/I701–758 ratio for Hg2+ as opposed to complete quenching observed for Cu2+.
2 stoichiometry (probe 47: Hg2+/Cu2+) for both ions through Job plot analysis, with binding constants of 1.0 × 109 M−2 (Hg2+) and 2.0 × 1010 M−2 (Cu2+), indicating stronger coordination with Cu2+. The response to Hg2+ was attributed to intramolecular charge transfer (ICT) inhibition, whereas Cu2+ prompted the quenching of photoinduced electron transfer (PET). The study demonstrated exceptional selectivity among 20 competing metal ions, including Pb2+ and Fe3+, with rapid response kinetics of less than five minutes. Cellular imaging conducted in HeLa cells confirmed dual-channel discrimination, indicating a 14-fold increase in the I640–700/I701–758 ratio for Hg2+ as opposed to complete quenching observed for Cu2+.
 | ||
Fig. 18 (a) Change in absorption spectra of probe 47 (6 μM) in CH3CN/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) upon addition of different metal ions (20 equiv.); (b) change in fluorescence spectra of probe 47 (6 μM) in CH3CN/H2O (5 1 v/v) upon addition of different metal ions (20 equiv.); (b) change in fluorescence spectra of probe 47 (6 μM) in CH3CN/H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) upon addition of different metal ions (20 equiv.); (c) the ratiometric and discriminative detection of Hg2+ and Cu2+ ions; Reproduced with permission from ref. 92. Copyright 2018, Elsevier. 1 v/v) upon addition of different metal ions (20 equiv.); (c) the ratiometric and discriminative detection of Hg2+ and Cu2+ ions; Reproduced with permission from ref. 92. Copyright 2018, Elsevier. | ||
3.5 Pyrrolyl-BODIPY-based mercury ion sensor
The pyrrolyl group, a structural unit derived from pyrrole (C4H4NH), is a five-membered aromatic heterocycle that contains a nitrogen atom in its conjugated π-electron system. This electron-rich structure, characterized by the partial delocalization of the nitrogen lone pair into the ring, facilitates strong coordination with metal ions and enhances charge–transfer interactions. These properties make the pyrrolyl group a versatile building block in developing chemosensors for environmental, biomedical, and industrial applications.In 2022, Chan et al. developed a mono-pyrrolyl substituted BODIPY chemosensor (probe 49) for ratiometric Hg2+ detection in aqueous-THF media (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v; HEPES buffer, pH 7.2) (Scheme 9).93 The sensor exhibited a distinct colorimetric response from pink to colorless alongside fluorescence quenching at 625 nm (quantum yield: 0.51 → 0) via Hg2+-induced perturbation of the intramolecular charge transfer (ICT) process between the pyrrole donor and BODIPY core (Fig. 19). Spectrophotometric analyses revealed hyperchromic absorption shifts at 505 nm and 346 nm with a detection limit of 0.05 μM, supported by a binding constant of 5.2 × 103 M−1 derived from Benesi–Hildebrand plots. Probe 49 demonstrated exceptional selectivity among 14 competing metal ions, including Pb2+ and Cu2+, with stable performance across pH 4–9. Job plot and 1H NMR titrations confirmed a 1
1 v/v; HEPES buffer, pH 7.2) (Scheme 9).93 The sensor exhibited a distinct colorimetric response from pink to colorless alongside fluorescence quenching at 625 nm (quantum yield: 0.51 → 0) via Hg2+-induced perturbation of the intramolecular charge transfer (ICT) process between the pyrrole donor and BODIPY core (Fig. 19). Spectrophotometric analyses revealed hyperchromic absorption shifts at 505 nm and 346 nm with a detection limit of 0.05 μM, supported by a binding constant of 5.2 × 103 M−1 derived from Benesi–Hildebrand plots. Probe 49 demonstrated exceptional selectivity among 14 competing metal ions, including Pb2+ and Cu2+, with stable performance across pH 4–9. Job plot and 1H NMR titrations confirmed a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry, where Hg2+ coordinates to pyrrole nitrogen atoms, evidenced by NH proton signal disappearance at 8.87 ppm. Practical utility was validated through paper test strips showing visible color transitions for Hg2+ concentrations ≥1 μM.
2 binding stoichiometry, where Hg2+ coordinates to pyrrole nitrogen atoms, evidenced by NH proton signal disappearance at 8.87 ppm. Practical utility was validated through paper test strips showing visible color transitions for Hg2+ concentrations ≥1 μM.
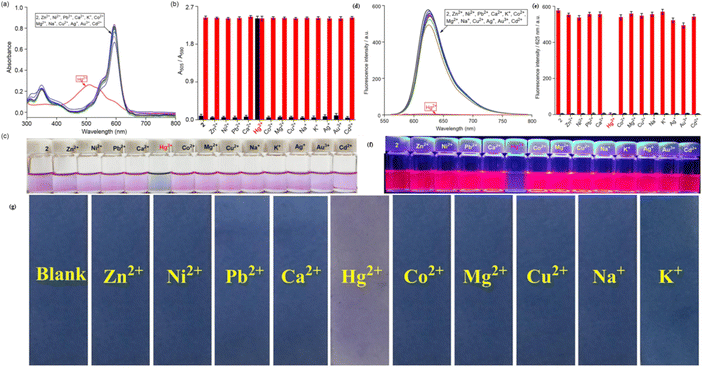 | ||
Fig. 19 (a) Change of UV-vis absorption spectra of 49 (c = 5.5 μM) mixed with different metal cations (25 eq.). (b) The absorbance ratios between A505/A591 responses of chemosensor 49 containing of 25 eq. Hg2+ ion to the selected different kinds of cations (25 eq.). (c) Changes of the solution color of 49 mixed with different metal cations. (d) Emission spectra (excitation at 587 nm) of 49 (c = 5.5 μM) measured in a THF–H2O solution (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1; HEPES 10 mM, pH = 7.2) with the presence of Hg2+ ion and other metal ions (25 eq.). (e) Fluorescence intensity at 625 nm (I625) of probe 49 before and after the addition of 25 eq. of Hg2+ ion and other metal cations solution (25 eq.); (f) photos of solution colors for 49 in the presence of different metal cations illuminated with UV lamp at 360 nm (g) photographs of the probe 49 based test strips colorimetric detect different cations. Reproduced with permission from ref. 93. Copyright 2021, Elsevier. 1; HEPES 10 mM, pH = 7.2) with the presence of Hg2+ ion and other metal ions (25 eq.). (e) Fluorescence intensity at 625 nm (I625) of probe 49 before and after the addition of 25 eq. of Hg2+ ion and other metal cations solution (25 eq.); (f) photos of solution colors for 49 in the presence of different metal cations illuminated with UV lamp at 360 nm (g) photographs of the probe 49 based test strips colorimetric detect different cations. Reproduced with permission from ref. 93. Copyright 2021, Elsevier. | ||
3.6 Catechol-BODIPY-based mercury ion sensor
Catechol, also known as pyrocatechol or 1,2-dihydroxybenzene, is a versatile organic compound with a broad array of biological and chemical applications. Its structure, featuring two hydroxyl groups attached to a benzene ring in an ortho configuration, imparts unique redox properties and strong metal ion chelating abilities.94,95 These characteristics make catechol an essential building block in various fields, including agrochemicals, pharmaceuticals, and materials science.96–98 Catechol is particularly valuable in sensing applications because it can form stable complexes with metal ions and undergo oxidation–reduction reactions, which can generate detectable optical or electrochemical signals.In 2021, Saiyasombat and Kiatisevi developed a bis-BODIPY-linked triazole chemosensor (probe 53) anchored on a catechol core for dual detection of Ag+ and Hg2+ in methanol media (Scheme 10).99 The probe exhibited a detection limit (LOD) of 1 μM for Hg2+ ions. Upon the addition of Hg2+ ions in methanol, the UV-visible absorption spectra displayed a slight red shift, accompanied by a hyperchromic shift in fluorescence spectra (Fig. 20). The increase in fluorescence intensity is due to the chelation-enhanced fluorescence (CHEF) effect, which occurs when intramolecular rotations of the BODIPY fluorophore are restricted after it complexes with Hg2+ ions. Job's plot analysis indicated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between probe 53 and Hg2+ ions. The probe's binding affinity for Hg2+ ions was measured at 1.41 × 105 M−1 through the Benesi–Hildebrand equation.
1 binding stoichiometry between probe 53 and Hg2+ ions. The probe's binding affinity for Hg2+ ions was measured at 1.41 × 105 M−1 through the Benesi–Hildebrand equation.
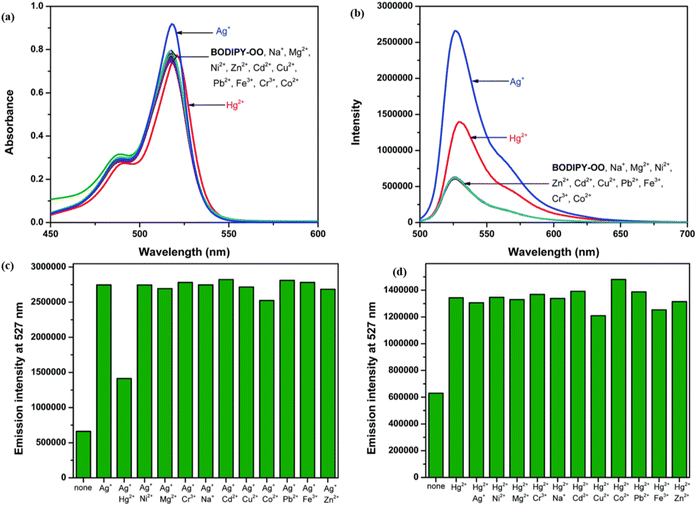 | ||
| Fig. 20 (a) Absorption spectra of probe 53 (5 mM) in the presence of various metal ions (20 equiv.) in MeOH. (b) Emission spectra of probe 53 (0.5 mM) in the presence of various metal ions (20 equiv.) in MeOH (c) competitive selectivity of probe 53 (0.5 mM) toward Ag+ (10 equiv.) with other metal cations, and (d) probe 53 (0.5 mM) toward Hg2+ (10 equiv.) with other metal cations. Reproduced with permission from ref. 99. Copyright 2021, Elsevier. | ||
3.7 Dithia-azacrown-BODIPY-based mercury ion sensor
Dithia-azacrown ethers represent a group of macrocyclic compounds characterized by the incorporation of sulfur (thioether) and nitrogen (aza) donor atoms within their cyclic framework. This unique combination of heteroatoms imparts exceptional binding properties towards specific metal ions, making dithia-azacrown ethers highly valuable in supramolecular chemistry and sensing applications. Their ability to create stable complexes with transition and heavy metal ions arises from the synergistic interaction between the soft sulfur donors and the nitrogen atoms, which provide complementary coordination environments. These compounds are also highly tunable, allowing for modifications that optimize their selectivity and sensitivity for targeted analytes.Chen and colleagues created a fluorescent probe based on near-infrared (NIR) BODIPY (probe 58) featuring a macrocyclic thioether receptor for selective Hg2+ detection across biological and environmental systems (Scheme 11).100 Upon the addition of Hg2+ ions in a DMF–water (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) solution, the UV-vis absorption spectrum of probe 58 exhibited a significant spectral change: the original absorption band in the 580–820 nm region disappeared, while a new intense peak emerged at 670 nm. The presence of an isobestic point at 701 nm further supports the specific interaction between the probe and Hg2+ ions. Concurrently, a distinct colorimetric change from light brown to green was observed, indicating the probe's suitability for colorimetric sensing of Hg2+ ions. Fluorescence studies indicated a modest red shift in the emission peak, shifting from 809 nm to 790 nm, with a significant increase in fluorescence intensity following the introduction of Hg2+ ions. The exciting “turn-on” fluorescence response occurs when photoinduced electron transfer (PET) from the ligand to the BODIPY core is inhibited after the formation of the Hg2+–probe complex (Fig. 21). We found that the binding constant between probe 39 and Hg2+ ions is a strong 6.4 × 104 M−1, with a LOD calculated at 26.6 μM. Job's plot analysis indicated a 1
7, v/v) solution, the UV-vis absorption spectrum of probe 58 exhibited a significant spectral change: the original absorption band in the 580–820 nm region disappeared, while a new intense peak emerged at 670 nm. The presence of an isobestic point at 701 nm further supports the specific interaction between the probe and Hg2+ ions. Concurrently, a distinct colorimetric change from light brown to green was observed, indicating the probe's suitability for colorimetric sensing of Hg2+ ions. Fluorescence studies indicated a modest red shift in the emission peak, shifting from 809 nm to 790 nm, with a significant increase in fluorescence intensity following the introduction of Hg2+ ions. The exciting “turn-on” fluorescence response occurs when photoinduced electron transfer (PET) from the ligand to the BODIPY core is inhibited after the formation of the Hg2+–probe complex (Fig. 21). We found that the binding constant between probe 39 and Hg2+ ions is a strong 6.4 × 104 M−1, with a LOD calculated at 26.6 μM. Job's plot analysis indicated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between the probe and Hg2+ ions, implying that a stable 1
1 binding stoichiometry between the probe and Hg2+ ions, implying that a stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex is likely formed through the fitting of the Hg2+ ion within the probe's macrocyclic ring.
1 complex is likely formed through the fitting of the Hg2+ ion within the probe's macrocyclic ring.
 | ||
Fig. 21 (a) UV-vis spectra of probe 58 in presence of different ions. (b) Emission spectra of probe 58 in presence of different ions. (c) Color change of probe 58 in daylight upon addition of 100 μM of different ions in DMF–H2O (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7). Reproduced with permission from ref. 100. Copyright 2022, Elsevier. 7). Reproduced with permission from ref. 100. Copyright 2022, Elsevier. | ||
3.8 Chitosan-BODIPY-based mercury ion sensor
Chitosan, a natural polysaccharide derived from chitin, is a highly versatile biopolymer known for its biocompatibility, biodegradability, non-toxicity, and antimicrobial properties. These characteristics have made it a valuable material in various fields, including medicine, food safety, environmental monitoring, and water treatment.Wang et al. developed a novel chitosan-based BODIPY macromolecular chemosensor (probe 65, 67 and 69) for the selective detection of mercury ions (Scheme 12).101 The initial chemosensor, probe 65, utilized a –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond formed via click chemistry as the primary recognition site for mercury ions. To enhance the sensing capabilities, probes 67 and 69 were subsequently synthesized by incorporating additional recognition sites into the chitosan backbone. These modified chemosensors demonstrated the ability to detect not only mercury ions but also iron ions (Fe2+ and Fe3+). In the absence of sulfur-containing moieties (as in probe 65), the chemosensor selectively recognized Hg2+ and Hg+ ions through the cleavage of the –C
N bond formed via click chemistry as the primary recognition site for mercury ions. To enhance the sensing capabilities, probes 67 and 69 were subsequently synthesized by incorporating additional recognition sites into the chitosan backbone. These modified chemosensors demonstrated the ability to detect not only mercury ions but also iron ions (Fe2+ and Fe3+). In the absence of sulfur-containing moieties (as in probe 65), the chemosensor selectively recognized Hg2+ and Hg+ ions through the cleavage of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond. Conversely, the incorporation of sulfur atoms (as in probes 67 and 69) facilitated the recognition of Fe3+ and Fe2+ ions, presumably through the formation of metal–sulfur bonds. These chemosensors exhibited a significant quenching effect in an acetic acid/water (1
N bond. Conversely, the incorporation of sulfur atoms (as in probes 67 and 69) facilitated the recognition of Fe3+ and Fe2+ ions, presumably through the formation of metal–sulfur bonds. These chemosensors exhibited a significant quenching effect in an acetic acid/water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99, v/v) solution, easily seen both by the naked eye and with UV light, due to the breaking of the –C
99, v/v) solution, easily seen both by the naked eye and with UV light, due to the breaking of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond when metal ions are present binding. Notably, the quenching effect was slightly more pronounced for Hg+ ions compared to Hg2+ ions. The cleavage of the –C
N bond when metal ions are present binding. Notably, the quenching effect was slightly more pronounced for Hg+ ions compared to Hg2+ ions. The cleavage of the –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond was further confirmed by Fourier Transform Infrared (FTIR) spectroscopy. Among probes 67 and 69, probe 67 demonstrated superior sensitivity towards both Hg2+ and Hg+ ions. The limit of detection (LOD) for these chemosensors was found to be 1.51 μM and 1.52 μM for Hg2+ and Hg+, respectively, for probe 67; 1.61 μM and 2.00 μM for probe 67; and 3.24 μM and 2.79 μM for probe 69.
N bond was further confirmed by Fourier Transform Infrared (FTIR) spectroscopy. Among probes 67 and 69, probe 67 demonstrated superior sensitivity towards both Hg2+ and Hg+ ions. The limit of detection (LOD) for these chemosensors was found to be 1.51 μM and 1.52 μM for Hg2+ and Hg+, respectively, for probe 67; 1.61 μM and 2.00 μM for probe 67; and 3.24 μM and 2.79 μM for probe 69.
In 2023, He et al. developed three chitosan-grafted fluorescent probes (probe 79, 80 and 81) via RAFT polymerization for selective Hg2+/Hg+ detection in organic media (Scheme 13).102 The probes exhibited “turn-on” fluorescence at 521 nm upon Hg2+/Hg+ coordination, with probe 81 showing the highest sensitivity and detection limits of 0.61 μM for Hg2+ and 0.47 μM for Hg+ in DMF (Fig. 22). Spectroscopic analyses revealed a photoinduced electron transfer (PET) inhibition mechanism through Hg-induced coordination to phenolic hydroxyl groups on the BODIPY fluorophore, supported by 1H NMR peak shifts at 5.56 ppm and FT-IR C–O bond attenuation at 1270 cm−1. The sensors demonstrated exceptional selectivity among the competing metal ions. Practical applications included flexible films and test strips for visual mercury ion detection.
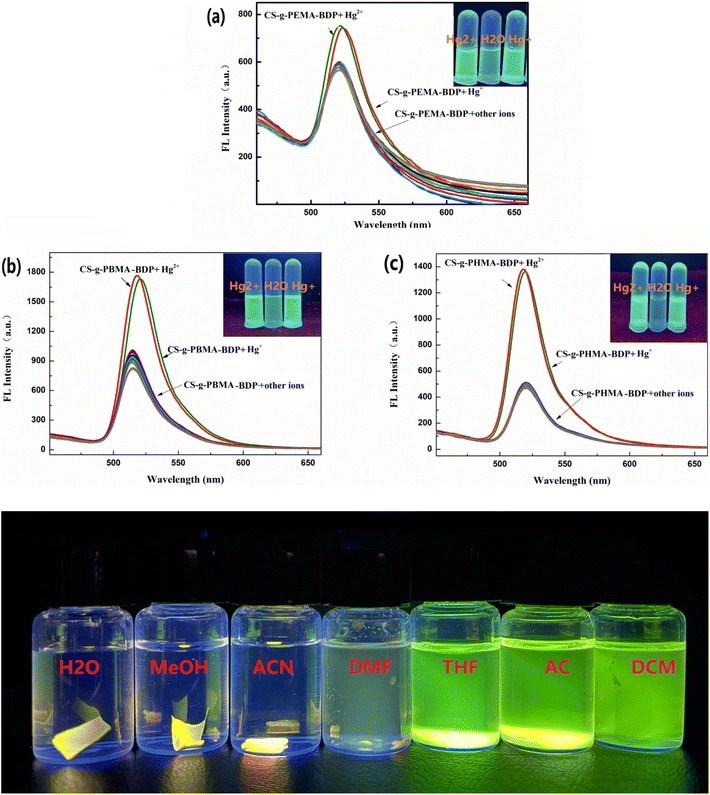 | ||
| Fig. 22 The fluorescent intensity of (a) 79, (b) 80 and (c) 81; and solubility of probe 81 is shown below. Reproduced with permission from ref. 102. Copyright 2018, Elsevier. | ||
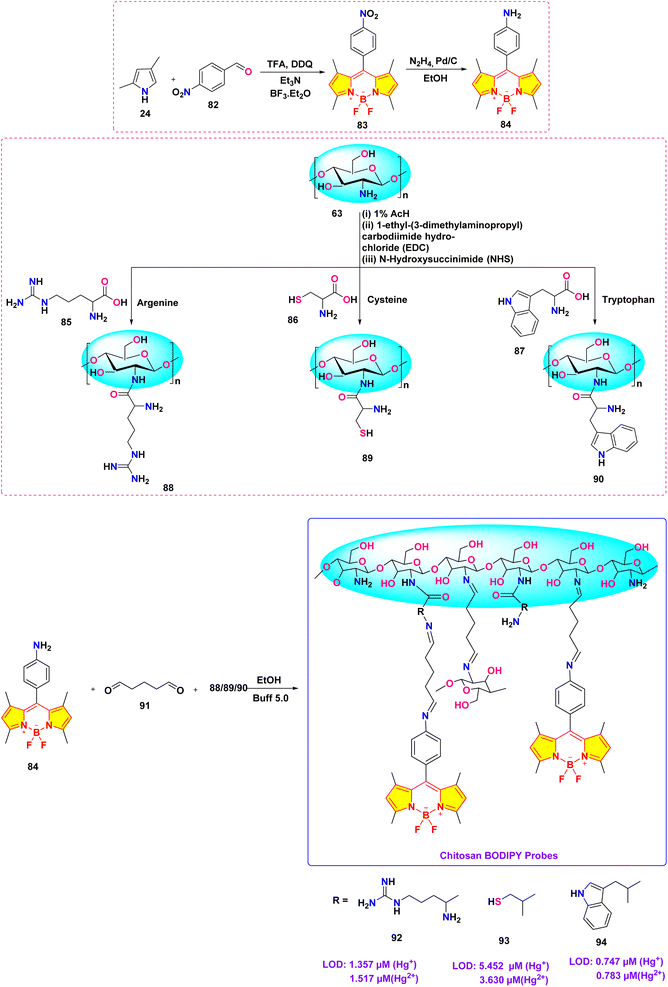
He et al. synthesized a series of chitosan-amino acid conjugated BODIPY fluorophores (probe 92, 93 and 94) for the selective detection of Hg+ and Hg2+ ions (Scheme 14).103 Spectroscopic characterization was performed in a 1% acetic acid (HAc) solution. The presence of Hg+ and Hg2+ ions led to a marked decrease in fluorescence intensity, which is linked to the disruption of intramolecular charge transfer (ICT) processes in BODIPY probes (Fig. 23). The limit of detection for Hg+ ions was determined to be 1.357 μM, 5.452 μM, and 0.747 μM, respectively, for probes 92, 93, and 94. Similarly, the LOD for Hg2+ ions was found to be 1.517 μM, 3.630 μM, and 0.783 μM for probes 92, 93, and 94, respectively.
 | ||
| Fig. 23 (a) Plausible binding mechanism of probe 92, 93, and 94 (b)FTIR spectra of 92, 92 + Hg+ and 92 + Hg2+; (c) 1H NMR of 92 and 92 + Hg (ClO4)2. Reproduced with permission from ref. 103. Copyright 2022, Elsevier. | ||
3.9 Quinoline-BODIPY-based mercury ion sensor
In 2024, Kumarasamy et al. reported the synthesis of novel quinoline-conjugated BODIPY derivatives (probe 97 and 99) for the selective detection of Hg2+ ions in a 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (v/v) acetonitrile–water mixture (Scheme 15).104 Upon the addition of Hg2+ ions, probe 97 exhibited a bathochromic shift in the absorption peak from 505 nm to 510 nm (Fig. 24). Concurrently, the emission spectrum displayed a notable decrease in the fluorescence intensity at 550 nm. The quenching effect occurs due to a photoinduced electron transfer (PET) process from the quinoline group to the BODIPY core, resulting in enhanced fluorescence quenching through chelation. Probe 99 exhibited analogous spectral behavior. Job's plot analysis revealed binding constants of 2.16 × 10−5 M−1 and 3.27 × 10−5 M−1 for probe 97 and probe 99, respectively. The probes showed a notable and precise reduction in fluorescence upon exposure to Hg2+ ions, achieving an impressively low detection limit of approximately 3.06 × 10−8 M. Additionally, Job's plot validated a 1
3 (v/v) acetonitrile–water mixture (Scheme 15).104 Upon the addition of Hg2+ ions, probe 97 exhibited a bathochromic shift in the absorption peak from 505 nm to 510 nm (Fig. 24). Concurrently, the emission spectrum displayed a notable decrease in the fluorescence intensity at 550 nm. The quenching effect occurs due to a photoinduced electron transfer (PET) process from the quinoline group to the BODIPY core, resulting in enhanced fluorescence quenching through chelation. Probe 99 exhibited analogous spectral behavior. Job's plot analysis revealed binding constants of 2.16 × 10−5 M−1 and 3.27 × 10−5 M−1 for probe 97 and probe 99, respectively. The probes showed a notable and precise reduction in fluorescence upon exposure to Hg2+ ions, achieving an impressively low detection limit of approximately 3.06 × 10−8 M. Additionally, Job's plot validated a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio between the chemosensors and Hg2+ ions.
1 binding ratio between the chemosensors and Hg2+ ions.
 | ||
Fig. 24 (a) Absorption and (b) emission spectra of probe 97 in acetonitrile/water (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) solution with 10 equivalents of various metal ions. Reproduced with permission from ref. 104. Copyright 2024, Elsevier. 3, v/v) solution with 10 equivalents of various metal ions. Reproduced with permission from ref. 104. Copyright 2024, Elsevier. | ||
3.10 Oxadiazol-BODIPY-based mercury ion sensor
In 2023, Kumar et al. synthesized a series of α/β isomeric oxadiazolyl-BODIPY derivatives (probe 105–112) for the selective detection of Hg2+ ions (Scheme 16).105 While no significant changes were observed in the absorption spectra of compounds 105–112 upon the addition of various metal ions, probe 108 exhibited a rapid and specific response towards Hg2+ ions in methanolic solution. Upon Hg2+ ion addition, the absorption spectra of all compounds displayed a shift towards longer wavelength in the absorption maxima accompanied by a decrease in the extinction coefficient. The probe 108 demonstrated a binding constant of 1.8 × 104 M−1 and a LOD of 2.1 μM. Job's plot analysis revealed a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry between the probe 108 and Hg2+ ions. Notably, compound 108 exhibited enhanced fluorescence intensity upon Hg2+ ion binding, attributed to the suppression of the photoinduced electron transfer (PET) process.
1 binding stoichiometry between the probe 108 and Hg2+ ions. Notably, compound 108 exhibited enhanced fluorescence intensity upon Hg2+ ion binding, attributed to the suppression of the photoinduced electron transfer (PET) process.
4. Conclusion and future outlook
The advancement in the development of BODIPY-based fluorescent sensors for the detection of mercury ions (Hg2+) signifies noteworthy progress in tackling the global issue of mercury pollution. Over the past six years (2019–2024), innovative synthetic strategies and photophysical mechanisms have enhanced the sensitivity, selectivity, and practicality of these sensors (Table 1). This analysis constitutes the first exhaustive review to systematically delineate the design principles, synthetic pathways, and sensing mechanisms of BODIPY-based architectures tailored for precise Hg2+ identification. Key advancements include the strategic incorporation of BODIPY fluorophores with macrocyclic receptors (e.g., pillararenes), dual-channel architectures (e.g., rhodamine-BODIPY hybrids), and sulfur-enriched ligands (e.g., thiosemicarbazides), achieving detection limits at ultratrace levels across aqueous and aqueous–organic matrices. Functional moieties such as thiosemicarbazides, crown ethers, and pillararenes enhance selectivity and binding affinity by exploiting Hg2+'s coordination preferences. These sensors leverage mechanisms such as photoinduced electron transfer (PET), Förster resonance energy transfer (FRET), and chelation-enhanced fluorescence (CHEF) to enable real-time, on-site monitoring with minimal interference from competing ions (Fig. 25). A key advantage of BODIPY-based probes is their structural tunability, which enables ratiometric signaling, colorimetric responses, and near-infrared (NIR) emission. For example, integrating soft donor atoms, such as sulfur or nitrogen, into macrocyclic or thiourea frameworks can exploit soft acid–soft base interactions, which are particularly beneficial for selectively binding mercury ions over other metal ions. Additionally, steric hindrance can be introduced around the binding site to minimize non-specific interactions. Meanwhile, ratiometric or dual-mode sensing approaches, which combine both colorimetric and fluorometric responses, provide built-in calibration that further discriminates against interfering species. Advances in computational modeling and machine learning also play a crucial role in predicting binding affinities and guiding the design of receptors that achieve a delicate balance between sensitivity and selectivity. Collectively, these approaches pave the way for developing high-performance sensors with enhanced selectivity in complex sample matrices.| Sl no. | Probe | Optical response | Solvent | LOD | Stoichiometric ratio | Colour change | Mechanism | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 |  |
Turn-off | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.2 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
— | PET | 78 |
| 2 |  |
Turn-off | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (1 H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
1.09 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Green-deep green | PET | 62 |
| 3 |  |
Turn-on | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.01 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Colourless-green | FRET | 81 |
| 0.44 μM | Colourless-orange | |||||||
| 4 |  |
Turn-on | MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (7 H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.3 μM | — | Yellow-pink | FRET | 82 |
| 5 |  |
Turn-on | H2O | 0.37 μM | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Blue-yellow | Complex formation | 84 |
| 6 |  |
Turn-off | H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMF (8 DMF (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
0.49 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Green-colourless | Complex formation | 44 |
| 7 |  |
Turn-off | Aq. THF | 0.7 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Green-light yellow | Inhibition of ICT | 91 |
| 8 |  |
Turn-on | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (9 H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0.09 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Green-blue green | Inhibition of PET | 92 |
| 9 |  |
Turn-off | Aq. THF | 0.05 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Pink-colourless | Inhibition of ICT | 93 |
| 10 | 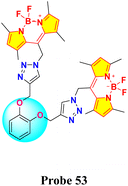 |
Turn-on | MeOH | 1 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | CHEF | 99 |
| 11 | 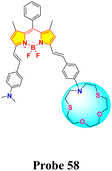 |
Turn-on | DMF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (3 H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
26.6 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Light Brown-green | Inhibition of PET | 100 |
| 12 |  |
Turn-off | Aq. Acetic acid | 1.51 μM (65-Hg2+) | — | — | PET | 101 |
| 1.53 μM (65-Hg+) | ||||||||
| 1.61 μM (67-Hg2+) | ||||||||
| 2.00 μM (67-Hg+) | ||||||||
| 3.24 μM (69-Hg2+) | ||||||||
| 2.79 μM (69-Hg+) | ||||||||
| 13 |  |
Turn-on | Organic solvent | 0.61 μM (Hg2+) | — | — | PET | 102 |
| 0.47 μM (Hg+) | ||||||||
| 14 |  |
Turn-off | 1% acetic acid | 1.517 μM (Hg2+) | — | Orange-red | Inhibition of ICT | 103 |
| 1.357 | ||||||||
| μM (Hg+) | ||||||||
| 3.630 μM (Hg2+) | ||||||||
| 15 |  |
Turn-off | CH3CN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (7 H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
0.03 μM | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | PET | 104 |
| 16 |  |
Turn-on | MeOH | 2.1 μM | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
— | Inhibition of PET | 105 |
 | ||
| Fig. 25 Different sensing mechanisms utilized by BODIPY-based chemosensors for the detection of mercury ions. | ||
Despite these breakthroughs, challenges persist in optimizing sensor stability across diverse pH ranges and mitigating matrix effects in complex samples. Furthermore, interference caused by coexisting ions (e.g., Cu2+, Fe3+) and organic compounds present in complex samples compromises selectivity, leading to erroneous signals or fluorescence quenching. Future research should focus on enhancing biocompatibility for in vivo imaging, developing recyclable sensor materials, and integrating these systems into portable devices for field applications. Integration of BODIPY-based sensors with intelligent devices, such as portable photometers,106–110 portable Raman meters,111–114 and mobile phones,19,115,116 can provide quick and precise data collection in real-time, streamline detection processes, and offer high practicality. Additionally, incorporating BODIPY-based sensors into testing papers offers an extremely cost-effective method for detecting metal ions.117–121 Furthermore, BODIPY-based sensors integrated into hydrogels122–126 or thin films127 could unlock better sensing capabilities and expand their practical applications. By addressing these challenges and building on existing knowledge, BODIPY-based sensors hold significant potential for meeting the growing demand for efficient and reliable metal ion detection.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.Acknowledgements
R. S. would like to thank SERB-POWER, Science & Engineering Research Board, India (Grant No. SPG/2021/002579), the Council of Scientific & Industrial Research, India (Grant No. 02(0308)/17/EMR-II), the Higher Education Department, Government of Odisha (Grant No. 26913/HED/HEPTC-WB-02-17 (OHEPEE)), Science and Technology Department, Government of Odisha (Grant No. ST-SCST-MISC.0036-2023), LSRB (Grant No. LSRB-431/BTB/2024) and OSHEC MRIP-2024-Chemistry (Grant No. 24EM/CH/31) for their financial support. R. S. also acknowledges DST, FIST-New Delhi (SR/FST/CSI-243/2012) for providing NMR facility to Ravenshaw University, Cuttack.References
- K. G. Pavithra, P. SundarRajan, P. S. Kumar and G. Rangasamy, Mercury Sources, Contaminations, Mercury Cycle, Detection and Treatment Techniques: A Review, Chemosphere, 2023, 312, 137314, DOI:10.1016/j.chemosphere.2022.137314.
- D. B. Peakall and R. J. Lovett, Mercury: Its Occurrence and Effects in the Ecosystem, BioScience, 1972, 22(1), 20–25, DOI:10.2307/1296180.
- D. Udhayakumari, Review on Fluorescent Sensors-Based Environmentally Related Toxic Mercury Ion Detection, J. Inclusion Phenom. Macrocyclic Chem., 2022, 102(5–6), 451–476, DOI:10.1007/s10847-022-01138-1.
- C. Tunsu and B. Wickman, Effective Removal of Mercury from Aqueous Streams via Electrochemical Alloy Formation on Platinum, Nat. Commun., 2018, 9(1), 4876, DOI:10.1038/s41467-018-07300-z.
- M. Basu, Impact of Mercury and Its Toxicity on Health and Environment: A General Perspective, in Mercury Toxicity, Environmental Science and Engineering, ed. N. Kumar, Springer Nature Singapore, Singapore, 2023, pp. 95–139, DOI:10.1007/978-981-99-7719-2_4.
- L. Mao, X. Liu, B. Wang, C. Lin, M. Xin, B.-T. Zhang, T. Wu, M. He and W. Ouyang, Occurrence and Risk Assessment of Total Mercury and Methylmercury in Surface Seawater and Sediments from the Jiaozhou Bay, Yellow Sea, Sci. Total Environ., 2020, 714, 136539, DOI:10.1016/j.scitotenv.2020.136539.
- S. Zhu, Z. Zhang and D. Žagar, Mercury Transport and Fate Models in Aquatic Systems: A Review and Synthesis, Sci. Total Environ., 2018, 639, 538–549, DOI:10.1016/j.scitotenv.2018.04.397.
- A. Carocci, N. Rovito, M. S. Sinicropi and G. Genchi, Mercury Toxicity and Neurodegenerative Effects, in Reviews of Environmental Contamination and Toxicology, ed. D. M. Whitacre, Springer International Publishing, Cham, 2014, vol. 229, pp. 1–18, DOI:10.1007/978-3-319-03777-6_1.
- V. Andreoli and F. Sprovieri, Genetic Aspects of Susceptibility to Mercury Toxicity: An Overview, Int. J. Environ. Res. Public Health, 2017, 14(1), 93, DOI:10.3390/ijerph14010093.
- B. Fernandes Azevedo, L. Barros Furieri, F. M. Peçanha, G. A. Wiggers, P. Frizera Vassallo, M. Ronacher Simões, J. Fiorim, P. Rossi De Batista, M. Fioresi, L. Rossoni, I. Stefanon, M. J. Alonso, M. Salaices and D. Valentim Vassallo, Toxic Effects of Mercury on the Cardiovascular and Central Nervous Systems, J. Biomed. Biotechnol., 2012, 2012, 1–11, DOI:10.1155/2012/949048.
- Y.-S. Wu, A. I. Osman, M. Hosny, A. M. Elgarahy, A. S. Eltaweil, D. W. Rooney, Z. Chen, N. S. Rahim, M. Sekar, S. C. B. Gopinath, N. N. I. Mat Rani, K. Batumalaie and P.-S. Yap, The Toxicity of Mercury and Its Chemical Compounds: Molecular Mechanisms and Environmental and Human Health Implications: A Comprehensive Review, ACS Omega, 2024, 9(5), 5100–5126, DOI:10.1021/acsomega.3c07047.
- R. A. Bernhoft, Mercury Toxicity and Treatment: A Review of the Literature, J. Environ. Public Health, 2012, 2012, 1–10, DOI:10.1155/2012/460508.
- F. Zahir, S. J. Rizwi, S. K. Haq and R. H. Khan, Low Dose Mercury Toxicity and Human Health, Environ. Toxicol. Pharmacol., 2005, 20(2), 351–360, DOI:10.1016/j.etap.2005.03.007.
- M. C. Houston, The Role of Mercury and Cadmium Heavy Metals in Vascular Disease, Hypertension, Coronary Heart Disease, and Myocardial Infarction, Alt. Ther. Health Med., 2007, 13(2), S128–S133 Search PubMed.
- J. T. Ackerman, T. E. C. Kraus, J. A. Fleck, D. P. Krabbenhoft, W. R. Horwath, S. M. Bachand, M. P. Herzog, C. A. Hartman and P. A. M. Bachand, Experimental Dosing of Wetlands with Coagulants Removes Mercury from Surface Water and Decreases Mercury Bioaccumulation in Fish, Environ. Sci. Technol., 2015, 49(10), 6304–6311, DOI:10.1021/acs.est.5b00655.
- L. Meyer, S. Guyot, M. Chalot and N. Capelli, The Potential of Microorganisms as Biomonitoring and Bioremediation Tools for Mercury-Contaminated Soils, Ecotoxicol. Environ. Saf., 2023, 262, 115185, DOI:10.1016/j.ecoenv.2023.115185.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Fluorescent Chemosensors: The Past, Present and Future, Chem. Soc. Rev., 2017, 46(23), 7105–7123, 10.1039/C7CS00240H.
- P. Kumar, K.-H. Kim, V. Bansal, T. Lazarides and N. Kumar, Progress in the Sensing Techniques for Heavy Metal Ions Using Nanomaterials, J. Ind. Eng. Chem., 2017, 54, 30–43, DOI:10.1016/j.jiec.2017.06.010.
- R. Jain, A. Thakur, P. Kaur, K.-H. Kim and P. Devi, Advances in Imaging-Assisted Sensing Techniques for Heavy Metals in Water: Trends, Challenges, and Opportunities, TrAC, Trends Anal. Chem., 2020, 123, 115758, DOI:10.1016/j.trac.2019.115758.
- G. Aragay, J. Pons and A. Merkoçi, Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection, Chem. Rev., 2011, 111(5), 3433–3458, DOI:10.1021/cr100383r.
- K. Li, H. Yang, X. Yuan and M. Zhang, Recent Developments of Heavy Metals Detection in Traditional Chinese Medicine by Atomic Spectrometry, Microchem. J., 2021, 160, 105726, DOI:10.1016/j.microc.2020.105726.
- S.-H. Chen, Y.-X. Li, P.-H. Li, X.-Y. Xiao, M. Jiang, S.-S. Li, W.-Y. Zhou, M. Yang, X.-J. Huang and W.-Q. Liu, Electrochemical Spectral Methods for Trace Detection of Heavy Metals: A Review, TrAC, Trends Anal. Chem., 2018, 106, 139–150, DOI:10.1016/j.trac.2018.07.005.
- P. Silveira and T. Falcade, Applications of Energy Dispersive X-Ray Fluorescence Technique in Metallic Cultural Heritage Studies, J. Cult. Herit., 2022, 57, 243–255, DOI:10.1016/j.culher.2022.09.008.
- G. S. Ustabasi, M. Ozcan and I. Yilmaz, Review—Voltammetric Determination of Heavy Metals with Carbon-Based Electrodes, J. Electrochem. Soc., 2021, 168(9), 097508, DOI:10.1149/1945-7111/ac253e.
- N. S. Patil, R. B. Dhake, M. I. Ahamed and U. Fegade, A Mini Review on Organic Chemosensors for Cation Recognition (2013-19), J. Fluoresc., 2020, 30(6), 1295–1330, DOI:10.1007/s10895-020-02554-7.
- M. Rajasekar, V. Ranjitha and K. Rajasekar, Recent Advances in Fluorescent-Based Cation Sensors for Biomedical Applications, Results Chem., 2023, 5, 100850, DOI:10.1016/j.rechem.2023.100850.
- S. Sargazi, I. Fatima, M. Hassan Kiani, V. Mohammadzadeh, R. Arshad, M. Bilal, A. Rahdar, A. M. Díez-Pascual and R. Behzadmehr, Fluorescent-Based Nanosensors for Selective Detection of a Wide Range of Biological Macromolecules: A Comprehensive Review, Int. J. Biol. Macromol., 2022, 206, 115–147, DOI:10.1016/j.ijbiomac.2022.02.137.
- Y. Shi, W. Zhang, Y. Xue and J. Zhang, Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives, Chemosensors, 2023, 11(4), 226, DOI:10.3390/chemosensors11040226.
- K. P. Carter, A. M. Young and A. E. Palmer, Fluorescent Sensors for Measuring Metal Ions in Living Systems, Chem. Rev., 2014, 114(8), 4564–4601, DOI:10.1021/cr400546e.
- X. Fang, Y. Zheng, Y. Duan, Y. Liu and W. Zhong, Recent Advances in Design of Fluorescence-Based Assays for High-Throughput Screening, Anal. Chem., 2019, 91(1), 482–504, DOI:10.1021/acs.analchem.8b05303.
- N. I. Georgiev, V. V. Bakov, K. K. Anichina and V. B. Bojinov, Fluorescent Probes as a Tool in Diagnostic and Drug Delivery Systems, Pharmaceuticals, 2023, 16(3), 381, DOI:10.3390/ph16030381.
- R. Kumar, S. Ravi, C. Immanuel David and R. Nandhakumar, A Photo-Induced Electron Transfer Based Reversible Fluorescent Chemosensor for Specific Detection of Mercury (II) Ions and Its Applications in Logic Gate, Keypad Lock and Real Samples, Arab. J. Chem., 2021, 14(1), 102911, DOI:10.1016/j.arabjc.2020.11.017.
- H. Liu, L. Ma, C. Ma, J. Du, M. Wang and K. Wang, Quencher-Free Fluorescence Method for the Detection of Mercury(II) Based on Polymerase-Aided Photoinduced Electron Transfer Strategy, Sensors, 2016, 16(11), 1945, DOI:10.3390/s16111945.
- D. Singharoy, S. Chowdhury, S. S. Mati, S. Ghosh, K. Chattopadhyay and S. C. Bhattacharya, Photoinduced Electron Transfer Switching Mechanism of a Naphthalimide Derivative with Its Solvatochromic Behaviour: An Experimental and Theoretical Study within Cell Investigations, Chem.–Eur. J., 2017, 23(65), 16516–16524, DOI:10.1002/chem.201702414.
- H. Lee, H.-S. Lee, J. H. Reibenspies and R. D. Hancock, Mechanism of “Turn-on” Fluorescent Sensors for Mercury(II) in Solution and Its Implications for Ligand Design, Inorg. Chem., 2012, 51(20), 10904–10915, DOI:10.1021/ic301380w.
- D. Singhal, N. Gupta and A. K. Singh, Chromogenic ‘Naked Eye’ and Fluorogenic ‘Turn on’ Sensor for Mercury Metal Ion Using Thiophene-Based Schiff Base, RSC Adv., 2015, 5(81), 65731–65738, 10.1039/C5RA11043B.
- S. Das, S. Goswami, K. Aich, K. Ghoshal, C. K. Quah, M. Bhattacharyya and H.-K. Fun, ESIPT and CHEF Based Highly Sensitive and Selective Ratiometric Sensor for Al3+ with Imaging in Human Blood Cells, New J. Chem., 2015, 39(11), 8582–8587, 10.1039/C5NJ01468A.
- C. Ma, F. Zeng, L. Huang and S. Wu, FRET-Based Ratiometric Detection System for Mercury Ions in Water with Polymeric Particles as Scaffolds, J. Phys. Chem. B, 2011, 115(5), 874–882, DOI:10.1021/jp109594h.
- B. Liu, F. Zeng, G. Wu and S. Wu, A FRET-Based Ratiometric Sensor for Mercury Ions in Water with Multi-Layered Silica Nanoparticles as the Scaffold, Chem. Commun., 2011, 47(31), 8913, 10.1039/c1cc12544c.
- B. Zhang, P. Ma, D. Gao, X. Wang, Y. Sun, D. Song and X. Li, A FRET-Based Fluorescent Probe for Mercury Ions in Water and Living Cells, Spectrochim. Acta, Part A, 2016, 165, 99–105, DOI:10.1016/j.saa.2016.04.037.
- L. Liu, H. Shi, R. Li, C. Liu, J. Cheng and L. Gao, Highly Sensitive Detection for Mercury Ions Using Graphene Oxide (GO) Sensors, Micromachines, 2021, 12(9), 1070, DOI:10.3390/mi12091070.
- B. Gu, L. Huang, N. Mi, P. Yin, Y. Zhang, X. Tu, X. Luo, S. Luo and S. Yao, An ESIPT-Based Fluorescent Probe for Highly Selective and Ratiometric Detection of Mercury(ii) in Solution and in Cells, Analyst, 2015, 140(8), 2778–2784, 10.1039/C5AN00273G.
- J. Wu, W. Liu, J. Ge, H. Zhang and P. Wang, New Sensing Mechanisms for Design of Fluorescent Chemosensors Emerging in Recent Years, Chem. Soc. Rev., 2011, 40(7), 3483, 10.1039/c0cs00224k.
- H. Chen, X. Li, P. Gao, Y. Pan and J. Liu, A BODIPY-Based Turn-off Fluorescent Probe for Mercury Ion Detection in Solution and on Test Strips, J. Mol. Struct., 2022, 1262, 133015, DOI:10.1016/j.molstruc.2022.133015.
- G. Aragay, H. Montón, J. Pons, M. Font-Bardía and A. Merkoçi, Rapid and Highly Sensitive Detection of Mercury Ions Using a Fluorescence-Based Paper Test Strip with an N-Alkylaminopyrazole Ligand as a Receptor, J. Mater. Chem., 2012, 22(13), 5978, 10.1039/c2jm15717a.
- A. Khatoon, J. A. Syed, A. R. Solangi and A. Mallah, Sirajuddin. Mechanistic Insights of Mercury Ion Detection and Its Influence on Time Monitored Hydrodynamic Size of Capsicum Annumm C Derived Silver Nanoparticles during Colorimetric Nano-Sensing, Optik, 2025, 325, 172237, DOI:10.1016/j.ijleo.2025.172237.
- M. Bayrak, A. Cimen and A. Bilgic, Synthesis, Characterization, and Application of Novel Fluorescent Sporopollenin for Effective Detection of Mercury (II) Ions from Aqueous Media, Int. J. Biol. Macromol., 2024, 281, 135754, DOI:10.1016/j.ijbiomac.2024.135754.
- J. Yin, Z. Wu, H. Li, B. Cao and W. Wang, Monitoring of Mercury Ion in Environmental Media and Biological Systems Using a Red Emissive Fluorescent Probe with a Large Stokes Shift, Spectrochim. Acta, Part A, 2025, 326, 125272, DOI:10.1016/j.saa.2024.125272.
- A. Dutta, W. Begum, S. Sarkar, S. Dam and U. Mandal, Highly Luminescent Nitrogen Doped Carbon Quantum Dots for Mercury Ion Sensing with Antibacterial Activity, J. Fluoresc., 2025 DOI:10.1007/s10895-024-04119-4.
- A. Bilgic, A. Cimen, M. Bayrak and A. N. Kursunlu, Novel Optical Microcapsule Sensor for Effective Recognition of Hg(II) and Ag(I) Ions in the Aquatic Environments, Opt. Laser Technol., 2025, 181, 111839, DOI:10.1016/j.optlastec.2024.111839.
- A. Elie, Y. Niberon, A. Bruyant, L. Arnaud, C. Cosio and J. Proust, Detection of Mercury Ions at Ng/L Scale by Surface Plasmon Resonance (SPR) on DNA Aptamer Biochips, J. Phys. Chem. C, 2025, 129(3), 1779–1788, DOI:10.1021/acs.jpcc.4c04764.
- R. Ziessel, G. Ulrich and A. Harriman, The Chemistry of Bodipy: A New El Dorado for Fluorescence Tools, New J. Chem., 2007, 31(4), 496, 10.1039/b617972j.
- S. Banfi, G. Nasini, S. Zaza and E. Caruso, Synthesis and Photo-Physical Properties of a Series of BODIPY Dyes, Tetrahedron, 2013, 69(24), 4845–4856, DOI:10.1016/j.tet.2013.04.064.
- A. Treibs and F. Kreuzer, Difluorboryl-Komplexe von Di- Und Tripyrrylmethenen, Adv. Cycloaddit., 1968, 718(1), 208–223, DOI:10.1002/jlac.19687180119.
- J. Bañuelos-Prieto and R. S. Llano, BODIPY Dyes: A Privilege Molecular Scaffold with Tunable Properties, BoD – Books on Demand, 2019 Search PubMed.
- M. Poddar and R. Misra, Recent Advances of BODIPY Based Derivatives for Optoelectronic Applications, Coord. Chem. Rev., 2020, 421, 213462, DOI:10.1016/j.ccr.2020.213462.
- E. V. Antina, N. A. Bumagina, A. I. V’yugin and A. V. Solomonov, Fluorescent Indicators of Metal Ions Based on Dipyrrоmethene Platform, Dyes Pigments, 2017, 136, 368–381, DOI:10.1016/j.dyepig.2016.08.070.
- N. A. Bumagina, E. V. Antina, A. A. Ksenofontov, L. A. Antina, A. A. Kalyagin and M. B. Berezin, Basic Structural Modifications for Improving the Practical Properties of BODIPY, Coord. Chem. Rev., 2022, 469, 214684, DOI:10.1016/j.ccr.2022.214684.
- M. Liu, S. Ma, M. She, J. Chen, Z. Wang, P. Liu, S. Zhang and J. Li, Structural Modification of BODIPY: Improve Its Applicability, Chin. Chem. Lett., 2019, 30(10), 1815–1824, DOI:10.1016/j.cclet.2019.08.028.
- L. Wang, H. Ding, X. Ran, H. Tang and D. Cao, Recent Progress on Reaction-Based BODIPY Probes for Anion Detection, Dyes Pigments, 2020, 172, 107857, DOI:10.1016/j.dyepig.2019.107857.
- N. A. Bumagina and E. V. Antina, Review of Advances in Development of Fluorescent BODIPY Probes (Chemosensors and Chemodosimeters) for Cation Recognition, Coord. Chem. Rev., 2024, 505, 215688, DOI:10.1016/j.ccr.2024.215688.
- A. N. Kursunlu, E. Bastug and E. Guler, Importance of BODIPY-Based Chemosensors for Cations and Anions in Bio-Imaging Applications, Curr. Anal. Chem., 2022, 18(2), 163–175, DOI:10.2174/1573411017666201215105055.
- B. Zhu, X. Yang, L. Jiang, T. Chen, S. Wang and L. Zeng, A Portable and Versatile Fluorescent Platform for High-Throughput Screening of Toxic Phosgene, Diethyl Chlorophosphate and Volatile Acyl Chlorides, Chin. Chem. Lett., 2025, 36(1), 110222, DOI:10.1016/j.cclet.2024.110222.
- W.-Q. Meng, A. C. Sedgwick, N. Kwon, M. Sun, K. Xiao, X.-P. He, E. V. Anslyn, T. D. James and J. Yoon, Fluorescent Probes for the Detection of Chemical Warfare Agents, Chem. Soc. Rev., 2023, 52(2), 601–662, 10.1039/D2CS00650B.
- Q. Chen, Y. Sun, S. Liu, J. Zhang, C. Zhang, H. Jiang, X. Han, L. He, S. Wang and K. Zhang, Colorimetric and Fluorescent Sensors for Detection of Nerve Agents and Organophosphorus Pesticides, Sens. Actuators, B, 2021, 344, 130278, DOI:10.1016/j.snb.2021.130278.
- J. Ordóñez-Hernández, J. G. Planas and R. Núñez, Carborane-Based BODIPY Dyes: Synthesis, Structural Analysis, Photophysics and Applications, Front. Chem., 2024, 12, 1485301, DOI:10.3389/fchem.2024.1485301.
- S. Chakraborty, The Advent of Bodipy-Based Chemosensors for Sensing Fluoride Ions: A Literature Review, J. Fluoresc., 2024 DOI:10.1007/s10895-024-03619-7.
- T. Kowada, H. Maeda and K. Kikuchi, BODIPY-Based Probes for the Fluorescence Imaging of Biomolecules in Living Cells, Chem. Soc. Rev., 2015, 44, 4953–4972, 10.1039/C5CS00030K.
- A. V. Raveendran, P. A. Sankeerthana, A. Jayaraj and P. Chinna Ayya Swamy, Recent Developments on BODIPY Based Chemosensors for the Detection of Group IIB Metal Ions, Results Chem., 2022, 4, 100297, DOI:10.1016/j.rechem.2022.100297.
- J. Janata, Optical Sensors, in Principles of Chemical Sensors, Springer, US, Boston, MA, 2009, pp. 267–311, DOI:10.1007/b136378_9.
- D. Khan, Shaily. Coumarin-based Fluorescent Sensors, Appl. Organomet. Chem., 2023, 37(7), e7138, DOI:10.1002/aoc.7138.
- S. Dadashi-Silab, S. Doran and Y. Yagci, Photoinduced Electron Transfer Reactions for Macromolecular Syntheses, Chem. Rev., 2016, 116(17), 10212–10275, DOI:10.1021/acs.chemrev.5b00586.
- S. Sasaki, G. P. C. Drummen and G. Konishi, Recent Advances in Twisted Intramolecular Charge Transfer (TICT) Fluorescence and Related Phenomena in Materials Chemistry, J. Mater. Chem. C, 2016, 4(14), 2731–2743, 10.1039/C5TC03933A.
- D. Udhayakumari, P. Jerome, N. Vijay and T. Hwan Oh, ESIPT: An Approach and Future Perspective for the Detection of Biologically Important Analytes, J. Lumin., 2024, 267, 120350, DOI:10.1016/j.jlumin.2023.120350.
- H. C. Joshi and L. Antonov, Excited-State Intramolecular Proton Transfer: A Short Introductory Review, Molecules, 2021, 26(5), 1475, DOI:10.3390/molecules26051475.
- A. C. Sedgwick, L. Wu, H.-H. Han, S. D. Bull, X.-P. He, T. D. James, J. L. Sessler, B. Z. Tang, H. Tian and J. Yoon, Excited-State Intramolecular Proton-Transfer (ESIPT) Based Fluorescence Sensors and Imaging Agents, Chem. Soc. Rev., 2018, 47(23), 8842–8880, 10.1039/C8CS00185E.
- H. Li, Y. Yang, F. Xu, T. Liang, H. Wen and W. Tian, Pillararene-Based Supramolecular Polymers, Chem. Commun., 2019, 55(3), 271–285, 10.1039/C8CC08085B.
- E. Yemisci, A. Nuri Kursunlu, M. Oguz, A. Oguz and M. Yilmaz, A Useful Macrocyclic Combination of Pillar[5]Arene and Bodipy for Fluorometric Analysis of Hg2+: High-Resolution Monitoring in Fish Sample and Living Cells, J. Mol. Liq., 2023, 369, 120940, DOI:10.1016/j.molliq.2022.120940.
- Y. Wang, X. Wang, W. Ma, R. Lu, W. Zhou and H. Gao, Recent Developments in Rhodamine-Based Chemosensors: A Review of the Years 2018–2022, Chemosensors, 2022, 10(10), 399, DOI:10.3390/chemosensors10100399.
- F. Abebe, P. Perkins, R. Shaw and S. Tadesse, A Rhodamine-Based Fluorescent Sensor for Selective Detection of Cu2+ in Aqueous Media: Synthesis and Spectroscopic Properties, J. Mol. Struct., 2020, 1205, 127594, DOI:10.1016/j.molstruc.2019.127594.
- W. Shi, J. Liu, P. Lo and D. K. P. Ng, Selective Detection of Hg2+ Ions with Boron Dipyrromethene-Based Fluorescent Probes Appended with a Bis(1,2,3-triazole)Amino Receptor, Chem.–Asian J., 2019, 14(7), 1059–1065, DOI:10.1002/asia.201900166.
- D. Wen, X. Deng, G. Xu, H. Wu and Y. Yu, A Novel FRET Fluorescent Probe Based on BODIPY- Rhodamine System for Hg2+ Imaging in Living Cells, J. Mol. Struct., 2021, 1236, 130323, DOI:10.1016/j.molstruc.2021.130323.
- R. A. Vithal, R. Kishore, D. S. Janardan, N. S. Chundawat, N. Srivastava and G. P. Singh, Novel Thiosemicarbazone Based Sensors for Transition Metals, Sens. Diagn., 2024, 3(11), 1822–1826, 10.1039/D4SD00266K.
- U. Haldar and H. Lee, BODIPY-Derived Polymeric Chemosensor Appended with Thiosemicarbazone Units for the Simultaneous Detection and Separation of Hg(II) Ions in Pure Aqueous Media, ACS Appl. Mater. Interfaces, 2019, 11(14), 13685–13693, DOI:10.1021/acsami.9b00408.
- P. M. O. Gomes, P. M. S. Ouro, A. M. S. Silva and V. L. M. Silva, Styrylpyrazoles: Properties, Synthesis and Transformations, Molecules, 2020, 25(24), 5886, DOI:10.3390/molecules25245886.
- F. Kournoutas, A. Fihey, J.-P. Malval, A. Spangenberg, M. Fecková, P. Le Poul, C. Katan, F. Robin-le Guen, F. Bureš, S. Achelle and M. Fakis, Branching Effect on the Linear and Nonlinear Optical Properties of Styrylpyrimidines, Phys. Chem. Chem. Phys., 2020, 22(7), 4165–4176, 10.1039/C9CP06476A.
- N. I. Wickramasinghe, B. Corbin, D. Y. Kanakarathna, Y. Pang, C. S. Abeywickrama and K. J. Wijesinghe, Bright NIR-Emitting Styryl Pyridinium Dyes with Large Stokes' Shift for Sensing Applications, Biosensors, 2023, 13(8), 799, DOI:10.3390/bios13080799.
- M.-C. Ríos, N.-F. Bravo, C.-C. Sánchez and J. Portilla, Chemosensors Based on N-Heterocyclic Dyes: Advances in Sensing Highly Toxic Ions Such as CN− and Hg2+, RSC Adv., 2021, 11(54), 34206–34234, 10.1039/D1RA06567J.
- K. Supabowornsathit, K. Faikhruea, B. Ditmangklo, T. Jaroenchuensiri, S. Wongsuwan, S. Junpra-ob, I. Choopara, T. Palaga, C. Aonbangkhen, N. Somboonna, J. Taechalertpaisarn and T. Vilaivan, Dicationic Styryl Dyes for Colorimetric and Fluorescent Detection of Nucleic Acids, Sci. Rep., 2022, 12(1), 14250, DOI:10.1038/s41598-022-18460-w.
- V. Le, J.-E. Jeong, H. Huynh, J. Lee and H. Woo, An Ionic 1,4-Bis(Styryl)Benzene-Based Fluorescent Probe for Mercury(II) Detection in Water via Deprotection of the Thioacetal Group, Sensors, 2016, 16(12), 2082, DOI:10.3390/s16122082.
- Z. Xue, T. Liu and H. Liu, Naked-Eye Chromogenic and Fluorogenic Chemosensor for Mercury (II) Ion Based on Substituted Distyryl BODIPY Complex, Dyes Pigments, 2019, 165, 65–70, DOI:10.1016/j.dyepig.2019.01.061.
- Y. Huang, C.-F. Li, W.-J. Shi, H.-Y. Tan, Z.-Z. He, L. Zheng, F. Liu and J.-W. Yan, A Near-Infrared BODIPY-Based Fluorescent Probe for Ratiometric and Discriminative Detection of Hg2+ and Cu2+ Ions in Living Cells, Talanta, 2019, 198, 390–397, DOI:10.1016/j.talanta.2019.02.012.
- C. Chan, H. Liu and Z. Xue, Chromogenic and Fluorescent Probe for Detection of Mercury (II) Ion Based on Mono-Pyrrolyl Substituted BODIPY, Microchem. J., 2021, 166, 106247, DOI:10.1016/j.microc.2021.106247.
- H. Meskher and F. Achi, Electrochemical Sensing Systems for the Analysis of Catechol and Hydroquinone in the Aquatic Environments: A Critical Review, Crit. Rev. Anal. Chem., 2022, 1–14, DOI:10.1080/10408347.2022.2114784.
- K. Chetankumar, B. E. Kumara Swamy, S. C. Sharma and S. A. Hariprasad, An Efficient Electrochemical Sensing of Hazardous Catechol and Hydroquinone at Direct Green 6 Decorated Carbon Paste Electrode, Sci. Rep., 2021, 11(1), 15064, DOI:10.1038/s41598-021-93749-w.
- N. Schweigert, A. J. B. Zehnder and R. I. L. Eggen, Chemical Properties of Catechols and Their Molecular Modes of Toxic Action in Cells, from Microorganisms to Mammals: Minireview, Environ. Microbiol., 2001, 3(2), 81–91, DOI:10.1046/j.1462-2920.2001.00176.x.
- Z. Xu, Mechanics of Metal-Catecholate Complexes: The Roles of Coordination State and Metal Types, Sci. Rep., 2013, 3(1), 2914, DOI:10.1038/srep02914.
- S. Razaviamri, K. Wang, B. Liu and B. P. Lee, Catechol-Based Antimicrobial Polymers, Molecules, 2021, 26(3), 559, DOI:10.3390/molecules26030559.
- W. Saiyasombat and S. Kiatisevi, Bis-BODIPY Linked-Triazole Based on Catechol Core for Selective Dual Detection of Ag+ and Hg2+, RSC Adv., 2021, 11(6), 3703–3712, 10.1039/D0RA09686E.
- W. Chen, Y. Guan, Q. Chen, J. Ren, Y. Xie and J. Yin, The Mark of Mercury(II) in Living Animals and Plants through Using a BODIPY-Based near-Infrared Fluorescent Probe, Dyes Pigments, 2022, 200, 110134, DOI:10.1016/j.dyepig.2022.110134.
- D. Wang, L. Marin and X. Cheng, Fluorescent Chitosan-BODIPY Macromolecular Chemosensors for Detection and Removal of Hg2+ and Fe3+ Ions, Int. J. Biol. Macromol., 2022, 198, 194–203, DOI:10.1016/j.ijbiomac.2021.12.075.
- J. He, L. Yun and X. Cheng, Organic-Soluble Chitosan-g-PHMA (PEMA/PBMA)-Bodipy Fluorescent Probes and Film by RAFT Method for Selective Detection of Hg2+/Hg+ Ions, Int. J. Biol. Macromol., 2023, 237, 124255, DOI:10.1016/j.ijbiomac.2023.124255.
- J. He, C. Li and X. Cheng, Water Soluble Chitosan-Amino Acid-BODIPY Fluorescent Probes for Selective and Sensitive Detection of Hg2+/Hg+ Ions, Mater. Chem. Phys., 2023, 295, 127081, DOI:10.1016/j.matchemphys.2022.127081.
- K. Kumarasamy, T. Devendhiran, W.-J. Chien, M.-C. Lin, S. K. Ramasamy and J.-J. Yang, Bodipy-Based Quinoline Derivative as a Highly Hg2+-Selective Fluorescent Chemosensor and Its Potential Applications, Methods, 2024, 223, 35–44, DOI:10.1016/j.ymeth.2024.01.002.
- B. Kumar, P. Saraf, M. Sarkar and D. Kumar, Efficient Synthesis of α/β-Isomeric Oxadiazolyl and Triazolopyridyl BODIPYs for Sensing of Hg2+ Ions and pH Sensors, Tetrahedron, 2023, 137, 133380, DOI:10.1016/j.tet.2023.133380.
- T. Hu, Q. Lai, W. Fan, Y. Zhang and Z. Liu, Advances in Portable Heavy Metal Ion Sensors, Sensors, 2023, 23(8), 4125, DOI:10.3390/s23084125.
- I. Hussain, K. U. Ahamad and P. Nath, Low-Cost, Robust, and Field Portable Smartphone Platform Photometric Sensor for Fluoride Level Detection in Drinking Water, Anal. Chem., 2017, 89(1), 767–775, DOI:10.1021/acs.analchem.6b03424.
- M. Moreira, J. A. De Franca, D. De Oliveira Toginho Filho, V. Beloti, A. K. Yamada, M. De, M. B. Franca and L. De Souza Ribeiro, A Low-Cost NIR Digital Photometer Based on InGaAs Sensors for the Detection of Milk Adulterations With Water, IEEE Sens. J., 2016, 16(10), 3653–3663, DOI:10.1109/JSEN.2016.2530873.
- W. Luo, J. Deng, J. He, Z. Han, C. Huang, Y. Li, Q. Fu and H. A. Chen, Smartphone-Based Multi-Wavelength Photometer for on-Site Detection of the Liquid Colorimetric Assays for Clinical Biochemical Analyses, Sens. Actuators, B, 2021, 329, 129266, DOI:10.1016/j.snb.2020.129266.
- S.-H. Li, A. Jain, T. Tscharntke, T. Arnold and D. W. Trau, Hand-Held Photometer for Instant On-Spot Quantification of Nucleic Acids, Proteins, and Cells, Anal. Chem., 2018, 90(4), 2564–2569, DOI:10.1021/acs.analchem.7b04031.
- S. Gupta, C. H. Huang, G. P. Singh, B. S. Park, N.-H. Chua and R. J. Ram, Portable Raman Leaf-Clip Sensor for Rapid Detection of Plant Stress, Sci. Rep., 2020, 10(1), 20206, DOI:10.1038/s41598-020-76485-5.
- J. Guo, Y. Liu, H. Ju and G. Lu, From Lab to Field: Surface-Enhanced Raman Scattering-Based Sensing Strategies for on-Site Analysis, TrAC, Trends Anal. Chem., 2022, 146, 116488, DOI:10.1016/j.trac.2021.116488.
- J. Jehlička and A. Culka, Critical Evaluation of Portable Raman Spectrometers: From Rock Outcrops and Planetary Analogs to Cultural Heritage – A Review, Anal. Chim. Acta, 2022, 1209, 339027, DOI:10.1016/j.aca.2021.339027.
- O. Bandeliuk, A. Assaf, M. Bittel, M.-J. Durand and G. Thouand, Development and Automation of a Bacterial Biosensor to the Targeting of the Pollutants Toxic Effects by Portable Raman Spectrometer, Sensors, 2022, 22(12), 4352, DOI:10.3390/s22124352.
- T. H. Bui, B. Thangavel, M. Sharipov, K. Chen and J. H. Shin, Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements, Chemosensors, 2023, 11(9), 468, DOI:10.3390/chemosensors11090468.
- T.-H. Ulep and J.-Y. Yoon, Challenges in Paper-Based Fluorogenic Optical Sensing with Smartphones, Nano Convergence, 2018, 5(1), 14, DOI:10.1186/s40580-018-0146-1.
- D. D. Liana, B. Raguse, J. J. Gooding and E. Chow, Recent Advances in Paper-Based Sensors, Sensors, 2012, 12(9), 11505–11526, DOI:10.3390/s120911505.
- C. Sicard, C. Glen, B. Aubie, D. Wallace, S. Jahanshahi-Anbuhi, K. Pennings, G. T. Daigger, R. Pelton, J. D. Brennan and C. D. M. Filipe, Tools for Water Quality Monitoring and Mapping Using Paper-Based Sensors and Cell Phones, Water Res., 2015, 70, 360–369, DOI:10.1016/j.watres.2014.12.005.
- E. Lemaire, R. Moser, C. J. Borsa and D. Briand, Green Paper-Based Piezoelectronics for Sensors and Actuators, Sens. Actuators, A, 2016, 244, 285–291, DOI:10.1016/j.sna.2016.04.024.
- Q. Mei, H. Jing, Y. Li, W. Yisibashaer, J. Chen, B. Nan Li and Y. Zhang, Smartphone Based Visual and Quantitative Assays on Upconversional Paper Sensor, Biosens. Bioelectron., 2016, 75, 427–432, DOI:10.1016/j.bios.2015.08.054.
- S. K. Mahadeva, K. Walus and B. Stoeber, Paper as a Platform for Sensing Applications and Other Devices: A Review, ACS Appl. Mater. Interfaces, 2015, 7(16), 8345–8362, DOI:10.1021/acsami.5b00373.
- F. Pinelli, L. Magagnin and F. Rossi, Progress in Hydrogels for Sensing Applications: A Review, Mater. Today Chem., 2020, 17, 100317, DOI:10.1016/j.mtchem.2020.100317.
- G. Ge, Y. Lu, X. Qu, W. Zhao, Y. Ren, W. Wang, Q. Wang, W. Huang and X. Dong, Muscle-Inspired Self-Healing Hydrogels for Strain and Temperature Sensor, ACS Nano, 2020, 14(1), 218–228, DOI:10.1021/acsnano.9b07874.
- Y. Yang, Y. Yang, Y. Cao, X. Wang, Y. Chen, H. Liu, Y. Gao, J. Wang, C. Liu, W. Wang, J.-K. Yu and D. Wu, Anti-Freezing, Resilient and Tough Hydrogels for Sensitive and Large-Range Strain and Pressure Sensors, Chem. Eng. J., 2021, 403, 126431, DOI:10.1016/j.cej.2020.126431.
- Y. Ma, Y. Gao, L. Liu, X. Ren and G. Gao, Skin-Contactable and Antifreezing Strain Sensors Based on Bilayer Hydrogels, Chem. Mater., 2020, 32(20), 8938–8946, DOI:10.1021/acs.chemmater.0c02919.
- D. Zhang, J. Jian, Y. Xie, S. Gao, Z. Ling, C. Lai, J. Wang, C. Wang, F. Chu and M.-J. Dumont, Mimicking Skin Cellulose Hydrogels for Sensor Applications, Chem. Eng. J., 2022, 427, 130921, DOI:10.1016/j.cej.2021.130921.
- V. S. Vaishnav, S. G. Patel and J. N. Panchal, Development of ITO Thin Film Sensor for Detection of Benzene, Sens. Actuators, B, 2015, 206, 381–388, DOI:10.1016/j.snb.2014.07.037.
| This journal is © The Royal Society of Chemistry 2025 |