Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis of thiopyran derivatives via [4 + 2] cycloaddition reactions
Maryam Mousavi-Ebadiaa,
Javad Safaei-Ghomi *a and
Masoumeh Jadidi Nejad
*a and
Masoumeh Jadidi Nejad b
b
aDepartment of Organic Chemistry, Faculty of Chemistry, University of Kashan, Kashan, 51167, I. R. Iran. E-mail: safaei@kashanu.ac.ir
bDepartment of Chemistry, Isfahan University of Technology, Isfahan, 84156-83111, Iran
First published on 9th April 2025
Abstract
In this review, we provide a comprehensive overview of the synthesis of thiopyran family compounds via cycloaddition reactions, with examples spanning from the year 2000 to the present. We have categorized the [4 + 2] cycloaddition processes using several criteria, particularly distinguishing between intermolecular and intramolecular types based on the Diels–Alder partners. Additionally, from a mechanism standpoint, we differentiate between concerted and stepwise [4 + 2] processes, offering an analysis of these mechanisms based on the current literature.
1 Introduction
For decades, sulfur-containing heterocycles have been a central focus of research in various branches of chemistry due to their unique properties, which facilitate the conversion of functional groups for the synthesis of bioactive compounds.1–8 These heterocycles are integral to everyday life, as many are key components of natural products, such as penicillin, one of the greatest achievements of the twentieth century. Over twenty sulphur derivatives of amino acids have been identified, and examples can be found in fossil fuels and petroleum products. Additionally, sulphur-containing heterocycles have broad applications in the synthesis of disinfectants, antibiotics, antioxidants, dyes, pigments, and especially in the pharmaceutical industry.9,10 Thiocarbonyl compounds act as versatile intermediates in the synthesis of polycyclic compounds. The increasing interest in thiocarbonyl chemistry highlights their potential to generate a wide range of products. The electronegativity of sulphur is only slightly higher than that of carbon, resulting in a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond polarity opposite to that of the carbonyl bond, with a lower overall polarity. As a result, the positive charge on the carbon atom is reduced, making the C
S bond polarity opposite to that of the carbonyl bond, with a lower overall polarity. As a result, the positive charge on the carbon atom is reduced, making the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond less stable than the carbonyl bond and favouring sp3 hybridization.11,12 Thiocarbonyls can function both as dienes and dienophiles, allowing for the formation of [4 + 2] cycloadducts depending on their molecular positioning.13,14 Due to their elevated HOMO and reduced LUMO energy levels compared to carbonyls, these compounds are often activated as dienophiles through electron acceptor binding to the carbon atom, enhancing orbital overlap in the Diels–Alder reaction.15,16 The D–A reaction is considered one of the most transformative processes in organic chemistry, known for its efficiency in forming two carbon–carbon bonds and up to four new stereocenters, which underscores its versatility in natural product and pharmaceutical synthesis.17–19 In classical literature, D–A reactions are generally described as concerted. Lewis's acids, such as BF3·OEt2, are commonly used as catalysts to activate cycloaddition partners by narrowing the energy gap between frontier orbitals.20,21 Based on DFT and the ELF calculations, Domingo has proposed a detailed mechanism for [4 + 2] cycloaddition reactions, distinguishing between concerted and stepwise types depending on intermolecular interactions. Both polar and non-polar stepwise reactions can occur; however, a zwitterionic intermediate is favoured in polar interactions, while a diradical intermediate predominates in non-polar conditions.22–29 Understanding reaction mechanisms offers valuable insights into reaction dynamics and molecular stability, which are crucial for advancing synthetic methodologies and understanding fundamental chemical processes. Pericyclic reactions are celebrated for their ability to synthesize complex compounds under mild conditions with high atom economy, making them essential tools in synthetic chemistry.30–34 Despite more than 80 years since the discovery of the Diels–Alder reaction as one of the most renowned pericyclic reactions, it remains a key concept in organic chemistry. The Hetero-Diels–Alder reaction offers a straightforward approach to synthesizing important six-membered heterocycles with high stereoselectivity.35–41 While oxo D–A and aza D–A reactions have received significant attention, the thio D–A reaction is fundamental in forming various medicinal polycyclic compounds based on the thiopyran scaffold.42 The therapeutic potential of thiopyrans has recently gained recognition for their importance in medicinal chemistry.43 For instance, chlorprothixene, a member of the thioxanthene class, serves as both an antidepressant and an antipsychotic drug. Additionally, tricyclic thiopyran-2-ones have proven effective in combating viral infections, while fragrant compounds containing thiopyran-carbaldehyde further demonstrate the practical significance of these compounds in various real-world applications.44 Woodward's groundbreaking work in 1982 demonstrated the utility of thiopyrans in simplifying the preparation of polypropionates during the total synthesis of rythromycin A.45,46 Thiopyrans are crucial building blocks in the structures of various bioactive agents, including antibacterial, anti-inflammatory, anticancer, and anti-atherosclerotic structures.47,48 Several biologically active derivatives of these compounds are shown in Fig. 1.49–52
S bond less stable than the carbonyl bond and favouring sp3 hybridization.11,12 Thiocarbonyls can function both as dienes and dienophiles, allowing for the formation of [4 + 2] cycloadducts depending on their molecular positioning.13,14 Due to their elevated HOMO and reduced LUMO energy levels compared to carbonyls, these compounds are often activated as dienophiles through electron acceptor binding to the carbon atom, enhancing orbital overlap in the Diels–Alder reaction.15,16 The D–A reaction is considered one of the most transformative processes in organic chemistry, known for its efficiency in forming two carbon–carbon bonds and up to four new stereocenters, which underscores its versatility in natural product and pharmaceutical synthesis.17–19 In classical literature, D–A reactions are generally described as concerted. Lewis's acids, such as BF3·OEt2, are commonly used as catalysts to activate cycloaddition partners by narrowing the energy gap between frontier orbitals.20,21 Based on DFT and the ELF calculations, Domingo has proposed a detailed mechanism for [4 + 2] cycloaddition reactions, distinguishing between concerted and stepwise types depending on intermolecular interactions. Both polar and non-polar stepwise reactions can occur; however, a zwitterionic intermediate is favoured in polar interactions, while a diradical intermediate predominates in non-polar conditions.22–29 Understanding reaction mechanisms offers valuable insights into reaction dynamics and molecular stability, which are crucial for advancing synthetic methodologies and understanding fundamental chemical processes. Pericyclic reactions are celebrated for their ability to synthesize complex compounds under mild conditions with high atom economy, making them essential tools in synthetic chemistry.30–34 Despite more than 80 years since the discovery of the Diels–Alder reaction as one of the most renowned pericyclic reactions, it remains a key concept in organic chemistry. The Hetero-Diels–Alder reaction offers a straightforward approach to synthesizing important six-membered heterocycles with high stereoselectivity.35–41 While oxo D–A and aza D–A reactions have received significant attention, the thio D–A reaction is fundamental in forming various medicinal polycyclic compounds based on the thiopyran scaffold.42 The therapeutic potential of thiopyrans has recently gained recognition for their importance in medicinal chemistry.43 For instance, chlorprothixene, a member of the thioxanthene class, serves as both an antidepressant and an antipsychotic drug. Additionally, tricyclic thiopyran-2-ones have proven effective in combating viral infections, while fragrant compounds containing thiopyran-carbaldehyde further demonstrate the practical significance of these compounds in various real-world applications.44 Woodward's groundbreaking work in 1982 demonstrated the utility of thiopyrans in simplifying the preparation of polypropionates during the total synthesis of rythromycin A.45,46 Thiopyrans are crucial building blocks in the structures of various bioactive agents, including antibacterial, anti-inflammatory, anticancer, and anti-atherosclerotic structures.47,48 Several biologically active derivatives of these compounds are shown in Fig. 1.49–52
Over time, there has been growing interest in developing innovative strategies to transform organosulfur precursors into thiopyran motifs for pharmaceutical applications. Numerous studies on thiopyrans have been conducted to date. Existing reviews typically explore topics such as Diels–Alder reactions, the clinical applications of thiopyrans, and the synthesis and utility of thiopyran-linked polycyclic systems.53–58 These comprehensive reviews offer valuable insights into the advancements in thiopyran research, highlighting their chemical synthesis and diverse applications. By shedding light on these developments, they inspire researchers to further explore new frontiers in the field. However, we have noted that these advancements have been somewhat fragmented in the synthesis literature. To address this gap, we have organized the synthesis methods of thiopyran structures via cycloaddition into a coherent framework, reviewing them from multiple perspectives. This review focuses on the synthesis of six-membered sulfur heterocycles through the [4 + 2] cycloaddition strategy, utilizing thiocarbonyl-functionalized precursors, which can act as either dienophiles or diene components. The review covers a broad spectrum of organosulfur compounds, ranging from common to rare, both cyclic and linear. We aim to provide a comprehensive overview of unique studies published from 2000 to 2025 in this area.
1.1 Intermolecular cycloaddition reactions
Intermolecular cycloaddition reactions involve the formation of rings from reactants derived from separate molecules. The conversion of two distinct entities into a cycloadduct typically occurs in opposition to entropy, resulting in a reaction rate and efficiency that are generally lower than those observed in intramolecular processes.591.1.2.1 Dithioesters. Dithioesters are unique esters in which sulfur atoms replace the oxygen atoms typically found in conventional structures. Among thiocarbonyl derivatives, dithioesters exhibit remarkable nucleophilic strength, a characteristic attributed to the high polarizability of the sulfur groups. Numerous studies have documented the involvement of dithioesters in nucleophilic substitution, cycloaddition and radical reactions and polymerization processes.65–68
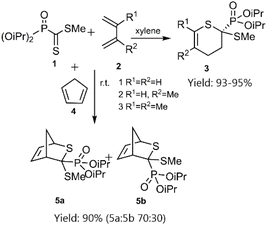
In a notable investigation, Masson et al. demonstrated the application of the D–A reaction for synthesizing phosphonic-substituted dihydrothiopyran derivatives (Schemes 1–3). In this account, phosphonic-substituted dithioformates 1, 9 were reacted with usual dienes such as functionalized butadiene 2, 6 and cyclopentadiene 4, yielding a with high yield. It is noteworthy that the product featuring an exo-phosphonate group 5a was the preferred cycloadduct (Scheme 1), and the use of Bu3SnH as a Lewis acid facilitated the selective desulfanylation of the thio D–A products without disrupting the thiopyran ring (Scheme 2).69,70
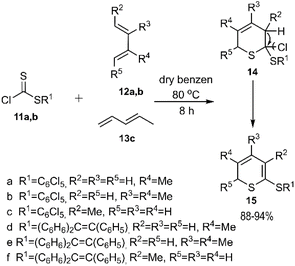
In that same year, El-Sayed and colleagues unveiled an intriguing pathway for thio D–A reactions aimed at the regioselective synthesis of 2H-thiopyran derivatives. The authors noted that the observed regioselectivity of the products was unexpected, yet it could be rationalized by steric effects. In their study, thioesters 11a-b reacted with dienes 12a-b and 13 to yield compound 15, which was subsequently transformed into the final product after spontaneous elimination and the removal of HCl. Dithioester 14b followed a similar pathway under identical conditions, leading to the formation of heterocycles 14e-f and 15e-f (Scheme 4).71
Shortly after this groundbreaking work, El-Sayed et al. reported a similar synthetic route for the preparation of (epithiomethano)anthracene sulfanediones 18 (Scheme 5). In their study, two anthracene derivatives (R3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H, methyl) were employed as 1,3-diene species, reacting with various C-sulfonyldithioformate derivatives 17 to achieve excellent stereoselectivity. This reaction subsequently led to the elimination of arenesulfinic acid from the D–A cycloadducts.72
H, methyl) were employed as 1,3-diene species, reacting with various C-sulfonyldithioformate derivatives 17 to achieve excellent stereoselectivity. This reaction subsequently led to the elimination of arenesulfinic acid from the D–A cycloadducts.72
El-Sayed and colleagues proposed a hetero D–A reaction that facilitated the construction of dihydrothiopyran derivatives. This innovative approach utilized C-sulfonyldithioformates 19 as super hetero dienophiles in combination with 1,3-dienes 4, 22, 24, and 13. Notably, when employing 1,3-pentadiene 21, the resulting products exhibited an endo preference at room temperature (Scheme 6).
It is worth mentioning that upon chromatography on silica gel using methylene chloride as the eluent, endo-20a was quantitatively converted to exo-20b. This transformation highlights the stability of exo-22b due to its lower steric hindrance, facilitated through ion pair 21 (Scheme 7). The products 25a, 25b, and 26a demonstrated favourable regioselectivity (Scheme 8).73
Gulea and her team explored the impact of pyridinedithioesters 27 on enhancing the reaction progress with common dienes, ultimately yielding new derivatives of the biologically relevant aprikalim 31 scaffold (Scheme 9). To further increase the reactivity of the hetero dienophile, N-oxide pyridine thioester 32 was employed in place of 27, resulting in satisfactory outcomes (Scheme 10).74
In 2008, Sinnwell and colleagues introduced a novel approach by employing the thio D–A reaction to synthesize dihydro-2H-thiopyran functionalized poly ethylene glycol 36. In this innovative reaction, the terminal diene group is situated on the polyethylene glycol, facilitating RAFT polymerization with functionalized polystyrene (see Scheme 11).75
 | ||
| Scheme 11 Preparation of dihydro-2H-thiopyran functionalized poly (ethylene glycol) 36 via HD–A reaction. | ||
Timoshenko et al. reported the thio D–A reaction involving chiral polyfluoroalkylthionocarboxylates 37 and butadiene derivatives 2. The resulting compounds exhibited moderate diastereoselectivity in the Diels–Alder reactions of thioesters (refer to Scheme 12).76
Goldmann and collaborators successfully combined RAFT polymerization with a hetero D–A reaction involving a 2-pyridinedithioester derivative 42 and cyclopentadiene grafted onto cellulose 43 (see Scheme 13).77
Gulea's research team unveiled novel dithioester substrates as dienophiles, derived from the addition–elimination of a sulfonyl molecule (Scheme 14). The observed trans-selectivity at room temperature can be attributed to the significant electron-withdrawing effect of the pyridyl or quinolyl group on the sulfonyl moiety, as other thioester substrates yielded a mixture of both isomers. Expanding upon their previous work, this research team explored the reaction of three distinct chiral dithioesters with simple dienes to synthesize valuable compounds bearing novel chiral stereocenters (Scheme 15). The employment of an asymmetric Cu(II)-bis(oxazoline) catalyst facilitated chelate formation during the reaction, leveraging its impressive stereo control to achieve high diastereomeric excess (90% de). DFT studies indicated that the Si-face approach was preferred due to reduced steric hindrance (Scheme 16).78,79
Lederer and colleagues reported a thermo-sensitive thio D–A reaction, utilizing the synthesis and application of polyfunctional dithiooxalate derivatives 49 as thiophilic dienophiles under ambient conditions. This reaction has been explored with a variety of dienes, encompassing cyclic and acyclic structures, as well as those exhibiting s-cis or s-trans conformations (Scheme 17).80
Gulea et al. described a distinct synthetic pathway for the preparation of organosulfur compounds. Exo-bicyclic 1,3-dienes were accessed via a metal-catalyzed intermolecular Sonogashira coupling of 2-bromocyclohex-2-en-1-ylsulfane derivatives 52a–c. Subsequently, the exo-bicyclic thio dienes 53a–c were subjected to cycloaddition reactions with various dienophiles to investigate their reactivity The thio D–A reaction of methyl pyridine-2-carbodithioate 32b yielded regioisomeric products 54 and 55 (Scheme 18).3
 | ||
| Scheme 18 H D–A reaction of methyl pyridine-2-carbodithioate 32b and 2, 3-exo-bicyclic dienes 53a–c. | ||
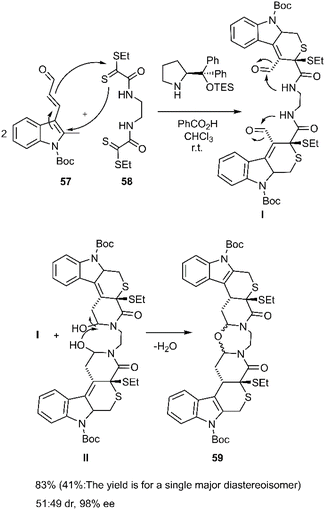
Cruz Cruz et al. broadened the scope of polycyclic synthesis by demonstrating a hetero-Diels–Alder/intramolecular cycloaddition cascade catalyzed by trienamines, affording nine-membered ring systems with excellent enantioselectivity. This strategy involved a double [4 + 2] cycloaddition reaction between (E)-oxopropenylindole carboxylate derivative 57 and bis-dithioamide 58 (Scheme 19). The group further extended this methodology to the synthesis of diverse polycycles, employing dienals and bis 2-oxoethanedithioates 58 as starting materials.81
The Gulea research group has recently demonstrated a novel and practical application of the thio D–A reaction, extending its utility into the realm of biological imaging. This work leverages the D–A reaction for the synthesis of novel fluorescent peptides, enabling the visualization of living cells. The approach involves a straightforward, uncatalyzed click reaction between phosphodiester peptides 60 and fluorophore-decorated dienes 61 via a thio D–A cycloaddition (Scheme 20).82
1.1.2.2 Thioaldehydes. Thioaldehydes, featuring the H–C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functional group, are sulfur analogs of conventional aldehydes. They serve as valuable intermediates in the construction of complex polycyclic systems, particularly within the context of cycloaddition reactions. However, thioaldehydes are inherently unstable, readily undergoing dimerization or polymerization, and often yielding a mixture of products in their reactions. Isolation of thioaldehydes is challenging, leading to their prevalent use as in situ generated and consumed reagents. Nevertheless, research has demonstrated that electron-donating substituents can enhance the stability of thioaldehydes, thereby facilitating their isolation.83–86
S functional group, are sulfur analogs of conventional aldehydes. They serve as valuable intermediates in the construction of complex polycyclic systems, particularly within the context of cycloaddition reactions. However, thioaldehydes are inherently unstable, readily undergoing dimerization or polymerization, and often yielding a mixture of products in their reactions. Isolation of thioaldehydes is challenging, leading to their prevalent use as in situ generated and consumed reagents. Nevertheless, research has demonstrated that electron-donating substituents can enhance the stability of thioaldehydes, thereby facilitating their isolation.83–86Sakakibara and Watanabe reported an efficient and straightforward synthesis of dihydro-2H-thiopyran-2,5-diyl diacetate 64 through a thio-D–A reaction between thioaldehydes 63 and 1,3-butadiene derivatives (Scheme 21).87
In situ generated thiobenzaldehyde 66, prepared via reaction with phenyl Grignard reagent, underwent a highly selective endo-thio D–A cycloaddition with cyclopentadiene, affording the corresponding cycloadducts as major products (Scheme 22). This rapid and innovative reaction, reported by Murai and coworkers, enabled the efficient synthesis of valuable heterocyclic compounds.88
Tanini and coworkers reported the thionation of aldehydes through mild conditions with different catalyst systems. Thio D–A cycloadducts were produced in situ by trapping the corresponding thioaldehydes by diene using cobalt salt in ionic liquids or silylated catalysts (Scheme 23). Finally, endo-isomer dihydrothiopyran derivatives 69 and 70 were obtained with good yields.89
Furthermore, the Nakamura research group explored the in situ generation of thioacrolein 72 from alysin, leveraging this reactive intermediate in a thio D–A reaction to synthesize antitumor dihydrothiopyran derivatives 73. Notably, this highly reactive intermediate, when reacted with a pair of Danishefsky's diene molecules 71, exhibited dual nucleophilic character, participating in a two-fold D–A cycloaddition (Scheme 24).90
1.1.2.3 Thioamides. Thioamides, also known as thioureylenes, are characterized by the presence of the N–C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functional group. These compounds have found applications in both drug development and biosynthesis. The enhanced resonance stabilization of thioamides compared to their amide counterparts results in a higher rotational barrier. This unique property has enabled synthetic chemists to utilize thioamides as versatile building blocks in the construction of diverse heterocyclic frameworks.91–94
S functional group. These compounds have found applications in both drug development and biosynthesis. The enhanced resonance stabilization of thioamides compared to their amide counterparts results in a higher rotational barrier. This unique property has enabled synthetic chemists to utilize thioamides as versatile building blocks in the construction of diverse heterocyclic frameworks.91–94In a pioneering study conducted Bouillon et al. reported the first successful synthesis of novel dihydrothiopyran derivatives via a highly efficient thio-D–A reaction employing a polyfluorothioamide derivative 74. While conventional approaches, such as increasing pressure or temperature, and the use of basic catalysts, were explored for analogous thioamides, these methods proved ineffective in achieving comparable outcomes (Scheme 25).95
1.1.2.4 Thioketones. Thioketones constitute a prominent class of organosulfur compounds. Characteristically exhibiting orange or brown coloration, these highly reactive species serve as valuable dienophiles in cycloaddition reactions. Notable examples of thioketones include thiobenzophenone, thioxanthone, and thioisatin.96,97
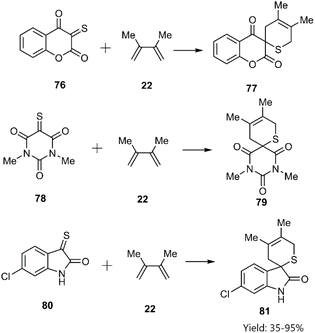
Shermolovich et al. reported the synthesis of a novel series of heterocyclic compounds featuring spirocyclic thione substituents through a thio D–A cycloaddition strategy. Their approach involved the initial selection of heterocycles bearing carbonyl functional groups. Subsequent via thionation of these precursors yielded α-oxo thioketones (e.g., compounds 76, 78, and 80), which were subsequently reacted with 2,3-dimethylbutadiene 22 (Scheme 26).98
Mlostoń, Kowalski, and their colleagues developed a protocol for the synthesis of heterocycles integrated within multi-redox systems. These multi-ferrocenyl-functionalized organometallic structures were synthesized via a hetero D–A reaction between diferrocenyl-substituted thioketone 82 and diene 22 in the presence of an organic acid (Scheme 27).99
Skiba and co-workers reported a facile synthetic route to two ferrocenyl-thymine-3,6-dihydro-2H-thiopyran derivatives 85, exhibiting anticancer activity, through a thio D–A cycloaddition. This approach involved the reaction of reagent 84 with diene 22 (Scheme 28).100
Mlostoń, Albrecht, and other coworkers reported enantioselective amine catalyzed thio D–A cycloaddition of thioketones 86 with dienales 87 (Scheme 29).101,102
Sperry research group reported a total synthesis of Alkaloids derived from isatine indigotica via thio D–A reaction. Insatindigothiadiazole-derived 89 as diene synthesized in several consecutive steps, starting from natural sources, and 3-thioxoindolin-2-one 88 was generated from oxindole by the sulfonation and performed in situ cycloaddition. The structures of the products and TSs were investigated using DFT. The interaction of π–π in the transition state has caused the high selectivity of the final product (Scheme 30).103
In 2018, Mlostoń's research group achieved the successful synthesis of novel oxathiazine derivatives 95, demonstrating promising potential for future applications. The presence of nitrogen, oxygen, and sulfur atoms within their structure renders these compounds attractive candidates for development as novel ligands. This synthetic pathway involved the cyclization of ferrocenyl thiones 94 with 1-nitroso-1-arylethene derivatives 93, originating from α-halogenacetophenone oxime substrate 92 (Scheme 31).
To demonstrate the versatility of this methodology, the reaction was further investigated with a range of thioketones, including thiochalcones 96, 5-(thiophene-2-carbonothioyl)thiophen-2-ylium 98, and adamantine-2-thione 100 (Scheme 32).104
The scope of this reaction was further expanded by employing α-nitrosoalkenes 102, 105 and thioxo cyclobutanone derivative 103. Symmetrical products 107 were synthesized via a novel double hetero D–A reaction between dithione 103 and α-nitroso alkene 105. Mechanistic studies revealed that steric effects govern the reaction pathway, with trans-selectivity favoured due to minimized steric hindrance. Notably, all reactions proceeded under mild conditions (Scheme 33).105
Zhou et al. reported the utilization of carbonyl sulfide for the organocatalytic sulfonation of isatine derivatives. In situ generated thioxoindolin-2-ones 88 underwent thio D–A reactions with diene 22, leading to the formation of spiro compounds featuring indoline thiopyran-2-one skeletons 108. A key aspect of this work was the judicious selection of solvent. DMSO proved to be an effective solvent, preventing the formation of dimeric products (Scheme 34).106
1.1.2.5 N-Sulfinyl dienophiles. N-Sulfinyl dienophiles, highly reactive dipolar ionic compounds, have garnered significant attention from synthetic chemists as versatile substrates for cycloaddition reactions, particularly [3 + 2] and [4 + 2] cycloaddition reactions.102,107,108
Gautun et al. reported efficient D–A cycloadditions employing N-sulfinyl compounds 109 as highly activating heterodienophiles with 1,3-cyclohexadiene 110 in the presence of chiral Ti(IV)-based Lewis's acids. These reactions afforded predominantly endo-isomers 111a-b with high enantioselectivity (Scheme 35). Subsequently, the same group explored the use of chiral organometallic catalysts to promote this reaction.109,110
Wang and coworkers developed a diastereoselective and regioselective [4 + 2] cycloaddition of dipolar N-sulfinyl dienophiles 109 with various dienes. A key aspect of this reaction is the ability of the substrate to chelate to Ti2+ or Sn2+, which significantly enhances the regioselectivity of the cycloaddition (Fig. 4).111
1.1.2.6 Thiophosgene. Thiophosgene is a highly reactive and versatile precursor characterized by two labile carbon–chlorine bonds. Its toxicity arises from its role as a toxicophore, similar to those found in fungicides like Captan and Folpet. While thiophosgene hydrolyzes more slowly than its oxygen analogue, phosgene, its pronounced reactivity makes it a valuable reagent in organic synthesis. Notably, the Diels–Alder reaction offers an efficient and practical route for converting toxic thiophosgene into useful thiopyran derivatives.112–114
Föhlisch and colleagues successfully synthesized a novel thio-tricyclic scaffold through a [4 + 2] cycloaddition reaction between thiophosgene 112 and spiro[2.4]hepta-4,6-diene 113, yielding the corresponding adduct 114 (Scheme 36).115
Subsequently, Nakayama and coworkers demonstrated the utility of (R)-4,5-dibutyl-2H-thiopyran 1-oxide 115 as an efficient trapping agent in thio D–A reactions with thiophosgene 112. This reaction exhibited excellent π-face selectivity, with cycloaddition occurring exclusively from the syn-π-face of the diene relative to the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond, leading to the formation of the endo-cycloadduct 116 via attack at the less hindered face (Scheme 37).116
O bond, leading to the formation of the endo-cycloadduct 116 via attack at the less hindered face (Scheme 37).116
1.1.3.1 Reactions of acyclic thiodines. Acyclic thiodienes encompass a diverse range of examples, including unsaturated thioketones, thioesters, thioaldehydes, and thioamides, among others.
Li et al. investigated the thio D–A reactions of thiochalcone derivatives 96 with cyclopentadiene (Scheme 38). Notably, the substitution pattern at R1 and R2 was found to exert spatial control over the dimerization process. For instance, when R1 and R2 are hydrogen, head-to-head dimerization is favoured. However, when R1 is phenyl and R2 is hydrogen, a preference for head-to-tail orientation is observed.117
D–A reactions of unsaturated 1, 3-oxathiolane derivative 117 and aliphatic and aromatic dienophiles were examined by Kerverdo and coworkers. The Lewis acidic TiCl4 facilitated the regioselective ring-opening of (E)-2-methyl-2-styryl-1,3-oxathiolane 129 via coordination, generating a reactive thio-dienophile intermediate (Schemes 39 and 40). NOESY data indicated that the major products were cis-cycloadducts. Notably, the masked thioketone 119 effectively served as a thiodiene in these reactions with various alkenes, as depicted in Scheme 40.118,119
According to report of Capperucci and coworkers, the cycloadducts 130 and 131 were obtained via the D–A reaction of thio diene 128 and ethanethial 129 which in situ formed from allene silanes (Scheme 41). Notably, self-dimerization of 131 was observed as the major product under these conditions.120
Subsequently, Bogdanowicz-Szwed and Budzowski successfully developed a diastereoselective thio D–A reaction between the unsaturated amino thioketone 161 and 1H-pyrrole-2,5-dione 162 to afford tetrahydrothiopyrans 163 (Scheme 42). NOESY analysis was employed to determine the stereochemistry of the products. Interestingly, previous studies by this research group had demonstrated the ineffectiveness of this approach with EWG-substituted alkenes, including maleic acid, fumaric acid, maleimide, oxo-4-(phenylamino)but-2-enoic acid, and furan-2,5-dione.121–123
Harrison-Marchand 's group pioneered the first asymmetric synthesis of thiazine derivatives. Their approach involved investigating the cycloaddition reaction of benzothioamide derivative 135 with dienophiles 136 under various conditions. Optimal results were achieved using samarium triflate as a catalyst. A stereoselectivity study of the cycloadducts revealed a temperature-dependent isomerization of 137a to 137b. Specifically, the cis-isomer underwent thermal conversion to the corresponding trans-isomer (Scheme 43).124
Schenk et al. explored the stabilization of thioaldehydes within organometallic structures through coordination of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S group. They investigated the hetero D–A reaction of thiocinnamaldehydes, employing a strategy involving the formation of ruthenium complexes of thiocinnamaldehydes 138. Two dienophiles, norbornadiene 139 and ethyl propiolate 141, were successfully trapped by the organometallic intermediate during its formation. Steric effects were found to play a dominant role in controlling these reactions (Scheme 44).125
S group. They investigated the hetero D–A reaction of thiocinnamaldehydes, employing a strategy involving the formation of ruthenium complexes of thiocinnamaldehydes 138. Two dienophiles, norbornadiene 139 and ethyl propiolate 141, were successfully trapped by the organometallic intermediate during its formation. Steric effects were found to play a dominant role in controlling these reactions (Scheme 44).125
The dihydro-1, 3-thiazinume derivatives 146 were produced using microwave-promoted MCRs of aryl aldehyde 143, thiourea 144, and vinyl aryls 145 (Scheme 45). This work was reported by Wan and coworkers. In the following, it is stated that the reaction of simple cyclohexene gave no product.126
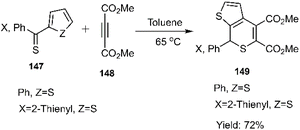
Mlostoń and co-workers related the synthesis of a series of polycyclic compounds through the D–A reaction of various diarylthioketones 147 with dimethyl but-2-ynedioate 148 in toluene at 65 °C (Scheme 46). This group also succeed to establish a mild reaction of thiobenzophenone 86 with dimethyl but-2-ynedioate (Scheme 47). Expanding upon the work of Mlostoń et al. on thio D–A reactions, this research group explored the regioselective cycloaddition of aryl thioketones 180a-b with dimethyl but-2-ynedioate 177. This reaction proceeded smoothly under the same conditions when employing thioketones bearing heteroaryl substituents. Subsequently, the cycloadducts underwent oxidation to yield the final sulfone structures. Furthermore, they successfully established a mild reaction protocol for thiobenzophenone 98 with dimethyl but-2-ynedioate (Scheme 48).105,106,127
Gulea and her research team developed a microwave-assisted three-component hetero D–A reaction to synthesize optically active 1,3-thiazine derivatives 156. This innovative method involves the amination of aromatic thioamide derivatives with arylaldehydes, leading to the formation of the unsaturated thioamide 154. This intermediate is subsequently reacted with various alkenes, including norbornene, hex-1-ene, chalcone, allyl benzene, and (Z)-cyclooctene. The synthetic strategy was validated through comprehensive COSY, HSQC, HMBC, and NOESY experiments. The resulting products were obtained in varying endo/exo ratios, with a preference for the exo-approach observed in the cases of norbornene and hex-1-ene (see Scheme 49).128–130
Richichi et al. conducted a series of elegant studies that elucidated a stereoselective inverse electron demand [4 + 2] cycloaddition. This innovative approach involved the in situ generation of reactive α-dioxothiones 158, which subsequently reacted with glycosyl substrates 159, yielding KDO-based glycosyloxathiins 160 as a promising biological scaffold (see Scheme 50).131
Mlostoń's group investigated the cycloaddition of thiochalcone derivatives 161 with acetylene carboxylates 162. These reactions were explored under two distinct conditions: in the presence of LiClO4 in THF at 65 °C and under microwave irradiation. Notably, microwave irradiation led to enhanced yields and a significant reduction in reaction time compared to conventional heating. Subsequently, Mlostoń, Albrecht, and colleagues101 explored the synthesis of bioactive compounds via an asymmetric inverse-electron-demand thio D–A reaction of the thiochalcone derivatives with electron-deficient alkenes 165 as reaction partners, affording the desired products in excellent yields. The results demonstrated a high degree of ortho-regioselectivity, a phenomenon previously unreported in the literature. In 2018, an unprecedented synthesis of thiochromenedione derivatives 168 was achieved via a D–A reaction. Notably, the anticipated oxo D–A reaction pathway was not observed. The proposed mechanism identified intramolecular hydrogen bond formation in the enol form of 1, 4-quinones, which amplified the electrophilicity of the C (3) position and accelerated the reaction. Then, they investigated the cycloaddition of α-nitroso alkene 169 with thiochalcones. Despite the possibility of eight isomeric products, the reaction exclusively produced 170, incorporating all three heteroatoms within the ring. A combination of experimental evidence and theoretical insights suggests that the reaction proceeds under kinetic control (See all three cases respectively in Scheme 51).
In continuation of Mlostoń's research in 2018, the scope and versatility of thiodienes were broadened under conventional conditions through the utilization of heteroaryl thiones bearing a ferrocenyl moiety 171 affording products with perfect regioselectivity. Importantly, attempts to synthesize isomeric thiochalcones by altering the aryl group at the C (1) position and the ferrocenyl (Fc) group at the C (3) position proved unsuccessful (Scheme 52).101,104,105,132
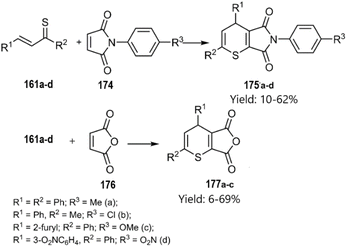
Merkulova et al. extended the scope of hetero-D–A reactions by employing in situ generated thiochalcones 161a–d as thio dienes in reactions with N-aryl maleimides 174 and furan-2,5-dione 176, leading to the synthesis of thiopyran heterocycles. Lawesson's reagent served as a sulfurization agent for α,β-unsaturated ketones, enabling the in situ generation of thiochalcones, which acted as active thiodienes in the D–A reaction (Scheme 53) According of their next report, catalyst-free thio D–A cycloadditions of in situ generated thiochalcones with itaconic, maleic, and 5-norbornene-2,3-dicarboxylic anhydride 178 in organic media afforded novel dihydrothiopyran scaffolds in excellent yields. Spectral data confirmed the predominance of the exo-product in the cycloaddition with the norbornene derivative (Scheme 54).133,134
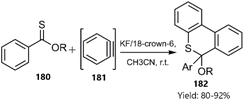
Du et al. described a tandem thio D–A reaction/aromatization strategy for the synthesis of 6H-benzo[c]thiochromene derivatives 182 without the need for a metal catalyst. The proposed mechanism involves the reaction of aryl thionoester derivatives 180 as dienes with in situ generated benzyne 181 (Scheme 55).135
In 2022, the Merkulova group reported a double in situ thio D–A reaction employing a 1,4-diene-3-thione derivative 183 with various dienophiles to construct a series of fused polycyclic structures based on thiopyran. The electronic nature of the dienophile proved crucial, as the use of electron-donating dienophiles resulted in a single cycloaddition with moderate to low yields. When norbornene 126 was employed as the dienophile, spectral data indicated a preference for the exo-product (Scheme 56).136
Recently, Dotsenko's research group unveiled the synthesis of executable access dihydro-2H-thiopyran-3-carbothiamide derivatives 188 via a thio D–A reaction of (E)-2-cyano-3-phenylprop-2-enethioamide 187 During this process, the E-isomer of the Knoevenagel product 215 underwent dimerization through cycloaddition in the presence of iodide or bromide (see Scheme 57).137
In a groundbreaking project, Mlostoń's research team endeavoured to synthesize rare structures from thiopyran scaffolds. They successfully prepared a P,S-cycloadduct exhibiting an endo-attack preference through a thiophilic D–A cycloaddition involving thiochalcones 96 and phosphinine 1-oxides 189 (see Scheme 58).138
In 2024, Huang et al. introduced an innovative thio D–A process. Utilizing a pentagonal 1,2,3-thiadiazole ring intermediate, they generated in situ alkene-1-thiones 191 as vibrant thiodienophiles. These compounds underwent thio D–A cycloaddition with 4-hydroxy-2-pyrones 192 in dimethylacetamide solvent, resulting in the formation of 2-methyl-6-(4-(trifluoromethyl)benzyl)-4H-thiopyran-4-one 193 (see Scheme 59).139
1.1.3.2 Cyclic thio dienes. These compounds typically consist of unsaturated derivatives of notable thiocycles, including thiochromenes, indoline-2-thiones, o-thioquinones, and thiazolones. Alternatively, they may represent unsaturated transition state formed in situ from unstable precursors.
Menichetti and coworkers have investigated stereospecific inverse electron demand D–A reactions of o-thio quinones (o-TQs) 194 with various trapping agents containing cyclic and acyclic dienes 195a–g, over the years consecutively. Interestingly, this research team has introduced (o-TQs) in both the role of diene and dienophile which are named [4 + 2] and [2 + 4] cycloadditions respectively. In Scheme 60, an illustrative example is given for this purpose (Scheme 60).140–142
 | ||
| Scheme 60 [4 + 2] and [2 + 4] cycloadditions of o-thio quinones (o-TQ) 194 in both the role of diene and dienophile 195a–g. | ||
Biehl and coworkers earned an available π-bond to participate in the Knoevenagel-thio D–A reaction by synthesizing arynes in situ. This approach allows for the use of various C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S functionalized molecules, such as unsaturated indoline-2-thione 198, in the synthesis of fascinating polycyclic structures (Scheme 61).143
S functionalized molecules, such as unsaturated indoline-2-thione 198, in the synthesis of fascinating polycyclic structures (Scheme 61).143
Research conducted by Matloubi Moghaddam 's team revealed that Knoevenagel-thio D–A dimerization facilitated the catalyst-free synthesis of functionalized dihydrothiopyran 201 in an aqueous medium. The self-cycloaddition of unsaturated indoline-2-thione 198 was rationalized through resonance interactions involving the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond and the nitrogen lone pair, with the unsaturated position acting as the dienophile in the corresponding substrate (Scheme 62).144 In a subsequent report, this group explored the reaction of unsaturated indoline-2-thiones with fullerene, resulting in a macromolecular D–A reaction that effectively functionalized the fullerene.145
S bond and the nitrogen lone pair, with the unsaturated position acting as the dienophile in the corresponding substrate (Scheme 62).144 In a subsequent report, this group explored the reaction of unsaturated indoline-2-thiones with fullerene, resulting in a macromolecular D–A reaction that effectively functionalized the fullerene.145
In a report by Meier and colleagues, thio bicyclooctatriene 202 was converted into 6-methylenecyclohexa-2,4-diene-1-thione 203 upon heating. Subsequently, the D–A reaction of the resulting thio diene with norbornene derivatives 126 and 205 yielded polycyclic products 204 and 206, favouring an exo-approach in the process (see Scheme 63).146 Earlier, Hegab's group had documented the regioselective reaction of indene-1-thione derivatives with 3,4-dichlorofuran-2,5-dione via a similar methodology.147
Viglianisi's group successfully synthesized o-TQs 194 as heterodienes in situ, leading to the formation of biologically active 2,3-dihydrobenzo[b][1,4]oxathiines 209. Notably, these heterocycles exhibit sweetness significantly surpassing that of sucrose and possess antioxidant properties (see Scheme 64).148
After that, Khan's group reported the synthesis of heterocycles 211 and 212 through a domino Knoevenagel thio Diels–Alder reaction (KTDA) (see Scheme 65). This reaction was catalyzed by Yb(OTf)3, demonstrating good regioselectivity as confirmed by 2D-NOE spectra. Various benzaldehyde derivatives were employed in condensation reactions with 4-hydroxydithiocoumarin 210, although reactions involving aliphatic and heteroaromatic substrates proved unsuccessful. Following this, the addition of ammonium acetate or primary amines to benzaldehyde resulted in the formation of imines, which subsequently underwent cycloaddition with the generated thio diene.149
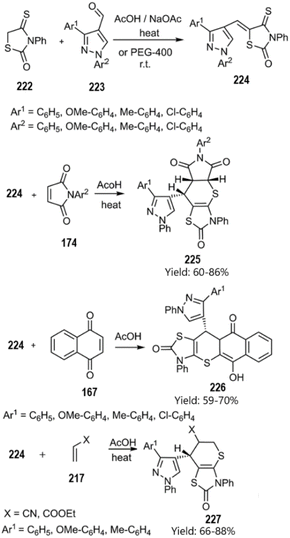
Metwally and colleagues successfully synthesized a series of thiopyrano[2,3-d]thiazole derivatives featuring a pyrazole moiety through a domino Knoevenagel thio Diels–Alder reaction. Their findings suggest that these compounds may hold significant potential in cancer treatment. According to their report, the catalyst-free KTD–A reaction of unsaturated thiazolidine-2-one derivatives 216 and 224 with various Michael acceptors, including ethyl acrylate, acrylonitrile, 3-nitroprop-1-ene 217, pyrrole-2,5-dione 162, triazole-3,5(4H)-dione 220, and 1,4-naphthoquinone 167 yielded the corresponding products (see Schemes 66 and 67).150,151
The Lesyk team selected maleic acid 229, fumaric acid 230, and furan-2,5-dione 176 as dienophiles to engage in thio D–A reactions with EWG substituted (hydroxybenzylidene)-4-thioxothiazolidin-2-ones 228. Over the subsequent years of research on unsaturated thiazolidinones, it was noteworthy that all reactions consistently produced the same product 231, with maleic acid yielding the highest output (see Scheme 68).152,153 In the continuation of research on the thioxothiazolidin-2-ones by Leski and his colleagues. A series of thiopyranothiazole structures have been elegantly synthesized through a tandem acylation thio D–A reaction facilitated by hydroquinone. The heightened reactivity of the unsaturated position in (E)-2-oxo-4-arylbut-3-enoic acid 233 renders it an ideal dienophile, resulting in its reaction with 5-ylidene-4-thioxo-2-thiazolidinone 228 to produce a mixture of endo/exo-adducts in a diastereomeric ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Scheme 69).154 In Further research under Lesyk's supervision, they successfully synthesized anticancer and antiviral thiopyranothiazoles 237, 238 (see Scheme 70).155–158 In 2017, the research team led by Lesyk investigated the D–A reaction of o-phenolic 4-thioxo-2-thiazolidinone derivatives 228 with a variety of unsaturated aldehydes 239, as illustrated in Scheme 71.159 Subsequently, they investigated D–A reactions of 4-thioxo-2-thiazolidinone 235 with aconitic acid derivatives (Scheme 72).160,161 Also, delineated two efficient synthetic pathways for constructing thiopyranothiazole skeletons and succeeded in the synthesis of spiro-substituted thiopyranothiazoles by devising a multi-component reaction involving unsaturated thioxo-2-thiazolidinones 235, trans-aconitic acid 245, and aniline derivatives 246, as illustrated in Scheme 73 (Method A). Remarkably, identical products were also obtained using arylpyrrolidin-3-ylidene derivatives 265a–c (Method B).160 In 2021, Lesyk et al. reported a thio D–A reaction of unsaturated thiooxazolones 228 with citraconic acid 249, achieving high regio- and stereoselectivity followed by intramolecular lactonization. This approach yielded the same products utilizing 3-methylfuran-2,5-dione 251 as the diene, as depicted in Scheme 74.162
1 (Scheme 69).154 In Further research under Lesyk's supervision, they successfully synthesized anticancer and antiviral thiopyranothiazoles 237, 238 (see Scheme 70).155–158 In 2017, the research team led by Lesyk investigated the D–A reaction of o-phenolic 4-thioxo-2-thiazolidinone derivatives 228 with a variety of unsaturated aldehydes 239, as illustrated in Scheme 71.159 Subsequently, they investigated D–A reactions of 4-thioxo-2-thiazolidinone 235 with aconitic acid derivatives (Scheme 72).160,161 Also, delineated two efficient synthetic pathways for constructing thiopyranothiazole skeletons and succeeded in the synthesis of spiro-substituted thiopyranothiazoles by devising a multi-component reaction involving unsaturated thioxo-2-thiazolidinones 235, trans-aconitic acid 245, and aniline derivatives 246, as illustrated in Scheme 73 (Method A). Remarkably, identical products were also obtained using arylpyrrolidin-3-ylidene derivatives 265a–c (Method B).160 In 2021, Lesyk et al. reported a thio D–A reaction of unsaturated thiooxazolones 228 with citraconic acid 249, achieving high regio- and stereoselectivity followed by intramolecular lactonization. This approach yielded the same products utilizing 3-methylfuran-2,5-dione 251 as the diene, as depicted in Scheme 74.162
 | ||
| Scheme 68 Synthesis of thiopyrano [2,3-d] thiazole-5-carboxylic acid bearing chromen derivatives 232. | ||
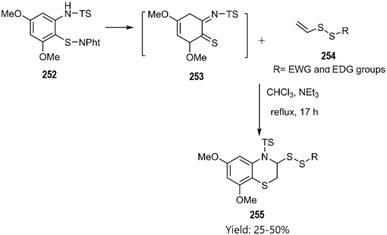
In the same year, Witt research team conducted an investigation that led to an inverse electron-demand [4 + 2] cycloaddition. The corresponding benzo[b][1,4]thiazines 255 were synthesized through the cycloaddition of o-iminothioquinone intermediate 253 with vinyl disulfide derivatives 254 the under moderate conditions, as illustrated in Scheme 75.163
In 2022, Lesyk et al. explored a novel multicomponent reaction involving 4-thioxothiazolidin-2-one 214 3-phenylpropanal 255 and pyrrole-2,5-dione 174. These chemical motifs were efficiently generated through a domino KTDA reaction, resulting in the successful synthesis of polycycle 256 in a single pot with commendable diastereoselectivity (Scheme 76).164
1.2 Step-wise reactions
While the Woodward-Hoffmann rules apply exclusively to concerted reactions, certain orbital interactions can prompt a reaction to follow a stepwise pathway, ultimately producing the allowed cycloadduct. The term “stepwise” refers to reactions that yield products through two or more sequential steps (Fig. 6). The stepwise mechanism be accomplished by passing through the of either zwitterion or diradical intermediates.165,166 Distinguishing between concerted and stepwise mechanisms remains a key challenge in cycloaddition chemistry, a subject that has been extensively debated by researchers. Numerous theories have been proposed to elucidate the factors governing the progression of cycloaddition reactions along single-step or stepwise pathways. Stereoselectivity has often been highlighted as a critical indicator for determining the reaction mechanism167 while steric hindrance can occasionally obstruct concerted pathways.168,169 Additionally, the polarity of reaction media to stabilize zwitterionic intermediates and the presence of specific substituents on precursors can push the mechanism toward a stepwise route.170,171 Computational chemistry has also provided valuable insights into distinguishing reaction mechanisms.172 By calculating the concert energy—defined as the activation energy difference between concerted and stepwise pathways—scientists can predict the preferred reaction pathway.173 These computational studies have also identified the presence of anti-transition states in two-step reactions.67,174,175 One proposed criterion suggests that if the formation of the second carbon–carbon bond during cycloaddition exceeds 30 femtoseconds, the reaction is classified as stepwise. This body of research underscores the intricate interplay of stereo electronic factors, substituent effects, and computational models in advancing our understanding of cycloaddition mechanisms.176–178 Overall, the stepwise cycloaddition exhibits lower stereospecificity than its concerted counterpart.179 Over the past two decades, organic chemists have made significant strides in the stepwise synthesis of thiopyran, demonstrating success in both experimental and computational methodologies.180Nelson and co-workers reported an in situ thio D–A reaction between ketene derivatives and N-thioacyl imines, facilitated by an alkaloid catalyst. This catalyst promoted the reaction through coordination to the TS, stabilizing a pseudo-chain-conformer geometry 263 (Scheme 77).186
Toma and colleagues employed computational methods to investigate HD–A reaction of o-thioquinoline 194 with methyl vinyl ether 265. Their findings suggest a two-step mechanism involving the formation of a zwitterionic intermediate 266. Furthermore, they explored the cycloaddition of 3-thioxo-2H-pyran-2,4(3H)-dione 268 as a heterodiene, comparing its reactivity to that of o-thioquinolines. These studies relied on DFT calculations to elucidate the reaction pathway (Scheme 78). Notably, they identified a unique intermediate 270 along the reaction coordinate, a feature potentially attributed to the enhanced electron density on the sulfur atom.187
Dory and co-workers investigated the mechanism and regioselectivity of the [4 + 2] cycloaddition reactions of thiono-analogs, specifically acetic 2-methoxy-2-oxoethanethioic anhydride 272 and thio-Meldrum's acid 275, with 1,3-dienes. They proposed a stepwise mechanism involving the formation of a zwitterionic intermediate 278, accessed via an inverse [2 + 1] cycloaddition pathway (Scheme 79).188
 | ||
| Scheme 79 Examples of [4 + 2] cycloaddition of thio-carbonyl derivatives through zwitterionic intermediate. | ||
Jørgensen et al. developed a novel approach to enhance the enantioselectivity and reactivity of dithioesters 49 with hexadienal derivatives 87 through the in situ generation of catalyst-bound dienes. This methodology facilitates the formation of thio D–A adducts via a zwitterionic intermediate. The authors demonstrated that EWG-activated thiodienophiles significantly improves both diastereo- and enantioselectivity in the D–A reaction (Scheme 80).189
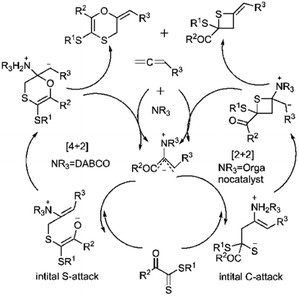
Wei and coworkers reported the first example of a controllable and stereoselective [4 + 2] and [2 + 2] cycloaddition reaction between allenoates 282 and dithioesters 49 (Scheme 81). They demonstrated that the choice of nucleophilic amine catalyst controlled the selectivity between these two cyclization pathways. The formation of a zwitterionic intermediate enables both [4 + 2] and [2 + 2] cycloadditions, depending on the site of attack. For instance, DABCO catalysis Favors the formation of the [4 + 2] cycloadduct 283, proceeding through an initial sulfur (S)-attack. Conversely, chiral amine catalysts promote an initial carbon (C)-attack, leading to the [2 + 2] cycloaddition reaction (Scheme 82).190
Hoye and coworkers explored alternative pathways for [4 + 2] cycloaddition reactions. Their approach utilized the HDDA strategy, generating a reactive benzyne intermediate 288. Subsequent reaction of this intermediate with a thioamide derivative 286 facilitated the formation of the [4 + 2] cycloadduct through electron transfer. Initially, a competing [2 + 2] cycloaddition pathway yielded a four-membered heterocycle 290. A subsequent 1,3-hydrogen atom shift in diene 291 led to the formation of product 287 (Scheme 83).191
An unprecedented approach to forming important thiopyranoindolethione compounds via [4 + 2] cycloaddition and C–S bond formation was reported by the research team of Vyalyh. This approach used in situ trapping of lithium indole-2, 3-dienolate mediates 293 with carbon disulfide, followed by aromatization and the release of lithium hydroxide. The authors estimated the reaction mechanism to be stepwise cycloaddition with ion pair mediate relied on the evaluation of several possible mechanisms through DFT studies (Scheme 84).192
A highly efficient method for the synthesis of valuable 1,5,2-oxathin derivatives has been developed through the thio D–A reaction of phenylvinyldiazene structures 296 thioketones. DFT revealed that, within this protocol, thioketone derivatives with steric hindrance perform the reaction in two steps by proceeding through a zwitterionic intermediate. In contrast, derivatives free from steric pressure perform a single-step D–A reaction. Key advantages of this approach include mild reaction conditions, excellent yields, and the regioselective synthesis of a Hexagon ring incorporating three heteroatoms (Scheme 85).193
Miranda's research group elucidated the thio D–A reaction of thiobenzophenone 86 with diverse aryl alkenes 300, encompassing both EWGs and EDGs. This reaction proceeds through the intermediate with a radical nature, as depicted in Scheme 86. Subsequent, comprehensive DFT studies have predicted a stepwise mechanism initiated by an ion-molecule complex.206
An elegant approach for the enantioselective synthesis of thiopyran derivatives 349 via a step-wise mechanism was reported by Mlostoń et al. A detailed mechanism was shown to two diradicals intermediate in equilibrium. Chiral organocatalysts based on pyrrolidine promote the cycloaddition of related thioketones 303 with diene 87, affording a diverse array of thiopyrans incorporating heteroaryl moieties (Scheme 87).207
According to a report released by Mlostoń et al., the hetero-D–A reaction of heteroaryl thioketones 303 with dimethyl butadiene isomers likely proceeds through a stepwise diradical mechanism. Heteroaryl thioketones exhibit exceptional dienophile character, readily reacting with inactivated dienes. The observed restriction of rotation around the C–C bond leading to the exclusive formation of the cis-thiopyran isomer 308 at ambient temperature, strongly supports this mechanism and facilitates the synthesis of more sustainable products (Scheme 88).103
Schneider and colleagues employed an innovative approach for the in situ generation of the desired thiodienophile from its active precursor 309. This strategy involved the photochemical synthesis of a thioaldehyde, which was subsequently trapped intramolecularly via a thio D–A reaction, enabling the efficient and large-scale preparation of 3,6-dihydro-2H-thiopyrans 312 (Scheme 89).207 Owning to expand their idea, this group evaluated reactions compounds 309 exposed various electron-deficient dienes. Ultraviolet irradiation and the presence of a diphenyl phosphate acid catalyst accelerated the reaction, leading to the synthesis of diverse product derivatives in high yields. Although for the authors, the step-by-step reaction was not proven. But according to the reaction conditions and relying on similar works in the literature, the existence of diradical intermediates is more probable.208
2 Intramolecular cycloaddition reaction
Intramolecular [4 + 2] cycloaddition reactions are characterized by their rapid reaction kinetics, enhanced frontier orbital overlap, and favourable stereoselectivity, owing to the inherent proximity of the D–A partners within the same molecule. This process affords the concerted formation of two rings in a single step. In addition to the six-membered ring arising from the [4 + 2] cycloaddition, the product incorporates a second ring whose size is dictated by the tether length connecting the diene and dienophile moieties. The intramolecular D–A reaction is typically irreversible and proceeds efficiently in the absence of significant steric hindrance. However, in certain instances, bond formation and cleavage are not strictly concerted, and the intermediary of radical species has been validated. This reaction constitutes a pivotal step in numerous total syntheses and the preparation of complex heterocyclic frameworks, frequently featuring as a key component in domino Knoevenagel thio D–A sequences.188,189,208–215A novel pentacyclic scaffold was synthesized via a domino Knoevenagel-thio D–A employing indoline-2-thione 312 and o-alkylated aromatic aldehydes 313. The formation of the endo-cycloadduct as the major product 316 is attributed to a favoured TS (path b). In contrast, the exo-addition was hindered by the sp2-geminal effect and 1,3-allylic strain, as reported by Majumdar and co-worker (Scheme 90).216,217
Moghaddam's research group has extensively investigated the synthesis of thiopyran derivatives through KTDA reactions. They harnessed the reactivity of unsaturated indoline-2-thione to access this motif. Treatment of indoline-2-thione 312 with o-propargylated salicylaldehydes 317 and o-acrylated salicylaldehydes 319 generated the Knoevenagel adducts Subsequent intramolecular cycloaddition of this intermediates afforded the pentacyclic products 318 and 320 in satisfactory yields (Scheme 91).21,218
Moghaddam and coworkers explained a green and convenient Knoevenagel thio D–A reaction to achieve pentacyclic products. In the current process, the 4-hydroxydithiocoumarin 210, while subjected to a reaction with o-acrylated salicylaldehyde 319 gave a mixture of diastereomers 321a and 321b (Scheme 92).219
Majumdar's group reported the synthesis of benzopyran-fused thiopyrano[2,3-b]thiochromen-5(4H)-ones from 4-hydroxy-2H-thiochromene-2-thione 210 via an intramolecular thio D–A reaction (Scheme 93).220,221 This reaction proceeds through a catalyst-free endo-selective cycloaddition of a condensate intermediate in an aqueous medium. The authors attributed the endo-selectivity to the sp2-geminal effect and 1,3-allylic strain, favouring the endo-orientation (path b).
Research Obushak et al., conducted a series of continuous studies exploring KTDA reaction thiazolidin-2-ones. Based on this, they reported the synthesis of biologic polycyclic systems incorporating a thiopyran moiety. Their approach involved the condensation of 4-thioxo-1,3-thiazolidin-2-one 214 with functionalized aldehydes, followed by an intramolecular cycloaddition via a half-chair TS. This strategy preferentially yielded the stereoselective products (Scheme 94).222–224 However, during successive research activities, Lesyk and colleagues focused on the Investigating the therapeutic properties of heterocycles with thiopyrano[2,3-d]thiazole scaffolds. They proved that N–H proton facilitated the functionalization of this position, which was found to influence the anticancer activity of the resulting fused thiopyrano[2,3-d]thiazole compounds.160,223
Moghaddam et al. employed 2-formylphenyl (E)-2-phenylethenyl sulfonates 334 to the synthesize novel thiopyranoindole-fused benzo-d-sultone derivatives 335. The observed diastereoselectivity indicated a preference for the endo-approach (Scheme 95)225
Parmar and colleagues developed KTDA reactions involving pyrazol-5-thiones 324 and functionalized salicylaldehydes in an ionic liquid. They noted that excellent diastereoselectivity is governed by the stereobarrier imposed on the rotational TS (Scheme 96).226,227
Kiamehr et al. have designed an efficient one-pot stereo and regioselective domino KTDA reaction. In this manner, synthesis of pentacyclic thiopyranoindol fused [3, 4-c] quinolone structures 399 has been accomplished from indoline-2-thions 365 and provided N-acrylated anthranilaldehyde derivatives 398 under direction of ZnBr2 in refluxing ethanol (Scheme 97).228
Lesyk and colleagues developed a concise synthesis of amide-substituted isothiochromenothiazole derivatives 346 through base-promoted alkylation of the D–A cycloadduct led to the reconnaissance of an anticancer agent featuring the functionalized isothiochromenothiazole moiety (Scheme 98).56,229
Expanding upon pervious their work, Kiamehr research group employed formylphenylsulfonamides 347 to synthesize novel benzosultam-annulated thiopyranoindole derivatives 348. These products are conveniently generated through a KTDA reaction (Scheme 99).230
3 Conclusion
The widespread use of sulfur-containing heterocyclic drugs, ranging from penicillin to olanzapine, has inspired organic synthesis chemists to explore novel structures of sulfur-based pharmaceuticals. The therapeutic potential of sulfur heterocycles underscores the need for innovative strategies to optimize the production of these compounds. Among these, thiopyran derivatives have demonstrated significant medicinal properties, making their synthesis an appealing endeavour. The development of new strategies for synthesizing thiopyran derivatives represents a promising frontier in organic chemistry. One of the most effective methods for transforming organosulfur compounds into thiopyran derivatives is through cycloaddition reactions. These reactions provide a controllable and accessible means of forming heterocycles, with [4 + 2] cycloaddition standing out as a particularly attractive route for organochemists seeking to synthesize six-membered thiocycles. In this review, we present a comprehensive overview of the synthesis of thiopyran compounds, categorizing and analyzing the subject from multiple perspectives. Our approach encompasses both intramolecular and intermolecular [4 + 2] cycloaddition reactions, and we further classify these processes into concerted and stepwise mechanisms based on their reaction pathways. It seems that intramolecular Diels–Alder reactions often proceed by a concerted mechanism. While the Diels–Alder reaction is traditionally known for its concerted nature, recent literature has introduced the concept of stepwise D–A reactions. Understanding reaction pathway allows chemists to design new synthetic pathways for complex molecules, including natural products and pharmaceuticals. Concerted mechanisms have high stereospecificity. Depending on the nucleophilic and electrophilic characteristics of the D–A partners, reaction condition and the steric hindrance present in the precursors, the reaction pathway may deviate from the conventional D–A mechanism. Any factor that increases concerted energy leads the reaction to a stepwise mechanism. Our objective is to elucidate the formation of thiopyran derivatives through both concerted and stepwise pathways, facilitating an indirect comparison between these two approaches in the literature. The stepwise D–A mechanism may involve zwitterionic intermediates or diradicals, contingent upon the polarity and steric effects within the TS. Notably, the mechanisms governing these reactions necessitate more extensive computational analyses than those required for concerted processes. It seems that the burgeoning interest in stepwise cycloaddition reactions has captivated researchers in the field. Scientists are actively seeking to manipulate these reaction processes through various substitutions, adjustments to reaction polarity, and the application of different catalysts— a pursuit that remains ripe for exploration and realization.Abbreviations
| HOMO | Highest occupied molecular orbital |
| LUMO | Least unoccupied molecular orbital |
| D–A | Diels–Alder |
| HD–A | Hetero-Diels–Alder |
| HDDA | Hexadehydro-Diels–Alder |
| ELF | Electron localization function |
| DFT | Density functional theory |
| TS | Transient transition state |
| RAFT | Radically mediated chain transfer |
| EWG | Electron-withdrawing group |
| EDG | Electron-donating group |
| MCRs | Multi component reactions |
| COSY | COrrelation spectroscopY |
| HSQC | Heteronuclear single quantum coherence |
| HMBC | Heteronuclear multiple bond coherence |
| NOESY | Nuclear overhauser spectroscopy |
| KDO | Deoxy manno octulosonic acid |
| o-TQs | o-Thio quinones |
| KTD–A | Knoevenagel thio Diels–Alder reaction |
Data availability
All data analyzed in this review are publicly available from various sources, including published articles and books. The specific studies and datasets referenced in this review can be found in the citations provided throughout the manuscript. No new data were generated during this review, apart from the conclusion and analysis of the collected data set.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project is supported by Kashan University.Notes and references
- J. Yuan, Z. Xu and M. O. Wolf, Chem. Sci., 2022, 13, 5447–5464 RSC ..
- W. Liu, J. Ke and C. He, Chem. Sci., 2021, 12, 10972–10984 RSC.
- T. Castanheiro, A. Schoenfelder, M. Donnard, I. Chataigner and M. Gulea, J. Org. Chem., 2018, 83, 4505–4515 CrossRef CAS PubMed.
- T. Erdogan, J. Iran. J. Iran. Chem. Soc., 2019, 16, 899–912 CrossRef CAS.
- J. Dong, Q. Pei, P. Wang, Q. Ma and W. Hu, Arab. J. Chem., 2022, 15, 103712 CrossRef CAS.
- L. G. Ardón-Muñoz and J. L. Bolliger, Phosphorus, Sulfur, Silicon Relat. Elem., 2023, 198, 1–6 CrossRef.
- Z.-B. Dong, Z. Gong, Q. Dou, B. Cheng and T. Wang, Org. Biomol. Chem., 2023, 21, 6806–6829 RSC.
- A. Deepthi, S. S. Leena and D. Krishnan, Org. Biomol. Chem., 2024, 22, 5676–5717 RSC.
- K. Mahato, P. R. Bagdi and A. T. Khan, RSC Adv., 2015, 5, 48104–48111 RSC.
- M. Shankar, A. Saha, S. Sau, A. Ghosh, V. Gandon and A. K. Sahoo, Chem. Sci., 2021, 12, 6393–6405 RSC.
- R. Guo, L. Jiang, X. Zhao, H. Lu, M. Li and H. Shan, Langmuir, 2010, 26, 12017–12025 CrossRef PubMed.
- O. S. Kanishchev, M. Sanselme and J.-P. Bouillon, Tetrahedron, 2013, 69, 1322–1336 CrossRef CAS.
- H. Jiang, D. C. Cruz, Y. Li, H. V. Lauridsen and K. A. Jørgensen, J. Am. Chem. Soc., 2013, 135, 5200–5207 CrossRef CAS PubMed.
- V. Jaiswal, B. Mondal and J. Saha, Asian J. Org. Chem, 2020, 9, 1466–1477 CrossRef CAS.
- T. Murai, Top. Curr. Chem., 2018, 376, 1–21 CrossRef CAS PubMed.
- S. Jiang, Theoretical studies of pericyclic reactions of thio- and selenocarbonyl compounds, Northern Illinois University, 1998 Search PubMed.
- F. Peudru, F. Le Cavelier, J. F. Lohier, M. Gulea and V. Reboul, Org. Lett., 2013, 15, 5710–5713 CrossRef CAS PubMed.
- M. Ghandi, S. Sheibani, M. Sadeghzadeh, F. J. Daha and M. Kubicki, J. Iran. Chem. Soc., 2013, 10, 1057–1065 CrossRef CAS.
- R. Aguilar, B. M. Santoyo, D. Zarate-Zarate, M. A. Vazquez, R. M. Padilla, H. Jimenez-Vazquez and A. J. Tamariz, Arab. J. Chem., 2020, 13, 900–915 CrossRef CAS.
- Y. H. Li, J. H. Chen and Z. Yang, Chem.–Eur. J., 2024, 30, e202304371 CrossRef CAS PubMed.
- S. Yamabe and T. A. Minato, J. Org. Chem., 2000, 65, 1830–1841 CrossRef CAS PubMed.
- L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, J. Org. Chem., 2024, 89, 12349–12359 CrossRef CAS PubMed.
- L. R. Domingo, M. José Aurell, P. Pérez and R. Contreras, J. Org. Chem., 2003, 68, 3884–3890 CrossRef CAS PubMed.
- L. R. Domingo and J. Sáez, Org. Biomol. Chem., 2009, 7, 3576–3583 RSC.
- L. R. Domingo, E. Chamorro and P. Pérez, Org. Biomol. Chem., 2010, 8, 5495–5504 RSC.
- S. Berski, J. Andrés, B. Silvi and L. R. Domingo, J. Phys. Chem. A, 2006, 110, 13939–13947 CrossRef CAS PubMed.
- A. Ajaz, A. Z. Bradley, R. C. Burrell, W. H. Hong-Li, K. J. Daoust, L. B. Bovee, K. J. DiRico and R. P. Johnson, J. Org. Chem., 2011, 76, 9320–9328 CrossRef CAS PubMed.
- I. Hammoudan, A. Aboulmouhajir, M. Dakir, D. R. Temsamani, M. Bakhouch, D. E. Bazi and S. Chtita, Struct. Chem., 2023, 34, 959–969 CrossRef CAS.
- S. Lin, L. Xiang, C. Liu, X. Chen and Y. Ye, Arab. J. Chem., 2024, 17, 105845 CrossRef CAS.
- M. Mousavi-Ebadi and J. Safaei-Ghomi, Front. Chem., 2024, 12, 1395008 CrossRef CAS PubMed.
- I. Fleming, Pericyclic Reactions, Oxford University Press, 2015 Search PubMed.
- J. Safaei Ghomi, Asymmetric Synthesis of Conformationally Restricted Amino Acids, University of Wollongong, 1995 Search PubMed.
- J. Safaei-Ghomi and S. Zahedi, Tetrahedron Lett., 2016, 57, 1071–1073 CrossRef CAS.
- J. Safaei-Ghomi, R. Masoomi, M. Hamadanian and S. Naseh, New J. Chem., 2016, 40, 3289–3299 RSC.
- B. Richichi, J. McKimm-Breschkin, V. Baldoneschi and C. Nativi, Arkivoc, 2014, 3, 65–79 Search PubMed.
- G. Mlostoń, P. Grzelak, A. Linden and H. Heimgartner, Chem. Heterocycl. Compd., 2017, 53, 518–525 CrossRef.
- Y. S. Patel and H. S. Patel, Arab. J. Chem., 2017, 10, 1373–1380 CrossRef.
- T. Zhan, L. Zhou, Y. Zhou, B. Yang, X. Feng and X. Liu, Chem. Sci., 2024, 15, 4797–4803 RSC.
- M. Hou, M. Xu, B. Yang, H. He and S. Gao, Chem. Sci., 2021, 12, 7575–7582 RSC.
- S. Ghosh, J. E. Erchinger, R. Maji and B. List, J. Am. Chem. Soc., 2022, 144, 6703–6708 CrossRef CAS PubMed.
- L. Yin, T. Ge, C. Zuo, M. Wang, G. Cui, Y. Li and L. Zhang, Arab. J. Chem., 2024, 17, 105696 CrossRef CAS.
- S. Xu, L. Zeng and S. Cui, Org. Lett., 2022, 24, 2689–2693 CrossRef CAS.
- E. L. Ellsworth, J. Domagala, J. V. Prasad, S. Hagen, D. Ferguson, T. Holler and E. A. Lunney, Bioorg. Med. Chem. Lett., 1999, 9, 2019–2024 CrossRef CAS PubMed.
- R. Cannon, A. J. Janczuk, D. O. Agyemang and Z. Chen, US Pat. 9,510,612, US Patent and Trademark Office, 2016.
- (a) R. B. Woodward, E. Logusch, K. P. Nambiar, K. Sakan, D. E. Ward, B. W. Au-Yeung and C. H. Chen, J. Am. Chem. Soc., 1981, 103, 3210–3213 CrossRef; (b) S. A. Siry, V. M. Timoshenko and J.-P. Bouillon, J. Fluor. Chem., 2012, 137, 6–21 CrossRef CAS.
- F. Tavakolinia, T. Baghipour, Z. Hossaini, D. Zareyee, M. A. Khalilzadeh and M. Rajabi, Nucleic Acid Ther., 2012, 22, 265–270 CrossRef CAS PubMed.
- R. Shaw, A. Elagamy, I. Althagafi, A. K. Srivastava and R. Pratap, Curr. Org. Chem., 2021, 25, 2331–2377 CrossRef CAS.
- A. Kryshchyshyn, D. Kaminskyy, I. Nektegayev, P. Grellier and R. Lesyk, Sci. Pharm., 2018, 86, 47 CrossRef PubMed.
- G. F. Pasha, S. Asghari, M. Tajbakhsh and M. Mohseni, J. Chin. Chem. Soc., 2019, 66, 660–667 CrossRef CAS.
- A. Deepthi, S. S. Leena and D. Krishnan, Org. Biomol. Chem., 2024, 22, 5676–5717 RSC.
- A. Ahamed, I. A. Arif, R. SurendraKumar, I. Akbar and B. Shaik, J. King Saud Univ. Sci., 2023, 35, 102588 CrossRef.
- F. M. Moghaddam, M. R. Khodabakhshi, M. Kiamehr and Z. Ghahremannejad, Tetrahedron Lett., 2013, 54, 2685–2689 CrossRef CAS.
- C. H. Chen, G. A. Reynolds and J. A. Van Allan, J. Org. Chem., 1977, 42, 2777–2778 CrossRef CAS.
- A. Deepthi, S. S. Leena and D. Krishnan, Org. Biomol. Chem., 2024, 22, 5676–5717 RSC.
- M. Wang, Y. Su, H. Guo, W. Chen, Z. Zhou, S. Liu and G. Zhou, J. Org. Chem., 2024, 89, 7758–7769 CrossRef CAS PubMed.
- M. I. Hegab, Phosphorus, Sulfur, Silicon Relat. Elem., 2025, 200, 1–11 CrossRef CAS.
- L. Z. El-Agha, M. M. El-Abadelah, M. R. Kamal, S. S. Sabri, R. M. Al-As' ad and W. Voelter, Z. für Naturforsch. B., 2018, 73, 109–113 CrossRef CAS.
- H. Jiang, D. C. Cruz, Y. Li, V. H. Lauridsen and K. A. Jørgensen, J. Am. Chem. Soc., 2013, 135, 5200–5207 CrossRef CAS PubMed.
- N. D. Epiotis, J. Am. Chem. Soc., 1972, 94, 1924–1934 CrossRef CAS.
- S. Sankararaman, Pericyclic Reactions-A Textbook: Reactions, Applications and Theory, John Wiley & Sons, 2005 Search PubMed.
- J. Héron and D. Balcells, ACS Catal., 2022, 12, 4744–4753 CrossRef.
- S. Chen, T. Hu and K. N. Houk, J. Org. Chem., 2021, 86, 6840–6846 CrossRef CAS PubMed.
- K. N. Houk, F. Liu, Z. Yang and J. I. Seeman, Angew. Chem., 2021, 60, 12660–12681 CrossRef CAS PubMed.
- M. Ganesh and S. Suraj, Org. Biomol. Chem., 2022, 20, 5651–5693 RSC.
- P. Gosselin, S. Masson and A. Thuillier, J. Org. Chem., 1979, 44, 2807–2809 CrossRef CAS.
- D. Yang, Y. Wang, T. Wang, Q. Dou, K. Zhou, H. Zhai and B. Cheng, Adv. Synth. Catal., 2023, 365, 88–95 CrossRef CAS.
- M. M. Cerda, T. D. Newton, Y. Zhao, B. K. Collins, C. H. Hendon and M. D. Pluth, Chem. Sci., 2019, 10, 1773–1779 RSC.
- B. Heuzé, R. Gasparova, M. Heras and S. Masson, Tetrahedron Lett., 2000, 41, 7327–7331 CrossRef.
- M. Heras and M. G. S. Masson, Chem. Commun., 2001, 611–612 RSC.
- E. Pfund, T. Lequeux, M. Vazeux and S. Masson, Tetrahedron Lett., 2002, 43, 2033–2036 CrossRef CAS.
- I. El-Sayed and A. Senning, Sulfur Lett., 2002, 25, 263–267 CrossRef CAS.
- I. El-Sayed, O. M. Ali, A. Fischer and A. Senning, Heteroat. Chem., 2003, 14, 170–174 CrossRef CAS.
- K. M. Hilmy, I. El-sayed, S. M. El-kousy and H. S. Slem, Sulfur Lett., 2003, 26, 187–193 CrossRef CAS.
- R. Bastin, H. Albadri, A.-C. Gaumont and M. Gulea, Org. Lett., 2006, 8, 1033–1036 CrossRef CAS PubMed.
- S. Sinnwell, A. J. Inglis, T. P. Davis, M. H. Stenzel and C. Barner-Kowollik, Chem. Commun., 2008, 1, 2052–2054 RSC.
- V. M. Timoshenko, S. A. Siry, A. B. Rozhenko, Y. G. Shermolovich and J. Fluor, J. Fluor. Chem., 2010, 131, 172–183 CrossRef CAS.
- A. S. Goldmann, T. Tischer, L. Barner, M. Bruns and C. Barner-Kowollik, J. Biol. Macromol., 2011, 12, 1137–1145 CAS.
- H. Dentel and M. Gulea, J. Org. Chem., 2010, 6, 62–73 Search PubMed.
- H. Dentel, I. Chataigner, J.-F. Lohier and M. Gulea, Tetrahedron, 2012, 68, 2326–2335 CrossRef CAS.
- K. Pahnke, N. L. Haworth, J. Brandt, U. Paulmann, C. Richter, F. G. Schmidt, A. Lederer, M. L. Coote and C. Barner-Kowollik, Polym. Chem., 2016, 7, 3244–3250 RSC.
- S. B. Mitkari, A. Medina-Ortíz, J. L. Olivares-Romero, M. A. Vázquez, E. Peña-Cabrera, C. Villegas Gomez and D. Cruz Cruz, Chem.–Eur. J., 2021, 27, 618–621 CrossRef CAS PubMed.
- T. Maujean, P. Marchand, P. Wagner, S. Riché, F. Boisson, N. Girard, J. Karpenko, D. Bonnet and M. Gulea, Phosphorus, Sulfur, Silicon Relat. Elem., 2023, 198, 532–535 CrossRef CAS.
- E. Vedejs and J. Stults, J. Org. Chem., 1988, 53, 2226–2232 CrossRef CAS.
- E. Vedejs, T. H. Eberlein, D. J. Mazur, C. K. McClure, D. A. Perry, R. Ruggeri and D. L. Varie, J. Org. Chem., 1986, 51, 1556–1562 CrossRef CAS.
- J. E. Baldwin and R. G. Lopez, Tetrahedron, 1983, 39, 1487–1498 CrossRef CAS.
- A. U. Augustin, M. Sensse, P. G. Jones and D. B. Werz, Angew. Chem., Int. Ed., 2017, 56, 14293–14296 CrossRef CAS PubMed.
- Y. Watanabe and T. Sakakibara, Tetrahedron, 2009, 65, 599–606 CrossRef CAS.
- T. Murai, K. Morikawa and T. Maruyama, Chem.–Eur. J., 2013, 19, 13112–13119 CrossRef CAS PubMed.
- D. Tanini, A. Angeli and A. Capperucci, Phosphorus, Sulfur, Silicon Relat. Elem., 2016, 191, 156–158 CrossRef CAS.
- T. Yoneda, N. Kojima, T. Matsumoto, D. Imahori, T. Ohta, T. Yoshida and S. Nakamura, Org. Biomol. Chem., 2022, 20, 196–207 RSC.
- D. M. Ziegler, Biologic. React. Intermed, IV, 1991, pp. 41–50 Search PubMed.
- N. Mahanta, D. M. Szantai-Kis, E. J. Petersson and D. A. Mitchell, ACS Chem. Biol., 2019, 14, 142–163 CrossRef CAS PubMed.
- T. S. Jagodziński, Chem. Rev., 2003, 103, 197–228 CrossRef PubMed.
- L. A. Camacho III, Thioamide protection and use against the dragon of solid-phase peptide synthesis, Doctoral dissertation, Iowa State University, 2022.
- S. S. Mikhailichenko, J.-P. Bouillon, T. Besson and Y. G. Shermolovich, Tetrahedron Lett., 2010, 51, 990–993 CrossRef CAS.
- P. Cassoux, L. Valade, H. Kobayashi, A. Kobayashi, R. Clark and A. E. Underhill, Coord. Chem. Rev., 1991, 110, 115–160 CrossRef CAS.
- G. Mlostoń, P. Grzelak and H. Heimgartner, J. Sulphur Chem., 2017, 38, 1–10 CrossRef.
- Y. G. Shermolovich, S. Emets and A. Tolmachev, Chem. Heterocycl. Compd., 2003, 39, 1076–1078 CrossRef CAS.
- K. Kowalski, R. Karpowicz, G. Mlostoń, D. Miesel, A. Hildebrandt, H. Lang, R. Czerwieniec and B. Therrien, Dalton Trans., 2015, 44, 6268–6276 RSC.
- J. Skiba, R. Karpowicz, I. Szabó, B. Therrien and K. Kowalski, J. Organomet. Chem., 2015, 794, 216–222 CrossRef CAS.
- J. Hejmanowska, M. Jasiński, G. Mlostoń and Ł. Albrecht, Eur. J. Org. Chem., 2017, 2017, 950–954 CrossRef CAS.
- E. K. Davison, P. A. Hume and J. Sperry, Org. Lett., 2018, 20, 3545–3548 CrossRef CAS PubMed.
- G. Mlostoń, K. Urbaniak, P. Urbaniak, A. Marko, A. Linden and H. Heimgartner, Beilstein J. Org. Chem., 2018, 14, 1834–1839 CrossRef PubMed.
- G. Mlostoń, R. Hamera-Fałdyga and H. Heimgartner, J. Sulphur Chem., 2018, 39, 322–331 CrossRef.
- W. Chen, H. Zhou, B. H. Ren, W. M. Ren and X. B. Lu, Org. Biomol. Chem., 2022, 20, 678–685 RSC.
- W. Chen, H. Zhou, B. H. Ren, W. M. Ren and X. B. Lu, Org. Biomol. Chem., 2022, 20, 678–685 RSC.
- S. W. Remiszewski, The Pennsylvania State University, 1986.
- A. Bayer and O. R. Gautun, Tetrahedron Lett., 2000, 41, 3743–3746 CrossRef CAS.
- A. Bayer and O. R. Gautun, Tetrahedron: Asymmetry, 2001, 12, 2937–2939 CrossRef CAS.
- A. Bayer, L. K. Hansen and O. R. Gautun, Tetrahedron: Asymmetry, 2002, 13, 2407–2415 CrossRef CAS.
- X.-J. Wang and J.-T. Liu, Tetrahedron, 2005, 61, 6982–7698 CrossRef CAS.
- S. Sharma, Sulfur. Rep., 1986, 5, 1–87 CrossRef.
- O. D. Salahdin, H. H. Kzar, M. J. C. Opulencia, A. H. Abdulkadhim, A. T. Hammid and A. G. Ebadi, Semicond. Sci. Technol., 2022, 37, 095015 CrossRef.
- B. Krebs and H. Beyer, Z. Anorg. Allg. Chem., 1969, 365, 199–210 CrossRef CAS.
- B. Föhlisch, D. Abu Bakr and P. Fischer, J. Org. Chem., 2002, 67, 3682–3686 CrossRef PubMed.
- J. Takayama, S. Fukuda, Y. Sugihara, A. Ishii and J. Nakayama, Tetrahedron Lett., 2003, 44, 5159–5162 CrossRef CAS.
- G. M. Li, S. Niu, M. Segi, K. Tanaka, T. Nakajima, R. A. Zingaro, J. H. Reibenspies and M. B. Hall, J. Org. Chem., 2000, 65, 6601–6612 CrossRef CAS PubMed.
- S. Kerverdo, L. Lizzani-Cuvelier and E. Duñach, Tetrahedron Lett., 2003, 44, 8841–8844 CrossRef CAS.
- S. Kerverdo, L. Lizzani-Cuvelier and E. Duñach, Tetrahedron Lett., 2003, 44, 853–856 CrossRef CAS.
- A. Capperucci, A. Degl'Innocenti, T. Nocentini, S. Biondi and F. Dini, J. Organomet. Chem., 2003, 686, 363–367 CrossRef CAS.
- K. Bogdanowicz-Szwed and A. Budzowski, Monatsh. Chem., 2001, 132, 947–957 CrossRef CAS.
- K. Bogdanowicz-Szwed and A. Z. Budzowski, Z. Naturforsch. B., 2002, 57, 637–644 CrossRef CAS.
- K. Bogdanowicz-Szwed and A. Budzowski, Monatsh. Chem., 2004, 135, 97–108 CrossRef CAS.
- A. Harrison-Marchand, S. Collet, A. Guingant, J. P. Pradère and L. Toupet, Tetrahedron, 2004, 60, 1827–1839 CrossRef CAS.
- W. A. Schenk, T. Beucke, N. Burzlaff, M. Klüglein and M. Stemmler, Chem.–Eur. J., 2006, 12, 4821–4834 CrossRef CAS PubMed.
- J.-P. Wan, Y.-H. Pan, H. Mao, Y.-H. Y. Chen and J. Pan, Synth. Commun., 2010, 40, 709–716 CrossRef CAS.
- G. Mlostoń, P. Grzelak, M. Mikina, A. Linden and H. Heimgartner, Beilstein J. Org. Chem., 2015, 11, 576–582 CrossRef PubMed.
- F. Peudru, R. Legay, J.-F. Lohier, V. Reboul and M. Gulea, Tetrahedron, 2012, 68, 9016–9022 CrossRef CAS.
- F. Peudru, J.-F. Lohier, M. Gulea and V. Reboul, Phosphorus, Sulfur, Silicon Relat. Elem., 2016, 191, 220–229 CrossRef CAS.
- F. Peudru, F. Le Cavelier, J. F. Lohier, M. Gulea and V. Reboul, Org. Lett., 2013, 15, 5710–5713 CrossRef CAS PubMed.
- B. Richichi, J. McKimm-Breschkin, V. Baldoneschi and C. Nativi, Arkivoc, 2014, 3, 65–79 Search PubMed.
- G. Mlostoń, P. Pipiak, R. Hamera-Fałdyga and H. Heimgartner, Phosphorus, Sulfur, Silicon Relat. Elem., 2017, 192, 204–211 CrossRef.
- E. Merkulova, A. Kolobov and K. Ovchinnikov, Russ. Chem. Bull., 2019, 68, 606–609 CrossRef CAS.
- E. A. Merkulova, A. V. Kolobov, K. L. Ovchinnikov, V. N. Khrustalev and V. G. Nenajdenko, Chem. Heterocycl. Compd., 2021, 57, 245–252 CrossRef CAS.
- Y. An, F. Zhang, G. Du, Z. Cai and L. He, Org. Chem. Front., 2021, 8, 6979–6984 RSC.
- E. A. Merkulova, A. V. Kolobov, K. A. Lyssenko and V. G. Nenajdenko, Mendeleev Commun., 2022, 32, 384–385 CrossRef CAS.
- B. S. Krivokolysko, V. V. Dotsenko, N. A. Pakholka, P. G. Dakhno, V. D. Strelkov, N. Aksenov and A. G. Krivokolysko, J. Iran. Chem. Soc., 2023, 20, 609–628 CrossRef CAS.
- G. Mlostoń, K. Urbaniak, M. Palusiak, E. Łastawiecka, S. Frynas, K. M. Pietrusiewicz and H. Heimgartner, Molecules, 2024, 29, 2036 CrossRef PubMed.
- N. A. Huang, W. Wen, K. Laughlin and S. T. Escorihuela, Org. Biomol. Chem., 2024, 22, 8285–8292 RSC.
- S. Menichetti and C. Viglianisi, Tetrahedron, 2003, 59, 5523–5530 CrossRef CAS.
- S. Menichetti, C. Nativi, P. Sarri and C. Viglianisi, J. Sulfur Chem., 2004, 25, 317 CrossRef CAS.
- S. Menichetti, C. Faggi, G. Lamanna, A. Marrocchi, L. Minuti and A. Taticchi, Tetrahedron, 2006, 62, 5626–5631 CrossRef CAS.
- S. Kamila, B. Koh, H. Zhang and E. R. Biehl, Arkivoc, 2006, 2, 1–14 Search PubMed.
- F. Matloubi Moghaddam, L. Hojabri, S. Taheri and P. Pirani, J. Iran. Chem. Soc., 2010, 7, 781–790 Search PubMed.
- F. Matloubi Moghaddam, B. Ghanbari, M. Behzadi and M. H. Baghersad, J. Heterocycl. Chem., 2017, 54, 911–915 CrossRef CAS.
- H. Meier, M. Schmidt, A. Mayer, D. Schollmeyer and B. Beile, J. Heterocycl. Chem., 2012, 49, 516–520 CrossRef CAS.
- M. I. Hegab, T. A. Elmalah and F. A. Gad, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 1665–1675 CrossRef CAS.
- C. Viglianisi, A. Sinni and S. Menichetti, Heteroat. Chem., 2014, 25, 361–366 CrossRef CAS.
- K. Mahato, P. R. Bagdi and A. T. Khan, RSC. Adv., 2015, 5, 48104–48111 RSC.
- N. H. Metwally, Phosphorus, Sulfur Silicon Relat. Elem., 2008, 183, 2073–2085 CrossRef CAS.
- N. H. Metwally, M. A. Badawy and D. S. Okpy, Chem. Pharm. Bull., 2015, 63, 495–503 CrossRef PubMed.
- D. Kaminskyy, O. Vasylenko, D. Atamanyuk, A. Gzella and R. Lesyk, Synlett, 2011, 2011, 1385–1388 CrossRef.
- N. Zelisko, D. Atamanyuk, O. Vasylenko, A. Bryhas, V. Matiychuk, A. Gzella and R. Lesyk, Tetrahedron, 2014, 70, 720–729 CrossRef CAS.
- N. Zelisko, D. Atamanyuk, Y. Ostapiuk, A. Bryhas, V. Matiychuk, A. Gzella and R. Lesyk, Tetrahedron, 2015, 71, 9501–9508 CrossRef CAS.
- R. Lesyk, B. Zimenkovsky, D. Atamanyuk, F. Jensen, K. Kieć-Kononowicz and A. Gzella, Bioorg. Med. Chem., 2006, 2011, 5230–5240 CrossRef PubMed.
- D. Havrylyuk, B. Zimenkovsky, O. Vasylenko, L. Zaprutko, A. Gzella and R. Lesyk, Eur. J. Med. Chem., 2009, 44, 1396–1404 CrossRef CAS PubMed.
- A. Lozynskyi, S. Golota, B. Zimenkovsky, D. Atamanyuk, A. Gzella and R. Lesyk, Phosphorus, Sulfur Silicon Relat. Elem., 2016, 191, 1245–1249 CrossRef CAS.
- A. Lozynskyi, V. Matiychuk, O. Karpenko, A. K. Gzella and R. Lesyk, Biopolym. Cell, 2017, 33, 183–205 Search PubMed.
- A. Lozynskyi, V. Matiychuk, O. Karpenko, A. K. Gzella and R. Lesyk, Heteroat. Chem., 2017, 23, 1–5 CAS.
- N. Zelisko, O. Karpenko, V. Muzychenko, A. Gzella, P. Grellier and R. Lesyk, Tetrahedron Lett., 2017, 58, 1751–1754 CrossRef CAS.
- A. Kryshchyshyn, O. Roman, A. Lozynskyi and R. Lesyk, Sci. Pharm., 2018, 86, 26 CrossRef PubMed.
- N. Zelisko, O. Karpenko, V. Muzychenko, A. Gzella and R. Lesyk, Synth. Commun., 2021, 51, 964–970 CAS.
- B. Jędrzejewski, M. Musiejuk, J. Doroszuk and D. Witt, Materials, 2021, 14, 1342 CrossRef PubMed.
- A. Lozynskyi, A. Karkhut, S. Polovkovych, O. Karpenko, S. Holota, A. K. Gzella and R. Lesyk, Results Chem., 2022, 4, 100464 CrossRef CAS.
- T. L. Gilchrist and R. C. Storr, Organic reactions and orbital symmetry, Cambridge University Press, 1979 Search PubMed.
- R. Jasiński, Symmetry, 2021, 13, 1911 CrossRef.
- A. Throup, L. H. Patterson and H. M. Sheldrake, Org. Biomol. Chem., 2016, 14, 9554–9559 RSC.
- Y. Zhu, J. Borstelmann, C. Neiss, W. Zheng, A. Görling, M. Kivala and M. A. Petrukhina, Chem. Sci., 2025, 6, 5762 Search PubMed.
- J. S. Chen, K. Houk and C. S. Foote, J. Am. Chem. Soc., 1998, 120, 12303–12309 CrossRef CAS.
- A. Kilaj, J. Wang, P. Straňák, M. Schwilk, U. Rivero, L. Xuvon, O. A. Lilienfeld, J. Küpper and S. Willitsch, Nat. Commun., 2021, 12, 6047 CrossRef CAS PubMed.
- T. Bekele, C. F. Christian, M. A. Lipton and D. A. Singleton, J. Am. Chem. Soc., 2005, 127, 9216–9223 CrossRef CAS PubMed.
- Q. Y. Cai, H. Zheng, Z. H. Li, L. K. Dong, J. S. Zhou and Y. Zhang, J. Chem. Educ., 2024, 101, 5068–5076 CrossRef CAS.
- K. N. Houk, Y. Li, J. Storer, L. Raimondi and B. Beno, J. Chem. Soc. Faraday Trans., 1994, 90, 1599–1604 RSC.
- L. R. Domingo, E. Chamorro and P. Pérez, Org. Biomol. Chem., 2010, 8, 5495–5504 RSC.
- B. R. Beno, S. Wilsey and K. Houk, J. Am. Chem. Soc., 1999, 121, 4816–4826 CrossRef CAS.
- D. McLeod, M. K. Thøgersen, N. I. Jessen, K. A. Jørgensen, C. S. Jamieson, X.-S. P. D. Xue and J. J. Mallet, J. Am. Chem. Soc., 1976, 98, 143 CrossRef.
- K. Bartlett, F. Houk and L. R. Hoffmann, Acc. Chem. Res., 2019, 52, 3488–3501 CrossRef PubMed.
- W. Fudickar and T. Linker, J. Phys. Org. Chem., 2019, 32, e3951 Search PubMed.
- X. He, I. Kevlishvili, K. Murcek, P. Liu and A. Star, ACS Nano, 2021, 15, 4833–4844 CrossRef CAS PubMed.
- T. Afshari and M. Mohsennia, Comput. Theor. Chem., 2018, 1140, 117–124 CrossRef CAS.
- C. Valentin, M. Freccero, R. Gandolfi and A. Rastelli, J. Org. Chem., 2000, 65, 6112–6120 Search PubMed.
- L. R. Domingo, M. Ríos-Gutiérrez and P. Pérez, J. Org. Chem., 2024, 89, 12349–12359 CrossRef CAS PubMed.
- N. Byrne, P. C. Howlett, D. R. MacFarlane and M. Forsyth, Adv. Mater., 2005, 17, 2497–2501 CrossRef CAS.
- R. Jasiński, M. Kubik, A. Łapczuk-Krygier, A. Kącka, E. Dresler and A. Boguszewska-Czubara, React. Kinet. Mech. Catal., 2014, 113, 333–345 CrossRef.
- E. Dresler, A. Wróblewska and R. Jasiński, Molecules, 2023, 28, 5289 CrossRef CAS PubMed.
- X. Xu, K. Wang and S. G. Nelson, J. Am. Chem. Soc., 2007, 129, 11690–11691 CrossRef CAS PubMed.
- L. Legnani, C. Lunghi, F. M. Albini, C. Nativi, B. Richichi and L. Toma, Eur. J. Org Chem., 2007, 21, 3547–3554 CrossRef.
- S. Perreault, M. Poirier, P. Léveillé, O. René, P. Joly, Y. Dory and C. Spino, J. Org. Chem., 2008, 73, 7457–7466 CrossRef CAS PubMed.
- H. Jiang, D. C. Cruz, Y. Li, H. V Lauridsen and K. A. Jørgensen, J. Am. Chem. Soc., 2013, 135, 5200–5207 CrossRef CAS PubMed.
- H. B. Yang, Y.-C. Yuan, Y. Wei and M. Shi, Chem. Commun., 2015, 51, 6430–6433 RSC.
- V. Palani, J. Chen and T. R. Hoye, Org. Lett., 2016, 18, 6312–6315 CrossRef CAS PubMed.
- K. F. Suzdalev, J. V. Vyalyh, V. V. Tkachev, E. A. Lysenko, O. N. Burov, A. V. Lisovin and S. V. Kurbatov, J. Org. Chem., 2021, 86, 11698–11707 CrossRef CAS PubMed.
- M. G. Mlostoń, K. Urbaniak, M. Sobiecka, H. Heimgartner, E.-U. Würthwein, R. Zimmer and H. U. Reissig, Molecules, 2021, 26, 2544 CrossRef PubMed.
- D. R. A. Firestone, Int. J. Chem. Kinet., 2013, 45, 415–428 CrossRef.
- D. J. Hastings and A. C. Weedon, J. Org. Chem., 1991, 56, 6326–6331 Search PubMed.
- C. Wentrup, Acc. Chem. Res., 2011, 44, 393–404 CrossRef CAS PubMed.
- R. K. Mohamed, P. W. Peterson and I. V. Alabugin, Chem. Rev., 2013, 113, 7089–7129 CrossRef CAS PubMed.
- M. Abe, Chem. Rev., 2013, 113, 7011–7088 CrossRef CAS PubMed.
- S. Comandini, N. Abid and J. Chaumeix, J. Phys. Chem. A., 2017, 121, 5921–5931 CrossRef PubMed.
- J. S. Tan, V. Hirvonen and R. S. Paton, Org. Lett., 2018, 20, 2821–2825 CrossRef CAS PubMed.
- M. Zhu, X. Zhang, C. Zheng and S. L. You, Acc. Chem. Res., 2022, 55, 2510–2525 CrossRef CAS PubMed.
- G. Tay, S. Nishimura and H. Oguri, Chem. Sci., 2024, 15, 15599–15609 RSC.
- X. He, I. Kevlishvili, K. Murcek, P. Liu and A. Star, ACS Nano, 2021, 15, 4833–4844 CrossRef CAS PubMed.
- J. Ma, S. Chen, P. Bellotti, R. Guo, F. Schäfer, A. Heusler and F. Glorius, Science, 2021, 371, 1338–1345 CrossRef CAS PubMed.
- L. K. Montgomery, K. Schueller and P. D. Bartlett, J. Am. Chem. Soc., 1964, 86, 622–628 CrossRef CAS.
- J. E. Argüello, R. Pérez-Ruiz and M. A. Miranda, Org. Lett., 2007, 9, 3587–3590 Search PubMed.
- F. Sachse, K. Gebauer and C. Schneider, Eur. J. Org Chem., 2021, 2021, 64–71 CrossRef CAS.
- F. Sachse and C. Schneider, Org. Lett., 2021, 23, 2682–2686 CrossRef CAS PubMed.
- W. Oppolzer, Synthesis, 1987, 1978, 793–802 CrossRef.
- G. Brieger and J. N. Bennett, Chem. Rev., 1980, 80, 63–97 CrossRef CAS.
- M. Bao, A. R. R. Bohórquez, H. Arman and M. P. Doyle, Chem. Sci., 2024, 15, 12042–12046 RSC.
- K. I. Takao, R. Munakata and K. I. Tadano, Chem. Rev., 2005, 105, 4779–4807 CrossRef CAS PubMed.
- M. Juhl and D. Tanner, Chem. Soc. Rev., 2009, 38, 2983–2992 RSC.
- H. V. Pham, R. S. Paton, A. G. Ross, S. J. Danishefsky and K. N. Houk, J. Am. Chem. Soc., 2014, 136, 2397–2403 CrossRef CAS PubMed.
- M. Ghandi, S. Feizi, M. T. Nazeri, B. Notash and J. Iran, J. Iran. Chem. Soc., 2017, 14, 177–187 CrossRef CAS.
- K. Majumdar, A. Taher and K. Ray, Tetrahedron Lett., 2009, 50, 3889–3891 CrossRef CAS.
- M. Kiamehr and F. M. Moghaddam, Tetrahedron Lett., 2009, 50, 6723–6727 CrossRef CAS.
- F. M. Moghaddam, M. Kiamehr, S. Taheri and Z. Mirjafary, Helv. Chim. Acta, 2010, 93, 964–973 CrossRef CAS.
- F. M. Moghaddam, M. Kiamehr, M. R. Khodabakhshi, Z. Mirjafary, S. Fathi and H. Saeidian, Tetrahedron, 2010, 66, 8615–8622 CrossRef CAS.
- K. Majumdar, A. Taher and S. Ponra, Synthesis, 2010, 2010, 4043–4050 CrossRef.
- K. Majumdar, A. Taher and S. Ponra, Tetrahedron Lett., 2010, 51, 2297–2300 CrossRef CAS.
- V. S. Matiychuk, R. B. Lesyk, M. D. Obushak, A. Gzella, D. V. Atamanyuk, Y. V. Ostapiuk and A. P. Kryshchyshyn, Tetrahedron Lett., 2008, 49, 4648–4651 CrossRef CAS.
- A. O. Bryhas, Y. I. Horak, Y. V. Ostapiuk, M. D. Obushak and V. S. Matiychuk, Tetrahedron Lett., 2011, 52, 2324–2326 CrossRef CAS.
- A. Kryshchyshyn, D. Atamanyuk and R. Lesyk, Sci. Pharm., 2012, 80, 509–530 CrossRef CAS PubMed.
- F. M. Moghaddam, B. K. Foroushani, M. Sobhani, M. R. Khodabakhshi and N. S. Weng, Tetrahedron, 2013, 69, 8169–8173 CrossRef CAS.
- N. J. Parmar, B. D. Parmar, T. R. Sutariya, R. Kant and V. K. Gupta, Tetrahedron Lett., 2014, 55, 6060–6064 CrossRef CAS.
- B. D. Parmar, T. R. Sutariya, G. C. Brahmbhatt, N. J. Parmar, R. Kant, V. K. Gupta, P. R. Murumkar, M. K. Sharma and M. R. Yadav, Mol. Divers., 2016, 20, 639–657 CrossRef CAS PubMed.
- M. Kiamehr, B. Alipour, L. Mohammadkhani, B. Jafari and P. Langer, Tetrahedron, 2017, 73, 3040–3047 CrossRef CAS.
- A. Lozynskyi, A. Karkhut, S. Polovkovych, O. Karpenko, S. Holota, A. K. Gzella and R. Lesyk, Results Chem., 2022, 4, 100464 CrossRef CAS.
- M. Kiamehr, F. Khademi, B. Jafari and P. Langer, Chem. Heterocycl. Compd., 2020, 56, 392–398 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |