 Open Access Article
Open Access ArticleResistive nanostructured W18O49 gas sensors: an overview
M. Hjiri a,
I. Najeh*b,
Fatemah M. Barakatc and
G. Nerid
a,
I. Najeh*b,
Fatemah M. Barakatc and
G. Nerid
aDepartment of Physics, College of Sciences, Imam Mohammad Ibn Saud Islamic University (IMSIU), Riyadh, 11623, Saudi Arabia
bLaboratory of Physics of Materials and Nanomaterials Applied at Environment (LaPhyMNE), Faculty of Sciences in Gabes, Gabes University, Gabes, Tunisia. E-mail: Imededdine.Najeh@gmail.com
cPhysics and Astronomy Department, Faculty of Science, King Saud University, Riyadh, Saudi Arabia
dDepartment of Engineering, University of Messina, Messina, 98166, Italy
First published on 25th April 2025
Abstract
The detection of toxic gases by resistive gas sensors, which are mainly fabricated using semiconducting metal oxides, is of importance from a safety point of view. These sensors have outstanding electrical and sensing properties as well as are inexpensive. W18O49 (WO2.72), which is a non-stoichiometric tungsten oxide, possesses abundant oxygen vacancies, which are beneficial for the adsorption of oxygen gas molecules and act as sites for sensing reactions. Thus, through the rational design of W18O49-based gas sensors using strategies such as morphology engineering, doping, decoration, formation of composites or their combination, the fabrication of high-performance W18O49 gas sensors is feasible. Herein, we present the gas-sensing features of pristine W18O49, doped W18O49, decorated W18O49 and composite-based W18O49 sensors. Moreover, focusing on the sensing mechanism of W18O49 sensors, this review provides an in-depth understanding on the working principles of the sensing of toxic gases using W18O49.
1. Introduction to resistive gas sensors
Owing to the rapid industrialization and extensive emission of toxic and dangerous gases from automobiles, chemical plants, food industry, mines, etc., air pollution is becoming one of the main causes of human death in most countries.1 Air pollution is linked to COVID-19 severity and mortality2 and causes dizziness, reduced oxygen content in the blood, difficulty in breathing, as well as cardiovascular and respiratory issues.3Traditionally, the presence of toxic gases can be demonstrated by techniques such as gas and ion chromatography.4,5 However, these methods have the disadvantage of being expensive, bulky and complicated, which makes their application difficult for real-time and low power consumption gas detection.6 Thus, the development of sensitive and reliable devices to detect toxic and harmful gases is essential. Gas sensors are sensitive electrical devices that can detect the presence of surrounding gases via the generation of an electrical signal due to a change in one of their physical properties such as capacitance or resistance. Gas sensors should have merits over traditional detection methods, such as portability and small size, low power consumption, on-line response, ease of fabrication and low cost.7 To date, various types of sensors such as piezo-resistive,8 electrochemical,9 catalytic combustion,10 thermal conductivity,11 infrared absorption12 and resistive gas sensors13 based on different materials and principles have been introduced. Each type of gas sensor has its own merits and drawbacks. Among them, resistive sensors are the most widely used owing to their high stability, good response, cost-effectiveness, simple operation, fast dynamics, and ease of fabrication.14 However, high sensing temperature and weak selectivity are the main drawbacks of this type of sensors.15,16
There are two types of sensor configurations: planar and tubular.1 In both cases, resistive gas sensors are fabricated via the deposition of a sensing layer over a substrate equipped with electrons. Generally, alumina and SiO2-coated Si are used as the substrate, and electrodes are fabricated using Pt, Au, and Pd–Ag. Moreover, on the back side of a planar sensor, a microheater is attached to the substrate, which is used for heating the sensor to the desired temperature, whereas in the case of tubular sensors, a resistive nichrome wire within the tubular substrate is used for heating the sensor.1 Fig. 1(a)–(c) display a schematic of a planar sensor.
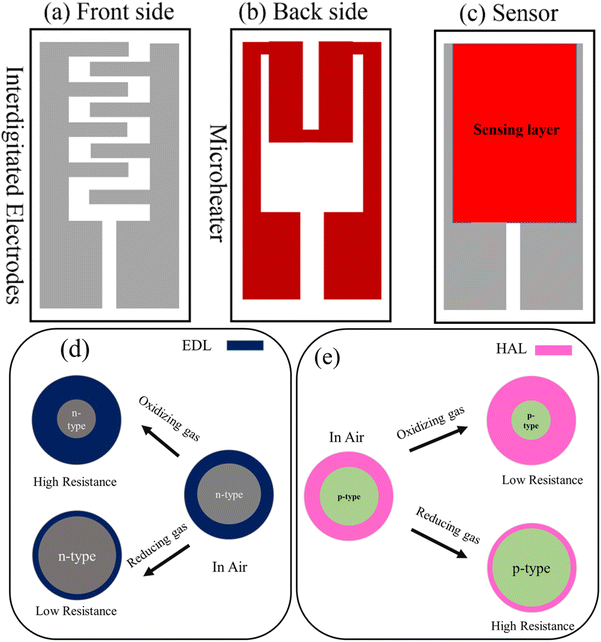 | ||
| Fig. 1 Schematic of (a) the front side, (b) back side and (c) fabricated resistive gas sensor. General gas sensing mechanism of (d) n-type and (e) p-type gas sensors. | ||
Various factors such as chemical composition, surface area, morphology, structure, and number of active sites significantly affect the sensing characteristics of gas sensors.17 Therefore, the choice of sensing material is very important to bring about a high performance sensor. For the realization of resistive gas sensors, semiconducting metal oxides such as SnO2 and ZnO are extensively employed,18 owing to their abundance and high stability and electron mobility.19 However, they have a high sensing temperature and relatively weak selectivity, especially in pristine form, similar to most other semiconducting-based sensors.20 In this regards, the development of metal oxide gas sensors using other materials such as non-stoichiometric tungsten oxides (WO3−x) can overcome some disadvantages associated with resistive gas sensors and may open some new doors in the exploration and design of high-performance gas sensors.
1.1 General gas sensing mechanism of resistive gas sensors
Resistance modulation is the key factor for the generation of a sensing signal in resistive gas sensors. Fig. 1(d) and (e) schematically illustrates the general gas sensing mechanism of n-type and p-type gas sensors, respectively. When a resistive gas sensor is exposed to fresh air, oxygen molecules with high electron affinity take electron from the sensor surface and become adsorbed on the surface. The abstraction of electrons by oxygen changes the resistance of the gas sensor relative to vacuum conditions, where there is no air. In the case of an n-type gas sensor, in which the main charge carriers are electrons, the abstraction of electrons causes the formation of an electron depletion layer, where the concentration of electrons is less than that in the inner parts, resulting in a high electrical resistance in air. In contrast, in a p-type gas sensor, the abstraction of electrons causes the formation of a hole accumulation layer (HAL), where the concentration of holes is much higher than that in the core region, resulting in a decrease in electrical resistance in air. When an n-type gas sensor is exposed to a reducing gas, electrons are released on the sensor surface, resulting in a decrease in the thickness of the EDL, which decreases the electrical resistance. However, in an oxidizing gas, further abstraction of electrons causes an increase in the thickness of the EDL, resulting in an increase in the sensor resistance. In the case of p-type gas sensors, the trends of resistance change are inverse, where the resistance increases and decreases in the presence of reducing and oxidizing gases, respectively.21–232. W18O49: an introduction
Tangiest oxide (WO3) is a semiconductor with unique electrical properties and multifunctional applications.24 Besides stoichiometric WO3, non-stoichiometric WO3−x, including WO2.72 (W18O49),25 WO2.8 (W5O14)26 and WO2.9 (W20O58),27 with abundant oxygen vacancies, is becoming an attractive candidate for the development of gas sensors owing to their abundance, low cost, nontoxicity, suitable band gap and high chemical stability.28 In WO3−x, the presence of oxygen vacancies results in the formation of shallow donors, increasing the number of adsorption sites and the electrical conductivity.29 In particular, owing to the high conductivity30 and abundant oxygen vacancies in W18O49 (WO2.72), it has attracted significant attention compared to other WO3−x materials.31,32 In addition to gas sensors,33–38 W18O49 has applications in energy storage,39 medicine,40 supercapacitors,41 sonodynamic therapy,42 and photocatalysts.43Bhavani et al.,24 Yan et al.,39 and Zhou et al.44 discussed the use of W18O49-based materials for energy and environmental use, energy storage conversion and photocatalysts, respectively. Although there are many reviews in the field of semiconductor gas sensors,1,35,45,46 a review on W18O49 as a gas sensor is lacking. Inspired by this lack of review papers related to the gas sensing features of W18O49, herein, we discuss the sensing properties and mechanisms of pristine, doped and composite W18O49-based gas sensors.
W18O49 has the most reduced form among the WO3−x oxides.28 The crystal structure of W18O49 as a sub-stoichiometric type of WO3 is formed by crystallographic shearing based on WO3. WO3 has a perovskite structure (ABO3), in which the “A” site is missing.31 In fact, it represents a repeated network consisting of WO6 octahedra arranged across corners. The oxygen and W ions are placed in the center and corners of the octahedra, respectively, and each oxygen ion connects two octahedra (Fig. 2(a)).47 Fig. 2(b) exhibits the corner- and edge-sharing modes as well as pentagonal columns mode. The ordered and coordinated structure may result in arrangement possibilities in the WO3 lattice (Fig. 2(b)).29 The band structure of a semiconductor can be altered due to the formation of oxygen deficiencies. The conduction band (CB) of WO3 is formed by empty W 5d orbitals, while the valence band (VB) is formed by filled O 2p orbitals (Fig. 2(e)).40 In W18O49, due to the formation of oxygen vacancies and subsequent ionization, new energy bands will be formed in its band gap, leading to higher conductivity relative to WO3. Moreover, due to the formation of oxygen vacancies, the reduction of some W6+ ions occurs, resulting in the formation of shallow states below the CB due to formation of W5+/W4+ energy levels (Fig. 2(e)). W5+/W4+ would capture the electrons excited by the oxygen vacancies, leading to polarization of the surrounding lattice and producing polarons.31 The polarons will lead to the localized surface plasmon resonance (LSPR) effect. Owing to the special band structure of W18O49, it possesses photosensitive capability, and thus it can respond to almost the whole spectrum because of (i) the direct transition of electrons between its VB and CB; (ii) VB to W5+/W4+ state transition, and (iii) LSPR effect. The special characteristics of W18O49 include the LSPR effect, photochromic effect, and narrow band gap. In particular, as stated before, the presence of oxygen vacancies leads to the generation of more free electrons, resulting in high electrical conductivity of W18O49, and thus it is a potential material for the fabrication of gas sensing devices.14 The performance of W18O49-based gas sensors can be boosted by doping, decoration, formation of composites and morphological engineering. In the next sections, we discuss the gas sensing features of W18O49 gas sensors.
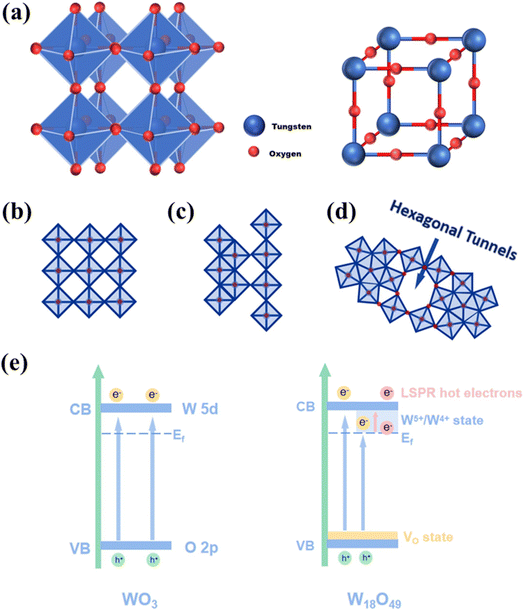 | ||
| Fig. 2 (a) Structure of WO3. (b) Corner- and (c) edge-sharing modes. (d) Pentagonal column mode. (e) Electronic band structure of WO3 and W18O49.44 Reproduced with permission from ref. 34. Copyright 2023, Elsevier. | ||
3. Pristine W18O49 gas sensors
In the solvothermal or hydrothermal methods, high temperature and pressure are used to accelerate the rate of reactions. Thus, they are highly promising synthesis methods for generating various nanostructures such as one-dimensional (1D) nanostructures. In this context, 1D W18O49 nanoneedles were produced via the solvothermal route at 160–220 °C for 3–9 h. Also, the solvent type (ethanol, propanol, butanol and cyclohexanol) and WCl6 amount (60, 80, 100 and 120 mg) were changed to find the optimal conditions to achieve the highest response to NO2 gas.48 Regarding the solvent type, the highest sensing output was obtained when cyclohexanol was used due to the formation of uniform 1D nanoneedles with excellent crystallinity and substantial surface area of ∼31 m2 g−1, which were beneficial for surface redox reactions, hence enhancing the sensing performance. The response of W18O49 nanostructures was also affected by the WCl6 dosage. Small WCl6 dosages led to the accelerated the growth of W18O49 nanostructures along the (010) direction. Finally, this resulted in the generation of thin W18O49 nanoneedles, where the sample prepared using 100 mg WCl6 possessed outstanding crystallinity and the best sensing properties. At a high WCl6 dosage (120 mg), not only short/thick W18O49 nanostructures were formed but also their crystallinity decreased, which resulted in a decrease in their gas response. Furthermore, the solvothermal temperature affected the gas response. At 160 °C and 180 °C, thin W18O49 nanoneedles with agglomeration and poor crystallinity were synthesized. At 200 °C, almost perfect W18O49 nanoneedles with high crystallinity were formed, which exhibited an enhanced gas response. At 220 °C, W18O49 nanorods (NRs) or even block-shaped nanostructures were synthesized because of the accelerated growth rate at high temperature and the NO2 response decreased. Regarding the solvothermal time, after 3 h reaction, long and thin W18O49 nanoneedles with poor crystallinity were synthesized. A longer solvothermal time to 5 h led to the formation of nanoneedles with high crystallinity, which eventually caused the highest response to NO2 gas. A further increase in solvothermal time to 7 h resulted in the formation of short rod-like nanostructures with low crystallinity and low response to NO2 gas. Overall, the sample solvothermally synthesized at 200 °C for 5 h showed that the use of cyclohexanol and WCl6 dosage of 100 mg resulted in an enhanced gas response (Rg/Ra) of ∼17 to 10 ppm NO2 gas at 160 °C. Also, it exhibited excellent selectivity to NO2 gas, which was related to the low bond dissociation energy of NO2 gas (305 kJ mol−1), which facilitated reactions with the absorbed oxygen ions. The improved response to NO2 gas was related to following reasons: (i) due to the nonstoichiometric nature of W18O49, plenty of oxygen vacancies were generated in sensing materials, which acted as powerful adsorption sites for oxygen gas and (ii) due to the 1D nature of the W18O49 nanoneedles, not only a high surface area was created, but also the mobility of the charge carriers increased, while the recombination rate of charge carriers decreased. Fig. 3 schematically exhibits the sensing mechanism of the W18O49 sensor, in which initially an electron depletion layer (EDL) is formed in the presence of air by the adsorption of oxygen species and abstraction of electrons. Upon subsequent exposure to NO2 gas, more electrons are extracted on the sensor surface, resulting in the expansion of the EDL and increase in the sensor resistance. | ||
| Fig. 3 Schematic of the NO2 gas sensing mechanism of W18O49 nanoneedles.48 Reproduced with permission from ref. 48. Copyright 2023, Elsevier. | ||
The detection of ammonia at low concentrations and RT is of high importance for clinical diagnosis and food safety.49 In this regard, ultra-thin (less than 5 nm) W18O49 nanowires (NWs) with a high surface area of 151 m2 g−1 were produced via the solvothermal method. Also, based on the photoluminescence (PL) study, a large amount of oxygen vacancies was formed during the hydrothermal synthesis of NWs. The sensor revealed a conductivity-type change with a change in the NH3 amount. The n-to p-type behavior of the sensor was explained as follows: when the bulk donor density (ND) was neither too high nor too low, then the surface state density (NA) could reach the minimum with a variation in the gas concentration and a change in sign with a variation in conductivity was expected. Given that the diameter of the NWs was smaller than the Debye length of W18O49 at RT, when the bulk donor density of the NWs was in the appropriate range, an abnormal conductivity change was recorded. This sensor could detect sub ppm concentrations of NH3 gas. The high response of the W18O49 NW sensor was due to the small diameter of the W18O49 NWs, creating a high surface area and non-stoichiometric nature due to the presence of a large amount of oxygen vacancies, which facilitated the chemisorption of oxygen at RT, resulting in a good response to NH3 gas at RT.50
The reliable sensing of volatile organic compounds (VOCs) at RT is vital due to the toxic effect of some VOCs.51 In this sense, 1D single crystalline W18O49 NRs with an aspect ratio of 20 were produced using a colloidal synthesis method for the detection of VOCs at RT.52 The response ([ΔR/Ra] × 100) to 130 ppm ethanol was 3.5% at RT. However, the trec was too long, which was related to the RT sensing temperature. They used UV irradiation during the recovery period of the sensor and the trec significantly decreased. Also, the effect of the thickness of the sensing layer (1.2, 4.6, and 7.0 μm) was investigated and the 4.6 μm-thick sensor manifested the highest response. Also, the response time (tres) and recovery time (trec) were not significantly different, which was related to the highly porous nature of W18O49 NRs.
Given that various crystal planes lead to various atomic surface structures and chemical and physical features, the sensing features of W18O49 with well-defined crystalline planes should be investigated. In this regards, Zhang et al.,53 synthesized ultra-fine W18O49 NWs via a chemical synthesis approach, using WCl6 and ethanol as the solvent. When the content of WCl6 was 0.4 g, ultra-fine NW bundles with a large surface area of 194.7 m2 g−1 and good crystallinity together with exposed (010) plane were formed. By increasing the content of WCl6, sea urchin-like nanostructures were formed with a lower surface area and poor crystallinity. The response (Ra/Rg) of the ultra-fine NW bundle sensor to 50 ppm acetone was 48.6 at 280 °C, which was higher than that of other gas sensors, mainly owing to its high surface area and presence of abundant oxygen vacancies. According to DFT studies, the total adsorption energies for acetone gas are 80.60, 31.53 and 38.29 kJ mol−1 on the (010), (100) and (001) crystal planes, respectively. Thus, the (010) crystal plane had the greatest adsorption ability for acetone. Furthermore, acetone had the highest adsorption energy on the W18O49 (010) surface relative to other VOCs, which justified its good selectivity for acetone.
Mesocrystals can be formed by the oriented growth of pre-synthesized, well-defined building blocks, where the fusion of the building blocks results in the formation of crystalline structures with a porous nature,54 which are highly promising for sensing studies. In an interesting study, W18O49 mesocrystals with a narrow size distribution (the diameter of ∼250 nm and length of ∼200 nm) were prepared by dissolving 200 mg WC6 in 30 mL 1-butanol and subsequent solvothermal synthesis at 200 °C for 24 h. Alternatively, W18O49 NWs were prepared by dissolving the same amount WCl6 in double the amount of 1-butanol, followed by the same solvothermal process.55 The W18O49 mesocrystals had an average diameters of ∼250 nm and average length of 200 nm. The mesocrystal sensor exhibited the maximum response (Rg/Ra) of 24.5 to 1 ppm NO2, which was 5-times that of W18O49 NWs. The surface area of W18O49 mesocrystals was ∼79 m2 g−1, while that of W18O49 NWs was 71 m2 g−1. Also, according to PL studies, more oxygen vacancies were present in the W18O49 mesocrystals than in the W18O49 NWs. Thus, although no significant difference between their surface areas was recorded, the higher amount of oxygen vacancies led to a noticeable difference in their sensing properties.
Given that in resistive gas sensors, gas molecules need to be absorbed on their surface, increasing the surface area of W18O49 is a good strategy to improve its properties as a sensor. Hence, it is expected that different morphologies of W18O49 will lead to different sensing properties due to their different surface areas.56 In this regards, Qin et al.57 solvothermally synthesized short and thick 1D NRs W18O49 using cyclohexane as the solvent at 200 °C/6 h. Also, long and thin W18O49 NWs were produced using 1-propanol as the solvent at 200 °C/9 h (Fig. 4).
 | ||
| Fig. 4 SEM and TEM micrographs of the W18O49 (a) and (b) NRs and (c) and (d) NWs.57 Reproduced with permission from ref. 57. Copyright 2011, Elsevier. | ||
The response (Rg/Ra) of W18O49 NWs and NRs were 140.7 and 131.5 to 5 ppm NO2 at 150 °C, respectively. Also, W18O49 NWs exhibited faster dynamics relative to W18O49 NRs. The NWs had a larger surface area (∼83.60 m2 g−1) than the NRs (∼69.25 m2 g−1), which led to the availability of more adsorption sites on NWs for gases. Therefore, the NWs showed higher resistance modulation when exposed to NO2 relative to W18O49 NRs.
Gu et al.58 reported that isolated tungsten oxide NWs can be directly grown on a metallic W layer. In this context, aligned arrays of W18O49 NWs were directly synthesized via the in situ oxidation of a sputtered W layer on a substrate equipped with Pt electrodes (Fig. 5).59 After the deposition of a tungsten film with a thickness of 150 nm, they were thermally oxidized at 550–750 °C under a continuous flow of argon and oxygen gases. At 550 °C, complete W18O49 NWs were not formed, while at 650 °C well-formed W18O49 NWs with small diameters (10 to 20 nm) were formed. Also at 750 °C, much thicker and longer NWs were formed.
 | ||
| Fig. 5 Schematic of the synthesis of W18O49 NW arrays.59 Reproduced with permission from ref. 59. Copyright 2016, Elsevier. | ||
Also, the effect of oxygen exposure time (0–60 min) during thermal oxidation at 650 °C was explored (Fig. 6(a)–(d)) and it was found that after exposure to oxygen for 60 min, the NWs possessed a length of 500 nm and diameter of 10–20 nm.
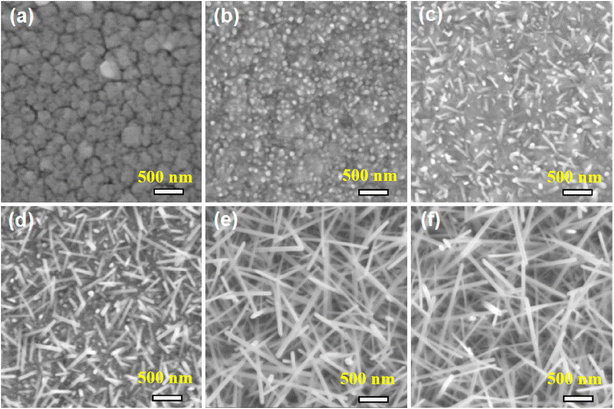 | ||
| Fig. 6 SEM micrographs of W18O49 grown at 650 °C for various oxidation times under (a) no oxygen and oxygen flow time for (b) 10, (c) 20, (d) 40 and (e) 60 min and (f) in Ar/O2 flow during growth.59 Reproduced with permission from ref. 59. Copyright 2016, Elsevier. | ||
The sensor fabricated using W18O49 NW arrays at 650 °C for 60 min revealed a response (Rg/Ra) of 4.4 to 1 ppm NO2 gas at 150 °C, with relatively fast tres and trec of 78 and 32 s, respectively, which was attributed to the good diffusion of gas molecules in the NWs owing to their structural porosity. The rough alignment of the NWs resulted in formation of numerous NW/NW junctions, which served as electrical bridges between the electrodes. Also, in contact areas between NWs, potential barriers were formed in air, and upon subsequent exposure to NO2 gas, great resistance modulation occurred.
As explained in this section, pristine W18O49 gas sensors are being used for detection of toxic gases; however, their performance can be further improved by noble metal decoration, which is explained in the next section.
4. Noble metal decorated W18O49 gas sensors
Decoration of the sensing layer using noble metals is a good technique to boost the sensing performance, where the improved performance is related to the electronic and chemical sensitization impacts of noble metals.60 In this regards, Pd-decorated flower-like W18O49 with an average diameter of 500 nm was prepared by Wang et al.61 for the detection of formaldehyde (HCHO). Firstly, WCl6 was added to an ethanol solution and sonicated. Then, PdCl2 (0, 20, 40, and 60 mg) was added, and after ultra-sonication for 0.5 h, the final products were synthesized by hydrothermal reaction at 180 °C/10 h (Fig. 7(a)). Both W18O49 and Pd (20 mg)-decorated W18O49 showed a flower-like morphology (Fig. 7(b)–(d)). The Pd (20 mg)-decorated W18O49 sensor displayed a high response (Ra/Rg) of 25.1 to 15 ppm formaldehyde at 180 °C, while the pristine sensor displayed a response of 12. The tres was 16 s for the W18O49 sensor, while it was only 1 s for the Pd-decorated W18O49 sensor. Also, DFT calculations confirmed that the adsorption energy of formaldehyde on the Pd-sensitized W18O49 was −1.362 eV, which was stronger than that of W18O49 (−0.835 eV), implying that the adsorption of HCHO on the surface of W18O49 increased after Pd decoration. Furthermore, the superior sensor capacity of the Pd-decorated sensor was mainly related to the fact that this sensor had the highest amount of the oxygen vacancies, which acted as active sites for oxygen gas. In addition, the catalytic and electronic effects of Pd NPs, together with the flower-like morphology of W18O49 contributed to the sensing response.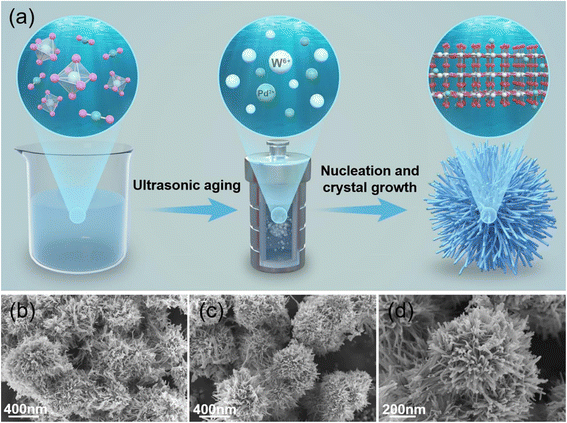 | ||
| Fig. 7 (a) Synthesis procedure and SEM images of (a) W18O49 nanoflowers and (b–d) Pd (20 mg)-decorated W18O49 nanoflowers.61 Reproduced with permission from ref. 61. Copyright 2023, Elsevier. | ||
Due to the highly explosive nature of H2 gas, there is an urgent need to monitor the leakage of hydrogen to ensure human safety.62 In this case, Pd is an excellent noble metal for H2 sensing. It can not only easily dissociate H2 gas molecules, but also adsorb up to 900-times its own volume of H2 gas.45 In this respect, urchin-like W18O49 was hydrothermally synthesized at 160 °C/24 h (Fig. 8(a) and (b)). Then, Pd NPs were decorated on urchin-like W18O49 using a chemical method, and subsequently the samples were annealed at 300 °C and 400 °C.63 Based on the thermogravimetry analysis, at 400 °C, owing to the oxidation of W18O49 in the air, W18O49 was oxidized to WO3 and Pd-decorated WO3 was obtained. However, annealing at 300 °C resulted in the formation of Pd-decorated urchin-like W18O49 (Fig. 8(c) and (d)). The Pd-decorated W18O49 sensor had a high response (Ra/Rg) of 1600 to 0.1 vol% H2 at 100 °C, while the Pd-decorated WO3 sensor revealed a response of ∼30. The boosted H2 sensing performance was related to the 3D urchin-like hierarchical structure, which was beneficial for the easy and fast diffusion of H2 gas in the in-depth parts of the sensor furthermore, W18O49 had many oxygen vacancies, which enhanced the adsorption of oxygen on the sensing layer. Finally, the spillover effect of Pd was responsible for the enhanced response. Firstly, in the presence of Pd NPs, H2 molecules were dissociated into H species, and then they moved to W18O49, and finally reacted with the adsorbed oxygen ions on W18O49 (Fig. 8(e)),64 as follows:
 | (1) |
| 2H(ads) + O2− → H2O + e− | (2) |
 | ||
| Fig. 8 TEM micrographs of (a) and (b) W18O49 and (c) Pd–W18O49. (d) HRTEM image of Pd–W18O49. (e) Schematic of the spillover effect.63 Reproduced with permission from ref. 63. Copyright 2018, Elsevier. | ||
Networked W18O49 NWs with a diameter of ∼50 nm and length of 2 μm were produced via the thermal evaporation of W powder at 1400–1450 °C for 10 min in the presence of oxygen.65 Then, a Pt layer was sputtered on the W18O49 NW. The fabricated sensor was used in self-heating mode, and to minimize the power consumption, the samples were suspended with the bonding wires to reduce the dissipation of heat (Fig. 9(a)). Upon the application of a voltage, heat was generated inside the sensor due to the Joule heating effect (Fig. 9(b)). Accordingly, the response increased by applying a voltage due to the large amount of heat generated inside the sensor at high voltages. The selectivity of the sensor to H2 gas was due to the dissociation of H2 into H species by catalytic effect of Pt, and then their spill-over to the sensing layer.
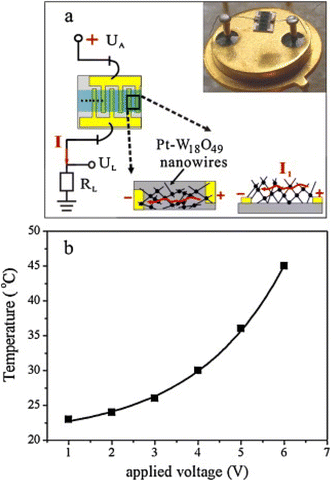 | ||
| Fig. 9 (a) Schematic of the electrical set up for gas sensing measurement together with digital photo of the sensor. (b) Induced temperature of networked Pt–W18O49 NW versus the applied voltage.65 Reproduced with permission from ref. 65. Copyright 2011, Elsevier. | ||
Urchin-like W18O49 microstructures were synthesized via the hydrothermal route at 160 °C for 24 h. Later, Pt NPs were deposited on W18O49 via the UV illumination of an H2PtCl6·6H2O solution. Under UV illumination, some W–O bonds were broken and the number of oxygen vacancies increased relative to the pristine W18O49. The surface area of W18O49 and Pt/W18O49 was ∼48.90 and 53.70 m2 g−1, respectively. Therefore, the surface area slightly increased after Pt decoration, which is beneficial for the sensing of gases. The pristine W18O49 displayed a response (Ra/Rg) of 7 to 10 ppm ethanol at 325 °C, while the Pt/W18O49 sensor displayed a response of 23 to the same amount of ethanol at 300 °C. Also, both sensors showed good selectivity to ethanol. According to DFT studies, the adsorption energies of ethanol on the W18O49 and Pt/W18O49 sensors (−0.85 and −1.18 eV) were higher than that for interfering gases, resulting in the better adsorption of ethanol relative to interfering gases. Furthermore, the higher adsorption energy for ethanol on W18O49 indicated the superior adsorption ability of the Pd-decorated sensor for ethanol. Moreover, the adsorption energy for O2 molecules on W18O49 and Pt/W18O49 was −2.31 and −3.41 eV, respectively, implying that Pt/W18O49 had higher adsorption ability for oxygen than W18O49. Due to the difference in work function between W18O49 and Pt, the Ohmic junctions were formed at the interface between W18O49 and Pt. Thus, in comparison with the W18O49 sensor, the flow of charge carriers in the Pt-decorated sensor was more favorable. To further study the behavior of the sensors, a fixed concentration of gas was extracted, and then injected into a gas chromatograph. It was revealed that the Pt/W18O49 sensor consumed more ethanol and generated more CO2 relative to the W18O49 sensor. This was related to the catalytic effect of Pt towards the easy oxidation of ethanol.66
The major gases in a coal mines are CO, H2S and CH4.67 Thus, it is important to realize gas sensors for the reliable sensing of these gases. In this context, Zhang et al.,68 prepared W18O49 NWs using the solvothermal approach at 180 °C/24 h. Subsequently, Pd@Au core–shell bimetallic NPs (BNPs) were decorated on W18O49 NWs (Fig. 10) for sensing coal mine gases. The concentration of HAuCl4 was set to 0.8, 1.3 and 1.8 mM, while that of Pd was fixed and the samples with the above-mentioned Au precursor concentrations were labelled as NWs/BNPs-1, NWs/BNPs-2, NWs/BNPs-3, respectively.
 | ||
| Fig. 10 Schematic representation of the synthesis of W18O49 NWs and W18O49 NWs/BNPs.68 Reproduced with permission from ref. 68. Copyright 2022, Elsevier. | ||
Owing to the interaction between Pd and W18O49 NWs, a small shift in the XPS spectrum was observed, which led to the regulation of the surface electronic characteristics of the W18O49 NW, and enhanced sensing features. The response (Ra/Rg) of the W18O49 NWs/BNP-2 sensor to H2S (50 ppm) at 100 °C was about 55.5 with a weak response to CH4 gas (Fig. 11(a)), while at 320 °C it had a response (Ra/Rg) of ∼7.8 to 1000 ppm CH4 (Fig. 11(b)). Thus, it was possible to tune the selectivity of the sensor to these gases by changing the temperature. In particular, CH4 is a stable molecule because of its high molecule symmetry, accordingly its oxidation is difficult at low temperatures. With an increase in the working temperature, and due to the higher catalytic activity of BNPs, the oxidation of CH4 molecules was accelerated. Furthermore, the sensor could successfully detect H2S and CH4 gases in mixed gases (50 ppm H2S, NO, CO, and NH3 as well as 1000 ppm CH4) at their optimum temperature. The enhanced features of the sensor were related to (i) the ultra-fine 1D NWs with diameters less than 1 nm, which provided more adsorption sites and fast transport channels, and the presence of a large amount of oxygen vacancies, (ii) catalytic effects of Au and Pd NPs towards the target gases and (iii) formation of potential barriers between the bimetallic NPs and sensing materials and modulation of the potential barriers in an atmosphere containing target gases.
 | ||
| Fig. 11 Selectivity of W18O49 NWs/BNPs-2 at (a) 320 °C and (b) 100 °C to various gases.68 Reproduced with permission from ref. 68. Copyright 2022, Elsevier. | ||
Hollow nanostructures offer a higher surface area relative to bulk materials due to the availability of both inner and outer walls for gas adsorption. Therefore, they should possess a high response to target gases. Xu et al.69 constructed hollow W18O49 spheres via the hydrothermal route at 180 °C/16 h. Subsequently, Co3O4/hollow W18O49 spheres were prepared via the chemical route by adding a Co precursor to hollow W18O49 spheres. Finally, Au was decorated on Co3O4/hollow W18O49 spheres using a chemical method. The hollow structure consisted of lamellar flakes, which formed porous and large spheres (Fig. 12).
 | ||
| Fig. 12 (a) and (b) SEM micrographs of W18O49 hollow spheres. TEM images of (c) W18O49, (d) Co3O4/W18O49, and (e) Au-decorated Co3O4/W18O49 hollow spheres.69 Reproduced with permission from ref. 69. Copyright 2019, Elsevier. | ||
The response (Ra/Rg) of the Au-decorated Co3O4/W18O49 sensor to 2 ppm TEA at 270 °C was to 16.7, which was significantly higher than that of Co3O4/W18O49 (11.3) and W18O49 (3.9). Firstly, the porous and hollow structure provided a high surface area (76.12 m2 g−1), which led to the availability of more active sites for the adsorption of oxygen molecules. Besides, the porous and hollow nature contributed to fast gas diffusion (Fig. 13(a)). Secondly, the Au NPs were a highly active catalyst for oxygen dissociation due to the spillover effect (Fig. 13(b)). Therefore, TEA easily reacted with the adsorbed oxygen ions and electrons were released to the sensor surface (Fig. 13(c)). Thirdly, the formation of p–n heterojunctions at the interface between p-type Co3O4 and n-type W18O49 contributed to the enhancement in the sensing performance. The work function of Co3O4 and W18O49 was 6.1, and 4.6 eV, respectively.70–72 Hence, their Fermi levels were equal due to the flow of electrons from W18O49 to Co3O4. This resulted in the widening of the EDL, and at the interface of heterojunctions, potential barriers were created with band bending. Therefore, owing to the large baseline resistance, a remarkable reduction in resistance occurred upon exposure to TEA gas.
 | ||
| Fig. 13 Schematic of (a) pristine W18O49 in air, (b) and (c) Au-decorated Co3O4/W18O49 in air and TEA gas.70 Reproduced with permission from ref. 70. Copyright 2019, Elsevier. | ||
Sun et al.,73 initially prepared W18O49 NRs via a solvothermal reaction at 200 °C for 8 h, and then Ag/AgCl NPs were decorated on the W18O49 NRs using AgNO3 as the precursor and irradiation with an Xe lamp. The response (Ra/Rg) of the sensor was 115.5 to 100 ppm H2S at 300 °C and tres and trec were 21 and 26 s, respectively. The improved gas-sensing capacity relative to the pristine sensor was attributed to catalytic effect of Ag NPs owing to the spillover effect,74 as well as the electronic effect due to the formation of potential barriers in the contact areas between Ag/AgCl and W18O49.
Alloy nanocrystals not only have the unique characteristics of each metal, but also additional features due to the synergy between different atoms. In particular, they provide boosted catalytic properties for gases, which can significantly enhance the catalytic performance of the sensor. In the study conducted by Bai et al.,75 initially urchin-like W18O49 was hydrothermally prepared at 180 °C/24 h. Then, the Au39Rh61 alloy was synthesized via the co-precipitation method, and finally Au39Rh61 alloy-decorated W18O49 was prepared using a chemical method for n-butanol sensing. At 260 °C, the response (Ra/Rg) of the Au39Rh61–W18O49 sensor was ∼11 to 50 ppm n-butanol, which was 4-fold higher than that of Au–W18O49 (∼2.2) and 2-times higher than Rh–W18O49 (∼5.1) sensor. The increased gas sensing properties of the AuxRh1−x-decorated W18O49 was related to (i) its large surface area due to its urchin-like morphology, (ii) spillover effect of Au39Rh61 alloy, where the dissociation of molecular oxygen due to the catalytic effect of the noble metals resulted in activated oxygen species, which were spilt over on the sensing layer and (iii) formation of Schottky barriers between the noble metals and sensing layer.76
5. Doped W18O49 gas sensors
Doping with metal atoms is an efficient approach to modify the electronic characteristics of materials via the introduction of additional energy levels, creation of oxygen vacancies and increasing the surface adsorption/desorption capability.77,78 Doping of W18O49 with metals such as Mo, Fe, Pd and Ni led to the generation of oxygen vacancies, which eventually led to an enhanced gas response.63,79–82Acetone is a biomarker gas and the amount of acetone in exhaled breath can be used for the diagnosis of diabetic patients, where its content in healthy people is ∼0.3–0.9 ppm and increases to 1.8 ppm in diabetic patients. In this regards, PdxW18O49 (x = 1.83%, 3.60%, 7.18%, and 11.69%) NWs, were synthesized via the hydrothermal method. All the Pd-doped samples showed an NW morphology without a significant difference in surface morphology. Also, based on the XPS study, the Pd7.18%W18O49 NWs had the largest oxygen vacancy content among the samples. Also, this sample displayed the highest response (Ra/Rg) of ∼146 to 50 ppm acetone at 175 °C, with the fast tres and trec of 5 and 10 s, respectively.81 The short dynamics of the optimized gas sensor was related to its relatively high sensing temperature, which provided more energy to facilitate the flow of electrons. Also, this sensor exhibited a reliable response to acetone in a mixed gas comprised of ethanol and acetone, which is important for medical diagnosis applications. The improved gas sensing was related to the formation of Schottky barriers between Pd and W18O49 NWs and existence of the highest amount of oxygen vacancies, which favored the adsorption of oxygen on the sensor surface. Accordingly, the reactions between acetone and adsorbed oxygen ions lead to the generation of a higher sensing signal.
In an interesting study, rambutan-like Ni-doped W18O49 was fabricated via a one-step solvothermal route. The specific surface area of W18O49 and Ni0.05W18O49 was ∼76 m2 g−1 and 130.5 m2 g−1, respectively. The NixW18O49 sensor with x = 0.05 Ni-doping displayed a response (Rg/Ra) of 182 to 50 ppm n-butanol at 160 °C with a short tres and trec (14 s/241 s), respectively, while the response of the pristine sensor was 26.7. The improved response of the doped sensor was correlated with the increase in the amount of oxygen vacancies due to Ni-doping and high surface area (130.5 m2 g−1).83
Simultaneously doping two metals in tungsten oxides has rarely been reported. The introduction of two dopants with different sizes and valences into host materials can change their electronic features, create more oxygen vacancies, and improve their catalytic activity.84 In this context, the impact of Co and Ni co-doping in W18O49 was investigated for the detection of TEA.85 Co and Ni-doped porous W18O49 nano-urchins with a surface area of ∼165.85 m2 g−1 were achieved using the solvothermal method at 180 °C/24 h. At 250 °C, the sensor with 1 wt% Co and 2 wt% Ni W18O49 displayed a response (Ra/Rg) of 114 to 50 ppm TEA, which was higher than that of the single-doped and undoped W18O49 sensors. The enhanced response was related to the hierarchical urchin-like structure of the sensor, its high surface area and effects of the co-doped metal ions.86 As p-type dopants, Co and Ni replaced W in the W18O49 lattice and created oxygen vacancies. Thus, more O2 molecules were adsorbed on the surface, and therefore more reactions with TEA occurred. Besides, the oxidization of Co and Ni dopants reversibly transferred electrons at the interface of W18O49 with NiO or Co2O3 as (Ni ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) NiO and/or Co
NiO and/or Co ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) Co2O3), forming a p–n heterojunction, resulting in high electrical resistance, and upon exposure to TEA, the release of electrons resulted in a remarkable change in resistance. Furthermore, dopants lowered the band gap and promoted the charge flow kinetics, and hence increased the reactivity to TEA molecules. Also, the C–N bond in TEA had the lowest bonding energy and easily broke and participated in redox reactions at the sensing temperature. Extra doping of Co and Ni (>3 wt%) resulted in intensive contraction/deformation in the W18O49 lattice and reduced the electrical conductivity or even caused the segregation of the dopant cations and formation of NiO and Co3O4. Accordingly, the sensing performance decreased when high amounts of dopant were used.
Co2O3), forming a p–n heterojunction, resulting in high electrical resistance, and upon exposure to TEA, the release of electrons resulted in a remarkable change in resistance. Furthermore, dopants lowered the band gap and promoted the charge flow kinetics, and hence increased the reactivity to TEA molecules. Also, the C–N bond in TEA had the lowest bonding energy and easily broke and participated in redox reactions at the sensing temperature. Extra doping of Co and Ni (>3 wt%) resulted in intensive contraction/deformation in the W18O49 lattice and reduced the electrical conductivity or even caused the segregation of the dopant cations and formation of NiO and Co3O4. Accordingly, the sensing performance decreased when high amounts of dopant were used.
Prior studies revealed that the d-band center of materials is related to their adsorption activity, where a higher energy level of the d-band center results in enhanced activity.87 Based on DFT results, the density of state (DOS) indicated that the doping of Ni and Co led to a shift in DOS from a low to high energy level, indicating an upward shifting of the d-band center (Fig. 14(a) and (b)). Thus, the adsorption ability of the sensor increased after co-doping. Also, based on the DFT analysis, the adsorption energy of TEA on Ni and Co co-doped W18O49 (Ead = −1.53 eV) was much higher than the pristine W18O49 (Ead = −0.79 eV) (Fig. 14(c) and (d)), which was consistent with the experimental results.
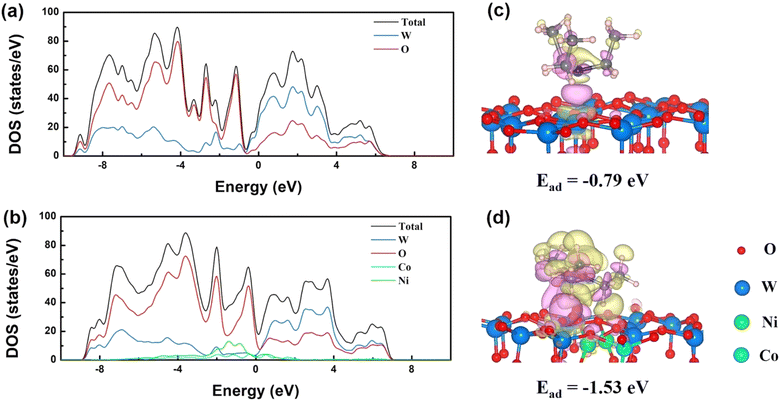 | ||
| Fig. 14 DOS of (a) the W18O49 and (b) Co and Ni co-doped W18O49 (010) surfaces. Charge density difference of the adsorption configuration of TEA on (c) W18O49 and (d) Co and Ni co-doped W18O49 (010) surface.85 Reproduced with permission from ref. 85. Copyright 2023, Elsevier. | ||
Ti was added to W18O49 after and during the growth of W18O49 NWs. Before the hydrothermal growth of the W18O49 NWs, Ti (2 at%) was added as TiCl4 to the final solution. Also, after the hydrothermal growth of the W18O49 NWs, using physical impregnation, Ti (0.6, 2, and 6 at%) was added to NWs using TiCl4.88 The pristine W18O49 NWs had an average diameter of ∼80 nm and average length of 900 nm (Fig. 15(a)). The Ti-added W18O49 NWs prepared via the physical impregnation method retained the morphology of the pristine W18O49 NWs, whereas the addition of Ti during solvothermal growth inhibited the growth of the W18O49 NWs, and finally W18O49 bundles were formed (Fig. 15(b)). Both Ti-added W18O49 sensors showed a p- to n-type transition with an increase in the temperature to 90 °C (Fig. 15(c)). The abnormal p-type feature observed at low temperature was due to the generation of an inversion layer at the surface of the n-type W18O49 NWs, originating from the intensive surface adsorption of oxygen due to the high density of surface states. In fact, the surface of nonstoichiometric W18O49 NWs was very active owing to the existence of a large amount of oxygen vacancies. When the additive was added to the W18O49 NWs a new energy level was created inside the band gap. Thus, the gas adsorption improved because of the presence of additional surface states. The intensive adsorption of oxygen on the NW surface caused a change in the Fermi level energy of the W18O49 NWs, resulting in the appearance of an inversion layer. Among the sensors, the sensor with 2% Ti prepared via physical impregnation displayed the highest response to NO2 gas at RT (Fig. 15(d)) because the physical impregnation method led to the formation of a random dispersion of small TiO2 particles, which increased the adsorption activity for oxygen on the surface of the sensing material. Also, the Ti-doped sample prepared via chemical synthesis revealed a lower response due to the fact that the W18O49 bundles inhibited the fast diffusion of NO2 to the in-depth parts of the bundles.
 | ||
| Fig. 15 SEM images of (a) pristine W18O49 NWs and (b) Ti-added W18O49 NWs prepared using a chemical synthesis method. (c) Sensor responses vs. temperature for pristine and Ti-added W18O49 NWs to 2 ppm NO2 and (d) corresponding calibration curves.88 P stands for physical impregnation and C stands for chemical addition of Ti. Reproduced with permission from ref. 88. Copyright 2012, Elsevier. | ||
6. Composite-based W18O49 gas sensors
In composite gas sensors, numerous heterojunctions between different materials can be formed, which cause a remarkable modulation in their electrical resistance.13 2D graphene, which consists of C atoms with sp2 hybridization, together with abundant defects and functional groups, high carrier mobility and high electrical conductivity,89 is a promising material for sensing studies. Qiu et al.90 prepared W18O49/graphene nanocomposites (with the graphene concentration of 0.005, 0.01, and 0.015 mg L−1) using the hydrothermal technique at 180 °C/24 h. The composites were comprised of interlaced NWs with diameters of 30–60 nm and lengths of 1–2 μm. The response (Ra/Rg) of the W18O49/graphene (0.01 mg L−1) sensor was 16.47 to 100 ppm ethanol at 340 °C, with tres and trec of 1 and 11 s, respectively. A higher amount of graphene in the composite resulted in high conductivity, which eventually suppressed the variation in resistance in the fabricated sensor. The high sensing performance of the optimal sensor was related to its high active surface area (∼42.35 m2 g−1), which provided abundant active sites for ethanol molecules. Also, due to the n- and p-type nature of W18O49 and graphene, p–n heterojunctions were formed at the interfaces between the two semiconductors, resulting in the significant modulation of the resistance. Furthermore, double Schottky barriers were created in the contact areas between the W18O49 grains, where the flow of electrons was difficult between the grains in air (Fig. 16(a)). In an ethanol atmosphere and due to the release of electrons, the height of the double Schottky barriers decreased and caused a remarkable change in resistance (Fig. 16(b)). Also, because of the difference between the work functions of graphene and W18O49, localized p–n heterojunctions were created at the interface of the composite materials, with formation of potential barriers in air, which are considered powerful sources of resistance modulation,91,92 remarkably modulating the resistance in an ethanol atmosphere. Furthermore, some structural defects were produced in the interface areas between the two materials, which acted as potential sites for the adsorption of ethanol. | ||
| Fig. 16 (a) Schematic of the sensing mechanism of W18O49/graphene in air (b) and (c) ethanol.90 Reproduced with permission from ref. 90. Copyright 2021, Elsevier. | ||
Transition metal dichalcogenides (TMDs) with the general formula of MX2 (M = transition metal; X= S, Se, and Te) have a small band gap, good conductivity, and edge-exposed sites, leading to good binding interactions with gas molecules.93 In this respect, a WS2/W18O49 heterojunction was synthesized via the pyrolysis of WO3 in the presence of sulfur (S) powder, thiourea (CH4N2S), and a mixture of “S” and CH4N2S at 850 °C for 1–4 h in an Ar atmosphere. The pyrolysis of WO3 using a mixture of “S” and CH4N2S yielded WS2 and W18O49 heterojunctions and exhibited superior gas-sensing properties. After 1 h of pyrolysis, agglomerated WS2 covered the W18O49 nanorods. By performing pyrolysis for 2 h, the amount of NRs increased because of the diffusion of “S” vapor into the core parts of WO3. After pyrolysis for 3 and 4 h, the formation of W18O49 NRs increased, with less agglomeration of WS2. The sensor fabricated from the sample after 3 h pyrolysis exhibited an enhanced response [(ΔR/Ra) × 100] of 25.7% and 35.67% to 5 ppm NH3 and NOx gases, respectively, at RT. The sensor displayed p-type behavior because of the p-type nature of WS2. The edge-exposed W atoms in WS2 were highly reactive to gases due to the high electron density of the d-orbitals in W atoms. Furthermore, the formation of p–n heterojunctions between W18O49 and WO3 resulted in the modulation of the electrical resistance of the sensor in the presence of gases.94
MXenes are 2D transition metal carbides/nitrides with the formula of Mn+1XnTx, where M is a transition metal, X is a carbon or N atom and Tx stands for surface functional groups. Due to their high conductivity, high surface area and presence of functional groups, they are very promising sensing materials.95 In this case, W18O49/Ti3C2Tx MXene nanocomposites with 1, 1.5,2 and 2.5 wt% MXene were fabricated via the in situ growth of 1D W18O49 NRs on the surface of 2D Ti3C2Tx MXene NSs through the solvothermal route at 150 °C/24 h.96 The W18O49/Ti3C2Tx composite containing 2 wt% Ti3C2Tx displayed a response (Rg/Ra) of 11.6 to 20 ppm acetone gas at 300 °C with fast tres and trec of ∼10.5 and ∼26 s, respectively. Furthermore, it could detect 170 ppb acetone, which was lower than the concentration of acetone in the exhaled breath of diabetic people.97 The enhanced gas sensing performance was related to the presence of MXene with a high amount of surface groups such as –O and –OH, which are considered potential sites for the adsorption of acetone molecules. Also, Schottky junctions were formed between MXene and W18O49, and therefore the sensor experienced a larger resistance variation than that of the W18O49 sensor when it was exposed to acetone. The decrease in response with a higher content of MXene was due to the presence of –F groups on the MXene surface, which had negative effects on the gas sensing. Furthermore, a high amount of MXene resulted in stacking of the MXene NSs, which decreased the surface area for the adsorption of gas molecules.
Conducting polymers (CPs) have high conductivity, good flexibility, tunable properties and can be used for the detection of gases at RT.98 In this regards, polypyrrole (PPy)@W18O49 C–S NRs were fabricated via the in situ polymerization of pyrrole monomer on solvothermally synthesized W18O49 NRs.99 The thickness of PPy was 5, 10 and 18 nm over W18O49 NRs with a diameter of ∼60 nm. The response (Ra/Rg) of the fabricated sensor (Fig. 17(a)) with a PPy shell thickness of 5 nm was 4.1 to 20 ppm NH3 at 15 °C. Furthermore, it could also detect 1 ppm NH3, which is below the human toxicity level, i.e. 25 ppm.100 Three types of junctions were present in the sensor, i.e., homojunctions between PPy in the contact areas between C–S NRs, PPy shell boundary and PPy-W18O49 heterojunctions (Fig. 17(b)). Owing to the modulation of the potential barriers in these junctions in the presence of NH3, and particularly heterojunctions (Fig. 17(c) and (d)), a high response was observed. In fact, when NH3 was adsorbed on the PPy shell, proton transfer and electron transfer occurred between the PPy and NH3 molecules, leading to a decrease of the hole concentration in the p-type PPy shell, which resulted in an increase in the height of the potential barriers and increase in the resistance of the PPy shell. The larger response of the sensor with a thinner shell was related to the complete depletion of the shell layer from charge carriers, and upon subsequent exposure to NH3, significant modulation of the resistance occurred (Fig. 17(e)).
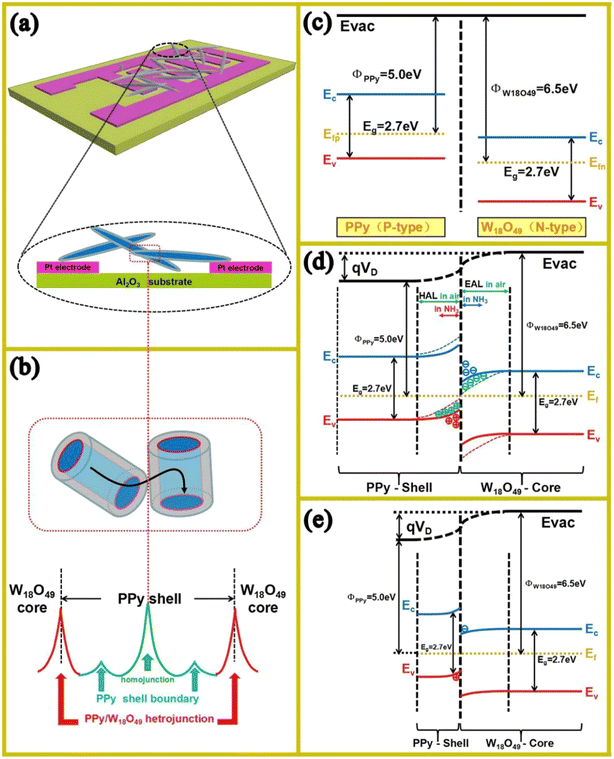 | ||
| Fig. 17 (a) Schematic of the C–S NR sensor. (b) Various potential barriers in the PPy-W18O49 C–S NR sensor. (c) Energy band levels of the PPy-W18O49 C–S NRs before contact, (d) change in energy band upon exposure to NH3 and (e) influence of thinner PPy shell on the energy levels.99 Reproduced with permission from ref. 99. Copyright 2017, Elsevier. | ||
The fabrication of 1D C–S arrays with a uniform shell layer and high alignment over their entire surface is attractive for realizing gas sensors with an excellent performance. In the study by Qin et al.,101 a W layer with a thickness of ∼100 nm was first sputtered on a substrate. Then, roughly aligned W18O49 NWs (15 to 20 nm) were selectively grown on the substrate via thermal oxidation at 650 °C for 1 h. The intercrossing of NWs formed numerous NW–NW junctions, which acted as potential barriers and were considered powerful sources of resistance modulation. Then, a Ti film with thicknesses of 3 and 6 nm was sputter-deposited on W18O49. Upon annealing at 450 °C for 1 h, crystalline W18O49–TiO2 C–S NWs were synthesized. The sensor with a TiO2 shell thickness of 6 nm revealed a higher response (Rg/Ra) of 36.5 to 5 ppm NO2 gas at RT. Interestingly, both C–S sensors exhibited a p-type feature at RT, despite the n-type semiconductor behavior of both the W18O49 and TiO2 materials, which was observed in other metal oxides.102,103 In fact, the significant adsorption of oxygen occurred due to (i) the presence of unstable surface states and (ii) catalytic activity of the TiO2 shell. Hence, the EDL inside the shell significantly expanded and upward band bending occurred, causing the formation of an inversion layer, in which holes were the main charge carriers. Thereby, the sensors exhibited p-type behavior. The resistance of the sensor was determined by the presence of TiO2–TiO2 homojunctions, TiO2–W18O49 heterojunctions and potential barriers as the grain boundaries of the TiO2 shell. However, the effect of the grain boundary potential barrier was negligible in the thin shell layers. Fig. 18(b) and (c) exhibit the energy band levels of the W18O49–TiO2 heterojunction before and after contact, respectively. The electron affinity, work function and band gap of TiO2 are 4.6, and 4.7 eV, 3.6, and that of W18O49 are 6.5, 6.7 and 2.7 eV, respectively.104 Thus, the electrons moved from TiO2 to W18O49 to balance their Fermi levels, leading to the formation of heterojunctions at the interface of TiO2 and W18O49 (Fig. 18(b)). In an NO2 atmosphere, more electrons were extracted from the sensor, and the resistance increased.
 | ||
| Fig. 18 (a) Schematic of the W18O49/TiO2 C–S NW gas sensor mechanism. Energy band levels of the W18O49/TiO2 C–S NW before (b) and after (c) contact.101 Reproduced with permission from ref. 101. Copyright 2017, Elsevier. | ||
Xiong et al.105 synthesized a WO3–W18O49 composite via a hydrothermal reaction at 200 °C for 24 h. Pristine W18O49 consisted of spindle-like particles (150–200 nm) (Fig. 19(a) and (c)), while the WO3–W18O49 samples had a loose spindle-shaped structure composed of numerous well-aligned NWs with a diameter of 10–20 nm and length of 150–200 nm in (Fig. 19(b) and (d)), which were more suitable for gas adsorption.
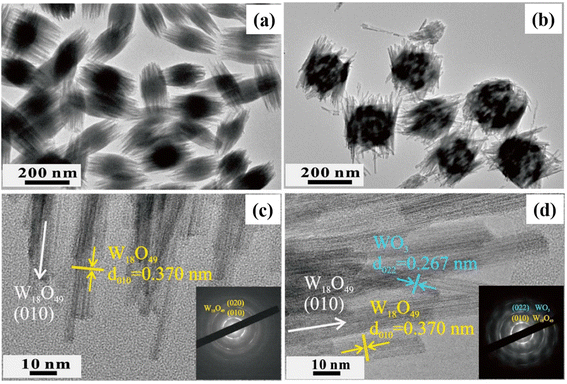 | ||
| Fig. 19 TEM images of (a) the W18O49 and (b) WO3–W18O49 composites and HRTEM images of (c) W18O49 and (d) WO3–W18O49 composites (insets reveal the corresponding SAED patterns).105 Reproduced with permission from ref. 105. Copyright 2018, Elsevier. | ||
The response (Ra/Rg) of WO3–W18O49 was 23.3 to 500 ppm NH3 at 250 °C, whereas the maximum response of the W18O49 sensor to 500 ppm NH3 was ∼5 at 200 °C. The selectivity of the composite sensor to NH3 was related to the different optimal operating temperatures for gases. Given that the work functions of the two materials were different (Fig. 20(a)), in the contact areas and to match their Fermi levels, electrons moved from WO3 to W18O49, resulting in band bending and the formation of an expanded depletion layer on WO3 (Fig. 20(a) and (b)). Upon the injection of NH3 gas and by release of electrons, the thickness of the EDL decreased, leading to a change in the resistance of the sensor. Secondly, the surface areas of WO3–W18O49 and pristine W18O49 were ∼95 and ∼37 m2 g−1, with pore volumes 0.2501 and 0.071 cm3 g−1, respectively. Thus, the composite sensor offered a greater surface area and larger pores for gas molecules. Also, based on the XPS study, more oxygen ions were adsorbed on WO3–W18O49 relative to the pristine W18O49, indicating the probability of higher reactions between them and gas molecules.
 | ||
| Fig. 20 Schematic of the energy band levels of WO3 and W18O49 (a) before contact and after junction formation in (b) vacuum, (c) air and (d) NH3 gas.105 Reproduced with permission from ref. 105. Copyright 2018, Elsevier. | ||
Hierarchical structures with a 3D morphology are very promising for the fabrication of gas sensors. In this regards, hierarchical WO3 NS-W18O49 NW composites were prepared through the hydrothermal technique at 150 °C for 4 h using WCl6 as the precursor, polyethylene–polypropylene glycol (P123) as the surfactant, and H2O and ethanol as solvents.106 In a fixed amount of ethanol (27.4 mL), WCl6 (0.666 g) and P123 (0.167 g), depending on the amount of water, urchin-like W18O49 (no water), hierarchical WO3/W18O49 (0.175 mL), and flower-like WO3 (0.25 mL) were synthesized. The response (Rg/Ra) of the hierarchical WO3/W18O49 to 1 ppm NO2 gas was 1687 at 160 °C, which was much higher than that of the urchin-like W18O49 (826) and flower-like WO3 (94). Furthermore, the tres and trec of the hierarchical WO3/W18O49 sensor were 36 s and 13 s, respectively. The fast dynamics of the sensor was related to the presence of abundant channels, leading to rapid diffusion and fast gas sensing reactions. Based on PL and electron paramagnetic resonance (EPR) studies, the sensors had almost the same amount of oxygen vacancies. Therefore, the difference between the sensing results was related to the synergistic nature of the NWs and NSs due to the fast electron separation and transport by the W18O49 NWs and abundant adsorption sites offered by the WO3 NSs. Also, the NSs acted as a current collector, receiving electrons from the NWs to react with oxygen or NO2 gas.
W18O49 NWs grown on SnO2 NWs with hierarchical structures were prepared for sensing application. Initially SnO2 NWs were grown via the vapor–liquid–solid (VLS) mechanism at 900 °C for 1 h. The SnO2 NWs had diameters in the range of ∼70–300 nm and lengths of ∼5–100 μm. Next, W18O49 NWs with a diameter of ∼50 nm and length of more than 2 μm were grown on the SnO2 NWs via the thermal evaporation of W. The effect of the growth temperature (1850 °C and 2050 °C) and growth pressure (5 × 10−5 mbar and 7 × 10−4 mbar) on the nature of the W18O49 NWs was investigated. Given that higher pressure led to a higher oxidation rate and increased evaporation rate, the density, diameter and length of the W18O49 NWs increased with an increase in pressure. At a fixed pressure, an increase in temperature resulted in the same trend as mentioned above.
The single wire SnO2/W18O49 heterojunction sensor revealed a response (Ig/Ia) of 11.0 to 6 ppm Cl2 gas, while the tres and trec were long, namely, 4.6 min and 17 min, respectively. Abnormally, the resistance decreased on exposure to Cl2 gas which is an oxidizing gas. Based on the comparison of the sensing behavior of the networked wire and single wire gas sensors, it was reported that the grain boundaries had a normal response to Cl2 gas, whereas the intragrain contribution was anomalous. Also, in the single wire gas sensor, the sensing reactions mainly occurred with lattice oxygen and oxygen adsorbed on its surface, resulting in an anomalous response, and additional sites for adsorption were not available due to the low defect density of the single wire gas sensor. The networked sensor had a large number of defect sites between the grains, on which additional Cl2 was adsorbed, leading to a normal response to Cl2.107
W18O49 hollow spheres were hydrothermally prepared at 180 °C for 16 h. Later, different amounts of FeCl3 (0.01, 0.02, 0.04, and 0.06 g) were added to the W18O49 suspension and the mixed suspension was heated at 120 °C/1 h under hydrothermal conditions. After annealing at 400 °C for 2 h, the final W18O49-α-Fe2O3 composites with 3.99, 6.3, 13.98, and 18.98 wt% α-Fe2O3, were prepared.108 The nanocomposites had a spherical hollow structure with an average diameter of ∼450 nm. The hollow shells were thicker and solid after the addition of α-Fe2O3 to W18O49. However, the composite with the highest amount of α-Fe2O3 lost the hollow spherical morphology. The surface area of the W18O49 hollow spheres was 78 m2 g−1, while that for the composite with 6.3 wt% α-Fe2O3 slightly decreased to 67 m2 g−1 due to the coverage of the pores in the W18O49 hollow spheres. However, both samples showed a mesoporous nature, in which the target gas could penetrate the in-depth parts of the sensor easily (Fig. 21(a)–(c)). The sensor fabricated using the composite with 6.3 wt% α-Fe2O3 exhibited a response (Ra/Rg) of 5.61 to 100 ppm acetone at 260 °C, while the pristine sensor revealed a much lower response. Also, its tres and trec were 10 and ∼30 s, respectively. The selectivity to acetone was related to the oxygen-deficient nature of W18O49, where the W atoms in W18O49 had unsaturated coordination to oxygen, manifesting dangling bonds and bringing about the highly polarized state of W18O49. Given that acetone has a larger dipole moment (2.88 D) than the interfering gases, it was easily adsorbed on the surface of W18O49. In air, the thickness of the EDL further increased because of the adsorption of chemisorbed oxygen species (Fig. 21(b)) and subsequent exposure to acetone decreased the thickness of the EDL (Fig. 21(c)). The creation of heterojunctions between different materials is one of the most powerful sources of resistance modulation in resistive gas sensors. Due to the difference in the work function of α-Fe2O3 (5.88 eV)109 and W18O49 (4.6 eV) (Fig. 21(d)), electrons flowed from W18O49 to α-Fe2O3 to match the Fermi levels at both sides of the interfaces (Fig. 21(e)).
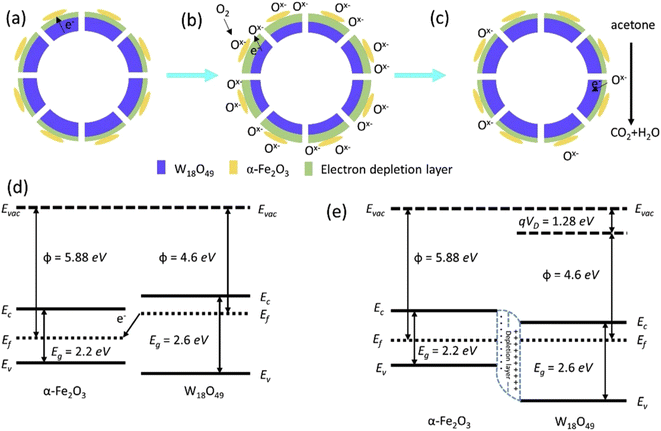 | ||
| Fig. 21 Schematic of the W18O49/α-Fe2O3 hollow sphere (a) in vacuum, (b) in air and (c) in acetone. (d and e) Energy band diagram of the W18O49/α-Fe2O3 heterojunction before and after contact.108 Reproduced with permission from ref. 108. Copyright 2019, Elsevier. | ||
The effect of plasma treatment on the gas sensing features of W18O49 was investigated. Initially, W18O49 NWs were hydrothermally synthesized at 180 °C for 5 h. Then, some of the powder was treated with H2 plasma at a fixed flow rate for 30 and 50 min, and the remaining powder was subjected to the same treatment conditions with Ar plasma. The 1D W18O49 along the (010) direction had accelerated anisotropic growth behavior, which provided surface defects as active sites for gas adsorption. Then, PANI with different thicknesses adjusted by amount of aniline monomer was applied on W18O49 via a chemical polymerization process. Oxygen vacancies were created at the interface of W18O49-PANI during plasma treatment, which acted as electron acceptors and promoted PANI to generate more protons to react with the target NH3 (Fig. 22(a)).110 Based on the EPR analysis, the concentration of oxygen vacancies in the Ar plasma-treated sample was lower than that in the H-plasma treated sample owing to their different atomic mass. Hence, Ar plasma could seriously destroy the surface lattice, resulting in the creation of various lattice defects, while the H2 plasma mostly generated oxygen vacancies. The response (Rg/Ra) of the W18O49-PANI sensor after 50 min exposure to H2 plasma was 35 to 100 ppm NH3 at 25 °C, which was the highest response among the sensors. In fact, at a fixed plasma treatment time, the hydrogen plasma-treated sensor exhibited a better performance than the Ar-treated sensor due to the higher concentration of oxygen vacancies generated by hydrogen plasma treatment, consistent with another study.111 Also with an increase in the plasma treatment time, the sensor showed a higher response due to the generation of more defects with longer times. At the same time, the H2 plasma-treated sample had a better performance than the Ar plasma-treated sample, which may be due to the greater concentration of oxygen vacancies generated by the H2 plasma treatment, in agreement with previous studies Also, the trec of the hydrogen plasma-treated sensor was shorter than that of the Ar plasma-treated sensor, which was again related to the presence of more oxygen defects in this sensor, accelerating the flow of electrons during the recovery period. Upon the interaction between PANI and NH3 molecules, PANI lost an H atom, thus gaining an electron left by the H atom (Fig. 22(b)), causing the low response by the sensor. The free electrons were adsorbed by oxygen vacancies, permitting the occurrence of gas sensing reactions at a deeper level. Notably, due to the higher potential difference between the introduced oxygen vacancy energy level and the CB of PANI, the electron transfer in path 2 was more efficient than path 1 (Fig. 22(c)). Obviously, the presence of more oxygen vacancies in the hydrogen plasma-treated sensor led to better efficiency and a higher gas response.
 | ||
| Fig. 22 (a) Schematic of W18O49-PANI C–S, (b) interaction of NH3 molecules with PANI and (c) heterojunction band structure and electron flow paths.110 Reproduced with permission from ref. 110. Copyright 2020, Elsevier. | ||
7. Other sub-stoichiometric WO3−x gas sensors
In comparison with W18O49, other sub-stoichiometric tungsten oxide nanostructures have been less studied for sensing purposes. Sub-stoichiometric WO2.9 nanostructures have also been used for the detection of gases both experimentally and theoretically.112,113 For example, Zhang et al.114 prepared WO2.9 flowers via the acid etching of W/Cu alloy powder. The sensor with the W/Cu ratio of 1/9 displayed an enhanced sensing performance to trimethylamine at 220 °C. The improved sensing output was related to the presence of high amounts of oxygen vacancies, a high surface area owing to its hierarchical flower structure and presence of energetic reaction sites on its surface. In another study,115 button-shaped porous CeO2/WO2.9 composites with different amounts of Ce/W were synthesized via a hydrothermal reaction at 170 °C for 12 h. The optimal sensor displayed the highest response (Ra/Rg) of 23.68 to 100 ppm n-butanol at RT, which was related to the formation of double oxygen defects on the surface of the CeO2/WO2.9 heterostructure, the large surface area, and unique surface/interface conduction modes of the sensor. However, there are no reports on the fabrication of high-performance gas sensors based on WO2.8 (W5O14). Overall, among the sub-stoichiometric WO3−x, W18O49 is more favorable for sensing studies owing to its simple synthesis together with excellent semiconducting features.Table 1 summarizes the gas sensing properties of the gas sensing performance of W18O49-based gas sensors. It can be seen that various toxic gases and VOCs can be detected by W18O49-based gas sensors. Also, their sensing temperatures are in the range of RT to 340 °C.
| Sensing material | Synthesis method | Gas and | Conc. (ppm) | T (°C) | Response (Ra/Rg) or (Rg/Ra) | Ref. |
|---|---|---|---|---|---|---|
| Pristine sensors | ||||||
| W18O49 nanoneedles | Solvothermal | NO2 | 10 | 160 | 17 | 48 |
| W18O49 NWs | Solvothermal | NH3 | 45 | RT | 1.13 (Ia/Ig) | 50 |
| Single crystalline W18O49 NRs | Colloidal synthesis method | Ethanol | 130 | RT | 3.5% [ΔR/Ra] × 100 | 52 |
| W18O49 NWs | Chemical synthesis | Acetone | 50 | 280 | 48.6 | 53 |
| W18O49 mesocrystals | Solvothermal | NO2 | 1 | 90 | 24.5 | 55 |
| W18O49 NRs | Solvothermal | NO2 | 5 | 150 | 131.5 | 57 |
| W18O49 NWs | In situ oxidation of sputtered W layer | NO2 | 1 | 150 | 4.4 | 59 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Noble metal-decorated sensors | ||||||
| Pd-decorated flower-like W18O49 | Ultrasonic-solvothermal method | HCHO | 15 | 180 | 25.1 | 61 |
| Pd-decorated urchin-like W18O49 | Hydrothermal and impregnation | H2 | 1000 | 100 | 1600 | 63 |
| Pt-decorated networked W18O49 NWs | Thermal evaporation and sputtering | H2 | 1000 | ∼48 | 1.6 | 65 |
Pt-decorated urchin-like W18O49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) microstructures microstructures |
Hydrothermal and chemical reduction | Ethanol | 10 | 23 | 300 | 66 |
| Pd@Au core–shell bimetallic NPs-decorated W18O49 NWs | Solvothermal and chemical reduction | H2S | 50 | 100 | 55.5 | 68 |
| Au-decorated Co3O4/W18O49 hollow spheres | Hydrothermal and chemical reduction | TEA | 2 | 270 | 16.7 | 69 |
| Ag/AgCl-decorated W18O49 NRs | Solvothermal and UV reduction | H2S | 100 | 300 | 115.5 | 73 |
| Au39Rh61-decorated urchin-like W18O49 | Hydrothermal and co-precipitation | n-Butanol | 50 | 260 | 11 | 75 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Doped sensors | ||||||
| Pd-doped W18O49 NWs | Hydrothermal | Acetone | 50 | 175 | 146 | 81 |
| Ni-doped rambutan-like W18O49 | Solvothermal | n-Butanol | 50 | 160 | 182 | 83 |
| Co and Ni-doped porous W18O49 nano-urchins | Hydrothermal | TEA | 50 | 250 | 114 | 86 |
| 0.6 at% Ti-doped W18O49 NWs | Hydrothermal | NO2 | 4 | 25 | 23 | 88 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Composite sensors | ||||||
| W18O49/graphene nanocomposites | Hydrothermal | Ethanol | 100 | 340 | 16.47 | 90 |
| WS2/W18O49 heterojunction | Pyrolysis of WO3 in sulfur atmosphere | NH3 | 5 | RT | 25.7% [ΔR/Ra]×100 | 94 |
| W18O49/Ti3C2Tx MXene nanocomposite | Solvothermal | Acetone | 20 | 300 | 11.6 | 96 |
| PPy@W18O49 C–S NRs | Solvothermal and in situ polymerization | NH3 | 20 | 15 | 4.1 | 100 |
| W18O49–TiO2 C–S NWs | Thermal oxidation and sputtering | NO2 | 5 | RT | 36.5 | 101 |
| WO3–W18O49 | Hydrothermal | NH3 | 500 | 250 | 23.3 | 105 |
| WO3 NS-W18O49 NW composite | Hydrothermal | NO2 | 1 | 160 | 1687 | 106 |
| SnO2/W18O49 heterojunction | VLS growth | Cl2 | 6 | RT | 11 (Ig/Ia) | 107 |
| W18O49-α-Fe2O3 composite | Hydrothermal | Acetone | 100 | 260 | 5.61 | 108 |
| W18O49-PANI | Hydrothermal and in situ polymerization | NH3 | 100 | RT | 35 | 109 |
| CeO2/WO2.9 composite | Hydrothermal | n-Butanol | 100 | RT | 23.68 | 115 |
8. Conclusion and outlooks
Herein, the gas sensing characteristics of W18O49-based sensors were explained. There are various strategies for the synthesis of W18O49, among which the most common are hydrothermal synthesis and VLS growth. Hydrothermal synthesis has advantages such as the possibility of the synthesis of various morphologies, simple preparation procedures, and good control of the process variables such as temperature and time. However, generally it needs high synthesis pressures and long synthesis times. Alternatively, VLS growth is a simple synthesis method for the growth of 1D nanostructures such as NWs with good control of their diameters and length by control of the synthesis temperature and time. However, it often needs high temperatures for the growth of W18O49.Pristine W18O49 gas sensors with various morphologies such as NWs have been successfully used for the detection of various toxic gases. However, their performance can be improved using different techniques such as doping, decoration with noble metals and composite formation. In all cases, optimization of the amount of added materials is required to achieve the highest sensing properties. Doping is often used with the addition of a small amount of metal cations into the W18O49 lattice. This results in the formation of structural defects and expansion or contraction of the lattice, which lead to changes in the surface and adsorption properties of doped W18O49. Decoration with noble metals such Pd, Pt and Au is another popular strategy to enhance the gas sensing features of W18O49 due to the catalytic and electronic effects of noble metals. Also, due to the catalytic effect of noble metals to some gases, the selectivity of W18O49 for a particular gas can be significantly increased. However, more studies are necessary to further explore the performance of W18O49 when it is decorated with noble metals. For example, no study related to Ag decoration on W18O49 has been reported to date. Another approach is bimetal decoration such as AuPt, AuPd, and PdPt on W18O49 to further increase its selectivity and response, owing to the synergistic effects of noble metals.
Composite formation is one the most reliable strategies to boost the sensing features of W18O49 because of the generation of a huge amount of heterojunctions at the interface areas of two different components. Composites of W18O49 with metal oxides, MXenes, TMDs and CPs have been reported for enhancing its gas sensing performance. Although MXenes, TMDs and CPs have lower sensing properties relative to metal oxides, they can work at lower temperatures, and therefore the sensing temperature of the fabricated sensors can be significantly decreased. In future works, composites of W18O49 with g-C3N4 and a combination of three sensing materials such as W18O49/TMD/MXene should be explored for gas sensing purposes.
Sensitivity is defined as the slope of the “response versus concentration” plot.116 Obviously, a higher response to a certain concentration of gas will lead to higher sensitivity. W18O49 gas sensors have relatively good response and sensitivity. However, compared with SnO2 and ZnO gas sensors, their performances are weaker. Also, a common shortage of resistance gas sensors is their poor selectivity. In this regards, W18O49 gas sensors also have poor selectivity to gases. However, their selectivity can be improved by noble metal decoration and formation of composites. The limit of detection is the minimum concentration of gas that can produce a sufficiently different resistance from the base resistance of a gas sensor.117 Obviously, a lower LOD is better for practical application. Unfortunately, most papers related to W18O49 gas sensors did not report the LOD of the fabricated sensors. In some cases, they can have an LOD in the range of ppb.
Often W18O49 gas sensors work at high temperatures, resulting in significant power consumption in the range of mW or W. This limits their applications in remote area or areas with energy shortage. Accordingly, in future studies, the power consumption of W18O49 sensors can be significantly decreased by (i) illumination of W18O49 with UV light during exposure to gas, (ii) employing of a gas sensor in self-heating mode by directly applying an external voltage to the sensor electrodes and generation of heat within the sensor and (iii) fabrication of single NW W18O49 gas sensor, which needs only negligible power for working at the sensing temperature.
Also, to increase the sensitivity of W18O49 sensors, irradiation with high energy beams including an electron beam or gamma rays can be applied to W18O49, resulting in the generation of defects. The created defects act as favorable sites for gas adsorption, resulting in an enhanced sensing response. Furthermore, the implantation of W18O49 by high energy ions not only generates structural defects in W18O49 but also simultaneously dope them in the W18O49 lattice. Another approach is to increase the SSA of W18O49 using the nanotube morphology or synthesis of highly porous morphologies derived from metal organic frameworks (MOFs). In particular, MOF-derived W18O49 has a highly porous structure, which allows the easy diffusion of gas molecules inside the sensing layer, resulting in a high response to gas.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.Author contributions
M. Hjiri: formal analysis, writing and editing; I. Najeh: data curation; Fatemah M. Barakat: methodology; G. Neri: reviewing; supervision.Ethical statement
All authors declare that the presented work was original research that has not been published previously, and not under consideration for publication elsewhere, in whole or in part.Conflicts of interest
Authors have a responsibility to disclose interests that might appear to affect their ability to present data objectively. Readers will benefit from transparency, including knowing authors' and contributors' affiliations and interests. Sources of funding for research were disclosed.Acknowledgements
This work was supported and funded by the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University (IMSIU) (grant number IMSIU-DDRSP2502).References
- A. Mirzaei, J.-H. Lee, S. M. Majhi, M. Weber, M. Bechelany, H. W. Kim and S. S. Kim, Resistive gas sensors based on metal-oxide nanowires, J. Appl. Phys., 2019, 126, 241102 CrossRef.
- O. Bronte, F. García-García, D.-J. Lee, I. Urrutia, A. Uranga and M. Nieves, et al., Impact of outdoor air pollution on severity and mortality in COVID-19 pneumonia, Sci. Total Environ., 2023, 894, 164877 CrossRef CAS PubMed.
- E. Merkus, Hold your breath! Air pollution and cognitive performance in Colombia, World Dev. Sustainability, 2024, 4, 100123 CrossRef.
- T. Wyttenbach and M. T. Bowers, Gas-phase conformations: the ion mobility/ion chromatography method, Modern Mass Spectrom., 2003, 225, 207–232 CAS.
- K. D. Bartle and P. Myers, History of gas chromatography, TrAC, Trends Anal. Chem., 2002, 21, 547–557 CrossRef CAS.
- Z. Wang, L. Zhu, S. Sun, J. Wang and W. Yan, One-dimensional nanomaterials in resistive gas sensor: from material design to application, Chemosensors, 2021, 9, 198 CrossRef CAS.
- A. Mirzaei and G. Neri, Microwave-assisted synthesis of metal oxide nanostructures for gas sensing application: a review, Sens. Actuators, B, 2016, 237, 749–775 CrossRef CAS.
- J. Pu, Y. Gao, Q. Cao, G. Fu, X. Chen, Z. Pan and C. Guan, Vanadium metal-organic framework-derived multifunctional fibers for asymmetric supercapacitor, piezoresistive sensor, and electrochemical water splitting, SmartMat, 2022, 3, 608–618 CrossRef CAS.
- B. Guruprasad and M. Shwetha, Design and fabrication of cantilever MEMS sensor model for electro-chemical gas sensor, Int. J. Eng. Res. Technol., 2020, 9, 704–715 Search PubMed.
- S. Okazaki, H. Kawada, Y. Koshiba, N. Kasai, Y. Maru and T. Mizutani, et al., Catalytic combustion type optical fiber Bragg grating hydrogen gas sensor using platinum-loaded fumed silica powder, Int. J. Hydrogen Energy, 2023, 48, 9512–9527 CrossRef CAS.
- E. L. Gardner, J. W. Gardner and F. Udrea, Micromachined thermal gas sensors—A review, Sensors, 2023, 23, 681 CrossRef CAS PubMed.
- Y. Li, L. Yu, C. Zheng, Z. Ma, S. Yang and F. Song, et al., Development and field deployment of a mid-infrared CO and CO2 dual-gas sensor system for early fire detection and location, Spectrochim. Acta, Part A, 2022, 270, 120834 CrossRef CAS PubMed.
- M. Bonyani, S. M. Zebarjad, K. Janghorban, J.-Y. Kim, H. W. Kim and S. S. Kim, Enhanced NO2 gas sensing properties of ZnO-PANI composite nanofibers, Ceram. Int., 2023, 49, 1238–1249 CrossRef CAS.
- N. Kaur, M. Singh and E. Comini, One-dimensional nanostructured oxide chemoresistive sensors, Langmuir, 2020, 36, 6326–6344 CrossRef CAS PubMed.
- M. Bonyani, S. M. Zebarjad, K. Janghorban, J.-Y. Kim, H. W. Kim and S. S. Kim, Au-decorated polyaniline-ZnO electrospun composite nanofiber gas sensors with enhanced response to NO2 gas, Chemosensors, 2022, 10, 388 CrossRef CAS.
- M. Bonyani, S. M. Zebarjad, K. Janghorban, J.-Y. Kim, H. W. Kim and S. S. Kim, Au sputter-deposited ZnO nanofibers with enhanced NO2 gas response, Sens. Actuators, B, 2022, 372, 132636 CrossRef CAS.
- A. Mirzaei, Z. Kordrostami, M. Shahbaz, J.-Y. Kim, H. W. Kim and S. S. Kim, Resistive-based gas sensors using quantum dots: a review, Sensors, 2022, 22, 4369 CrossRef CAS PubMed.
- H.-J. Kim and J.-H. Lee, Highly sensitive and selective gas sensors using p-type oxide semiconductors: overview, Sens. Actuators, B, 2014, 192, 607–627 CrossRef CAS.
- N. Yamazoe, G. Sakai and K. Shimanoe, Oxide semiconductor gas sensors, Catal. Surv. Asia, 2003, 7, 63–75 CrossRef CAS.
- J. Walker, P. Karnati, S. A. Akbar and P. A. Morris, Selectivity mechanisms in resistive-type metal oxide heterostructural gas sensors, Sens. Actuators, B, 2022, 355, 131242 CrossRef CAS.
- J. H. Lee, J. Y. Kim, A. Mirzaei, H. W. Kim and S. S. Kim, Significant enhancement of hydrogen-sensing properties of ZnO nanofibers through NiO loading, Nanomaterials, 2018, 8, 902 CrossRef PubMed.
- Y. Jian, W. Hu, Z. Zhao, P. Cheng, H. Haick, M. Yao and W. Wu, Gas sensors based on chemi-resistive hybrid functional nanomaterials, Nano-Micro Lett., 2020, 12, 1–43 CrossRef PubMed.
- M. D. El-Muraikhi, A. I. Ayesh and A. Mirzaei, Resistive gas sensors based on inorganic nanotubes: a review, J. Alloys Compd., 2025, 1020, 179585 CrossRef CAS.
- M. Zhang, C. Yang, Z. Zhang, W. Tian, B. Hui, J. Zhang and K. Zhang, Tungsten oxide polymorphs and their multifunctional applications, Adv. Colloid Interface Sci., 2022, 300, 102596 CrossRef CAS PubMed.
- Y. Qiu, Y. Wang and C. Song, Facile synthesis of W18O49/graphene nanocomposites for highly sensitive ethanol gas sensors, Colloids Surf., A, 2021, 616, 126300 CrossRef CAS.
- M. Saqib, J. Jelenc, L. Pirker, S. D. Škapin, L. D. Pietro and U. Ramsperger, et al., Field emission properties of single crystalline W5O14 and W18O49 nanowires, J. Electron Spectrosc. Relat. Phenom., 2020, 241, 146837 CrossRef CAS.
- F. R. Sale, Heat capacities of the tungsten oxides WO3, W20O58, W18O49 and WO2, Thermochim. Acta, 1979, 30, 163–171 CrossRef CAS.
- P. Bhavani, K.-J. J. D. Praveen Kumar, M. Hussain and Y.-K. Park, Recent advances in wide solar spectrum active W18O49-based photocatalysts for energy and environmental applications, Catal. Rev., 2023, 65, 1521–1566 CrossRef CAS.
- S. J. Park, S. M. Lee, J. Lee, S. Choi, G. B. Nam, Y. K. Jo, I. S. Hwang and H. W. Jang, Pd-W18O49 nanowire MEMS gas sensor for ultraselective dual detection of hydrogen and ammonia, Small, 2025, 21, 2405809 CrossRef CAS PubMed.
- Z. Huang, J. Song, L. Pan, X. Zhang, L. Wang and J. Zou, Tungsten oxides for photocatalysis, electrochemistry, and phototherapy, Adv. Mater., 2015, 27, 5309–5327 CrossRef CAS PubMed.
- H. Gu, C. Guo, S. Zhang, L. Bi, T. Li and T. Sun, et al., Highly efficient, near-infrared and visible light modulated electrochromic devices based on polyoxometalates and W18O49 nanowires, ACS Nano, 2018, 12, 559–567 CrossRef CAS PubMed.
- S. Sui, Y. Liao, Y. Xie, X. Wang, L. Li and Z. Luo, et al., High catalytic activity of W18O49 nanowire-reduced graphite oxide composite counter electrode for dye-sensitized solar cells, ChemistrySelect, 2017, 2, 8927–8935 CrossRef CAS.
- Z. Zhang, J. Liang, K. Liu, W. Tian, X. Liang, K. Zhao and K. Zhang, Defect-engineered WO3−x architectures coupled with random forest algorithm enables real-time seafood quality assessment, ACS Sens., 2024, 9, 4196–4206 CrossRef CAS PubMed.
- W. Ding, X. Wang, C. Yang, P. Wang, W. Tian, K. Zhao and K. Zhang, Interfacial photo-reduction of graphene oxide on defective WO3−x for multifunctional applications in sensor, catalyst and supercapacitor, Appl. Surf. Sci., 2022, 606, 154877 CrossRef CAS.
- W. Ding, M. Feng, Z. Zhang, F. Fan, L. Chen and K. Zhang, Machine learning-motivated trace triethylamine identification by bismuth vanadate/tungsten oxide heterostructures, J. Colloid Interface Sci., 2025, 682, 1140–1150 CrossRef CAS PubMed.
- Z. Zou, Z. Zhao, Z. Zhang, W. Tian, C. Yang, X. Jin and K. Zhang, Room-temperature optoelectronic gas sensor based on core–shell g-C3N4@WO3 heterocomposites for efficient ammonia detection, Anal. Chem., 2023, 95, 2110–2118 CrossRef CAS PubMed.
- M. Zhang, K. Liu, X. Zhang, B. Wang, X. Xu, X. Du, C. Yang and K. Zhang, Interfacial energy barrier tuning of hierarchical Bi2O3/WO3 heterojunctions for advanced triethylamine sensor, J. Adv. Ceram., 2022, 11, 1860–1872 CrossRef CAS.
- M. Zhang, Z. Zhao, B. Hui, J. Sun, J. Sun, W. Tian, Z. Zhang, K. Zhang and Y. Xia, Carbonized polymer dots activated hierarchical tungsten oxide for efficient and stable triethylamine sensor, J. Hazard. Mater., 2021, 416, 126161 CrossRef CAS PubMed.
- N.-F. Yan, H.-M. Cui, J.-S. Shi, S.-Y. You and S. Liu, Recent progress of W18O49 nanowires for energy conversion and storage, Tungsten, 2023, 5, 371–390 CrossRef.
- P. Zhao, S. Ren, Y. Liu, W. Huang, C. Zhang and J. He, PL–W18O49–TPZ nanoparticles for simultaneous hypoxia-activated chemotherapy and photothermal therapy, ACS Appl. Mater. Interfaces, 2018, 10, 3405–3413 CrossRef CAS PubMed.
- J. Jung and D. H. Kim, W18O49 nanowires assembled on carbon felt for application to supercapacitors, Appl. Surf. Sci., 2018, 433, 750–755 CrossRef CAS.
- P. Zheng, Y. Ami’erjiang, B. Liu, M. Wang, H. Ding and B. Ding, et al., Oxygen-vacancy-engineered W18O49−x nanobrush with a suitable band structure for highly efficient sonodynamic therapy, Angew. Chem., Int. Ed., 2024, e202317218 CAS.
- S. Li, J. Liu, W. Su, Y. Wang, J. Li, C. Ning, J. Ren, X. Wen, W. Zhang, Y. Tong and C. Wang, Tuning excited-state electronic structure in tungsten oxide for enhanced nitrogen photooxidation as fertilizer, Appl. Catal., B, 2024, 343, 123539 CrossRef CAS.
- Z. Zhou, R. Tang, L. Li, S. Xiong, H. Zeng and J. Zheng, et al., W18O49-based photocatalyst: enhanced strategies for photocatalysis employment, Sep. Purif. Technol., 2023, 12, 4028 Search PubMed.
- A. Sharma, S. B. Eadi, H. Noothalapati, M. Otyepka, H. D. Lee and K. Jayaramulu, Porous materials as effective chemiresistive gas sensors, Chem. Soc. Rev., 2024, 53, 2530–2577 RSC.
- L. Y. Zhu, L. X. Ou, L. W. Mao, X. Y. Wu, Y. P. Liu and H. L. Lu, Advances in noble metal-decorated metal oxide nanomaterials for chemiresistive gas sensors: overview, Nano-Micro Lett., 2023, 15, 89 CrossRef CAS PubMed.
- R. Diehl, G. Brandt and E. Salje, The crystal structure of triclinic WO3, Acta Crystallogr., Sect. B, 1978, 34, 1105–1111 CrossRef.
- Y. Qiu and Y. Wang, Controllable synthesis of W18O49 nanoneedles for high-performance NO2 gas sensors, J. Alloys Compd., 2023, 944, 169199 CrossRef CAS.
- D. Kwak, Y. Lei and R. Maric, Ammonia gas sensors: a comprehensive review, Talanta, 2019, 204, 713–730 CrossRef CAS PubMed.
- Y. Zhao and Y. Zhu, Room temperature ammonia sensing properties of W18O49 nanowires, Sens. Actuators, B, 2009, 137, 27–31 CrossRef.
- A. Mirzaei, S. G. Leonardi and G. Neri, Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: a review, Ceram. Int., 2016, 42, 15119–15141 CrossRef CAS.
- Y. S. Kim and K. Lee, Material and gas-sensing properties of tungsten oxide nanorod thin-films, J. Nanosci. Nanotechnol., 2009, 9, 2463–2468 CrossRef CAS PubMed.
- W. Zhang, Y. Fan, T. Yuan, B. Lu, Y. Liu and Z. Li, et al., Ultrafine tungsten oxide nanowires: synthesis and highly selective acetone sensing and mechanism analysis, ACS Appl. Mater. Interfaces, 2020, 12, 3755–3763 CrossRef CAS PubMed.
- L. Bahrig, S. G. Hickey and A. Eychmüller, Mesocrystalline materials and the involvement of oriented attachment – a review, CrystEngComm, 2014, 16, 9408–9424 RSC.
- D. Wang, J. Sun, X. Cao, Y. Zhu, Q. Wang and G. Wang, et al., High-performance gas sensing achieved by mesoporous tungsten oxide mesocrystals with increased oxygen vacancies, J. Mater. Chem. A, 2013, 1, 8653–8657 RSC.
- Y. Cui and C. M. Lieber, Functional nanoscale electronic devices assembled using silicon nanowire building blocks, Science, 2001, 291, 851–853 CrossRef CAS PubMed.
- Y. Qin, X. Li, F. Wang and M. Hu, Solvothermally synthesized tungsten oxide nanowires/nanorods for NO2 gas sensor applications, J. Alloys Compd., 2011, 509, 8401–8406 CrossRef CAS.
- G. Gu, B. Zheng, W. Q. Han, S. Roth and J. Liu, Tungsten oxide nanowires on tungsten substrates, Nano Lett., 2002, 2, 849–851 CrossRef CAS.
- Y. Qin, W. Xie, Y. Liu and Z. Ye, Thermal-oxidative growth of aligned W18O49 nanowire arrays for high performance gas sensor, Sens. Actuators, B, 2016, 223, 487–495 CrossRef CAS.
- S. Navale, M. Shahbaz, A. Mirzaei, S. S. Kim and H. W. Kim, Effect of Ag addition on the gas-sensing properties of nanostructured resistive-based gas sensors: an overview, Sensors, 2021, 21, 6454 CrossRef CAS PubMed.
- P. Wang, S. Guo, Z. Hu, T. Li, S. Pu and H. Mao, et al., W18O49 sensitized with Pd nanoparticles for ultrasensitive ppb-level formaldehyde detection, Chem. Eng. J., 2023, 456, 140988 CrossRef CAS.
- J.-H. Kim, A. Mirzaei, H. W. Kim, P. Wu and S. S. Kim, Design of supersensitive and selective ZnO-nanofiber-based sensors for H2 gas sensing by electron-beam irradiation, Sens. Actuators, B, 2019, 293, 210–223 CrossRef CAS.
- R. Zhou, X. Lin, D. Xue, F. Zong, J. Zhang and X. Duan, et al., Enhanced H2 gas sensing properties by Pd-loaded urchin-like W18O49 hierarchical nanostructures, Sens. Actuators, B, 2018, 260, 900–907 CrossRef CAS.
- A. Kolmakov, D. Klenov, Y. Lilach, S. Stemmer and M. Moskovits, Enhanced gas sensing by individual SnO2 nanowires and nanobelts functionalized with Pd catalyst particles, Nano Lett., 2005, 5, 667–673 CrossRef CAS PubMed.
- L. F. Zhu, J. C. She, J. Y. Luo, S. Z. Deng, J. Chen and X. W. Ji, et al., Self-heated hydrogen gas sensors based on Pt-coated W18O49 nanowire networks with high sensitivity, good selectivity and low power consumption, Sens. Actuators, B, 2011, 153, 354–360 CrossRef CAS.
- Y. Ou, G. Zhu, W. Zhang, S. Zhang, J. Gao and H. Lu, et al., Achieving rapid response and high sensitivity in ethanol gas sensing using a Pt/W18O49 ohmic contact via modulating the adsorption and activation properties: Theoretical and experimental insights, Sens. Actuators, B, 2021, 347, 130601 CrossRef CAS.
- X. Xiao, X. Zhou, J. Ma, Y. Zhu, X. Cheng and W. Luo, et al., Rational synthesis and gas sensing performance of ordered mesoporous semiconducting WO3/NiO composites, ACS Appl. Mater. Interfaces, 2019, 11, 26268–26276 CrossRef CAS PubMed.
- W. Zhang, T. Yuan, X. Wang, Z. Cheng and J. Xu, Coal mine gases sensors with dual selectivity at variable temperatures based on a W18O49 ultra-fine nanowires/Pd@ Au bimetallic nanoparticles composite, Sens. Actuators, B, 2022, 354, 131004 CrossRef CAS.
- L. Yu, X. Y. Yu and X. W. Lou, The design and synthesis of hollow micro-/nanostructures: present and future trends, Adv. Mater., 2018, 30, 1800939 CrossRef PubMed.
- Y. Xu, T. Ma, L. Zheng, L. Sun, X. Liu and Y. Zhao, et al., Rational design of Au/Co3O4-functionalized W18O49 hollow heterostructures with high sensitivity and ultralow limit for triethylamine detection, Sens. Actuators, B, 2019, 284, 202–212 CrossRef CAS.
- X. Wang, J. I. Feng, Y. Bai, Q. Zhang and Y. Yin, Synthesis, properties, and applications of hollow micro-/nanostructures, Chem. Rev., 2016, 116, 10983–11060 CrossRef CAS PubMed.
- Y. Wang, F. Liu, H. Yuan and T. Hu, Interface engineering of Co3O4—SmMn2O5 nanosheets for efficient oxygen reduction electrocatalysis, Front. Mater. Sci., 2021, 15, 567–576 CrossRef.
- S. Sun, X. Chang, L. Dong, Y. Zhang, Z. Li and Y. Qiu, W18O49 nanorods decorated with Ag/AgCl nanoparticles as highly-sensitive gas-sensing material and visible-light-driven photocatalyst, J. Solid State Chem., 2011, 184, 2190–2195 CrossRef CAS.
- N. Yamazoe, New approaches for improving semiconductor gas sensors, Sens. Actuators, B, 1991, 5, 7–19 CrossRef CAS.
- J. Bai, Y. Li, Y. Liu, H. Wang, F. Liu and F. Liu, et al., Au39Rh61 alloy nanocrystal-decorated W18O49 for enhanced detection of n-butanol, ACS Sens., 2019, 4, 2662–2670 CrossRef CAS PubMed.
- R. Xing, Q. Li, L. Xia, J. Song, L. Xu and J. Zhang, et al., Au-modified three-dimensional In2O3 inverse opals: synthesis and improved performance for acetone sensing toward diagnosis of diabetes, Nanoscale, 2015, 7, 13051–13060 RSC.
- V. Amiri, H. Roshan, A. Mirzaei, G. Neri and A. I. Ayesh, Nanostructured metal oxide-based acetone gas sensors: a review, Sensors, 2020, 20, 3096 CrossRef CAS PubMed.
- C. Wang, Y. Li, F. Gong, Y. Zhang, S. Fang and H. Zhang, Advances in doped ZnO nanostructures for gas sensor, Chem. Rec., 2020, 20, 1553–1567 CrossRef CAS PubMed.
- N. Zhang, A. Jalil, D. Wu, S. Chen, Y. Liu and C. Gao, et al., Refining defect states in W18O49 by Mo doping: a strategy for tuning N2 activation towards solar-driven nitrogen fixation, J. Am. Chem. Soc., 2018, 140, 9434–9443 CrossRef CAS PubMed.
- X. Zhong, Y. Sun, X. Chen, G. Zhuang, X. Li and J. Wang, Mo doping induced more active sites in urchin-like W18O49 nanostructure with remarkably enhanced performance for hydrogen evolution reaction, Adv. Funct. Mater., 2016, 26, 5778–5786 CrossRef CAS.
- P. Li, Z. Zhang, Z. Zhuang, J. Guo, Z. Fang and S. L. Fereja, et al., Pd-doping-induced oxygen vacancies in one-dimensional tungsten oxide nanowires for enhanced acetone gas sensing, Anal. Chem., 2021, 93, 7465–7472 CrossRef CAS PubMed.
- Z. Liu, B. Liu, W. Xie, H. Li, R. Zhou and Q. Li, et al., Enhanced selective acetone sensing characteristics based on Co-doped WO3 hierarchical flower-like nanostructures assembled with nanoplates, Sens. Actuators, B, 2016, 235, 614–621 CrossRef CAS.
- R. Wu, S.-Q. Guo, Y.-C. Li, M.-Y. Qi, B. Ge and J.-M. Song, Improving the sensing performance of rambutan-like W18O49 based gas sensor for n-butanol by Ni doping, Sens. Actuators, B, 2024, 410, 135671 CrossRef CAS.
- G. Li, Z. Cheng, Q. Xiang, L. Yan, X. Wang and J. Xu, Bimetal PdAu decorated SnO2 nanosheets based gas sensor with temperature-dependent dual selectivity for detecting formaldehyde and acetone, Sens. Actuators, B, 2019, 283, 590–601 CrossRef CAS.
- S. Zeb, Z. Yang, R. Hu, M. Umair, S. Naz and Y. Cui, et al., Electronic structure and oxygen vacancy tuning of Co & Ni co-doped W18O49 nanourchins for efficient TEA gas sensing, Chem. Eng. J., 2023, 465, 142815 CrossRef CAS.
- M. Shi, X. Tong, W. Li, J. Fang, L. Chen and C. Ma, Enhanced electrocatalytic oxygen reduction on NiWOx solid solution with induced oxygen defects, ACS Appl. Mater. Interfaces, 2017, 9, 34990–35000 CrossRef CAS PubMed.
- H. Xu, R. Hu, Y. Zhang, H. Yan, Q. Zhu and J. Shang, et al., Nano high-entropy alloy with strong affinity driving fast polysulfide conversion towards stable lithium sulfur batteries, Energy Storage Mater., 2021, 43, 212–220 CrossRef.
- Y. Qin, X. Sun, X. Li and M. Hu, Room temperature NO2-sensing properties of Ti-added nonstoichiometric tungsten oxide nanowires, Sens. Actuators, B, 2012, 162, 244–250 CrossRef CAS.
- A. R. Urade, I. Lahiri and K. S. Suresh, Graphene properties, synthesis and applications: a review, JOM, 2023, 75, 614–630 CrossRef CAS PubMed.
- Y. Qiu, Y. Wang and C. Song, Facile synthesis of W18O49/graphene nanocomposites for highly sensitive ethanol gas sensors, Colloids Surf., A, 2021, 616, 126300 CrossRef CAS.
- J.-H. Kim, A. Mirzaei, Y. Zheng, J.-H. Lee, J.-Y. Kim and H. W. Kim, et al., Enhancement of H2S sensing performance of p-CuO nanofibers by loading p-reduced graphene oxide nanosheets, Sens. Actuators, B, 2019, 281, 453–461 CrossRef CAS.
- H. W. Kim, Y. J. Kwon, A. Mirzaei, S. Y. Kang, M. S. Choi and J. H. Bang, et al., Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors, Sens. Actuators, B, 2017, 249, 590–601 CrossRef CAS.
- W. Zheng, X. Liu, J. Xie, G. Lu and J. Zhang, Emerging van der Waals junctions based on TMDs materials for advanced gas sensors, Coord. Chem. Rev., 2021, 447, 214151 CrossRef CAS.
- M. Manoharan, K. Govindharaj, K. Muthumalai, R. Pandian, Y. Haldorai and R. T. Rajendra Kumar, Highly selective room temperature detection of NH3 and NOx using oxygen-deficient W18O49-supported WS2 heterojunctions, ACS Appl. Mater. Interfaces, 2023, 15, 4703–4712 CrossRef CAS PubMed.
- A. Mirzaei, M. H. Lee, H. Safaeian, T.-U. Kim, J.-Y. Kim and H. W. Kim, et al., Room temperature chemiresistive gas sensors based on 2D MXenes, Sensors, 2023, 23, 8829 CrossRef CAS PubMed.
- S. Sun, M. Wang, X. Chang, Y. Jiang, D. Zhang and D. Wang, et al., W18O49/Ti3C2Tx Mxene nanocomposites for highly sensitive acetone gas sensor with low detection limit, Sens. Actuators, B, 2020, 304, 127274 CrossRef CAS.
- W. Li, Y. Liu, X. Lu, Y. Huang, Y. Liu and S. Cheng, et al., A cross-sectional study of breath acetone based on diabetic metabolic disorders, J. Breath Res., 2015, 9, 016005 CrossRef PubMed.
- V. Kumar, A. Mirzaei, M. Bonyani, K.-H. Kim, H. W. Kim and S. S. Kim, Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia, TrAC, Trends Anal. Chem., 2020, 129, 115938 CrossRef CAS.
- Y. Qin, T. Zhang and Z. Cui, Core-shell structure of polypyrrole grown on W18O49 nanorods for high performance gas sensor operating at room temperature, Org. Electron., 2017, 48, 254–261 CrossRef CAS.
- Y. Xiong, W. Xu, D. Ding, W. Lu, L. Zhu and Z. Zhu, et al., Ultra-sensitive NH3 sensor based on flower-shaped SnS2 nanostructures with sub-ppm detection ability, J. Hazard. Mater., 2018, 341, 159–167 CrossRef PubMed.
- Y. Qin, X. Zhang, Y. Wang and Y. Liu, Remarkable improvement of W18O49/TiO2 heteronanowires in ambient temperature-responsive NO2-sensing abilities and its unexpected np transition phenomenon, Sens. Actuators, B, 2017, 240, 477–486 CrossRef CAS.
- C. Zhang, M. Debliquy, A. Boudiba, H. Liao and C. Coddet, Sensing properties of atmospheric plasma-sprayed WO3 coating for sub-ppm NO2 detection, Sens. Actuators, B, 2010, 144, 280–288 CrossRef CAS.
- S. Vallejos, T. Stoycheva, P. Umek, C. Navio, R. Snyders and C. Bittencourt, Au nanoparticle-functionalised WO3 nanoneedles and their application in high sensitivity gas sensor devices, Chem. Commun., 2011, 47, 565–567 RSC.
- Y. Zhuo, L. Huang, Y. Ling, H. Li and J. Wang, A novel NO2 sensor based on TiO2 nanotubes array with in-situ Au decoration, J. Nanosci. Nanotechnol., 2013, 13, 1177–1181 CrossRef CAS PubMed.
- Y. Xiong, Z. Zhu, T. Guo, H. Li and Q. Xue, Synthesis of nanowire bundle-like WO3-W18O49 heterostructures for highly sensitive NH3 sensor application, J. Hazard. Mater., 2018, 353, 290–299 CrossRef CAS PubMed.
- B.-R. Wang, R.-Z. Wang, L.-Y. Liu, C. Wang, Y.-F. Zhang and J.-B. Sun, WO3 nanosheet/W18O49 nanowire composites for NO2 sensing, ACS Appl. Nano Mater., 2020, 3, 5473–5480 CrossRef CAS.
- S. Sen, P. Kanitkar, A. Sharma, K. P. Muthe, A. Rath and S. K. Deshpande, et al., Growth of SnO2/W18O49 nanowire hierarchical heterostructure and their application as chemical sensor, Sens. Actuators, B, 2010, 147, 453–460 CrossRef CAS.
- Y. Xu, T. Ma, L. Zheng, Y. Zhao, X. Liu and J. Zhang, Heterostructures of hematite-sensitized W18O49 hollow spheres for improved acetone detection with ultralow detection limit, Sens. Actuators, B, 2019, 288, 432–441 CrossRef CAS.
- M. Niu, F. Huang, L. Cui, P. Huang, Y. Yu and Y. Wang, Hydrothermal synthesis, structural characteristics, and enhanced photocatalysis of SnO2/α-Fe2O3 semiconductor nanoheterostructures, ACS Nano, 2010, 4, 681–688 CrossRef CAS PubMed.
- Y. Chen, C. Li, X. Ma, Q. Qiang, B. Liu and S. Cao, et al., Interface defect engineering induced drastic sensing performance enhancement of W18O49@ PANI nanowires for ammonia detection at room temperature, Appl. Surf. Sci., 2020, 506, 144816 CrossRef CAS.
- L. Xu, Q. Jiang, Z. Xiao, X. Li, J. Huo and S. Wang, et al., Plasma-engraved Co3O4 nanosheets with oxygen vacancies and high surface area for the oxygen evolution reaction, Angew. Chem., Int. Ed. Eng., 2016, 128, 5363–5367 CrossRef.
- F. Han, F. Li, S. Liu and L. Niu, Sub-stoichiometric WO2.9 as co-catalyst with platinum for formaldehyde gas sensor with high sensitivity, Sens. Actuators, B, 2018, 263, 369–376 CrossRef CAS.
- D. Wang, D. Han, L. Liu and L. Niu, Sub-stoichiometric WO2.9 for formaldehyde sensing and treatment: a first-principles study, J. Mater. Chem. A, 2016, 4, 14416–14422 RSC.
- W. Zhang, P. Xu, Y. Shen, J. Feng, Z. Liu, G. Cai, X. Yang, R. Guan, L. Su and L. Yue, Selective detection of trimethylamine utilizing nanosheets assembled hierarchical WO2.9 nanostructure, J. Environ. Chem. Eng., 2021, 9, 106493 CrossRef CAS.
- Q. Zhang, Q. Ma, X. Wang, Y. Wang and D. Zhao, Surface double oxygen defect engineering in button-shaped porous CeO2/WO2.9 heterostructures for excellent n-butanol detection at room temperature, Appl. Surf. Sci., 2023, 616, 156536 CrossRef CAS.
- Y. Wang, D. Liu, J. Yin, Y. Shang, J. Du, Z. Kang, R. Wang, Y. Chen, D. Sun and J. Jiang, An ultrafast responsive NO2 gas sensor based on a hydrogen-bonded organic framework material, Chem. Commun., 2020, 56, 703–706 RSC.
- K. W. Kao, M. C. Hsu, Y. H. Chang, S. Gwo and J. A. Yeh, A sub-ppm acetone gas sensor for diabetes detection using 10 nm thick ultrathin InN FETs, Sensors, 2012, 12, 7157–7168 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |
