Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Antibacterial, antioxidant, and scolicidal activity investigation of biosynthesized ZnO NPs using Zhumeria majdae essential oil and hydroalcoholic extract
Rana Kiania,
Zahra Rafiee *a,
Damoun Razmjoueb,
Ahmad Oryanc,
Mehrorang Ghaedia and
Hassan Abidid
*a,
Damoun Razmjoueb,
Ahmad Oryanc,
Mehrorang Ghaedia and
Hassan Abidid
aDepartment of Chemistry, Yasouj University, Yasouj, 75918-74831, Islamic Republic of Iran. E-mail: z.rafiee@yu.ac.ir; zahrarafiee2004@yahoo.com; Fax: +98-741-222-3048; Tel: +98-741-222-3048
bMedicinal Plants Research Center, Yasuj University of Medical Sciences, Yasuj, Iran. E-mail: d.razmjoue@gmail.com
cDepartment of Pathology, School of Veterinary Medicine, Shiraz University, Shiraz, Iran
dCellular and Molecular Research Center, Yasuj University of Medical Sciences, Yasuj, Iran
First published on 8th July 2025
Abstract
The biosynthesis of zinc oxide nanoparticles (ZnO NPs) from the Zhumeria majdae plant is reported in this study. Additionally, the essential oil and hydroalcoholic extract of this plant were manufactured and their antibacterial, antioxidant, and scolicidal activities were investigated. The essential oil was analyzed using GC/MS spectroscopy. The UV-vis spectrum of ZnO NPs exhibited an absorption maximum at 368 nm. The XRD and EDS confirmed the hexagonal structure of ZnO NPs and the presence of zinc. FE-SEM indicated well-defined cubic NPs. The FT-IR spectrum of ZnO NPs revealed the presence of functional groups, including flavonoids and polyphenolic biomolecules. The antibacterial activity of biosynthesized ZnO NPs, essential oil, and hydroalcoholic extract against Staphylococcus aureus and Pseudomonas aeruginosa was evaluated. Additionally, the scolicidal activity of various concentrations of these treatment regimens was investigated on protoscoleces of hydatid cysts at different time intervals. Furthermore, the antioxidant activity of these products was assessed using DPPH and FRAP tests. The antimicrobial effects of ZnO NPs, hydroalcoholic extract, and essential oil on Staphylococcus aureus and Pseudomonas aeruginosa were confirmed. The treatments demonstrated significant effects on protoscolexes of hydatid cysts at various time intervals, and their lethal effects were validated. From the findings of this study, it is believed that the essential oil of the Zhumeria majdae plant is more effective on some species of bacteria and also has significant scolicidal properties.
1. Introduction
Nanotechnology is a multidisciplinary science regarding production of materials at the nanoscale (1 to 100 nm) with promising properties such as a high surface area-to-volume, small size, charge, shape, crystal structure, thermal conductivity and surface morphology, which enables them to be used in the fields of biomedicine, and biotechnology.1,2 Nanoparticles (NPs) can be synthesized using chemical, physical, and biological methods. However, chemical and physical methods often produce hazardous byproducts due to the involvement of high temperatures, pressures, and toxic substances. Thus, there has been a growing interest in biological methods, also known as green nanotechnology.3 Green nanotechnology refers to environmentally friendly and cost-effective approaches for synthesizing NPs, which minimize or eliminate the use of hazardous materials.4 The green synthesis of NPs can be done using various biological sources, including bacteria, fungi, algae, and plants. Plant extracts containing compounds such as phenols, terpenoids, polypeptides, and starch serve as biological reducing agents and stabilizers in this process.5Metal oxide NPs have garnered substantial attention due to their appealing characteristics. Among these NPs, zinc oxide (ZnO) NPs are particularly noteworthy because of their eco-friendly, non-toxic nature and ease of production.6–8 Additionally, ZnO NPs are recognized for their antibacterial and antifungal properties9 as well as their ability to eliminate free radicals.10 One of the most common and chronic diseases affecting humans and herbivorous animals is hydatidosis, also known as echinococcosis.11 This disease is caused by the larval stage of the Echinococcus parasite, which is predominantly found in regions where sheep, goats, and cattle are raised.12 Carnivores serve as the definitive hosts, while humans usually act as intermediate hosts in the life cycle of this parasite.13 The eggs of the Echinococcus parasite, present in the feces of infected dogs and other carnivores, are ingested by humans and herbivores. The humans are infected by vegetables, fruits or water contaminated by eggs of the cestode in the excreta of infected carnivores. The protoscolices then attach to the small intestine, where they grow and mature. The oncosphere then penetrates the intestinal wall of these intermediate hosts, is transported via the bloodstream, and forms hydatid cysts in the liver, lungs, spleen, kidneys, brain, muscles and, in rare cases, other organs.14
The substances that degrade and inactivate protoscolices include hydrogen peroxide, formalin, hypertonic saline, ethyl alcohol, and silver nitrate. These agents are associated with many side effects, such as necrosis, fibrosis, and dysfunction of infected organs including the lungs, kidneys, spleen, liver, and gallbladder.15 Therefore, there is an urgent need for new substances that can serve as effective protoscolicidal agents with minimal side effects. Polyphenols, phenols, and triterpenoid compounds found in plants exhibit strong antimicrobial properties against various types of bacteria, fungi, and parasites. Their diversity, abundance, and lack of side effects suggest that they may be beneficial in treating diseases caused by these organisms.16
The mint family, comprising 236 genera and over 7000 species, primarily thrives in temperate regions. It is widely utilized in traditional medicine due to its antimicrobial, antifungal, antioxidant, and anti-inflammatory properties. Additionally, it plays a significant role in the perfume, food, and pharmaceutical industries.17 Zhumeria majdae, a species within this family, is characterized by its herbaceous, shrubby form, fragrant white or gray appearance, and blue-purple flowers, reaching a height of 50 cm. This plant is found in southern Iran and is employed in Iranian medicine to treat headaches, heart arrhythmia, and colds.18 The essential oil extracted from Zhumeria majdae is renowned for its two primary compounds: linalool (C10H18O) and camphor (C10H16O), which exhibit antimicrobial, anti-inflammatory, antioxidant, and antibacterial properties.19 Additionally, these compounds possess reducing and stabilizing capabilities, critical for NPs synthesis.
The green preparation of ZnO NPs using plant-based essential oils such as Zhumeria majdae leverages the inherent reducing and capping agents present in the oil.20 This approach minimizes environmental impact, offering a cost-effective and straightforward technique for producing NPs. The process typically involves mixing the essential oil with a zinc precursor, such as zinc nitrate hexahydrate. The bioactive compounds in the oil facilitate the reduction of zinc ions to form ZnO NPs. This method aligns with the growing trend of using natural sources including plant extracts, bacteria, fungi, and honey to produce NPs in an environmentally sustainable manner. The use of plant extracts introduces diverse phytochemicals including flavonoids, alkaloids, and polyphenols, which act as reducing and capping agents, influencing the size, shape, and stability of the resulting NPs. The advantages of using the Zhumeria majdae plant include: (1) reducing and stabilizing agent: the compounds in Zhumeria majdae essential oil, such as linalool and camphor, act as both reducing agents, converting zinc ions into zinc oxide, and stabilizing agents, preventing agglomeration of the NPs. (2) Eco-friendliness: green synthesis using plant extracts such as Zhumeria majdae essential oil reduces the need for hazardous chemicals, making the process more environmentally friendly. (3) Cost-effectiveness: plant-based synthesis is generally more cost-effective compared to traditional methods that require expensive chemical reagents and complex equipment. (4) Antimicrobial properties: Zhumeria majdae essential oil exhibits antimicrobial activity, which can be transferred to the synthesized ZnO NPs, enhancing their potential for biomedical applications. ZnO NPs synthesized using Zhumeria majdae plant have potential applications in various fields: active food packaging: the incorporation of Zhumeria majdae essential oil nanoemulsion into kefiran-gelatin films enhances their properties, making them suitable for active food packaging.21 Antimicrobial agents: these NPs can be used to control multidrug-resistant clinical pathogens, addressing the growing concern of nosocomial infections. Biomedical applications: ZnO NPs show promise in biomedicine, including wound healing, drug delivery, and antioxidant applications.22 Agriculture: ZnO NPs can be used to improve plant growth and development, leveraging the essential role of zinc in living organisms.23 Traditional chemical methods for producing ZnO NPs require high temperatures and pressures. These methods can be energy-intensive and may generate toxic byproducts. In contrast, green synthesis using the Zhumeria majdae plant offers a milder, more sustainable approach.
The purpose of the present study was to construct ZnO NPs from the Zhumeria majdae plant and to evaluate these NPs, along with the hydroalcoholic extract and essential oil, for their antibacterial, antioxidant, and scolicidal activities. Zhumeria majdae is utilized in the synthesis of ZnO NPs due to its unique chemical composition, eco-friendly nature, cost-effectiveness and bioactive properties, which make it a suitable candidate for green synthesis methods. Green synthesis offers an eco-friendly alternative to traditional chemical methods, which often involve hazardous reagents, complex equipment, and high energy consumption. The resulting NPs inherit beneficial properties from the essential oil, such as antimicrobial activity, making them suitable for a wide range of applications in food packaging, biomedicine, and agriculture. This green synthesis approach aligns with the growing demand for sustainable and environmentally conscious methods in nanotechnology.
2. Materials and methods
2.1. Chemicals
All chemicals, salts, and solvents required in this study were obtained from Merck with high purity. Ethanol (96%), methanol (80%), sodium acetate trihydrate (99%), acetic acid glacial (99%), 2,4,6-tri(2-pyridyl)-s-triazine (99%), hydrochloric acid (37%), ferric chloride (98%), ammonium iron(II) sulfate hexahydrate (98%), 2,2-diphenyl picrylhydrazyl (95%), sodium carbonate (99.5%), folin-Ciocalteu, gallic acid (assay > 97.5%), potassium acetate (99%), aluminium chloride hexahydrate (97%), zinc nitrate hexahydrate (99%), quercetin (95%), and dimethyl sulfoxide (99.9%).2.2. Collection, identification, and hydroalcoholic extraction
The aerial parts of the Zhumeria majdae plant (leaves, flowers, and stems) were harvested in May 2019 from an altitude of 700 meters above sea level on Mount Genu, located in Bandar Abbas city, Hormozgan Province, Iran. The plant was identified by a botanist at the Research Institute of Forests and Ranges and registered with the herbarium code TARI50023. Following collection, the aerial parts were extracted in a dry, shaded environment, using the hydroalcoholic method.242.3. Extraction of the essential oil analysis by GC/MS method and identification of chemical components
100 g of the aerial parts of the plant including flowers, leaves, and stems was extracted using the hydrodistillation method with a Clevenger apparatus for 3 h. The essential oil was dried with sodium sulfate (Na2SO4) and stored in a dark glass vial at 4 °C until needed.25 The main active compounds of the essential oil were identified using a GC-MS analysis. A Model 6890 chromatograph was coupled with an Agilent Mass Spectrometer (Model N-5973) for the qualitative analysis of the compounds. The temperature was programmed as follows: starting at 60 °C and increasing to 246 °C at a rate of 3 °C min−1. The injector and detector temperatures were maintained at 250 °C, with an injection volume of 1 μL and a split ratio of 1.50. Helium was used as the carrier gas, flowing at a rate of 1.5 mL min−1.262.4. Preparation and characterization of ZnO NPs
The biosynthesis of ZnO NPs was performed through the reduction of Zn(NO3)2·6H2O using an extract from the aerial parts of Zhumeria majdae. 10 mL of the filtered aqueous extract was mixed with 2 g of Zn(NO3)2·6H2O and heated to 60 °C while being continuously stirred at 500 rpm for 2 h. The color change from light brown to dark brown indicated the successful formation of NPs. The resulting solution was centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min and the precipitate was washed with 10 mL of double-distilled water. The obtained ZnO NPs were then placed in an oven at 100 °C for 1 h and subsequently calcined in a furnace to obtain crystalline NPs. The calcined ZnO NPs were stored in a cool, dry, and dark place.27 The formation of ZnO NPs was analyzed using a UV-vis spectrophotometer (UV-1900i, Shimadzu, Japan) over a wavelength range of 300 to 700 nm. The presence of phytochemical compounds and functional groups was examined through Fourier Transform Infrared (FTIR) spectroscopy (Alfa, Bruker, Germany) within the range of 4000 to 400 cm−1. The morphology and size of the ZnO NPs were assessed using a Field Emission Scanning Electron Microscope (FE-SEM) (Tescan Orsay Holding, Brno, Czech Republic). The presence and percentage of zinc element were determined using Energy Dispersive X-ray spectroscopy (EDS) analysis. The crystalline structure of the synthesized ZnO NPs was investigated with a Pert Power X-ray diffractometer (XRD), utilizing Cu Kα radiation over a 2θ range of 10 to 80°.
000 rpm for 30 min and the precipitate was washed with 10 mL of double-distilled water. The obtained ZnO NPs were then placed in an oven at 100 °C for 1 h and subsequently calcined in a furnace to obtain crystalline NPs. The calcined ZnO NPs were stored in a cool, dry, and dark place.27 The formation of ZnO NPs was analyzed using a UV-vis spectrophotometer (UV-1900i, Shimadzu, Japan) over a wavelength range of 300 to 700 nm. The presence of phytochemical compounds and functional groups was examined through Fourier Transform Infrared (FTIR) spectroscopy (Alfa, Bruker, Germany) within the range of 4000 to 400 cm−1. The morphology and size of the ZnO NPs were assessed using a Field Emission Scanning Electron Microscope (FE-SEM) (Tescan Orsay Holding, Brno, Czech Republic). The presence and percentage of zinc element were determined using Energy Dispersive X-ray spectroscopy (EDS) analysis. The crystalline structure of the synthesized ZnO NPs was investigated with a Pert Power X-ray diffractometer (XRD), utilizing Cu Kα radiation over a 2θ range of 10 to 80°.
2.5. Antibacterial activity
2.6. Antioxidant properties of the essential oil, hydroalcoholic extract and biosynthesized ZnO NPs
2.7. Scolicidal activity
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of the sample and protoscolex was then prepared with 0.1% eosin and incubated for an additional 15 min. Subsequently, the mixture was placed on a glass slide, and the survival and death of the protoscolices were examined using an ordinary light microscope at 40× magnification. The live protoscolices appeared green or gray due to their selective permeability, while the dead ones were red, indicating their inability to selectively permeate and allow eosin to pass through. In this study, 9% physiological saline with Tween 80 was used as the negative control, and saturated salt served as the positive control.35
1 mixture of the sample and protoscolex was then prepared with 0.1% eosin and incubated for an additional 15 min. Subsequently, the mixture was placed on a glass slide, and the survival and death of the protoscolices were examined using an ordinary light microscope at 40× magnification. The live protoscolices appeared green or gray due to their selective permeability, while the dead ones were red, indicating their inability to selectively permeate and allow eosin to pass through. In this study, 9% physiological saline with Tween 80 was used as the negative control, and saturated salt served as the positive control.353. Results and discussion
3.1. Preparation of ZnO NPs
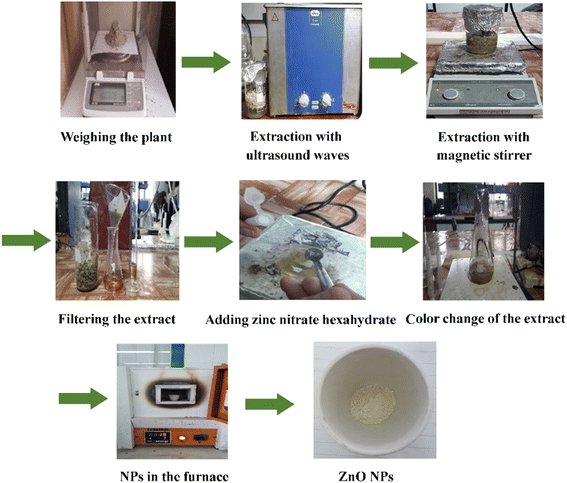
The steps for preparing ZnO NPs in the presence of the Zhumeria majdae plant are shown in Fig. 1.3.2. Chemical composition of the Zhumeria majdae essential oil
The efficiency of the essential oil was recorded at 5.52% based on analytical calculations, which aligns with the findings of Saeidi et al. (2019), who reported an efficiency of 6.30%.36 GC/MS analysis was conducted to identify the chemical compounds present in the Zhumeria majdae essential oil, resulting in the identification of a total of 23 compounds, as listed in Table 1. Among these, three compounds exhibited the highest peak intensities (Fig. 2). The predominant components were linalool (41.50%), camphor (28.25%), and camphene (4.38%). Fallah et al. (2019) identified 30 compounds in the essential oil of the Zhumeria majdae plant, with linalool and camphor being the most concentrated, and 16 of these compounds corresponded with the current findings.37 Additionally, Saidi et al. (2019) identified 42 compounds in the essential oil of Zhumeria majdae, of which 18 were consistent with the present results, with linalool and camphor again showing the highest concentrations.36 Furthermore, according to the results of Soltani Poor et al., linalool and camphor constituted the highest percentages of Zhumeria majdae essential oil, at 54.1% and 24.8%, respectively.38 Given that previous studies have already characterized the constituents of Zhumeria majdae using GC/MS and GC, the novelty could lie in several areas: properties of synthesized NPs, enhanced bioactivity and optimization of synthesis parameters.| No. | Name | RT (min) | Standard retention index | Conc. (%) | Formulation |
|---|---|---|---|---|---|
| 1 | α-Pinene | 11.50 | 939 | 2.24 | C10H16 |
| 2 | Camphene | 12.48 | 953 | 4.38 | C10H16 |
| 3 | 3-Octanone | 13.50 | 993 | 0.97 | C8H16O |
| 4 | Myrcene | 13.66 | 994 | 0.47 | C10H16 |
| 5 | α-Terpinene | 14.80 | 1018 | 0.35 | C10H16 |
| 6 | p-Cymene | 15.10 | 1026 | 1.30 | C10H14 |
| 7 | Limonene | 15.30 | 1031 | 3.70 | C10H16 |
| 8 | γ-terpinene | 16.32 | 1062 | 1.12 | C10H16 |
| 9 | cis-Linalool oxide | 16.83 | 1074 | 1.32 | C10H18O |
| 10 | trans-Linalool oxide | 17.42 | 1088 | 1.49 | C10H18O |
| 11 | Linalool | 18.19 | 1098 | 41.50 | C10H18O |
| 12 | Camphor | 20.10 | 1143 | 28.25 | C10H16O |
| 13 | Borneol | 20.83 | 1165 | 2.96 | C10H18O |
| 14 | Terpinen-4-ol | 21.05 | 1177 | 0.75 | C10H18O |
| 15 | α-Terpineol | 21.54 | 1189 | 1.34 | C10H18O |
| 16 | Neral | 22.92 | 1235 | 0.67 | C10H16O |
| 17 | Geraniol | 23.34 | 1255 | 2.38 | C10H18O |
| 18 | Geranial | 23.94 | 1270 | 0.78 | C10H16O |
| 19 | cis-Jasmone | 28.29 | 1394 | 0.42 | C11H16O |
| 20 | β-Caryophyllene | 29.39 | 1418 | 0.79 | C15H24 |
| 21 | Caryophyllene oxide | 34.49 | 1581 | 1.27 | C15H24O |
| 22 | β-Eudesmol | 36.58 | 1649 | 0.63 | C15H26O |
| 23 | Hexadecanoic acid | 44.18 | 1984 | 0.47 | C16H32O2 |
| Monoterpenes | %95 |
| Sesqueiterpenes | %2.69 |
| Ketone | %0.97 |
| 3-Octanone | |
| Palmic acid | %0.47 |
| Hexadecanoic acid | |
| Alcohol cis-Jasmone | %0.42 |
| Total | %99.55 |
3.3. Characterization of ZnO NPs
Biological reduction of the metal ions in a salt solution at regular intervals leads to the formation of NPs. The color change of the solution from light brown to dark brown indicates the formation of ZnO NPs. The biological conversion of Zn2+ ions to Zn0 was mainly studied due to the presence of chemicals in the hydroalcoholic extract of the plant. The appearance of the surface plasmon resonance (SPR) band in the wavelength range of 350–400 nm confirmed the formation of ZnO NPs. The maximum absorption peak was observed at 368 nm, attributed to the SPR of ZnO NPs, indicating their formation (Fig. 3). The results of this study are consistent with those reported by Chaudhuri et al. (2017), Khan et al. (2017), and Degefa et al. (2021), who identified absorption peaks at 355, 347, and 375 nm for ZnO NPs, respectively.39–41The determination of functional groups involved in the biosynthesis of ZnO NPs was investigated using FTIR spectroscopy (Fig. 4). The characteristic peak at 3404 cm−1 corresponds to the O–H groups. The peaks at 2923 and 2846 cm−1 represent the C–H stretching vibration mode found in alkanes. Additionally, the peak at 1730 cm−1 is due to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the bands at 1653 and 1602 cm−1 are related to the stretching vibration of C
O, and the bands at 1653 and 1602 cm−1 are related to the stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds. The peaks at 1461 and 1359 cm−1 contributed to bending vibrations of C–H bonds and the peak at 1051 cm−1 belonged to the stretching vibration of C–O. The peak at 846 cm−1 is associated with the stretching vibrations of ZnO synthesized by the active compounds from the plant. All these peaks indicate the presence of compounds such as flavonoids and polyphenolic phytomolecules. The results obtained from this FTIR spectrum are consistent with the findings of Basri et al. (2020), Ossai et al. (2020), and Saravanadevi et al. (2020).42–44
C bonds. The peaks at 1461 and 1359 cm−1 contributed to bending vibrations of C–H bonds and the peak at 1051 cm−1 belonged to the stretching vibration of C–O. The peak at 846 cm−1 is associated with the stretching vibrations of ZnO synthesized by the active compounds from the plant. All these peaks indicate the presence of compounds such as flavonoids and polyphenolic phytomolecules. The results obtained from this FTIR spectrum are consistent with the findings of Basri et al. (2020), Ossai et al. (2020), and Saravanadevi et al. (2020).42–44
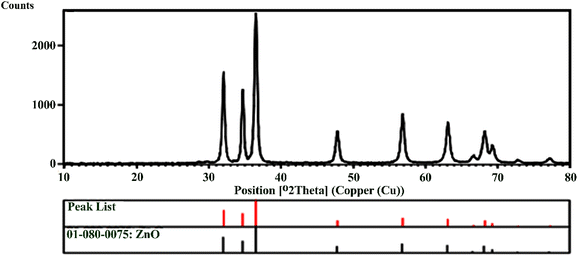
The XRD pattern for the synthesized ZnO NPs is presented in Fig. 5. The Bragg reflection peaks observed at 2θ = 32.02°, 34.69°, 36.51°, 47.81°, 56.85°, 63.11°, 66.63°, 68.21°, 69.27°, 72.82°, and 77.52° are indexed as (100), (002), (101), (102), (110), (103), (200), (112), (201), (004), and (202), respectively, and correspond to the hexagonal phase of the ZnO NPs structure. The characteristic peaks show a good correlation with JCPDS standard number 01-089-1397. These results are consistent with the findings of Khaghani Boroujeni et al. (2018) and Abdelkhalek et al. (2020).45,46 The XRD pattern shows characteristic peaks for ZnO, indicating its crystal structure.
The chemical composition of the ZnO NPs was determined through the EDS analysis (Fig. 6). The EDS spectrum of the synthesized ZnO NPs revealed weight percentages of zinc (78.17%) and oxygen (21.8%). Additionally, the energy absorption peak for zinc was observed at 1 keV, indicating successful production of the ZnO NPs, which aligns with the findings of Rad et al. (2019).47
The FE-SEM ultramicrographs of the ZnO NPs are presented in Fig. 7. The images reveal cubic polyhedral NPs with sizes ranging from 69.1 to 92.6 nm. Also, some agglomeration was observed, possibly due to the presence of plant-derived organic molecules. These findings are consistent with the results reported by Suresh et al. (2018), Nithya et al. (2019), Ogunyemi et al. (2019) and Modwi et al. (2021).48–51 The effect of particle size on the properties of ZnO NPs is a crucial area of study due to its wide-ranging applications in various fields such as electronics, optics, catalysis, and biomedicine. The size of ZnO NPs significantly influences their physical, chemical, and biological properties, which in turn dictate their performance in specific applications. The toxicity of ZnO NPs is a significant concern, especially in biomedical applications. Smaller particles tend to exhibit higher toxicity due to their ability to penetrate cells more easily and induce oxidative stress. The release of zinc ions from the NPs can also contribute to toxicity. ZnO NPs have antibacterial properties, making them useful in antimicrobial coatings and drug delivery systems.52 The antibacterial activity is size-dependent, with smaller particles generally exhibiting higher activity due to their increased surface area and ability to interact with bacterial cell membranes. Smaller particles can be more easily taken up by cells, facilitating drug delivery. However, the stability and biocompatibility of these particles need to be optimized to ensure effective and safe drug delivery.
3.4. Antibacterial activity
The antibacterial activity of the hydroalcoholic extract, essential oil, and ZnO NPs was investigated against the strain Staph. aureus and the strain Pseud. aeruginosa and the ZOI was measured (Fig. 8). Significant antibacterial activity was observed for Staph. aureus, with a MIC of 25.00 mg μL−1 for the hydroalcoholic extract, 3.12 μL for the essential oil, and 0.003 g μL−1 for the ZnO NPs. On the other hand, the minimum lethal concentration for the samples was 50.00 mg μL−1, 3.12 μL, and 0.003 g μL−1, respectively. Furthermore, the ZOI was measured 14, 31, and 7 mm, respectively, using well diffusion and disk diffusion methods. For Pseud. aeruginosa, the hydroalcoholic extract and essential oil exhibited MICs of 100 mg μL−1, and 12.5 μL, respectively. The minimum lethal concentrations were found to be 200 mg μL−1, and 50 μL, respectively. The ZOI for the hydroalcoholic extract and essential oil were 9 and 25 mm, respectively. This indicates that the essential oil possesses the strongest antibacterial properties against both bacterial strains, attributed to the presence of monoterpenes and terpenoids, including linalool and camphor, compared to the hydroalcoholic extract. Additionally, the ZnO NPs did not demonstrate a positive effect on Pseud. aeruginosa (Table 2). | ||
| Fig. 8 ZOI of the hydroalcoholic extract, essential oil and ZnO NPs against Staph. aureus (a–c), ZOI of hydroalcoholic extract and essential oil against Pseud. aeruginosa (d and e). | ||
| Organism | Essential oil (μL) | Hydroalcholic extract (mg mL−1) | ZnO NPs (mg mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MIC | MBC | ZOI (mm) | MIC | MBC | ZOI (mm) | MIC | MBC | ZOI (mm) | |
| Pseud. aeruginosa | 12.5 | 50 | 25 | 100 | 200 | 9 | — | — | — |
| Staph. aureus | 3.12 | 3.12 | 31 | 25 | 50 | 31 | 0.003 | 0.003 | 7 |
Based on the results of Table 2, the compounds present in the essential oil were correlated with the results obtained in this test. The constituents of the essential oil such as linalool, camphor, α-pinene, camphene, borneol, and α-terpineol, exhibit synergistic effects, with some demonstrating antibacterial properties. Notably, this test revealed differences in the effects on Gram-positive and Gram-negative bacteria. The cell wall of Gram-positive bacteria contains mucopeptides, while the cell wall of Gram-negative bacteria is composed of lipoproteins, lipopolysaccharides, and purines, which serve as barriers to the passage of large hydrophobic molecules, including most compounds found in the hydroalcoholic extract and the essential oil. These findings align with the results reported by Santos et al. (2019) regarding the sweet plant from the mint family, which showed greater efficacy against Gram-positive bacteria.53 Additionally, Soren et al. (2018) highlighted the effect of ZnO NPs on the Gram-positive bacterium Staph. epidermidis.54 Nasiri et al. (2014) utilized metal oxide NPs, including TiO2, ZnO, and CuO, to investigate their antibacterial properties. They found that Escherichia coli was eradicated at higher concentrations compared to Staph. aureus.55
3.5. Antioxidant activity
Based on the DPPH test, the antioxidant activity, expressed as milligrams of ascorbic acid per gram, was highest in the hydroalcoholic extract (738.24 mg g−1), followed by the essential oil (118.70 mg g−1) and ZnO NPs (37.41 mg g−1). The highest concentration of total phenols, measured as milligrams of gallic acid per gram, was also found in the hydroalcoholic extract (95.07 mg g−1), followed by the essential oil (70.01 mg g−1) and ZnO NPs (11.38 mg g−1). Additionally, the highest amount of flavonoids, quantified as milligrams of quercetin per gram, was present in the hydroalcoholic extract (37.01 mg g−1), followed by the essential oil (19.98 mg g−1) and ZnO NPs (4.61 mg g−1). The highest antioxidant activity, tested by the FRAP test, was recorded for the hydroalcoholic extract (0.68 mg FeSO4 per g), followed by the essential oil (0.63 mg FeSO4 per g) and ZnO NPs (0.17 mg FeSO4 per g). The results of the antioxidant activity are illustrated in Fig. 9. These diagrams indicate that the antioxidant effect of the hydroalcoholic extract was superior to that of the essential oil in all the tests. The lowest level of antioxidant activity was associated with the ZnO NPs, which may be attributed to the high distribution coefficient of ethanol used as the extraction solvent. The choice of organic reagent for extracting phenolic compounds, as well as the quantity and type of the extracted compounds in terms of polarity and solubility, is directly influenced by the extraction solvent. In the study by Oalde et al. (2021), ethanol and water were identified as effective solvents for mint species.56 These findings align with those of El Aanachi et al. (2020), which reported a total phenol content of 106.55 micrograms per milligram of gallic acid and a flavonoid content of 49 micrograms per milligram of quercetin in the hydroalcoholic extract. This indicates a higher concentration of total phenols compared to flavonoids.57 Furthermore, based on the findings of Mehdizadeh et al. (2019) regarding the essential oil and ethanolic extract of the Zhumeria majdae plant, the antioxidant activity of the hydroalcoholic extract was found to be greater than that of the essential oil in all the antioxidant activity tests. This result is consistent with the findings of the present study.58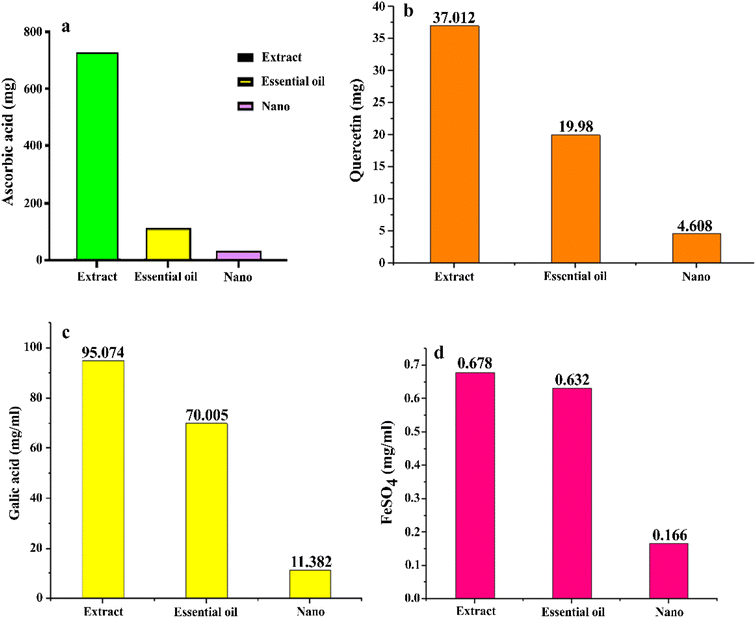 | ||
| Fig. 9 Antioxidant activity of the hydroalcoholic extract, essential oil and ZnO NPs, using DPPH (a), total flavonoid (b), total phenol (c) and FRAP (d). | ||
3.6. Scolicidal activity of the ZnO NPs
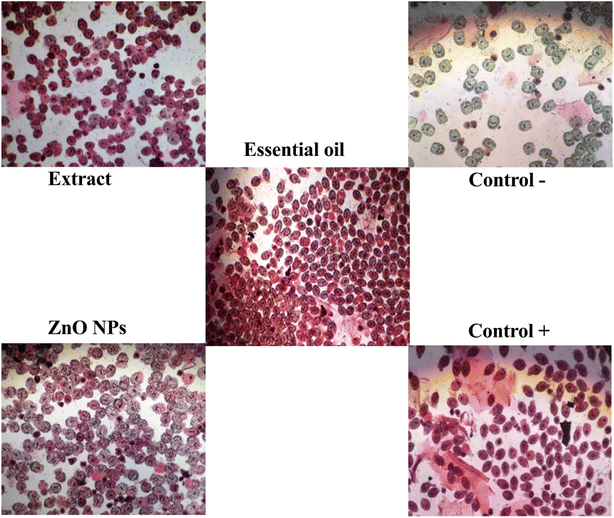
As shown in Fig. 10, the highest percentage of protoscolicidal activity was observed by the hydroalcoholic extract of the Zhumeria majdae plant at 200 mg mL−1 concentration, after 60 min. Additionally, exposure to 10 μL mL−1 concentration of the essential oil, for 10 min, resulted in a 100% mortality rate of the protoscolices. Furthermore, the highest percentage of protoscolicidal activity from ZnO NPs was attained with a concentration of 400 μg mL−1 after 60 min. The survival percentage of the protoscolices, examined using a light microscope, is illustrated in Fig. 11. The average lethality percentage and standard deviation are presented in Tables 3–5. | ||
| Fig. 10 Mortality rate of protoscoleces by different concentrations and duration of exposure of hydroalcoholic extract (a), ZnO NPs (b) and essential oil (c). | ||
| Exposure time (min) | Concentration (mg mL−1) | Number of protoscolices counted | Number of dead protoscolices | Rate of dead protoscolices |
|---|---|---|---|---|
| 15 | 200 | 503.33 ± 4.16 | 241.00 ± 16.37 | 47.87 ± 2.91 |
| 100 | 508.67 ± 3.06 | 204.00 ± 26.32 | 40.09 ± 4.96 | |
| 50 | 503.33 ± 3.51 | 120.67 ± 13.65 | 23.97 ± 2.69 | |
| 25 | 501.33 ± 1.53 | 92.00 ± 14.73 | 18.34 ± 2.88 | |
| Negative control | 512 | 4 | 0.78 | |
| Positive control | 506 | 506 | 100 | |
| 30 | 200 | 504.67 ± 4.16 | 354.33 ± 22.05 | 70.23 ± 4.72 |
| 100 | 505.33 ± 2.52 | 255.00 ± 25.00 | 50.48 ± 5.20 | |
| 50 | 503.00 ± 3.00 | 210.00 ± 15.62 | 41.74 ± 2.89 | |
| 25 | 507.67 ± 4.04 | 152.33 ± 27.57 | 29.99 ± 5.25 | |
| Negative control | 509 | 7 | 1.38 | |
| Positive control | 508 | 508 | 100 | |
| 60 | 200 | 506.67 ± 4.62 | 498.67 ± 16.65 | 98.41 ± 2.75 |
| 100 | 502.00 ± 1.73 | 409.33 ± 13.65 | 81.54 ± 2.61 | |
| 50 | 505.67 ± 5.13 | 363.67 ± 10.97 | 71.94 ± 2.91 | |
| 25 | 503.00 ± 3.00 | 252.67 ± 23.59 | 50.25 ± 4.99 | |
| Negative control | 506 | 12 | 2.37 | |
| Positive control | 504 | 504 | 100 |
| Exposure time (min) | Concentration (mg mL−1) | Number of protoscolices counted | Number of dead protoscolices | Rate of dead protoscolices |
|---|---|---|---|---|
| 5 | 10 | 502.00 ± 2.00 | 493.00 ± 17.35 | 98.2 ± 3.12 |
| 5 | 504.67 ± 5.69 | 344.33 ± 18.48 | 68.22 ± 3.18 | |
| 2.5 | 502.33 ± 2.08 | 235.00 ± 14.73 | 46.79 ± 13.3 | |
| Negative control | 523 | 7 | 1.34 | |
| Positive control | 514 | 385 | 74.90 | |
| 10 | 10 | 506.33 ± 5.69 | 506.33 ± 5.69 | 100.00 ± 0 |
| 5 | 504.33 ± 3.22 | 496.67 ± 14.74 | 98.48 ± 2.64 | |
| 2.5 | 504.67 ± 6.43 | 347.67 ± 18.77 | 74.23 ± 3.26 | |
| Negative control | 512 | 13 | 2.54 | |
| Positive control | 507 | 507 | 100 | |
| 15 | 10 | 505.67 ± 5.51 | 505.67 ± 5.51 | 100.00 ± 0 |
| 5 | 506.33 ± 5.69 | 506.33 ± 5.69 | 100.00 ± 0 | |
| 2.5 | 503.00 ± 3.00 | 503.00 ± 3.00 | 100.00 ± 0 | |
| Negative control | 12 | 12 | 2.37 | |
| Positive control | 504 | 504 | 100 |
| Exposure time (min) | Concentration (mg mL−1) | Number of protoscolices counted | Number of dead protoscolices | Rate of dead protoscolices |
|---|---|---|---|---|
| 15 | 400 | 506.00 ± 6/08 | 169.00 ± 15/72 | 33/38 ± 2/79 |
| 200 | 507.67 ± 4/04 | 127.67 ± 22.50 | 25.17 ± 4.63 | |
| 100 | 504.67 ± 2.89 | 57.67 ± 15.14 | 11.41 ± 2.93 | |
| 50 | 508.33 ± 7.37 | 32.33 ± 4.51 | 6.36 ± 0.83 | |
| Negative control | 507 | 8 | 1.58 | |
| Positive control | 502 | 502 | 100 | |
| 30 | 400 | 507.00 ± 1.00 | 254.33 ± 26.00 | 50.16 ± 5.03 |
| 200 | 506.00 ± 5.29 | 212.00 ± 15.62 | 41.89 ± 2.79 | |
| 100 | 510.00 ± 5.29 | 152.00 ± 28.05 | 29.81 ± 5.52 | |
| 50 | 510.00 ± 11.79 | 105.33 ± 26.10 | 20.62 ± 4.93 | |
| Negative control | 502 | 12 | 2.39 | |
| Positive control | 523 | 523 | 100 | |
| 60 | 400 | 506.33 ± 9.29 | 506.33 ± 9.29 | 100.00 ± 0 |
| 200 | 502.67 ± 3.06 | 494.00 ± 12.17 | 98.29 ± 2.97 | |
| 100 | 510.33 ± 5.77 | 423.67 ± 17.04 | 83.01 ± 2.62 | |
| 50 | 502.67 ± 4.62 | 360.33 ± 11.02 | 71.69 ± 2.54 | |
| Negative control | 502 | 24 | 4.78 | |
| Positive control | 507 | 507 | 100 |
Based on these results, it appears that the majority of the antimicrobial and antiparasitic activity in medicinal plants is attributed to phenolic compounds. These compounds induce degeneration and necrosis of the metacestodes by inhibiting protein or DNA synthesis and disrupting the metabolism of protoscolices. Among the factors contributing to the high antiparasitic activity of essential oils, the presence of compounds such as linalool and camphor is noteworthy. Moazeni et al. (2012) investigated the scolicidal effect of Khuzestani Marzeh essential oil at a concentration of 5 mg mL−1 over durations of 10, 20, 30, and 60 min, yielding results of 66.51%, 68.33%, 81.12%, and 100.00%, respectively. These findings indicate that the plant under study exhibits a significantly greater effect.59 Furthermore, Ranjber et al. (2020) demonstrated that the methanolic extract of Mentha species at a concentration of 200 mg mL−1 for 30 min. produced the most pronounced scolicidal effect.60 Additionally, Shnawa (2021) reported the scolicidal effect of ZnO NPs in the Mentha longifolia plant at a concentration of 400 ppm.61
4. Conclusion
The present study concluded that the phytochemical compounds found in the Zhumeria majdae plant serve as stabilizing and capping agents, playing a crucial role in the biological formation of ZnO NPs through an environmentally friendly, cost-effective, and straightforward process. The biosynthesized ZnO NPs were crystalline in nature and spherical, with an average size of 75 nm. The hydroalcoholic extract and essential oil derived from the Zhumeria majdae plant exhibited antibacterial, antioxidant, and scolicidal activities. The findings of this study showed that the essential oil of the Zhumeria majdae plant is more effective on some species of bacteria and also has significant scolicidal properties. These findings may have significant implications for the development of antiparasitic, antioxidant, and antibacterial drugs from the mint family. However, to achieve these objectives, further studies are necessary under in vivo conditions and through clinical trials.Data availability
All data generated or analyzed during this study are included in this published article.Author contributions
Rana Kiani: carried out the experiment, methodology, validation, investigation; Zahra Rafiee: conceptualization, data curation, visualization, supervision, writing – review & editing, project administration, funding acquisition; Damoun Razmjoue: conceptualization, data curation, writing – original draft, supervision, project administration, funding acquisition; Ahmad Oryan: data curation, writing – review & editing, visualization, Mehrorang Ghaedi: conceptualization, data curation, and Hassan Abidi: visualization, project administration.Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.Acknowledgements
The authors thank the Research Council of Yasouj University for the financial support.References
- D. H. Samak, Y. S. El-Sayed, H. M. Shaheen, A. H. El-Far, M. E. Abd El-Hack, A. E. Noreldin, K. El-Naggar, S. A. Abdelnour, E. M. Saied, H. R. El-Seedi, L. Aleya and M. M. Abdel-Daim, Developmental toxicity of carbon nanoparticles during embryogenesis in chicken, Environ. Sci. Pollut. Res. Int., 2020, 27, 19058–19072 CrossRef PubMed.
- M. S. Aref and S. S. Salem, Bio-callus synthesis of silver nanoparticles, characterization, and antibacterial activities via Cinnamomum camphora callus culture, Biocatal. Agric. Biotechnol., 2020, 27, 101689 CrossRef.
- T. I. Shaheen, S. S. Salem and S. Zaghloul, A new facile strategy for multifunctional textiles development through in situ deposition of SiO2/TiO2 nanosols hybrid, Ind. Eng. Chem. Res., 2019, 58, 20203–20212 CrossRef.
- A. Abdel-Azeem, A. A. Nada, A. ODonovan, V. K. Thakur and A. Elkelish, Mycogenic silver nanoparticles from endophytic Trichoderma atroviride with antimicrobial activity, J. Renewable Mater., 2020, 8, 171–185 Search PubMed.
- S. S. Salem and A. Fouda, Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview, Biol. Trace Elem. Res., 2021, 199, 344–370 CrossRef PubMed.
- J. A. Ruszkiewicz, A. Pinkas, B. Ferrer, T. V. Peres, A. Tsatsakis and M. Aschner, Neurotoxic effect of active ingredients in sunscreen products, a contemporary review, Toxicol Rep, 2017, 4, 245–259 CrossRef PubMed.
- M. D. Madhuwantha, H. Galagedara, Y. Y. Kannangara, S. Karunarathne, M. M. M. G. P. G. Mantilaka, H. C. S. Perera, R. Mahadeva, S. Arya, R. M. G. Rajapakse and W. P. S. L. Wijesinghe, Low-cost ZnO incorporated Carbonized Nitrile Butadiene Rubber (NBR) as a relative humidity monitoring sensor, Mater. Sci. Eng., B, 2023, 298, 116862 Search PubMed.
- M. Thakur, A. Singh, A. Dubey, A. K. Sundramoorthy, P. Kumar and S. Arya, Synthesis and characterization of ZnO/NiO nanocomposites for electrochemical sensing of p-Cresol in water, Emergent Mater., 2024, 7, 1805–1817 Search PubMed.
- R. Anitha, K. V. Ramesh, T. N. Ravishankar, K. H. Sudheer Kumar and T. Ramakrishnappa, Cytotoxicity, antibacterial and antifungal activities of ZnO nanoparticles prepared by the Artocarpus gomezianus fruit mediated facile green combustion method, J. Sci.:Adv. Mater. Devices, 2018, 3, 440–451 Search PubMed.
- K. A. Hammer, C. F. Carson and T. V. Riley, Antimicrobial activity of essential oils and other plant extracts, J. Appl. Microbiol., 1999, 86, 985–990 Search PubMed.
- A. Lymbery, Phylogenetic pattern, evolutionary processes and species delimitation in the genus Echinococcus, Adv. Parasitol., 2017, 95, 111–145 Search PubMed.
- M. Siles-Lucas, A. Casulli, R. Cirilli and D. Carmena, Progress in the pharmacological treatment of human cystic and alveolar echinococcosis: compounds and therapeutic targets, PLoS Neglected Trop. Dis., 2018, 12, e0006422 CrossRef PubMed.
- L. D. Singla, H. Singh, P. Kaur, N. D. Singh, N. K. Singh and P. D. Juyal, Serodetection of Ehrlichia canis infection in dogs from Ludhiana district of Punjab, India, J. Parasit. Dis., 2011, 35, 195–198 CrossRef PubMed.
- B. H. Shnawa, Advances in the use of nanoparticles as anti-cystic echinococcosis agents: a review article, J. Pharm. Res. Int., 2018, 24, 1–14 CrossRef.
- M. A. Rajabi, Fatal reactions and methaemoglobinaemia after silver nitrate irrigation of hydatid cyst, Surg. Pract., 2009, 13, 2–7 CrossRef.
- M. Gorzynik-Debicka, P. Przychodzen, F. Cappello, A. Kuban-Jankowska, A. M. Gammazza, N. Knap, M. Wozniak and M. Gorska-Ponikowska, Potential health benefits of olive oil and plant polyphenols, Int. J. Mol. Sci., 2018, 19, 686 CrossRef PubMed.
- S. Pignatti, Flora d'Italia, Edagricole, Bologna, Italy, 1982, vol. I, II, III Search PubMed.
- K. H. Rechinger and P. Wendelbo, Zhumeria majdae, Nytt Mag. Bot., 1967, 14, 39–43 Search PubMed.
- M. Valifard, N. Omidpanah, S. Farshad, M. A. Shahidi, S. Mohsenzadeh and O. Farshad, Antibacterial activity of extracts and essential oils of two iranian medicinal plants, Salvia mirzayanii and Zhumeria majdae, against Helicobacter pylori, Middle East, J. Sci. Res., 2014, 19, 1001–1006 Search PubMed.
- Z. Villagran, L. M. Anaya-Esparza, C. A. Velazquez-Carriles, J. M. Silva-Jara, J. M. Ruvalcaba-Gomez, E. F. Aurora-Vigo, E. Rodriguez-Lafitte, N. Rodriguez-Barajas, I. Balderas-Leon and F. Martinez-Esquivias, Plant-based extracts as reducing, capping, and stabilizing agents for the green synthesis of inorganic nanoparticles, Resources, 2024, 13, 70 CrossRef.
- S.-M. Hasheminya and J. Dehghannya, Development and characterization of kefiran-gelatin bio-nanocomposites containing Zhumeria majdae essential oil nanoemulsion to use as active food packaging in sponge cakes, Int. J. Biol. Macromol., 2024, 279, 135120 CrossRef PubMed.
- V. Puspasari, A. Ridhova, A. Hermawan, M. Ikhlasul Amal and M. Mansoob Khan, ZnO-based antimicrobial coatings for biomedical applications, Bioprocess Biosyst. Eng., 2022, 45, 1421–1445 CrossRef PubMed.
- H. Zhang, X. Zhao, J. Bai, M. Tang, W. Du, Z. Lv, K. H. M. Siddique and H. Mao, Effect of ZnO nanoparticle application on crop safety and soil environment: a case study of potato planting, Environ. Sci.: Nano, 2024, 11, 351–362 RSC.
- R. Fecker, V. Buda, E. Alexa, S. Avram, I. Z. Pavel, D. Muntean, I. Cocan, C. Watz, D. Minda, C. A. Dehelean, C. Soica and C. Danciu, Phytochemical and biological screening of oenothera biennis L. hydroalcoholic extract, Biomolecules, 2020, 10, 818 CrossRef PubMed.
- S. Raeisi, M. H. Mirjalili, F. Nadjafi and J. Hadian, Variability in the essential oil content and composition in different plant organs of Kelussia odoratissima Mozaff.(Apiaceae) growing wild in Iran, J. Essent. Oil Res., 2015, 27, 283–288 CrossRef.
- R. P. Adams, Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry, Allured publishing corporation Carol Stream, 2007, vol. 456 Search PubMed.
- B. Ahsan Abbasi, J. Iqbal, R. Ahmad, L. Zia, S. Kanwal, T. Mahmood, C. Wang and J.-T. Chen, Bioactivities of Geranium wallichianum leaf extracts conjugated with zinc oxide nanoparticles, Biomolecules, 2019, 10, 38 CrossRef PubMed.
- K. Zomorodian, M. Moein, K. Pakshir, F. Karami and Z. Sabahi, Chemical composition and antimicrobial activities of the essential oil from Salvia mirzayanii leaves, J. Evidence-Based Complementary Altern. Med., 2017, 22, 770–776 CrossRef PubMed.
- I. Cocan, E. Alexa, C. Danciu, I. Radulov, A. Galuscan, D. Obistioiu, A. A. Morvay, R. M. Sumalan, M.-A. Poiana, G. Pop and C. A. Dehelean, Phytochemical screening and biological activity of Lamiaceae family plant extracts, Exp. Ther. Med., 2018, 15, 1863–1870 Search PubMed.
- G. Miliauskas, P. Venskutonis and T. Van Beek, Screening of radical scavenging activity of some medicinal and aromatic plant extracts, Food Chem., 2004, 85, 231–237 CrossRef.
- I. F. Benzie and J. J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay, Anal. Biochem., 1996, 239, 70–76 CrossRef PubMed.
- N. Bhardwaj, S. Puri, A. Kumari, A. Chauhan and A. Kumar, Investigation on antioxidant, antimicrobial, anti-inflammatory, and neuropsychiatry potential of phyto-mediated ZnO NPs using Colebrookea oppositifolia, J. Drug Deliv. Technol., 2024, 97, 105748 CrossRef.
- B. Durga Lakshmi, B. V. Vamsi Krishna, P. Tirupathi Rao, A. Marukurti, K. Vasudha, E. Basha Sk and K. Ramachandra Rao, Novel synthesis and biophysical characterization of zinc oxide nanoparticles using virgin coconut oil, ACS Omega, 2024, 9, 38396–38408 CrossRef PubMed.
- M. Moazeni, S. Larki, A. Oryan and M. J. Saharkhiz, Preventive and therapeutic effects of Zataria multiflora methanolic extract on hydatid cyst: An in vivo study, Vet. Parasitol., 2014, 205, 107–112 CrossRef PubMed.
- R. Norouzi, M. Hejazy and A. Ataei, Scolicidal activity of zinc oxide nanoparticles against hydatid cyst protoscolices in vitro, Nanomed. Res. J., 2019, 4, 23–28 Search PubMed.
- M. Saeidi, J. Asili, S. A. Emami, N. Moshtaghi and S. Malekzadeh-Shafaroudi, Comparative volatile composition, antioxidant and cytotoxic evaluation of the essential oil of Zhumeria majdae from south of Iran, Iran, J. Basic Med. Sci., 2019, 22, 80–85 Search PubMed.
- M. Fallah, M. Farzaneh, M. Yousefzadi, M. Ghorbanpour and M. H. Mirjalili, In vitro mass propagation and conservation of a rare medicinal plant, Zhumeria majdae Rech. f & Wendelbo (Lamiaceae), Biocatal. Agric. Biotechnol., 2019, 17, 318–325 CrossRef.
- M. A. Soltani Poor, M. B. Rezaei and A. Moradshahi, Study on antimicrobial effects of essential oil of Zhumeria majdae Rech. f. & Wendelbo, Iran, J. Med. Aromat. Plant Res., 2004, 20, 277–289 Search PubMed.
- S. K. Chaudhuri and L. Malodia, Biosynthesis of zinc oxide nanoparticles using leaf extract of Calotropis gigantea: characterization and its evaluation on tree seedling growth in nursery stage, Appl. Nanosci., 2017, 7, 501–512 CrossRef.
- M. I. Khan, K. A. Bhatti, R. Qindeel, N. Alonizan and H. S. Althobaiti, Characterizations of multilayer ZnO thin films deposited by sol-gel spin coating technique, Results Phys., 2017, 7, 651–655 CrossRef.
- A. Degefa, B. Bekele, L. T. Jule, B. Fikadu, S. Ramaswamy, L. P. Dwarampudi, N. Nagaprasad and K. Ramaswamy, Green synthesis, characterization of zinc oxide nanoparticles, and examination of properties for dye-sensitive solar cells using various vegetable extracts, J. Nanomater., 2021, 2021, 3941923 Search PubMed.
- A. Ossai, S. Ezike and A. Dikko, Bio-synthesis of zinc oxide nanoparticles from bitter leaf (vernonia amygdalina) extract for dye-sensitized solar cell fabrication, J. Mater. Environ. Sci., 2020, 11, 444–451 Search PubMed.
- H. H. Basri, R. A. Talib, R. Sukor, S. H. Othman and H. Ariffin, Effect of synthesis temperature on the size of ZnO nanoparticles derived from pineapple peel extract and antibacterial activity of ZnO-starch nanocomposite films, Nanomaterials, 2020, 10, 1061 CrossRef.
- K. Saravanadevi, M. Kavitha, P. Karpagavinayagam, K. Saminathan and C. Vedhi, Biosynthesis of ZnO and Ag doped ZnO nanoparticles from Vitis vinifera leaf for antibacterial, photocatalytic application, Mater. Today: Proc., 2022, 48, 352–356 Search PubMed.
- A. Khaghani Boroujeni, H. Madani and S. Shakeri, Investigation of the antibactrial effect of cement matrix containing zinc oxide nanoparticles on bacillus cereus and pseudomonas aeruginosa, J. Water Wastewater, 2018, 29, 88–100 Search PubMed.
- A. Abdelkhalek and A. A. Al-Askar, Green Synthesized ZnO Nanoparticles Mediated by Mentha Spicata Extract Induce Plant Systemic Resistance against Tobacco Mosaic Virus, Appl. Sci., 2020, 10, 5054 CrossRef.
- S. S. Rad, A. M. Sani and S. Mohseni, Biosynthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from leaf extract of Mentha pulegium (L.), Microb. Pathog., 2019, 131, 239–245 CrossRef.
- A. Modwi, A. S. Al-Ayed, D. E. Mustafa, A. A. Bagabas, M. R. Elamin, F. K. Algethami, R. Arasheed, M. Q. Alfaifi, A. Alqarni, F. Alotaibi and K. K. Taha, Ultrasound-assisted green biosynthesis of ZnO nanoparticles and their photocatalytic application, Z. Naturforsch. A, 2021, 76, 535–547 CrossRef.
- S. O. Ogunyemi, Y. Abdallah, M. Zhang, H. Fouad, X. Hong, E. Ibrahim, M. M. I. Masum, A. Hossain, J. Mo and B. Li, Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. Oryzae, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 341–352 CrossRef.
- K. Nithya and S. Kalyanasundharam, Effect of chemically synthesis compared to biosynthesized ZnO nanoparticles using aqueous extract of C. halicacabum and their antibacterial activity, OpenNano, 2019, 4, 100024 CrossRef.
- J. Suresh, G. Pradheesh, V. Alexramani, M. Sundrarajan and S. I. Hong, Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities, Adv. Nat. Sci.:Nanosci. Nanotechnol., 2018, 9, 015008 Search PubMed.
- L. Palanikumar, S. Ramasamy, G. Hariharan and C. Balachandran, Influence of particle size of nano zinc oxide on the controlled delivery of Amoxicillin, Appl. Nanosci., 2013, 3, 441–451 CrossRef CAS.
- J. D. C. Santos, E. Coelho, R. Silva, C. P. Passos, P. Teixeira, I. Henriques and M. A. Coimbra, Chemical composition and antimicrobial activity of Satureja montana byproducts essential oils, Ind. Crops Prod., 2019, 137, 541–548 CrossRef CAS.
- S. Soren, S. Kumar, S. Mishra, P. K. Jena, S. K. Verma and P. Parhi, Evaluation of antibacterial and antioxidant potential of the zinc oxide nanoparticles synthesized by aqueous and polyol method, Microb. Pathog., 2018, 119, 145–151 CrossRef CAS PubMed.
- A. Nasiri, M. Malakootian and F. Tamaddon, Synthesis nano ZnO assisted by ultrasound irradiation and evaluation of antimicrobial properties, Toloo-e-Behdasht, 2014, 13, 115–128 Search PubMed.
- M. Oalde, S. Kolarevic, J. Zivkovic, A. A. Aradski, J. J. Maric, M. K. Kolarevic, J. Dordevic, P. D. Marin, K. Savikin, B. Vukovic-Gacic and S. Duletic-Lausevic, A comprehensive assessment of the chemical composition, antioxidant, genoprotective and antigenotoxic activities of Lamiaceae species using different experimental models in vitro, Food Funct., 2021, 12, 3233–3245 RSC.
- S. El Aanachi, L. Gali, S. Rammali, C. Bensouici, H. Aassila and K. Dari, In vitro study of the antioxidant, photoprotective, anti-tyrosinase, and anti-urease effects of methanolic extracts from leaves of six Moroccan Lamiaceae, J. Food Meas. Charact., 2021, 15, 1785–1795 CrossRef.
- Z. Alizadeh Amoli, T. Mehdizadeh, H. Tajik and M. Azizkhani, Shelf life extension of refrigerated, vacuum-packed rainbow trout dipped in an alginate coating containing an ethanolic extract and/or the essential oil of Mentha Aquatica, Chem. Pap., 2019, 73, 2541–2550 CrossRef CAS.
- M. Moazeni, S. Larki, M. J. Saharkhiz, A. Oryan, M. Ansary Lari and A. Mootabi Alavi, In vivo study of the efficacy of the aromatic water of Zataria multiflora on hydatid cysts, Antimicrob, Agents Chemother., 2014, 58, 6003–6008 CrossRef.
- M. Ranjbar, M. Kiani and A. Nikpay, Antioxidant and scolicidal activities of four Iranian Mentha species (Lamiaceae) in relation to phenolic elements, J. HerbMed Pharmacol., 2020, 9, 200–208 CrossRef CAS.
- B. H. Shnawa, S. M. Hamad, A. A. Barzinjy, P. A. Kareem and M. H. Ahmed, Scolicidal activity of biosynthesized zinc oxide nanoparticles by Mentha longifolia L. leaves against Echinococcus granulosus protoscolices, Emergent Mater., 2022, 5, 683–693 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |