 Open Access Article
Open Access ArticleAdvanced development of conductive biomaterials for enhanced peripheral nerve regeneration: a review
Jianguang Wang
a,
Jiaqi Fanga,
Zhijie Wenga,
Liping Nana,
Yunfeng Chena,
Junkuan Shana,
Feng Chen *b and
Junjian Liu
*b and
Junjian Liu *a
*a
aDepartment of Orthopedics, Shanghai Tenth People's Hospital, School of Medicine, Tongji University, Shanghai 200072, China. E-mail: jjliu@tongji.edu.cn
bShanghai Key Laboratory of Craniomaxillofacial Development and Diseases, Stomatological Hospital, School of Stomatology, Fudan University, Shanghai 201102, China
First published on 22nd April 2025
Abstract
Peripheral nerve injury (PNI), as a major cause of disability worldwide, makes it difficult to achieve effective repair and regeneration. Including autologous nerve transplantation, traditional therapies are restricted by surgical intricacy, donor scarcity, and inconsistent recovery effects. As to nerve guidance conduits (NGCs), conductive materials have brought novel pathways for PNI repair. Such materials boost nerve regeneration via electrical stimulation and bring key mechanical stability and biophysical signaling. This review summarizes the progress in conductive materials for PNI therapy while emphasizing their functions in electrical stimulation (ES), bioelectric signal transmission, and cell behavior guidance, as well as revealing the design and function needs of nerve conduits. Additionally, our review highlights the demand for follow-up studies to accentuate material optimization and improve real-time electrical signal supervision. Accordingly, this research is insightful and contributes to developing PNI repair. This results in more efficacious therapies and enhanced outcomes.
1 Introduction
Peripheral nerve injury (PNI) is a widespread clinical symptom and triggers acute disabilities. In addition, its regeneration and repair invariably encounter great challenges in the medical domain. Despite being extensively applied, traditional therapies, including autologous nerve transplantation, are confronted with several restrictions like surgical intricacy, donor scarcity, and incomplete rehabilitation. Recently, conductive materials have been a research focus in nerve generation. Conductive nerve guidance conduits (NGCs) can promote neuronal growth and migration by providing electrical stimulation, which brings hope to PNI repair.The simulated electrophysiological environment in vivo of nerves revealed that conductive materials have a vital advantage of promoting nerve cell progress within a natural-like microenvironment. These simulations were essential to facilitate the differentiation and growth of nerve cells. Hence, nerve repair would be accelerated.
Conductive biomaterials integrated with smart NGCs improved the functionality of ES during the whole active proliferation stage. In the preparation of NGCs, the application forms of conductive materials in the repair of peripheral nerve injury (PNI) are highly diverse (Fig. 1). Its core design strategy is to promote regeneration by simulating the electrophysiological microenvironment of nerve tissue and cooperating with physical guidance and bioelectrical signal regulation. At present, the mainstream technical paths include: conductive coating and composite catheter-enhancing the electrical activity of the catheter by surface modification (such as carbon nanotubes and polypyrrole coating) or matrix doping (such as graphene/PCL composite material) to guide the axon electrotaxis growth; in situ electro-responsive hydrogel-using graphene oxide/silk fibroin and other conductive networks to transmit endogenous electrical signals in real time, and carrying neurotrophic factors to realize chemical–electrical synergistic regulation; Mechanical–electrical coupling piezoelectric scaffold-based on the mechanical–electrical conversion characteristics of piezoelectric materials such as PVDF and ZnO, the mechanical energy of ultrasound or muscle contraction is converted into a local electric field to drive the directional migration of Schwann cells; bioelectronic interface-integrating flexible electrodes and intelligent catheters to realize closed-loop regulation of intraoperative electrophysiological monitoring (such as evoked potential feedback) and adaptive electrical stimulation. The above-mentioned multimodal strategy systematically optimizes the nerve regeneration efficiency by matching the injury types (such as short-distance defects requiring high-conductivity coatings and long-distance defects relying on wireless piezoelectric stimulation) and microenvironment characteristics (ischemia, inflammation, etc.).1–5 Suitable electrical performances were important to efficient ES during repair since they instructed cell progress and transfer via mechanisms, like electrotaxis and galvanotaxis. This has prospects in wound healing and tissue growth.6 Polycaprolactone (PCL), with its biodegradability, low melting point, and ease of molding, has become a popular material for nerve and bone tissue engineering, especially when combined with 3D printing technology.7–9
 | ||
| Fig. 1 Application forms and application scenarios of some conductive materials in peripheral nerve injury repair. Created in BioRender. Weng, Z. (2025) https://BioRender.com/r7xcmdn. | ||
NGCs, as critical scaffolds in neural tissue engineering, aim to bridge peripheral nerve gaps and promote nerve regeneration and functional recovery. Ideal nerve conduits should mimic the electrophysiological environment of natural nerves, provide a three-dimensional structure suitable for nerve regeneration, and exhibit excellent biomechanical properties and biocompatibility.
Though the peripheral nervous system (PNS) possesses some self-regenerative abilities, numerous patients undergo incomplete function recovery even after initial surgical therapy. In terms of nerve repair, the utilization of conductive materials encounters challenges such as improving conductivity, biocompatibility, and mechanical properties while controlling degradation rates. Furthermore, the effects of electrical stimulation parameters, encompassing frequency, intensity, and duration, on nerve regeneration remain unclear. Follow-up studies should identify the optimum parameters for the best effect of nerve repair. Our review emphasizes the innovative applications of diverse electroactive biomaterials to boost targeted nerve repair and comprehend the newest progress in conductive nerve conduits.
2 Mechanisms of peripheral nerve injury repair
Indeed, the PNS works as an important bridge between the central nervous system (CNS) and various body parts. The PNS takes the responsibility for transmitting signals from the CNS, including brains and spinal cords to target organs and tissues in the entire body.10 In the PNS, sensory neurons transmit the information from the sensory receptor to the CNS, whereas motor neurons express signals from the CNS to the effector organ, like skeletal muscles, to facilitate movement.11 Neurons consist of multiple components, including the cell body (soma), rough endoplasmic reticulum (responsible for protein synthesis), Golgi apparatus (storage of signaling molecules), mitochondria (energy production), dendrites (signal reception), and axons (signal transmission). Axons are covered by myelin sheaths, which enhance signal transmission speed. Signals travel along axons to their terminals, where neurotransmitters are released into the synaptic cleft to communicate with adjacent cells.12In the PNS, several non-neuronal cells interact with neurons to facilitate signal transmission and nerve repair. As the principal glial cells in the PNS, Schwann cells (SCs) release various growth factors, like nerve growth factor (NGF) and glial cell-derived neurotrophic factor (GDNF), in an effort to back up nerve regeneration. What's more, endothelial cells and fibroblasts maintain structural integrity and nutrition of peripheral nerves, whereas macrophages help to clear degraded myelin and axons.13
In light of the degree of demyelination and injury to axons and ambient tissues, the Seddon classification system categorizes peripheral neuron damages into neurapraxia, axonotmesis, and neurotmesis. Featured by localized demyelination with no axon or tissue damage, neurapraxia becomes the most appropriate form. Despite an intact epineurium, axonotmesis involves localized demyelination and axonal injury. Neurotmesis presents the acutest nerve damage, with complete transection of connective tissue layers and axons. Sunderland has expanded this categorization to distinguish the severity of connective tissue damage.
Although peripheral nerves have a natural repair mechanism, the process is highly slow at a speed of just 1 mm per day.14 Such a process includes three basic phases: Wallerian degeneration, axonal regeneration, and reinnervation of target organs. Wallerian degeneration is a critical process following axonal transection or nerve rupture, where nerve endings retract and inflammatory responses result in fibrin deposition and scar formation.15 This is accompanied by the clearance of debris by macrophages and Schwann cells (SCs), which phagocytose degenerated axons and myelin, initiating the Wallerian degeneration process. As axons regenerate from the proximal to the distal ends, they form growth cones to direct their growth, aided by SCs that proliferate to form “Bands of Bungner” and create an environment rich in extracellular matrix (ECM) proteins, cytokines, chemokines, and neurotrophic factors, all of which are essential for optimal axonal regeneration.16 Once new axonal sprouts regrow within these bands, SCs remyelinate the regenerated axons, restoring nerve impulse conduction and completing the nerve regeneration process, which is crucial for functional recovery.17
However, the slow rate of nerve regeneration and chronic denervation can lead to progressive muscle fiber atrophy in target organs, potentially preventing regenerated nerves from forming functional connections and resulting in permanent functional deficits.18 Nonetheless, functional recovery remains incomplete in some cases, with persistent motor or sensory deficits, chronic pain, and muscle atrophy, which can lead to lifelong disabilities. Additionally, autologous nerve grafting involves issues such as donor site morbidity, size mismatch between donor and injured nerves, and limited donor availability, constraining its clinical application.19 In consequence, there exists a pressing demand to formulate artificial nerve graft alternatives showing stable material origins and regeneration ratios relative to or surpassing those of autologous nerve transplantation.
3 Mechanisms of conductive materials in peripheral nerve injury repair
3.1 Electrophysiological simulation and promotion of nerve regeneration by conductive materials
Processing damaged nerves with ES becomes an underlying tactic for boosting peripheral nerve regeneration (PNR).20 Conductive materials are key to nerve regeneration since they can simulate the electrophysiological environment in vivo of nerves and hence formulate a microenvironment amounting to natural physiological conditions for neurons.21–25 These simulations are crucial to facilitate the progress and differentiation of neurons. Consequently, nerve repair will be accelerated. As an illustration, conductive polymers like polypyrrole (PPy) forge a microcurrent environment among nerve conduits. This boosts nerve cell progress and axonal extension. Such a microcurrent environment offers the imperative ES while mimicking the electrophysiological performances of nerves, therefore improving nerve repair.26It is demonstrated that electrical stimulation (ES) improves the early phases of regeneration, like axonal sprouting and neuronal survival.27 Rodent injury models prove that electrical stimulation can enhance regeneration across various types of nerve injuries, such as crush injuries,28 transections,29 and long-distance injuries.30 In vitro, such stimulation increases intracellular cAMP in dorsal root ganglia (DRG) neurons and NGF among Schwann cells.31 Sustained cAMP elevation enhances regenerative potential by increasing the expressions of cytoskeletal and neurotrophic proteins.32,33 Neurons need to revert to a “growth mode” characteristic of developmental stages to regenerate, and calcium influx serves as an early injury-induced signal triggering cell-autonomous mechanisms for axonal growth.34 McGregor et al. proposed that electrical stimulation mimics retrograde calcium waves following axonal injury, initiating regenerative cell-autonomous mechanisms.35 This activation of intrinsic regenerative mechanisms through ES involves ionic dynamics where sodium and calcium influx generates action potentials that travel retrogradely to the neuronal soma – a process mirroring the natural electrophysiological responses triggered by neural injury (Fig. 2).
 | ||
| Fig. 2 Electrical stimulation proximal to the injury site stimulates the upregulation of RAG through a calcium-dependent mechanism. Increased expression of BDNF and trkB drives increased expression of cAMP which activates CREB to maximize the pro-regenerative axon phenotype, stimulating axonal sprouting and neuron survival. BDNF = brain derived neurotrophic factor; cAMP = cyclic adenosine monophosphate; CREB = cAMP response element binding protein; trkB = tyrosine receptor kinase B; pKA = phosphokinase A; GAP-43 = growth-associated protein; MAPK = mitogen-activated protein kinase.36 Reproduced from ref. 36 with permission from Elsevier Inc., copyright 2020. | ||
Conductive materials provide these critical signals by promoting the expression of neurotrophic factors, modulating intracellular signaling pathways, and remodeling the extracellular matrix, thereby facilitating axonal regeneration. Accordingly, conductive materials become key players in mimicking the electrophysiological environment and facilitating nerve repair.
3.2 Role of conductive materials in guiding cellular behavior and bioelectric signal transmission
In terms of nerve regeneration, the function of conductive materials offers physical guidance and electrochemical signals. Besides, they remarkably affect cell behavior and bioelectric signal transfer. When directing cell behavior, conductive materials communicate with ion channels on the cell membrane and react sensitively to variations in the electric field while impacting cell progress, differentiation, and transfer.37 Research has indicated that neurons grow preferentially along conductive tracks, a property guiding nerve repair. Actually, it has been proved that conductive materials contribute to cell proliferation,7 recover cell functions,8 and also affect stem cell differentiation.9 In consequence, the significance of conductive materials is highlighted in terms of guiding cell behavior.As to bioelectric signal transfer, conductive materials as important players in promoting interactions among neurons, are crucial to recover nerve function. In practice, it has shown that electrical stimulation facilitates axonal repair via mechanisms, like adjusting intracellular signaling pathways, remodeling the extracellular matrix, etc. To be specific, research has manifested that electrical stimulation incorporated into nerve conduit technology remarkably improves the PNR and axonal myelination while raising the sciatic nerve function indicator.38
Including graphene, polyaniline, and polypyrrole, conductive materials have indicated outstanding biocompatibility and likely boost nerve repair.39 The effect of conductive materials optimizes the ES parameters (e.g., intensity, frequency, and duration), and improves nerve regeneration in depth. The application of conductive polymer microelectrodes has achieved minimally invasive peripheral nerve interfaces and shows the potential of conductive polymers in biomedicine.
In addition, research on conductive materials involves refining the ES strategies. Research has shown that discrepant ES paradigms have different effects on nerve cells. Although proper ES is capable of triggering axonal progress, excessive direct current can block fiber proliferation.40 It is shown that low-frequency and low-current ES boost the repair of more mature nerve structures.40
As a result, conductive materials are beneficial for nerve regeneration in many manners: directing cell behavior, delivering bioelectric signals, and promoting intercellular interaction. Therefore, nerve function improves. These discoveries offer scientific evidence for usable conductive materials in nerve regeneration and are fundamental for follow-up studies.
4 Conductive biomaterials in nerve conduit fabrication
Conductive biomaterials integrated with smart NGCs are critical to the ES functionality in the active proliferation stage. During the preparation of NGCs, discrepant conductive biomaterials have distinctive advantages and mechanisms of application, and they all serve to enhance the performance of NGCs and facilitate nerve injury regeneration. The following outlines the underlying conductive biomaterials for the preparation of smart NGCs.4.1 Conductive nanoparticles
On account of exceptional biocompatibility and electrical conductivity, conductive nanoparticles are extensively applied to the preparation of NGCs. Including gold and silver, metallic nanoparticles have been explored for their excellent stability and electrical performances. Specifically, gold nanoparticles make electrons go through molecules and show great electrical, magnetic, and optical performances.41,42 Carbon-based nanomaterials, especially carbon nanotubes, show great potential in nerve regeneration, on account of their distinctive mechanical performances and electrical conductivity.43 Graphene nanomaterials (GNs) are key to nerve regeneration owing to their strong bonding, large surface area, and excellent electron transport rates.44,45 Prior studies have manifested that electrospun PCL nerve conduits stained with Ti3C2Tx MXene show outstanding biocompatibility and likely facilitate the recovery of nerve functionality in vitro and in vivo research In vitro experiments there is no toxic effect on cell proliferation or morphology for NGCs with Ti3C2Tx MXene and 12 weeks after implantation of MXene/PCL composite in rats, no significant accumulation of its degradation products (Ti4) is observed in the liver and kidney.46These nanoparticles offer electron transmission pathways while improving signal transport and enhancement among neurons. Hence, nerve injury repair will be accelerated.
4.2 Piezoelectric polymers
Including poly(3,4-ethylenedioxythiophene) (PEDOT) and polyvinylidene fluoride (PVDF), piezoelectric polymers are key players in nerve conduit preparation since they are capable of converting mechanical stresses into electrical signals. PVDF's piezoelectric stimulation contributes to neuronal regeneration. Electrospun PVDF fibers are applied to nerve tissue regeneration to simulate biochemical, chemical, and physiological performances of the natural ECM. Reportedly, PVDF fibers are conducive to monkey neural stem cells differentiated into glial cells and neurons.47 In contrast with PPy, PEDOT has elevated electrical conductivity, and biocompatibility, as well as superior antioxidative and conductive properties, and supports to formulate conductive nerve conduits to enhance the environment of nerve repair.484.3 Piezoelectric nanoparticles
Piezoelectric nanoparticles are a particular type of biomaterials, which can produce electrical pulses for mechanical stress without requiring electrical overstimulation.49 Such distinctive characteristic contributes to the PNR. As a durable 2D material showing outstanding biocompatibility and conductivity, black phosphorus (BP) has been proven to recover injured nerve electrical activity while boosting neuronal recovery.50 As a piezoelectric ceramic showing elevated electromechanical and piezoelectric coupling coefficients, lead zirconate titanate (PZT) has been applied to analyze wireless nerve cuffs yielding electrical pulses through ultrasound in an attempt to stimulate nerve activity.51 Due to excellent biocompatibility and low cytotoxicity, barium titanate nanoparticles (BTNPs) have been widely investigated as biomedical scaffold materials for neural progress and neurite outgrowth.52These nanoparticles react to external mechanical forces, producing electrical signals without requiring external ES. This provides novel possibilities for producing wireless nerve conduits. It is expected that they are key players in neural tissue engineering.
4.4 Organic conductive polymers
Including polyaniline (PANI) and polypyrrole (PPy), organic conductive polymers have exhibited prospective results in PNI repair owing to their simple productive processes, adaptability, and exceptional cell compatibility.53 The electrical conductivity of these polymers is mainly ascribed to their conjugated π-electron mechanisms. With the development of the conjugation structure, the delocalization of electrons improves and thus makes the polymer conduct electricity. Due to such a property, organic conductive polymers are beneficial to fabricating nerve conduits. The reason is that they can directly yield electrical signals to stimulate neuron progress and repair.Our prior research has revealed that conductive nerve conduits with PPy integrated with human placenta-derived mesenchymal stem cells (PDMSCs) formed to improve the therapy of the PNI. Assessments in vitro verified the outstanding biocompatibility of these substances. Animal trials unveiled that PPy-containing nerve conduits remarkably boosted the PNR26 (Fig. 3). Moreover, PANI shows distinctive properties in facilitating cell growth and adhesion. Reportedly, scaffolds combined with PANI improve neuron development, back up neural stem cell growth and differentiation, and even stimulate neurite outgrowth for external ES. Such characteristics make PANI a prospective candidate for analyzing artificial NGCs to restore injured peripheral nerve tissues.54–56
 | ||
| Fig. 3 Illustration of the preparation of PC–PCL NGCs. Firstly, the PCL micropattern films were formulated via the electrostatic writing technique. Secondly, the PPY was stained on the surface of PCL micropattern films to produce the PCL/PPY scaffolds. PDMSCs triggered into blood-like cells were loaded on the scaffolds. Ultimately, the PC–PCL NGCs were seeded into a mouse model to restore the peripheral nerve deficits.26 Reproduced from ref. 26 with permission from RSC, copyright 2024. | ||
4.5 Wireless stimulation technology combined with piezoelectric materials
Using the characteristics of piezoelectric materials, combined with ultrasonic, magnetoelectric coupling, light control and other external excitation methods, can achieve wireless and controllable electrical stimulation has attracted wide attention. This technology can autonomously control the time node of stimulation, stimulation duration and stimulation intensity according to the demand. Recently, some researchers have found that biocompatible PVDF–TrFE scaffolds have been fabricated, which use ultrasonic waves to generate mechanical deformation to activate the scaffolds, and then generate electrical stimulation of local tissues. Mechanical vibration can be combined with piezoelectric materials, which has a positive impact on the nerve repair ability after PNI. In addition to ultrasound, Zhang et al. designed a loaded core–shell magnetoelectric nanoparticles (NPs) hyaluronic acid/collagen biomimetic composite hydrogel (HA/Col) to construct a wireless external magnetic field in response to the electro microenvironment to generate radio stimulation and regulate nerve regeneration.57,585 Conductive nerve conduits: design, materials, and challenges
5.1 Advantages of conductive nerve conduits
Conductive nerve conduits facilitate nerve cell growth, migration, and differentiation by providing physical guidance and electrochemical signals. These conduits can alter the charge density and potential difference at the interface, influence the biological behavior of nerve cells, and improve the transmission of regenerative signals, thereby promoting peripheral nerve regeneration. Research on nerve conduits for repairing peripheral nerve defects covers multiple aspects, including material optimization, bioactive substance carrier design, cellular scaffold development, conduit structure and functionality regulation, and clinical applications. These diverse approaches provide a broad range of strategies for neural tissue engineering.5.2 The conductive materials in conductive nerve conduits
As key scaffolds in neural tissue engineering, NGCs aim to diminish peripheral nerve defects and promote nerve repair and function rehabilitation. These conduits should have physical and spatial properties customized to the defect position while tacking difficulties, like limited donor availability and the hazard of secondary surgeries.A perfect nerve conduit should duplicate the electrophysiological environment of nerves and provide a 3D structure for nerve repair while exhibiting outstanding biocompatibility and biomechanical properties to decrease inflammatory reactions and facilitate cell adhesion. Through NGCs, both nutrient exchange and waste evacuation occur, with degeneration speeds matching the rate of nerve repair to safeguard sustainable support till the new nerve tissue forms.59–61 Noticeably, conductive nerve conduits reveal physical guidance and electrochemical signals to back up the progress, transfer, and differentiation of neurons (Fig. 4), which proves the potential for facilitating nerve repair.
 | ||
| Fig. 4 Elements of an ideal intelligent nerve guidance conduit. Created in BioRender. Weng, Z. (2025) https://BioRender.com/6p35qj3. | ||
In the quest for optimum biomimetic scaffolds, studies have progressively highlighted conductive materials. External stimuli, encompassing light,62–64 electricity,65–68 and magnetic fields69,70 can modulate cell viability while triggering tissue regeneration. For example, in alternating magnetic fields, superparamagnetic iron oxide nanoparticles (SPIONs) distributed within nerve conduits can react to the magnetic field while boosting the axonal extension.66 Electrical signals, being integral to neural communication, can guide axonal growth along the direction of stimulation.46,69 Conductive electrospun fibers made of polystyrene/polyaniline (PS–PANi), integrated with the NGF and ES, have been shown to accelerate axonal extension of PC12 cells along the electric field direction. This suggests the potential for functional nerve tissue repair and regeneration.71
Even without external electrochemical stimulation, conductive materials can promote the differentiation of stem cells into nerve cells, a phenomenon closely linked to their electrical properties.72 Polypyrrole (PPy), with its high conductivity, biocompatibility, and biodegradability, has increasingly been used in developing artificial NGCs.73–76 Vijayavenkataraman et al. successfully fabricated PCL/PPy composite conductive nerve conduits using electrohydrodynamic jet (E-jet) 3D printing technology, guiding nerve axonal growth along fiber directions.77 Similarly, Qian et al. utilized the advantages of PCL in 3D printing to construct a multilayer porous nerve conduit, combining dopamine, amino acids, and graphene oxide (GO) with PCL.78,79 The complex structure achieved a balance between cell adhesion, conductivity, and mechanical strength.
Recent advancements in fabrication technologies have further expanded the application of conductive materials. For instance, Tsinghua University's Center for Bio-Manufacturing developed a cross-scale 3D printing platform capable of fabricating conductive multiscale fiber nerve conduits (MF-NGCs) with hierarchical structures ranging from nano-to-millimeter-scale fibers (Fig. 5).80 These MF-NGCs mimic the electro-mechanical properties of natural tissues, integrating stochastic PCL/collagen nanofibers for nutrient diffusion, longitudinal PCL microfibers for anisotropic guidance, and reduced graphene oxide (rGO)/PCL microfibers for electroactive support. This design not only enhances mechanical stability but also accelerates macrophage recruitment and vascularization, demonstrating substantial potential in bridging long-gap peripheral nerve injuries.
 | ||
| Fig. 5 Outline of a multipurpose conductive MF-NGC showing hierarchical fibers for the PNI therapy. The conductive MF-NGC is comprised of hierarchical fibers showing diameters between 500 nm and 120 μm, which were printed by electrospinning technologies and MEW.80 Reproduced from ref. 80 with permission from Wiley-VCH GmbH, copyright 2023. | ||
Carbon-based nanomaterials have also shown promise in translational research. Li et al. applied carbon nanotube/sericin nerve conduits to a 10 mm sciatic nerve transection model in mice. Combined with electrical stimulation (ES), these conduits promoted functional repair and structural recovery within 12 weeks, achieving outcomes comparable to autologous nerve transplantation (Fig. 6).81 Similarly, Lu et al. designed a biodegradable polydopamine/graphene composite conduit with micro-patterned grooves via micro-imprinting. In a sciatic nerve crush model, the conduit integrated with ES significantly enhanced myelin formation and accelerated functional recovery by 20-fold compared to controls (Fig. 7).82 These studies highlight the synergistic effects of conductive materials and ES in optimizing the regenerative microenvironment.
 | ||
| Fig. 6 Targeting gastrocnemius muscle atrophy and histological analyses at 8 and 12 weeks following surgery. (A) Macroscopic investigation into the targeting gastrocnemius muscles as the groups exhibited (scale bar = 1 cm). The PNI illuminates the gastrocnemius muscles of the mouse suffering through the PNI in the absence of conduits implantation. (B) Quantification of the corresponding weight of gastrocnemius muscle of the groups exhibited in (A) (n = 4 animals in each group at 8 week postsurgery, n = 6 animals in each group at 12 week postsurgery). (C) Cross sections showing Masson trichrome staining of gastrocnemius muscles as the groups indicated (scale bar = 20 μm). (D) Quantitative analysis on the diameter of gastrocnemius muscles among the cross sections of the groups exhibited in (C) (n = 4 animals in each group at 8 week postsurgery, n = 6 animals in each group at 12 week postsurgery). (E) Quantitative measure of the ratio of the collagen fiber region among cross sections of the gastrocnemius muscles (n = 4 animals in each group at 8 week postsurgery, n = 6 animals in each group at 12 week postsurgery). *p < 0.05; **p < 0.01; ***p < 0.001; N.S., not significant; data are denoted as mean ± standard error of mean; one-way ANOVA.81 Reproduced from ref. 81 with permission from American Chemical Society, copyright 2020. | ||
 | ||
| Fig. 7 Diagram illuminating the production of poly(L-lactide-co-caprolactone) (PLCL) and graphene nanosheet (GN) films showing stripe micropatterns and polydopamine (PDA) modification and their adaptability in vitro and in vivo to boost nerve regeneration.82 Reproduced from ref. 82 with permission from John Wiley & Sons, copyright 2023. | ||
While rodent models remain the primary platform for rapid screening of conductive nerve conduits due to their cost-effectiveness and short experimental cycles, non-human primates offer critical insights for clinical translation.83 This emphasizes the importance of using primates in the disease model to bridge the gap between laboratory research and clinical application.
5.3 The challenges of conductive nerve conduits
Despite the significant potential of conductive nerve conduits, they face several challenges. These include difficulties in material processing, degradation control, optimization of electrical stimulation parameters, effectiveness in repairing long-distance nerve injuries, and the long-term biocompatibility of conductive materials.In vitro experiments have shown that conduits with electrical stimulation can effectively direct Schwann cell adhesion, migration, and elongation and facilitate the expression of nerve growth factors.84 For example, conductive electrospun fibers made of polystyrene/polyaniline (PS–PANi) combined with electrical stimulation have been shown to accelerate axonal extension in PC12 cells, demonstrating potential for functional neural tissue repair. Polypyrrole (PPy), with its biocompatibility, biodegradability, and high conductivity, has been increasingly applied to the progress of artificial nerve conduits.
For instance: a research team from Tsinghua University's School of Materials developed a biodegradable self-powered nerve repair conduit composed of a degradable galvanic cell and an artificial conduit. This conduit provides both structural and electrical field guidance, promoting peripheral nerve regeneration. In vivo experiments on rats demonstrated that the device could provide a sustained electric field for approximately three days, significantly enhancing nerve tissue repair and motor function85 (Fig. 8). However, its short-term electric field duration still has some limitations for long-distance nerve repair and clinical transformation. Future research must focus on the optimization of conductive material design and their ability to monitor electrical signals in real-time within the complex in vivo microenvironments. Interdisciplinary collaboration can accelerate the application and clinical translation of conductive materials in nerve repair.
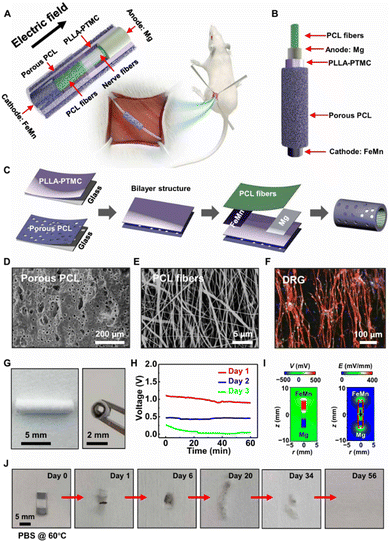 | ||
| Fig. 8 (A) Illustration of the experiment for sciatic nerve regeneration. The experiment consists of porous PCL (∼350 μm, 4.7 × 10 mm), PLLA–PTMC (∼300 μm, 4.7 × 10 mm), a Mg–FeMn galvanic cell (FeMn ∼ 1.5 μm, 4.7 × 3 mm; Mg ∼ 3.5 μm, 4.7 × 3 mm), and electrospun PCL fibers (∼30 μm, 4.7 × 10 mm). (B) Explored illustration of the experiment. (C) Preparation procedure of the experiment. (D) SEM representation of porous PCL. (E) SEM representation of electrospun orientational PCL fibers. (F) Confocal representation of the guided neurite growth of DRG neurons cultivated on the orientational PCL fibers (day 7). Immunohistochemical staining: nuclei (DAPI, blue), axons (β-tubulin, red), and Schwann cells (S100, green). (G) Representation of the electroactive experiment: front view (left) and side view (right). (H) The OCV measured in vivo of the experiment implanted. (I) Limited element analysis on voltage (left) and electric field (right) distribution surrounding the experiment on day 1 postoperatively. (J) Representations were gathered during diverse phases of the accelerative dissolution of the experiment (planar state) in PBS (pH 7.4, 60 °C). Photo credit: Liu Wang, Tsinghua University.84 Reproduced from ref. 84 with permission from Science Advances, copyright 2020. | ||
These examples illustrate the significant progress when conductive materials are applied to peripheral nerve regeneration. From MF-NGCs to carbon nanotube/sericin conduits and polydopamine/graphene composites, the research deepens our comprehension of nerve regeneration systems while offering new strategies for clinical treatment. With continued technological advancements, it is expected that conductive materials become a progressively crucial player in future neural restoration therapies.
6 Challenges and future outlook
In previous studies, we have designed a variety of bioactive materials, including non-conductive bioactive materials such as drug-loaded collagen hydrogel catheter and drug-loaded microsphere gel catheter, and also designed a conductive nerve scaffold of MXene–PCL to transmit physiological nerve electrical signals in damaged nerve tissues, induce angiogenesis, and stimulate nerve regeneration, with promising results.45 However, in terms of conductive materials, the utilization of conductive materials is confronted with several challenges, including improving the biocompatibility, conductivity, and mechanical performances of these materials while controlling their degradation rates. The effects of ES parameters, like frequency, intensity, and duration, on nerve repair are still insufficiently comprehended. Subsequent studies should gauge the optimum ES parameters for the best outcomes of nerve repair.In addition, the long-standing biosafety of conductive materials needs in-depth assessment through long animal research and clinical tests for their security and efficiency. The associated research and application need cooperation throughout disciplines, such as materials science and biomedical engineering. The interdisciplinary progresses can drive the translation and application of conductive materials in the domain of nerve regeneration.
A key future orientation includes the progress of intelligent nerve conduits. Such conduits should react to variations within the neural microenvironment while modulating their ES tactics to refine nerve repair.
7 Conclusions
With respect to the PNI repair, artificial electrically conductive nerve conduits have proved sole advantages of facilitating nerve repair, notably for long-gap nerve defects. On account of outstanding biocompatibility, conductive materials, like graphene, polyaniline, and polypyrrole have drawn remarkable attention. Integrated with polymer blends or surface modification technologies like coatings and chemical vapor deposition, these materials need conductivity to improve neural stem cell adhesion, growth, and differentiation and hence prominently enhance nerve repair outcomes, which accelerates the PNI recovery and decreases the treatment duration, while increasing cure rates and reducing patient burdens.Despite the great potential of conductive materials in the PNR, challenges persist all the time, like difficulties in processing materials, degeneration control, and long-ranging security assessment. Importantly, future research should pay more attention to refining material design while supervising electrical signals in real-time under intricate microenvironments in vivo. Interdisciplinary cooperation integrating knowledge from material science and biomedical engineering will drive the translation and application of conductive materials in nerve regeneration. In addition to the advancement of intelligent nerve conduits, the accurate modulation of the ES tactics will deeply facilitate this domain. These endeavors intend to formulate artificial nerve graft alternatives surpassing autologous nerve transplantation and present patients with more efficient therapies.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
All authors helped in performing the research. JW, JF and ZW wrote the paper and contributed equally to this work; LN, YC and JS collect the literature; FC and JL are corresponding authors and revised it critically for important intellectual content. All authors have read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
JW, JF and ZW thanked Tongji University for providing suitable infrastructure for document retrieval. The authors acknowledge financial support from the financial support from the Science and Technology Commission of Shanghai Municipality (23ZR1449000).Notes and references
- Y. Zhao, Y. Liu and S. Kang, et al., Peripheral nerve injury repair by electrical stimulation combined with graphene-based scaffolds, Front. Bioeng. Biotechnol., 2024, 12, 1345163 CrossRef PubMed.
- G. Song, Y. Wang and S. Wang, et al., Research progress in artificial nerve conduit prepared by carbon nanotube-doped polymer, J. Textil. Res., 2023, 44(11), 232–239 Search PubMed.
- F. Qi, R. Liao and Y. Shuai, et al., A conductive network enhances nerve cell response, Addit. Manuf., 2022, 52, 102694 CAS.
- W. Mao, E. Lee and W. Cho, et al., Cell-directed assembly of luminal nanofibril fillers in nerve conduits for peripheral nerve repair, Biomaterials, 2023, 301, 122209 CrossRef CAS PubMed.
- C. Hu, B. Liu and X. Huang, et al., Sea Cucumber-Inspired Microneedle Nerve Guidance Conduit for Synergistically Inhibiting Muscle Atrophy and Promoting Nerve Regeneration, ACS Nano, 2024, 18(22), 14427–14440 CrossRef CAS PubMed.
- J. Y. Wong, R. Langer and D. E. Ingber, Electrically Conducting Polymers Can Noninvasively Control the Shape and Growth of Mammalian-Cells, Proc. Natl. Acad. Sci. U. S. A., 1994, 91(8), 3201–3204 CrossRef CAS PubMed.
- J. X. Zhang, Z. Y. Liu and J. Wang, et al., 3D Coaxially Printing rGO Aerogel-Based Biocompatible Fiber for Peripheral Nerve Regeneration, Adv. Fiber Mater., 2024, 6(3), 713–726 CrossRef CAS.
- Z. Javkhlan, S. H. Hsu and R. S. Chen, et al., Original Article Interactions of neural-like cells with 3D-printed polycaprolactone with different inner diameters for neural regeneration, J. Dent. Sci., 2024, 19(2), 1096–1104 CrossRef PubMed.
- S. Zhou, J. Y. Xing and L. Sun, et al., Melt Electrowriting: A Promising 3D Printing Technology for Cartilage and Osteochondral Repair, Adv. Ther., 2024, 7(2), 1431–1444 Search PubMed.
- M. Catala and N. Kubis, Gross anatomy and development of the peripheral nervous system, Hand Clin., 2013, 115, 29–41 Search PubMed.
- C. R. Carvalho, J. M. Oliveira and R. L. Reis, Modern Trends for Peripheral Nerve Repair and Regeneration: Beyond the Hollow Nerve Guidance Conduit, Front. Bioeng. Biotechnol., 2019, 7, 337 CrossRef PubMed.
- A. S. Saab, Z. J. Looser and M. J. P. Barrett, et al., Activity-induced lactate increase in myelinated axons, Glia, 2017, 65, E214–E5 Search PubMed.
- Y. Qian, Z. Yan and T. Ye, et al., Decoding the regulatory role of ATP synthase inhibitory factor 1 (ATPIF1) in Wallerian degeneration and peripheral nerve regeneration, Exploration, 2024, 4(6), 20230098 CrossRef CAS PubMed.
- G. Stoll and H. W. Müller, Nerve injury, axonal degeneration and neural regeneration: basic insights, Brain Pathol., 1999, 9(2), 313–325 CrossRef CAS PubMed.
- K. Zhang, M. Jiang and Y. Fang, The Drama of Wallerian Degeneration: The Cast, Crew, and Script, Annu. Rev. Genet., 2021, 55, 93–113 CrossRef CAS PubMed.
- B. Zheng and M. H. Tuszynski, Regulation of axonal regeneration after mammalian spinal cord injury, Nat. Rev. Mol. Cell Biol., 2023, 24(6), 396–413 CrossRef CAS PubMed.
- T. Gordon, Peripheral Nerve Regeneration and Muscle Reinnervation, Int. J. Mol. Sci., 2020, 21(22), 8652 CrossRef CAS PubMed.
- L. H. Luo, Y. He and L. Jin, et al., Application of bioactive hydrogels combined with dental pulp stem cells for the repair of large gap peripheral nerve injuries, Bioact. Mater., 2021, 6(3), 638–654 CAS.
- B. Fogli, N. Corthout and A. Kerstens, et al., Imaging axon regeneration within synthetic nerve conduits, Sci. Rep., 2019, 9, 10095 CrossRef PubMed.
- Z. Saadat, Z. Rojhani-Shirazi and L. Abbasi, Dose postural control improve following application of transcutaneous electrical nerve stimulation in diabetic peripheral neuropathic patients? A randomized placebo control trial, Diabetol. Metab. Syndr., 2017, 11(suppl. 2), S755–S7 CrossRef PubMed.
- Z. Liu, X. Wan and Z. L. Wang, et al., Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications, Adv. Mater., 2021, 33(32), e2007429 CrossRef PubMed.
- J. Kim, J. Jeon and J. Y. Lee, et al., Electroceuticals for Regeneration of Long Nerve Gap Using Biodegradable Conductive Conduits and Implantable Wireless Stimulator, Adv. Sci., 2023, 10(24), e2302632 CrossRef PubMed.
- H. Xuan, S. Wu and Y. Jin, et al., A Bioinspired Self-Healing Conductive Hydrogel Promoting Peripheral Nerve Regeneration, Adv. Sci., 2023, 10(28), e2302519 CrossRef PubMed.
- Y. Sun, Y. Zhang and Y. Guo, et al., Electrical aligned polyurethane nerve guidance conduit modulates macrophage polarization and facilitates immunoregulatory peripheral nerve regeneration, J. Nanobiotechnol., 2024, 22(1), 244–259 CrossRef CAS PubMed.
- J. Fang, L. Nan and K. Song, et al., Application and progress of bionic scaffolds in nerve repair: a narrative review, Adv. Tech. Neurosci., 2024, 1(1), 43–50 Search PubMed.
- F. Zhang, L. Nan and J. Fang, et al., Nerve guide conduits promote nerve regeneration under a combination of electrical stimulation and RSCs combined with stem cell differentiation, J. Mater. Chem. B, 2024, 12(45), 11636–11647 RSC.
- D. Lal, L. T. Hetzler and N. Sharma, et al., Electrical stimulation facilitates rat facial nerve recovery from a crush injury, Otolaryngol. Head Neck Surg., 2008, 139(1), 68–73 CrossRef PubMed.
- B. Zorko, J. Rozman and A. Seliskar, Influence of electrical stimulation on regeneration of the radial nerve in dogs, Acta Vet. Hung., 2000, 48(1), 99–105 CAS.
- N. M. Geremia, T. Gordon and T. M. Brushart, et al., Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression, Exp. Neurol., 2007, 205(2), 347–359 CrossRef CAS PubMed.
- J. Huang, L. Lu and X. Hu, et al., Electrical stimulation accelerates motor functional recovery in the rat model of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally oriented microchannels, Neurorehabilit. Neural Repair, 2010, 24(8), 736–745 CrossRef PubMed.
- E. Udina, M. Furey and S. Busch, et al., Electrical stimulation of intact peripheral sensory axons in rats promotes outgrowth of their central projections, Exp. Neurol., 2008, 210(1), 238–247 CrossRef PubMed.
- N. J. Batty, K. K. Fenrich and K. Fouad, The role of cAMP and its downstream targets in neurite growth in the adult nervous system, Neurosci. Lett., 2017, 652, 56–63 CrossRef CAS PubMed.
- B. Y. Lau, S. M. Fogerson and R. B. Walsh, et al., Cyclic AMP promotes axon regeneration, lesion repair and neuronal survival in lampreys after spinal cord injury, Exp. Neurol., 2013, 250, 31–42 CrossRef CAS PubMed.
- F. M. Mar, A. Bonni and M. M. Sousa, Cell intrinsic control of axon regeneration, EMBO Rep., 2014, 15(3), 254–263 CrossRef CAS PubMed.
- C. E. McGregor and A. W. English, The Role of BDNF in Peripheral Nerve Regeneration: Activity-Dependent Treatments and Val66Met, Front. Cell. Neurosci., 2018, 12, 522–543 CrossRef CAS PubMed.
- K. J. Zuo, T. Gordon and K. M. Chan, et al., Electrical stimulation to enhance peripheral nerve regeneration: update in molecular investigations and clinical translation, Exp. Neurol., 2020, 332, 113397 CrossRef PubMed.
- J. Wang, H. Wang and X. Mo, et al., Reduced Graphene Oxide-Encapsulated Microfiber Patterns Enable Controllable Formation of Neuronal-Like Networks, Adv. Mater., 2020, 32(40), e2004555 CrossRef PubMed.
- S. N. Iwasa, X. Liu and H. E. Naguib, et al., Electrical Stimulation for Stem Cell-Based Neural Repair: Zapping the Field to Action, Eneuro, 2024, 11(9), 0183 CrossRef PubMed.
- C. Dong, A. Carnicer-Lombarte and F. Bonafe, et al., Electrochemically actuated microelectrodes for minimally invasive peripheral nerve interfaces, Nat. Mater., 2024, 23(7), 969–976 CrossRef CAS PubMed.
- S. Vijayavenkataraman, Nerve guide conduits for peripheral nerve injury repair: a review on design, materials and fabrication methods, Acta Biomater., 2020, 106, 54–69 CrossRef CAS PubMed.
- Y. Tauran, A. Brioude and A. W. Coleman, et al., Molecular recognition by gold, silver and copper nanoparticles, World J. Biol. Chem., 2013, 4(3), 35–63 CrossRef PubMed.
- M. Rahman, T. M. Dip and R. Padhye, et al., Review on electrically conductive smart nerve guide conduit for peripheral nerve regeneration, J. Biomed. Mater. Res., Part A, 2023, 111(12), 1916–1950 Search PubMed.
- H. Zare, S. Ahmadi and A. Ghasemi, et al., Carbon Nanotubes: Smart Drug/Gene Delivery Carriers, Int. J. Nanomed., 2021, 16, 1681–1706 CrossRef PubMed.
- X. Fang, H. Guo and W. Zhang, et al., Reduced graphene oxide-GelMA-PCL hybrid nanofibers for peripheral nerve regeneration, J. Mater. Chem. B, 2020, 8(46), 10593–10601 RSC.
- Y. Hui, Z. Yan and H. Yang, et al., Graphene Family Nanomaterials for Stem Cell Neurogenic Differentiation and Peripheral Nerve Regeneration, ACS Appl. Bio Mater., 2022, 5(10), 4741–4759 CrossRef CAS PubMed.
- L. P. Nan, Z. Lin and F. Wang, et al., Ti3C2Tx MXene-coated electrospun PCL conduits for enhancing neurite regeneration and angiogenesis, Front. Bioeng. Biotechnol., 2022, 10, 850650 CrossRef PubMed.
- O. Gryshkov, F. Al Halabi and A. I. Kuhn, et al., PVDF and P(VDF-TrFE) Electrospun Scaffolds for Nerve Graft Engineering: A Comparative Study on Piezoelectric and Structural Properties, and In Vitro Biocompatibility, Int. J. Mol. Sci., 2021, 22(21), 11373 CrossRef CAS PubMed.
- A. Magaz, B. F. Spencer and J. G. Hardy, et al., Modulation of Neuronal Cell Affinity on PEDOT-PSS Nonwoven Silk Scaffolds for Neural Tissue Engineering, ACS Biomater. Sci. Eng., 2020, 6(12), 6906–6916 CrossRef CAS PubMed.
- S. Chen, X. Tong and Y. Huo, et al., Piezoelectric Biomaterials Inspired by Nature for Applications in Biomedicine and Nanotechnology, Adv. Mater., 2024, 36(35), e2406192 CrossRef PubMed.
- W. Qi, R. Zhang and Z. Wang, et al., Advances in the Application of Black Phosphorus-Based Composite Biomedical Materials in the Field of Tissue Engineering, Pharmaceuticals, 2024, 17(2), 242–281 CrossRef CAS PubMed.
- D. K. Piech, B. C. Johnson and K. Shen, et al., A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication, Nat. Biomed. Eng., 2020, 4(2), 207–222 CrossRef PubMed.
- M. Candito, E. Simoni and E. Gentilin, et al., Neuron Compatibility and Antioxidant Activity of Barium Titanate and Lithium Niobate Nanoparticles, Int. J. Mol. Sci., 2022, 23(3), 1761 CrossRef CAS PubMed.
- X. Yao, Y. Qian and C. Fan, Electroactive nanomaterials in the peripheral nerve regeneration, J. Mater. Chem. B, 2021, 9(35), 6958–6972 RSC.
- F. F. F. Garrudo, R. J. Linhardt and F. C. Ferreira, et al., Designing Electrical Stimulation Platforms for Neural Cell Cultivation Using Poly(aniline): Camphorsulfonic Acid, Polymers, 2023, 15(12), 2674 CrossRef CAS PubMed.
- M. Mohseni, A. R. SA and F. H. Shirazi, et al., Preparation and characterization of self-electrical stimuli conductive gellan based nano scaffold for nerve regeneration containing chopped short spun nanofibers of PVDF/MCM41 and polyaniline/graphene nanoparticles: physical, mechanical and morphological studies, Int. J. Biol. Macromol., 2021, 167, 881–893 CrossRef CAS PubMed.
- S. Shrestha, S. R. Jang and B. K. Shrestha, et al., Engineering 2D approaches fibrous platform incorporating turmeric and polyaniline nanoparticles to predict the expression of betaIII-Tubulin and TREK-1 through qRT-PCR to detect neuronal differentiation of PC12 cells, Mater. Sci. Eng., C, 2021, 127, 112176 CrossRef CAS PubMed.
- Y. Zhang, S. Chen and Z. Xiao, et al., Magnetoelectric nanoparticles incorporated biomimetic matrix for wireless electrical stimulation and nerve regeneration, Adv. Healthcare Mater., 2021, 10(16), 2100695 CrossRef CAS PubMed.
- X. Zhou, A. Tang and C. Xiong, et al., Oriented graphene oxide scaffold promotes nerve regeneration in vitro and in vivo, Int. J. Nanomed., 2024, 2573–2589 CrossRef PubMed.
- A. Omidinia-Anarkoli, J. W. Ephraim and R. Rimal, et al., Hierarchical fibrous guiding cues at different scales influence linear neurite extension, Acta Biomater., 2020, 113, 350–359 CrossRef CAS PubMed.
- S. E. Thomson, C. Charalambous and C. A. Smith, et al., Microtopographical cues promote peripheral nerve regeneration via transient mTORC2 activation, Acta Biomater., 2017, 60, 220–231 CrossRef CAS PubMed.
- H. Gregory and J. B. Phillips, Materials for peripheral nerve repair constructs: natural proteins or synthetic polymers?, Neurochem. Int., 2021, 143, 104953 CrossRef CAS PubMed.
- Y. He, J. Leng and K. Li, et al., A multifunctional hydrogel coating to direct fibroblast activation and infected wound healing via simultaneously controllable photobiomodulation and photodynamic therapies, Biomaterials, 2021, 278, 121164 Search PubMed.
- Z. Y. Zhang, M. L. Jorgensen and Z. G. Wang, et al., 3D anisotropic photocatalytic architectures as bioactive nerve guidance conduits for peripheral neural regeneration, Biomaterials, 2020, 253, 120108 CrossRef CAS PubMed.
- F. Qi, R. Liao and L. Yang, et al., Photoexcited wireless electrical stimulation elevates nerve cell growth, Colloids Surf., B, 2022, 220, 112890 CrossRef CAS PubMed.
- G. Choe, U. G. Han and S. Ye, et al., Effect of Electrical Stimulation on Nerve-Guided Facial Nerve Regeneration, ACS Biomater. Sci. Eng., 2023, 9(6), 3512–3521 CrossRef CAS PubMed.
- F. Jin, T. Li and Z. D. Wei, et al., Biofeedback electrostimulation for bionic and long-lasting neural modulation, Nat. Commun., 2022, 13(1), 5302 CrossRef CAS PubMed.
- F. Jin, T. Li and T. Yuan, et al., Physiologically Self-Regulated, Fully Implantable, Battery-Free System for Peripheral Nerve Restoration, Adv. Mater., 2021, 33(48), 2104175 CrossRef CAS PubMed.
- H. H. Yan, Y. Wang and L. L. Li, et al., A micropatterned conductive electrospun nanofiber mesh combined with electrical stimulation for synergistically enhancing differentiation of rat neural stem cells, J. Mater. Chem. B, 2020, 8(13), 2673–2688 RSC.
- J. L. Funnell, A. M. Ziemba and J. F. Nowak, et al., Assessing the combination of magnetic field stimulation, iron oxide nanoparticles, and aligned electrospun fibers for promoting neurite outgrowth from dorsal root ganglia in vitro, Acta Biomater., 2021, 131, 302–313 Search PubMed.
- P. Ghaderinejad, N. Najmoddin and Z. Bagher, et al., An injectable anisotropic alginate hydrogel containing oriented fibers for nerve tissue engineering, Chem. Eng. J., 2021, 420, 130465 CrossRef CAS.
- J. G. Zhang, K. X. Qiu and B. B. Sun, et al., The aligned core-sheath nanofibers with electrical conductivity for neural tissue engineering, J. Mater. Chem. B, 2014, 2(45), 7945–7954 RSC.
- E. A. Ostrakhovitch, J. C. Byers and K. D. O'neil, et al., Directed differentiation of embryonic P19 cells and neural stem cells into neural lineage on conducting PEDOT-PEG and ITO glass substrates, Arch. Biochem. Biophys., 2012, 528(1), 21–31 CrossRef CAS PubMed.
- R. Trueman, O. Guillemot-Legris and H. Lancashire, et al., Aligned and Conductive 3d Collagen/Ppy Scaffolds for Peripheral Nerve Tissue Engineering, Tissue Eng., Part A, 2023, 29(11–12), 4040–4053 Search PubMed.
- C. J. Shuai, F. Ding and X. S. Chen, et al., Electromagnetic induction drives electron-hole separation in an optoelectronic nerve conduit to accelerate nerve repair, Mater. Chem. Front., 2024, 8(22), 3758–3769 Search PubMed.
- I. Ozcicek, N. Aysit and Z. Balcikanli, et al., Development of BDNF/NGF/IKVAV Peptide Modified and Gold Nanoparticle Conductive PCL/PLGA Nerve Guidance Conduit for Regeneration of the Rat Spinal Cord Injury, Macromol. Biosci., 2024, 24(5), e2300453 Search PubMed.
- S. Farshid, P. Mofazali and A. Samadi, et al., Revitalizing the nervous system: Exploring polypyrrole-based composites in nerve regeneration, Mater. Today Commun., 2024, 41, 110685 Search PubMed.
- S. Vijayavenkataraman, S. Kannan and T. Cao, et al., 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair, Front. Bioeng. Biotechnol., 2019, 7, 266 Search PubMed.
- Y. Qian, X. T. Zhao and Q. X. Han, et al., An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration, Nat. Commun., 2018, 9(1), 323 CrossRef PubMed.
- X. Yao, Z. Yan and X. Wang, et al., The influence of reduced graphene oxide on stem cells: a perspective in peripheral nerve regeneration, Regen. Biomater., 2021, 8(4), rbab032 CrossRef PubMed.
- Y. Fang, C. Wang and Z. Liu, et al., 3D Printed Conductive Multiscale Nerve Guidance Conduit with Hierarchical Fibers for Peripheral Nerve Regeneration, Adv. Sci., 2023, 10(12), e2205744 CrossRef PubMed.
- X. Li, W. Yang and H. Xie, et al., CNT/Sericin Conductive Nerve Guidance Conduit Promotes Functional Recovery of Transected Peripheral Nerve Injury in a Rat Model, ACS Appl. Mater. Interfaces, 2020, 12(33), 36860–36872 CrossRef CAS PubMed.
- S. Lu, W. Chen and J. Wang, et al., Polydopamine-Decorated PLCL Conduit to Induce Synergetic Effect of Electrical Stimulation and Topological Morphology for Peripheral Nerve Regeneration, Small Methods, 2023, 7(2), e2200883 CrossRef PubMed.
- S. Wakao, T. Hayashi and M. Kitada, Long-term observation of auto-cell transplantation in non-human primate reveals safety and efficiency of bone marrow stromal cell-derived Schwann cells in peripheral nerve regeneration., Exp. Neurol., 2010, 223(2), 537–547 CrossRef CAS PubMed.
- S. Wu, Y. Qi and W. Shi, et al., Electrospun conductive nanofiber yarns for accelerating mesenchymal stem cells differentiation and maturation into Schwann cell-like cells under a combination of electrical stimulation and chemical induction, Acta Biomater., 2022, 139, 91–104 CrossRef CAS PubMed.
- L. Wang, C. Lu and S. Yang, et al., A fully biodegradable and self-electrified device for neuroregenerative medicine, Sci. Adv., 2020, 6(50), eabc6686 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |

