Open Access Article
Open Access ArticleEnzyme-immobilized graphene oxide-based electrochemical biosensor for glutathione detection
Neeta Ukiradeab,
Upasana Choudharic,
Sunil Bhapkard,
Umesh Jadhavd,
Shweta Jagtap *c and
Sunit Rane
*c and
Sunit Rane *a
*a
aCentre for Materials for Electronics Technology (C-MET), Panchawati, Off. Dr Bhabha Road, Pune-411008, India. E-mail: sunitrane@yahoo.com
bPratibha College of Commerce and Computer Studies, Pune, India
cDepartment of Electronic and Instrumentation Science, Savitribai Phule Pune University, Pune-411007, India. E-mail: shweta.jagtap@gmail.com
dDepartment of Microbiology, Savitribai Phule Pune University, Pune-411007, India
First published on 22nd April 2025
Abstract
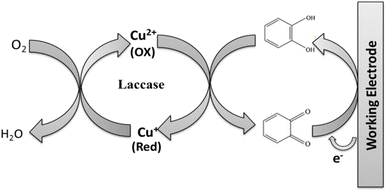
Glutathione acts as a natural antioxidant in the human body and the reduction of its content is a sign of oxidative stress. In this study, a sensitive electrochemical sensor was developed using laccase enzyme immobilized onto graphene oxide (GO) for detection of glutathione. The surface of the indium tin oxide (ITO) was modified with GO via a drop casting method. Subsequently, laccase was immobilized onto the modified ITO decorated with GO. The modified electrode was characterized using field-emission scanning electron microscopy (FESEM), X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The FTIR spectra of laccase/GO confirmed the successful immobilization of laccase onto GO sheets. FESEM analysis revealed the transformation from a layered, wrinkled structure to a compact, smooth surface with spherical laccase, confirming successful enzyme integration. Raman analysis confirmed successful laccase immobilization onto GO, as evidenced by structural changes in the D and G bands, highlighting the modification of the material. The cyclic voltammetry measurements revealed that laccase/GO/ITO exhibited better electrocatalytic activity toward oxidation of GSH in acetate buffer solution than the bare ITO electrode. This newly developed electrode exhibited a good response to glutathione with a wide linear range from 1 μM to 100 μM, a limit of detection of 0.89 μM and high sensitivity (6.51 μA μM−1). Furthermore, it exhibited excellent selectivity, repeatability, and long-term stability. The modified electrode was successfully used for the detection of GSH in a real sample, offering satisfactory results.
Introduction
Development of biosensors is receiving a lot of attention in the biomedical and healthcare fields owing to its extensive use in medicine, clinical care and food processing. A biosensor is an analytical device that measures the changes in biological elements or materials, such as cells, tissues, enzymes, and microorganisms, and converts them into electrical signals. The output of the transducer will either be current or voltage, depending on the kind of enzyme and components utilized in the biological element.1–3 Biosensors are made up of various elements, including an analyser, bio receptor, transducer, electronics, and a reader display. Novel nanobiosensors can be developed by combining biological sensing components with organic, inorganic or hybrid nanomaterials to detect chemical or biological substances. Rapid advances in nanotechnology have led to the development of innovative nanomaterials and nanodevices that hold promise for use in biomedical and healthcare applications in the future.4–8 The first biosensor was created in 1916 when proteins were immobilized on activated charcoal. Later, Clark furthered the technology for the amperometric detection of glucose.9 The advancement of nanomaterial-based biosensors opens a new way for the identification and real-time monitoring of biomarkers linked to a variety of diseases and their treatments. Electrochemical biosensors based on nanomaterials have changed the outlook for traditional biosensors by enhancing their performance in terms of sensitivity, stability, and selectivity for the detection of different biomolecules.10–12 Nanomaterials with special chemical and physical properties are advantageous for developing efficient biosensors owing to the high surface area for loading enzymes. Carbon-based nanomaterials, particularly graphene, have contributed to improved insights in the fields of bioengineering and medicine.13,14 In comparison with other materials, graphene is recognised as the lightest material, and it is used in practically every aspect of life owing to its exceptional qualities, including its high surface area, excellent electrical conductivity, extensive strength, simplicity of functionalization, superior thermal conductivity, chemical inertness, and gas impermeability.15–17 Graphene oxide (GO) has a significantly better capability for protein adsorption compared with other carbonaceous materials with a wide surface area. Its physicochemical characteristics and structure are said to be advantageous for alterations in enzyme activity. Enzymatic electrochemical biosensors have gained significant interest in recent times owing to their efficaciousness and stability.18,19 The human body is made up of several biomolecules that control physiological processes, including proteins, carbohydrates, nucleic acids, and amino acids. Abnormal concentrations of these biomolecules cause a number of diseases. As a result, selective and sensitive detection of these biomolecules is crucial for pharmacological compositions and clinical diagnostics.20,21 Glutathione (GSH) is a tripeptide of L-glutamyl-L-cysteinyl-glycine thiol molecule with a low intracellular molar mass and is an essential antioxidant cofactor for the metabolism of living cells. Glutathione concentration changes to low levels are connected to diseases such as leukaemia, pre-mature arteriosclerosis, diabetes, Alzheimer's, and AIDS-related dementia. As a result, there has been a growing interest in and demand for this biomarker's accurate detection during the past ten years.22 The preservation of enzyme activity, involvement in bioreductive reactions, transport of amino acids, and detoxification of free radicals, hydrogen peroxide, and toxins are all significant functions of reduced glutathione (GSH).23,24 Glutathione inhibits laccase, a frequently utilised multicopper oxidase for oxygen reduction.In this research, the developed biosensor utilizes the catalytic activity of laccase combined with the unique properties of GO to achieve high sensitivity and selectivity. The interaction between the analyte (glutathione) and the enzyme leads to measurable changes in the solution's properties, which are then detected by the biosensor. We focused on laccase's potential application as a glutathione sensor.25,26 In this study, we used an electrochemical biosensor based on laccase to examine the electrochemical behaviour of GSH. First, we synthesized GO which was deposited on the surface of bare indium tin oxide (ITO). Then, laccase was immobilized onto the electrode surface using a gelatin membrane, which was cross-linked by glutaraldehyde. Furthermore, the electrochemical behavior of the modified electrode was investigated by cyclic voltammetry. The results of this study indicate that GO may be used as a potential sensing material in conjunction with an enzyme to detect GSH. The modified electrode showed high selectivity for the determination of GSH in real samples.
Experimental
Materials
Glutathione (GSH), cysteine (CYS), dopamine (DA), ascorbic acid (AA), uric acid (UA), glutaraldehyde, and laccase were purchased from Sigma-Aldrich. Graphite powder, potassium permanganate, sulfuric acid (99.99%), hydrochloric acid, and sodium nitrate were purchased from Merck Company. All other compounds were analytical reagent grade and all solutions were made with deionized water. 0.1 M phosphate buffer solution (PBS, pH 7) was used as the background electrolyte. A stock solution of 1 mM GSH was prepared.Preparation of laccase/GO/ITO electrode
An ITO-coated piece of glass slide (0.5 × 0.5 cm2) was used as the substrate. Prior to usage, the substrate was cleaned using ultrasonic cleaning in deionized water and ethanol. GO was prepared using Hummer's method.27–29 Furthermore, GO was electrodeposited on the surface of bare ITO by the drop casting method. The electrode was dried under an infrared (IR) lamp until the solvent evaporated and the electrode surface was fully dry. In the next step, laccase was immobilized onto GO/ITO. For this purpose, 20 mg of laccase was diluted in 100 μL of phosphate buffer solution (pH 7.0). Then, 25 U of laccase and 12 mg of gelatin were mixed at 38 °C in potassium phosphate buffer (pH 7.0, 100 μL). Next, 25 μL of the mixed solution was spread over the modified electrode surface and allowed to dry at 4 °C for 1 h. Finally, it was immersed in 2.5% glutaraldehyde in phosphate buffer (50 mM, pH 7.0) for 5 min for cross-linkage.30 To evaluate the electrochemical behavior of the developed sensors, cyclic voltammetry was used with a three-electrode system with a working electrode of 0.25 cm2 electrochemical active surface area.Characterization
The crystallographic structure of the samples were studied using X-ray diffraction (XRD) analysis using a Bruker D8 Advance with a monochromator of CuKα radiation (λ = 1.541 87 Å) over a 2θ range of 5–80°. The surface morphology and microstructural analysis of the samples were performed using a NOVA NanoSEM 450 (Thermo Fisher Scientific, USA). The instrument was operated at an accelerating voltage ranging from 1 to 30 kV, depending on the sample's conductivity. Elemental composition analysis was performed using an energy-dispersive X-ray spectroscopy (EDS) system (Bruker XFlash 6I30). Fourier transform infrared spectroscopy (FTIR) was performed using a Shimadzu FTIR-8900 to identify the chemical functional groups present in all synthesized materials, covering a spectral range of 4000–400 cm−1. The K-Lyte potentiostat is used for conducting cyclic voltammetry (CV) experiments as part of electrochemical analyses. For the electrochemical measurements, a standard three-electrode cell assembly made up of an Ag/AgCl as reference electrode, a Pt counter electrode and an ITO working electrode.Results and discussion
(A) Physical characterization of the nanocomposite
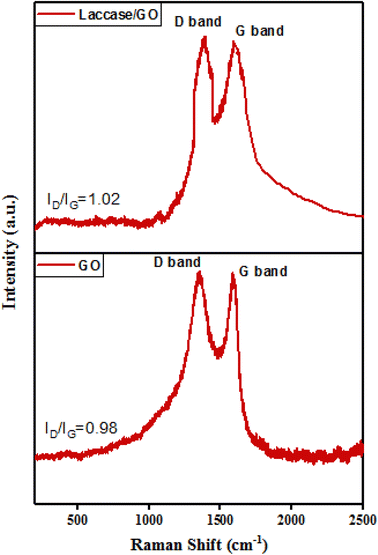
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching, and C–O stretching vibration, indicating that the graphene was oxidized and the GO product could be successfully dispersed in water.31 For laccase, the peak at 3284 cm−1 was caused by O–H stretching in laccase. The spectrum also showed an amide I band at 1636 cm−1 due to C
O stretching, and C–O stretching vibration, indicating that the graphene was oxidized and the GO product could be successfully dispersed in water.31 For laccase, the peak at 3284 cm−1 was caused by O–H stretching in laccase. The spectrum also showed an amide I band at 1636 cm−1 due to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching and a band at 1165–948 cm−1 characteristic of laccase.32 The FTIR spectrum of laccase/GO showed that laccase was successfully immobilized onto GO due to presence of characteristic peaks of laccase and the GO.
O stretching and a band at 1165–948 cm−1 characteristic of laccase.32 The FTIR spectrum of laccase/GO showed that laccase was successfully immobilized onto GO due to presence of characteristic peaks of laccase and the GO.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C at 283.3 eV, C–C at 284.1, the C atom in the C–OH bond at 285.6 eV, C–O at 286 eV, and the carbonyl C (C
C at 283.3 eV, C–C at 284.1, the C atom in the C–OH bond at 285.6 eV, C–O at 286 eV, and the carbonyl C (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) at 287.13 eV. Similarly, the oxygen 1s (O 1s) spectrum of GO showed two functional groups in the spectra: O–C
O) at 287.13 eV. Similarly, the oxygen 1s (O 1s) spectrum of GO showed two functional groups in the spectra: O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 530.5 eV and C
O at 530.5 eV and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 531.2 eV, indicating that the graphene was oxidized (Fig. 5c). XPS spectra for C 1s core levels of laccase as deposited layers on GO contain peaks corresponding to C–C or C–H bonds at 283.4 eV, C–C bonds at 284.6 eV, C–O bonds at 285.8 eV, and C–O–C bonds at 286.5 eV (Fig. 5e). The peak areas indicate that the laccase layer deposited on GO is characterized by a higher content of C–C or C–H. Similarly, O 1s core levels of spectra showed three O components such as O–C
O at 531.2 eV, indicating that the graphene was oxidized (Fig. 5c). XPS spectra for C 1s core levels of laccase as deposited layers on GO contain peaks corresponding to C–C or C–H bonds at 283.4 eV, C–C bonds at 284.6 eV, C–O bonds at 285.8 eV, and C–O–C bonds at 286.5 eV (Fig. 5e). The peak areas indicate that the laccase layer deposited on GO is characterized by a higher content of C–C or C–H. Similarly, O 1s core levels of spectra showed three O components such as O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 530.7 eV, C
O at 530.7 eV, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 531.5 eV (Fig. 5f). XPS spectra for the N 1s core levels of laccase as-deposited layers on GO show two peaks that come from amino groups: amide at 398.5 eV and imide at 400.06 eV (Fig. 5g), confirming the presence of laccase molecules.38,39
O at 531.5 eV (Fig. 5f). XPS spectra for the N 1s core levels of laccase as-deposited layers on GO show two peaks that come from amino groups: amide at 398.5 eV and imide at 400.06 eV (Fig. 5g), confirming the presence of laccase molecules.38,39
(B) Electrochemical behavior
The surface characteristics of the fabricated electrode surface at various stages of modification were examined using the CV technique in 5 mM [Fe(CN)6]3−/4− solution at a scan rate of 40 mV s−1. Fig. 6 shows the CVs of the bare ITO electrode and ITO modified with GO and GO/ITO modified with laccase. Well-defined redox peaks were observed for bare ITO. Due to GO, the redox peak currents increase after the ITO was modified with GO.40 Laccase catalyses the single electron oxidation of various phenolic compounds. The performance of laccase-based electrochemical biosensors is improved by the introduction of graphene-based nanomaterial owing to its high conductivity, large specific surface area, flexibility and chemical inertness. Furthermore, the redox peak current increases by placing laccase on modified ITO. | ||
| Fig. 6 Cyclic voltammograms of bare ITO, GO/ITO and laccase/GO/ITO in a 1.0 mM [Fe(CN)6]3−/4− solution containing 0.1 M KCl. | ||
Effect of pH. It is generally recognised that the electrolyte's pH plays a significant role in electrochemical techniques. The electrocatalytic capabilities and pH of the modified electrode are inseparably connected. Electrode materials often exhibit stable behaviour near neutral pH, making it suitable for studying fundamental electrochemical processes without interference from extreme pH conditions. Neutral pH (around pH 7) is relevant to many practical applications.41 The pH value for the detection of glutathione plays a key role due to disulphide bond formation in the oxidation of glutathione. As a result, the electrochemical behaviour of the modified electrode for different pH values was investigated. The peak potential (Ep) shifts towards less positive values as the pH increases (Fig. 7a). This shift suggests that the oxidation process is influenced by proton concentration, which affects the redox potential. The shift in peak potential is indicative of proton-dependent reactions occurring at the electrode surface.42,43 As the pH increased, glutathione's oxidation peak current first gradually increased, then gradually reduced. The highest value was obtained at pH 7. Therefore, pH 7 was selected as the ideal value for the following experiment.
Catalytic effect. The electrochemical oxidation of 4 μM GSH using various modified electrode surfaces at a scan rate of 0.4 V s−1 was investigated by cyclic voltammetry (CV) (Fig. 8). In the presence of 4 μM GSH, the bare ITO exhibits a small oxidation peak current (0.08 μA) during the anodic scan, which is caused by the oxidation of GSH. However, a dramatic change in the oxidation peak current (0.1 μA) is observed when the ITO electrode surface is modified with GO. The GO/ITO electrode shows a pair of quasi-reversible redox peaks,44,45 due to the electrocatalytic activity of oxygen functional species on GO towards the GSH oxidation. Furthermore, the modified electrode enhanced the catalytic activity when laccase is immobilized on GO-modified ITO. However, the oxidation peak current (0.18 μA) is higher than the other electrodes when the modified electrode laccase/GO/ITO is tested in the same condition due to the effect of laccase.39,40
 | ||
| Fig. 9 (a) CV of laccase/Go/ITO in solutions containing different concentrations of GSH in PBS (pH = 7). (b) Linear curves of the anodic peak currents vs. GSH concentration. | ||
| Sr. no. | Modified electrodes | Linear range | LOD | Ref. |
|---|---|---|---|---|
| 1 | GO/GCE | 5–875 μM; 875 μM to 4.08 mM | 5 μM | 46 |
| 2 | 4-Amino-TEMPO/ERGO/GCE | 1–254 μM | 0.6 μM | 47 |
| 3 | AgNPs(TMSPED)–rGO/GC | 0.1–2.75 μM | 0.1 μM | 48 |
| 4 | GO/CdTe QDs | 24–214.0 μM | 8.3 μM | 49 |
| 5 | CuCoHCF/GO | 5–90 μM | 0.25 μM | 50 |
| 6 | NiHCF/AuNPs/CPE | 0.1 μM to 1.4 mM | 0.5 μM | 51 |
| 7 | Co–MOCP/CPE | 2.5 μM–0.95 mM | 2.5 μM | 52 |
| 8 | Cu–CoHCF/GCE | 5–90 μM | 2.5 μM | 53 |
| 9 | MWCNTs/SPE | 5–20 μM | 2 μM | 54 |
| 10 | NiONPs/GCE | 12.5 μM to 2.3 mM | 2 μM | 55 |
| 11 | Ni/ITO | 5–840 μM | 5 μM | 56 |
| 12 | Lacasse/GO/ITO | 1–100 μM | 0.89 μM | This work |
Furthermore, the reproducibility of electrochemical sensors is a crucial aspect in deciding their practical application. As a result, we used seven different electrodes (a new one used each time) for the detection of 5 μM GSH under similar experimental conditions. Each individual sensor's electrochemical response was the average of 10 consecutive experiments. The result showed that a relative standard deviation RSD% of 3.2%. In addition, the repeatability was checked using five consecutive measurements on a single electrode. The results showed an RSD% of 2.7%. The long-term stability of the modified electrode was evaluated through repetitive CV scans over multiple cycles: the modified electrode was tested for a month in 5 μM GSH. The storage stability test demonstrated that the electrode retained over 95% of its initial response after 30 days of storage at 4 °C. The current response (μA) vs. time (days) showed minimal degradation for the modified electrode over 30 days, indicating good stability (Fig. 12). The above results show that the laccase/GO/ITO electrode has excellent stability and reproducibility.
Conclusion
An electrochemical biosensor based on the immobilization of enzymes was developed for the detection of glutathione. The CV and EIS analyses performed on laccase/GO/ITO exhibited high electrocatalytic activity and the best selectivity toward GSH along with a linear dynamic range of 1 μM to 100 μM. In addition, the lower limit of detection was 0.89 μM and the limit of quantification was 2.70 μM with high sensitivity (6.51 μA μM−1). The modified electrode has a high potential for the development of novel sensors due to its inexpensive cost of electrode material, simple fabrication method, and high sensitivity to GSH. The findings of this work suggest that GO could be employed as a promising sensing material along with enzyme for the detection of GSH. Also, this developed sensor can be successfully used to determine GSH in pharmaceutical and biological samples.Data availability
The data sets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.Author contributions
Mrs Neeta Ukirade: conceptualization, investigation, methodology, explanation of results, writing of the manuscript. Mrs Upasana Choudhari: conceptualization, investigation, visualization, explanation of the results. Dr Sunil Bhapkar: conceptualization, investigation, visualization, format analysis. Dr Umesh Jadhav: conceptualization, investigation, visualization format analysis. Dr Shweta Jagtap: conceptualization, methodology, resources, visualization, supervision, reviewing and editing. Dr Sunit Rane: conceptualization, methodology, resources, visualization, supervision, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
One of the authors (Neeta Ukirade) is grateful to C-MET for providing the facility to carry out the research work for her PhD degree.References
- N. Varnakavi and N. Lee, Sensors, 2021, 21(4), 1109 CrossRef.
- R. Gaia, A. Spanu, S. Babudieri, G. Latte, G. Madeddu, G. Galleri, S. Nuvoli, P. Bagella, M. Demartis, V. Fiore, R. Manetti and P. Serra, Sensors, 2016, 16(6), 780 CrossRef PubMed.
- K. Nur Melis, S. Singh, G. Keles, S. Cinti, S. Kurbanoglu and D. Odaci, Biosensors, 2023, 13(6), 622 CrossRef PubMed.
- P. Mohankumar, J. Ajayan, T. Mohanraj and R. Yasodharan, Measurement, 2021, 167, 108293 CrossRef.
- A. Haleem, M. Javaid, R. Singh, R. Suman and S. Rab, Sensors International, 2021, 100100 CrossRef.
- S. Malik, K. Muhammad and Y. Waheed, Molecules, 2023, 28(18), 6624 CrossRef CAS PubMed.
- V. Singh, C. Singh, H. Ranjan and S. K. Pandey, International Conference on Smart Grid and Energy (ICSGE), 2024, pp. 38–42 Search PubMed.
- V. Singh, C. P. Singh, H. Ranjan, G. Kumar, J. Jaiswal and S. Pandey, Curr. Appl. Phys., 2024, 63, 48–55 CrossRef.
- C. Revathi, Fundamentals and Sensing Applications of 2D Materials, Woodhead Publishing, 2019, pp. 259–300 Search PubMed.
- N. Chauhan, K. Saxena, M. Tikadar and U. Jain, Nanotechnol. Precis. Eng., 2021, 045003 CrossRef CAS.
- J. Yoon, M. Shin, T. Lee and J. W. Choi, Materials, 2020, 13(2), 299 CrossRef CAS PubMed.
- U. Choudhari, S. Jagtap, N. Ramgir, A. K. Debnath and K. P. Muthe, Rev. Chem. Eng., 2023, 39(7), 1227–1268 CrossRef CAS.
- D. Holmannova, P. Borsky, T. Svadlakova, L. Borska and Z. Fiala, Appl. Sci., 2022, 12(15), 7865 CrossRef CAS.
- C. Cha, S. R. Shin, N. Annabi, M. R. Dokmeci and A. Khademhosseini, ACS Nano, 2013, 7(4), 2891–2897 CrossRef CAS PubMed.
- T. Mathew, R. A. Sree, S. Aishwarya, K. Kounaina, A. G. Patil, P. Satapathy, S. Hudeda, S. More, K. Muthucheliyan, T. Kumar and A. Raghu, FlatChem, 2020, 23, 100184 CrossRef CAS.
- D. Papageorgiou, I. Kinloch and R. Young, Prog. Mater. Sci., 2017, 90, 75–127 CrossRef CAS.
- U. Choudhari, N. Ramgir, D. Late, S. Jagtap, A. K. Debnath and K. P. Muthe, Microchem. J., 2023, 190, 108728 CrossRef CAS.
- S. Malik, J. Singh, R. Goyat, Y. Saharan, V. Chaudhry, A. Umar, A. A. Ibrahim, S. Akbar, S. Ameen and S. Baskoutas, Heliyon, 2023, 19929 CrossRef PubMed.
- J. Zhang, J. Lei, Z. Liu, Z. Chu and W. Jin, Environ. Res., 2022, 214, 113858 CrossRef CAS PubMed.
- B. Kaur, R. Srivastava and B. Satpati, RSC Adv., 2015, 5(115), 95028–95037 RSC.
- V. Singh, C. Singh, H. Ranjan, K. Harikrishnan and S. Pandey, IEEE Trans. Electron Devices, 2024, 5744–5753 CAS.
- P. Lee, K. Ward, K. Tschulik, G. Chapman and R. Compton, Electroanalysis, 2014, 26(2), 366–373 CrossRef CAS.
- H. J. Forman, H. Zhang and A. Rinna, Mol. Aspects Med., 2009, 30(1–2), 1–12 CrossRef CAS PubMed.
- N. Nesakumar, S. Berchmans and S. Alwarappan, Sens. Actuators, B, 2018, 264, 448–466 CrossRef CAS.
- P. Baldrian, Appl. Microbiol. Biotechnol., 2004, 63, 560–563 CrossRef CAS PubMed.
- C. Wang, X. Sun, M. Su, Y. Wang and Y. Lv, Analyst, 2020, 145(5), 1550–1562 RSC.
- W. S. Hummers Jr and R. E. Offeman, J. Am. Chem. Soc., 1958, 80(6), 1339 CrossRef.
- U. Anik, M. Çubukçu and F. Ertas, Artif. Cells, Nanomed., Biotechnol., 2016, 971–977 CAS.
- M. Bera, P. Gupta and P. K. Maji, J. Nanosci. Nanotechnol., 2018, 18(2), 902–912 CrossRef CAS PubMed.
- S. Malinowski, J. Jaroszyńska-Wolińska and P. Herbert, J. Mater. Sci., 2019, 54(15), 10746–10763 CrossRef CAS.
- Y. Shin, Y. Sa, S. Park, J. Lee, K. Shin, S. Joo and H. Ko, Nanoscale, 2014, 6(16), 9734–9741 RSC.
- Y. Li, S. Y. Yang and S. M. Chen, Int. J. Electrochem. Sci., 2011, 6(9), 3982–3996 CrossRef CAS.
- W. Zhou, W. Zhang and Y. Cai, Sep. Purif. Technol., 2022, 294, 121178 CrossRef CAS.
- G. Greczynski and L. Hultman, Prog. Mater. Sci., 2020, 107, 100591 CrossRef CAS.
- D. Krishna and J. Philip, Applied Surface Science Advances, 2022, 12, 100332 Search PubMed.
- N. Hidayah, W. Liu, C. Lai, N. Noriman, C. Khe, U. Hashim and H. Lee, AIP Conf. Proc., 2017, 1892, 150002 CrossRef.
- Y. Hongxia, Y. Yuxiang, H. Yan, Z. Yi and N. I. Chaoying, J. East China Univ. Sci. Technol., 2019, 45(6), 873–882 Search PubMed.
- S. Cheraghi, M. A. Taher, H. Karimi-Maleh, F. Karimi, M. Shabani-Nooshabadi and M. Alizadeh, Chemosphere, 2022, 287, 132187 CrossRef CAS PubMed.
- B. Yuan, C. Xu, L. Liu, Q. Zhang, S. Ji, L. Pi, D. Zhang and Q. Huo, Electrochim. Acta, 2013, 104, 78–83 CrossRef CAS.
- B. Liu, M. Wang and B. Xiao, J. Electroanal. Chem., 2015, 757, 198–202 CrossRef CAS.
- J. C. Fornaciari, L. C. Weng, S. M. Alia, C. Zhan, T. A. Pham, A. T. Bell, T. Ogitsu and N. Danilovic, Electrochim. Acta, 2022, 405, 139810 CrossRef CAS.
- S. Alharthi, H. A. Batakoushy, S. A. Alharthy, M. O. Abd El-Magied and W. M. Salem, Toxicol. Res., 2022, 11(1), 245–254 CrossRef PubMed.
- Y. Cai, Y. Zhang, S. Su, S. Li and Y. Ni, Front. Biosci., 2007, 12, 1946–1955 CrossRef CAS PubMed.
- J. Qu, Y. Wang, J. Guo, T. Lou and Y. Dong, Anal. Lett., 2014, 47(15), 2537–2547 CrossRef CAS.
- S. Lzaod and T. Dutta, ACS Omega, 2022, 51, 47434–47448 CrossRef PubMed.
- B. Yuan, X. Zeng, C. Xu, L. Liu, Y. Ma, D. Zhang and Y. Fan, Sens. Actuators, B, 2013, 184, 15–20 CrossRef CAS.
- B. Yuan, C. Xu, D. Zhang, R. Zhang, H. Su, P. Guan, J. Nie and C. Fernandez, Electrochem. Commun., 2017, 81, 18–23 CrossRef CAS.
- V. Vinoth, J. Wu, A. Asiri and S. Anandan, Ultrason. Sonochem., 2017, 39, 363–373 CrossRef CAS PubMed.
- Y. Wang, J. Lu, L. Tang, H. Chang and J. Li, Anal. Chem., 2009, 81(23), 9710–9715 CrossRef CAS PubMed.
- V. V. Sharma, L. Guadagnini, M. Giorgetti and D. Tonelli, Sens. Actuators, B, 2016, 228, 16–24 CrossRef CAS.
- P. C. Pandey and A. K. Pandey, Analyst, 2012, 137(14), 3306–3313 RSC.
- B. Yuan, R. Zhang, X. Jiao, J. Li, H. Shi and D. Zhang, Electrochem. Commun., 2014, 40, 92–95 CrossRef CAS.
- V. V. Sharma, L. Guadagnini, M. Giorgetti and D. Tonelli, Sens. Actuators, B, 2016, 228, 16–24 CrossRef CAS.
- P. T. Lee and R. G. Compton, Sens. Actuators, B, 2015, 209, 983–988 CrossRef CAS.
- B. Yuan, X. Zeng, D. Deng, C. Xu, L. Liu, J. Zhang, Y. Gao and H. Pang, Anal. Methods, 2013, 5(7), 1779–1783 RSC.
- H. Tian, T. Wang, Y. Fu, Y. Yu, C. Guo and J. Hu, J. Electrochem. Soc., 2014, 161(9), 191 CrossRef.
- U. Choudhari and S. Jagtap, AIP Adv., 2021, 11(12), 125327 CrossRef CAS.
- J. Chen, Z. He, H. Liu and C. Cha, J. Electroanal. Chem., 2006, 588(2), 324–330 CrossRef CAS.
- N. Ukirade, S. Jagtap and S. Rane, Hybrid Advances, 2023, 3, 100042 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |