 Open Access Article
Open Access ArticleReview on the synergistic effect of adsorption and photocatalytic degradation of patulin by functionalized graphitic carbon nitride nanomaterials and hydrogels
Meie Zheng†
,
Wenwen Li†,
Fei Ma *,
Yujia Shao,
Mengru Guo,
Xinyue Gao and
Junbo Du
*,
Yujia Shao,
Mengru Guo,
Xinyue Gao and
Junbo Du
Hubei Key Laboratory of Resource Utilization and Quality Control of Characteristic Crops, College of Life Science and Technology, Hubei Provincial Engineering Research Center of Key Technologies in Modern Paper and Hygiene Products Manufacturing, School of Mechanical Engineering, Hubei Engineering University, Xiaogan, Hubei Province 432000, P. R. China. E-mail: 376144258@qq.com
First published on 14th July 2025
Abstract
The efficient removal of patulin (PAT) contamination in food is an important challenge in the field of food safety, and the traditional single adsorption or photocatalytic techniques have bottlenecks such as low efficiency and difficult regeneration. The integration of adsorption–photocatalytic degradation by utilizing the synergistic effect of functionalized graphite-phase carbon nitride (g-C3N4) nanomaterials and hydrogels has proved to be a highly promising solution. However, how to construct a bifunctional material system with both high adsorption capacity and long-lasting photocatalytic activity is still a current research challenge. In this paper, we systematically review the recent progress of g-C3N4-based nanocomposites and hydrogel materials and their recent advances for enhancing the removal efficiency of PAT. This review provides a theoretical basis and technical reference for the development of “adsorption–degradation” smart material systems.
1 Introduction
In 1943, Harold Raistrick isolated PTA for the first time from P. gray-yellowiasis. After identification, the British Medical Research Center conducted a study on PAT, which then used PAT as an antibacterial agent for some specific bacteria with either Gram-positive or Gram-negative characteristics. Although, in 1944, the research center staff discovered its toxic effects.1 PAT has been detected in numerous foods. It is prevalent in fruits, vegetables, and their respective products. Among them, apples and apple-based products are particularly notable sources,2 and it is employed as a quality benchmark for the apples used in juice-making or compost-making.3 Research findings indicate that the extent of PAT accumulation primarily hinges on the specific strain. In contrast, it has relatively little connection with the various storage stages that apples go through during extended storage periods.4 Therefore, the key to preventing and controlling PAT in food is to control strains that can produce PAT toxins. To achieve this goal, a series of control measures can be taken, including preharvest control (such as selecting resistant varieties and implementing pest management), control during harvest (such as timely and manual harvesting) and postharvest control measures (such as high-pressure cleaning and cold chain storage), to maximize the prevention of contamination of food by strains carrying PAT toxins.5 However, since contamination may take place at each and every link within the food supply chain it is not possible to ensure that there is no strain or zero PAT in the food chain that produces PAT toxins. Furthermore, PAT possesses notable physical and chemical steadiness. Due to this characteristic, its structure is not easily damaged during the food processing stage. Moreover, PAT can build up through the food chain, exerting considerable influences on the health of mammals. These impacts involve symptoms such as headaches, nausea, pulmonary edema, injuries to the liver and kidneys, along with neurotoxicity and immunotoxicity. PAT can trigger a variety of acute and chronic diseases and complex responses at the cellular level and has been listed as the third category of suspected carcinogen by the International Cancer Research Institute.6–9 In this context, the World Health Organization (WHO, 2005), the U.S. Food and Drug Administration (FDA) and the European Union (EU) consistently stipulate that the maximum acceptable content of PAT in apple juice is 50 μg L−1 and that in children and babies. The PAT content was limited to 10 μg L−1.10 Therefore, putting into practice powerful and effective detoxification strategies to regulate PAT tainting in fruits and their derivatives is of great significance. In recent times, a variety of techniques have been employed for the elimination of mycotoxins (Fig. 1). These encompass ionizing radiation methods like gamma-ray irradiation and electron-beam bombardment, optical techniques such as ultraviolet illumination and pulsed-light emission, aqueous-based treatments represented by electrolytic water application, plasma-related technologies typified by cold-plasma utilization, and degradation processes based on catalysis, with photocatalytic degradation being a prime example,11–13 where photocatalytic degradation occurs because of the recovery rate of mycotoxins. The characteristics of simplicity, greenness, and high efficiency have attracted widespread attention.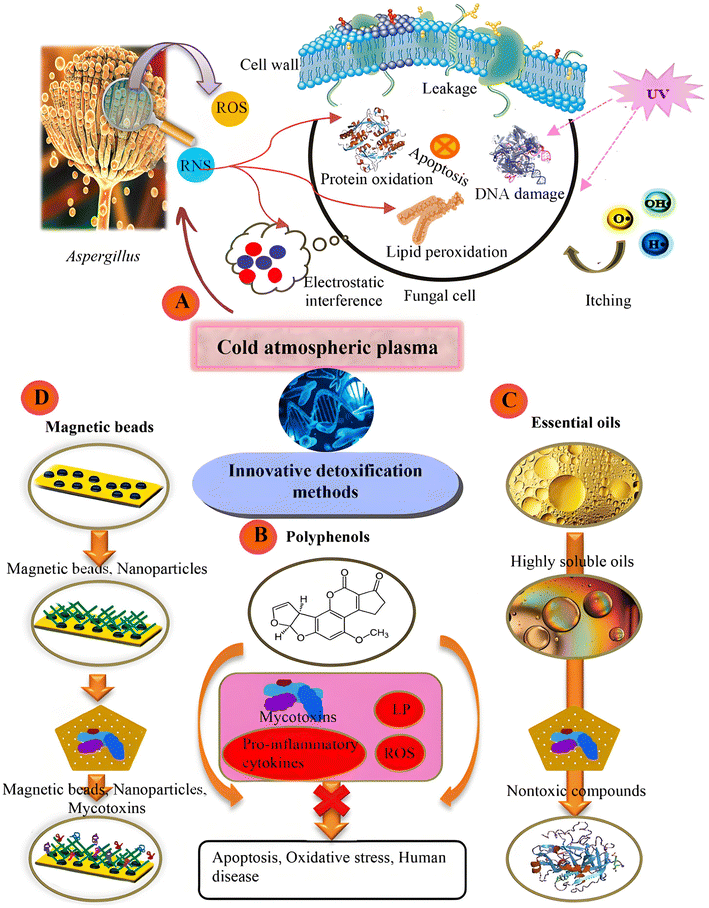 | ||
| Fig. 1 Methods for controlling mycotoxin contamination. (A) Cold atmospheric plasma method, (B) polyphenols, (C) natural essential oils, and (D) magnetic materials and nanoparticles.14 | ||
The abnormal stability of PAT under acidic environment and heat treatment leads to the difficulty of effective degradation by conventional means, while the physical adsorption method is limited by the bottleneck of poor selectivity and high regeneration cost. The traditional g-C3N4 materials still face the core challenges of high photogenerated carrier complexity, limited specific surface area, and insufficient adsorption selectivity in complex food matrices, which seriously limit their practical application efficacy. Recent studies have shown that the carrier separation efficiency can be significantly improved (by 3–5 times) through interfacial engineering strategies, such as heterostructure construction and elemental doping; the combination of 3D hydrogel network and molecular imprinting technology optimises the material's adsorption capacity (>200 mg g−1) and photocatalytic active site accessibility, resulting in a PAT degradation rate of more than 90%. Nevertheless, the analysis of the conformational relationship of the materials is mostly limited to a single performance index, and there is still a lack of systematic understanding of the key scientific issues such as the mechanism of adsorption–photocatalysis synergy, the interference effect of food components, and the cyclic stability of the materials.
In this review, we present a comprehensive overview of the recent progress of functionalized g-C3N4-based materials for PAT removal, focusing on: (1) the regulation of adsorption–photocatalytic synergistic performance by multidimensional nanostructural design; (2) the influence of coexisting components such as polysaccharides and pigments on the degradation pathway of food matrices; and (3) regeneration strategies and life-cycle assessment of the materials for large-scale applications. By clarifying the current technological bottlenecks and theoretical gaps in knowledge, we will provide prospective guidance for the development of new photocatalytic materials that are highly efficient, stable and food-compatible.
2 Overview of graphite-phase carbon nitride
2.1 Structure of graphite-phase carbon nitride
g-C3N4 is a 2D nanomaterial using a stratified graphite formation. Its basic structural units are stacked with atomic layers formed linked by the covalent bonds between C atoms and N atoms, within which tri-s-triazine represents the most stable unit (Fig. 2A). The topological structure of g-C3N4 is a 2D graphitic structure connected by a tertiary amine. The built-in functional groups include –NH2/–NH–/![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–, which provide many surface defects and reactive sites for g-C3N4. The five varieties of graphite-phase carbon nitride structures encompass α-C3N4, β-C3N4, g-C3N4, cubic-C3N4 and pseudocubic-C3N4. Among them, g-C3N4 has the lowest binding energy, the lowest hardness and the most stable structure.15,16
N–, which provide many surface defects and reactive sites for g-C3N4. The five varieties of graphite-phase carbon nitride structures encompass α-C3N4, β-C3N4, g-C3N4, cubic-C3N4 and pseudocubic-C3N4. Among them, g-C3N4 has the lowest binding energy, the lowest hardness and the most stable structure.15,16
 | ||
| Fig. 2 Photocatalytic reactions of carbon–nitride-based materials. (A) g-C3N4 has two structures, namely the triazine (a) and the tri-s-triazine.17 (B) Mechanism and application of g-C3N4 photocatalytic reaction.18 | ||
The C atoms and N atoms in g-C3N4 possess lone pairs of electrons within the pz orbital. These electrons can interact with each other, resulting in the formation of a large π bond that bears resemblance to the benzene ring. Moreover, through sp2 hybridization, a highly delocalized conjugated system is established. This conjugated system endows graphite-phase carbon nitride with a unique energy band structure, it is divided into three parts: a bonding band, a free-electron band and a band gap. The energy separation between the bonding band and the free-electron band amounts to 1.9 eV, while the width of the band gap is 0.4 eV. The interlayer distance of g-C3N4 is approximately 0.345 nm, which has a value that is slightly greater than the layer spacing of graphene (0.34 nm) and has better photoelectric and mechanical properties. In addition, g-C3N4 has a 2D structure, therefore, an appropriate external force can be applied to overcome the intermolecular van der Waals force between layers. Subsequently, by carefully peeling off the bulk g-C3N4, a 2D g-C3N4 nanosheet that has a significantly large specific surface can be successfully obtained. These nanosheets are rich in active sites and have excellent photoelectric properties, which are conducive to the progress of photocatalytic reactions (Fig. 2B). At present, scientific researchers have obtained g-C3N4 nanosheets through different methods, including top-down peeling of blocked g-C3N4 and assembling the precursor from bottom to top in a 2D manner. These two preparation methods can be further subdivided into ultrasonic-assisted liquid peeling,19 acid etching,20 thermal oxidation peeling,21 etc., in order to synthesize g-C3N4 nanosheets with low cost, simplicity and rapidity. Among them, the thermal oxidation peeling method is low in cost and environmentally friendly and is considered to be an effective method for obtaining g-C3N4 nanoscale sheets.22 Furthermore, adjusting the synthesis parameters allows for the production of g-C3N4 photocatalysts that exhibit varying sizes and shapes, ranging from 0D quantum dots, 1D nanotubes, 2D nanoscale sheets, and 3D porous frameworks, as well as diverse nanoscale dimensions. The material exhibits excellent electron transfer properties, stable optical properties, porosity, and distinctive structural characteristics marked by a significant specific surface area that endow it with substantial potential for utilization in the area of photocatalytic degradation.16
2.2 The properties inherent in graphite-phase carbon nitride
g-C3N4 has a variety of excellent physical and chemical properties, such as electrochemical properties, thermal stability, chemical stability and catalytic activity. These properties give g-C3N4 broad application prospects in ambient photocatalytic degradation, energy conversion, electrochemical energy conversion and storage (Fig. 3). | ||
| Fig. 3 Multifaceted applications and properties of g-C3N4.23 | ||
First, g-C3N4 has high conductivity and electron mobility, which results in good electrochemical activity. Through doping and surface modification, its electrical properties can be controlled, expanding its applicability in devices based on electronics, sensors, and various other domain. Second, g-C3N4 has good thermal stability and can exist stably below 600 °C, and its structure and mass do not change significantly. When the temperature increases above 600 °C, g-CNQDs gradually decompose; at 750 °C, g-C3N4 completely decomposes. Furthermore, g-C3N4 has good chemical stability and strong corrosion resistance to chemical substances such as acids, alkalis, oxidants and reducing agents. It can remain stable in air, water, acid and alkali. After that, g-C3N4 possesses superior adsorption capabilities, making it suitable for adsorbing and purifying gases, pollutants, and other substances. Its edges are rich in basic groups, such as –NH and –NH2, which can provide rich binding sites and achieve the adsorption and elimination of acidity pollutants through electrostatic attraction. Furthermore, the conjugated π region in its plane can adsorb aromatic pollutants by means of π–π conjugation.
Finally, g-C3N4 has high catalytic activity and can be used in catalytic reactions, environmental purification and other fields. With its role as an effective photocatalyst, it finds wide-spread uses in the domains of splitting water via photocatalysis and reducing carbon dioxide through photocatalysis. In 1924, Baur and Ferret reported through an experiment that silver salt can be reduced to metallic silver under the action of ZnO. This discovery first proposed the concept of photocatalysis.24 Since Fujishima and Honda made groundbreaking research results in 1972, researchers have developed a strong interest in photocatalytic technology.25 On the one hand, researchers are working hard to develop efficient semiconductor photocatalysts to enhance the efficiency of photocatalytic reactions. On the other hand, we are also constantly expanding the application of photocatalytic technology, from initial water cracking to all aspects of life, such as medical care, environmental governance, and agricultural production. In terms of pollutant removal, in 1976, Carey et al. used TiO2 to photocatalyze the removal of PCBs under ultraviolet light, which has since inspired much related research.26 At present, photocatalytic technology has also made certain progress in the process of catalytically oxidizing a wide range of organic contaminants, for instance colorants, pesticides, antibiotics, and mycotoxins. The core mechanism behind semiconductor photocatalytic reactions involves the effective conversion of solar energy into electrical or chemical energy, and this is of vital importance for diverse applications within energy-related domains. In this process, semiconductor catalysts play a key role, but they themselves remain unchanged before and after the reaction. When a semiconductor photocatalyst absorbs light energy equal to or exceeding its band-gap energy, electrons in the valence band (VB) become energized and transition to the conduction band (CB). Meanwhile, holes are left behind in the VB. Afterward, the energized electrons and the corresponding holes travel to the surface of the material, initiating redox reactions in the presence of species adsorbed on the surface of the catalyst.27 In the aqueous-phase reaction system, photogenerated electrons also undergo a free radical chain reaction with solvent molecules. This process generates a variety of reactive oxygen species. This includes, among others, hydroxyl radicals (·OH), superoxide anion radicals (·O2−), and singlet oxygen (O2−), but is not restricted to these species. Owing to their strong oxidative properties, these reactive oxygen species can further participate in the oxidation process on outer layer, thereby enhancing the effect of the photocatalytic conversion.28
Research by Wang et al. demonstrates that the particle size of g-C3N4 exerts a profound influence on its adsorption capacity and overall catalytic efficiency.33 Specifically, reducing the particle size of g-C3N4 significantly enhances its specific surface area, thereby exposing a greater density of active sites on the material surface. This augmentation elevates the probability of pollutant encounter and adsorption by amplifying the accessible interface between the catalyst and environmental contaminants. The expanded specific surface area not only accelerates pollutant sequestration but also provides abundant reactive interfaces for subsequent catalytic reactions.
However, particle size reduction, while increasing the specific surface area, may concurrently compromise the in-plane piezoelectric polarization strength of g-C3N4, thereby undermining its piezocatalytic performance. Piezoelectric polarization is a critical determinant in piezocatalysis, governing the segregation and migration of charge carriers across the material surface to facilitate the generation of reactive oxygen species (ROS). These ROS serve as pivotal mediators for pollutant degradation. Consequently, the rational design of g-C3N4-based catalysts necessitates meticulous particle size optimization to reconcile surface area expansion (for enhanced adsorption) with the preservation of adequate piezoelectric polarization. This integrated optimization framework offers a novel strategy for developing high-efficiency, eco-friendly g-C3N4 catalysts. Beyond environmental remediation, such advancements hold transformative potential for clean energy technologies and related interdisciplinary applications, underscoring their strategic value in sustainable innovation.
Wang and colleagues conducted a systematic research into the adsorption behavior of g-C3N4 prepared from different precursors and explained the formation mechanism, influencing factors and relative contributions of various interactions in the surface adsorption process of humus on g-C3N4. Due to the combined influence of the amino group of g-C3N4 and the heptazine ring, along with the aromatic rings and oxygen-containing functional groups within humus, the adsorption process of humus on g-C3N4 is caused mainly through electrostatic attractions, π–π stacking, and hydrogen bonding interaction.34 Cai and colleagues further confirmed that g-C3N4 nanosheets, as adsorbents with excellent performance, have unique adsorption properties. For example, Cd2+ can achieve an adsorption capacity of 94.40 mg g−1 by coordinating with C and N atoms within the triazine ring unit of g-C3N4. At pH = 7.0, the capacity of MB adsorption by g-C3N4 can reach 42.19 mg g−1 owing to π–π conjugation interactions and electrostatic gravity. Moreover, the composite materials of g-C3N4 also demonstrate favorable adsorption capabilities. For instance, Guo and his/her team managed to synthesize a g-C3N4/MnO2 composite abundant in active sites through an in situ deposition approach. The maximum adsorption of Pb(II) in water by this composite material is as high as 204.10 mg g−1. This result fully demonstrates that g-C3N4. As an adsorbent, it has broad application prospects.35
g-C3N4 possesses a band gap of roughly 2.70 eV, enabling it to react to visible light.37 Furthermore, the preparation process of g-C3N4 is relatively simple. Through a heat condensation reaction, g-C3N4 can be prepared using several low-cost and nitrogen-rich precursors as raw materials. At present, urea and melamine are the two most commonly used precursors. In addition, thiourea and dicyanide can likewise be employed in the synthesis of g-C3N4.38 Apart from the heat condensation technique, g-C3N4 can also be synthesized through the processes of self-assembly of molecules,39 heating synthesis assisted by microwave,40 and solvent thermal method.39 In recent years, photocatalytic technology has emerged in the field of purifying mycotoxins in food and the environment.41,42 g-C3N4, as an innovative nonmetal semiconductor material, is capable of stimulating the production of photogenerated electrons and holes when exposed to visible light and then trigger a redox reaction. This property has garnered considerable interest in the field of photocatalytic studies. Through its ability to combine with other functional materials, g-C3N4 can form nanogel composites with strong synergistic effects, which perform excellently in improving the adsorption and photocatalytic degradation capabilities of PAT.
Additionally, particle size significantly influences the photocatalytic performance of g-C3N4. Smaller particle sizes enhance the dispersibility of g-C3N4, exposing a higher density of active sites and facilitating the separation/migration of photogenerated charge carriers. This synergistic effect substantially boosts its capacity for photocatalytic hydrogen peroxide (H2O2) generation and organic pollutant degradation. As demonstrated by Zhang et al., g-C3N4-KCl synthesized via a salt-templating approach exhibits the smallest particle size and superior dispersibility compared to its non-templated counterpart. Notably, its H2O2 production rate reaches 6.32 mmol g−1 h−1, 32-fold higher than that of non-salt-templated g-C3N4.43 Moreover, g-C3N4-KCl achieves a degradation rate constant (k) of 0.1707 min−1 for rhodamine B (RhB) under visible light irradiation, doubling the performance of non-templated g-C3N4 and demonstrating exceptional photocatalytic degradation efficiency.
There have been reports of methods for degrading mycotoxins in food via photocatalytic techniques, with PAT being mentioned as one of the target toxins.44,45 For example: under ultraviolet irradiation, N-TiO2 nanoparticles (Nps) exhibit excellent photocatalytic activity, which can completely remove PAT in simulated juice and natural juice within 1 hour. Ultraviolet irradiation promotes the generation of degradation active sites in N-TiO2Nps, and the properties of these active sites are heterogeneous.45 A La-ZnFe2O4@Fe3O4@carbon magnetic hybrid material was successfully prepared through a straightforward absorption–pyrolysis method, utilizing a MOF (Zn, Fe) as the starting material. Under ultraviolet light, the composite material possesses the advantage of a large surface area per unit mass and superior photocatalytic capabilities and has a particularly significant degradation effect on aflatoxin B1, PAT and zearalenone, with degradation efficiencies as high as 98.37%, 97.35% and 98.52%, respectively.46
Compared with other treatment methods, photocatalytic technology has shown unparalleled advantages in removing mycotoxins, including the use of clean and pollution-free solar energy, efficient degradation of pollutants under mild conditions, and complete harmlessness of pollutants. Treatment, as well as the high efficiency, stability and recycling of photocatalysts. Therefore, photocatalytic technology provides a completely novel approach for the purification of mycotoxins in food and the environment.47 Nevertheless, one major drawback of photocatalytic reactions lies in their lack of selectivity. This implies that should photocatalytic technology be directly utilized in the food industry, it will likely lead to the destruction of food nutrients unavoidably. Photocatalysts, as core elements in the photocatalytic process, have a decisive influence on the reaction performance. The early research regarding the photocatalytic regeneration of adsorbents in the environmental domain has offered fresh concepts and inspiration for the transformation of photocatalysts.48,49
However, because g-C3N4 is hydrophobic, agglomeration is very likely to occur in practical applications. This agglomeration not only exerts an adverse effect on the photocatalytic properties of the material but also exacerbates the recombination of electrons and holes.50 To solve this problem, we can learn from the modification ideas of graphene oxide, and through a series of modification methods, hydroxyl and carboxyl groups are introduced at the exterior of g-C3N4 to increase its hydrophilicity and effectively reduce agglomeration in water, further improving its photocatalytic performance.51–53
Although g-C3N4 has certain advantages in the field of photocatalysis, it inevitably has some inherent defects. For example, its electron–hole recombination rate is high, its absorption capacity is insufficient for observing light, its surface reaction kinetics are slow, and the surface area in contact with the catalyst and the reactants is limited, resulting in incomplete adsorption of pollutants and a relatively large number of active sites wait less.22 To overcome the above shortcomings, it is particularly important to explore appropriate strategies to improve the photocatalytic performance of g-C3N4.
Mycotoxins, as a class of highly toxic secondary metabolites, pose significant risks to food safety, ecological safety and public health. Nano semiconductor photocatalytic technology is regarded as a highly promising degradation strategy, and its application in g-C3N4-based composites is particularly noteworthy (Table 1). Due to the high performance, cost-effectiveness, and environmental friendliness of g-C3N4-based composites, they exhibit unique advantages in the field of mycotoxin abatement. In order to enhance the practical application of the adsorption and degradation system, it is necessary to systematically analyse the key influencing factors, such as the energy band structure of the photocatalysts, the molecular characteristics of the pollutants and the reaction conditions, so as to optimize the degradation pathway and reaction kinetics.
| Materials | Findings | Advantage | Disadvantage | Ref. |
|---|---|---|---|---|
| g-C3N4/TiO2 | It has a good degradation effect on antibiotics such as tetracycline in water; it has shown certain potential in photocatalytic water splitting to produce hydrogen | The addition of TiO2 broadened the photoresponse range of g-C3N4 and improved the photocatalytic activity. It has good stability and can be reused many times | The photocatalytic efficiency under visible light still needs to be improved. The degradation selectivity for some contaminants is not high enough | 54 |
| ZnO: Sr/g-C3N4 | The band gap was reduced from 3.5 eV to 1.9 eV, and the efficiency of photocatalytic degradation of cationic dye MB (99%) and anionic dye MO (95%) was significantly improved | Green synthesis (using plant extracts) with both antioxidant and anti-diabetic biomedical potential | Toxicity assessment for biomedical applications is inadequate, and further validation of safety is needed | 55 |
| Ce/P functionalized g-C3N4/ABS nanocomplexes | Significantly improved flame retardancy, thermal stability and mechanical properties for use in engineering plastics | Multi-functional composite materials, breaking through the performance limitations of traditional flame retardants | The effects of environmental ageing in long-term use can be costly for industrial applications | 56 |
| Precursor modulation of g-C3N4 (U550) | The urea precursor prepared g-C3N4 (U550) with the highest N/C ratio (1.22) and RhB degradation efficiency of 80.27% (100 min) | The performance was optimised by precursor selection and the photocatalytic mechanism was well defined (·OH and h+ dominated) | Strong dependence on precursor purity and possible feedstock consistency challenges in industrial production | 57 |
| Ag-0.8/LaCN-1 | The adsorption capacity for methyl orange (MO) was 49.6 times higher than that of bulk g-C3N4 (BCN), and the photocatalytic degradation rate was 13.1 times higher than that of BCN.The synergistic effect of La doping and Ag NPs significantly enhanced the adsorption and photocatalytic performance | High specific surface area (48.1 m2 g−1) and mesoporous structure providing more active sites. Visible light absorption range extended beyond 550 nm (band gap 2.50 eV). Good cycling stability (only 3% decrease in total removal after 5 cycles) | The preparation process involves multiple steps (pyrolysis + wet impregnation) and is more complex. The leaching of trace amounts of Ag (0.25%) and La (0.01%) may pose an environmental risk | 58 |
| g-C3N4/FTO glass composite film and g-C3N4/Si composite film | Powdered g-C3N4 degraded 96.3% of rhodamine B (Rh B) in 8 h, while LB film degraded 73% in the same time. The LB film after repeated use (after 24 h) maintained 73% degradation efficiency | Remarkable photocatalytic performance, material stability Lianghao FTO glass on the LB film coverage uniform, after repeated use still maintains stable activity. LB technology can be accurately regulate the film thickness and structure, suitable for photoelectric devices and chemical conversion system integration, synthesis of raw materials is cheap and easy to obtain, suitable for large-scale applications | Incomplete coverage (presence of voids) on a silicon substrate limits its application on that substrate. The degradation efficiency of the LB film was reduced compared to the powder (73% vs. 96.3%), probably due to the reduction of active sites as a result of unilateral contact of the film with the substrate | 59 |
| Co2SiO4/g-C3N4 | The highest degradation rate of EB under UV light (90%, within 120 min) was significantly better than that of single components (57.7% for pure g-C3N4 and 63.6% for pure Co2SiO4). The catalytic efficiency only decreased by 11.1% after 5 cycles of use | Simple preparation process, low cost, suitable for large-scale production. The performance remains stable after many cycles, suitable for long-term application. It can effectively remove organic dye pollutants in water and is suitable for sewage treatment | Low utilisation of visible light, limiting practical application scenarios. Degradation of anionic dyes is significantly better than that of cationic dyes, limiting the scope of application. The optimal degradation requires 70 mg of catalyst (0.7 g L−1), which may increase the cost, and the long-term use of cobalt in Co2SiO4 may pose a risk of metal leaching | 60 |
| Pt/CuPc/g-C3N4 Z-type heterojunction | Under visible light, the Pt/CuPc/g-C3N4 catalysts selectively reduced CO2 to CH4 in yields of 39.8 μmol g−1 h−1, with selectivities of up to 90%, which were significantly better than those of the single components (pure g-C3N4 and CuPc yields of 15.9 μmol g−1 h−1 and 12.8 μmol g−1 h−1, respectively). After 12 h of continuous reaction and 5 cycles, the catalytic activity only decreased by 11.1% without significant structural degradation | The introduction of CuPc extends the light absorption range to the near-infrared region (800 nm) and enhances the solar energy utilisation, while the Z-type heterojunction structure significantly reduces the charge complexation rate and enhances the photocurrent intensity | The high cost of precious metal Pt as a co-catalyst limits the economics of large-scale applications. Involving multiple steps (ultrasonic dispersion, photodeposition of Pt, etc.), the process conditions need to be precisely controlled, which may increase production costs. The optimal CuPc loading is 5 wt%, and excessive amounts (e.g., 10 wt%) can lead to active site blockage and performance degradation. Long-term use of Pt and Cu may lead to metal leaching, and the environmental safety needs to be further evaluated | 61 |
| MoS2@g-C3N4 | MoS2@g-C3N4 adsorption efficiency: RhB (pH 4.0): 96%, SLD (pH 8.0): 96%, FLX (pH 9.0): 85%, with good photocatalytic degradation ability, and the photocatalytic efficiency remained 99–100% after 5 cycles. The BET surface area amounted to 69.53 m2 g−1, with a significant increase in pore volume | Combined adsorption and photocatalytic degradation capabilities, enhanced adsorption capacity and reactive sites. The performance does not decay after many cycles and is suitable for long-term application. g-C3N4 is a metal free material with high chemical stability. Adsorption efficiency for different pollutants can be optimised by adjusting pH | Excess MoS2 covers the g-C3N4 surface, leading to loss of photocatalytic activity. The adsorption efficiency (85%) for FLX is lower than that of RhB and SLD. the degradation time is longer and the synthesis is complicated, which requires precise control of the ratio of MoS2 to g-C3N4 and the synthesis conditions | 62 |
| Pd SAs/g-C3N4 | The photocatalytic hydrogen production efficiency reached 0.24 mmol h−1 mg−1 Pd at a Pd loading of only 0.05 wt%, which is 55 times higher than that of conventional Pd nanoparticles (1.5 wt%). After five cycle tests, the catalytic activity remained stable (99–100%) without Pd atom agglomeration or deactivation | Only 0.05 wt% Pd loading is required for significant cost reduction. The single-atom active site maximises atom utilisation and avoids agglomeration of nanoparticles. The Pd–N coordination structure significantly reduces Rct and reduces photogenerated carrier complexation. The Pd loading is adjusted by precursor concentration, and the process is simple and reproducible. Efficient hydrogen production under visible light (365 nm) for practical photocatalytic systems | Only Pd(NH3)4Cl2 achieves efficient monoatomic dispersion, and other precursors (e.g. PdCl2) are prone to nanoparticle formation or incomplete reaction. Pd loading above 0.26 wt% decreases the activity due to atomic agglomeration. Excellent performance under laboratory conditions, but the problems of homogeneous dispersion and cost control need to be solved in large-scale production | 63 |
| CuO/Fe3O4@ g-C3N4 double-Z ternary composites | The use of Aloe vera extract as a reducing and stabilising agent resulted in the formation of a double Z-type heterojunction, which significantly reduced the band gap energy (from 2.66 eV to 1.67 eV for g-C3N4) and broadened the visible light absorption range. The photogenerated electron–hole pair complexation rate of the composites was significantly reduced, the charge transfer resistance (Rct) decreased, and the specific surface area was 74.5 m2 g−1 with a pore size distribution of 12.1 nm, providing abundant active sites | Using an environmentally friendly process, the quantum dot sizes of CuO and Fe3O4 (∼3.34 nm) enhance light absorption and surface reactivity. The charge separation is facilitated by a double Z-type heterojunction mechanism to enhance the photocatalytic redox capability. Absorption in the UV to visible light range, suitable for practical environmental applications | The synthesis conditions are harsh and the composites are stuck in the laboratory stage. Data on photocatalytic efficiency or material structural stability after multiple cycles of use are not available, and performance comparisons with traditional non-biobased materials (e.g., Pt/g-C3N4) are lacking, making it difficult to comprehensively assess competitiveness | 64 |
| Ternary CuO/ZrO2@S-doped g-C3N4 hybrid nanocomposites | The introduction of S doping and CuO/ZrO2 significantly optimised the grain size of g-C3N4 and enhanced the crystallinity, with a significant reduction in the photogenerated electron–hole pair complexation rate, excellent electrocatalytic performance, and a low limit of detection (LOD) as low as 1.7 μM and a limit of quantification (LOQ) as high as 2.1 μM when used for bisphenol A (BPA) detection | High electrocatalytic activity and selectivity, inexpensive raw materials, simple synthesis method and high yield, both photocatalytic and electrochemical sensing potential, suitable for environmental remediation and pollutant detection, improved thermal stability | The proportion of sulphur doping and metal oxide loading needs to be precisely controlled, and the metal oxides may agglomerate in long-term use, affecting the performance | 65 |
| Heterojunction of S-type NiMn2O4/g-C3N4 nanocomposites | Under visible light, 1% NiMn2O4/g-C3N4 degraded EB at 10 ppm by 96.41% (120 min) with a rate constant k = 0.0241 min−1. The degradation efficiency decreased by only 10.17% after 5 cycles, showing good stability | The degradation rate of EB under acidic condition (pH = 4) was up to 96.41%, and showed degradation potential for many organic dyes (e.g. rhodamine B, methyl violet, etc.), with inexpensive raw materials (e.g. nickel nitrate, manganese nitrate, melamine) and simple process | Elevated pollutant concentration (e.g., 30 ppm EB) led to a significant decrease in degradation (from 96.41% to 47.46%), a substantial decrease in degradation efficiency (63.09%) under alkaline conditions (pH = 10), and a decrease in the photocatalytic performance after NiMn2O4 loading of more than 1% | 66 |
| SiO2@g-C3N4/Au NPs | Au NP broadens the visible light absorption range and promotes photogenerated electron–hole pair separation through the formation of Schottky barriers. Silicon dioxide nanotubes provide high specific surface area (BET surface) and mesoporous structure to enhance contaminant adsorption. The band gap decreased from 2.71 eV in pure g-C3N4 to 2.63–2.69 eV (after modification by Au NPs), but was still higher than the ideal semiconductor band gap (2.0 eV) | The degradation kinetic constant of SiO2@g-C3N4/Au 0.5% (0.0068 min−1) was significantly higher than that of pure g-C3N4 (0.0019 min−1), and silica nanotubes were derived from the natural eolianite clay, the synthesis process is green and less costly, and the LSPR effect of Au NPs enhances visible light absorption and reduces the dependence on UV light | The use of gold nanoparticles increases the material cost and the loading needs to be strictly optimised. The band gap is still high (2.63–2.71 eV) and the efficiency of utilising the solar spectrum is limited. Multi-step synthesis is involved and the process is difficult. Both SiO2 and g-C3N4 are negatively charged at near-neutral pH, inhibiting the adsorption of negatively charged pollutants | 67 |
Adsorption enriches the target molecules on the catalyst surface, shortening the mass transfer distance between the photogenerated carriers and the contaminants and improving the reaction kinetics. Adsorbed contaminants can act as electron acceptors (or donors) to facilitate photogenerated carrier separation. For example, adsorbed O2 molecules trap electrons to generate ·O2−, while organic pollutants act as hole traps to reduce e−–h+ complexation. High surface concentration of pollutants can accelerate the chain reaction process of photocatalytic reaction, which is especially effective for difficult-to-degrade pollutants (e.g., antibiotics, perfluorinated compounds).
Photocatalysis enhances the adsorption capacity, under light, photogenerated holes (h+) positively charge the surface of the material and enhance the adsorption of anionic pollutants, while photogenerated electrons (e−) reduce the adsorption sites and promote the adsorption of cations. The photocatalytic reaction may change the molecular structure of pollutants to generate intermediates that are easier to adsorb, or change the valence state of pollutants through redox to enhance the subsequent adsorption efficiency. After photocatalytic degradation of adsorbed pollutants, the adsorption sites are released to achieve in situ regeneration of the material, avoiding the saturation problem of traditional adsorbents. For example, phenol adsorption was photocatalytically mineralised to CO2 and H2O, and the surface active sites were restored.
Fig. 4A illustrates a typical photocatalytic process. Following the efficient transfer of photogenerated charge carriers to the semiconductor surface, electrons (e−) interact with oxygen (O2) to produce superoxide radical anions (·O2−). These radicals can undergo protonation leading to the formation of superoxide hydroxyl radicals (–HOO) and the subsequent production of hydrogen peroxide (H2O2). Superoxide and hydroxyl radicals contribute significantly to the degradation of mycotoxins. Meanwhile, holes (hVB+) can directly oxidise mycotoxins to form degradation products or react with water (H2O) to produce hydroxyl radicals (·OH). g-C3N4-SH@KG achieves efficient removal of PAT and regeneration of the material through the synergistic effect of efficient adsorption of thiol groups and photocatalytic regeneration by the mechanism shown in Fig. 4B. The thiol functionalisation not only the thiol functionalization not only enhances the adsorption selectivity, but also optimizes the photocatalytic activity, and the “dark adsorption–photoregeneration” strategy takes into account the food safety and sustainability of the material, which provides a new idea for the control of food contaminants.68
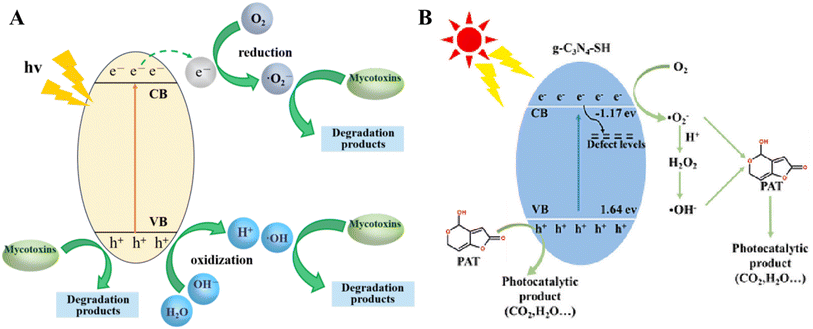 | ||
| Fig. 4 Schematic of a typical photocatalytic mechanism. (A) Schematic representation of a typical photocatalytic mechanism;68 (B) schematic representation of the photocatalytic mechanism of KG-5 photocatalyst. | ||
Synergistic structural modulation strategies to construct meso/macroporous structures by templating or thermal stripping to enhance mass transfer efficiency and adsorption capacity (three-dimensional porous g-C3N4). Introducing electron-rich groups to enhance the selective adsorption of specific pollutants. Load co-catalysts to facilitate photogenerated carrier separation while providing additional adsorption sites. Formation of heterojunctions with semiconductors (TiO2, BiOBr) or conductive materials (graphene) to optimise the light absorption range and enhance interfacial adsorption-catalysis synergies.
Porous materials (e.g., graphene hydrogel, g-C3N4-HEC hydrogel, TiO2/g-C3N4) enrich pollutants (e.g., BPA, PAT) through physical and chemical adsorption, and shorten the mass-transfer distance between the photogenerated carriers and the pollutants; heterojunction structures (e.g., BiOI/porous g-C3N4, TiO2/g-C3N4) promote the charge separation through energy band matching, the photogenerated electrons are transferred to the conducting substrate (graphene) or another semiconductor conduction band, and holes are retained in the valence band, inhibiting compounding and enhancing photocatalytic activity; adsorbed pollutants act as electron acceptors (O2 to generate ·O2−) or hole trappers (direct oxidation), and efficient degradation is achieved by ROS; pollutants are mineralised into harmless products after photocatalytic reaction (CO2, H2O) after the photocatalytic reaction, releasing the adsorption sites to achieve in situ regeneration of the material, while the dynamic flow system (continuous photoreactor) maintains long-term stability through adsorption–degradation rate matching. The synergistic effect is essentially a coupled process of “adsorption enrichment – in situ transformation – dynamic recycling”, and the efficient removal of pollutants is achieved through the design of multi-stage structure of the material and interface regulation.69–71
The synergistic effect of carbon nitride adsorption and photocatalysis is essentially a dynamic coupling process of “enrichment–reaction–regeneration”, the core of which lies in the efficient capture and rapid transformation of pollutants on the surface through the design of materials and interface regulation. In the future, we need to further combine in situ characterisation to reveal the synergistic mechanism at the molecular level and to promote the precise design for practical applications.
| Modification method | Performance parameter | Performance data | Ref. |
|---|---|---|---|
| Nitrogen doping | Band gap (eV) | 2.7 | 78 |
| Nitrogen doping | Specific surface area (m2 g−1) | 1500 | 79 |
| Nitrogen doping | Photocatalytic H2 evolution rate (μmol g−1 h−1) | 100 | 79 |
| Nitrogen doping + metal doping | Photocatalytic H2 evolution rate (μmol g−1 h−1) | 220 | 78 |
| Carbon doping | Band gap (eV) | 2.2 | 78 |
| Carbon doping | Photocatalytic H2 evolution rate (μmol g−1 h−1) | 180 | 78 |
| Co, Ni doping | Electrocatalytic HER activity (mV) | 150 | 79 |
| Co, Ni doping | Electrocatalytic HER activity (current density, mA cm−2) | 10 | 79 |
| Molybdenum doping (Mo) | Photocatalytic H2 evolution rate (μmol g−1 h−1) | 300 | 79 |
| Molybdenum doping (Mo) | Photocatalytic CO2 reduction rate (μmol g−1 h−1) | 70 | 78 |
| Surface modification (polymer) | Specific surface area (m2 g−1) | 1700 | 79 |
| Surface modification (oxidation) | Photocatalytic H2 evolution rate (μmol g−1 h−1) | 150 | 78 |
| Nanostructuring | Photocatalytic H2 evolution rate (μmol g−1 h−1) | 500 | 78 |
| Nanostructuring | Photocatalytic CO2 reduction rate (μmol g−1 h−1) | 90 | 78 |
 | ||
| Fig. 5 Energy band structure of doped g-C3N4 synthesized by thermal polycondensation. (A) g-C3N4 was synthesized from several precursors by thermal polycondensations.80 (B) Energy band diagrams of TiO2 and g-C3N4, along with their commonly used dopant species.80 (C) Illustrative representation of the energy band configure uration of various types of heterojunctions.81 | ||
(1) Energy band regulation
The most prevalent modification approach for enhancing the capability of g-C3N4 to absorb visible light is to carry out energy-band engineering. By adjusting the components or morphology, the position of the semiconductor catalyst bottom or apex of the valence energy band is capable of being adjusted so that the visible light absorption band edge of the catalyst redshifts, thereby expanding its visible light response wavelength range. Furthermore, energy band engineering has the capacity to enhance the oxidation–reduction capabilities of the catalyst. By doing so, it offers a more potent driving force for the target oxidation–reduction reaction, thereby augmenting the catalytic activity of the catalyst.82
In the current energy band regulation research on g-C3N4, element doping is one of the most in-depth and widely used methods, covering two directions: metal element doping and nonmetal element doping. At this stage, in terms of bandgap regulation, the incorporation of metal elements is the most effective method and is widely used to regulate the arrangement of electrons and optical attributes of g-C3N4. The catalytic ability of g-C3N4 can be significantly improved by incorporating different transition metal ions into its structure. These ions of transition metals encompass Zn2+, Mn3+,Fe3+, Ni3+, Co3+, as well as Cu2+.83–86
As depicted in Fig. 5B, apart from the doping of metal elements, the doping of non-metallic elements like S, B, O, and C can also decrease the energy gap of g-C3N4. This reduction in the band gap leads to an improvement in its light absorption performance and an enhancement in the degree of delocalization of conjugated electrons.87–90 Through a blend of experimental and theoretical approaches, Liu and colleagues verified that the incorporation of P can substitute for C1 or N2 positions, effectively narrowing the forbidden bandwidth of g-C3N4 to 2.20 eV. Moreover, the doping of P is highly important for the movement and partitioning of light-induced electrons.
(2) Porous structure design
Creating a porous structure for g-C3N4 provides numerous advantages. For one thing, it can increase the catalyst's specific surface area directly and enhance its pore volume. Consequently, its adsorption performance is notably enhanced; on the other hand, it also has the ability to elevate the count of reactive sites. This effectively propels the progression of surface reactions, speeds up the electron migration and separation process, and at the same time, elevates the light collection efficiency.91 When preparing porous g-C3N4 nanophotocatalysts, a variety of soft template methods have been used, such as ionic liquids,92 bubble templates,93 etc. However, ionic liquids have obvious disadvantages, their cost is high, their carbon residue is high, and they are insoluble in water. These disadvantages seriously restrict their promotion in large-scale practical applications.
To overcome these shortcomings of the soft template method, researchers have conducted many in-depth studies on the hard template method.94 Theoretically, from the perspective of principles, any material featuring an ultra-high specific surface area can be utilized as a hard template for the preparation of g-C3N4. At present, many related studies have reported successful preparation methods, such as the use of porous alumina,95 SiO2,96 SBA15,97 etc. as hard templates. Among these materials, the mesoporous SiO2 is the most commonly used hard template. Due to its distinctive structure and characteristics, it serves as an outstanding template to facilitate the formation of porous structures in g-C3N4.
(3) Shape adjustment
Nanosized g-C3N4 exhibits more prominent advantages in photocatalysis compared to bulk g-C3N4. Due to its larger specific surface area, it can offer a greater number of reaction sites; shorter charge migration distances, allowing photogenerated carriers to reach the reaction site faster; and higher solubility, which helps in the reaction system. Evenly dispersed and has an adjustable energy band structure.98 In particular, the distance that photogenerated carriers need to travel in g-C3N4 is significantly shorter compared to bulk material, allowing them to readily reach the catalyst surface for reaction, thus enabling efficient separation of photogenerated electron–hole pairs. This resulted in a substantial enhancement in photocatalytic efficiency.
To date, in the domain of photocatalysis, a wide variety of g-C3N4 nanostructures with diverse morphologies have undergone extensive development and utilization. These encompass 0D quantum dots or nanoparticles, 1D nanowires, nanorods, or nanotubes, 2D nanosheets or nanothin films, as well as 3D hierarchical architectures.99–103 Owing to their unique surface properties, 2D ultrathin g-C3N4 nanosheets and 0D nanoparticles provide many adsorption and reaction sites for reactant molecules. 0D and 2D materials, due to the influence of dimensional effects, not only have an increased specific surface area, enabling them to offer more adsorption and reactive centers, but also lead to a reduction in the interface energy barrier. This reduction improves the carrier transmission efficiency and significantly shortens the transmission path; thus, the overall photocatalytic activity has been comprehensively improved, making broader application prospects in the field of environmental purification.104
(4) Constructing heterojunctions
The construction of heterojunctions with other materials effectively enhances the catalytic performance of g-C3N4 semiconductors under light illumination. This happens due to the fact that, upon the recombination of two semiconductors, an electrical field inside arises between them. This internal electrical field aids in the swift dissociation of electrons and holes. Consequently, the rate at which photogenerated charge carriers recombine is reduced, and their lifetime is prolonged. As presented in Fig. 5C, the mechanisms by which charge carriers are separated in heterojunctions can be categorized as follows: type I, type II, p–n junctions, Schottky junctions, and Z-scheme heterojunctions.105
In the structure of a type I heterojunction that is established, both electrons and holes accumulate in one of the semiconductors, which decreases the likelihood of electron–hole recombination occurring in the other semiconductor. In the case of a p–n heterojunction, electrons migrate from the conduction band of the p-type semiconductor to that of the n-type semiconductor, while concurrently, holes shift from the valence band of the n-type semiconductor to the valence band of the p-type semiconductor, thereby realizing efficient electron–hole separation. The mechanism for charge transfer involving type II heterojunctions is similar to that of type p–n heterojunctions. Schottky junctions are usually heterogeneous interfaces formed by metals and semiconductors. The Z-type heterojunction not only efficiently achieves charge carrier isolation but also preserves the strong oxidation and reduction potentials of the two semiconductors and has excellent oxidation and reduction powers.
Cai and colleagues managed to synthesize g-C3N4/CuS p–n-type heterojunction photocatalysts via an in situ synthesis method. Experimental results indicated that the degradation rates of RhB and MB for this combination were 8.91 times and 13.54 times higher, respectively, compared to pure g-C3N4, and 3.02 times and 6.37 times higher, respectively, compared to pure CuS. The key factor for boosting the efficiency of catalysis of g-C3N4 under light exposure the lies in the adaptation of the energy band position of CuS to that of g-C3N4. Additionally, it offers more interfaces in order to facilitate the high-efficiency transfer of photo-created electron–hole pairs, thus suppressing recombination.81
For all kinds of heterojunctions, tight interface contact is an important guarantee for the effective transmission of electrons.106 Cao et al. used a high-energy ball mill-assisted calcination method to prepare a heterojunction composed of multilayer Ti3C2 as a support loaded with g-C3N4 and oxidized TiO2 in situ. The degradation rate of methyl orange in the ternary Z-type heterostructure is 3.62 times higher in comparison to g-C3N4, it is higher, and it is 6.60 times greater than that of Ti3C2. These authors hold the view that the improvement in photocatalytic performance stems from the compact Z-shaped heterointerface established among multilayer Ti3C2, the enhancement in photocatalytic performance is attributed to porous g-C3N4 sheets possessing substantial specific surface areas, as well as TiO2. This heterointerface promotes the creation of photogenerated carriers within the composite materials. Furthermore, it enhances the dissociation of carriers generated by light and accelerates the advancement of catalytic reactions.107
Overall, building heterojunctions with other materials is still the most effective way to optimize g-C3N4 photocatalytic properties. On the one hand, the establishment of heterojunctions helps to improve the stability of the catalyst and reduce photocorrosion and agglomeration; on the other hand, there is a synergistic effect between different semiconductors, thereby expanding the spectrum of light absorption and enhancing the efficiency of sunlight utilization. In addition, semiconductor crystal structures used to prepare heterojunctions are crucial to the high quantum efficiency of photocatalysts. The difference in the lattice spacing between the two semiconductors may lead to a lattice mismatch at the interface, thereby inhibiting the recombination of carriers.108
In promoting the splitting of light-induced charge carriers, photogenerated electrons can be provided by regulating the dielectric properties of the semiconductor catalyst or by constructing an interface electric field or local electric field or perturbing the local space charge distribution. To enhance the separation efficiency of light-excited electron–hole pairs, the driving force for holes to migrate in opposite directions is increased.109,110 By adjusting the micromorphology of the catalyst, the migration distance for the transfer of light-induced charge carriers can be shortened, or the electrical properties of the catalyst can be optimized to reduce the migration resistance, such as increasing the crystallinity of semiconductor catalysts and forming new common Price bonds or adjusting the electronic structure.111,112
The influencing factors of the catalyst surface redox reaction mainly include the quantity of surfactant binding locations on the catalyst, the overpotential of the material, and the adsorption and activation ability of reactant molecules. By loading or doping components with catalytic efficiency, the overpotential of the catalytic agent can be reduced, the surface area can be expanded, and the count of reactive sites can be increased, and the adsorption and activation ability of reactant molecules can be improved, thereby enhancing the surface redox reaction. activity.113,114
3 Overview of graphite-phase carbon nitride-based hydrogels
3.1 Overview of hydrogels
Hydrogels are polymeric materials composed of three-dimensional crosslinked networks that can absorb and retain large amounts of water without dissolving, and their properties can be modulated by the preparation process.115–121 Among them, cellulose-based hydrogels have attracted much attention due to their good hydrophilicity, biocompatibility and degradability.122,123 By introducing functional molecules such as cell adhesion peptides, enzyme-responsive sequences, or growth factors (Fig. 6A), its cytocompatibility can be further significantly enhanced to achieve specific cell adhesion and controlled degradation, thus overcoming the limitations of traditional PEG hydrogels in cellular microenvironment construction.124 In addition, cellulose nanocrystals (CNCs) can significantly enhance the comprehensive performance of hydrogels by virtue of their high hydrophilicity, crystallinity, and environmentally friendly properties,125 as well as two-dimensional materials, such as g-C3N4, through hydrogen bonding.126–129 | ||
| Fig. 6 Structure, modification and application of gels. (A) Chemical structure of chitosan.130 (B) The structures, characteristics and utilizations of hydrogels. (C) Bioactive modification of PEG hydrogels.125 | ||
Synthetic hydrogels are prepared by physical or chemical cross-linking.124,131,132 Chemically cross-linked hydrogels form permanent networks due to covalent bonding.133 The preparation methods include direct crosslinking of water-soluble polymers or hydrophilic modification of hydrophobic polymers followed by crosslinking. In contrast, physical gels usually have inferior mechanical properties to chemical gels due to weaker dynamic cross-linking, but their reversible sol–gel transition properties are more advantageous for specific applications.134 The crosslinked structure determines the viscoelastic or elastoplastic behavior of hydrogels, while the elasticity of the polymer network directly affects their sol–gel capacity (Fig. 6B).122,134 The swelling rate, mechanical properties, and degradation behavior of hydrogels can be optimized by adjusting the hydrophilic to hydrophobic ratio, initiator concentration, and reaction conditions (e.g., time, temperature) (Table 3). Semi-synthetic hydrogels combine the bioactivity of natural polymers with the tunability of synthetic materials, which broadens their applications. Among them, smart hydrogels can respond to environmental stimuli such as temperature and pH. Chitosan hydrogels are produced by deacetylation of chitin and their structure contains glucosamine and N-acetylglucosamine units (Fig. 6C). Their properties can be optimized by modifications such as ionic cross-linking, graft polymerization, or polyelectrolyte complex (PEC) formation. For example, PMVC/NVP composite hydrogel particles prepared by ionic gelation improved insulin release in acidic environments.135
| Macromolecule | Alteration in properties subsequent to modification | Employment | Ref. |
|---|---|---|---|
| Gelatin | Enhancement of rigidity | Caffeine-specific carriers in the human organism | 136 |
| Starch | The flexibility and elasticity of the patch were enhanced, along with the manifestation of favorable bioadhesive properties | The controlled-release delivery of α-hydroxy acid present in the extract of tamarind fruit pulp | 137 |
| Hydroxyethyl cellulose | Constructs firm network | Sustained-release delivery of chlorothiazide | 138 |
| Polyethylene glycol | Enhances heparin's blood-compatibility and anti-coagulation ability | Research on compatibility for diverse biomedical uses | 139 |
| Polyvinyl pyrrolidone | Boosts pH-dependent swelling, creates a firm network | A sustained-release system for delivering amoxicillin | 140 |
| Poly(ethylene-graft-acrylamide) | Constructs a firm network | Sustained release of capecitabine | 141 |
| Poly(acrylic acid-co-acrylamide) | Enhances mechanical strength, mucoadhesive ability and solubility | Promising muco-adhesive systems for oral administration of peptide and protein drugs | 142 |
| Pluronic F127 | Network structure turns more inflexible, leading to enhanced drug retention | Regulated delivery of 5-fluorouracil | 143 |
| Alginate and N, O carboxymethy chitosan | Electrostatic repulsion among ionized acidic groups enhances swelling characteristic | Targeted protein delivery in the intestinal tract | 144 |
| Carboxymethyl hexanoyl chitosan | Level of hexanoyl replacement alters the swelling capacity and solubility | Entrapment of slightly water – soluble drug | 145 |
| Carboxymethyl cellulose and chitosan | Generation of polyion complex offers high strength and steadiness | As a drug delivery vehicle | 146 |
| Chitosan and poly(vinyl alcohol) | Alteration of chitosan amount in the grafted polymer enhances cell survival probability | Prospective application in diverse biomedical fields | 147 |
| Chitosan and gelatin | Stiffness of the matrix went up | Biomedical utilization | 148 |
| Chitosan and polyethylene amide | Reduction in drug release speed as cross-linking density rises | Drug delivery utilization | 149 |
| Chitin and chitosan | Offers a beneficial environment for the growth of cartilage cells | Culture of bovine knee chondrocytes | 150 |
| Chitosan and gelatin | Rise in the pore-size ratio of the scaffolds | Articular cartilage tissue construction | 151 |
Hydrogels have shown great potential in the fields of agriculture, biomaterials, food industry and regenerative medicine due to their unique properties.115,134 Among them, polysaccharide-based hydrogels are prominent in wastewater treatment,123 for example, by embedding functional particles in hydrogel beads for efficient adsorption of heavy metals, dyes or radioactive pollutants.152,153 In addition, the responsiveness of hydrogels to external stimuli (e.g., temperature, light, pH, enzymes) makes them valuable in environmental sensing, actuation, and self-assembly systems.134,154 By precisely tuning the mechanical strength, electrical conductivity or self-repairing ability, hydrogel systems based on natural/synthetic polymers can fulfill diverse applications.115
3.2 Preparation of graphite phase carbon–nitride based hydrogels
(1) Construction of g-C3N4-based hydrogels with a three-dimensional hierarchical framework through nanostructure engineering.155It has rich pore structure with different sizes of voids, which is conducive to the adsorption and transport of substances; it can absorb and retain a large amount of water, and the water content can be as high as 90% or more; it also has good flexibility; using the efficient electron transport ability of g-C3N4, and the close contact interface between it and the active substances, it enhances the adsorption, catalytic and photocatalytic activities of g-C3N4.156 The researchers dispersed g-C3N4 nanosheets in sodium alginate hydrogels by ionic cross-linking and found that the photodegradation efficiency of the composite hydrogels for organic pollutants was significantly enhanced, indicating that a reasonable embedding method can effectively regulate the dispersibility and interfacial interactions of g-C3N4, which can provide a basis for the design of multifunctional hydrogels.157 For ternary hybrid aerogels containing g-C3N4, GO, metal oxides or layered hydroxides in multicomponent hydrogel composites.158 DPCN and NiFe-LDH were prepared by calcining urea in argon and hydrogen atmospheres at 550 and 540 °C, respectively, and hydrothermally treating with Ni-based and Fe-based nitrate solutions at 150 °C for 20 h. DPCN/NRGO/NiFe-LDH aerogels were then synthesized by a hydrothermal method in a PTFE-lined autoclave at 180 °C for 6 hours. The hydrogen bonding of amino or hydroxyl functional groups on the surface of g-C3N4 with acrylic monomers can be utilized to achieve its homogeneous dispersion and in situ polymerization in polyacrylamide hydrogels. In another study, the self-assembly of g-C3N4 nanosheets with chitosan was driven by π–π stacking and electrostatic interactions, resulting in the formation of a composite hydrogel with a hierarchical porous structure, which significantly enhances the adsorption capacity for heavy metal ions.
(2) Gelation of g-C3N4 derivatives using co-assembly or self-assembly methods.159,160
It can be combined with g-C3N4 and other materials to form a three-dimensional porous structure while retaining its unique lamellar sp2 structure. Jiang et al. worked on interconnecting GA/GH with g-C3N4 using co-assembly or self-assembly methods with the help of internal hydrogen bonding as well as π–π interactions between GA/GH and g-C3N4.148 GA/GH with a three-dimensional porous structure not only provides a large specific surface area, high adsorption capacity, and excellent mechanical strength, but also forms robust connections and conductive paths during assembly with g-C3N4, which in turn enables efficient charge transfer. Qu By calcining hydrothermally treated urea-derived graphene oxide reticulated composites under argon flow at a temperature of 600 °C. A reticulated g-C3N4/graphene interconnected mesh network was developed.144 These samples exhibited mesoporosity with a BET specific surface area as high as 352 m2 g−1 and a pore volume of 1.63 cm3 g−1, which amply demonstrated that these mesh online structures could significantly facilitate the transfer of water molecules and charges through the porous channels, thus enhancing the activity of hydrogen precipitation reaction. In addition to the hydrothermal assembly of GO with g-C3N4, reduced graphene oxide (rGO) also exhibits strong π–π conjugation interactions with g-C3N4, which can lead to the formation of g-C3N4/rGH hydrogels through the addition of sodium ascorbate as a reducing agent.150 The BET specific surface area, pore volume, and pore diameter of the 90%-g-C3N4/rGH sample were 302.6 m2 g−1, 1.48 cm3 g−1, and 3.9 nm, respectively, which suggests that the three-dimensional porous structure facilitates the enhancement of the removal of Cr(VI) by adsorption. Wu et al. utilized cetyltrimethylammonium bromide and ethylenediamine with g-C3N4 and GO suspension, heated at 95 °C for 7 h, and freeze-dried to obtain functionalized g-C3N4/GOA aerogels.151 This g-C3N4/GOA exhibits strong hydrogen bonding interactions, forming a stable structure and abundant three-dimensional connectivity channels for fast charge separation.
(3) The photopolymerisation reaction was used to prepare hydrogels using g-C3N4 as an initiator to provide free radicals.
Antonietti et al. reported that exfoliated g-C3N4 nanosheets can be irradiated with Xenon lamp (lambda >420 nm, 50 mW cm−2) to drive N-isopropylacrylamide (NIPAm) cross-linking for free radical polymerization, leading to the construction of PNIPAm/CNNS hydrogels.139 The concentration of NIPAm and CNNS, radiation duration and energy source (i.e., light or heat) strongly modulated the hydrogelation process. The results showed that the nitrogen atoms of the basic amine used as a co-initiator reacted with the free radicals on the surface of g-C3N4, transferring the free radicals directly from the g-C3N4 sites to the co-initiator sites, thus initiating the photoinitiated polymerization.
3.3 Application of graphite-phase carbon nitride-based hydrogels
In recent years, several studies have shown that graphite-phase carbon–nitride-based hydrogels combine the advantages of semiconductor photocatalysis and adsorption with porous materials, and exhibit unique potential in the field of adsorption and photocatalytic degradation of PAT.161–163 Its three-dimensional network structure not only provides abundant active sites, but also significantly improves the photocatalytic performance by enhancing the photogenerated carrier separation efficiency. The swelling property of the hydrogel further enlarges the internal porosity, resulting in a significant increase in the pollutant adsorption capacity compared with that of the conventional powder catalysts.164 Li et al. investigated a novel 3D–2D–3D BiOI/porous g-C3N4/graphene hydrogel (BPG) composite photocatalyst synthesized via a two-step hydrothermal method, which showed synergistic effects of adsorption and photocatalysis (Fig. 6A).165 A hydrogel exhibiting selective pollutant adsorption properties was prepared by Sun et al. (Fig. 6B).163,165–167 Fernandes et al. proposed the immobilization of g-C3N4-T-N in sodium alginate hydrogels to significantly improve the efficiency of photocatalytic synthesis of vitamin B3 (VB3). This strategy is promising for the synthesis of VB3, eliminating the need for a photocatalyst separation step, simplifying the reaction flow, efficiently oxidizing the process, targeting the conversion of the intermediate 3PC, and enhancing the pathway controllability. Thurston's group prepared self-supported g-C3N4/PVA composite hydrogels by direct casting method. The molecular interactions between the semiconductor particles and PVA chains significantly enhanced the mechanical strength and photophysical properties of the hydrogels when the g-C3N4 loading was 0.67% (38% reduction in PL intensity). The dense arrangement of g-C3N4 particles at higher loading enlarged the light absorption cross-section by 1.8-fold, and the rate constant of photocatalytic degradation of RhB was increased to 4.3-fold of pure PVA.4 Application of graphite-phase carbon nitride-based materials
4.1 Application of graphitic carbon nitride-based materials in patulin removal
g-C3N4 has become a research hotspot in the field of photocatalysis due to its unique electronic structure and chemical stability, but its specific surface area limitation and charge compounding problems constrain its practical application. g-C3N4 is an ideal carrier for modifying g-C3N4 by virtue of its high electrical conductivity, large specific surface area, and abundance of functional groups on the surface. Porous g-C3N4/GO developed by Sun et al. sodium alginate hydrogel microspheres (CN/GO/SA), although focusing on the degradation of aflatoxin B1 (AFB1) in peanut oil, the constructed synergistic system of adsorption and photocatalysis provides a universal idea for mycotoxin removal.157 The g-C3N4/MoS2 composite membrane and magnetic g-C3N4/Fe3O4 nanomaterials, on the other hand, have been prepared by electrostatic spinning technique to enhance the degradation of AFB1 in complex food matrices through the enhancement of visible-light responsiveness and the convenient magnetic separation properties, respectively (Fig. 7A and B).158 Zhang et al. studied a controlled magnetic adsorbent Fe3O4/ZIFs (Fig. 7C), and found that Fe3O4/ZIFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) had the highest adsorption efficiency for two precursors of AFB1 (AVN, ST) and that the material was also somewhat reusable.159 On this basis, the TiO2/g-C3N4 composite designed by Yang et al. was directly targeted for the efficient removal of PAT, and experiments showed that this heterojunction structure could achieve the complete degradation of 0.5 μg mL−1 PAT under 30 °C and 10 mg addition with only 3 min of dark adsorption in conjunction with 5 min of photocatalysis, which is significantly superior to that of the single-component. It was significantly better than the adsorption or catalytic performance of single component, revealing the synergistic mechanism of adsorption enrichment and photogenerated carrier separation.168
8) had the highest adsorption efficiency for two precursors of AFB1 (AVN, ST) and that the material was also somewhat reusable.159 On this basis, the TiO2/g-C3N4 composite designed by Yang et al. was directly targeted for the efficient removal of PAT, and experiments showed that this heterojunction structure could achieve the complete degradation of 0.5 μg mL−1 PAT under 30 °C and 10 mg addition with only 3 min of dark adsorption in conjunction with 5 min of photocatalysis, which is significantly superior to that of the single-component. It was significantly better than the adsorption or catalytic performance of single component, revealing the synergistic mechanism of adsorption enrichment and photogenerated carrier separation.168
 | ||
Fig. 7 Characterization, mechanism, catalytic activity diagram of different materials for mycotoxin removal. (A) SEM images of electrospun membranes anchored with g-C3N4/MoS2 prepared by different processes: (a) S1 ∼ mostly wrapped, (b) S2 ∼ partially exposed, and (c) S3 ∼ fully exposed.158 (B) (a) Photocatalytic degradation efficiencies of AFB1 with as-prepared S1, S2, and S3 under visible light irradiation. (b) HPLC chromatogram of AFB1 photocatalytic degradation with S3 under visible light irradiation at different times. (c) The photocatalytic activity of S3 for degradation of AFB1 at different pH values. (d) The photocatalytic activity of S3 for degradation of AFB1 with different initial concentrations. (e) The photocatalytic activity of S3 for degradation of AFB1 for five cycles. (f) Photocatalytic activities of S3 for the degradation of AFB1 in the presence of different scavengers.158 (C) Extraction mechanism of Fe3O4/ZIFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8) for AVN and ST.159 (D) TEM images of Fe3O4 (a), g-C3N4 (b) and g-C3N4/Fe3O4 (c) nanocomposites.160 8) for AVN and ST.159 (D) TEM images of Fe3O4 (a), g-C3N4 (b) and g-C3N4/Fe3O4 (c) nanocomposites.160 | ||
To address the complex contamination scenario of PAT in food matrices such as fruit juices, the nitrogen-doped titanium dioxide nanoparticles (N-TiONps) developed by Chen et al. demonstrated unique advantages under UV light. 1 hour was sufficient for the complete degradation of PAT in simulated and natural fruit juices, and the reaction mechanism study showed that superoxide radicals (·O2−) and hydroxyl radicals (·OH) The reaction mechanism study showed that ·O2− and ·OH are the main reactive species, and combined with the density functional theory (DFT) calculation confirmed that ester group is the key site of radical attack, which provides theoretical support for the degradation pathway at molecular level.169
In the same period, in another study, La-ZnFeO@FeO@carbon magnetic composites prepared with Fe/Zn metal–organic framework (MOF) as precursor showed 97.35% degradation of PAT under UV light, and the improved performance was attributed to the charge separation efficiency promoted by La doping and the multilevel active interfaces constructed with multifaceted components, which was further verified by electron spin resonance (ESR) analysis ·OH and ·O2− dominant roles.170
In the optimization of photocatalytic material structure, TiO2 nanotubes (TNTs) prepared by hydrothermal method combined with calcination showed unique advantages. Calcined at 450 °C, TNTs-450 can completely degrade 1000 μg L−1 PAT in simulated juice in 25 min with the high surface hydroxyl density of the amorphous structure, the mass transfer advantage of mesoporous nanotube structure, and the enhanced UV absorption, and the kinetic study shows that the degradation process is in accordance with the one-stage reaction model and Langmuir–Hinshelwood adsorption–surface reaction mechanism, which provided an efficient solution to the problem of PAT exceeding the standard in juice industry.168
The functionalized design of g-C3N4 matrix composites further expands the application scenarios. The g-C3N4-SH@KG aerogel prepared by glutaraldehyde cross-linking to introduce sulfhydryl groups (–SH) and immobilized with konjac glucomannan (KG) was constructed in a “dark adsorption–light regeneration” cyclic mode. The specific adsorption of PAT by sulfhydryl groups was rapidly accomplished in the dark environment, and the photocatalytic activity of g-C3N4 in the light achieved the regeneration of the adsorption sites, which was both highly efficient in removal and reuse.41
The g-C3N4 composites are also widely used in other applications. Ma et al. prepared a multifunctional magnetic g-C3N4/Fe3O4 nanocomposite (Fig. 7D), which exhibited a strong adsorption capacity for substances in complex matrices and realized a convenient magnetic separation from the sample solution.160 According to the research results published by Shi and other scholars, when the cross-linking reaction between g-C3N4 and sodium alginate occurs, which leads to the formation of nanocomposite hydrogels, the thermal stability of the gel is greatly enhanced, and the mechanical properties are also significantly improved, which demonstrates a greater advantage in practical applications.171 In contrast, Gahlot et al. showed that a significant enhancement of thermal stability could be achieved by adding 0.5 wt% graphene to PVA nanocomposites.172 This phenomenon is attributed to the strong interfacial interactions between graphene and polymers, which are essential for enhancing the physical properties of hydrogels.173 Notably, the composite system of g-C3N4 with hydrophilic polymers (e.g., sodium alginate, polyvinyl alcohol) not only enhances the mechanical strength and thermal stability of the hydrogel through interfacial interactions, but its abundant functional groups also synergize with the photocatalytic active sites to form a multi-trapping, directed degradation network. Combining g-C3N4 with hydrophilic polymers can also significantly enhance the mechanical properties, thermal stability and adsorption capacity of hydrogels.174 The successful application of this structural design strategy in dye decolorization and heavy metal removal further confirms its potential value in food toxin management.175 Future research could focus on the development of visible light-responsive composites, the optimization of the fitness of interfering factors in real food matrices, and the in-depth analysis of the degradation pathway based on in situ characterization techniques, to promote the key leap of photocatalytic technology from the laboratory to industrial applications.
In addition to being used for PAT removal, graphite-phase carbon nitride-based materials have been widely used to treat other pollutants. Zinc-enriched g-C3N4 was prepared in the experiments of Chen et al. When the salinity increased from 0.1 wt% to 2.3 wt%, the removal efficiency of 2,4-DCP by the material slightly increased. This material exhibited exceptional efficiency in eliminating 2,4-DCP, achieving a removal rate exceeding 75.6%, this particular quantity was two times as large as what was observed in the case of pure g-C3N4 and g-C3N4 that had been treated with Co, Ag, Mo, and Bi. Once 0.1 g L−1 of g-C3N4 having a significant amount of zinc was introduced, the percentages by which 2,4-DCP, 2-chlorohydroquinone, chloroacetophenone, and 2-chloropropionic acid were removed reached the values of 99.3%, 99.8%, 98.2%, and 99.9% respectively.176 Liu et al. succeeded in constructing Ag3PO4/Ag/g-C3N4 heterojunctions, which have a wide range of light absorption, via a hydrothermal method. Under visible light, Ag3PO4/Ag/g-C3N4-1.6 demonstrated a superior methyl orange removal rate (∼90%) compared to Ag3PO4 or Ag/g-C3N4 alone. Its degradation rate of 0.04126 min−1 was 4.23 and 6.53 times higher than that of Ag3PO4/Ag and g-C3N4, respectively. The enhanced photocurrent intensity of Ag3PO4/Ag/g-C3N4 suggests improved the efficiency of separating the electron–hole pairs that are generated by photo-excitation, attributed to the material's heterojunction structure. Even after five cycles, the photocatalyst retained high activity. The exceptional performance under UV-visible light is linked to the effective carrier separation enabled by the Ag3PO4/Ag/g-C3N4 heterostructure.177 In the research conducted by Mahmoudi and colleagues, g-C3N4/Fe3O4 nanocomposites were employed as catalysts through a straightforward hydrothermal approach. The influence of critical operational factors, including initial pH, catalyst dosage, contact duration, and initial oxytetracycline (OTC) concentration in aqueous solution, was examined under UV light. Optimal OTC removal efficiency (99.8%) was attained under neutral pH conditions (pH 7) with a catalyst loading of 0.7 g L−1 and an initial OTC concentration of 5 mg L−1.178
4.2 Graphite-phase carbon nitride-based materials for energy storage applications
Renowned for its outstanding chemical and physical stability, environmental-friendliness, and non-pollution, g-C3N4 is a widely-acknowledged 2D semiconductor material. Due to its exceptional characteristics, g-C3N4 has found widespread use in various energy storage arrangements, encompassing lithium-ion, lithium-sulfur, sodium-ion, and potassium-ion accumulators, in addition to supercondensers.179 Electrochemical energy storage systems are those that are designed to store energy through electrochemical processes. They include two main types of components. One type is batteries, which are devices that convert chemical energy into electrical energy and vice versa. The other type is supercapacitors (SCs), which store energy electrostatically and can charge and discharge much faster than batteries, have significantly impacted the field of energy applications (Fig. 8A). Hussain et al. demonstrated a new approach to fabricate g-C3N4 for supercapacitor devices using spinel oxide materials. They synthesized MnFe2O4, g-C3N4, and MnFe2O4@g-C3N4 nanocomposites through a hydrothermal method to study their electrochemical properties. The MnFe2O4@g-C3N4 nanohybrids showed a particular capacitance (Cs) of 1414 F g−1 at 1 A g−1 and excellent durability after the 5th, 5000th cycle, as determined from the Nyquist plot. The small charge transfer impedance (Rct = 0.08 Ω) suggested that this material performs well in supercapacitor applications. In addition, they performed symmetric two-electrode tests on MnFe2O4@g-C3N4 nanohybrids possessing a Cs of 392.02 F g−1 at 1 A g−1, this phenomenon can be ascribed to the robust interfacial interactions existing between MnFe2O4 and g-C3N4.161 Wu et al. developed nitrogen-doped graphitic carbon nanosheets (NGCNs) through a straightforward reaction involving g-C3N4 and CaC2. The resulting NGCN exhibits a elevated nitrogen content (3.98 at%), a substantial specific surface area (72.96 m2 g−1), and a high degree of graphitization (interlayer spacing of 0.335 nm). As an negative electrode material for lithium-ion batteries, NGCN demonstrates a promising reversible capacity of 475 mA h g−1 at 500 mA g−1, maintaining 98.5% capacity retention after 1000 cycles. Additionally, it shows a respectable rate capability (238 mA h g−1 at 5000 mA g−1). This investigation presents a plausible tactic to convert renewable carbon sources into nitrogen-incorporated graphitic carbon nanoplates with remarkable electrochemical characteristics.180 | ||
| Fig. 8 g-C3N4-based materials and electrocatalytic mechanisms for energy conversion applications. (A) Various composites of g-C3N4 for supercapacitor applications.166 (B) Feasible mechanism for the HER. (C) Feasible mechanism for the OER.165 | ||
4.3 Graphite-phase carbon nitride-based materials for energy conversion applications
The rational development and sustainable preparation of g-C3N4-based materials enable their wide-ranging applications in energy conversion and storage fields. These applications cover various aspects, like photocatalytic H2 release, photocatalytic O2 release, photocatalytic total water decomposition, CO2 photoreduction, electrocatalytic H2 release, O2 release, and O2 reduction.163 The photocatalytic fuel cell (PFC) system is a major innovation within the domain of energy transformation technology, which uses the potential of organic waste to generate electricity. A new method for simultaneous power generation and pollutant removal using a PFC system consisting of Bi2O3/TiO2 heterojunction nanotubes modified with g-C3N4 as the photoanode material was presented By Zhao and colleagues. The g-C3N4/Bi2O3/TiO2 (CBT) heterostructure has a large specific surface region, better visible photonic absorption and enhanced photogenerated charge-carrier segregation. In the case where tetracycline hydrochloride was employed as the pollutant that was specifically targeted, the ability of the CBT heterostructures to perform photocatalytic degradation was 3.2 times as great as the corresponding ability of pure TiO2 nanotubes. In addition, the PFC system consisting of CBTs as photoanodes significantly improved the pollutant degradation and current output compared with those of the PFC system with pure TiO2. This study presents a cost-effective method for organic waste treatment and simultaneous power generation.164 In 2023, Sowmya and Vijaikanth introduced an economical modified electrode incorporating g-C3N4/chlorocobalt oxime composites, showcasing their electrocatalytic performance for hydrogen evolution reaction (HER) (Fig. 8B) and oxygen evolution reaction (OER) (Fig. 8C), alongside their energy storage potential. Hydrolysis experiments of the g-C3N4/ClCo(dpgH)2 (pyridine-3,5-dicarboxylic acid) composite in 0.5 M KOH demonstrated sustained hydrogen and oxygen generation for up to 120 hours. The composite was characterized by a high level of hydrogen and oxygen release over a period of up to 120 h. The composite was then subjected to an electrocatalytic reaction (HER) and oxygen release (OER), which was characterized by a high level of hydrogen and oxygen. Supercapacitor application studies via cyclic voltammetry and charge/discharge studies revealed that the g-C3N4/ClCo(dpgH)2 (isonicotinic acid) nanocomposite has a high specific capacity of 236 F g−1 at 0.5 A g−1.165The high dispersibility of g-C3N4 can make its separation from solutions challenging, often requiring time-consuming centrifugation. In contrast, Fe3O4 particles can be easily isolated using an external magnet. Thus, incorporating Fe3O4 onto the g-C3N4 surface presents a practical solution to address this issue effectively. In 2021, Ma et al. prepared a multifunctional magnetic g-C3N4/Fe3O4 nanocomposite material to be used as a modified QuEChERS adsorbent through ultra-high-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). This precise quantification method combines multiple methods. The g-C3N4/Fe3O4 composite not only strongly adsorbs substances on complex substrates but also achieves convenient magnetic separation from the sample solution.160
Fe3O4/ZIFs possess a large specific surface area and a hierarchical porous structure comprising micropores and mesopores, along with abundant π–π and Zn-Nx groups. These characteristics render Fe3O4/ZIFs highly effective adsorbents, offering numerous adsorption sites and multifunctional groups that align with the analyte's chemical structure, leading to excellent adsorption performance. In 2023, a controlled magnetic adsorbent of Fe3O4/ZIFs was studied by Zhang et al. Through altering the proportion of Fe2+ to Zn2+, the specific surface area of Fe3O4/ZIFs is tunable, thereby affecting performance. Research has revealed that for Fe3O4/ZIFs (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8), the adsorption efficiency of the two precursor substances of AFB1 (AVN, ST) is the highest, and this material has a certain degree of reusability.159
8), the adsorption efficiency of the two precursor substances of AFB1 (AVN, ST) is the highest, and this material has a certain degree of reusability.159
Many aspects of hydrogels, including a high water content, excellent biocompatibility and controllable network structure, have been obtained. Extremely extensive and in-depth application. According to research results published by Shi and other scholars, when g-C3N4 reacts with sodium alginate and then forms a nanocomposite hydrogel, the thermal stability of the gel is greatly enhanced, and the mechanical properties are significantly improved. This approach has advantages in practical applications.142 Research by Gahlot and associates demonstrated that within PVA nanocomposites, only 0.5 wt% graphene is required to achieve a significant increase in thermal stability.143 This effect is due to the robust interfacial interaction between graphene and the polymer, playing a key role in enhancing the hydrogel's physical properties.144 Considering the structural resemblance between g-C3N4 and graphene, we hypothesize that incorporating g-C3N4 with hydrophilic polymers can remarkably enhance the mechanical characteristics, thermal endurance, and adsorption capability of the hydrogel.145 In addition, some researchers have grafted SH with visible light catalytic properties via glutaraldehyde cross-linking and immobilized it with KG in order to acquire a composite aerogel g-C3N4-SH (Gl)@KG-5 material, this material was employed for the elimination of PAT in juice (Fig. 9A and B).35 Moreover, hydrogels are additionally employed for the removal of dyes and heavy-metal ions, antibacterial applications, and photocatalytic hydrogen generation, among other purposes (Fig. 9C).146 These studies suggest that the removal efficiency of g-C3N4/GO/Fe3O4/ZIF-8 nanohydrogel composites for PAT is significantly greater than that of traditional methods.
 | ||
| Fig. 9 Composite gel materials are used for PAT removal, as well as other applications. (A), (a) Photos of g-C3N4-SH(Gl), (b) a pure konjac aerogel sample, and (c) g-C3N4-SH (Gl)@KG-5 are presented. Scanning Electron Microscopy (SEM) pictures of (d) g-C3N4-SH(Gl), (e) the pure konjac aerogel, and (f) g-C3N4-SH (Gl)@KG-5 are shown at various magnifications. Moreover, (g) elemental mappings of sulfur (S) for g-C3N4-SH (Gl)@KG-5 using SEM-Energy-Dispersive X-ray (SEM-EDX) analysis are provided. Energy-Dispersive X-ray (EDX) spectra of (h) g-C3N4 and (i) g-C3N4-SH (Gl)@KG-5 are also included.35 (B), (a) Variations in the duration of photocatalytic treatment and the concentration of –SH groups; (b) the reusability of g-C3N4-SH(Gl)@KG-5 for the adsorption of PAT in PCBs. (c and d) Fourier-Transform Infrared (FT-IR) spectral profiles of g-C3N4, g-C3N4-SH(Gl)-PAT, and g-C3N4-SH(Gl)-PAT subsequent to light exposure. (e) Overall XPS spectral scans of g-C3N4, g-C3N4-SH(Gl)-PAT, and g-C3N4-SH(Gl)-PAT following light exposure. High-resolution XPS spectral analyses of carbon (C) 1s (f), nitrogen (N) 1s (g), and sulfur (S) 2p (h) For g-C3N4, g-C3N4-SH(Gl)-PAT, and g-C3N4-SH(Gl)-PAT after being subjected to light.35 (C) Hydrogel photocatalytic application.146 | ||
Composite materials such as GO, Fe3O4 and ZIF-8 possess extensive application potential in the domains of food safety and environmental conservation. The composite material can be used to remove PAT and contaminate fruits and their products, as well as a variety of mycotoxins, such as deoxyfusarium enol (DON), AFB1, zearalenone (ZEN), and ochratoxin A (OTA), to improve the safety of food; at the same time, it can also be used to treat wastewater, soil and other environmental pollutants containing mycotoxins to protect the ecological environment.
5 Conclusion and prospects
The g-C3N4-based materials show remarkable potential in the field of adsorption and photocatalytic synergistic removal of PAT due to their unique two-dimensional layered structure, visible-light-responsive properties and excellent chemical stability. It has been shown that its large specific surface area and abundant surface functional groups can realize efficient adsorption and enrichment of PAT through electrostatic interaction, hydrogen bonding and π–π stacking, while the photocatalytic process can gradually degrade PAT into harmless small molecules through the generation of reactive oxygen species (e.g., ·OH and ·O2−) by stimulating electron–hole pairs. The synergistic effect of adsorption and photocatalysis not only improves the PAT removal efficiency, but also maintains the adsorption capacity of the material through dynamic equilibrium. Currently, this technology has been validated in food matrices such as fruit juice and fruit wine, and has shown promising applications in environmental treatment scenarios such as wastewater purification and soil remediation. However, the high complexity of photogenerated carriers, the lack of stability in complex environments, and the lack of a scale-up preparation process remain the core challenges limiting its practical application.In recent years, emerging research trends have focused on exploring interdisciplinary means to optimize material properties and expand application boundaries. For example, heterostructure construction modulates carrier separation efficiency through energy band engineering, and Yang et al. achieved 100% removal rate of PAT through TiO2/g-C3N4 heterojunction with significantly better synergistic effect than single component (Fig. 7D).160 Multi-dimensional structural design can simultaneously enhance the adsorption capacity and photoresponsive activity, and the CN/GO/SA hydrogel microspheres developed by Sun et al. achieved up to 98.4% removal of AFB1 from peanut oil with excellent cycling stability.157 In addition, the rise of intelligent composites provides new ideas for PAT control, such as light-responsive hydrogels for targeted adsorption and degradation of pollutants through in situ polymerization.41 These advances indicate that the performance boundaries of g-C3N4-based materials are being broken through the deep integration of materials science and environmental engineering.
Future studies need to deepen the exploration in the following directions: first, the refinement of mechanistic studies needs to combine in situ characterization techniques with theoretical calculations to clarify the PAT degradation pathway and the toxicity evolution of intermediates. Liu et al. revealed the promotion of carrier migration by nitrogen vacancies in P-doped g-C3N4 through DFT,179 and this kind of study can provide atomic-level guidance. Second, the development of functional composite systems needs to focus on multi-technology synergies, combining g-C3N4 with magnetic materials to simplify the recycling process by using magnetic separation technology, or coupling with biodegradation technology to achieve the harmless transformation of pollutants. Recently, Zhang et al. designed Fe3O4/ZIFs magnetic adsorbents to achieve efficient capture of mycotoxin precursors by modulating the pore structure,159 a strategy that can be extended to the field of PAT governance. In addition, the suitability for practical application scenarios needs to be focused on, such as the development of flexible photocatalytic membranes (e.g., electrostatically spun g-C3N4/MoS2 membranes) to suit the continuous operation in food processing.158
At the EHS level, the long-term ecological risks of g-C3N4-based materials need to be systematically assessed. For example, the potential bioaccumulation of nanoparticles and their effects on non-target organisms have not been clarified.174 In addition, the synergistic removal mechanism of multifunctional composites for composite pollutants still needs to be deeply analyzed. Optimization of the preparation process through life cycle analysis and green chemistry principles will be the key to achieve sustainable development of the materials.180
In conclusion, g-C3N4-based materials have demonstrated the potential to transition from laboratory research to industrialization in PAT pollution control. Through interdisciplinary innovation and engineering exploration, the future is expected to build an efficient, green and economical technology system for PAT management, providing more competitive solutions for food safety and environmental protection.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was supported by the Natural Science Foundation Project of Hubei Province (2022CFB533) and the Scientific Research Plan of Education Department of Hubei Province (D20222702), and the Natural Science Project of Xiaogan City (XGKJ2024030002).References
- I. Chalmers and M. Clarke, Int. J. Epidemiol., 2004, 33, 253–260 CrossRef PubMed
.
- G. L. Ngolong Ngea, Q. Yang, R. Castoria, X. Zhang, M. N. Routledge and H. Zhang, Compr. Rev. Food Sci. Food Saf., 2020, 19, 2447–2472 CrossRef
.
- L. Zhong, J. Carere, Z. Lu, F. Lu and T. Zhou, Toxins, 2018, 10, 475 CrossRef CAS
.
- N. De Clercq, G. Vlaemynck, E. Van Pamel, D. Colman, M. Heyndrickx, F. Van Hove, B. De Meulenaer, F. Devlieghere and E. Van Coillie, World Mycotoxin J., 2016, 9, 379–388 CAS
.
- S. Barad, E. Sionov and D. Prusky, Fungal Biol. Rev., 2016, 30, 24–32 CrossRef
.
- H. Dai, Y. Huang and H. Huang, Carbohydr. Polym., 2018, 185, 1–11 CrossRef
.
- J. Niu, B. Ma, J. Shen, H. Zhu, Y. Lu, Z. Lu, F. Lu and P. Zhu, Food Microbiol., 2025, 126, 104676 CrossRef CAS
.
- S. Ramalingam, A. Bahuguna and M. Kim, Trends Food Sci. Technol., 2019, 83, 99–113 CrossRef CAS
.
- X. Zheng, W. Wei, W. Zhou, H. Li, S. Rao, L. Gao and Z. Yang, Food Res. Int., 2021, 140, 110034 CrossRef CAS
.
- I. Saleh and I. Goktepe, Food Chem. Toxicol., 2019, 129, 301–311 CrossRef CAS PubMed
.
- P. Udomkun, A. N. Wiredu, M. Nagle, J. Müller, B. Vanlauwe and R. Bandyopadhyay, Food Control, 2017, 76, 127–138 CrossRef CAS PubMed
.
- S. K. Pankaj, H. Shi and K. M. Keener, Trends Food Sci. Technol., 2018, 71, 73–83 CrossRef CAS
.
- J. Wan, B. Chen and J. Rao, Compr. Rev. Food Sci. Food Saf., 2020, 19, 928–953 CrossRef PubMed
.
- R. Khan, F. Anwar and F. M. Ghazali, Heliyon, 2024, 10, e28361 CrossRef CAS PubMed
.
- E. Kroke, M. Schwarz, E. Horath-Bordon, P. Kroll, B. Noll and A. D. Norman, New J. Chem., 2002, 26, 508–512 RSC
.
- Q. Hao, G. Jia, W. Wei, A. Vinu, Y. Wang, H. Arandiyan and B.-J. Ni, Nano Res., 2020, 13, 18–37 CrossRef CAS
.
- G. Liao, Y. Gong, L. Zhang, H. Gao, G.-J. Yang and B. Fang, Energy Environ. Sci., 2019, 12, 2080–2147 RSC
.
- M. Ismael, J. Alloys Compd., 2020, 846, 156446 CrossRef CAS
.
- S. Yang, Y. Gong, J. Zhang, L. Zhan, L. Ma, Z. Fang, R. Vajtai, X. Wang and P. M. Ajayan, Adv. Mater., 2013, 25, 2452–2456 CrossRef CAS
.
- F. Cheng, H. Wang and X. Dong, Chem. Commun., 2015, 51, 7176–7179 RSC
.
- P. Niu, L. Zhang, G. Liu and H. Cheng, Adv. Funct. Mater., 2012, 22, 4763–4770 CrossRef CAS
.
- S. Yin, J. Han, T. Zhou and R. Xu, Catal. Sci. Technol., 2015, 5, 5048–5061 RSC
.
- S. K. Selvam, R. A. Kumar, N. Balasubramanian, P. S. Kumar, A. Muthukrishnaraj, S. S. Kalaivani, R. Vinayagam, R. S. Azarudeen, M. A. R. Ahamed, A. Harikrishnan, S. Josephine, G. A and G. Rangasamy, Desalin. Water Treat., 2024, 318, 100385 CrossRef CAS
.
- E. Baur and A. Perret, Helv. Chim. Acta, 1924, 7, 910–915 CrossRef CAS
.
- A. Fujishima and K. Honda, Nature, 1972, 238, 37–38 CrossRef CAS
.
- J. H. Carey, J. Lawrence and H. M. Tosine, Bull. Environ. Contam. Toxicol., 1976, 16, 697–701 CrossRef CAS
.
- Y. Shi, Q. Zhao, J. Li, G. Gao and J. Zhi, Appl. Catal., B, 2022, 308, 121216 CrossRef CAS
.
- O. S. Keen, S. Baik, K. G. Linden, D. S. Aga and N. G. Love, Environ. Sci. Technol., 2012, 46, 6222–6227 CrossRef CAS PubMed
.
- J. K. Glenn and J. Goldman, Am. J. Public Health, 1976, 66, 64–66 CrossRef CAS
.
- E. Haque, J. W. Jun, S. N. Talapaneni, A. Vinu and S. H. Jhung, J. Mater. Chem., 2010, 20, 10801 RSC
.
- Y. Cui, Z. Ding, X. Fu and X. Wang, Angew. Chem., 2012, 124, 11984–11988 CrossRef
.
- Q. Liao, W. Pan, D. Zou, R. Shen, G. Sheng, X. Li, Y. Zhu, L. Dong, A. M. Asiri, K. A. Alamry and W. Linghu, J. Mol. Liq., 2018, 261, 32–40 CrossRef CAS
.
- W. Wang, Q. He, Y. Yi, Y. Xiao, X. Xiao, H. Yang and X. Dong, J. Cleaner Prod., 2023, 415, 137818 CrossRef CAS
.
- B. Zhu, P. Xia, W. Ho and J. Yu, Appl. Surf. Sci., 2015, 344, 188–195 CrossRef CAS
.
- J. Wang, D. Yue, D. Cui, L. Zhang and X. Dong, Environ. Sci. Technol., 2021, 55, 7910–7919 CrossRef CAS
.
- H. Wu, W. Zhang, H. Zhang, Y. Pan, X. Yang, Z. Pan, X. Yu and D. Wang, Colloids Surf., A, 2020, 607, 125517 CrossRef CAS
.
- X. Li, J. Yu, J. Low, Y. Fang, J. Xiao and X. Chen, J. Mater. Chem. A, 2015, 3, 2485–2534 RSC
.
- Y. Gong, M. Li and Y. Wang, ChemSusChem, 2015, 8, 931–946 CrossRef CAS
.
- Y. Jun, E. Z. Lee, X. Wang, W. H. Hong, G. D. Stucky and A. Thomas, Adv. Funct. Mater., 2013, 23, 3661–3667 CrossRef CAS
.
- P. A. Poole-Wilson and G. A. Langer, Am. J. Physiol., 1975, 229, 570–581 CrossRef CAS
.
- Y. Qiu, J. Yan, X. Liu, Y. Pang, Y. Ding and F. Lyu, Food Chem., 2024, 451, 139421 CrossRef CAS
.
- R. A. M. Magzoub, A. A. A. Yassin, A. M. Abdel-Rahim, E. A. Gubartallah, M. Miskam, B. Saad and S. Sabar, Food Control, 2019, 95, 206–214 CrossRef CAS
.
- H. Zhang, P. Wu, J. He, W. Jiang and C. Liu, J. Environ. Chem. Eng., 2021, 9, 106430 CrossRef CAS
.
- D. Sun, J. Mao, L. Cheng, X. Yang, H. Li, L. Zhang, W. Zhang, Q. Zhang and P. Li, Chem. Eng. J., 2021, 418, 129417 CrossRef CAS
.
- C. Hu, C. Huang and B. Peng, Food Chem., 2023, 426, 136592 CrossRef CAS PubMed
.
- X. Yang, J. Pan, J. Hu, S. Zhao and K. Cheng, Chem. Eng. J., 2023, 467, 143381 CrossRef CAS
.
- Q. Sun, Z. Li, J. Li, N. Liu, M. Zhang and T. Le, J. Alloys Compd., 2023, 955, 170234 CrossRef CAS
.
- R. Dhodapkar, N. N. Rao, S. P. Pande, T. Nandy and S. Devotta, React. Funct. Polym., 2007, 67, 540–548 CrossRef CAS
.
- Y. Xing, D. Liu and L.-P. Zhang, Desalination, 2010, 259, 187–191 CrossRef CAS
.
- T. Kahn, J. Bosch, M. F. Levitt and M. H. Goldstein, Am. J. Physiol., 1975, 229, 746–753 CrossRef CAS PubMed
.
- S. Y. Kim, J. Oh, S. Park, Y. Shim and S. Park, Chem.–Eur. J., 2016, 22, 5142–5145 CrossRef CAS PubMed
.
- J. Liu, W. Li, L. Duan, X. Li, L. Ji, Z. Geng, K. Huang, L. Lu, L. Zhou, Z. Liu, W. Chen, L. Liu, S. Feng and Y. Zhang, Nano Lett., 2015, 15, 5137–5142 CrossRef CAS
.
- H.-J. Li, B.-W. Sun, L. Sui, D.-J. Qian and M. Chen, Phys. Chem. Chem. Phys., 2015, 17, 3309–3315 RSC
.
- J.-S. Chen, T.-C. Lo, Y.-C. Hsieh, C.-H. Chen, M. Lin, H.-Y. Lin, M.-W. Hung, H.-R. Wu and S.-Y. Luo, ACS Omega, 2023, 8, 8885–8893 CrossRef CAS PubMed
.
- A. Sudha, I. Manimehan, B. Sundaresan, R. Arivuselvi, S. Manimaran, M. Ayyanar, P. Gangapriya and K. Ravichandran, Appl. Phys. A, 2025, 131, 393 CrossRef CAS
.
- G. Huang, S. Huo, J. Ren, W. Chen, H. Yang, S. Xiao, T. Wang, H. Peng and P. Song, Appl. Mater. Today, 2024, 38, 102191 CrossRef
.
- L. Nguyen Thi, N. N. Tri, L. N. Tan, N. M. Vuong, T.-L. Thi Le, D. Thi To Nu, L. T. Nguyen, D. T. Khan, L. V. T. Son, T. N. Dat, V. T. Nguyen, Q. T. H. Ta, V. A. Tran, T. T. Trang Phan and V. Vo, J. Alloys Compd., 2025, 1018, 179245 CrossRef CAS
.
- K. I. John, T. B. Issa, G. Ho, A. N. Nikoloski and D. Li, Water Cycle, 2025, 6, 151–175 CrossRef
.
- É. W. A. Almeida, C. M. C. Dazon, M. D. V. R. Rodriguez, T. M. Nobre, M. C. Pereira and D. S. Monteiro, ACS Omega, 2025, 10, 17024–17032 CrossRef PubMed
.
- M. Hosseini, M. Ghanbari, M. A. Mahdi, M. H. Almaamori, Z. A. A. Alhassan and M. Salavati-Niasari, Appl. Water Sci., 2025, 15, 41 CrossRef CAS
.
- J. Chen, J. Lu, R. Lang, C. Wang, S. Bao, Y. Li, K. Li and M. Fan, Green Energy Environ., 2025, 10, 1348–1358 CrossRef CAS
.
- S. Marouch, G. Sarp, M. Soylak and E. Yilmaz, ACS Omega, 2025, 10, 11961–11971 CrossRef CAS
.
- V. Jeyalakshmi, S. Wu, S. Qin, X. Zhou, B. B. Sarma, D. E. Doronkin, J. Kolařík, M. Šoóš and P. Schmuki, Chem. Sci., 2025, 16, 4788–4795 RSC
.
- N. Mamba, T. A. Makhetha, B. S. Mbuli and S. P. Malinga, Inorg. Chem. Commun., 2025, 176, 114215 CrossRef CAS
.
- N. Alebachew, T. B. Demissie, H. C. A. Murthy, B. A. Gonfa, K. G. Von Eschwege, E. Coetsee, E. H. G. Langner, Jayadev and B. H. Doreswamy, RSC Adv., 2025, 15, 6441–6456 RSC
.
- M. Yaqoubi, M. Ghanbari, R. W. Maya, H. A. Jasim and M. Salavati-Niasari, Results Eng., 2025, 25, 104037 CrossRef CAS
.
- L. Santamaría, S. A. Korili, A. Gil, J. M. López-de-Luzuriaga and M. Monge, Appl. Surf. Sci., 2024, 673, 160906 CrossRef
.
- Y. Qiu, J. Yan, X. Liu, Y. Pang, Y. Ding and F. Lyu, Food Chem., 2024, 451, 139421 CrossRef CAS PubMed
.
- S. Sun, J. Yang, M. Ku, W. Zhang, Y. Xie and R. Zhao, SSRN, 2024, 42, 679–692 Search PubMed
.
- X. Ruan, L. Wang, D. Liang and Y. Shi, Langmuir, 2023, 39, 3371–3379 CrossRef CAS PubMed
.
- Y. Yang, Z. Liu, R. Jiang, R. Li and X. Li, J. Alloys Compd., 2019, 811, 151991 CrossRef CAS
.
- J. Zhang, M. Zhang, R. Sun and X. Wang, Angew. Chem., 2012, 124, 10292–10296 CrossRef
.
- X. Wang, K. Maeda, X. Chen, K. Takanabe, K. Domen, Y. Hou, X. Fu and M. Antonietti, J. Am. Chem. Soc., 2009, 131, 1680–1681 CrossRef CAS PubMed
.
- M. Wang, J. Cheng, X. Wang, X. Hong, J. Fan and H. Yu, Chin. J. Catal., 2021, 42, 37–45 CrossRef CAS
.
- Y. Xu, H. Xu, J. Yan, H. Li, L. Huang, J. Xia, S. Yin and H. Shu, Colloids Surf., A, 2013, 436, 474–483 CrossRef CAS
.
- J. Zhang, G. Zhang, X. Chen, S. Lin, L. Möhlmann, G. Dołęga, G. Lipner, M. Antonietti, S. Blechert and X. Wang, Angew. Chem., 2012, 124, 3237–3241 CrossRef
.
- J. Zhang, X. Chen, K. Takanabe, K. Maeda, K. Domen, J. D. Epping, X. Fu, M. Antonietti and X. Wang, Angew. Chem., 2010, 122, 451–454 CrossRef
.
- H. Li, C. Liu, A. Saini, Y. Wang, H. Jiang, T. Yang, L. Chen, C. Pan and H. Shen, J. Power Sources, 2019, 438, 226974 CrossRef CAS
.
- Y. Wang, H. Wang, J.-S. He and X. Feng, Nat. Commun., 2017, 8, 15972 CrossRef CAS
.
- S. Cao, J. Low, J. Yu and M. Jaroniec, Adv. Mater., 2015, 27, 2150–2176 CrossRef CAS
.
- Z. Cai, Y. Zhou, S. Ma, S. Li, H. Yang, S. Zhao, X. Zhong and W. Wu, J. Photochem. Photobiol., A, 2017, 348, 168–178 CrossRef CAS
.
- B. Modak and S. K. Ghosh, J. Phys. Chem. C, 2016, 120, 6920–6929 CrossRef CAS
.
- J. Tian, Q. Liu, A. M. Asiri, A. H. Qusti, A. O. Al-Youbi and X. Sun, Nanoscale, 2013, 5, 11604 RSC
.
- Z. Li, C. Kong and G. Lu, J. Phys. Chem. C, 2016, 120, 56–63 CrossRef CAS
.
- B. Yue, Q. Li, H. Iwai, T. Kako and J. Ye, Sci. Technol. Adv. Mater., 2011, 12, 034401 CrossRef PubMed
.
- S. Hu, R. Jin, G. Lu, D. Liu and J. Gui, RSC Adv., 2014, 4, 24863 RSC
.
- L. Cao, R. Wang and D. Wang, Mater. Lett., 2015, 149, 50–53 CrossRef CAS
.
- J. Liu, J. Alloys Compd., 2016, 672, 271–276 CrossRef CAS
.
- J. Li, B. Shen, Z. Hong, B. Lin, B. Gao and Y. Chen, Chem. Commun., 2012, 48, 12017 RSC
.
- G. Dong, K. Zhao and L. Zhang, Chem. Commun., 2012, 48, 6178 RSC
.
- X. Li, J. Yu and M. Jaroniec, Chem. Soc. Rev., 2016, 45, 2603–2636 RSC
.
- J. Xu, Y. Wang and Y. Zhu, Langmuir, 2013, 29, 10566–10572 CrossRef CAS PubMed
.
- Y. Zhang, J. Liu, G. Wu and W. Chen, Nanoscale, 2012, 4, 5300 RSC
.
- J. Zhang, F. Guo and X. Wang, Adv. Funct. Mater., 2013, 23, 3008–3014 CrossRef CAS
.
- X.-H. Li, J. Zhang, X. Chen, A. Fischer, A. Thomas, M. Antonietti and X. Wang, Chem. Mater., 2011, 23, 4344–4348 CrossRef CAS
.
- Y. Cui, J. Huang, X. Fu and X. Wang, Catal. Sci. Technol., 2012, 2, 1396 RSC
.
- X.-H. Li, X. Wang and M. Antonietti, Chem. Sci., 2012, 3, 2170 RSC
.
- X. Chen, C. Li, M. Grätzel, R. Kostecki and S. S. Mao, Chem. Soc. Rev., 2012, 41, 7909 RSC
.
- J. Ding, Q. Liu, Z. Zhang, X. Liu, J. Zhao, S. Cheng, B. Zong and W.-L. Dai, Appl. Catal., B, 2015, 165, 511–518 CrossRef CAS
.
- X. Wang, G. Sun, N. Li and P. Chen, Chem. Soc. Rev., 2016, 45, 2239–2262 RSC
.
- Y. Zhao, F. Zhao, X. Wang, C. Xu, Z. Zhang, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2014, 53, 13934–13939 CrossRef CAS PubMed
.
- J. Liu, H. Wang and M. Antonietti, Chem. Soc. Rev., 2016, 45, 2308–2326 RSC
.
- W. Wang, J. C. Yu, Z. Shen, D. K. L. Chan and T. Gu, Chem. Commun., 2014, 50, 10148–10150 RSC
.
- C. Hu, R. Chen and N. Zheng, Adv. Mater., 2021, 33, 2006159 CrossRef CAS PubMed
.
- Y. Lu, Y. Wang and J. Zhang, J. Phys. D: Appl. Phys., 2021, 54, 313002 CrossRef CAS
.
- G. Gardos and J. O. Cole, Am. J. Psychiatry, 1976, 133, 32–36 CrossRef CAS PubMed
.
- Z. Cao, J. Su, Y. Li, J. Li, Z. Wang, M. Li, B. Fan, G. Shao, H. Wang, H. Xu, R. Zhang and H. Lu, J. Alloys Compd., 2021, 889, 161771 CrossRef
.
- W. Wang, R. Yang, T. Li, S. Komarneni and B. Liu, Composites, Part B, 2021, 205, 108512 CrossRef CAS
.
- Z. Zhang, Q. Qian, B. Li and K. J. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 17419–17426 CrossRef CAS PubMed
.
- J. H. Bombile, M. J. Janik and S. T. Milner, Phys. Chem. Chem. Phys., 2019, 21, 11999–12011 RSC
.
- W. Li, Y. Bai, C. Liu, Z. Yang, X. Feng, X. Lu, N. K. Van Der Laak and K.-Y. Chan, Environ. Sci. Technol., 2009, 43, 5423–5428 CrossRef CAS PubMed
.
- Y. Sun, B. Sun, J. He and C. Wang, InfoMat, 2019, 1, 496–524 CrossRef CAS
.
- Q. Hua, X. Zhou, B. Zhang, M. Wang, J. Liu, Y. Wang and L. Jiang, ACS Sustainable Chem. Eng., 2020, 8, 2919–2930 CrossRef CAS
.
- Q. Wang, Q. Gao, A. M. Al-Enizi, A. Nafady and S. Ma, Inorg. Chem. Front., 2020, 7, 300–339 RSC
.
- J. R. McKee, S. Hietala, J. Seitsonen, J. Laine, E. Kontturi and O. Ikkala, ACS Macro Lett., 2014, 3, 266–270 CrossRef CAS PubMed
.
- X. Zhang, L. Xu, S. Wei, M. Zhai and J. Li, J. Biomed. Mater. Res., Part A, 2013, 101, 2191–2201 CrossRef PubMed
.
- A. Saarai, T. Sedlacek, V. Kasparkova, T. Kitano and P. Saha, J. Appl. Polym. Sci., 2012, 126(S1), E79–E88 CrossRef CAS
.
- J. Shin, J. S. Lee, C. Lee, H. Park, K. Yang, Y. Jin, J. H. Ryu, K. S. Hong, S. Moon, H. Chung, H. S. Yang, S. H. Um, J. Oh, D. Kim, H. Lee and S. Cho, Adv. Funct. Mater., 2015, 25, 3814–3824 CrossRef CAS
.
- M. He and C. Chu, J. Appl. Polym. Sci., 2013, 130, 3736–3745 CrossRef CAS
.
- P. V. O. Toledo, D. P. C. Limeira, N. C. Siqueira and D. F. S. Petri, Cellulose, 2019, 26, 597–615 CrossRef CAS
.
- J. Ma, Y. Xu, B. Fan and B. Liang, Eur. Polym. J., 2007, 43, 2221–2228 CrossRef CAS
.
- Z. Tong, D. Yang, J. Shi, Y. Nan, Y. Sun and Z. Jiang, ACS Appl. Mater. Interfaces, 2015, 7, 25693–25701 CrossRef CAS PubMed
.
- Q. Han, Z. Cheng, J. Gao, Y. Zhao, Z. Zhang, L. Dai and L. Qu, Adv. Funct. Mater., 2017, 27, 1606352 CrossRef
.
- N. S. V. Capanema, A. A. P. Mansur, H. S. Mansur, A. C. De Jesus, S. M. Carvalho, P. Chagas and L. C. De Oliveira, Environ. Technol., 2018, 39, 2856–2872 CrossRef CAS PubMed
.
- Y. S. Zhang and A. Khademhosseini, Science, 2017, 356, eaaf3627 CrossRef PubMed
.
- D. Yang, Chem. Mater., 2022, 34, 1987–1989 CrossRef CAS
.
- T.-C. Ho, C.-C. Chang, H.-P. Chan, T.-W. Chung, C.-W. Shu, K.-P. Chuang, T.-H. Duh, M.-H. Yang and Y.-C. Tyan, Molecules, 2022, 27, 2902 CrossRef CAS PubMed
.
- F. M. Croisfelt, L. L. Tundisi, J. A. Ataide, E. Silveira, E. B. Tambourgi, A. F. Jozala, E. M. B. Souto and P. G. Mazzola, J. Mater. Sci., 2019, 54, 10963–10983 CrossRef CAS
.
- P. Mohammadzadeh Pakdel and S. J. Peighambardoust, Carbohydr. Polym., 2018, 201, 264–279 CrossRef CAS PubMed
.
- T. K. Giri, A. Thakur, A. Alexander, Ajazuddin, H. Badwaik and D. K. Tripathi, Acta Pharm. Sin. B, 2012, 2, 439–449 CrossRef CAS
.
- Q. Tang, J. Lin and J. Wu, J. Appl. Polym. Sci., 2008, 108, 1490–1495 CrossRef CAS
.
- J. W. Yu, J. Jung, Y.-M. Choi, J. H. Choi, J. Yu, J. K. Lee, N.-H. You and M. Goh, Polym. Chem., 2016, 7, 36–43 RSC
.
- H. Kasap, R. Godin, C. Jeay-Bizot, D. S. Achilleos, X. Fang, J. R. Durrant and E. Reisner, ACS Catal., 2018, 8, 6914–6926 CrossRef CAS
.
- H. Xie, S. Tang, D. Li, S. Vongehr and X. Meng, ChemSusChem, 2017, 10, 2301–2308 CrossRef CAS PubMed
.
- V. Van Tran, D. Park and Y.-C. Lee, Environ. Sci. Pollut. Res., 2018, 25, 24569–24599 CrossRef CAS PubMed
.
- F.-L. Mi, H.-W. Sung and S.-S. Shyu, Carbohydr. Polym., 2002, 48, 61–72 CrossRef CAS
.
- S. S. Silva, A. Motta, M. T. Rodrigues, A. F. M. Pinheiro, M. E. Gomes, J. F. Mano, R. L. Reis and C. Migliaresi, Biomacromolecules, 2008, 9, 2764–2774 CrossRef CAS PubMed
.
- B.-L. Guo and Q.-Y. Gao, Carbohydr. Res., 2007, 342, 2416–2422 CrossRef CAS PubMed
.
- M. V. Risbud and R. R. Bhond, Drug Delivery, 2000, 7, 69–75 CrossRef CAS PubMed
.
- S. Sajeesh and C. P. Sharma, Drug Delivery, 2011, 18, 227–235 CrossRef CAS PubMed
.
- T. Buranachai, N. Praphairaksit and N. Muangsin, AAPS PharmSciTech, 2010, 11, 1128–1137 CrossRef CAS PubMed
.
- W.-C. Lin, D.-G. Yu and M.-C. Yang, Colloids Surf., B, 2005, 44, 143–151 CrossRef CAS PubMed
.
- A. P. Rokhade, N. B. Shelke, S. A. Patil and T. M. Aminabhavi, J. Microencapsulation, 2007, 24, 274–288 CrossRef CAS PubMed
.
- Y. Chen and H. Tan, Carbohydr. Res., 2006, 341, 887–896 CrossRef CAS PubMed
.
- J. You, F.-Q. Hu, Y.-Z. Du and H. Yuan, Biomacromolecules, 2007, 8, 2450–2456 CrossRef CAS PubMed
.
- P. Marcasuzaa, S. Reynaud, F. Ehrenfeld, A. Khoukh and J. Desbrieres, Biomacromolecules, 2010, 11, 1684–1691 CrossRef CAS PubMed
.
- I. M. El-Sherbiny, Carbohydr. Polym., 2010, 80, 1125–1136 CrossRef CAS
.
- H. Cai, Z. P. Zhang, P. Chuan Sun, B. Lin He and X. Xia Zhu, Radiat. Phys. Chem., 2005, 74, 26–30 CrossRef CAS
.
- I. M. El-Sherbiny and H. D. C. Smyth, Carbohydr. Res., 2010, 345, 2004–2012 CrossRef CAS PubMed
.
- J. H. Hamman, Mar. Drugs, 2010, 8, 1305–1322 CrossRef CAS PubMed
.
- J. Zarate, L. Virdis, G. Orive, M. Igartua, R. M. Hernández and J. L. Pedraz, J. Microencapsulation, 2011, 28, 614–620 CrossRef CAS PubMed
.
- X. Wang, Y. Liang, W. An, J. Hu, Y. Zhu and W. Cui, Appl. Catal., B, 2017, 219, 53–62 CrossRef CAS
.
- J. Chen, X. Shi, L. Ren and Y. Wang, Carbon, 2017, 111, 18–27 CrossRef CAS
.
- D. Chen, J. Yang, Y. Zhu, Y. Zhang and Y. Zhu, Appl. Catal., B, 2018, 233, 202–212 CrossRef CAS
.
- L. Tang, C. Jia, Y. Xue, L. Li, A. Wang, G. Xu, N. Liu and M. Wu, Appl. Catal., B, 2017, 219, 241–248 CrossRef CAS
.
- X. Wu, S. Li, B. Wang, J. Liu and M. Yu, Microporous Mesoporous Mater., 2017, 240, 216–226 CrossRef CAS
.
- S. Sun, J. Yang, Y. Liu, Y. Xie and F. Mwabulili, Food Chem., 2023, 417, 135964 CrossRef CAS PubMed
.
- R. Song, L. Yao, C. Sun, D. Yu, H. Lin, G. Li, Z. Lian, S. Zhuang and D. Zhang, Toxins, 2023, 15, 133 CrossRef CAS PubMed
.
- Y. Zhang, Y. Man, J. Li, Y. Sun, X. Jiang, L. He and S. Zhang, Talanta, 2023, 259, 124534 CrossRef CAS PubMed
.
- S. Ma, L. G. Pan, T. You and K. Wang, J. Agric. Food Chem., 2021, 69, 4874–4882 CrossRef CAS PubMed
.
- M. Hussain, A. M. Idris, J. Fatima, S. Chandra and A. Kumar, J. Mater. Sci. Eng. B, 2025, 313, 117937 CrossRef CAS
.
- Z. Wu, Z. Wang and X. Jin, J. Energy Storage, 2023, 72, 108604 CrossRef
.
- O. Iqbal, H. Ali, N. Li, A. I. Al-Sulami, K. F Alshammari, H. S. M. Abd-Rabboh, Y. Al-Hadeethi, I. U. Din, A. I. Alharthi, R. Altamimi, A. Zada, Z. Wang, A. Hayat and M. Zahid Ansari, Mater. Today Phys., 2023, 34, 101080 CrossRef CAS
.
- S.-Z. Zhao, R.-D. Shi, G.-T. Xiang, Y.-D. Hu and J.-J. Chen, Fuel, 2025, 386, 134306 CrossRef CAS
.
- S. Sowmya and V. Vijaikanth, ACS Omega, 2023, 8, 32940–32954 CrossRef CAS PubMed
.
- P. Chaluvachar, Y. N. Sudhakar, G. T. Mahesha, V. G. Nair, N. Desai and D. K. Pai, J. Energy Chem., 2025, 103, 498–524 CrossRef CAS
.
- J. Yang, D. Liu, X. Song, Y. Zhao, Y. Wang, L. Rao, L. Fu, Z. Wang, X. Yang, Y. Li and Y. Liu, Gels, 2022, 8, 270 CrossRef CAS PubMed
.
- S. Sun, J. Yang, M. Ku, W. Zhang, Y. Xie and R. Zhao, Food Addit. Contam.,:Part A, 2024, 42, 679–692 CrossRef PubMed
.
- D. Jeon, J. Park, C. Shin, H. Kim, J.-W. Jang, D. W. Lee and J. Ryu, Sci. Adv., 2020, 6, eaaz3944 CrossRef CAS PubMed
.
- X. Yang, J. Pan, J. Hu, S. Zhao and K. Cheng, Chem. Eng. J., 2023, 467, 143381 CrossRef CAS
.
- Y. Shi, S. Jiang, K. Zhou, C. Bao, B. Yu, X. Qian, B. Wang, N. Hong, P. Wen, Z. Gui, Y. Hu and R. K. K. Yuen, ACS Appl. Mater. Interfaces, 2014, 6, 429–437 CrossRef CAS PubMed
.
- S. Gahlot, V. Kulshrestha, G. Agarwal and P. K. Jha, Macromol. Symp., 2015, 357, 173–177 CrossRef CAS
.
- H. Zhang, L. Zhao, F. Geng, L.-H. Guo, B. Wan and Y. Yang, Appl. Catal., B, 2016, 180, 656–662 CrossRef CAS
.
- Y. Zheng, L. Lin, B. Wang and X. Wang, Angew. Chem., 2015, 127, 13060–13077 CrossRef
.
- J. Yang, D. Liu, X. Song, Y. Zhao, Y. Wang, L. Rao, L. Fu, Z. Wang, X. Yang, Y. Li and Y. Liu, Gels, 2022, 8, 270 CrossRef CAS
.
- H. Chen, Y. Wang, S. Zhu, X. Wang, J. Liu, L. Wang, W. Fan and Y. Yu, Water, 2024, 16, 2756 CrossRef CAS
.
- Q. Liu, Y. Meng, Q. Liu, M. Xu, Y. Hu and S. Chen, Molecules, 2023, 28, 6082 CrossRef CAS PubMed
.
- K. Mahmoudi, M. Farzadkia, R. Rezaei Kalantary, H. R. Sobhi, M. Yeganeh and A. Esrafili, Heliyon, 2024, 10, e30604 CrossRef CAS PubMed
.
- X. Yang, J. Peng, L. Zhao, H. Zhang, J. Li, P. Yu, Y. Fan, J. Wang, H. Liu and S. Dou, Carbon Energy, 2024, 6, e490 CrossRef CAS
.
- Z. Wu, Z. Wang and X. Jin, J. Energy Storage, 2023, 72, 108604 CrossRef
.
Footnote |
| † These authors contributed equally to the work and are co-first authors. |
| This journal is © The Royal Society of Chemistry 2025 |
