 Open Access Article
Open Access ArticleMagnetic mesoporous silica nanoparticles as advanced polymeric scaffolds for efficient cancer chemotherapy: recent progress and challenges
Sara Payamifara,
Yasaman Khalilib,
Amin Foroozandeh*a,
Majid Abdouss*a and
Mohammad Hasanzadeh *c
*c
aDepartment of Chemistry, Amirkabir University of Technology, Tehran, Iran. E-mail: aminforoozandeh94@gmail.com; phdabdouss44@aut.ac.ir
bSchool of Chemistry, Faculty of Science, University of Tehran, Iran
cPharmaceutical Analysis Research Center, Tabriz University of Medical Sciences, Tabriz, Iran. E-mail: hasanzadehm@tbzmed.ac.ir
First published on 14th May 2025
Abstract
Magnetic mesoporous silica nanoparticles (MMS NPs) stand out as excellent options for targeted chemotherapy owing to their remarkable features, such as extensive surface area, substantial pore volume, adjustable and uniform pore size, facile scalability, and versatile surface chemistry. This review comprehensively explores the latest developments in MMS NPs, emphasizing their design, functionalization, and application in cancer therapy. Initially, we discuss the critical need for targeted and controlled drug delivery (DD) in oncology, highlighting the role of magnetic and MMs in addressing some challenges. Subsequently, the key features of MMS NPs, such as their high surface area, pore structure, and functionalization strategies, are examined for their impact on their DD performance for efficient cancer chemotherapy. The integration of chemotherapy methods such as photothermal therapy and photodynamic therapy with MMS NPs is also explored, showcasing multifunctional platforms that combine imaging and therapeutic capabilities. Finally, we identify the current challenges and provide future perspectives for the development and clinical translation of MMS NPs, underscoring their potential to reshape CT paradigms.
1. Introduction
Despite the tremendous progress in nanomaterials to develop novel cancer therapeutic approaches, cancer is still a serious threat to human health globally owing to the heterogeneity, variety, and sophistication of tumors.1–3 The prompt advancements in cancer treatments (CTs), comprising chemotherapy, immunotherapy, photodynamic therapy (PDT), radiotherapy, and photothermal therapy (PTT), have recently been investigated in clinical trials to inhibit tumor multiplication and extend the endurance life of patients.4 Among these, chemotherapy is still the most widely employed cancer therapy and has been strongly promoted. Combination treatment utilizing two or more therapeutic techniques has been examined since the 1960s5 to decrease the toxicity and increase the nanotherapeutic impact in diverse cancer therapies. According to the effective super-additive therapeutic impacts of two or more therapies, the combination of non-invasive PDT, PTT, and chemotherapy within a single nanosystem can overpower the limitations of mono-chemotherapy.6–9 For example, the drawbacks of conventional chemotherapy against cancer include nonspecific drug agglomeration, low bioavailability, and problems in overcoming the heterogeneity and multidrug resistance of cancers, which induce intense systemic side effects and reoccurrence of tumors.10,11Targeted CTs can effectively differentiate between cancerous and healthy cells, making them significantly more effective than traditional treatment approaches. These methods also tend to result in fewer adverse side effects. In conventional systemic drug delivery (DD), the lack of specificity often leads to rapid drug clearance, necessitating the use of higher doses to achieve therapeutic effects. Consequently, this approach is both costly and frequently associated with severe toxicity. Recent studies have demonstrated the potential of nanoscale materials in enhancing tumor targeting, diagnosis, and treatment, offering more efficient and precise alternatives.12 Nanoparticles (NPs), with dimensions ranging between 1 nm and 100 nm, are capable of encapsulating drugs, imaging agents, and genetic materials. Their nanometric dimensions and extensive surface area confer distinctive chemical, physical, and biological characteristics compared with bulk materials.13–16 These characteristics enable NPs to deliver high concentrations of therapeutic agents directly to tumor cells, while sparing healthy tissues. The structural design of NPs supports the attachment of drugs and imaging agents, while their surfaces are adaptable for functionalization with ligands to improve their biodistribution and enable targeted delivery to tumor-specific biomarkers.17 By addressing the challenges associated with conventional chemotherapy, such as non-specific drug distribution, drug resistance, and undesirable side effects, NPs have demonstrated significant potential. Over the past two decades, these remarkable properties have paved the way for several NP-based treatments to progress to clinical trials.12 The ability to modulate various properties of NPs has made them highly effective as therapeutic vectors in CT. Nanocarriers enhance the half-life of drugs in the circulation and promote their accumulation at the tumor site. This effect is partly attributed to the small size of NPs, abnormalities in the vascular architecture, and increased permeability and retention effects.18 The physicochemical properties of nanocarriers significantly impact their biodistribution and circulation half-life.13,19 The size of NPs plays a pivotal role in their biological fate; for instance, NPs with a hydrodynamic diameter smaller than 7 nm are typically cleared through renal filtration and excreted in the urine,17,20 while those larger than 100 nm are generally removed from the circulation by phagocytic cells.21,22 Additionally, a positively charged surface facilitates the internalization of NPs into cancer cells (C cells). Accordingly, modifying NPs with polymers such as polyethylene glycol (PEG) further extends their circulation time by preventing their clearance through the reticuloendothelial system and enhancing their accumulation in tumor regions.23
The introduction highlights the growing burden of cancer and the limitations of conventional chemotherapy, such as systemic toxicity and drug resistance. It underscores the transformative potential of nanotechnology in addressing these challenges, with nanoparticles offering improved drug targeting, controlled release, and enhanced therapeutic outcomes. This section sets the stage for magnetic mesoporous silica nanoparticles (MMS NPs) as versatile carriers, emphasizing the need for innovative DDSs that can precisely deliver therapeutic agents to the tumor site, while minimizing harm to healthy tissues.
Bharti et al. (2015)24 introduced MSNs as potential carriers for targeted DD, emphasizing their stability, biocompatibility, and versatility in diagnostics and therapeutics. The early focus was on exploring the chemical and physical properties of MSNs and establishing their feasibility in DDS. Song et al. (2017)25 provided a comprehensive overview of stimuli-responsive MSNs, focusing on internal (e.g., pH, enzymes) and external (e.g., light, temperature) triggers to achieve controlled drug release. Vallet-Regí et al. (2022)26 delivered a 20 year retrospective on MSNs, reviewing the advancements in their synthesis, functionalization, and application, making it a foundational work on the evolution of MSNs. Florek et al. (2017)27 assessed the oral administration potential of MSNs, addressing their absorption and bioavailability challenges, expanding MSN research beyond intravenous or localized delivery. Li et al. (2019)28 reviewed the application of MSNs in cancer therapy, emphasizing the therapeutic outcomes and biological interactions of MSN-drug systems in oncology. Tella et al. (2022)29 focused on MSNs as carriers for anti-tubercular agents, exploring their drug loading, solubility, and release profiles tailored to tuberculosis treatment. Iranshahy et al. (2023)30 examined curcumin-loaded MSNs, evaluating their synthesis, functionalization, and potential in treating multiple diseases. Knežević et al. (2013)31 were one of the first groups to review MMS NPs, discussing their dual utility in diagnostics (imaging) and therapy (e.g., hyperthermia and targeted DDS). The review by Godakhindi et al. (2024)32 explored MSNs in cancer immunotherapy, focusing on remodeling the tumor microenvironment and their role in combination immuno-therapies, a novel emerging frontier. Alternatively, in this review, for the first time, we summarize the recent advances in magnetic mesoporous silica nanoparticles as advanced polymeric scaffolds for efficient cancer chemotherapy.
1.1. Importance of targeted and controlled DD in CT
Cancer is a substantial health menace and one of the main causes of mortality annually. Cancer is distinguished by unusual and uncontrolled cell proliferation, leading to changes in metabolic processes and disrupting natural cell signaling routes. Cancer is regarded as an acute global health problem.33,34 Thus, due to the devastating impact of this life-threatening disease, researchers across the globe are focused on developing innovative carrier systems that can deliver anticancer agents specifically to the tumor site, while minimizing harm to healthy tissues. Various treatment approaches, including surgery, immunotherapy, radiotherapy, chemotherapy, and hormone therapy, are employed to combat cancer.35 Among them, chemotherapy remains one of the most widely used methods, relying on the administration of anticancer drugs. However, its efficacy is often limited by the severe toxicity associated with these drugs, which results in significant side effects. The primary challenges hindering the success of cancer therapies include poor selectivity, lack of controlled DD, and inefficiencies in therapeutic agent administration.36 Significant strides are being made to create advanced DDSs capable of precisely targeting C cells, while reducing harm to surrounding healthy tissues. Leveraging advancements in nanotechnology, extensive research has focused on investigating the capabilities of various nanomaterials, such as polymeric micelles, liposomes, CNT, and dendrimers, as DDS for CT.37 Recently, drug-loaded NPs have garnered substantial interest for their potential to achieve localized and controlled drug release (DR) in CT.Nanotechnology has been vigorously employed as a drug carrier in the last few years to cure different cancer diseases.38 A key obstacle in cancer therapy lies in overcoming multidrug resistance, which limits the effectiveness of chemotherapeutic agents. Thus, to address the limitations of conventional chemotherapy, researchers have focused on advancing innovative approaches to enhance monotherapy by facilitating the targeted delivery of chemotherapeutic agents at higher drug concentrations.39 Numerous nano-DDSs designed for tumor targeting have undergone preclinical and clinical evaluations, yielding promising outcomes. Several studies have highlighted the critical role of nanocarriers in treating different types of cancer, emphasizing their potential to revolutionize DD in CT.40
Nanocarriers loaded with chemotherapeutic agents can perform active targeting by conjugating them with molecules that specifically interact with antigens overexpressed on C cells. Advanced nanoscale DDSs, such as liposomes, polymeric NPs, and micelles, have demonstrated substantial promise for clinical use. Several NP-based chemotherapeutic formulations are currently approved, while numerous others are undergoing clinical or preclinical evaluation. However, nanocarriers face significant limitations, including limited bioavailability, systemic instability, uneven distribution within tissues, and possible toxicity, raising significant safety challenges, particularly for prolonged applications. Chemoresistance, a major obstacle in CT, occurs when C cells initially sensitive to chemotherapy eventually become resistant to drugs. Thus, to overcome these challenges, next-generation DDSs with enhanced targeting capabilities are critical for improving CT outcomes, reducing side effects, and alleviating chemotherapy-associated discomfort. Researchers have developed a variety of delivery systems with diverse sizes, structures, and surface properties, incorporating advanced targeting methodologies. In CT, a diverse array of nanoscale materials, such as artificial polymers, biomolecules such as proteins and lipids, as well as organic and inorganic nanoparticles, are utilized. Encapsulating chemotherapeutic agents within nanocarriers offers numerous advantages over administering free drugs. These benefits include protection against degradation in the bloodstream, enhanced solubility and stability of the drugs, targeted delivery to specific tissues, reduced toxicity to healthy cells, and improved pharmacokinetic and pharmacodynamic profiles.41
A critical advantage of MMS nanoparticles is their ability to provide controlled and sustained drug release, an essential feature for maximizing the therapeutic efficacy, while minimizing systemic toxicity. The integration of mesoporous channels and surface-modifiable chemistries allows precise tuning of the drug loading and release kinetics. Moreover, the magnetic core enables externally guided localization, and in some designs, thermally triggered release under an alternating magnetic field. To better illustrate the performance of MMS-based systems, Table 1 presents a comparison of the drug release profiles of MMS nanoparticles with other widely used nanocarriers. Parameters such as time to 50% drug release (t50) and cumulative release over 48 h highlight the superiority of MMS in modulating drug discharge, especially under tumor-mimicking acidic conditions (pH 5.0–6.5).
| DDS type | Carrier material | Drug | Release medium | Time for 50% release (t50) | Cumulative release (%) at 48 h | Notable features | Ref. |
|---|---|---|---|---|---|---|---|
| MMS | Fe3O4@SiO2 | Dox | pH 5.0 | ∼12 h | ∼92% | Magnetic targeting, pH-responsive release | 42 |
| MSNs | Mesoporous silica (non-magnetic) | Dox | pH 7.4 | ∼4 h | ∼85% | Fast burst release, lack targeting ability | 43 |
| Polymeric NP | PLGA | Paclitaxel | pH 7.4 | ∼8 h | ∼70% | Enzymatic degradation required | 44 |
| Liposome | PEGylated lipid bilayer | Dox | Serum (pH 7.4) | ∼6 h | ∼65% | Prone to leakage under acidic stress | 45 |
| Functionalized MMS | Fe3O4@SiO2@PEG | Cisplatin | pH 6.5 | ∼15 h | ∼88% | PEG gatekeeping; responsive to acidic pH | 46 |
This section outlines the critical importance of targeted and controlled DD in enhancing the effectiveness of cancer therapy. It explains how conventional therapies often lack specificity, leading to systemic side effects and reduced efficacy. Advances in nanocarriers, including liposomes, dendrimers, and polymeric nanoparticles, have improved the drug bioavailability and tumor localization. However, challenges such as chemoresistance, toxicity, and uneven biodistribution remain, reinforcing the need for next-generation DDSs with better targeting and controlled release capabilities.
1.2. Role of MMS NPs in advancing chemotherapy
MMS NPs, with their hierarchically structured core–shell architecture, have emerged as leading nanocarriers in the field of chemotherapy due to their multifunctionality and engineered precision. These nanostructures consist of a magnetic iron oxide core surrounded by a mesoporous silica shell, enabling them to combine magnetic responsiveness with high surface area and tunable pore structure, which are critical properties for drug loading, controlled release, and targeting.Unlike conventional magnetic or mesoporous materials used in isolation, MMS NPs uniquely integrate drug storage, transport, and targeting functionalities within a single nanoplatform. Their mesoporous shell facilitates a high loading of various therapeutic agents, including hydrophilic and hydrophobic drugs, nucleic acids, and proteins, while also allowing their surface functionalization with targeting ligands or imaging agents. Meanwhile, their magnetic core permits external-field-guided delivery, enhancing their accumulation at tumor sites and reducing systemic toxicity.
Recent strategies have advanced the performance of MMS NPs through innovative structural designs such as hollow MMS NPs, which offer increased pore volume and internal surface area for higher drug payloads and the possibility of co-delivery or combination therapy. These structures also serve as promising nanoreactors or carriers in cancer theranostics.
To broaden the biomedical utility of MMS NPs, they are often modified through physical and chemical surface engineering approaches. Physical doping with imaging agents or fluorescent dyes allows the integration of therapeutic and diagnostic (theranostic) capabilities, while chemical functionalization via co-condensation or post-grafting provides reactive functional groups that enhance the drug conjugation efficiency, release kinetics, and biocompatibility.
The ability of MMS NPs to combine targeted delivery, stimuli-responsive release, and real-time imaging positions them as one of the most promising nanocarriers for next-generation chemotherapy. Their performance depends not only on their structural features but also on their rational surface engineering, which enhances their interaction with biological systems and therapeutic agents. Several well-established strategies are used to synthesize MMS NPs, each offering distinct advantages in terms of structure, control, and scalability.
These synthetic pathways offer flexibility in designing MMS NPs tailored for specific DD applications. The appropriate method is selected based on the desired properties, such as particle size, pore architecture, surface functionality, and biocompatibility.
Magnetic and mesoporous materials offer a synergistic platform for cancer DD. Magnetic nanoparticles (MNPs) allow site-specific targeting through an external magnetic field, while mesoporous silica provides a high surface area and tunable pores for efficient drug loading and release. This section explores the various synthesis strategies for magnetic mesoporous composites and highlights their superior biocompatibility, responsiveness, and functionalization capabilities, establishing their significance in the design of effective, multifunctional DD systems.
1.3. Overview of magnetic mesoporous silica nanoparticles (MMS NPs) as carriers
The procedures for the synthesis of magnetic mesoporous silica NPs (MMS NPs) have become a topic of attention to address significant issues in various fields, such as the pharmaceutical, biomedical, industrial, and environmental fields. Their unique, non-toxic nature and outstanding features such as biocompatibility, stability, easy surface modifiability, mesopore structure, and large volume and surface area make them versatile platforms and potential candidates in different biomedical applications. Nowadays, among the diverse NPs, MSNs are regarded as one of the most efficacious delivery systems for delivering different drugs. Table 2 summarizes the various MMS NP systems reported as carriers in CT.| MSNs | Size of MSNs | Drug | Target cancer | Outcomes | Reference |
|---|---|---|---|---|---|
| Folate-conjugated Fe3O4@SiO2 hollow mesoporous spheres | 400–600 nm | Doxorubicin (DOX) | Cervical cancer (CC) | Due to enhanced cell uptake, DR from DOX-loaded spheres displayed a sustained pattern, and their cytotoxicity was higher compared to free DOX and non-folate-conjugated spheres. The system demonstrates potential for targeted anticancer therapy | 47 |
| Fe3O4 cluster@QD-embedded mesoporous SiO2 | 122 nm | Breast cancer (BC) | This NPF probe exhibits outstanding magnetic, fluorescent, and photothermal characteristics, making it ideal for a range of biomedical uses. Specifically, it serves as a dual-purpose diagnostic and therapeutic agent, effectively labeling and eradicating C cells in vitro via fluorescence and PTT | 48 | |
| Fe3O4@SiO2@SBA15 | 11–97 nm | DOX | BC | The targeted MMNPs nanocarrier demonstrated strong potential for controlled DD, with DOX showing a pH-dependent release, releasing faster in acidic conditions than in neutral ones. The nanocarrier enhanced the cellular toxicity of DOX and effectively delivered it to MCF-7 C cells | 49 |
| Fe3O4@SiO2-CDs | 155 nm | Gambogic acid (GA) | Liver cancer | Fe3O4@SiO2-CDs loaded with GA effectively inhibited VX2 cells, reducing their survival rate to below 20% at 100 μg mL−1, demonstrating the pharmacological activity of GA. In VX2 tumor-bearing mice, the NPs showed a strong magnetic targeting effect, significantly reducing tumor volume in the magnetic targeting group. This highlights their potential for targeted liver cancer therapy | 50 |
| Fe2O3/Fe3O4@mSiO2-HA | 25.5 nm | Apigenin | Lung cancer | The Fe2O3/Fe3O4@mSiO2-HA magnetic nanosystem demonstrated effective magnetic and hyaluronic acid-based targeting, showing promise as a platform for targeted delivery of lung tumor A549 cells | 51 |
| Fe3O4@SiO2@MIL-100(Fe) | 50 nm | Celecoxib | CC | The Fe3O4@SiO2@MIL-100(Fe) system demonstrated pH-responsive DR, showing biocompatibility with normal NIH-3T3 cells and effective delivery of celecoxib to cancerous HeLa cells in acidic conditions. These findings highlight its potential for smart DD and biomedical applications | 52 |
| Fe3O4@SiO2-Glu | 50 nm | DOX | The Fe3O4@SiO2-Glu system shows significant potential for developing effective and stable drug carriers for targeted and controlled DR | 53 | |
| Citric acid functionalized MMS (MMS NP-NCO-CA) | 107–118 nm | BC | The CA-functionalized core–shell magnetic mesoporous NPs are promising drug carriers for BC therapy | 54 | |
| Fe3O4@MSN/PEI-FA | 100–200 nm | DOX | BC | The results offer a controlled, targeted DDS to decrease the side effects of anti-cancer drugs in BC therapy | 55 |
| Fe3O4@SiO2@mSiO2-SiCDs | 600 nm | Calvarial tumors | The results showed that based on the properties of Fe3O4@SiO2@mSiO2-SiCDs, they have the potential for use in sensor technologies and DD carriers | 56 | |
| Fe3O4@Au@SiO2 | 100 to 130 nm | Etoposide | This nanosphere would be a hopeful nanocarrier for magnetic-targeted and NIR irradiation-controlled DD, extending a new route for in vivo medical diagnostics and efficacious treatment | 57 | |
| MnFe3O4@SiO2-PEG-RGD | 20–30 nm | Liver cancer | The research demonstrated that silica NPs functionalized with PEG and RGD peptides were effective carriers for anticancer complexes. These multifunctional NP-loaded anticancer complexes have the potential to serve as specialized agents for CT monitoring | 58 | |
| M@SiO2@amino | 100–200 nm | DOX | BC | This research presented the developed multifunctional IO/MS/amino core/shell (M/silica/amino), by sonochemical approach, loaded with DOX as a DDS for BC therapy | 59 |
| Fe3O4/SiO2 | — | DOX | BC | This nanocomposite was introduced as an efficient NP for DD and combating BC. Protease/DOX decorated Fe3O4/SiO2 nanocomposite exhibited 7.5% cell viability as measured by the MTT test | 60 |
| Fe3O4@SiO2@SBA-15 | 11–97 nm | DOX | BC | The impact of the drug-loaded nanocarrier on BC cells was evaluated using MCF7 cell cultures. Cell viability was assessed via an MTT assay, and the half-maximal IC50 of the drug was determined. The IC50 refers to the concentration required to reduce cell viability by 50% compared to the untreated control group | 49 |
| Fe3O4@SiO2 | 50 nm | DOX | BC | This study demonstrated that DOX-Fe3O4@SiO2 induces dose-dependent cell death and exhibits greater cytotoxicity under an exterior magnetic field compared to free DOX, underscoring the potential of Fe3O4@SiO2 for targeted DD | 61 |
| Fe3O4@SiO2-ZIF-8@N-Chit-FA | 43 nm | Cisplatin | CC | The finding illustrated that Fe3O4@SiO2-ZIF-8@N-Chit-FA nanocomposites had antitumor effects in CC cells. Also, it could be served in targeted nanomedicine | 62 |
This section presents MMS NPs as promising drug carriers for cancer therapy, highlighting their unique structural and functional attributes, including large surface area, uniform mesopores, and magnetic responsiveness. Various MMS systems are reviewed for their efficacy in delivering drugs such as doxorubicin, apigenin, and cisplatin across different cancer types. The findings underline the potential of MMS NPs to enhance the uptake and prolong the circulation time of drugs and achieve targeted delivery, with positive results in both in vitro and in vivo studies.
1.4. Impact of API molecular type and physicochemical properties on DDS
The physicochemical properties of active pharmaceutical ingredients (APIs), including their molecular type, size, polarity, hydrophobicity, and stability, play a pivotal role in determining the appropriate DDS design and performance. APIs can be broadly categorized into several classes including small molecules, peptides, proteins, nucleic acids, monoclonal antibodies (mAbs), antibody–drug conjugates (ADCs), and gene therapy products. Each category presents distinct challenges and opportunities regarding encapsulation, delivery, and therapeutic efficacy.Small molecules (typically <900 Da) are the most widely used APIs in clinical settings due to their well-established pharmacokinetics, synthetic accessibility, and oral bioavailability. These molecules vary in hydrophobicity or hydrophilicity, influencing their solubility, biodistribution, and interaction with nanocarriers. MMS NPs can be engineered to load both hydrophobic and hydrophilic small molecules through surface functionalization and pore size optimization.
Peptides (500 Da to ∼10 kDa) and proteins (>10 kDa) offer high specificity and potency but are limited by their enzymatic degradation and poor oral bioavailability. These macromolecules typically require parenteral administration and protection via nanocarrier encapsulation. Thus, the porous architecture of mesoporous silica provides a promising platform for their stabilization, while surface modification, such as PEGylation and incorporation of responsive gatekeepers, can enhance their circulation time and reduce their proteolytic degradation.
Nucleic acids, such as siRNA and mRNA, are highly hydrophilic and prone to degradation by nucleases. Thus, they require sophisticated delivery strategies involving surface-charged nanoparticles or lipid-based systems. In this case, MMS NPs functionalized with cationic polymers or modified with targeting ligands offer an effective scaffold for the intracellular delivery of nucleic acids, while ensuring their endosomal escape and controlled release.
Monoclonal antibodies (mAbs) and antibody–drug conjugates (ADCs) are large biomolecules (∼150–200 kDa) with complex tertiary structures. Their delivery relies on surface conjugation rather than encapsulation. Thus, the mesoporous silica framework can act as a robust platform for site-specific antibody conjugation, enabling the active targeting of tumor antigens and controlled release of payloads in the tumor microenvironment.
Gene therapy products, such as DNA and viral vectors, have emerged as transformative therapies but are hampered by their instability and delivery challenges. In this case, the magnetic cores in MMS NPs enable their targeted delivery via an external magnetic field, while the silica shell supports the structural integrity and co-delivery of gene-editing components or transcription factors.
2. Structural features and functionalization
The application of core–shell porous NPs has emerged as a promising strategy in DD due to their tailored architecture, which integrates a highly porous core with a stabilizing outer shell.63,64 Their internal porous structure offers a high surface area and large void volume, allowing the efficient encapsulation of diverse bioactive agents such as peptides, nucleic acids, and small-molecule drugs. Their external shell provides structural integrity and protects their cargo from premature degradation or release. Furthermore, these systems can be engineered to exhibit controlled and stimuli-responsive DR behavior under specific environmental triggers such as temperature, enzymatic activity, and light exposure.65,66 Surface functionalization plays a crucial role in enhancing the pharmacokinetics and biocompatibility of these nanocarriers.67 Modifying the surface of NPs with ligands, targeting moieties, or imaging agents enables site-specific delivery and real-time tracking of the therapeutic distribution.68,69 Table 3 displays some examples of integrated systems for diagnostic imaging and therapeutic applications. The coating layer, whether composed of polymers, lipids, or silica, further improves the stability, prevents aggregation, and regulates the release kinetics.50,73 For instance, mesoporous MSNs are frequently coated with functional polymers or molecules to create gatekeeping structures, which prevent premature drug leakage and allow triggered release.74,75 Chemical functionalization techniques introduce groups such as amino (–NH2), thiol (–SH), and carboxyl (–COOH) on the surface of NPs using agents such as 3-aminopropyltrimethoxysilane and fluorescein isothiocyanate.50,73 These groups provide reactive sites for covalent bonding with therapeutic molecules or polymers, enhancing the drug retention and targeting efficiency. Additionally, physical interactions including hydrogen bonding, van der Waals forces, and electrostatic attractions support the non-covalent immobilization of biomolecules.74–76 Stable polymer coatings can also be developed by chemically grafting polymers, such as PEG and poly(lactic-co-glycolic acid), on the surface of NPs. The resulting durable coating enhances the retention of the encapsulated drugs within the NPs, improving their stability and enabling their controlled release.53,77| Structure | Applications | Advantages | Ref. |
|---|---|---|---|
| Fe3O4@SiO2@mSiO2 structure with AuNPs | Targeted DD and real-time imaging of tumor progression | Enhanced therapeutic efficacy with combined chemotherapy and PTT and real-time DD and tumor response monitoring | 70 |
| Fe3O4@SiO2 core–shell structure functionalized with QDs | Breast and liver CT | Enhanced imaging accuracy due to dual-mode capabilities, controlled DR in acidic tumor environments, and reducing side effects on healthy tissues | 48 |
| Fe3O4@mSiO2 functionalized with PSs like chlorin e6 (Ce6) | Treatment of hypoxic tumor regions resistant to standard therapies | Synergistic effects of PDT and chemotherapy for higher efficacy, non-invasive imaging and targeted treatment | 71 |
| MnFe2O4@SiO2-PEG-RGD | Effective in liver and BC models | High tumor specificity due to RGD-targeting peptide reducing off-target toxicity | 58 |
| Fe3O4@SiO2 core–shell with temperature-responsive polymers | Cancer therapy where localized hyperthermia enhances chemotherapeutic effects | Non-invasive hyperthermia activation and precise control of DR | 72 |
2.1. Description of core–shell structures
Magnetic DD is a promising emerging technology that utilizes MAPs to deliver drugs precisely to target sites. MNPs, a type of NP, exhibit magnetic behavior in response to an external magnetic field. They are composed of magnetic materials such as pure elements (Fe, Co, Mn, and Ni), composites, oxides, and alloys. Typically, MNPs feature an IO core (e.g., Fe3O4 or γ-Fe2O3) surrounded by an organic or inorganic shell coating.78 Their notable physicochemical properties include superparamagnetism and a large surface area (LSA), especially at the nanoscale. MNPs are used extensively in biomedicine, including DD, MRI, biosensing, and magnetic hyperthermia.79 In DD utility, MNPs present the benefit of targeted delivery to particular cells and tissues, consequently enhancing the effectiveness and safety of drugs. Magnetic hyperthermia employs MNPs to produce heat when exposed to an exterior magnetic field, promoting the selective devastation of C cells.80Magnetite (Fe3O4) has a cubic reverse spinel structure with the general formula Fe3O4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Fe3+ (A) (Fe3+Fe2+) BO4. Fe3+ ions occupy both the tetrahedral (A) and octahedral (B) sites, while Fe2+ ions are confined to the octahedral sites. The anti-parallel alignment of Fe3+ spins across these sites cancels out the net magnetization, whereas Fe2+ contributes to the net magnetic moment due to its spin alignment with the adjacent Fe3+ ions. These magnetic properties make Fe3O4 NPs highly responsive to an external magnetic field.81
Fe3+ (A) (Fe3+Fe2+) BO4. Fe3+ ions occupy both the tetrahedral (A) and octahedral (B) sites, while Fe2+ ions are confined to the octahedral sites. The anti-parallel alignment of Fe3+ spins across these sites cancels out the net magnetization, whereas Fe2+ contributes to the net magnetic moment due to its spin alignment with the adjacent Fe3+ ions. These magnetic properties make Fe3O4 NPs highly responsive to an external magnetic field.81
Magnetite NPs have attracted significant attention from researchers in various fields, such as magnetic fluids, catalytic processes, and biotechnology. Due to the wide application of these particles, various methods have been developed to synthesize particles with suitable stability for use in various fields. The suitable particle size is typically in the range of 10 to 20 nm in many applications. Two key factors influencing the properties of magnetic NPs are finite size and surface effects, both of which contribute to the distinct characteristics of these particles. The finite size effect arises due to the quantum confinement of electrons, while surface effects are linked to the disruption of crystal symmetry at the particle boundaries.82,83 Despite their promising properties, nano-sized particles face challenges related to their inherent instability. These particles often undergo agglomeration and aggregation as they attempt to minimize the energy associated with their reactive surface.
Alternatively, bare metal NPs are chemically very active and easily oxidized in the presence of air, which decreases their magnetic properties and dispersion. As a result, the protection of these NPs is crucial for many applications. This process is carried out using organic coatings containing surface-active materials, polymers or inorganic coatings such as silica and carbon. Of course, in addition to protecting the NPs, these coatings also allow them to be functionalized. Functionalized NPs are very important and are used in catalysis, biolabeling, and bio-separation processes. Also, in catalytic reactions in the liquid phase, these NPs can exhibit quasi-homogeneous system behavior due to their properties such as high dispersibility, suitable interaction with reactants, and ease of separation.84–88
Silica (SiO2), a silicon oxide, is highly suitable for targeted DD. It efficiently encapsulates and delivers therapeutic agents with precision, making it an essential material for controlled-release applications. Through polymerization, silica forms MSNs, which offer numerous advantages, including an LSA, the ability to release drugs in a controlled fashion, and the potential to incorporate various functional groups and ligands for targeted DD. Furthermore, the pore size of NPs can be adjusted, boosting their drug-loading capacity. Consequently, photosensitizers (PSs) can be effectively conjugated to silica NPs for PDT, providing notable benefits in CT. Over the last decade, the investigation of NPs, particularly the construction of SiNPs with a controlled morphology and various uses, has attracted attention researchers in many fields.89
Recent advances in nanotechnology have immensely influenced the investigation of silica materials for biological applications. Traditionally utilized as tablet fillers and absorbents owing to their large surface space and heightened adsorption capability, silica particles (nano and micro-sized) have been employed in novel applications with the expansion of MS compounds.90,91 These substances, engineered with accurate control of their pore volume and size, suggest improved DD traits.92
The photothermal and hyperthermal properties of Fe3O4-SiO2 NPs have garnered significant attention in scientific DD, offering diverse strategies for controlled DR. When Fe3O4 NPs are exposed to an alternating magnetic field (AMF), they produce hyperthermia, leading to localized tissue heating, which facilitates the controlled release of encapsulated therapeutic agents.93–95 This thermal effect can activate the release of thermosensitive drugs or destabilize the structure of DDSs, offering precise control of the timing and location of DR.96–98 The addition of a silica shell to Fe3O4 NPs enhances their stability and biocompatibility, while also enabling their functionalization with stimuli-responsive or targeting ligands, thereby improving their effectiveness in DD.99,100 The photothermal properties of Fe3O4-SiO2 NPs induce hyperthermia, enhancing their drug-release capabilities. These NPs efficiently capture near-infrared (NIR) light and convert it into thermal energy. This thermal energy can be utilized to trigger localized hyperthermia, boosting the therapeutic outcomes or activating DR from carriers responsive to photothermal cues.101,102
The integration of photothermal effects with hyperthermia presents a synergistic strategy for regulating DR, allowing precise control of the release kinetics in response to external stimuli. Coating Fe3O4 NPs with a silica shell significantly improves their stability and biocompatibility, while offering a versatile platform for functionalization with imaging agents or targeting ligands.103,104 These enhancements make NPs highly promising candidates for clinical use, facilitating their application in targeted DDSs or non-invasive tumor ablation through remote activation of DR from thermosensitive carriers.105,106
Various types of MSNs are commonly employed in DD. Among them, MCM-48, SBA-15, MCM-41, and SBA-16 are extensively utilized due to their well-defined pore structures and tunable properties. Beyond DD, these materials have been explored for applications such as adsorption, catalysis, and biosensing. Additionally, SBA-11, MCM-50, and SBA-12 have demonstrated potential as ideal adsorbents for catalytic and pharmaceutical applications.107
2.2. Importance of surface area, pore size, and functional groups
Employing nanoplatforms for loading anticancer drugs is a cutting-edge procedure in DD to treat tumors and reduce poisonous effects on healthy cells. According to the literature, MNP nanoplatforms are the most commonly utilized in DDS. Their benefits include their simplicity and inexpensive synthesis, capacity to efficiently adsorb drugs, and biocompatibility, making them excellent nominees for the reconstruction of DD scaffolds. Even if the body can readily assimilate the various ionic states generated by IONs after their degradation,108 no clear inference regarding the biocompatibility of IONs exists.109 This promotes additional investigation in the area of the biological utilities of IONs.110,111 A further benefit of employing IONs is their superparamagnetic attributes, which make it possible to control DD utilizing an exterior magnetic field.111,112 IONs are additionally used in magnetic resonance imaging (MRI), cancer diagnostics, molecular imaging, and hyperthermia.113,114 These appealing biomedical aspects present a route for merging the characteristics of DD and MRI, which increases the efficacy of antiCT.115–117 Owing to their extended surface, IONs tend to accumulate and oxidize. Therefore, their surface is covered with organic, inorganic, and polymeric substances that execute the operations of surface protection and selective functionalization, as investigated by Zhu et al.118The use of MNPs in biomedical research, both in vitro and in vivo, has garnered significant interest due to their versatile surface properties, nanoscale dimensions, and excellent magnetic field stability.119 In vitro, MNPs are employed in a range of applications, including diagnostic separation, cell selection processes, and magneto-relaxometry techniques. Moreover, therapeutic studies have showcased their potential in diagnostics, such as nuclear MRI, hyperthermia-based treatments, and facilitating targeted DD.120
The dimensions of NPs play a crucial role in determining the biomedical effectiveness of MSNs as DDSs. Consequently, fine-tuning the size of NPs is essential for achieving the optimal DD performance. One of the primary factors affecting the dimensions of MSNs is the pH of the reaction medium. The inclusion of specific additive reagents, such as alcohols, inorganic bases, amines, and inorganic salts, is crucial for controlling the size of NPs. These additives influence the hydrolysis and condensation steps of the SiO2 precursor, accelerating the reaction rate and promoting the formation of smaller NPs.
The development of mesoscopic materials dates back to the 1970s, but a major breakthrough took place in 1992 when a team at Mobil Research and Development Corporation pioneered the creation of mesoporous solids using aluminosilicate gels and a liquid crystal templating technique. These materials were named Mobil Composition of Matter (MCM-41). According to IUPAC, MMs are characterized by a pore size in the range of 2 to 50 nm and a highly organized pore structure.121 By selecting specific surfactants, the pore size of these materials can be precisely adjusted. MCM-41 typically adopts a hexagonal structure, with pore diameters in the range of 2.5 to 6 nm, where cationic surfactants serve as structural templates. MCM-41 has become one of the most extensively studied materials in DD applications. Furthermore, mesoporous structures can be modified by altering their precursor materials or reaction conditions, which can lead to variations in both their pore size and the overall structural arrangement. For example, MCM-48 exhibits a cubic structure, while MCM-50 is characterized by a lamellar or layered configuration.122
MSNs, silica NPs with mesopores, have become increasingly popular in recent years. Their unique advantages include a uniform and adjustable pore size, the ability to functionalize both their internal and external pore surfaces independently, and a gating mechanism for controlling their pore openings. These features make MSNs highly distinctive and promising as DD carriers. Researchers have effectively utilized these NPs to load various types of cargo, including drugs and larger macromolecules such as proteins,123 DNA,124 and RNA.125 A lot of studies have been accomplished in this field, and research continues to explore new possibilities for using MSNs in DD. Numerous reviews have been reported, highlighting the role of MSNs in enhancing drug solubility,126 serving as systems for controlled or sustained DR,127 and their utility in biomedicine.128 MSNs, with their unique properties, have found widespread applications across various fields, including biomedicine,129–147 catalysis,148–153 environmental protection,154–159 and optics.160,161
2.3. Techniques for functionalization and their impact on drug loading/release
Various types of silica, including porous, fumed, and non-porous variants, have shown promise as effective DDSs, yielding promising outcomes in preclinical trials. However, their successful clinical translation requires a thorough understanding of their in vivo behavior, such as biodistribution and potential toxicity. Silica NPs intended for biomedical use are primarily amorphous and are classified as either porous or non-porous. Unlike crystalline silica, amorphous silica is cleared more rapidly from the lungs, reducing its potential toxicity.162 MSNs exhibit rapid dissolution when their concentration remains below saturation thresholds. According to Martin's findings,128 silica undergoes dissolution in bodily fluids, and subsequently absorbed or excreted as silicic acid through fecal routes. The degradation of silica NPs into non-toxic silicic acid occurs via three primary mechanisms including ion exchange, hydration, and hydrolysis. This degradation behavior is influenced by the properties of the surrounding environment and the NP concentration. Various approaches have been investigated to modulate the degradation rates of silica NPs, including the non-covalent attachment of organic groups to improve their hydrolytic breakdown, covalent linkage with organic silsesquioxane-based NPs, and the incorporation of degradable organic silsesquioxane linkers into silica NPs to promote their degradation through biological stimuli. Notably, their degradation process is more intricate than that of other silica NPs due to the variations in their structural composition. This complexity arises from the variations in the rate and extent of silica condensation achieved through different sol–gel synthesis methods used for the fabrication of MSNs. The study by Croissant et al. demonstrated that the degradation rate of MSNs depends on their degree of condensation, where MSNs with lower condensation degrade within days, while MSNs with higher condensation require several weeks, and calcined MSNs may take months to fully degrade.163This section elaborates on the architectural design and strategies for the functionalization of core–shell MMS NPs. We emphasize the importance of structural features such as high surface area, tunable pore size, and the presence of functional groups that facilitate effective drug loading and release. Functionalization techniques, including chemical and physical modifications, are discussed for improving biocompatibility, drug retention, and targeted delivery. These advancements provide the foundation for creating responsive and stable DDS tailored for cancer treatment.
3. Mechanisms of DD
Nanotechnology has emerged as a cornerstone of modern science, revolutionizing various fields in the 21st century. In recent decades, its integration into biomedicine has marked a significant breakthrough in disease diagnosis and treatment. The advent of green technology and its application in nanomedicine have introduced transformative approaches in medical treatments and regenerative medicine, primarily due to the benefits provided by nanostructures. These include an elevated LSA, the capacity to engineer and modify surfaces, and the potential to produce nanoparticles with diverse sizes, shapes, and chemical properties. Nanocarriers are recognized for their biocompatibility, biodegradability, and non-toxicity, making them highly favorable for biomedical applications.164–167 Lipid-based nanocarriers,168,169 polymeric NPs,170,171 and dendrimers172 have revolutionized treatment approaches for a variety of diseases, particularly cancers and infections. Both natural and synthetic NPs have seen widespread use in medical applications. Noteworthy examples include QDs and iron(II,III) oxide NPs, which are readily accessible. Furthermore, carbon dots, Au NPs, Ag NPs, different metal oxides, layered double hydroxide NPs, and silica NPs have been utilized for diverse diagnostic and therapeutic purposes.173–175 Targeted DDSs, in particular, facilitate precise drug transport to diseased tissues or C cells, minimizing damage to surrounding healthy tissues.The concept of using IONPs for magnetic drug targeting (MDT) was first introduced by Frei.176 This approach entails conjugating anticancer drugs to Fe3O4 NPs, administering the NP-based system intravenously, and guiding it to the tumor site via the use of an external magnetic field (Fig. 1). By utilizing this technique, a higher concentration of chemotherapeutic agents can be delivered directly to the tumor, thereby reducing the overall dosage required and minimizing systemic exposure.178 Numerous in vivo investigations and clinical trials have since confirmed the potential of MDT in improving the therapeutic efficacy, while decreasing adverse effects.179,180
 | ||
| Fig. 1 In MDT, anticancer drugs are loaded onto Fe3O4 NPs, injected into the bloodstream, and guided to the tumor site using an external magnetic field. Reproduced from ref. 177 with permission from Wiley, Copyright 2016. | ||
3.1. pH-responsive systems
Stimuli-responsive DDS encompass a range of designs, including those that respond to pH, enzymes, DNA/RNA, temperature, magnetic fields, ultrasound, or light. Among them, pH-sensitive systems are particularly popular due to the natural variations in pH across tissues and cellular compartments. These systems are designed to release drugs rapidly upon reaching the acidic tumor environment or being internalized by cells via endocytosis. They operate through various mechanisms, such as breaking chemical bonds, undergoing phase transformations, structural changes, assembly or disassembly, molecular release, and material dissolution. With their ability to deliver drugs in a controlled manner, pH-responsive systems hold significant potential for applications in diverse biomedical fields.181In many biomedical applications, it is essential for drugs to be released in response to the pH levels in the body. Thus, achieving controlled release that matches the physiological requirements at specific locations, with predetermined release rates for particular durations, will be highly beneficial. Different tissues, organs, and cellular compartments have varying pH values, making the pH level an ideal trigger for regulating DR. pH-responsive DDSs are gaining significant attention as a “smart” approach to overcoming the limitations of conventional drug formulations. These systems enable precise control of DD in both time and location, leading to enhanced therapeutic outcomes. The benefits of pH-responsive DD are as follows:
(I) Targeted release: ensures that drugs are released primarily in the target tissue (e.g., tumor site and acidic intracellular compartments).
(II) Reduced side effects: minimizes systemic toxicity by avoiding drug leakage in healthy tissues.
(III) Improved stability: protects sensitive drugs (e.g., proteins and nucleic acids) from degradation under physiological conditions.
(IV) Enhanced efficacy: increases drug concentration at the target site, improving the therapeutic outcomes.182
The pH levels within different regions of the digestive system, various body organs, tissues, and cellular environments vary significantly. For instance, the pH is in the range of 1.5–3.5 in the stomach, 5.5–6.8 in the small intestine, and 6.4–7.0 in the colon.183 Tumors and inflamed tissues present a more acidic environment compared to the bloodstream and healthy tissues, which maintain a pH of around 7.4. Inside cells, the acidity increases, with endosomes having a pH in the range of 5.5–6.0 and lysosomes in the range of 4.5–5.0.184 These pH differences across organs, tissues, and cellular compartments provide a promising physiological trigger for pH-sensitive DDS. The drug-release mechanism in these systems, which is highly responsive to pH changes, offers significant potential for targeted therapy. By preventing DR in the bloodstream at the normal physiological pH of 7.4, the drug is only released in the acidic environment found in C cells.185 Although most research focused on pH-responsive DDSs based on organic polymers, recent trends have seen increasing interest in inorganic and hybrid inorganic/organic composite systems. These systems are gaining attention due to their benefits, including enhanced biocompatibility, thermal stability, and versatility, as well as better control of morphology, size, and structure. pH-responsive DDSs can be categorized into four types, as follows: (1) systems based on organic materials, (2) systems utilizing inorganic nanostructured materials, (3) systems made from inorganic/inorganic nanocomposites, and (4) systems comprised of inorganic/organic composites.186 DOX-loaded pH-responsive NPs were reported by reserchers in 2020.186 They developed multifunctional theranostic nanoplatforms, DOX/PB@Ce6NPs, which demonstrated outstanding reactive oxygen species (ROS) generation and promise as a potential therapeutic system for combined chemo-PDT. The MSNs functionalized with an acid-sensitive hydrazone linker release DOX selectively in the acidic tumor microenvironment. Thus, this system enhances the therapeutic efficiency while reducing the cardiotoxicity associated with DOX.
pH-responsive DDSs have gained significant attention in recent years, with considerable progress achieved over the last few decades. A wealth of experimental data has been published, with numerous studies focusing on organic polymer-based systems. Recently, there has been a notable increase in interest in inorganic and inorganic/organic composite pH-responsive DDSs. These systems are highly regarded for their advantages, including excellent thermal and chemical resilience, biocompatibility, versatility, and precise control of their morphology, size, and structure. However, despite these advancements, several challenges persist. Although many drug carriers have been developed, most have not achieved wide practical to date. An ideal DDS must integrate multifunctionality, including improved site-specific targeting, responsive pH-controlled DR, and diagnostic capabilities. Further progress is needed to incorporate additional functional components and enhance their overall performance. One of the key obstacles remains the efficient, cost-effective, and precise fabrication of pH-responsive DDSs with consistent structures, sizes, morphologies, and molecular characteristics.
Creating innovative pH-sensitive systems with biocompatible and biodegradable inorganic or hybrid inorganic/organic nanostructured materials is essential for practical use; however, this has been insufficiently explored in current research. Beyond organic substances, inorganic nanostructures, particularly hybrid inorganic/organic composites, offer substantial potential for pH-sensitive DD. Materials such as precious metals, metal oxides, silica, and carbon-based materials (including CNTs, CQDs, and graphene) exhibit remarkable properties, such as exceptional thermal and chemical durability, making them ideal for a wide range of biomedical uses. However, their limited biodegradability presents challenges regarding their in vivo use. Biodegradable inorganic materials, such as calcium phosphate-based nanostructures and their composites, show considerable potential for pH-responsive DD applications. Nevertheless, continued research is essential to further refine their structure, size, and morphology control, as well as to improve their surface modification and functionalization for future clinical use.187–189
3.2. Magnetic field and photothermal activation
Magnetic DD is a promising technology that employs MNPs to deliver medicines to specific target areas. MNPs are a type of NPs that show magnetic behavior in response to a magnetic field. They include diverse magnetic materials, comprising pure elements such as iron, cobalt, Mn, and nickel, as well as their oxides, composites, and alloys. They include a core created of IO, such as magnetite (Fe3O4) and maghemite (γ-Fe2O3), covered by a surface coating, which can be organic and inorganic.78 Magnetic field and photothermal activation are two distinct but related concepts often applied in various scientific and technological fields, particularly in biomedicine and materials science. A magnetic field is a vector field surrounding a magnet, electric currents, or changing electric fields characterized by the force it exerts on other magnetic materials. It is typically represented by magnetic field lines that show the direction and strength of the field at various points. Magnetic fields can be produced by magnets, coils carrying electrical currents, or other sources.190Photothermal activation involves the absorption of light (usually in the form of infrared or visible wavelengths) by a material, which then converts the absorbed energy into heat. This phenomenon is based on the absorption of photons (light particles) by a material, causing electrons and atoms within the material to vibrate and generate thermal energy. Subsequently, the material experiences an increase in temperature, which can be controlled by adjusting the intensity, wavelength, and exposure time to the light source.
3.3. Magnetic-field-assisted photothermal therapy of cancer
When a magnetic substance is subjected to an external magnetic field, it can generate heat through hysteresis losses or magnetic relaxation processes. In contrast, photothermal activation involves absorbing light and converting that energy into heat. By combining these mechanisms, researchers can create materials that respond to either a magnetic field or light (or both) to induce heating. Combining magnetic field activation with photothermal activation in MMS NPs offers a powerful dual-trigger system for precise and efficient CT. These systems utilize the magnetic and photothermal properties of MMS NPs to achieve controlled DR and localized tumor ablation, making them a promising tool in nanomedicine. The integration of a magnetic field and photothermal activation in MMS NPs is a cutting-edge approach with tremendous potential in cancer therapy. Ongoing research aims to optimize these dual-responsive systems for clinical applications, focusing on improving delivery precision, enhancing therapeutic efficacy, and ensuring safety. These advancements can revolutionize personalized CT, offering highly controlled and effective therapies with minimal side effects.191,192Magnetic cores (e.g., IO) within MMS NPs heat up when exposed to an AMF. The localized heat generated can induce thermal ablation of C cells. Then, the release of therapeutic agents encapsulated in the mesopores is triggered by disrupting their heat-sensitive bonds or pore caps. The heat also sensitizes tumor cells to chemotherapy, enhancing the treatment efficacy. An external magnetic field can guide MMS NPs to tumor sites, increasing their local concentration and reducing off-target effects. Functionalization with photothermal agents (e.g., gold nanostructures, carbon materials, and upconversion NPs) allows MMS NPs to absorb light, especially in the NIR range. These agents generate localized heat upon NIR irradiation, enabling tumor cell apoptosis or necrosis via hyperthermia and stimuli-responsive DR. Photo-responsive linkers (e.g., azobenzene) and thermosensitive polymers on the surface of MMS NPs can release drugs upon light exposure, further enhancing the precision. Combining magnetic and photothermal effects generates higher and more controllable heat levels at the tumor site. This dual activation improves the efficacy of hyperthermia therapy and reduces the treatment time. Magnetic hyperthermia and photothermal activation can work together to disrupt bonds or mechanisms holding drugs within the MMS NPs. Controlled heating ensures precise and efficient DR only in targeted regions. Magnetic targeting ensures that the NPs are concentrated at the tumor site before activation. Light-based activation adds a layer of spatial control, given that the photothermal effect is localized to the irradiated area.193–195
3.4. Dual or multi-responsive delivery systems
Responsive nanomedicines with dual and multi-functional capabilities offer significant promise in enhancing site-targeted DD, providing a strategic approach to reduce side effects and boost the therapeutic efficacy in CT. These advanced nanomedicines have significantly advanced nanoparticulate drug formulations by enhancing various factors such as ease of preparation, stability, retention at the tumor site, tissue penetration, and overall therapeutic performance. Their capability to undergo transformations such as charge reversal, alterations in size, PEG deshielding, and controlled DR facilitates the incorporation of diverse therapeutic strategies, thereby boosting the effectiveness of chemotherapy, phototherapy, immunotherapy, and combination therapies. With their precisely controlled DR mechanisms, these nanomedicines can significantly reduce the systemic toxicity and immune-related side effects, resulting in superior anticancer outcomes both in vitro and in vivo. Conventional nanomedicines lacking stimuli-responsive capabilities release therapeutic drugs continuously through a diffusion-driven mechanism, leading to an “always-on” delivery effect, which can pose significant risks to healthy tissues.196,197 Thus, to address these limitations, the development of intelligent nanomedicines capable of delivering drugs in a controlled manner specific to time, location, and dosage has become essential.198–200 Stimuli-responsive nanomedicines, which activate their therapeutic effects only in response to external triggers or tumor-specific biomarkers, have garnered substantial interest in recent years owing to their potential to enable highly targeted CT, while minimizing toxicity concerns.201,202 The release of drugs and the antitumor effects of nanomedicines can be modulated by various intrinsic conditions within the tumor microenvironment, such as low pH,203–205 specific enzymes,206–208 ROS,209–211 glutathione,212,213 and hypoxic conditions,214,215 leading to enhanced therapeutic outcomes. Furthermore, non-invasive external triggers such as NIR light offer the advantage of precise spatiotemporal control of DR.216–219 However, most stimuli-responsive nanomedicines are designed to react to a single type of stimulus, which often limits their efficiency. This can result in inadequate DR during circulation or unintended drug leakage into healthy tissues, owing to the constraints of relying on just one endogenous or exogenous trigger.220–222 For instance, although nanomedicines responsive to external stimuli offer advantages in controllability through the precise application of physical triggers, their effectiveness is hindered by the limited penetration of the stimuli, restricting their use primarily to superficial tumors.223–226 Internal stimuli-responsive nanomedicines show significant potential for targeting both surface and deep tumors. However, their ability to effectively adjust to the intricate and fluctuating physiological conditions in the body is constrained. This challenge arises mainly from the irreversibility of their responses, which are dependent on the depletion of endogenous substances.198 Alternatively, dual- and multi-responsive nanomedicines, engineered to react to a combination of internal and external stimuli, or multiple internal signals, provide superior flexibility. These systems are more capable of preventing premature DR, thereby minimizing the systemic toxicity and enhancing the efficacy of antitumor therapies.223,227,228 These advanced nanomedicines enable innovative controlled DD mechanisms by allowing concurrent multi-step reactions at a single location or sequential reactions across various environments and compartments. This design approach significantly boosts the antitumor effectiveness both in vitro and in vivo.229–232Dual- and multi-stimuli responsive nanomedicines hold great promise for enhancing the precision and personalization of CT, owing to their ability to deliver drugs in a controlled manner and their versatile, modular design. Despite the challenges in advancing stimuli-responsive metallodrugs, this fast-developing area shows considerable potential. In addition to CT, these innovative nanomedicines may have applications in managing cardiovascular diseases, neurodegenerative disorders, and autoimmune conditions.
The DD mechanisms enabled by MMS nanoparticles have been explored in detail, focusing on the use of external and internal stimuli to trigger drug release. The key approaches include magnetic drug targeting, pH-responsive systems, photothermal activation, and dual- or multi-responsive platforms. These smart delivery systems have been designed to release drugs only at the tumor site, reducing their systemic toxicity and improving their therapeutic precision. Thus, the integration of multiple stimuli-responsive mechanisms demonstrates great promise for advancing personalized cancer therapy.
4. Applications in chemotherapy
4.1. Specific applications of MMS NPs in DD
MMS NPs have emerged as multifunctional nanocarriers for the targeted delivery of anticancer drugs such as DOX and cisplatin. Building on their structural advantages, including tunable pore size, large surface area, high loading capacity, and functionalization versatility, recent studies have focused on the performance of MMS NPs in specific DDSs and cancer models.Zhu and colleagues developed Fe3O4@SiO2 hollow mesoporous spheres with a rattle-type structure using carbon spheres as templates. They varied the particle size, mesoporous shell thickness, and Fe3O4 content to assess their influence on cellular uptake and cytotoxicity in HeLa cells. The spheres exhibited rapid cellular internalization. At concentrations up to 150 mg mL−1, they showed no toxic effects, while a mild cytotoxic effect was observed at 200 mg mL−1 after 48 h of incubation. When loaded with the anticancer drug DOX hydrochloride, the spheres exhibited slightly higher cytotoxicity compared to the free DOX. These results underscore the promise of Fe3O4@SiO2 hollow mesoporous spheres as effective drug carriers for the targeted delivery and treatment of C cells.233
Ehsanimehr et al. conducted a comprehensive study on the synthesis and surface functionalization of Fe3O4@SiO2@SBA-15 with a biodegradable, cationic, and biocompatible copolymer (Fig. 2). The process began with the preparation of MMNPs through sol–gel methods applied to Fe3O4 NPs. Subsequently, these particles were modified with L-cysteine, creating VMMNP-L-cysteine. In a separate process, PEI was modified by introducing CM-β-CD and FA, with FA being encapsulated within the cyclodextrin cavity through host–guest interactions, forming the PEI/CM-β-CD/FA complex. Subsequently, this complex was conjugated to VMMNP-L-cysteine, resulting in the creation of a novel hollow mesoporous structure designed as a nanocarrier. The drug loading and release profiles were assessed using UV-visible spectroscopy. To examine the impact of the nanocarrier on BC cells, the MCF7 cell line was cultured, and the cell viability was evaluated via MTT assays and IC50 values, representing the drug concentration required to inhibit 50% of cell growth relative to the control group. Furthermore, fluorescence microscopy was employed to observe potential the morphological changes in the C cell nuclei.49
 | ||
| Fig. 2 Synthetic path for the preparation of the targeted MMNPs nanocarrier. Reproduced from ref. 49 with permission from Elsevier, Copyright 2021. | ||
Iranpour et al. proposed an aptamer-functionalized DDS using SPION@MSNs capped with gold gatekeepers for DOX release. Its surface was further modified with heterofunctional PEG and EpCAM-targeting aptamers, yielding Apt-PEG-Au@NP-DOX. This system achieved selective uptake in colorectal cancer cells, improved in vivo tumor suppression, and minimized systemic toxicity in HT-29 xenograft mouse models.234
Abedi et al. developed monodisperse carboxylic acid-functionalized MMS NPs using two different approaches. i.e., a two-phase sol–gel method and post-modification techniques involving a CA-functionalized isocyanate silane coupling agent (MMS NP-NCO-CA) or succinic anhydride-functionalized MMS (MMS NP-NH-SA). Fig. 3 shows the TEM micrograph and FESEM image of MMS NP-NH-SA and MMS NPNCO-CA. The cytotoxicity of these nanoparticles, including both general toxicity and cisplatin (cis-Pt)-specific effects, was assessed using an MTT assay with the MDA-MB-231 BC cell line. Apoptosis was further analyzed using acridine orange/ethidium bromide dual staining, followed by observation with fluorescence microscopy. The in vitro anti-cancer activity of cis-Pt-loaded MMS NP-NCO-CA and MMS NP-NH-SA was significantly improved compared to free cis-Pt, with MMS NP-NCO-CA inducing a higher level of specific apoptotic cell death. These results suggest that the CA-functionalized core–shell magnetic mesoporous hybrid nanoparticles represent a promising strategy for in vivo DD in cancer therapy.54
 | ||
| Fig. 3 (A) TEM micrographs of (a–c) MMS NP-NH-SA and (d) MMS NPNCO-CA. (B) FESEM images of (e and f) MMS NP-NH-SA and (g and h) MMS NP-NCO-CA samples. Reproduced from ref. 54 with permission from Elsevier, Copyright 2019. | ||
Shao and colleagues designed a simple method for the synthesis of core–shell MMS NPs (Fe3O4@mSiO2 NPs) in an aqueous solution, utilizing cetyltrimethylammonium bromide as a template under alcohol-free conditions. This method is faster, more economical, and environmentally friendly compared to the conventional techniques, given that it eliminates the need for organic solvents and allows a single-step synthesis process that takes only five minutes. The Fe3O4@mSiO2 NPs loaded with DOX exhibited significant selectivity for liver C cells, facilitated by pH-sensitive DR, and enhanced cellular uptake in C cells relative to normal liver cells. These NPs offer significant benefits as therapeutic delivery systems, including exceptional biocompatibility, high payload capacity, protection of active compounds, selective toxicity towards C cells, and efficient cellular uptake. Thus, the pH-responsive release of DOX from these nanoparticles presents a potential strategy for enhancing targeted CT, while minimizing the DOX-related toxicity to healthy tissues and cells.235
This section highlights the application of MMS NPs in delivering specific chemotherapeutic agents, such as doxorubicin and cisplatin, across different cancer models including breast and cervical cancers. Case studies demonstrate the ability of these systems to enhance cellular uptake, provide sustained and pH-responsive drug release, and induce greater cytotoxic effects on cancer cells compared to free drugs. The findings underscore the potential of MMS-based platforms to overcome the limitations of traditional chemotherapy and offer more targeted and efficient cancer treatments.
5. Synergistic therapies
5.1. Combining chemotherapy with PTT and PDT
Recently, the research focus has shifted from monotherapy to combination treatments in the fight against cancer. Phototherapies are fast-evolving cancer therapy modalities that use the light of diverse wavelengths to cause photochemical and photothermal shifts in a target organ. Phototherapy comprised of PTT and PDT has demonstrated excellent prospects as an efficacious therapeutic procedure against cancer. These treatments have attracted significant scientific attention for the therapy of diverse illnesses, particularly cancer, owing to their distinctive advantages, such as minimum invasiveness, restricted side effects, excellent efficiency, and insignificant drug resistance. To overcome the limitations of single treatments, the combination of PTT and PDT can produce a synergistic effect, enhancing the therapeutic efficacy. PTT and PDT treatments harness light to destroy C cells with spatiotemporal accuracy via either the production of active oxygen species or increased temperatures.236–238 The combination of immunotherapy and the combined use of PDT with other therapy methods has also been developed to demonstrate excellent antitumor effects (Fig. 4(A)).239 Fig. 4(B) 8 illustrates the underlying mechanism of PDT. During PDT, a photosensitizer (PS) absorbs a photon, transitioning to an excited singlet state (1PS*). This state may convert into a longer-lived triplet state (3PS*), which drives the therapeutic effects through two distinct pathways. In the Type I reaction, 3PS* transfers electrons to biomolecules or oxygen, leading to the formation of reactive radicals such as O2˙−, H2O2, and OH˙. Alternatively, in the Type II reaction, 3PS* transfers energy directly to molecular oxygen, generating singlet oxygen (1O2), a highly cytotoxic species responsible for inducing cellular damage and apoptosis in targeted C cells. | ||
| Fig. 4 (A) Combination of diverse treatment procedures such as immunotherapy, magnetic therapy, biotherapy, sonodynamic therapy, radiation therapy, and chemotherapy with PTT and PDT (reproduced from ref. 239 with permission from Wiley, Copyright 2021). (B) Mechanism of PDT: a photosensitizer absorbs photons, generating ROS (Type I) or singlet oxygen (Type II) to induce targeted cytotoxicity (reproduced from ref. 240 with permission from Elsevier, Copyright 2019 (adapted and redrawn using the Corel Draw 5.6 software)). | ||
In PDT, NPs show significant potential within DDS due to their ability to improve the absorption of PSs by C cells. By enhancing the encapsulation efficiency of PSs, NPs enable their focused delivery and internalization by C cells through either passive or active transport pathways. This is due to the high surface-area-to-volume ratio of NPs. Additionally, conjugating PSs with NPs increases their solubility and stability, reduces their toxic effects under low-light environments, and boosts their selective delivery. As a result, this approach reduces adverse side effects, while yielding more effective PDT results.
NPs, due to their small size, can evade the immune system defenses by resembling biological molecules. This feature enables both the passive and active targeting of PSs to C cells.241 To enhance the targeted uptake and biocompatibility of PS-loaded nanocarriers for active targeting, specific ligands are incorporated, which selectively bind to overexpressed receptors on tumor cells.242 As shown in Fig. 5, NPs demonstrate their effectiveness in chemotherapy by targeting tumor-associated receptors, efficiently directing NPs to the tumor site. Once at the tumor, NPs can penetrate the plasma membrane and release the chemotherapeutic agents. Furthermore, when exposed to a specific wavelength of light, these NPs generate ROS, leading to tumor cell death. Over time, researchers have developed various carrier systems, composed of both organic and inorganic NPs, to enhance the uptake of PSs and promote PDT in the treatment of BC.243
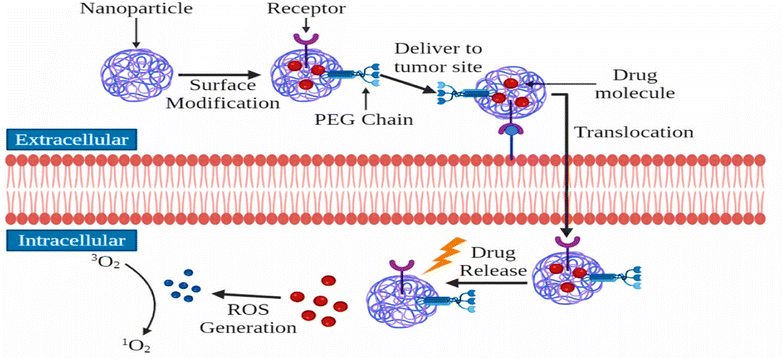 | ||
| Fig. 5 Illustration of the chemotherapeutic efficacy of NPs: NPs deliver drugs to tumor-associated receptors, effectively directing them to the tumor site for targeted therapy (reproduced from ref. 243 with permission from Wiley, Copyright 2021 and redrawn using the Corel Draw 5.6 software). | ||
PTT relies on photothermal agents to convert absorbed photon energy into heat, inducing localized hyperthermia, which results in cell death through necrosis and apoptosis.244,245 In contrast, PDT works by generating ROS through PSs, which transfer energy to oxygen within tissues when exposed to light, leading to the destruction of C cells.246–248 Although the individual application of PDT and PTT has demonstrated promising outcomes in CT,249,250 there is growing interest in combining both therapies in a unified system to enhance their therapeutic effectiveness.251,252 A key challenge in PDT is the reduced oxygen levels in tissues, which exacerbates local hypoxia, diminishing the efficiency of ROS generation due to insufficient oxygen.253,254 The integration of PTT with PDT offers a potential solution to this issue by improving the delivery of oxygen. Localized hyperthermia from PTT can enhance the blood circulation to the tumor site, thus increasing the availability of oxygen.255 PTT typically requires high-intensity laser irradiation (often exceeding 0.5 W cm−2) to achieve sufficient hyperthermia.256 However, excessive light exposure can result in damage to surrounding healthy tissues.257 Thus, by combining PDT with PTT, lower laser intensities can be used, reducing the risk of side effects. Moderate hyperthermia can also sensitize C cells to various therapeutic approaches, promoting drug uptake and alleviating tissue hypoxia.258,259 Therefore, combining PDT and PTT provides an opportunity to achieve effective therapeutic outcomes with minimal adverse effects. The main advantages and limitations of both PDT and PTT are summarized in Table 4.
| Phototherapies | Chief advantages | Chief disadvantages | Mechanism | Ref. |
|---|---|---|---|---|
| PTT | Local therapy, spatiotemporal selectivity, oxygen independence, thermal ablation, immunogenic | Restricted light penetration, heat resistance, heat-shock answer, and restricted tissue penetration | It uses NIR light to heat NPs, leading to localized tumor hyperthermia | 260 and 261 |
| PDT | Local therapy, spatiotemporal selectivity, minimal harm to normal tissues, limited or no potential for resistance | Restricted light penetration and tissue penetration and oxygen dependence | Activates PSs with light to produce ROS, causing oxidative damage to tumor cells | 260 and 261 |
6. Conclusion
Among the various nanomaterials developed for cancer therapy and diagnosis, MSNs and iron IONPs stand out owing to their remarkable potential. These materials offer several advantages, such as responsiveness to an external magnetic field, high drug payload capacity, the ability to easily modify their surface to prevent undesirable biological interactions, and excellent biocompatibility. These attributes make them valuable scaffolds to address some of the major challenges in CT today. IONPs, in particular, possess unique properties that allow them to function as nano-heaters, contrast agents for imaging, and DDS, playing a crucial role in cancer therapy. Research has demonstrated that IONPs can help mitigate the harmful side effects commonly associated with conventional chemotherapy. Meanwhile, MMS NPs are emerging as a highly promising platform for chemotherapy owing to their multifunctionality, biocompatibility, and effectiveness in DD. Their mesoporous structure offers an LSA and pore volume for the efficient loading of chemotherapeutic agents. Also, the functionalization of their pores allows precise control of DR, minimizing the systemic toxicity and improving the therapeutic outcomes. The magnetic cores of MMS can generate heat under an AMF, inducing localized hyperthermia to destroy C cells selectively, while sparing healthy tissue. MMS NPs enable combination therapy, integrating DD with hyperthermia or other treatments such as PDT, enhancing their efficacy. Functionalization allows the incorporation of imaging agents for theranostics, combining therapy and diagnostics in a single platform. Surface functionalization with ligands or antibodies enables targeted delivery to C cells, reducing off-target effects. Magnetic targeting via an external field can further guide NPs to tumor sites. Silicate materials are generally biocompatible and degrade into harmless byproducts, addressing concerns of their long-term toxicity. Magnetic cores, typically made of IO, are also biocompatible and have been used in FDA-approved applications. MMS NPs enhance imaging modalities such as MRI for the real-time monitoring of DD and treatment efficacy. We strongly believe that the interaction of diverse fields such as biology, chemistry, and medicine will provide the essential means to overcome the main challenges preventing this technology from being transferred into the clinic.The section on biocompatibility and toxicity concerns highlighted the key aspects of ensuring the safe and effective use of MMS NPs. The key points associated with biocompatibility and toxicity include core materials, surface functionalization, toxicity challenges, controlled degradation, and regulatory and long-term safety. IONPs, commonly used in MMS systems, are biocompatible and non-toxic at appropriate concentrations. They degrade into ionic states that the body can assimilate. Silica coatings enhance their biocompatibility and stability, providing a protective barrier against aggregation and premature degradation. Thus, coating MMS NPs with organic, inorganic, and polymeric substances ensures compatibility with biological environments and prevents aggregation. Functional groups such as amino, thiol, and carboxyl allow targeted interaction with biological molecules, reducing the non-specific uptake. Bare magnetic NPs may oxidize and generate ROS, leading to potential cytotoxic effects. Smaller NPs risk rapid degradation, which may affect their stability and effectiveness. Some surfactants and coatings used to modify NPs for improved dispersion and biocompatibility may introduce biotoxicity. MMS NPs degrade into non-toxic byproducts such as silicic acid, but the rate and mechanism depend on their synthesis methods and structural properties. Surface modifications can tune the degradation kinetics, enabling a balance between stability and bioavailability.
The scalability and clinical translation of magnetic silicate MMS NPs are critical for their transition from research to advanced applications. The main challenges in scalability and clinical translation include large-scale synthesis, complex functionalization, clinical translation, and economic feasibility. Achieving consistent size, shape, and magnetic properties during large-scale production is challenging. Small-scale synthesis methods often result in variations between batches, which can affect reproducibility. The high cost of raw materials and complex functionalization processes are barriers to scaling up. Surface modifications (e.g., addition of targeting ligands or polymers) often require multi-step chemical processes. Also, ensuring that these processes are scalable, while maintaining the integrity and functionality of NPs is difficult. The safety and efficacy of MMS NPs must be rigorously tested to meet stringent regulatory requirements. Ensuring biocompatibility and minimizing long-term side effects are crucial. Maintaining stability during storage and handling requires robust designs, particularly for clinical-grade materials. Cost-effective synthesis, functionalization, and purification methods are essential to make MMS NPs viable for widespread use. Reducing costs associated with high-quality starting materials (such as silica precursors and IO) is necessary. Future directions for scalability and clinical applications can be involved in using MMS NPs as adjuncts to existing treatments (e.g., enhancing the efficacy of chemotherapy or radiotherapy by integration with existing therapies), developing low-cost functionalization strategies, such as using biologically derived ligands instead of synthetic chemicals and conducting multicenter clinical trials to establish safety and efficacy across diverse patient populations.
Superparamagnetic Fe3O4 NPs, a type of magnetic nanomaterial, offer numerous benefits, including excellent biocompatibility, non-toxicity, and strong absorption properties. These advantages have led to their widespread use in bio-separation, cell labeling, and cancer therapy. Fe3O4 NPs used as photothermal agents have also been the focus of several studies in previous research.262,263 Fe3O4 NPs have several notable disadvantages. Firstly, their inherent magnetism makes them prone to agglomeration. Secondly, conventional Fe3O4 NPs often exhibit significant mass and increased dimensions, resulting in decreased photothermal efficiency. Thirdly, pure Fe3O4 has limited functional properties and poses challenges in modification, restricting its potential for broader applications. Finally, smaller Fe3O4 NPs may degrade rapidly in biological environments. Thus, to address these issues, strategies such as creating smaller NPs, co-loading with other materials, and developing composite NPs can be used to enhance the performance of Fe3O4 NPs.
For Fe3O4 NPs to perform optimally as nanoprobes in biological tissues, they must exhibit both chemical and structural stability and remain well-dispersed in aqueous solutions.264,265 Although hydrophilic surfactants can improve the dispersibility and performance of Fe3O4 NPs, their potential biotoxicity raises concerns regarding their safety in biological and medical applications. Thus, to address these issues, the application of a silica coating on the surface of Fe3O4 NPs not only enhances their aqueous dispersion but also significantly improves their mechanical and chemical integrity.266–269 The hydroxyl groups present on the surface of silica can react with different crosslinking agents, allowing the attachment of specific ligands to the NPs. Furthermore, the large pores within the silica structure offer several benefits. They can accommodate a wide variety of compounds, offer an expanded surface area, and decrease the particle density, thereby improving the loading capacity for multiple compounds and boosting the stability in aqueous environments. In biosensing applications, QDs have emerged as a superior alternative to conventional organic dyes due to their exceptional optical properties, including broad continuous absorption spectra, sharp emission peaks, and outstanding resistance to photodegradation.270,271 The layer-by-layer (LbL) approach is frequently used to integrate QDs into the silica matrix through electrostatic interactions between the silica and QDs. Nevertheless, this method results in the limited attachment of QDs to the silica surface, leading to relatively weak photoluminescence (PL) and increased toxicity of the QDs, which can be problematic for biomedical applications.
Mesoporous NPs represent the latest advancement in their category and are utilized in targeted DD to enhance the delivery efficiency. Diverse MMs have been employed as DDS. Siliceous porous materials can be categorized based on two key characteristics, their porous properties and the structure of their cell walls. In addition to these factors, these NPs can also be compared according to how easily fluid materials can access their pores. By incorporating intelligent polymers into modified MMs, researchers can deliver drugs to target tissues at a specific time and location with controlled release rates.272 These materials feature dual-component structures with distinct hydrophilic and hydrophobic properties, known as amphiphilic structures.273–275
MS materials feature an abundant number of silanol groups on their surface, which can be chemically altered to incorporate various functional groups. This modification is typically achieved using monomers containing RO3 and SiR′ structures, where R represents an organic functional group. By attaching diverse functional groups and organic molecules to the surface of MMs, their properties, such as water affinity, repulsion, and capacity to interact with guest molecules, can be customized to fulfill specific requirements.276 Drug loading within the pores of MS can be carried out through various strategies, as illustrated in Fig. 6.
 | ||
| Fig. 6 Schematic of the various approaches utilized to load drugs into MMS NPs based on solvent-free procedures and solvent-based procedures. | ||
The surface of MMs can be modified with organic compounds through three primary methods, i.e., grafting, coating, and compaction, together with structural modification via the rotational method.277 The use of MS as a drug carrier in DDSs began in 2001. MMs are highly suitable for DD due to their distinct properties. The key features of MS include (1) a well-ordered pore structure, which ensures a uniform distribution of pore sizes, enabling precise control of the loading and release of drugs in the body; (2) an LSA, which enhances the drug absorption and loading capacity due to the abundant pore volume; and (3) the ability to functionalize its surface with various organic molecules, allowing the incorporation of diverse drugs. These properties make MS an ideal candidate for the delivery of genes, drugs, and peptides to targeted tissues.278 However, MS alone cannot provide the necessary conditions for controlled DR, particularly in cancer therapy. Thus, to address this limitation, polymers and organic compounds are incorporated to enable controlled release and targeted delivery.279 MS must shield the drug until it reaches the targeted tissue, avoiding premature release. In chemotherapy, drugs are highly toxic and can affect both healthy and cancerous cells, sometimes leading to severe side effects or even fatal outcomes. In mesoporous systems, controlled DR is often triggered by specific environmental conditions, which can be categorized as intrinsic and extrinsic triggers. Intrinsic triggers include biological agents such as enzymes, reducing agents, and the acidic or alkaline environments found in lysosomes or cancerous cells. Alternatively, extrinsic triggers involve external influences such as magnetic fields and light exposure. The pH in the human body fluctuates significantly, ranging from 1–2 in the stomach to approximately 8 in the intestines.280 At the cellular level, extracellular fluid pH is about 7.4, but it decreases to 4–5 in lysosomes. C cells, due to their increased activity, metabolism, and lactic acid production, have a pH of around 5.8. These pH variations have been leveraged in DDSs to enable targeted and controlled drug release within the body.281
7. Future perspective
Although MMS NPs have great potential, challenges such as their large-scale synthesis, uniformity, long-term stability, and comprehensive safety evaluations remain. Research is ongoing to optimize these materials for clinical applications. In conclusion, MMS NPs represent a versatile and powerful tool in cancer therapy, offering targeted, multimodal, and less invasive treatment options. Their ability to integrate therapy with diagnostics makes them a key focus for advancing personalized medicine. Future research on MMS NPs should focus on integrating cutting-edge materials science, biology, and engineering to create versatile and clinically viable solutions. By addressing these opportunities, MMS NPs can move closer to becoming a cornerstone of personalized and precision-based cancer therapies. We present some suggestions for future research in the area of MMS NPs.➢ Designing MMS NPs to detect biomarkers within the tumor microenvironment for early diagnosis and tailored treatment.
➢ Utilizing environmentally friendly synthesis methods to improve sustainability and reduce the environmental impact of MMS NP production.
➢ Research into methods for the large-scale, reproducible synthesis of MMS NPs with consistent size, shape, and magnetic properties.
➢ Reducing costs associated with raw materials and functionalization to facilitate widespread clinical use.
➢ Advancing designs for MMS NPs that respond to multiple stimuli (e.g., pH, temperature, and enzymes) present in the tumor microenvironment.
➢ Incorporating smart polymers for highly precise and tunable DR profiles.
Abbreviations
| DDS | Drug delivery system |
| DR | Drug release |
| CT | Cancer treatment |
| CC | Cervical cancer |
| BC | Breast cancer |
| C cell | Cancer cell |
| GA | Gambogic acid |
| CA | Citric acid |
| LSA | Large surface area |
| DOX | Doxorubicin |
| DD | Drug delivery |
| MDT | Magnetic drug targeting |
| AMF | Alternating magnetic field |
| MMs | Mesoporous materials |
| MS | Mesoporous silica |
| MSNs | Mesoporous silica nanoparticles |
| MMS NPs | Magnetic mesoporous silica nanoparticles |
| MNPs | Magnetic nanoparticles |
| IO | Iron oxide |
| SPIOs | Superparamagnetic iron oxide nanoparticles |
| QDs | Quantum dots |
| CNTs | Carbon nanotubes |
| MRI | Magnetic resonance imaging |
| Ce6 | Chlorin e6 |
| PTT | Photothermal therapy |
| PDT | Photodynamic therapy |
| ROS | Reactive oxygen species |
| PSs | Photosensitizers |
| EPR | Permeability and retention |
| PEGylation | Polyethylene glycol |
| NIR | Near-infrared |
Data availability
All relevant data supporting the findings of this study are available within the article. Access to some data is restricted due to privacy or ethical restrictions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to express their gratitude to the technical staff of the Chemistry Department laboratory at Amirkabir University of Technology and the Pharmaceutical Analysis Research Center, Tabriz University of Medical Sciences in Tabriz, Iran (76115). Their invaluable support and contributions to this research are sincerely appreciated.References
- W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed.
- A. Foroozandeh, M. Abdouss, H. SalarAmoli, M. Pourmadadi and F. Yazdian, Process Biochem., 2023, 127, 82–91 CrossRef CAS.
- A. Foroozandeh, H. SalarAmoli, M. Abdouss and M. Pourmadadi, Sens. Actuators Rep., 2024, 7, 100195 CrossRef.
- A. S. Thakor and S. S. Gambhir, Ca-Cancer J. Clin., 2013, 63, 395–418 CrossRef PubMed.
- M. C. Li, JAMA, 1960, 174, 1291 CrossRef CAS PubMed.
- S.-Y. Qin, Y.-J. Cheng, Q. Lei, A.-Q. Zhang and X.-Z. Zhang, Biomaterials, 2018, 171, 178–197 CrossRef CAS PubMed.
- C.-Y. Zhao, R. Cheng, Z. Yang and Z.-M. Tian, Molecules, 2018, 23(4), 826 CrossRef.
- W. Sang, Z. Zhang, Y. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 3771–3810 RSC.
- Z. Zhang, W. Sang, L. Xie and Y. Dai, Coord. Chem. Rev., 2019, 399, 213022 CrossRef CAS.
- S. M. Hosseini, M. Abdouss, S. Mazinani, A. Soltanabadi and M. Kalaee, Composites, Part B, 2022, 231, 109557 CrossRef CAS.
- S. M. Hosseini, S. Mazinani, M. Abdouss, H. Kalhor, K. Kalantari, I. S. Amiri and Z. Ramezani, Polym. Bull., 2022, 79, 541–554 CrossRef CAS.
- F. B. Bombelli, C. A. Webster, M. Moncrieff and V. Sherwood, Lancet Oncol., 2014, 15, e22–e32 CrossRef CAS.
- F. Jadidi-Niaragh, F. Atyabi, A. Rastegari, N. Kheshtchin, S. Arab, H. Hassannia, M. Ajami, Z. Mirsanei, S. Habibi, F. Masoumi, F. Noorbakhsh, F. Shokri and J. Hadjati, J. Controlled Release, 2017, 246, 46–59 CrossRef CAS.
- A. Hatami kaleshtari, S. Farjaminejad, M. Hasani, R. Farjaminejad, A. Foroozandeh, M. Abdouss and M. Hasanzadeh, Carbohydr. Polym. Technol. Appl., 2025, 9, 100692 CAS.
- S. Payamifar, A. Foroozandeh, M. Abdouss and A. Poursattar Marjani, Sci. Rep., 2024, 14, 28493 CrossRef CAS PubMed.
- A. Foroozandeh, M. Pourmadadi, H. SalarAmoli and M. Abdouss, Sens. Bio-Sens. Res., 2024, 45, 100669 CrossRef.
- S. Najafi-Hajivar, P. Zakeri-Milani, H. Mohammadi, M. Niazi, M. Soleymani-Goloujeh, B. Baradaran and H. Valizadeh, Biomed. Pharmacother., 2016, 83, 1365–1378 CrossRef CAS PubMed.
- H. Qi, Z. Li, K. Du, K. Mu, Q. Zhou, S. Liang, W. Zhu, X. Yang and Y. Zhu, Nanoscale Res. Lett., 2014, 9, 595 CrossRef PubMed.
- B. Haley and E. Frenkel, Urol. Oncol.:Semin. Orig. Invest., 2008, 26, 57–64 CrossRef CAS.
- H. Soo Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef.
- M. E. Davis, Z. (Georgia) Chen and D. M. Shin, Nat. Rev. Drug Discov., 2008, 7, 771–782 CrossRef CAS PubMed.
- P. Decuzzi, R. Pasqualini, W. Arap and M. Ferrari, Pharm. Res., 2009, 26, 235–243 CrossRef CAS.
- S. D. Perrault, C. Walkey, T. Jennings, H. C. Fischer and W. C. W. Chan, Nano Lett., 2009, 9, 1909–1915 CrossRef CAS PubMed.
- C. Bharti, N. Gulati, U. Nagaich and A. Pal, Int. J. Pharm. Invest., 2015, 5, 124 CrossRef CAS PubMed.
- Y. Song, L. Yihong, X. Qien and Z. Liu, Int. J. Nanomed., 2017, 12, 87–110 CrossRef CAS.
- M. Vallet-Regí, F. Schüth, D. Lozano, M. Colilla and M. Manzano, Chem. Soc. Rev., 2022, 51, 5365–5451 RSC.
- J. Florek, R. Caillard and F. Kleitz, Nanoscale, 2017, 9, 15252–15277 RSC.
- T. Li, S. Shi, S. Goel, X. Shen, X. Xie, Z. Chen, H. Zhang, S. Li, X. Qin, H. Yang, C. Wu and Y. Liu, Acta Biomater., 2019, 89, 1–13 CrossRef CAS PubMed.
- J. O. Tella, J. A. Adekoya and K. O. Ajanaku, R. Soc. Open Sci., 2022, 9, 220013 CrossRef CAS.
- M. Iranshahy, M. Y. Hanafi-Bojd, S. H. Aghili, M. Iranshahi, S. M. Nabavi, S. Saberi, R. Filosa, I. F. Nezhad and M. Hasanpour, RSC Adv., 2023, 13, 22250–22267 RSC.
- N. Ž. Knežević, E. Ruiz-Hernández, W. E. Hennink and M. Vallet-Regí, RSC Adv., 2013, 3, 9584–9593 RSC.
- V. Godakhindi, M. Tarannum, S. K. Dam and J. L. Vivero-Escoto, Adv. Healthcare Mater., 2024, 13, 2400323 CrossRef CAS PubMed.
- I. Foster, Radiography, 2008, 14, 144–149 CrossRef.
- C. Schiliro and B. L. Firestein, Cells, 2021, 10(5), 1056 CrossRef CAS.
- R. Kaur, A. Bhardwaj and S. Gupta, Mol. Biol. Rep., 2023, 50, 9663–9676 CrossRef PubMed.
- U. Anand, A. Dey, A. K. S. Chandel, R. Sanyal, A. Mishra, D. K. Pandey, V. De Falco, A. Upadhyay, R. Kandimalla, A. Chaudhary, J. K. Dhanjal, S. Dewanjee, J. Vallamkondu and J. M. Pérez de la Lastra, Genes Dis., 2023, 10, 1367–1401 CrossRef CAS PubMed.
- M. Sohail, W. Guo, Z. Li, H. Xu, F. Zhao, D. Chen and F. Fu, Curr. Med. Chem., 2021, 28, 3753–3772 CAS.
- O. Afzal, A. S. A. Altamimi, M. S. Nadeem, S. I. Alzarea, W. H. Almalki, A. Tariq, B. Mubeen, B. N. Murtaza, S. Iftikhar, N. Riaz and I. Kazmi, Nanomaterials, 2022, 12(24), 4494 CrossRef CAS PubMed.
- L. Huang, S. Zhao, F. Fang, T. Xu, M. Lan and J. Zhang, Biomaterials, 2021, 268, 120557 CrossRef CAS PubMed.
- M. C. Phillips and S. A. Mousa, Nanomedicine, 2022, 17, 405–421 CrossRef CAS PubMed.
- S. Senapati, A. K. Mahanta, S. Kumar and P. Maiti, Signal Transduction Targeted Ther., 2018, 3, 7 CrossRef.
- N. Ž. Knežević, B. G. Trewyn and V. S. -Y. Lin, Chem. – Eur. J., 2011, 17, 3338–3342 CrossRef.
- N. V Roik and L. A. Belyakova, Interfaces: Focus, 2016, 6, 20160041 Search PubMed.
- D. K. Sahana, G. Mittal, V. Bhardwaj and M. N. V. R. Kumar, J. Pharm. Sci., 2008, 97, 1530–1542 CrossRef CAS.
- Y. (Chezy) Barenholz, J. Controlled Release, 2012, 160, 117–134 CrossRef.
- M. Mikeska, A. Burečcek, O. Dutko, P. Peikertová, J. Kupková, L. Hružík and D. Plachá, J. Nanosci. Nanotechnol., 2019, 19, 2567–2574 CrossRef CAS.
- Y. Zhu, Y. Fang and S. Kaskel, J. Phys. Chem. C, 2010, 114, 16382–16388 CrossRef CAS.
- N. Yin, X. Wang, T. Yang, Y. Ding, L. Li, S. Zhao, P. Li, X. Xu and L. Zhu, Ceram. Int., 2021, 47, 8271–8278 CrossRef CAS.
- S. Ehsanimehr, P. N. Moghadam, W. Dehaen and V. S. Irannejad, Colloids Surf., A, 2021, 615, 126302 CrossRef CAS.
- Y. Guan, Y. Yang, X. Wang, H. Yuan, Y. Yang, N. Li and C. Ni, J. Mol. Liq., 2021, 327, 114783 CrossRef CAS.
- R. Liu, G. Rong, Y. Liu, W. Huang, D. He and R. Lu, Mater. Sci. Eng., C, 2021, 120, 111719 CrossRef CAS PubMed.
- A. Lajevardi, M. Hossaini Sadr, A. Badiei and M. Armaghan, J. Mol. Liq., 2020, 307, 112996 CrossRef CAS.
- W. Cai, M. Guo, X. Weng, W. Zhang, G. Owens and Z. Chen, Mater. Sci. Eng., C, 2020, 112, 110900 CrossRef CAS.
- M. Abedi, S. S. Abolmaali, M. Abedanzadeh, S. Borandeh, S. M. Samani and A. M. Tamaddon, Mater. Sci. Eng., C, 2019, 104, 109922 CrossRef CAS PubMed.
- N. Avedian, F. Zaaeri, M. P. Daryasari, H. Akbari Javar and M. Khoobi, J. Drug Delivery Sci. Technol., 2018, 44, 323–332 CrossRef CAS.
- X. Li, W. Wang, Q. Li, H. Lin, Y. Xu and L. Zhuang, Mater. Des., 2018, 151, 89–101 CrossRef CAS.
- X. Liu, Y. Tao, H. Mao, Y. Kong, J. Shen, L. Deng and L. Yang, Ceram. Int., 2017, 43, 5061–5067 CrossRef CAS.
- Q.-Y. Chen, G.-P. Tao, Y.-Q. Liu and X. Yang, Spectrochim. Acta, Part A, 2012, 96, 284–288 CrossRef CAS PubMed.
- H. M. Azab, S. M. Shawky, M. M. El-Sheikh, E.-Z. M. Ebeid, A. Abd-elhamid, S. Shalaby and A. Elshal, Egypt. J. Cancer Biomed. Res., 2024, 8, 1–14 CrossRef.
- L. Vahabi, P. Rashidi Ranjbar and F. Davar, J. Drug Delivery Sci. Technol., 2023, 80, 104144 CrossRef CAS.
- Y. Zhou, Y. Zeng, S. Huang, Q. Xie, Y. Fu, L. Tan, M. Ma and S. Yao, Sens. Actuators, B, 2012, 173, 433–440 CrossRef CAS.
- M. Darroudi, S. E. Nazari, F. Asgharzadeh, N. Khalili-Tanha, G. Khalili-Tanha, T. Dehghani, M. Karimzadeh, M. Maftooh, G. A. Fern, A. Avan, M. Rezayi and M. Khazaei, Cancer Nanotechnol., 2022, 13, 36 CrossRef CAS.
- D.-X. Zhang, T. Tieu, L. Esser, M. Wojnilowicz, C.-H. Lee, A. Cifuentes-Rius, H. Thissen and N. H. Voelcker, ACS Appl. Mater. Interfaces, 2022, 14, 54539–54549 CrossRef CAS PubMed.
- Y. Zhang, R. Wen, J. Hu, D. Guan, X. Qiu, Y. Zhang, D. S. Kohane and Q. Liu, Nat. Commun., 2022, 13, 5927 CrossRef CAS PubMed.
- L. García, E. Garaio, A. López-Ortega, I. Galarreta-Rodriguez, L. Cervera-Gabalda, G. Cruz-Quesada, A. Cornejo, J. J. Garrido, C. Gómez-Polo and J. I. Pérez-Landazábal, Langmuir, 2023, 39, 211–219 CrossRef PubMed.
- G. Zhang, W. Han, P. Zhao, Z. Wang, M. Li, X. Sui, Y. Liu, B. Tian, Z. He and Q. Fu, Nanoscale, 2023, 15, 1937–1946 RSC.
- V. A. Tran and S.-W. Lee, J. Colloid Interface Sci., 2018, 510, 345–356 CrossRef CAS PubMed.
- N. B. Fernandes, Y. Nayak, S. Garg and U. Y. Nayak, Coord. Chem. Rev., 2023, 478, 214977 CrossRef CAS.
- B. Siddiqui, A. ur. Rehman, I. Haq, A. A. Al-Dossary, A. Elaissari and N. Ahmed, Int. J. Pharm.:X, 2022, 4, 100116 CAS.
- M. Khosroshahi, I. M. Tehrani and A. Nouri, Adv. Nano-Bio-Mat. Devices, 2018, 2, 230 Search PubMed.
- L. Zhang, Z. Yang, J. Ren, L. Ba, W. He and C.-Y. Wong, Nano Res., 2020, 13, 1389–1398 CrossRef CAS.
- R. C. S. Azevedo, R. G. Sousa, W. A. A. Macedo and E. M. B. Sousa, J. Sol-Gel Sci. Technol., 2014, 72, 208–218 CrossRef CAS.
- M. Kulpa-Greszta, A. Tomaszewska, A. Dziedzic, I. Rzeszutek and R. Pązik, Mater. Today Commun., 2023, 35, 105513 CrossRef CAS.
- S. Liu, B. Yu, S. Wang, Y. Shen and H. Cong, Adv. Colloid Interface Sci., 2020, 281, 102165 CrossRef CAS PubMed.
- C. Tao, F. Zhao, Z.-W. Tang, L. Zhang, Q. Niu, G. Cao, L.-M. Zhao, W. Huang and P. Zhao, J. Solid State Chem., 2021, 303, 122489 CrossRef CAS.
- M. A. Mohammadi, S. Asghari and B. Aslibeiki, Surf. Interfaces, 2021, 25, 101271 CrossRef CAS.
- C. Jia, J. Guo, Y. Hu, T. Li, T. Zhou, X. Liang and S. Wang, Colloids Surf., A, 2023, 675, 132077 CrossRef CAS.
- J. Chomoucka, J. Drbohlavova, D. Huska, V. Adam, R. Kizek and J. Hubalek, Pharmacol. Res., 2010, 62, 144–149 CrossRef CAS PubMed.
- S. M. Krishna, J. V Moxon, R. J. Jose, J. Li, A. Sahebkar, M. R. Jaafari, M. Hatamipour, D. Liu and J. Golledge, J. Cell. Physiol., 2018, 233, 6951–6964 CrossRef CAS PubMed.
- I. M. Obaidat, V. Narayanaswamy, S. Alaabed, S. Sambasivam and C. V. V Muralee Gopi, Magnetochemistry, 2019, 5(4), 67 CrossRef.
- M. B. Gawande, P. S. Branco and R. S. Varma, Chem. Soc. Rev., 2013, 42, 3371–3393 RSC.
- A. Lu, E. L. Salabas and F. Schüth, Angew. Chem., Int. Ed., 2007, 46, 1222–1244 CrossRef CAS PubMed.
- B. Karimi, F. Mansouri and H. M. Mirzaei, ChemCatChem, 2015, 7, 1736–1789 CrossRef CAS.
- D. C. Jiles, Acta Mater., 2003, 51, 5907–5939 CrossRef CAS.
- O. V Salata, J. Nanobiotechnol., 2004, 2, 3 CrossRef.
- G. Barratt, Cell. Mol. Life Sci., 2003, 60, 21–37 CrossRef CAS.
- T. Neuberger, B. Schöpf, H. Hofmann, M. Hofmann and B. von Rechenberg, J. Magn. Magn. Mater., 2005, 293, 483–496 CrossRef CAS.
- H. Gu, K. Xu, C. Xu and B. Xu, Chem. Commun., 2006, 941–949 RSC.
- A. Al Ragib, R. Chakma, J. Wang, Y. M. Alanazi, M. El-Harbawi, G. A. Arish, T. Islam, M. A. B. Siddique, A. R. M. T. Islam and T. Kormoker, Nano-Struct. Nano-Objects, 2024, 40, 101395 CrossRef.
- R. Narayan, U. Y. Nayak, A. M. Raichur and S. Garg, Pharmaceutics, 2018, 10(3), 118 CrossRef CAS.
- Z. Jamalpoor, H. Ahmadi, M. Abdous and A. Rahdar, J. Mol. Liq., 2024, 408, 125407 CrossRef CAS.
- A. El-Aneed, J. Controlled Release, 2004, 94, 1–14 CrossRef CAS PubMed.
- R. Huang, Y.-W. Shen, Y.-Y. Guan, Y.-X. Jiang, Y. Wu, K. Rahman, L.-J. Zhang, H.-J. Liu and X. Luan, Acta Biomater., 2020, 116, 1–15 CrossRef CAS.
- Z. Fu, L. Li, Y. Wang, Q. Chen, F. Zhao, L. Dai, Z. Chen, D. Liu and X. Guo, Chem. Eng. J., 2020, 382, 122905 CrossRef CAS.
- C. von Baeckmann, H. Kählig, M. Lindén and F. Kleitz, J. Colloid Interface Sci., 2021, 589, 453–461 CrossRef CAS PubMed.
- S. M. Fotukian, A. Barati, M. Soleymani and A. M. Alizadeh, J. Alloys Compd., 2020, 816, 152548 CrossRef CAS.
- X. Wang, Z. Li, Y. Ding, K. Wang, Z. Xing, X. Sun, W. Guo, X. Hong, X. Zhu and Y. Liu, Chem. Eng. J., 2020, 381, 122693 CrossRef CAS.
- E. Juère, G. Del Favero, F. Masse, D. Marko, A. Popat, J. Florek, R. Caillard and F. Kleitz, Eur. J. Pharm. Biopharm., 2020, 151, 171–180 CrossRef PubMed.
- W. F. Elmobarak and F. Almomani, Chemosphere, 2021, 265, 129054 CrossRef CAS.
- J. A. Flood-Garibay and M. A. Méndez-Rojas, Colloids Surf., A, 2021, 615, 126236 CrossRef CAS.
- J. H. Cha, H.-H. Choi, Y.-G. Jung, S.-C. Choi and G. S. An, Ceram. Int., 2020, 46, 14384–14390 CrossRef CAS.
- C. Wang, L. Yang, X. Yuan, W. Zhou, M. Xu and W. Yang, Colloid Interface Sci. Commun., 2021, 45, 100521 CrossRef CAS.
- M. Asgari, T. Miri, M. Soleymani and A. Barati, J. Mol. Liq., 2021, 324, 114731 CrossRef CAS.
- H. Lin, L. Yin, B. Chen and Y. Ji, Colloids Surf., B, 2022, 219, 112814 CrossRef CAS PubMed.
- Z. Dai, W. Wen, Z. Guo, X.-Z. Song, K. Zheng, X. Xu, X. Qi and Z. Tan, Colloids Surf., B, 2020, 195, 111274 CrossRef CAS.
- Q. Zhu, J. Song, Z. Liu, K. Wu, X. Li, Z. Chen and H. Pang, J. Colloid Interface Sci., 2022, 623, 992–1001 CrossRef CAS.
- P. S. Shinde, P. S. Suryawanshi, K. K. Patil, V. M. Belekar, S. A. Sankpal, S. D. Delekar and S. A. Jadhav, J. Compos. Sci., 2021, 5(3), 75 CrossRef CAS.
- O. V Kuznetsova, I. S. Reshetnikova, S. N. Shtykov, V. K. Karandashev, B. K. Keppler and A. R. Timerbaev, Chem. Commun., 2019, 55, 4270–4272 RSC.
- N. Malhotra, J.-S. Lee, R. A. D. Liman, J. M. S. Ruallo, O. B. Villaflores, T.-R. Ger and C.-D. Hsiao, Molecules, 2020, 25(14), 3159 CrossRef CAS PubMed.
- R. V Mehta, Mater. Sci. Eng., C, 2017, 79, 901–916 CrossRef.
- O. L. Gobbo, K. Sjaastad, M. W. Radomski, Y. Volkov and A. Prina-Mello, Theranostics, 2015, 5, 1249–1263 CrossRef CAS.
- U. Kanwal, I. B. Nadeem, O. Muhammad, A. Nasir, H. Khalid and A. Raza, J. Drug Targeting, 2018, 26, 296–310 CrossRef CAS PubMed.
- L. Zhu, Z. Zhiyang, M. Hui and L. Yang, Nanomedicine, 2017, 12, 73–87 CrossRef CAS PubMed.
- P. Eslami, M. Albino, F. Scavone, F. Chiellini, A. Morelli, G. Baldi, L. Cappiello, S. Doumett, G. Lorenzi, C. Ravagli, A. Caneschi, A. Laurenzana and C. Sangregorio, Nanomaterials, 2022, 12(3), 303 CrossRef CAS.
- T. T. H. Le, T. Q. Bui, T. M. T. Ha, M. H. Le, H. N. Pham and P. T. Ha, J. Mater. Sci., 2018, 53, 13826–13842 CrossRef CAS.
- Y. Yang, Q. Guo, J. Peng, J. Su, X. Lu, Y. Zhao and Z. Qian, J. Biomed. Nanotechnol., 2016, 12, 1963–1974 CrossRef CAS.
- S. Chandra, S. Mehta, S. Nigam and D. Bahadur, New J. Chem., 2010, 34, 648–655 RSC.
- N. Zhu, H. Ji, P. Yu, J. Niu, M. U. Farooq, M. W. Akram, I. O. Udego, H. Li and X. Niu, Nanomaterials, 2018, 8(10), 810 CrossRef.
- S. G. Grancharov, H. Zeng, S. Sun, S. X. Wang, S. O'Brien, C. B. Murray, J. R. Kirtley and G. A. Held, J. Phys. Chem. B, 2005, 109, 13030–13035 CrossRef CAS.
- Y. Piao, J. Kim, H. Bin Na, D. Kim, J. S. Baek, M. K. Ko, J. H. Lee, M. Shokouhimehr and T. Hyeon, Nat. Mater., 2008, 7, 242–247 CrossRef CAS PubMed.
- B. G. Trewyn, I. I. Slowing, S. Giri, H.-T. Chen and V. S.-Y. Lin, Acc. Chem. Res., 2007, 40, 846–853 CrossRef CAS.
- G. Øye, J. Sjöblom and M. Stöcker, Adv. Colloid Interface Sci., 2001, 89–90, 439–466 CrossRef PubMed.
- I. I. Slowing, B. G. Trewyn and V. S.-Y. Lin, J. Am. Chem. Soc., 2007, 129, 8845–8849 CrossRef CAS.
- W. Cha, R. Fan, Y. Miao, Y. Zhou, C. Qin, X. Shan, X. Wan and J. Li, Molecules, 2017, 22(5), 782 CrossRef.
- M. Y. Hanafi-Bojd, A. Legha and B. Malaekeh-Nikouei, Ther. Delivery, 2016, 7, 649–655 CrossRef CAS.
- J. Riikonen, W. Xu and V.-P. Lehto, Int. J. Pharm., 2018, 536, 178–186 CrossRef CAS PubMed.
- Y. Wang, Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, E. Che, L. Hu, Q. Zhang, T. Jiang and S. Wang, Nanomedicine, 2015, 11, 313–327 CrossRef CAS.
- Z. Tao, RSC Adv., 2014, 4, 18961–18980 RSC.
- A. Saadati, H. N. Baghban, M. Hasanzadeh and N. Shadjou, RSC Adv., 2024, 14, 8810–8818 RSC.
- H. Kholafazad Kordasht, M. Pazhuhi, P. Pashazadeh-Panahi, M. Hasanzadeh and N. Shadjou, TrAC, Trends Anal. Chem., 2020, 124, 115778 CrossRef CAS.
- M. Hasanzadeh, A. S. Nahar, S. Hassanpour, N. Shadjou, A. Mokhtarzadeh and J. Mohammadi, Enzyme Microb. Technol., 2017, 105, 64–76 CrossRef CAS PubMed.
- N. Shadjou and M. Hasanzadeh, Mater. Technol., 2016, 31, 806–811 CrossRef.
- M. Hasanzadeh, M. H. Pournaghi-Azar, N. Shadjou and A. Jouyban, J. Anal. Chem., 2016, 71, 386–395 CrossRef CAS.
- M. Hasanzadeh, M. H. Pournaghi-Azar, N. Shadjou and A. Jouyban, J. Anal. Chem., 2016, 71, 386–395 CrossRef CAS.
- N. Shadjou and M. Hasanzadeh, Mater. Sci. Eng., C, 2015, 55, 401–409 CrossRef CAS.
- L. Abbasy, A. Mohammadzadeh, M. Hasanzadeh, M. Ehsani and A. Mokhtarzadeh, Int. J. Biol. Macromol., 2020, 154, 584–595 CrossRef CAS PubMed.
- J. Soleymani, M. Hasanzadeh, M. H. Somi, N. Shadjou and A. Jouyban, Biosens. Bioelectron., 2019, 132, 122–131 CrossRef CAS PubMed.
- M. Hasanzadeh, N. Shadjou, M. de la Guardia, M. Eskandani and P. Sheikhzadeh, TrAC, Trends Anal. Chem., 2012, 33, 117–129 CrossRef CAS.
- M. Hasanzadeh and N. Shadjou, TrAC, Trends Anal. Chem., 2016, 80, 167–176 CrossRef CAS.
- A. Mohammadzadeh, A. Jouyban, M. Hasanzadeh, V. Shafiei-Irannejad and J. Soleymani, Anal. Methods, 2021, 13, 4280–4289 RSC.
- M. B. Behyar, F. Farshchi and M. Hasanzadeh, J. Mol. Recognit., 2022, 35, e2960 CrossRef CAS PubMed.
- M. Shaban and M. Hasanzadeh, RSC Adv., 2020, 10, 37116–37133 RSC.
- H. Navay Baghban, M. Hasanzadeh, Y. Liu and F. Seidi, Biosensors, 2022, 12(10), 911 CrossRef.
- A. Mirzaie, A. Saadati, S. Hassanpour, M. Hasanzadeh, M. Siahi-Shadbad and A. Jouyban, Anal. Methods, 2019, 11, 4609–4619 RSC.
- H. Kholafazad kordasht, M.-H. Moosavy, M. Hasanzadeh, J. Soleymani and A. Mokhtarzadeh, Anal. Methods, 2019, 11, 3910–3919 RSC.
- J. Soleymani, M. Hasanzadeh, M. H. Somi, N. Shadjou and A. Jouyban, Biosens. Bioelectron., 2018, 115, 61–69 CrossRef CAS.
- M. Hasanzadeh, S. Hassanpour, A. Saadati, N. Shadjou and A. Mokhtarzadeh, Nano LIFE, 2017, 07, 1750006 CrossRef CAS.
- H. Navay Baghban, M. Baghal Behyar, A. Nilghaz, R. Ebrahimi, M. Hasanzadeh and N. Shadjou, Microchem. J., 2024, 199, 110102 CrossRef CAS.
- M. Hasanzadeh, K. Balal and N. Shadjou, Nanocomposites, 2016, 2, 76–83 CrossRef CAS.
- S. Azizi, J. Soleymani and M. Hasanzadeh, Appl. Organomet. Chem., 2020, 34, e5440 CrossRef CAS.
- S. Azizi, S. Jafar and M. Hasanzadeh, Nanocomposites, 2020, 6, 31–40 CrossRef CAS.
- S. Azizi, N. Shadjou and M. Hasanzadeh, Appl. Organomet. Chem., 2020, 34, e5321 CrossRef CAS.
- S. Azizi, S. Nasrin and M. Hasanzadeh, Nanocomposites, 2019, 5, 124–132 CrossRef CAS.
- H. K. Kordasht, M. Pazhuhi, M. Hasanzadeh, N. Shadjou, N. H. Voelcker and A. Nilghaz, TrAC, Trends Anal. Chem., 2024, 181, 117998 CrossRef CAS.
- J. Werner, R. Frankowski, T. Grześkowiak and A. Zgoła-Grześkowiak, TrAC, Trends Anal. Chem., 2024, 176, 117772 CrossRef CAS.
- K. Dashtian, F. Zahedpour, A. Foroozandeh, R. Karimi, M. Abdouss and S. Hajati, TrAC, Trends Anal. Chem., 2025, 189, 118271 CrossRef CAS.
- M. Mahmudi, N. Shadjou and F. A. M. Hasanzadeh, J. Electroanal. Chem., 2019, 848, 113272 CrossRef CAS.
- M. Hasanzadeh, F. Farzad, S. Nasrin and A. Jouyban, Environ. Technol., 2015, 36, 36–44 CrossRef CAS PubMed.
- A. Foroozandeh, M. A. Babazad, S. Jouybar, M. Abdouss, H. Salar Amoli, K. Dashtian and M. Hasanzadeh, TrAC, Trends Anal. Chem., 2025, 183, 118119 CrossRef CAS.
- J. Soleymani, M. Hasanzadeh, N. Shadjou, M. H. Somi and A. Jouyban, J. Pharm. Biomed. Anal., 2020, 180, 113077 CrossRef CAS.
- A. Foroozandeh, M. A. Babazad, S. Jouybar, M. Abdouss, H. SalarAmoli, K. Dashtian and M. Hasanzadeh, TrAC, Trends Anal. Chem., 2024, 118119 Search PubMed.
- D. Napierska, L. C. Thomassen, D. Lison, J. A. Martens and P. H. Hoet, Part. Fibre Toxicol., 2010, 7, 39 CrossRef CAS PubMed.
- J. G. Croissant, Y. Fatieiev and N. M. Khashab, Adv. Mater., 2017, 29, 1604634 CrossRef.
- M. G. Krukemeyer, V. Krenn, F. Huebner, W. Wagner and R. Resch, J. Nanomed. Nanotechnol., 2015, 06, 336 Search PubMed.
- S. R. Mudshinge, A. B. Deore, S. Patil and C. M. Bhalgat, Saudi Pharm. J., 2011, 19, 129–141 CrossRef CAS.
- R. K. Kankala, Y. S. Zhang, S. Wang, C. Lee and A. Chen, Adv. Healthcare Mater., 2017, 6, 1700433 CrossRef.
- R. K. Kankala, K. Zhu, X.-N. Sun, C.-G. Liu, S.-B. Wang and A.-Z. Chen, ACS Biomater. Sci. Eng., 2018, 4, 800–818 CrossRef CAS PubMed.
- M. Alhariri, A. Ali and A. Omri, Expert Opin. Drug Delivery, 2013, 10, 1515–1532 CrossRef CAS.
- P. P. Deshpande, B. Swati and V. P. Torchilin, Nanomedicine, 2013, 8, 1509–1528 CrossRef CAS PubMed.
- C. J. Cheng, G. T. Tietjen, J. K. Saucier-Sawyer and W. M. Saltzman, Nat. Rev. Drug Discov., 2015, 14, 239–247 CrossRef CAS.
- Y.-H. Han, R. K. Kankala, S.-B. Wang and A.-Z. Chen, Nanomaterials, 2018, 8(6), 360 CrossRef PubMed.
- K. Madaan, S. Kumar, N. Poonia, V. Lather and D. Pandita, J. Pharm. Bioallied Sci, 2014, 6(3), 139–150 CrossRef.
- J. J. Giner-Casares, M. Henriksen-Lacey, M. Coronado-Puchau and L. M. Liz-Marzán, Mater. Today, 2016, 19, 19–28 CrossRef CAS.
- R. K. Kankala, P.-Y. Tsai, Y. Kuthati, P.-R. Wei, C.-L. Liu and C.-H. Lee, J. Mater. Chem. B, 2017, 5, 1507–1517 RSC.
- Y. Kuthati, R. K. Kankala and C.-H. Lee, Appl. Clay Sci., 2015, 112–113, 100–116 CrossRef CAS.
- E. H. Frei, J. Appl. Phys., 1969, 40, 955–957 CrossRef.
- M. P. Alvarez-Berríos, N. Sosa-Cintron, M. Rodriguez-Lugo, R. Juneja and J. L. Vivero-Escoto, J.Chem, 2016, 2016, 2672740 Search PubMed.
- J. Estelrich, E. Escribano, J. Queralt and M. A. Busquets, Int. J. Mol. Sci., 2015, 16(4), 8070–8101 CrossRef CAS.
- A. S. Lübbe, C. Alexiou and C. Bergemann, J. Surg. Res., 2001, 95, 200–206 CrossRef.
- B. Polyak and G. Friedman, Expert Opin. Drug Delivery, 2009, 6, 53–70 CrossRef CAS.
- R. A. Verkhovskii, A. N. Ivanov, E. V. Lengert, K. A. Tulyakova, N. Y. Shilyagina and A. V. Ermakov, Pharmaceutics, 2023, 15(5), 1566 CrossRef CAS PubMed.
- Y. Zhu and F. Chen, Chem. - Asian J., 2015, 10, 284–305 CrossRef CAS PubMed.
- V. Balamurali, T. M. Pramodkuma, N. Srujana, M. P. Venkatesh, N. V. Gupta, K. L. Krishna and H. V. Gangadhara, Am. J. Drug Discovery Dev., 2010, 1, 24–48 CrossRef.
- Q. Yang, S. Wang, P. Fan, L. Wang, Y. Di, K. Lin and F.-S. Xiao, Chem. Mater., 2005, 17, 5999–6003 CrossRef CAS.
- M. Kanamala, W. R. Wilson, M. Yang, B. D. Palmer and Z. Wu, Biomaterials, 2016, 85, 152–167 CrossRef CAS.
- Z. Chen, W. Xiaoxiao, Z. Na, C. Haifeng and G. Guo, Expert Opin. Drug Delivery, 2023, 20, 1623–1642 CrossRef CAS.
- S. Abdella, F. Abid, S. H. Youssef, S. Kim, F. Afinjuomo, C. Malinga, Y. Song and S. Garg, Drug Discovery Today, 2023, 28, 103414 CrossRef CAS.
- A. Nazli, M. Z. Irshad Khan, Á. Rácz and S. Béni, Eur. J. Med. Chem., 2024, 276, 116699 CrossRef CAS.
- L. Liu, Y. WenDong, R. YueFeng, L. XiaoYang and J. Gao, Drug Delivery, 2017, 24, 569–581 CrossRef CAS.
- L. Zhang, G. Alimu, Z. Du, T. Yan, H. Li, R. Ma, Z. Lan, Z. Yu, N. Alifu and K. Sun, ACS Omega, 2023, 8, 21793–21801 CrossRef CAS PubMed.
- M. Sharifi, A. Hasan, N. M. Q. Nanakali, A. Salihi, F. A. Qadir, H. A. Muhammad, M. S. Shekha, F. M. Aziz, K. M. Amen, F. Najafi, H. Yousefi-Manesh and M. Falahati, Sci. Rep., 2020, 10, 5925 CrossRef CAS.
- A. T. Shivanna, B. S. Dash and J.-P. Chen, Micromachines, 2022, 13(8), 1279 CrossRef.
- J.-W. Lu, F. Yang, Q.-F. Ke, X.-T. Xie and Y.-P. Guo, Nanomedicine, 2018, 14, 811–822 CrossRef CAS PubMed.
- Q. Fu, Z. Li, J. Ye, Z. Li, F. Fu, S.-L. Lin, C. A. Chang, H. Yang and J. Song, Theranostics, 2020, 10, 4997–5010 CrossRef CAS.
- A. Espinosa, J. Reguera, A. Curcio, Á. Muñoz-Noval, C. Kuttner, A. Van de Walle, L. M. Liz-Marzán and C. Wilhelm, Small, 2020, 16, 1904960 CrossRef CAS PubMed.
- W. H. De Jong and P. J. A. Borm, Int. J. Nanomed., 2008, 3, 133–149 CrossRef CAS.
- T. Thambi and D. S. Lee, in Woodhead Publishing Series in Biomaterials, ed. A. S. H. Makhlouf and D. D. A. Abu-Thabit, Woodhead Publishing, 2019, pp. 413–438, DOI:10.1016/B978-0-08-102548-2.00001-9.
- J. Liao, Y. Jia, Y. Wu, K. Shi, D. Yang, P. Li and Z. Qian, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 2020, 12, e1581 CrossRef CAS.
- Y. Lee and D. H. Thompson, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 2017, 9, e1450 CrossRef PubMed.
- H. Priya James, R. John, A. Alex and K. R. Anoop, Acta Pharm. Sin. B, 2014, 4, 120–127 CrossRef PubMed.
- K. Zhang, P.-P. Yang, J.-P. Zhang, L. Wang and H. Wang, Chin. Chem. Lett., 2017, 28, 1808–1816 CrossRef CAS.
- J. Shi, P. W. Kantoff, R. Wooster and O. C. Farokhzad, Nat. Rev. Cancer, 2017, 17, 20–37 CrossRef CAS PubMed.
- C. Xu, Y. Yan, J. Tan, D. Yang, X. Jia, L. Wang, Y. Xu, S. Cao and S. Sun, Adv. Funct. Mater., 2019, 29, 1808146 CrossRef.
- Y. Zhang, C. Teh, M. Li, C. Y. Ang, S. Y. Tan, Q. Qu, V. Korzh and Y. Zhao, Chem. Mater., 2016, 28, 7039–7050 CrossRef CAS.
- T. Zhou, X. Zhou and D. Xing, Biomaterials, 2014, 35, 4185–4194 CrossRef CAS PubMed.
- J. Liu, B. Zhang, Z. Luo, X. Ding, J. Li, L. Dai, J. Zhou, X. Zhao, J. Ye and K. Cai, Nanoscale, 2015, 7, 3614–3626 RSC.
- S. J. Lee, J. Young-Il, P. Hyung-Kyu, K. Dae Hwan, O. Jong-Suk, L. Sam-Gyu and H. C. Lee, Int. J. Nanomed., 2015, 10, 5489–5503 CAS.
- Q. Hu, P. S. Katti and Z. Gu, Nanoscale, 2014, 6, 12273–12286 RSC.
- S. H. Lee, M. K. Gupta, J. B. Bang, H. Bae and H. Sung, Adv. Healthcare Mater., 2013, 2, 908–915 CrossRef CAS.
- W. Yin, W. Ke, W. Chen, L. Xi, Q. Zhou, J. F. Mukerabigwi and Z. Ge, Biomaterials, 2019, 195, 63–74 CrossRef CAS.
- G. Saravanakumar, J. Kim and W. J. Kim, Adv. Sci., 2017, 4, 1600124 CrossRef.
- J. Chen, J. Ding, Y. Wang, J. Cheng, S. Ji, X. Zhuang and X. Chen, Adv. Mater., 2017, 29, 1701170 CrossRef PubMed.
- Y. Chi, X. Yin, K. Sun, S. Feng, J. Liu, D. Chen, C. Guo and Z. Wu, J. Controlled Release, 2017, 261, 113–125 CrossRef CAS PubMed.
- Y. Li, A. Lu, M. Long, L. Cui, Z. Chen and L. Zhu, Acta Biomater., 2019, 83, 334–348 CrossRef CAS PubMed.
- H. Liu, Y. Xie, Y. Zhang, Y. Cai, B. Li, H. Mao, Y. Liu, J. Lu, L. Zhang and R. Yu, Biomaterials, 2017, 121, 130–143 CrossRef CAS PubMed.
- J. Li, H. Duan and K. Pu, Adv. Mater., 2019, 31, 1901607 CrossRef.
- J. Li and K. Pu, Acc. Chem. Res., 2020, 53, 752–762 CrossRef CAS.
- M. Karimi, P. Sahandi Zangabad, S. Baghaee-Ravari, M. Ghazadeh, H. Mirshekari and M. R. Hamblin, J. Am. Chem. Soc., 2017, 139, 4584–4610 CrossRef CAS.
- Q. Xiong, Y. Lim, D. Li, K. Pu, L. Liang and H. Duan, Adv. Funct. Mater., 2020, 30, 1903896 CrossRef CAS.
- R. J. Sanderson, M. A. Hering, S. F. James, M. M. C. Sun, S. O. Doronina, A. W. Siadak, P. D. Senter and A. F. Wahl, Clin. Cancer Res., 2005, 11, 843–852 CrossRef CAS.
- M. Dorywalska, P. Strop, J. A. Melton-Witt, A. Hasa-Moreno, S. E. Farias, M. Galindo Casas, K. Delaria, V. Lui, K. Poulsen, C. Loo, S. Krimm, G. Bolton, L. Moine, R. Dushin, T.-T. Tran, S.-H. Liu, M. Rickert, D. Foletti, D. L. Shelton, J. Pons and A. Rajpal, Bioconjugate Chem., 2015, 26, 650–659 CrossRef CAS PubMed.
- B. Gorovits and C. Krinos-Fiorotti, Cancer Immunol. Immunother., 2013, 62, 217–223 CrossRef CAS PubMed.
- R. Cheng, F. Meng, C. Deng, H.-A. Klok and Z. Zhong, Biomaterials, 2013, 34, 3647–3657 CrossRef CAS.
- W. Zhen, S. An, S. Wang, W. Hu, Y. Li, X. Jiang and J. Li, Adv. Mater., 2021, 33, 2101572 CrossRef CAS.
- L. Zhu and V. P. Torchilin, Integr. Biol., 2013, 5, 96–107 CrossRef CAS.
- S. Bai, Y. Zhang, D. Li, X. Shi, G. Lin and G. Liu, Nano Today, 2021, 36, 101038 CrossRef CAS.
- A. M. Pethe and K. S. Yadav, Artif. Cells, Nanomed., Biotechnol., 2019, 47, 395–405 CrossRef CAS PubMed.
- R. Cheng, F. Meng, S. Ma, H. Xu, H. Liu, X. Jing and Z. Zhong, J. Mater. Chem., 2011, 21, 19013–19020 RSC.
- S. Chen, F. Jiang, Z. Cao, G. Wang and Z.-M. Dang, Chem. Commun., 2015, 51, 12633–12636 RSC.
- M. S. Shim and Y. J. Kwon, Adv. Drug Delivery Rev., 2012, 64, 1046–1059 CrossRef CAS.
- Y. Zhang, J. Li and K. Pu, Biomaterials, 2022, 291, 121906 CrossRef CAS.
- B. Yu, N. Song, H. Hu, G. Chen, Y. Shen and H. Cong, Biochim. Biophys. Acta, Rev. Cancer, 2018, 106, 3203–3210 CAS.
- Y. Zhu, T. Ikoma, N. Hanagata and S. Kaskel, Small, 2010, 6, 471–478 CrossRef CAS.
- S. Iranpour, A. R. Bahrami, S. Nekooei, A. Sh. Saljooghi and M. M. Matin, J. Nanobiotechnol., 2021, 19, 314 CrossRef CAS.
- D. Shao, Z. Wang, W. Dong, X. Zhang, X. Zheng, X. Xiao, Y. Wang, X. Zhao, M. Zhang, J. Li, Q. Huo and L. Chen, Chem. Biol. Drug Des., 2015, 86, 1548–1553 CrossRef CAS.
- L. Zhao, Y. Liu, R. Xing and X. Yan, Angew. Chem., 2020, 132, 3821–3829 CrossRef.
- A. Wiehe, J. M. O'Brien and M. O. Senge, Photochem. Photobiol. Sci., 2019, 18, 2565–2612 CrossRef CAS PubMed.
- B. C. Wilson and R. A. Weersink, Photochem. Photobiol., 2020, 96, 219–231 CrossRef CAS.
- X. Deng, Z. Shao and Y. Zhao, Adv. Sci., 2021, 8, 2002504 CrossRef CAS PubMed.
- C. Donohoe, M. O. Senge, L. G. Arnaut and L. C. Gomes-da-Silva, Biochim. Biophys. Acta, Rev. Cancer, 2019, 1872, 188308 CrossRef CAS PubMed.
- J. Zhao, L. Duan, A. Wang, J. Fei and J. Li, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol., 2020, 12, e1583 CrossRef CAS.
- B. Du, S. Jia, Q. Wang, X. Ding, Y. Liu, H. Yao and J. Zhou, Biomacromolecules, 2018, 19, 1026–1036 CrossRef CAS PubMed.
- S. Rajput, R. Malviya and S. B. Sridhar, Nano-Struct. Nano-Objects, 2024, 40, 101405 CrossRef CAS.
- D. Jaque, L. Martínez Maestro, B. del Rosal, P. Haro-Gonzalez, A. Benayas, J. L. Plaza, E. Martín Rodríguez and J. García Solé, Nanoscale, 2014, 6, 9494–9530 RSC.
- K. F. Chu and D. E. Dupuy, Nat. Rev. Cancer, 2014, 14, 199–208 CrossRef CAS.
- H. Kim, S. Beack, S. Han, M. Shin, T. Lee, Y. Park, K. S. Kim, A. K. Yetisen, S. H. Yun, W. Kwon and S. K. Hahn, Adv. Mater., 2018, 30, 1701460 CrossRef.
- S. S. Lucky, K. C. Soo and Y. Zhang, Chem. Rev., 2015, 115, 1990–2042 CrossRef CAS.
- M. S. Baptista, J. Cadet, P. Di Mascio, A. A. Ghogare, A. Greer, M. R. Hamblin, C. Lorente, S. C. Nunez, M. S. Ribeiro, A. H. Thomas, M. Vignoni and T. M. Yoshimura, Photochem. Photobiol., 2017, 93, 912–919 CrossRef CAS.
- M. Aioub and M. A. El-Sayed, J. Am. Chem. Soc., 2016, 138, 1258–1264 CrossRef CAS PubMed.
- A. Amirshaghaghi, L. Yan, J. Miller, Y. Daniel, J. M. Stein, T. M. Busch, Z. Cheng and A. Tsourkas, Sci. Rep., 2019, 9, 2613 CrossRef.
- M. Yang, J. Deng, H. Su, S. Gu, J. Zhang, A. Zhong and F. Wu, Mater. Chem. Front., 2021, 5, 406–417 RSC.
- M. Li, H. Lin and F. Qu, Chem. Eng. J., 2020, 384, 123374 CrossRef CAS.
- A. Looft, M. Pfitzner, A. Preuß and B. Röder, Photodiagn. Photodyn. Ther., 2018, 23, 325–330 CrossRef CAS.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS.
- P. Liu, H. Zheng, Z. Yang, L. Ba, W. Zhu, L. Lin, Y. Xiong, Z. Xu and J. Ren, J. Mater. Chem. B, 2018, 6, 1688–1698 RSC.
- D. de Melo-Diogo, R. Lima-Sousa, C. G. Alves and I. J. Correia, Biomater. Sci., 2019, 7, 3534–3551 RSC.
- Z. Xie, T. Fan, J. An, W. Choi, Y. Duo, Y. Ge, B. Zhang, G. Nie, N. Xie, T. Zheng, Y. Chen, H. Zhang and J. S. Kim, Chem. Soc. Rev., 2020, 49, 8065–8087 RSC.
- D. Gao, X. Guo, X. Zhang, S. Chen, Y. Wang, T. Chen, G. Huang, Y. Gao, Z. Tian and Z. Yang, Mater. Today Bio, 2020, 5, 100035 CrossRef CAS PubMed.
- D.-K. Ji, C. Ménard-Moyon and A. Bianco, Adv. Drug Delivery Rev., 2019, 138, 211–232 CrossRef CAS.
- X. Li, J. F. Lovell, J. Yoon and X. Chen, Nat. Rev. Clin. Oncol., 2020, 17, 657–674 CrossRef.
- C. Kong and X. Chen, Int. J. Nanomed., 2022, 17, 6427–6446 CrossRef.
- B. Ding, S. Shen, L. Wu, X. Qi, H. Ni and Y. Ge, Chem. Lett., 2015, 44, 858–860 CrossRef CAS.
- J. Hu, H. Wang, F. Dong and Z. Wu, Appl. Catal., B, 2017, 204, 584–592 CrossRef CAS.
- I. Monaco, F. Arena, S. Biffi, E. Locatelli, B. Bortot, F. La Cava, G. M. Marini, G. M. Severini, E. Terreno and M. Comes Franchini, Bioconjugate Chem., 2017, 28, 1382–1390 CrossRef CAS PubMed.
- F. Wang, L. Xu, Y. Zhang, V. A. Petrenko and A. Liu, J. Mater. Chem. B, 2017, 5, 8209–8218 RSC.
- A. Das, K. Panigrahi, S. Saha, B. K. Das, N. S. Das, S. Sarkar, R. Chatterjee and K. K. Chattopadhyay, Mater. Res. Bull., 2020, 131, 110966 CrossRef CAS.
- R. Jin, Z. Qiu, W. Cheng and X. Jin, Chem. Phys. Lett., 2020, 755, 137747 CrossRef CAS.
- S. Khammar, N. Bahramifar and H. Younesi, Mater. Chem. Phys., 2020, 252, 123195 CrossRef CAS.
- M. Ma, W. Li, Z. Tong, W. Huang, R. Wang, P. Lyu, Y. Ma, G. Wu, Q. Yan, P. Li and X. Yao, J. Alloys Compd., 2020, 843, 155199 CrossRef CAS.
- A. Radchanka, A. Iodchik, T. Terpinskaya, T. Balashevich, T. Yanchanka, A. Palukoshka, S. Sizova, V. Oleinikov, A. Feofanov and M. Artemyev, Nanotechnology, 2020, 31, 435102 CrossRef CAS PubMed.
- S. Şimşek, A. A. Şüküroğlu, D. Yetkin, B. Özbek, D. Battal and R. Genç, Sci. Rep., 2020, 10, 13880 CrossRef.
- C. Liu, X. Wang, S. Lee, L. D. Pfefferle and G. L. Haller, Microporous Mesoporous Mater., 2012, 147, 242–251 CrossRef.
- S. Khoee and N. Abedini, Polymer, 2014, 55, 5635–5647 CrossRef CAS.
- A. Shakeri-Zadeh, M.-B. Shiran, S. Khoee, A. M. Sharifi, H. Ghaznavi and S. Khoei, J. Biomater. Appl., 2014, 29, 548–556 CrossRef.
- S. Khoee and M. Kardani, Eur. Polym. J., 2014, 58, 180–190 CrossRef CAS.
- N. Shevchenko, V. Zaitsev and A. Walcarius, Environ. Sci. Technol., 2008, 42, 6922–6928 CrossRef CAS PubMed.
- A. Vinu, K. Z. Hossain and K. Ariga, J. Nanosci. Nanotechnol., 2005, 5, 347–371 CrossRef CAS.
- D. Niu, Z. Liu, Y. Li, X. Luo, J. Zhang, J. Gong and J. Shi, Adv. Mater., 2014, 26, 4947–4953 CrossRef CAS.
- S. B. Hartono, N. T. Phuoc, M. Yu, Z. Jia, M. J. Monteiro, S. Qiao and C. Yu, J. Mater. Chem. B, 2014, 2, 718–726 RSC.
- S. Khoee and A. Kavand, Eur. J. Med. Chem., 2014, 73, 18–29 CrossRef CAS PubMed.
- S. Khoee and K. Hemati, Polymer, 2013, 54, 5574–5585 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |
