Open Access Article
Open Access ArticleBacterial responses to Ephedra aphylla stem extract and green-synthesized Ag-TiO2 and Ag-SeO2 core/shell nanocomposites: unveiling antimicrobial and antioxidant properties†
Mahmood Razzaq Mashar Askar
Department of Special Education Sciences, Imam Alkadhim University College, Iraq. E-mail: Lecdhi118@iku.edu.iq; mahmoodaskar78@gmail.com
First published on 24th April 2025
Abstract
This study reports an efficient and green protocol for the green synthesis of Ag-TiO2 and Ag-SeO2 nanocomposites using the extracted stems of Ephedra aphylla. Results of spectroscopic and analytical analyses confirmed the successful synthesis, stability, and crystalline nature of the nanomaterials. The phytochemical profile and antioxidant and antimicrobial activities of the E. aphylla extract and the nanocomposites were also studied. E. aphylla extract and both the nanomaterials exhibited significant levels of active phytochemical compounds. These compounds contributed to their potent antioxidant activity, with E. aphylla extract and Ag-TiO2 NC demonstrating the highest antioxidant activity. Besides, Ag-SeO2 NC displayed remarkable antibacterial properties against different pathogenic bacteria with 31.0 ± 1.27 mm against K. pneumonia, 31.0 ± 1.72 mm against S. aureus, and 44.0 ± 1.09 mm against B. subtilis, and antifungal properties against Candida glabrata and Aspergillus niger. The enhanced antimicrobial activity of Ag-SeO2 NC can be attributed to the synergistic effects of silver and selenium nanoparticles, which can disrupt cell membranes, induce oxidative stress, and interfere with essential cellular processes. The minimum inhibitory concentration values of Ag-SeO2 NC against S. aureus and K. pneumoniae were found to be 0.2956 mg mL−1 and 4.73 mg mL−1, respectively. The mechanism of action of Ag-SeO2 NC against both fungal strains was investigated using FTIR and HR-TEM analyses.
1. Introduction
Antibiotic resistance is increasing worldwide along with high mortality caused by new bacteria strains, which drives the necessity to develop novel antimicrobial agents.1 Traditional medicines use plant extracts containing bioactive compounds because they have been used to treat infections for several years according to scientific research.2,3 The remarkable antimicrobial activities come from a combination of alkaloids, flavonoids, terpenoids and other related compounds.Traditional medicines have employed the plant species Ephedra aphylla as a medicinal herb for various health purposes for several centuries.4 Ephedrine and pseudoephedrine along with quercetin, rutin, gallic acid and caffeic acid are bioactive compounds that make this plant a valuable source.5–7 The increasing importance of E. aphylla as an herbal pharmaceutical plant derives from its utility in providing natural bioactive substances that demonstrate multiple pharmaceutical effects.8 Recent studies focus on the development of metal and metal oxide nanoparticles through eco-friendly plant extract methods.9
Nanomaterials present distinct physico-chemical behavior that scientists find useful in antimicrobial studies.10 Many scientists prefer metal and metal oxide nanoparticles as antimicrobial agents owing to their excellent reactivity levels, high surface area properties and proven action against microbes.11 As these materials exhibit adverse effects on the environment and toxicity issues, scientists have developed plant extract-based natural synthesis procedures as an alternative solution.12 When nanoparticles release silver ions, they show lethal effects against bacteria by disrupting several bacterial processes.13–15 When exposed to the visible light spectrum, the photocatalytic activity of TiO2 is initiated to produce ROS, which include hydroxyl and oxygen radicals.16 ROS particles created via visible light exposure degrade the bacterial cell wall and membrane until the microorganisms become inactive.17 Nanoparticles have the capability to penetrate bacterial cell walls and establish direct interactions, resulting in cell damage that leads to bacterial death.18
Research conducted by multiple investigators in recent times has proven that Ag-TiO2 and Ag-SeO2 nanocomposites show strong antibacterial effects against various bacterial strains.13–15 Research conducted by scientists established that nanomaterials incorporating Ag-TiO2 and Ag-SeO2 demonstrated powerful antibacterial effects on Gram-positive and Gram-negative bacteria along with E. coli, S. aureus, and P. aeruginosa19 while showing effective antifungal properties against fungal microorganisms.20
Different industries can utilize Ag-TiO2 and Ag-SeO2 nanocomposites owing to their wide array of potential applications. Nanomaterials show promise as water decontamination agents that eliminate bacterial and viral microorganisms.21,22 Nanocomposite particles improve food packaging materials while maintaining product stability and preventing the transmission of contagious diseases in foods.23 These nanocomposites enable medical professionals to create antimicrobial surface coatings for medical tools that defend against patient infections.24 Ag-TiO2 NC integration with textiles allows fabric development to block bacterial growth, including odor-causing bacteria.25 The assessment of the long-term safety and environmental sustainability of Ag-TiO2 NCs requires further research. Sustainable and eco-friendly nanocomposite production through green synthesis provides a dual protection mechanism against antibiotic resistance while improving human wellness.
AgNPs (silver nanoparticles), TiO2 NPs (titanium dioxide nanoparticles) and SeNPs (selenium nanoparticles) demonstrate antimicrobial properties because they damage membranes and produce reactive oxygen species (ROS), which disrupt metabolic activities. Bacterial death results from AgNP activity owing to Ag+ ion production, which breaks both the membrane structure and protein formation within pathogens.26 Research has shown that AgNPs can be used for wastewater treatment because they successfully prevent microorganisms from multiplying.27 TiO2 NPs release ROS under both UV illumination conditions that destroy microbial cells, thus making them effective agents against plant pathogens, including Alternaria alternata.28 The combination of Ag or Se with TiO2 surfaces serves as an effective method for bacterial inhibition and enhances osteoblastic cell growth, which makes these materials suitable for biomedical device applications.29 SeNPs, which are less toxic than AgNPs, are metabolic inhibitors that can inhibit bacterial growth, biofilm formation, and foodborne bacteria.30,31 Their potential use as antimicrobial agents in chitosan solutions has been demonstrated in recent studies, suggesting dental and wound healing applications.32 The synergistic action of employing Ag and Se nanoparticles combined on Ti surfaces has been documented to enhance antimicrobial action. These observations indicate that nanoparticle blends might strengthen microbial defense mechanisms and could be exploited in medicine, agriculture, and food security applications.28,29
Ag-TiO2 NC proved effective because TiO2 demonstrates known photocatalytic capabilities to generate reactive oxygen species (ROS) under light, which enhances antimicrobial sterilization.33 The combination of Ag NPs with TiO2 produces enhanced antibacterial and antioxidant properties, which makes the material suitable for various biomedical applications, water purification systems, and antimicrobial packaging needs.34 Ag-SeO2 NC shows promise because selenium plays a vital biological role in cell metabolic processes and demonstrates antimicrobial behavior at lower toxic levels than other heavy metals.35 Ag-SeO2 NC was reported to verify enhanced microbial suppression through membrane-bursting activity along with the production of oxidative stress during bacterial dysfunction and cellular function blockade.36 The pharmaceutical and biomedical industries require such materials to meet their requirements of both antimicrobial breadth and biological compatibility. Ephedra aphylla extract enabled the first-time green synthesis of Ag-TiO2 and Ag-SeO2 NCs by enabling both stability improvement and bioactivity enhancement of the nanocomposites through their phytochemical properties.
The main goal of this work is to establish an efficient and green protocol for the preparation of Ag-TiO2 and Ag-SeO2 nanocomposites using the stem extract of E. aphylla. This novel green synthesis method also eliminates the further use of dangerous chemicals and toxic solvents, which lowers the environmental drawbacks of nanomaterial production and processing. Furthermore, this study employs different techniques to determine the properties of the synthesized nanocomposites, assess their antimicrobial and antioxidant properties, and determine the mode of action and possible uses of nanocomposites in diverse areas of application. The improved antimicrobial activity of the nanomaterials produced can be attributed to the synergistic interaction between silver and selenium NPs and their interaction with bacterial cell membranes and different intracellular targets that lead to cell death.
2. Materials and methods
2.1. Reagents
The reagents used in the study were of analytical grade (for details, see the ESI file, Section S1†).2.2. Instruments
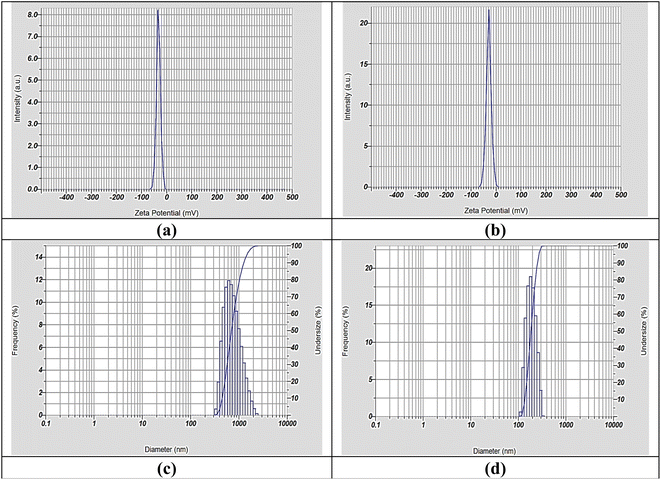
UV-visible spectroscopy was employed using a Spectrophotometer UV2 (Uni-cam UV-vis, USA). Fourier Transform Infrared (FTIR) spectroscopy (Thermo-Fisher Nicolet IS10, USA) was used to identify functional groups with a resolution of 4 cm−1 across a wavenumber range of 500–4000 cm−1. The surface charge of the nanoparticles was determined using zeta potential analysis (HORIBA SZ-100). High-resolution-transmission electron microscopy (HR-TEM) analysis (Thermoscientific, Talos F200i) provided insights into the size, shape, aggregation behavior, and crystallinity of nanoparticles. Scanning electron microscopy (SEM) equipped with energy-dispersive X-ray (EDX) spectroscopy was employed to investigate the surface features, morphology, and elemental composition. This analysis was performed using a Czech FEI-type SEM instrument at an accelerating voltage of 25 kV. X-ray diffraction (XRD) analysis using a Panalytical Philips instrument helped determine the crystal structure of the nanomaterials. A spectrophotometer (Spekol 11, Analytik Jena AG, Jena, Germany) was employed to assess the antioxidant and phytochemical profiles.2.3. Preparation of E. aphylla extract
E. aphylla stems were collected from further banks of the Tigris and Euphrates rivers. The collected stems were washed with cold water, dried with the help of paper towels, and left to dry for a few hours. Alhammadi (2012) used 20 grams of dried plant material to prepare 100 mL of 70% ethanol, which was macerated at 30 °C for 24 hours with a view to making bioactive compounds more soluble. The mass was subsequently filtered through Whatman no 1 filter paper and further subjected to centrifugation for 15 minutes at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for further pellet removal. The purified extract was used immediately for nanocomposite solutions and other analysis and application-related studies.8,37
000 rpm for further pellet removal. The purified extract was used immediately for nanocomposite solutions and other analysis and application-related studies.8,37
2.4. Green synthesis of nanocomposites
A plant-mediated green synthesis technique38–40 was employed to prepare nanocomposites using the ethanol extract of E. aphylla. Silver-selenium dioxide nanoparticles were prepared using (25 mL, 1 mM) silver nitrate solution and the prepared extract. A solution container included selenium dioxide (50 mg in 10 mL ethanol) besides titanium dioxide (50 mg in 10 mL ethanol). The solutions underwent sonication treatment at 30 °C for 15 minutes. The researchers mixed 1 mM silver nanoparticle solution (25 mL) with sonicated titanium dioxide solution under 30 °C continuous stirring for 15 minutes to prepare a silver-titanium dioxide nanocomposite. A combined mixing of silver nanoparticle solution with appropriate selenium dioxide solution continued while being stirred for 15 minutes at a temperature of 30 °C. Both Ag-TiO2 and Ag-SeO2 NC solutions underwent heat treatment at 60 °C while maintaining continuous stirring for 5 hours before subjecting them to 2 hours of sonication at 60 °C. A spectrophotometer helped confirm the formation of nanoparticles by observing the color transformations. Ag-TiO2 NC solution underwent dark yellow to dark green color transformation, while Ag-SeO2 NC progressed from pale yellow to dark brown coloration. The extraction of nanoparticles took place through centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 20 minutes using each solution. A washing routine involving distilled water, followed by ethanol, purified the solid nanoparticles from their contaminants. The dry process was finished by placing the solid nanoparticles in an oven heated to 60 °C. The researchers preserved part of the solution at −70 °C in dark storage to prevent the degradation of solution constituents.38
000 rpm for 20 minutes using each solution. A washing routine involving distilled water, followed by ethanol, purified the solid nanoparticles from their contaminants. The dry process was finished by placing the solid nanoparticles in an oven heated to 60 °C. The researchers preserved part of the solution at −70 °C in dark storage to prevent the degradation of solution constituents.38
2.5. Phytochemical analysis
Analysis of the plant extract and nanocomposites involved the identification of their phytochemical profiles together with antioxidant activity assessment. The analysis of phenolic content used the Folin–Ciocalteu (F–C) assay41 and flavonoid content evaluation, followed by the aluminum chloride assay.42 The tannin content in the samples was evaluated via a vanillin–hydrochloride reaction method.43 The research data analysis utilized a gallic acid standard curve relationship with y = 0.0062x and R2 = 0.987 and obtained results as mg g−1 of dry sample. The evaluation of the flavonoid and tannin content in extracts used catechin as a standard to develop the calibration curve (y = 0.0028x, R2 = 0.988) as well as tannic acid (y = 0.0009x, R2 = 0.955). A supplemental file contains complete procedures for conducting phytochemical tests.2.6. Biological assessment
| Inhibition (%) = [(A“control” − A“sample”)/A“control”] × 100, |
2.6.2.1. Antibacterial activity assessment. The antimicrobial activity of the samples was evaluated using an agar well diffusion assay. Distilled water served as a negative control, while gentamicin, a standard antibiotic, was used as a positive control. Mueller–Hinton agar plates were prepared according to standard protocols.46 The bacterial inoculum was evenly spread across the agar plate using a sterile swab to create a bacterial lawn. A sterile cork borer was used to create a well in the center of the agar plate, approximately 6 mm in diameter and 5 mm deep. Each sample solution (100 μL) was carefully pipetted into the well. The agar plates were incubated at 37 °C for 24 hours to allow for bacterial growth and inhibition by the samples. The diameter of the clear zone of inhibition surrounding the well was measured, indicating the antimicrobial activity of the sample. Larger zones of inhibition signify greater antimicrobial potency.
2.6.2.2. Broth microdilution assay. To determine the MIC of the sample against S. aureus and K. pneumoniae, serial dilutions were prepared in nutrient broth medium at concentrations ranging from 16.58 to 0.0324 mg mL−1 for Ag-TiO2 NC and from 9.46 to 0.0185 mg mL−1 for Ag-SeO2 NC. A control containing only inoculated broth was incubated for 24 hours at 37 °C. The MIC was identified as the lowest concentration at which no visible bacterial growth was observed in the tubes. The turbidity of the tubes was visually assessed before and after incubation to confirm the MIC value, and the optical density (OD) was measured at 600 nm for further verification.47
2.6.2.3. Antifungal activity assessment. To assess the antifungal activity of the plant extract and nanocomposites against Candida glabrata and Aspergillus niger, a disc diffusion assay was employed.48 Sterile filter paper discs were impregnated with 10 μL of the plant extract, nanocomposite solution, standard antifungal drug (fluconazole), or sterile distilled water (negative control). The researchers set the fungal culture on agar plates before adding the prepared discs. After placing the plates into an incubator at 37 °C, they remained there for 24–48 hours. The antifungal activity determination involved measuring the clear zone diameter that formed around each disc following the incubation period. A larger zone of inhibition shows an increased antifungal capacity of the applied substances.
2.7. Statistical analysis
The results are presented as mean values ± standard deviation (SD). All experimental phytochemical analyses and antibacterial assessments of the Ephedra aphylla extract and the synthesized nanocomposites were conducted in triplicate. The Statistical Package for Social Sciences (SPSS, version 21) provided an evaluation of the mean values obtained from the samples. A one-way analysis of variance (ANOVA) provided statistical analyses, and differences at or below p ≤ 0.05 were considered significant.3. Results and discussion
3.1. Plausible mechanism for the formation of nanocomposites
The chemical reaction process for green nanocomposite synthesis uses E. aphylla extract to perform various stages of the synthetic procedure. An explanation of the Ag-TiO2 and Ag-SeO2 NC green synthesis with E. aphylla extract follows in this section, as displayed in Fig. 1. E. aphylla goes through two processes to extract ethanol-based bioactive compounds: polyphenols and flavonoids together with tannins. Silver nitrate is then dissociated in the solution to generate silver ions (Ag+) and nitrate ions (NO3−). The phytochemical compounds donate electrons to the Ag+ ions, resulting in the bioreduction, nucleation, and growth of silver nanoparticles.49 Subsequently, the silver nanoparticles interact with TiO2 or SeO2 nanoparticles, resulting in the formation of Ag-TiO2 or Ag-SeO2 nanocomposites.13 The interactions of silver nanoparticles with metal oxides, such as TiO2 or SeO2, can cause several changes in the characteristics of the final solution. First, it can alter the position of the SPR of the silver nanoparticles, meaning that it alters the absorption and scattering properties of light as well as the color of the solution.50 Second, charge transfer processes are possible at the interface between silver nanoparticles and metal oxide, affecting the electronic structure of the nanoparticles and their plasmonic response.51 Finally, the adsorption process of Ag nanoparticles on the surface of the formed metal oxide (TiO2 or SeO2) nanoparticles alters the optical characteristics of the solution, leading to the formation of Ag-TiO2 or Ag-SeO2 nanocomposites.52The synthesis of nanoparticles through plant extracts depends on bioactive molecules, such as phenolics, flavonoids, and tannins, along with proteins, terpenoids, and polysaccharides that function as reducing and stabilizing agents. Metal ion reduction occurs through phenolics and flavonoids, resulting in smaller nanoparticles, but tannins provide stability improvements.53 Enzymes and proteins accelerate reduction while enhancing crystallinity, but terpenoids along with polysaccharides inhibit agglomeration and adjust surface charge levels.54 The concentration of biochemical compounds influences nanoparticle size together with their shape characteristics and stability because flavonoid-containing extracts create spherical shapes, while tannin extract solutions develop non-spherical structures.55 The effectiveness of reduction agents leads to higher crystallinity, and surface charge control stems from capping agents, which stabilize dispersion.56 Laboratory control of nanoparticle fabrication methods becomes possible through a comprehensive understanding of the synthesis mechanisms, which leads to numerous practical applications in medicine, catalysis, and environmental remediation.
In addition, the stability and antimicrobial properties of nanocomposites strongly respond to environmental conditions, including pH and temperature measurements. When pH values change, they affect nanoparticle dispersion factors and surface charge distribution, which determine the rate of ion release and aggregation patterns.57 Silver-based nanocomposites leverage acidic solutions to increase Ag+ ion release, which drives up their antimicrobial effects, but alkaline conditions result in reduced ion release, which lowers their effectiveness.58 Temperature functions as an essential determinant that influences the synthesis processes of both nanocomposites while affecting their stability throughout their existence. Synthesis of proper nanoparticles occurs when heating is controlled at 60 °C for five continuous hours. The prolonged existence of high-temperature conditions causes nanoparticle aggregation along with oxidation, which pulls down their stability and biological effectiveness.59 The nanocomposites remain stable when they are preserved at −70 °C under dark conditions, which protects their structure together with their antimicrobial properties. The long-term usage and effectiveness of nanocomposites in biomedical and environmental areas require exact pH and temperature management.
3.2. Characterization of nanocomposites
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and aromatic rings with heteroatomic lone pairs (e.g., nitrogen or oxygen).60 The color manifestation occurs from transitions observed in the visible spectrum owing to chemical processes. The specific absorbance of Ag-TiO2 nanomaterials appeared at 562 nm owing to their silver nanoparticle-specific surface plasmon resonance characteristics.61 The SPR band red shift from silver bulk to silver nanoparticles demonstrates the existence of large-sized silver nanoparticles.62
O) and aromatic rings with heteroatomic lone pairs (e.g., nitrogen or oxygen).60 The color manifestation occurs from transitions observed in the visible spectrum owing to chemical processes. The specific absorbance of Ag-TiO2 nanomaterials appeared at 562 nm owing to their silver nanoparticle-specific surface plasmon resonance characteristics.61 The SPR band red shift from silver bulk to silver nanoparticles demonstrates the existence of large-sized silver nanoparticles.62
The Ag-SeO2 nanomaterial showed its absorbance peak at 480 nm, and this observation could be explained by the SPR of the silver nanoparticles (Fig. 2a). The wavelength position shifts toward the red region of the silver spectrum along with its observation, indicating that moderate-sized silver nanoparticles can be formed. The placement of SPR bands in nanoparticles depends on factors that incorporate their physical dimensions together with their shapes and dielectric composition.61 The optical properties of the composite materials could be affected by TiO2 and SeO2 when combined with the materials, possibly influencing light scattering along with electronic transitions. The UV-visible spectroscopy data show the verification of nano-material synthesis when silver-based nanomaterials show different detectable optical characteristics. The absorbance peaks demonstrate that silver nanoparticles exist in the sample with TiO2 and SeO2 semiconductor components.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching bond (1709 cm−1) is accompanied by the aromatic vinyl C
O stretching bond (1709 cm−1) is accompanied by the aromatic vinyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretch (1601 cm−1).
C stretch (1601 cm−1).The peaks found at 1078, 1044, 897, 868, 762, and 718 cm−1 show C–O stretching vibrations, which indicate the presence of compounds, including alcohols and ethers together with esters. Several characteristic absorption bands appeared in the FTIR spectrum of the Ag-TiO2 NC (Fig. 2b). The C–H stretching vibrations appear at 2913 and 2845 cm−1, demonstrating the persisting organic materials introduced by the plant extract in the synthesis process. The Ti–O–Ti stretching bonds appear at 1105 cm−1 in the spectrum, which indicates that the TiO2 phase exists within the sample structure.63 Vibrations at 414 and 378 cm−1 correspond to metal–oxygen bonds, which most probably involve Ti–O and Ag–O bonding patterns, respectively.64 The main bands in the spectrum indicate the successful creation of Ag-TiO2 NC by combining TiO2 and silver nanoparticles. The synthesis process of nanomaterials was aided by plant extract residual compounds that simultaneously played the roles of reducers and surface stabilizers.65
The FTIR spectrum of Ag-SeO2 NC displayed various distinct peak bands. The FTIR spectrum shows two peaks at 2914 and 2846 cm−1 from the C–H stretching vibrations because the spectrum contains residual organic compounds from the plant extract. The FTIR spectrum exhibits Se–O and Ag–O bond vibrations through the various bands present at 1731, 1604, 1507, 1442, 1104, 1034, 822, 762, 709, 665, 490, 430, and 378 cm−1.66 Ag-SeO2 NC formation was successful because of the absorption bands observed in the spectrum. Ag-TiO2 NC produced a significantly weakened biomolecule peak intensity in its spectral analysis. The biomolecules from the extract participated during synthesis by reducing metal ions and serving to stabilize them. New peaks indicating Ti–O–Ti bond formation and metal–oxygen bond formation identify the production of Ag-TiO2 NC. An intensity decrease in the biomolecular peaks appears in the spectrum of Ag-SeO2 NC. New peaks indicating Se–O together with Ag–O bonds appear in the spectrum to verify the synthesis of Ag-SeO2 NC.67 The FTIR examination provides compelling proof that biomolecules from E. aphylla extract take part in the green nanomaterial formation method of Ag-TiO2 and Ag-SeO2. Analysis through FTIR showed that new peaks linked to metal–oxygen bonds in combination with vanished functional groups proved that the nanocomposite synthesis was successful.
The variation in the dispersion behavior of the two types of nanomaterials can be explained in terms of the surface chemistry of the nanoparticles, the type of capping agents, and the conditions of synthesis.74 The nanoparticle aggregation together with stability exhibits signs of change owing to temperature effects, pH conditions, and exposure to light. Future investigations should focus on studying the storage behavior to understand completely the long-term stability performance of these nanocomposites in different environmental settings.
Generally, the HR-TEM image of Ag-SeO2 NC (Fig. 4b) indicates that the samples display a non-uniform nature of mostly spherical and irregularly shaped nanoparticles. The size range of the particles is large, and some of them are aggregated. The observed presence of spherical and irregularly shaped particles confirms the synthesis of a composite material of silver and selenium dioxide nanoparticles. The HR-TEM images give a clear indication of well-dispersed Ag-TiO2 and Ag-SeO2 nanocomposites with a relatively narrow size distribution of the nanocomposites. The observed nanoparticles are spherical and have a relatively smooth surface.13 The size distribution of the observed particles represents a relatively narrow size distribution, which improves the properties of the synthesized nanoparticles, such as catalytic properties and stability. This has been a result of the appropriate choice of capping agents during the synthesis process for the nanoparticles in question. The capping agents can also form a layer at the nanoparticle surface and thus reduce agglomeration and enhance stability.15
For Ag-TiO2 NC (Fig. 5a), the spectrum revealed the presence of carbon (16.13 wt%, 20.43 at%), oxygen (43.64 wt%, 52.53 at%), titanium (15.02 wt%, 16.01 at%), and silver (25.21 wt%, 12.03 at%). Similarly, for Ag-SeO2 NC (Fig. 5b), the spectrum showed the presence of carbon (33.63 wt%, 45.29 at%), oxygen (28.50 wt%, 34.02 at%), selenium (12.63 wt%, 11.33 at%), and silver (25.24 wt%, 14.36 at%). The presence of these elements in the expected ratios confirms the successful incorporation of silver nanoparticles into the TiO2 and SeO2 matrices. The presence of carbon in both samples can be attributed to the use of organic capping agents during the synthesis process.75 The capping agents help to stabilize the nanoparticles and prevent agglomeration.76 However, the presence of carbon can also affect the properties of the nanomaterials, such as their surface area and catalytic activity.77
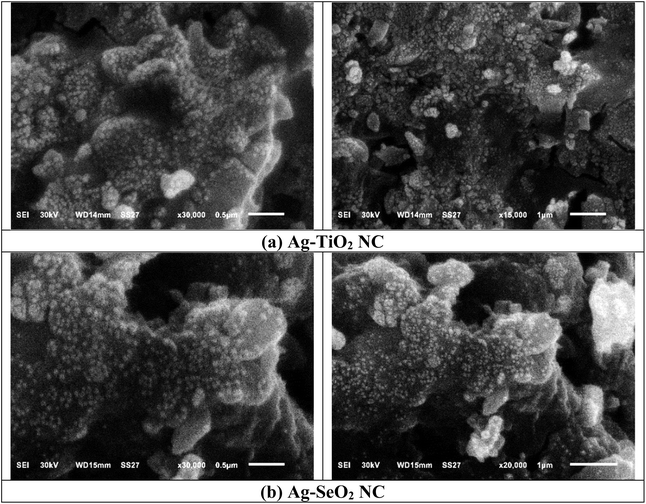
The nanoparticles have an inhomogeneous shape and porous structure that allows for increasing the material's surface and improves the catalytic or adsorbent properties. It is possible to form a composite nanomaterial with spherical and irregular-shaped particles, such as a combination of TiO2 and silver nanoparticles. Aggregation is encountered often in nanoparticle synthesis and has a significant impact on the properties of the resulting materials, including the mass surface area, porosity, and catalytic activity.78
The SEM of Ag-SeO2 NC (Fig. 6b) shows that the sample has irregular and rough surface morphology and agglomerated particles with sizeable variations in the size and shape of the NCs. The particles show a high level of irregularity in the surface structure, which may cause increased surface area and corresponding improvements in the catalytic or adsorptive material. However, owing to the porosity of the nanoparticles, there may be other active sites for interactions with target molecules or microorganisms.79
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) using typical values of K = 0.9 and X-ray wavelength λ = 1.5406 Å for Cu Kα radiation, where β represents fractional maximum peak width in radians and θ represents Bragg angle. The crystallite size measurement of the anatase TiO2 phase relies on applying the Scherrer equation to XRD-generated data. The material achieves enhanced reactivity and increased surface area because nanocrystalline TiO2 formation produces small crystals.80
θ) using typical values of K = 0.9 and X-ray wavelength λ = 1.5406 Å for Cu Kα radiation, where β represents fractional maximum peak width in radians and θ represents Bragg angle. The crystallite size measurement of the anatase TiO2 phase relies on applying the Scherrer equation to XRD-generated data. The material achieves enhanced reactivity and increased surface area because nanocrystalline TiO2 formation produces small crystals.80
Scientists used XRD analysis to study the synthesized Ag-SeO2 NCs illustrated in Fig. 7b and Table S5† for phase identification. The XRD pattern exhibited peaks that corresponded to the crystalline structures of silver selenide (Ag2Se) with JCPDS card no. 01-087-0719 and selenium dioxide (SeO2) with JCPDS card no. 01-085-0567. The successful production of Ag-SeO2 NC material becomes evident through the detection of the identified phases in the synthesized structure. The prominent peaks at 32.06°, 38.37°, 44.67°, 53.70°, 64.83°, and 77.63° 2θ correspond to the characteristic diffraction peaks of silver selenide.81 The additional peaks at 28.11°, 36.95°, 46.53°, and 63.43° 2θ can be attributed to the presence of selenium dioxide. The XRD analysis confirmed the successful synthesis of both Ag-TiO2 and Ag-SeO2 nanomaterials. The diffraction patterns exhibited characteristic peaks corresponding to the respective crystalline phases, indicating the formation of well-defined nanostructures.
3.3. Phytochemical analysis
The phytochemical analysis (Fig. 8 and Table S7†) revealed that E. aphylla extract and Ag-TiO2 NC are rich sources of phenolic compounds, flavonoids, and tannins. For instance, E. aphylla extract contained 193.28 ± 1.73 mg gallic acid equivalent/g dry weight of phenolic compounds, 76.55 ± 1.48 mg catechin equivalent/g dry weight of flavonoids, and 123.8 ± 1.77 mg tannic acid equivalent/g dry weight of tannins. The phenolic compounds found in Ag-TiO2 NC were 129.53 ± 1.09 gallic acid/g of dry weight, flavonoids were 66.19 ± 1.18 catechin/g dry weight and tannins were 90.2 ± 1.86 tannic acid/g dry weight. These compounds are known to have very high antioxidant activity. They can mop up free radicals, thus preventing cell injury from oxidative processes. Higher concentrations of these compounds in the E. aphylla extract and Ag-TiO2 NC are attributed to their antioxidant effects. Ag-SeO2 NC, although it contains comparatively lower concentrations of these bioactive compounds (82.889 ± 1.51 mg GAE/g DW of phenolic compounds, 17.89 ± 1.89 mg CE/g DW of flavonoids, and 28.83 ± 1.31 mg TAE/g DW of tannins), still possesses antioxidant features owing to the presence of silver nanoparticles. The capping agents of these nanoparticles can also act as electron donors and free radical scavengers, which may also make them antioxidants. These findings agree with earlier findings,13,15,38,39 which established that phytochemical compounds are involved in the formation of nanoparticles and are thus depleted in the final nanocomposite.3.4. Antioxidant activity
The antioxidant activity of the E. aphylla extract and the nanomaterials was assessed by the DPPH assay using ascorbic acid as a standard. The results, as shown in Fig. 9a and Table S8,† verified that a lower IC50 value indicates higher antioxidant activity. Based on the results, E. aphylla extract and ascorbic acid exhibited the highest antioxidant activity, with IC50 values of 0.024 and 0.022 mg mL−1, respectively. Ag-TiO2 NC showed moderate antioxidant activity with an IC50 value of 0.044 mg mL−1. The antioxidant activity in Ag-SeO2 NC reached the lowest level when tested across all samples because they demonstrated an IC50 value of 0.092 mg mL−1. The antioxidant properties of E. aphylla extract stem from multiple bioactive elements that contain flavonoids along with phenolic acids and alkaloids. The bioactive compounds in these substances give them the ability to neutralize free radicals by accepting electrons or donating hydrogen atoms, thus stopping oxidative destruction. Owing to their silver nanoparticle structure, Ag-TiO2 and Ag-SeO2 NCs demonstrate antioxidant functionality by functioning as electron donors that eliminate free radicals. Nanoparticles achieve higher antioxidant potential by utilizing their large surface area to facilitate more contact with free radicals.The evaluation of E. aphylla extract and Ag-TiO2 NC and Ag-SeO2 NC antioxidant activity occurred through DPPH assay analysis. All the tested samples showed antioxidant activity that increased with higher concentrations, as illustrated in Fig. 9b. The DPPH scavenging activity of E. aphylla extract and ascorbic acid reached a maximum inhibition rate of over 80% when used at 0.094 and 0.06 mg mL−1, respectively. Antioxidant properties were detected in Ag-TiO2 NC and Ag-SeO2 NCs, but these materials demonstrated reduced activity compared to E. aphylla plant extract and ascorbic acid. Under the study conditions, Ag-TiO2 NC achieved a 79.25% inhibition rate at 0.13 mg mL−1 concentration while Ag-SeO2 NC demonstrated 83.66% inhibition at 0.591 mg mL−1 concentration. E. aphylla extract exhibits antioxidant properties because it contains multiple bioactive compounds, including flavonoids, phenolic acids, and alkaloids. Both nanomaterials, Ag-TiO2 and Ag-SeO2 NCs, have antioxidant properties because silver nanoparticles in them function as electron donors and radical-scavenging agents.82 The antioxidants found in nanoparticles become more effective owing to their extended surface areas, which increase free radical interactions.83
The results in this study concur with those reported by El-Zayat et al.,8 highlighting the significant contribution of metal-based nanocomposites to antioxidant activity. Al-Barri et al.84 investigated the antioxidant activity of E. aphylla and Bassia muricata extracts, confirming their high antioxidant capacity. These results were consistent with the results of this study, confirming E. aphylla extract as a strong antioxidant source. Ahmad et al.85 prepared selenium-silver nanostructures and discovered enhanced antioxidant activity. Their findings are consistent with the results in this work concerning the results of Ag-SeO2 NC. Alghonaim et al.86 documented the antioxidant activity of Se@TiO2 NPs embedded in biopolymers, which displayed high antioxidant activity. This is in concurrence with your research on Ag-TiO2 NC, focusing on the free radical scavenging activity of silver and titanium dioxide nanoparticles. In short, the results in this research uphold the significant antioxidant activity of the E. aphylla extract and the moderate activity of both nanocomposites. Nanomaterials based on metals exhibit suitable potential for antioxidant usage, while their effectiveness depends on synthesis protocols and material compositions.
E. aphylla extract shows antioxidant activity because it contains different bioactive compounds, such as flavonoids, phenolic acids and alkaloids, according to ref. 8. Oxidative damage prevention occurs when these compounds provide either electrons or hydrogen atoms to neutralize free radicals.87 Ag-TiO2 and Ag-SeO2 NCs demonstrate antioxidant activity because silver nanoparticles serve as electron donors that eliminate free radicals through their scavenging mechanism.88 Nanoparticles possess enhanced free radical contact because of their enlarged surface area, which strengthens their antioxidant capabilities.89 This study proves that E. aphylla extract and Ag-TiO2 NC show high antioxidant capabilities.
3.5. Antimicrobial activity
| Microorganisms | Inhibition zones in mm | |||
|---|---|---|---|---|
| E. aphylla extract | Ag-TiO2 NC | Ag-SeO2 NC | Gentamycin | |
| Gram-negative bacteria | ||||
| Escherichia coli (ATCC 10536) | −ve | −ve | 30.0 ± 1.20 | 17.0 ± 1.29 |
| Salmonella typhimurium (ATCC 25566) | −ve | −ve | 30.0 ± 1.88 | 14.0 ± 1.81 |
| Klebsiella pneumonia (ATCC 10031) | −ve | 15.6 ± 1.43 | 31.0 ± 1.27 | 16.0 ± 1.03 |
| Enterobacter cloacae (DMS 30054) | −ve | −ve | 11.0 ± 1.35 | 14.0 ± 1.35 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Gram-positive bacteria | ||||
| Bacillus subtilis (DMS 1088) | −ve | −ve | 44.0 ± 1.09 | 17.0 ± 1.41 |
| Bacillus cereus (EMCC number 1080) | −ve | 14 | 12.0 ± 1.16 | 21.0 ± 1.56 |
| Staphylococcus aureus (ATCC 6538) | 17.0 ± 1.58 | 15.3 ± 1.06 | 31.0 ± 1.72 | 15.0 ± 1.90 |
| Staphylococcus epidermidis (EMCC number 1353 t) | 12.0 ± 1.59 | −ve | 11.0 ± 1.34 | 17.0 ± 1.14 |
Both the plant extract and Ag-TiO2 NC were ineffective against B. subtilis. Ag-SeO2 NC displayed significant activity with a zone of 44.0 ± 1.09 mm, greatly surpassing the effectiveness of gentamicin (17.0 ± 1.41 mm). Ag-TiO2 NC exhibited moderate activity against B. cereus, showing a zone of 14 mm, while Ag-SeO2 NC presented a zone of 12.0 ± 1.16 mm, and gentamicin demonstrated better performance with a zone of 21.0 ± 1.56 mm. The plant extract showed moderate activity against S. aureus, achieving a zone of 17.0 ± 1.58 mm. Ag-TiO2 NC and Ag-SeO2 NC exhibited similar activity, with zones of 15.3 ± 1.06 mm and 31.0 ± 1.72 mm, respectively, while gentamicin revealed a zone of 15.0 ± 1.90 mm. The plant extract displayed moderate activity against S. epidermidis, yielding a zone of 12.0 ± 1.59 mm. Although Ag-TiO2 NC was ineffective, Ag-SeO2 NC showed a zone of 11.0 ± 1.34 mm. Gentamicin proved more effective with a zone of 17.0 ± 1.14 mm.
The observed antibacterial activity of Ag-SeO2 NC can be attributed to its unique nanostructure and the synergistic effect of silver and selenium nanoparticles.67 The high surface area of nanoparticles enhances their interaction with the bacterial cell wall and membrane, leading to disruption and cell death.90–92 Silver nanoparticles are known to possess strong antimicrobial properties through their interaction with thiol groups in proteins and phospholipids, leading to membrane damage and cell death.93,94 Selenium dioxide can induce oxidative stress in cells and interfere with essential cellular processes.95 The combination of silver and selenium nanoparticles in Ag-SeO2 NC enhanced the antimicrobial activity through a synergistic effect.
The antibacterial properties of the prepared nanocomposites in the current research were also compared with previously reported Ag-TiO2, Ag-SeO2 NCs, and selenium-based NPs. The results in this work showed promising inhibition against S. aureus and B. cereus comparable to Ag-TiO2 NC (15.3 and 14 mm, respectively) and Ag-SeO2 NC (31 and 12 mm, respectively).96 The strong inhibition of B. subtilis by Ag-SeO2 NC (44 mm) agrees with the enhanced antibacterial activity of selenium-based nanoparticles reported by Tran et al.97 Similarly, K. pneumonia exhibited moderate inhibition (15.6 mm) against Ag-TiO2 NC, while Ag-SeO2 NC (31 mm) and previously studied selenium nanoparticles were more effective.98,99 Against Gram-negative bacteria such as E. typhimurium, Ag-SeO2 NC showed intense inhibition (30 mm), which is consistent with the superb antimicrobial potential of selenium nanoparticles observed in earlier research.96,98 The data show the antimicrobial effectiveness of our nanocomposites against a wide range of bacteria.
However, the morphology of nanomaterials is crucial in determining their antimicrobial properties.100 Antimicrobial action is based on numerous factors, including particle size, shape, surface area, and porosity.101 Particles of smaller sizes are likely to be more antimicrobial in character owing to their greater surface area and hence more sites of microbial interaction.102 Nanoparticles of spherical shape are likely to be characterized by good antimicrobial properties owing to their greater surface area and ease of penetrating cell membranes.103 Rod-shaped nanoparticles are more active antimicrobials because they have pointed ends, which cause cell membranes to break more effectively.104 Irregular shapes can generate more surface area and active sites, which can be utilized to enhance antimicrobial activity.105 A greater surface area means more interaction with microorganisms, which also translates to more antimicrobial activity.106 Porous structures can trap and kill microorganisms, which enhances antimicrobial activity.107 The antimicrobial action of Ag-TiO2 and Ag-SeO2 nanomaterials is multifaceted and involves multiple parameters, such as the release of silver ions, the generation of ROS, and direct interaction with microbes. The results of this study show the potential of Ag-SeO2 NC as a potent antibacterial agent. The optimization of the synthesis and formulation of Ag-SeO2 NC should be further researched to achieve clinical efficacy.
Ag-TiO2 and Ag-SeO2 NCs use three distinct antibacterial mechanisms: ROS production, cell membrane penetration and intracellular damage. Bacterial cells induce silver (Ag+) and selenium (Se2+) ion release from these nanocomposites until the ions penetrate the cell membrane to disrupt microbial functions.108 The production of reactive oxygen species (ROS) is enabled by TiO2 under light exposure. Oxidative stress generates detrimental effects on bacterial cells, resulting in lipid peroxidation, protein oxidation, and DNA fragmentation, which causes bacterial cell death.109 The ROS attacks bacterial cell membranes structurally while simultaneously time making membranes more permeable until the cells eventually rupture.110 The antimicrobial activity of Ag-SeO2 NC receives additional enhancement through its ability to block vital cellular processes and disrupt metabolic pathways.13,67 The scope of ROS generation with ion release and membrane disruption events produces bacterial dysfunction and cell death, making these nanocomposites promising antimicrobial agents.
| Sample | Bacterial species | Concentration (mg mL−1) | O.D600 |
|---|---|---|---|
| Ag-TiO2 NC | S. aureus | 8.29 | 0.033 |
| Ag-SeO2 NC | S. aureus | 0.2956 | 0.034 |
| Ag-TiO2 NC | K. pneumonia | 4.145 | 0.050 |
| Ag-SeO2 NC | K. pneumonia | 4.73 | 0.002 |
Increased antimicrobial efficacy of Ag-SeO2 NCs is due to the synergistic action between silver and selenium dioxide nanoparticles. Silver nanoparticles are strong antimicrobials owing to their interaction with the bacterial cell membrane, leading to damage and disruption.111 Silver nanoparticles can also generate reactive oxygen species (ROS), which induce oxidative stress and cellular structure destruction.112 Additionally, silver nanoparticles interfere with vital cell processes, such as DNA replication and protein synthesis.113 Selenium dioxide (SeO2), however, is a potent oxidant that can produce reactive oxygen species to impose oxidative stress on bacterial cells.114 Reactive oxygen species can inflict damage on cell components, such as DNA, proteins, and lipids, which can lead to cell death in the long term.115 The synergism of these two very active antimicrobial agents in Ag-SeO2 NCs enhances their antimicrobial activity to a high extent, with decreased MIC values and higher efficacy against bacterial infection.
| Strains | Inhibition zone diameter in mm of the tested samples | ||
|---|---|---|---|
| E. aphylla extract | Ag-TiO2 NC | Ag-SeO2 NC | |
| Candida glabrata | −ve | −ve | 12 |
| Aspergillus niger | −ve | −ve | 12 |
3.6. Studying the mechanism of action of antifungal activity
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively. A new band emerged at 774 cm−1, which represents the formation of metal–oxygen (M–O) bonds. These spectral changes confirm that the Ag-SeO2 NC had interacted with the constituents of the fungal cell wall, leading to a shift in their structure and function.120 The alterations observed in the FTIR spectra are good indications of the interaction between the Ag-SeO2 NC and the cell wall of the fungus, which may be the reason for the antifungal activity of the nanoparticles.121 Cell wall integrity disruption and new bond formation can lead to cell death and fungal growth inhibition. Similar to C. glabrata, the FTIR spectrum of untreated A. niger also displayed characteristic absorption bands for O–H stretching vibrations at 3526 and 3275 cm−1; C–H stretching vibrations at 2968, 2930, and 2872 cm−1; amide I band at 1688 cm−1; and other bands for C–C, C–N, and C–O bonds (Fig. S11†). Following treatment with Ag-SeO2 NC, there were significant changes in the FTIR spectrum of A. niger (Fig. S12†).
C bonds, respectively. A new band emerged at 774 cm−1, which represents the formation of metal–oxygen (M–O) bonds. These spectral changes confirm that the Ag-SeO2 NC had interacted with the constituents of the fungal cell wall, leading to a shift in their structure and function.120 The alterations observed in the FTIR spectra are good indications of the interaction between the Ag-SeO2 NC and the cell wall of the fungus, which may be the reason for the antifungal activity of the nanoparticles.121 Cell wall integrity disruption and new bond formation can lead to cell death and fungal growth inhibition. Similar to C. glabrata, the FTIR spectrum of untreated A. niger also displayed characteristic absorption bands for O–H stretching vibrations at 3526 and 3275 cm−1; C–H stretching vibrations at 2968, 2930, and 2872 cm−1; amide I band at 1688 cm−1; and other bands for C–C, C–N, and C–O bonds (Fig. S11†). Following treatment with Ag-SeO2 NC, there were significant changes in the FTIR spectrum of A. niger (Fig. S12†).
| Wavenumber (cm−1) | Untreated C. glabrata | Treated C. glabrata | Untreated A. niger | Treated A. niger | Functional group | Interpretation |
|---|---|---|---|---|---|---|
| 3353–3526 | 3353, 3273 | 3366, 3276 | 3526, 3275 | 3365, 3275 | O–H | O–H stretching vibration |
| 2935–2968 | 2935 | 2933, 2873 | 2968, 2930, 2872 | 2934, 2873 | C–H | C–H stretching vibration (aliphatic) |
| 1674–1688 | 1674 | 1674, 1642, 1579 | 1688 | 1674, 1578 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
Amide I band (carbonyl stretching) |
| 1550–1516 | 1550, 1516 | 1516 | 1503, 1452 | 1516, 1450 | C–N | Amide II band (N–H bending and C–N stretching) |
| 1452–1452 | 1452 | 1451 | 1452 | 1450 | C–H | C–H bending vibration (methylene) |
| 1405–1412 | 1405, 1398 | 1406, 1399 | 1412, 1385 | 1406, 1399 | C–H | C–H bending vibration (methyl) |
| 1308–1399 | 1308 | 1338, 1309 | 1236 | 1309, 1238 | C–N | C–N stretching vibration |
| 1238–1238 | 1238 | 1238 | 1236 | 1238 | C–O | C–O stretching vibration (phenolic) |
| 1120–1122 | 1120 | 1120 | 1122 | 1120 | C–O | C–O stretching vibration (alcoholic) |
| 1078–1078 | 1078 | 1078 | 1078 | 1079 | C–O | C–O stretching vibration (alcoholic) |
| 1037–1032 | 1037 | 1038 | 1032 | 1037 | C–O | C–O stretching vibration (alcoholic) |
| 981–990 | 981 | 990 | 979 | 923 | C–H | C–H bending vibration (aromatic) |
| 924–924 | 924 | 923 | 924 | 923 | C–H | C–H bending vibration (aromatic) |
| 851–850 | 851 | 850 | 849 | 850 | C–H | C–H bending vibration (aromatic) |
| 774–775 | 774 | 775 | 775 | M–O | Metal–oxygen bond (Ag–O or Se–O) | |
| 653–400 | 530 | 530, 400 | 702, 653, 531, 398 | 425, 400 | C–C, C–N, C–O | Skeletal vibrations |
New absorption bands occurred at 1674 and 1578 cm−1, corresponding to the existence of new C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and C
N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds, respectively. New absorption at 775 cm−1 also emerged, indicating the formation of metal–oxygen (M–O) bonds. The spectral changes prove that the Ag-SeO2 NCs react with the constituents of the fungal cell wall, leading to structural and functional changes.122 New bond formation and disruption of cell wall integrity may lead to cell death and inhibition of the growth of the fungus. The red shift of the O–H stretching band from 3526 cm−1 to 3365 cm−1 for treated A. niger verifies new hydrogen bond formation between the constituents of the fungal cell wall and Ag-SeO2 NCs. This interaction further enhances the disruption of the cell wall structure and enhances the antifungal action of the nanoparticles.
C bonds, respectively. New absorption at 775 cm−1 also emerged, indicating the formation of metal–oxygen (M–O) bonds. The spectral changes prove that the Ag-SeO2 NCs react with the constituents of the fungal cell wall, leading to structural and functional changes.122 New bond formation and disruption of cell wall integrity may lead to cell death and inhibition of the growth of the fungus. The red shift of the O–H stretching band from 3526 cm−1 to 3365 cm−1 for treated A. niger verifies new hydrogen bond formation between the constituents of the fungal cell wall and Ag-SeO2 NCs. This interaction further enhances the disruption of the cell wall structure and enhances the antifungal action of the nanoparticles.
The analysis of the changes in the morphology of A. niger after exposure to tracer Ag-SeO2 NCs is based on the data of the HR-TEM images. As presented in Fig. 12c, untreated A. niger presented a typical fungal morphology, including defined hyphae and clear cell walls. However, upon treatment with Ag-SeO2 NC, the extent of cellular damage varied significantly. The treated fungal cells (Fig. 12d) revealed damage to the cell wall and cell membrane, the cytoplasm leaked, and the cells died.
The disruptions in the cell walls and membrane structure probably contributed to the disruption of cellular equilibrium and prerequisite cellular processes. Possible factors that might have contributed to the anti-fungal activity of Ag-SeO2 NC against A. niger may be summarized as follows: the silver nanoparticles (Ag NPs) could directly come into contact with the cell membrane, SMR damage, and cell membrane disruption.116 They may also produce ROS, which causes cellular oxidative stress and leads to damage to cellular structures. Additionally, owing to their small size, Ag NPs can pose a threat to the cell's processes, including DNA replication and protein synthesis.124 The selenium dioxide present in the nanocomposite may complement its antimicrobial activity through the imposition of oxidative stress. The combined effect of Ag NPs and SeO2 in Ag-SeO2 NCs probably increases the effectiveness of antimicrobial activity against A. niger. Through the synergistic action of the two effective antimicrobial components, Ag-SeO2 NC could inhibit the growth of the fungal cell wall and membrane, leading to the cell death of the fungi.
4. Conclusion
This study provides an efficient and green protocol for the synthesis of Ag-TiO2 and Ag-SeO2 nanocomposites. The nanocomposites are well-characterized by different techniques, and the proposed mechanism for the formation of nanocomposites was investigated. UV-vis spectroscopy confirmed the presence of silver nanoparticles; FTIR spectroscopy indicated the involvement of plant extract biomolecules in the synthesis, and zeta potential analysis showed good colloidal stability. DLS analysis revealed differences in particle size distribution between the two nanomaterials. HR-TEM images visualized the morphology and particle size, while EDX analysis confirmed the elemental composition. SEM analysis showed the surface morphology and particle agglomeration. Finally, XRD analysis confirmed the crystalline phases present in the nanocomposites. The current study also provides valuable insights into the potential of natural and nanomaterial-based antimicrobial agents. E. aphylla extract and Ag-SeO2 NC demonstrated significant antioxidant and antimicrobial activities, respectively. A high concentration of bioactive compounds in the plant extract, such as phenolic compounds, flavonoids, and tannins, explains the high antioxidant activity identified in this study. These compounds can reduce free radical concentrations and preserve the structure of cells from oxidative effects. The improvement in the antimicrobial properties of Ag-SeO2 NC can be explained using the dual effect of the silver and selenium particles. Silver nanoparticles can encourage or impose membrane disruption and damage as a result of interactions with cells. They can also produce ROS, which, in turn, can cause oxidative stress and alter the cellular ultrastructure. Furthermore, it was found that silver nanoparticles can interfere with cell division and other operations, as basic as the protein synthesis process. Selenium dioxide is, in contrast, a powerful oxidative agent that may cause oxidative damage to cells through the production of ROS. Integrating these two highly effective antimicrobial agents in Ag-SeO2 NC results in a composed enhanced antimicrobial effect. Characterization studies that involved FTIR spectroscopy coupled with HR-TEM imaging supported the working mechanism of Ag-SeO2 NC. From the obtained FTIR spectra, it can be observed that there are considerable changes in the functional groups of the fungal cell wall of the treated cells with Ag-SeO2 NC that pointed towards interaction and possible disruption of the cell wall. The analysis of the HR-TEM images of C. glabrata and A. niger cells after interaction with Ag-SeO2 NC revealed cell damage demonstrated by cell wall deformation, membrane disorganization, and cytoplasmic leakage. All these results add more evidence to the belief that Ag-SeO2 NC could be a potential material for use as a broad-spectrum antimicrobial agent. Subsequent studies, which will examine these mechanisms of action in greater depth, could supply useful information in creating new classes of antimicrobial agents.Data availability
The data supporting the findings of this study are available in the ESI† file and can also be obtained from the corresponding author upon reasonable request.Conflicts of interest
No conflict of interest was declared by the authors.Acknowledgements
This work is not funded by any funding agencies.References
- D. Chinemerem Nwobodo, M. C. Ugwu, C. Oliseloke Anie, M. T. Al-Ouqaili, J. Chinedu Ikem, U. Victor Chigozie and M. Saki, J. Clin. Lab. Anal., 2022, 36, e24655 CrossRef PubMed.
- S. Mickymaray, Antibiotics, 2019, 8, 257 CrossRef CAS PubMed.
- M. T. El-Saadony, N. M. Zabermawi, N. M. Zabermawi, M. A. Burollus, M. E. Shafi, M. Alagawany, N. Yehia, A. M. Askar, S. A. Alsafy, A. E. Noreldin and A. F. Khafaga, Food Rev. Int., 2023, 39, 2138–2160 CrossRef CAS.
- D. E. González-Juárez, A. Escobedo-Moratilla, J. Flores, S. Hidalgo-Figueroa, N. Martínez-Tagüeña, J. Morales-Jiménez, A. Muñiz-Ramírez, G. Pastor-Palacios, S. Pérez-Miranda, A. Ramírez-Hernández and J. Trujillo, Molecules, 2020, 25, 3283 CrossRef PubMed.
- A. S. Dousari, N. Satarzadeh, B. Amirheidari and H. Forootanfar, Rev. Bras. Farmacogn., 2022, 32, 883–899 CrossRef CAS.
- M. Kumar, P. Kumar, A. Kaur, S. Kaur and S. Kaur, Thai J. Pharm. Sci., 2024, 47, 7 Search PubMed.
- W. Al-Awaida, B. J. Al-Hourani, M. Akash, W. H. Talib, S. Zein, R. R. Falah and Z. Aburubaiha, J. Cancer Res. Ther., 2018, 14, 1350–1354 CrossRef CAS PubMed.
- M. M. El-Zayat, M. M. Eraqi, H. Alrefai, A. Y. El-Khateeb, M. A. Ibrahim, H. M. Aljohani, M. M. Aljohani and M. M. Elshaer, Biomolecules, 2021, 11, 470, DOI:10.3390/biom11030470.
- M. Ashour, A. T. Mansour, A. M. Abdelwahab and A. E. Alprol, Processes, 2023, 11, 3356 CrossRef CAS.
- E. O. Ogunsona, R. Muthuraj, E. Ojogbo, O. Valerio and T. H. Mekonnen, Appl. Mater. Today, 2020, 18, 100473 CrossRef.
- A. Raghunath and E. Perumal, Int. J. Antimicrob. Agents, 2017, 49, 137–152 CrossRef CAS PubMed.
- S. Ying, Z. Guan, P. C. Ofoegbu, P. Clubb, C. Rico, F. He and J. Hong, Environ. Technol. Innovation, 2022, 26, 102336 CrossRef CAS.
- K. M. Elattar, F. O. Al-Otibi, M. S. El-Hersh, A. A. Attia, N. M. Eldadamony, A. Elsayed, F. Menaa and W. I. Saber, Heliyon, 2024, 10, e28359 CrossRef CAS PubMed.
- Y. A. Hameed, A. Almahri, A. I. Alalawy, S. F. Ibarhiam, N. D. Alkhathami, H. Mattar, W. M. Alamoudi and N. M. El-Metwaly, Inorg. Chim. Acta, 2025, 574, 122390 CrossRef CAS.
- K. M. Elattar, A. A. Ghoniem, F. O. Al-Otibi, M. S. El-Hersh, Y. A. Helmy and W. I. Saber, Appl. Sci., 2023, 13, 10110 CrossRef CAS.
- P. Ribao, J. Corredor, M. J. Rivero and I. Ortiz, J. Hazard. Mater., 2019, 372, 45–51 CrossRef CAS PubMed.
- Y. Gu, J. Dong, J. Li, Q. Luo, X. Dong, G. Tang, J. Zhang, X. Du, Q. Pu, L. He and K. Zhao, Front. Vet. Sci., 2023, 10, 1121082 CrossRef PubMed.
- A. Chwalibog, E. Sawosz, A. Hotowy, J. Szeliga, S. Mitura, K. Mitura, M. Grodzik, P. Orlowski and A. Sokolowska, Int. J. Nanomed., 2010, 5, 1085–1094 CrossRef PubMed.
- M. Alavi and N. Karimi, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 399–413 CrossRef PubMed.
- G. R. Khalaf, K. N. Abbas and A. M. Abbas, Braz. J. Phys., 2024, 54, 75 CrossRef CAS.
- M. Jariyaboon, P. Masrinoul, J. Komaikul, K. Muenkaew, S. Juntarapornchai, K. Ketsuwan, E. Rodpai, S. Ruangdachsuwan, S. Palabodeewat and C. Chitichotpanya, Emergent Mater., 2023, 6, 1259–1272 CrossRef CAS.
- S. Kang, J. Choi, G. Y. Park, H. R. Kim and J. Hwang, Appl. Surf. Sci., 2022, 599, 153930 CrossRef CAS.
- M. M. Abutalib and A. J. P. T. Rajeh, Polym. Test., 2021, 93, 107013 CrossRef CAS.
- C. Liu, L. Geng, Y. Yu, Y. Zhang, B. Zhao and Q. Zhao, Biofouling, 2018, 34, 190–199 CrossRef CAS PubMed.
- M. M. Rashid, B. Tomšič, B. Simončič, I. Jerman, D. Štular and M. Zorc, Appl. Surf. Sci., 2022, 595, 153521 CrossRef.
- L. Juan, Z. Zhimin, M. Anchun, L. Lei and Z. Jingchao, Int. J. Nanomed., 2010, 5, 261–267 CrossRef PubMed.
- A. García, L. Delgado, J. A. Torà, E. Casals, E. González, V. Puntes, X. Font, J. Carrera and A. Sánchez, J. Hazard. Mater., 2012, 199, 64–72 CrossRef PubMed.
- N. El-Gazzar and A. M. Ismail, Biocatal. Agric. Biotechnol., 2020, 27, 101708 CrossRef.
- K. Staats, M. Pilz, J. Sun, T. Boiadjieva-Scherzer, H. Kronberger, S. Tobudic, R. Windhager and J. Holinka, Sci. Rep., 2022, 12, 8298 Search PubMed.
- G. M. Khiralla and B. A. El-Deeb, LWT--Food Sci. Technol., 2015, 63, 1001–1007 CrossRef CAS.
- E. Zonaro, S. Lampis, R. J. Turner, S. J. S. Qazi and G. Vallini, Front. Microbiol., 2015, 6, 584 Search PubMed.
- M. Darroudi, A. Rangrazi, K. Ghazvini, H. Bagheri and A. Boruziniat, Pesqui. Bras. Odontopediatr. Clin. Integr., 2021, 21, e0121 CrossRef CAS.
- Y. Guo, C. Cheng, J. Wang, Z. Wang, X. Jin, K. Li, P. Kang and J. Gao, J. Hazard. Mater., 2011, 192, 786–793 CrossRef CAS PubMed.
- M. Skiba and V. Vorobyova, Appl. Nanosci., 2023, 13, 5185–5198 CrossRef CAS.
- F. Ahmed, D. Zhang, X. Tang and P. K. Malakar, Foods, 2024, 13, 4026 Search PubMed.
- M. Surya, S. Sampath, S. B. Vairamuthu, P. G. Sravanthy, B. Ramachandran, M. M. Al-Ansari, T. Asmelash and M. Saravanan, Mater. Technol., 2024, 39, 2331899 CrossRef.
- A. A. Ghoniem, K. M. Elattar, A. S. Alotaibi, H. Ghabban, M. S. El Hersh, A. Y. El-Khateeb, Y. A. El-Amier, H. M. El-Gendy, N. M. Eldadamony, W. I. Saber and A. Elsayed, Eur. J. Plant Pathol., 2024, 170, 567–591 CrossRef CAS.
- A. A. Alanazi, W. I. Saber, M. A. AlDamen and K. M. Elattar, Int. J. Biol. Macromol., 2024, 280, 135862 CrossRef CAS PubMed.
- M. M. Hammouda, A. A. Alanazi and K. M. Elattar, ChemistrySelect, 2024, 9, e202401385 CrossRef CAS.
- M. E. Mert, B. D. Mert and K. M. Elattar, ChemistrySelect, 2025, 10, e202403151 Search PubMed.
- M. Platzer, S. Kiese, T. Herfellner, U. Schweiggert-Weisz and P. Eisner, Antioxidants, 2021, 10, 811 CrossRef CAS PubMed.
- A. M. Shraim, T. A. Ahmed, M. M. Rahman and Y. M. Hijji, LWT--Food Sci. Technol., 2021, 150, 111932 Search PubMed.
- S. Perumalla and N. Nayeem, Int. J. Phytother. Res., 2012, 2, 16–19 Search PubMed.
- İ. Gulcin and S. H. Alwasel, Processes, 2023, 11, 2248 CrossRef.
- M. Asadujjaman, M. A. Hossain and U. K. Karmakar, Pharmacologyonline, 2013, 1, 161–165 Search PubMed.
- B. Athanassiadis, P. V. Abbott, N. George and L. J. Walsh, Aust. Dent. J., 2009, 54, 141–146 CrossRef CAS PubMed.
- M. A. Islam, M. M. Alam, M. E. Choudhury, N. Kobayashi and M. U. Ahmed, Bangladesh J. Vet. Med., 2008, 6, 121–126 Search PubMed.
- R. Dabur, H. Singh, A. K. Chhillar, M. Ali and G. L. Sharma, Fitoterapia, 2004, 75, 389–391 CrossRef PubMed.
- M. A. Sobi, M. R. Bindhu, D. Usha, R. Rajagopal, A. Alfarhan, S. Arokiyaraj and M. Umadevi, J. Cryst. Growth, 2024, 637, 127708 CrossRef.
- V. K. Sharma, C. M. Sayes, B. Guo, S. Pillai, J. G. Parsons, C. Wang, B. Yan and X. Ma, Sci. Total Environ., 2019, 653, 1042–1051 CrossRef CAS PubMed.
- S. T. Kochuveedu, Y. H. Jang and D. H. Kim, Chem. Soc. Rev., 2013, 42(21), 8467–8493 RSC.
- M. M. Abutalib and A. Rajeh, Phys. B, 2020, 578, 411796 CrossRef CAS.
- S. M. Amini, Mater. Sci. Eng., C, 2019, 103, 109809 CrossRef CAS PubMed.
- A. Barhoum, J. Jeevanandam, A. Rastogi, P. Samyn, Y. Boluk, A. Dufresne, M. K. Danquah and M. Bechelany, Nanoscale, 2020, 12, 22845–22890 Search PubMed.
- A. Albanese, P. S. Tang and W. C. Chan, Annu. Rev. Biomed. Eng., 2012, 14, 1–16 CrossRef CAS PubMed.
- F. Dumur, A. Guerlin, E. Dumas, D. Bertin, D. Gigmes and C. R. Mayer, Gold Bull., 2011, 44, 119–137 CrossRef.
- V. S. Sousa and M. R. Teixeira, Environ. Chem., 2013, 10(4), 313–322 Search PubMed.
- H. P. Nagaiah, M. B. Samsudeen, A. R. Augustus and K. P. Shunmugiah, Discover Nano, 2025, 20, 14 Search PubMed.
- S. Shrestha, B. Wang and P. Dutta, Adv. Colloid Interface Sci., 2020, 279, 102162 CrossRef CAS PubMed.
- C. N. Dipunadas and V. B. Jothy, J. King Saud Univ., Sci., 2019, 31, 372–383 Search PubMed.
- V. Sharma, D. Verma and G. S. Okram, J. Phys.: Condens. Matter, 2020, 32, 145302 CrossRef CAS PubMed.
- C. S. Seney, B. M. Gutzman and R. H. Goddard, J. Phys. Chem. C, 2009, 113, 74–80 CrossRef CAS.
- H. T. Liao and C. S. Wu, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 4272–4280 CrossRef CAS.
- T. Ivanova, A. Harizanova, T. Koutzarova and R. Closset, Molecules, 2024, 29, 5156 CrossRef CAS PubMed.
- N. Nazar, I. Bibi, S. Kamal, M. Iqbal, S. Nouren, K. Jilani, M. Umair and S. Ata, Int. J. Biol. Macromol., 2018, 106, 1203–1210 Search PubMed.
- A. Bachvarova-Nedelcheva, R. Iordanova, K. L. Kostov, V. Ganev and Y. Dimitriev, J. Non-Cryst. Solids, 2018, 481, 138–147 CrossRef CAS.
- A. A. Ghoniem, K. M. Elattar, F. O. Al-Otibi, A. Elsayed, M. S. El-Hersh, A. Y. El-Khateeb, Y. A. Helmy and W. I. Saber, RSC Adv., 2024, 14, 7088–7111 Search PubMed.
- E. Illés and E. Tombácz, Colloids Surf., A, 2003, 230, 99–109 CrossRef.
- J. Jiang, G. Oberdörster and P. Biswas, J. Nanopart. Res., 2009, 11, 77–89 CrossRef CAS.
- A. Serrano-Lotina, R. Portela, P. Baeza, V. Alcolea-Rodríguez, M. Villarroel and P. Ávila, Catal. Today, 2023, 423, 113862 Search PubMed.
- J. M. Zook, V. Rastogi, R. I. MacCuspie, A. M. Keene and J. Fagan, ACS Nano, 2011, 5, 8070–8079 Search PubMed.
- Y. Min, M. Akbulut, K. Kristiansen, Y. Golan and J. Israelachvili, Nat. Mater., 2008, 7, 527–538 Search PubMed.
- B. Krause, M. Mende, P. Pötschke and G. Petzold, Carbon, 2010, 48, 2746–2754 CrossRef CAS.
- C. M. Phan and H. M. Nguyen, J. Phys. Chem. A, 2017, 121, 3213–3219 CrossRef CAS PubMed.
- N. M. Eldadamony, A. A. Ghoniem, A. A. Al-Askar, A. A. Attia, M. S. El-Hersh, K. M. Elattar, H. Alrdahi and W. I. Saber, Int. J. Biol. Macromol., 2024, 269, 132109 Search PubMed.
- M. M. Hammouda, K. Shalabi, A. A. Alanazi, K. M. Elattar, M. A. Azzam and M. M. Rashed, RSC Adv., 2023, 13, 32532–32546 RSC.
- J. Zhu, A. Holmen and D. Chen, ChemCatChem, 2013, 5, 378–401 Search PubMed.
- E. Antolini, Appl. Catal., B, 2016, 181, 298–313 CrossRef CAS.
- Q. Mu, G. Jiang, L. Chen, H. Zhou, D. Fourches, A. Tropsha and B. Yan, Chem. Rev., 2014, 114, 7740–7781 Search PubMed.
- M. S. Hossain and S. Ahmed, Results Mater., 2023, 20, 100492 Search PubMed.
- D. A. García, L. Mendoza, K. Vizuete, A. Debut, M. T. Arias, A. Gavilanes, T. Terencio, E. Ávila, C. Jeffryes and S. A. Dahoumane, Molecules, 2020, 25(21), 5193 CrossRef PubMed.
- Z. Bedlovičová, I. Strapáč, M. Baláž and A. Salayová, Molecules, 2020, 25, 3191 Search PubMed.
- M. Misawa and J. Takahashi, Nanomed. Nanotechnol. Biol. Med., 2011, 7, 604–614 CrossRef CAS PubMed.
- S. N. Al-Barri, T. M. Al-Deeb, A. T. Al-Fawwaz, M. M. Al-Omari and K. M. Al-Qaoud, Plant Cell Biotechnol. Mol. Biol., 2021, 22, 47–64 Search PubMed.
- W. Ahmad, S. Shams, A. Ahmad, Y. Wei, Q. Yuan, A. U. Khan, M. S. Khan, A. Ur Rahman and M. Iqbal, Appl. Nanosci., 2020, 10, 1191–1204 Search PubMed.
- M. I. Alghonaim, S. A. Alsalamah, A. M. Mohammad and T. M. Abdelghany, Biomass Convers. Biorefin., 2025, 15, 6767–6779 CrossRef CAS.
- G. A. Engwa, Free Radicals and the Role of Plant Phytochemicals as Antioxidants against Oxidative Stress-Related Diseases, Phytochemicals: Source of Antioxidants and Role in Disease Prevention, BoD–Books on Demand, 2018, vol. 7, pp. 49–74 Search PubMed.
- S. Firoozi, M. Jamzad and M. Yari, J. Nanostruct. Chem., 2016, 6, 357–364 Search PubMed.
- Z. Nie, K. J. Liu, C. J. Zhong, L. F. Wang, Y. Yang, Q. Tian and Y. Liu, Free Radicals Biol. Med., 2007, 43, 1243–1254 CrossRef CAS PubMed.
- B. Ramalingam, T. Parandhaman and S. K. Das, ACS Appl. Mater. Interfaces, 2016, 8, 4963–4976 CrossRef CAS PubMed.
- M. A. Ansari, H. M. Khan, A. A. Khan, M. K. Ahmad, A. A. Mahdi, R. Pal and S. S. Cameotra, J. Basic Microbiol., 2014, 54, 905–915 CrossRef CAS PubMed.
- A. Ahmad, Y. Wei, F. Syed, K. Tahir, A. U. Rehman, A. Khan, S. Ullah and Q. Yuan, Microb. Pathog., 2017, 102, 133–142 CrossRef CAS PubMed.
- A. S. Joshi, P. Singh and I. Mijakovic, Int. J. Mol. Sci., 2020, 21, 7658 CrossRef CAS PubMed.
- A. Roy, O. Bulut, S. Some, A. K. Mandal and M. D. Yilmaz, RSC Adv., 2019, 9, 2673–2702 RSC.
- M. Bébien, G. Lagniel, J. Garin, D. Touati, A. Verméglio and J. Labarre, J. Bacteriol., 2002, 184, 1556–1564 CrossRef PubMed.
- O. Bilek, Z. Fohlerova and J. Hubalek, PLoS One, 2019, 14, e0214066 CrossRef CAS PubMed.
- P. A. Tran, N. O'Brien-Simpson, J. A. Palmer, N. Bock, E. C. Reynolds, T. J. Webster, A. Deva, W. A. Morrison and A. J. O'Connor, Int. J. Nanomed., 2019, 14, 4613–4624 CrossRef CAS PubMed.
- L. D. Geoffrion, T. Hesabizadeh, D. Medina-Cruz, M. Kusper, P. Taylor, A. Vernet-Crua, J. Chen, A. Ajo, T. J. Webster and G. Guisbiers, ACS Omega, 2020, 5, 2660–2669 CrossRef CAS PubMed.
- Q. Wang, L. M. Barnes, K. I. Maslakov, C. A. Howell, M. J. Illsley, P. Dyer and I. N. Savina, Mater. Sci. Eng., C, 2021, 121, 111859 CrossRef CAS PubMed.
- R. Kumar, A. Umar, G. Kumar and H. S. Nalwa, Ceram. Int., 2017, 43, 3940–3961 CrossRef CAS.
- J. Sawai, H. Igarashi, A. Hashimoto, T. Kokugan and M. Shimizu, J. Chem. Eng. Jpn., 1996, 29, 251–256 Search PubMed.
- B. Lallo da Silva, M. P. Abuçafy, E. Berbel Manaia, J. A. Oshiro Junior, B. G. Chiari-Andréo, R. C. R. Pietro and L. A. Chiavacci, Int. J. Nanomed., 2019, 14, 9395–9410 CrossRef PubMed.
- M. Inam, J. C. Foster, J. Gao, Y. Hong, J. Du, A. P. Dove and R. K. O'Reilly, J. Polym. Sci., Part A: Polym. Chem., 2019, 57, 255–259 CrossRef CAS.
- F. A. Z. Sayed, N. G. Eissa, Y. Shen, D. A. Hunstad, K. L. Wooley and M. Elsabahy, J. Nanobiotechnol., 2022, 20, 536 CrossRef CAS PubMed.
- B. Abebe, E. A. Zereffa, A. Tadesse and H. A. Murthy, Nanoscale Res. Lett., 2020, 15, 1–19 CrossRef PubMed.
- R. Kaur and S. Liu, Prog. Surf. Sci., 2016, 91, 136–153 CrossRef CAS.
- T. M. S. U. Gunathilake, Y. C. Ching, K. Y. Ching, C. H. Chuah and L. C. Abdullah, Polymers, 2017, 9, 160 Search PubMed.
- N. F. Fahmy, M. M. Abdel-Kareem, H. A. Ahmed, M. Z. Helmy and E. A. R. Mahmoud, Microb. Cell Fact., 2025, 24, 6 CrossRef CAS PubMed.
- V. I. Lushchak, Oxidative stress and mechanisms of protection against it in bacteria, Biochemistry, 2001, 66, 476–489 CAS.
- T. Y. Wang, M. D. J. Libardo, A. M. Angeles-Boza and J. P. Pellois, ACS Chem. Biol., 2017, 12, 1170–1182 CrossRef CAS PubMed.
- B. Ahmed, A. Hashmi, M. S. Khan and J. Musarrat, Adv. Powder Technol., 2018, 29, 1601–1616 CrossRef CAS.
- Y. H. Lee, F. Y. Cheng, H. W. Chiu, J. C. Tsai, C. Y. Fang, C. W. Chen and Y. J. Wang, Biomaterials, 2014, 35, 4706–4715 CrossRef CAS PubMed.
- P. V. AshaRani, S. Sethu, H. K. Lim, G. Balaji, S. Valiyaveettil and M. P. Hande, Genome Integr., 2012, 3, 1–14 Search PubMed.
- P. S. Bapte, S. S. Pansambal and S. Borgave, J. Nanostruct., 2022, 12, 178–193 Search PubMed.
- C. A. Juan, J. M. Pérez de la Lastra, F. J. Plou and E. Pérez-Lebeña, Int. J. Mol. Sci., 2021, 22, 4642 CrossRef CAS PubMed.
- L. Wang, L. Liu and X. Zhou, Food Bioprocess Technol., 2024, 17, 2304–2325 CrossRef CAS.
- Y. H. Lee, F. Y. Cheng, H. W. Chiu, J. C. Tsai, C. Y. Fang, C. W. Chen and Y. J. Wang, Biomaterials, 2014, 35, 4706–4715 CrossRef CAS PubMed.
- N. Filipović, D. Ušjak, M. T. Milenković, K. Zheng, L. Liverani, A. R. Boccaccini and M. M. Stevanović, Front. Bioeng. Biotechnol., 2021, 8, 624621 CrossRef PubMed.
- Y. Xiao, X. Zhang and Q. Huang, Int. J. Biol. Macromol., 2022, 213, 339–351 Search PubMed.
- R. M. Elamawi, R. E. Al-Harbi and A. A. Hendi, Egypt. J. Biol. Pest Control, 2018, 28, 1–11 Search PubMed.
- P. Kanhed, S. Birla, S. Gaikwad, A. Gade, A. B. Seabra, O. Rubilar, N. Duran and M. Rai, Mater. Lett., 2014, 115, 13–17 CrossRef CAS.
- F. Faghihzadeh, N. M. Anaya, L. A. Schifman and V. Oyanedel-Craver, Nanotechnol. Environ. Eng., 2016, 1, 1–16 Search PubMed.
- M. A. Wallig and E. B. Janovitz, Morphologic manifestations of toxic cell injury, in Haschek and Rousseaux's Handbook of Toxicologic Pathology, Academic Press, 2022, pp. 113–148 Search PubMed.
- L. Wei, J. Lu, H. Xu, A. Patel, Z. S. Chen and G. Chen, Drug Discovery Today, 2015, 20, 595–601 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00936g |
| This journal is © The Royal Society of Chemistry 2025 |