Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of Eu3+ ion concentration on optical and magnetic properties of oriented Gd2O3/CTAB nanoparticles as multifunctional optical-magnetic probes in biomedicine
Thi Lien Pham a,
Cong Quang Tonga,
Ngoc Phan Vub,
Thi Hong Ha Vu*b,
Thi Anh Hoc,
Duc Thang Phamd,
Thi Hoi Lee,
Manh Tien Dinha,
Thanh Huong Nguyena,
Thi Khuyen Hoanga,
Thi Kieu Giang Lam
a,
Cong Quang Tonga,
Ngoc Phan Vub,
Thi Hong Ha Vu*b,
Thi Anh Hoc,
Duc Thang Phamd,
Thi Hoi Lee,
Manh Tien Dinha,
Thanh Huong Nguyena,
Thi Khuyen Hoanga,
Thi Kieu Giang Lam a,
Vu Nguyena,
Hong Nam Phama and
Tien Ha Le
a,
Vu Nguyena,
Hong Nam Phama and
Tien Ha Le *f
*f
aInstitute of Materials Science, Vietnam Academy of Science and Technology, 18 Hoang Quoc Viet, Cau Giay, Hanoi, Vietnam
bFaculty of Biotechnology, Chemistry and Environmental Engineering, Phenikaa University, Hanoi 12116, Vietnam. E-mail: ha.vuthihong@phenikaa-uni.edu.vn
cFaculty of Engineering Physics and Nanotechnology, VNU University of Engineering and Technology, Vietnam National University, Hanoi, 144 Xuan Thuy, Cau Giay, Hanoi 11310, Vietnam
dFaculty of Physics, VNU University of Science, Vietnam National University, Hanoi, 334 Nguyen Trai, Thanh Xuan, Hanoi 11416, Vietnam
eHanoi Medical University, 01 Ton That Tung, Dong Da, Hanoi, Vietnam
fInstitute of Science and Technology, TNU-University of Sciences, Thai Nguyen, 250000, Vietnam. E-mail: letienha@tnu.edu.vn
First published on 28th March 2025
Abstract
The Gd2O3:Eu3+ nanoparticles were synthesized using a multi-step chemical method with urea as a reactant to control the ratio of different Eu3+ activation centers: 2, 4, 6, 8, 10, and 12 mol% combined with CTAB surfactant to improve surface quality. The study aimed to determine the optimal concentration of Eu3+ in the presence of CTAB to increase biocompatibility and achieve the best fluorescence. The structure, surface morphology, optical properties, and magnetic properties of the materials were analyzed through FSEM, XRD, HRTEM, XPS, UV-vis, fluorescence, fluorescence excitation, time-resolved fluorescence, vibrating sample magnetometry (VSM), and magnetic heating measurements. The obtained material had a diameter of 180–280 nm, and it emitted red light with characteristic shifts from 5D0 to 7FJ (J = 0–4). The strongest emission peak occurred at the transition of 5D0 to 7F2, corresponding to a wavelength of 611 nm. The crystal is in the cubic phase. The highest lifetime of the samples is 2.1 ms, and the highest calculated quantum efficiency is 91% for the Gd2O3:8% Eu3+ sample. The M–H hysteresis curve revealed that the highest magnetic field obtained was 1.83 emu g−1. Experimental induction heating of samples reached temperatures in the range of 44–49 °C, which is an appropriate temperature range for destroying cancer cells without affecting healthy cells. These findings demonstrate that the material has great potential in cancer diagnosis and treatment.
1. Introduction
Rare earth elements (RE) have garnered significant interest in both basic and applied research over recent decades due to their unique physical and chemical properties.1–4 This interest is reflected in the growing number of applications, as RE elements have become indispensable for important technologies.5–7 Nanostructured materials containing RE elements, either as the main component or dopant phase, have opened up new avenues for various biomedical applications, including bioimaging, biosensors, targeted drug delivery, and other therapies.8–11The fluorescence properties of inorganic fluorescent materials depend closely on their substrate, dopant ions, size, and morphology.12 Researchers have thus explored the synthesis of inorganic luminescent materials with different substrates, dopant ions, and shapes.12,13 The choice of substrate significantly impacts the luminescent properties of materials, making substrate exploration a focal point for researchers.
Gd2O3 (gadolinium sesquioxide) is a well-known material widely used in cathode ray tubes, phosphors, bioimaging, and biosensors.1–3 Among various rare earth oxide materials, Gd2O3 offers several advantages, including physical, chemical, and thermal stability, low phonon energy, high refractive index, high dielectric constant, paramagnetism, and high density.10,14,15 When doped with europium (Eu3+), Gd2O3 becomes a red luminescent material with potential applications in fluorescent lamps, white light-emitting diodes, plasma display panels, flat screens, cathode ray tubes, MRI contrast agents, biosensors, and bioimaging.16 The long excitation level of Gd3+ ions produces emission lines in the UV region, and luminescence changes occur when other rare earth ions are added.17 Importantly, the ionic radius of Eu3+ matches that of Gd3+, allowing easy incorporation into the Gd2O3 substrate without distorting the crystal structure.11,18
On the other hand, gadolinium (Gd) and Gd3+ ions possess seven unpaired electrons, resulting in strong superparamagnetic properties.19 Organic hybrid compounds containing Gd3+ ions are commonly used as contrast agents in magnetic resonance imaging (MRI).20–22 In modern medicine, there is a growing emphasis on combining diagnosis and treatment.23 Therapies such as targeted drug delivery, chemotherapy, thermotherapy, or radiotherapy are increasingly integrated with imaging diagnostics, both in vitro and in vivo. Various imaging methods, including MRI and fluorescent labeling, can be combined with treatment agents like thermotherapy, chemotherapy, or drug delivery. This combined imaging approach allows for better control, information gathering, and understanding of process dynamics, ultimately enhancing treatment effectiveness.
In our study, we focus on evaluating the luminescent properties of Gd2O3 material at different Eu3+ doping concentrations, while also investigating the magnetic properties and thermotherapy potential of Gd2O3:Eu3+. Luminescent Gd2O3:Eu3+ materials have been synthesized using various methods, such as sol–gel, hydrothermal, co-precipitation, and multi-step chemistry.11,24–26 Among these methods, the multi-step chemical synthesis stands out as an easy and cost-effective approach to produce uniform-sized nanoparticles in a shorter time and at lower temperatures. Therefore, we will use this method to synthesize Gd2O3:Eu3+ material combined with CTAB surfactant to enhance surface quality. Using CTAB not only improves the dispersion of nanoparticles but also enhances their stability in biological environments. This research can lead to improved biocompatibility, making the nanoparticles more effective for in vivo applications. Investigating the relationship between optical properties and hyperthermia allows for the design of nanoparticles with tunable heating profiles. This can optimize treatment protocols, providing controlled thermal doses to target tissues while minimizing damage to surrounding healthy cells. The novelty of this research lies in the intersection of improved optical properties, enhanced stability through CTAB, and the potential for real-time imaging during hyperthermia treatments.
2. Materials and methods
2.1. Materials
Chemicals used in material synthesis Gd2O3:Eu3+: Gd(NO3)3·6H2O from Sigma-Aldrich, 99.9%, Eu(NO3)3·5H2O from Sigma-Aldrich 99.9%, urea (CO(NH2)2) from Sigma-Aldrich, hexadecyltrimethylammonium bromide (CTAB) from Sigma-Aldrich.2.2. Synthesis process
Prepare “Solution 1” by mixing 0.05 M Eu(NO3)3·5H2O and 0.05 M Gd(NO3)3·6H2O in a 100 mL beaker, adjusting the Eu3+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Gd3+ ratio to 2%, 4%, 6%, 8%, 10%, or 12%.
Gd3+ ratio to 2%, 4%, 6%, 8%, 10%, or 12%.
In a 500 mL flask, prepare a 0.5 M urea solution and gradually add “Solution 1,” stirring for 2 hours. Add 0.01 M CTAB and heat the mixture to 85 °C for 70 minutes to produce a white precipitate of Gd(OH)CO3·H2O.
Centrifuge the precipitate with deionized water and ethanol, then dry at 70 °C for 24 hours. Finally, calcine the material at 700 °C for 5 hours (Scheme 1).
2.3. Characterization techniques
The crystal structure of the nanoparticles was analyzed using X-ray diffraction (XRD) with a Bruker D8 Advance instrument with CuKα radiation (λ = 0.154 nm) at fine steps of 0.02°. The morphology of the synthesized material was analyzed using Field Emission Scanning Electron Microscopy (FESEM) on a Hitachi S-4800 machine. High-resolution transmission electron microscopy (HR-TEM) spectra were measured by a JEM2100 system (Jeol, Japan). X-ray photoelectron spectroscopy (XPS, Nexsa G2) analyzed the chemical bonding configurations. The bandgap energy was estimated using the UV-vis absorption spectrum obtained by JASCO V-750 spectrophotometer. Photoluminescence (PL) spectra were recorded with a Nanolog spectrophotometer (Horiba Jobin Yvon) excited by a 450 W xenon lamp. The saturation magnetization (MS) values were characterized by a vibrating sample magnetometer (VSM, MicroSense EZ9). The inductive heating experiment was conducted using an RDO-HFI device with an output power of 5 kW. All measurements were done in ambient air.3. Results and discussion
3.1. Characterization of the Gd2O3 nanoparticles
 | ||
| Fig. 1 FESEM images of Eu-doped Gd2O3/CTAB with concentrations of 2% (a), 4% (b), 6% (c), 8% (d), 10% (e) and 12% (f). | ||
These results indicate that variations in the Eu3+ doping ratio significantly affect both the shape and size of the material, with a clear trend of increasing size as the Eu3+ doping ratio rises.
 | (1) |
 | ||
| Fig. 4 XPS spectrum of Gd2O3 and Gd2O3:8% Eu nanorods: (a) survey spectrum, (b) Gd 3d, (c) O 1s and (d) Eu 3d levels. | ||
However, the half-width of the peak was broadened, proving that when Eu was doped into the Gd2O3 lattice, it affected the local crystal field at the position where the Eu3+ ion replaced the Gd3+ ion. The effect of this substitution also changed the bonding of the O atom with other ions. Fig. 4c shows the bonding state of O 1s, and the result shows that when there is a circular shoulder of the Eu3+ ion in the lattice, the half-width of the O 1s peak narrows towards low energy. This result is believed to be due to the Eu3+ ion radius and its electronegativity (1.0) being smaller than the Gd3+ ion radius and electronegativity (1.2), narrowing the half-width towards this low binding energy region. To compare this phenomenon, we conducted a survey of the high-resolution XPS spectrum of the O 1s state and fitted the peaks corresponding to the characteristic bonds in Fig. 5. At the same time, the high-resolution XPS spectrum in the range from 1125 eV to 1160 eV in Fig. 4d shows that in the Gd2O3:Eu3+/CTAB sample, peaks appeared at 1134.25 eV and 1155.15 eV corresponding to the Eu 3d5/2 and Eu 3d3/2 states with a separation of 20.9 eV of the Eu3+ ion, while in the Gd2O3/CTAB sample, these two bond peaks were not present.
3.2. Optical studies
In this study, the band gap of Gd2O3/CTAB and Gd2O3:Eu3+/CTAB materials was deduced from the UV-vis spectrum according to the Kubelka–Munk eqn (2):| (F(R∞)hν)γ = A(hν − Eg) | (2) |
 | ||
| Fig. 6 UV-vis spectra of Gd2O3:Eu3+ with different mol concentrations (a and b) and energy band gap of Gd2O3:Eu3+ for different dopant concentrations (c and d) using K–M theory. | ||
3.3. Optical properties
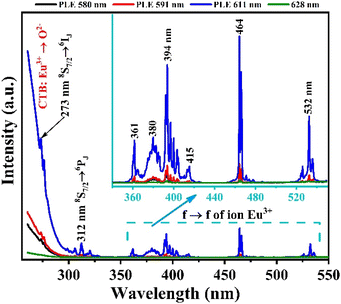
To study the optical properties of the Gd2O3:Eu3+/CTAB material, we measured the fluorescence spectrum of the Gd2O3:Eu3+/CTAB sample doped with 8% Eu3+ ions. The results obtained in Fig. 7 show that the material emits strongly in the red light region with characteristic emission peaks of Eu3+ ions corresponding to the transition from the 5D0 state to the 7FJ state (J = 0–4): 5D0 → 7F0 (580 nm), 5D0 → 7F1 (588–600 nm), 5D0 → 7F2 (607–620 nm), 5D0 → 7F3 (620–632 nm) and 5D0 → 7F4 (701–712 nm) in which the emission peak at 611 nm has the most vigorous intensity.27–29 This emission peak is a transition from the 5D0 state to the 7F2 state, where the parity rule does not forbid the electric dipole state of the Eu3+ ion. The characteristic properties of this fluorescence spectrum once again demonstrate that the Gd2O3 matrix has a cubic structure.Based on the fluorescence analysis results of the material, we measured the fluorescence excitation spectrum of the Gd2O3/CTAB material sample doped with 8% Eu3+ ions with different emission peaks at 580, 591, 611, and 628 nm. The results obtained in Fig. 8 show that the material strongly absorbs in the ultraviolet and visible regions with excitation peaks at 252, 273, 312, 360, 395, 464, and 532 nm. The strong absorption band in the UV region at 252 nm is attributed to the charge transfer transition (CTB) between Eu3+ → O2−. The absorption peaks at 273 nm and 312 nm are the characteristic absorption peaks of Gd3+ ions corresponding to the 8S → 6I and 8S → 6P transitions, respectively.27–29 Meanwhile, the excitation peaks at 360, 394, 464, and 532 nm are the characteristic absorption peaks of Eu3+ ions corresponding to the f–f transition. Among these absorption bands, the CTB band between Eu3+ → O2− is the strongest, and the emission peak for fluorescence excitation has the highest intensity at 611 nm. This result shows that when excited at different wavelengths, the energy level transition of Eu3+ ions from the 5D0 state to the 7F2 state has the highest transition probability.
To evaluate the emission ability of the material with different excitation wavelengths obtained in Fig. 8. We measured the fluorescence spectrum of Gd2O3:8% Eu3+/CTAB with excitation wavelengths of 273, 394, 464, and 512 nm.
The results obtained in Fig. 9 show that the positions of the characteristic emission peaks of Eu3+ ions in the Gd2O3 matrix do not change, but only the intensity of the peaks changes, and no strange peaks are emitted when excited at different wavelengths. This shows that the emission process of the material only includes the energy level transitions of Eu3+ ions from the excited state 5D0 to the state 7FJ without including the emission process of Gd3+ ions. With this different excitation wavelength, the material emits best when excited at 273 nm (corresponding to the energy level transition of Gd3+ ion from 8S–6I state), followed by 394 nm. This shows that the absorption process of the Gd2O3 matrix and Gd3+, Eu3+ ions, when moving to high energy excited states, all tend to shift without emission to the 5D0 state of Eu3+ ion before shifting to the 7FJ state for characteristic emission of Eu3+ ion. The energy transfer process from the 8S excited state of Gd3+ ion to Eu3+ ion does not lead to an emission process that is worth studying. The mechanism of these energy transfer processes is shown in Fig. 10.
With the results of this study, we will investigate the effect of Eu3+ doping concentration on the energy transfer mechanism between Gd3+ ions and Eu3+ ions by measuring the fluorescence spectrum depending on Eu concentration with an excitation wavelength of 273 nm.
The analysis results in Fig. 11, when excited at 273 nm with Gd2O3/CTAB samples doped with Eu3+ ions at concentrations from 2 to 12%, show that when the Eu3+ ion concentration is at 2%, the intensity of the fluorescence peaks is very low. This shows that with low Eu concentrations, when excited at 273 nm, the absorbed electrons move to the 6I7/2 state and recombine without emission. Theoretically, Gd3+ ions can transfer energy to Eu3+ ions when absorbed to a high energy level. However, in the fluorescence spectra of Gd2O3/CTAB samples doped with Eu3+ ions at different concentrations, we did not observe the emission band of Gd3+ ions at the 312 nm emission peak corresponding to the transition of the 6P excited state to the 8S state. This result can be explained by the fact that the Gd3+ ion has a stable electron structure in the 4f7 electron configuration.30 Therefore, the host environment has almost no effect on the energy level of Gd3+ ions. In addition, considering the very high excitation energy of Gd3+ ion, the instantaneous energy transfer from Gd3+ ion to Eu3+ ion is almost impossible in Gd2O3/CTAB material doped with low concentration Eu3+ ion. This leads to the lifetime of Gd2O3/CTAB samples doped with low-concentration Eu3+ ions being usually longer than that of high-concentration doped samples. When the Eu concentration increases, the charge transfer process between Gd3+ ions and Eu3+ ions increases, causing the fluorescence intensity in the emission band of Eu3+ ions from the 5D0 state to the 7FJ state to increase and reach a maximum value at a doping concentration of 8% Eu. The fluorescence quenching phenomenon occurs when the doping concentration increases above 8% in the cubic structure of Gd2O3 nanoparticles. This fluorescence quenching result at high Eu concentration is attributed to the fact that Eu3+ ion has a similar radius to Gd3+ ion, and at the same time, Gd3+ ion acts as a photobleach that enhances the luminescence of Eu3+ ion when replacing Gd3+ ion in general substrate lattices. The concentration of Eu3+ ion doping in this Gd2O3 substrate lattice is much higher than that in some other substrate lattices such as Sr6P5BO20,31 Sr5(PO4)3Cl,32 or with monoclinic Gd2O3 structure.
 | ||
| Fig. 11 Fluorescence spectra of Gd2O3:Eu3+/CTAB at different concentrations with an excitation wavelength of 273 nm, measured at room temperature. | ||
To investigate the crystal symmetry, we analyzed peak splitting in the Gd2O3:8% Eu3+ sample (Fig. 12). The transition from 5D0 to 7F1 is identified as an electric dipole transition that is unaffected by local crystal field symmetry. In cubic Gd2O3, two symmetry positions exist: C2 and S6, occurring in a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The C2 position lacks inversion symmetry, while the S6 position possesses it. When Eu ions occupy the C2 positions, electric dipole transitions from 5D0 to 7F2 follow the selection rule ΔJ = 2. In contrast, when occupying S6 positions with inversion symmetry, the electric dipole transition from 5D0 to 7F1 adheres to ΔJ = 1.14 To determine this issue, we have fitted the fluorescence spectra of the Gd2O3:8% Eu3+ sample at different emission peak positions.
1. The C2 position lacks inversion symmetry, while the S6 position possesses it. When Eu ions occupy the C2 positions, electric dipole transitions from 5D0 to 7F2 follow the selection rule ΔJ = 2. In contrast, when occupying S6 positions with inversion symmetry, the electric dipole transition from 5D0 to 7F1 adheres to ΔJ = 1.14 To determine this issue, we have fitted the fluorescence spectra of the Gd2O3:8% Eu3+ sample at different emission peak positions.
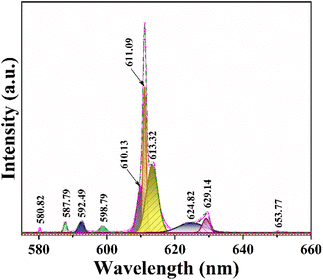 | ||
| Fig. 12 Fluorescence spectrum of Gd2O3:8% Eu3+/CTAB excited at 273 nm fits at different peak positions. | ||
Between 575 nm and 660 nm, several prominent peaks are observed in the luminescence spectrum of Gd2O3 samples doped with Eu3+. These peaks correspond to transitions between energy states, specifically from 5D0 to 7F0, 5D0 to 7F1, 5D0 to 7F2, 5D0 to 7F3, and 5D0 to 7F4. Here's a detailed explanation of the key transitions:
• 5D0 to 7F1 transition (around 587.79, 592.49, and 598.77 nm): in highly symmetric crystal fields, the electric dipole transition from J = 1 typically does not split into further sublevels. However, in these samples with a monoclinic crystal structure and reduced symmetry, the J = 1 state of 7F1 splits into three sublevels. This splitting is likely influenced by the concentration of impurities.
• 5D0 to 7F2 transition (around 610.13, 611.09, 613.32, 624.82, and 629.14 nm): the characteristic red luminescence of Eu3+ arises from this transition, typically occurring between 610 and 630 nm. Due to the Cs monoclinic symmetry positions of Eu3+, the J = 2 state of 7F2 splits into five sublevels. These sublevels are represented by five distinct peaks, indicating transitions from 5D0 to these 7F2 sublevels.
To compare the difference between the energy transfer mechanism from Gd3+ ions to Eu3+ ions, we also investigated the emission ability of Gd2O3/CTAB samples doped with Eu3+ at an excitation wavelength of 394 nm, corresponding to the preferential transition of Eu3+ ions from the ground state 7F0 to the state 5L6.
The results shown in Fig. 13 show that the material emits strongly in the red light region with the position of the characteristic emission peaks of Eu3+ ions from the excited state 5D0 to the state 7FJ almost unchanged compared to when excited at 273 nm. However, we observed that with the 2% doped sample, the intensity of the 611 nm emission peak is relatively large compared to this sample when excited at 273 nm.
 | ||
| Fig. 13 Fluorescence spectra of Gd2O3:Eu3+/CTAB at different concentrations with an excitation wavelength of 394 nm, measured at room temperature. | ||
This result indicates that, for the energy transfer phenomenon between Gd3+ ions and Eu3+ ions to occur, the Eu concentration must be large enough to receive this energy transfer process. To compare the above results, we have established the ratio between the 611 nm peak intensity of samples with different concentrations, with the 8% doped Gd2O3/CTAB sample giving the most vigorous intensity; the results are shown in Table 1.
| Eu3+ concentration | Excitation 273 nm | Excitation 394 nm |
|---|---|---|
| 2 | 1.16 | 14.40 |
| 4 | 52.35 | 67.08 |
| 6 | 73.57 | 92.12 |
| 8 | 100 | 100 |
| 10 | 48.61 | 25.39 |
| 12 | 33.01 | 16.23 |
Based on the results obtained in Table 1, we have drawn a graph showing this intensity ratio in Fig. 14. The analysis results show that with low doping concentrations, the energy transfer process between Gd3+ ions and Eu3+ ions is more complex, making the intensity ratio of the 611 nm peak of the samples compared to the sample with the highest intensity when excited at 273 nm. At the same time, when excited at 394 nm, the intensity ratio of this 611 nm peak to the sample with the highest intensity is stronger. However, when the phenomenon of fluorescence quenching due to concentration occurs, when excited at 273 nm, the quenching process occurs more slowly. This shows that the energy transfer process from Gd3+ ions to Eu3+ ions reduces the non-radiative recombination process in the material when the doping concentration is high. To supplement this study, we measured the time-resolved fluorescence spectra of Gd2O3/CTAB samples doped with Eu3+ ions with different doping concentrations, with an excitation wavelength of 273 nm and an emission wavelength of 611 nm.
 | ||
| Fig. 14 Ix/I0 intensity ratio of Gd2O3/CTAB samples with different Eu concentrations, with excitation wavelengths of 273 nm and 394 nm. | ||
3.4. Effect of Eu concentration on PL lifetime
To investigate the influence of the concentration of Eu3+ ions doped into the matrix of the material, we also measured the time-resolved fluorescence spectra of Gd2O3/CTAB samples doped with Eu3+ ions with doping concentrations from 2 to 12% corresponding to the fluorescence excitation peak at 273 nm and the emission peak at 611 nm (Fig. 15) The results showed that the curves fit a quadratic, exponential function, indicating two separate origins of the emission process in Gd2O3/CTAB materials doped with Eu3+ ions eqn (3):
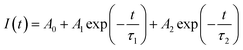 | (3) |
 | (4) |
Table 2 shows the average lifetime of Gd2O3:x% Eu3+/CTAB (x = 2–12%) samples calculated by formula (3).
| Eu3+ concentration | Average lifetime, τ* (ms) | Chromaticity diagram (x, y) | |
|---|---|---|---|
| 2 | 1.18 | 0.61 | 0.31 |
| 4 | 1.29 | 0.62 | 0.34 |
| 6 | 1.36 | 0.59 | 0.33 |
| 8 | 2.1 | 0.64 | 0.34 |
| 10 | 1.42 | 0.63 | 0.33 |
| 12 | 1.38 | 0.64 | 0.33 |
With the results of fluorescence lifetime analysis of the materials listed in Table 2, it can be seen that, as the Eu concentration increases, the average lifetime of electrons in the excited state tends to increase from 1.18 ms with the 2% Eu doped sample and reaches a maximum of 2.1 ms with the 8% Eu doped Gd2O3/CTAB sample and then tends to decrease. The results of the lifetime analysis of these Eu-doped Gd2O3/CTAB samples with different concentrations are consistent with the fluorescence analysis results in Section 3.3, as we have analyzed the energy transfer mechanism shown in Fig. 10. The energy transfer mechanisms in Eu3+-doped Gd2O3/CTAB materials when the material is excited with a wavelength of 273 nm can include: T1(O2− → Eu3+) related to the direct energy transfer between the CTB band to the Eu3+ ions; T2(Gd3+ → Eu3+) related to the energy transfer between the Gd3+ ions in the excited state 6IJ to the Eu3+ ions; T3(Eu3+ → Eu3+) related to the direct energy transfer of the Eu3+ ions in the excited state to each other. This is one of the most important mechanisms for concentration-dependent fluorescence quenching. Finally, T4(Eu3+ → O2−) is the back transfer between Eu3+ ions to O2−; in this process, the back transfer of energy of Eu3+ ions from the excited state to the CTB band. During the fluorescence excitation process at 273 nm, the T1 transition is limited because this energy is only enough to excite Gd3+ ions to the 6IJ state. With low-concentration Eu3+ doped Gd2O3/CTAB samples, the T2 process is less likely to occur than with high-concentration samples because the density of Eu3+ ion emission centers in the material is low, so the energy transfer phenomenon between Gd3+ ions in the 6IJ excited state is less likely to occur. We analyzed this result in Section 3.3 when comparing the fluorescence intensity of this sample with the sample with the highest intensity, which is only 1.16%.
Meanwhile, when excited at 394 nm, this ratio is 14.40%. When the Eu doping concentration increases, the density of the emission center increases, this process increases the non-radiative recombination when the Gd3+ ion transfers energy from the excited state 6IJ to the excited state 5DJ of the Eu3+ ion, increasing the lifetime of electrons in the excited state, while increasing the electron density in the 5D0 state. This process increases the transition of electrons in the 5D0 state to the 7FJ state, increasing the fluorescence intensity when the concentration increases. When the Eu concentration increases, the T3 energy level transfer process increases, reducing the electron density in the excited state by the reverse energy transfer process between Eu3+ ions, reducing the fluorescence lifetime and intensity due to concentration-dependent fluorescence quenching. The results of the fluorescence lifetime analysis of the material with an excitation wavelength of 273 nm, with an emission peak of 611 nm, are very consistent with the fluorescence analysis results developed in Section 3.3.
3.5. Calculation of Judd–Ofelt parameters
The probability of electric dipole transitions from the 5D0 state to the 7FJ state (where J = 2, 4, 6) is determined by the following formula eqn (5):19
 | (5) |
 | (6) |
For the transitions 5D0 → 7F2, the matrix elements are U(2) = 0.0033, U(4) = U(6) = 0. For the 5D0 → 7F4 transitions, U(2) = 0, U(4) = 0.0023, and U(6) = 0. Lastly, for the 5D0 to 7F6 transitions, U(2) = U(4) = 0 and U(6) = 0.003. The total area of the absorption bands for 5D0 to 7FJ (with J = 2, 4, 6) and 5D0 → 7F1 is also considered.
The intensity parameters Ωλ provide valuable insights into the local environment surrounding the Eu3+ ion. The parameter Ω2 is particularly sensitive to changes in ligand asymmetry and the covalency of the Eu3+–ligand bond: a high Ω2 value indicates significant ligand asymmetry and high covalency in the Eu3+–ligand bond. On the other hand. Ω4 reflects the rigidity of the environment embedding the rare-earth ion; a high Ω4 value corresponds to lower environmental rigidity (Table 3).18
| Samples | Ω2 (×10−20 cm2) | Ω4 (×10−20 cm2) | Ω6 (×10−20 cm2) |
|---|---|---|---|
| Gd2O3:2% Eu3+ | 6.63 | 1.15 | 0 |
| Gd2O3:4% Eu3+ | 8.1 | 3.22 | 0 |
| Gd2O3:6% Eu3+ | 12.2 | 3.29 | 0 |
| Gd2O3:8% Eu3+ | 14.2 | 4.01 | 0 |
| Gd2O3:10% Eu3+ | 11.2 | 3.94 | 0 |
| Gd2O3:12% Eu3+ | 11 | 3.95 | 0 |
The transition probability from the excited state J to a lower state J′ determines the fluorescence intensity of the J to J′ transition eqn (7):
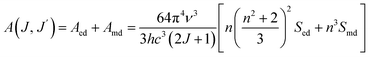 | (7) |
Total transition probability and lifetime of the excited state J eqn (8) and (9):
 | (8) |
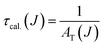 | (9) |
Branching ratio: used to predict the relative intensity of a fluorescence band from an excited state. The theoretical branching ratio is calculated using the formula eqn (10):
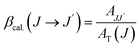 | (10) |
Quantum efficiency is determined using the following formula eqn (11):
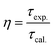 | (11) |
Quantum efficiency calculations of Eu-doped Gd2O3/CTAB materials show that the quantum efficiency ranges from 40% to 91% depending on the Eu doping concentration. The sample with the lowest quantum efficiency is the Gd2O3/CTAB sample doped with 2% Eu3+ ions; the quantum efficiency gradually increases and reaches a maximum value of 91% with the Gd2O3:8% Eu3+ sample and tends to decrease when the doping concentration is above 8%. The results obtained based on the theoretical model are consistent with the fluorescence survey results. When the Eu concentration is low, the energy transfer phenomenon between Gd3+ ions and Eu3+ ions is more challenging, so the material absorbs and leads to large non-radiative recombination. When the concentration of Eu3+ ions increases, the density of emission centers increases, and the energy transfer process between Gd3+ ions and Eu3+ ions is more effective, so the quantum efficiency increases. When the doping concentration reaches 8%, the fluorescence quenching phenomenon occurs, so the quantum efficiency tends to decrease. The result obtained for the highest quantum efficiency is 91%, which is larger than the result we obtained.33 When synthesizing this material in the environment, only urea solution and TEOS or TOPO were used (Table 4).
| Samples | βexp. (%) | βcal. (%) | AT | τcal. (ms) | τexp. (ms) | η (%) |
|---|---|---|---|---|---|---|
| Gd2O3:2% Eu3+ | 76.2 | 76 | 334.569 | 2.9 | 1.18 | 40.1 |
| Gd2O3:4% Eu3+ | 80.1 | 80 | 587.111 | 1.7 | 1.29 | 75.8 |
| Gd2O3:6% Eu3+ | 76.5 | 75 | 678.170 | 1.5 | 1.36 | 90.1 |
| Gd2O3:8% Eu3+ | 76.5 | 72 | 426.650 | 2.3 | 2.1 | 91 |
| Gd2O3:10% Eu3+ | 77.2 | 77 | 561.172 | 1.7 | 1.42 | 83 |
| Gd2O3:12% Eu3+ | 75.1 | 74 | 527.372 | 1.8 | 1.38 | 76 |
3.6. Magnetic properties
The magnetic properties of the Gd2O3:Eu3+ material with [Eu3+]/[Gd3+] ratios of 2, 4, 6, 8, 10, and 12 mol% were analyzed using a vibrating sample magnetometer (VSM) on a MicroSense EZ9 (USA), as shown in Fig. 16. The magnetism of the samples was measured at room temperature under an applied magnetic field of 20 kOe using the VSM system.The paramagnetic properties of Gd2O3:Eu3+/CTAB arise from the presence of seven unpaired electrons in the 4f shell of Gd3+. These unpaired electrons are shielded from the crystal field by the outer 5s25p6 shell electrons.14 The shape of the hysteresis curve (M–H) varies across all samples with different concentrations of Gd and Eu ions, likely due to changes in the size of the synthesized particles. As shown in Fig. 16, the magnetism of the Gd2O3 material reaches a peak value of 1.83 emu g−1. This value is twice as high as that reported by Zhang et al.34 and comparable to the results obtained by Xu et al.35 Although the addition of Eu3+ leads to a decrease in magnetic value, the Gd2O3:12% Eu3+ sample exhibits the lowest magnetism at 1.23 emu g−1, which aligns with the values reported by Zhang et al.34 Consequently, Gd2O3:Eu3+/CTAB material demonstrates significant potential for enhancing the contrast in magnetic resonance imaging (MRI).
To evaluate the potential application of the material's magnetothermal effect in targeting cancer cell destruction, we conducted an investigation of the material's heat generation capability. The inductive heating experiment was conducted in an alternating magnetic field with a frequency of 390 kHz and an intensity of 300 Oe. This magnetic field was generated by an induction coil (7 turns, 3 cm in diameter and 11.5 cm long) connected to a commercial RDO-HFI generator with an output power of 5 kW. The magnetic field intensity was calculated using the formula: H = nI, where n is the number of coil turns per unit length, and I is the amplitude of the alternating current flowing through the coil. The samples for measurement were dissolved in a water solution and thermally insulated from the external environment using a vacuum-drawn glass bottle maintained at 10−3 to 10−4 torr. Temperature readings were taken using an optical thermometer (GaAs sensor, Opsens) with an accuracy of ±0.3 °C within the range of 0 to 250 °C. The specific loss power (SLP) was calculated using the following formula eqn (12):
 | (12) |
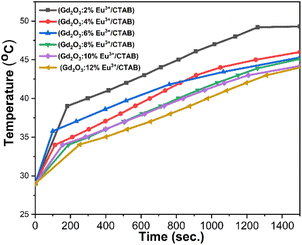
The results indicate that the samples achieve temperatures ranging from 44 to 49 °C. Physiological studies on cancer cells have demonstrated their limited heat tolerance, identifying a suitable temperature range of 42 to 49 °C for effectively destroying cancer cells without harming healthy ones.36 Therefore, in the research and development of nanomaterials for magnetic heating, it is essential to establish conditions that meet these criteria. With Eu3+ doped at molar ratios of 2%, 4%, 6%, 8%, 10%, and 12%, the observed temperatures were 49 °C, 46 °C, 45.3 °C, 45.1 °C, 44.2 °C, and 44 °C, respectively. While increasing the Eu3+ doping concentration enhances luminescent properties, it simultaneously reduces the material's magnetic properties. Thus, it is crucial to select an optimal medium doping ratio of Eu3+ that balances both luminescent and magnetic properties. These findings confirm that these material systems possess magnetism and can be effectively utilized in magnetic hyperthermia applications (Fig. 17).
To provide a clearer overview, Table 5 presents the parameters from the magnetic induction heating experiment with Gd2O3:Eu3+/CTAB samples at molar ratios of 2%, 4%, 6%, 8%, 10%, and 12%. The table includes magnetic field intensity, saturation temperature at 1500 seconds, initial heating rate, specific absorption power, and material concentration.
| Sample | (H, Oe)–(f, kHz) | Ts (°C) | dT/dt (°C s−1) | SAR (W g−1) |
|---|---|---|---|---|
| Gd2O3:2% Eu3+ | 300 Oe | 49.3 | 0.054 | 11.29 |
| Gd2O3:4% Eu3+ | 46.0 | 0.044 | 18.40 | |
| Gd2O3:6% Eu3+ | 45.3 | 0.068 | 9.20 | |
| Gd2O3:8% Eu3+ | 45.1 | 0.027 | 5.53 | |
| Gd2O3:10% Eu3+ | 44.2 | 0.032 | 6.69 | |
| Gd2O3:12% Eu3+ | 44.0 | 0.020 | 4.18 |
The specific absorption rate (SAR) values for Gd2O3 doped with Eu3+ at various molar concentrations in the presence of CTAB reveal that SAR increases at lower doping concentrations, while it gradually decreases at higher concentrations. This trend indicates that an increase in the Eu3+ ratio within the composite nanoparticles leads to a reduction in the magnetization saturation (MS) value, which subsequently decreases heat generation from magnetic induction. However, the SAR remains adequate to achieve temperatures above 42 °C, ensuring its suitability for magnetic induction heating applications.
4. Conclusions
In this study, Gd2O3:Eu3+/CTAB material was synthesized by chemical method through many steps in the presence of CTAB. The obtained material has a spherical shape, an average size distribution from 40 to 220 nm, and a typical cubic structure of Gd2O3. The material's band gap depends on the doping concentration of Eu3+ ions. The band gap tends to decrease when the Eu doping ratio increases and reaches the smallest value of about 3.44 eV when the doping ratio is 8%. Then, the band gap tends to increase when the concentration increases above 8%. This result is because when the Eu concentration increases, Eu3+ ions replace Gd3+ ions in the matrix of the material, forming emission centers in the band gap of the Gd2O3 material, causing the band gap to decrease. When the Eu ratio increases, these ions tend to cluster together and escape from the Gd2O3 material's matrix, causing the material's band gap to increase when the doping ratio increases above 8%.The Gd2O3:Eu3+/CTAB material strongly absorbs in the ultraviolet region, giving strong emission in the red light region with characteristic emissions of Eu3+ ions from the 5D0 excited state to the 7FJ state (J = 0–4). The fluorescence spectrum shows that when the Eu doping ratio is low, the energy transfer process between the bright Gd3+ ions and the Eu3+ ions is low. The fluorescence quenching phenomenon due to the concentration of this material system corresponds to the doping ratio of 8%. With the best-emitting sample, Gd2O3:Eu3+/CTAB has a quantum efficiency of about 91%.
In addition, the Gd2O3:Eu3+/CTAB material also exhibits weak ferromagnetic properties, with a maximum magnetic field of 1.83 emu g−1. This result is twice as high as the maximum magnetic field obtained by other groups using different precursors when synthesizing the material instead of CTAB. Magneto-thermal experiments show that the material can reach temperatures from 43 to 49 °C, within the optimal range for effectively killing cancer cells without harming surrounding healthy cells.
Overall, the results obtained for the Eu3+ ion-doped Gd2O3/CTAB material show that it possesses optical and magnetic properties, making it a promising candidate for multifunctional applications in diagnosis and treatment.
Data availability
All data are presented in the article.Author contributions
Pham Thi Lien: writing – original draft, methodology, investigation, formal analysis, data curation, conceptualization. Tong Quang Cong: methodology, investigation. Vu Ngoc Phan: methodology, investigation. Vu Thi Hong Ha: writing – original draft, methodology. Ho Thi Anh: methodology, investigation. Pham Duc Thang: methodology, investigation, Le Thi Hoi: methodology, investigation. Dinh Manh Tien: methodology, formal analysis. Nguyen Thanh Huong: methodology, investigation. Hoang Thi Khuyen: methodology, investigation. Lam Thi Kieu Giang: methodology, investigation. Nguyen Vu: methodology, formal analysis. Pham Hong Nam: methodology, formal analysis. Le Tien Ha: writing – review & editing, and editing the final manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was carried out with financial support from the project source ĐTĐL.CN-26/23.References
- A. Escudero, et al., Rare earth based nanostructured materials: Synthesis, functionalization, properties and bioimaging and biosensing applications, Nanophotonics, 2017, 6, 881–921 CrossRef CAS.
- H. Dong, et al., Lanthanide Nanoparticles: From Design toward Bioimaging and Therapy, Chem. Rev., 2015, 115, 10725–10815 CrossRef CAS PubMed.
- A. Garrido-Hernandez, Synthesis by hydrothermal process of lanthanide orthophosphates for optical applications, Other, Master’s thesis, Université Blaise Pascal - Clermont-Ferrand II, 2015.
- B. K. Gupta, et al., Bifunctional Luminomagnetic Rare-Earth Nanorods for High-Contrast Bioimaging Nanoprobes, Sci. Rep., 2016, 6, 1–12 Search PubMed.
- A. R. Rajan, A. Rajan, A. John, V. Vilas and D. Philip, Biogenic synthesis of nanostructured Gd2O3: Structural, optical and bioactive properties, Ceram. Int., 2019, 45, 21947–21952 CAS.
- G. Zhang, et al., Oxygen-enriched Fe3O4/Gd2O3 nanopeanuts for tumor-targeting MRI and ROS-triggered dual-modal cancer therapy through platinum (IV) prodrugs delivery, Chem. Eng. J., 2020, 388, 124269 CAS.
- W. Cai, et al., Engineering the surface of Gd2O3 nanoplates for improved T1-weighted magnetic resonance imaging, Chem. Eng. J., 2020, 380, 2–8 Search PubMed.
- H. Liu and J. Liu, Hollow mesoporous Gd2O3:Eu3+ spheres with enhanced luminescence and their drug releasing behavior, RSC Adv., 2016, 6, 99158–99164 RSC.
- Y. Wu, et al., Synthesis of bifunctional Gd2O3:Eu3+ nanocrystals and their applications in biomedical imaging, J. Rare Earths, 2015, 33, 529–534 CrossRef CAS.
- X. Mao, J. Xu and H. Cui, Functional nanoparticles for magnetic resonance imaging, Wiley Interdiscip. Rev.:Nanomed. Nanobiotechnol., 2016, 8, 814–841 CAS.
- A. Jain, et al., Functionalized rare earth-doped nanoparticles for breast cancer nanodiagnostic using fluorescence and CT imaging, J. Nanobiotechnol., 2018, 16, 1–18 Search PubMed.
- B. Qian, et al., Columnar Gd2O3:Eu3+/Tb3+ phosphors: preparation, luminescence properties and growth mechanism, CrystEngComm, 2018, 20, 7322–7328 CAS.
- G. Singh, et al., Synthesis of gadolinium oxide nanodisks and gadolinium doped iron oxide nanoparticles for MR contrast agents, J. Mater. Chem. B, 2017, 5, 418–422 CAS.
- R. Priya and O. P. Pandey, Structural, morphological, luminescent and magnetic studies of CTAB and TOPO assisted Gd2O3:Eu phosphors synthesized via co-precipitation route, J. Alloys Compd., 2020, 847, 156388 CAS.
- K. M. Riyas, P. Prasannan and P. Jayaram, Multiple deep-level defect correlated emissions and phosphorescence in Eu3+ doped Gd2O3 compound systems, Mater. Lett., 2020, 273, 127925 CAS.
- P. Serna-Gallén, H. Beltrán-Mir and E. Cordoncillo, Practical guidance for easily interpreting the emission and physicochemical parameters of Eu3+ in solid-state hosts, Ceram. Int., 2023, 49, 41078–41089 CrossRef.
- R. K. Tamrakar, D. P. Bisen and K. Upadhyay, Effect of Different Excitations on Photoluminescence Behaviour of the Tb3+ Gd2O3 Phosphor, International Journal of Luminescence and applications, 2017, 7, 359–363 Search PubMed.
- L. Chunxu, L. Junye and D. Kai, Judd-ofelt intensity parameters and spectral properties of Gd 2O3:Eu3+ nanocrystals, J. Phys. Chem. B, 2006, 110, 20277–20281 CrossRef PubMed.
- E. Blumfield, D. W. Swenson, R. S. Iyer and A. L. Stanescu, Gadolinium-based contrast agents — review of recent literature on magnetic resonance imaging signal intensity changes and tissue deposits, with emphasis on pediatric patients, Pediatr. Radiol., 2019, 49, 448–457 CrossRef PubMed.
- Y. D. Xiao, et al., MRI contrast agents: Classification and application (Review), Int. J. Mol. Med., 2016, 38, 1319–1326 CAS.
- K. A. Layne, P. I. Dargan, J. R. H. Archer and D. M. Wood, Gadolinium deposition and the potential for toxicological sequelae – A literature review of issues surrounding gadolinium-based contrast agents, Br. J. Clin. Pharmacol., 2018, 84, 2522–2534 CrossRef CAS PubMed.
- Z. Sahraei, M. Mirabzadeh, D. Fadaei Fouladi, N. Eslami and A. Eshraghi, Magnetic Resonance Imaging Contrast Agents: A Review of Literature, Journal of Pharmaceutical Care, 2014, 2, 177–182 Search PubMed.
- X. J. Chen, X. Q. Zhang, Q. Liu, J. Zhang and G. Zhou, Nanotechnology: A promising method for oral cancer detection and diagnosis, J. Nanobiotechnol., 2018, 16, 1–17 CrossRef.
- G. Zhu, R. Zhao, Y. Li and R. Tang, Multifunctional Gd,Ce,Tb co-doped β-tricalcium phosphate porous nanospheres for sustained drug release and bioimaging, J. Mater. Chem. B, 2016, 4, 3903–3910 RSC.
- F. J. Nicholls, et al., DNA-gadolinium-gold nanoparticles for in vivo T1 MR imaging of transplanted human neural stem cells, Biomaterials, 2016, 77, 291–306 CrossRef CAS.
- W. Song, W. Di and W. Qin, Synthesis of mesoporous-silica-coated Gd2O3:Eu@silica particles as cell imaging and drug delivery agents, Dalton Trans., 2016, 45, 7443–7449 CAS.
- S. Majeed and S. A. Shivashankar, Rapid, microwave-assisted synthesis of Gd2O3 and Eu:Gd2O3 nanocrystals: characterization, magnetic, optical and biological studies, J. Mater. Chem. B, 2014, 2, 5585–5593 CAS.
- R. Priya, O. P. Pandey and S. J. Dhoble, Review on the synthesis, structural and photo-physical properties of Gd2O3 phosphors for various luminescent applications, Opt. Laser Technol., 2021, 135, 106663 CrossRef CAS.
- D. Wawrzynczyk, M. Nyk, A. Bednarkiewicz, W. Strek and M. Samoc, Morphology- and size-dependent spectroscopic properties of Eu3+-doped Gd2O3 colloidal nanocrystals, J. Nanopart. Res., 2014, 16, 2690 CrossRef PubMed.
- M. Que, W. Que, T. Zhou, J. Shao and L. Kong, Photoluminescence and energy transfer of YAG: Ce3+, Gd3+, Bi3+, J. Adv. Dielectr., 2016, 6, 1650029 CAS.
- L. T. Ha, N. Duc, T. Kien and P. T. Huy, Structral characterizations and optical properties of Eu2+ doped Sr6B5PO20 phosphor powders prepared via co-precipitation method, J. Electron. Mater., 2012, 5, 165–169 Search PubMed.
- L. T. Ha, et al., Effect of doping concentration and sintering temperature on structure and photoluminescence properties of blue/red emitting bi-phase Eu3+/Eu2+-doped Sr5(PO4)3Cl/Sr3(PO4)2 phosphors, Mater. Res. Express, 2018, 5(7), 1–11 Search PubMed.
- P. T. Lien, et al., Characterization of Gd2O3:Eu3+ nanocomplexes conjugate with IgG for the identification of CEA tumor cells, Mater. Trans., 2020, 61, 1575–1579 CrossRef CAS.
- L. Zhang, et al., Mutifuntional GdPO4:Eu3+ hollow spheres: Synthesis and magnetic and luminescent properties, Inorg. Chem., 2011, 50, 10608–10613 CrossRef CAS.
- X. Xu, X. Zhang and Y. Wu, Folic acid-conjugated GdPO4:Tb3+@SiO2 Nanoprobe for folate receptor-targeted optical and magnetic resonance bi-modal imaging, J. Nanopart. Res., 2016, 18, 334 CrossRef.
- J. Beik, et al., Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications, J. Controlled Release, 2016, 235, 205–221 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |