Open Access Article
Open Access ArticleRecent advances in catalytic approaches for the synthesis of 3-substituted indoles: mechanisms and strategies
Ebraheem Abdu Musad Saleh *a,
Kakul Hussin Firoza,
Subasini Uthirapathy
*a,
Kakul Hussin Firoza,
Subasini Uthirapathy *b,
Mohammed Asiri
*b,
Mohammed Asiri c,
Rekha M Md,
Mayank Kundlase,
V. Ramesh Kumarf,
Subhashree Rayg,
Zainab Sadeq Yousifh and
Hayder Ridha-Salman
c,
Rekha M Md,
Mayank Kundlase,
V. Ramesh Kumarf,
Subhashree Rayg,
Zainab Sadeq Yousifh and
Hayder Ridha-Salman i
i
aDepartment of Chemistry, College of Science and Humanities in Al-Kharj, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia. E-mail: eabdumusadsaleh@gmail.com; e.saleh@psau.edu.sa
bPharmacy Department, Tishk International University, Erbil, Kurdistan Region, Iraq. E-mail: subasini.uthirapathy@tiu.edu.iq
cDepartment of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia
dDepartment of Chemistry and Biochemistry, School of Sciences, JAIN (Deemed to be University), Bangalore, Karnataka, India
eCentre for Research Impact & Outcome, Chitkara University Institute of Engineering and Technology, Chitkara University, Rajpura 140401, Punjab, India
fDepartment of Biotechnology, Sathyabama Institute of Science and Technology, Chennai, Tamil Nadu, India
gSiksha ‘O’ Anusandhan (Deemed to be University), Bhubaneswar, Odisha-751003, India
hMedical Laboratories Department, Mazaya University College, Dhiqar, Iraq
iCollege of Pharmacy, Al-Mustaqbal University, 51001 Babylon, Iraq
First published on 17th April 2025
Abstract
This review provides a comprehensive overview of recent advances in the synthesis of 3-substituted indoles, highlighting various catalytic methodologies employed to improve the reaction efficiency, selectivity, and sustainability. This article discusses base-catalyzed methods, amino acid catalysts, Brønsted acid catalysts, and Lewis acids and their unique roles in enhancing the synthesis of these valuable compounds. Additionally, the application of ionic liquids, surfactants, and heteropolyacid-based catalysts was explored for their green chemistry benefits, demonstrating reduced environmental impact and improved reaction outcomes. Electrochemical approaches using simple electrodes and phase-transfer catalysts are also examined as eco-friendly and efficient alternatives. This review underscores the broad versatility and applicability of these catalytic systems in synthesizing 3-substituted indoles, which are important intermediates in pharmaceuticals, material sciences, and natural product synthesis while emphasizing the need for continued innovation toward more sustainable and efficient synthesis methods.
1 Introduction
Heterocyclic compounds, particularly nitrogen-containing heterocycles (N-heterocycles), are vital components of organic materials, serving as fundamental building blocks in natural products and synthetic pharmaceuticals.1 Their broad applications across diverse fields of materials science and medicinal chemistry underscore their importance. Indole and its derivatives, which are characterized by a bicyclic structure with a nitrogen atom at the 1-position, represent a prominent and extensively studied class of N-heterocycles.2,3 The vast body of research on both naturally occurring and synthetic indoles has significantly advanced organic synthesis and continues to drive the development of indole chemistry.4–7The structural diversity of indoles and their 3-substituted derivatives is crucial for their pharmaceutical applications.8–19 Strategic modifications at the 3-position can modulate biological activity by targeting the receptors, enzymes, and transporters, highlighting the privileged nature of the indole scaffold in medicinal chemistry.20–24 As shown in Fig. 1, some examples of pharmacologically relevant 3-substituted indoles include triptans like sumatriptan and naratriptan for migraine,25–27 ergine, also known as lysergic acid diethylamide (LSD) (a psychotherapeutic adjunct and experimental tool that was first synthesized by Albert Hofmann),28 natural product-inspired compounds like gelliusine E and hyrtinadine A,29–31 anti-inflammatory drug indomethacin,32 and antimigraine agent donitriptan.33
 | ||
| Fig. 1 Selected examples of bioactive 3-substituted indole derivatives used in pharmaceutical applications. | ||
The site-selective functionalization of the indole ring presents a synthetic challenge, requiring specialized techniques and reaction conditions.34 The functionalization of the indole 3-position is particularly valuable owing to its enhanced electron density, making it highly nucleophilic. This facilitates electrophilic aromatic substitutions, resulting in a variety of 3-substituted indole derivatives.34 Various researches have led to the development of various methodologies for synthesizing 3-substituted indoles, with carbonyl compounds and α,β-unsaturated derivatives frequently used as electrophiles. These approaches expand the chemical diversity of indole derivatives, broadening their applications in pharmaceuticals and materials science.34 Among these, the active methylene-based three-component Yonemitsu condensation, which was initially reported in 1978, offers a promising route to 3-substituted indole derivatives.35 However, traditional implementations of this three-component reaction involving indole, an aldehyde, and an active methylene compound are often hampered by extended reaction times and harsh conditions, such as elevated temperatures and the use of specialized catalysts or solvents.36 Although some studies have reported a sequential approach involving the reaction of indole with pre-synthesized Knoevenagel adducts, others have employed a one-pot procedure wherein indole reacts directly with the aldehyde and active methylene compound. Such sequential reactions can be challenging to control, often leading to the formation of byproducts. In general, the condensation of an aldehyde with two distinct nucleophiles (indole and an active methylene compound) presents a challenge in the synthesis of aldehydes. Selective three-component condensation typically relies on nucleophiles possessing specific reactive functional groups that facilitate their mutual compatibility during the reaction. In the absence of such directing groups, achieving selectivity is considerably more difficult. Consequently, nucleophiles lacking these functionalities are seldom used in three-component aldehyde condensations. Several innovative research efforts have therefore focused on the rational design of catalysts capable of promoting such transformations with high selectivity.
To date, several studies have investigated the synthesis of 3-substituted indoles through three-component reactions, resulting in a diverse library of their derivatives. These reactions involve a wide range of aldehydes, including aliphatic, aromatic, and heteroaromatic aldehydes, as well as various indole derivatives, such as 1-substituted, 2-substituted, and 5-substituted indoles. In addition, various active methylene compounds have been employed, including Meldrum's acid, barbituric acid, 4-hydroxycoumarin, malononitrile, alkyl cyanoacetate, malonic esters, and 1,3-diketones (e.g., dimedone and 1,3-cyclohexadione). A wide array of catalysts, ranging from homogeneous to heterogeneous, has been used to facilitate these transformations.
In general, homogeneous catalysts offer precise control over the reaction conditions and selectivity, making them valuable for fine-tuning the reaction outcomes. These catalysts can also be tailored to specific substrates to enhance their efficiency in the promotion of desired transformations. On the other hand, heterogeneous catalysts, including those supported on various materials, offer the advantage of easy separation and recyclability, making them more sustainable and cost-effective.37–39 The use of such catalysts is of great importance in green chemistry, as they help to minimize waste and reduce the need for harsh solvents or extreme reaction conditions.40–43 The development of efficient and selective catalytic systems is therefore critical for advancing the synthesis of complex organic compounds, including 3-substituted indoles, with broader applications in pharmaceuticals, materials science, and other fields.
Numerous reviews have been published on the synthesis reactivity and applications of indoles.3,8,16,18,44,45 This review focuses on recent advances in the synthesis of 3-substituted indoles via three-component reactions. This review discusses the diverse chemical methodologies employed to synthesize a broad range of 3-substituted indoles, utilizing diverse catalytic systems, ranging from base and acid catalysts (including Brønsted and Lewis acids) and base–acid combinations to amino acid catalysts, electrochemical methods, ionic liquids, deep eutectic solvents (DES), polyethylene glycols (PEGs), heteropolyacid (HPA) catalysts, and surfactants.
2 Chemical methodologies in the synthesis of 3-substituted indoles
2.1. Base-catalyzed methods
The synthesis of 3-substituted indoles is important due to their broad application in fields such as medicinal chemistry and material science. Base-catalyzed methods are particularly effective in their synthesis because they use simple, accessible catalysts to promote reactions such as Knoevenagel condensation, Michael addition, and Friedel–Crafts alkylation. These reactions are key to the formation of 3-substituted indoles and highlight the efficiency and versatility of base catalysis. This section focuses on recent advances in base-catalyzed approaches, including the development of new catalysts and a mechanistic understanding of the driving factors of these reactions.Knoevenagel condensation is a reaction involving the dehydrogenation of active hydrogen compounds such as methylene derivatives.46 This generates an ionic intermediate that is often stabilized by resonance. This active carbanion subsequently attacks a carbonyl group via nucleophilic addition, followed by a dehydration reaction, typically yielding an α,β-unsaturated ketone (a conjugated enone).40 Common classes of active methylene compounds that undergo Knoevenagel condensation include 1,3-dicarbonyl, nitrile, and cyanoacetate. The key requirement for these compounds is the presence of a sufficiently acidic methylene group, which is typically facilitated by two electron-withdrawing groups. Although these materials exhibit some inherent acidity, the addition of a base significantly enhances the rate and efficiency of deprotonation.47–49 Building on this premise, researchers have developed a diverse array of approaches, each employing unique base catalysts, to facilitate reactions.
For example, in 2013, Singh and colleagues reported the successful utilization of tetrabutylammonium fluoride trihydrate (TBAF·3H2O) as an organobase catalyst for the solvent-free synthesis of 3-substituted indoles.50 This inexpensive, non-toxic, and air-stable quaternary ammonium salt serves as a convenient source of naked fluoride ions, making it a valuable base for transformation. This occurs through the direct generation of nucleophiles via deprotonation under mild conditions. Their investigations demonstrated that catalytic amounts of several bases, such as triethylamine (TEA), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and KOH, catalyzed the reaction, albeit with moderate yields ranging from 55–67%. This highlights the crucial role of the base in this transformation. However, TBAF provided a remarkably higher product yield under solvent-free conditions at 80 °C (Scheme 1).
Employing the reported methodology, a diverse array of indoles, aromatic aldehydes, and active methylene compounds, including malononitrile, ethyl cyanoacetate, diethyl malonate, and acetylacetone, were efficiently transformed into their corresponding 3-substituted indoles with excellent yields. The observed reactivity trends indicate that both the electronic and steric effects of all reactants significantly influence the reaction rate. Among the active methylene compounds, the following reactivity order was observed: ethyl cyanoacetate > malononitrile > diethyl malonate > acetylacetone. Indoles bearing electron-withdrawing substituents exhibited relatively lower product yields. Conversely, aromatic aldehydes substituted with halogens and electron-withdrawing functional groups reacted more rapidly and afforded higher product yields than those bearing electron-donating groups. Surprisingly, single-crystal X-ray diffraction analysis revealed that when salicylaldehyde derivatives combined with ethyl cyanoacetate and malononitrile undergo an unexpected cyclization pathway, affording 2-amino-4-(indol-3-yl)-4H-chromenes.50 Mechanistically, this transformation likely proceeds through a cascade of reactions involving the Knoevenagel, Pinner, and Friedel–Crafts steps.50,51
Based on the proposed mechanism, the strong hydrogen-bonding ability of the fluoride ion, as supported by extensive literature, plays a crucial role in these transformations (Scheme 2). Furthermore, the unique nature of TBAF arises from its electrophilic and bulky quaternary ammonium cation, which exhibits Lewis acidic character and can activate carbonyl groups through coordination with the oxygen atom.50 This initiates a cascade of reactions, including a Knoevenagel condensation between aldehydes and active methylene compounds, followed by subsequent Michael addition50 or Friedel–Crafts reaction51 of indole with the resulting arylidene or heterocyclic intermediates.
In the same year, Wang et al. reported a KH2PO4-promoted three-component synthesis of 3-indole derivatives using indoles, aldehydes, and malononitrile in a mixture of water with PEG-200 as an eco-friendly co-solvent and phase transfer co-catalyst52 (Scheme 3). They found that electron-poor aromatic aldehydes provided higher yields than electron-rich aldehydes, such as 4-methoxybenzaldehyde, which suffer from side reactions. Steric effects were also crucial; ortho-substituted aldehydes reacted more efficiently than para-substituted aldehydes. Additionally, the necessity of the N–H proton in indoles was confirmed, as 2-methyl indole gave excellent yields, whereas N-methyl indole failed to produce the desired products. This result indicates that the N–H proton activates the nitrile moiety through hydrogen bonding, significantly improving the reaction rate and yield. The optimal conditions included a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of PEG-200 to water and ambient temperature, delivering high product yields with minimal purification steps. This method also demonstrated excellent recyclability in the reaction system, further enhancing its green chemistry appeal. By combining sustainability, simplicity, and high efficiency, this protocol provides a valuable strategy for synthesizing diverse 3-indole derivatives.
1 ratio of PEG-200 to water and ambient temperature, delivering high product yields with minimal purification steps. This method also demonstrated excellent recyclability in the reaction system, further enhancing its green chemistry appeal. By combining sustainability, simplicity, and high efficiency, this protocol provides a valuable strategy for synthesizing diverse 3-indole derivatives.
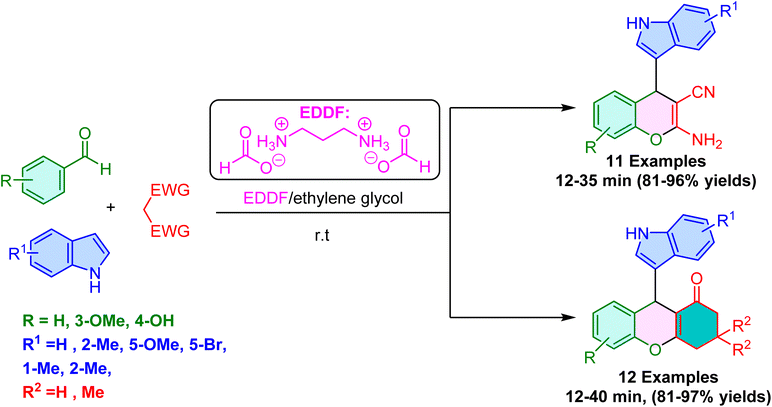
In 2015, Rawat et al. reported the EDDF(ethylene diammonium-diformate) as a base catalyst for the synthesis of 3-indolochromene and 3-indoloxanthene scaffolds via a one-pot three-component reaction of substituted salicylaldehyde, substituted indoles and active methylene compounds in ethylene glycol as a promoter and reusable solvent at ambient temperatures.53 The optimization results demonstrate that while ethylene diamine alone slows the reaction, the addition of formic acid not only slows the reaction down but also leads to significant side product formation. However, EDDF selectively produced the desired product, emphasizing its role as an effective catalyst in this reaction. Furthermore, the addition of ethylene glycol enhances EDDF's catalytic activity through a synergistic effect.
The EDDF–ethylene glycol cooperative catalytic system demonstrated high efficacy in promoting the synthesis of diverse 3-substituted indoles with excellent yields (Scheme 4). Notably, reactions involving ortho-vanillin and 2-methylindole exhibited faster kinetics than those employing salicylaldehyde. Furthermore, expanding the substrate scope to include 1,3-dicarbonyls such as dimedone and 1,3-cyclohexadione instead of malononitrile resulted in the efficient formation of the corresponding products with high yields. Importantly, this methodology exhibited high selectivity, minimizing the formation of by-products. A key strength of this method is its simplicity. In many cases, the desired product precipitates directly from the reaction mixture, enabling straightforward purification via recrystallization from ethanol. This combined with the recyclability of the EDDF–ethylene glycol system for at least six cycles significantly enhances the environmental sustainability and scalability of this method.
One year later, Deb et al. reported another convenient base-promoted method for the synthesis of 3-(α,α-diarylmethyl)indoles via a three-component reaction involving aromatic nucleophiles, aldehydes, and indoles.54 While several attempts employing Brønsted or Lewis acids, as well as organocatalysts, resulted in the undesired formation of bis(indolyl)methanes, the authors demonstrated that alkaline bases, specifically NaOH and KOH, effectively promoted the desired transformation. Despite the initial expectation that water would be a suitable solvent due to the ionic nature of the base, the reaction failed in pure water, likely due to the poor solubility of the starting materials. A mixture of ethanol and water was found to be the optimal medium. Using NaOH, a variety of β-naphthols, phenols, aldehydes, and indoles were successfully condensed to afford the corresponding 3-(α,α-diarylmethyl)indoles in moderate to excellent yields (Scheme 5). The electron-withdrawing groups on aldehydes enhanced the reaction efficiency, whereas electron-donating groups on nucleophiles (phenols) generally resulted in lower product yields. Additionally, 2-methoxynaphthalene lacking OH did not form a product, indicating the crucial role of the hydroxyl group. This suggests that deprotonation by NaOH enhances nucleophilicity, although O-alkylation remains possible. N-Alkylindoles were incompatible substrates due to the absence of an N–H proton, which is crucial for the formation of the 3-indolylalcohol intermediate. The results also highlight the formation of ionic intermediates during this transformation. Some significant advantages of this method are its green reaction conditions, selectivity and lack of chromatographic purification. Highly pure products were readily isolated through a simple trituration procedure using an EtOH/hexane mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), as confirmed by spectroscopic analysis (FT-IR, HRMS, 1H NMR, and 13C NMR).
1), as confirmed by spectroscopic analysis (FT-IR, HRMS, 1H NMR, and 13C NMR).
Based on these observations, the authors proposed a mechanism in which NaOH deprotonates indole, generating an indole anion that subsequently attacks the aldehyde to form 3-indolylalcohols (Scheme 6). Further deprotonation of the indole by NaOH and subsequent water elimination leads to the formation of an alkylideneindolenine intermediate. This reactive intermediate can then undergo various pathways, including a reaction with aryl alkoxides to yield the desired 3-(α,α-diarylmethyl)indoles products, a reaction with ethanol to form an ether (which can be reconverted to alkylideneindolenine intermediates by NaOH), and a reaction with excess indole to produce minor amounts of bisindolylmethanes as side products (Scheme 6). Water likely plays a significant role in this process. In polar protic solvents such as water, the naphthoxide/phenoxide ions generated under basic conditions are stabilized by hydrogen bonding with solvent molecules. This solvation reduces the nucleophilicity of the oxygen atom, favoring C-alkylation over O-alkylation in subsequent reactions.54
 | ||
| Scheme 6 Plausible mechanism for the synthesis of 3-(α,α-diarylmethyl)indoles via arylation of 3-indolylalcohols. | ||
Two years later, this group attempted to expand the scope of this NaOH-catalyzed functionalization of indoles by employing a range of nucleophiles in nucleophilic substitution reactions with in situ generated 3-indolylalcohol intermediates.55 However, these efforts exclusively yielded bisindolylmethane, even when using Brønsted or Lewis acids. When employing a sequential three-component approach involving the base-catalyzed generation of 3-indolylalcohol followed by acidification and subsequent addition of nucleophile, the desired nucleophilic substituted 3-indolylalcohol products were obtained in good to excellent yields. This method demonstrated excellent activity across various aldehydes and nucleophiles. While malononitrile and imidazole showed slightly lower yields, high yields were obtained with 1,3-dimethyl-6-aminouracil, N-substituted indoles, and naphthol nucleophiles (Scheme 7). In comparison with their previous method using only NaOH,54 the sequential three-component base–acid-promoted approach demonstrated enhanced efficacy with naphthol nucleophiles. This novel strategy significantly reduced the reaction time and consistently yielded higher product yields. Nevertheless, a significant limitation of this method is the necessity for the chromatographic purification of the products. This study employed a diverse set of nucleophiles for each substituted aldehyde, precluding direct comparisons of their relative reactivities. Consequently, the influence of substituents on aldehyde reactivity remains an underexplored area that warrants further investigation.
As previously reported,54 the proposed mechanism for this reaction involves an initial base-catalyzed deprotonation of the indole N–H bond by sodium hydroxide. This reaction generates a 3-indolylalcohol intermediate upon reaction with aldehyde. Subsequently, the reaction mixture is acidified to pH 5 with acetic acid, facilitating the conversion of 3-indolylalcohol into an alkylideneindolenine intermediate by the removal of a water molecule.55 This highly reactive intermediate then undergoes nucleophilic attack by various species present in the reaction medium, leading to the formation of the desired product. Concurrently, a side reaction involving indole can occur, resulting in the formation of symmetrical bisindolylmethanes as a byproduct in some cases (Scheme 8).
 | ||
| Scheme 8 Plausible mechanism for the one-pot sequential base–acid promoted synthesis of 3-substituted indoles. | ||
In 2023, Banerjee et al. discovered that a common household ingredient, sodium lauryl sulfate (SDS), could act as a surprisingly effective organocatalyst for the synthesis of 3-substituted indoles. This green and efficient method catalyzes the condensation of various salicylaldehyde, malononitrile and substituted indoles in water at room temperature with excellent yields (Scheme 9).56
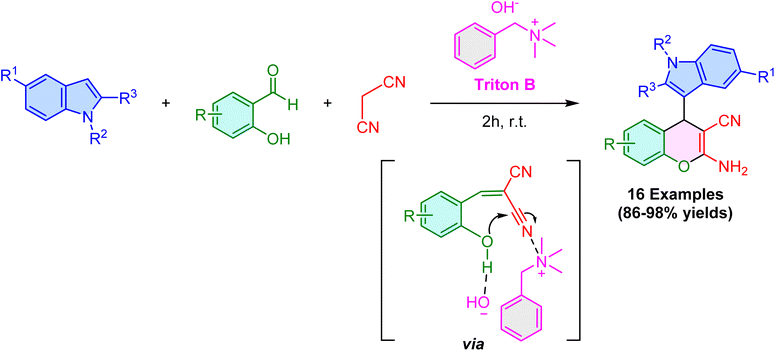
In 2023, Talukdar and coworkers demonstrated the effectiveness of benzyltrimethylammonium hydroxide (Triton B) as a base promoter for synthesizing 3-substituted indoles under environmentally friendly conditions (Scheme 10). In this approach, the quaternary ammonium hydroxide salt serves as both a catalyst and solvent, facilitating the reaction between salicylaldehyde, malononitrile, and a range of indoles. This method exhibits a broad substrate scope and delivers excellent yields of 4H-chromenes regardless of whether electron-withdrawing or electron-donating groups are present on aromatic aldehyde or indole substrates.57
In 2015, Rai et al. reported an efficient and sustainable synthesis of tetrahydro-1H-xanthen-1-one and 4H-chromene-3-carbonitrile derivatives via another innovative base-catalyzed sequential method (Scheme 11).58 Their method used a sequential multi-component one-pot approach involving the initial reaction of salicylaldehyde with an active methylene compound (malononitrile/dimedone/1,3-cyclohexanedione) in water under the catalysis of a DABCO base as an efficient medium for Knoevenagel condensation followed by cyclization to a 2-iminochrome intermediate (Schemes 2 and 11) and further addition of indole nucleophiles. The key innovation lies in the synergistic catalysis of DABCO (an organic catalyst) and beta-cyclodextrin (a biomimetic phase transfer catalyst). The combined catalytic system demonstrated remarkable versatility, accommodating several indole and active methylene substrates and consistently delivering high yields. It is worth mentioning that the screening of acidic (iodine and indium chloride) and basic catalysts (NaOH, KOH, Et3N, DMAP and DABCO) revealed DABCO to be the most effective, particularly in combination with cyclodextrin, leading to optimized yields and reaction times. The different substituents on salicylaldehyde did not significantly affect the reaction efficiency. The recyclability of the biomimetic beta-cyclodextrin surfactant enhances the sustainability and economic viability of the method.
 | ||
| Scheme 11 One-pot sequential synthesis of 3-substituted indoles using the beta-cyclodextrin/DABCO catalytic system. | ||
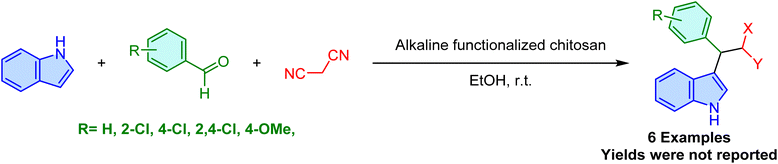
Continuing the research on heterogeneous base catalysts for these reactions, in 2017, Dekamin et al. reported the use of alkaline-functionalized chitosan as a base catalyst for the synthesis of 3-substituted indole derivatives (Scheme 12).59 This approach involves the condensation of indoles, aldehydes, and malononitrile in ethanol at room temperature. While this method offers a heterogeneous base catalyst with a safe and easy workup procedure, this conference paper lacked crucial details, such as the yields, reaction times, type of alkaline used, the exact structure of the catalyst and its preparation procedure. The lack of these crucial details necessitates further investigation to thoroughly characterize and evaluate the methodology. Despite these limitations, the salient features of this protocol include its environmental friendliness, simplicity, readily accessible catalyst, and mild reaction conditions.
In 2018, Bahuguna et al. successfully developed a novel nanocomposite material by combining polyaniline with graphitic carbon nitride (GCN) nanosheets, resulting in polyaniline-graphitic carbon nitride (PGCN). This material was subsequently used to dope ammonia (NPGCN), creating a novel heterogeneous base catalyst.60 This innovative catalyst exhibited exceptional catalytic properties in the synthesis of diverse indole-substituted 4H-chromenes through the one-pot reaction of substituted salicylaldehyde, malononitrile/ethyl cyanoacetate/methyl cyanoacetate, and substituted indoles in water. Notably, acidic PGCN led to the formation of a bis-indole product while leaving malononitrile unreacted. In contrast, ammonia-doped NPGCN effectively catalyzed the desired 4H-chromenes formation (Scheme 13).
 | ||
| Scheme 13 Synthesis of PGCN and NPGCN, and their effects on the synthesis of 3-substituted indoles and 4H-chromenes. | ||
In this method, various substituted indoles were successfully reacted in high yields. However, 7-azaindole did not participate in the reaction, likely due to the electron-withdrawing effect of the nitrogen atom at the 7 position, which deactivates the C3 position of the indole ring. Similar to Rai et al.'s research,58 the reaction of substituted salicylaldehyde bearing various electron-donating and electron-withdrawing groups showed minimal impact on the reaction's reactivity. In contrast, replacing the cyano groups in malononitrile with different esters (ethyl cyanoacetate or methyl cyanoacetate) significantly decreased the reaction performance.
The control experiments by varying the reactant ratios revealed key mechanistic insights. The use of only one equivalent of malononitrile with salicylaldehyde formed an unstable intermediate. However, two equivalents of malononitrile produced a different product, suggesting an altered pathway. Crucially, the presence of indole directed the reaction towards the desired 4H-chromene. Similar results for ethyl cyanoacetate confirmed an intermediate's formation. These findings highlighted the crucial role of indole and provided valuable mechanistic information. Additionally, the developed NPGCN catalyst demonstrated excellent recoverability and recyclability in water without any significant loss of activity, making it an ideal green chemistry catalyst.
In 2024, Cheng et al. developed a novel method for the synthesis of 3-substituted indoles via the condensation of different aldehydes and indoles over the catalysis of piperidine through tiny droplets and thin films procedure (Scheme 14).61 This method worked well with several substituted aromatic aldehydes and indoles bearing both electron-donating and electron-withdrawing groups, demonstrating its versatility. Surprisingly, ortho-substituted reacted slightly better than their para-substituted counterparts. Importantly, this approach is efficient, requires mild conditions, and can produce significant amounts of the desired products, making it an attractive and environmentally friendly option for creating such molecules.
2.2. Amino acid catalysts
Amino acids, which have reactive amino and carboxyl groups, are versatile catalysts for organic synthesis.62–64 Their natural abundance and structural diversity make them ideal for a range of reactions, particularly the synthesis of 3-substituted indoles. Recent advances have focused on boosting catalytic activity and recyclability by immobilizing amino acids on supports or incorporating them into hybrid materials like nanoparticles.37,65 This section explores the use of these catalysts in 3-substituted indole synthesis, examining the reaction mechanisms, catalytic systems, and optimized conditions.In 2013, Khalafi-Nezhad et al. reported the immobilization of L-cysteine onto magnetic nanoparticles, yielding a magnetically recoverable amino acid nanocatalyst.66 In the initial step, silica-coated magnetic nanoparticles were functionalized with vinyl groups via a reaction with trimethoxy(vinyl)silane. Subsequently, the thiol groups at the terminus of L-cysteine engaged in a thiol–ene reaction with these vinyl groups, facilitated by the presence of azobisisobutyronitrile (AIBN) as an initiator (Scheme 15). The obtained L-cysteine-functionalized Fe3O4 was abbreviated as LCMNP and applied to the synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives, leading to the formation of targeted products from the reaction of various substituted indoles and salicylaldehyde with malononitrile in water as a green solvent. The reaction yield was affected by both the electronic and steric effects of the substituents on the indole and aldehyde derivatives. Electron-withdrawing groups on substrates generally enhanced reactivity, whereas sterically hindered indoles, such as 2-methyl-1H-indole, exhibited lower reactivity.
 | ||
| Scheme 15 Synthesis of LCMNP, and its catalytic effect on the synthesis of 3-substituted indoles and 4H-chromenes. | ||
The main difference between this method and the sequential base–acid catalyzed pathway is the participation of the amine groups of the amino acid catalysts in the reaction mechanism through bond formation reactions (Scheme 16). In this way, the amine group of LCMNPs reacts with the aldehyde to form an imine intermediate. The subsequent addition of malononitrile to this imine generates another intermediate. The carboxylate group of LCMNPs also facilitates the formation of a double bond (III) by abstracting a proton. Concurrently, the amino acid moiety activates the nitrile group, enabling Michael addition to the nucleophile (IV). Moreover, the catalyst assists in the cyclization process by promoting the nucleophilic addition of the OH group to intermediate V. Finally, a proton exchange and tautomerization event culminates in the formation of the target molecule.66
Nongthombam et al. developed another hybrid catalyst for the same process. They immobilized glutathione onto Fe3O4 MNPs to create a novel amino acid-based catalyst. This hybrid catalyst demonstrated excellent activity in the ultrasound-assisted synthesis of indolylchromenes in water, achieving high yields of the desired products (Scheme 17).67
 | ||
| Scheme 17 Nano magnetic SPION@glutathione-catalyzed synthesis of 3-substituted indolyl chromenes and indolo xanthenes. | ||
Several studies have explored the catalytic potential of other amino acids, such as L-proline, in such condensation reactions. For instance, in 2015, He et al. reported the efficient room-temperature condensation of malononitrile, aliphatic and aromatic aldehydes as well as kerones and indoles to yield 3-substituted indoles with excellent yields (up to 98%) in the presence of L-proline as a catalyst in ethanol. This methodology has also been demonstrated to be effective when using pyrrole as the nucleophile and 2,2,2-trifluoroacetophenone as the electrophile (Scheme 18).68
In 2024, Burra et al. reported a highly efficient L-proline catalyzed method for the regioselective synthesis of a novel series of substituted indolyl-4H-chromene scaffolds (Scheme 19).69 This approach uses a simple reaction between 4-hydroxy-1-methyl-2-oxo-1,2-dihydroquinoline-3-carbaldehyde, active methylene compounds (malononitrile or ethyl cyanoacetate), and substituted indoles. Notably, the reaction proceeds smoothly in water under the catalytic influence of L-proline at room temperature, affording excellent yields of the desired products after a simple, facile workup and elimination of column chromatography. The authors proposed a plausible mechanism for this process, suggesting a sequential base–acid promotion. This mechanism is similar to the mechanism illustrated in Scheme 8.
In 2021, Baharfar et al. developed a new biocatalytic method for synthesizing indol-3-yl-4H-chromene derivatives.70 Their approach involves the functionalization of silica-modified magnetic nanoparticles with a dendrimer and further attachment of trypsin enzyme to the structure. This unique nanocomposite biocatalyst (Fe3O4@SiO2@D-NHCS-Tr) promoted the condensation of several indole derivatives, salicylaldehyde, and various active methylene compounds, to produce the desired product under solvent-free conditions in excellent yields (Scheme 20). Several active methylene compounds, including 1,3-dicarbonyl derivatives, malononitrile, and alkyl cyanoacetate, were efficiently transformed into the desired products under optimized conditions. The catalyst exhibits excellent reusability up to five times, confirming its stability.
2.3. Brønsted acid catalysts
Brønsted acid catalysts play a crucial role in organic synthesis due to their ability to facilitate a wide variety of reactions with high efficiency and selectivity. Their significance is particularly evident in the synthesis of indole derivatives, in which they help to activate nucleophilic species and promote protonation, which are key steps in numerous transformation pathways. Known for their ability to accelerate reactions under mild conditions and to adapt to diverse substrates, Brønsted acids have become a preferred choice in many catalytic processes.71 This section highlights the application of Brønsted acid catalysts in the synthesis of 3-substituted indoles, focusing on how their unique properties enable enhanced yields, selectivity, and sustainability in indole-related reactions, demonstrating their remarkable versatility across different synthetic routes.72,73For example, in 2016, Ganesan et al. developed an innovative approach for synthesizing 4H-chromenes in the presence of oleic acid as a lipophilic Brønsted acid catalyst.74 Interestingly, initial attempts to synthesize 4H-chromene through a one-pot sequential condensation of acetophenone, salicylaldehyde, and indole were unsuccessful. Instead, the reaction predominantly yielded bis(indolyl)methane as a byproduct, likely due to the high nucleophilicity of indole toward the aldehyde group. However, when pre-synthesized various substituted 2-hydroxychalcone derivatives were used instead, the reaction proceeded smoothly and produced the corresponding 4H-chromenes in good yields via Michael addition, followed by an intramolecular cyclization mechanism (Scheme 21). Furthermore, when acetophenone was replaced with malononitrile, the reaction successfully produced 4H-chromene derivatives in good yields at room temperature. This reaction probably occurs through the formation of an iminochromene intermediate with limited solubility in water. However, the lipophilic oleic acid effectively interacts with this intermediate in the emulsion, facilitating the Michael addition step and ultimately leading to the desired 4H-chromene products.74
2.4. Ionic liquids
Ionic liquids (ILs) have gained significant attention in recent years as efficient and sustainable catalysts for the synthesis of 3-substituted indoles. These unique solvents and catalysts can provide green, versatile, and highly selective reaction conditions, often under mild temperatures, without the need for toxic solvents. Their tunable properties, including their potential to act as solvents and catalysts, have made ILs ideal candidates for promoting various reactions, including Knoevenagel condensation, Michael addition, and Pinner cyclization. This section explores the role of ionic liquids in the synthesis of 3-substituted indoles, highlighting the development of novel IL-based catalytic systems, their environmental advantages, and the mechanism behind their catalytic activity in various synthetic routes.Rawat et al. reported a novel quasi-homogeneous catalytic system in which tetrabutylammonium valinate ionic liquid ([NBu4][Val]) is immobilized onto superparamagnetic Fe3O4 nanoparticles functionalized with 3-chloropropyltriethoxysilane (VSF) for the selective synthesis of 2-amino-4-(indol-3-yl)-4H-chromenes employing water as a mild condition75 (Scheme 22). The results revealed that electron-donating groups on the indole or aldehyde significantly enhanced the reaction efficiency, yielding higher product amounts in shorter reaction times, whereas electron-withdrawing groups slightly reduced the efficiency, resulting in moderate yields and longer reaction times. The high catalytic activity was attributed to a synergistic effect between the active sites on the Fe3O4 nanoparticles (e.g., surface vacancies, low-coordinated sites) and the distinctive properties of the immobilized ionic liquid. The carboxylate anion and ammonium ions in [NBu4][Val] likely act as a conjugate acid–base pair, facilitating the sequential Knoevenagel–Pinner reaction with efficient catalytic turnover.
In 2015, Rawat et al. developed an efficient and eco-friendly method for synthesizing 3-substituted indoles and indolyl-4H-chromenes using the biodegradable organocatalyst tetrabutylammonium glycinate ([TBA][Gly]) under solvent-free conditions at 60 °C, achieving with excellent yields.76 The reactivity of aliphatic/aromatic aldehydes significantly varied according to their electronic properties (Scheme 23). Aldehydes with electron-donating groups exhibited faster reaction times and higher yields due to their increased nucleophilicity. In contrast, aldehydes bearing electron-withdrawing groups require longer reaction times and yield slightly fewer products because their reduced nucleophilicity impedes intermediate formation. Additionally, steric effects were found to influence the reaction outcomes. Aromatic aldehydes or indole derivatives with bulky substituents, such as tert-butyl groups, led to lower yields and longer reaction times due to steric hindrance during the condensation and addition steps. Similarly, bulky active methylene compounds such as dimedone and cyclohexanedione derivatives showed reduced efficiency, producing moderate product yields alongside minor by-products. The mechanistic insights provided in the manuscript demonstrate that the ionic liquid facilitates key steps such as Knoevenagel condensation, Michael addition, and Pinner cyclization. In addition, [TBA][Gly] demonstrated impressive reusability, maintaining its catalytic activity over six cycles without degradation. This study highlights the potential of [TBA][Gly] as a sustainable and efficient catalyst for organic synthesis.
 | ||
| Scheme 23 [TBA][Gly]-catalyzed solvent-free synthesis of 3-substituted indoles and indolyl-4H-chromenes. | ||
Dige et al. highlighted the design and synthesis of a novel task-specific ionic liquid (TSIL), 1-(ethylaceto acetate)–1-(2-hydroxyethyl) piperidinium tetrachloroaluminate [EAHEPiPY]+[AlCl4]− and its application as an efficient catalyst for the synthesis of oxindoles77 (Scheme 24). This procedure uses cascade reactions of various substituted isatins and indoles with malononitrile to efficiently produce the corresponding oxindoles in high yields under mild conditions. The reaction was performed in a green solvent system comprising a water–ethanol mixture at 80 °C, significantly reducing the reaction time to just 7 hours compared to the 120 hours required in earlier methods, thereby demonstrating the TSIL's superior catalytic efficiency. The dual functionality of TSIL, attributed to its hydroxyl group for hydrogen bonding and its [AlCl4]− anion for enhanced catalytic activity, was critical to its effectiveness. Comparative analyses with other catalysts revealed that the absence of these functional groups significantly diminished the catalyst performance, further emphasizing the innovative design of this TSIL. Beyond efficiency, the study demonstrated the method's alignment with green chemistry principles. The use of a sustainable water–ethanol solvent system, high atom economy, straightforward product isolation without complex purification, and the TSIL's recyclability over multiple cycles underscore its environmental and economic advantages.
In 2018, Cheng-Bin Li et al. developed an efficient and eco-friendly method for the synthesis of biologically significant 2-amino-4-(indol-3-yl)-4H-chromene through a three-component tandem Knoevenagel–Pinner cyclization–Michael reaction, utilizing dual nature (acid and base) 1,4-diazabicyclo[2.2.2]octane (DABCO)-based ionic liquids (ILs) as catalysts78 (Scheme 25). This approach achieved excellent yields under mild conditions with short reaction times and minimal solvent use, employing ethanol as a sustainable reaction medium. Among the ILs tested, protic [DABCO-H][HSO4] was identified as the most effective, and it demonstrated exceptional reusability, retaining high catalytic activity over five cycles. The method's broad applicability was demonstrated through the successful synthesis of various 2-amino-4H-chromene derivatives, as well as 1-oxo-hexahydroxanthene derivatives, using diverse salicylaldehyde, 1H-indoles, malononitrile and pyrazolones, respectively. Among the substituted indoles, 5-nitro-1H-indole and 1-methyl-1H-indole produced slightly lower yields. Similarly, reactions involving 1H-indole and salicylaldehyde containing sterically bulky multi-substituent groups often resulted in lower yields. Additionally, mechanistic studies suggested that the IL plays multiple roles as both an acid and a base catalyst as well as a reaction medium, enabling a streamlined process. This innovative strategy, offering high efficiency, simplified product isolation via recrystallization, reusability of catalyst and alignment with green chemistry principles, holds significant potential for pharmaceutical and agrochemical applications, reinforcing its relevance in the development of sustainable synthetic methodologies.
In 2020, Ying et al. developed magnetically recoverable DABCO-functionalized mesoporous poly(ionic liquids) (MPILs) with ferric oxide nanoparticles as an efficient catalyst for the one-pot synthesis of indole derivatives and seven-membered fused indoles using indole (or its substituted derivatives), malononitrile (or ethyl cyanoacetate), and aldehydes as the key reagents79 (Scheme 26). The optimized conditions involved 20 mg of the MPIL catalyst P(4DVB-[AD][Br]) (1.28 mmol%) in ethanol at 60 °C. Under these conditions, the reactions proceeded efficiently, producing yields of 70–95% depending on the reagents used. Under the optimized conditions, aromatic aldehydes with electron-withdrawing groups generally afforded higher yields than those with electron-donating substituents. When ethyl cyanoacetate was used instead of malononitrile, yields of 86–91% were obtained. Substituted indoles, such as those bearing methyl or methoxy groups, also reacted successfully, although they required slightly longer reaction times due to reduced reactivity. Additionally, the use of 4-hydroxyindole as a reactant enabled the catalyst to selectively facilitate the synthesis of seven-membered fused indoles with moderate to good yields (70–91%) (Scheme 26). Compared to 4-H indole derivatives, this cyclization process included an additional intramolecular cyclization step, followed by isomerization, which resulted in the formation of a thermally stabilized amine as the final product. The recyclability of the catalyst up to 10 times, combined with its magnetic properties for easy recovery, underscores its potential for sustainable and practical applications in organic synthesis.
 | ||
| Scheme 26 P(4DVB-[AD][Br]) ionic liquid-catalyzed synthesis of 3-substituted indoles and seven-membered fused indoles. | ||
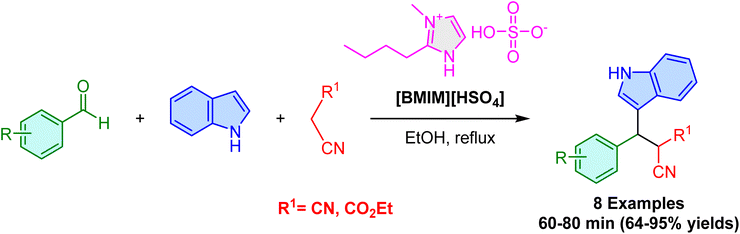
To avoid the use of above mentioned traditional halogenated ionic liquids, which are toxic and environmentally problematic. In 2021, Shekarchi et al. reported a green and efficient methodology for the selective synthesis of 3-substituted indoles via a one-pot, three-component reaction involving aldehydes, malononitrile or ethyl cyanoacetate, and indole in the presence of 1-butyl-3-methylimidazolium hydrogen sulfate [BMIM][HSO4], a non-halogenated acidic ionic liquid, in ethanol under reflux conditions (Scheme 27).80 This catalytic system providing good-to-excellent yields for a variety of 3-substituted indoles, including electron-withdrawing and electron-donating substituents on the aldehyde shows that both classes can be effectively used, offering versatility and high reaction efficiency. Electron-withdrawing groups at the meta or para positions can result in lower yields compared to electron-donating groups. Notably, the process avoids side reactions typical under acidic conditions, such as the formation of bis(indolyl)methanes, which are common in traditional methods. Additionally, [BMIM][HSO4] demonstrates excellent reusability, maintaining high reaction efficiency across up to four cycles, which further enhances the sustainability and cost-effectiveness of the methodology.
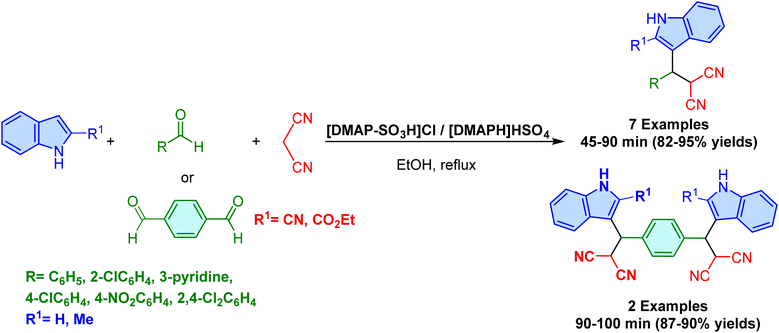
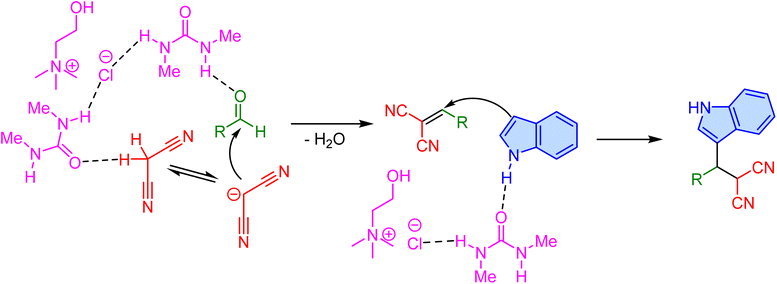
Two years later, Omidi et al. compared the synthesis of 3-substituted indoles in aqueous media using two different Brønsted acid ionic liquid catalysts: [DMAP-SO3H]Cl and [DMAP-H]HSO4, representing halogenated and halogen-free ionic liquid catalysts, respectively (Scheme 28).81 The reaction involves a one-pot three-component reaction between aldehyde, malononitrile, and indole in aqueous media, providing high yields in short reaction times under mild conditions. The results revealed that the catalytic activity of [DMAP-SO3H]Cl is superior to [DMAP-H]HSO4, offering better yields across various substrates. Notably, the reaction efficiency is largely unaffected by the type of substituent on the aromatic aldehyde, indicating the robustness of the catalytic system. Both catalysts enable the synthesis of targeted 3-substituted indoles without the need for toxic solvents or complex workup procedures, demonstrating their practical utility and sustainability. Additionally, a comparison of these ionic liquids with other acidic catalysts, such as [PySO3H]Cl and NH4HSO4, underscores the effectiveness of the DMAP-based catalysts. The ability to conduct reactions under mild, environmentally friendly conditions while maintaining high yields makes this methodology highly attractive for both laboratory and industrial applications, marking a notable advancement in the synthesis of bioactive indoles.
2.5. DES solvents
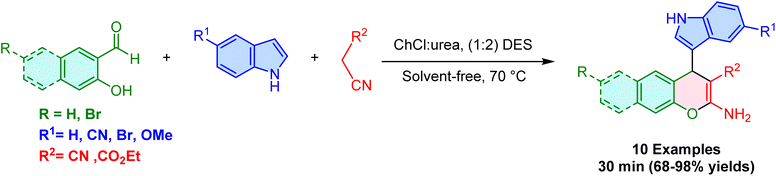
Deep Eutectic Solvents (DESs) are an interesting class of ionic liquids that are prepared by combining ammonium halides with metal salts and hydrogen bonds. These mixtures show unique properties such as salt change ability, hydrogen bond donation, low toxicity, high biodegradability, low cost, and simple synthesis, all of which make them attractive materials for chemists.82–85 In this regard, several studies have focused on using DES as a suitable catalyst and reaction medium for the reaction between indole, benzaldehyde, and malononitrile.For example, in 2018, Ruan et al. reported that choline chloride–dimethylurea (ChCl–DMU) DES catalyzed the Yonemitsu-type reaction of indole, aryl aldehydes and malononitrile or Meldrum's acid in a mixture of EtOH–H2O as a cheap and environmentally friendly method to afford the 3-substituted indoles in satisfactory yields (Scheme 29).86 The ChCl–DMU showed good activity for ortho-, meta-, and para-substituted aldehydes bearing electron-withdrawing groups. In contrast, lower yields are generated with electron-donating substituents. Accordingly, the results implied that electronic effects have a higher impact on reaction efficiency than steric hindrance, probably owing to the conjugation effect. The authors propose a particular activating pathway for this process in which both the malononitrile and aldehyde are activated by hydrogen bonding interaction with the DES, which leads to generation from the Knoevenagel adduct, followed by Michael addition reaction with indole to form the desired final product from the arylidenemalononitrile intermediate. The nucleophilicity of the indole in this step is likely enhanced by hydrogen bonding interactions with the DES (Scheme 30). One of the most significant advantages of this method is the simple purification procedure, which involves washing the precipitated products with 75% or 50% cold aqueous ethanol.
In 2019, Tran et al. reported a zinc chloride and choline chloride [cholineCl][ZnCl2]3 DES-promoted synthesis of 3-substituted indoles (Scheme 31).87 In this method, several substituted indoles and aryl aldehydes and two different active methylene compounds, including diethyl malonate or malononitrile, undergo a one-pot, three-component condensation reaction under solvent-free sonication to form the corresponding products using 30 mol% of [cholineCl][ZnCl2]3 DES as the catalyst. Both the electronic and steric effects of substituents on the benzaldehydes have controlled the reactivity and efficiency of the method. Electron-donating groups on the aryl aldehyde ring provided the desired products in good yields, while bearing electron-withdrawing ones, such as 4-NO2 and 4-F derivatives, produced the desired products in lower yields (35% and 49%, respectively). The investigation of the influence of C5-substituted indoles shows that the substitution of this position leads to an improvement in reaction yield, and the electron-rich methyl group provides a higher yield as compared to the halogens, which are deactivating. Malononitrile exhibits higher reactivity and shorter reaction times than diethyl malonate. This method offers several advantages, including broad substrate scope, low catalyst loadings, solvent-free conditions, and reusability of the DES catalyst for up to four cycles.
Nagre et al. (2021) also demonstrated that choline chloride–urea (ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, 1
urea, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) can effectively catalyze a similar three-component transformation under solvent-free conditions (Scheme 32).88 The ultrasound-assisted method significantly improved the performance of the above-mentioned ChCl–DMU catalyst. This versatile method exhibited high efficiency for a diverse array of substituted aldehydes, consistently affording high yields after recrystallization from absolute ethanol regardless of the electronic and resonance effects of substituent groups. This procedure not only eliminates the need for alkylated urea (DMU) and solvents but is also very fast and provides higher yields of targeted products.
2) can effectively catalyze a similar three-component transformation under solvent-free conditions (Scheme 32).88 The ultrasound-assisted method significantly improved the performance of the above-mentioned ChCl–DMU catalyst. This versatile method exhibited high efficiency for a diverse array of substituted aldehydes, consistently affording high yields after recrystallization from absolute ethanol regardless of the electronic and resonance effects of substituent groups. This procedure not only eliminates the need for alkylated urea (DMU) and solvents but is also very fast and provides higher yields of targeted products.
 | ||
Scheme 32 ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea, (1 urea, (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES-catalyzed solvent-free synthesis of 3-substituted indoles under ultrasound irradiation. 2) DES-catalyzed solvent-free synthesis of 3-substituted indoles under ultrasound irradiation. | ||
Two years later, Alvi et al. reported ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES as a green solvent and catalyst for the synthesis of indole-based 4H-chromenes.89 The synthesis involved the initial reaction of a mixture of choline chloride and urea with 1
2) DES as a green solvent and catalyst for the synthesis of indole-based 4H-chromenes.89 The synthesis involved the initial reaction of a mixture of choline chloride and urea with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratios at 70 °C for 30 min to obtain ChCl
2 molar ratios at 70 °C for 30 min to obtain ChCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) urea (1
urea (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) DES as a clear melting mixture, followed by the addition of substituted indoles, active methylene compounds (malononitrile and ethyl cyanoacetate), and ortho-hydroxyaryl aldehydes (2-hydroxy-1-naphthaldehyde and salicylaldehyde) (Scheme 33). The reaction was affected by the substituent effects of the 5-substituted indoles. Electron-donating groups at this position of the indole ring enhanced its nucleophilicity, leading to higher reaction yields than electron-withdrawing groups. Furthermore, while replacing malononitrile with ethyl cyanoacetate had a minimal effect on the reaction efficiency, salicylaldehyde consistently afforded higher yields than 2-hydroxy-1-naphthaldehyde and its derivatives. The reusability study indicated that this inexpensive solvent/catalyst system could be operated for four cycles with no appreciable loss in activity.
2) DES as a clear melting mixture, followed by the addition of substituted indoles, active methylene compounds (malononitrile and ethyl cyanoacetate), and ortho-hydroxyaryl aldehydes (2-hydroxy-1-naphthaldehyde and salicylaldehyde) (Scheme 33). The reaction was affected by the substituent effects of the 5-substituted indoles. Electron-donating groups at this position of the indole ring enhanced its nucleophilicity, leading to higher reaction yields than electron-withdrawing groups. Furthermore, while replacing malononitrile with ethyl cyanoacetate had a minimal effect on the reaction efficiency, salicylaldehyde consistently afforded higher yields than 2-hydroxy-1-naphthaldehyde and its derivatives. The reusability study indicated that this inexpensive solvent/catalyst system could be operated for four cycles with no appreciable loss in activity.
2.6. Heteropolyacid-based methodologies
Heteropolyacid (HPA)-based catalysts are of significant interest due to their high acidity, thermal stability, and ability to catalyze diverse reactions under mild, environmentally friendly conditions. Combining HPAs with supports or nanoparticles creates novel catalytic systems with improved reaction rates, recyclability, and sustainability.90,91 They are particularly effective for synthesizing 3-substituted indoles with high efficiency and selectivity. This section examines the role of HPA-based catalysts in 3-substituted indole synthesis, highlighting their versatility in various pathways, including solvent-free and ultrasound-assisted methods, and their potential in green chemistry.In 2016, Amrollahi and Kheilkordi developed phosphotungstic acid as an efficient catalyst for the synthesis of bis(indoles) via a one-pot pseudo-four-component reaction involving aldehydes, active methylene compounds, and indoles (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio) in aqueous media under both silent and ultrasonic conditions (Scheme 34).92 This method produces the desired products in moderate to excellent yields under green conditions. The reaction exhibited good scope for various substituents in the benzaldehyde, including both electron-donating and -withdrawing groups. However, with 2- and 3-pyridinecarboxaldehydes, only the corresponding indolyl derivatives were obtained in high yields. The authors attributed this observation to the potential oxidative dehydrogenation of intermediate 2-((indolyl)(pyridyl)methylene)malononitriles and 3-(indolyl)(pyridyl) acrylates catalyzed by H3PW12O40. The results demonstrate that ultrasonication significantly accelerates the reaction rate, leading to product formation in a considerably shorter timeframe compared to the silent method.
2 molar ratio) in aqueous media under both silent and ultrasonic conditions (Scheme 34).92 This method produces the desired products in moderate to excellent yields under green conditions. The reaction exhibited good scope for various substituents in the benzaldehyde, including both electron-donating and -withdrawing groups. However, with 2- and 3-pyridinecarboxaldehydes, only the corresponding indolyl derivatives were obtained in high yields. The authors attributed this observation to the potential oxidative dehydrogenation of intermediate 2-((indolyl)(pyridyl)methylene)malononitriles and 3-(indolyl)(pyridyl) acrylates catalyzed by H3PW12O40. The results demonstrate that ultrasonication significantly accelerates the reaction rate, leading to product formation in a considerably shorter timeframe compared to the silent method.
 | ||
| Scheme 34 H3PW12O40-catalyzed solvent-free synthesis of bis(indoles) under silent and ultrasound irradiation. | ||
In 2017, Mahdizadeh Ghohe and co-workers developed a new heterogeneous catalyst for the synthesis of 3-substituted indole.93 First, they synthesized vanadium-doped SBA-15 (VOx/SBA-15) by the sol–gel method. This material was subsequently functionalized with 3-(triethoxysilyl)propylamine acting as an amine-containing linker molecule for the non-covalent immobilization of the H5PW10V2O40 HPA to obtain a new SBA-15 supported Keggin-type catalyst (PW10V2@VOx/SBA-15-NH2) with both Lewis and Brønsted acid sites. PW10V2@VOx/SBA-15-NH2 was found to be an efficient catalyst for the one-pot synthesis of 3-substituted indoles under solvent-free and mild conditions (50 °C) (Scheme 35). The efficiency of the catalytic system was evidenced by its wide substrate scope and consistently high yields. In particular, the conversion of the aldehydes containing electron-withdrawing substituents provided slightly higher yields (78–95%) than those with electron-donating groups (73–76%). Remarkably, the catalyst exhibited good recyclability, maintaining its catalytic activity across a range of reaction cycles, with no significant decrease in activity.
 | ||
| Scheme 35 H3PW12O40-catalyzed solvent-free synthesis of bis(indoles) under silent and ultrasound irradiation. | ||
Tayebee et al. developed a similar method for synthesizing 3-substituted indoles using highly dispersed H3PW12O40@SBA-15.94 This approach involves the facile ligand-free immobilization of H3PW12O40 onto SBA-15 under ultrasonic irradiation (Scheme 36). The resulting supported catalyst shows excellent performance in the conversion of different electron-withdrawing and donating substituted aromatic aldehydes to the corresponding 3-substituted indole products under solvent-free conditions, at 60 °C in high yields for most cases, with electron-withdrawing groups showing slightly superior yields compared to electron-donating ones. The hot filtration test indicated low H3PW12O40 leaching from the SBA-15 surface, making it an excellent choice for such reactions.
Wang et al. synthesized a novel heterogeneous nanohybrid material by combining a tetraphenylporphyrin (TPP) with nickel oxide nanoparticles and H5PW10V2O40 (HPA).95 This innovative inorganic–organic nanohybrid demonstrated high catalytic activity in the solvent-free synthesis of 3-substituted indoles via the condensation of indole with malononitrile and various aldehydes in excellent yields due to the synergistic interplay between the TPP, HPA, and NiO components via their ability to function as electron acceptors, facilitating efficient charge separation between electrons and holes. Interestingly, benzaldehydes bearing electron-withdrawing substituents tended to produce higher yields than their counterparts with electron-donating groups (Scheme 37).
2.7. Lewis acid catalysts
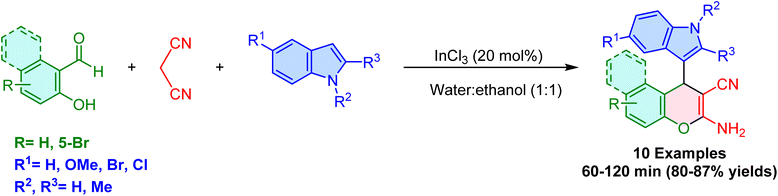
Lewis acid catalysts are widely used in organic synthesis because of their ability to activate electrophilic species, making them highly effective in facilitating various reactions, including the synthesis of 3-substituted indoles. These catalysts, such as copper, zinc, and indium-based compounds, have shown remarkable efficiency in promoting multicomponent reactions, often under mild and environmentally friendly conditions. This section focuses on the use of Lewis acid catalysts in the synthesis of 3-substituted indoles, highlighting their versatility, reaction efficiency, and selectivity. Key examples include copper and zinc catalysis, which have demonstrated broad substrate compatibility and high yields, making them valuable tools for synthesizing bioactive indole derivatives.In 2007, Perumal and Shanthi reported an efficient method for the room-temperature synthesis of indolyl chromenes via a three-component condensation of salicylaldehyde derivatives, malononitrile, and indoles, catalyzed by indium(III) chloride (InCl3) in aqueous ethanol media, achieving up to 88% yield (Scheme 38).96 This protocol was extended to 2-hydroxynaphthalene-1-carboxaldehyde, indole, and malononitrile. Electron-donating groups (e.g., –CH3 and –OCH3) at the 1, 2, and 5 positions of the indole ring enhance its nucleophilicity, leading to higher yields and faster reactions. In contrast, electron-withdrawing groups (e.g., –Br and –Cl) reduce nucleophilicity, resulting in slower reactions and lower yields. This method highlights the increasing importance of indium-based Lewis acids in organic synthesis, particularly in sustainable methodologies.
In 2011, Qu et al. developed a straightforward and efficient method for synthesizing 3-substituted indoles through a three-component reaction involving indoles, aldehydes, and malononitrile, catalyzed by a copper(II) sulfonato salen complex in water (Scheme 39).97 The addition of KH2PO4 significantly improved both the reaction efficiency and selectivity, likely by regulating the pH of the aqueous solution, as demonstrated by the superior results compared to other bases like K2CO3 and KOAc. The methodology proved to be highly versatile, accommodating a wide range of aldehydes and 1,2-methyl indole derivatives, yielding excellent results across various substrates. The use of N-methyl indole resulted in slightly lower yields than 1H-indoles. On the other hand, substituents on the aldehyde, particularly electron-withdrawing groups, enhanced the reaction rate, whereas steric effects had minimal influence on the outcomes. This robust approach, with its high yield, selectivity, and broad functional group tolerance, stands as an efficient and environmentally friendly strategy for synthesizing 3-substituted indoles, which are valuable intermediates in the production of bioactive compounds and pharmaceuticals.
In 2012, Chandrasekhar et al. reported a ligand-free, copper(II)-catalyzed three-component reaction for synthesizing 3-indole derivatives using indole, aldehydes, and malononitrile or ethyl 2-cyano acetate in PEG-400 at 70 °C (Scheme 40).98 This eco-friendly method eliminates the need for complex ligands using PEG-400 as both a solvent and a potential co-ligand, along with Cu(OAc)2 as the catalyst and KH2PO4 as an additive. Mechanistic studies reveal that the reaction proceeds via an uncatalyzed Knoevenagel condensation, followed by a copper-catalyzed Michael addition of indole to the Knoevenagel adduct. This process demonstrates high efficiency, with aldehydes bearing electron-withdrawing groups resulting in the highest yields (up to 98%), followed by halogen-substituted aldehydes with good yields ranging from 70% to 88%. In contrast, aldehydes with electron-donating groups produce the lowest yields (48% to 68%). Aliphatic aldehydes, such as isobutyraldehyde, provide moderate yields (62%). This indicates that electron-withdrawing groups enhance reaction efficiency, while electron-donating groups lower yields. Additionally, ethyl 2-cyano acetate and substituted indoles, including N-methyl and 5-methoxy indoles, also participate effectively in the reaction. This method provides a simple, efficient, and sustainable route for preparing a diverse range of 3-indole derivatives, with both the PEG-400 solvent and copper catalyst being recyclable up to five times with minimal loss in activity.
 | ||
| Scheme 40 Ligand-free copper(II) acetate-catalyzed aqueous synthesis of 3-substituted indoles in PEG-400. | ||
A 2016 study by Jiang et al. presents a highly efficient, environmentally friendly method for synthesizing 3-substituted indoles via a base-free copper-catalyzed three-component reaction in water.99 Using a 2-pyrrolecarbaldiminato–Cu(II) complex as the catalyst, the reaction involves indole, an aldehyde, and malononitrile (or ethyl 2-cyanoacetate) at 30 °C, offers high yields (80–96%) within 12 hours without the need for any additional base or organic solvents (Scheme 41). The reaction demonstrates broad substrate compatibility, efficiently converting both electron-withdrawing and electron-donating aldehydes to the desired products, with electron-withdrawing groups typically yielding higher results. The approach also accommodates sterically hindered aldehydes, such as 2-methoxybenzaldehyde, and aliphatic aldehydes like cyclohexanecarbaldehyde. Additionally, substituted indoles such as 2-methylindole, 5-methoxyindole, and 5-bromoindole provide good yields (77–78%). Ethyl 2-cyanoacetate also performs well under the same conditions, yielding a diastereomeric mixture at 77% yield. The use of water as the solvent, combined with the base-free conditions, makes this reaction eco-friendly and sustainable, offering a simple and efficient route for synthesizing bioactive 3-indole derivatives.
 | ||
| Scheme 41 2-Pyrrolecarbaldiminato–Cu(II) complex-catalyzed aqueous synthesis of 3-substituted indoles. | ||
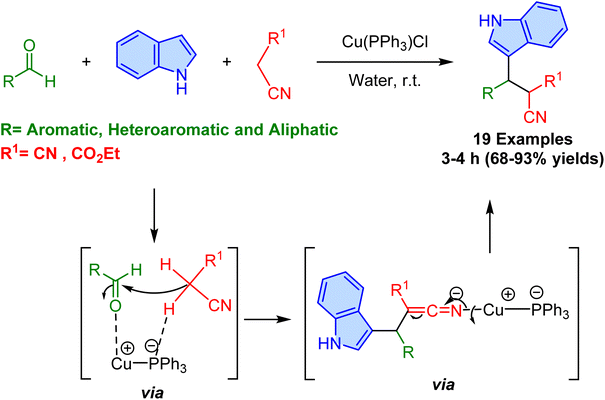
In 2017, Prasad et al. reported a highly efficient method for room temperature synthesizing 3-substituted indoles through a one-pot multicomponent reaction catalyzed by a copper(I)–phosphine complex, Cu(PPh3)Cl, in water as environmentally friendly conditions (Scheme 42).100 This approach utilizes aldehydes (aromatic, aliphatic, and heteroaromatic), indole, and active methylene compounds (such as malononitrile and ethyl 2-cyano acetate) to form 3-substituted indole derivatives in good to excellent yields (68–93%). The copper(I)-phosphine catalyst was found to be more effective than other copper salts and copper metal powder, showing significant catalytic activity without the need for external ligands or additives, which is a notable advantage. The method works efficiently with broad substrate compatibility and the influence of both electronic and steric factors on the reaction efficiency. Notably, aldehydes with electron-withdrawing groups were more reactive, leading to higher yields, whereas electron-donating groups resulted in lower yields. Additionally, steric effects had minimal influence on the reaction, as demonstrated by the moderate yields obtained from substrates like 2-fluoro and 2-trifluoromethyl benzaldehyde. The method was also effective with heteroaromatic aldehydes (e.g., 2-thiophenecarboxaldehyde) and aliphatic aldehydes (e.g., nonanal), which provided good yields of the 3-substituted indoles. Interestingly, the reaction of aldehydes with ethyl 2-cyano acetate gave excellent yields, albeit as an inseparable diastereomeric mixture. Based on the proposed mechanism, the reaction proceeds through a Knoevenagel condensation followed by a Michael addition of indole, facilitated by the Cu(I)-phosphine catalyst.
In the same year, Das et al. developed a novel strategy for constructing unsymmetrical 3-substituted indole-based triarylmethanes via Cu(II)-catalyzed dual C–C coupling method, which efficiently assembles indoles, aldehydes, and arylboronic acids into triarylmethane derivatives (Scheme 43).101 This method overcomes the challenge of the nucleophilicity of indoles, which often leads to the formation of undesired bis(indolyl)methanes. The approach demonstrates excellent functional group tolerance, high selectivity, and the use of inexpensive and readily available reagents, making it a promising solution for synthesizing diverse indole-based compounds. Key to the success of this methodology is the use of Cu(OTf)2 as a catalyst, which ensures high yields and efficient reactions under mild conditions, even with sterically hindered aldehydes. This strategy not only expands the scope of indole synthesis but also facilitates the creation of compounds with potential applications in drug design, including anti-cancer and anti-viral agents.
In 2024, Gogoi et al. Reported the CuSB-GO/FO heterogeneous nanocomposite, a novel magnetically retrievable Schiff base copper complex grafted onto amino-functionalized graphene oxide, as an efficient catalyst for the synthesis of 3-substituted indoles via a three-component reaction with indole, aldehydes, and malononitrile (Scheme 44).102 Under optimized conditions (ethanol–water solvent, 35 °C, and 10 wt% catalyst loading), the catalyst achieved high yields (up to 98%) in just 10–20 minutes. Its performance is significantly enhanced by the presence of electron-withdrawing groups on the aldehyde. The catalyst is easily recovered via magnetic separation and can be reused up to four times with minimal loss of activity. The system also exhibits remarkable stability and high catalytic turnover compared to other catalysts reported in the literature.
In 2011, Chen et al. reported a highly efficient method for synthesizing enantioenriched 2-amino-4-(indol-3-yl)-4H-chromenes via a one-pot, three-component domino Knoevenagel/Pinner/Friedel–Crafts reaction catalyzed by an N,N′-dioxide Zn(II) complex as a chiral Lewis acid (Scheme 45).51 Using an N,N′-dioxide ligand derived from L-proline and Zn(ClO4)2·6H2O, along with NaBArF and 3 Å molecular sieves in dichloromethane, they achieved good yields and enantioselectivities (up to 90% ee) with various indoles and salicylaldehyde under mild conditions. The reaction was sensitive to the substitution pattern on the indole, with electron-withdrawing groups, particularly 5-bromoindole, leading to higher enantioselectivities. Although various salicylaldehydes were tolerated, 4-methoxy substitution led to a decrease in yield, and the sterically hindered 3,5-di-tert-butyl derivative, while maintaining high enantioselectivity, also resulted in reduced yield. 2-Hydroxy-1-naphthaldehyde was identified as a suitable alternative, providing both good yield and enantioselectivity. The method demonstrated practical applicability through successful gram-scale synthesis, where the reactivity and enantioselectivity were not compromised. Additionally, the products could be easily purified by recrystallization, underscoring the method's potential for large-scale applications. Overall, the study provides a valuable approach to the synthesis of optically active chromenes and could be useful for future catalytic asymmetric syntheses.
 | ||
| Scheme 45 N,N′-Dioxide Zn(II) complex-catalyzed enantioselective one-pot synthesis of 2-amino-4-(indol-3-yl)-4H-chromenes. | ||
In 2012, Kleij et al. developed another Zn(II)-mediated multi-component synthesis of 3-substituted indoles using a zinc(salphen) complex. The reaction involved benzaldehydes, indole, and malononitrile as substrates, with diisopropylethylamine (DIPEA) as the base in dichloromethane (Scheme 46).103 The product formation was significantly affected by the formation of side products, resulting from the intermediate benzylidene-1,1-dicyanomethane. These intermediates play a critical role in the reaction's selectivity, as it can further react with malononitrile, leading to undesired by-products and reducing the yield of the desired 3-substituted indoles. Notably, the selectivity for the desired product is influenced by the type of aldehyde used, with electron-deficient aldehydes yielding more favorable results. This study provides valuable insights into the complexities of 3-substituted indole synthesis, emphasizing the importance of reaction intermediates in shaping product distribution.
In 2013, Ghosh and Das reported a green synthesis of indolyl chromenes using ZnO nanoparticles, synthesized via a bottom-up approach, in aqueous media at 55 °C through the “NOSE” method (Scheme 47).104 The reaction efficiently produced the desired products in 90–91% yields within 40–50 minutes by reacting 2-hydroxybenzaldehyde and indole with active methylene compounds including; dimedone, malononitrile, and N,N-dimethylbarbituric acid. The study highlights nano-ZnO as a highly effective catalyst, outperforming alternatives like PTSA, L-proline, and zeolite in terms of yield, reaction time, and selectivity. Additionally, the catalyst can be recycled for up to six cycles without significant loss of activity. This environmentally benign method does not require toxic solvents or chromatography, making it a cleaner and more sustainable approach for the synthesis.
In 2014, Rajesh et al. developed a highly effective and environmentally friendly heterogeneous catalyst by combining reduced graphene oxide (RGO) and zinc oxide (ZnO), known as the RGO/ZnO composite (Scheme 48).105 This catalyst was used for the efficient synthesis of physiologically active 3-substituted indoles in water, offering a reusable solution for sustainable chemical processes. The study demonstrated that RGO/ZnO facilitated the rapid and selective synthesis of a diverse range of 3-substituted indoles, achieving good to excellent yields at low times. This process was effective for various substrates, including aromatic aldehydes, substituted indoles, secondary amines and active methylenes such as malononitrile and ethyl cyanoacetate. The study highlighted that the reactions with aryl aldehydes containing electron-withdrawing groups resulted in higher yields and faster reaction times, whereas those with electron-donating groups showed lower yields and required longer reaction durations. Additionally, reactions involving cyano (CN) groups exhibited enhanced yields and faster reaction times compared to those with ethyl cyanoacetate (CO2Et). Importantly, the RGO/ZnO catalyst demonstrated remarkable recyclability, maintaining its catalytic activity across six consecutive runs without any significant loss in efficiency.
In the same year, Renzetti et al. reported a Yonemitsu-type trimolecular condensation for the room-temperature synthesis of 3-substituted indoles using L-proline and europium triflate (Eu(OTf)3) as catalysts in methanol (Scheme 49).106 The reaction is compatible with various active methylene compounds, although their reactivity varies. Methyl acetoacetate provided moderate to high yields, whereas ethyl nitroacetate showed superior reactivity. Conversely, ethyl methylsulfonylacetate and ethyl benzoylacetate yielded lower conversions, likely due to enolate stabilization by the metal catalyst. These differences stem from the electronic effects of carbonyl substituents, with more acidic compounds reacting more efficiently. This method, while not enantioselective, exhibits notable diastereo-selectivity in some cases, with yields ranging from 15% to 78%, depending on the substrate. The proposed mechanism involves a combination of Knoevenagel condensation and Michael addition, where L-proline activates both the nucleophile and electrophile, while Eu(OTf)3 enhances electrophilicity, facilitating the final addition step. This catalytic system is a versatile and efficient approach to synthesizing polyfunctionalized indoles under mild conditions.
Another noteworthy catalytic approach for the synthesis of 3-substituted indoles was introduced by Pradhan et al. in 2017, employing phosphate-grafted SnO2–ZrO2 nanocomposite oxides as a catalyst, synthesized through a hydrolysis method followed by phosphate grafting, exhibit enhanced acidic properties due to the interaction between phosphate ions and metal centers107 (Scheme 50). The resulting materials efficiently catalyze the one-pot multicomponent condensation reaction of indole, malononitrile, and aryl aldehydes under mild conditions, yielding 3-substituted indoles in excellent purity and high yields in a short reaction time. The substitution pattern of the aryl aldehyde plays a crucial role in the reaction efficiency. Aldehydes with electron-withdrawing groups, such as 4-NO2 and 2-NO2, result in higher yields (92% and 90%, respectively) than electron-donating groups like 4-CH3 (82%) and 4-OCH3 (81%). The position of the substituent further influences the reaction outcomes, with para-substituents like 4-chlorobenzaldehyde leading to a yield of 91%, while meta-substituents like 2-chloro result in a slightly lower yield (88%) due to steric effects. In general, electron-withdrawing groups and para-substituents improve the reaction efficiency. Compared to traditional catalytic systems, phosphate-grafted SnO2–ZrO2 composites offer several advantages, including improved recyclability, high catalytic activity, and significantly reduced reaction times, making them an attractive choice for both laboratory and industrial applications.
In 2017, Omidi and Amrollahi developed an efficient, environmentally friendly Zn-catalyzed methodology to synthesize a library of 3-substituted indoles containing highly polarized double bonds with good yields (Scheme 51).108 Their approach utilized a Zn(OAc)2/NaOAc-catalyzed three-component reaction of an aldehyde, malononitrile, and indole to generate (indolylmethyl)malononitriles. The study demonstrated that the reaction could proceed under both silent and ultrasound conditions, with the best results achieved at 60 °C in ethanol under ultrasound irradiation, which significantly accelerated the reaction and reduced the reaction time to mere minutes. Electron-withdrawing groups enhanced the reaction efficiency, whereas electron-donating groups reduced the reactivity. Additionally, sterically bulky groups, such as 2,4-diCl, slowed down the reaction due to spatial constraints. The oxidative elimination of these compounds under solvent-free conditions in the presence of calcium hypochlorite as the oxidative agent led to the formation of indolylacrylonitriles in high yields and short reaction times.
 | ||
| Scheme 51 Zn(OAc)2/NaOAc-catalyzed synthesis of 3-substituted indoles and oxidative elimination of (indolylmethyl)malononitriles using Ca(OCl). | ||
2.8. Surfactants
Surfactants play a crucial role in enhancing the efficiency of organic reactions, particularly in aqueous media, by facilitating the dispersion of organic molecules and reducing interfacial tension. The use of surfactants in catalysis not only improves substrate interaction and helps control reaction pathways, promoting greener and more sustainable chemistry. The incorporation of surfactants in water facilitates the dispersion of organic molecules and lowers the interfacial tension within the aqueous medium.109 In this regard, in 2018, Mirhashemi and Amrollahi, developed the β-cyclodextrin (β-CD) inclusion complexes with surfactants, specifically cetyltrimethylammonium bromide (CTAB) and sodium dodecyl sulfate (SDS), as catalysts for the selective synthesis of mono- and bis-(indolyl)methylmalononitriles via a one-pot, three-component reaction involving aldehydes, malononitrile, and indole in an aqueous medium (Scheme 52).110 The encapsulation of surfactant molecules within β-CD cavities creates hydrophobic microenvironments that enhance substrate interaction and reaction efficiency. The results of this study underscore the versatility of β-CD inclusion systems in controlling reaction pathways and promoting green chemistry. The β-CD/CTAB complex was optimized for mono-product formation using a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of indole, aldehyde, and malononitrile, facilitating a single Michael addition. Conversely, the β-CD/SDS complex was more effective for bis-product synthesis by increasing the indole ratio to 2
1 molar ratio of indole, aldehyde, and malononitrile, facilitating a single Michael addition. Conversely, the β-CD/SDS complex was more effective for bis-product synthesis by increasing the indole ratio to 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, enabling a second conjugate Michael addition of indole with (indolyl)methylenemalononitriles in higher times. Electron-withdrawing substituents on aldehydes (e.g., nitro, fluoro) enhanced reaction efficiency in the synthesis of both mono- and bis-(indolyl)methylmalononitriles by increasing yields (up to 95%) and reducing reaction times. In contrast, electron-donating substituents, like methoxy and hydroxy groups resulted in slightly lower yields and longer reaction times due to the decreased electrophilicity of the aldehyde. These inclusion complexes significantly outperformed individual surfactants, offering shorter reaction times, high product selectivity, and mild, environmentally friendly conditions. Furthermore, the ICs were reusable with minimal activity loss, thereby reinforcing their eco-friendly potential.
1, enabling a second conjugate Michael addition of indole with (indolyl)methylenemalononitriles in higher times. Electron-withdrawing substituents on aldehydes (e.g., nitro, fluoro) enhanced reaction efficiency in the synthesis of both mono- and bis-(indolyl)methylmalononitriles by increasing yields (up to 95%) and reducing reaction times. In contrast, electron-donating substituents, like methoxy and hydroxy groups resulted in slightly lower yields and longer reaction times due to the decreased electrophilicity of the aldehyde. These inclusion complexes significantly outperformed individual surfactants, offering shorter reaction times, high product selectivity, and mild, environmentally friendly conditions. Furthermore, the ICs were reusable with minimal activity loss, thereby reinforcing their eco-friendly potential.
2.9. Electrochemical approach
Electrochemical methods have gained attention as an efficient and sustainable approach for synthesizing 3-substituted indoles. By utilizing electrodes and phase-transfer catalysts, these methods offer a unique, environmentally friendly alternative to traditional catalytic systems. In 2017, Vinay K. Singh and coworkers have developed an electrochemical method for synthesis of 3-substituted indoles.111 This method involved the condensation of various aldehydes, indole, and malononitrile in the presence of LiClO4 as the phase-transfer catalyst or supporting electrolyte, using inexpensive graphite and iron plate electrodes under an open-air atmosphere. The electrolysis was performed in ethanol at an applied potential of 1.5 V and a current density of 10 mA cm−2. This procedure worked well for a broad range of substrates including; both aromatic and aliphatic aldehydes and various N-substituted indoles. Electron-withdrawing substituted aldehydes generally favored the reaction, while steric hindrance appeared to have little effect (Scheme 53). They suggested that the reaction proceeds at the anode by deprotonating malononitrile via its reaction with in situ generated ethoxide ions in ethanol solvent. After the formation of a desired active anion, the process is followed with a Knoevenagel condensation and subsequent Michael addition sequence.3 Conclusion
In conclusion, the synthesis of 3-substituted indoles has undergone remarkable evolution driven by the development of diverse and innovative catalytic methodologies. These advances offer a range of advantages regarding efficiency, selectivity, and sustainability, demonstrating the potential of modern synthetic chemistry. From traditional base-catalyzed reactions and bio-inspired amino acid catalysts to the innovative use of ionic liquids, surfactants, Lewis acids, and even electrochemical techniques, the field has consistently pushed boundaries. The emergence of heteropolyacid-based catalysts and surfactant inclusion complexes further underscores the growing emphasis on green chemistry principles. This impressive array of methods, coupled with their broad substrate compatibility, positions 3-substituted indoles as crucial building blocks in both academic and industrial settings. Looking ahead, key areas for future research include catalyst recyclability, reaction optimization (particularly concerning reaction times), and the continued pursuit of truly sustainable synthetic strategies. These efforts will undoubtedly enhance the utility of 3-substituted indoles and solidify their importance across diverse fields, including medicinal chemistry, materials science, and the synthesis of complex natural products.Data availability
No primary research results, software or code have been included, and no new data were generated or analyzed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the large research group program under grant number (R.G.P.02/519/44).References
- R. S. Keri, S. Budagumpi and V. Adimule, ACS Omega, 2024, 9, 42630–42667 CrossRef CAS PubMed.
- G. Bartoli, R. Dalpozzo and M. Nardi, Chem. Soc. Rev., 2014, 43, 4728–4750 RSC.
- A. Porwal, A. Rajendiran, P. Alam, H. Singh, K. Singh and A. Dubey, Int. J. Pharm. Invest., 2024, 14, 1052–1060 CrossRef CAS.
- A. T. Brown, S. J. Cotterell, M. R. Denny and N. K. Downer-Riley, Copper-Based Nanomaterials in the Synthesis of Heterocycles, in Copper-Based Nanomaterials in Organic Transformations, American Chemical Society, 2024, pp. 193–230 Search PubMed.
- J. Huang and G. Yu, Chem. Mater., 2021, 33, 1513–1539 CrossRef CAS.
- N. Kaur, Synth. Commun., 2015, 45, 1145–1182 CrossRef CAS.
- B. Paul, D. Panja and S. Kundu, Nat. Protoc., 2024, 19, 3640–3676 CrossRef CAS PubMed.
- B. Robinson, Chem. Rev., 1969, 69, 227–250 CrossRef CAS.
- G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045–1075 RSC.
- F. Dumitrascu and M. A. Ilies, Recent advances in the Nenitzescu indole synthesis (1990–2019), Adv. Heterocycl. Chem., 2021, 133, 65–157 CrossRef.
- T. Guo, F. Huang, L. Yu and Z. Yu, Tetrahedron Lett., 2015, 56, 296–302 CrossRef CAS.
- D. F. Taber and P. K. Tirunahari, Tetrahedron, 2011, 67, 7195–7210 CrossRef CAS PubMed.
- T. Ahmad, S. Khan and N. Ullah, ACS Omega, 2022, 7, 35446–35485 CrossRef CAS PubMed.
- M. Shiri, M. A. Zolfigol, H. G. Kruger and Z. Tanbakouchian, Chem. Rev., 2010, 110, 2250–2293 CrossRef CAS PubMed.
- S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108–7149 CrossRef CAS PubMed.
- M. M. Heravi, S. Rohani, V. Zadsirjan and N. Zahedi, RSC Adv., 2017, 7, 52852–52887 RSC.
- D. I. Bugaenko, A. V. Karchava and M. A. Yurovskaya, Russ. Chem. Rev., 2019, 88, 99–159 CrossRef CAS.
- J. Ma, R. Feng and Z. Dong, Asian J. Org. Chem., 2023, 12(6), e202300092 CrossRef CAS.
- R. Dalpozzo and G. Bartoli, Curr. Org. Chem., 2005, 9, 163–178 CrossRef CAS.
- E. Mahmoud, A. M. Hayallah, S. Kovacic, D. Abdelhamid and M. Abdel-Aziz, Pharmacol. Rep., 2022, 74, 570–582 CrossRef CAS PubMed.
- N. Tennoune, M. Andriamihaja and F. Blachier, Microorganisms, 2022, 10, 930 CrossRef CAS PubMed.
- E. Barresi, E. Baglini, V. Poggetti, J. Castagnoli, D. Giorgini, S. Salerno, S. Taliani and F. Da Settimo, Molecules, 2024, 29, 2127 CrossRef CAS PubMed.
- A. A. Singh, D. Yadav, F. Khan and M. Song, Brain Sci., 2024, 14, 674 CrossRef CAS PubMed.
- B. T. Sridhar, N. Gunavanthrao Yernale, R. S. Gani, N. Gupta, S. V. Ganachari and B. Suliphuldevara Mathada, J. Indian Chem. Soc., 2024, 101, 101282 CrossRef CAS.
- A. Menshawy, H. Ahmed, A. Ismail, A. I. Abushouk, E. Ghanem, R. Pallanti and A. Negida, Neurol. Sci., 2018, 39, 31–44 CrossRef PubMed.
- P. D. Charles, T. L. Davis and N. J. Brown, Am. J. Med. Sci., 1994, 307, 445–447 CrossRef CAS PubMed.
- P. Tfelt-Hansen, Cephalalgia, 2021, 41, 1499–1505 CrossRef PubMed.
- T. Passie, J. H. Halpern, D. O. Stichtenoth, H. M. Emrich and A. Hintzen, CNS Neurosci. Ther., 2008, 14, 295–314 CrossRef CAS PubMed.
- C. Chantana, U. Sirion, P. Iawsipo and J. Jaratjaroonphong, J. Org. Chem., 2021, 86, 13360–13370 CrossRef CAS PubMed.
- J. Dong, H. Ma, B. Wang, S. Yang, Z. Wang, Y. Li, Y. Liu and Q. Wang, Molecules, 2022, 27, 8439 CrossRef CAS PubMed.
- T. Endo, M. Tsuda, J. Fromont and J. Kobayashi, J. Nat. Prod., 2007, 70, 423–424 CrossRef CAS PubMed.
- M. Hull, S. Gardner and G. Hawcroft, Cancer Treat. Rev., 2003, 29, 309–320 CrossRef CAS PubMed.
- G. W. John, M. Perez, P. J. Pauwels, B. Le Grand, Y. Verscheure and F. C. Colpaert, CNS Drug Rev., 2000, 6, 278–289 CrossRef CAS.
- G. Bartoli, G. Bencivenni and R. Dalpozzo, Chem. Soc. Rev., 2010, 39, 4449 RSC.
- Y. Oikawa, H. Hirasawa and O. Yonemitsu, Tetrahedron Lett., 1978, 19, 1759–1762 CrossRef.
- A. Viola, L. Ferrazzano, G. Martelli, S. Ancona, L. Gentilucci and A. Tolomelli, Tetrahedron, 2014, 70, 6781–6788 CrossRef CAS.
- M. Mohammadi, M. Khodamorady, B. Tahmasbi, K. Bahrami and A. Ghorbani-Choghamarani, J. Ind. Eng. Chem., 2021, 97, 1–78 CrossRef CAS.
- M. Mohammadi and G. Mansouri, Langmuir, 2024, 40, 22773–22786 CrossRef CAS PubMed.
- A. Nikseresht, F. Ghoochi and M. Mohammadi, ACS Omega, 2024, 9, 28114–28128 CrossRef CAS PubMed.
- F. Ghobakhloo, D. Azarifar, M. Mohammadi, H. Keypour and H. Zeynali, Inorg. Chem., 2022, 61, 4825–4841 CrossRef CAS PubMed.
- A. Nikseresht, M. Karami and M. Mohammadi, Langmuir, 2024, 40, 18512–18524 CrossRef CAS PubMed.
- F. Ghobakhloo, M. Mohammadi, M. Ghaemi and D. Azarifar, ACS Appl. Nano Mater., 2024, 7, 1265–1277 CrossRef CAS.
- M. Mohammadi, A. Ghorbani-Choghamarani and S. M. Ramish, J. Mol. Struct., 2023, 1292, 136115 CrossRef CAS.
- A. J. Rago and G. Dong, Green Synth. Catal., 2021, 2, 216–227 CrossRef PubMed.
- V. Nigam, S. Singh, S. Kasana, S. Kumar, B. Das Kurmi, G. Das Gupta and P. Patel, ChemistrySelect, 2024, 9(23), e202402171 CrossRef CAS.
- K. van Beurden, S. de Koning, D. Molendijk and J. van Schijndel, Green Chem. Lett. Rev., 2020, 13, 349–364 CrossRef CAS.
- M. M. Heravi, F. Janati and V. Zadsirjan, Monatsh. Chem., 2020, 151, 439–482 CrossRef CAS.
- V. Campisciano, F. Giacalone and M. Gruttadauria, ChemCatChem, 2022, 14, e202200696 CrossRef CAS.
- R. H. Vekariya and H. D. Patel, Synth. Commun., 2014, 44, 2756–2788 CrossRef CAS.
- N. Singh, B. K. Allam, D. S. Raghuvanshi and K. N. Singh, Adv. Synth. Catal., 2013, 355, 1840–1848 CrossRef CAS.
- W. Chen, Y. Cai, X. Fu, X. Liu, L. Lin and X. Feng, Org. Lett., 2011, 13, 4910–4913 CrossRef CAS PubMed.
- L. Wang, M. Huang, X. Zhu and Y. Wan, Appl. Catal., A, 2013, 454, 160–163 CrossRef CAS.
- A. Thakur, P. Linga Reddy, M. Tripathi and D. S. Rawat, New J. Chem., 2015, 39, 6253–6260 RSC.
- M. Deb, P. Borpatra, P. J. Saikia and P. Baruah, Synthesis, 2016, 49, 1401–1409 CrossRef.
- P. J. Borpatra, B. Deka, B. K. Rajbongshi, M. L. Deb and P. K. Baruah, Synth. Commun., 2018, 48, 2074–2082 CrossRef CAS.
- B. Banerjee, A. Priya, M. Kaur, A. Sharma, A. Singh, V. K. Gupta and V. Jaitak, Catal. Lett., 2023, 153, 3547–3560 CrossRef CAS.
- M. Talukdar, N. Islam and E. Begari, Results Chem., 2023, 6, 101215 CrossRef CAS.
- P. Rai, M. Srivastava, S. Yadav, J. Singh and J. Singh, Catal. Lett., 2015, 145, 2020–2028 CrossRef CAS.
- E. Valiey, M. Dekamin and Z. Alirezvani, in Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, MDPI, Basel, Switzerland, 2017, p. 4740 Search PubMed.
- A. Bahuguna, P. Choudhary, T. Chhabra and V. Krishnan, ACS Omega, 2018, 3, 12163–12178 CrossRef CAS PubMed.
- Y. Wu, J. Li, J. Sun, Y. Wang, J. Liu and H. Cheng, J. Org. Chem., 2024, 89, 18493–18503 CrossRef CAS PubMed.
- J. Paradowska, M. Stodulski and J. Mlynarski, Angew. Chem., Int. Ed., 2009, 48, 4288–4297 CrossRef CAS PubMed.
- X. Liu, S. Dong, L. Lin and X. Feng, Chin. J. Chem., 2018, 36, 791–797 CrossRef CAS.
- F. S. Prout, J. Org. Chem., 1953, 18, 928–933 CrossRef CAS.
- C. Qi, J. Ye, W. Zeng and H. Jiang, Adv. Synth. Catal., 2010, 352, 1925–1933 CrossRef CAS.
- A. Khalafi-Nezhad, M. Nourisefat and F. Panahi, Org. Biomol. Chem., 2015, 13, 7772–7779 RSC.
- G. S. Nongthombam and R. Nongkhlaw, Synth. Commun., 2018, 48, 541–552 CrossRef CAS.
- Y.-H. He, J.-F. Cao, R. Li, Y. Xiang, D.-C. Yang and Z. Guan, Tetrahedron, 2015, 71, 9299–9306 CrossRef CAS.
- S. K. Burra, V. R. Lakinani, R. Akula and C. Yankkanti, Synth. Commun., 2024, 54, 207–215 CrossRef CAS.
- R. Baharfar, S. Peiman and B. Maleki, J. Heterocycl. Chem., 2021, 58, 1302–1310 CrossRef CAS.
- A. Pramanik and S. Bhar, New J. Chem., 2021, 45, 16355–16388 RSC.
- N. A. Kazin, N. S. Demina, R. A. Irgashev, E. F. Zhilina and G. L. Rusinov, Tetrahedron, 2019, 75, 4686–4696 CrossRef CAS.
- F. Hammoud, A. Hijazi, M. Ibrahim-Ouali, J. Lalevée and F. Dumur, Eur. Polym. J., 2022, 172, 111218 CrossRef CAS.
- A. Ganesan, J. Kothandapani and S. G. Subramaniapillai, RSC Adv., 2016, 6, 20582–20587 RSC.
- U. C. Rajesh, D. Divya and D. S. Rawat, RSC Adv., 2014, 40, 41323–41330 RSC.
- U. C. Rajesh, R. Kholiya, A. Thakur and D. S. Rawat, Tetrahedron Lett., 2015, 56, 1790–1793 CrossRef CAS.
- N. C. Dige, S. N. Korade and D. M. Pore, Res. Chem. Intermed., 2017, 43, 7029–7040 CrossRef CAS.
- C.-B. Li, Y.-W. Li and D.-Z. Xu, Synthesis, 2018, 50, 3708–3714 CrossRef CAS.
- A. Ying, S. Li, X. Liu, J. Wang, Y. Liu and Z. Liu, J. Catal., 2020, 391, 312–326 CrossRef CAS.
- M. Shekarchi, F. K. Behbahani and M. Shekarchi, Monatsh. Chem., 2021, 152, 659–664 CrossRef CAS.
- M. Omidi, A. Mobinikhaledi and N. Ahadi, Org. Prep. Proced. Int., 2023, 55, 372–378 CrossRef CAS.
- J. Wang, S. Zhang, Z. Ma and L. Yan, Green Chem. Eng., 2021, 2, 359–367 CrossRef.
- D. O. Abranches and J. A. P. Coutinho, Curr. Opin. Green Sustainable Chem., 2022, 35, 100612 CrossRef CAS.
- T. El Achkar, H. Greige-Gerges and S. Fourmentin, Environ. Chem. Lett., 2021, 19, 3397–3408 CrossRef CAS.
- A. Prabhune and R. Dey, J. Mol. Liq., 2023, 379, 121676 CrossRef CAS.
- H. Ruan, L. Yue, Y. Shijun, L. Chengwei and A. Yue, Water assisted and choline chloride-dimethylurea deep eutectic salts as catalyst towards the attractive reaction of indole, benzaldehyde, and malononitrile, Heterocycles, 2018, 96(7), 1266–1274 CrossRef CAS.
- M.-N. T. Tran, X.-T. T. Nguyen, H. T. Nguyen, D.-K. N. Chau and P. H. Tran, Tetrahedron Lett., 2020, 61, 151481 CrossRef CAS.
- D. T. Nagre, A. U. Khandebharad, S. R. Sarda, B. K. Dhotre and B. R. Agrawal, Org. Prep. Proced. Int., 2021, 53, 278–283 CrossRef CAS.
- S. Alvi, M. Alam and R. Ali, J. Mol. Liq., 2023, 390, 122951 CrossRef CAS.
- M. J. da Silva, A. A. Rodrigues and N. P. G. Lopes, Inorganics, 2023, 11(4), 162 CrossRef CAS.
- A. M. Escobar, G. Blustein, R. Luque and G. P. Romanelli, Catalysts, 2021, 11, 1–36 CrossRef.
- M. A. Amrollahi and Z. Kheilkordi, J. Iran. Chem. Soc., 2016, 13, 925–929 CrossRef CAS.
- N. M. Ghohe, R. Tayebee, M. M. Amini, A. Osatiashtiani, M. A. Isaacs and A. F. Lee, Tetrahedron, 2017, 73, 5862–5871 CrossRef CAS.
- R. Tayebee, A. F. Lee, L. Frattini and S. Rostami, Catalysts, 2019, 9, 409 CrossRef CAS.
- R. Wang, S. Abbaspour, N. Vahabi and R. Tayebee, Polycyclic Aromat. Compd., 2023, 43, 7381–7398 CrossRef CAS.
- G. Shanthi and P. T. Perumal, Tetrahedron Lett., 2007, 48, 6785–6789 CrossRef CAS.
- Y. Qu, F. Ke, L. Zhou, Z. Li, H. Xiang, D. Wu and X. Zhou, Chem. Commun., 2011, 47, 3912 RSC.
- S. Chandrasekhar, V. Patro, G. Pavan Kumar Reddy and R. Grée, A ligand-free copper(II)-catalyzed three-component reaction in poly(ethylene glycol) medium: a versatile protocol for the preparation of selected 3-indole derivatives, Tetrahedron Lett., 2012, 53(46), 6223–6225 CrossRef CAS.
- H. Jiang, L. Wang and J. Xie, J. Chem. Res., 2016, 40, 338–340 CrossRef CAS.
- A. N. Prasad, F. C. Braga, R. da S. Lopes, G. A. Casagrande, D. P. de Lima and A. Beatriz, Synth. Commun., 2018, 48, 104–114 CrossRef CAS.
- T. Das, S. Debnath, R. Maiti and D. K. Maiti, J. Org. Chem., 2017, 82, 688–700 CrossRef CAS PubMed.
- H. P. Gogoi, N. Goswami and P. Barman, React. Chem. Eng., 2024, 9, 2569–2583 RSC.
- D. Anselmo, E. C. Escudero-Adán, M. Martínez Belmonte and A. W. Kleij, Eur. J. Inorg. Chem., 2012, 2012, 4694–4700 CrossRef CAS.
- P. P. Ghosh and A. R. Das, J. Org. Chem., 2013, 78, 6170–6181 CrossRef CAS PubMed.
- U. C. Rajesh, J. Wang, S. Prescott, T. Tsuzuki and D. S. Rawat, ACS Sustain. Chem. Eng., 2015, 3, 9–18 CrossRef CAS.
- A. Renzetti, E. Boffa, M. Colazzo, S. Gérard, J. Sapi, T.-H. Chan, H. Nakazawa, C. Villani and A. Fontana, RSC Adv., 2014, 4, 47992–47999 RSC.
- S. Pradhan, J. Saha and B. G. Mishra, New J. Chem., 2017, 41, 6616–6629 RSC.
- M. Omidi and M. A. Amrollahi, C. R. Chim., 2017, 20, 549–553 CrossRef CAS.
- F. Mirhashemi and M. Shirali, Results Chem., 2023, 5, 100945 CrossRef CAS.
- F. Mirhashemi and M. A. Amrollahi, Turk. J. Chem., 2018, 42, 988–996 CrossRef CAS.
- V. K. Singh, R. Dubey, A. Upadhyay, L. K. Sharma and R. K. P. Singh, Tetrahedron Lett., 2017, 58, 4227–4231 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |