Open Access Article
Open Access ArticleBiofuel production from waste residuals: comprehensive insights into biomass conversion technologies and engineered biochar applications
Esraa M. El-Fawala,
Ahmed M. A. El Naggar
 *a,
Adel A. El-Zahhar
*a,
Adel A. El-Zahhar
 b,
Majed M. Alghandi
b,
Asmaa S. Morshedy
b,
Majed M. Alghandi
b,
Asmaa S. Morshedy a,
Hussien A. El Sayed
a and
Ard elshifa M. E. Mohammed
a,
Hussien A. El Sayed
a and
Ard elshifa M. E. Mohammed c
c
aEgyptian Petroleum Research Institute (EPRI), 1 Ahmed El-Zomor st. Nasr City, Cairo, Egypt. E-mail: drmeto1979@yahoo.com
bDepartment of Chemistry, Faculty of Science, King Khalid University, Abha 9004, Saudi Arabia
cDepartment of Chemistry, College of Science, Qassim University, Al-Qassim, Saudi Arabia
First published on 22nd April 2025
Abstract
Biomass-derived residuals represent a vital renewable energy source, offering sustainable alternatives to mitigate fossil fuel dependency, address climate change, and manage waste. Although biomass generally has a lower calorific value (10–20 MJ kg−1) compared to fossil fuels (40–50 MJ kg−1), its energy recovery potential can be enhanced through advanced conversion technologies such as torrefaction, pyrolysis, and gasification. Additionally, biomass is considered carbon neutral when sourced sustainably, as the CO2 released during combustion is reabsorbed by plants during their regrowth cycle, maintaining a balanced carbon flux in the atmosphere. This review explores the diverse sources of biomass and examines their chemical compositions and inherent properties, emphasizing their transformation into valuable energy carriers and bio-products. It provides a comprehensive analysis of thermochemical, biochemical, and physicochemical conversion technologies, detailing their mechanisms, efficiencies and applications. Special attention is given to biochar, a product of biomass pyrolysis, highlighting its potential in pollution mitigation, carbon sequestration, and as a catalyst in industrial applications. The review delves into synthesis processes of biochar and performance-enhancing modifications, illustrating its significant role in sustainable environmental management. Additionally, the economic and ecological advantages of biomass-derived energy, including reduced greenhouse gas emissions and waste reutilization, are critically evaluated, underscoring its superiority over conventional fossil fuels. Challenges limiting the scalability of biomass energy, such as technology costs, process efficiency, and market dynamics, are addressed, alongside prospective solutions. By consolidating extensive research on biomass conversion technologies and engineered biochar applications, this review serves as a valuable resource for researchers and policymakers. It aims to guide advancements in biomass utilization, fostering a transition toward sustainable energy systems and addressing global energy and environmental challenges.
1. Introduction
The reliance on conventional fossil fuels, such as coal, oil, and natural gas, has been the cornerstone of global power generation for decades. However, this dependency has led to significant environmental challenges, primarily due to the extensive release of greenhouse gases (GHGs), including carbon dioxide (CO2), into the atmosphere. Fossil fuel combustion remains the largest contributor to anthropogenic CO2 emissions, accounting for approximately 23% of global emissions.1–3 Alarmingly, projections suggest that CO2 emissions from petroleum fuels could rise by 80% by 2030 if current trends persist4,5 Supporting this forecast, the United States Environmental Protection Agency (US-EPA) has reported that the combustion of gasoline and diesel fuels results in the emission of approximately 8.887 × 10−3 and 1.0180 × 10−2 metric tons of CO2 per gallon, respectively.In response to these alarming trends, there is a growing imperative to transition from fossil fuels to low-carbon and renewable energy sources, such as hydrogen, solar energy, and bio-energy derived from biomass.6–9 Such a shift holds the promise of significantly reducing CO2 emissions to levels below approximately 8 kg CO2 per unit of energy produced. In this context, it means that for every unit of energy generated (for example, 1 kW h of electricity or 1 MJ of heat), 8 kilograms of CO2 are emitted or less. This suggests that a shift in energy production methods could significantly lower CO2 emissions, aiming for a reduction to below 8 kg of CO2 per unit of energy. To achieve this goal, there is increasing momentum behind the utilization of biomass residuals for energy generation, marking a rapid departure from hydrocarbon-based energy systems. Biomass emerges as a particularly promising renewable energy source due to its widespread availability globally and the existence of cost-effective conversion technologies.10 The global transition toward renewable energy sources is essential. Biomass, as a renewable and widely available energy resource, has gained significant attention due to its potential to reduce GHG emissions and mitigate climate change.11 Unlike fossil fuels, biomass energy operates within a balanced carbon cycle; the CO2 emitted during biomass combustion is reabsorbed by plants during their growth. This biogenic origin of CO2 positions biomass as a carbon-neutral energy source, provided that sustainable cultivation and harvesting practices are maintained (Fig. 1).
 | ||
| Fig. 1 The carbon cycle in biomass production and utilization.12 | ||
The escalating demand for clean energy sources amid a global push for sustainability has underscored the increasing attractiveness of biomass-based fuels as viable alternatives to traditional fossil fuels.13 Despite its promise, the large-scale adoption of biomass-derived energy faces several challenges, including technological limitations, economic barriers, and the efficient conversion of biomass into high-value products. While existing reviews have extensively discussed biomass conversion technologies, few have provided a synchronized perspective on their integration with the emerging applications of engineered biochar—a material that offers solutions for pollution mitigation, carbon sequestration, and catalytic processes.
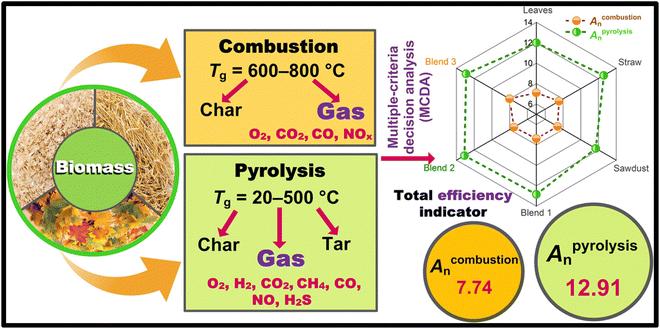
Among the various processing pathways for biomass conversion, pyrolysis emerges as a particularly effective method due to its ability to yield a range of valuable products simultaneously, including bio-oil, biochar, and combustible gases. Leveraging agricultural, industrial, and municipal solid residuals as feedstock during pyrolysis holds significant environmental promise, contributing to greenhouse gas emission reduction, solid waste minimization, and carbon sequestration. Moreover, upgrading the bio-oil derived from solid residuals pyrolysis through thermochemical processes offers additional economic incentives for bio-energy production. Catalytic hydrotreatment of bio-oils with hydrogen at elevated temperatures yields green diesel and gasoline-like fuels, which exhibit reduced emissions during combustion owing to their predominantly hydrocarbon composition. Consequently, biomass-derived fuels represent an environmentally favorable energy source, offering a pathway towards sustainable energy production.14–18
This review uniquely integrates a comprehensive analysis of biomass conversion technologies with the advanced applications of engineered biochar, emphasizing its potential in pollution mitigation, carbon sequestration, and catalytic processes. By critically evaluating the economic and environmental advantages of biomass-derived energy over fossil fuels, the study provides novel insights into optimizing biochar synthesis and its modifications for enhanced functionality.19 Furthermore, the manuscript highlights emerging opportunities and challenges in scaling biomass energy solutions, offering a holistic perspective that bridges technological advancements and sustainable environmental management. This integrated approach establishes the review as a valuable resource for guiding future research and policy development in renewable energy systems (Fig. 2). Unlike prior reviews that discuss biomass conversion pathways or biochar applications in isolation, this study uniquely integrates both aspects, emphasizing the transformation of biomass-derived residuals into engineered biochar for high-value applications. While earlier works,20,21 have focused on biochar's role in agriculture and carbon sequestration, this review extends beyond conventional uses to highlight biochar's potential as a precursor for energy storage materials, catalysts for biofuel upgrading, and functional adsorbents for pollutant removal. Furthermore, compared to studies that assess biochar's physicochemical properties without a direct link to energy applications,22 this article presents a detailed discussion on optimizing biochar characteristics for enhanced electrochemical performance in super-capacitors, hydrogen storage, and catalysis. This comprehensive approach provides deeper insights into the development of biochar-based functional materials that can support the transition toward sustainable energy solutions. Moreover, this review cover expanded perspectives with regard to the introduction of recent methods of biochar synthesis, elaborating the unique innovative aspects of these methods in terms of process and environmental benefits. Policy environment faced by biomass energy development in different regions worldwide as well as the challenges faced by the large-scale application of biomass energy is also discussed through this review. Thus, the novelty of this article is based on simultaneously presenting versatile aspects in correlation to biomass as an energy source and its potential sub-products (such as biochar) for use in advanced applications.
 | ||
| Fig. 2 Transforming various biomass sources into valuable products.19 | ||
2. Conversion of solid residuals to carbonaceous structures
Thermal conversion methods stand as the predominant means for repurposing biomass-derived solid residues into valuable bio-based products, particularly within the waste-to-energy sector. Recent years have witnessed a surge in research endeavors focused on harnessing thermal techniques to produce efficient carbon-based structures capable of capturing heavy metals and other contaminants from liquid waste media. Notably, between 2019 and 2021, several studies have documented the conversion of rice straw into biochar via pyrolysis. Subsequent activation of the produced biochar was achieved through two distinct chemical treatments. In the first approach, biochar underwent immersion in various mineral acids followed by thermal treatment at elevated temperatures to generate active carbon species. Conversely, the second treatment involved doping biochar with nanoparticles of co-metal hydroxides, succeeded by calcination at high temperatures. Both methodologies yielded activated biochar structures exhibiting significant adsorption capacities for precious heavy metals and organic pollutants present in industrial-grade phosphoric acids.23–27In a separate investigation, El Saied et al.28 introduced a novel methodology for transforming sawdust into activated carbon particles possessing a high specific surface area. This one-step conversion process employed a chemical activator under a nitrogen gas stream at moderate operating temperatures. The resulting activated carbon demonstrated remarkable catalytic prowess, facilitating the conversion of cellulose, an additional solid waste stream, into biofuel feedstock via hydrothermal hydrolysis reactions. Further expanding the repertoire of biochar synthesis methods, Moneim et al.10 pioneered the synthesis of a specialized grade of biochar utilizing a low-temperature pyrolysis process. Pre-treatments of the solid waste precursor, including ball-milling and UV irradiation, were undertaken prior to pyrolysis. Additionally, variations in the operating time during pyrolysis enabled the acquisition of biochar grades characterized by high levels of crystallinty and commendable specific surface areas.
These multifaceted approaches to biochar synthesis underscore the versatility of thermal conversion methods in transforming diverse biomass residues into functional carbonaceous materials, thus contributing to the sustainable management of organic waste streams and the development of valuable bio-based products.29,30
2.1. Utilizing nitrogen-free biomass for hydro-char production
 | ||
| Fig. 3 Structure and key components of lignocellulosic biomass: a systematic overview.31 | ||
Gao et al.38 conducted a comprehensive analysis of biochar derived from cellulose at a hydrothermal carbonization (HTC) temperature of 250 °C utilizing different characterization techniques. Their investigation revealed distinct core–shell architecture within the biochar, with the core characterized by ketone and ether groups, while the shell predominantly featured carboxylic and carbonyl functionalities. This structural elucidation provided a foundation for understanding the cellulose-to-biochar conversion mechanism, as depicted in Fig. 3. Further insights into biochar formation mechanisms can be gleaned from previous studies.39,40 Sevilla et al.41 similarly examined biochar, identifying a core–shell structure with a hydrophobic aromatic core and a hydrophilic shell rich in reactive oxygen functional groups, such as hydroxyl/phenolic, carbonyl, or carboxylic moieties. Their investigations, conducted on biochar derived from glucose at temperatures ranging from 170 to 240 °C, emphasized the presence of stable oxygen-containing groups within the aromatic nucleus. Consistent findings were observed by other researchers investigating cellulose-derived biochar within the temperature range of 220–250 °C, where a carbonaceous scaffold comprising condensed benzene rings was identified.42 Chuntanapum et al.43 proposed a structural model for biochar derived from 5-HMF at hydrothermal temperatures of 350 °C and 450 °C, denoted as “tarry material” elucidating the presence of furan units coupled with benzene rings. The diverse structural features observed across these studies are attributed to variations in hydrothermal conditions, particularly temperature and residence time. Additionally, Falco et al.44 proposed a model for cellulose hydrothermal carbonization under mild temperatures (180–280 °C), elucidating the formation of extensive aromatic networks or polyfuranic structures through interchangeable mechanisms dependent on temperature.45
In a summary, the intricate pathways governing biochar formation from cellulose are illustrated in Fig. 3 and 4.
 | ||
| Fig. 4 Mechanism of biochar preparation via cellulose decomposition.32 | ||
 | ||
| Fig. 5 Formation pathways of hemicellulose (D-xylose) into biochar.55 | ||
Zhang et al.63 investigated the hydrothermal treatment of Kraft pine lignin, observing a two-phase mechanism characterized by rapid lignin solubilization followed by a slower repolymerization phase. Fang et al.64 consolidated prior research and proposed distinct reaction pathways for dissolved and non-dissolved lignin in homogeneous and heterogeneous environments. Dissolved lignin undergoes hydrolysis and dealkylation, yielding phenolic intermediates that subsequently undergo cross-linking and repolymerization into phenolic char.64 In contrast, non-dissolved lignin, primarily in heterogeneous environments, undergoes solid–solid formation akin to pyrolysis, resulting in highly condensed char with polyaromatics.64 However, during the HTC process, solid–solid formation may prevail with observation for low production of soluble intermediates, leading to diminished phenolic char. As hydrothermal severity escalates, solid–solid conversion may augment polyaromatic char production. A simplified schematic of lignin-derived hydrochar formation mechanisms is depicted in Fig. 6.
 | ||
| Fig. 6 Basic mechanism of lignin-derived biochar formation in hydrothermal carbonization55,64 | ||
Hydrothermal carbonization (HTC) mirrors this approach by breaking down lignocellulosic biomass structures for enhanced degradation. Hydrolysis step is a key in such process to attain damage structure of lignocellulose component and reducing biomass polymerization degree.69 The complexity of lignocellulose necessitates higher activation energy for its disruption which is explicitly reflected in setting HTC conditions.70 As shown in Fig. 7, presence of lignin markedly influences degradation of lignocellulosic biomass during hydrochar formation, preserving the initial biomass' macrostructure.71
 | ||
| Fig. 7 Hydrothermal carbonization reaction pathways for lignocellulosic biomass, with emphasis on liquid biocrude formation.70 | ||
2.2. Using nitrogen-rich biomass for hydro-char production
Researchers often employ amino acids as model compounds to investigate hydrothermal reactions, with decarboxylation and deamination being prominent reactions observed in studies utilizing glycine and alanine.77 Moreover, the decomposition of mixed amino acids can be mutually influenced, yielding ammonia, amines, CO2, and organic acids as primary products of decomposition stage.78
Saccharides play a dual role in the hydrothermal process by not only serving as reactants but also via influencing the pH, thereby impacting the degradation of amino acids, as highlighted by E. Danso-Boateng et al.79 Moreover, He et al.73 observed that the hydrothermal carbonization (HTC) of SS yielded uniform brown hydrochars emitting a nut-like aroma, indicative to substantial Maillard reactions. Danso-Boateng et al.79 further elucidated the Maillard reaction products, including aldehydes, furans, pyrroles, pyrazines, and pyridines, in liquid samples derived from primary sewage sludge that is subjected to HTC at temperatures ranging from 180 °C to 200 °C, with reaction times spanning from 30 to 240 minutes. Intriguingly, their findings revealed a stark contrast, with no discernible Maillard reaction products observed when the primary sewage sludge was carbonized at lower temperatures (140 °C for 240 min and 160 °C for 60–120 min). However, the prevalence of Maillard reactions became prominent when HTC was conducted at temperatures exceeding 180 °C for reaction times surpassing 15 minutes.
Hence, it can be inferred that the formation of hydrochar is contingent upon various factors, including the composition of sewage sludge (SS), the types of proteins present, the composition of amino acids, and the hydrothermal temperature. Achieving hydrochars with reduced nitrogen (N) content is imperative when considering their application as solid fuel.74 Seiichi Inoue et al.80 conducted a comprehensive investigation into the nitrogen dynamics in SS, revealing that solubilization and decomposition commence at temperatures exceeding 150 °C, leading to the transformation of N into the aqueous phase.79,81 Furthermore, He et al.82 observed substantial conversions of initial proteins into ammonium in the liquid phase with increasing hydrothermal temperatures, exerting a significant influence on the distribution of N. Hydrothermal carbonization (HTC) process of sewage sludge (SS) to produce hydrochar can be delineated into two distinct approaches, as given through Fig. 8 and 9.
 | ||
| Fig. 8 Schematic representation of biochar formation mechanism from sewage sludge.83 | ||
 | ||
| Fig. 9 Biochar formation pathways from nitrogen-rich or nitrogen-free biomass.84 | ||
In summary, the principal hydrochar structure from nitrogen-rich biomass is attributed to the N-containing aromatic network. Nevertheless, elucidating the chemical mechanisms underlying the introduction of N from proteins, amino acids, and carbohydrate-derived hydrochar matrices remains a challenge. Advanced technologies are warranted to delineate the specific framework of hydrochar, with future research focusing on exploring complex compounds and the utilization of N doping materials.83
2.3. Chemical properties of biochar
The pyrolysis process entails the conversion of biomass into biochar, characterized by a spectrum of properties intricately linked to both the type of feedstock and the temperature at which pyrolysis occurs. Synthesizing data aids in predicting the properties and functionalities of biochar.85 Generally, an exponential decrease in biochar yield accompanies an increase in pyrolysis temperature, while its alkalinity (pH) exhibits a linear rise.86 This elevation in pH stems from the thermal degradation of hydroxyl bonds and other weak linkages within the biochar matrix under high-temperature conditions. Conversely, the cation exchange capacity of biochar demonstrates an inverse correlation with pyrolysis temperature due to the depletion of acidic functional groups.86,87 Notably, biochars derived from biosolids typically boast the highest cation exchange capacity, owing to the mineral-rich composition of biosolids that fosters the generation of oxygen-containing functional groups during pyrolysis.88In contrast to ash content, the volatile matter content of biochar exhibits a linear decrease with increasing pyrolysis temperature. The formation of ash can be attributed to residual inorganic minerals resulting from the decomposition of hydrogen, oxygen, and carbon within the biomass.86 Concomitantly, reductions in these elements occur due to the breakdown of hydroxyl, azanide, and other weakly bonded groups as temperature escalates. However, the carbon content (excluding some volatile carbon) undergoes a gradual decline, resulting in higher carbon proportions in the resultant biochar at elevated pyrolysis temperatures.87
The thermal breakdown of lignocellulosic biomass during pyrolysis initiates a series of well-defined stages of transformation, encompassing dehydration, pyrolysis, graphene nucleation, and carbonization, each characterized by distinct temperature ranges, typically occurring under near-atmospheric pressure conditions.89 Initially, dehydration, coupled with a mild depolymerization of cellulosic constituents, initiates below 250 °C, leading to minimal mass loss, approximately 3 wt% at 150 °C.89 During this phase, there is a gradual augmentation in carbon concentration as the biomass temperature exceeds 150 °C. Notably, a detailed 13C-NMR spectral analysis of biochar produced at 200 °C indicates a reduction in signal intensity associated with hemicellulose and cellulose structures (O-alkyl C and di-O-alkyl C), compared with heightened signals attributed to lignin (aryl C and O-aryl C).90 Further analysis discerns a transition from O-alkyl to aryl C structures in biochar generated at 250 °C. The transition to the second phase (250–350 °C) is marked by a decline in the hydrogen-to-carbon (H-to-C) ratio, primarily due to the disproportionate loss of oxygen and hydrogen relative to carbon constituents, as noted by Baldock and Smernik.91 This decline in H-to-C ratios signifies the formation of structures rich in unsaturated carbon, such as aromatic rings. Concurrently, within this phase, cellulose experiences complete depolymerization, triggering biomass pyrolysis, substantial mass reduction, and the formation of an amorphous carbon framework. For instance, pyrolysis conducted at 300 °C can lead to a substantial reduction of up to 81 wt% in the initial biomass mass, as documented by Baldock and Smernik.91 The evolution from amorphous carbon to aromatic carbon and polyaromatic graphene sheets typically occurs at temperatures around 330 °C and surpassing 350 °C, respectively, representing the third phase of transformation. Following these stages, at temperatures exceeding 600 °C, most non-carbon atoms are eliminated through the process of carbonization, as discussed by Amonette and Joseph.92 The process of pyrolysis transforms lignocellulosic biomass into biochar, with distinct phases of transformation: dehydration, pyrolysis, graphene nucleation, and carbonization, occurring at transition temperatures approximately at 250 °C, 350 °C, and 600 °C, respectively.92 During pyrolysis, graphene sheets undergo lateral growth, absorbing amorphous carbon and amalgamating with neighboring packets to establish electrical connectivity within the biochar matrix.93 This transformation enhances microporosity by generating voids within the biochar structure, as aligned graphene packets have greater density compared to amorphous carbon. At elevated pyrolysis temperatures, carbonized biochars are predominantly composed of a solid amalgamation of graphene packets and amorphous carbon, forming the structural core.94
The reduction in biomass mass during volatilization, induced by dehydration and pyrolysis, primarily involves the loss of oxygen, hydrogen, and, to a lesser extent, carbon constituents.95 These elements are expelled in various forms, including water vapor, hydrocarbons, gases such as CO2, CO, H2, and tarry vapors. Typically, the carbon content in biochar undergoes a significant enhancement, transitioning from approximately 40 to 50 wt% in the initial biomass to 70–80 wt% in the resultant biochar following pyrolysis within the temperature range of 250–600 °C. It is noteworthy that, through carbonization, biochar can attain carbon contents exceeding 90 wt%, excluding chars with high mineral ash content.96
The fate of inorganic constituents within biomass during pyrolysis encompasses diverse trajectories, including volatilization, incorporation into biochar, or retention as distinct mineral phases.97 This intricate process is significantly shaped by both the elemental composition of the biomass feedstock and the specific parameters governing the pyrolysis operation. Biomass origins such as forest residues, scrap wood, and sawmill byproducts typically boast minimal ash content, typically falling below 1 wt%. In contrast, agricultural remnants like grain husks, straw, and grasses, which are rich in silica, may yield biochars characterized by elevated ash content, potentially reaching up to 24 wt%.98 It is noteworthy that the depletion of hydrogen, oxygen, and carbon during biomass pyrolysis leads to the concentration of mineral constituents within the biochar matrix. This phenomenon underscores the intricate interplay between biomass composition, pyrolysis conditions, and the resultant mineral entrainment within the biochar structure.
Control over the mineral ash content in biochar involves nuanced manipulation of several key parameters within the pyrolysis process, foremost among them being reaction temperature and the partial pressures of CO2, steam, and O2.99 At lower temperatures, the thermal breakdown of biomass constituents triggers the vaporization of highly mobile ions like chlorine (Cl) and potassium (K).100 Similarly, nitrogen (N), frequently associated with diverse organic molecules, displays volatility at lower temperatures.100 In contrast, sulfur (S) and phosphorus (P) form stable bonds with intricate organic compounds present within biomass cells, rendering them resistant to thermal decomposition at lower temperatures. Conversely, certain ions, such as calcium (Ca) and silicon (Si), sequestered within biomass cell walls, including those bound to organic acids or encapsulated within opal phytoliths and silica, are liberated at considerably higher temperatures during thermal decomposition.101
The presence of inherent minerals within biomass profoundly impacts the properties of resultant biochar, as these minerals engage in intricate interactions with organic constituents during the pyrolysis process.102 Pre-pyrolysis removal of these minerals can elevate the optimal pyrolysis temperature for biomass conversion into biochar, raising it from 330 to 370 °C compared to unprocessed biomass containing inherent minerals. Notably, the absence of biomass minerals can lead to the sequestration of up to 30.1% more carbon content from biomass into biochar while concurrently emitting lower levels of low-molecular-weight organic compounds during pyrolysis.103 Furthermore, the removal of biomass minerals prior to pyrolysis augments the carbonaceous structure of biochar by fostering aromatization, thereby promoting the formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and/or C–C bonds at the expense of oxygen-containing functional groups. Thus, the type and quantity of minerals present in biomass demand careful optimization based on the targeted environmental application of biochar.
C and/or C–C bonds at the expense of oxygen-containing functional groups. Thus, the type and quantity of minerals present in biomass demand careful optimization based on the targeted environmental application of biochar.
2.3.1.1. Structural and compositional analyses using SEM-EDS and XRD. Understanding the microstructural and crystalline properties of biomass fuels is crucial for optimizing their conversion processes and enhancing energy efficiency. Scanning Electron Microscopy (SEM), coupled with Energy Dispersive X-ray Spectroscopy (EDS), provides detailed insights into the surface morphology and elemental composition of biomass materials. For instance, SEM analysis of Sengon wood pellets revealed a porous structure with varying densities across different sections, which influences combustion efficiency. EDS analysis further identified the elemental composition, predominantly carbon (C) and oxygen (O), with trace elements such as silicon (Si) and calcium (Ca), which can impact ash formation and slagging behavior during combustion.104 X-ray Diffraction (XRD) analysis complements these findings by identifying the crystalline phases present in biomass samples. XRD patterns of various biomass residues, such as corn cobs, have shown characteristic peaks corresponding to cellulose, hemicellulose, and lignin. The degree of crystallinity, indicated by these peaks, affects the biomass's thermal degradation behavior and reactivity during conversion processes.105 For example, a higher crystallinity index suggests a more ordered cellulose structure, which can influence the efficiency of enzymatic hydrolysis and subsequent biofuel production. Integrating SEM-EDS and XRD analyses offers a comprehensive understanding of biomass fuels' physical and chemical properties. This combined approach aids in tailoring pre-treatment methods and optimizing conversion technologies, thereby improving the efficiency and sustainability of biomass as a renewable energy source.106
3. Techniques for biomass conversion into energy
As previously noted, biomass represents a renewable energy source that has potential to significantly mitigate the associated environmental impacts to usage of fossil fuel. Through various treatment and conversion processes, biomass can be transformed into diverse energy carriers. The selection of an appropriate production process hinges on several key factors, including the desired end-product, biomass quality and quantity, and process economics.107Biomass conversion typically yields two primary types of energy carriers, as outlined by McKendry:108 electrical/heat energy and transportation fuels. The physicochemical properties of biomass play a pivotal role in determining the suitability of the feedstock for each energy domain or both. The properties of biomass, particularly moisture content, caloric value, proportions of fixed carbon and volatile substances, ash content and alkali metal content significantly influence the conversion processes, especially for dry biomass, with moisture content and cellulose/lignin ratio being particularly crucial for wet biomass conversion.108
To render lignocellulosic biomass suitable for conversion into transportation fuels and value-added co-products, pretreatment processes are indispensable.109 These pretreatment steps aim to enhance cellulose hydrolysis efficiency by reducing cellulose crystallinity and increasing its surface area through the removal of lignin and hemicellulose layers.110
Biomass can be converted into useful forms of energy with a wide range of technology and process options. The conversion techniques of biomass are divided into five classes as presented in Fig. 10. Advantages and disadvantages for each of these techniques are provided through Table 1.
 | ||
| Fig. 10 Different conversion techniques of biomass111 | ||
| Technology | Benefit | Limitation |
|---|---|---|
| Thermochemical | The process occurs rapidly, typically within seconds or minutes, but requires significant energy consumption and operates under potentially dangerous conditions | Significant energy demand and potentially unsafe operating conditions |
| Biological | Operates at standard pressure and temperature, handles diverse waste materials, avoids CO2 buildup, and remains economically efficient | A gradual process that may require hours, days, weeks, or even years to reach completion |
| Biochemical | Needs minimal external energy supply | The complex structural integrity of plant cell wall materials presents a challenge for microbial degradation |
| Physical | Simple to handle with reduced chemical usage | It involves significant energy consumption and lacks economic viability |
The conversion technology option is determined by the type of biomass feedstock, the nature of the anticipated energy (i.e., endues requirements), commercial settings and environmental values. Biomass may be turned to three categories: chemical feedstock, transportation fuels, and power/heat generation.
3.1. Physical conversion technique
Physical or mechanical conversion of biomass involves size reduction/commination, drying, and densification of the introduced solid biomass species. Briquetting, extraction, and distillation are the most utilized physical conversion technologies,111 as shown in Table 2.| Tools | Type of biomass | Products | Benefits | Limitations |
|---|---|---|---|---|
| Briquetting | Agricultural and forestry residues | Fuel briquettes | Offering benefits such as extended transportation distances, reliable energy supply, and storage potential | The process involves costly technology and high energy requirements |
| Extraction | Seeds | Oil | Eco-friendly, safe and energy-efficient | Faces challenges in large-scale implementation |
| Distillation | Seeds | Bio-oil | Effectively separates components with maximum yield | Requires substantial energy consumption |
3.2. Biological conversion
Biological conversion techniques are deemed as eco-friendly, however, their prolonged operating time, usage of expensive hydrolytic enzymes and susceptibility of microorganisms to different factors may stand as major restrictions for such route. These factors include growth conditions, contaminants, sugar substrates, nutrients, and inhibitors.120• All types of biomass, including lignin, can be included in such process regardless of their grades
• Increased bio-catalytic selectivity is provided
• Bioreactor functioning is done at typical non-severe conditions
• There is no involvement of metal toxicity.122
3.3. Biochemical conversion technique
Biochemical conversion technologies include hydrolysis, transesterification, and supercritical water gasification, which combine biological and chemical mechanisms. This method employs microbes and biological catalysts to convert biomass into gas (CO2/CH4), trash (compost or manure), and H2O.119 | ||
| Fig. 11 Thermo-chemical biomass processes and products.134 | ||
3.3.2.1. Feedstock. Combustion process can be performed for biomass feedstock having water content up to 60%. Ingredients other than C, H, and O in biomass feedstock are undesirable because they contribute in formation of pollutants and ash. Wood is usually the best feedstock for combustion process since it has low ash and nitrogen contents. Herbaceous biomass such as straw and grass include high levels of N, S, K, and Cl, resulting in production of increased emissions of NOX and sulfur oxides.15,136
3.3.2.2. Staged combustion. Two-staged combustion is based on primary air injection in a fuel bed followed by secondary air injection in the combustion chamber, as displayed in Fig. 12. This sequence allows efficient mixing of combustion air with the produced combustible gases by de-volatilization and gasification in the fuel bed. Effective mixing can reduce unburned pollutants to near-zero concentrations (e.g., CO < 50 mg m−3).137,138
 | ||
| Fig. 12 Main reactions during two-stage combustion of biomass.137 | ||
 | ||
| Fig. 13 Types of gasifiers (A) entrained bed, (B) fixed bed, and (C) fluidized bed.142 | ||
During the gasification process, water content in a feedstock plays key-role in the efficiency of biomass conversion since that huge amount of energy is consumed to evaporate water molecules. Furthermore, excessive moisture content in the biomass feedstock affects composition of syngas and its quality due to elevated content of CO2 which significantly reduces the calorific value of syngas during energy and power generation.143
On the other hand, produced syngas from gasification process can be used for manufacture of liquid fuels through three different routes as follows:
(1) Conversion of coal-derived syngas and natural gas into liquid fuels through Fischer–Tropsch synthesis, (2) methanol synthesis from syngas via catalytic steam reforming, (3) aerobic fermentation of syngas in presence of bacteria to produce ethanol.
 | ||
| Fig. 14 Scheme of biomass pyrolysis reactions.144 | ||
Pyrolysis process is classified into three subcategories based on the rate of heating: fast, flash, and slow/conventional pyrolysis.147 However, the relative distribution of products is determined by the pyrolysis type and operating parameters148 including solid residence time and biomass particle size.
3.3.4.1. Fast pyrolysis. In this process biomass is rapidly heated to a high temperature in the absence of oxygen. Fast pyrolysis yields 60–75% oily products, 15–25% solids (biochar) and 10–20% gaseous phase liquids in such process are often produced from biomass at high heating rates, short residence times at temperatures ranging from 400 to 800 °C. The production of liquids fractions from biomass is typically dependent on the rapid cooling of pyrolysis vapors, controlled reaction temperatures, and extremely high heat transfer which are the primary characteristics of a fast pyrolysis process.149
3.3.4.2. Flash pyrolysis. Flash pyrolysis of biomass is regarded as a promising, improved, and modified method of fast pyrolysis for the manufacture of solid, liquid, and gaseous fuel from biomass. The flash pyrolysis process requires high reaction temperatures (700–900 °C), a high heating rate (1000 °C s−1), small biomass particle size and a comparatively short gas residence time of roughly 0.5 s.150 The conversion efficiency of biomass to oil in flash pyrolysis can approach 70%. However, the creation of pyrolysis water poses a significant barrier to the quality and stability of the resulting oil.151 Generally speaking, bio-oil produced by standard flash pyrolysis is of low quality and requires costly improvement before it can be utilized as a transportation fuel.
3.3.4.3. Slow pyrolysis. Slow pyrolysis is performed at a heating rate of approximately 10 °C min−1 and at temperatures ranging from 350 to 500 °C for an operational time starting from few minutes and up to 30 min. This kind of pyrolysis normally yields 25–30% bio-char, 35–45% bio-oil and 25–40% as gaseous products.152 The composition of the obtained bio-products in this process varies depending on operating parameters including feedstock residence time, heating rate and temperature.153 The main disadvantages of this technique are the limited up-scalability and high operating costs due to elongated processing time.154
3.3.4.4. Pyrolysis reactors. There are several types of reactors that can be employed in pyrolysis process; however, each of them has unique properties. The most common types of reactors are discussed as follows:
3.3.4.4.1. Fixed bed reactor. This type of pyrolysis reactor is equipped with a gas cooling and cleaning system. In this sort of reactor, solids flow vertically and come into contact with the counter-current upward moving gas stream. A fixed bed reactor is made of steel and includes a fuel feeding unit, an ash removal unit and a gas exit, as presented in Fig. 15. Fixed bed reactors typically operate with long residence time of solid feedstock, low gas velocity and low ash carryover. These reactors are designed for small-scale heat and electricity applications.
 | ||
| Fig. 15 Fixed bed reactor for biomass pyrolysis155 | ||
3.3.4.4.2. Fluidized-bed reactor. The fluidized-bed reactor is composed of a fluid–solid combination with fluid-like characteristics. Such type of reactor appears to be popular for fast pyrolysis because they provide rapid heat transfer, good control over the pyrolysis reaction, extensive high surface contact between fluid and solid per unit bed volume, good thermal transport within the system and a high relative velocity between the fluid and solid phases.156 There are two types of fluidized-bed reactors which are:
3.3.4.4.3. Bubbling fluidized-bed reactors. These reactors offer improved temperature control, solid-to-gas contact, and heat transmission. Heated sand is utilized as the solid phase to rapidly heat the biomass in an oxygen-free environment to be decomposed into char, gases and aerosols. Fluidizing gas stream is then introduced to transport the decomposed biomass, as displayed in Fig. 16.157 Bubbling fluidized-bed pyrolysis is widely used because it produces high-quality bio-oil with a yield of around 70–75% of biomass.158
 | ||
| Fig. 16 Bubbling fluidized-bed reactor.157 | ||
3.3.4.4.4. Circulating fluidized-bed reactors. Circulating fluidized beds are identical to bubbling fluidized bed reactors, with the exception of shorter residence durations for chars and gases. This leads to increased gas velocity and char content in bio-oil than in bubbling fluidized bed reactors. One advantage for such sort of reactor that it can handle very large throughputs,158 as illustrated in Fig. 17.
 | ||
| Fig. 17 Schematic of circulating fluidized bed pyrolysis.155 | ||
Recent advancements in biochar synthesis technologies have led to more efficient, environmentally friendly, and performance-enhanced biochar compared to traditional slow pyrolysis and torrefaction methods. Conventional pyrolysis typically operates at moderate to high temperatures (400–700 °C) in an oxygen-limited environment, yielding biochar with moderate porosity and limited functionalization. However, emerging synthesis techniques such as plasma-assisted pyrolysis, hydrothermal carbonization (HTC), and catalytic pyrolysis have demonstrated superior efficiency, tailored physicochemical properties, and lower environmental impact.159–161
Plasma-assisted pyrolysis, for instance, utilizes high-energy plasma fields to rapidly decompose biomass under an inert gas atmosphere, leading to ultrahigh surface area biochar with well-defined pore structures. This technique enhances biochar's applicability in electrocatalysis, energy storage devices, and pollutant adsorption due to its high graphitization degree and tailored functional groups.162 Meanwhile, HTC operates at subcritical water conditions (180–250 °C), converting wet biomass feedstocks into hydrochar, which retains oxygen-containing functional groups that improve biochar's cation exchange capacity (CEC) and adsorption efficiency for heavy metals.22 HTC also enables high carbon sequestration potential, making it an attractive approach for climate mitigation 163 and soil amendment applications.164
Furthermore, catalytic pyrolysis, which integrates metallic and acid catalysts, has been developed to selectively tailor biochar properties for biofuel upgrading and syngas purification. The inclusion of zeolites, metal oxides, and alkaline earth metals in pyrolysis systems improves the deoxygenation of biochar, leading to high-value carbonaceous materials with applications in green hydrogen production and bio-refinery processes.165,166 Compared to traditional pyrolysis, catalytic pyrolysis reduces the formation of toxic polycyclic aromatic hydrocarbons (PAHs) while enhancing bio-oil quality and biochar stability.167,168
These emerging biochar synthesis techniques significantly contribute to industrial applications by offering customizable carbon materials for batteries, fuel cells, and catalytic converters, thereby promoting a more sustainable bio-economy. As the demand for renewable carbon materials grows, these innovations will play a crucial role in scaling up biochar production with optimized energy efficiency and minimal environmental impact.169,170
3.3.4.5. Char separation. Char, the intermediate solid residue formed during pyrolysis processes, poses a challenge due to its role as a vapor cracking and its contribution to the formation of polycyclic aromatic hydrocarbons (PAHs), especially at lower temperatures.171 Traditionally, char separation from reactors has relied on cyclone methods. However, this approach has limitations, as fine particles may pass through the cyclone and collect in the liquid product, causing aging and instability issues. Alternative methods, such as in-bed vapor filtration and rotary particle separation, have been explored to address this challenge.172 Nonetheless, these techniques encounter difficulties due to the complex interaction between char and the pyrolytic liquid, often resulting in the formation of a gel-like phase that rapidly blocks the filter. Efforts to mitigate this problem have involved the use of solvents like methanol or ethanol to modify the liquid microstructure.173 However, this approach leads to solvent dilution of the liquid product and increases process costs. Consequently, ongoing research aims to identify suitable mechanisms for char separation in pyrolysis processes, considering both effectiveness and cost-efficiency.174
3.3.4.6. Liquids collection. Pyrolysis gaseous products typically contain true vapor, aerosols, and non-condensable gases, all of which necessitate swift cooling to minimize secondary reactions and facilitate the condensation of true vapors, while also requiring aerosol coalescence or agglomeration.175 However, the utilization of high-temperature heat recovery for this purpose is not ideal due to the severe fouling observed in recuperators. Commonly employed methods to address these challenges include cooling with a simple heat exchanger, quenching in product oil or an immiscible hydrocarbon solvent, or the use of conventional aerosol capture devices such as demisters and electrostatic precipitators. Nevertheless, these approaches often exhibit limited effectiveness, with electrostatic precipitation emerging as a preferable method for liquid separation, particularly at smaller scales up to pilot plant operations.175
Contemporary research in fast or flash pyrolysis has illuminated the potential for enhanced yields of primary, non-equilibrium liquids, and gases from biomass feedstocks. These yields encompass a diverse array of valuable products, spanning from chemicals and petrochemicals to essential fuel components and intermediates. Notably, the shift from traditional slow pyrolysis to fast pyrolysis heralds a transformation in product composition and value. Slow pyrolysis typically yields solid char of lower value, fast pyrolysis generates higher-value products such as fuel gas, fuel oil, and assorted chemicals. This transition underscores the versatility and economic viability of fast pyrolysis as a pivotal technology in biomass conversion processes.176
Exhibiting heating values typically ranging from 40 to 50% of conventional hydrocarbon fuels, pyrolysis liquid fuels offer several notable advantages. Firstly, they contribute positively to the CO2 balance, aligning with the sustainable nature of biomass-derived fuels. Secondly, their adaptability extends from small-scale power generation systems to integration within larger power stations.177 Commonly referred to as bio-oil or bio-crude, pyrolysis oils manifest as intricate blends of oxygenated compounds, encompassing various chemical groups such as carbonyls and carboxyls.175 Table 3 provides a comparative analysis of fuel properties between standard diesel and pyrolysis bio-oil across different feedstocks, offering insights into their respective characteristics and potential applications in the energy landscape.
| Moisture content (wt%) | pH | Viscosity mm2 s−1 | HHV (MJ kg−1) | |
|---|---|---|---|---|
| Diesel | — | 1 | 2.39 | 44.7 |
| Wood | 15–30 | 2.5 | 40–100 | 16 |
| Straw | 47.4 | 3.45 | 17.2 | 13.6 |
| Lonium perenne | 48 | 3.16 | 6.5 | 15.8 |
| Rice husk | 27.2 | 2.8 | 128 | — |
| Cotton talk | 26.7 | 2.6 | 156 | — |
| Palm shells | 10 | 2.7 | 14.6 | — |
Table 3 presents a comparative analysis of bio-oil properties derived from various biomass feedstocks, highlighting differences in moisture content, pH, viscosity, and heating value (HHV/LHV). Wood-derived bio-oil exhibits a moderate moisture content (15–30 wt%) and a higher HHV (∼16 MJ kg−1), making it suitable for direct combustion. In contrast, straw-based bio-oil has a significantly higher moisture content (47.4 wt%), which reduces energy efficiency and increases the need for drying or upgrading processes before utilization.
Rice husk and cotton stalk bio-oils have exceptionally high viscosities (128 mm2 s−1 and 156 mm2 s−1, respectively), indicating a greater need for catalytic hydrodeoxygenation or blending with lower-viscosity fuels to improve flow properties. The pH of all bio-oils (2.5–3.45) reflects their acidic nature, which can contribute to corrosion in storage tanks and pipelines, necessitating upgrading or neutralization treatments.
Notably, palm shell-derived bio-oil, with the lowest moisture content (∼10 wt%), demonstrates higher energy efficiency and lower water-induced energy losses, making it a promising candidate for power generation and co-firing applications. These findings underscore the importance of feedstock selection and refining strategies to enhance bio-oil quality and optimize its feasibility for transportation fuels and energy sector applications.179
3.3.4.7. Catalysts in pyrolysis. In general; catalysts are used to enhance pyrolysis reaction kinetics by cracking high molecular weight compounds into hydrocarbon products, however; distributions of obtained products are strongly dependent to catalyst type and differences in operating conditions. Catalysts in pyrolysis can be classified into three different groups, according to the way they are introduced during the process itself. First group is meant for catalysts that are added to the biomass feedstock before being fed into the thermal conversion reactor. The second group belongs to the catalysts that are added into the reactor to attain immediate contact with generated vapors, solid and tar from biomass degradation. For the third group, it contains the catalysts which can be placed in a secondary reactor located downstream from the pyrolysis reactor. Another classification for catalysts in pyrolysis, according to their compositions, was done by Han and Kim.180 This classification includes: dolomite catalyst, Ni-based catalysts, alkali metal catalysts and novel metal catalysts. Use of catalysts in fixed-bed reactor for pyrolysis of solid waste was found to greatly influence the yields of obtained products.180 Some types of catalysts have been studied in biomass pyrolysis such as: Ni–Al2O3,181 sodium feldspar,182 CeO2/Rh- SiO2,183 carbonates of Li, Na or K,184 zeolite185 and ZrO2.186 Ni and alkali metal-based catalysts were confirmed to be effective in heavy tar elimination and achieving more than 99% tar destruction, however, these catalysts were found to become inactive due to carbon deposition.184 Catalytic processing of pyrolysis bio-oil or liquid biomass (raw vegetable oils, waste cooking oils, animal fats and algal oils) can offer great flexibility in filling the increasing demands of the bio-fuels market. In general, catalytic process allows the conversion of triglycerides and lipids (liquid biomass) into paraffins and iso-paraffins within the range of naphtha, kerosene and diesel. Such products have improved characteristics compared to fossil fuels in terms of having high heating value, increased oxidation stability and negligible acidity. Besides, this route is effective for upgrading the released intermediate products (pyrolysis oil) during the process of solid biomass conversion,187,188 as exhibited in Fig. 18.
 | ||
| Fig. 18 Catalytic processing for biomass conversion towards fuels production.19 | ||
3.3.4.7.1. Catalytic cracking of bio-oil to produce light olefins and aromatics. Light olefins, including ethylene, propylene, and butenes, serve as fundamental precursors in the petrochemical industry, essential for synthesizing a wide array of products such as polyethylene, polypropylene, butadiene, acrylonitrile, and epoxyethane, among others.189 Traditionally, these compounds are predominantly derived from steam cracking of naphtha or light-alkane feedstocks, a process characterized by high energy consumption and significant CO2 emissions.190 Complementing these olefins are aromatics, comprising benzene, toluene, and xylenes, constituting approximately one-third of the market for commodity petrochemicals. The primary sources of aromatics include petroleum naphtha (70%), pyrolysis gasoline by-products from ethylene plants (23%), and coal liquids from coke ovens (7%).191
Gayubo et al.192 explored the selective production of C2–C4 olefins from bio-oil using a two-step (thermal-catalytic) reaction system employing HZSM-5 zeolite (SiO2/Al2O3 = 80) as a hydrothermally stable catalyst. Their investigation focused on analyzing the impact of various operating conditions, including methanol content in the raw bio-oil, temperature, and space-time, culminating in achieving a bio-oil conversion rate of 94% with a notable olefins selectivity of 48 wt% by employing a bio-oil/methanol feed ratio of 50/50 wt% at 500 °C and a space-time of 0.37 g catalyst h(gfeed)−1. These findings serve as a pivotal starting point for further exploration into the co-processing of raw bio-oil with methanol, particularly within the methanol-to-olefins (MTO) process paradigm.
Gong et al.193 reported another noteworthy study focusing on the selective production of light olefins through the catalytic cracking of raw bio-oil utilizing La-modified HZSM-5 catalysts. Incorporation of La into the zeolite structure led to a moderate increase in medium acid sites, effectively enhancing selectivity toward light olefins while concurrently improving catalyst stability. Their investigations yielded promising results, with the highest light olefins yield reaching 0.28 kgolefins (kgbio-oil)−1, accompanied by nearly complete bio-oil conversion. Subsequent studies by the same researchers, employing Mg-modified HZSM-5 catalysts to adjust acidity, further corroborated these findings, demonstrating enhanced olefins selectivity and improved catalyst stability, with an olefins yield of 0.25 kgolefins (kgbio-oil)−1 and near-complete bio-oil conversion. A burgeoning area of interest lies in the integrated approach of catalytic cracking–oligomerization, aiming to produce gasoline-range hydrocarbons from light olefins. This innovative strategy entails catalytic cracking of bio-oil to generate light olefins, followed by the subsequent synthesis of higher C5+ olefins through oligomerization, typically achieved via two reactors in series. This directional synthesis pathway facilitates the production of C6–C12 olefins, offering promising prospects for advancing the utilization of bio-based feedstocks in the production of valuable hydrocarbon products.
Puértolas et al.194 demonstrated that hierarchically structured HZSM-5 zeolites, with increased accessibility of Brønsted acid sites on the mesoporous surface, facilitated a notable up to 50% increase in the production of aromatics compared to bulk zeolites with moderate Si/Al ratios (25–40). These catalysts favor decarboxylation/decarbonylation reactions, yielding selectively cycloalkanes and alkyl monoaromatic hydrocarbons, whereas microporous zeolites predominantly promote dehydration reactions, resulting in the formation of cyclic olefins and polyaromatic hydrocarbons.
Effective conversion of bio-oil into aromatics necessitates deoxygenation over both metal and acid sites to form olefins, cyclization of olefins over acid sites to produce cycloalkanes, and dehydrogenation of cycloalkanes over metal sites to yield final aromatics. For bio-oils rich in phenolic compounds, aromatics can also form through direct deoxygenation (hydrogenolysis). Selection of appropriate metals has been shown to efficiently control selectivity between aromatics/cycloalkanes via hydrogenolysis/hydrogenation–dehydration routes. ZSM-5 modified with Ga, Zn, and Ni, as well as Ru, Cu, and Fe/zeolite catalysts, exhibited promising behavior in converting bio-oil into BTX aromatics through hydrogenolysis. Additionally, metal-doped (Zn, Ce, Ni) mesoporous rod-like Al2O3 catalysts have shown encouraging results in selectively producing aromatics from raw bio-oil, with Ni/Al2O3 demonstrating the highest hydrocarbon content, mainly comprising BTX aromatics.195,196
Shafaghat et al.197 investigated the conversion of a simulated phenolic bio-oil over Ni and Fe supported H-beta catalysts. The Fe/H-beta catalyst enhanced hydrogenolysis of phenolic compounds, yielding aromatic hydrocarbons as the main products, while the Ni/H-beta catalyst exhibited a higher capability for hydrogenation–dehydration–hydrogenation of benzene rings, resulting in cycloalkanes as the primary products. Other studies have shown that ZSM-5 modified with metals such as Zn and Ga effectively promote aromatization via dehydrogenation of cycloalkanes. For instance, Cheng et al. reported a 40% increase in aromatics yield when Ga was added to ZSM-5 in the catalytic upgrading of furan, with Ga/ZSM-5 facilitating decarbonylation and olefin aromatization reactions, while ZSM-5 catalyzed other reactions leading to aromatics production, such as oligomerization and cracking.198
3.3.4.7.2. Production of bio-gasoline. Table 4 below delineates the types of biomasses utilized for the production of bio-oil through pyrolysis conversion, which holds promise for subsequent conversion into bio-gasoline.199 Previous investigations into biomass-derived bio-oil have consistently highlighted the significance of low moisture content, high carbon content, and low oxygen content in enhancing its heating value. The heating value of bio-oil derived from biomass, reaching a maximum of 20.28 MJ kg−1 (as observed in Jatropha seed shell), underscores its potential for conversion into biofuels, given its comparability to ethanol (23 MJ L−1) and gasoline (43.1 MJ kg−1) in terms of heating value.200
| Feedstock | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| Rice straw | 37.81 | 26.89 | 13.1 |
| Empty fruit bruches | 51.2 | 22.5 | 21.3 |
| Mesocarp fibre of a palm tree | 23.7 | 30.5 | 27.3 |
| Jatropha seed shell | 36.64 | 4.82 | 39.61 |
| Wheat shell | 10–15 | 30 | 4–8 |
| Mesquite sawdust | 40–45 | 25–30 | 11–30 |
| Sawdust | 41.94 | 19.33 | 29.63 |
| Beach wood | 46.4 | 31.7 | 21.9 |
| Corn cob | 41.7 | 30.84 | 12.44 |
The low moisture content, typically ranging from 1.1% to 18.8%, underscores the efficiency of biomass sources for thermal conversion into liquid fuels, with values consistently below 50%. Ash content measurement is essential to ascertain the residual incombustible solids post-thermal processing, which comprise mineral and inorganic components such as calcium, potassium, magnesium, and phosphorus, serving as potential nutrients for other processes.201
The fixed carbon percentage, determined based on data obtained from physical analysis including moisture, ash, and volatile matter, provides insights into the remaining mass of carbon. Volatile matter and fixed carbon contents play pivotal roles in determining the ease of ignition and subsequent gasification or oxidation of biomass as an energy source. Higher volatile matter values indicate the potential for solid transformation into other products, either in liquid or gas phases. The elevated proportions of carbon and hydrogen contribute to the high C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H ratio, underscoring the suitability of waste with abundant amounts of these elements for biofuel production.129,202
H ratio, underscoring the suitability of waste with abundant amounts of these elements for biofuel production.129,202
The commercialization of biomass-to-biofuel technologies, for example the production of bioethanol from sugar substrates and biodiesel from rapeseed or sunflowers, marks significant progress in the field. Biomass, harnessing approximately 0.1–1.0% of solar energy, predominantly comprises sugar polymers constituting 75–90 wt% of biomass, with lignin accounting for 10–25% of its weight. Cellulosic biomass, the primary type, consists of cellulose (35–50%), hemicelluloses (15–25%), and lignin (15–30%). By dry weight, biomass typically contains 30–40% oxygen, 30–60% carbon, 5–6% hydrogen, and minimal amounts of nitrogen, sulfur, and chlorine, with inorganic components concentrated in the ash.203
Fructose, glucose, and xylose serve as precursors for liquid alkanes, the constituents of gasoline, synthesized through microbial fermentation or catalytic processes. In a wood chips design case, fast pyrolysis, hydro-treatment, and hydrocracking yield 23% bio-gasoline and 32% biodiesel. Molybdenum zeolite-catalyzed gasification of woody biomass also contributes to bio-gasoline production, facilitating its use in unmodified motor vehicles. Direct conversion of cellulosic biomass into bio-gasoline via hydrothermal processes, employing in situ hydrogen under various conditions, presents a promising avenue.204,205 Table 4 provides a comprehensive overview of biomass types suitable as feedstock for bio-gasoline production, underpinned by exhaustive ultimate and proximate analyses.
Table 4 summarizes the physicochemical properties of biochar obtained from various biomass feedstocks, highlighting differences in surface area, carbon content, and ash composition. Lignocellulosic biomass-derived biochar (e.g., wood, sawdust) generally possesses higher fixed carbon content (>80%) and lower ash content (<5%), making it more suitable for energy storage and catalytic applications. Conversely, agricultural residues (e.g., rice straw, corn stover) produce biochar with higher ash content (>20%), which can affect adsorption efficiency in pollutant removal applications.206 Hydrochar synthesized via hydrothermal carbonization retains higher oxygen functional groups, enhancing cation exchange capacity (CEC) for soil amendment purposes. These variations underscore the importance of selecting appropriate biomass precursors and conversion methods to tailor biochar properties for specific industrial applications.106,207
3.3.4.7.3. Enhancement of bio-oil to biofuel. Mortensen et al. 208 implemented a hydrodeoxygenation (HDO) technique, employing a conventional hydrocracking catalyst, to enhance the bio-oil, yielding approximately 0.33–0.64 g oil per g of bio-oil, with hydrocracking facilitating the formation of a pure hydrocarbon product. Conversely, findings from Eboibi et al. 209 demonstrated that vacuum distillation, without catalyst utilization, significantly increased the biocrude yield, reaching 62–67 wt% (from 36 to 42 wt%) for Spirulina sp. and 70–73 wt% (from 34 to 58 wt%) for Tetraselmis sp. Furthermore, the higher heating value (HHV) of the resultant biocrude escalated from 32 MJ kg−1 to 40 MJ kg−1 through this method. In a recent investigation by Tian et al.,210 the hydrodeoxygenation process was explored as a means to refine biocrude oil under relatively moderate conditions, resulting in a notable reduction of oxygen content to 1% and nitrogen content to 0.3%. Subsequent processing in the hydrotreater yielded distinct fractions, including C4 minus, gasoline, diesel, and heavy oil. Conversely, Zhang et al.211 highlighted the challenges posed by bio-oil, generated via fast pyrolysis, characterized by oxygenated compounds exhibiting unfavorable properties such as high viscosity, limited thermal and chemical stability, as well as poor miscibility with hydrocarbon fuels, leading to corrosion concerns. Consequently, the focus shifted towards refining bio-oil to diminish its oxygen content through hydrodeoxygenation and hydrodesulphurization processes. Notably, hydrotreatment of pyrolysis oil derived from pine sawdust not only raised the pH value from 2.27 to 4.07 but also augmented the hydrogen content from 6.28 to 7.01 wt%. Tao et al.212 proposed an innovative approach to integrate bio-oil into the transportation sector by directly blending it with diesel fuel through an emulsification process employing a surfactant. Optimal emulsifier concentrations ranging from 0.5% to 2.0% were identified, resulting in bio-oil ratios of 25%, 50%, and 75% by weight, yielding viscosities conducive to effective utilization. Meanwhile, Samolada et al.213 investigated the utilization of biomass flash pyrolysis liquids (BFPLs) in the FCC process. Employing ReUSY catalysts with a Re2O3 content of 0.6 and a pore size of 36 Å, a nominal coke catalyst formation of 1 wt% and a notable gasoline yield ranging from 23% to 25% were achieved.
4. Waste cooking oils conversion to paraffinic biofuels
Waste Cooking Oils (WCO) is a type of residual biomass resulting usually from frying (e.g. soybean-oil, corn-oil and olive-oil, etc.) and they have specific problems. Catalytic hydroprocessing of WCO was studied as an alternative route for producing 2nd generation of biofuels. The catalytic hydrocracking was investigated over commercial hydrocracking catalysts leading not only to production of biodiesel but also to obtain lighter products such as biogasoline, employing continuous-flow catalytic hydroprocessing plant with a fixed-bed reactor.214 During such technique several parameters should be considered, including hydrocracking temperature (350–390 °C) and liquid hourly space velocity (0.5–2.5 h−1) and under high pressure (60–100 bar). The efficient conversion of WCO to bio-gasoline was found to be favored at high reaction temperature and low LHSV. On the other hand, implementation of quite low temperatures is more preferred for biodiesel production.215–2174.1. WCO transesterification
Transesterification is a series of reversible reactions that can be used for conversion of WCO into biofuels. In such process the present lipids/triglycerides in oil specimen combine with alcohol, typically methanol, to produce monoglycerides, diglycerides glycerol and biodiesel. Factors that may affect the efficiency of transesterification include alcohol-to-oil molar ratio, temperature, stirring speed, moisture content, reaction duration, and catalyst type. Various catalysts, including acidic or alkaline (homogeneous or heterogeneous), enzymes, ionic liquids and carbon-based catalysts can speed up this process. However, homogeneous alkaline catalysts are the most widely utilized in the process of biodiesel synthesis.218 The superiority of such type of catalysts is due to their ability to accelerate the reaction at low temperatures and ambient pressure, resulting in high conversion yields. The most typically used alkaline catalysts for this process are potassium hydroxide (KOH) and sodium hydroxide (NaOH).2195. Comparing biomass to alternative renewable energy sources
The recent conclusion of COP28 stands as a pivotal moment in the global fight against climate change. The agreement reached, aimed at “transitioning away from fossil fuels,” coupled with the firm commitment to “triple renewable energy capacities and double energy efficiency by 2030,” heralds a promising era for sustainable energy transitions. Despite coal and gas continuing to dominate global electricity generation in 2020, collectively constituting nearly 60% of the total, there have been notable advancements in renewable energy sources such as solar and wind power, which accounted for 29% of global electricity production.220The latest data in 2023 from world bioenergy association (WBA) statistics report showed in 2020,221,222 global biomass-based electricity generation amounted to 685 terawatt-hours (TWh). A substantial majority of this biopower, comprising 69%, originated from solid biomass sources, with municipal and industrial waste contributing 11% (Fig. 19).223
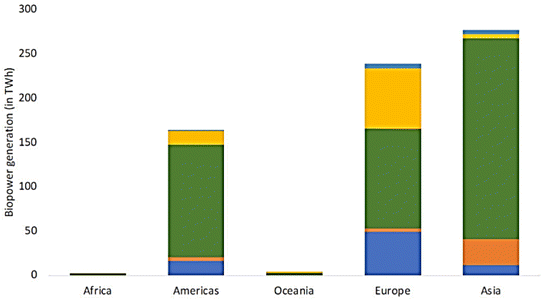
Moreover, the transport sector witnessed the consumption of 4.23 EJ of renewable energy in 2020, where liquid and gaseous biofuels dominated, accounting for 90% of all renewable energy utilized in this domain. Notably, the share of renewable electricity in the transport sector, calculated based on the overall electricity sector, stood at 10% (Fig. 20). Regionally, Asia and Europe continued to lead in biopower generation, with Asia contributing 276 TW h and Europe closely following at 238 TW h in 2020. Europe notably excelled in biopower production from municipal waste, constituting 64% of global production, while Asia led in utilizing industrial waste for electricity, commanding a global share of 79%. Furthermore, Europe emerged as a frontrunner in biopower from biogas, claiming a global share of 75% in this domain.
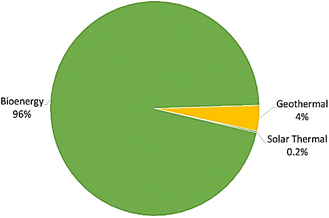
Regionally, Asia emerged as a significant player, accounting for 40% of global biopower production (Fig. 21), generating 276 TW h, followed closely by Europe at 35%. Notably, electricity-only plants, designed exclusively for power generation, utilized 5.3 joules (EJ) of biomass in 2020. Additionally, renewable heat production, totaling 1.26 EJ in 2020, primarily relied on biomass, contributing 96% of all renewable heat generated (Fig. 22), with geothermal and solar thermal technologies making minor contributions.
5.1. Policy environments influencing biomass energy development
The advancement of biomass energy is profoundly shaped by regional policy frameworks, which vary significantly across different countries and regions. In the United States, the Inflation Reduction Act (IRA) of 2022 introduced substantial tax incentives to bolster renewable energy production, including biomass.224 For instance, biodiesel blenders registered with the Internal Revenue Service (IRS) are eligible for a tax credit of $1.00 per gallon of pure biodiesel or renewable diesel blended with petroleum diesel. Additionally, the Biomass Crop Assistance Program (BCAP) offers financial assistance to producers establishing biomass feedstock crops, reimbursing up to 50% of establishment costs and providing annual payments for up to five years for herbaceous feedstocks and up to 15 years for woody feedstocks. In the European Union, the Renewable Energy Directive II (RED II) sets forth stringent sustainability criteria for biomass used in energy production. These criteria encompass greenhouse gas emission reductions and sustainable forest management practices, ensuring that biomass contributes effectively to the EU's renewable energy targets. The directive also introduces sustainability requirements for forestry feedstocks and greenhouse gas criteria for solid and gaseous biomass fuels.225China has demonstrated remarkable progress in expanding its renewable energy capacity, with biomass energy playing a vital role in its strategy to peak carbon emissions before 2030 and achieve carbon neutrality before 2060. The country's policies focus on integrating biomass energy into its broader renewable energy expansion plans, contributing to a significant portion of global renewable capacity growth. Brazil has implemented the RenovaBio program, a policy designed to promote the decarbonization of the transport sector by encouraging the use of biofuels with superior energy-environmental efficiency compared to fossil fuels. This program has led to the production of 77 billion liters of biodiesel, avoiding emissions of 240 million tons of CO2 and saving approximately USD 38 billion in imports.226
These examples illustrate how diverse policy environments influence the development and application of biomass energy. While the United States and Brazil emphasize financial incentives and market-based approaches,227 the European Union focuses on stringent sustainability criteria, and China integrates biomass energy into its centralized planning for carbon neutrality. Understanding these regional policy differences is crucial for comprehensively assessing the global landscape of biomass energy development.
5.2. Challenges and solutions in scaling biomass energy and overcoming technical and market barriers
The large-scale deployment of biomass energy faces significant challenges, notably high technical costs and volatile market dynamics. Addressing these issues requires a multifaceted approach encompassing technological innovation, strategic industrial planning, and supportive policy frameworks.Technological innovation: advancements in biomass processing technologies can substantially reduce production costs. For instance, the implementation of biomass torrefaction—a thermal pre-treatment process—enhances the energy density of biomass, leading to more efficient transportation and storage. A case study analyzing biomass transport from Indonesia to Japan demonstrated that torrefied biomass resulted in 6.7% energy savings and a 10.3% reduction in greenhouse gas emissions compared to traditional methods. Such improvements not only lower operational costs but also enhance the environmental sustainability of biomass energy production.228
Strategic industrial planning: optimizing the industrial layout of biomass facilities is crucial for cost efficiency. Locating biomass plants near abundant feedstock sources minimizes transportation expenses and supply chain disruptions. For example, Sweden's Värmevärden heating plant achieved energy self-sufficiency and reduced operating costs by approximately 30% by utilizing locally sourced forestry waste in its biomass boilers. This strategic siting not only cuts costs but also supports local economies and promotes sustainable resource utilization.229
Policy support: government policies play a pivotal role in shaping the biomass energy landscape. Incentive structures such as feed-in tariffs, subsidies, and tax credits can make biomass projects more financially viable. In the United States, the Biomass Crop Assistance Program (BCAP) provides financial assistance to producers establishing biomass feedstock crops, covering up to 50% of establishment costs and offering annual payments for up to five years for herbaceous feedstocks.230 Such policies lower the economic barriers to entry and encourage investment in biomass energy.231 However, reliance on policy incentives necessitates stability and clarity to prevent market volatility. The case of Braya Renewable Fuels in Canada illustrates this point; the refinery was idled due to low profit margins and market disruptions linked to uncertainties in U.S. tax-credit policies for biofuels.232 This example underscores the importance of consistent and transparent policy frameworks to foster investor confidence and industry growth. In summary, overcoming the challenges of scaling biomass energy requires an integrated approach that combines technological advancements, strategic industrial positioning, and robust, stable policy support. Learning from practical cases worldwide can provide valuable insights into effective strategies for reducing costs and navigating market dynamics in the biomass energy sector.
6. Assessing the economic and environmental impacts of energy production from biomass
Biomass stands out among diverse renewable resources and technologies as a potentially paramount economic driver for developing nations, concurrently fostering employment opportunities and environmental preservation. This assertion necessitates a thorough economic evaluation of various biomass production technologies, focusing on the generation of primary biomass products such as electricity and biofuels. Key factors under scrutiny include production costs, conversion efficiencies, and the scale of processing. It's noteworthy that the manufacturing costs and conversion efficiencies vary significantly depending on the raw materials utilized and the specific conversion methodologies employed. Typically, electricity production costs range from 0.03 to 0.24 USD per kW per h, whereas biofuel production costs span from 0.13 to 0.99 USD per L.233,234 Despite these costs, conventional electricity and fossil fuels currently maintain competitive pricing compared to their bio-based counterparts. However, biomass holds a substantial environmental advantage over fossil fuels for several reasons. Firstly, biomass serves as a renewable energy source, ensuring its perpetual availability. Secondly, biomass energy predominantly originates from continually accumulating waste streams from various sectors, mitigating environmental pollution that would otherwise occur. Thirdly, biomass utilization has the potential to significantly curtail greenhouse gas (GHG) emissions, thereby addressing pressing environmental challenges like climate change and global warming. Notably, empirical studies have demonstrated substantial reductions in GHG emissions, exceeding 90% in certain instances, through the substitution of fossil fuels with biomass-derived energy.207 Fig. 23 provides a comparative analysis of CO2 emissions from conventional energy sources compared with biomass-derived energy sources, showcasing their respective GHG emission savings.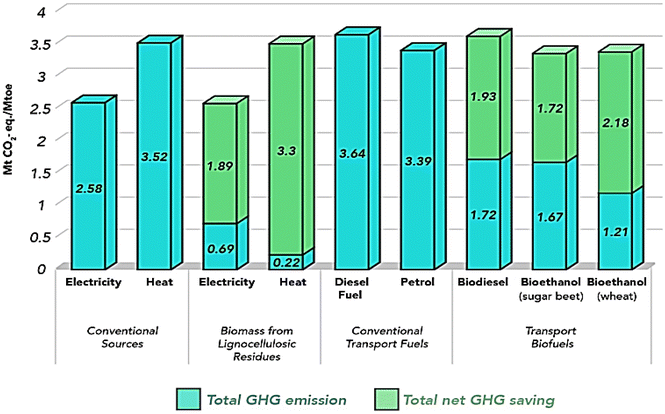 | ||
| Fig. 23 Comparison of CO2 emissions from conventional energy sources biomass-derived energy sources along with their saving in GHGs emission.235 | ||
7. Final reflections and prospects ahead
The substitution of fossil energy sources with biomass-derived alternatives offers multifaceted benefits spanning economic, environmental, and public health domains. Additionally, the ubiquity of biomass resources worldwide renders biomass-derived energy production viable across diverse geographical landscapes, concurrently facilitating the effective management of various waste streams. However, to realize the full potential of biomass energy, significant technological advancements are imperative to enhance productivity and mitigate production costs. This necessitates a concerted focus on developing user-friendly and cost-effective technologies across different scales to stimulate greater investment in the sector. Furthermore, there is a pressing need to foster greater awareness regarding the advantages of biomass utilization for renewable energy generation. Importantly, the production of biomass-derived energy carriers, including bioelectricity and biofuels, aligns with several Sustainable Development Goals (SDGs) outlined by the United Nations General Assembly in 2015, notably contributing to the goal of Affordable and Clean Energy by 2030.8. Future research directions in biomass energy and engineered biochar
The potential of biomass energy and engineered biochar is vast, but several key areas require further research to maximize their efficiency, environmental benefits, and economic feasibility. The following aspects should be considered in future studies:8.1. Optimization of biochar production processes
One of the primary challenges in biochar research is improving the efficiency and sustainability of production techniques. While pyrolysis remains the most common method, low-temperature slow pyrolysis yields biochar with higher stability, whereas fast pyrolysis favors bio-oil production. Future studies should focus on:• Catalytic pyrolysis and gasification: the use of heterogeneous catalysts such as metal oxides (ZnO, Fe2O3) and zeolites can enhance biochar's surface area and adsorption capabilities while reducing unwanted byproducts.
• Microwave-assisted pyrolysis: this emerging technology offers energy-efficient heating, leading to higher carbon retention and improved pore structure.
• Hydrothermal carbonization (HTC): an alternative to pyrolysis that operates under subcritical water conditions, allowing wet biomass to be converted into hydrochar with oxygen-rich functional groups, improving its cation exchange capacity for soil applications.
• Advanced process optimization through machine learning and AI-based modeling can further refine reaction conditions, maximizing biochar yield and physicochemical properties while reducing energy consumption.
8.2. Functionalization for environmental remediation
Biochar's ability to adsorb heavy metals, organic pollutants, and greenhouse gases can be significantly enhanced through chemical and structural modifications. Future research should focus on:• Surface activation techniques: acid (H3PO4, H2SO4) and alkaline (KOH, NaOH) treatments increase porosity and introduce oxygen-containing functional groups, improving adsorption of pollutants.236
• Metal-doped biochar: incorporating transition metals (Fe, Co, Ni) and carbon-based nanostructures (graphene, carbon nanotubes) enhances biochar's catalytic efficiency in wastewater treatment and CO2 capture.
• Photoactive biochar composites: ZnO and TiO2-modified biochar can function as efficient photocatalysts for degrading organic pollutants under visible light.
Further investigation into synergistic effects between biochar and nanomaterials is essential for developing high-performance adsorbents and catalysts with broad environmental applications.
8.3. Integration into agricultural systems
Biochar has demonstrated significant potential in improving soil fertility, carbon sequestration, and plant growth. However, long-term field studies are needed to understand its impact on:• Microbial communities: biochar's effect on soil microbiota, enzyme activity, and nutrient cycling must be evaluated across different climatic and soil conditions.
• Crop-specific applications: different plant species respond differently to biochar amendments. Research should optimize application rates based on biochar composition, soil pH, and crop type.
• Carbon sequestration modeling: more research is needed to quantify long-term carbon stability and net carbon sequestration rates in biochar-amended soils under various land-use scenarios.
Future research should also assess biochar's role in climate resilience strategies, particularly in mitigating drought stress in agricultural systems.
8.4. Life cycle and techno-economic analyses
While biochar and biomass energy present environmental benefits, their large-scale deployment requires economic and sustainability assessments. Future research should address:• Life cycle assessment (LCA): studies should evaluate the energy balance, carbon footprint, and environmental trade-offs of biochar production from various feedstocks.
• Techno-economic feasibility: assessing the costs of biomass preprocessing, transportation, and biochar activation will determine the commercial viability of different biochar applications.
• Integration with carbon credit markets: investigating how biochar projects can generate carbon credits and contribute to decarbonization policies will enhance investment attractiveness in the sector.
Developing standardized cost–benefit analysis models will further aid policymakers and investors in decision-making processes.
8.5. Policy and market development
Policy frameworks play a crucial role in shaping biomass and biochar commercialization. Future research should examine:• Regulatory standards for biochar quality and safety: harmonized international guidelines are needed to define biochar grading, certification, and application limits.
• Economic incentives: policies such as carbon tax credits, feed-in tariffs, and subsidies could significantly drive biomass and biochar market expansion.
• Supply chain optimization: investigating regional feedstock availability and logistics can improve market scalability and reduce production costs.
A better understanding of policy interventions and market mechanisms will facilitate the widespread adoption of biochar as a sustainable energy solution.
9. Conclusion
Biomass combustion represents a promising avenue for optimizing heater design in future applications, with a critical focus on enhancing conversion efficiencies from biomass combustion to heat. One key parameter crucial for achieving optimal combustion performance is the excess air ratio. However, it's imperative to acknowledge the inherent challenges associated with mitigating emissions such as nitrogen oxides (NOx) and particulate matter. These challenges stem from the fundamental physical constraints imposed by the high temperatures and oxygen presence characteristic of combustion processes. In the quest for sustainable energy solutions, the utilization of both wood and non-wood biomass in gasification processes has garnered significant attention. Employing thermochemical models, researchers have evaluated various biomass feedstocks to assess their suitability for gasification. This evaluation encompasses the examination of produced syngas and the gasification process itself, serving as pivotal metrics for comparing different biomass types. Simulation studies have been conducted using a diverse array of biomass sources, including oats, wheat straw, rapeseed by-products from bio-fuel production, and various wood biomass varieties. Furthermore, biomass pyrolysis has emerged as a focal point in energy research due to its potential for converting agricultural residues, wood wastes, and solid waste into valuable energy products. Particularly noteworthy is the catalytic hydrotreatment of liquid biomass, a proven technology capable of transforming feedstocks characterized by low hydrogen-to-carbon (H/C) ratios and high oxygen and water content into a range of alternative fuels. These fuels, including bio-naphtha, bio-jet, and biodiesel, exhibit a paraffinic nature and boast high heating values, underscoring the versatility and potential of catalytic hydrotreatment in advancing sustainable fuel production methodologies. This review distinguishes itself from prior studies by bridging biomass conversion technologies with advanced biochar engineering, offering a multi-faceted perspective on biochar's transformation into high-value energy and industrial products. Unlike previous studies that primarily assess biomass-derived biochar for soil enhancement and carbon sequestration,237 this review underscores biochar's potential in fuel cell electrodes, energy storage devices, and catalytic upgrading of bio-fuels. Additionally, the review explores recent advancements in biochar-supported metal catalysts for hydrogen production, syngas purification, and biodiesel refinement. By critically analyzing biochar's evolving role in super-capacitors, batteries, and green hydrogen production, this study provides a framework for future innovations that enhance biomass valorization and decarbonization efforts in the global energy sector.Data availability
The data that support the findings of this study are openly available in [Technologies and Innovations for Biomass Energy Production] at [https://colab.ws/articles/10.3390%2Fsu151612121] and [Recent progress on catalytic pyrolysis of lignocellulosic biomass to high-grade bio-oil and bio-chemicals] at [https://www.sciencedirect.com/science/article/pii/S1364032116310589], references number [111 and 205]. The authors confirm that the data supporting the findings of this study are available within the article.Author contributions
Esraa El-Fawal: literature survey, writing original draft, Ahmed El Naggar: literature survey, writing and preparing article for submission, Adel El-Zahhar: literature survey, and revising the article before submission, Majed M. Alghamdi: literature survey, and revising the article before submission, Asmaa Morshedy: literature survey, Hussien El Sayed: literature survey and took part in writing, and Ard elshifa Mohammed: revising the article before submissionConflicts of interest
The authors of this article would like to confirm that all of them have no conflict/competing of interests with any organization or any person and the funding body is listed.Acknowledgements
The authors extend their appreciation to the Deanship of Research and Graduate studies at King Khalid University for funding this work through Large Research project under grant number RGP 2/74/45.References
- W. A. Aboutaleb, A. M. A. El Naggar, M. A. Sayed, A. I. Zahran, H. S. Ahmed, M. A. Mekewi and A. A. El-Zahhar, Mater. Chem. Phys., 2022, 276, 125165 CrossRef CAS.
- A. M. A. El Naggar, A. I. Zahran, W. A. Aboutaleb, M. A. Sayed, H. S. Ahmed, A. A. El-Zahhar and M. A. Mekewi, Int. J. Environ. Anal. Chem., 2024, 104, 231–250 CrossRef CAS.
- A. M. A. El Naggar, H. A. El Sayed, A. A. Salem and N. E. A. AbdEl-Sattar, Ind. Eng. Chem. Res., 2020, 59, 7277–7290 CrossRef CAS.
- D. R. Abd El-Hafiz, S. A. El-Temtamy, M. A. Ebiad, R. A. El-Salamony, S. A. Ghoniem, A. M. A. El Naggar and T. S. Gendy, Int. J. Hydrogen Energy, 2020, 45, 9783–9794 CrossRef CAS.
- L. C. Goveas, S. Nayak, R. Vinayagam, R. Selvaraj and A. Pugazhendhi, Fuel, 2023, 348, 128460 CrossRef CAS.
- R. A. El-Salamony, S. A. El-Temtamy, A. M. A. El Naggar, S. A. Ghoneim, D. R. Abd El-Hafiz, M. A. Ebiad, T. Gendy and A. M. Al-Sabagh, Int. J. Energy Res., 2021, 45, 3899–3912 CrossRef CAS.
- A. M. A. El Naggar and C. Kazak, Sep. Purif. Technol., 2016, 160, 73–80 CrossRef CAS.
- A. S. Morshedy, H. M. Abd El Salam, A. M. A. El Naggar and T. Zaki, Energy Fuels, 2020, 34, 11660–11669 CrossRef CAS.
- H. A. El Sayed, A. M. A. El Naggar, B. H. Heakal, N. E. Ahmed, S. Said and A. A. H. Abdel-Rahman, Fuel, 2017, 209, 127–131 CrossRef CAS.
- M. A. Moneim, A. M. A. El Naggar, H. A. El Sayed, M. S. Mostafa, N. M. Khalil and M. E. D. Hassan, J. Petrol. Geol., 2018, 27, 991–995 Search PubMed.
- S. Hussain, Z. Ulhassan, M. Brestic, M. Zivcak, W. Zhou, S. I. Allakhverdiev, X. Yang, M. E. Safdar, W. Yang and W. Liu, Photosynth. Res., 2021, 150, 5–19 CrossRef CAS.
- X. Wei, X. Zhang, L. Jin, X. Yang, W. Zou, B. Gao and L. Dong, Appl. Catal., B, 2024, 351, 123957 CrossRef CAS.
- J. Ben-Iwo, V. Manovic and P. Longhurst, Renew. Sustain. Energy Rev., 2016, 63, 172–192 CrossRef.
- L. J. R. Nunes, J. C. O. Matias and J. P. S. Catalão, Renew. Sustain. Energy Rev., 2014, 40, 153–160 CrossRef CAS.
- V. Kirsanovs and A. Žandeckis, Agron. Res., 2015, 13, 500–510 Search PubMed.
- C. Huang, S. Chen, X. Fei, D. Liu and Y. Zhang, Catalysts, 2015, 5, 1846–1861 CrossRef CAS.
- E. M. El-Fawal and T. Zaki, J. Polym. Environ., 2022, 30, 971–987 CrossRef CAS.
- E. M. El-Fawal, L. Saad and Y. M. Moustafa, Water Environ. Res., 2020, 92, 1293–1305 CrossRef CAS.
- R. A. Quevedo-Amador, B. P. Escalera-Velasco, A. M. R. Arias, H. E. Reynel-Ávila, J. C. Moreno-Piraján, L. Giraldo and A. Bonilla-Petriciolet, Clean Technol. Environ. Policy, 2024, 26, 943–997 CrossRef CAS.
- M. Tripathi, J. N. Sahu and P. Ganesan, Renew. Sustain. Energy Rev., 2016, 55, 467–481 CrossRef CAS.
- J. Lehmann and S. Joseph, Biochar for Environmental Management: Science, Technology and Implementation, Taylor & Francis, 2024 Search PubMed.
- H. Chaudhary, J. Dinakaran and K. S. Rao, J. Environ. Chem. Eng., 2024, 12, 113003 CrossRef CAS.
- A. S. Morshedy, M. H. Taha, D. M. Abd El-Aty, A. Bakry and A. M. A. El Naggar, Environ. Technol. Innov., 2021, 21, 101363 CrossRef CAS.
- M. H. Taha, S. A. Abdel Maksoud, M. M. Ali, A. M. A. El Naggar, A. S. Morshedy and A. A. Elzoghby, Int. J. Environ. Anal. Chem., 2019, 99, 1211–1234 CrossRef CAS.
- A. M. A. El Naggar, M. M. Ali, S. A. Abdel Maksoud, M. H. Taha, A. S. Morshedy and A. A. Elzoghby, J. Radioanal. Nucl. Chem., 2019, 320, 741–755 CrossRef CAS.
- E. M. El-Fawal and O. A. A. El-Shamy, Int. J. Environ. Sci. Technol., 2019, 16, 6827–6838 CrossRef CAS.
- E. M. El-Fawal, S. A. Younis and T. Zaki, J. Photochem. Photobiol., A, 2020, 401, 112746 CrossRef CAS.
- M. El Saied, A. M. A. El Naggar and F. I. Elhosiny, J. Clean. Prod., 2019, 218, 157–166 CrossRef CAS.
- K. Wang, J. Remón, Z. Jiang and W. Ding, Polymers, 2024, 16, 851 CrossRef CAS PubMed.
- P. Prabakar, K. Mustafa Mert, L. Muruganandam and K. Sivagami, Front. Energy Res., 2024, 12, 1448520 CrossRef.
- S. Brethauer, R. L. Shahab and M. H. Studer, Appl. Microbiol. Biotechnol., 2020, 104, 5201–5212 CrossRef CAS PubMed.
- W.-J. Liu, H. Jiang and H.-Q. Yu, Chem. Rev., 2015, 115, 12251–12285 CrossRef CAS.
- L. Laureano-Perez, F. Teymouri, H. Alizadeh and B. E. Dale, Appl. Biochem. Biotechnol., 2005, 124, 1081–1099 CrossRef.
- Y. Luo, T. Lu, S. Jin, K. Ye, S. Yu, X. Zhang, X. Wu, P. Ma, J. W. Tester and K. Wang, J. Supercrit. Fluids, 2024, 106290 CrossRef CAS.
- M. Sasaki, Z. Fang, Y. Fukushima, T. Adschiri and K. Arai, Ind. Eng. Chem. Res., 2000, 39, 2883–2890 CrossRef CAS.
- S. Kumar, in Advanced Biofuels and Bioproducts, Springer, 2012, pp. 147–183 Search PubMed.
- P. Modugno and M. Titirici, ChemSusChem, 2021, 14, 5271–5282 CrossRef CAS PubMed.
- Y. Gao, X.-H. Wang, H.-P. Yang and H.-P. Chen, Energy, 2012, 42, 457–465 CrossRef CAS.
- Y. Gao, P. Wu, P. Jeyakumar, N. Bolan, H. Wang, B. Gao, S. Wang and B. Wang, J. Environ. Manage., 2022, 313, 114973 CrossRef CAS.
- X. Zhou, Y. Zhu, Q. Niu, G. Zeng, C. Lai, S. Liu, D. Huang, L. Qin, X. Liu and B. Li, Chem. Eng. J., 2021, 416, 129027 CrossRef CAS.
- M. Sevilla and A. B. Fuertes, Chem.–Eur. J., 2009, 15, 4195–4203 CrossRef CAS PubMed.
- I. Güdücü, K. Alper, T. Evcil, K. Tekin, H. Ohtani and S. Karagöz, J. Energy Inst., 2021, 99, 299–306 CrossRef.
- A. Chuntanapum and Y. Matsumura, Ind. Eng. Chem. Res., 2009, 48, 9837–9846 CrossRef CAS.
- C. Falco, N. Baccile and M.-M. Titirici, Green Chem., 2011, 13, 3273–3281 RSC.
- S. Bertella and J. S. Luterbacher, Trends Chem., 2020, 2, 440–453 CrossRef CAS.
- Z. Fang, B. Li, Y. Liu, J. Zhu, G. Li, G. Hou, J. Zhou and X. Qiu, Matter, 2020, 2, 1000–1014 CrossRef.
- N. Gigli-Bisceglia, T. Engelsdorf and T. Hamann, Cell. Mol. Life Sci., 2020, 77, 2049–2077 CrossRef CAS PubMed.
- J. Rao, Z. Lv, G. Chen and F. Peng, Prog. Polym. Sci., 2023, 101675 CrossRef CAS.
- A. M. Borrero-López, E. Masson, A. Celzard and V. Fierro, Ind. Crops Prod., 2018, 124, 919–930 CrossRef.
- C. Wang, S. Huang, Y. Zhu and S. Zhang, J. Anal. Appl. Pyrolysis, 2022, 161, 105408 CrossRef CAS.
- M. He, Y. Sun and B. Han, Angew. Chem., 2022, 134, e202112835 CrossRef.
- J. Qi and L. Ü. Xiuyang, Chin. J. Chem. Eng., 2007, 15, 666–669 CrossRef.
- N. Paksung and Y. Matsumura, Ind. Eng. Chem. Res., 2015, 54, 7604–7613 CrossRef CAS.
- J. Poerschmann, B. Weiner, R. Koehler and F.-D. Kopinke, ACS Sustain. Chem. Eng., 2017, 5, 6420–6428 CrossRef CAS.
- S. Kang, X. Li, J. Fan and J. Chang, Ind. Eng. Chem. Res., 2012, 51, 9023–9031 CrossRef CAS.
- C. Wang, S. Xia, C. Cui, S. Kang, A. Zheng, Z. Yu and Z. Zhao, Fuel, 2022, 329, 125215 CrossRef CAS.
- J. M. L. Penninger, R. J. A. Kersten and H. C. L. Baur, J. Supercrit. Fluids, 1999, 16, 119–132 CrossRef CAS.
- M. Sasaki and M. Goto, Fuel, 2009, 88, 1656–1664 CrossRef.
- Wahyudiono, T. Kanetake, M. Sasaki and M. Goto, Chemical Engineering & Technology: Industrial Chemistry-Plant Equipment-Process Engineering-Biotechnology, 2007, vol. 30, pp. 1113–1122 Search PubMed.
- X. Besse, Y. Schuurman and N. Guilhaume, Catal. Today, 2015, 258, 270–275 CrossRef CAS.
- J. Barbier, N. Charon, N. Dupassieux, A. Loppinet-Serani, L. Mahé, J. Ponthus, M. Courtiade, A. Ducrozet, A.-A. Quoineaud and F. Cansell, Biomass Bioenergy, 2012, 46, 479–491 CrossRef CAS.
- M. Saisu, T. Sato, M. Watanabe, T. Adschiri and K. Arai, Energy Fuel., 2003, 17, 922–928 CrossRef CAS.
- B. Zhang, H.-J. Huang and S. Ramaswamy, in Biotechnology for Fuels and Chemicals: Proceedings of the Twenty-Ninth Symposium on Biotechnology for Fuels and Chemicals Held April 29–May 2, 2007, Springer, Denver, Colorado, 2008, pp. 487–499 Search PubMed.
- Z. Fang, T. Sato, R. L. Smith Jr, H. Inomata, K. Arai and J. A. Kozinski, Bioresour. Technol., 2008, 99, 3424–3430 CrossRef CAS PubMed.
- A. Yousuf, D. Pirozzi and F. Sannino, in Lignocellulosic Biomass to Liquid Biofuels, Elsevier, 2020, pp. 1–15 Search PubMed.
- T.-C. Hsu, G.-L. Guo, W.-H. Chen and W.-S. Hwang, Bioresour. Technol., 2010, 101, 4907–4913 CrossRef CAS PubMed.
- M. Anjum, A. Khalid, T. Mahmood and I. Aziz, J. Clean. Prod., 2016, 117, 56–63 CrossRef CAS.
- C. Rémond, N. Aubry, D. Crônier, S. Noël, F. Martel, B. Roge, H. Rakotoarivonina, P. Debeire and B. Chabbert, Bioresour. Technol., 2010, 101, 6712–6717 CrossRef PubMed.
- W.-H. Chen, B.-L. Pen, C.-T. Yu and W.-S. Hwang, Bioresour. Technol., 2011, 102, 2916–2924 CrossRef CAS PubMed.
- A. Kruse, A. Funke and M.-M. Titirici, Curr. Opin. Chem. Biol., 2013, 17, 515–521 CrossRef CAS PubMed.
- M. T. Reza, J. Andert, B. Wirth, D. Busch, J. Pielert, J. G. Lynam and J. Mumme, Appl. Bioenergy, 2014, 1, 11–29 Search PubMed.
- Y. Zhai, H. Chen, B. Xu, B. Xiang, Z. Chen, C. Li and G. Zeng, Bioresour. Technol., 2014, 159, 72–79 CrossRef CAS PubMed.
- C. He, C.-L. Chen, A. Giannis, Y. Yang and J.-Y. Wang, Renew. Sustain. Energy Rev., 2014, 39, 1127–1142 CrossRef CAS.
- A. A. Peterson, F. Vogel, R. P. Lachance, M. Fröling, M. J. Antal Jr and J. W. Tester, Energy Environ. Sci., 2008, 1, 32–65 RSC.
- T. Rogalinski, K. Liu, T. Albrecht and G. Brunner, J. Supercrit. Fluids, 2008, 46, 335–341 CrossRef CAS.
- W. Abdelmoez, T. Nakahasi and H. Yoshida, Ind. Eng. Chem. Res., 2007, 46, 5286–5294 CrossRef CAS.
- D. Klingler, J. Berg and H. Vogel, J. Supercrit. Fluids, 2007, 43, 112–119 CrossRef CAS.
- M. Li, Z. Yu, Y. Bin, Z. Huang, H. He, Y. Liao, A. Zheng and X. Ma, J. Anal. Appl. Pyrolysis, 2022, 167, 105666 CrossRef CAS.
- E. Danso-Boateng, G. Shama, A. D. Wheatley, S. J. Martin and R. G. Holdich, Bioresour. Technol., 2015, 177, 318–327 CrossRef CAS PubMed.
- S. Inoue, S. Sawayama, Y. Dote and T. Ogi, Biomass Bioenergy, 1997, 12, 473–475 CrossRef CAS.
- F. Merzari, M. Langone, G. Andreottola and L. Fiori, Crit. Rev. Environ. Sci. Technol., 2019, 49, 947–988 CrossRef CAS.
- C. He, K. Wang, Y. Yang, P. N. Amaniampong and J.-Y. Wang, Environ. Sci. Technol., 2015, 49, 6872–6880 CrossRef CAS PubMed.
- C. He, A. Giannis and J.-Y. Wang, Appl. Energy, 2013, 111, 257–266 CrossRef CAS.
- T. Wang, Y. Zhai, Y. Zhu, C. Li and G. Zeng, Renew. Sustain. Energy Rev., 2018, 90, 223–247 CrossRef CAS.
- S. Li, S. Harris, A. Anandhi and G. Chen, J. Clean. Prod., 2019, 215, 890–902 CrossRef CAS.
- B. Liu, Z. Cai, Y. Zhang, G. Liu, X. Luo and H. Zheng, Chemosphere, 2019, 224, 151–161 CrossRef CAS PubMed.
- A. Nazir, U. Laila, F. Bareen, E. Hameed and M. Shafiq, Sustainability, 2021, 13, 13796–13810 CrossRef CAS.
- E. Agrafioti, G. Bouras, D. Kalderis and E. Diamadopoulos, J. Anal. Appl. Pyrolysis, 2013, 101, 72–78 CrossRef CAS.
- H. Shi, K. K. Ramasamy, R. Ma and H. Wang, in Nanoporous Materials for Molecule Separation and Conversion, Elsevier, 2020, pp. 387–440 Search PubMed.
- N. Gorbach, V. Startsev, A. Mazur, E. Milanovskiy, A. Prokushkin and A. Dymov, Sustainability, 2022, 14, 16772 CrossRef CAS.
- J. A. Baldock and R. J. Smernik, Org. Geochem., 2002, 33, 1093–1109 CrossRef CAS.
- J. E. Amonette and S. Joseph, in Biochar for Environmental Management, Routledge, 2012, pp. 65–84 Search PubMed.
- X. He and X. Zhang, J. Energy Storage, 2022, 56, 106023 CrossRef.
- G. Ischia, M. Cutillo, G. Guella, N. Bazzanella, M. Cazzanelli, M. Orlandi, A. Miotello and L. Fiori, Chem. Eng. J., 2022, 449, 137827 CrossRef CAS.
- J. Liu, X. Chen, W. Chen, M. Xia, Y. Chen, H. Chen, K. Zeng and H. Yang, Proc. Combust. Inst., 2023, 39, 3157–3181 CrossRef CAS.
- Y. X. Seow, Y. H. Tan, N. M. Mubarak, J. Kansedo, M. Khalid, M. L. Ibrahim and M. Ghasemi, J. Environ. Chem. Eng., 2022, 10, 107017 CrossRef CAS.
- A. Raheem, Q. He, F. H. Mangi, C. Areeprasert, L. Ding and G. Yu, Energy Fuels, 2022, 36, 2351–2368 CrossRef CAS.
- M. Cavali, N. L. Junior, J. D. de Sena, A. L. Woiciechowski, C. R. Soccol, P. Belli Filho, R. Bayard, H. Benbelkacem and A. B. de Castilhos Junior, Sci. Total Environ., 2023, 857, 159627 CrossRef CAS PubMed.
- A. R. Aghamiri and P. Lahijani, Biomass Bioenergy, 2024, 183, 107120 CrossRef CAS.
- B. Peng, X. Li, J. Luo and X. Yu, Energy Fuel., 2019, 33, 9272–9279 CrossRef CAS.
- R. Zhang, H. Liu, E. Sariola-Leikas, K.-Q. Tran and C. He, Water Res., 2024, 255, 121524 CrossRef CAS PubMed.
- F. Qin, C. Zhang, G. Zeng, D. Huang, X. Tan and A. Duan, Renew. Sustain. Energy Rev., 2022, 157, 112056 CrossRef CAS.
- H. Nan, F. Yang, L. Zhao, O. Mašek, X. Cao and Z. Xiao, ACS Sustain. Chem. Eng., 2018, 7, 1591–1599 CrossRef.
- E. Setyawan, R. Napitupulu, S. Djiwo, P. Djoko and A. Nugroho, in Proceedings of the 2nd Universitas Kuningan International Conference on System Engineering, and Technology, UNISET 2021, 2022 Search PubMed.
- B. Sunkar and B. Bhukya, Front. Energy Res., 2022, 10, 802522 CrossRef.
- H. Suryanto, U. Yanuhar, H. W. Wijaya, J. S. Binoj, A. F. Osman, P. Puspitasari, J. Maulana, N. R. Caesar, F. Nusantara and K. Komarudin, J. Korean Wood Sci. Technol., 2024, 52, 504–523 CrossRef.
- F. Dalena, A. Senatore, A. Tursi and A. Basile, in Bioenergy Systems for the Future, Elsevier, 2017, pp. 559–599 Search PubMed.
- P. McKendry, Bioresour. Technol., 2002, 83, 47–54 CrossRef CAS PubMed.
- V. Ashokkumar, R. Venkatkarthick, S. Jayashree, S. Chuetor, S. Dharmaraj, G. Kumar, W.-H. Chen and C. Ngamcharussrivichai, Bioresour. Technol., 2022, 344, 126195 CrossRef CAS PubMed.
- H. Zhang, L. Han and H. Dong, Renew. Sustain. Energy Rev., 2021, 140, 110758 CrossRef CAS.
- A. Tshikovhi and T. E. Motaung, Sustainability, 2023, 15, 12121 CrossRef CAS.
- C. K. W. Ndiema, P. N. Manga and C. R. Ruttoh, Energy Convers. Manag., 2002, 43, 2157–2161 CrossRef CAS.
- S. Velusamy, A. Subbaiyan and R. S. Thangam, Environ. Sci. Pollut. Res., 2021, 28, 21471–21485 CrossRef CAS PubMed.
- P. Fajfrlíková, A. Brunerová and H. Roubík, Sustainability, 2020, 12, 8153 CrossRef.
- B. V. Bot, J. G. Tamba and O. T. Sosso, Biomass Convers. Biorefin., 2024, 14, 1905–1917 CrossRef.
- G. D. Sorita, S. P. Favaro, A. Ambrosi and M. Di Luccio, Trends Food Sci. Technol., 2023, 133, 99–113 CrossRef CAS.
- A. Kumar, N. Kumar, P. Baredar and A. Shukla, Renew. Sustain. Energy Rev., 2015, 45, 530–539 CrossRef.
- H. Thatoi, P. K. Dash, S. Mohapatra and M. R. Swain, Int. J. Sustain. Energy, 2016, 35, 443–468 CrossRef.
- A. Omojola, A. Ekiti and D. Kallon, Proceedings of the SAIIE32 Steps, Muldersdrift, South Africa, 2021, pp. 4–6 Search PubMed.
- T. Kuthiala, K. Thakur, D. Sharma, G. Singh, M. Khatri and S. K. Arya, Int. J. Biol. Macromol., 2022, 209, 1956–1974 CrossRef CAS PubMed.
- P. C. Munasinghe and S. K. Khanal, Bioresour. Technol., 2010, 101, 5013–5022 CrossRef CAS PubMed.
- S. M. Z. Hossain, Chem. Eng. Technol., 2019, 42, 2594–2607 CrossRef CAS.
- P. Choudhary, P. P. Assemany, F. Naaz, A. Bhattacharya, J. de Siqueira Castro, E. D. A. do Couto Couto, M. L. Calijuri, K. K. Pant and A. Malik, Sci. Total Environ., 2020, 726, 137961 CrossRef CAS PubMed.
- T. H. Pham, K. Rabaey, P. Aelterman, P. Clauwaert, L. De Schamphelaire, N. Boon and W. Verstraete, Eng. Life Sci., 2006, 6, 285–292 CrossRef CAS.
- N. J. Themelis and P. A. Ulloa, Renew. Energy, 2007, 32, 1243–1257 CrossRef CAS.
- R. M. Jingura and R. Matengaifa, Renew. Sustain. Energy Rev., 2009, 13, 1116–1120 CrossRef CAS.
- A. Roopnarain and R. Adeleke, Renew. Sustain. Energy Rev., 2017, 67, 1162–1179 CrossRef CAS.
- M. Vakili, M. Rafatullah, M. H. Ibrahim, B. Salamatinia, Z. Gholami and H. M. Zwain, Environ. Dev. Sustain., 2015, 17, 691–709 CrossRef.
- K. Tekin, S. Karagöz and S. Bektaş, Renew. Sustain. Energy Rev., 2014, 40, 673–687 CrossRef CAS.
- W. Lu, M. A. Alam, W. Luo and E. Asmatulu, Bioresour. Technol., 2019, 271, 59–65 CrossRef CAS PubMed.
- Z. Yu, H. Jameel, H. Chang and S. Park, Bioresour. Technol., 2011, 102, 9083–9089 CrossRef CAS PubMed.
- Z. Yu, K. Gwak, T. Treasure, H. Jameel, H. Chang and S. Park, ChemSusChem, 2014, 7, 1942–1950 CrossRef CAS PubMed.
- F. Guo, Z. Fang, C. C. Xu and R. L. Smith Jr, Prog. Energy Combust. Sci., 2012, 38, 672–690 CrossRef CAS.
- H. C. Ong, W.-H. Chen, A. Farooq, Y. Y. Gan, K. T. Lee and V. Ashokkumar, Renew. Sustain. Energy Rev., 2019, 113, 109266 CrossRef CAS.
- A. A. Babatunde, K. C. Egmba, A. J. Kehinde and O. A. Falode, in SPE Nigeria Annual International Conference and Exhibition, SPE, 2013, SPE-167542 Search PubMed.
- A. M. A. El Naggar, H. A. El Sayed, R. A. Elsalamony and A. Abd Elrazak, RSC Adv., 2015, 5, 77897–77905 RSC.
- D. O. Glushkov, G. S. Nyashina, R. Anand and P. A. Strizhak, Process Saf. Environ. Prot., 2021, 156, 43–56 CrossRef CAS.
- H. Kumar, S. K. Mohapatra and R. I. Singh, J. Inst. Eng. (India): C, 2018, 99, 449–474 Search PubMed.
- Q. Dang, X. Zhang, Y. Zhou and X. Jia, Fuel Process. Technol., 2021, 212, 106604 CrossRef CAS.
- F. N. Sidek, N. A. F. A. Samad and S. Saleh, IOP Conf. Ser. Mater. Sci. Eng., 2020, 863, 012028 CrossRef.
- A. A. Ahmad, N. A. Zawawi, F. H. Kasim, A. Inayat and A. Khasri, Renew. Sustain. Energy Rev., 2016, 53, 1333–1347 CrossRef CAS.
- Z. Lian, Y. Wang, X. Zhang, A. Yusuf, L. Famiyeh, D. Murindababisha, H. Jin, Y. Liu, J. He and Y. Wang, J, 2021, 4, 266–287 CAS.
- Y. Richardson, J. Blin and A. Julbe, Prog. Energy Combust. Sci., 2012, 38, 765–781 CrossRef CAS.
- T. R. Carlson, Y.-T. Cheng, J. Jae and G. W. Huber, Energy Environ. Sci., 2011, 4, 145–161 RSC.
- H. Suopajärvi, K. Umeki, E. Mousa, A. Hedayati, H. Romar, A. Kemppainen, C. Wang, A. Phounglamcheik, S. Tuomikoski and N. Norberg, Appl. Energy, 2018, 213, 384–407 CrossRef.
- M. V Rodionova, A. M. Bozieva, S. K. Zharmukhamedov, Y. K. Leong, J. C.-W. Lan, A. Veziroglu, T. N. Veziroglu, T. Tomo, J.-S. Chang and S. I. Allakhverdiev, Int. J. Hydrogen Energy, 2022, 47, 1481–1498 CrossRef.
- Y. Shen, J. Wang, X. Ge and M. Chen, Renew. Sustain. Energy Rev., 2016, 59, 1246–1268 CrossRef CAS.
- J. Cai, Y. He, X. Yu, S. W. Banks, Y. Yang, X. Zhang, Y. Yu, R. Liu and A. V. Bridgwater, Renew. Sustain. Energy Rev., 2017, 76, 309–322 CrossRef CAS.
- H. V. Ly, S.-S. Kim, H. C. Woo, J. H. Choi, D. J. Suh and J. Kim, Energy, 2015, 93, 1436–1446 CrossRef CAS.
- J. G. Brammer and A. V. Bridgwater, Renew. Sustain. Energy Rev., 1999, 3, 243–289 CrossRef.
- M.-F. Li, S.-N. Sun, F. Xu and R.-C. Sun, in Biomass Conversion: the Interface of Biotechnology, Chemistry and Materials Science, Springer, 2012, pp. 341–379 Search PubMed.
- T. Kan, V. Strezov and T. J. Evans, Renew. Sustain. Energy Rev., 2016, 57, 1126–1140 CrossRef CAS.
- S. Mondal, A. K. Mondal, V. Chintala, S. M. Tauseef, S. Kumar and J. K. Pandey, Biofuels, 2021, 12, 125–134 CrossRef CAS.
- Y. Lee, C. Ryu, Y.-K. Park, J.-H. Jung and S. Hyun, Bioresour. Technol., 2013, 130, 345–350 CrossRef CAS PubMed.
- M. Verma, S. Godbout, S. K. Brar, O. Solomatnikova, S. P. Lemay and J.-P. Larouche, Int. J. Chem. Eng., 2012, 2012, 542426 Search PubMed.
- H. D. Beyene, A. A. Werkneh and T. G. Ambaye, Renew. Energy Focus, 2018, 24, 1–11 CrossRef.
- S. Sadaka and A. A. Boateng, FSA1052, available online: http://www. uaex. edu/Other_Areas/publications/PDF/FSA-1052. pdf, accessed on 5 August 2010 Search PubMed.
- T. Rasheed, M. T. Anwar, N. Ahmad, F. Sher, S. U.-D. Khan, A. Ahmad, R. Khan and I. Wazeer, J. Environ. Manage., 2021, 287, 112257 CrossRef CAS PubMed.
- M. T. Safian, U. S. Haron and M. N. M. Ibrahim, Bioresources, 2020, 15, 9756 Search PubMed.
- H. Rezaei, F. Soltani-Mohammadi, H. Dogari, H. Ghafuri and R. Peymanfar, Nanoscale, 2024, 16, 18962–18975 RSC.
- R. Madaka, B. Pandey, D. K. Sahoo, M. Peddigari, J. R. Dasari, B. D. Boruah and J. K. Rath, in Waste-Derived Carbon Nanostructures: Synthesis and Applications, Springer, 2025, pp. 307–340 Search PubMed.
- B. Takam, J.-B. Tarkwa, E. Acayanka, S. Nzali, D. M. Chesseu, G. Y. Kamgang and S. Laminsi, Environ. Sci. Pollut. Res., 2020, 27, 20500–20515 CrossRef CAS PubMed.
- E. N. de Freitas, J. C. S. Salgado, R. C. Alnoch, A. G. Contato, E. Habermann, M. Michelin, C. A. Martínez, M. de and L. T. M. Polizeli, Biology, 2021, 10, 1277 CrossRef PubMed.
- S. Tan, G. Zhou, Q. Yang, S. Ge, J. Liu, Y. W. Cheng, P. N. Y. Yek, W. A. W. Mahari, S. H. Kong and J.-S. Chang, Sci. Total Environ., 2023, 864, 160990 CrossRef CAS PubMed.
- A. Elhambakhsh, N. V. D. Long, P. Lamichhane and V. Hessel, Renew. Energy, 2023, 218, 119307 CrossRef CAS.
- D. Zhao, Y. Liang, L. Gou, Y. Cui, H. Wang, C. Wang, H. Liu, S. Guo and S. Li, Chem. Eng. J., 2024, 502, 157923 CrossRef CAS.
- Y. Wu, S. Xu, Y. Jiang, S. Fan, T. Wen, S. Wang, W. Chen and L. Ding, Sep. Purif. Technol., 2024, 332, 125784 CrossRef CAS.
- A. K. Vuppaladadiyam, S. S. V. Vuppaladadiyam, A. Sahoo, S. Murugavelh, E. Anthony, T. Bhaskar, Y. Zheng, M. Zhao, H. Duan and Y. Zhao, Sci. Total Environ., 2023, 857, 159155 CrossRef CAS PubMed.
- B. Díaz, A. Sommer-Márquez, P. E. Ordoñez, E. Bastardo-González, M. Ricaurte and C. Navas-Cárdenas, Resources, 2024, 13, 8 CrossRef.
- V. Rajput, I. Saini, S. Parmar, V. Pundir, V. Kumar, V. Kumar, B. Naik and S. Rustagi, Discover Appl. Sci., 2024, 6, 408 CrossRef CAS.
- X. Li, H. Zhang, M. Liu, L. Zhi, B. A. I. Jin, Z. Bai and L. I. Wen, J. Fuel Chem. Technol., 2020, 48, 897–907 CrossRef CAS.
- E. Lester, C. Avila, C. H. Pang, O. Williams, J. Perkins, S. Gaddipatti, G. Tucker, J. M. Barraza, M. P. Trujillo-Uribe and T. Wu, Fuel, 2018, 232, 845–854 CrossRef CAS.
- Y. Zhang, L. Wan, J. Guan, Q. Xiong, S. Zhang and X. Jin, Energy Fuels, 2020, 34, 13438–13455 CrossRef CAS.
- Y. Chen, Z. Deng, Q. Ren, D. Ren, S. Su, S. Hu, Y. Wang and J. Xiang, Fuel, 2022, 312, 122994 CrossRef CAS.
- P. Ibarra-Gonzaleza and B.-G. Rong, Chin. J. Chem. Eng., 2019, 27, 1523–1535 CrossRef.
- P. Ibarra-Gonzalez, L. P. Christensen and B.-G. Rong, Chem. Eng. Commun., 2022, 209, 529–554 CrossRef CAS.
- A. M. da Costa Lopes and R. M. Łukasik, ChemSusChem, 2018, 11, 1099–1107 CrossRef CAS PubMed.
- M. I. Jahirul, M. G. Rasul, A. A. Chowdhury and N. Ashwath, Energies, 2012, 5, 4952–5001 CrossRef CAS.
- G. Ravindiran, S. Rajamanickam, G. Janardhan, G. Hayder, A. Alagumalai, O. Mahian, S. S. Lam and C. Sonne, Biochar, 2024, 6, 62 CrossRef CAS.
- J. Han and H. Kim, Renew. Sustain. Energy Rev., 2008, 12, 397–416 CrossRef CAS.
- I. F. Elbaba, C. Wu and P. T. Williams, Int. J. Hydrogen Energy, 2011, 36, 6628–6637 CrossRef CAS.
- C. S. K. Lin, L. A. Pfaltzgraff, L. Herrero-Davila, E. B. Mubofu, S. Abderrahim, J. H. Clark, A. A. Koutinas, N. Kopsahelis, K. Stamatelatou and F. Dickson, Energy Environ. Sci., 2013, 6, 426–464 RSC.
- L. H. Tamborini, M. E. Casco, M. P. Militello, J. Silvestre-Albero, C. A. Barbero and D. F. Acevedo, Fuel Process. Technol., 2016, 149, 209–217 CrossRef CAS.
- A. V Bridgwater, Environ. Prog. Sustain. Energy, 2012, 31, 261–268 CrossRef.
- P. Parthasarathy and K. S. Narayanan, Renew. Energy, 2014, 66, 570–579 CrossRef CAS.
- A. Zheng, Z. Zhao, S. Chang, Z. Huang, H. Wu, X. Wang, F. He and H. Li, J. Mol. Catal. A Chem., 2014, 383, 23–30 CrossRef.
- A. Gonzalez-Quiroga, M. R. Djokic, K. M. Van Geem and G. B. Marin, Energy Fuels, 2016, 30, 8292–8303 CrossRef CAS.
- F. Mafakheri and F. Nasiri, Energy Policy, 2014, 67, 116–126 CrossRef.
- I. Amghizar, L. A. Vandewalle, K. M. Van Geem and G. B. Marin, Engineering, 2017, 3, 171–178 CrossRef CAS.
- A. M. Niziolek, O. Onel and C. A. Floudas, AIChE J., 2016, 62, 1531–1556 CrossRef CAS.
- S. M. Sadrameli, Fuel, 2016, 173, 285–297 CrossRef CAS.
- A. G. Gayubo, B. Valle, A. T. Aguayo, M. Olazar and J. Bilbao, Energy Fuels, 2009, 23, 4129–4136 CrossRef CAS.
- F. Gong, Z. Yang, C. Hong, W. Huang, S. Ning, Z. Zhang, Y. Xu and Q. Li, Bioresour. Technol., 2011, 102, 9247–9254 CrossRef CAS PubMed.
- B. Puértolas, A. Veses, M. S. Callén, S. Mitchell, T. García and J. Pérez-Ramírez, ChemSusChem, 2015, 8, 3283–3293 CrossRef PubMed.
- I. Y. Mohammed, Y. A. Abakr, S. Yusup, P. A. Alaba, K. I. Morris, Y. M. Sani and F. K. Kazi, J. Clean. Prod., 2017, 162, 817–829 CrossRef CAS.
- L. Nie, P. M. de Souza, F. B. Noronha, W. An, T. Sooknoi and D. E. Resasco, J. Mol. Catal. A Chem., 2014, 388, 47–55 CrossRef.
- H. Shafaghat, P. S. Rezaei and W. M. A. W. Daud, J. Ind. Eng. Chem., 2016, 35, 268–276 CrossRef CAS.
- Y.-T. Cheng, J. Jae, J. Shi, W. Fan and G. W. Huber, Angew. Chem., Int. Ed., 2012, 51, 1387–1390 CrossRef CAS PubMed.
- G. Kabir and B. H. Hameed, Renew. Sustain. Energy Rev., 2017, 70, 945–967 CrossRef CAS.
- P. Knuuttila, Fuel, 2013, 104, 101–108 CrossRef CAS.
- J. Street, F. Yu, J. Wooten, E. Columbus, M. G. White and J. Warnock, Fuel, 2012, 96, 239–249 CrossRef CAS.
- K. Jacobson, K. C. Maheria and A. K. Dalai, Renew. Sustain. Energy Rev., 2013, 23, 91–106 CrossRef CAS.
- S. Yin, A. K. Mehrotra and Z. Tan, Biomass Bioenergy, 2012, 47, 228–239 CrossRef CAS.
- S. Yin and Z. Tan, Appl. Energy, 2012, 92, 234–239 CrossRef CAS.
- B. L. Joyce and C. N. Stewart Jr, Biotechnol. Adv., 2012, 30, 1011–1022 CrossRef CAS PubMed.
- H. B. Sharma, K. R. Vanapalli, D. Bhatia, S. Singh, G. Arora, S. Panigrahi, B. K. Dubey, P. C. Ramamurthy and B. Mohanty, Clean Technol. Environ. Policy, 2024, 1–35 Search PubMed.
- H. B. Sharma, K. R. Vanapalli, D. Bhatia, S. Singh, G. Arora, S. Panigrahi, B. K. Dubey, P. C. Ramamurthy and B. Mohanty, Clean Technol. Environ. Policy, 2024, 1–35 Search PubMed.
- P. M. Mortensen, J.-D. Grunwaldt, P. A. Jensen, K. G. Knudsen and A. D. Jensen, Appl. Catal., A, 2011, 407, 1–19 CrossRef CAS.
- B. E. Eboibi, O. Eboibi, O. L. Okan, E. C. Udochukwu, P. E. Uku and S. E. Agarry, Environ. Prog. Sustain. Energy, 2024, 43, e14440 CrossRef CAS.
- C. Tian, B. Li, Z. Liu, Y. Zhang and H. Lu, Renew. Sustain. Energy Rev., 2014, 38, 933–950 CrossRef CAS.
- L. Zhang, R. Liu, R. Yin and Y. Mei, Renew. Sustain. Energy Rev., 2013, 24, 66–72 CrossRef CAS.
- J. Tao, Y. Ge, R. Liang, Y. Sun, Z. Cheng, B. Yan and G. Chen, Appl. Energy Combust. Sci., 2022, 10, 100070 Search PubMed.
- M. C. Samolada and I. A. Vasalos, Fuel, 1991, 70, 883–889 CrossRef CAS.
- N. Neveux, A. K. L. Yuen, C. Jazrawi, M. Magnusson, B. S. Haynes, A. F. Masters, A. Montoya, N. A. Paul, T. Maschmeyer and R. De Nys, Bioresour. Technol., 2014, 155, 334–341 CrossRef CAS PubMed.
- J. Kosinkova, A. Doshi, J. Maire, Z. Ristovski, R. Brown and T. J. Rainey, Renew. Sustain. Energy Rev., 2015, 49, 1271–1285 CrossRef.
- D. L. Barreiro, W. Prins, F. Ronsse and W. Brilman, Biomass Bioenergy, 2013, 53, 113–127 CrossRef.
- R. Tiwari, B. S. Rana, R. Kumar, D. Verma, R. Kumar, R. K. Joshi, M. O. Garg and A. K. Sinha, Catal. Commun., 2011, 12, 559–562 CrossRef CAS.
- V. D. Tsavatopoulou, A. F. Aravantinou and I. D. Manariotis, Biomass Convers. Biorefin., 2021, 11, 1301–1309 CrossRef CAS.
- R. Karpagam, K. Jawaharraj and R. Gnanam, Sci. Total Environ., 2021, 766, 144236 CrossRef CAS PubMed.
- F. R. Pazheri, M. F. Othman and N. H. Malik, Renew. Sustain. Energy Rev., 2014, 31, 835–845 CrossRef.
- M. Shokri, G. Isapour, S. Shamsvand and B. Kavousi, J. Mater. Environ. Sci., 2016, 7, 2843–2851 CAS.
- WBA, WBA Global Bioenergy Statistics 2018, Summary Report, World Bioenergy Association, 2018, https://www.worldenergy.org Search PubMed.
- World Bioenergy Association, Global Bioenergy Statistics Report 2023, World Bioenergy Association, Stockholm, 10th edn, 2023 Search PubMed.
- J. A. Ferguson, J. Bus. Entrepreneurship & L., 2023, 17, 91 Search PubMed.
- T. Mai-Moulin, R. Hoefnagels, P. Grundmann and M. Junginger, Renew. Sustain. Energy Rev., 2021, 138, 110645 CrossRef.
- H. Shen, X. Wen and E. Trutnevyte, Energy Econ. Clim. Change, 2023, 4, 100111 CAS.
- L. A. H. Nogueira, R. S. Capaz, S. P. Souza and J. E. A. Seabra, Biofuels Bioprod. Biorefining, 2016, 10, 728–737 CrossRef CAS.
- M. Ivanovski, D. Goricanec, J. Krope and D. Urbancl, Energy, 2022, 240, 122483 CrossRef CAS.
- S. Sharma, M. Sharma, D. Mudgal and H. Bhowmick, Corros. Rev., 2021, 39, 387–408 CrossRef CAS.
- G. Hochman, M. Traux and D. Zilberman, Handbook of Bioenergy Economics and Policy: Volume II: Modeling Land Use and Greenhouse Gas Implications, 2017, pp. 15–38 Search PubMed.
- C. S. Galik, M. E. Benedum, M. Kauffman and D. R. Becker, Biomass Bioenergy, 2021, 148, 106035 CrossRef.
- W. Antweiler, Scaling up: the Promise and Perils of Canada's Biofuels Strategy, CD Howe Institute e-Brief, 2024, vol. 364 Search PubMed.
- Irena, Renewables Readiness Assessment: Azerbaijan, International Renewable Energy Agency, Abu Dhabi, 2019 Search PubMed.
- X. Chen, M. Khanna and S. Yeh, Environ. Res. Lett., 2012, 7, 045907 CrossRef.
- A. Tursi, Biofuel Res. J., 2019, 6, 962–979 CrossRef CAS.
- K. Nadarajah, T. Asharp and Y. Jeganathan, Water Sci. Technol., 2024, 89, 1211–1239 CrossRef CAS PubMed.
- X. Tan, Y. Liu, G. Zeng, X. Wang, X. Hu, Y. Gu and Z. Yang, Chemosphere, 2015, 125, 70–85 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |