Open Access Article
Open Access ArticleTextile dye removal using diatomite nanocomposites: a metagenomic study in photosynthetic microalgae-assisted microbial fuel cells†
Anshuman Raiab,
Vandana Sirotiyac,
Ankesh Ahirwarc,
Gurpreet Singhc,
Rajeev Kawatrab,
Anil K. Sharma d,
Harishe and
Vandana Vinayak
d,
Harishe and
Vandana Vinayak *c
*c
aMMU, Deemed University, School of Engineering, Department of Biotechnology, Ambala, Haryana 133203, India
bForensic Science Laboratory, Haryana, Madhuban, 132037, India
cDiatom Nanoengineering and Metabolism Laboratory, Dr Harisingh Gour Central University, School of Applied Science, Sagar, M.P, India 470003. E-mail: kapilvinayak@gmail.com
dDepartment of Biotechnology, Amity School of Biological Sciences, Amity University Punjab, Mohali 140306, Punjab, India
eDepartment of Botany, Mohanlal Sukhadia University, Udaipur, Rajasthan 313001, India
First published on 17th March 2025
Abstract
In this study, Coomassie brilliant blue (CBB), brilliant green (BG), and rhodamine (Rh) dyes were used to simulate dye-rich wastewater. Adsorption and degradation of these dyes (2 μM, 10 μM, and 30 μM) on diatomite (DE) were evaluated under light (L) and dark (D) conditions. The adsorption of dye–DE composites followed pseudo-second-order kinetics at all concentrations and conditions had R2 > 0.99, thus showing a good fit. The calculated equilibrium adsorption amount qe,(cal) was coherent with the value of experimental qe,(exp). The poorest adsorption and photocatalysis occurred at 30 μM, prompting the functionalization of dyes with TiO2 and Fe3O4 nanoparticles (NP(s)). The highest dye degradation efficiencies (DGeff) for 30 μM dyes were 86.79% (CBB–DE–Fe3O4, 72 h), 96.10% (BG–DE–TiO2, 52 h), and 81.74% (Rh–DE–TiO2, 48 h), with Rh–DE–TiO2 showing the fastest degradation. Functionalized DE–dye (30 μM) nanocomposites were further tested in a photosynthetic microalgae-assisted microbial fuel cell with dye-simulated wastewater at the anode (PMA-MFC-1 with CBB–DE–Fe3O4, PMA-MFC-2 with BG–DE–TiO2 and PMA-MFC-3 with Rh–DE–TiO2) and Asterarcys sp. GA4 microalgae at the cathode. In dark anode chambers, PMA-MFC-3 achieved the highest DGeff value of Rh dye as 88.23% and a polarization density of 30.06 mW m−2, outperforming PMA-MFC-2 with BG dye and PMA-MFC-1 with CBB dye. The molecular identifier analysis of microbes in wastewater at the anode showed the dominance of Sphingobacteria and Proteobacteria in PMA-MFC-3 (Rh–DE–TiO2) and COD removal of 61.36%, highlighting its potential for efficient dye degradation and bioelectricity generation. Furthermore, PMA-MFC-3 simultaneously demonstrated a superior microalgal lipid yield of 3.42 μg g−1 and an algal growth of 8.19 μg g−1 at the cathode.
1 Introduction
Dyes from textile industries contribute to the largest amount of pollution to water resources, and they are one of the major health concerns.1 India alone generates 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 354 million liters per day (MLD) of textile wastewater with a sewage treatment capacity of only 11
354 million liters per day (MLD) of textile wastewater with a sewage treatment capacity of only 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 786 MLD.2 Among the significant amount of colored wastewater generated, the major portion is from textile and tannery industries, which are rich in dyes and heavy metals. Besides causing water pollution, the colored substances of the dyes are resistant to biodegradation via conventional wastewater treatment strategies.3 Based on the color index, there are 10
786 MLD.2 Among the significant amount of colored wastewater generated, the major portion is from textile and tannery industries, which are rich in dyes and heavy metals. Besides causing water pollution, the colored substances of the dyes are resistant to biodegradation via conventional wastewater treatment strategies.3 Based on the color index, there are 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different dyes, which result in the production of ∼700
000 different dyes, which result in the production of ∼700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons of dye waste across the world, of which 1–15% are released into wastewaters during the dyeing process.4 There are many structural varieties of dyes like acidic, basic, azo, metal complex and synthetic dyes.5 These dyes are carcinogenic and cause respiratory, skin, and other health hazards.6 While numerous physical and chemical methods exist for removing these dyes, they are often not cost-effective and lead to secondary chemical pollution, harming aquatic life and indirectly impacting the human health.7,8 Numerous adsorbents and nanocomposites have gained attention for their photocatalytic properties, enabling the removal of dyes and colors through adsorption and degradation. The commonly used adsorbents are activated carbon, zeolites, and noble metals.9
000 tons of dye waste across the world, of which 1–15% are released into wastewaters during the dyeing process.4 There are many structural varieties of dyes like acidic, basic, azo, metal complex and synthetic dyes.5 These dyes are carcinogenic and cause respiratory, skin, and other health hazards.6 While numerous physical and chemical methods exist for removing these dyes, they are often not cost-effective and lead to secondary chemical pollution, harming aquatic life and indirectly impacting the human health.7,8 Numerous adsorbents and nanocomposites have gained attention for their photocatalytic properties, enabling the removal of dyes and colors through adsorption and degradation. The commonly used adsorbents are activated carbon, zeolites, and noble metals.9
Alternatively, treating such effluents with freely available diatomite is very economical.10 DE, which is also known as diatomaceous earth, is formed from dead frustules of diatoms.11 Diatoms are unicellular microalgae rich in silica naturally present in all open water bodies.12 They are natural reservoirs of organic silica, which varies from species to species.13 Their walls are made up of a specific pattern of hole arrays, which are known as areola in their frustules.14 The size of each pore in diatoms ranges from 1 to 100 nm.15,16 The pores are further arranged in a nanopore array architecture, which is unique for each diatom species giving each diatom a different genus and species.13 The nanoporous architecture increases the surface area, thus helping in removing heavy metals via different mechanisms such as biotransformation, biosorption, bioaccumulation and biomineralization.17,18 They can adsorb heavy metals, pollutants, dyes and drugs on their surface, thus enhancing their removal to clean wastewater from industries.10 A study demonstrated that amine-functionalized mesoporous diatom silica xerogels are efficient for the removal of Eriochrome black T (EBT) dye.19 It was found that 100 mg of DE alone removed 50 mg L−1 dye by 58% and DE-modified xerogel by 99% in ∼1 h.19 Various metal organic frameworks can be easily functionalized on diatom's surface to form strong metal bound interactions on the surface of diatom frustules.20 It can be a choice over expensive materials such as activated carbon for the adsorption, removal and degradation of textile dyed wastewater.20 The present study involves the use of DE nanocomposites for the adsorption, removal, and degradation of three textile industrial dyes under light and dark conditions.
Another way of efficiently removing dyed wastewater is the use of microbial fuel cells (MFCs).21 Microbial fuel cell techniques are efficient ways of removing dyes and other pollutants from various types of wastewater via a biochemical oxidation process.22 Therefore, these dye–DE nanocomposites are utilized in a PMA-MFC to treat and degrade dyed wastewater while concurrently generating bioelectricity.23,24 The use of live microalgae at the cathode in a PMA-MFC system offers the advantage of prolonged operation while ensuring a continuous supply of oxygen to the cathode.25,26 In addition to safely sanitizing wastewater, microalgae can be used to produce value-added products such as its biomass, lipids, carotenoids, proteins, carbohydrates, biofuel, and bioplastics.27,28 In the present study, oleaginous microalgae, cultured from the local water site, has been used at the cathode in PMA-MFCs projected to remediate dyed wastewater functionalised with DE nanocomposites at the anode. The dye–DE nanocomposites coated on the anode surface in the PMA-MFC not only facilitated nanocatalytic degradation but also underwent organic oxidation by anaerobic microbes in the anode chamber of PMA-MFC. The novelty of this study is that the metagenomic study of microbial community, their metabolism, role in the degradation of three different dyes using different sets of diatom nanocomposites in a PMA-MFC yielding algal biomass, value-added components, bioelectricity and remediated textile-dyed wastewater.
2 Material and methods
2.1 Adsorption of textile dyes on diatomite and nanoparticles
The DE and different industrial synthetic dyes, namely Coomassie brilliant blue (CBB), brilliant green (BG), and rhodamine (Rh), were purchased from HiMedia. Dye solutions with concentrations of 2 μM, 10 μM, and 30 μM for each of CBB, BG, and Rh were prepared, with their pH maintained at 7 using a phosphate buffer solution. These dyes were prepared at different concentrations in 5 mL of distilled water and used as controls in test tubes. The dye solutions were then mixed with 100 mg of diatomite (DE), which were washed with deionized water and dried at 100 °C, to create various test samples. The control and test samples were incubated at room temperature in light (0.45 lux) (L) and darkness (D) to observe their rate of adsorption and degradation using a UV-VIS spectrophotometer (LabIndia 3000) at different time intervals, 30 min till 1 h, and then at intervals of 24 h till 72 h.The dye was first adsorbed onto DE and then exposed to light (a physical catalyst) or nanoparticles (a chemical catalyst) to measure how efficiently the dyes were degraded. The dye–DE combinations with the lowest dye DGeff were further treated with NP(s) to see if they improved, slowed down, or had no effect on the dye removal or degradation rate. The NP(s) used in this study were titanium dioxide (TiO2) and magnetite (Fe3O4) purchased from Sigma. These NP(s) were doped on DE via simple incubation in UV light and tested at a very low concentration of 0.2 μg mL−1 to be economic and environment-friendly.29 Both the TiO2–DE and Fe3O4–DE nanocomposites were checked for their nano photocatalytic degradation of the above-mentioned three dyes. TiO2 is photocatalytic30 and environment-friendly with a strong oxidative potential, and hence, it is popularly used for the degradation of pollutants in wastewater.31 The magnetite (Fe3O4) NP(s) have electromagnetic properties, chemical stability and biocompatibility to remove heavy metals and organic pollutants from wastewater.32 These NP(s) can generate reactive oxygen species (ROS) such as hydroxyl radicals (˙OH) and superoxide anions  which degrade dye molecules into smaller, non-toxic compounds.33
which degrade dye molecules into smaller, non-toxic compounds.33
The amount of dye adsorbed (qe) onto the adsorbent at time (t), which is the amount of dye adsorbed by DE (g) measured in mg g−1, is presented as eqn (1):
 | (1) |
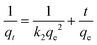 | (2) |
Using the calculated qt, plot the data 1/qt versus t to determine the value of qe and k2.
The DGeff of dyes at various compositions and concentrations was calculated using eqn (3):
 | (3) |
2.2 Photosynthetic microalgae-assisted microbial fuel cells for the remediation of dyes and electricity generation
The three dyes (CBB, BG, and Rh) were tested at concentrations of 2 μM, 10 μM, and 30 μM in combination with DE and NP in test tubes, under both light and dark conditions. The nanocomposite concentrations exhibiting poor DGeff in the test tubes were subsequently evaluated in a PMA-MFC setup for further degradation. Accordingly, three distinct PMA-MFC configurations were designed, each corresponding to the nanocomposites with one of the three dyes. Three microbial fuel cells were thus constructed with a ceramic plate acting as proton exchange membrane as per the protocol suggested by Das et al., 2020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34 with modification of living microalgae at the cathode and thus known as PMA-MFCs. These three PMA-MFCs contained CBB, BG, and Rh dyes individually, along with DE and NP(s) (TiO2 and Fe3O4) at the anode and were thus labeled as PMA-MFC-1, PMA-MFC-2, and PMA-MFC-3, respectively. The dyes in all the three PMA-MFCs were mixed with synthetic wastewater (SWW) (recipe ESI Table 1,†35) and activated sludge at anode chamber. The wastewater sludge was collected from Green hostel at Dr Harisingh Gour Vishwavidyalaya (A Central university), Sagar, M.P, India. The cathode and anodes were made of graphite felt purchased from Marine lines, Mumbai. The cell potential of PMA-MFC reactors 1, 2 and 3 were tested for resistance ranging from 20
34 with modification of living microalgae at the cathode and thus known as PMA-MFCs. These three PMA-MFCs contained CBB, BG, and Rh dyes individually, along with DE and NP(s) (TiO2 and Fe3O4) at the anode and were thus labeled as PMA-MFC-1, PMA-MFC-2, and PMA-MFC-3, respectively. The dyes in all the three PMA-MFCs were mixed with synthetic wastewater (SWW) (recipe ESI Table 1,†35) and activated sludge at anode chamber. The wastewater sludge was collected from Green hostel at Dr Harisingh Gour Vishwavidyalaya (A Central university), Sagar, M.P, India. The cathode and anodes were made of graphite felt purchased from Marine lines, Mumbai. The cell potential of PMA-MFC reactors 1, 2 and 3 were tested for resistance ranging from 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Ω to 5 Ω using a resistance box (Model Bexco, total resistance 21
000 Ω to 5 Ω using a resistance box (Model Bexco, total resistance 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 Ω, manganin coil, 9/16′′, thick brass blocks, resistance in ohms: 1–10000 Ω). The anode chamber contained SWW as the anolyte of volume ∼400 mL simulated with the dye of concentration, which showed least DGeff in a test tube (L/D). The graphite felt at the anode was 11 cm in length and 3 cm in breadth. It was further coated with DE–NP (Fe3O4/TiO2) depending upon which DE and NP combination showed the best DGeff for the respective dyes as in the case of WO3 as a novel catalyst in an MFC.36 The doping of Fe3O4 and TiO2 with DE on the graphite felt at the anode of PMA-MFCs was checked using a scanning electron microscope (SEM; Model NOVA NANO SEM 450 FEI CO. OF USA (S.E.A.) PTE LTD, SINGAPORE). Fe3O4 and TiO2 NP elemental analysis was performed by SEM-EDX. The cathode was further filled with green microalgae collected from a water site in Sagar, Madhya Pradesh, India (24°45′46′′N 78°14′02′′E) and identified via Molecular Identifier (MID) Analysis.37 Then about 69 × 106 mL/400 mL microalgal cells were inoculated in classical BG-11 media,38 which acted as the catholyte at the cathode and was exposed to light (0.72 Klux) for a photoperiod of 12 h light
110 Ω, manganin coil, 9/16′′, thick brass blocks, resistance in ohms: 1–10000 Ω). The anode chamber contained SWW as the anolyte of volume ∼400 mL simulated with the dye of concentration, which showed least DGeff in a test tube (L/D). The graphite felt at the anode was 11 cm in length and 3 cm in breadth. It was further coated with DE–NP (Fe3O4/TiO2) depending upon which DE and NP combination showed the best DGeff for the respective dyes as in the case of WO3 as a novel catalyst in an MFC.36 The doping of Fe3O4 and TiO2 with DE on the graphite felt at the anode of PMA-MFCs was checked using a scanning electron microscope (SEM; Model NOVA NANO SEM 450 FEI CO. OF USA (S.E.A.) PTE LTD, SINGAPORE). Fe3O4 and TiO2 NP elemental analysis was performed by SEM-EDX. The cathode was further filled with green microalgae collected from a water site in Sagar, Madhya Pradesh, India (24°45′46′′N 78°14′02′′E) and identified via Molecular Identifier (MID) Analysis.37 Then about 69 × 106 mL/400 mL microalgal cells were inoculated in classical BG-11 media,38 which acted as the catholyte at the cathode and was exposed to light (0.72 Klux) for a photoperiod of 12 h light![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 h darkness in PMA-MFCs 1 to 3. Here we compared the three PMA-MFCs with different dye nanocomposites at the anode, hence the control PMA-MFC without dye nanocomposite was not used.
8 h darkness in PMA-MFCs 1 to 3. Here we compared the three PMA-MFCs with different dye nanocomposites at the anode, hence the control PMA-MFC without dye nanocomposite was not used.
| P = V × I | (4) |
The coulombic efficiency (CE) was calculated using eqn (5):
 | (5) |
 | (6) |
2.3 Identification of microorganisms at the anode and cathode
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 240–50).44 All samples were quantified using a Qubit DNA HS Assay (Invitrogen, Cat#Q32854). The DNA sample was diluted to 5 ng (For “Too Low” sample, 5 μL DNA was taken for PCR) and was amplified for the 16s region (1500 bp) followed by V3V4 (460 bp) using 16s and V3V4 primers, respectively, in a nested PCR approach. Positive and no template controls were included in the PCR. These PCR products were checked on agarose gel. All amplified PCR products were then cleaned up (1X) using AMPure XP beads (Beckman Coulter, Cat#A63882) to get rid of non-specific fragments before proceeding with library preparations. The PCR products were further taken for DNA library preparation using a NEB Next Ultra DNA Library Prep Kit for Illumina (NEB, Cat# E7370L). The amplicons were end repaired and mono-adenylated at 3′ end in a single enzymatic reaction. NEB hairpin-loop adapters are ligated to the DNA fragments in a T4-DNA ligase-based reaction. Following ligation, the loop containing Uracil is linearized using a USER Enzyme (a combination of UDG and Endo VIII), to make it available as a substrate for PCR-based indexing in the next step. During PCR, barcodes were incorporated using unique primers for each of the samples, thereby enabling multiplexing. All prepared libraries were checked for fragment distribution using a 5300 fragment analyser system (Agilent). Prepared libraries were quantified using a Qubit HS Assay (Invitrogen, Cat# Q32854). The obtained libraries were pooled and diluted to a final optimal loading concentration using a MiSeq instrument (2 × 250 cycles, Cat# MS1022003). The generated sequence data were assessed for quality control and processed to generate FASTQ files. The primer details used were 16SF 5′ AGAGTTTGATCCTGGCTCAG'3, 16SR 5'GGTTACCTTGTTACGACTT'3, V3V4F 5'CCTACGGGNGGCWGCAG'3 and V3V4R 5'GACTACHVGGGTATCTAATCC'3.45
240–50).44 All samples were quantified using a Qubit DNA HS Assay (Invitrogen, Cat#Q32854). The DNA sample was diluted to 5 ng (For “Too Low” sample, 5 μL DNA was taken for PCR) and was amplified for the 16s region (1500 bp) followed by V3V4 (460 bp) using 16s and V3V4 primers, respectively, in a nested PCR approach. Positive and no template controls were included in the PCR. These PCR products were checked on agarose gel. All amplified PCR products were then cleaned up (1X) using AMPure XP beads (Beckman Coulter, Cat#A63882) to get rid of non-specific fragments before proceeding with library preparations. The PCR products were further taken for DNA library preparation using a NEB Next Ultra DNA Library Prep Kit for Illumina (NEB, Cat# E7370L). The amplicons were end repaired and mono-adenylated at 3′ end in a single enzymatic reaction. NEB hairpin-loop adapters are ligated to the DNA fragments in a T4-DNA ligase-based reaction. Following ligation, the loop containing Uracil is linearized using a USER Enzyme (a combination of UDG and Endo VIII), to make it available as a substrate for PCR-based indexing in the next step. During PCR, barcodes were incorporated using unique primers for each of the samples, thereby enabling multiplexing. All prepared libraries were checked for fragment distribution using a 5300 fragment analyser system (Agilent). Prepared libraries were quantified using a Qubit HS Assay (Invitrogen, Cat# Q32854). The obtained libraries were pooled and diluted to a final optimal loading concentration using a MiSeq instrument (2 × 250 cycles, Cat# MS1022003). The generated sequence data were assessed for quality control and processed to generate FASTQ files. The primer details used were 16SF 5′ AGAGTTTGATCCTGGCTCAG'3, 16SR 5'GGTTACCTTGTTACGACTT'3, V3V4F 5'CCTACGGGNGGCWGCAG'3 and V3V4R 5'GACTACHVGGGTATCTAATCC'3.45| Total weight of biomass = weight of tube with sample − weight of empty tube | (7) |
The lipid yield was measured following the sulfo-phospho-vanillin method48 using linseed oil as the standard purchased from Sigma.
3 Results and discussion
3.1 Dyes adsorption
The dye adsorption kinetics was estimated for CBB, BG and Rh dyes on DE. The pseudo-second-order kinetics of dye adsorption, showing t/qt versus time, is represented in Fig. 1. The two curves in one plot of Fig. 1 correspond to the adsorption of dye under light (L) and dark (D) conditions at different concentrations (2 μM, 10 μM and 30 μM). The qe,(cal) and qe,(exp) show a perfect correlation, as shown in Fig. 1. The linear regression (R2) in this pseudo-second-order kinetics is more than 0.99 in all plots (Fig. 1A to I), showing a good straight-line slope for the adsorption of all dyes in all concentrations onto DE. The lower concentration dyes at 2 μM showed almost same qe,(cal) and qe,(exp) for all dyes, which were ∼0.07 mg g−1 in L and 0.09 mg g−1 in D for CBB, ∼0.04 in L and 0.03 mg g−1 in D for BG, and ∼0.05 mg g−1 in L and 0.04 mg g−1 in D for Rh. However, the qe,(cal) and qe,(exp) under light conditions were slightly higher for all the three dyes compared to that under dark conditions at higher concentrations, i.e. at 10 μM (∼0.07 mg g−1 in L and 0.04 mg g−1 in D for CBB, ∼0.07 mg g−1 in L and 0.05 mg g−1 in D for BG, and ∼0.08 mg g−1 in L and 0.04 mg g−1 in D for Rh). The dyes at 30 μM of dye concentration followed the same pattern under light and dark conditions, and the qe,(cal) and qe,(exp) values were almost similar with no significant change. The kinetic constant k2, qe,(cal) and qe,(exp) and R2 for all dyes are shown in ESI Fig. S1.† Hence, in comparison, the pseudo-second-order kinetics represents and fits the adsorption kinetics data of CBB, BG and Rh dyes on DE. A study on the adsorption of malachite green on tamarind seeds also followed the linear regression plots, where qe,(cal) and qe,(exp) values were coherent and the R2 values were above 0.98.49 Similarly, the adsorption of methylene blue dye on a zinc-derived (ZD) porous nanosystem showed the pseudo-second-order rate model (rate constant = 0.00011 g mg−1 min−1; adsorption capacity (qe,(cal)) = 386.1 mg g−1; R2 = 0.990)503.2 Dyes degradation
3.2.1.1 Coomassie brilliant blue nanocomposites. Since CBB at a concentration of 30 μM showed the least DGeff, even under light conditions, the 30 μM CBB–DE mixture was thus functionalised with TiO2 and Fe3O4 NP(s). The NP(s) were added at a minimal concentration of 0.2 μg mL−1 to assess whether there was any enhancement in degradation through nano catalytic activity. The CBB–DE–Fe3O4 nanocomposites facilitated nano photocatalytic degradation of dyes with the remaining amount of dyes in supernatant reduced to 5.35 μg mL−1 from its initial concentration of 39.37 μg mL−1 in 24 h (Fig. 2G). On the offset with CBB–DE–TiO2 nanocomposites, the dye showed a remaining concentration of 15.39 μg mL−1 in the supernatant at 24 h from its initial concentration of 31.81 μg mL−1 (Fig. 2G). This is better than CBB (30 μM) degradation without Fe3O4/TiO2 NP(s), where the initial concentration dropped from 50.38 μg mL−1 to 28.35 μg mL−1 in 24 h and to 15.3 μg mL−1 in 72 h upon exposure to light (Fig. 2D). The TiO2 NP-doped CBB–DE in light showed a DGeff value of 60.27% and 86.79% with Fe3O4 in 72 h and 69.61% without NP(s) in 72 h, showing that TiO2 did not improve CBB degradation and was poor in performance compared to Fe3O4 (Fig. 2H). Other studies have shown the use of chitin nanoparticles for the effective removal of a fixed concentration of CBB, methylene blue and bromophenol blue dyes.56 They also showed that the increasing dye concentration has decreased the rate of adsorption and increasing chitin NP(s) content increased the adsorption of dyes.56 Similarly, the thiourea-modified Fe3O4/graphene oxide nanocomposite showed 86% efficient removal of CBB from aqueous solutions.57
3.2.2.1 Evaluating brilliant green nanocomposites. To enhance the adsorption and degradation of BG dye on DE, nanoparticles, TiO2 and Fe3O4 NP(s) (0.2 μg mL−1) were added individually to the BG dye at 30 μM concentration because it showed the poorest DGeff (40.96% in light, since light outperformed degradation than dark conditions). The BG dye–DE–TiO2 nanocomposite showed 88.18 μg mL−1 dye at 0 min and 3.43 μg mL−1 dye left in supernatant in 52 h (Fig. 3G). Furthermore, the BG dye–DE–Fe3O4 nanocomposite dye content decreased from 90.65 μg mL−1 to 14.08 μg mL−1 in 52 h (Fig. 3G).
Among the two NP(s) used, TiO2 showed enhanced degradation of the BG (30 μM)–DE complex with a DGeff value of 96.10% and Fe3O4 showed degradation of BG (30 μM)–DE with a DGeff value of 84.45% in 48 h (Fig. 3H). In comparison to the DGeff value of 40.96% in BG dye–DE without NP(s) exposed to light in 48 h (Fig. 3E), NP(s) definitely increased DGeff. In a recent study, copper sulfide nanoparticles helped in 99% photocatalytic degradation of BG dye using 5 g L−1 of the nanoparticles in 20 min.68 The quantity of NP(s) used in this study is, however, far less, about 200 μg L−1, and further, this quantity of NP(s) was tested on the highest concentration of dye (30 μM). Therefore, the estimated time of degradation is longer in the present study compared to Andrew et al.‘s (2024) study (20 min).68 A study has shown that CaO nanospheres from egg shells have better adsorption properties for BG (98%) compared to phenol red (78%).69 This shows that the adsorbent material is selective for dye removal, and in the present case, diatomite had nanopores and developed multiple adsorption sites and stability with strong van der Waals, hydrogen bonds, electrostatic and π–π stacking force with ionic dye molecules.70
3.2.3.1 Evaluating rhodamine nanocomposites. When 30 μM of Rh–DE was functionalised with 0.2 g mL−1 of TiO2 NP(s) and Fe3O4 NP(s) individually, the DGeff value with TiO2 NP(s) was better (81.74%) than that of Fe3O4 NP(s) (75.19%) in 52 h. The TiO2 NP(s) catalyzed the adsorption of Rh on the surface of DE with the dye concentration in the supernatant falling from 32.33 μg mL−1 initially at 0 min to 5.90 μg mL−1 in 52 h (Fig. 4G). However, with the Fe3O4 NP(s) as a catalyst functionalized on the Rh–DE mixture, the initial concentration of the Rh dye was 49.23 μg mL−1 in 0 min and was left to 12.21 μg mL−1 in 52 h (Fig. 4H). This was much better than the DGeff value of 49.11% with only Rh–DE without NP(s) in 72 h. This study is concordant with previous studies where TiO2 diatomite has been used to remove Rh dye.72
3.3 Application: photosynthetic microalgae-assisted microbial fuel cells
The three different dyes CBB, BG and Rh were simulated to make dye-rich synthetic wastewater and used for testing in situ removal via electrochemical oxidation. This involved the setup of photosynthetic microalgae-assisted microbial fuel cells (PMA-MFCs) along with graphite electrodes. The dye–DE–NP(s) composite was brushed on anode graphite surface and dried. The graphite anode had dye-simulated SWW as electrolytes at the anode and microalgal cells as catholytes at the cathode.The cathode had BG-11 media with microalgae coded as DNM_X, which was identified as Asterarcys sp. GA4 using primers 32F: TTGGATTCAAAGCTGGTGTT 634R: GAAACGGTCTCTCCAACGCAT based on nucleotide homology and phylogenetic analysis, as shown in ESI Fig. S5.† The evolutionary history was inferred by using the maximum likelihood method based on the Kimura 2-parameter model.73 The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analysed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentages of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) are shown next to the branches. Initial tree(s) for the heuristic search were obtained automatically by applying the Neighbour-Joining tree and BioNJ algorithms to a matrix of pairwise distances estimated using the maximum composite likelihood (MCL) approach, and then selecting the topology with a superior log likelihood value. The analysis involved 11 nucleotide sequences. The codon positions included were 1st + 2nd + 3rd + noncoding. All positions containing gaps and missing data were eliminated. There were a total of 1434 positions in the final dataset. Evolutionary analyses were performed in MEGA X.
All the PMA-MFCs having an equal concentration of DE (100 g) and respective dyes (30 μM) (CBB, BG, and RH) and their respective NP(s) which acted as better nano catalysts in test tubes, were functionalized with DE on the graphite felt of anodes of each of the three PMA-MFCs and were run for 33 days with each cycle comprising 3 days. All the PMA-MFC's 1–3 reactors with microalgae Asterarcys sp. GA4 at the cathode and diatomite nanocomposites coated on its graphite felt at the anode rich in dye-simulated SWW showed enhanced DGeff than their respective dye DE nanocomposites in test tubes under light and dark conditions.
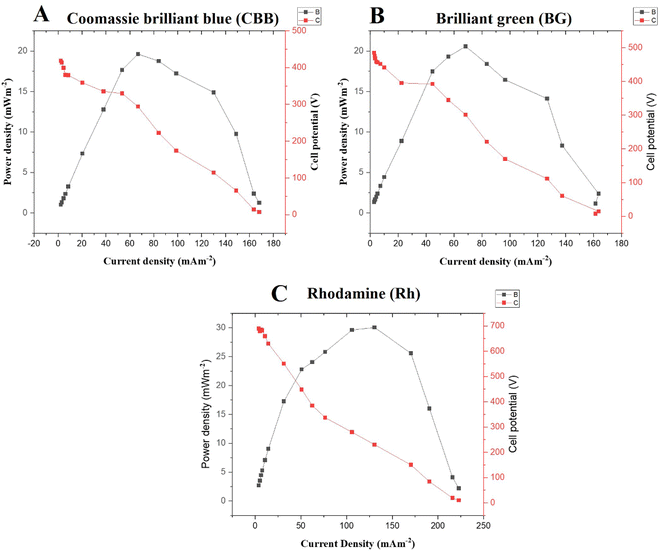
It was seen that PMA-MFC reactor 1 with CBB (30 μM) dye–DE–Fe3O4 nanocomposites in anode containing SWW kept under total dark conditions generated electrons and underwent nanocatalytic and organic degradation generating electrons and protons, transitioning dye molecules into its lower forms of chemical moieties. The protons are transferred to the cathode via a proton exchange membrane, which combines with oxygen released during photosynthesis by microalgae and gets converted to water. Such photosynthesis-assisted microalgal microbial fuel cell (PMA-MFC) generated a total current density of 168.18 mA m−2 and maximum power density (PDmax) of 19.63 mW m−2, on an average, for PMA-MFC-1 enriched with CBB–DE–Fe3O4 nanocomposites. The COD of PMA-MFC-1 was 55.71%, with a coulombic efficiency (CE%) of 6.66%, while the NER was 0.046 kW h m−3 (Fig. 5A). Fe NP(s) can improve the performance of PMA-MFC as an electron shuttle carrier.74 Further Fe3O4 can stimulate the metabolism of electroactive microbes by acting as an electron sink or source, thus promoting their growth and electricity generation.
Similarly, PMA-MFC-2 rich in BG 30 μM dye–DE–TiO2 nanocomposites at the anode with Asterarcys sp. GA4 at the cathode showed a total current density of 127.27 mA m−2 and a PDmax of 20.58 mW m−2. The COD of PMA-MFC-2 was 57.55%, CE was 6.96% and NER was 0.057 kW h m−3 (Fig. 5B). PMA-MFC-1 and 2 had nearly the same maximum polarization density and COD. Microalgae at the cathode in PMA-MFC 1–3 play the role of oxygen producers while simultaneously producing their byproducts and biomass, which is an additional benefit of using such hybridized MFC set ups.
PMA-MFC-3, where the Rh dye at 30 μM DE–TiO2 NP was used, showed maximum polarization density and COD. A total current density of 222.75 mA m−2 and a PDmax of 30.06 mW m−2 were observed in PMA-MFC-3 (Fig. 5C). The COD of PMA-MFC-3 was 61.36%, CE was 8.92% and NER was 0.046 kW h m−3. The volt ampere curves of these PMA-MFCs 1, 2 and 3 showed a drop in voltage upon increase in the current with PMA-MFC-3, showing the lowest voltage drop (7.221 mV) and an increase in current (1.44 mA) (ESI Fig. S6A–C†).
PMA-MFC reactor 1 (CBB–Fe3O4), 2 (BG-TiO2) and 3 (Rh–TiO2) in darkness with dye (30 μM)–DE–NP(s) underwent biochemical and nanocatalytic oxidation and showed DGeff values of 72.31%, 65.71% and 88.23%, respectively (ESI Fig. S7A and B†). On the offset, DGeff for dye and DE without NP(s) in test tube kept in dark i.e., CBB (30 μM)–DE, BG (30 μM)–DE and Rh (30 μM)–DE was 49.58%,; 22.82% and 21.4%, respectively, where only biochemical oxidation is taking place. This shows that nanocatalytic oxidation of dye–DE-nanocomposites at the anode in darkness in PMA-MFC is stronger than the dye functionalized with DE under dark conditions. Among, PMA-MFC better degradation of dye in anode simulated as dye rich SWW, under dark conditions was shown by PMA-MFC-3 with Rh dye (88.23%) as seen in ESI Fig S7A and B†. The overall efficiency of PMA-MFC is also dependent upon the microbiota of wastewater at the anode during the initial and final days of its operation.75 The DGeff value of PMA-MFC is less than the individual photo-nanocatalysis for CBB (30 μM)–DE–Fe3O4, BG (30 μM)–DE–TiO2 and Rh (30 μM)–DE–TiO2, which was 84.46%, 96.10% and 81.74%, respectively. Photocatalysis is always better than catalysis in darkness, but PMA-MFC has yielded multiple value-added products such as bioelectricity, biomass, lipids and remediation of wastewater performing reasonably good even in darkness.
The purpose of analyzing three PMA-MFCs was to check which PMA-MFC supports the highest removal of dyes and multiple bioproduct production to make the overall process of remediation economic and efficient. Since all three PMA-MFCs had 30 μM of dye concentration as the threshold limit, which was tested in test tubes, it is essential to check other parameters too. The microbial consortium, which may be responsible for the organic degradation of dyes under dark conditions at the anode with microalgae at the cathode, is a very important factor to analyze.
3.4 Metagenomics of microbes in synthetic wastewater at the anode
The wastewater on the initial and final days of PMA-MFCs run in all three PMA-MFCs i.e. 1, 2 and 3, was taken for MID, and hence, extracted DNA was amplified using primers V3V4 forward and reverse, as observed in the PCR gel pictures, showing amplification at 460 bp present in PMA-MFCs 1, 2 and 3 on the initial and final days (ESI Table 2 and Fig. S8†). The MID analysis of the amplicon for PMA-MFC-1, PMA-MFC-2 and PMA-MFC-3 showed that different dyes affect the microbial diversity at the anode in a PMA-MFC. In PMA-MFCs 1 and 2 with CBB and BG dyes, the microbial diversity decreased on the final day of polarization cycle (Fig. 6 and S9†). However, PMA-MFC-3 with Rh dye and DE–Fe3O4 nanocomposite showed stable maintenance of existing microbial diversity, thus indicating its higher PDmax (30.06 mW m−2) compared to PMA-MFCs 1 and 2 (∼20 mW m−2). In the present study, Proteobacteria, Bacteroidetes and Firmicutes remained dominant in PMA-MFCs 1 to 3 on the initial and final days of polarization cycle. The Venn diagram of all the three PMA-MFCs showed the shared species diversity among different sets on their first and final days of polarization (ESI Fig. S10†). The anode had different microbes changing their percentage population on the first and final days of the run of microbial fuel cells showing the dominance of Proteobacteria on the first day and Planctomycetes on the final day. PMA-MFC-2 with BG–DE dye showed Proteobacteria on both initial and final days and PMA-MFC-3 with Rh–DE nanocomposites showed dominance of Sphingobacteria and Proteobacteria. PMA-MFC-1 enriched with CBB at the anode had 25 out of 126 species in common, PMA-MFC-2 had 20 among 98 species in common and PMA-MFC-3 had 16 among 96 species in common present on the first and final days of polarization cycles.3.5 Biochemical analysis of microalgae at the cathode
The microalgae Asterarcys sp. GA4 at the cathode of PMA-MFC showed maximum growth in terms of optical density on the 23rd day in all PMA-MFCs and was highest in PMA-MFC-1 (1.40) followed by PMA-MFC-2 (1.74) and PMA-MFC-3 (1.99) at 740 nm (Fig. 7A). The biomass of Asterarcys sp. GA4 was highest on the 30th day in PMA-MFC-3 (8.19 μg g−1), followed by PMA-MFC-1(5.38 μg g−1) and PMA-MFC-2 (4.28 μg g−1) (Fig. 7B). The lipid from this microalga at the cathode was, however, highest in PMA-MFC-3 on the 21st day (4.278 μg g−1), followed by 3.09 μg g−1 on the 33rd day in PMA-MFC-2 and 2.99 μg g−1 on the 21st day in PMA-MFC-1. Biomass of microalgae at cathode was, however, 2.769 μg g−1, 3.09 μg g−1 and 4.054 μg g−1 on the 33rd day in PMA-MFCs 1, 2 and 3 with CBB, BG and Rh–DE-nanocomposite dyes at the anode respectively (Fig. 7C). The microalgal cell growth, biomass and lipids although showed higher shift for PMA-MFC 3, followed by PMA-MFC-2 and PMA-MFC-1 throughout its growth pattern. This is coherent with the high PDmax of PMA-MFC 3 followed by PMA-MFCs 2 and PMA-MFC-1. Many studies have demonstrated the use of microalgae at the cathode in a PMA-MFC, showing the production of lipids, biomass and other value-added products besides producing bioelectricity and remediating wastewater.28,76,77 | ||
| Fig. 7 Microalgae (Asterarcys sp.GA4) at the cathode for 40 days and measuring its (A) cell density, (B) biomass and (C) lipid yield on different days at the cathode of PMA-MFC 1–3. | ||
The above study shows that PMA-MFC 3 with rhodamine dye and diatomite as the adsorbent and TiO2 as the NP catalyst proved to be the most efficient microbial fuel cell in terms of remediating dye-rich wastewater. The overall efficiency has further confirmed PMA-MFC-3 as best in comparison with otherwise well-performed PMA-MFC-1 and PMA-MFC-2. A table of comparison evaluating the different parameters performed and analyzed at both chambers of all the three PMA-MFCs is seen in ESI Table S3.† ESI Table S4† shows the comparison of the present study with the previous studies, proving the present study's significant output in terms of dye degradation, bioelectricity production and value-added products in a PMA-MFC.
4 Conclusions
Dye removal is a very important need of the growing industrialization. In the current study, a comparison of three different ionic dyes, namely Coomassie brilliant blue, brilliant green and rhodamine, for their adsorption and degradation ability was carried out. All the three textile dyes followed the pseudo-second-order kinetics successfully, describing the adsorption process. Among these three, the dye–diatomite–TiO2 nanocomposite has yielded better photocatalytic degradation efficiency. Further, the degradation efficiency of these dyes is economic in a photosynthetic microalgae-assisted microbial fuel cell. Among the different dyes tested in different fuel cells with the diatomite–Fe3O4 nanocomposite at the anode, with rhodamine dye has yielded the highest PDmax. The PDmax of PMA-MFC-1 and PMA-MFC-2 was less than that of PMA-MFC-3 but effective when observed individually with Asterarcys sp.GA4 microalgae. The high electrogenicity in PMA-MFC-3 is also associated with microbial population comprising Sphingobacteria and Proteobacteria identified by MID. Lipid production in PMMFC-1 (CBB), 2 (BG) and 3 (Rh) on the 33rd day of PMA-MFC, respectively, was in coherence with the growth pattern and efficiency of the fuel cell. PMA-MFC-3 yielded the highest lipid productivity on the 21st day. Future studies may need to focus on the degradation potential of these nanocomposites for other industrial dyes and pollutants. Indeed, the investigation of multi-dye systems or wastewater containing a mix of dyes could have impact on real-world applications.Data availability
The data supporting this article have been uploaded as part of the ESI.†Authors contribution
AR; VS: experimentation, data curation, and writing original draft; AA: data curation and analysis, GS; RK; AKS; H: review and editing; VV: supervision, conceptualization, writing original draft, funding, review, and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
AR is thankful to Maharishi Markandeshwar Deemed University Haryana, India, VS is thankful to CEFIPRA Indo French Project for project fellowship, AA is thankful to CEFIPRA Indo French Project for senior research fellowship, GS is thankful to UGC for junior research fellowship, and VV is thankful to CEFIPRA Indo French Project No. PPMB 7133/2020 for financial support.References
- T. Akter, A. T. Protity, M. Shaha, M. Al Mamun and A. Hashem, in Nanohybrid Materials for Treatment of Textiles Dyes, Springer, 2023, pp. 401–431 Search PubMed.
- R. Kaur, S. Wani, A. Singh and K. Lal, Wastewater production, treatment and use in India, In National Report presented at the 2nd regional workshop on Safe Use of Wastewater in Agriculture, 2012, pp. 1–13 Search PubMed.
- A. B. Isaev, N. S. Shabanov, A. G. Magomedova, P. Nidheesh and M. A. Oturan, Environ. Chem. Lett., 2023, 21, 2863–2911 CrossRef CAS.
- G. Moussavi and M. Mahmoudi, J. Hazard. Mater., 2009, 168, 806–812 CrossRef CAS PubMed.
- R. Khanum, R. S. Ali, H. Rangaswamy, S. S. Kumar, A. Prashantha and A. Jagadisha, Results Chem., 2023, 5, 100890 CrossRef CAS.
- T. Islam, M. R. Repon, T. Islam, Z. Sarwar and M. M. Rahman, Environ. Sci. Pollut. Res., 2023, 30, 9207–9242 CrossRef CAS PubMed.
- R. Al-Tohamy, S. S. Ali, F. Li, K. M. Okasha, Y. A.-G. Mahmoud, T. Elsamahy, H. Jiao, Y. Fu and J. Sun, Ecotoxicol. Environ. Saf., 2022, 231, 113160 CrossRef CAS PubMed.
- N. Sheraz, A. Shah, A. Haleem and F. J. Iftikhar, RSC Adv., 2024, 14, 11284–11310 RSC.
- Y. Fei and Y. H. Hu, J. Mater. Chem. A, 2022, 10, 1047–1085 RSC.
- M. J. Khan, A. Rai, A. Ahirwar, V. Sirotiya, M. Mourya, S. Mishra, B. Schoefs, J. Marchand, S. K. Bhatia and S. Varjani, Bioengineered, 2021, 12, 9531–9549 CrossRef CAS PubMed.
- M. M. Ghobara, M. M. Ghobara, M. M. Ghobara and A. Mohamed, Diatoms: Fundamentals and Applications, 2019, pp. 471–509 Search PubMed.
- R. Patrick, The Biology of Diatoms, 1977, vol. 13, pp. 284–332 Search PubMed.
- V. Kumar, M. Kashyap, S. Gautam, P. Shukla, K. B. Joshi and V. Vinayak, J. Biosci., 2018, 43, 717–729 CrossRef CAS.
- M. J. Khan, D. Mathys and V. Vinayak, Diatom Microscopy, 2022, pp. 179–219 Search PubMed.
- M. Hildebrand, E. York, J. I. Kelz, A. K. Davis, L. G. Frigeri, D. P. Allison and M. J. Doktycz, J. Mater. Res., 2006, 21, 2689–2698 CrossRef CAS.
- D. Losic, G. Rosengarten, J. G. Mitchell and N. H. Voelcker, J. Nanosci. Nanotechnol., 2006, 6, 982–989 CrossRef CAS PubMed.
- T. T. Rahman, T. Jiang, C. Zhang, Y. Rao, R. C. Sims and L. Hou, Crit. Rev. Environ. Sci. Technol., 2024, 54, 557–580 CrossRef CAS.
- V. Vinayak, K. B. Joshi, R. Gordon and B. Schoefs, Nanoengineering of diatom surfaces for emerging applications, Nanoscience & Nanotechnology Series, 2017, pp. 55–78 Search PubMed.
- G. Sriram, M. P. Bhat, M. Kigga, U. Uthappa, H.-Y. Jung, T. Kumeria and M. D. Kurkuri, Mater. Chem. Phys., 2019, 235, 121738 CrossRef.
- V. Hegde, U. Uthappa, S. S. Han, H.-Y. Jung, T. Altalhi and M. D. Kurkuri, Mater. Today Commun., 2022, 32, 103887 CrossRef CAS.
- B. E. Logan, B. Hamelers, R. Rozendal, U. Schröder, J. Keller, S. Freguia, P. Aelterman, W. Verstraete and K. Rabaey, Environ. Sci. Technol., 2006, 40, 5181–5192 CrossRef CAS PubMed.
- B. Logan, In Microbial fuel cells, John Wiely & Sons Inc, 2008 Search PubMed.
- M. J. Khan, N. Singh, S. Mishra, A. Ahirwar, F. Bast, S. Varjani, B. Schoefs, J. Marchand, K. Rajendran and J. R. Banu, Chemosphere, 2022, 288, 132589 CrossRef CAS PubMed.
- J. Greenman, I. Gajda and I. Ieropoulos, Sustainable Energy Fuels, 2019, 3, 2546–2560 RSC.
- A. Ahirwar, S. Das, S. Das, Y.-H. Yang, S. K. Bhatia, V. Vinayak and M. M. Ghangrekar, Algal Res., 2023, 70, 102973 CrossRef.
- M. J. Khan, V. J. Suryavanshi, K. B. Joshi, P. Gangadharan and V. Vinayak, in Handbook of Algal Biofuels, Elsevier, 2022, pp. 363–384 Search PubMed.
- M. Shukla and S. Kumar, Renewable Sustainable Energy Rev., 2018, 82, 402–414 CrossRef CAS.
- S. Arun, A. Sinharoy, K. Pakshirajan and P. N. Lens, Renewable Sustainable Energy Rev., 2020, 132, 110041 CrossRef CAS.
- S. Gupta, M. Kashyap, V. Kumar, P. Jain, V. Vinayak and K. B. Joshi, J. Mol. Liq., 2018, 249, 600–608 CrossRef CAS.
- Y. Nam, J. H. Lim, K. C. Ko and J. Y. Lee, J. Mater. Chem. A, 2019, 7, 13833–13859 RSC.
- D. Chen, Y. Cheng, N. Zhou, P. Chen, Y. Wang, K. Li, S. Huo, P. Cheng, P. Peng and R. Zhang, J. Cleaner Prod., 2020, 268, 121725 CrossRef CAS.
- R. Taghavi, S. Rostamnia, M. Farajzadeh, H. Karimi-Maleh, J. Wang, D. Kim, H. W. Jang, R. Luque, R. S. Varma and M. Shokouhimehr, Inorg. Chem., 2022, 61, 15747–15783 CrossRef CAS.
- N. Thakur, N. Thakur, A. Kumar, V. K. Thakur, S. Kalia, V. Arya, A. Kumar, S. Kumar and G. Z. Kyzas, Sci. Total Environ., 2024, 169815 CrossRef CAS.
- I. Das, S. Das, R. Dixit and M. Ghangrekar, Ionics, 2020, 26, 3061–3072 CrossRef CAS.
- S. Das, A. Mishra and M. Ghangrekar, Chem. Phys. Lett., 2020, 759, 137986 CrossRef CAS.
- S. Das and M. Ghangrekar, Environ. Technol., 2020, 41, 2546–2553 CrossRef CAS.
- J. L. Sanz and T. Köchling, Process Biochem., 2007, 42, 119–133 CrossRef CAS.
- A. Behle, Recipe for standard BG-11 media, 2019 Search PubMed.
- M. J. Khan, S. Das, V. Vinayak, D. Pant and M. Ghangrekar, Chemosphere, 2022, 291, 132841 CrossRef CAS.
- L. Xiao, Z. Ge, P. Kelly, F. Zhang and Z. He, Bioresour. Technol., 2014, 157, 77–83 CrossRef CAS.
- K. Obileke, H. Onyeaka, E. L. Meyer and N. Nwokolo, Electrochem. Commun., 2021, 125, 107003 CrossRef CAS.
- B. E. Logan, Nat. Rev. Microbiol., 2009, 7, 375–381 CrossRef CAS.
- S. Ghosh and A. P. Das, Frontiers in soil and environmental microbiology, 2020, 247–254 Search PubMed.
- L. Karstens, N. Y. Siddiqui, T. Zaza, A. Barstad, C. L. Amundsen and T. A. Sysoeva, Sci. Rep., 2021, 11, 6186 CrossRef CAS.
- K. K. Yadav, Y. Nimonkar, B. J. Poddar, L. Kovale, I. Sagar, Y. Shouche, H. J. Purohit, A. A. Khardenavis, S. J. Green and O. Prakash, Microbiol. Spectrum, 2022, 10, e00007–e00022 Search PubMed.
- N. A. Stover and A. R. Cavalcanti, Curr. Protoc. Essent. Lab. Tech, 2017, 14, 111111–111134 Search PubMed.
- S. Kumar, G. Stecher, M. Li, C. Knyaz and K. Tamura, Mol. Biol. Evol., 2018, 35, 1547–1549 CrossRef CAS.
- M. J. Khan, N. Bawra, A. Verma, V. Kumar, A. Pugazhendhi, K. B. Joshi and V. Vinayak, Bioresour. Technol., 2021, 319, 124129 CrossRef CAS.
- S. Chowdhury and P. Saha, Desalin. Water Treat., 2011, 30, 229–236 CAS.
- S. Devi and S. Tyagi, RSC Adv., 2022, 12, 34951–34961 RSC.
- M. Pirilä, M. Saouabe, S. Ojala, B. Rathnayake, F. Drault, A. Valtanen, M. Huuhtanen, R. Brahmi and R. L. Keiski, Top. Catal., 2015, 58, 1085–1099 CrossRef.
- M. Rauf and S. S. Ashraf, Chem. Eng. J., 2009, 151, 10–18 CrossRef CAS.
- W. Zhu and A. R. Kamali, J. Water Process Eng., 2023, 53, 103903 CrossRef.
- B. M. Thamer, A. Aldalbahi, M. Moydeen, H. El-Hamshary, A. M. Al-Enizi and M. H. El-Newehy, Mater. Chem. Phys., 2019, 234, 133–145 CrossRef CAS.
- N. Liu, Y. Wu and H. Sha, Sep. Sci. Technol., 2020, 55, 234–246 CrossRef CAS.
- S. Dhananasekaran, R. Palanivel and S. Pappu, J. Adv. Res., 2016, 7, 113–124 Search PubMed.
- M. Sun, J. Ma, M. Zhang, Y. Xiao, Y. Zhu and S. Zhang, Mater. Chem. Phys., 2020, 241, 122450 CrossRef CAS.
- B. K. Nandi, A. Goswami and M. K. Purkait, J. Hazard. Mater., 2009, 161, 387–395 CrossRef CAS.
- V. S. Mane, I. D. Mall and V. C. Srivastava, J. Environ. Manage., 2007, 84, 390–400 CrossRef CAS.
- V. S. Mane, I. D. Mall and V. C. Srivastava, Dyes Pigm., 2007, 73, 269–278 CrossRef CAS.
- T. Calvete, E. C. Lima, N. F. Cardoso, S. L. Dias and E. S. Ribeiro, Clean:Soil, Air, Water, 2010, 38, 521–532 Search PubMed.
- R. O. Gembo, S. Odisitse, T. A. Msagati and C. K. King’ondu, Next Sustainability, 2024, 4, 100054 CrossRef.
- M. S. U. Rehman, M. Munir, M. Ashfaq, N. Rashid, M. F. Nazar, M. Danish and J.-I. Han, Chem. Eng. J., 2013, 228, 54–62 CrossRef CAS.
- K. S. Baidya and U. Kumar, S. Afr. J. Chem. Eng., 2021, 35, 33–43 Search PubMed.
- N. Laskar and U. Kumar, Int. J. Environ. Sci. Technol., 2019, 16, 1649–1662 Search PubMed.
- M. Asadullah, M. Asaduzzaman, M. S. Kabir, M. G. Mostofa and T. Miyazawa, J. Hazard. Mater., 2010, 174, 437–443 CrossRef CAS.
- M. K. Khan, A. S. Abdulhameed, H. Alshahrani and S. Algburi, Int. J. Biol. Macromol., 2024, 263, 130465 Search PubMed.
- F. P. Andrew, T. R. Papo and P. A. Ajibade, Environ. Adv., 2024, 17, 100575 CrossRef CAS.
- S. Thakur, S. Singh and B. Pal, J. Nanostruct. Chem., 2022, 12, 207–221 Search PubMed.
- Y. Fang, M. Wu, Q. Zhang, F. Zhou, C. Deng, Y. Yan, H.-H. Shen, Y. Tang and Y. Wang, Sep. Purif. Technol., 2022, 298, 121611 CrossRef CAS.
- T. A. Saleh, M. Tuzen and A. Sarı, Environ. Sci. Pollut. Res., 2021, 28, 55655–55666 CrossRef CAS.
- S. K. Padmanabhan, S. Pal, E. U. Haq and A. Licciulli, Appl. Catal., A, 2014, 485, 157–162 CrossRef CAS.
- E. B. Wong, N. Kamaruddin, M. Mokhtar, N. Yusof and R. F. R. Khairuddin, J. Genet. Eng. Biotechnol., 2023, 21, 104 CrossRef.
- S. Zhao, S. Liu, T. Sumpradit, J. Zhou and J. Qu, Int. J. Hydrogen Energy, 2024, 63, 163–172 CrossRef CAS.
- M. Li, M. Zhou, X. Tian, C. Tan, C. T. McDaniel, D. J. Hassett and T. Gu, Biotechnol. Adv., 2018, 36, 1316–1327 CrossRef CAS.
- J. K. Nayak and U. K. Ghosh, Biomass Bioenergy, 2019, 131, 105415 CrossRef CAS.
- A. Khandelwal, A. Vijay, A. Dixit and M. Chhabra, Bioresour. Technol., 2018, 247, 520–527 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00793c |
| This journal is © The Royal Society of Chemistry 2025 |