Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New zinc(II) metalloporphyrin: molecular structure, spectroscopic characterization, electrochemical sensing of dopamine, and catalytic dye degradation†
Mohamed Achraf Bouichaa,
Chama Mabrouk b,
Bouzid Gassoumib,
Houcine Barhoumib,
Florian Moltonc,
Frédérique Loiseauc,
Thierry Roisneld,
Aracely Serrano Medinae,
Jose Manuel Cornejo Bravo
b,
Bouzid Gassoumib,
Houcine Barhoumib,
Florian Moltonc,
Frédérique Loiseauc,
Thierry Roisneld,
Aracely Serrano Medinae,
Jose Manuel Cornejo Bravo f,
Eduardo Alberto Lopez-Maldonado
f,
Eduardo Alberto Lopez-Maldonado *f and
Habib Nasri*a
*f and
Habib Nasri*a
aUniversity of Monastir, Laboratory of Physical Chemistry of Materials (LR01ES19), Faculty of Sciences of Monastir, Avenue de l'environnement, 5019 Monastir, Tunisia. E-mail: Habib.Nasri@fsm.rnu.tn
bUniversity of Monastir, Laboratory of Interfaces and Advanced Materials, Faculty of Sciences of Monastir, Avenue de l'environnement, 5019 Monastir, Tunisia
cDépartement de Chimie Moléculaire, Université Grenoble Alpes, 301 rue de la Chimie, CS 40700, 38058 Grenoble, Cedex 9, France
dInstitute of Chemical Sciences of Rennes, UMR 6226, University of Rennes 1, Beaulieu Campus, 35042 Rennes, France
eFacultad de Medicina y Psicología, Universidad Autónoma de Baja California, Tijuana 22390, Mexico
fFaculty of Chemical Sciences and Engineering, Autonomous University of Baja California, 22424, Mexico. E-mail: elopez92@uabc.edu.mx; Tel: +216 73 500 278
First published on 1st April 2025
Abstract
This work is a continuation of the series of studies aimed at studying the electronic and structural properties of divalent metal porphyrin complexes, especially zinc(II) metalloporphyrins. In this perspective, we have prepared the [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) coordination compound, where TMPP is the meso-tetra(para-methoxyphenyl)porphyrinate and 4,4′-bpy is the 4,4′-bipyridine. The UV/Vis, fluorescence, IR and 1H NMR spectroscopic techniques, ESI-HRMS mass spectrometry investigation as well as a single crystal X-ray diffraction study were used to characterize the title compound. Notably, we demonstrated the ability of this zinc(II) metalloporphyrin to degrade the methylene blue (MB) dye, examining several influencing factors, including pH, temperature and initial dye concentration. Additionally, complex I exhibited remarkable efficiency in degrading MB under blue LED irradiation. Beyond catalytic applications, this compound was successfully employed as an electrochemical sensor for the detection of dopamine (DA) using the square wave voltammetry (SWV) method, showcasing its multifunctional capabilities.
1 Introduction
Since the pioneering work of Hans Fischer in the early 20th century, metalloporphyrins have captivated the attention of researchers worldwide due to their intriguing electronic, magnetic, and catalytic properties. Among these, zinc(II) porphyrin complexes stand out as a cornerstone in the field, owing to their unique structural features and diverse applications.The most used metalloporphyrins as models for hemoproteins such as hemoglobin, myoglobin, and cytochromes P450 are iron porphyrin complexes because of the presence of the iron as a center ion in hemoproteins. Just to have an idea about the most investigated metals in porphyrin complexes, we used the Cambridge Structural Database updated to November 2023 (ref. 1) to classify the reported crystal structures of porphyrin complexes for several metal ions.
According to Table S1,† the most important reported metalloporphyrin crystal structures are those with zinc(II) metal ion. These porphyrin complexes are among the most studied metalloporphyrins due to several key reasons:
(1) The insertion of Zn(II) ion into the porphyrin cavity is very easy compared to the insertion of other divalent M(II) center metals by using the zinc acetate common synthetic route.2 The zinc(II) metallation leads to very stable metalloporphyrins.
(2) Zn(II) metalloporphyrins provide much simpler coordination compounds than those of cobalt, iron or other d transition metals to study the influence of different types of axial ligands on the physical chemistry properties of metal porphyrins. Indeed, the zinc metal ion is unambiguously in the II oxidation state.
(3) Zinc(II) porphyrins exhibit unique photophysical properties due to their closed-shell properties that make them attractive for various applications. They have strong absorption in the visible region, high fluorescence quantum yields, and long-lived excited states.3
Despite the electrochemical inactivity of Zn(II) due to its fully filled d-orbitals, Zn(II) porphyrin complexes can still participate in redox processes. This is made possible through the delocalized π-electron system of the porphyrin and its interactions with axial ligands, which enhance electron transfer. Consequently, Zn(II) porphyrins have proven valuable for electrochemical sensing applications.
Moreover, various molecular electrocatalysts have been widely explored for detecting glucose, pesticides, and pharmaceutical compounds, demonstrating broad applicability in electroanalysis.4,5 These findings underscore the versatility of metal porphyrin systems, not only in sensing but also in more complex catalytic applications. In this context, a review highlighted the use of indium tin oxide electrodes modified with materials such as ZnO nanowire arrays and graphene foam for the electrochemical determination of L-Dopa, a drug molecule structurally similar to dopamine.6
Furthermore, small molecular d10 metal complexes, particularly Zn-based systems, have attracted considerable interest in electrocatalysis due to their unique electronic properties and various applications, including water splitting and sensing technologies.7–10 Although these complexes are electrochemically inactive in their pure metal form, they exhibit catalytic activity when their electronic structure is modified through ligand interactions. This behavior allows them to facilitate reactions such as oxygen and hydrogen evolution while also enhancing their efficiency in sensor applications. These findings highlight the broad potential of d10 metal systems in advancing energy and environmental technologies.
On the other hand, 4,4′-bipyridine is a heterocyclic compound widely used in various materials and compounds.11
4,4′-Bipyridine and its derivatives have numerous applications in chemistry and materials science. They are used as ligands in coordination chemistry. These compounds are widely used as ligands in coordination chemistry as demonstrated by the very important number of molecular structures of 4,4′-bpy-metal complexes reported in the CCDC Cambridge database (more than 680 hits) (Cambridge Structural Database updated to November 2023). Among 4,4′-bpy metal complexes, 4,4′-bpy metalloporphyrins present an important class of coordination compounds including a large variety of center ions such as Fe(II), Fe(III), Ru(II), Os(II), Ni(II), Mn(III), V(IV) and mainly Zn(II). Several investigations of these zinc(II) metalloporphyrins have been reported especially this last decade.12–15 It is noteworthy that in solid state 4,4′-bpy zinc(II) metalloporphyrins crystallized as monomers such as [Zn(TEBOP)(4,4′-bpy)] (TEBOP = meso-(tetraethyl-4(4-butyryl)oxyphenyl)porphyrinate),16 dimers such as [{Zn(TPP)}2(μ2-4,4′-bpy)] (TPP = meso-tetraphenylporphyrinate),17 trimers such as [{Zn(TPP)}3(μ2-4,4′-bpy)]15 and as polymers such as {[Zn(TMP)(4,4′-bpy)]}n (TMP = meso-tetramesitylporphyrinate)18(Scheme 1).
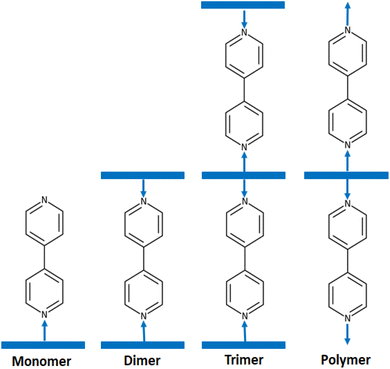 | ||
| Scheme 1 Schematic illustrations of the various structural types of zinc porphyrins with 4,4′-bipyridine axial ligand. | ||
The widespread use of organic dyes, such as methylene blue (MB), presents a significant environmental challenge due to their resistance to conventional degradation methods.19,20 As a result, there is a growing need for eco-friendly and efficient strategies to degrade these dyes. Oxidative and photocatalytic degradation methods19 have emerged as promising solutions to address water contamination caused by toxic pollutants. Synthetic metalloporphyrins, particularly Zn(II) porphyrins, have gained attention as effective photocatalysts in such degradation processes. These catalysts utilize reactive oxygen species (ROS) to break down contaminants in a manner similar to peroxidase-like catalytic activity. For instance, the degradation of methylene blue (MB) using Zn(II) porphyrin in the presence of H2O2 involves the generation of hydroxyl radicals (˙OH), which accelerates the breakdown of the dye. This mechanism relates on hydroxylation and oxidative cleavage pathways, eventually leading to the complete mineralization of the dye molecules.21
Recent studies, including those conducted by our research team, have demonstrated the successful degradation of various organic dyes using meso-arylporphyrins and metalloporphyrins as catalysts.13,16,22–25 Building on this work, we aim to investigate the dual functionality of Zn(II) porphyrin complexes in both environmental and biomedical applications. Specifically, we focus on the degradation of methylene blue dye and the detection of dopamine (DA), highlighting the redox-active nature of these complexes and their ability to facilitate charge transfer processes in both oxidative and photocatalytic degradation, as well as in electrochemical sensing. This dual functionality, stemming from the ability of Zn(II) porphyrins to modulate electron density and engage in redox reactions, positions them as promising candidates for both environmental remediation and biosensing technologies.
In this context, we present the synthesis and characterization of the (4,4′-bipyridine)[meso-tetra(para-methoxyphenyl)porphyrinato]zinc(II) chloroform monosolvate complex [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I). The structure of this complex was determined using UV-Vis, fluorescence, IR, 1H NMR, cyclic voltammetry, and ESI-HRMS techniques. The crystal structure was analyzed, and the intermolecular interactions contributing to its stability were studied using surface Hirshfeld analysis. We also investigated the degradation of methylene blue dye through photodegradation and oxidative degradation, using complex I as a catalyst. The activation energy and thermodynamic variables for the oxidative degradation were determined using the pseudo-first-order model. Furthermore, we explored the electrochemical detection of dopamine (DA) in human urine samples using the square wave voltammetry (SWV) technique.
2 Experimental section
2.1 Synthetic procedures
UV-Vis: λmax (nm, CH2Cl2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 423 (6.24), 519 (4.95), 557(4.82), 596 (4.65), 651 (4.70) nm. FT-IR (solid, cm−1): 3320 (w) [ν(NH) pyrrole], 2990–2839 (m) [ν(CH) Porph.], 1243 (s) [ν(CO) methoxy], 964 (m) [δ(CCH) Porph] 1H NMR (400 MHz, CDCl3 δ(ppm)): 8.87 (s, 8H, Hβ), 8.11 (d, 8H, Ho,o′), 7.29 (d, 8H, Hm), 4.11 (s, 12H, Ha), −2.74 (s, 2H, NHpy).
ε): 423 (6.24), 519 (4.95), 557(4.82), 596 (4.65), 651 (4.70) nm. FT-IR (solid, cm−1): 3320 (w) [ν(NH) pyrrole], 2990–2839 (m) [ν(CH) Porph.], 1243 (s) [ν(CO) methoxy], 964 (m) [δ(CCH) Porph] 1H NMR (400 MHz, CDCl3 δ(ppm)): 8.87 (s, 8H, Hβ), 8.11 (d, 8H, Ho,o′), 7.29 (d, 8H, Hm), 4.11 (s, 12H, Ha), −2.74 (s, 2H, NHpy).
The reaction mixture was then cooled to 50 °C, and 50 mL of water was added to precipitate the product. The solid obtained was filtered, washed with n-hexane, and dried under vacuum for 30 minutes, yielding the [Zn(TMPP)] complex with an 83% yield (450 mg) (Scheme 2).
UV-Vis: λmax (nm, CH2Cl2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 425 (6.36), 551 (5.17) 592 (4.92) nm. FT-IR (solid, cm−1): 3000–2825 (m) [ν(CH) Porph.], 1264 (s) [ν(CO) methoxy], 1001 (m) [δ(CCH) Porph] 1H NMR (400 MHz, CDCl3 δ(ppm)): 8.95 (s, 8H, Hβ), 8.14 (d, 8H, Ho,o′), 7.30 (d, 8H, Hm), 4.10 (s, 12H, Ha).
ε): 425 (6.36), 551 (5.17) 592 (4.92) nm. FT-IR (solid, cm−1): 3000–2825 (m) [ν(CH) Porph.], 1264 (s) [ν(CO) methoxy], 1001 (m) [δ(CCH) Porph] 1H NMR (400 MHz, CDCl3 δ(ppm)): 8.95 (s, 8H, Hβ), 8.14 (d, 8H, Ho,o′), 7.30 (d, 8H, Hm), 4.10 (s, 12H, Ha).
UV-Vis: λmax (nm, CH2Cl2, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε): 432 (6.07), 558 (4.96), 604 (4.84) nm. FT-IR (solid, cm−1): 3109 (w) [ν(CH) bpy], 3033–2834 (m) [ν(CH) Porph.], 1507 (s) [ν(CN) bpy], 1234 (s) [ν(CO) methoxy], 995 (m) [δ(CCH) Porph] 1H NMR (400 MHz, CDCl3 δ(ppm)): 8.81 (s, 8H, Hβ), 8.01 (d, 8H, Ho,o′), 7.21 (d, 8H, Hm), 4.06 (s, 12H, Ha).
ε): 432 (6.07), 558 (4.96), 604 (4.84) nm. FT-IR (solid, cm−1): 3109 (w) [ν(CH) bpy], 3033–2834 (m) [ν(CH) Porph.], 1507 (s) [ν(CN) bpy], 1234 (s) [ν(CO) methoxy], 995 (m) [δ(CCH) Porph] 1H NMR (400 MHz, CDCl3 δ(ppm)): 8.81 (s, 8H, Hβ), 8.01 (d, 8H, Ho,o′), 7.21 (d, 8H, Hm), 4.06 (s, 12H, Ha).
2.2 X-ray molecular structure
High-quality crystals of the title compound were grown by the slow diffusion of n-hexane into a chloroform solution of Complex I. For the X-ray diffraction analysis, a dark purple prism-shaped single crystal with dimensions of 0.29 × 0.20 × 0.07 mm3 was selected. Data collection was performed at 150(2) K using a Bruker AXS D8 VENTURE diffractometer27 with Mo Kα radiation (λ = 0.71073 Å), and the structure was solved using direct methods via the SIR-2014 program28 and refined through full-matrix least-squares techniques on F2 with the SHELXL-2014 program.29 Hydrogen atoms were incorporated into the refinement using the riding model. Intermolecular interactions were analyzed using the PLATON program,30 and packing diagrams were generated with MERCURY software.31 Crystallographic and structural parameters are summarized in Table 1.| a R1 = Σ‖Fo|–|Fc‖/Σ|Fo|.b wR2 = {Σ[w(|Fo|2–|Fc|2)2]/Σ[w(|Fo|2)2]}1/2. | |
|---|---|
| Formula | C59H45Cl3N6O4Zn |
| M.W. | 1073.73 |
| Crystal system | Monoclinic |
| Crystal | P21/c |
| a (Å) | 13.0739(14) |
| b (Å) | 14.9439(16) |
| c (Å) | 11.6472(12) |
| α (°) | 90 |
| β (°) | 98.352(4) |
| γ (°) | 90 |
| V (Å3) | 4972.2(9) |
| Z | 4 |
| ρcalc./g cm−3 | 1.434 |
| μ/mm−1 | 0.712 |
| F(000) | 2216 |
| Crystal size (mm3) | 0.29 × 0.20 × 0.07 |
| Crystal color | Violet |
| Crystal shape | Prism |
| T (K) | 150 (2) |
| θmin – θmax (°) | 1.872–27.488 |
| Limiting indices | −16 ≤ h ≤ 16, −42 ≤ k ≤ 38, −15 ≤ l ≤ 15 |
| R (int) | 0.0209 |
| Total/unique data | 68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 934/11 934/11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 367 367 |
| Observed data [Fo > 4σ(Fo)] | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
| Parameters/rest | 658/0 |
| S [goodness of fit] | 1.021 |
| R1a, wR2b [Fo > 4σ(Fo)] | R1 = 0.0415; wR2 = 0.1066 |
| R1 a,wR2 b [all data] | R1 = 0, 0.0471; wR2 = 0.1103 |
| Min./max. Res. (e Å−3) | 0.899/−0.737 |
| CCDC | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 358 358![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 252 252 |
3 Results and discussion
3.1 Mass spectrometry
The [Zn(TMPP)] starting material and complex I were characterized using high-resolution mass spectrometry ESI, as depicted in Fig. S1 and S2,† respectively. In Fig. S2,† fragments corresponding to [Zn(TMPP) + H]+ and [Zn(TMPP)(4,4′-bpy) + H]+ are observed with m/z values of 797.2119 and 953.2794, respectively. These values are consistent with the theoretical m/z values of 797.2101 and 953.2700. These results from high-resolution ESI mass spectrometry confirm the stability of the [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) complex in dichloromethane solution.3.2 1H NMR and IR spectroscopy
The 1H NMR spectra of the H2TMPP and the [Zn(TMPP)] complex, recorded in CDCl3, are shown in Fig. S3 and S4.† In the spectrum of H2TMPP, the NH-pyrrolic inner protons exhibit strong shielding, appearing at a chemical shift of −2.74 ppm. The β-pyrrolic protons and aromatic phenyl protons (Ho,o′ and Hm,m′) resonate within the range of 8.87 to 7.29 ppm. A singlet at 4.11 ppm corresponds to the methoxy protons. For [Zn(TMPP)], the disappearance of the −2.74 ppm signal unequivocally confirms the insertion of the Zn(II) ion into the porphyrin core. The β-pyrrolic and phenyl protons in [Zn(TMPP)] exhibit slight shifts compared to those in the free-base H2TMPP, reflecting the diamagnetic nature of the [Zn(TMPP)] complex and the subtle electronic changes caused by metallation.The 1H NMR spectrum of [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) which is shown in Fig. S5† is characteristic of a diamagnetic zinc(II) meso-arylporphyrin complex. The chemical shift values of the β pyrrolic, Ho,o′, Hm,m′ and H(OCH3) protons of the TMPP porphyrinate are 8.81, 8.01, 7.21 and 4.06 ppm, respectively (Table S2†). The spectrum further reveals distinct signals corresponding to the axial 4,4′-bipyridine ligand. The aromatic protons (C–H) of the 4,4′-bpy ring appear downfield, with chemical shifts observed at 6.17 and 5.57 ppm. These protons are significantly more shielded compared to those in the non-coordinated 4,4′-bipyridine molecule, which exhibit chemical shift values of 8.74 and 7.53 ppm, respectively.
The IR spectra of H2TMPP, [Zn(TMPP)] and [Zn(TMPP)(4,4′-bpy)]·CHCl3 depicted in Fig. S6, S7 and S8,† respectively, were recorded in solid state in the range of [4000–500 cm−1].
The IR spectrum of the H2TMPP exhibits (Fig. S6†) (i) a weak absorption band at 3320 cm−1 attributed to the stretching frequency ν(N–H) of the pyrrole rings, (ii) a multiple weak bands between 2990 and 2839 cm−1 corresponding to ν(C–H) of the TMPP porphyrinate, (iii) a strong absorption band at 1243 cm−1 attributed to the ν(C–O) stretching frequency of the OMe groups of the TMPP porphyrinate and (iv) a strong band corresponding to the deformation frequency δ(CCH) with a wavenumber value of 864 cm−1 of the porphyrin macrocycle. Upon the insertion of the zinc(II) ion into the porphyrin ring ([Zn(TMPP)] complex), the band attributed to the N–H vibration frequency of the free base porphyrin disappears and the band corresponding to δ(CCH) of the porphyrin macrocycle is shifted toward the high frequencies (![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) = 999 cm). For this starting material (Fig. S7†), the ν(C–H) of the porphyrin core and the ν(C–O) stretching frequency of the OMe groups present
= 999 cm). For this starting material (Fig. S7†), the ν(C–H) of the porphyrin core and the ν(C–O) stretching frequency of the OMe groups present ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) values very close to those of the H2TMPP free base porphyrin. For complex I, the weak absorption band with
values very close to those of the H2TMPP free base porphyrin. For complex I, the weak absorption band with ![[small nu, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0ce.gif) value of 3108 cm−1 and the strong IR band at 1493 cm−1 (Fig. S8†) are attributed to the ν(C–H) and ν(C
value of 3108 cm−1 and the strong IR band at 1493 cm−1 (Fig. S8†) are attributed to the ν(C–H) and ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) stretching frequencies of the 4,4′-bpy axial ligand. The characteristic porphyrinate frequencies of complex I resonate at wavenumber values very close to those of H2TMPP and [Zn(TMPP)].
N) stretching frequencies of the 4,4′-bpy axial ligand. The characteristic porphyrinate frequencies of complex I resonate at wavenumber values very close to those of H2TMPP and [Zn(TMPP)].
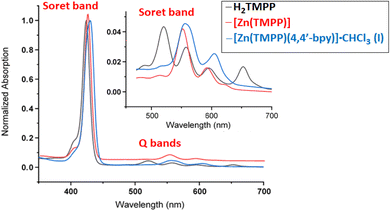
3.3 UV-visible absorption spectroscopy
The UV-Vis absorption spectra of H2TMPP, [Zn(TMPP)] and complex I recorded in dichloromethane solvent are shown in Fig. 1 and in Table 2 are given the λmax values of the Soret and Q bands of these three porphyrinic species along with those of a selection of meso-arylporphyrins and zinc(II) metalloporphyrins.| Compound | Solvent | λmax (nm) | Ref. | |
|---|---|---|---|---|
| Soret band | Q bands | |||
| a H2(TEBOP) = meso-(tetraethyl-4(4-butyryl)oxyphenyl)porphyrin.b TTP = meso-tetra-p-tolylporphyrin.c H2(TAzp-HVP) = meso-tetrakis(3-methoxy-4-((1-phenyl-1H-1,2,3-triazol-4-yl)methoxy)phenyl)porphyrin.d TPP = meso-tetraphenylporphyrinate.e TPBP = meso-{tetrakis-[4-(benzoyloxy)phenyl] porphyrin.f DABCO = 1,4-diazabicyclo[2.2.2]octane.g 4,4′-mda = 4,4′-diaminodiphenylmethane. | ||||
| H2(TEBOP)a | CH2Cl2 | 422 | 517, 554, 593, 651 | 16 |
| H2TTPb | CH2Cl2 | 420 | 518, 554, 594, 650 | 32 |
| H2TMPP | CH2Cl2 | 423 | 519, 557, 596, 651 | t.w. |
| H2(TAzP-HVP)c | CH2Cl2 | 420 | 517, 554, 593, 650 | 33 |
| [Zn(TEBOP)]a | CH2Cl2 | 424 | 552![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 594 594 |
16 |
| [Zn(TMPP)] | CH2Cl2 | 425 | 551![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 592 592 |
t.w. |
| [Zn(TAzP-HVP)]c | CH2Cl2 | 424 | 551![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 592 592 |
33 |
| [Zn(TPP)(py)]d | CH2Cl2 | 428 | 562![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 602 602 |
34 |
| [Zn(TPBP)(DABCO)]e,f | C6H5Cl | 431 | 564![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
12 |
| [Zn(TPBP)(4,4′-mda)]e,g | CHCl3 | 431 | 563![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 604 |
12 |
| [{Zn(TPBP)}2(μ2-4,4′-bpy)]e | CHCl3 | 430 | 563![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 603 603 |
12 |
| [{Zn(TPP)}3(μ2-4,4′-bpy)]d | CHCl3 | 425 | 562![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 601 601 |
15 |
| [Zn(TEBOP)(4,4′-bpy)]e | CHCl3 | 430 | 563![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 604 |
16 |
| [Zn(TMPP)(4,4′-bpy)]·CHCl3 | CH2Cl2 | 432 | 558![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 604 604 |
t.w. |
The electronic spectra of H2TMPP exhibit a prominent absorption band (B band) at 423 nm known as the Soret band, which corresponds to the allowed transition from the ground state So to the second excited state S2 (So ← S2). Additionally, there are four less intense absorption bands observed at 519, 557, 596, and 651 nm, which are attributed to Qy(1,0), Qy(0,0), Qx(1,0) and Qx(0,0) absorption bands corresponding to the forbidden transitions from the ground state So to the first excited state S1 (So ← S1), respectively. Upon the insertion of the zinc(II) metal ion into the porphyrin ring, a reduction in both the number and intensity of Q bands was observed, alongside a redshift of up to four nm of the intense Soret band. Thus, the [Zn(TMPP)] obtained complex exhibits electronic spectra with Soret and Q bands at 427 nm, 557 nm and 598 nm, respectively.
For [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I), the Soret band displays a noticeable red shift with a λmax value of 423 nm which is very close to those of the related Zn(II)-(4,4′-bpy) meso-aryl porphyrin complexes such as [{Zn(TPBP)}2(μ2-4,4′-bpy)],12 [{Zn(TPP)}3(μ2-4,4′-bpy)],15 and [Zn(TPBP)(4,4′-bpy)]16 (Table 2). These Soret band values are also very close to those with N-donor neutral axial ligands such as pyridine and DABCO (1,4-diazabicyclo[2.2.2]octane) ligands. This bathochromic shift for the Soret of the later species compared to those of the [Zn(Porph)] starting materials may be attributed to an increase in the π-conjugation resulting from the addition of the N-donor axial ligand.
The optical gap energy (Eg-opt) values of H2TMPP, [Zn(TMPP)] and [ZnII(TMPP)(4,4′-bpy)]·CHCl3 (I) calculated using the Tauc method35 are 1.848, 1.946 and 1.957 eV, respectively (Fig. S9†). These values indicate that our three porphyrinic compounds are considered semi-conductors, which is typical for all porphyrins and metalloporphyrins.
3.4 Fluorescence spectroscopy
The fluorescence emission spectrum of free base porphyrin H2TMPP recorded in dichloromethane solvent at room temperature upon photoexcitation at 420 nm exhibits two emission bands Q(0,0) and Q(0,1) corresponding to the S1 ← So transition (Fig. 2). These emission bands exhibit maximum emission wavelengths values of 655 nm and 721 nm corresponding to Q(0,0) and Q(0,1), respectively. The metalloporphyrin [Zn(TMPP)] gave two split emission bands Q(0,0) and Q(0,1) with values of 602 and 650 nm, respectively. Axially ligated metalloporphyrin (complex I) give rise to two emission from the S2 excited state to the fundamental state S0, with one centered at 601 nm (resulting from S2 [Q(0,0)] ← S0) and the other at 649 nm (stemming from S2 [Q(0,1)] ← S0) when excited at 430 nm for [Zn(TMPP)(4,4′-bpy)]·CHCl3 (complex I). The fluorescence quantum yields (Φf) for H2TMPP, [Zn(TMPP)] and [Zn(TMPP)(4,4′-bpy)]·CHCl3 are 0.082, 0.033 and 0.028, respectively, with lifetimes of 7.15 ns for H2TMPP, 1.5 ns for [Zn(TMPP)] and 1.3 ns for complex I (Table 3). These values fall within the typical range for meso-arylporphyrins and their Zn(II) complexes. | ||
| Fig. 2 The fluorescence spectra of H2TMPP, [Zn(TMPP)] and [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) in CH2Cl2 (ca. 10−6 M). | ||
| Compound | λmax (nm) | Φf | τf (ns) | Ref. | |
|---|---|---|---|---|---|
| Q(0,0) | Q(0,1) | ||||
| a H2TPP = meso-tetraphenylporpyrin.b H2TClPP = meso-tetra(4-chlorophenyl)porphyrin.c H2TTP = meso-tetra(p-tolyl)porphyrin.d TPBP = meso-tetrakis(4-tert-butylphenyl)porphyrinate.e DABCO = 1,4-diazabicyclo[2.2.2]octane.f pyz = pyrazine.g 4-CNpy = 4-cyanopyridine.i TEBOP = meso-(tetraethyl-4(4-butyryl)oxyphenyl)porphyrin. | |||||
| meso-Arylporphyrins | |||||
| H2TPPa | 653 | 722 | 0.12 | 9.60 | 36 |
| H2TClPPb | 652 | 714 | 0.089 | 7.42 | 37 |
| H2TTPc | 657 | 721 | 0.098 | 7.90 | 37 |
| H2TMPP | 655 | 721 | 0.082 | 7.15 | t.w. |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Zinc(II) meso-arylporphyrins | |||||
| [Zn(TMPP)] | 602 | 650 | 0.033 | 1.5 | t.w. |
| [Zn(TTP)]c | 600 | 648 | 0.030 | 1.6 | 37 |
| [Zn(TPBP)]d | 606 | 654 | 0.027 | 1.6 | 12 |
| [Zn(TPBP)(DABCO)]d,e | 612 | 660 | 0.039 | 1.3 | 12 |
| [Zn(TPBP)(pyz)2]d,f | 596 | 644 | 0.049 | 1.6 | 12 |
| [Zn(TPBP)(4-CNpy)]d,g | 596 | 645 | 0.041 | 1.5 | 12 |
| [{Zn(TPBP)}2(μ2-4.4-bpy)]d | 596 | 645 | 0.044 | 1.5 | 12 |
| [Zn(TEBOP)(4,4′-bpy)]i | 603 | 651 | 0.028 | 1.2 | 16 |
| [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) | 601 | 649 | 0.035 | 1.3 | t.w. |
3.5 Cyclic voltammetry of complex I
The cyclic voltammetry spectrum of complex I is depicted in Fig. S10† while in Table 4 is given the half-potential (E1/2) values of the oxidation and reduction waves of complex I and several zinc(II) porphyrin complexes.| Complex | Oxidations 1st porph oxid | 2nd porph oxid | Reductions 3rd porph oxid | 1st porph red | Ref. 2nd porph red | |
|---|---|---|---|---|---|---|
| (O1,R1) | (O2,R2) | (R3,O3) | (R4,O4) | (R5,O5) | ||
| E1/2b | E1/2 | E1/2 | E1/2 | E1/2 | ||
| a Potentials are reported versus SCE.b E1/2 = half wave potential.c TPP = meso-tetraphenylporphyrinate.d HIm = imidazole.e TMP = meso-tetramesitylporphyrin.f 2-MeIm = 2-methylimidazole.g TPBP = meso-tetrakis(4-tert-butylphenyl)porphyrinate.i DABCO = 1,4-diazabicyclo[2.2.2]octane.j pyz = pyrazine.k 4,4′-mda = 4,4′-diaminodiphenylmethane.l 4-CNpy = 4-cyanopyridine, *: irreversible wave. | ||||||
| [Zn(TPP)(HIm)]c,d | 0.65 | 1.35 | — | −1.34* | −1.67* | 34 |
| [Zn(TMP)(2-MeIm)]e,f | 0.58* | 1.18* | — | −1.55* | — | 34 |
| [Zn(TPP)(CN)]−c | 0.65 | 1.06 | 1.38 | −1.51 | −1.77 | 37 |
| [Zn(TPBP)(DABCO)]g,i | 0.84 | 1.12 | — | −1.33* | — | 12 |
| [Zn(TPBP)(pyz)2]g,j | 0.82 | 1.12 | 1.38* | −1.34* | — | 12 |
| [Zn(TPBP)(4,4′-mda)]g,k | 0.81 | 1.28* | — | −1.31* | −1.69 | 12 |
| [Zn(TPBP)(4-CNpy)]g,l | 0.81 | 1.10 | 1.36 | −1.52 | −1.74 | 12 |
| [{Zn(TPBP)}2(μ2-4,4′-bpy)]g | 0.81 | 1.13 | 1.38* | −1.30* | — | 12 |
| [Zn(TMPP)(4,4′-bpy)] (I) | 0.82 | 0.98 | 1.39 | −1.38 | — | This work |
Zinc(II) metalloporphyrins exhibit two or three one-electron reversible or quasi-reversible oxidation waves and one or two reversible or quasi-reversible reduction waves corresponding to the oxidation and the reduction of the porphyrin macrocycle.34 For our Zn(II)-4,4′-bpy-TMPP derivative, the E1/2 values of the first, second and third oxidation waves are 0.82, 0.98 and 1.39 V which are very close to the related Zn(II) metalloporphyrins reported in Table 4. In the anodic region of the voltammogram of complex I, the quasi-reversible wave with E1/2 value of −1.38 V is attributed to the first reduction of the porphyrin core of the TMPP porphyrinate. This value is also in the range observed for pentacoordinated and hexacoordinated zinc(II) porphyrin coordination compounds. Notably, the UV-Vis data and those of the cyclic voltammetry of zinc(II) metalloporphyrins are very close regardless the nature of the meso-arylporphyrins and the axial ligands.
3.6 X-ray structures of [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I)
Complex I crystallizes in the monoclinic space group with the centrosymmetric P21/c space group. The number of formula units by cell is Z = 4, and the asymmetric unit is made by one [Zn(TMPP)(4,4′-bpy)] molecule and one chloroform solvent molecule leading to the formula [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I). An Ortep view of I is depicted in Fig. 3 while a selection of distances and angles of complex I is given in Table S3.† | ||
| Fig. 3 ORTEP drawing of [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) with thermal ellipsoids drawn at 40% probability. | ||
The hydrogen atoms are removed for clarity.
As mentioned in the introduction, 4,4′-bpy zinc(II) metalloporphyrins exhibit four types of solid-state molecular structures which are: monomers, dimers, trimmers and polymers. The majority of these 4,4′-bpy zinc(II) porphyrin complexes are dimers type [{Zn(Porph)}2(μ2-4,4′-bpy)], e.g. [{Zn(TPP)}2(μ2-4,4′-bpy)],17 only one trimer complex is reported which is [{Zn(TPP)}3(μ2-4,4′-bpy)]15 and one polymer type is known which is {[Zn(TMP)(4,4′-bpy)]}n (TMP = meso-tetrakis(2,4,6-trimethylphenyl)porphyrin).32 For monomers type [Zn(Porph)(4,4′-bpy)], besides our [Zn(TMPP)(4,4′-byp)] (I) species, only one example is reported in the literature which is [Zn(TEBOP)(4,4′-bpy)] (TEBOP = meso-(tetraethyl-4(4-butyryl)oxyphenyl)porphyrin).16 It is clear from these examples that Zn(II)-(4,4′-bpy)-Porph complexes can adopt either monomer, dimer, trimer or polymer structure types. The prediction of the types of these Zn(II)-(4,4′-bpy) metalloporphyrins based on the nature of the substituents on the para-positions of a meso-arylporphyrin or on the β-pyrrolic positions of a porphyrin is not possible. Kinetic and theoretical investigations are needed to understand this phenomenon.
The average equatorial distance between the zinc(II) central ion and the nitrogen atoms of the porphyrin ring (Zn__Np) of complex I is 2.0682(17) Å which is very close to those of the related hexacoordinated and pentacoordinated 4,4′-bipyrine zinc(II) metalloporphyrins (Table S3†). The Zn__N(4,4′-bpy) distance value of 2.1441(17) Å for complex I is very close to that of the related zinc(II) porphyrin complexes reported in Table 5. The reported dihedral angle φ (Figure S11†) values between the two pyridyl groups of the 4,4′-bpy axial ligand range between 0 and 38°, while for complex I, the φ angle is 41.78(4)° which is slightly higher. Nevertheless, for the magnesium(II) 4,4′-bipyrine complex {[Mg(TPBP)(4,4′-bpy)2]}n the φ value is quite higher than that of complex I with a value of 59.60°.42
| Complex | M__Npa | M__Nb | M__PCc | φ d(°) | Ref. |
|---|---|---|---|---|---|
| a M__Np = average equatorial M–N pyrrole bond length.b M__NL = distance between the metal atom and the nitrogen atom of axial ligand.c M__PC = distance between Mg and the mean plane made by the 24-atom core of the porphyrin (PC).d φ = the diedral angle between the two pyridyl groups of the 4,4′-bpy.e TEBOP = meso-(tetraethyl-4(4-butyryl)oxyphenyl)porphyrin.f TPBP = meso-tetrakis(4-tert-butylphenyl)porphyrinate.g TPP = meso-tetraphenylporphyrinate.i T(OH)PP = meso-tetrakis(4-hydroxyphenyl)porphyrinate.j TMP = meso-tetramesitylporphyrinate.k TpivPP = α,α,α,α-tetrakis(o-pivalamidophenyl)porphinate.l TCPP = 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrinato. | |||||
| (4,4′-Bipyridine) zinc(II) metalloporphyrins | |||||
| [Zn(TEBOP)(4,4(-bpy)]e | 2.0675(3) | 2.151(2) | 0.29 | 38) | 16 |
| [Zn(TMPP)(4,4′-bpy)]] (I) | 2.0682(17) | 2.1441(17) | 0.3083(4) | 41.78(4) | This work |
| [{Zn(TPBP)}2(μ2-4,4′-bpy)]f | 2.063(6) | 2.178(6) | 0.329(2) | 0 | 12 |
| [{Zn(TPP)}2(μ2-4,4′-bpy)]g | 2.081 | 2.169 | 0.333 | 37.84 | 17 |
| [{Zn(T(OH)PP}2(μ2-4,4′-bpy)]i | 2.047/2.041 | 2.134/2.144 | 0.306/0.308 | 0.0 | 38 |
| [{Zn(TPP)}3(μ2-4,4′-bpy)]g | 2.036/2.054 | 2.185/2.490 | 0.319/0.003 | 23.61/23.65 | 15 |
| 2.050 | 2.185 | 0.317 | |||
| {[Zn(TMP)(4,4′-bpy)]}nj | 2.059 | 2.371 | 0.0 | 0.0 | 35 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| (4,4′-Bipyridine) metalloporphyrins | |||||
| [CoIII(TpivPP)Cl(4,4′-bpy)]k | 1.983 | 2.028 | — | 29.78 | 39 |
| {[CoII(TPP)(4,4′-bpy)]}ng | 1.993 | 2.342 | — | 37.72 | 40 |
| {[Fe(TPP)(4,4′-bpy)]}ng | 1.990 | 1.985 | — | 29.44 | 41 |
| {[Mg(TPBP)(4,4′-bpy)2]}nf | 2.065 | 2.319/2.290 | — | 59.60 | 42 |
| [Ni(TCPP)(4,4′-bpy)2]l | 2.050 | 2.197 | — | 24.67 | 43 |
Fig. S12† illustrates the crystal packing of complex I along the b direction which is stabilized by intermolecular interactions types C__H⋯Cl, C__H⋯O, C__H⋯N and C__H⋯Cg (Cg are the centroids of a pyrrole ring, a phenyl ring or a pyridyl ring) including the [Zn(TMPP)(4,4′-bpy)] complexes and the chloroform solvent molecules (Fig. 4 and Table S4†).
3.7 Hirshfeld surface analysis
The Hirshfeld Surface (HS) and 2D fingerprint plots of complex I was investigated using The Crystal Explorer 17.5 program.44 Eqn (1) gives the normalized contact distance dnorm as a function of the nearest atom outside (de), the inside (di) the Hirshfeld surface and the van der Waals radii (rvdW):
 | (1) |
The shorter contacts than the sum of the van der Waals radii are shown in red in the Hirshfeld surface, while the longer and closer to the van der Waals contacts are indicated in blue and white colors (Fig. 5).
 | ||
| Fig. 5 Representation of the Hirshfeld surface for complex I plotted over dnorm in the range −0.1949 to 1.5547 a.u. | ||
We notice that by using the Crystal Explorer 17.5 program or the PLATON program (see the X-ray molecular structure section) we got practically the same types of the intermolecular interactions. In Fig. S13† are depicted the two-dimensional fingerprint plots for complex I showing that the major types of intermolecular contacts responsible of the stability of the crystal lattice of I are: H⋯H (45.8%), H⋯C (25.5%), H⋯Cl (8.7%) H⋯O (7.2%) and H⋯N (6.4%).
The sharp index mapped on HS of complex I (Fig. S14-a†) show the absence blue and red triangles indicating that there are no π⋯π stacking interactions in the crystal lattice. The absence of flat surfaces patches in the curvedness plot (Fig. S14-b†) is an indication of the absence of planar stacking.
The absence of planar stacking is confirmed by the fact that there are no flat surfaces patches in the curvedness plot (Fig. S14-b†).
3.8 Photocatalytic degradation of methylene blue
We investigated the photocatalytic degradation of the MB dye using our Zn(II)-TMPP-4,4′-bpy species (I) as photocatalyst at room temperature, pH = 6 and a pure blue led lamp irradiation (emitting monochromatic light, λ = 450 nm). The masse of complex I used is 5 mg (0.0052 mmol), the initial dye concentration is 20 mg L−1. As shown by Fig. 6, the MB concentration started decreasing after 30 min of reaction indicating that a small quantity of the dye is adsorbed by complex I. | ||
| Fig. 6 Change in absorbance intensity of the MB dye at λmax (664 nm) under visible light in the presence of compound I (5 mg). The MB concentration was 20 mg l−1 with a pH of 6. | ||
The UV-Vis spectroscopy was used to monitored the photodegradation of the MB dye using complex I as photocatalyst and especially the variation of the absorption at λmax equal to 664 nm (corresponding to the highest absorption band of the MB organic species) in function of time. After 180 min of reaction, the decomposition efficiency for the MB dye reaches 63% (Fig. 7).
 | ||
| Fig. 7 Variation of the photodegradation yield (%R) of the MB dye under visible light in the presence of compound I as function of time. | ||
The degradation yield (R%) is calculated using the eqn (2) provided below.
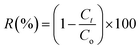 | (2) |
The kinetic investigation shows that degradation reaction of the MB dye reaction fellows the pseudo-second order kinetic model and the k was calculated using the following Langmuir–Hinshelwood eqn (3):
 | (3) |
The calculated rate constant k value is 0.00546 min−1 with R2 = 0.9760 (Fig. 8).
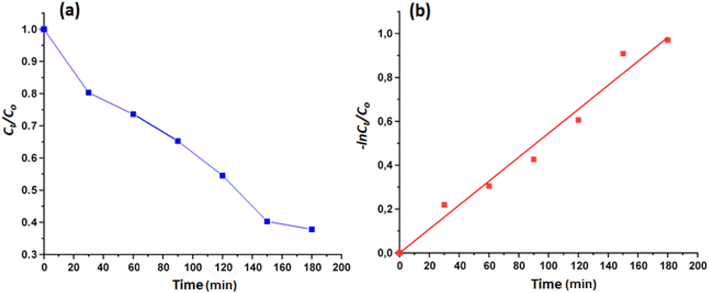 | ||
| Fig. 8 (a): Evolution of Ct/Co as a function of time, (b): variation of −ln(Ct/Co) as function of time. | ||
The reusability of [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) as a photocatalyst for the degradation of methylene blue (MB) dye was assessed. After each catalytic cycle, complex I was recovered via filtration, thoroughly rinsed with distilled water, and dried in an oven at 60 °C. The photocatalyst demonstrated consistent performance over three successive cycles under identical conditions (Fig. S15†).
The mechanism of heterogeneous photocatalysis, illustrated in Fig. S16,† involves the activation of complex I through the absorption of light energy with a wavelength equal to or greater than the material's band gap energy (hν ≥ Eg). Upon activation, an electron–hole pair (e−/h+) is generated via the excitation of an electron from the valence band to the conduction band. The excited electron reacts with oxygen molecules adsorbed on the surface of the porphyrinic complex, while the hole (h+) interacts with surface hydroxyl ions (OH−), producing highly reactive hydroxyl radicals (OH˙). These radicals are primarily responsible for degrading the MB dye. The generation of (OH˙) as reactive oxygen species (ROS) is also facilitated by the efficient electron transfer in the Zn–porphyrin complex, which enhances their photocatalytic activity. The reaction between hydroxyl radicals and the MB dye results in the formation of carbon dioxide (CO2) and water (H2O), the main mineralization products.
3.9 Catalytic oxidative degradation of MB dye
The Langmuir–Hinshelwood is given by the following eqn (4):
 | (4) |
The experimental data were analyzed using this formula at various reaction temperatures. Fig. 10 depicts the plots of Ct/Co and −ln(Ct/Co) as functions of time, both demonstrating high correlation coefficients. These findings indicate that the degradation reaction adheres to a pseudo-first-order kinetic model. The apparent rate k constants were derived from the slopes of the linear regression curves at 298 K, 308 K, and 318 K. The calculated k values for the degradation of MB dye were 0.00449 min−1 (R2 = 0.9832), 0.00458 min−1 (R2 = 0.9667) and 0.00493 min−1 (R2 = 0.9639) at 298, 308 and 318 K, respectively.
| Degradation system | Degradation method | Degradation yield, time reaction | Ref. |
|---|---|---|---|
| System used: MnTCPPOAc@ MWCNT | Catalytic oxidation by H2O2 | 98% (720 min) | 45 |
| System used: FeTCPPCl@MWCNT | Catalytic oxidation by H2O2 | 30% (720 min) | 45 |
| System used: [CoII(TMAPP)] | Catalytic oxidation by H2O2 | 44% (240 min) | 46 |
| System used: BiFeO3 | Photodegrdation | 70.8% (360 min) | 47 |
| System used: BiFeO3/GdFeO3 | Photodegrdation | 52% (360 min) | 48 |
| System used: [NiII(TAMPP)] | Catalytic oxidation by H2O2 | 73% (90 min) | 49 |
| System used: [ZnII(TMPP)(4,4′-bpy)] (I) (H2O2 solution) | Photodegradation | 63% (180 min) | t.w. |
| System used: [ZnII(TMPP)(4,4′-bpy)]·CHCl3 (I) | Catalytic oxidation by H2O2 | 53.84% (180 min) | t.w. |
The catalytic degradation of MB dye, using [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I) along with an aqueous hydrogen peroxide solution, yields approximately 54%. While this yield is deemed acceptable in comparison to other H2O2 oxidation methods for this dye as listed in Table 6, it is understandably lower than the yields achieved by photocatalytic systems, as expected.
 | (5) |
 | (6) |
| ΔG* = ΔH − TΔS* | (7) |
| Temperature (K) | 298 | 308 | 318 |
|---|---|---|---|
| k (min−1) | 000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 449 449 |
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 458 458 |
000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 493 493 |
| Ea (J mol−1) | 368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274 274 |
368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274 274 |
368![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 274 274 |
| ΔS* (J mol−1 K−1) | −28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 608 |
−28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 608 |
−28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 608 608 |
| ΔH* (J mol−1) | 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 383 383 |
112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 383 383 |
112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 383 383 |
| ΔG* (J mol−1) | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 637 637![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 567 567 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 923 923![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 647 647 |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 209 209![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 727 727 |
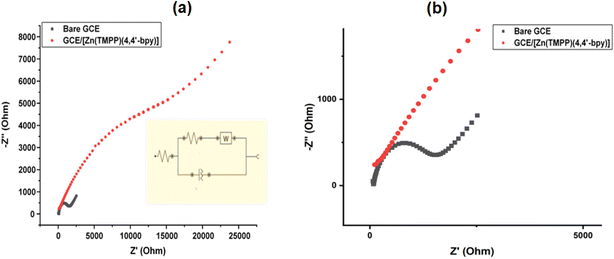
3.10 Electrochemical sensor application of [Zn(TMPP)(4,4′-bpy)]·CHCl3 (I)
| Electrodes | RS (Ω) | CPE (μF) | Rtc (kΩ) | W(μF) |
|---|---|---|---|---|
| Bare GCE | 81.841 | 13.15 | 5.04 | 325 |
| GCE/[Zn(TMPP)(4,4′-bpy)]·CHCl3 | −150.8 | 12.76 | 28.7 | 222 |
- Effect of drop volume (μL): The relationship between current response and drop volume was investigated by applying varying amounts of [Zn(TMPP)(4,4′-bpy)]·CHCl3 film suspension (ranging from 5 to 20 μL) onto the GCE. As shown in Fig. S20,† the current response to dopamine (DA) increased with volumes from 5 μL to 7 μL, but further increases in volume led to a suppression of the current. Therefore, 7 μL was selected as the optimal drop volume. This behavior can likely be attributed to the thickening of the composite layer on the GCE surface, which diminishes the electrical conductivity of the modified film.
- Effect of drying time: drying time is a crucial factor that influences both the detection limit and sensitivity of the sensor membrane. To optimize this parameter, the effect of drying time was assessed using a fixed dopamine concentration (10−3 M) over a range from 30 minutes to 24 hours, as shown in Fig. S21.† A significant increase in current was observed as the drying time was extended from 30 minutes to 2 hours. However, beyond 2 hours, the current increase slowed, likely due to rapid surface saturation. As a result, 2 hours was selected as the optimal drying time.
- Effect of pH: the effect of pH on the sensor's electrochemical response was examined in the pH range from 4 to 8 using CV and SWV techniques. As shown in Fig. 14, the anodic peak potentials for dopamine (DA) shift negatively with increasing pH. The anodic peak currents increase as the pH rises up to 7, after which a slight decrease is observed. Therefore, a 0.1 M phosphate buffer solution (PBS) with a pH of 7 was selected for further studies. Additionally, the relationship between pH and anodic peak potential was analyzed. Fig. S22† illustrates a clear linear correlation between the anodic peak potentials (Ea) of DA and the pH values within the range of 5 to 8. The linear regression equation for DA is expressed as Ea (DA) = −0.127 pH + 0.531.
- Effect of incubation time: The interaction between the matrix and dopamine (DA) is influenced by the incubation time, which can affect the electrochemical performance. To optimize this, the accumulation time was varied between 5 and 20 minutes. As shown in Fig. S23,† the optimal accumulation time was found to be around 5 minutes. The optimal experimental conditions were determined to be a drop volume of 7 μL, a drying time of 2 hours, a pH of 7, and a DA incubation time of 5 minutes.
| Electrode | Method | Detection limit (M) | Linear range (M) | Reference |
|---|---|---|---|---|
| a MIP = molecularly Imprinted Polymer.b PPY-CTS = polypyrrole-chitosan composites.c EG = electrodeposited graphene oxide.d GCE = glassy carbon electrode.e Au(NPS) = gold nanoparticles.f rGO = reduced graphene oxide.g GR = graphene. | ||||
| MIP-GCE/TiO2/(PPY-CTS)a,b | DPV | 2.81 × 10−7 M | 10−6–10−5 M | 57 |
| GCE/EG-Ni-Au(NPs)c,d,e | SWV | 10−7 M | 2 × 10−7–10−4 M | 50 |
| Aggregation of AuNPs induced by copper ions | Colorimetric | 2 × 10−7 M | 5 × 10−7–10−6 M | 58 |
| GCE/rGO/AuNPsf | DPV | 2 × 10−5 M | 10−6–6 × 10−5 M | 59 |
| NiO–CuO/GR/GCEg | SWV | 1.67 × 10−7 M | 5 × 10−7–2 × 10−5 M | 62 |
| Complex I | SWV | 5 × 10−8 M | 5 × 10−8–10−4 M | t. w. |
 | (8) |
| Sample | Added dopamine (M) | Found dopamine (M) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Urine | 10−6 M | 0.98 × 10−6 M | 98 | 0.970 |
| 5 × 10−6 M | 5.03 × 10−6 M | 100.6 | 1.179 | |
| 10−5 M | 9.75 × 10−6 M | 97.5 | 1.032 |
The significant detection and recovery results show that our modified GCE is promising for the determination of DA in urine human urine samples.
4 Conclusion
The [Zn(TMPP)(4,4′-bpy)]·CHCl3 coordination compound (complex I), where TMPP is meso-tetra(para-methoxyphenyl)porphyrinate and 4,4′-bpy is 4,4′-bipyridine, was characterized using UV-Vis spectroscopy, fluorescence, IR, 1H NMR, cyclic voltammetry, and ESI-HRMS techniques. X-ray crystallographic analysis revealed that complex I contains a single 4,4′-bpy axial ligand coordinated to the Zn(II) ion. Additionally, the molecular packing is stabilized by various intermolecular interactions, including C–H⋯Cl, C–H⋯O, C–H⋯N, and C–H⋯Cg (where Cg represents the centroid of a pyrrole, phenyl, or pyridyl ring), involving both the [Zn(TMPP)(4,4′-bpy)] complexes and chloroform solvent molecules. Photocatalytic degradation and H2O2 oxidative degradation of MB dye using complex I as a catalyst, under optimized conditions, achieved degradation yields of 63% and 53%, respectively. Complex I was further evaluated as an electrochemical sensor for dopamine (DA) detection via square wave voltammetry (SWV). The GCE/[Zn(TMPP)(4,4′-bpy)]·CHCl3 electrode demonstrated excellent sensitivity (1.072 μA mol−1 L) and a low detection limit (5 × 10−8 M). Application of this sensor to human urine samples for dopamine analysis highlighted its potential as a reliable electrochemical tool for quantifying dopamine in biological systems.Ethical statement
Human urine samples were collected from voluntary donor after obtaining informed consent, following ethical guidelines.Data availability
Data are contained within the article.Author contributions
Conceptualization, M. A. B., C. M., E. A. L.-M and B. G.; methodology, M. A. B., B. G. and H. N.; validation, M. A. B, C. M., B. G. and H. N.; formal analysis, M. A. B., C. M. and E. A. L.-M; investigation, M. A. B., C. M., F. L., H. B. and H. N.; data curation, M. A. B., T. R. and F. M.; resources, H. N.; writing—original draft preparation, M. A. B, C. M. and H. N.; writing—review and editing, M. A. B., E. A. L.-M and H. N.; investigation, visualitation, A. S-. M. and J. C.-B.; visualization, E. A. L.-M, M. A. B. and H. N.; supervision, H. N. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This research was funded by the Ministry of Higher Education and Scientific Research of Tunisia.References
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, The Cambridge Structural Database, Acta Crystallogr., 2016, B72, 171–179, DOI:10.1107/S2052520616003954.
- J. W. Buchler, in The Porphyrins, ed. D. Dolphin, Academic Press, New-York, 1978, pp. 390–474 Search PubMed.
- J. Karolczak, D. Kowalska, A. Lukaszewicz, A. Maciejewski and R. P. Steer, Photophysical Studies of Porphyrins and Metalloporphyrins: Accurate Measurements of Fluorescence Spectra and Fluorescence Quantum Yields for Soret Band Excitation of Zinc Tetraphenylporphyrin, J. Phys. Chem. A, 2004, 108, 4570–4575, DOI:10.1021/jp049898v.
- G. Deng, H. Chen, Q. Shi, L. Ren, K. Liang, W. Long, W. Lan, X. Han, Y. She and H. Fu, Colorimetric assay based on peroxidase like activity of dodecyl trimethylammonium bromide tetramethyl zinc (4 pyridinyl) porphyrin for detection of organophosphorus pesticides, Microchim. Acta, 2022, 189, 375, DOI:10.1007/s00604-022-05430-2.
- D. C. da Silva Martins, I. T. Resende and B. J. R. da Silva, Degradation features of pesticides: a review on (metallo)porphyrin mediated catalytic processes, Environ. Sci. Pollut. Res., 2022, 29, 42384–42403, DOI:10.1007/s11356-022-19737-3.
- R. Georgescu State and J. F. van Staden, Review. Electrochemical sensors used in the determination of L-Dopa, Electrochem. Sci. Adv., 2022, 2, e2100040, DOI:10.1002/elsa.202100040.
- D. K. Jangid, S. G. Dastider, S. Mandal, P. Kumar, P. Kumari, K. K. Haldar, K. Mondal and R. S. Dhayal, Ferrocenyl Dithiophosphonate Ag(I) Complexes: Synthesis, Structures, Luminescence, and Electrocatalytic Water Splitting Tuned by Nuclearity and Ligands, Chem. - Eur. J., 2024, 30, e202402900, DOI:10.1002/chem.202402900.
- W. Zhang, W. Lai and R. Cao, Energy-Related Small Molecule Activation Reactions: Oxygen Reduction and Hydrogen and Oxygen Evolution Reactions Catalyzed by Porphyrin- and Corrole-Based Systems, Chem. Rev., 2017, 117(4), 3717–3797, DOI:10.1021/acs.chemrev.6b00299.
- Z. Liu, W. He and Z. Guo, Metal coordination in photoluminescent sensing, Chem. Soc. Rev., 2013, 42, 1568–1600, 10.1039/C2CS35363F.
- E. M. Nolan and S. J. Lippard, Small-Molecule Fluorescent Sensors for Investigating Zinc Metalloneurochemistry, Acc. Chem. Res., 2009, 42(1), 193–203, DOI:10.1021/ar8001409.
- Y. Yamanoi, Recent Progress on the Synthesis of Bipyridine Derivatives, Molecules, 2024, 29, 576, DOI:10.3390/molecules29030576.
- S. Nasri, I. Zahou, I. Turowska-Tyrk, T. Roisnel, F. Loiseau, E. Saint-Amant and H. Nasri, Synthesis, Electronic Spectroscopy, Cyclic Voltammetry, Photophysics, Electrical Properties and X-ray Molecular Structures of meso-{Tetrakis[4-(benzoyloxy)phenyl]porphyrinato} zinc(II) Complexes with Aza Ligands, Eur. J. Inorg. Chem., 2016, 5004–5019, DOI:10.1002/ejic.201600575.
- R. Soury, M. Jabli, T. A. Saleh, W. S. Abdul-Hassan, E. Saint-Aman, F. Loiseau, C. Philouze, A. Bujacz and H. Nasri, Synthesis of the (4,4′-bipyridine)(5,10,15,20-tetratolylphenylporphyrinato)zinc(II) bis(4,4-bipyridine) disolvate dehydrate and evaluation of its interaction with organic dyes, J. Mol. Liq., 2018, 264, 134–142, DOI:10.1016/j.molliq.2018.05.050.
- J. Brahmi, S. Nasri, H. Saidi, K. Aouadi, R. Sanderson, M. Winter, D. Cruickshank, S. Najmudini and H. Nasri, Optical and photoelectronic properties of a new material: Optoelectronic application, C. R. Chim., 2020, 23, 403–414, DOI:10.5802/crchim.20.
- A. D. Shukla, P. C. Dave, E. Suresh, A. Das and P. Dastidar, Multicomponent Zn-tetraphenylporphyrins: syntheses, characterization and their self assembly in the solid state, J. Chem. Soc., Dalton Trans., 2000, 4459–4463, 10.1039/B004211K.
- R. Soury, M. Jabli, T. A. Saleh, W. S. Abdul-Hassan, E. Saint-Aman, F. Loiseau, C. Philouze and H. Nasri, Tetrakis(ethyl-4(4-butyryl)oxyphenyl)porphyrinato zinc complexes with 4,4′-bpyridin: synthesis, characterization, and its catalytic degradation of Calmagite, RSC Adv., 2018, 8, 20143, 10.1039/C8RA01134F.
- Q. Zeng, J. Lu, S. Xu, D. Wu, C. Liu, Y. Li, C. Wang and C. Bai, Solid-State Supramolecular Chemistry of Zn-Tetraphenylporphyrins with 4,4'-Dipyridyl N,N'-Dioxide and Hexamethylenetetramine, Bentham Science Publishers, in Letters in Organic Chemistry, Bentham Science Publishers, 2005, vol. 2, DOI:10.2174/1570178054406011.
- R. Soury, J.-C. Daran, R. Rein, N. Solladie and H. Nasri, CSD Communication (Private Communication), 2015, CCDC 1060290 Search PubMed.
- H. Aliyan, R. Fazaeli and R. Jalilian, Fe3O4@mesoporous SBA-15: a magnetically recoverable catalyst for photodegradation of malachite green, Appl. Surf. Sci., 2013, 276, 147–153, DOI:10.1016/j.apsusc.2013.03.049.
- Y. Li, L. Wang, Y. Gao, W. Yang, Y. Li and C. Guo, Porous metalloporphyrinic nanospheres constructed from metal 5,10,15,20- tetraksi(4-ethynylphenyl)porphyrin for efficient catalytic degradation of organic dyes, RSC Adv., 2018, 8, 7330–7339, 10.1039/C7RA12701D.
- K. S. Min, R. S. Kumar, J. H. Lee, K. S. Kim, S. G. Lee and Y.-A. Son, Synthesis of new TiO2/porphyrin-based composites and photocatalytic studies on methylene blue degradation, Dyes Pigm., 2018, 160, 37–47, DOI:10.1016/j.dyepig.2018.07.045.
- R. Soury, M. Jabli, T. A. Salehd, A. Kechich, F. Loiseau, E. Saint-Aman and H. Nasri, Degradation of Calmagite by dichloride (5,10,15,20-tetraphenylporphyrinato)antimony hexachloridoantimonate:[Sb(TPP)Cl2] SbCl6, Inorg. Chem. Commun., 2019, 104, 54–60, DOI:10.1016/j.inoche.2019.03.033.
- M. Guergueb, S. Nasri, J. Brahmi, F. Loiseau, F. Molton, T. Roisnel, V. Guerineau, I. Turowska-Tyrk, K. Aouadi and H. Nasri, Effect of the coordination of π-acceptor 4-cyanopyridine ligand on the structural and electronic properties of meso-tetra(para-methoxy) and meso-tetra(para-chlorophenyl) porphyrin cobalt(II) coordination compounds. Application in the catalytic degradation of methylene blue dye, RSC Adv, RSC Adv., 2020, 10, 6900, 10.1039/c9ra08504a.
- M. Guergueb, F. Loiseau, F. Molton, H. Nasri and A. Klein, CO2 to CO Electroreduction, Electrocatalytic H2 Evolution, and Catalytic Degradation of Organic Dyes Using a Co(II) meso-Tetraarylporphyrin, Molecules, 2022, 27, 1705, DOI:10.3390/molecules27051705.
- T. Fradi, O. Noureddine, A. Kechiche, M. Guergueb, F. Molton, F. Loiseau, T. Roisnel, I. Turowska-Tyrk and H. Nasri, Bis(κ N-DABCO) magnesium(II) meso-arylporphyrin: Characterization, DFT calculations, catalytic degradation of rhodamine B dye and inhibiting activity of the COVID-19 virus and oxidase enzymes using molecular docking study, Polyhedron, 2024, 255, 116980, DOI:10.1016/j.poly.2024.116980.
- A. D. Adler, F. R. Longo, J. D. Finarelli, J. Goldmacher, J. Assour and L. Korsakoff, A Simplified Synthesis for meso-Tetraphenylporphin, J. Org. Chem., 1967, 32, 476, DOI:10.1021/jo01288a053.
- Bruker, SAINT, APEX2 and SADABS, Bruker AXS Inc., Madison, Wisconsin, USA, 2004 Search PubMed.
- M. C. Burla, R. Caliandro, B. Carrozzini, G. L. Cascarano, C. Cuocci, C. Giacovazzo, M. Mallamo, A. Mazzone and G. Polidori, Crystal structure determination and refinement via SIR2014, J. Appl. Crystallogr., 2015, 48, 306–309, DOI:10.1107/S1600576715001132.
- G. M. Sheldrick, SHELXT – Integrated space-group and crystal-structure determination, Acta Crystallogr., 2015, C71, 3–8, DOI:10.1107/S2053273314026370.
- A. L. Spek, Structure validation in chemical crystallography, Acta Crystallogr., 2009, D65, 148–155, DOI:10.1107/S090744490804362X.
- C. F. Macrae, I. J. Bruno, J. A. Chisholm, P. R. Edgington, P. McCabe, E. Pidcock, L. Rodriguez-Monge, R. Taylor, J. van de Streek and P. A. Wood, Mercury CSD 2.0 - new features for the visualization and investigation of crystal structures, J. Appl. Crystallogr., 2008, 41, 466–470, DOI:10.1107/S0021889807067908.
- M. A. Bouicha, S. Hrichi, R. Chaabane-Banaoues, H. Ghalla, M. Guergueb, H. Babba, T. Roisnel and H. Nasri, Spectroscopic, X-ray structure, radical scavenging and in vitro antifungal activities and catalytic degradation of methyl orange dye investigation of the Meso-tetrakis(p-tolyl) diprotonated porphyrin, J. Mol. Struct., 2024, 1304, 137650, DOI:10.1016/j.molstruc.2024.137650.
- M. Guergueb, J. Brahmi, S. Nasri, F. Loiseau, K. Aouadi, V. Guerineau and H. Nasri, Zinc(II) triazole meso-arylsubstituted porphyrins for UV-visible chloride and bromide detection. Adsorption and catalytic degradation of malachite green dye, RSC Adv., 2020, 10, 22712, 10.1039/D0RA03070H.
- C.-I. Lin, M.-Y. Fang and S.-H. Cheng, Substituent and axial ligand effects on the electrochemistry of zinc porphyrins, J. Electroanal. Chem., 2002, 531, 155, DOI:10.1016/S0022-0728(02)01056-2.
- K. Colladet, M. Nicolas, L. Goris, L. Lutsen and D. Vanderzande, Low-band gap polymers for photovoltaic applications, Thin Solid Films, 2004, 451, 7–11, DOI:10.1016/j.tsf.2003.10.085.
- J. W. Owens, R. Smith, R. Robinson and M. Robins, Photophysical properties of porphyrins, phthalocyanines, and benzochlorins, Inorg. Chim. Acta, 1998, 279, 226–231, DOI:10.1016/S0020-1693(98)00137-6.
- Z. Denden, K. Ezzayani, E. Saint-Aman, F. Loiseau, S. Najmudin, C. Bonifácio, J.-C. Daran and H. Nasri, Insights on the UV-Vis, Fluorescence, and Cyclic Voltammetry Properties and the Molecular Structures of ZnII Tetraphenylporphyrin Complexes with Pseudohalide Axial Azido, Cyanato-N, Thiocyanato-N, and Cyanido Ligands, Eur. J. Inorg. Chem., 2015, 2596, DOI:10.1002/ejic.201403214.
- Y. Diskin-Posner, G. K. Patra and I. Goldberg, Crystal engineering of metalloporphyrin assemblies. New supramolecular architectures mediated by bipyridylligands, Chem. Commun., 2002, 1420 10.1039/B202791G.
- A. Mansour, M. Zaied, I. Ali, S. Soliman and M. Othmani, Synthesis, molecular structure, spectroscopic characterization and antibacterial activity of the Co(III) (chlorido)(pyridine) and (chlorido)(4,4′-bipyridine) “picket fence” porphyrin complexes, Polyhedron, 2017, 2017, 496, DOI:10.1016/j.poly.2016.10.031.
- A. Mansour, Y. Belghith, M. S. Belkhiria, A. Bujacz, V. Guerineau and H. Nasri, Synthesis, crystal structures and spectroscopic characterization of Co(II) bis(4,4′-bipyridine) with meso-porphyrins α,β,α,β-tetrakis(o-pivalamidophenyl) porphyrin (α,β,α,β-TpivPP) and tetraphenylporphyrin (TPP), J. Porphyrins Phthalocyanines, 2013, 17, 1094, DOI:10.1142/S1088424613500843.
- A. Fidalgo-Marijuan, G. Barandika, B. Bazan, M. K. Urtiaga, L. Lezama and M. I. Arriortua, Fe–TPP Coordination Network with Metalloporphyrinic Neutral Radicals and Face-to-Face and Edge-to-Face π–π Stacking, Inorg. Chem., 2013, 2013, 8074, DOI:10.1021/ic4007372.
- N. Amiri, M. Hajji, T. Roisnel, G. Simonneaux and H. Nasri, Synthesis, molecular structure, photophysical properties and spectroscopic characterization of new 1D-magnesium(II) porphyrin-based coordination polymer, Res. Chem. Intermed., 2018, 44, 5583, DOI:10.1007/s11164-018-3442-9.
- M. Morshedi, J. S. Ward, P. E. Kruger and N. G. White, Supramolecular frameworks based on 5,10,15,20-tetra(4-carboxyphenyl)porphyrins, Dalton Trans., 2018, 47, 783, 10.1039/C7DT04162D.
- M. Turner, J. McKinnon, S. Wolff, S. D. Grimwood, P. Spackman, D. Jayatilaka and M. Spackman, CrystalExplorer (Version 17.5), University of Western Australia, Crawley, Australia, 2017 Search PubMed.
- S. Rayati and Z. Sheybanifard, Catalytic activity of Mn(III) and Fe(III) porphyrins supported onto multi-walled carbon nanotubes in the green oxidation of organic dyes with hydrogen peroxide: a comparative study, J. Iran. Chem. Soc., 2016, 13, 541, DOI:10.1007/s13738-015-0763-0.
- N. Amiri, M. Guergueb, M. S. Al-Fakeh, M. Bourguiba and H. Nasri, A new cobalt(II) meso-porphyrin: synthesis, characterization, electric properties and application in the catalytic degradation of dyes, RSC Adv., 2020, 44920–44932, 10.1039/D0RA08786F.
- S. Mohan, B. Subramanian, I. Bhaumik, P. K. Gupta and S. N. Jaisankar, Nanostructured Bi(1−x)Gd(x)FeO3 – a multiferroic photocatalyst on its sunlight driven photocatalytic activity, RSC Adv., 2014, 4, 16871–16878, 10.1039/C4RA00137K.
- Y. Subramanian, V. Ramasamy, R. Karthikeyan, G. R. Srinivasan, D. Arulmozhi, R. K. Gubendiran and M. Sriramalu, Investigations on the enhanced dye degradation activity of heterogeneous BiFeO3–GdFeO3 nanocomposite photocatalyst, Heliyon, 2019, 5, 01831, DOI:10.1016/j.heliyon.2019.e01831.
- M. A. Bouicha, N. Moulahi, M. Guergueb, R. Ben Chaabane and H. Nasri, A new Ni(II) metalloporphyrin: characterization, theoretical sensing calculations and catalytic degradation of methylene blue and methyl orange dyes, J. Iran. Chem. Soc., 2024, 10, 1007, DOI:10.1007/s13738-024-03022-w.
- C. B. A. Hassine, H. Kahri and H. Barhoumi, Enhancing Dopamine Detection Using Glassy Carbon Electrode Modified with Graphene Oxide, Nickel and Gold Nanoparticles, J. Electrochem. Soc., 2020, 167, 027516, DOI:10.1149/1945-7111/ab6971.
- X. Liu and J. Liu, Biosensors and sensors for dopamine detection, View, 2021, 2, 20200102, DOI:10.1002/VIW.20200102.
- S. Lakard, I. A. Pavel and B. Lakard, Electrochemical Biosensing of Dopamine Neurotransmitter: A Review, Biosensors, 2021, 11, 179, DOI:10.3390/bios11060179.
- J. Ma, W. Bai, X. Liu and J. Zheng, Electrochemical dopamine sensor based on bi metallic Co/Zn porphyrin metal–organic framework, Microchim. Acta, 2022, 189, 20, DOI:10.1007/s00604-021-05122-3.
- O. I. Torres-Soto, A. Vega-Rios, R. B. Dominguez and V. Osuna, Electrochemical Detection of Dopamine with Graphene Oxide Carbon Dots Modified Electrodes, Chemosensors, 2019, 13(1), 7, DOI:10.3390/chemosensors13010007.
- A. Dhaffouli, P. A. Salazar-Carballo, S. Carinelli, M. Holzinger, B. V. M. Rodrigues and H. Barhoumi, Electrochemical Detection of Dopamine with a Non-Enzymatic Sensor Based on Au@SiO2-APTES Composite, Chemosensors, 2021, 13(3), 87, DOI:10.3390/chemosensors13030087.
- S. Slimi, C. Mabrouk, H. Barhoumi and N. Jaffrezic-Renault, A Simple Over-Oxidized Molecularly Imprinted Polypyrrole for the Sensitive Detection of Dopamine in Human Serum, J. Sens. Sci. Technol., 2022, 12, 33–44 CrossRef CAS . https://www.scirp.org/journal/jst.
- C. Mabrouk, H. Barhoumi and N. Jaffrezic-Renault, Electrochemical Dopamine-Imprinted Sensor Based on TiO2 Nanoparticles and Polypyrrole-Chitosan Composites Modified Glassy Carbon Electrode, Port. Electrochim. Acta, 2024, 42, 455–474, DOI:10.4152/pea.2024420605.
- J. M. Liu, X. X. Wang, M. L. Cui, L. P. Lin, S. L. Jiang SL, L. Jiao L and L. H. Zhang, A promising non-aggregation colorimetric sensor of AuNRs–Ag+ for determination of dopamine, Sens. Actuators, B, 2013, 176, 97, DOI:10.1016/j.snb.2012.08.083.
- H. Teymourian, A. Salimi and S. Khezrian, Fe3O4 magnetic nanoparticles/reduced graphene oxide nanosheets as a novel electrochemical and bioeletrochemical sensing platform, Biosens. Bioelectron., 2013, 49, 1, DOI:10.1016/j.bios.2013.04.034.
- B. D. Liu, X. Q. Ouyang, Y. P. Ding, L. Q. Luo, D. Xu and Y. Q. Ning, Electrochemical preparation of nickel and copper oxides-decorated graphene composite for simultaneous determination of dopamine, acetaminophen and tryptophan, Talanta, 2016, 146, 114–121, DOI:10.1016/j.talanta.2015.08.034.
- J. Wang, B. Yang, J. Zhong, B. Yan, K. Zhang, C. Zhai, Y. Shiraishi, Y. Dua and P. Yang, Dopamine and uric acid electrochemical sensor based on a glassy carbon electrode modified with cubic Pd and reduced graphene oxide nanocomposite, J. Colloid Interface Sci., 2017, 497, 172, DOI:10.1016/j.jcis.2017.03.011.
- X. Gong, L. Lin and K. Ye, et al., Tuning Charge Transport Properties in Porphyrin-Based Molecular Materials for Organic Electronics, J. Mater. Chem. A, 2018, 6(37), 18083–18092, 10.1039/C8TA04847A.
- M. E. R. Diniz, L. S. Vilhena, B. P. Paulo, T. C. C. Barbosa and E. C. Mateoa, Simultaneous Determination of Catecholamines and Metanephrines in Urine by Liquid Chromatography Electrospray Ionization Tandem Mass Spectrometry: Successful Clinical Application, J. Braz. Chem. Soc., 2015, 26, 1684–1691, DOI:10.5935/0103-5053.20150142.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2358252. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra00762c |
| This journal is © The Royal Society of Chemistry 2025 |