Open Access Article
Open Access ArticleCoumarin–naphthalene conjugate for rapid optical detection of OCl− and Y3+ in a cascade manner: combined experimental and theoretical studies†
T. Shyam,
N. Jahan and
D. Das *
*
Department of Chemistry, The University of Burdwan, Burdwan-713104, WB, India. E-mail: ddas100in@yahoo.com; Fax: +91-342-2530452; Tel: +91-342-2533913 (ext. 424)
First published on 23rd April 2025
Abstract
The coumarin–naphthalene conjugate (A3), an ESIPT-active probe, selectively recognized OCl− in a ratiometric manner in DMSO–water media. The recognition was associated with sky-blue emission (under UV light) as well as yellow emission (under visible light). The OCl− assisted inhibition of the ESIPT process via H-bonding resulted in an intense emission at 484 nm (λex = 365 nm). It allowed for the detection of OCl− as low as 18.42 nM with a strong association constant, K = 1.08 × 105 M−1, around physiological pH. Furthermore, the A3-OCl− adduct (Ad1) ratiometrically detected Y3+ via bright orange emission at 556 nm (λex = 440 nm) under both UV and visible light. Detection up to 98.51 nM was achieved with a binding constant, K = 1.38 × 105 M−1, at physiological pH. Density functional theory (DFT) and lifetime decay measurements substantiated the interactions. Real sample analysis were also achieved with the developed method.
Introduction
Nowadays, the detection and quantification of various environmental and biological contaminants is a primary concern.1,2 Different types of pollutants, such as cationic, anionic, and molecular species,3,4 exhibit different types of interactions. Nitrate, fluoride, phosphate, hypochlorite, bromate, arsenate, nitrite, chloride, sulfide, and cyanide are common anionic contaminants. Among them, hypochlorite (OCl−) is a contaminant frequently encountered due to its application in household bleaching,5,6 particularly for drinking water disinfection.7 This facilitates its easy entry into biological systems. Being one of the bio-relevant reactive oxygen species (ROS),8 OCl− plays an important role in several natural processes. For example, endogenous OCl− is generated from the myeloperoxidase (MPO) enzyme-catalyzed reaction between H2O2 and Cl−.9 It is a powerful in vivo oxidant with effective antibacterial activity during microbial invasion.10 Excess OCl− is detrimental to various biomolecules, including DNA, RNA, fatty acids, cholesterol, and proteins,11 and it is also responsible for tissue damage and diseases such as atherosclerosis, arthritis, and cancer.12–14 It is employed as a safe disinfectant for drinking water and swimming pools. It is also used as a bleaching agent in industrial applications. These are potential external sources of OCl−.15 Thus, easy and instant detection, as well as quantification of OCl−, is highly relevant and in demand.Yttrium (Y) is a second-row transition metal, but its property similarities with the lanthanides make it unique. It has diverse applications in various fields such as materials, medicine, and catalysis. In the materials industry, synthetic garnets,16 cathode ray tubes for color televisions,17 near-IR lasers,18 white LEDs,19 spark plugs,20 superconductors,21 and material enhancers22 are some common applications. Y has extensive use in organic catalytic reactions, such as intermolecular aminoalkene hydroamination,23 o-selective C–H addition,24 lactide polymerization,25 acylation of alcohols, Diels–Alder reactions,26 asymmetric hydroamination,27 and the Tishchenko reaction, among others.28 In the medicinal field, it is used for the treatment of cancer as a source of powerful β radiation. For example, Y90-DOTA-tyr3-octreotide29 and Y90 ibritumomab tiuxetan30 are two drugs used to treat various cancers, including lymphoma, leukemia, liver cancer, and bone cancer.
Optical probes, such as colorimetric and fluorescent probes, are very useful and popular due to their selectivity, rapidity, sensitivity, simplicity, direct visual perception, non-invasiveness, and low-cost methodology.31–36 The literature indicates that most dual probes for assaying OCl− as one of the analytes suffer from background fluorescence, solvent dependence, and time-consuming synthesis.37,38 Thus, there is ample scope to contribute to overcoming these limitations. Similarly, the number of optical sensors for Y3+ is limited.
These facts led us to design and develop a simple, inexpensive, highly selective probe, A3. A simple receptor-linker-fluorophore-type compound (A3), where coumarin and naphthalene are connected via hydrazine, was developed. This type of structure was a potential candidate for excited-state intramolecular proton transfer (ESIPT), intramolecular charge transfer (ICT), photo induced electron transfer (PET), chelation-enhanced fluorescence (CHEF), through-bond energy transfer (TBET), chelation-enhanced quenching (CHEQ), and excimer/exciplex formation. The A3 probe was found to be a fluorescence probe for the trace-level recognition and determination of OCl− and Y3+ in a cascade manner through the ESIPT and CHEF processes in a green solvent system, such as DMSO–water.
Upon interaction with OCl−, the colorless probe emitted sky-blue fluorescence, turning the solution yellow to the naked eye, allowing the detection of OCl− as low as 18.42 nM. Subsequent addition of Y3+ turned the fluorescence to orange, allowing its detection up to 98.51 nM.
Materials and methods
Synthesis
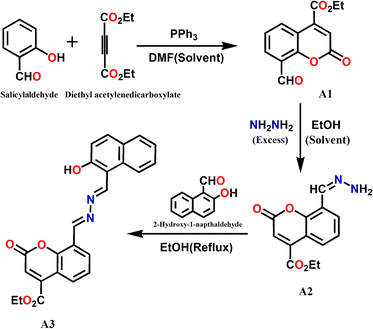
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio in EtOH with overnight stirring. The resultant product was crystallized and characterized by mass spectrometry (Fig. S1a, ESI†), FTIR (Fig. S1b, ESI†), and 1H-NMR (Fig. S1c and d, ESI†) spectra. Finally, the coumarin–naphthalene conjugate, A3, was prepared by refluxing a mixture of A2 (130 mg, 0.50 mmol) and 2-hydoxy-1-naphthaldehyde (86 mg, 0.50 mmol) in EtOH for 4 h (Scheme 1). The resulting pale yellow solution yielded pure A3 after a few days via crystallization. The A3 was characterized by different spectroscopic techniques, namely, mass spectrometry (Fig. S2a, ESI†), FTIR (Fig. S2b, ESI†), and 1H-NMR (Fig. S2c and d, ESI†) spectra.
1 mole ratio in EtOH with overnight stirring. The resultant product was crystallized and characterized by mass spectrometry (Fig. S1a, ESI†), FTIR (Fig. S1b, ESI†), and 1H-NMR (Fig. S1c and d, ESI†) spectra. Finally, the coumarin–naphthalene conjugate, A3, was prepared by refluxing a mixture of A2 (130 mg, 0.50 mmol) and 2-hydoxy-1-naphthaldehyde (86 mg, 0.50 mmol) in EtOH for 4 h (Scheme 1). The resulting pale yellow solution yielded pure A3 after a few days via crystallization. The A3 was characterized by different spectroscopic techniques, namely, mass spectrometry (Fig. S2a, ESI†), FTIR (Fig. S2b, ESI†), and 1H-NMR (Fig. S2c and d, ESI†) spectra.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio. Stirring was continued for 15 min. The resultant mixture was subjected to filtration to remove any suspended particles, and the filtrate was kept undisturbed for slow evaporation of the solvent. After two days, a pale yellow solid was collected and characterized as Ad1 by spectroscopic techniques such as mass spectrometry (Fig. S3a, ESI†) and FTIR (Fig. S3b, ESI†).
1 mole ratio. Stirring was continued for 15 min. The resultant mixture was subjected to filtration to remove any suspended particles, and the filtrate was kept undisturbed for slow evaporation of the solvent. After two days, a pale yellow solid was collected and characterized as Ad1 by spectroscopic techniques such as mass spectrometry (Fig. S3a, ESI†) and FTIR (Fig. S3b, ESI†).Likewise, Ad2 was obtained by stirring the mixture of Ad1 and Y3+ (ethanol solution) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio and characterized by mass spectrometry (Fig. S4a, ESI†) and FTIR (Fig. S4b, ESI†) spectra.
1 mole ratio and characterized by mass spectrometry (Fig. S4a, ESI†) and FTIR (Fig. S4b, ESI†) spectra.
Results and discussion
The optical response of the probe, A3, towards common ROS/RNS (such as O2, H2O2, NO, O2−, OH−, ONOO−, and ROO−) and anions (such as F−, Cl−, Br−, I−, SO42−, SO32−, H2PO4−, ClO4−, SCN−, NO3−, NO2−, H2AsO4−, and BzO−) was tested. Interestingly, A3 selectively detected OCl− in PBS-buffered (10 mM, pH 7.4) DMSO–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) media.
7) media.
Absorption spectroscopic studies
UV-Vis absorption spectra of A3 [20 μM] in the presence of common ROS/RNS and anions were recorded (Fig. 1A). Interestingly, only OCl− significantly changed the spectrum of A3. A3 showed two intense absorption peaks, at 313 nm and 360 nm, along with a relatively weak peak at 413 nm. Upon addition of OCl− [0–1800 μM], the absorbance at 313 nm and 360 nm decreased, while it increased at 413 nm in a ratiometric manner. The colorless solution turned yellow (Fig. 1B). In contrast, other tested common ROS/RNS and anions did not significantly affect the absorption spectra of A3. | ||
Fig. 1 Changes in the absorption spectra of A3: (A) in the presence of common ROS/RNS and anions; (B) with [OCl−] (media: PBS-buffered DMSO–water (10 mM, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v, pH 7.4)). 7, v/v, pH 7.4)). | ||
Emission spectroscopic studies
The emission studies were performed in the same media as mentioned above. A3 exhibited a weak emission at 394 nm (λex = 365 nm). Except for OCl−, other tested common ROS/RNS and anions did not significantly affect its emission spectra (Fig. 2A). Upon gradual addition of OCl−, a new emission peak at 484 nm gradually increased, accompanied by intense sky-blue fluorescence (Fig. 2B). Interference studies with different common anions and ROS/RNS (listed above) were performed at varying concentrations. No interference was observed, though (Fig. S5, ESI†). The quantum yields of A3 in the absence and presence of OCl− were 0.0164 and 0.1415, respectively. The effect of pH on the emission of A3, in the absence and presence of OCl− was also checked (Fig. S6, ESI†). The change in emission intensity was maximum in the pH range of 6 to 8.5, making it functional at physiological pH 7.4. | ||
| Fig. 2 Changes in the emission spectra of A3: (A) upon addition of common ROS/RNS and anions; (B) with [OCl−]. Media and pH are mentioned above. | ||
The Job's plot showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mole ratio) stoichiometry for the A3-OCl− adduct (Ad1) (Fig. S7, ESI†). The stoichiometry of the A3-OCl− adduct was also supported by the mass spectrum (Fig. S3a, ESI†). The binding constant of A3 for OCl− was determined using the Benesi–Hildebrand40–43 equation, assuming a 1
1 (mole ratio) stoichiometry for the A3-OCl− adduct (Ad1) (Fig. S7, ESI†). The stoichiometry of the A3-OCl− adduct was also supported by the mass spectrum (Fig. S3a, ESI†). The binding constant of A3 for OCl− was determined using the Benesi–Hildebrand40–43 equation, assuming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry and was found to be 1.08 × 105 M−1 (Fig. S8, ESI†). Emission intensities at 484 nm were used to determine the lowest detection limit.44,45 The limit of detection (LOD) of A3 for OCl− was 18.42 nM (Fig. S9, ESI†). The plot of emission intensity of A3 vs. OCl− was linear up to 21 μM OCl−, which was useful for measuring unknown OCl− concentrations (Fig. S10, ESI†). Addition of water to DMSO slightly lowered the emission maxima with a minute blue shift (Fig. 3).
1 stoichiometry and was found to be 1.08 × 105 M−1 (Fig. S8, ESI†). Emission intensities at 484 nm were used to determine the lowest detection limit.44,45 The limit of detection (LOD) of A3 for OCl− was 18.42 nM (Fig. S9, ESI†). The plot of emission intensity of A3 vs. OCl− was linear up to 21 μM OCl−, which was useful for measuring unknown OCl− concentrations (Fig. S10, ESI†). Addition of water to DMSO slightly lowered the emission maxima with a minute blue shift (Fig. 3).
Sequential recognition of yttrium ion (Y3+)
The Ad1 (A3-OCl−) [20 μM] acted as a proficient candidate for sequentially recognizing yttrium ion (Y3+) ratiometrically by both absorption and emission spectroscopy in the same medium. In response to Y3+ ion [0–1800 μM], the Ad1 altered its emission with a red shift from blue (λem = 489 nm) to yellowish-orange (λem = 555 nm), along with a visible color change from yellow to orange (Fig. 4).The other rare earth cations (such as La3+, Ce4+, Nd3+, Sm3+, Gd3+, and DY3+) along with common cations (such as Na+, K+, Ca2+, Mg2+, Ni2+, Al3+, Co2+, Zn2+, Mn2+, Cu2+, Fe2+, Hg2+, and Fe3+) remained inert toward Ad1. Only Y3+ responded selectively (Fig. S11, ESI†). Other tested cations showed negligible interference when tested at different concentrations (Fig. S12, ESI†). The LOD for Y3+ was 98.51 nM (Fig. S13–S14, ESI†), with a binding constant of 1.38 × 105 M−1 (Fig. S15, ESI†). It functioned well at physiological pH (Fig. S16, ESI†). The quantum yields of Ad1 and Ad2 were 0.1415 and 0.536, respectively. The Job's plot showed 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (mole ratio) stoichiometry for Ad2 (Fig. S17, ESI†).
1 (mole ratio) stoichiometry for Ad2 (Fig. S17, ESI†).
Proposed sensing mechanism
ESIPT active46 A3 (Fig. S18, ESI†) contains a –OH moiety ortho to the imine group, which easily undergoes tautomerization. Upon addition of OCl− to A3, the ESIPT proton is arrested via an H-bond, resulting in fluorescence enhancement (Fig. 5). It is noteworthy that direct interaction between Y3+ and A3 did not lead to fluorescence recognition of Y3+, probably because A3 failed to chelate Y3+.However, in the presence of OCl−, A3 could recognize Y3+ via a change in its emission profile. This means the [A3+OCl−] adduct (Ad1) acts as a fluorescence sensor for Y3+. This may be due to the fact that OCl− aids in the deprotonation of the –OH group of A3, facilitating chelation to Y3+, which results in a change in its emission profile. Thus, fluorescence recognition of Y3+ can be termed as OCl−-assisted CHEF process (Fig. 5). 1H-NMR studies supported the proposed binding interaction (Fig. 6), which was also corroborated by mass (Fig. S3a and S4a, ESI†) and FTIR (Fig. S3b and S4b, ESI†) spectral results. The proposed binding stoichiometry matched that predicted from Job's studies (Fig. S7 and S17, ESI†).
 | ||
| Fig. 6 Changes in the 1H-NMR spectra of A3 upon addition of OCl− and Y3+ in a cascade manner (solvent, CDCl3). | ||
1H-NMR studies
1H-NMR studies were performed to demonstrate the binding interaction of A3 with OCl− and Y3+ in a cascade manner. Upon addition of 0.5 equiv. OCl−, the highly de-shielded phenol –OH proton shifted downfield from 13.158 ppm to 13.615 ppm, while its intensity significantly diminished. Two imine protons also experienced a slight downfield shift from 10.302 ppm and 10.830 ppm to 10.314 ppm and 10.836 ppm, respectively. Addition of 1.0 equiv. OCl− to A3 further enhanced the intensity of the phenol proton, along with its downfield shift from 13.615 ppm to 14.014 ppm. In addition, the imine protons further shifted downfield from 10.314 ppm and 10.836 ppm to 10.324 ppm and 10.844 ppm, respectively. All these observations suggest H-bonding interaction between phenol–OH with OCl−.Upon addition of 1.0 equiv. Y3+ to the [A3+OCl−] adduct (Ad1), the phenol–OH peak disappeared, and the two imine proton peaks further shifted downfield to 10.384 ppm and 10.886 ppm, respectively (Fig. 6). The data is provided in ESI (Table S1).†
Fluorescence life time decay studies
The change in emission characteristics of A3 in the presence of OCl− (at 484 nm) was also reflected (IRF 336 nm) in its fluorescence lifetime decay profile (Fig. 7). The average lifetime of A3 increased from 1.91 ns to 3.78 ns (1.98 times) in the presence of OCl−, indicating an interaction between A3 and OCl−.DFT studies
TD-SCF/DFT/B3LYP/6-311G level of theory was employed to optimize the energy levels of A3 and Ad1. The lowering of the HOMO–LUMO energy gap from A3 (0.10093 eV) to Ad1 (0.09699 eV) indicated an interaction between A3 and OCl−, leading to the formation of Ad1 (Fig. 8). The oscillator strength (fb) for the S0 → S1 transition increased in Ad1 (f = 0.0019) compared to A3 (f = 0.0001). The oscillator strength (fb) and transitions involved, along with their energy levels, are listed in Table S2 (ESI).†Application
Real sample analysis for OCl−
Water samples collected from local source were analyzed to determine OCl− concentration employing A3, following the standard addition method.47 The concentration was measured utilizing the calibration plot, which involved the emission intensity of A3 vs. [OCl−].For this purpose, 0.1 mL of a 4 × 10−4 M solution of A3 in DMSO was mixed with 1 mL of the unknown water sample, and the volume was adjusted to 4 mL such that the final solution had the composition DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 (v/v). Similarly, four known OCl− solutions with concentrations of 20 μM, 50 μM, 100 μM, and 200 μM were prepared. Emission intensities of both known and unknown solutions were measured. The concentration of the unknown solution was then calculated from the calibration graph, which was found to be 57.44 μM (Fig. 9).
7 (v/v). Similarly, four known OCl− solutions with concentrations of 20 μM, 50 μM, 100 μM, and 200 μM were prepared. Emission intensities of both known and unknown solutions were measured. The concentration of the unknown solution was then calculated from the calibration graph, which was found to be 57.44 μM (Fig. 9).
Real sample analysis for Y3+
The Ad1 was successfully applied to determine the concentration of Y3+ in real water samples following the standard addition method.45 For this purpose, a known amount of Y3+ (as nitrate salt) was added to the collected water samples, namely, river (R) and tap (T) water from the Durgapur–Asansol industrial area (West Bengal). The total Y3+ concentration was then measured using the calibration graph (Fig. S19, ESI†). The following results (Table 1) indicate the efficiency of the developed method.| Water sample | Added (10–2 M) | Found (10–2 M) | Recovery (%) |
|---|---|---|---|
| R1 | 5.20 | 4.93 | 94.23 ± 1.38 |
| R2 | 10.25 | 9.81 | 95.71 ± 1.13 |
| R3 | 15.10 | 14.64 | 96.95 ± 1.06 |
| T1 | 5.15 | 4.87 | 94.56 ± 1.41 |
| T2 | 10.35 | 9.9 | 96.13 ± 1.36 |
| T3 | 15.22 | 14.85 | 97.57 ± 1.05 |
Comparison of A3 with reported pioneering probes
The efficiency of A3 was compared with reported pioneering probes (Table 2). The facile synthesis, instantaneous response, low detection limit, and prompt visualization of both a cation and an anion in a cascade manner make A3 a very useful probe for practical applications.| Sl. No. | System (probe) | Type | Limit of detection (LOD) | Ref. |
|---|---|---|---|---|
| 1 |  |
Fluorescence sensor | 15.3 × 10−9 M (for OCl−) | 48 |
| 2 |  |
Fluorescence sensor | 5 × 10–8 M (for OCl−) | 49 |
| 3 |  |
Fluorescence sensor | 1.41 × 10−7 M (for OCl−) | 50 |
| 4 |  |
Fluorescence sensor | 1.10 × 10−7 M (for OCl−) | 51 |
| 5 |  |
Fluorescence sensor | 2.23 × 10−7 M (for Y3+) | 52 |
| 6 |  |
Fluorescence sensor | 3 × 10−7 M (for Y3+) | 53 |
| 7 |  |
Fluorescence sensor | 5.5 × 10−7 M (for Y3+) | 54 |
| 8 |  |
Fluorescence sensor | 8 × 10−7 M (for Y3+) | 54 |
| 9 |  |
Fluorescence sensor | 18.4 × 10−9 M (for OCl−) | This work |
| 98.5 × 10−9 M (for Y3+) |
Conclusion
The coumarin–naphthalene conjugate (A3) is a potential ESIPT-active ratiometric probe for the selective recognition of OCl− and Y3+ in a cascade manner, with strong sky-blue emission at 484 nm (λex = 365 nm) and orange emission at 556 nm (λex = 440 nm), respectively. The probe was characterized by FTIR, ESI-MS, and 1H-NMR spectra. The LODs for OCl− and Y3+ were 18.42 nM and 98.51 nM, respectively. The corresponding binding constants were 1.08 × 105 M−1 and 1.38 × 105 M−1, respectively. The interference from other common tested analytes was insignificant. Both DFT studies and lifetime decay studies substantiated these interactions.Data availability
Please note that all data related to the above-mentioned manuscript are available and can be shared upon request. All related data are available in the ESI,† in addition to the main text.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to Dr I. Ansary of our department for helpful discussions and suggestions.Notes and references
- S. D. Richardson and T. A. Ternes, Anal. Chem., 2014, 86, 2813–2848 CrossRef CAS PubMed.
- J. Zhu, S. Liu, Z. Liu, Y. Li, M. Qiao and X. Hu, RSC Adv., 2014, 4, 5990–5994 RSC.
- M. Thakur, A. Singh, A. Dubey, A. K. Sundramoorthy, P. Kumar and S. Arya, Emergent Mater., 2024, 7, 1805–1817 CrossRef CAS.
- B. Padha, Z. Ahmed, S. Dutta, A. Pandey, N. Padha, M. Tomar, A. Sharma, I. Yadav and S. Arya, J. Alloys Compd., 2025, 1010, 177673 CrossRef CAS.
- A. Dutta and W. P. Saunders, J. Endod., 2012, 38, 1395–1398 CrossRef PubMed.
- J. Park, H. Kim, Y. Choi and Y. Kim, Analyst, 2013, 138, 3368–3371 RSC.
- J. Zhang and X. R. Yang, Analyst, 2013, 138, 434–437 RSC.
- K. Setsukinai, Y. Urano, K. Kakinuma, H. J. Majima and T. Nagano, J. Biol. Chem., 2003, 278, 3170–3175 CrossRef CAS PubMed.
- A. Hammer, G. Desoye, G. Dohr, W. Sattler and E. Malle, Lab. Invest., 2001, 81, 543–554 CrossRef CAS PubMed.
- K. Cui, D. Q. Zhang, G. X. Zhang and D. B. Zhu, Tetrahedron Lett., 2010, 51, 6052–6055 CrossRef CAS.
- S. Goswami, S. Das, K. Aich, P. K. Nandi, K. Ghoshal, C. K. Quah, M. Bhattacharyya, H. K. Fun and H. A. Abdel-Aziz, RSC Adv., 2014, 4, 24881–24886 RSC.
- Y. K. Yang, H. J. Cho, J. Lee, I. Shin and J. Tae, Org. Lett., 2009, 11, 859–861 CrossRef CAS PubMed.
- S. M. Wu and S. V. Pizzo, Arch. Biochem. Biophys., 2001, 391, 119–126 CrossRef CAS PubMed.
- N. R. Stanley, D. I. Pattison and C. L. Hawkins, Chem. Res. Toxicol., 2010, 23, 1293–1302 Search PubMed.
- Y. Wang, X. C. Xia, J. Han, X. Bao, Y. Y. Li, X. Tang, L. Ni, L. Wang and M. M. Gao, Talanta, 2016, 161, 847–853 CrossRef CAS PubMed.
- R. Fopase, V. Saxena, P. Seal, J. P. Borah and L. M. Pandey, Mater. Sci. Eng., C, 2020, 116, 111163 CrossRef CAS PubMed.
- V. Innocenzi, I. De Michelis, F. Ferella and F. Vegliò, Waste Manage., 2013, 33, 2390–2396 CrossRef CAS PubMed.
- J. S. Slimi, H. Yu, H. Zhang, C. Kränkel, P. Loiko, R. M. Solé, M. Aguiló, F. Díaz, W. Chen, U. Griebner, V. Petrov and X. Mateos, Opt. Express, 2025, 33, 2529–2541 CrossRef PubMed.
- M. Kottaisamy, P. Thiyagarajan, J. Mishra and M. S. Ramachandra Rao, Mater. Res. Bull., 2008, 43, 1657–1663 CrossRef CAS.
- F. Bisetto, J. Toniolo and R. Menezes, SAE Technical paper, 2006, 2006-01–2630 Search PubMed.
- H. Liu, I. I. Naumov, R. Hoffmann, N. W. Ashcroft and R. J. Hemley, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6990–6995 CrossRef CAS PubMed.
- H. J. Lee, K. P. Kim, G. Y. Hong and J. S. Yoo, J. Lumin., 2010, 130, 941–946 CrossRef CAS.
- S. Tobisch, Dalton Trans., 2012, 41, 9182–9191 RSC.
- G. Song, G. Luo, J. Oyamada, Y. Luo and Z. Hou, Chem. Sci., 2016, 7, 5265–5270 RSC.
- D. Patel, S. T. Liddle, S. A. Mungur, M. Rodden, A. J. Blake and P. L. Arnold, Chem. Commun., 2006, 1124–1126 RSC.
- A. Keshavaraja, V. R. Hegde, B. Pandey, A. V. Ramaswamy, P. Kumar and T. Ravindranathan, Angew. Chem., 1995, 34, 2143–2145 CrossRef CAS.
- J. Hannedouche, I. Aillaud, J. Collin, E. Schulz and A. Trifonov, Chem. Commun., 2008, 3552–3554 RSC.
- M. R. Bürgstein, H. Berberich and P. W. Roesky, Chem.–Eur. J., 2001, 7, 3078–3085 CrossRef.
- F. Bertagna, R. Giubbini, G. Savelli, C. Pizzocaro, C. Rodella, G. Biasiotto, S. Lucchini, R. Maroldi, J. Rosenbaum and A. Alavi, Hell. J. Nucl. Med., 2009, 12, 161–164 Search PubMed.
- T. E. Witzig, Drugs Today, 2004, 40, 111 CrossRef CAS PubMed.
- L. Yuan, W. Y. Lin, Y. N. Xie, B. Chen and J. Z. Song, Chem.–Eur. J., 2012, 18, 2700–2706 CrossRef CAS PubMed.
- J. Deng, P. Yu, Y. Wang and L. Mao, Anal. Chem., 2015, 87, 3080–3086 CrossRef CAS PubMed.
- Z. Yao, H. Bai, C. Li and G. Shi, Chem. Commun., 2011, 47, 7431–7433 RSC.
- D. Tan, X. He, X. Xing, Y. Zhao, H. Tang and D. Pang, Talanta, 2013, 113, 26–30 CrossRef CAS PubMed.
- V. V. Kumar and S. P. Anthony, RSC Adv., 2014, 4, 18467–18472 RSC.
- M. Banerjee, S. Ta, M. Ghosh, A. Ghosh and D. Das, ACS Omega, 2019, 4, 10877–10890 CrossRef CAS PubMed.
- C. Li, Y. Zhou, Y. Li, X. Kong, C. Zou and C. Weng, Anal. Chim. Acta, 2013, 774, 79–84 CrossRef CAS PubMed.
- J. Zha, B. Fu, C. Qin, L. Zeng and X. Hu, RSC Adv., 2014, 4, 43110–43113 RSC.
- K. C. Majumdar, I. Ansary, S. Samanta and B. Roy, Synlett, 2011, 5, 694–698 CrossRef.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- A. Banerjee, A. Sahana, S. Das, S. Lohar, S. Guha, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Analyst, 2012, 137, 2166–2175 RSC.
- S. Das, A. Sahana, A. Banerjee, S. Lohar, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia, I. Hauli, S. K. Mukhopadhyay and D. Das, Dalton Trans., 2013, 42, 4757–4763 RSC.
- S. Das, S. Lohar, J. S. Matalobos and D. Das, Chin. J. Chem., 2015, 33, 1173–1177 CrossRef CAS.
- D. Karak, S. Das, S. Lohar, A. Banerjee, A. Sahana, I. Hauli, S. K. Mukhopadhyay, D. A. Safin, M. G. Babashkina, M. Bolte, Y. Garcia and D. Das, Dalton Trans., 2013, 42, 6708–6715 RSC.
- S. Ta, S. Das, M. Ghosh, M. Banerjee, S. K. Hira, P. P. Manna and D. Das, Spectrochim. Acta. A. Mol. Bimol. Spectrosc., 2019, 209, 170–185 CrossRef CAS PubMed.
- H. Guan, A. Zhang, P. Li, L. Xia and F. Guo, ACS Omega, 2019, 4, 9113–9119 CrossRef CAS PubMed.
- M. Ghosh, S. Ta, M. Banerjee and D. Das, ACS Omega, 2018, 3, 16089–16098 CrossRef CAS PubMed.
- M. Q. Zhang, W. H. Guan, J. Wang, B. Zhao, J. Y. Zeng, J. C. Lu, M. Gao and X. B. Wang, Spectrochim. Acta. A. Mol. Bimol. Spectrosc., 2025, 330, 125738 CrossRef CAS PubMed.
- X. Zhong, L. Zhou, C. Jin, B. Wang, Y. Jiang and J. Shen, Talanta, 2019, 202, 369–374 CrossRef CAS PubMed.
- F. Yan, X. Sun, Y. Jiang, R. Wang, Y. Zhang and Y. Cui, Dyes Pigm., 2020, 182, 108531 CrossRef CAS.
- Z. Ma, X. Wang, C. Wang, X. Chen and Q. Lv, Spectrochim. Acta. A. Mol. Bimol. Spectrosc., 2019, 213, 370–374 CrossRef CAS PubMed.
- S. Jiang, S. Chen, H. Guo and F. Yang, Dyes Pigm., 2020, 183, 108717 CrossRef CAS.
- Y. Qin, Q. Meng, J. Yao, M. Chen, Y. Dong, D. Chen, S. He, C. Bai, L. Zhang, B. Wei, H. Miao, C. Qu and R. Qiao, J. Fluoresc., 2023, 33, 731–737 CrossRef CAS PubMed.
- S. Chatterjee, S. Ta, S. Khanra and D. Das, RSC Adv., 2022, 12, 33293–33303 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00689a |
| This journal is © The Royal Society of Chemistry 2025 |