 Open Access Article
Open Access ArticleA sustainable framework for advancing circular practices for polyethylene terephthalate textiles: optimized recycling of depolymerization byproducts and LCA validation†
Ye-eun Wooa,
Kyungha Baika,
Sujin Jeonga,
Suhyun Lee ab and
Jooyoun Kim
ab and
Jooyoun Kim *ab
*ab
aDepartment of Fashion and Textiles, Seoul National University, Seoul 08826, Republic of Korea. E-mail: jkim256@snu.ac.kr
bResearch Institute of Human Ecology, Seoul National University, Seoul 08826, Republic of Korea
First published on 7th March 2025
Abstract
This study demonstrates a novel circular approach for discarded polyethylene terephthalate (PET) textiles with life cycle assessment (LCA) validation, proposing an optimized recycling method for PET depolymerization byproducts. The experimental approach emphasizes sourcing the metal–organic framework ingredients including metal and linker components from a single PET depolymerization reaction. For depolymerization, a metal salt-based deep eutectic solvent (DES) composed of p-toluene sulfonic acid and iron(III) chloride hexahydrate was employed acting as both solvent and catalyst. Sequential recovery processes for multiple depolymerization products were established, and their recyclability was demonstrated. LCA was performed on the varied scenarios of the developed process, and the results provided solid grounds to conclude that the suggested process produces relatively less burden on the environment, attributed to the effective catalytic action of DES and the maximized use of byproducts generated from the depolymerization reactions. This work pioneers a comprehensive approach integrating novel experimental methods with LCA validation, establishing a robust proof-of-concept for maximizing the recycling potential of PET depolymerization products.
Introduction
Metal–organic frameworks (MOFs), composed of inorganic metal nodes and organic linkers, have drawn rising attention in a wide range of applications including foulant adsorption, drug delivery, photocatalysis, gas separation and storage due to their high porosity, large surface area, adjustable pore size, and chemical tailorability.1–3 Among the numerous organic linkers forming MOFs, terephthalic acid (TA, or benzenedicarboxylic acid, BDC) or bis(2-hydroxyethyl) terephthalate (BHET) are well suited to construct useful MOFs including HKUST-1, UiO-66, and MIL-88B, which exhibit notable stability in aqueous environments and serve as effective adsorbents for pollutant removal.4–7 Since TA is one of the monomeric constituents of polyethylene terephthalate (PET), which comprises the largest proportion of synthetic textile materials, using PET waste as a source of MOF linkers has been explored as a green synthesis strategy to solve fiber waste pollution by producing high-value products.8–12Regarding PET depolymerization, various methods have been suggested toward efficient and eco-friendly processes to lower the environmental burden such as process temperature, time, and byproduct waste.13–15 Most of the prior studies employed catalysts to reduce the activation energy in the depolymerization reaction.16–23 With the catalytic effect of metal salts, metal salt-based deep eutectic solvents (DES) have gained attention.24,25 DES is a mixture of two or three-component chemicals, which are associated with hydrogen bonds to form a liquid with a single melting point lower than the individual components. When the metal salt-based DES is used as a solvent for PET depolymerization, the chain disassociation is accelerated by the synergistic effect, whereby the metal salt acts as a Lewis acid and the other component acts as a Brønsted acid. Despite the benefit of metal-based DES, they often leave the unwanted metal complex and byproducts in the reaction system, contaminating the solvent.26,27 Therefore, measures to effectively remove and/or utilize the generated metal byproducts need to be studied to overcome the current challenges of using metal-based catalysts in a depolymerization system.
Most of the prior studies for PET-derived MOF synthesis were performed for (1) obtaining a high-purity TA from depolymerization of uncolored PET plastic bottles or textiles, and (2) synthesizing a well-defined MOF of interest. While numerous studies have progressed in this area, most of earlier studies have rarely paid attention to the discarded byproducts generated from the depolymerization process other than TA, and the metal nodes of MOF were still sourced from commercially purchased materials. As a prior attempt to source both metal and ligand components from waste, Song et al.28 sourced the metal node and TA from the nickel-containing electroplating sludge (EPS) and PET waste, respectively, and synthesized Ni-MOF. While this study demonstrated a way to source the main MOF ingredients from the waste, it involved independent processes to extract the metal and ligand ingredients from each EPS and PET, which may require high cost and environmental burden due to the complex processes. Therefore, to conceive a significantly sustainable recycling strategy, it is essential to account for simple and environmentally responsible processes with minimized generation of process byproducts and waste.
Herein, a novel strategy of recycling the TA and reaction byproducts from PET depolymerization is suggested by developing a series of processes to extract and separate TA (or TA in NaOH aq. solution), metal compounds, and dye, as shown in Fig. 1. A particular emphasis lies in sourcing the MOF ingredients including both metal and linker components from a single reaction system of PET depolymerization. Furthermore, dye from the discarded PET fabric was also simultaneously recovered from the depolymerization system, and it was recycled for dyeing an uncolored PET fabric. Aiming at developing an environmentally responsible PET depolymerization process, a metal salt-based DES, comprised of p-toluene sulfonic acid (PTSA) and iron(III) chloride hexahydrate (FeCl3·6H2O), was employed for an efficient reaction with lowered temperature and time. Sequential processes of recovering TA, FeCl3·6H2O, and dye from the reaction solution were developed, and a series of recycling approaches were developed for: recycling TA and FeCl3 for the synthesis of MIL-88B(Fe), and recycling the dye.
 | ||
| Fig. 1 Schematic overview of processes for PET depolymerization with catalytic activity of DES, chemicals recovery, and MOF synthesis. | ||
Life cycle assessment (LCA) was performed on the developed depolymerization process and the suggested process was compared to others from prior studies.29,30 Furthermore, LCA was tested for varied scenarios in which certain byproducts were credited as useful byproducts. The LCA results provided solid grounds to conclude that the developed process produces relatively less burden on the environment, attributed to the effective catalytic action of DES and the optimized use of byproducts. This study offers an innovative circular strategy for discarded PET by conceiving a series of sequential processes to recover reaction byproducts and by recycling them. To our knowledge, it is the first time to report a comprehensive discussion including the novel experimental approach and the LCA validation. The significance of this study lies in demonstrating a novel proof-of-concept recycling approach and offering an integrated assessment method to substantiate the experimental results. Ultimately, this study contributes to a sustainable framework for advancing circular practices in PET recycling.
Materials and methods
Materials
Undyed PET fabric was purchased from Testfabrics, Inc. (USA), and discarded dyed PET fabric was obtained elsewhere. Iron(III) chloride hexahydrate (FeCl3·6H2O), p-toluene sulfonic acid (PTSA), terephthalic acid (TA), hydrochloric acid (HCl), sodium hydroxide (NaOH), N,N-dimethylformamide (DMF), and ethanol were purchased from Daejung Chemicals (Korea) and used without further purification. Rhodamine B (RhB) was supplied by SCI Seoul Chemical (Korea).PET depolymerization
The DES was synthesized by heating a mixture of FeCl3·6H2O and PTSA at 60 °C until a homogeneous dark red liquid was obtained. PET fabrics were cut into 1 cm × 1 cm pieces, and 5 g of fabric pieces were immersed in 25 g of DES. The mixture was heated at varied reaction temperatures (80, 90, 100, and 110 °C) for varied reaction times (15, 30, 45, and 60 min) to determine the optimal depolymerization condition.After the depolymerization reaction, powdery TA, iron oxides, extracted dyes, and PET fragments with incomplete degradation were precipitated in DES solution. These precipitates were separated from the DES solution by passing through a glass filter (pore size of 15–40 μm) at least twice. Among the separated precipitates, visibly apparent PET fragments were easily removed. After cleaning the PET fragments, their weight was measured as  . The remaining precipitates, which were TA, Fe-compound, and dyes, were mixed with 20 mL of 2 M NaOH aq. solution to selectively dissolve the TA. Fe-compound and dyes were filtered out of a glass filter (pore size of 15–40 μm), leaving TA/NaOH eluent. This TA/NaOH solution was used as is for MOF synthesis step without further purification.
. The remaining precipitates, which were TA, Fe-compound, and dyes, were mixed with 20 mL of 2 M NaOH aq. solution to selectively dissolve the TA. Fe-compound and dyes were filtered out of a glass filter (pore size of 15–40 μm), leaving TA/NaOH eluent. This TA/NaOH solution was used as is for MOF synthesis step without further purification.
To calculate TA yield (%) and crystal analysis, it was necessary to obtain TA solid particles. For this, 8 mL of 10 M HCl aq. solution was added to the TA/NaOH solution to sediment TA precipitate and NaCl. After NaCl was removed by washing with distilled water, high-purity TA particles were collected. The depolymerization efficiency (%) and TA yield (%) for varied process conditions were calculated using eqn (1) and (2); where WPET is the weight of PET fabric,  is the weight of PET fragments with incomplete degradation, and WTA is the weight of TA obtained from the reaction.31 The coefficient of 0.85 represents the theoretical ratio of the molecular weight of TA units (C8H4O4, 164.11 g mol−1) to that of ethylene terephthalate repeating units (C10H8O4, 192.17 g mol−1).32
is the weight of PET fragments with incomplete degradation, and WTA is the weight of TA obtained from the reaction.31 The coefficient of 0.85 represents the theoretical ratio of the molecular weight of TA units (C8H4O4, 164.11 g mol−1) to that of ethylene terephthalate repeating units (C10H8O4, 192.17 g mol−1).32
 | (1) |
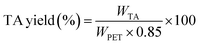 | (2) |
The remaining precipitates of Fe-compound and dyes were mixed with 4 mL of 10 M HCl aq. solution. At this time, the Fe-compound was dissolved in aq. HCl solution, presumably forming FeCl3·6H2O, whereas the dye is precipitated. The dye particles were separated by the glass filter and dried. This dye was recycled later for dyeing the uncolored PET fabric. The Fe-compound (presumably FeCl3·6H2O) in 10 M HCl aq. solution was concentrated to about 60 wt%, evaporating the solvent at 100 °C, and 14 mL of concentrated solution was stored at 5 °C to precipitate FeCl3·6H2O.
Synthesis of MIL-88B(Fe)
The TA dissolved in 2 M NaOH aq. solution and FeCl3·6H2O (s), which were recovered from PET depolymerization process, were used for MIL-88B(Fe) synthesis. A 0.635 g of FeCl3·6H2O and 4 mL of TA/NaOH aq. solution were added in 50 mL of DMF under sonication. The solution was then placed in a hydrothermal reactor and heated at 100 °C for 12 h. The resulting MIL-88B(Fe) was washed with DMF and ethanol, and then oven-dried at 60 °C for 24 h for activation.Characterization
Thermal property of dyed PET sample was examined by differential scanning calorimetry (DSC) (DSC Q100, Ta Instruments, USA) with heating and cooling rates of 5 °C min−1 under nitrogen (N2) gas flow (50 mL min−1). The precipitated Fe-containing compound was analyzed for its composition by the wavelength dispersive X-ray fluorescence spectrometer (WD-XRF) (ZSX Primus, Rigaku, Japan). The collected TA and dye were analyzed by the Fourier transform infrared assembled with attenuated total reflectance spectroscopy (FT-IR) (Vertex-80V, Bruker Corp., Germany). Morphology of MIL-88B(Fe) was observed by a field-emission scanning electron microscopy (FE-SEM) (JSM-7800F Prime, JEOL Ltd, Japan) with prior Pt-coating using a sputter coater (108 Auto Sputter Coater, Ted Pella, USA). The crystalline phase of MIL-88B(Fe) was analyzed using X-ray diffraction (XRD) (SmartLab, Rigaku Corp., Japan) with a Cu-targeted X-ray generator. Brunauer–Emmett–Teller (BET) adsorption analysis was conducted for MIL-88B(Fe) by the nitrogen (N2) sorption–desorption isotherm (Tristar ll 3020, Micromeritics, USA).Adsorption property of MIL-88B(Fe)
The adsorption performance of MIL-88B(Fe) was examined using RhB dye as a model water pollutant. In this test, 0.015 g of MIL-88B(Fe) was immersed in 50 mL of aq. dye solution (20 mg L−1), and the concentration of RhB solution was measured over time. The dye concentration was determined by measuring the absorbance at 553 nm of RhB using a multi-mode microplate reader (BioTek Synergy H1, Agilent, USA).Dyeing performance of recycled dye
A 2 g of uncolored PET fabric was treated with 100 mL of 0.8 g L−1 dye solution (4% on the weight of fabric), in which the dye was the one recovered from PET depolymerization process. The pH of the dyeing solution was adjusted to 4.5 using acetic acid, and the dyeing procedure was conducted at 130 °C for 1 h, followed by washing with distilled water to remove unfixed dyes. The color of the fabric dyed with the recycled dye was compared with that of the originally dyed PET fabric before depolymerization, using the CIE L* a* b* color system. The color difference between the samples ΔE was calculated as eqn (3). In this eqn, L* indicates the lightness of the sample, a* indicates the redness/greenness, and b* indicates yellowness/blueness. The ΔL*, Δa*, and Δb* represent the lightness and color difference between the originally dyed PET sample and the PET dyed with recycled dye. The L*, a*, b* measurements were done at least five times.
 | (3) |
Life cycle assessment
LCA was performed on the processes including PET depolymerization and the byproduct recovery. The system boundary of the LCA was defined as ‘cradle-to-gate’ including transport (Fig. S7†). To perform LCA, the ‘cut-off’ method was applied. Ecoinvent version 3.10 database and OpenLCA software version 2.3.0 were used to implement LCA. ReCiPe 2016 version 1.03 (Hierarchist) method was utilized to calculate the impact categories. The functional unit of 1 kg sorted waste PET was inserted into the depolymerization process. The assumptions applied to accomplish the LCA are summarized in Table S2.† The scenarios analyzed in this research are summarized in Table S3.† The input and output data were calculated from lab-scale data of this work, which recycled 5 g of PET (Tables S4–S6†). This LCA aimed to find major contributors to impacts and suggest a better scenario for further research and industrialization. The detailed information for LCA implementation including assumptions, inputs, outputs, inventories, and providers is summarized in ESI Section 2.†Results and discussion
Optimization of PET depolymerization
As shown in eqn (1), PET depolymerization efficiency (%) was defined as the extent to which PET was depolymerized into smaller molecular chains until the apparent fiber pieces were not observed. The depolymerization of various PET-based materials, including PET bottles and fibers, using FeCl3·6H2O/PTSA confirmed that the solvent effectively depolymerizes PET (Table S1†). In Fig. 2, depolymerization efficiency (%) and TA yield (%) of dyed PET were examined with respect to reaction parameters, including the PET to DES mass ratio, the molar ratio of FeCl3·6H2O to PTSA, process time and temperature. Fig. 2a shows the depolymerization efficiency as a function of PET to DES (solvent and catalyst) mass ratio when the reaction was carried out at 100 °C for 30 min in DES with a molar ratio of FeCl3·6H2O to PTSA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. When PET
1. When PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DES mass ratio was 1
DES mass ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the amount of solvent was insufficient to fully immerse the PET mass (Fig. S1†), inhibiting the proper depolymerization. As the PET
3, the amount of solvent was insufficient to fully immerse the PET mass (Fig. S1†), inhibiting the proper depolymerization. As the PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DES mass ratio increased to 1
DES mass ratio increased to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 or higher, PET was sufficiently immersed in DES, reaching 100% depolymerization efficiency and 95% TA yield. Thus, the optimized mass ratio of PET to DES was determined to be 1
5 or higher, PET was sufficiently immersed in DES, reaching 100% depolymerization efficiency and 95% TA yield. Thus, the optimized mass ratio of PET to DES was determined to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.
5.
 | ||
| Fig. 2 Depolymerization efficiency and TA yield with varied conditions. (a) PET to DES mass ratio, (b) FeCl3·6H2O to PTSA molar ratio, (c) reaction temperature, and (d) reaction time. | ||
Fig. 2b shows the depolymerization efficiency and TA yield with varied molar ratios of FeCl3·6H2O to PTSA when the experiment was conducted at 100 °C for 30 min with a PET to DES mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. The FeCl3·6H2O or PTSA alone was in the solid phase, thus, neither could be used as a solvent. To test the depolymerization efficiency by the single component-solvent (FeCl3·6H2O
5. The FeCl3·6H2O or PTSA alone was in the solid phase, thus, neither could be used as a solvent. To test the depolymerization efficiency by the single component-solvent (FeCl3·6H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTSA of 1
PTSA of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 or 0
0 or 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), an 80% aqueous solution of either FeCl3·6H2O or PTSA was prepared. In either case of FeCl3·6H2O or PTSA aq. solution, PET fabrics remained intact with 0% depolymerization efficiency. Yang et al.33 reported that PET was depolymerized by PTSA solution (80%) at 150 °C within 90 min, but under the conditions of this experiment at 100 °C for 30 min, depolymerization was very limited. When a DES with FeCl3·6H2O and PTSA was used as the solvent, 100% depolymerization efficiency and 95% TA yield were attained at 100 °C, regardless of the molar ratios tested in this study. In the depolymerization process, FeCl3·6H2O acts as a Lewis acid by accepting electron pairs from the oxygen in the ester bonds, and it weakens the ester linkage. In the meanwhile, PTSA acts as a Brønsted acid by donating H+ ions and cleaves the ester bonds.34 By the dual activation of PTSA and FeCl3·6H2O, the reaction is facilitated, allowing the depolymerization at a lower temperature. After the reaction, a partial amount of FeCl3·6H2O was precipitated and it was recovered by the subsequent process to be used for MOF synthesis. As the amount of FeCl3·6H2O in DES gradually reduced with precipitation, the molar ratio of 2
1), an 80% aqueous solution of either FeCl3·6H2O or PTSA was prepared. In either case of FeCl3·6H2O or PTSA aq. solution, PET fabrics remained intact with 0% depolymerization efficiency. Yang et al.33 reported that PET was depolymerized by PTSA solution (80%) at 150 °C within 90 min, but under the conditions of this experiment at 100 °C for 30 min, depolymerization was very limited. When a DES with FeCl3·6H2O and PTSA was used as the solvent, 100% depolymerization efficiency and 95% TA yield were attained at 100 °C, regardless of the molar ratios tested in this study. In the depolymerization process, FeCl3·6H2O acts as a Lewis acid by accepting electron pairs from the oxygen in the ester bonds, and it weakens the ester linkage. In the meanwhile, PTSA acts as a Brønsted acid by donating H+ ions and cleaves the ester bonds.34 By the dual activation of PTSA and FeCl3·6H2O, the reaction is facilitated, allowing the depolymerization at a lower temperature. After the reaction, a partial amount of FeCl3·6H2O was precipitated and it was recovered by the subsequent process to be used for MOF synthesis. As the amount of FeCl3·6H2O in DES gradually reduced with precipitation, the molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with a higher ratio of FeCl3·6H2O, was chosen as an optimal condition, in consideration of recycling the DES for the next PET depolymerization.
1, with a higher ratio of FeCl3·6H2O, was chosen as an optimal condition, in consideration of recycling the DES for the next PET depolymerization.
The optimal reaction temperature was examined in Fig. 2c, fixing the other process conditions as follows; PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DES mass ratio of 1
DES mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, FeCl3·6H2O
5, FeCl3·6H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTSA molar ratio of 2
PTSA molar ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, reaction time 30 min). At 80 °C, only 20% of PET depolymerization was attained. The glass transition temperature (Tg) of the dyed PET sample, analyzed by DSC, was approximately 85 °C (Fig. S2†). This suggests that polymer chains will have segmental motions above this temperature, at least in the amorphous region, allowing efficient DES penetration into the polymer chains. For that reason, a substantial portion of PET began to depolymerize from 90 °C onward, and at 100 °C and above, about 95% TA yield was attained. From Fig. 2d, under the optimized conditions (PET
1, reaction time 30 min). At 80 °C, only 20% of PET depolymerization was attained. The glass transition temperature (Tg) of the dyed PET sample, analyzed by DSC, was approximately 85 °C (Fig. S2†). This suggests that polymer chains will have segmental motions above this temperature, at least in the amorphous region, allowing efficient DES penetration into the polymer chains. For that reason, a substantial portion of PET began to depolymerize from 90 °C onward, and at 100 °C and above, about 95% TA yield was attained. From Fig. 2d, under the optimized conditions (PET![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DES of 1
DES of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, FeCl3·6H2O
5, FeCl3·6H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PTSA of 2
PTSA of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 100 °C), a minimum reaction time of 30 min of reaction time was required to achieve a 95% TA yield. A longer reaction time did not contribute to a higher TA yield. The discrepancy between depolymerization efficiency and TA yield at 15 min reaction time demonstrates that the random hydrolysis reaction occurs first, producing shorter chains and oligomers, and as the degradation proceeds, it further degrades to TA and ethylene glycol (EG).
1, 100 °C), a minimum reaction time of 30 min of reaction time was required to achieve a 95% TA yield. A longer reaction time did not contribute to a higher TA yield. The discrepancy between depolymerization efficiency and TA yield at 15 min reaction time demonstrates that the random hydrolysis reaction occurs first, producing shorter chains and oligomers, and as the degradation proceeds, it further degrades to TA and ethylene glycol (EG).
This study aimed to design a PET depolymerization process with minimal environmental impacts with lower reaction temperature, less reaction time, and less use of solvent. By using DES as a solvent, the reaction temperature was lowered compared to that of conventional acid or alkaline hydrolysis. The optimal depolymerization condition for obtaining 95% TA yield (%) was chosen as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 PET to DES ratio, 2
5 PET to DES ratio, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 FeCl3·6H2O to PTSA, 100 °C reaction temperature, and 30 min reaction time, and those process parameters were employed for later experiments. The comparative LCA of the depolymerization process is discussed further in the later section.
1 FeCl3·6H2O to PTSA, 100 °C reaction temperature, and 30 min reaction time, and those process parameters were employed for later experiments. The comparative LCA of the depolymerization process is discussed further in the later section.
Byproduct recovery
In the depolymerization reaction, precipitates including TA, dye from PET fabric, and Fe-compound from FeCl3·6H2O were formed in the solution. Each component of the precipitates was separated and recovered as described in the ‘methods’ section. When the precipitates were added to a 2 M of aq. NaOH solution, only TA dissolved, while the dye and Fe-impurities remained as solid precipitates. The TA/NaOH solution was separated and then the solution was directly used for the synthesis of MIL-88B(Fe) without further purification of TA. To confirm the presence of TA in the NaOH solution (denoted as ‘recovered TA/NaOH’), FT-IR analysis was performed on the TA/NaOH solution, and the resulting peaks were compared with those of commercially purchased TA dissolved in 2 M of aq. NaOH solution (denoted as ‘commercial TA/NaOH’). From Fig. 3, the FT-IR peaks of recovered TA/NaOH matched those of commercial TA/NaOH, presenting: 3440 cm−1 for OH stretching due to hydrogen bonding with hydroxide ions (OH−);35 1652 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching;36,37 1580 cm−1 for C
O stretching;36,37 1580 cm−1 for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C of the benzene ring;37,38 1411 for OH in-plane deformation.39 From the UV absorbance in Fig. S3,† the commercial TA (purity >97%) and the recovered TA showed a similar trend with varying concentrations. While an exact purity of the recovered TA was not analyzed, the experiment suggests the recovered TA has a purity as close to the commercial one.
C of the benzene ring;37,38 1411 for OH in-plane deformation.39 From the UV absorbance in Fig. S3,† the commercial TA (purity >97%) and the recovered TA showed a similar trend with varying concentrations. While an exact purity of the recovered TA was not analyzed, the experiment suggests the recovered TA has a purity as close to the commercial one.
The remaining precipitates after TA separation were analyzed by XRF (Table 1) to examine the compositions. From the analysis, the precipitates contained 80 wt% Fe in the Fe2O3 form; Cl and SO3 from the dyes of PET fabric;40,41 and TiO2, a common pigment added in PET fiber manufacturing.42–44 The Fe2O3 was generated through the following process. When PET was depolymerized in DES, TA precipitated, binding with Fe3+ ions in the solution. The Fe3+ containing precipitate reacts with OH− of NaOH solution, and Fe(OH)3 (s) is precipitated. Subsequently, Fe(OH)3 is oxidized to Fe2O3 in the air,45 and Fe2O3 remains as a solid byproduct.
| Fe3+ + 3OH− → Fe(OH)3 (s)↓ |
| 4Fe(OH)3 + 3O2 → 2Fe2O3 + 12 OH− |
| Oxide | |
|---|---|
| Component | Mass (%) |
| Fe2O3 | 80 |
| Cl | 12 |
| SO3 | 5 |
| TiO2 | 3 |
The precipitates consisting of Fe2O3 and dye are typically treated as reaction waste; however, in this study, Fe2O3 was reacted with HCl aq. solution to produce FeCl3·6H2O, which was then used for the synthesis of MIL-88B(Fe). When the precipitates containing Fe2O3 and dye were treated with HCl aq. solution, only dye precipitated. After the dye was separated by filtration, it was recycled for dyeing an uncolored PET fabric. For the recovered crystals of FeCl3·6H2O,46 the morphology and elemental compositions were analyzed by SEM-EDS (Fig. S4†), where the atomic ratio of Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cl was found to be about 1
Cl was found to be about 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The XPS analysis of Fig. 4a–c was performed to verify the hydration status of FeCl3. In Fig. 4a, the peak at the binding energy of 710.97 eV and 724.07 eV were attributed to Fe(III) 2p3/2 and Fe(III) 2p1/2, while the peak at 715.12 eV and 729.47 eV corresponded to the satellite peak of Fe(III) 2p3/2 and Fe(III) 2p1/2.47,48 In Fig. 4b, Fe–Cl bonds and Cl 2p1/2 at binding energies of 198.6 and 200.6 eV.49,50 In Fig. 4c, the presence of H2O was observed at 532.3 eV and O–Fe at 533.4 eV.51,52 The XRD analysis shown in Fig. 4d supports the identical crystal structures of FeCl3·6H2O and r-FeCl3·6H2O with distinct peaks at 15.2°, 20.1°, 25.38°, 28.18°, 36.8°, 46.48° and 50.56°.53 This confirms the successful recovery of FeCl3·6H2O in the PET recycling process. The recycled FeCl3·6H2O could be used as an addition to the DES component, but we used it as a source of metal component for MIL-88B(Fe) synthesis. Although the purity of the recycled FeCl3·6H2O was not directly calculated, it exhibited XRD peaks very similar to those of commercially purchased FeCl3·6H2O, and the successful synthesis of the MOF suggests that a high yield of FeCl3·6H2O was obtained.
3. The XPS analysis of Fig. 4a–c was performed to verify the hydration status of FeCl3. In Fig. 4a, the peak at the binding energy of 710.97 eV and 724.07 eV were attributed to Fe(III) 2p3/2 and Fe(III) 2p1/2, while the peak at 715.12 eV and 729.47 eV corresponded to the satellite peak of Fe(III) 2p3/2 and Fe(III) 2p1/2.47,48 In Fig. 4b, Fe–Cl bonds and Cl 2p1/2 at binding energies of 198.6 and 200.6 eV.49,50 In Fig. 4c, the presence of H2O was observed at 532.3 eV and O–Fe at 533.4 eV.51,52 The XRD analysis shown in Fig. 4d supports the identical crystal structures of FeCl3·6H2O and r-FeCl3·6H2O with distinct peaks at 15.2°, 20.1°, 25.38°, 28.18°, 36.8°, 46.48° and 50.56°.53 This confirms the successful recovery of FeCl3·6H2O in the PET recycling process. The recycled FeCl3·6H2O could be used as an addition to the DES component, but we used it as a source of metal component for MIL-88B(Fe) synthesis. Although the purity of the recycled FeCl3·6H2O was not directly calculated, it exhibited XRD peaks very similar to those of commercially purchased FeCl3·6H2O, and the successful synthesis of the MOF suggests that a high yield of FeCl3·6H2O was obtained.
After the 1st depolymerization (dep'n) process with the subsequent byproduct recovery, the remaining DES solution was recycled for the 2nd PET dep'n. It is noted that after the 1st dep'n, EG was not separated from the DES solution. Thus, the DES reused for the 2nd dep'n certainly contains EG; however, in this case, EG remaining in DES may be beneficial as functions as a catalyst for PET dep'n.54,55 For the 2nd dep'n experiment, 5 g of discarded PET fabric was reacted with the recycled DES solvent, and it produced the same depolymerization efficiency (100%) and TA yield (95%) as the 1st dep'n reaction (Fig. S5†). This result demonstrates the efficient recyclability of DES at least in the 2nd dep'n process.
MIL-88B(Fe) synthesis with recycled compounds
The TA/NaOH and FeCl3·6H2O obtained from the PET depolymerization process were used for the synthesis of MIL-88B(Fe), which is denoted as r-MIL-88B(Fe). The characteristics of r-MIL-88B(Fe) were compared with those of MIL-88B(Fe) synthesized from commercial chemicals of TA, FeCl3·6H2O and NaOH aq. solution (Fig. 5). The crystal morphologies for MIL-88B(Fe) and r-MIL-88(Fe) were similar in their shape and size, suggesting that TA/NaOH and FeCl3·6H2O recovered from the PET depolymerization process formed the well-defined MIL-88B(Fe) crystals. The XRD analysis of Fig. 5c also supports the identical crystal structures of MIL-88B(Fe) and r-MIL-88B(Fe) with distinct peaks at 9.2°, 10.1°, 10.6°, 16.7°, 18.1°, and 21.3°, corresponding to the (002), (100), (101), (103), (200), and (202) crystal planes, respectively.56In Fig. 5d, the adsorption of RhB dye was compared for MIL-88B(Fe) and r-MIL-88B(Fe) samples. The comparable adsorption performances between the two samples, or slightly better adsorption performance of r-MIL-88B(Fe), confirms that r-MIL-88B(Fe) can be well applied as an adsorbent against a water-soluble pollutant. Fig. 5e shows the average pore diameter and specific surface area of the two MOF samples, which demonstrates that the pore characteristics of r-MIL-88B(Fe) are not inferior to those of MIL-88B(Fe); the data shows a slightly larger surface area for r-MIL-88B(Fe) than MIL-88B(Fe).
The results demonstrate that MIL-88B(Fe) was successfully synthesized using the TA/NaOH solution recovered from the developed process, without further purification steps for obtaining TA powder. Notably, FeCl3·6H2O, one of the DES components, was obtained from the byproduct of the same depolymerization system, and it was recycled as a main component of MIL-88B(Fe) synthesis. To our knowledge, it is the first time to report that both MOF ingredients of metal and ligand components were simultaneously resourced from a single PET depolymerization system.
Recyclability of recovered dye
The recyclability of the dye obtained from the PET depolymerization reaction was investigated. Since the experiments were conducted on the discarded dyed fabrics, the chemical formula of the dye was unknown, except that PET is commonly dyed with azo- or anthraquinone-type dispersive dye. The recovered dye was dissolved in benzyl alcohol and was analyzed by FT-IR (Fig. 6a). The peaks were observed at: 1454 cm−1 corresponding to N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching57–59 a characteristic vibrational mode of azo compounds; and 1264 cm−1 for S–O stretching in the sulfonate group.60 From the analysis, the dye recovered from the depolymerization process was characterized as a dispersive dye containing the azo and sulfite groups. To assess the recyclability of the recovered dye, the color difference between the PET fabric dyed with the recycled dye (noted as r-Dyed PET) and the discarded PET fabric originally dyed (noted as Discarded PET) was examined. Since the dyeing condition of the original fabric was unknown, the process conditions such as dye concentration, time, and temperature were arbitrarily adjusted to attain a similar color to the original fabric (Fig. S6†). From Fig. 6b, the CIE L*, a*, b*, and the color difference value (ΔE ∼1.4) presented the comparable color between the original fabric and the fabric treated with recycled dye, indicating the dye extracted from the depolymerization process can be effectively recycled for PET dyeing, as similar colors were maintained even after three washing cycles. The proposed dye recycling process minimizes the essential purification and separation steps required in previous studies, presenting a highly efficient method for recovering and recycling the dye through simple filtration during the waste recovery process.
N stretching57–59 a characteristic vibrational mode of azo compounds; and 1264 cm−1 for S–O stretching in the sulfonate group.60 From the analysis, the dye recovered from the depolymerization process was characterized as a dispersive dye containing the azo and sulfite groups. To assess the recyclability of the recovered dye, the color difference between the PET fabric dyed with the recycled dye (noted as r-Dyed PET) and the discarded PET fabric originally dyed (noted as Discarded PET) was examined. Since the dyeing condition of the original fabric was unknown, the process conditions such as dye concentration, time, and temperature were arbitrarily adjusted to attain a similar color to the original fabric (Fig. S6†). From Fig. 6b, the CIE L*, a*, b*, and the color difference value (ΔE ∼1.4) presented the comparable color between the original fabric and the fabric treated with recycled dye, indicating the dye extracted from the depolymerization process can be effectively recycled for PET dyeing, as similar colors were maintained even after three washing cycles. The proposed dye recycling process minimizes the essential purification and separation steps required in previous studies, presenting a highly efficient method for recovering and recycling the dye through simple filtration during the waste recovery process.
 | ||
| Fig. 6 (a) FT-IR analysis of recovered dye. (b) Comparison of L*, a*, b* values between Discarded PET and r-Dyed PET. | ||
To conclude, novel and efficient processes sequentially linked with PET depolymerization, byproducts recovery, and their recycling/upcycling were developed in this study. Applying the developed processes, PET was efficiently depolymerized at a lower reaction temperature than other hydrolysis processes, due to the synergistic catalytic effect of DES. From the recovery processes, TA/NaOH and FeCl3·6H2O, the main ingredients of MIL-88B(Fe) synthesis, and the dye from the discarded PET fabric were appropriately recovered. The analytical results showed that all the recovered byproducts functioned well enough for proper MIL-88B(Fe) synthesis and PET fabric dyeing, demonstrating that the proposed process is a relevant option for circular practices of discarded PET textiles.
Life cycle assessment
LCA was performed on four case scenarios for our developed process including PET depolymerization (dep'n) and byproduct recovery. Case 1 analysis was conducted assuming that DES is not reused and all products (TA/NaOH(aq.), FeCl3·6H2O, and dye) are intended products from this process, without giving any credits for the obtained products. From the life cycle impact analysis (LCIA), Case 1 recorded 13.62 kg CO2 eq. of global warming potential (GWP), where the major causes for this value were: electricity (34.81%), PTSA (26.52%), and FeCl3·6H2O (23.76%) (Tables 2 and S7†).| Electricity and materialsa | Contribution (%) |
|---|---|
| a As mentioned in ESI, since materials in the LCI DB (life cycle inventory database) were not exactly same concentration with our process, compensation value of water was added in the LCA to meet the total materials inserted. | |
| Electricity | 34.81 |
| PTSA | 26.52 |
| FeCl3·6H2O | 23.76 |
| HCl | 7.84 |
| NaOH | 4.30 |
| Sorted waste PET | 2.76 |
| Water | 0.02 |
| Global warming potential | 13.6242 kg CO2-eq. (100%) |
Case 2 reflects the scenario where DES solvent/catalyst (FeCl3·6H2O and PTSA) is used two times, assuming that all DES is regained after the 1st dep'n process and a similar amount of DES is used in the 2nd dep'n. The analysis in Fig. 7 showed that GWP for Case 2 was 75% of Case 1, attributed to the full recycle of FeCl3·6H2O and PTSA. Since FeCl3·6H2O and PTSA were the major contributors to the environmental impacts, the reuse of DES (Case 2) significantly reduced the GWP. The analysis demonstrates that DES, simultaneously functioning as solvent and catalyst, needs to be reused as much as possible to reduce GWP, and an efficient solvent recovery process needs to be considered in developing an industrial-level process.
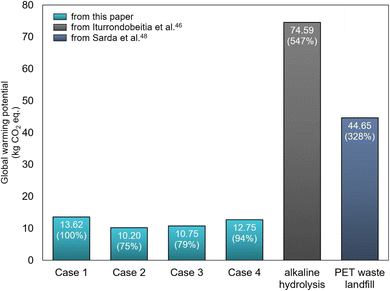 | ||
| Fig. 7 Global warming potential of each scenario and referred methods. The relative impact ratio compared to Case 1 is presented in the parenthesis. Note. Iturrondobeitia et al.30 also referred PET waste landfill data of Sarda et al.62's study. Alkaline hydrolysis route's functional unit was 1 kg of as-received post-consumer PET, while our study used 1 kg of sorted waste PET. The functional unit of PET waste landfill was 1 kg of PET bottle. | ||
In Fig. 7, additional scenarios of Case 3 and Case 4 were analyzed, where the credits were given for the avoided production of virgin products. In Case 3, all the recovered materials (TA/NaOH, FeCl3·6H2O, dye, and NaOH(aq.)) are recognized as useful byproducts, thus credits are given accordingly as it allows to avoid the production of virgin materials for TA/NaOH, FeCl3·6H2O, dye, and NaOH(aq.) and to avoid the associated environmental burden as detailed in Table S3.† Case 4 considers the whole process as a TA production process, and the obtained byproducts except TA (that is, byproducts of FeCl3·6H2O, dye, and NaOH(aq)) are considered as additional benefits, avoiding the production of FeCl3·6H2O, dye, and NaOH(aq). Thus, in this case, credits are given to the avoided production of FeCl3·6H2O, dye, and NaOH(aq.). Case 4 focuses on the TA production only to reflect the others' approach of using recycled TA only for MOF synthesis, even though our process and LCA does not include separation between TA and NaOH(aq.). From the analysis, Case 3 and Case 4 produced 79% and 94% of the GWP in Case 1, respectively. As the recovery of TA was much larger than the other recovered materials, Case 3 showed significantly lower environmental impacts than Case 4. The analysis offers evidence-based justification for our efforts to recover TA and other byproducts for the maximized recycling of materials. Detailed data for other impact categories are summarized in Fig. S8.†
The scenarios from our developed process (Cases 1–4) were compared to other polyester depolymerization and waste PET handling methods for the GWP (Fig. 7). The compared data of alkaline hydrolysis was referred from Iturrondobeitia et al.30 which used the same impact assessment method with this study (ReCiPe 2016(Hierarchist)). PET waste landfill was referred from other references,61,62 and Iturrondobeitia et al.30 also referred Sarda et al.62 It should be noted that all data from Iturrondobeitia et al.30 are the prospective LCA by the up-scale process while our results are calculated from the lab-scale process; the functional unit of Iturrondobeitia et al.30 was 1 kg of as-received post-consumer PET, while our result was obtained from 1 kg of sorted waste PET. The functional unit of PET waste landfill was 1 kg of PET bottle. From Fig. 7, “alkaline hydrolysis” route which recovers TA like our study generated 74.59 kg CO2 eq., which is much higher than any of the scenarios from our developed process. The “PET waste landfill” route showed 44.65 kg CO2 eq., which was also much higher than our scenarios (Cases 1–4). This environmental impact analysis demonstrates the competitiveness of our depolymerization approach as a sustainable process, attributable to DES that allowed accelerated depolymerization by the effective catalytic action.
Conclusions
This study demonstrated an integrated framework for circular practices of PET textiles proposing an optimized recycling method and providing LCA validation. For depolymerization, a metal salt-based DES comprised of PTSA and FeCl3·6H2O was employed as both solvent and catalyst, and the reaction produced TA with a high yield of 95% in just 30 min at 100 °C. Sequential processes for multiple byproduct recovery were established, where the experimental approach emphasizes sourcing MOF ingredients of both metal and linker components from a single PET depolymerization reaction. The TA and FeCl3·6H2O regained from the depolymerization system were used to synthesize MIL-88B(Fe), which demonstrated an excellent adsorption performance against a water-soluble foulant, RhB. Furthermore, dye from the discarded PET fabric was also simultaneously recovered from the depolymerization system, and it was recycled for dyeing an uncolored PET fabric. Life cycle assessment (LCA) was performed on the varied scenarios of the developed process, and the results provided a solid ground to conclude that the developed process produced relatively less burden on the environment, attributed to the effective catalytic action of DES and the maximized use of byproducts. This work pioneers a comprehensive approach integrating novel experimental methods with LCA validation, establishing a novel proof-of-concept for maximizing the recycling potential of PET depolymerization byproducts. This work intends to demonstrate potential recovery processes for the materials that have been wasted or ignored before and their recyclability as useful materials, rather than testing the economic feasibility of recycling processes. Further study is suggested to examine the economic feasibility of the process with the recyclability of solvent and ethylene glycol. In response to the urgent need for sustainable practices for discarded textiles, this study provides insightful ideas on the circular development of value-added materials and suggests practical considerations for designing environmentally responsible processes.Data availability
All data related to this publication are available either in the main manuscript or its ESI.†Author contributions
Y. Woo and K. Baik designed and ran the experiments, analyzed the data, and wrote the original draft; S. Jeong ran the characterization experiments and analyzed the data; S. Lee and J. Kim analyzed the data and supervised the work. All authors reviewed and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (RS-2024-00405333; RS-2025-00523094).References
- Y. Peng, S. Sanati, A. Morsali and H. García, Angew. Chem., Int. Ed., 2023, 62, e202214707 CrossRef CAS PubMed.
- D. Chakraborty, A. Yurdusen, G. Mouchaham, F. Nouar and C. Serre, Adv. Funct. Mater., 2023, 34, 2309089 CrossRef.
- H. D. Lawson, S. P. Walton and C. Chan, ACS Appl. Mater. Interfaces, 2021, 13, 7004–7020 CrossRef CAS PubMed.
- D. A. Cabrera-Munguia, M. I. León-Campos, J. A. Claudio-Rizo, D. A. Solís-Casados, T. E. Flores-Guia and L. F. Cano Salazar, Bull. Mater. Sci., 2021, 44, 1–9 CrossRef.
- Z. Li, L. Wang, L. Qin, C. Lai, Z. Wang, M. Zhou, L. Xiao, S. Liu and M. Zhang, Chemosphere, 2021, 285, 131432 CrossRef CAS PubMed.
- N. Abigail K, J. Ronald T, M. Phillip J and S. Jin, ACS Sustain. Chem. Eng., 2022, 11, 18–22 Search PubMed.
- M. Jussara Lopes de, A. Tatiana Pereira de, N. João Mário Brito, S. Dejair de Pontes, C. Igor, S. Fernando, O. Suzane de Sant’ Ana and M. Luiza Cristina de, Sustainable Mater. Technol., 2023, 37, e00689 CrossRef.
- P. Marino, P. R. Donnarumma, H. A. Bicalho, V. Quezada-Novoa, H. M. Titi and A. J. Howarth, ACS Sustain. Chem. Eng., 2021, 9, 16356–16362 CrossRef CAS.
- R. Hardian, Z. Liang, X. Zhang and G. Szekely, Green Chem., 2020, 22, 7521–7528 RSC.
- H. Li, J. Lei, L. Zhu, Y. Yao, Y. Li, T. Li and C. Qiu, Green Energy Environ., 2024, 9, 1650–1665 CrossRef CAS.
- M. Shanmugam, C. Chuaicham, A. Augustin, K. Sasaki, P. J. Sagayaraj and K. Sekar, New J. Chem., 2022, 46, 15776–15794 RSC.
- S. Cheng, Y. Li, Z. Yu, R. Gu, W. Wu and Y. Su, Sep. Purif. Technol., 2024, 339, 126490 CrossRef CAS.
- Z. Cao, X. Fu, H. Li, S. Pandit, F. M. Amombo Noa, L. Öhrström, A. Zelezniak and I. Mijakovic, ACS Sustain. Chem. Eng., 2023, 11, 15506–15512 CrossRef CAS.
- P. Waribam, T. R. Katugampalage, P. Opaprakasit, C. Ratanatawanate, W. Chooaksorn, L. P. Wang, C.-H. Liu and P. Sreearunothai, Chem. Eng. J., 2023, 473, 145349 CrossRef CAS.
- C.-Y. Wang, H.-Y. Chu and C.-C. Wang, Coord. Chem. Rev., 2024, 518, 216106 CrossRef CAS.
- E. Barnard, J. J. R. Arias and W. Thielemans, Green Chem., 2021, 23, 3765–3789 RSC.
- F. Cao, L. Wang, R. Zheng, L. Guo, Y. Chen and X. Qian, RSC Adv., 2022, 12, 31564–31576 RSC.
- S. Javed, J. Fisse and D. Vogt, Ind. Eng. Chem. Res., 2023, 62, 4328–4336 CrossRef CAS.
- Z. Fehér, J. Kiss, P. Kisszékelyi, J. Molnár, P. Huszthy, L. Kárpáti and J. Kupai, Green Chem., 2022, 24, 8447–8459 RSC.
- R. Sharma, H. Kumar, G. Kumar, S. Sharma, R. Aneja, A. K. Sharma, R. Kumar and P. Kumar, J. Chem. Eng., 2023, 468, 143706 CrossRef CAS.
- W. Chen, M. Li, X. Gu, L. Jin, W. Chen and S. Chen, Polym. Degrad. Stab., 2022, 206, 110168 CrossRef CAS.
- A. K. Nason, W. Phamonpon, T. A. Pitt, R. T. Jerozal, P. J. Milner, N. Rodthongkum and J. Suntivich, Chem. Mater., 2024, 36, 10319–10326 CrossRef CAS.
- R.-X. Yang, Y.-T. Bieh, C. H. Chen, C.-Y. Hsu, Y. Kato, H. Yamamoto, C.-K. Tsung and K. C.-W. Wu, ACS Sustain. Chem. Eng., 2021, 9, 6541–6550 CrossRef CAS.
- A. Khalid, S. Tahir, A. R. Khalid, M. A. Hanif, Q. Abbas and M. Zahid, Green Chem., 2024, 26, 2421–2453 RSC.
- M. Rollo, F. Raffi, E. Rossi, M. Tiecco, E. Martinelli and G. Ciancaleoni, Chem. Eng. J., 2023, 456, 141092 CrossRef CAS.
- S. Marullo, C. Rizzo, N. T. Dintcheva and F. D'Anna, ACS Sustain. Chem. Eng., 2021, 9, 15157–15165 CrossRef CAS.
- M. M. Najafpour, Acc. Chem. Res., 2022, 55, 2260–2270 CrossRef CAS PubMed.
- K. Song, X. Qiu, B. Han, S. Liang and Z. Lin, Environ. Sci. Nano, 2021, 8, 390–398 RSC.
- W. T. Lang, S. A. Mehta, M. M. Thomas, D. Openshaw, E. Westgate and G. Bagnato, J. Environ. Chem. Eng., 2023, 11, 110585 CrossRef CAS.
- M. Iturrondobeitia, L. Alonso and E. Lizundia, Resour. Conserv. Recycl., 2023, 198, 107182 CrossRef CAS.
- Ü. Sibel, M. V. G. Kevin, D. Ruben, R. Martijn, M. Nicolas, R. Kim and D. M. Steven, Green Chem., 2020, 22, 5376–5394 RSC.
- K. Leila, M. Arianna, S. Stefania, E. H. Nissrine, M. Pascale and R. Julien, Mater. Adv., 2021, 2, 2750–2758 RSC.
- W. Yang, J. Wang, L. Jiao, Y. Song, C. Li and C. Hu, Green Chem., 2022, 24, 1362–1372 RSC.
- M. Rollo, M. A. Perini, A. Sanzone, L. Polastri, M. Tiecco, A. Torregrosa-Chinillach, E. Martinelli and G. Ciancaleoni, RSC Sustainability, 2024, 2, 187–196 RSC.
- T. Cai, H. Du, X. Liu, B. Tie and Z. Zeng, Environ. Technol. Innov., 2021, 24, 102031 CrossRef CAS.
- H. Lin, S. Bean, M. Tilley, K. Peiris and D. Brabec, Food Anal. Methods, 2021, 14, 268–279 CrossRef.
- T. H. Nguyen and K.-Y. Chiang, Sustain. Environ. Res., 2024, 34, 16 CrossRef CAS.
- V. Ţucureanu, A. Matei and A. M. Avram, Crit. Rev. Anal. Chem., 2016, 46, 502–520 CrossRef PubMed.
- Y. Ko, T. J. Azbell, P. Milner and J. P. Hinestroza, Ind. Eng. Chem. Res., 2023, 62, 5771–5781 CrossRef CAS.
- Y. Li, H.-Y. Bi, Y.-S. Jin and X.-Q. Shi, RSC Adv., 2014, 4, 58307–58314 RSC.
- R. Chu, Y. Zhang, T. Xing and G. Chen, RSC Adv., 2020, 10, 42633–42643 RSC.
- J. H. Braun, A. Baidins and R. E. Marganski, Prog. Org. Coat., 1992, 20, 105–138 CrossRef CAS.
- X. Lv, B. Yan, Y. Shao, H. Zhang, H. Zhang and J. Zhu, Particuology, 2022, 67, 18–26 CrossRef CAS.
- U. Gesenhues, J. Photochem. Photobiol., A, 2001, 139, 243–251 CrossRef CAS.
- M. Salmanion and M. M. Najafpour, J. Phys. Chem. C, 2023, 127, 18340–18349 CrossRef CAS.
- W. Yang, Z. Mei, S. Feng, C. Li, J. Guo, H. Bian, H. Xiao, H. Dai, C. Hu and J. Han, ACS Sustain. Chem. Eng., 2023, 11, 10172–10182 CrossRef CAS.
- Z. Xu, S. Gu, Z. Sun, D. Zhang, Y. Zhou, Y. Gao, R. Qi and W. Chen, Environ. Sci. Pollut. Res., 2020, 27, 11012–11025 CrossRef CAS PubMed.
- Z. Hou, P. Yan, B. Sun, H. Elshekh and B. Yan, Results Phys., 2019, 14, 102498 CrossRef.
- L. Wei, Y. Zhang, Y. Yang, M. Ye and C. C. Li, Small, 2022, 18, 2107667 CrossRef CAS PubMed.
- D. Pradhan and K. Leung, J. Phys. Chem. C, 2008, 112, 1357–1364 CrossRef CAS.
- Y. Jiang, M. Dai, F. Yang, I. Ali, I. Naz and C. Peng, Water, 2022, 14, 894 CrossRef CAS.
- H. S. Casalongue, S. Kaya, V. Viswanathan, D. J. Miller, D. Friebel, H. A. Hansen, J. K. Nørskov, A. Nilsson and H. Ogasawara, Nat. Commun., 2013, 4, 2817 CrossRef.
- N. Louvain, A. Fakhry, P. Bonnet, M. El-Ghozzi, K. Guérin, M.-T. Sougrati, J.-C. Jumas and P. Willmann, CrystEngComm, 2013, 15, 3664–3671 RSC.
- Z. Wang, Y. Jin, Y. Wang, Z. Tang, S. Wang, G. Xiao and H. Su, ACS Sustain. Chem. Eng., 2022, 10, 7965–7973 CrossRef CAS.
- S. Shirazimoghaddam, I. Amin, J. A. Faria Albanese and N. R. Shiju, ACS Eng. Au, 2023, 3, 37–44 CrossRef CAS PubMed.
- M. Ma, A. Bétard, I. Weber, N. S. Al-Hokbany, R. A. Fischer and N. Metzler-Nolte, Cryst. Growth Des., 2013, 13, 2286–2291 CrossRef CAS.
- H. Shaki, K. Gharanjig and A. Khosravi, Biotechnol. Prog., 2015, 31, 1086–1095 CrossRef CAS PubMed.
- A. Mohammadi, M. R. Yazdanbakhsh and L. Farahnak, Spectrochim. Acta, Part A, 2012, 89, 238–242 CrossRef CAS PubMed.
- N. Biswas and S. Umapathy, J. Phys. Chem. A, 2000, 104, 2734–2745 CrossRef CAS.
- S. Ramesh and L. J. F. Yi, Ionics, 2009, 15, 413–420 CrossRef CAS.
- A. Ncube and Y. Borodin, 7th International Forum On Strategic Technology (IFOST) 2012, 2012, pp. 1–6 Search PubMed.
- P. Sarda, J. C. Hanan, J. G. Lawrence and M. Allahkarami, J. Polym. Sci., 2022, 60, 7–31 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00572h |
| This journal is © The Royal Society of Chemistry 2025 |



