Open Access Article
Open Access ArticlePreparation of nanoemulsions from Elsholtzia kachinensis and Elsholtzia ciliata essential oils via ultrasonic homogenization and their antibacterial and anticancer activities†
Nguyen Quang Tinha,
Dang Van Thanh b,
Nguyen Van Thub,
Bui Thi Quynh Nhungb,
Pham Ngoc Huyenb,
Nguyen Phu Hungc,
Nguyen Thi Thuy
b,
Nguyen Van Thub,
Bui Thi Quynh Nhungb,
Pham Ngoc Huyenb,
Nguyen Phu Hungc,
Nguyen Thi Thuy de,
Pham Dieu Thuya,
Nguyen Hoa Mif and
Khieu Thi Tam
de,
Pham Dieu Thuya,
Nguyen Hoa Mif and
Khieu Thi Tam *g
*g
aThai Nguyen University of Agriculture and Forestry, Thai Nguyen 25000, Vietnam
bThai Nguyen University of Pharmacy and Medicine, Thai Nguyen 25000, Vietnam
cCenter for Interdisciplinary Science and Education, Thai Nguyen University, Tan Thinh Ward, Thai Nguyen 25000, Vietnam
dSchool of Chemical and Environmental Engineering, International University, Quarter 6, Linh Trung Ward, Thu Duc City, Ho Chi Minh City, Vietnam
eVietnam National University Ho Chi Minh City, Linh Trung Ward, Thu Duc City, Ho Chi Minh City, Vietnam
fCenter for Computational Chemistry, Faculty of Chemistry, VNU University of Science, 19 Le Thanh Tong, Hoan Kiem, Hanoi City, Vietnam
gFaculty of Chemistry, TNU-University of Sciences, Tan Thinh Ward, Thai Nguyen 25000, Vietnam. E-mail: tamkt@tnus.edu.vn
First published on 9th April 2025
Abstract
Plant essential oils can function as effective antibacterial and anticancer agents, but their low solubility and hydrophobic nature limit their practical applications. In this study, we report the preparation of nanoemulsions of Elsholtzia kachinensis and Elsholtzia ciliata via ultrasonic homogenization and the characterization of their antibacterial and anticancer activities for the first time. The product characteristics were evaluated based on turbidity, droplet size, polydispersion index, zeta potential and electrophoretic mobility. The activities were evaluated based on their ability to inhibit the growth of bacteria and HepG2 cancer cells. The Elsholtzia kachinensis and Elsholtzia ciliata nanoemulsions exhibited good stabilities, narrow size distributions with droplet sizes of 72.81 nm and 32.13 and zeta potentials of −27.8 mV and −11.2 mV, respectively. The Mulliken atomic charge analysis demonstrated that the E. kachinensis nanoemulsion had greater stability than the E. ciliata nanoemulsion. In vitro anti-bacterial studies using strains of Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Bacillus subtilis and Staphylococcus epidermidis showed that both nanoemulsions exhibited higher growth inhibition efficiency than the respective essential oils. The inhibition efficiency of the Elsholtzia ciliata nanoemulsion against Bacillus subtilis and Staphylococcus epidermidis was 5 times higher than those of the corresponding essential oils. The HepG2 cell inhibition efficiency was about 80% for both nanoemulsions at a concentration of 500 μg mL−1, while the commercial essential oils inhibited only about 60% of HepG2 cells. Therefore, Elsholtzia kachinensis and Elsholtzia ciliata nanoemulsions can be potential candidates for modern biopharmaceuticals in the future.
1 Introduction
Elsholtzia, an important genus of aromatic herbs with a total of 42 species, is widely distributed all over the world and used in folk medicine to treat respiratory infectious diseases, such as fever, pneumonia and cold.1,2 Some species in this genus are renowned for their essential oils, which possess various aromatic and medicinal properties.2 The essential oils of Elsholtzia species typically contain a range of volatile compounds, such as aliphatic compounds, terpenoids and aromatic compounds, of which terpenoids are the main components.3–5 The compositions of the essential oils of Elsholtzia species depend on the distribution of the species and the plant part used. Reports indicate that their essential oils possess a range of pharmacological activities with antibacterial, antioxidant, anti-inflammatory, and anticancer properties.3–5 For example, these essential oils are considered key antibacterial components, with strong inhibitory effects on Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae (K. pneumoniae) and Staphylococcus aureus (S. aureus).6,7 Elsholtzia kachinensis (E. kachinensis) and Elsholtzia ciliata (E. ciliata) are two species that belong to the Elsholtzia genus. E. kachinensis with notable aromatic and medicinal qualities is used to support digestion and alleviate symptoms of bloating and pain.8 The essential oils of this species mainly contain carvone and dehydroelsholtzia ketone, which have inhibitory effects against S. aureus and Salmonella enterica.8 In addition, these essential oils are effective against insects, and hence, they have the potential to be developed into environmentally friendly pesticides.8 E. ciliata is a popular species that is distributed widely all over the world. Previous studies on the volatile oils extracted from this species have shown that dehydrogenanone and elsholtzia ketone are the main components.3,9 However, applications of these essential oils are limited due to issues, such as low stability, poor water solubility and susceptibility to oxidation.10,11To overcome these limitations, essential oils can be formulated into nanoemulsions, which offer improved stability, increased surface area, and solubility and controlled release of their components, thereby increasing their biological efficacy.12–15 Nanoemulsions maintain the quality of essential oils over time by protecting them from degradation and oxidation, improving their solubility and enabling the controlled release of their constituent compounds.16 Moreover, the small droplet sizes of nanoemulsions increase the surface area for interactions and allow essential oils to reach deeper cell membranes, thereby increasing their biological efficacy.12,13 As a result, nanoemulsions of essential oils exhibit higher bioactivity than the parent essential oils. Therefore, nanoemulsions offer a promising platform for enhancing the biological activity of essential oils by improving their characteristics. Various studies have demonstrated that incorporation of essential oils as nanoemulsions can enhance their antimicrobial and anticancer activities.17–19 However, up to date, there are no reports on nanoemulsions of E. kachinensis and E. ciliata essential oils.
This study reports, for the first time, the preparation and characterization of nanoemulsions of E. kachinensis and E. ciliata essential oils. Additionally, the mechanism of nanoemulsion formation is proposed. Moreover, the antibacterial activities of these nanoemulsions against strains of Gram-negative (E. coli, P. aeruginosa, and K. pneumoniae) and Gram-positive (S. aureus, B. subtilis, S. epidermidis) bacteria were examined and compared with their corresponding essential oils. Finally, the anticancer activities of the nanoemulsions were investigated using the 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT) assay.
2 Methods
2.1 Materials
All chemicals, including n-hexane (≥99%), Na2SO4 (≥99%), and Tween 80 (99%) were purchased from Merck Chemicals. Fresh E. kachinensis was collected from Cao Bang Province, Vietnam (22°71′9.54′′ N, 106°33′9.39′′ E), and E. ciliata was obtained from Thai Nguyen Province, Vietnam (21°55′9.17′′ N, 105°83′5.14′′ E) in April 2024.2.2 Extraction of essential oils from E. kachinensis and E. ciliata
Essential oils from the aerial parts of E. kachinensis (EKEO) and E. ciliata (ECEO) were extracted using the steam distillation method using the Clevenger apparatus. Ten kilograms of E. kachinensis or E. ciliata were washed with distilled water, then chopped into small pieces and subjected to distillation. The E. kachinensis or E. ciliata samples were placed in the distillation apparatus, and water was added to submerge the samples. The obtained essential oils of E. kachinensis and E. ciliata were removed from the water using Na2SO4. The essential oils were stored at 4 °C for analyzing their chemical compositions, identifying their bioactivities and synthesizing their nanoemulsions.2.3 Analysis of chemical constituents of essential oils using gas chromatography/mass spectra (GS/MS)
The chemical compositions of the essential oils were analysed using an Agilent 7890A gas chromatograph coupled with an Agilent MSD5975C mass spectrometer equipped with an HP-5MS fused silica capillary column (60 m × 0.25 mm x 0.25 μm). Helium was applied as the carrier gas at a flow rate of 1.0 mL min−1. One microliter of the essential oil was diluted to a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 with n-hexane and injected manually such that the flow ratio was 1
50 with n-hexane and injected manually such that the flow ratio was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. The temperature of the oven was initially set at 60 °C for 5 min, then heated gradually to 240 °C at the rate of 4 °C min−1 and was kept constant for 10 min. The temperature of the detector and interface was 270 °C. The mass spectra were recorded at 70 eV in the spectral range of 35–450 Da at 1.0 scan per s. The identification of essential oil constituents was carried out based on retention indices (RI) and the comparison of their mass spectra in the spectrogram library.20 Essential oil components were reported in the form of relative concentrations of each peak area per total area in the gas chromatogram.
100. The temperature of the oven was initially set at 60 °C for 5 min, then heated gradually to 240 °C at the rate of 4 °C min−1 and was kept constant for 10 min. The temperature of the detector and interface was 270 °C. The mass spectra were recorded at 70 eV in the spectral range of 35–450 Da at 1.0 scan per s. The identification of essential oil constituents was carried out based on retention indices (RI) and the comparison of their mass spectra in the spectrogram library.20 Essential oil components were reported in the form of relative concentrations of each peak area per total area in the gas chromatogram.
2.4 Preparation of E. kachinensis and E. ciliata nanoemulsions
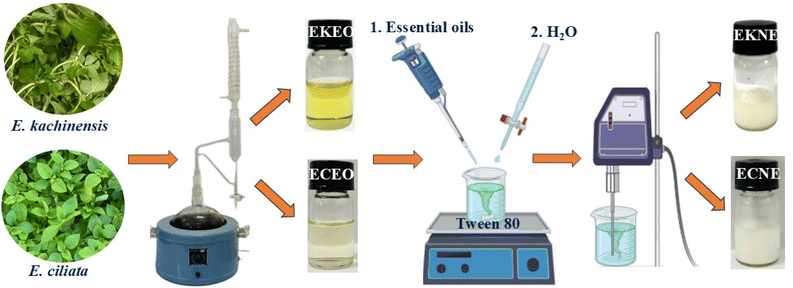
The nanoemulsions of E. kachinensis (EKNE) and E. ciliata essential oils (ECNE) were prepared according to the method described by X. Fu et al.,13 with slight modifications to obtain nanoemulsions with droplet sizes below 100 nm, narrow distributions, and good stabilities. According to X. Fu, Tween 80 is a suitable surfactant for the preparation of stable nanoemulsions. Tween 80 (15% (w/w)) and essential oils (10% (w/w)) were mixed until homogeneous using a magnetic stirrer at 1000 rpm for 15 min. Distilled water was then added dropwise to the mixture at a rate of 1 mL min−1 while stirring continuously at 800 rpm for 10 min. The mixture was then made up to a total volume of 50 mL using distilled water and subjected to ultrasonic treatment for 15 min at 2 kHz and 300 W operation power using an ultrasonic homogenizer (Scientz, Ningbo Xinzhi Biotechnology, China). The turbidity of the obtained nanoemulsions were measured and stored at 4 °C for 0 and 30 days in order to determine their droplet sizes, dispersion indexes, and zeta potentials. Their FTIR spectra and bioactivities were also evaluated. Fig. 1 displays the schematic representation of the synthesis of E. kachinensis and E. ciliata nanoemulsions.2.5 Characteristics of the nanoemulsions
The optical density absorption of the nanoemulsions was measured at 600 nm using a UV-Vis spectrophotometer (Double Beam Spectrophotometer UH5300). The turbidity values of the nanoemulsions were calculated as follows:21| T = (2.023 × A)/L |
The droplet size, polydispersion index (PDI), zeta potential and electrophoretic mobility of the nanoemulsions were determined using dynamic light scattering (DLS) (Horiba SZ-100, Japan) in the particle size measurement range of 0.3 nm to 10 μm and zeta potential range from −500 to +500 mV. The nanoemulsions were diluted to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio with distilled water before measurement. The compositions of the nanoemulsions were identified using FTIR (Perkin Spectrum Two, Japan) by scanning in the wavenumber range of 4000–450 cm−1.
50 ratio with distilled water before measurement. The compositions of the nanoemulsions were identified using FTIR (Perkin Spectrum Two, Japan) by scanning in the wavenumber range of 4000–450 cm−1.
2.6 Bioactivity
| % Cell proliferation = (ODtreated samples/ODcontrol) × 100 |
The IC50 values, which represent the concentration of the sample that inhibits 50% cell growth, were calculated based on the optical density data and analyzed using the GraphPad Prism 5.0 software. The Mann–Whitney test was employed to identify the statistical significance of the data. Each experiment was repeated 3 times.
2.7 Computational investigations
Complementing the experimental findings, computational analysis was performed to explain the stability of nanoemulsions by evaluating their electrostatic interactions and zeta potentials. The geometrical structures and Mulliken atomic charges of molecules, including Tween 80, dehydroelsholtzia, trans-β-ocimene, and β-farnesene, were optimized at the B3LYP/6-31G* level. All computations were implemented using the Gaussian 16 software package.253 Results and discussion
3.1 Chemical compositions of the E. kachinensis and E. ciliata essential oils
The essential oils of E. kachinensis and E. ciliata were obtained by steam distillation with yields of 0.015% and 0.036%, respectively, compared with the weights of fresh samples. The results of the compositional analysis of E. kachinensis and E. ciliata essential oils are shown in Table S1,† Fig. 2a and b. Clearly, 36 compounds were identified in the essential oils of E. kachinensis and E. ciliata, representing 99.6% and 99.9% of their total compositions, respectively.The major components of the E. kachinensis essential oil included dehydroelsholtzia ketone (62.86%), D-limonene (6.75%), β-caryophyllene (5.42%), 1-octen-3-ol-acetate (4.8%), and α-humulene (4.4%). The structures of these compounds are shown in Fig. 3. However, 36 components accounting for 98.767% of its composition were identified in the essential oil of this species harvested from Yunnan Province, China, with carvone (32.298%), dehydroelsholtzia ketone (31.540%), E-β-farnesene (10.098%), [2,2-dimethyl-4-(3-methylbut-2-enyl)-6-methylidenecyclohexyl]methanol (4.781%) and 1-octen-3-ol-acetate (4.123%)8 as major constituents. While the main compounds of E. ciliata were trans-β-ocimene (29.21%), β-farnesene (24.25%), α-citral (11.15%), β-citral (8.99%) and β-caryophyllene (6.24%). The structures of these compounds are presented in Fig. 4. These components are distinct from those reported in previous works. For example, β-farnesene (10.8–11.7%), neral (15.2–20.5%), geranial (19.5–26.5%) and D-limonene (10.9–14.2%) were the major constituents of E. ciliata essential oils extracted from samples in south Vietnam,26 whereas dehydroelsholtzia ketone (71.34%) and elsholtzia ketone (24.94%) were the main components of E. ciliata essential oils from Vilnius, Lithuania.3 These differences in the essential oil components could be the result of factors such as location, climate, harvesting time and extraction method.27 As a result, the quality and bioactivities of these essential oils may vary. Furthermore, previous studies have shown that most of the compounds in both essential oils possess promising therapeutic effects. Dehydroelsholtzia ketone, a terpene ketone, demonstrates inhibitory effects on various cancer cells, indicating its potential use for cancer treatment.3 β-Caryophyllene is a natural compound with significant anticancer activity against several cancer cell types, and it can induce apoptosis and inhibit the growth of cancer cells.28,29 These compounds are capable of donating electrons to free radicals, neutralizing their reactivity and preventing them from causing oxidative damage to cells. In addition, α and β-citral and monoterpenes, exhibit notable antibacterial, antioxidant and anticancer activities,30,31 while ocimene shows cytotoxic effects against cancer cells, as well as antibacterial properties against various pathogens.32 Therefore, the essential oils of E. kachinensis and E. ciliata exhibit great application scope in medicinal and pharmaceutical fields. However, it is necessary to formulate these oils into nanoemulsions for practical applications due to the poor solubility and stability of these essential oils.
3.2 Characteristics of the nanoemulsions of essential oils
The characteristics of nanoemulsions of essential oils are influenced by factors, such as the chemical composition of the essential oil, the surfactant used and the synthesis method.16 Among surfactants, Tween 80 and Tween 20 are commonly used in the synthesis of essential oil-based nanoemulsions as they provide high stability, simplicity and safety without the need for a co-surfactant. Furthermore, Tween 80 has been demonstrated to be effective and is considered the optimal choice for achieving nanoemulsions with desired properties.16 Thus, in the current study, Tween 80 was selected as the surfactant for the preparation of E. kachinensis and E. ciliata nanoemulsions. Ultrasonic homogenization is one of the most efficient techniques for preparing nanoemulsions from essential oils.16 Thus, in this study, ultrasonic homogenization was used for the preparation of nanoemulsions. Additionally, the composition of the essential oil plays a crucial role in determining the stability and properties of the nanoemulsion, as different chemical constituents in the oil can interact with the surfactant and aqueous phase in different ways, influencing droplet size, stability, and polydispersity. Thus, the selection of essential oils with appropriate chemical profiles in conjunction with Tween 80 is key to the successful formation and stabilization of nanoemulsions.In this study, the nanoemulsions of E. kachinensis and E. ciliata were synthesized using Tween 80 as the surfactant. The characteristics of these nanoemulsions, including turbidity, average drop size, polydispersity, zeta potential and electrophoretic mobility, were identified, as given in Table 1. Since the turbidity of a nanoemulsion is an important parameter for identifying its quality and stability, monitoring this parameter can be useful for optimizing the formulation for intended applications. As seen in Table 1, the turbidity of the E. kachinensis nanoemulsion was higher than that of the E. ciliata nanoemulsion, resulting in stronger light scattering. This result is consistent with their average droplet sizes given in Fig. 5. Accordingly, the average droplet size of the E. kachinensis nanoemulsion was larger compared to that of the E. ciliata nanoemulsion, which may be due to the chemical composition of the essential oils. Dehydroelsholtzia ketone is the major component of the E. kachinensis essential oil with larger polarity and molecular weight than monoterpenes, such as trans-β-ocimene and β-farnesene found in the E. ciliata essential oil, leading to the formation of larger droplets. This result is in agreement with previous reports,33,34 which suggested that compounds with higher molecular weight tend to form larger sizes.
| Sample | Storage time (days) | Turbidity (cm−1) | Average drop size (nm) | Polydispersity | Zeta potential (mV) | Electrophoretic mobility (cm2 V−1 s−1) |
|---|---|---|---|---|---|---|
| E. kachinensis nanoemulsion | 0 | 1.757 ± 0.765 | 72.81 ± 2.12 | 0.281 ± 0.023 | −27.8 ± 0.9 | −0.000215 ± (−1.52 × 10−6) |
| 30 | 1.654 ± 0.546 | 95.16 ± 3.25 | 0.189 ± 0.015 | −20.1 ± 1.2 | −0.000155 ± (−1.34 × 10−6) | |
| E. ciliata nanoemulsion | 0 | 1.525 ± 0.643 | 32.13 ± 1.65 | 0.336 ± 0.042 | −11.2 ± 0.7 | −0.000087 ± (−0.98 × 10−6) |
| 30 | 1.456 ± 0.651 | 73.05 ± 1.89 | 0.231 ± 0.035 | −7.9 ± 0.8 | −0.000061 ± (−1.00 × 10−6) |
 | ||
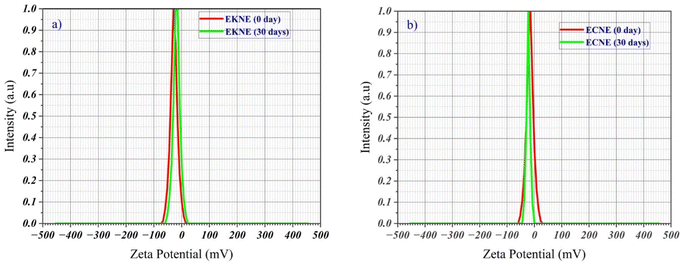
| Fig. 5 Size distribution based on the DLS intensity of (a) E. kachinensis nanoemulsion and (b) E. ciliata nanoemulsion. | ||
We further considered the PDI value, which is a dimensionless indicator of the size distribution of droplets. Typically, a low PDI value (<0.3) signifies a narrow size distribution, whereas values above 0.7 indicate a broad size distribution.35 In this study, the PDI value of E. kachinensis (0.281) was lower than that of E. ciliata (0.336), which indicates a narrow size distribution in the E. kachinensis nanoemulsion. This is possibly due to the dominance of dehydroelsholtzia ketone in the E. kachinensis essential oil, resulting in a more uniform droplet size distribution in its nanoemulsion compared with that of the E. ciliata nanoemulsion. Since zeta potential is a main factor that represents the electrical charge of particles and defines the stability of nanoemulsions, this parameter was also monitored. The results showed that both nanoemulsions displayed negative zeta potential values, probably due to the non-ionic surfactant (Tween 80) and the negative charge of the essential oils. The ionization of hydroxyl groups in Tween 80 during dispersion into the medium and the presence of terpene in the essential oils may have contributed to the negative zeta potential values. In previous studies, nanoemulsions of the essential oils of eugenol,13 garlic,12 and green tea18 prepared with Tween 80 have also shown negative zeta potential values. In addition, the E. kachinensis nanoemulsion had a significantly higher absolute zeta potential value (−27.8 mV) than that of the E. ciliata nanoemulsion (−11.2 mV) (Fig. 6), suggesting that the E. kachinensis nanoemulsion was more stable.
The electrophoretic mobilities of the E. kachinensis and E. ciliata nanoemulsions were −0.000215 and −0.000087 cm2 V−1 s−1, respectively. These results indicate that the E. kachinensis nanoemulsion had greater stability, more uniform particle size distribution and less aggregation than the E. ciliata nanoemulsion. These findings align with the results of PDI and zeta potential mentioned above.
The stability of nanoemulsions is critical for their applications. Key parameters, including droplet size, PDI and zeta potential, are commonly utilized to examine nanoemulsion stability. In this study, these parameters were measured at the beginning (0 days) and after 30 days of storage at 4 °C. As shown in Table 1, Fig. 5, and 6, the droplet sizes of both E. kachinensis and E. ciliata nanoemulsions exhibited an increase over the storage period of 30 days, rising from 72.81 to 95.16 nm and 32.13 to 73.05 nm, respectively. This is possibly due to coalescence or Ostwald's ripening, which is an issue observed in oil-in-water emulsions.36,37
In the aqueous phase, oil molecules surrounding smaller droplets generally demonstrate greater water solubility than those surrounding larger droplets.36,37 Consequently, oil molecules can be transferred from smaller to larger droplets, resulting in an increase in droplet size. However, despite this increase, the droplet sizes of the E. kachinensis and E. ciliata nanoemulsions remained below 100 nm. Furthermore, the turbidity, PDI, zeta potential values and electrophoretic mobilities of both nanoemulsions decreased after 30 days of storage. The PDI values decreased from 0.281 to 0.189 for the E. kachinensis nanoemulsion and 0.336 to 0.231 for the E. ciliata nanoemulsion, indicating greater uniformity in droplet size distribution. This trend is consistent with changes in droplet size, which may be attributed to electrostatic repulsion among particles. Similarly, the zeta potential values also decreased from −27.8 to −20.1 mV for the E. kachinensis nanoemulsion and from −11.2 to −7.9 mV for the E. ciliata nanoemulsion. Previous reports have demonstrated similar changes in the droplet size, PDI and zeta potential values of a nanoemulsion formulated from citrus38 and Cymbopogon nardus39 essential oils. Based on the above analysis, the nanoemulsion maintained good stability during storage time.
FTIR spectroscopy was used to identify the characteristic functional groups of the essential oils and investigate the interactions between the essential oils, water, and surfactant by measuring the absorption peaks. As seen in Fig. 7, the spectra exhibited the characteristic peaks of Tween 80, E. kachinensis, and E. ciliata essential oils, respectively, without the appearance of any new peaks.
 | ||
| Fig. 7 FTIR spectra of the essential oils, nanoemulsions and Tween 80: (a) E. kachinensis, (b) E. ciliata. | ||
The FTIR spectra of E. kachinensis nanoemulsion presented characteristic peaks at 3447, 1738, 1645, 1045 and 1089 cm−1, corresponding to the stretching vibrations of O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–O groups, which are typical groups found in E. kachinensis essential oils and Tween 80. However, the intensity of the O–H peak and its broad band at 3447 cm−1 compared with the FTIR spectrum of E. kachinensis essential oils and Tween 80 can be due to the presence of a water phase. Furthermore, the presence of characteristic functional groups of the essential oils in the nanoemulsion suggested that the components of the essential oils were retained during the nanoemulsion formation process. The analysis of E. ciliata nanoemulsion yields similar results to that of E. kachinensis nanoemulsion. This result indicated that Tween 80 played the role as a surfactant, and no chemical interactions occurred between this compound and essential oils.
O and C–O groups, which are typical groups found in E. kachinensis essential oils and Tween 80. However, the intensity of the O–H peak and its broad band at 3447 cm−1 compared with the FTIR spectrum of E. kachinensis essential oils and Tween 80 can be due to the presence of a water phase. Furthermore, the presence of characteristic functional groups of the essential oils in the nanoemulsion suggested that the components of the essential oils were retained during the nanoemulsion formation process. The analysis of E. ciliata nanoemulsion yields similar results to that of E. kachinensis nanoemulsion. This result indicated that Tween 80 played the role as a surfactant, and no chemical interactions occurred between this compound and essential oils.
The mechanism of nanoemulsion formation can be proposed as follows. Tween 80, a non-ionic surfactant with a hydrophilic head and a lipophilic tail, functions to reduce interfacial tension between the essential oils and water. Initially, the hydrophobic tail of Tween 80 adheres to the oil droplets. With the addition of the aqueous phase, the hydrophilic head of Tween 80 interacts with water. As the interfacial tension between the oils and water phase decreases, mechanical processes, such as stirring and ultrasonication, cause the oil droplets to break into smaller droplets. These smaller oil droplets are then stabilized by a layer of Tween 80, which creates both electrostatic and steric repulsion, preventing the coalescence of these droplets into larger ones, thereby stabilizing the emulsion system.
Molecular activity is key to determining the chemical properties and structural positions during chemical reactions.40–42 The interactions of compounds can be significantly influenced by the distribution of atomic charges. The measurement of localized reactive regions is important because it enables the interpretation of reactive variations due to different atomic positions in a molecule.43,44 This information can be obtained from Mulliken atomic charges.43 Before calculating Mulliken atomic charges, the molecular structures of compounds, including Tween 80, dehydroelsholtzia ketone, trans-β-ocimene and β-farnesene, were optimized, as shown in Fig. 8. Mulliken atomic charges of Tween 80, dehydroelsholtzia ketone, trans-β-ocimene and β-farnesene were analyzed and are listed in Tables S1–S4† and depicted in Fig. 9a–d. The atomic charges of Tween 80 revealed that all hydrogen atoms had a positive charge; however, H67, H68, and H71 possessed a higher positive charge (from 0.390958 to −0.394601) than the other hydrogen atoms. These hydrogen atoms attack oxygen atoms and can form hydrogen bonds with other molecules. Among the carbon atoms, C36 (0.62954) had the highest positive charge, while C42 (−0.4413) had the highest negative charge. Furthermore, the charge distribution on the oxygen atoms (O6, O7, O8) indicated that these sites exhibited higher electron density (from −0.60662 to −0.60842), suggesting their potential as proton acceptors. The Mulliken atomic charge analysis of dehydroelsholtzia ketone demonstrated that two oxygen atoms (O1 and O2) had significant negative charges (−0.45101 and −0.52453), rendering them capable of forming hydrogen bonds with H67, H68, and H71 of Tween 80. As a result, this compound is effectively stabilized in aqueous environments through hydrogen bonding interactions with Tween 80.
 | ||
| Fig. 8 Optimized molecular structures of Tween 80, dehydroelsholtzia ketone, trans-β-ocimene and β-farnesene. | ||
 | ||
| Fig. 9 Mulliken atomic charges of (a) Tween 80, (b) dehydroelsholtzia ketone, (c) trans-β-ocimene and (d) β-farnesene. | ||
Additionally, carbon atoms C9 (−0.50462), C11 (−0.51198), and C12 (−0.53255) exhibited strong negative charges, indicating hydrophobicity. Consequently, the hydrophobic segment of Tween 80 may encapsulate these regions, thereby enhancing the solubility of dehydroelsholtzia ketone in the aqueous phase. This encapsulation prevents phase separation and aggregation, ensuring stable dispersion of the compound within the emulsion system. Meanwhile, the Mulliken atomic charges of the principal components in E. ciliata, such as trans-β-ocimene and β-farnesene, revealed that both molecules contained negatively charged carbon atoms. Among the carbon atoms, C6, C7, and C8 in trans-β-ocimene and C8, C11, and C12 in β-farnesene had higher negative charges. These hydrophobic regions exhibited a strong tendency to be encapsulated by Tween 80 via van der Waals interactions, further contributing to the stabilization of these compounds within the emulsion system. As a result, dehydroelsholtzia ketone, the main component of E. kachinensis essential oils, interacts more effectively with both the hydrophilic and lipophilic regions of Tween 80 via hydrogen bonds and van der Waals interaction, further contributing to the better stabilization of these compounds within the emulsion system than trans-β-ocimene and β-farnesene in E. ciliata essential oils. These results explain that the E. kachinensis nanoemulsion was more stable, with a higher absolute zeta potential value than the E. ciliata nanoemulsion.
3.3 Antibacterial activity of the E. kachinensis and E. ciliata nanoemulsions
To the best of our knowledge, there is no previous report on the antibacterial activity of nanoemulsions of E. kachinensis and E. ciliata essential oils. Therefore, for the first time, their antibacterial activities are reported here. The agar disc diffusion method revealed their effective inhibition of the two tested bacterial strains, namely P. aeruginosa and S. aureus (Fig. 10). The inhibition zone diameters were 12.0 ± 0.3 and 14.0 ± 0.2 mm for the E. ciliata essential oil, 14.3 ± 0.3 and 15.5 ± 0.1 mm for its nanoemulsion, 11.2 ± 04 and 12.5 ± 0.2 mm for the E. kachinensis essential oil, and 13.8 ± 04 and 15.0 ± 04 mm for its nanoemulsion. In comparison, ciprofloxacin displayed inhibition zones of 12.0 ± 0.2 and 15.2 ± 0.3 mm against P. aeruginosa and S. aureus, respectively, while Tween (15%) exhibited no antibacterial activity. These results indicate that the nanoemulsions of both essential oils possessed enhanced antibacterial efficacy against the tested strains compared with their corresponding essential oils. Similar results have been reported in previous studies.45,46 | ||
| Fig. 10 Antibacterial activity of E. kachinensis and E. ciliata essential oils and their nanoemulsions against (a) P. aeruginosa and (b) S. aureus. | ||
The results of the antibacterial activity of the samples based on the broth microdilution method are provided in Table 2 and Fig. 11. In general, both nanoemulsions had MIC values in the range of 0.0037 to 1.8750 mg mL−1 and were found to have a greater inhibitory effect on bacteria than their corresponding essential oils. Our findings are in accordance with previous antibacterial results of nanoemulsions prepared from thyme,47 Sichuan pepper,48 sage,49 Lavandula intermedia50 and garlic essential oils.12 Similarly, the nanoemulsion of laurel essential oil was more effective against Staphylococcus aureus and Enterococcus faecalis than laurel essential oils itself.27 In addition, these results show that the essential oil and nanoemulsion of E. kachinensis were more effective against Gram-negative bacteria than Gram-positive. Moreover, the inhibitory effect of E. kachinensis essential oil collected from Cao Bang, Vietnam on E. coli and P. aeruginosa was better than that collected from Guizhou, China.51 The MIC value of the E. kachinensis essential oil collected from Guizhou, China against both E. coli and P. aeruginosa was 1.3 mg mL−1. However, its effect on S. aureus and B. subtilis was lower, with MIC values of 0.64 and 0.32 mg mL−1, respectively.51 This difference in antibacterial activity is possible because of the composition of essential oil components and their quality. Meanwhile, the essential oil and nanoemulsion of E. ciliata exhibited more effective antibacterial activity against Gram-positive bacteria than Gram-negative bacteria. Especially, the essential oil and nanoemulsion of E. ciliata provided the best activity against S. aureus, with MIC values of 0.625 and 0.0372 mg mL−1, respectively. According to Tian 2013, E. ciliata essential oil collected from China showed strong inhibitory activity against S. aureus, B. subtilis and E. coli, with MIC values of 6.88, 0.02 and 1.08 μL mL−1, respectively.52 Besides, findings from previous studies indicate that Tween 80 might reduce the antibacterial efficacy of antibacterial agents, such as nanoemulsions.53,54
| Bacteria | MIC values (mg mL−1) | MIC values (μg mL−1) | ||||
|---|---|---|---|---|---|---|
| E. kachinensis essential oil | E. kachinensis nanoemulsion | E. ciliata essential oil | E. ciliata nanoemulsion | Tween 80 | Ciprofloxacin | |
| E. coli | 0.9375 ± 0.0000 | 0.6250 ± 0.2706 | 0.9375 ± 0.0000 | 0.4688 ± 0.0000 | 3.7500 ± 1.8334 | 0.0156 ± 0.0000 |
| P. aeruginosa | 0.9375 ± 0.0000 | 0.7813 ± 0.2706 | 0.9375 ± 0.0000 | 0.7813 ± 0.2706 | 2.8125 ± 0.0000 | 0.1875 ± 0.0000 |
| K. pneumoniae | 0.6250 ± 0.2706 | 0.6250 ± 0.2706 | 0.4688 ± 0.0000 | 0.3125 ± 0.1353 | 2.8125 ± 0.0000 | 0.5000 ± 0.0000 |
| S. aureus | 1.8750 ± 0.0000 | 0.9375 ± 0.0000 | 0.3906 ± 0.1353 | 0.1503 ± 0.0677 | 5.6250 ± 0.0000 | 0.2500 ± 0.0000 |
| B. subtilis | 0.7810 ± 0.2706 | 0.625 ± 0.2706 | 0.1953 ± 0.0677 | 0.0372 ± 0.0136 | 4.6875 ± 1.6231 | 0.0625 ± 0.0000 |
| S. epidermidis | 0.9375 ± 0.000 | 0.625 ± 0.2706 | 0.3906 ± 0.1105 | 0.0743 ± 0.0371 | 2.8125 ± 0.0000 | 0.1250 ± 0.0000 |
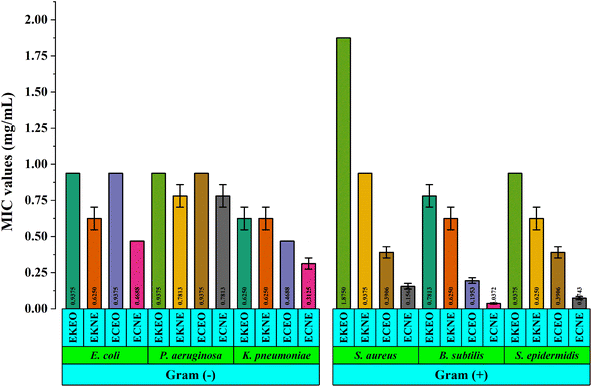 | ||
| Fig. 11 Antibacterial activity of the E. kachinensis and E. ciliata essential oils and their nanoemulsions (n = 3, error bars show the standard deviations). | ||
The results also showed a significant correlation between the concentration of Tween 80 in the nanoemulsions and their antibacterial effects. These findings emphasized the necessity for optimizing the Tween 80 concentration in these nanoemulsion formulations to maximize their antimicrobial effectiveness while maintaining stability. When only 15% Tween 80 was used, the antibacterial activity of the nanoemulsions did not change much, with MIC values remaining ≥2.8125 mg mL−1, proving no obvious side effects of 15% Tween 80 on the activity of the nanoemulsions. This finding is similar to that reported by Hou.55 Ciprofloxacin was used as the standard control in this study, and it was found to be highly active in comparison with the nanoemulsions.
3.4 Anticancer activity against HepG2 liver cancer cells
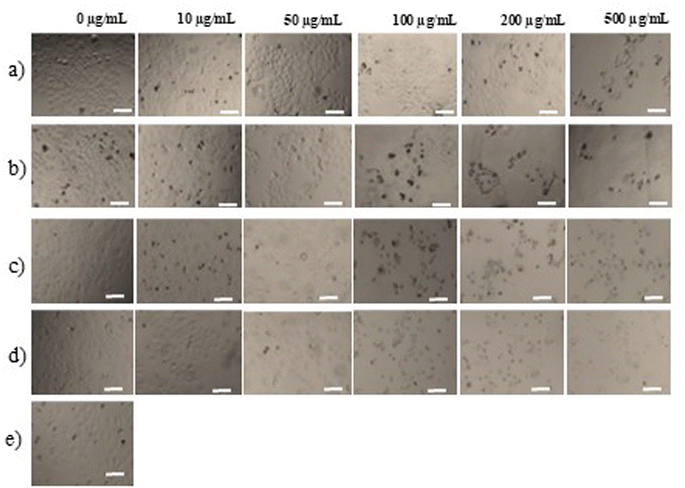
Although there have been some reports on the anticancer properties of essential oils or extracts from certain species of the Elshotzia genus, studies on nanoemulsions synthesized from these species, as well as their activity, are still limited.The effects of the essential oils and their nanoemulsions of two species belonging to the Elshotzia genus (i.e., E. kachinensis and E. ciliata) on HepG2 liver cancer cell morphology are shown in Fig. 12a–d. Fig. 12a demonstrates that at concentrations ranging from 10 to 50 μg mL−1, the E. kachinensis essential oil had minimal effects on cell density and morphology. At higher concentrations (100 to 500 μg mL−1), large gaps appeared, indicating that the essential oil reduced the cell proliferation capacity, while some dark-colored dead cells, which were not adherent to the culture plate surface, were observed. When cells were treated with the E. kachinensis essential oil nanoemulsion (Fig. 12b), a significant reduction in cell density was observed at concentrations ≥ 50 μg mL−1, and the appearance of dead cells was noted at concentrations ≥ 100 μg mL−1. The impact of the E. ciliata essential oil on HepG2 liver cancer cell morphology (as shown in Fig. 12c) was evident at concentrations above 50 μg mL−1, while the nanoemulsion of E. ciliata essential oil caused a noticeable reduction in cell density even at 10 μg mL−1. The nanoemulsion of E. ciliata essential oil (Fig. 12d) also led to the appearance of dead cells at concentrations as low as 50 μg mL−1. A quantitative analysis of the ability of the essential oils and nanoemulsions to inhibit cell proliferation is illustrated in Fig. 13a and b.
As seen in Fig. 13a, the nanoemulsion of E. kachinensis essential oil caused cell inhibition ranging from 20% to 80%, which was significantly higher than that elicited by the E. kachinensis essential oil at each concentration (p < 0.05). The IC50 values were determined to be 84.3 μg mL−1 for the E. kachinensis nanoemulsion and greater than 200 μg mL−1 for the E. kachinensis essential oil. Similar results are also given in Fig. 13b; the inhibition percentage of the E. ciliata essential oil ranged from 7% to 65%, while the inhibition percentage of the E. ciliata nanoemulsion ranged from 25% to 80% (p < 0.05). The IC50 values were determined to be 72.1 μg mL−1 for the E. ciliata nanoemulsion and 163.7 μg mL−1 for the E. ciliata essential oil. Thus, both nanoemulsions exhibited significantly stronger inhibitory effects than the corresponding essential oils. Meanwhile, in this cell line, 5-FU demonstrated IC50 values ranging from 117 to 128 μM. At a concentration of 100 μM, 5-FU achieved an approximate inhibition rate of 40%, which provides a sufficiently distinct reference point for comparative analysis with the nanoemulsions. Previous studies have indicated the anticancer activity of the Elshotzia genus against human glioblastoma, pancreatic cancer, and breast cancer.56 The nanoemulsion of Pinus morrisonicola essential oil with a droplet size of 41.1 nm and polydispersion index of 0.31 showed a stronger inhibitory effect on cancer cells than normal cells (HFF).17 Additionally, the nanoemulsion exhibited effective antioxidant activity by inhibiting ABTS and DPPH free radicals with IC50 values of 4 and 40 μg mL−1, respectively.17 In this study, we demonstrate that essential oils from E. ciliata and E. kachinensis could inhibit the proliferation of HepG2 liver cancer cells. Manaa reported that a nanoemulsion of oregano essential oil exhibited significantly reduced IC50 values against the A549 cell line compared with the free essential oil.57 Thus, the results from this study propose that using nanoemulsion form is an effective strategy for enhancing the anti-cancer efficacy of essential oils.
4 Conclusion
Nanoemulsions were successfully prepared from E. kachinensis and E. ciliata essential oils using ultrasonic homogenization in an aqueous medium containing Tween 80 as the surfactant. The prepared nanoemulsions exhibited good stability and droplet sizes in the nanoscale (i.e., 72.81 and 32.13 nm). In addition, the E. kachinensis nanoemulsion displayed better size distribution, PDI < 0.3 and better stability than the E. ciliata nanoemulsion. The Mulliken atomic charge analysis explained that the E. kachinensis nanoemulsion was more stable than the E. ciliata nanoemulsion. However, the nanoemulsion of E. ciliata displayed stronger antibacterial activity and anticancer activity than the nanoemulsion of E. kachinensis. Both nanoemulsions exhibited significantly higher antibacterial and anticancer activities than the corresponding essential oils, indicating the advantages of nanoemulsions. Based on the results of this study, we also propose that the development of an efficient and safe delivery system holds significant potential in advancing the utilization of essential oils for antibacterial and anticancer activities.Data availability
The datasets produced and analyzed during this study are available from the corresponding author upon reasonable request.Author contributions
Nguyen Quang Tinh: conceptualization, investigation, writing-original draft; Dang Van Thanh: conceptualization, resource, editing, Nguyen Van Thu: data curation, methodology, formal analysis, Bui Thi Quynh Nhung: resource, methodology, investigation, Pham Ngoc Huyen: conceptualization, investigation, data curation, Nguyen Phu Hung: formal analysis, writing-review, Nguyen Thi Thuy: formal analysis, validation and editing, Pham Dieu Thuy: data curation, methodology, formal analysis, Nguyen Hoa Mi: software, visualization, formal analysis, Khieu Thi Tam: conceptualization, resource, writing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Ministry of Education and Training, Vietnam, under the project number B2024-TNA-14. We would like to thank Professor Mori Seiji, the Institute of Quantum Beam Science, Ibaraki University, Japan, for the use of its server and software.Notes and references
- Z. Guo, Z. Liu, X. Wang, W. Liu, R. Jiang, R. Cheng and G. She, Chem. Cent. J., 2012, 6, 1–8 CrossRef.
- A.-L. Liu, S. M. Lee, Y.-T. Wang and G.-H. Du, J. Chin. Pharm. Sci., 2007, 16, 73 CAS.
- L. Pudziuvelyte, M. Stankevicius, A. Maruska, V. Petrikaite, O. Ragazinskiene, G. Draksiene and J. Bernatoniene, Ind. Crops Prod., 2017, 107, 90–96 CrossRef CAS.
- T. Sripahco, S. Khruengsai, R. Charoensup, J. Tovaranonte and P. Pripdeevech, Sci. Rep., 2022, 12, 2225 CrossRef CAS PubMed.
- J. Liang, Y. Shao, H. Wu, Y. An, J. Wang, J. Zhang and W. Kong, Foods, 2021, 10, 2304 CrossRef CAS PubMed.
- P. N. Paudel, P. Satyal, W. N. Setzer, S. Awale, S. Watanabe, J. Maneenet, R. Satyal, A. Acharya, M. Phuyal and R. Gyawali, Nat. Prod. Commun., 2023, 18, 1934578X231189325 CrossRef CAS.
- S. Chen, J. Chen, Y. Xu, X. Wang and J. Li, J. Ethnopharmacol., 2022, 297, 115549 CrossRef CAS PubMed.
- J.-W. Zhang, Y.-X. Feng, Y.-S. Du, X.-X. Lu, Y. Zheng, W. Dan and S.-S. Du, J. Oleo Sci., 2022, 71, 1075–1084 CrossRef CAS PubMed.
- F. Li, C. Wang, J. Xu, X. Wang, M. Cao, S. Wang, T. Zhang, Y. Xu, J. Wang and S. Pan, Front. Microbiol., 2023, 14, 1219004 CrossRef.
- J. Mendes, H. Martins, C. Otoni, N. Santana, R. Silva, A. Da Silva, M. Silva, M. Correia, G. Machado and A. Pinheiro, LWT, 2018, 93, 659–664 CrossRef CAS.
- Z. Lou, J. Chen, F. Yu, H. Wang, X. Kou, C. Ma and S. Zhu, Lwt, 2017, 80, 371–377 CrossRef CAS.
- M. Liu, Y. Pan, M. Feng, W. Guo, X. Fan, L. Feng, J. Huang and Y. Cao, Ultrason. Sonochem., 2022, 90, 106201 CrossRef CAS PubMed.
- X. Fu, Y. Gao, W. Yan, Z. Zhang, S. Sarker, Y. Yin, Q. Liu, J. Feng and J. Chen, RSC Adv., 2022, 12, 3180–3190 RSC.
- A. Gupta, H. B. Eral, T. A. Hatton and P. S. Doyle, Soft Matter, 2016, 12, 2826–2841 RSC.
- D. Renggli and P. S. Doyle, Soft Matter, 2025, 21, 652–669 RSC.
- I. R. Singh and A. K. Pulikkal, OpenNano, 2022, 8, 100066 CrossRef CAS.
- N. Khatamian, M. Soltani, B. Shadan, A. Neamati, M. H. Tabrizi and B. Hormozi, Inorg. Nano-Met. Chem., 2022, 52, 253–261 Search PubMed.
- A. B. Perumal, X. Li, Z. Su and Y. He, Ultrason. Sonochem., 2021, 76, 105649 CrossRef CAS PubMed.
- W. Wang, Z. Leng, Q. Liu, J. Zhao and S. Li, Ind. Crops Prod., 2024, 219, 118987 CrossRef CAS.
- R. P. Adams, Identification of Essential Oil Components by Gas Chromatography/mass Spectrometry, Allured Publishing Corporation Carol Stream, 2007 Search PubMed.
- M. D. Julian and R. Jiajia, Crit. Rev. Food Sci. Nutr., 2011, 51, 285–330 CrossRef.
- T. P. Lazou and S. C. Chaintoutis, J. Microbiol. Methods, 2023, 204, 106649 CrossRef CAS PubMed.
- S. Hussiny, A. Elissawy, O. Eldahshan, M. Elshanawany and A.-N. Singab, Arch. Pharm. Sci. Ain Shams Univ., 2020, 4, 207–214 Search PubMed.
- A. Russo, C. Formisano, D. Rigano, F. Senatore, S. Delfine, V. Cardile, S. Rosselli and M. Bruno, Food Chem. Toxicol., 2013, 55, 42–47 CrossRef CAS PubMed.
- M. Frisch, G. Trucks, H. Schlegel, G. Scuseria, M. Robb, J. Cheeseman, G. Scalmani, V. Barone, G. Petersson and H. Nakatsuji, Gaussian 16, Wallingford CT, 2016 Search PubMed.
- N. X. Dũng, L. Van Hac, L. H. Hái and P. A. Leclercq, J. Essent. Oil Res., 1996, 8, 107–109 CrossRef.
- Y. Özogul, N. El Abed and F. Özogul, Food Chem., 2022, 368, 130831 CrossRef PubMed.
- K. Fidyt, A. Fiedorowicz, L. Strządała and A. Szumny, Cancer Med., 2016, 5, 3007–3017 CrossRef CAS PubMed.
- S. S. Dahham, Y. M. Tabana, M. A. Iqbal, M. B. Ahamed, M. O. Ezzat, A. S. Majid and A. M. Majid, Molecules, 2015, 20, 11808–11829 CrossRef CAS PubMed.
- S. A. Singh, Y. A. Potdar, R. S. Pawar and S. V. Bhat, Nat. Prod. Commun., 2011, 6, 1934578X1100600902 CrossRef.
- S. Ben-Yehoshua and R. Ofir, 2009.
- A. I. Hussain, F. Anwar, S. Rasheed, P. S. Nigam, O. Janneh and S. D. Sarker, Rev. Bras. Farmacogn., 2011, 21, 943–952 CrossRef CAS.
- O. Campolo, G. Giunti, M. Laigle, T. Michel and V. Palmeri, Ind. Crops Prod., 2020, 157, 112935 CrossRef CAS.
- R. Pathania, R. Kaushik and M. A. Khan, Curr. Res. Nutr. Food Sci., 2018, 6, 626–643 Search PubMed.
- O. Gul, F. T. Saricaoglu, A. Besir, I. Atalar and F. Yazici, Ultrason. Sonochem., 2018, 41, 466–474 CrossRef CAS PubMed.
- S. Kumari, R. Kumaraswamy, R. C. Choudhary, S. Sharma, A. Pal, R. Raliya, P. Biswas and V. Saharan, Sci. Rep., 2018, 8, 6650 CrossRef PubMed.
- P. Pongsumpun, S. Iwamoto and U. Siripatrawan, Ultrason. Sonochem., 2020, 60, 104604 CrossRef CAS PubMed.
- Z. Kang, S. Chen, Y. Zhou, S. Ullah and H. Liang, Innovative Food Sci. Emerging Technol., 2022, 80, 103110 CrossRef CAS.
- N. Somala, C. Laosinwattana and M. Teerarak, Sci. Rep., 2022, 12, 10280 CrossRef CAS PubMed.
- O. Dagdag, A. El Harfi, L. El Gana, Z. S Safi, L. Guo, A. Berisha, C. Verma, E. E. Ebenso, N. Wazzan and M. El Gouri, J. Appl. Polym. Sci., 2021, 138, 49673 CrossRef CAS.
- O. Dagdag, Z. Safi, H. Erramli, N. Wazzan, L. Guo, C. Verma, E. Ebenso, S. Kaya and A. El Harfi, Mater. Today Commun., 2020, 22, 100800 CrossRef CAS.
- R. Ihamdane, M. Tiskar, B. Outemsaa, L. Zelmat, O. Dagdag, A. Berisha, E. Berdimurodov, E. E. Ebenso and A. Chaouch, Arabian J. Sci. Eng., 2023, 48, 7685–7701 CrossRef CAS.
- M. Manssouri, A. Chraka, I. Raissouni, A. Wahby and N. EL Aouad, Anal. Bioanal. Electrochem., 2024, 16, 507–536 Search PubMed.
- R. Solmaz, G. Kardaş, M. Çulha, B. Yazıcı and M. Erbil, Electrochim. Acta, 2008, 53, 5941–5952 CrossRef CAS.
- R. Moghimi, L. Ghaderi, H. Rafati, A. Aliahmadi and D. J. McClements, Food Chem., 2016, 194, 410–415 CrossRef CAS PubMed.
- E. Fachriyah, P. Wibawa and A. Awaliyah, J. Phys.: Conf. Ser., 2020, 1524, 012060 CrossRef CAS.
- Y. Ozogul, E. K. Boğa, I. Akyol, M. Durmus, Y. Ucar, J. M. Regenstein and A. R. Köşker, Food Biosci., 2020, 36, 100635 CrossRef CAS.
- Y. Shi, M. Zhang, K. Chen and M. Wang, Lwt, 2022, 154, 112779 CrossRef CAS.
- H. Yazgan, Lwt, 2020, 130, 109669 CrossRef CAS.
- S. Garzoli, S. Petralito, E. Ovidi, G. Turchetti, V. L. Masci, A. Tiezzi, J. Trilli, S. Cesa, M. A. Casadei and P. Giacomello, Ind. Crops Prod., 2020, 145, 112068 CrossRef CAS.
- C. F. Zhu, W. D. Ping, Y. Z. Chang, L. C. Ming, M. Lin and Y. X. Sheng, Guihaia, 2012, 32, 269–273 Search PubMed.
- G.-h. Tian, Chin. Herb. Med., 2013, 5, 104–108 CAS.
- Q. Ma, P. M. Davidson and Q. Zhong, Int. J. Food Microbiol., 2016, 226, 20–25 CrossRef CAS.
- S. Mansouri, M. Pajohi-Alamoti, N. Aghajani, B. Bazargani-Gilani and A. Nourian, J. Sci. Food Agric., 2021, 101, 3880–3888 CrossRef CAS PubMed.
- K. Hou, Y. Xu, K. Cen, C. Gao, X. Feng and X. Tang, Food Biosci., 2021, 43, 101232 CrossRef CAS.
- F. Wang, X. Liu, Y. Chen, Y. An, W. Zhao, L. Wang, J. Tian, D. Kong, Y. Xu and Y. Ba, Molecules, 2022, 27, 6411 CrossRef CAS PubMed.
- A. O. Manaa, H. H. Baghdadi, N. A. El-Nikhely, L. A. Heikal and L. S. El-Hosseiny, J. Drug Delivery Sci. Technol., 2022, 78, 103978 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00386e |
| This journal is © The Royal Society of Chemistry 2025 |