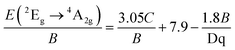DOI:
10.1039/D5RA00377F
(Paper)
RSC Adv., 2025,
15, 7039-7049
Novel efficient deep-red emitting phosphor SrCa2Ga2O6:Mn4+ with tululite-related structure†
Received
16th January 2025
, Accepted 18th February 2025
First published on 4th March 2025
Abstract
Mn4+-activated phosphors have been attracted to replace the rare-earth-activated phosphors in the use of deep-red optical devices. Owing to their low toxicity and wide applications, oxide materials are promising hosts for Mn4+ phosphors. Exploration into novel oxides is important for developing new Mn4+-doped phosphors with high luminescent efficiencies. In this study, we discovered the deep-red emitting phosphor SrCa2Ga2O6:Mn4+ in the Sr3Ga2O6–Ca3Ga2O6 solid solution system. From the single crystal X-ray diffraction analysis, SrCa2Ga2O6:Mn4+ was found to crystallize in a cubic unit cell with space group F432. Furthermore, SrCa2Ga2O6:Mn4+ was revealed to be a new member of tululite structure-related phosphors, such as Ca14Zn6Al10O35:Mn4+, Ca14Zn6Ga10O35:Mn4+, and Ca14Mg4Ga12O36:Mn4+. To study the fundamental luminescence properties, we synthesized SrCa2Ga2O6:Mn4+ powder samples via the conventional solid-state reaction method. SrCa2Ga2O6:Mn4+ has an absorption band in the region of 250–550 nm, and shows a deep-red emission band peaks at 712 nm. The excitation band is well matched to the emission wavelength of near-ultraviolet and blue light emitting diodes. The optimized sample exhibited high quantum efficiency and good thermal quenching properties. This study revealed SrCa2Ga2O6:Mn4+ has excellent potential as a deep-red emitting phosphor and is expected to be used for commercial applications, such as indoor plant cultivation and wavelength down-convertor for solar-cells.
Introduction
Tetravalent manganese ion (Mn4+)-activated phosphors are well known for deep-red emission in the wavelength range of 600 to 760 nm under near-ultraviolet (n-UV) or blue light excitation (250–550 nm).1–3 The major applications of these phosphors are solid-state lighting, indoor plant cultivation, and wavelength down-convertors for solar cells.4–9 Moreover, Mn4+-activated phosphors are considered to replace the major rare-earth (Eu3+, Eu2+, and Ce3+) -activated red-emitting phosphors due to their lower cost and longer emission wavelength.10–13 Therefore, Mn4+ takes an important role as a luminescent center in phosphors.
The most common host materials for Mn4+-doped phosphors are fluorides or oxides. Fluoride-based phosphors, such as K2SiF6:Mn4+ and K2TiF6:Mn4+, have been used as red components for optical devices like light-emitting diodes (w-LEDs). They show narrow band emission peak at approximately 630 nm with high quantum efficiencies (80–90%).14–16 However, fluoride phosphors have some problems, such as the use of highly toxic material HF in synthesis and low physical and chemical stability.17,18 In addition, their short emission wavelength and narrow spectrum are not suitable for applications like artificial plant growth, which requires deep-red emission in the 650–750 nm range.19 On the other hand, Mn4+-activated oxide phosphors typically exhibit deep-red broad emission peaking between 650 and 760 nm. Furthermore, they can be synthesized easier and safer than fluoride phosphors.1 The stronger covalency of Mn4+–O2− bonds compared to Mn4+–F− provide longer emission wavelength, which can fulfill the demands of indoor plant cultivation and wavelength down-converter for solar cells.20 From the both safety and practical perspectives, oxide hosts are favorable.
To improve the luminescence properties of Mn4+-doped phosphors, host lattice tuning by doping with different cations is generally considered. This approach can stabilize the Mn ion as tetravalent, cause structural distortion, and sensitize Mn4+ by energy transfer phenomenon.21–24 However, significant improvements to reach practical use are difficult in many cases. Thus, it is desirable to select phosphors with good luminescence properties as the starting materials. Based on these considerations, we focused on exploring novel oxide materials suitable as Mn4+ phosphors.
We have been searching for new materials suitable as phosphor hosts. For instance, we previously reported the Ba6La2Al1.5Fe2.5O15-type green-emitting phosphor Ba5La3MgAl3O15:Ce3+ and the Olivine-type red-emitting phosphor NaMgPO4:Eu2+.25,26 Similarly, Singh et al. discovered the red-emitting phosphor Li3RbGe8O18:Mn4+ with a novel crystal structure by a combinatorial synthesis within the A2Ge4O9 (A = Li, K, and Rb) series.5 Among these compounds, Li3RbGe8O18:Mn4+ displayed the highest emission intensity, which is a successful example of material discovery. These examples highlight that searching for novel host materials is effective.
In this study, we selected gallate compounds as potential host materials for Mn4+-activated phosphors. For instance of gallate-based Mn4+-doped phosphors, Ca14Zn6Ga10O35:Mn4+, Mg7GeGa2O12:Mn4+, Mg6ZnGeGa2O12:Mn4+, Mg3GeGa2O8:Mn4+, LaGaO3:Mn4+, SrLaGaO4:Mn4+, SrLaGa3O7:Mn4+, and Ca14Mg4Ga12O36:Mn4+ (Ca7Mg2Ga6O18:Mn4+) have been previously reported.27–34 Gallate compounds often make [GaO6] octahedra in these crystal lattices, which are essential for Mn4+-activated phosphors because Mn4+ can display its characteristic emission when placed in a six-fold octahedral coordination environment. Consequently, Mn4+-doped gallate phosphors have been widely studied. In this work, we aimed to discover novel compounds in oxide systems containing Ga2O3.
As a result, we successfully discovered a deep-red-emitting phosphor with the empirical formula SrCa2Ga2O6:Mn4+ in the Sr3Ga2O6–Ca3Ga2O6 solid solution system. SrCa2Ga2O6:Mn4+ exhibits a broad absorption band from n-UV to blue light (250–500 nm) and shows intense deep-red emission peaks at 712 nm. However, due to the unknown crystal structure of SrCa2Ga2O6:Mn4+, its luminescence mechanism is unidentified. To clarify the crystal structure, a single crystal X-ray diffraction analysis was performed. Herein, we report the crystal structure of the novel deep-red emitting phosphor SrCa2Ga2O6:Mn4+ and its fundamental luminescence properties.
Experimental section
Materials and synthesis methods
SrCa2Ga2O6:Mn4+ (SCG:Mn4+) single crystals were synthesized by a flux method. SrCO3 (Kanto Chemical Co., Inc, 99.9%), CaCO3 (Kanto Chemical Co., Inc, 99.99%), Ga2O3 (Kojundo Chemical Lab., 99.99%), and MnO2 (Wako Pure Chemical Co., 99.5%) were weighed in a stoichiometric ratio of Sr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca
Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 1
Mn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.99
1.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01. The raw materials were mixed with acetone in an agate mortar. After drying, 50 wt% of prepared weight of SrCl2 (Kojundo Chemical Lab., 99.9%) was added to the agate mortar as flux and re-mixed without solvent. The homogeneously mixed powder was mounted on an alumina boat, calcined at 1473 K for 6 h in ambient air, then cooled to 1123 K at 50 K h−1, and finally natural-cooled to ambient temperature. The yellow single crystals produced on the alumina boat were separated using a spatula.
0.01. The raw materials were mixed with acetone in an agate mortar. After drying, 50 wt% of prepared weight of SrCl2 (Kojundo Chemical Lab., 99.9%) was added to the agate mortar as flux and re-mixed without solvent. The homogeneously mixed powder was mounted on an alumina boat, calcined at 1473 K for 6 h in ambient air, then cooled to 1123 K at 50 K h−1, and finally natural-cooled to ambient temperature. The yellow single crystals produced on the alumina boat were separated using a spatula.
SrCa2Ga2(1−x)Mn2xO6 (0 ≤ x ≤ 0.07) powders were synthesized by a conventional solid-state reaction method. The same raw materials except SrCl2 flux were mixed using the agate mortar in a stoichiometric ratio of Sr![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca
Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 1
Mn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(1 − x)
2(1 − x)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2x (0 ≤ x ≤ 0.07). The mixture was mounted on the alumina boat and heated at 1473 K for 6 h in ambient air. The sintered samples were ground with agate mortar for several characterizations.
2x (0 ≤ x ≤ 0.07). The mixture was mounted on the alumina boat and heated at 1473 K for 6 h in ambient air. The sintered samples were ground with agate mortar for several characterizations.
Characterization
The crystallographic data of SCG:Mn4+ were collected for the single crystal X-ray diffraction (XRD). A single crystal was put on a glass capillary. The XRD data were measured using a single crystal X-ray diffractometer XtaLAB mini (Rigaku). Data collection, cell refinement, and data reduction were computed using the Crystal Clear-SM Auto 2.0 rl (Rigaku, 2009). The structural analysis was performed using a structural analysis software CrystalStructure 4.3 (Rigaku, 2019). The initial structure was obtained by a direct method using SIR2008.35 The structural parameters were refined using the SHELXL2014 program.36 The refined structural data was re-performed by a structural analysis software WinGX for twin refinement.37 The VESTA program was used to visualize the crystal structure.38 The semiquantitative measurement of the carbon-coated single crystal samples was performed using an electron probe microanalyzer EPMA-1720 (Shimadzu).
The XRD patterns of the powder samples were obtained using an X-ray diffractometer MiniFlex-600 (Rigaku) with a monochromatic Cu Kα radiation (λ = 1.54056 Å) under 10 mA and 30 kV. The XRD data for the Rietveld refinement were collected using an X-ray diffractometer D2 PHASER (Bruker) with a monochromatic Cu Kα radiation (λ = 1.54056 Å) under 10 mA and 30 kV. Rietveld refinement was carried out using the REITAN-FP program software.39 The major elements in the powders were determined by X-ray fluorescence (XRF) spectroscopy using an XRF spectrometer SEA1200VX (SII Nano Technology). The reflection and absorption spectra of the powder samples were measured using a UV-visible spectrometer V-750 (JASCO) based on BaSO4 white ceramics as a reference and were evaluated using a diffuse reflectance spectrum. The particle morphology of powder samples was observed using a scanning electron microscope (SEM) JSM-IT200 (JEOL). Photoluminescence (PL) and photoluminescence excitation (PLE) spectra were obtained using a spectrofluorometer FP-6500/FP-6600 (JASCO) with a 150 W Xenon lamp at 298 K. Near-infrared (NIR) photoluminescence was measured using a spectrometer RB4524-NIRC3 (OtO photonics) with an attached CCD detector. Absolute quantum efficiency was measured using an integrating sphere ISF-834 (JASCO) with a photoluminescence spectrometer FP-8500 (JASCO), and a standard halogen lamp ESC-842 (JASCO) was used for calibration of the measurement system. The PL decay curves were measured using the Quantaurus-tau (Hamamatsu Photonics). Thermal quenching PL spectra was measured using a PL equipment (FP-6500/FP-6600) with a heating attachment HPC-503 (JASCO).
Results and discussion
Crystal structure
The crystal structure of SCG:Mn4+ is illustrated in Fig. 1. SCG:Mn4+ crystallized in space group F432 (#209) with a cubic unit cell with a = 15.4894(15) Å, and the composition formula was determined to SrCa2Ga1.87Mn0.13O6. The detailed crystallographic data and atomic refinement parameters are listed in Tables 1 and 2. SCG:Mn4+ is a new member of tululite structure-related compounds (Ca14Zn6Al10O35, Ca14Zn6Ga10O35, Ca7Mg2Ga6O18, Ca7Co3Ga5O18, Ca6.3Mn3Ga4.4Al1.3O18, and Ca7Mn2.14Ga5.86O17.93).34,40–46 Notably, SCG:Mn4+ is the first material containing high-concentration strontium in the aforementioned series. The compositional identity between SCG and other materials with a fundamental formula [A2+7](B2+2){C3+6}O18 can be described to rewrite SrCa2Ga2O6 as [Sr3Ca4](Ca2){Ga6}O18.
 |
| | Fig. 1 The overview of refined crystal structure of SrCa2Ga2O6:Mn4+. | |
Table 1 Crystallographic data of the SrCa2Ga1.87Mn0.13O6 single crystal
| Chemical formula |
SrCa2Ga1.87Mn0.13O6 |
| Formula weight (g mol−1) |
401.26 |
| Crystal system |
Cubic |
| Space group |
F432 (#209) |
| Measurement temperature (K) |
296(2) |
| a (Å) |
15.4894(15) |
| V (Å3) |
3716.2(11) |
| Z |
24 |
| Density (g cm−3) |
4.303 |
| Radiation type and wavelength (Å) |
Mo Kα 0.71075 |
| μ (mm−1) |
18.54 |
| Crystal size (mm3) |
0.109 × 0.094 × 0.091 |
| Diffractometer |
Rigaku XtaLAB mini |
| Absorption correction |
Numerical (NUMABS; Rigaku, 1999) |
| Tmin, Tmax |
1, 1 |
| Measd, indep, obsd [I > 2σ(I)] reflns |
6448, 377, 377 |
| R [F2 > 2σ(F2)], wR(F2), S |
0.0317, 0.0795, 1.272 |
| Δρmax, Δρmin (e Å−3) |
1.25, −0.71 |
| Absolute structure parameter |
0.15(8) |
Table 2 Atomic positions, occupancies, and anisotropic displacement parameters for the SrCa2Ga1.87Mn0.13O6 single crystal
| Atom |
Site |
Occ.a |
x |
y |
z |
Uani (Å2) |
| Occ. means occupancy. |
| Sr1 |
24e |
1 |
0.20879(8) |
1 |
0 |
0.0134(4) |
| Ca1 |
32f |
1 |
0.38960(8) |
0.88960(8) |
0.11040(8) |
0.0068(5) |
| Ca2 |
32f |
0.5 |
0.1564(4) |
0.8436(4) |
0.1564(4) |
0.031(2) |
| Ga1/Mn1 |
4b |
0.20(8)/0.80(8) |
0.5 |
1 |
0 |
0.0037(16) |
| Ga2 |
32f |
0.5 |
0.17737(17) |
0.82263(17) |
0.17737(17) |
0.0172(9) |
| Ga3 |
24d |
1 |
0.25 |
1 |
0.25 |
0.0139(4) |
| Ga4 |
4a |
1 |
0 |
1 |
0 |
0.0136(8) |
| O1 |
24e |
1 |
0.3746(6) |
1 |
0 |
0.0077(17) |
| O2 |
8c |
1 |
0.25 |
0.75 |
0.25 |
0.027(4) |
| O3 |
96j |
1 |
0.2509(6) |
0.9334(6) |
0.1513(4) |
0.033(2) |
| O4 |
32f |
0.5 |
0.0681(6) |
0.9319(6) |
0.0681(6) |
0.017(4) |
The cation-centered polyhedra in SCG:Mn4+ are shown in Fig. 2. There are 11 crystallographically independent sites (one strontium, two calcium, three gallium and four oxygen, and one gallium disordered with manganese). The Sr1 atom is coordinated by nine O atoms and forms a monocapped polyhedron. The average bond length of Sr1–O is 2.6335 Å. Considering the 0.5 occupancy of the O4 atom, [Sr1O9] is a virtually seven-coordinated prism. The Ca1 atom is coordinated by six O atoms and forms a distorted octahedron. The average bond length for Ca1–O is 2.3849 Å. The Ca2 atom is coordinated by five O atoms and forms a trigonal bipyramidal. The average bond length for Ca2–O is 2.1885 Å. Ga2 and Ga3 are coordinated by four O atoms and form tetrahedra. The Ga4 is coordinated by eight O atoms with 0.5 occupancy and composes a cubic structure. Considering Ga3+ cannot make eight-coordinated configuration, [Ga4O8] is virtually four-coordinated tetrahedron. The average bond lengths for Ga2, Ga3, and Ga4 are 2.0610 Å, 1.8443 Å, and 1.8270 Å, respectively. The Ga1/Mn1 is coordinated by six O atoms and forms an octahedron, and its occupancy ratio is determined to 0.21(8)/0.79(8) for Ga1/Mn1. The average bond distance for (Ga1/Mn1)–O is 1.9424 Å. The introduction of Mn4+ ions into octahedral sites results in red emission.47,48 Therefore, the deep-red emission of the single crystal was observed, as shown in Fig. S1.†
 |
| | Fig. 2 Cation-centered polyhedra in SrCa2Ga2O6:Mn4+. | |
As shown in Fig. 3a, the tetrahedral structure is composed of [Ca2O5], [Ga2O4], [Ga3O4], and [Ga4O8] polyhedra via a vertex-sharing connection. [Ca2O5] and [Ga2O4] polyhedra with 0.5 occupancy formed cluster centered on the O2. [Ca2O5] is connected to [Ga3O4] and [Ga4O8]. On the other hand, [Ga2O4] is only connected to [Ga3O4]. As shown in Fig. 3b, the octahedral cluster of [Ca1O6] and [Sr1O9] centered on the [(Ga1/Mn1)O4] octahedron is also present, containing an aristotype perovskite-like structure (Fig. 3c). [Ca1O6] is connected to [Sr1O9] and [(Ga1/Mn1)O6] via an edge-sharing. In contrast, [Sr1O9] and [(Ga1/Mn1)O6] are connected via the vertex-sharing. The crystal structure of SCG is constructed in the way that octahedral fragments filled the voids in the framework formed by the tetrahedral fragments with [Ga4O8] cube as a vertex. On the SCG as a host, the complex structure isolates Mn4+ ions, and the long lattice separates octahedral sites, which is expected to protect Mn4+ ions from nonradiative perturbations.49
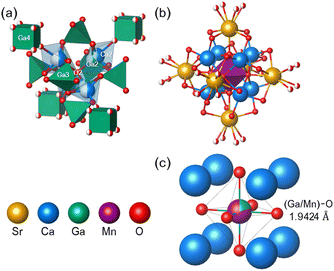 |
| | Fig. 3 (a) The tetrahedral cluster constructed by [Ga2O4], [Ca2O5], [Ga3O4], and [Ga4O8] centered on O2. (b) The octahedral cluster formed by [Sr1O9] and [Ca1O6] centered on [(Ga1/Mn1)O6] octahedron. (c) The aristotype perovskite-like structure composed of Ga1/Mn1, Ca1, and O1. | |
XRD measurements
The XRD patterns of SrCa2Ga2(1−x)Mn2xO6 (0 ≤ x ≤ 0.07) powders were measured, as shown in Fig. 4a. Most of the diffraction peaks are good agreement with simulation pattern calculated from the SCG:Mn4+ single crystal data. The undesirable peaks indexed to Sr3Ga2O6 and Ca5Ga6O14 are also detected. To confirm the crystal structure and phase purity, the Rietveld refinement was performed. Fig. 4b depicts the Rietveld refinement result for SCG:0.03Mn4+ powder using the SCG:Mn4+ single crystal structure data as a model. The crystallographic data and refined parameters of SCG:0.03Mn4+ are summarized in Tables S2 and S3.† The Mn4+ concentration in the SCG:0.03Mn4+ phase was fixed at 0.03 and all isotropic displacement parameters (Beq) were fixed at 1.000. The R factors converged to Rwp = 9.316, Rp = 6.556, Re = 3.705, and S = 2.515, indicating that the obtained result is reliable. The refined crystal structure is in good agreement with that of the single crystal data. Sr3Ga2O6 and Ca5Ga6O14 phases were successfully detected as impurities. The phase purity was found to be SCG:0.03Mn4+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr3Ga2O6
Sr3Ga2O6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca5Ga6O14 = 93.74
Ca5Ga6O14 = 93.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.40
0.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.86 in mass%. These impurities may affect the luminescence properties because they have the tetrahedral sites where Mn5+ ions could substitute. To suppress the production of these impurities is difficult because they were probably produced from charge imbalance by replacing Ga3+ ion to Mn4+ ion and compositional deviation from undesirable cation mixing of Sr2+ and Ca2+ ions. Therefore, in this study, we considered these impurities not affect the emission spectrum since they have no octahedral sites where Mn4+ ions can substitute. The XRF analysis was also performed to investigate the elements composed of the SCG:Mn4+ powder (Table S4†). The elemental amount was in good agreement with the stoichiometric ratio. In this study, SCG:Mn4+ powders were successfully synthesized.
5.86 in mass%. These impurities may affect the luminescence properties because they have the tetrahedral sites where Mn5+ ions could substitute. To suppress the production of these impurities is difficult because they were probably produced from charge imbalance by replacing Ga3+ ion to Mn4+ ion and compositional deviation from undesirable cation mixing of Sr2+ and Ca2+ ions. Therefore, in this study, we considered these impurities not affect the emission spectrum since they have no octahedral sites where Mn4+ ions can substitute. The XRF analysis was also performed to investigate the elements composed of the SCG:Mn4+ powder (Table S4†). The elemental amount was in good agreement with the stoichiometric ratio. In this study, SCG:Mn4+ powders were successfully synthesized.
 |
| | Fig. 4 (a) XRD patterns of SrCa2Ga2O6:xMn4+ (0 ≤ x ≤ 0.07) powders. (b) Rietveld refinement result for XRD pattern of the SrCa2Ga2O6:0.03Mn4+ sample. | |
UV-vis diffuse reflectance spectra
The UV-vis diffuse reflectance spectra of the SCG host powder and SCG:0.03Mn4+ were measured (Fig. 5). In the SCG host powder, the reflection intensity between 300 and 800 nm is higher than 85%; thus, the host material has a white body color. To determine an energy band gap (Eg) of the non-doped SCG, a Kubelka–Munk formula (eqn (1)) was used;50,51| |
 | (1) |
where h is a Plank's constant, ν is a frequency of light, K is an absorption coefficient, S is a scattering coefficient, and R is a reflectivity. By the Kubelka–Munk equation, the Eg is determined to be 4.89 eV, which is sufficiently large. On host materials with a large band gap, the interaction between the energy transition of Mn4+ and valence/conduction bands becomes less, and Mn4+ functions as an emission center.52 In the SCG:0.03Mn4+ powder, n-UV and blue light absorptions are clearly observed between 290 and 530 nm; hence, the body color of Mn4+-doped SCG powder is yellow. The characteristic absorption spectrum indicates that Mn4+ ions occupy octahedral sites. On the other hand, an absorption band from 500 to 800 nm was also observed. This absorption is mainly due to the 3A2 → 3T1 spin-allowed transition of Mn5+ in tetrahedral coordination environments, which overlaps with the emission of Mn4+. Thus, a decrease in luminescence efficiency is anticipated due to the energy transfer from Mn4+ to Mn5+.
 |
| | Fig. 5 UV-vis diffuse reflectance spectra of SrCa2Ga2O6 (black) and SrCa2Ga2O6:0.03Mn4+ (red). The insert photograph is the body color of SrCa2Ga2O6:0.03Mn4+ under fluorescent light. | |
PL properties
Fig. 6a shows the normalized PL and PLE spectra of the SCG:0.03Mn4+ powder monitored at λex = 353 nm and λem = 712 nm. PL and PLE spectra of SrCa2Ga2(1−x)Mn2xO6 (0.003 ≤ x ≤ 0.07) powders are also displayed in Fig. 6b. SCG:Mn4+ phosphors have a broad excitation band ranging from 250 to 550 nm. The emission spectrum peaked at 712 nm is observed, corresponding to 2Eg → 4A2g spin-forbidden transition of Mn4+. The PL measurement shows that the Mn4+ are successfully doped in octahedral sites. The optimal Mn4+ concentration is found to be x = 0.03, as shown in Fig. 6c. Moreover, the internal and external quantum efficiencies (QEs) of SCG:0.03Mn4+ under 365 nm excitation are 66% and 50%, and the absorbance is 76%. The QEs of SCG:0.03Mn4+ and other Mn4+-activated gallate compounds are listed in Table 3. SCG:0.03Mn4+ exhibits sufficiently high quantum efficiencies, and its emission intensity is almost comparable with Ca14Zn6Ga10O35:Mn4+ (Fig. S3†).
 |
| | Fig. 6 (a) PL and PLE spectra of SrCa2Ga2O6:0.03Mn4+. The insert photograph is emission color of SrCa2Ga2O6:0.03Mn4+ under 365 nm n-UV irradiation. (b) PL and PLE spectra of SrCa2Ga2O6:xMn4+ (0 ≤ x ≤ 0.07) excited at 476 nm. (c) Dependence of emission intensities of SrCa2Ga2O6:Mn4+ on the concentration of Mn4+ ion. (d) Deconvoluted PLE spectra by four components of Gaussian curves. Red line is fitting curve. | |
Table 3 Quantum efficiencies of SrCa2Ga2O6:Mn4+ and other Mn4+-activated gallate phosphors
| Phosphor |
IQE |
EQE |
Ref. |
| SCG:0.03Mn4+ |
66% |
50% |
This study |
| Ca14Zn6Ga10O35:Mn4+ |
51% |
45% |
24 |
| Mg7GeGa2O12:Mn4+ |
28% |
— |
28 |
| Mg3GeGa2O8:Mn4+ |
65% |
— |
30 |
| SrLaGa3O7:Mn4+ |
14% |
— |
33 |
| Ca7Mg2Ga6O18:Mn4+ |
51% |
— |
34 |
The high symmetrical octahedral site increases the covalent bond strength between Mn4+ and ligands, and the strong covalent binding for the Mn4+-ligand realizes deep-red emission by decreasing 2Eg → 4A2g transition energy.53,54 To consider the symmetry, a distortion index D was calculated using the following eqn (2);55
| |
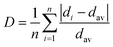 | (2) |
where
n is the coordination number,
di is the bond length between the central atom and
ith coordinating atom,
dav is the average bond length. For the SCG:Mn
4+ single crystal, all the bond lengths of [(Ga1/Mn1)O
6] octahedron are 1.942(10) Å, and the
D is 0. Therefore, SCG:Mn
4+ powders display the emission topped at 712 nm. The nephelauxetic effect that affect the covalency is discussed in Crystal Field Analysis and Nephelauxetic Effect Calculation section.
The excitation spectra deconvolution of SCG:0.03Mn4+ was also conducted, as shown in Fig. 6d. As a result, the excitation band was successfully deconvoluted into four components with Mn4+–O2− charge transfer (CT) transition (centered at 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293 cm−1 and 292 nm), 4A2g → 4T1g spin-allowed transition (centered at 27
293 cm−1 and 292 nm), 4A2g → 4T1g spin-allowed transition (centered at 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896 cm−1 and 358 nm), 4A2g → 2T2g spin-forbidden transition (centered at 22
896 cm−1 and 358 nm), 4A2g → 2T2g spin-forbidden transition (centered at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 208 cm−1 and 450 nm), and 4A2g → 4T2g spin-allowed transition (centered at 20
208 cm−1 and 450 nm), and 4A2g → 4T2g spin-allowed transition (centered at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 cm−1 and 486 nm). These characteristic excitation peaks are well matched to n-UV and blue LED chips. The spectral change of excitation bands, as shown in Fig. 6b, is caused by the change of intensity ratio between CT transition and 4A2g → 4T1g transition.
578 cm−1 and 486 nm). These characteristic excitation peaks are well matched to n-UV and blue LED chips. The spectral change of excitation bands, as shown in Fig. 6b, is caused by the change of intensity ratio between CT transition and 4A2g → 4T1g transition.
To study the mechanism of concentration quenching, the luminescence lifetime measurement was conducted. The PL decay curves of SrCa2Ga2(1−x)Mn2xO6 (0.005 ≤ x ≤ 0.07) powders are shown in Fig. 7. The luminescence lifetimes are calculated using the following first-order exponential decay model (eqn (3)) because the Mn4+ ion occupies one Ga3+ octahedral coordination environment in SCG:Mn4+;57
| |
 | (3) |
where
I(
t) is the emission intensity at time
t,
I1(0) is the initial emission intensity,
t is the time,
τ1 is the luminescence lifetime. However, the double-order decay model (
eqn (4)) can also be applied to high Mn
4+ concentration samples;
58| |
 | (4) |
Moreover, the average decay lifetimes
τave are calculated by the following integral decay model (
eqn (5));
59| |
 | (5) |
 |
| | Fig. 7 PL decay curves of SrCa2Ga2O6:xMn4+ (0.005 ≤ x ≤ 0.07) monitored at 712 nm excited at 365 nm. | |
The fitting results are listed in Table S5.† The measurement result is reasonable because microsecond-order PL lifetimes are usually observed in Mn4+ phosphors.60 The average lifetime decreases from 2.573 to 2.026 ms with increasing Mn4+ concentration, and the shape of decay curves become linear to non-linear. The decreased lifetime and concentration quenching are thought to be caused by nonradiative energy transfer among adjacent Mn4+ ions. The nonradiative energy transfer is due to exchange or electric multipole interactions.61 To determine the origin of the concentration quenching, a critical distance for energy transfer Rc was calculated by an eqn (6);62
| |
 | (6) |
where
V is the host lattice volume,
xc is a critical concentration of dopant, and
N is the number of sites available for the dopant in the unit cell. According to the equation,
Rc is determined to be 38.96 Å. The origin of concentration quenching is suggested to be the electric multipole interaction because
Rc is much larger than 5 Å.
61 Moreover, to specify the type of electric multipole interaction, the following formula (
eqn (7)) was used;
63| |
 | (7) |
where
I is the emission intensity,
x is the dopant concentration,
A is a constant, and
θ is the electric multipole index with
θ = 3, 6, 8, and 10 corresponding to nonradiative energy transfer among nearest-neighbor ions, electric dipole–dipole, dipole-quadrupole, and quadrupole–quadrupole interaction, respectively.
64 In the concentration range of 0.03 ≤
x ≤ 0.07, the
θ is determined to be 2.60, which is close to 3. This fact contradicts the result of
Rc calculation, and indicates that considering only Mn
4+ ions in octahedral sites is not appropriate to discuss the concentration quenching mechanism. According to Liao
et al., in the Ca
14Zn
6Ga
10O
35:Mn phosphor, doping high amount of Mn
4+ leads to existence of Mn
2+ and Mn
5+ in the host lattice.
64 Even in SCG:Mn
4+ phosphors, the other valent Mn ion behaved as a killer center for Mn
4+, and drastic quenching and non-linear rapid lifetime decay were observed in high Mn concentration samples. Therefore,
τ2 are derived from the energy transfer from Mn
4+ to other valence Mn ions, and the primary concentration quenching mechanism is considered to be that phenomenon. This result is in good agreement with the result of UV-vis diffuse reflectance spectra measurements. Fig. S4
† shows the NIR-PL spectra of the SCG:0.03Mn
4+ phosphor excited by a 568 nm LED light. A characteristic PL spectrum derived from
1E →
3A
2 spin-forbidden transition of tetrahedrally coordinated Mn
5+ was observed. This fact supports the presence of Mn
5+ in SCG:Mn
4+. In order to increase the luminescence efficiency of SCG:Mn
4+, the production of other valence Mn ions should be suppressed.
Crystal field analysis and nephelauxetic effect calculation
The Tanabe–Sugano energy diagram of six-fold octahedral Mn4+ ion with a 3 d3 electronic orbit can be described as Fig. 8. The crystal field strength Dq of Mn4+ in the SCG host lattice was calculated by an eqn (8);32| |
 | (8) |
where E(4A2g → 4T2g) is the peak energy of 4A2g → 4T2g transition (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 cm−1). The Racah parameter B is expressed by the following eqn (9);32
578 cm−1). The Racah parameter B is expressed by the following eqn (9);32| |
 | (9) |
where x is refined as following eqn (10) using energy gap between 4A2g → 4T1g and 4A2g → 4T2g transitions (7318 cm−1);32| |
 | (10) |
Moreover, the Racah parameter C can be represented by an eqn (11) using the peak energy of 2Eg → 4A2g transition (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 cm−1);32
045 cm−1);32| |
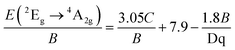 | (11) |
 |
| | Fig. 8 Tanabe–Sugano energy level diagram of Mn4+ at octahedrally coordinated environment. Red line indicates the crystal filed strength of Mn4+ in the SrCa2Ga2O6 host lattice. | |
From the above, Dq, B, and C were determined as 2058 cm−1, 707 cm−1, and 2927 cm−1, respectively. The crystal filed is considered as strong when Dq/B ≥ 2.2, and the Dq/B is 2.91. Therefore, Mn4+ has a strong crystal field in the SCG crystal lattice.
According to the Tanabe–Sugano energy diagram, the crystal field strength hardly affects the emission energy of Mn4+. As mentioned in the PL properties section, the emission of Mn4+ depends on the covalency of center metal and ligands. Therefore, the investigation of the nephelauxetic effect that is derived from chemical bonding is important. The nephelauxetic parameter β1 is calculated by an eqn (12);32
| |
 | (12) |
where
B0 and
C0 are the Racah parameters of free Mn
4+ ion, 1160 cm
−1 and 4303 cm
−1, respectively. From the calculation,
β1 was determined to be 0.9133, representing the covalency between Mn
4+ and ligands is strong.
Thermal quenching properties
The temperature-dependent PL measurement was conducted because the thermal quenching property is an important factor for phosphor applications. The temperature-dependent PL spectra of SCG:0.03Mn4+ between 298 K and 598 K are illustrated in Fig. 9a. At 423 K, the relative PL intensity is 78% of that at 298 K. To further study the thermal quenching property, an Arrhenius equation (eqn (13)) was used to calculate an activation energy (Ea);65| |
 | (13) |
where IRT and IT are the intensities at room temperature (298 K) and arbitrary temperatures, A is a constant, and k is the Boltzmann's constant. Fig. 9(b) and (c) depict PL intensities between 298 K and 598 K and the plot of ln[(IRT/IT) − 1] versus 1/(kT) for SCG:0.03Mn4+. Consequently, the Ea is found to be 0.278 eV. The reported activation energies for Ca14Zn6Al10O35:Mn4+ and Ca14Zn6Ga10O35:Mn4+ are 0.233 eV and 0.29 eV, respectively, and the activation energy of SCG:Mn4+ is found to be close to them.21,56 The results indicate that SCG:Mn4+ has good thermal stability.
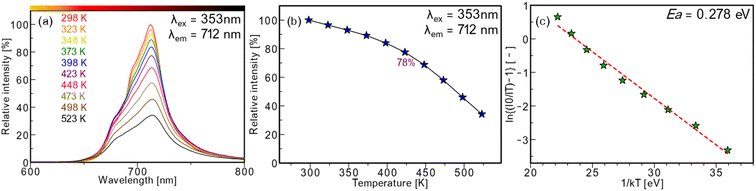 |
| | Fig. 9 (a) Temperature-dependent PL spectra of SrCa2Ga2O6:0.03Mn4+. (b) The plot of relative emission intensities at each temperature. (c) Arrhenius plot of relative emission intensities for SrCa2Ga2O6:0.03Mn4+. | |
LED application
To confirm the potential of SCG:Mn4+ as a LED device, a 470 nm blue LED chip and the SCG:0.03Mn4+ powder were fabricated into a deep-red emitting LED device. Fig. 10 shows the emission spectrum of the LED device under 10 mA current. The device exhibited two peaks at maxima of 470 and 712 nm, which originated from the blue LED chip and SCG:Mn4+. The result indicates SCG:Mn4+ can convert blue light into deep-red light, suggesting the potential of lighting applications.
 |
| | Fig. 10 Luminescence spectra of 470 nm blue LED chip packaged with SrCa2Ga2O6:0.03Mn4+ powder under 10 mA current. The insert photograph is the prepared LED device emitting blue and deep-red light. | |
Conclusion
In summary, the novel deep-red emitting phosphor SrCa2Ga2O6:Mn4+ was discovered in Sr3Ga2O6–Ca3Ga2O6 solid solution system and successfully synthesized by the conventional solid-state reaction method for the first time. SrCa2Ga2O6:Mn4+ was found to have the tululite-related structure belonging to space group F432 from the single crystal XRD analysis. Mn ion occupied on the Ga octahedral site, and SrCa2Ga2O6:Mn4+ single crystals exhibited deep-red emission. From UV-vis diffuse reflectance spectra measurement, the broad absorption band between 220 nm and 550 nm by Mn4+ in the six-fold octahedral coordination environment was observed from the SrCa2Ga2O6:0.03Mn4+ powder. SrCa2Ga2O6:Mn4+ powders exhibited deep-red emission peaked at 712 nm under the n-UV/blue light. The optimal Mn4+ concentration was x = 0.03, and the internal and external quantum efficiencies were 66% and 50%. The luminescence lifetime gradually decreased with increasing Mn4+ concentration, and the concentration quenching occurred in concentration range of 0.03 ≤ x ≤ 0.07. The causal mechanism of concentration quenching was estimated to be energy transfer from Mn4+ to other valence Mn ions. The PL intensity was 78% at 423 K, and the thermal activation energy was calculated as 0.278 eV. The LED device fabricated from the blue LED chip and SrCa2Ga2O6:0.03Mn4+ powder displayed two emission bands peaked at 470 and 712 nm. Therefore, SrCa2Ga2O6:Mn4+ was found to have excellent potential as a Mn4+-activated phosphor, and is expected to be used for commercial applications.
Data availability
Crystallographic data for SrCa2Ga1.87Mn0.13O6 (single crystal) has been deposited at the CCDC under deposition number 2346856.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgements
We thank for Prof. Dr Katsuyoshi Oh-ishi, Department of Applied Chemistry, Faculty of Science and Engineering, Chuo University, for his assistance with the quantum efficiencies measurement. Our study was supported by the equipment in Center for Coordination of Research Facilities (CCRF), Institute for Research Administration, Niigata University. We are grateful to Ayako Ikarashi, staff of CCRF, for her technical assistance with the EPMA analysis.
References
- S. Adachi, Photoluminescence properties of Mn4+-activated oxide phosphors for use in white-LED applications: A review, J. Lumin., 2018, 202, 263–281 Search PubMed.
- X. Wang, O. Li, M. G. Brik, X. Li, L. Li and M. Peng, Thermal quenching of Mn4+ luminescence in SrAl12O19:Mn4+, J. Lumin., 2019, 206, 84–90 CrossRef CAS.
- W. Lü, W. Lv, Q. Zhao, M. Jiao, B. Shao and H. You, A Novel Efficient Mn4+ Activated Ca14Al10Zn6O35 Phosphor: Application in Red-Emitting and White LEDs, Inorg. Chem., 2014, 53, 11985–11990 CrossRef PubMed.
- C. Yang, Z. Zhang, G. Hu, Ru. Cao, X. Liang and W. Xiang, A novel deep red phosphor Ca14Zn6Ga10O35:Mn4+ as color converter for warm W-LEDs: Structure, and luminescence properties, J. Alloys Compd., 2017, 694, 1201–1208 CrossRef CAS.
- S. P. Singh, M. Kim, W. B. Park, J.-W. Lee and K.-S. Sohn, Discovery of a Red-Emitting Li3RbGe8O18:Mn4+ Phosphor in the Alkali-Germanate System: Structural Determination, and Electronic Calculations, Inorg. Chem., 2016, 55, 10310–10319 CrossRef CAS PubMed.
- B. Wang, H. Lin, J. Xu, H. Chen and Y. Wang, CaMg2Al16O27:Mn4+-based Red Phosphor: A Potential Color Converter for High-Powered Warm W-LED, Appl. Mater. Interfaces, 2014, 6, 22905–22913 CrossRef CAS PubMed.
- B. Wang, H. Lin, F. Huang, J. Xu, H. Chen, Z. Lin and Y. Wang, Non-Rare-Earth BaMgAl10-2xO17:xMn4+, xMg2+: A Narrow-Band Red Phosphor for Use as a High-Power Warm w-LED, Chem. Mater., 2016, 28, 3515–3524 CrossRef CAS.
- S. Gu, M. Xia, C. Zhou, Z. Kong, M. S. Molokeev, L. Liu, W.-Y. Wong and Z. Zhou, Red shift properties, crystal filed theory and nephelauxetic effect on Mn4+-doped SrMgAl10-yGayO17 red phosphor for plant growth LED light, Chem. Eng. J., 2020, 396, 125208 Search PubMed.
- R. Cao, Z. Shi, G. Quan, T. Chen, S. Guo, Z. Hu and P. Liu, Preparation and luminescence properties of Li2MgZrO4:Mn4+ red phosphor for plant growth, J. Lumin., 2017, 188, 577–581 CrossRef CAS.
- T. Hasegawa, Y. Nishiwaki, F. Fujishiro, S. Kamei and T. Ueda, Quantitative Determination of the Effective Mn4+ Concentration in a Li2TiO3:Mn4+ Phosphor and Its Effect on the Photoluminescence Efficiency of Deep Red Emission, ACS Omega, 2019, 4, 19856–19862 CrossRef CAS PubMed.
- J. Zhong, D. Chen, S. Yuan, M. Liu, Y. Yuan, Y. Zhu, X. Li and Z. Ji, Tunable Optical Properties and Enhanced Thermal Quenching of Non-Rare-Earth Double-Perovskite (Ba1-xSrx)2YSbO6:Mn4+ Red Phosphors Based on Composition Modulation, Inorg. Chem., 2018, 57, 8978–8987 CrossRef CAS PubMed.
- L. Dang, L. Zhang, Y. Jia, B. Shao, W. Lü, S. Zhao and H. You, Site Occupation and Luminescence of Novel Orange-Red Ca3M2Ge3O12:Mn2+, Mn4+ (M = Al, Ga) Phosphors, ACS Sustain. Chem. Eng., 2020, 8, 3357–3366 CrossRef.
- M. Han, H. Tang, L. Liu, Y. Wang, X. Zhang and L. Lv, Tuning the Mn4+ Coordination Environment in Mg2TiO4 through a Codoping Strategy for Enhancing Luminescence Performance, J. Phys. Chem. C, 2021, 125, 15687–15695 CrossRef CAS.
- S. Adachi, Photoluminescence spectra and modeling analysis of Mn4+-activated fluoride phosphors: A review, J. Lumin., 2018, 197, 119–130 CrossRef CAS.
- S. Adachi and T. Takahashi, Direct synthesis and properties of K2SiF6:Mn4+ phosphor by wet chemical etching of Si wafer, J. Appl. Phys., 2008, 104, 023512 CrossRef.
- H. Zhu, C. C. Lin, W. Luo, S. Shu, Z. Liu, Y. Liu, J. Kong, E. Ma, Y. Cao, R.-S. Liu and X. Chen, Highly efficient non-rare-earth red emitting phosphor for warm white light-emitting diodes, Nat. Commun., 2014, 5, 4312 CrossRef CAS PubMed.
- G. Li, X. Shi, X. Lu, Q. Mao, L. Pei, Y. Zhu, M. Liu, L. Chu and J. Zhong, Local Structure Modulation-Induced Highly Efficient Red-Emitting Ba2Gd1-xYxNbO6:Mn4+ Phosphors for Warm WLEDs, Inorg. Chem., 2021, 60, 17398–17406 CrossRef CAS PubMed.
- B. Lan, R. Cao, T. Chen, L. Li, R. Liu, X. Yi, S. Nie and J. Wang, Far-red-emitting LaSrRO4:Mn4+ (R = Al and Ga) phosphor: Synthesis and optical properties, J. Mol. Struct., 2022, 1265, 133484 CrossRef CAS.
- S. Zhao, J. Xiang, M.-H. Fang, C. Chen, M. Jin and N. Zhang, A novel high thermal stability Ba2CaWO6:Mn4+ far-red emitting phosphor with a double-perovskite structure for plant growth LEDs, Opt. Mater., 2022, 124, 112052 Search PubMed.
- S. Adachi, Review—Mn4+-Activated Red and Deep Red-Emitting Phosphors, ECS J. Solid State Sci. Technol., 2020, 9, 016001 Search PubMed.
- Y. Zhong, S. Gai, M. Xia, S. Gu, Y. Zhang, X. Wu, J. Wang, N. Zhou and Z. Zhou, Enhancing quantum efficiency and tuning photoluminescence properties in far-red-emitting phosphor Ca14Ga10Zn6O35:Mn4+ based on chemical unit engineering, Chem. Eng. J., 2019, 374, 381–391 CrossRef.
- Y. Wu, Y. Lv, K. Ruan and Z. Xie, A far-red emission (Ca, Sr)14Zn6Ga10O35:Mn4+ phosphor for potential application in plant-growth LEDs, Dalton Trans., 2018, 47, 15574 Search PubMed.
- T. K. Kuttiat, M. Abraham, A. K. Kunti, N. Amador-Mendez, M. Tchernycheva and S. Das, Enriching the Deep-Red Emission in (Mg, Ba)3M2GeO8: Mn4+ (M = Al, Ga) Compositions for Light-Emitting Diodes, Appl. Mater. Interfaces, 2023, 15, 7083–7101 CrossRef PubMed.
- Y. Lv, Y. Jin, T. Sun, J. Su, C. Wang, G. Ju, L. Chen and Y. Hu, Visible to NIR down-shifting and NIR to visible upconversion luminescence in Ca14Zn6Ga10O35:Mn4+, Ln3+ (Ln = Nd, Yb, Er), Dyes Pigm., 2019, 161, 137–146 Search PubMed.
- M. Iwaki, K. Uematsu, M. Sato and K. Toda, Structure and Luminescence Studies of a Ce3+-Activated Ba5La3MgAl3O15 Green-Emitting Phosphor, Inorg. Chem., 2023, 62, 1250–1265 Search PubMed.
- S. W. Kim, T. Hasegawa, T. Ishigaki, K. Uematsu, K. Toda and M. Sato, Efficient Red Emission of Blur-Light Excitable new Structure Type NaMgPO4:Eu2+ Phosphor, ECS Solid State Lett., 2013, 2, R49 CrossRef CAS.
- K. Seki, K. Uematsu, K. Toda and M. Sato, Novel Deep Red Emitting Phosphors Ca14Zn6M10O35:Mn4+ (M = Al3+ and Ga3+), Chem. Lett., 2014, 43, 1213–1215 CrossRef CAS.
- C. Wu, J. Li, H. Xu, J. Wu, J. Zhang, Z. Ci, L. Feng, C. Cao, Z. Zhang and Y. Wang, Preparation, structural and photoluminescence characteristics of novel red emitting Mg7Ga2GeO12:Mn4+ phosphor, J. Alloys Compd., 2015, 646, 734–740 CrossRef CAS.
- X. Ding and Y. Wang, Structure and photoluminescence properties of rare-earth free narrow-band red-emitting Mg6ZnGeGa2O12: Mn4+ phosphor excited by NUV light, Opt. Mater., 2017, 64, 445–452 CrossRef CAS.
- X. Ding, G. Zhu, W. Geng, Q. Wang and Y. Wang, Rare-Earth-Free High-Efficiency Narrow-Band Red-Emitting Mg3Ga2GeO8:Mn4+ Phosphor Excited by Near-UV Light for White-Light-Emitting Diodes, Inorg. Chem., 2016, 55, 154–162 CrossRef CAS PubMed.
- A. M. Srivastava, S. J. Camardello and M. G. Brik, Luminescence of Mn4+ in the orthorhombic perovskite, LaGaO3, J. Lumin., 2017, 183, 437–441 CrossRef CAS.
- C. Jiang, X. Zhang, J. Wang, Q. Zhao, K.-L. Wong and M. Peng, Synthesis and photoluminescence properties of a novel red phosphor SrLaGaO4:Mn4+, J. Am. Ceram. Soc., 2019, 102, 1269–1276 CrossRef CAS.
- Q. Liu, P. Xiong, X. Liu, Y. Fu, S. Wu, Q. Dong, Y. Li, Y. Chen, Z. Ma and M. Peng, Deep red SrLaGa3O7:Mn4+ for near ultraviolet excitation of white light LEDs, J. Mater. Chem. C, 2021, 9, 3969–3977 RSC.
- G. Sivakumar, A. T. Muhammed Munthasir, P. Thilagar and S. Natarajan, Mn-Doped Ca14Mg4Ga12O36 with the Tululite Mineral Structure for Color-Tunable Emission, Chem. Mater., 2024, 36(11), 5356–5369 CrossRef CAS.
- M. C. Burla, R. Caliandro, M. Camalli, B. Carrozzini, G. L. Cascarano, L. De Caro, C. Giacovazzo, G. Polidori, D. Siliqi and R. Spagna, IL MILIONE: a suite of computer for crystal structure solution of proteins, J. Appl. Crystallogr., 2007, 40, 609–613 CrossRef CAS.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C:Cryst. Struct. Commun., 2015, 71, 3–8 CrossRef PubMed.
- L. J. Farrugia, WinGX and ORTEP for Windows: an update, J. Appl. Crystallogr., 2012, 45, 849–854 CrossRef CAS.
- K. Momma and F. Izumi, VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data, J. Appl. Crystallogr., 2011, 44, 1272–1276 CrossRef CAS.
- F. Izumi and K. Momma, Three-Dimensional Visualization in Powder Diffraction, Solid State Phenom., 2007, 130, 15–20 CAS.
- H. N. Khoury, E. V. Sokol, S. N. Kokh, Y. V. Seryotkin, E. N. Nigmatulina, S. V. Goryainov, E. V. Belogub and I. D. Clark, Tululite, Ca14(Fe3+, Al)(Al, Zn, Fe3+, Si, P, Mn, Mg)15O36: a new Ca zincate-aluminate from combustion metamorphic marbles, central Jordan, Mineral. Petrol., 2016, 110, 125–140 CrossRef CAS.
- V. D. Barbanyagre, T. I. Timoshenko, A. M. Ilyinets and V. M. Shamshurov, Calcium aluminozincates of CaxAlyZnkOn composition, Powder Diffr., 1997, 12, 22–26 CrossRef CAS.
- S. Y. Istomin, S. V. Chernov, E. V. Antipov and Y. A. Dobrovolsky, composition-induced phase transition in Ca14Zn6-xGa10+xO35+x/2 (x = 0.0 and 0.5), J. Solid State Chem., 2007, 180, 1882–1888 CrossRef CAS.
- J. Li, J. Huang, P. Jiang, W. Gao, R. Cong and T. Yang, Complex crystal structure and photoluminescence of Bi3+-doped and Bi3+/Eu3+ co-doped Ca7Mg2Ga6O18, Dalton Trans., 2021, 50, 6848–6856 RSC.
- J. Grins, S. Y. Istomin, G. Svensson, J. P. Attfield and E. V. Antipov, The disordered cubic structure of Ca7Co3Ga5O18, J. Solid State Chem., 2005, 178, 2197–2204 CrossRef CAS.
- A. M. Abakumov, J. Hadermann, A. S. Kalyuzhnaya, M. G. Rozova, M. G. Mikheev, G. Van Tendeloo and E. V. Antipov, Ca6.3Mn3Ga4.4Al1.3O18—A novel complex oxide with 3D tetrahedral framework, J. Solid State Chem., 2005, 178, 3137–3144 CrossRef CAS.
- A. S. Kalyuzhnaya, A. M. Abakumov, M. G. Rozova, H. D'Hondt, J. Hadermann and E. V. Antipov, Synthesis and crystal structure of the new complex oxide Ca7Mn2.14Ga5.86O17.93, Russ. Chem. Bull., 2010, 59, 706–711 CrossRef CAS.
- Q. Zhou, L. Dolgov, A. M. Srivastava, L. Zhou, Z. Wang, J. Shi, M. D. Dramićanin, M. G. Brik and M. Wu, Mn2+ and Mn4+ Red Phosphors: Synthesis, Luminescence and Applications in WLEDs. A Review, J. Mater. Chem. C, 2018, 6, 2652–2671 RSC.
- P. Li, L. Tan, L. Wang, J. Zheng, M. Peng and Y. Wang, Synthesis, Structure, and Performance of Efficient Red Phosphor LiNaGe4O9:Mn4+ and Its Application in Warm WLEDs, J. Am. Ceram. Soc., 2016, 99, 2029–2034 CrossRef CAS.
- M. Peng, X. Yin, P. A. Tanner, M. G. Brik and P. Li, Site Occupancy Preference, Enhancement Mechanism, and Thermal Resistance of Mn4+ Red Luminescence in Sr4Al14O25: Mn4+ for Warm WLEDs, Chem. Mater., 2015, 27, 2938–2945 CrossRef CAS.
- M. Medić, Z. Ristić, S. Kuzman, V. Đorđević, I. Vukoje, M. G. Brik and M. D. Dramićanin, Luminescence of Mn4+ activated Li4Ti5O12, J. Lumin., 2020, 228, 117646 CrossRef.
- M. Iwaki, H. Takahashi, K. Uematsu, K. Toda and M. Sato, Emission color shift from green yellow to reddish orange in Eu2+-activated Ca6BaP4O17 by doping high amount of activator ion, J. Lumin., 2022, 246, 118810 CrossRef CAS.
- X. Ding, Q. Wang and Y. Wang, Rare-earth free red-emitting K2Ge4O9: Mn4+ phosphor excited by blue light for warm white-LEDs, Phys. Chem. Chem. Phys., 2016, 18, 8088–8097 RSC.
- A. M. Srivastava, M. G. Brik, H. A. Comanzo, W. W. Beers, W. E. Cohen and T. Pocock, Spectroscopy of Mn4+ in Double Perovskites, La2LiSbO6 and La2MgTiO6: Deep Red Photon Generators for Agriculture LEDs, ECS J. Solid State Sci. Technol., 2018, 7, R3158–R3162 CrossRef CAS.
- A. M. Srivastava, H. A. Comanzo, D. J. Smith, J. W. Choi, M. G. Brik, W. W. Beers and S. A. Payne, Spectroscopy of Mn4+ in orthorhombic perovskite LaInO3, J. Lumin., 2019, 206, 398–402 CrossRef CAS.
- Y. Zhang, C. Zhou, Q. Zhang, P. Yin, X. Sun, K. Wang, J. Wang and X. Feng, Change from La2Ti2O7 to LaTiO3 induced by Li2CO3 addition: Higher local symmetry and particle uniformity achieved an efficient Mn4+ activated far red phosphor for agricultural cultivation, J. Lumin., 2022, 248, 119000 CrossRef CAS.
- N. Zhou, L. Liu, Z. Zhou, Y. Zhang, M. Li, Z. Zhou, M. Xia and Z. Zhou, Engineering cation vacancies to improve the luminescence properties of Ca14Al10Zn6O35: Mn4+ phosphors for LED plant lamp, J. Am. Ceram. Soc., 2019, 103, 1796–1808 Search PubMed.
- R. Cao, Q. Xiang, W. Luo, D. Wu, F. Xiao and X. Yu, Synthesis and luminescence properties of efficient red phosphors SrAl4O7:Mn4+, R+ (R+ = Li+, Na+, and K+) for white LEDs, Ceram. Int., 2015, 41, 7191–7196 Search PubMed.
- R. Cao, B. Zhong, J. Nie, L. Zhang, Y. Chen, L. Li, T. Chen and J. Wang, Synthesis, spectral characteristics and energy transfer of SrLa2Al2O7:Mn4+, Dy3+, J. Lumin., 2023, 264, 120163 CrossRef CAS.
- W. Sun, Y. Jia, R. Pang, H. Li, T. Ma, D. Li, J. Fu, S. Zhang, L. Jiang and C. Li, Sr9Mg1.5(PO4)7:Eu2+: A Novel Broadband Orange-Yellow-Emitting Phosphor for Blue Light-Excited Warm Ehite LEDs, ACS Appl. Mater. Interfaces, 2015, 7, 25219–25226 CrossRef CAS PubMed.
- J. Xiang, J. Chen, N. Zhang, N. Yao and C. Guo, Far red and near infrared double-wavelength emitting phosphor Gd2ZnTiO6:Mn4+, Yb3+ for plant cultivation LEDs, Dyes Pigm., 2018, 154, 257–262 CrossRef CAS.
- M. D. Dramićanin, B. Milićević, V. Đorđević, Z. Ristić, J. Zhou, D. Milivojević, J. Papan, M. G. Brik, C.-G. Ma, A. M. Srivastava and M. Wu, Li2TiO3:Mn4+ Deep-Red Phosphor for the Lifetime-Based Luminescence Thermometry, ChemistrySelect, 2019, 4, 7067–7075 CrossRef.
- R. Cao, T. Chen, Y. Ren, T. Chen, H. Ao, W. Li and G. Zheng, Synthesis and photoluminescence properties of Ca2LaTaO6:Mn4+ phosphor for plant growth LEDs, J. Alloys Compd., 2019, 780, 749–755 CrossRef CAS.
- S. Wang, Q. Sun, B. Devakumar, J. Liang, L. Sun and X. Huang, Mn4+-activated Li3Mg2SbO6 as an ultrabright fluoride-free red-emitting phosphor for warm white light-emitting diodes, RSC Adv., 2019, 9, 3429–3435 RSC.
- Z. Liao, H. Xu, W. Zhao, H. Yang, J. Zhong, H. Zhang, Z. Nie and Z.-K. Zhou, Energy transfer from Mn4+ to Mn5+ and near infrared emission with wide excitation band in Ca14Zn6Ga10O35:Mn phosphors, Chem. Eng. J., 2020, 395, 125060 CrossRef CAS.
- Z. Chen, Z. Tian, J. Zhang, J. Li, S. Du, W. Cui, X. Yuan, K. Chen and G. Liu, Deep-red-emitting Ca2ScSbO6:Mn4+ phosphors with a double perovskite structure: Synthesis, characterization and potential in plant growth lighting, J. Am. Ceram. Soc., 2022, 105, 2094–2104 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Masato Iwaki
a,
Masato Iwaki a,
Mizuki Watanabea,
Kazuyoshi Uematsub,
Mineo Satob and
Kenji Toda*a
a,
Mizuki Watanabea,
Kazuyoshi Uematsub,
Mineo Satob and
Kenji Toda*a
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca
Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 1
Mn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.99
1.99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.01. The raw materials were mixed with acetone in an agate mortar. After drying, 50 wt% of prepared weight of SrCl2 (Kojundo Chemical Lab., 99.9%) was added to the agate mortar as flux and re-mixed without solvent. The homogeneously mixed powder was mounted on an alumina boat, calcined at 1473 K for 6 h in ambient air, then cooled to 1123 K at 50 K h−1, and finally natural-cooled to ambient temperature. The yellow single crystals produced on the alumina boat were separated using a spatula.
0.01. The raw materials were mixed with acetone in an agate mortar. After drying, 50 wt% of prepared weight of SrCl2 (Kojundo Chemical Lab., 99.9%) was added to the agate mortar as flux and re-mixed without solvent. The homogeneously mixed powder was mounted on an alumina boat, calcined at 1473 K for 6 h in ambient air, then cooled to 1123 K at 50 K h−1, and finally natural-cooled to ambient temperature. The yellow single crystals produced on the alumina boat were separated using a spatula.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca
Ca![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ga
Ga![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn = 1
Mn = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2(1 − x)
2(1 − x)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2x (0 ≤ x ≤ 0.07). The mixture was mounted on the alumina boat and heated at 1473 K for 6 h in ambient air. The sintered samples were ground with agate mortar for several characterizations.
2x (0 ≤ x ≤ 0.07). The mixture was mounted on the alumina boat and heated at 1473 K for 6 h in ambient air. The sintered samples were ground with agate mortar for several characterizations.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sr3Ga2O6
Sr3Ga2O6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ca5Ga6O14 = 93.74
Ca5Ga6O14 = 93.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.40
0.40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.86 in mass%. These impurities may affect the luminescence properties because they have the tetrahedral sites where Mn5+ ions could substitute. To suppress the production of these impurities is difficult because they were probably produced from charge imbalance by replacing Ga3+ ion to Mn4+ ion and compositional deviation from undesirable cation mixing of Sr2+ and Ca2+ ions. Therefore, in this study, we considered these impurities not affect the emission spectrum since they have no octahedral sites where Mn4+ ions can substitute. The XRF analysis was also performed to investigate the elements composed of the SCG:Mn4+ powder (Table S4†). The elemental amount was in good agreement with the stoichiometric ratio. In this study, SCG:Mn4+ powders were successfully synthesized.
5.86 in mass%. These impurities may affect the luminescence properties because they have the tetrahedral sites where Mn5+ ions could substitute. To suppress the production of these impurities is difficult because they were probably produced from charge imbalance by replacing Ga3+ ion to Mn4+ ion and compositional deviation from undesirable cation mixing of Sr2+ and Ca2+ ions. Therefore, in this study, we considered these impurities not affect the emission spectrum since they have no octahedral sites where Mn4+ ions can substitute. The XRF analysis was also performed to investigate the elements composed of the SCG:Mn4+ powder (Table S4†). The elemental amount was in good agreement with the stoichiometric ratio. In this study, SCG:Mn4+ powders were successfully synthesized.



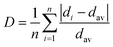
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 293 cm−1 and 292 nm), 4A2g → 4T1g spin-allowed transition (centered at 27
293 cm−1 and 292 nm), 4A2g → 4T1g spin-allowed transition (centered at 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896 cm−1 and 358 nm), 4A2g → 2T2g spin-forbidden transition (centered at 22
896 cm−1 and 358 nm), 4A2g → 2T2g spin-forbidden transition (centered at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 208 cm−1 and 450 nm), and 4A2g → 4T2g spin-allowed transition (centered at 20
208 cm−1 and 450 nm), and 4A2g → 4T2g spin-allowed transition (centered at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 cm−1 and 486 nm). These characteristic excitation peaks are well matched to n-UV and blue LED chips. The spectral change of excitation bands, as shown in Fig. 6b, is caused by the change of intensity ratio between CT transition and 4A2g → 4T1g transition.
578 cm−1 and 486 nm). These characteristic excitation peaks are well matched to n-UV and blue LED chips. The spectral change of excitation bands, as shown in Fig. 6b, is caused by the change of intensity ratio between CT transition and 4A2g → 4T1g transition.






![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 578 cm−1). The Racah parameter B is expressed by the following eqn (9);32
578 cm−1). The Racah parameter B is expressed by the following eqn (9);32

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 045 cm−1);32
045 cm−1);32