Open Access Article
Open Access ArticleFabrication of highly luminescent red-emissive carbon dots and their enhancement of cotton growth promoted by La3+†
Shuai Hanabc,
Yunhan Qind,
Dandan Zhangc,
Cuifang Lia,
Xiaoxue Duc,
Lijuan Liuc,
Songbo Yuana,
Fangfang Weic,
Baoxin Yang *a and
Zhiying Ma*b
*a and
Zhiying Ma*b
aHebei Key Laboratory of Crop Hybrid Advantage Research and Utilization, Handan Academy of Agricultural Sciences, Handan, P. R. China. E-mail: BXYangHD@163.com
bSchool of Agriculture, Agricultural University of Hebei, Baoding, Hebei, P. R. China. E-mail: mzhy@hebau.edu.cn
cHandan Key Laboratory of Novel Nanobiomaterials, College of Materials Science and Engineering, Hebei University of Engineering, Handan, Hebei, P. R. China
dSchool of Chemistry and Chemical Engineering, Shandong University, Jinan, P. R. China
First published on 2nd April 2025
Abstract
Red-emissive carbon dots (RCDs) were fabricated using a simple solvothermal method with Nile blue A sulphate and malic acid. The obtained RCDs exhibited good water solubility, excellent stability and a high luminescent quantum yield. The effect of RCDs on the growth of cotton sprouts was further explored, and it was found that the RCDs could effectively promote growth by enhancing root vitality. Furthermore, it was found that adding lanthanum to the RCD solutions could achieve better promotion, with the optimum concentration measured to be 0.02 mg mL−1 of RCDs for mixed solutions, which was much lower than that of the RCD solution alone (0.03 mg mL−1). Further analysis results showed that adding lanthanum could enhance the absorption of RCDs by the cotton sprouts, which might be induced by the enhancement of endocytosis in root cells triggered by lanthanum. This work not only provides novel long-wavelength emissive carbon dots with excellent biological activity but also holds positive significance for plant physiology and agricultural production.
1. Introduction
Due to their versatile surface modification, excellent photo stability and unique fluorescence characteristics, quantum dots have garnered widespread attention in biological studies, such as fluorescence imaging, ion detection and DNA delivery.1–3 In the past decade, quantum dots have become a powerful tool in botanical research.4 For example, Parrish et al. fabricated novel light conversion films based on CuInS2/ZnS quantum dots to improve photosynthetic efficiency in lettuce.5 Yin et al. synthesized novel selenium carbon quantum dots and investigated their effect on the growth and fruit quality of tomatoes.6Carbon dots (CDs), which possess good aqueous solubility and excellent fluorescent properties, have become a fascinating rising star in the quantum dot family.7 Their high biocompatibility provides the possibility for CDs to replace semiconductor quantum dots. However, most of the reported CDs exhibit intense short-wavelength luminescence (blue and green) under excitation or possess relatively low quantum yield (QY) for long-wavelength emissions (orange and red). These drawbacks limit them in biological field applications to a certain degree. Therefore, there is still a strong desire to develop simple and efficient methods for synthesizing highly luminescent red-emitting CDs.8 On the other hand, CDs have been found to possess a positive influence on plant growth in recent years,9 providing a new option for growth regulators beyond traditional hormone substances, such as indoleacetic acid and naphthylacetic acid. However, it remains a great challenge to develop effective strategies to enhance plant physiological activity using CDs, while the mechanism of CDs in promoting growth still needs further exploration.10 Moreover, as an emerging artificial nanomaterial, the high cost of CDs limits their potential for commercial applications.11,12 Improving the utilization efficiency of CDs for organisms has become one of the urgent problems to be solved in commercialization. It is worth mentioning that the research by Huang et al. showed that lanthanum could promote endocytosis in root cells, thereby contributing to the uptake of nutrients by the plant roots.13 However, the effect of rare earth elements on the uptake of nanoparticles by plants is still rarely reported.14,15
In this research, novel red-emissive carbon dots (RCDs) were fabricated using a simple solvothermal method with Nile blue A sulphate and malic acid. The obtained CDs exhibited good water solubility, excellent stability and a highly luminescent quantum yield (53.8%). The effect of CDs on the growth of plant sprouts was further explored, and it was found that the RCDs could effectively promote growth by enhancing root vitality. It is worth mentioning that cotton was chosen as the model plant, as it is an important cash crop with a short germination time. Furthermore, it was found that adding lanthanum to the cultivation system with RCDs could more significantly promote growth, which might be induced by the enhancement of endocytosis in root cells triggered by lanthanum. This work not only provides a novel strategy to obtain biologically applicable CDs with long-wavelength emissions but also is considered to possess considerable potential in agricultural nano-fertilizers.
2. Experimental
2.1 Chemicals and materials
Malic acid, Nile blue A sulphate, and ethanol were purchased from Aladdin Reagent Co., Ltd (Shanghai, China). Lanthanum chloride (analytical grade) and 2,3,5-triphenyltetrazolium chloride (TTC) were obtained from Nanjing Chemical Reagent Co., Ltd (Jiangsu, China). Cotton seeds were obtained from Handan Academy of Agricultural Sciences (Handan, China). Deionized water (DI water) was obtained using a leading micro super-pure water machine (Shanghai, China) and was applied throughout all the experiments.2.2 Measurements
Transmission electron microscopy (TEM) and high-resolution TEM were performed for observing the morphology of the CDs samples using a Tecnai G2-20 TEM instrument (Netherlands, 200 kV). Fourier transform infrared (FTIR) spectroscopy was performed to analyse the surface functional groups of the CDs using a Shimadzu IR Tracer-100 spectrometer (Japan). X-ray photoelectron spectroscopy (XPS) was further applied for the surface observations using a Shimadzu AXIS SUPRA+ spectrometer (Japan, AlKα radiation, 1486.6 eV). The optical characteristics of the CDs solutions were observed by UV-vis absorption (PerkinElmer Lambda 850, USA) and fluorescence spectroscopy (Hitachi F-7000 fluorescence spectrophotometer, Japan). The fluorescence quantum yield (QY) of the CDs was recorded using a Horiba FluoroMax-4 fluorescence spectrometer (Japan).16 Confocal microscopy images were obtained using a Leica DM2500 confocal fluorescence microscope (Germany).2.3 Fabrication of RCDs
RCDs were fabricated by the solvothermal method. Briefly, malic acid (1.2 g) and Nile blue A sulfate (10 mg) were dissolved in 20.0 mL of ethanol. Then, the mixed solution was treated at 180 °C for 6 h in a Teflon-lined autoclave (50 mL). After ultra-filtration, the obtained brown-red solution was treated by evaporating ethanol, dissolving in ultrapure water, and then dialyzing (MWCO: 3500D) overnight. The dry RCD samples were finally collected by the freeze-drying method (yield: ca. 21.6%). Afterward, aqueous solutions with various concentrations were obtained by redispersing the RCDs in DI water.2.4 Plant growth
After depilating and sterilizing cotton seeds using concentrated sulfuric acid, the cotton seeds with similar plumpness and sizes were randomly allocated to six groups and arranged by a sandy culture method. Briefly, the divided cotton seeds were sown in six culture basins filled with fine sand (0.3–1.0 mm), and watered by the same amount of deionized water or RCDs solutions with different concentrations (0.01, 0.02, 0.03, 0.04, 0.05 mg mL−1), respectively. The basins were further transferred into a growth chamber and cultured at 30 °C. After five days, cotton sprouts were harvested. The root length, stem length and wet weight of the sprouts were recorded. The culture experiments were repeated five times. Experimental data were analyzed by statistical methods as previously.17 Furthermore, the root vitality of the cotton seedlings was determined by the tetrazolium trichloride (TTC) reduction method as per the previous literature.18 Cotton germination tests were performed with treatment with mixed solutions (CRCDs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CLaCl3 = 5
CLaCl3 = 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with different concentrations (0.01 mg mL−1 + 0.002 mg mL−1, 0.02 mg mL−1 + 0.004 mg mL−1, 0.03 mg mL−1 + 0.006 mg mL−1, 0.04 mg mL−1 + 0.008 mg mL−1, and 0.05 mg mL−1 + 0.01 mg mL−1) and analyzed similarly to the above experiments. For comparison, cultivation experiments with different concentrations of LaCl3 (0.002 mg mL−1, 0.004 mg mL−1, 0.006 mg mL−1, 0.008 mg mL−1, 0.01 mg mL−1) were also performed.
1) with different concentrations (0.01 mg mL−1 + 0.002 mg mL−1, 0.02 mg mL−1 + 0.004 mg mL−1, 0.03 mg mL−1 + 0.006 mg mL−1, 0.04 mg mL−1 + 0.008 mg mL−1, and 0.05 mg mL−1 + 0.01 mg mL−1) and analyzed similarly to the above experiments. For comparison, cultivation experiments with different concentrations of LaCl3 (0.002 mg mL−1, 0.004 mg mL−1, 0.006 mg mL−1, 0.008 mg mL−1, 0.01 mg mL−1) were also performed.
3. Results and discussion
3.1 Physicochemical characterization of the RCDs
RCDs were fabricated by a simple solvothermal method as described before. The formation of RCDs was considered to involve chelation, dehydration and carbonization of the raw materials, similar to the previous studies.19 As shown in Fig. 1, the TEM image showed that the obtained RCDs comprised uniformly dispersed and spherical nanoparticles, with the particles sizes measured to be 3–5 nm. Further from the HRTEM image (inset of Fig. 1), it could be observed that the obtained RCDs had an obvious lattice structure with a crystal plane spacing of 0.206 nm, which was attributed to the (101) crystal plane of graphitic carbon.20 The FTIR spectrum of RCDs is shown in Fig. 2a, showing that the surface of the RCDs held a large amount of –OH and –NH2, corresponding to the broad and strong absorption bands at 3700–3100 cm−1.21 The spectrum also displayed a typical absorption peak at 1680 cm−1, corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration. The absorption peaks from C
O vibration. The absorption peaks from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H bending were considered to be indicated by the peaks at 1420 and 1570 cm−1, respectively. The peak detected at 1055 cm−1 represented the stretching of C–O–C bonds. XPS characterization was further performed to gain more information on the elemental composition and surface groups of the RCDs. As shown in Fig. 2b, the full-scan analysis of XPS revealed the presence of three major peaks at 284.8, 398.9, and 531.8 eV, which corresponded to C 1s (80.5%), N 1s (3.8%) and O 1s (15.7%). Furthermore, the C 1s high-resolution spectrum (Fig. S1†) indicated the presence of four types of C element: C–C (284.4 eV), C–O/C–N (285.7 eV), C
C and N–H bending were considered to be indicated by the peaks at 1420 and 1570 cm−1, respectively. The peak detected at 1055 cm−1 represented the stretching of C–O–C bonds. XPS characterization was further performed to gain more information on the elemental composition and surface groups of the RCDs. As shown in Fig. 2b, the full-scan analysis of XPS revealed the presence of three major peaks at 284.8, 398.9, and 531.8 eV, which corresponded to C 1s (80.5%), N 1s (3.8%) and O 1s (15.7%). Furthermore, the C 1s high-resolution spectrum (Fig. S1†) indicated the presence of four types of C element: C–C (284.4 eV), C–O/C–N (285.7 eV), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (286.7 eV), respectively, verifying the presence of water soluble polar functional groups, such as hydroxyl, carbonyl and carboxylic acid groups on the surface of the RCDs.22
O (286.7 eV), respectively, verifying the presence of water soluble polar functional groups, such as hydroxyl, carbonyl and carboxylic acid groups on the surface of the RCDs.22
3.2 Optical properties of the RCDs
The optical properties of the RCDs were evaluated by UV-vis absorption and fluorescence spectroscopy. The absorption spectrum of RCDs is displayed in Fig. 3a. The significant absorption peak at 276 nm could likely be assigned to the π–π* transition of aromatic groups on the surface.23 In addition, the broad absorption band at 565 nm might be attributed to the n → π* transition of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–N bonds. The fluorescence spectrum indicated that 621 nm was the maximum emission wavelength of the RCDs (red line in Fig. 3b, when excited at 520 nm). With the increase in excitation wavelength from 460 to 560 nm (measured every 20 nm), the fluorescence emission peak was gradually redshifted, which could be considered an excitation-dependent phenomenon. The QY of the RCDs was measured and calculated to be 52.6%, using rhodamine B as the reference. Additionally, the RCDs showed excellent photo-bleaching resistance, whereby their PL intensity did not decrease significantly after 6 h of excitation with a Xe lamp (365 nm, Fig. S2a†). It was also found that the photoluminescence of the RCDs did not change significantly even in high ionic strength solutions (NaCl, 1.0 mol L−1, Fig. S2b†). The PL quenching effect of La3+ ions on the RCDs was further investigated. Notably, it was found that the presence of La3+ led to only slight changes in the PL intensity, even at 0.2 mol L−1 (Fig. S2c†). The MTT colorimetric assay was further used to evaluate the cytotoxicity of the RCDs. As shown in Fig. S3,† the RCDs exhibited low toxicity against HeLa cells, even at a high concentration of 300 μg mL−1 with 36 h of incubation. Therefore, the RCDs are considered to possess good compatibility for biological applications.24
O/C–N bonds. The fluorescence spectrum indicated that 621 nm was the maximum emission wavelength of the RCDs (red line in Fig. 3b, when excited at 520 nm). With the increase in excitation wavelength from 460 to 560 nm (measured every 20 nm), the fluorescence emission peak was gradually redshifted, which could be considered an excitation-dependent phenomenon. The QY of the RCDs was measured and calculated to be 52.6%, using rhodamine B as the reference. Additionally, the RCDs showed excellent photo-bleaching resistance, whereby their PL intensity did not decrease significantly after 6 h of excitation with a Xe lamp (365 nm, Fig. S2a†). It was also found that the photoluminescence of the RCDs did not change significantly even in high ionic strength solutions (NaCl, 1.0 mol L−1, Fig. S2b†). The PL quenching effect of La3+ ions on the RCDs was further investigated. Notably, it was found that the presence of La3+ led to only slight changes in the PL intensity, even at 0.2 mol L−1 (Fig. S2c†). The MTT colorimetric assay was further used to evaluate the cytotoxicity of the RCDs. As shown in Fig. S3,† the RCDs exhibited low toxicity against HeLa cells, even at a high concentration of 300 μg mL−1 with 36 h of incubation. Therefore, the RCDs are considered to possess good compatibility for biological applications.24
 | ||
| Fig. 3 UV-vis absorption spectrum (a) and luminescence spectra at different excitations (b) of the RCDs. | ||
3.3 Effects of the RCDs on the growth of cotton
In order to study the effect of the RCDs on the growth and development of cotton, different concentrations of RCDs and pure water were used to culture the plants. After five days of incubation, the cotton sprouts were harvested, and the elongations of the root and stem, as well as the fresh weight, were further determined. As shown in Fig. 4a–c, it could be observed that the growth indices depended on the concentration of RCDs. When the concentration was lower than 0.03 mg mL−1, an increase in concentration showed a positive effect on growth. Beyond the optimal concentration, the growth indices showed a decreasing trend. When the concentration was 0.05 mg mL−1, the RCDs no longer stimulated the growth of cotton.In order to explore the synergistic effect of lanthanum ions and carbon dots on plants, an RCD solution mixed with lanthanum ions was used to cultivate the cotton seedlings, while the lanthanum ion solution was used for the control experiments. It could be seen that lanthanum ions alone had no significant effect on plant growth, as shown in Fig. S4(a–c).† Interestingly, the mixed solutions of lanthanum ions and carbon dots showed excellent positive effects on plant growth, as shown in Fig. 4d–f. The optimal concentration of RCDs was determined to be 0.02 mg mL−1, which was much lower than that of the RCD solution without lanthanum addition.
4. Discussion
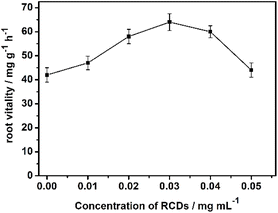
Root dehydrogenase is an important enzyme in plant roots, playing a crucial role in the aerobic respiration process of plants. In general, the stronger the activity of root dehydrogenase, the more vigorous aerobic respiration is, and the stronger the root vitality.25 On the other hand, root vitality is considered to be one of the key factors that can promote the absorption of water and nutrients, and thus promote plant growth. In order to investigate the reasons for the positive effect of RCDs on the growth of cotton, the activity of root dehydrogenase was measured in the cotton seedlings using the TTC method. It can be seen from Fig. 5 that the RCDs can effectively enhance the activity of dehydrogenase, with the optimal concentration of RCDs being 0.03 mg mL−1, which corresponds to the statistical results from the growth promotion investigations. Thus, it is possible that the positive impacts of the RCDs on the growth of cotton are caused by root vitality, more precisely, the activity of root dehydrogenase.The enhancement of the growth-promoting activity of RCDs by the addition of lanthanum was further explored. As we know, lanthanum, at proper concentrations, can promote endocytosis in root cells, thereby contributing to the uptake of nutrients by the plant roots. So, we assumed that lanthanum could induce plant roots to take up more RCDs, which would be beneficial for plant growth. Finally, the RCD solution mixed with lanthanum could promote plant growth at a much lower concentration than that of the RCD solution alone. In order to verify the above conjecture, cotton sprouts were cultured at the same RCD concentration in both RCD solutions (CRCDs = 0.02 mg mL−1) and mixed solutions (CRCDs = 0.02 mg mL−1, CLaCl3 = 0.004 mg mL−1) for the same duration (48 h), and the fluorescent photographs of the roots were taken for comparison. It was found that the fluorescence of the cotton roots cultured with the mixed solution was significantly stronger than that of those cultured with RCDs alone (Fig. 6). Considering that lanthanum ions had little effect on the fluorescence of RCDs, it was assumed that more RCDs were taken up by the roots of cotton, which were stimulated by lanthanum in the mixed solutions. To further verify the promoting effect of lanthanum on plant cell endocytosis, the cotton seeds were cultivated with FITC-dextran 40 kDa solution, with and without the addition of lanthanum. FITC-dextran 40 kDa is a fluorescein derivative labeled with pectin, with an average molecular weight of about 40 kDa. Due to its large molecular weight, it can only penetrate the cell membranes via endocytosis. The fluorescence staining effect on the roots was compared after cultivation (Fig. S5†), which showed that the staining effect was much stronger for the mixed solution. This proved that more fluorescent molecules were absorbed by the cotton roots cultivated in the mixed solution, thus proving that lanthanum could promote endocytosis in plant root cells.
5. Conclusion
RCDs were fabricated by a simple one-step solvothermal method, which exhibited good water solubility, excellent stability and strong fluorescence. The obtained RCDs could effectively promote cotton growth by enhancing root vitality. Furthermore, it was found that the addition of lanthanum to the RCD solutions could more significantly promote growth, with the optimum concentration measured to be 0.02 mg mL−1 of RCDs for the mixed solutions, much lower than that of the RCD solution alone (0.03 mg mL−1). Further analysis showed that more RCDs were absorbed by the cotton roots for the mixed solutions, which might be induced by the enhancement of endocytosis in the root cells triggered by lanthanum. This work presents a novel carbon nanomaterial with excellent physiological activities and reveals that lanthanum might be an effective factor in improving the bioavailability of nano fertilizers for plants, thus providing a new perspective for agricultural chemistry and plant physiology.Data availability
The authors confirm that the data supporting the findings of this manuscript are available within the article and its ESI.†Author contributions
SH and YQ fabricated the novel RCDs and drafted the manuscript. DZ and CL supplied the cotton seeds and performed the germination tests. XD and LL handled the experimental data. SY performed the fluorescence microscopy tests of the cotton sprouts. WS and SQ planned the entire work and revised the manuscript. All authors read and approved the final manuscript.Conflicts of interest
The authors declare that they have no competing interests.Acknowledgements
The research work was supported by the Science & Technology Innovation 2030 of China-Major Project in Agricultural Biological Breeding (2023ZD04039-02), Science Research Project of Hebei Education Department (BJ2025063) and the Program Foundation of Hebei University of Engineering (SJ2201901053).References
- S. Wang, X. Zhang, T. Guo and S. Gao, ACS Appl. Mater. Interfaces, 2024, 16, 29402 CAS.
- F. Liu, M. Wang, Y. He, G. Song and J. Zhao, Mikrochim. Acta, 2022, 189, 113 Search PubMed.
- Y. Chen, Y. Zhao, Y. Liu, H. Xing, X. Zhu, J. Feng, Y. Zong, C. Liao, X. Li and X. Zheng, Int. J. Hydrogen Energy, 2024, 95, 43 CAS.
- S. Balasurya, M. K. Okla, H. AbdElgawad, A. A. Al-Ghamdi, M. A. Abdel-Maksoud, S. S. Al-Amri, M. M. Y. Madany and S. S. Khan, Chemosphere, 2023, 313, 137286 CAS.
- C. H. Parrish, D. Hebert, A. Jackson, K. Ramasamy, H. McDaniel, G. A. Giacomelli and M. R. Bergren, Commun. Biol., 2021, 4, 124 Search PubMed.
- K. Yin, Q. Bao, J. Li, M. Wang, F. Wang, B. Sum, Y. Gaong and F. Lian, Chemosphere, 2024, 364, 143175 Search PubMed.
- A. Hasan and M. Susan, RSC Adv., 2024, 14, 7616 Search PubMed.
- H. Wang, M. Zhang, Y. Song, H. Li, H. Huang, M. Shao, Y. Liu and Z. Kang, Carbon, 2018, 136, 94–102 Search PubMed.
- A. Guirguis, W. Yang, X. A. Conlan, L. X. Kong, D. M. Cahill and Y. C. Wang, Small, 2023, 19, 2300671 CAS.
- S. Milliken, I. T. Cheong, K. Cui and J. G. Veinot, ACS Appl. Nano Mater., 2022, 5, 15785 CrossRef CAS.
- M. Saikia, A. Singh, A. Dihingia, P. Khare, J. Kalita and B. K. Saikia, Chem. Eng. J., 2022, 433, 133633 Search PubMed.
- S. Han, J. Wang, Y. Ren, B. Yang, X. Liu, J. Yang, L. Liu, K. Meng, W. Shan and S. Qin, Micro Nano Lett., 2022, 17, 149 CrossRef CAS.
- M. Cheng, L. Wang, Q. Zhou, D. Chao, S. Nagawa, D. He, J. Zhang, H. Li, T. Li, Z. Gu, X. Huang and Z. Yang, Nat. Commun., 2021, 12, 4327 Search PubMed.
- H. Ou, Z. Zhang and D. Yao, J. Rare Earths, 2014, 32, 672 Search PubMed.
- Y. Liu, Y. Wang, Y. Liu, J. Cui, L. Hu and K. Mu, J. Rare Earths, 2008, 26, 115 CrossRef.
- W. Zhu, X. Gao, X. Yu, Q. Li, Y. Zhou, H. Qiu, B. Xing and Z. Zhang, Mikrochemie, 2022, 189, 223 CAS.
- V. Singh, C. K. Pallaghy and D. Singh, Field Crops Res., 2006, 9, 191 Search PubMed.
- T. Shaima, M. Ajisha, S. Menon and A. Mohammad Kunhi, Int. J. Plant Soil Sci., 2020, 32, 31 CrossRef.
- M. Liu, X. Du, J. Liu, G. Wang, J. Yang, X. Wang, S. Han and Z. Huo, Micro Nano Lett., 2024, 19, 12190 CrossRef.
- T. K. Purkait, M. Iqbal, M. A. Islam, M. H. Mobarok, C. M. Gonzalez, L. Hadidi and J. G. Veinot, J. Am. Chem. Soc., 2016, 138, 7114 CrossRef CAS PubMed.
- P. Shanmuga and S. Suseem, RSC Adv., 2024, 14, 17471 RSC.
- K. Alhazzani, A. Alanazi, A. Mostafa, B. James and E. MohamedM, RSC Adv., 2024, 14, 5609 RSC.
- B. Dutta, A. Waghmare, S. K. Das, Y. Bhargava, A. Kumar, A. K. Debnath, K. C. Barick and P. A. Hassan, Nanoscale, 2025, 17, 4502 Search PubMed.
- Y. Meng, Z. Zhang, H. Zhao, Y. Jiao, J. Li, S. Shuang and C. Dong, J. Hazard. Mater., 2022, 424, 127289 CrossRef CAS.
- J. Hu, W. Jia, X. Wu, H. Zhang, Y. Wang, J. Liu, Y. Yang, S. Tao and X. Wang, Environ. Sci., 2022, 9, 1530 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00209e |
| This journal is © The Royal Society of Chemistry 2025 |