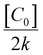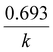Open Access Article
Open Access ArticleResidue determination and decline pattern of abamectin and fenpyroximate in okra fruits: a prelude to risk assessment
Farag Malhat *,
Osama I. Abdallah
*,
Osama I. Abdallah *,
El-Sayed Saber,
Nevein S. Ahmed and
Shokr Abdel Salam Shokr
*,
El-Sayed Saber,
Nevein S. Ahmed and
Shokr Abdel Salam Shokr
Pesticide Residues and Environmental Pollution Department, Central Agricultural Pesticide Laboratory, Agricultural Research Center, Dokki, Giza, 12618, Egypt. E-mail: shebin_osama@yahoo.com; farag_malhat@yahoo.com; Tel: +20 1096800994 Tel: +20 1553398868
First published on 3rd March 2025
Abstract
This study developed and validated an LC-MS/MS analytical method for determining abamectin and fenpyroximate residues in okra fruits. The method optimization focused on chromatographic separation and ionization conditions, adding formic acid and ammonium formate to enhance ionization efficiency and signal sensitivity. Validation was performed according to SANTE guidelines, demonstrating good selectivity, linearity (R2 > 0.998), precision, recovery, and minimal matrix effects (14.6% for abamectin and 5.2% for fenpyroximate). The limit of detection (LOD) was set at 0.0006 mg kg−1 for abamectin and 0.0002 mg kg−1 for fenpyroximate, while the limit of quantification (LOQ) was 0.002 mg kg−1 and 0.001 mg kg−1, respectively. Precision was within acceptable limits, with intra-day RSD of 11.4% for abamectin and 7.6% for fenpyroximate. Recovery ranged from 84.2% to 98.6%, meeting the acceptable 70–120% range. Persistence studies indicated that abamectin and fenpyroximate residues dissipated over time, with half-lives of 2.3 and 2.45 days, respectively. The pre-harvest interval (PHI) required for residues to fall below the maximum residue limit (MRL) was estimated to be 2.6 days for abamectin and 6.9 days for fenpyroximate. The risk quotient was assessed based on the Egyptian adult consumers' consumption of okra, ensuring a negligible risk.
1. Introduction
Okra, also known as lady's finger, is a nutritious vegetable with soluble and insoluble dietary fiber, making it an effective natural laxative.1,2 It is a good source of minerals, such as calcium, magnesium, and iron, and vitamins, including B1, B2, B6, C, and folate, which help manage weight, reduce cancer risk, boost the immune system, lower cholesterol, prevent diabetes, and alleviate asthma symptoms.3,4 Okra can be eaten raw and is particularly effective for digestive issues, coughs, and excessive sweating.1 Today, okra is an essential crop in Egypt, thriving in warm summer temperatures. The growing global demand, especially in Europe, drives its exports. As both domestic and international demand rise, Egypt's competitive-quality okra presents promising export opportunities for the future. In modern agriculture, chemical insecticides play a crucial role in cost-effective pest control for essential field crops, revolutionizing the management of insect pests and diseases.5 One such crop, okra, has a relatively short shelf life and is an annual plant, meaning it completes its lifecycle within a single growing season. Since okra does not regrow after its lifecycle ends, replanting is necessary each season, especially when harvested fresh.6Fenpyroximate (α-(4-phenoxyphenyl)-α,α-dimethyl-1H-pyrazole-3-propanenitrile) is a pyrazole acaricide and insecticide that belongs to the chemical class of sulfonanilides.7 It possesses acaricidal and insecticidal properties due to inhibiting quinol oxidation in the mitochondria at complex III in its target organisms.8 Fenpyroximate is used in horticultural crops, indoors, and in ornamental plants. In Egypt, it is registered for use on various crops, including cotton, brassica leafy greens, grapes, head and stem brassica, stone fruit, and pome fruit, specifically for controlling spider mites.9 Additionally, it is applied to various fruiting vegetables, except for cucurbits, as a selective acaricide against Tetranychidae and Brevipalpidae. The use of pesticides remains the primary means of controlling most insect pests that attack okra crops. Among the various pests that attack okra, fenpyroximate is used in integrated pest management, mainly against fruit-sucking bugs. Concerns regarding fenpyroximate residues and their effects on environmental and food safety have garnered significant attention.
Abamectin is a natural pesticide derived from a product called avermectin.10 It is highly effective and has low toxicity for mammals and other non-target organisms, making it a promising option for biological pest control. Since its discovery in the 1980s, the use of abamectin has rapidly increased. Avermectins have two main variations—B1a and B1b—that differ in their methylation. Abamectin, a chemical derived from the same organism that produces avermectin, has similar effects. It effectively targets a wide range of insects and mites, penetrates leaves easily, and quickly impacts pests. Abamectin is commonly used as an insecticide, acaricide, and nematicide, particularly against caterpillar pests.10 Regarding safety and environmental impact, abamectin is compelling while protecting the environment.
The objective of this study is to establish and validate an analytical method using LC-MS/MS for detecting abamectin and fenpyroximate residues in okra fruits, optimize chromatographic and ionization conditions to enhance detection sensitivity, assess the persistence and dissipation patterns of abamectin and fenpyroximate in okra fruits, establish pre-harvest intervals (PHI), and finally conduct a risk assessment to evaluate consumer safety and support safe agricultural practices. The results of this study can benefit farmers, buyers, guideline implementers, lawmakers, and the general public. Furthermore, this study addresses several research gaps in the literature.
2. Materials and methods
2.1. Chemical reagents and standards
The reference standards used in this study included fenpyroximate (99.5% purity) and abamectin (97.2% purity), both of which were obtained from Chem Service Inc. (West Chester, PA, USA). The commercial pesticide formulations Fenpyroximate (Volitan Extra®, 5% suspension concentrate (SC), Fine Seeds International, Egypt) and abamectin (Opaltin®, 5% emulsifiable concentrate (EC), Agrien Serve for Services and Consultants, Egypt) were obtained from a local supplier. HPLC-grade acetonitrile, methanol, and glacial acetic acid were acquired from Fisher Scientific (Loughborough, UK). Additionally, LC-MS grade formic acid and ammonium formate, as well as analytical-grade anhydrous sodium acetate (CH3COONa) and magnesium sulfate (MgSO4), were sourced from Chem-Lab NV (Zedelgem, Belgium). The primary secondary amine (PSA) was obtained from Macherey-Nagel (Düren, Germany). Multi-walled carbon nanotubes (MWCNT) were acquired from Shilpa Enterprises (Shilpent®, Maharashtra, India). A ceramic homogenizer was purchased from Chrom Tech, Inc. (Copure®, Apple Valley, MN, USA). Ultrapure water was produced using an Evoqua Ultra Clear system (Evoqua Water Technologies LLC, Günzburg, Germany).2.2. Pesticide standard preparation
Primary stock solutions of fenpyroximate and abamectin were prepared at concentrations of 1000 μg mL−1 by dissolving 0.0503 g of fenpyroximate and 0.0514 g of abamectin, respectively, in 50 mL of HPLC-grade acetonitrile. An intermediate 50 μg mL−1 solution was prepared from the primary stock using HPLC-grade acetonitrile. A working standard mixture of fenpyroximate and abamectin at 10 μg mL−1 was prepared by diluting the intermediate stock solution with acetonitrile. The standard solutions were kept refrigerated at −20 °C.2.3. Preparation of calibration standards
The blank okra samples were processed using the proposed extraction and purification method to create matrix blank extracts. These extracts and acetonitrile were then used to serially dilute a standard working solution mixture (10 μg mL−1) of fenpyroximate and abamectin. Serial dilutions were prepared at concentrations of 0.5, 0.25, 0.1, 0.05, 0.025, 0.01, and 0.005 μg mL−1 to create both in-solvent and matrix-matched calibration curves.2.4. LC-MS/MS
A Dionex Ultimate 3000 RS Ultra-High-Performance Liquid Chromatography (UHPLC) separation module was coupled with a TSQ (Triple Stage Quadrupole) Altis tandem mass spectrometer (Thermo Fisher Scientific, Austin, TX, USA) for chromatographic analysis. The electrospray ionization (ESI) interface operated in selective reaction monitoring (SRM) and positive ionization modes. The interface conditions were set: capillary voltage at 3.8 kV, source temperature at 275 °C, and desolvation temperature at 325 °C. Sheath and auxiliary gas flows were adjusted to 40 and 10 Arb, respectively.An Accucore Reversed-Phase Mass Spectrometry (RP-MS) C18 column (2.6 μm, 2.1 × 100 mm) maintained at 40 °C was used for analyte separation. The mobile phase consisted of two solutions: mobile phase A (methanol and water, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v, with 0.1% formic acid and 10 mM ammonium formate) and mobile phase B (water and methanol, 95
5 v/v, with 0.1% formic acid and 10 mM ammonium formate) and mobile phase B (water and methanol, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v/v, also containing 0.1% formic acid and 10 mM ammonium formate). The flow rate and injection volume were set at 0.3 mL min−1 and 5 μL, respectively. Gradient elution was programmed as follows: 0–1 min at 2% B, 1–5 min at 35% A, 5–10 min at 98% B, 10–14 min at 98% B, and 14.1–20 min returning to 2% B. Initial tuning was performed using a Harvard infusion pump (Harvard Apparatus, South Natick, MA, USA), with a total run time of 20 minutes. Quantification and confirmation were performed using multiple reaction monitoring modes, with data acquisition and system control handled by Trace Finder software v4.1 (Thermo Fisher Scientific Inc., Waltham, MA, USA).
5 v/v, also containing 0.1% formic acid and 10 mM ammonium formate). The flow rate and injection volume were set at 0.3 mL min−1 and 5 μL, respectively. Gradient elution was programmed as follows: 0–1 min at 2% B, 1–5 min at 35% A, 5–10 min at 98% B, 10–14 min at 98% B, and 14.1–20 min returning to 2% B. Initial tuning was performed using a Harvard infusion pump (Harvard Apparatus, South Natick, MA, USA), with a total run time of 20 minutes. Quantification and confirmation were performed using multiple reaction monitoring modes, with data acquisition and system control handled by Trace Finder software v4.1 (Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.5. Field experiment
The field experiments were conducted in the Belbies region of El-Sharkia Governorate, Egypt, during the spring season in April 2024. The experiment utilized a completely randomized block design, comprising three replicates on 100 m2 plots (10 m × 10 m) each. Untreated control plots were positioned at an adequate distance from those treated with fenpyroximate and abamectin to minimize pesticide drift.2.6. Dissipation experiment
For the dissipation study, abamectin (5% EC) and fenpyroximate (5% SC) were applied in okra following the guideline of the Ministry of Agriculture in Egypt,11 at the recommended doses of 10 g a.i. ha−1 and 25 g a.i. ha−1, respectively. The applications were made using a manual backpack sprayer with a 20 liter capacity, with water used for formulation dilution at a rate of 1000 L ha−1. Representative random samples (1 kg) were collected from each treated plot at 2 hours (initial residue) and 1, 3, 7, 10, 14, and 21 days following application. All samples were promptly transported to the laboratory, processed immediately, and stored at −20 °C until analysis.2.7. Terminal residue experiment
To evaluate the terminal residues of the tested pesticides, abamectin and fenpyroximate were uniformly applied to okra two or three times. The recommended rates were 25 g a.i. ha−1 for fenpyroximate and 10 g a.i. ha−1 for abamectin, with treatments also applied at double the recommended doses of 50 g a.i. ha−1 and 20 g a.i. ha−1, respectively. These treatments were executed at 14 day intervals during the 2024 farming season. Samples were analyzed 4, 7, and 14 days after the final treatment. The okra samples were packed in labeled, vented polyethylene bags, stored at low temperatures, transported to the laboratory, and stored at −20 °C until analysis.2.8. Extraction and cleanup
A 10 ± 0.1 g portion of the finely ground frozen sample was weighed into a 50 mL centrifuge tube. For extraction, 10 mL of acidified acetonitrile (1% acetic acid) and a ceramic homogenizer were added, then the tube was vortexed for 2 minutes. To the extraction tube, 4 g of anhydrous MgSO4 and 1 g of CH3COONa were added, followed by vortexing and centrifugation at 5000 rpm for 5 minutes. After centrifugation, 1 mL of the clear supernatant was transferred to a 2 mL tube containing 150 mg of anhydrous MgSO4, 25 mg of PSA, and 2.5 mg of Multi-Walled Carbon Nanotubes (MWCNTs). The mixture was vortexed for 1 min, centrifuged for 5 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, and filtered through a 0.22 μm syringe filter into an LCMS/MS vial for analysis. When necessary, diluted solutions of real samples were made up using blank extracts (Fig. 1).
000 rpm, and filtered through a 0.22 μm syringe filter into an LCMS/MS vial for analysis. When necessary, diluted solutions of real samples were made up using blank extracts (Fig. 1).
2.9. Method validation
The method was validated according to the SANTE guideline9 for linearity, limits of detection (LOD) and quantification (LOQ), recovery, and precision. Blank okra samples collected from untreated fields were used to validate the method. Linearity was studied using a six-point standard calibration graph by plotting the detector response against standard concentration within the 0.001 to 0.5 μg mL−1 range. The LOD was estimated at a signal-to-noise ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The LOQ was estimated by considering a value of 3.3 times the LOD and then confirmed by calculating the recovery and repeatability,12 which should be within 70–120% and <20%, respectively.
1. The LOQ was estimated by considering a value of 3.3 times the LOD and then confirmed by calculating the recovery and repeatability,12 which should be within 70–120% and <20%, respectively.
The method recovery study was assessed at four spiking concentrations of 0.005, 0.01, 0.1, and 1 mg kg−1, with five replications. The spiked samples were equilibrated and processed using the above extraction and clean-up procedure. The method's repeatability was estimated through the relative standard deviation (RSD%) at the LOQ level in one day (intra-day repeatability, RSDr, n = 6) and three different days (inter-days repeatability, RSDR, n = 18).
Calibration curves for the tested analytes, constructed in pure solvent and matrix-matched solutions, were used to assess the matrix effect (ME) by comparing their slopes using eqn (1).
| ME (%) = (Smatrix − Ssolvent)/Ssolvent × 100 | (1) |
An ME value between −20% and 20% indicates no significant matrix effect. Values from −20% to −50% or 20% to 50% suggest a moderate effect, while values below −50% or above 50% indicate a strong effect.13
2.10. Calculations
The dissipation kinetics of abamectin and fenpyroximate residues in okra were best described by a first-order kinetic model, represented by the equation: Ct = C0 × exp−kt, where Ct is the concentration (mg kg−1) of abamectin or fenpyroximate at time t (days), C0 is the initial concentration (mg kg−1), and k is the dissipation rate constant (per day). The goodness of fit was evaluated based on the correlation coefficient (R2).
The half-life (t1/2) was calculated using: t1/2 = ln(2)/k, while the pre-harvest interval (PHI), or safe waiting period, was determined using: PHI = (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C0 − ln
C0 − ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MRL)/k.
MRL)/k.
| NEDI = Σ(STMRi × Fi) | (2) |
| HQc = NEDI/ADI × bw | (3) |
3. Results and discussion
3.1. Optimization of LC-MS/MS conditions
Data acquisition parameters for the analytes in selective reaction monitoring (SRM) mode were automatically optimized using Trace Finder software v4.1. Each analyte was directly infused at a concentration of 0.5 mg L−1. Optimal precursor ions, fragment voltages, precursor-product ion pairs, and collision energies were selected for the target compounds (Fig. 2). Two product ions were chosen for each analyte: one with a higher abundance for quantification and another with a lower abundance for confirmation (Table 1).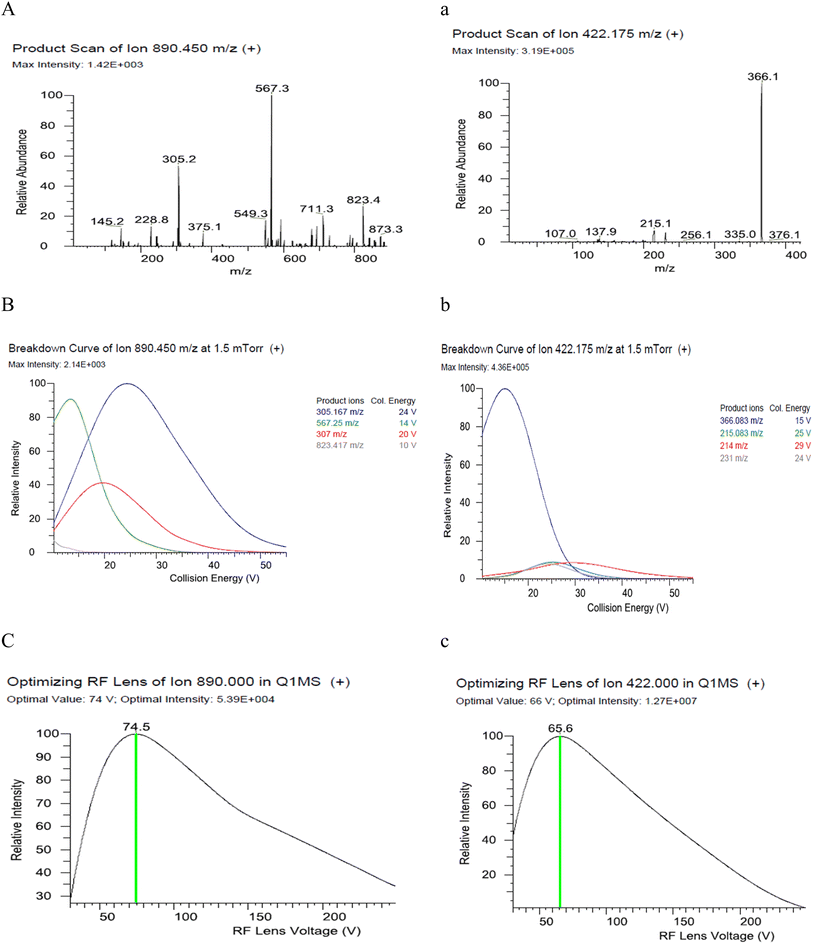 | ||
| Fig. 2 Product ions scan, product ion collision energy, and RF lens optimization of abamectin (A)–(C) and fenpyroximate (a)–(c). | ||
| Pesticide | tR (min) | Precursor ion (m/z) | Product ion (m/z) | Collision energy (v) | RF lens (v) |
|---|---|---|---|---|---|
| a The underlined ions were used for quantitation. | |||||
| Abamectin | 14.77 | 890.4 | 305.1 | 24 | 75 |
| 567.2 | 14 | 75 | |||
| Fenpyroximate | 14.53 | 422.1 | 366 | 15 | 66 |
| 231 | 24 | 66 | |||
Optimizing chromatographic separation and ionization conditions is essential to ensure accuracy and sensitivity in analysis. This study evaluated different gradient elution programs using water and methanol as the mobile phase. Adding formic acid to the water/methanol phase enhanced ionization, particularly for fenpyroximate. Ammonium formate was added to enhance abamectin detection during electrospray ionization (ESI). It promoted the formation of ammonium adducts [M + NH4]+, which improved ionization efficiency, leading to high signal intensity and greater detection sensitivity.20 In this study, adding 10 mM ammonium formate improved the chromatographic peak shape and sensitivity for abamectin without significantly affecting fenpyroximate detection. Adding 0.1% formic acid to the water/methanol mobile phase enhanced the signal response of fenpyroximate considerably.
This improvement contributed to a lower limit of detection (LOD) and limit of quantification (LOQ) for abamectin. The mass transitions m/z 890.4 > 305.1 for abamectin and 422.1 > 366 for fenpyroximate were used for quantification, as they showed higher intensities and stability than other transitions. Fenpyroximate and abamectin were eluted under standardized chromatographic conditions at 14.53 min and 14.77 min, respectively (Fig. 3).
 | ||
| Fig. 3 Product ion chromatograms (m/z) for abamectin (A) and fenpyroximate (B) in the spiked okra matrix at 0.005 mg kg−1. | ||
3.2. Method validation
The validation process assessed linearity, limit of quantification (LOQ), matrix effect (ME), precision, and recovery. Results are provided in Tables 2 and 3.| Abamectin | Fenpyroximate | |
|---|---|---|
| a RSDr: the relative standard deviation (intra-day repeatability).b RSDR: the relative standard deviation (inter-days repeatability). | ||
| Range (mg kg−1) | 0.002–0.1 | 0.001–0.1 |
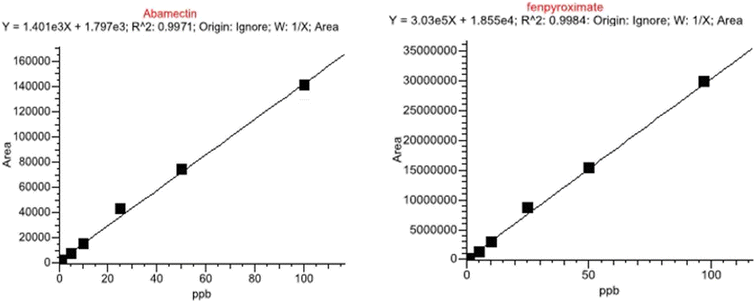
| Regression equation | Y = 1.401 × 103 + 1.797 × 103 | Y = 3.03 × 105 + 1.855 × 104 |
| R2 | 0.9971 | 0.9984 |
| RSDr (n = 6)a | 11.4 | 7.6 |
| RSDR (n = 18)b | 16.8 | 10.3 |
| ME (%) | −14.6 | −5.2 |
| LOD (mg kg−1) | 0.0006 | 0.0002 |
| LOQ (mg kg−1) | 0.002 | 0.001 |
| Pesticides | Spiked levels (mg kg−1) | Average recoveries (%) | RSDs (%) |
|---|---|---|---|
| Abamectin | 0.005 | 84.2 | 5.8 |
| 0.01 | 91.4 | 3.8 | |
| 0.1 | 93.7 | 4.1 | |
| 1 | 96.2 | 6.5 | |
| Fenpyroximate | 0.005 | 89.4 | 8.7 |
| 0.01 | 96.3 | 5.8 | |
| 0.1 | 98.6 | 7.1 | |
| 1 | 97.1 | 8.4 |
A linear correlation between detector response (y) and analyte concentration (x) in mg kg−1 was determined from calibration curves prepared for abamectin and fenpyroximate standards. The signal responses of the target analytes were evaluated by injecting 5 μL of the analytical solution prepared in acetonitrile across nine concentration levels (0.001, 0.002, 0.005, 0.01, 0.02, 0.05, 0.1, 0.2, and 0.5 mg kg−1). Calibration curves for abamectin and fenpyroximate demonstrated strong linearity within the ranges of 0.002–0.1 mg kg−1 and 0.001–0.1 mg kg−1, with correlation coefficients (R2) of 0.9971 and 0.9984, respectively (Fig. 4). Back-calculating the area (y) for each concentration level (x) resulted in deviations from the ideal response of ≤13.8%, well within the acceptable deviation limit of ±20%.12
The matrix effect (ME) was assessed by comparing the calibration curve slopes for abamectin and fenpyroximate in a pure solvent with those in okra extracts, and they showed weak signal suppression with ME values of 14.6% and 5.2%, respectively. The ME values were <20%, indicating no significant effect on the current analysis. Nevertheless, the analytes tested were quantified with matrix-matched calibration curves by an external standard method to mitigate possible matrix effects (Table 4).
| Analyte | Matrix | Instrument | Sample weight (g) | Extraction solvent | Salts | Cleanup | LOQ (mg kg−1) | Recovery (%) | Precision (RSD%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| a Abamectin was determined among other analytes. | ||||||||||
| Abamectin | Perilla leaves | HPLC-FLD (derivatization) | 10 | Acetonitrile (30 mL) | MgSO4 (6 g) | Silica cartridge (SPE) | 0.01 | 82.11–93.03 | ≤8 | 21 |
| Abamectin | Apples | HPLC-FLD (derivatization) | 5 | Acetonitrile (20 mL) | — | C18 cartridge (SPE) | 0.002–0.005 | 88–106 | Satisfactory | 23 |
| Pears | ||||||||||
| Tomatoes | ||||||||||
| Abamectina | Eggplant | LC-MS/MS | 10 | Acidified (1%), acetonitrile (10 mL) | MgSO4 (4g), CH3COONa (1g) | 2× dilution | 0.02 | 88.6–94.8 | ≤14.8% | 20 |
| Fenpyroximate | Grapes | HPLC-UV | 10 | Acidified (1%), acetonitrile (10 mL) | MgSO4 (4g), NaCl (1g) | MgSO4 (150 mg), PSA (25 mg), GCB (5 mg) | 0.05 | 88.5–100.4 | ≤8 | 22 |
| Fenpyroximate | Apple | HPLC-UV | 10 | Acidified (1%), acetonitrile (15 mL) | MgSO4 (4g), NaCl (1g), sodium citrate | MgSO4 (166.6 mg), PSA (25 mg) | 0.003 | 92–103 | ≤9 | 24 |
| Citrus | ||||||||||
| Grape | Dehydrate (1g), disodium hydrogen citrate sesquihydrate (0.5 g) | |||||||||
| Fenpyroximate | Guava | LC-MS/MS | 10 | Acetonitrile (10 mL) | MgSO4 (4g), NaCl (1g) | 20× dil | 0.01 | 92.4–107.3 | ≤15.4 | 9 |
| Orange | ||||||||||
| Eggplant | ||||||||||
| Abamectin | Okra | LC-MS/MS | 10 | Acidified (1%), acetonitrile (10 mL) | MgSO4 (4g), CH3COONa (1g) | MgSO4 (150 mg), PSA (25 mg), MWCNTs, (2.5 mg) | 0.002 | 84.2–96.2 | ≤16.8 | This study |
| Fenpyroximate | 0.001 | 89.4–98.6 | ≤10.3 | |||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. LOD values were determined to be 0.0006 mg kg−1 for abamectin and 0.0002 mg kg−1 for fenpyroximate. The limit of quantification (LOQ) represented the lowest concentration quantifiable with an acceptable recovery of 70–120% and precision of ≤20%. LOQ values were 0.002 mg kg−1 for abamectin and 0.001 mg kg−1 for fenpyroximate, yielding mean recoveries (n = 3) of 74 ± 8% and 81 ± 6%, respectively. LOD and LOQ values were below the maximum residue limit (MRL) of 0.01 mg kg−1 set by the European Commission Regulation (EU MRL) for the tested pesticides in okra.
1. LOD values were determined to be 0.0006 mg kg−1 for abamectin and 0.0002 mg kg−1 for fenpyroximate. The limit of quantification (LOQ) represented the lowest concentration quantifiable with an acceptable recovery of 70–120% and precision of ≤20%. LOQ values were 0.002 mg kg−1 for abamectin and 0.001 mg kg−1 for fenpyroximate, yielding mean recoveries (n = 3) of 74 ± 8% and 81 ± 6%, respectively. LOD and LOQ values were below the maximum residue limit (MRL) of 0.01 mg kg−1 set by the European Commission Regulation (EU MRL) for the tested pesticides in okra.The validation results demonstrate the method's reliability for determining abamectin and fenpyroximate in okra samples.
3.3. Comparison of extraction, cleanup, and analytical performance with previous studies
This study optimized the determination of abamectin and fenpyroximate in okra using LC-MS/MS, achieving improved sensitivity, precision, and cleanup efficiency compared to previous methodologies (Table 1). The limit of quantification (LOQ) achieved was 0.002 mg kg−1 for abamectin and 0.001 mg kg−1 for fenpyroximate, lower than previous methods such as 0.01 mg kg−1 in perilla leaves,21 0.02 mg kg−1 in eggplant,20 and 0.05 mg kg−1 in grapes.22 This highlights the enhanced sensitivity of our method. The recovery rates, 84.2–96.2% for abamectin and 89.4–98.6% for fenpyroximate (in this study), are comparable to or better than previous studies, including 82.11–93.03% for abamectin in perilla leaves21 and 88.6–94.8% in eggplant.20 Precision (RSD%) was ≤16.8% for abamectin and ≤10.3% for fenpyroximate (in this study), aligning with values reported in other studies, such as ≤14.8% for eggplant20 and ≤8% for grapes.22This study extraction and cleanup procedures reduced matrix interference and improved method robustness. Acidified (1%) acetonitrile with MgSO4 (4 g) and CH3COONa (1 g) was used for extraction, improving analyte solubilization over methods relying solely on acetonitrile and MgSO4, such as perilla leaves.21 Cleanup included MgSO4 (150 mg), PSA (25 mg), and multi-walled carbon nanotubes (MWCNTs, 2.5 mg), which enhanced matrix removal compared to PSA alone or C18 cartridges.23,24 MWCNTs improved selectivity, reducing background noise and enhancing sensitivity. LC-MS/MS provided superior specificity and lower detection limits than HPLC-FLD21,23 or HPLC-UV,22,24 making it more suitable for trace pesticide detection. The combined improvements in LOQ, extraction efficiency, and cleanup selectivity make this method highly effective for determining abamectin and fenpyroximate residues.
3.4. Persistence and dissipation kinetics
The persistence of abamectin and fenpyroximate residues in okra fruits was investigated over the experimental time (Fig. 5). The initial residue of abamectin after applying the recommended dose of 10 g a.i. ha−1 was 0.0437 mg kg−1, which gradually decreased to 0.0111 mg kg−1 after 1 day (74.56% reduction), 0.0051 mg kg−1 after 3 days (88.28% reduction), and 0.0037 mg kg−1 after 7 days (91.60% reduction). By day 10, abamectin residues had fallen below the detection limit (BDL) of 0.0006 mg kg−1. Fenpyroximate had a higher initial residue of 0.134 mg kg−1 after applying the recommended dose of 25 g a.i. ha.−1 It decreased after 1 day to 0.044 mg kg−1 (67.41% reduction), after 3 days to 0.0233 mg kg−1 (82.59% reduction), and after 7 days to 0.0103 mg kg−1 (92.31% reduction). By day 10, the residue had fallen further to 0.0033 mg kg−1 (97.51% reduction) and reached 0.0025 mg kg−1 (98.12% reduction) after 14 days. On day 21, the residues of fenpyroximate were below the detection limit of 0.0003 mg kg−1. The results showed that both pesticides degraded over the experimental period, with residues of abamectin falling below the detection limit within 10 days, while fenpyroximate persisted for up to 21 days.Abamectin and fenpyroximate dissipation patterns in okra fruits were evaluated by fitting the experimental data to zero-order, first-order, and second-order kinetic models to identify the best-fitting model (Table 5).
The zero-order model was unsuitable for analyzing abamectin, yielding an R2 value of only 0.4937. In contrast, the first-order model provided a considerably better fit, with an R2 value of 0.7186. The second-order model, however, achieved the highest accuracy, producing an optimal R2 value of 0.9217, indicating the best fit to the data. For fenpyroximate, the first-order model provided the best fit, with an R2 value of 0.9389. The zero-order model had an R2 value of 0.5351, while the second-order model had an R2 value of 0.9144. Simpler models are generally preferred when they minimize overfitting and enhance interpretability, provided they offer reasonable accuracy. Consequently, we selected the first-order model. The experimental results indicated an exponential decay pattern consistent with first-order kinetics, showing a sharp reduction in concentration between days 0 and 1, followed by a gradual, steady decline.
The results indicate that the calculated half-life of abamectin was 2.30 days, whereas fenpyroximate has a slightly longer half-life of 2.45 days. Abamectin dissipates at a rate of 0.3014 per days compared to 0.2832 per days for fenpyroximate. A higher dissipation rate indicates a faster decline, which means that abamectin is dissipated more quickly than fenpyroximate in okra fruits. Fenpyroximate achieved a significantly higher initial deposition on okra fruits (0.134 mg kg−1) than abamectin (0.0437 mg kg−1). This impressive difference in initial deposition indicates that fenpyroximate adheres very effectively or deposits more efficiently on the okra surface. Fenpyroximate has a higher log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value (5.7) than abamectin (4.4),25 which evaluates the high lipophilicity of fenpyroximate compared to abamectin, which will facilitate the binding of fenpyroximate to the waxy surface of okra and increases its persistence. This increased binding affinity could explain the higher initial deposition observed for fenpyroximate, as it is less likely to be washed off or rapidly degraded.
P value (5.7) than abamectin (4.4),25 which evaluates the high lipophilicity of fenpyroximate compared to abamectin, which will facilitate the binding of fenpyroximate to the waxy surface of okra and increases its persistence. This increased binding affinity could explain the higher initial deposition observed for fenpyroximate, as it is less likely to be washed off or rapidly degraded.
Abamectin and fenpyroximate are formulated as 5% EC and 5% SC, respectively. The formulation type of a pesticide can influence how long the active ingredient persists on plants.26 In the case of abamectin, the EC formulation, which involves dissolving the active ingredient in a solvent with an emulsifier, may lead to higher and more persistent residues on okra surfaces compared to the SC formulation of fenpyroximate due to the solvent's penetration properties in the EC formulation. However, abamectin is also highly susceptible to rapid photodegradation27 and enzymatic breakdown28 within plants, leading to a faster metabolism and quicker dissipation. In contrast, fenpyroximate in its SC form is more resistant to plant metabolic degradation.18 The lipophilic nature of fenpyroximate25 allows it to adhere longer to plant surfaces, making it less prone to rapid metabolic breakdown and, therefore, more persistent within plant tissues. The vapor pressure is critical in determining a pesticide's tendency to volatilize. Fenpyroximate has a higher vapor pressure (0.00921 mPa) than abamectin (0.0037 mPa),25 indicating that fenpyroximate may be more likely to volatilize under favorable climatic conditions. Temperature and humidity significantly influence vapor pressure on volatilization but may not significantly affect the overall dissipation rate in this context. Another critical factor influencing persistence and dissipation is the chemical structure. Fenpyroximate is photostable,29 i.e., it is more resistant to sunlight degradation than abamectin,30 increasing its persistence on the plant surface.25
The half-life of abamectin determined in this study was 2.3 days, slightly longer than the documented values of 1.0, 1.06, 1.02, and 1.75 days for green beans, tomatoes, strawberries, and tomatoes, respectively.31–34 However, it is similar to the half-lives of 2.38 days in cucumbers and 2.1 to 2.4 days in eggplants reported by other studies.20,35 The half-life of fenpyroximate was 2.45 days, longer than the previously reported values of 1.7, 2.2, and 1.9 days for eggplants, guavas, and oranges9 but within the range of 1.56 to 2.75 days observed for other crops.24 However, it was shorter than the longer half-life of 3.5 days in grapes.22
The differences in dissipation rates across crops are influenced by morphological factors, including surface structure, wax content, and transpiration rates, which affect pesticide retention and absorption since okra's higher trichome density and cuticle thickness than strawberries or tomatoes might slow the penetration and breakdown of pesticide residues, leading to a slightly extended persistence. Environmental conditions such as temperature, humidity, and UV exposure are crucial in pesticide dissipation. Additionally, microbial degradation is another critical factor influencing pesticide dissipation. The presence of pesticide-degrading bacteria and fungi in soil and plant surfaces can contribute to faster degradation in warmer climates with higher microbial activity. The climatic conditions in Egypt, characterized by med-high temperatures and intense solar radiation in the spring-summer seasons, likely enhanced the dissipation rates of both compounds compared to studies conducted in cooler environments. The formulation type significantly affects pesticide adherence, penetration, and persistence on plant surfaces. Furthermore, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P (partition coefficient) values influence the solubility and persistence of pesticides.36
P (partition coefficient) values influence the solubility and persistence of pesticides.36
The pre-harvest interval (PHI), defined as the time required for abamectin and fenpyroximate residue levels to decrease below the established maximum residue limit (MRL) of 0.01 mg kg−1 (as per the EU-MRL database), was calculated to be 2.6 days for abamectin and 6.9 days for fenpyroximate.
3.5. Terminal residues and risk assessment
The results of fenpyroximate and abamectin terminal residues in okra fruits are shown in Tables 6 and 7. Various doses, spraying frequencies, and residue measurements at varied intervals were used to evaluate the associated risk. Abamectin dosages of 10 and 20 g a.i. ha−1 showed residue levels ranging from 0.0020 mg kg−1 to 0.0170 mg kg−1, with concentrations decreasing significantly between day 3 and day 7. This trend highlights the fast dissipation of abamectin terminal residues in okra fruits.| Dosage (g a.i. ha−1) | Number of times sprayed | Days after spraying | Mean residues (mg kg−1) | SD | NEDI (mg kg−1 bw) | HQc (%) |
|---|---|---|---|---|---|---|
| 10 | 2 | 3 | 0.0073 | 0.0006 | 1.92 × 10−7 | 0.016 |
| 7 | 0.0022 | 0.0010 | 2.36 × 10−8 | 0.002 | ||
| 3 | 3 | 0.0083 | 0.0021 | 2.19 × 10−7 | 0.018 | |
| 7 | 0.0028 | 0.0001 | 7.41 × 10−8 | 0.006 | ||
| 20 | 2 | 3 | 0.0170 | 0.0010 | 4.45 × 10−7 | 0.037 |
| 7 | 0.0021 | 0.0000 | 2.62 × 10−8 | 0.002 | ||
| 3 | 3 | 0.0120 | 0.0017 | 3.14 × 10−7 | 0.026 | |
| 7 | 0.0043 | 0.0006 | 1.13 × 10−7 | 0.009 |
| Dosage (g a.i. ha−1) | Number of times sprayed | Days after spraying | Mean residues (mg kg−1) | SD | NEDI (mg kg−1 bw) | HQ (%) |
|---|---|---|---|---|---|---|
| 25 | 2 | 3 | 0.040 | 0.002 | 1.05 × 10−3 | 10.47 |
| 7 | 0.011 | 0.001 | 2.70 × 10−4 | 2.70 | ||
| 14 | 0.002 | 0.001 | 6.11 × 10−5 | 0.61 | ||
| 3 | 3 | 0.042 | 0.005 | 1.09 × 10−3 | 10.86 | |
| 7 | 0.009 | 0.002 | 2.46 × 10−4 | 2.46 | ||
| 14 | 0.002 | 0.000 | 5.74 × 10−5 | 0.57 | ||
| 50 | 2 | 3 | 0.060 | 0.003 | 1.58 × 10−3 | 15.79 |
| 7 | 0.022 | 0.000 | 5.76 × 10−4 | 5.76 | ||
| 14 | 0.004 | 0.000 | 1.05 × 10−5 | 0.10 | ||
| 3 | 3 | 0.097 | 0.006 | 2.53 × 10−3 | 25.25 | |
| 7 | 0.027 | 0.006 | 7.07 × 10−4 | 7.07 | ||
| 14 | 0.003 | 0.000 | 6.63 × 10−5 | 0.66 |
The estimated national daily intake (NEDI) values for abamectin, ranging from 2.36 × 10−8 to 4.45 × 10−7 mg kg−1 body weight, and the corresponding chronic hazard quotient (HQc) values, all well below the 100% threshold, provide reassurance of the minimal health risk from abamectin residues in okra fruits.
For fenpyroximate, 25 and 50 g a.i. ha−1 dosages showed terminal residue levels between 0.002 mg kg−1 and 0.097 mg kg−1, decreasing over time. The highest residue (0.097 mg kg−1) was observed at 50 g a.i. ha−1 with three sprays three days after application. NEDI values ranged from 6.11 × 10−5 to 2.53 × 10−3 mg kg−1 body weight, while HQc values ranged from 0.10% to 25.25%, all remaining below the 100% threshold, indicating a low risk associated with fenpyroximate-treated okra.
Both abamectin and fenpyroximate residues consistently decreased concentrations over time, influenced by dosage and spraying frequency. Nevertheless, the HQc values remained below 100%, which means a negligible risk for the adults, even with high dosages and repeated applications. The dissipation pattern underlines the importance of appropriate pre-harvest intervals to reduce residue levels before consumption. Our results provide essential insights for safely using these acaricides in okra cultivation.
4. Conclusion
The validated QuEChERS (Quick, Easy, Cheap, Effective, rugged, and Safe) method combined with LC-MS/MS was developed in this study for determining abamectin and fenpyroximate residues in okra fruits. The method was successfully validated according to SANTE guidelines and was characterized by high accuracy and reliability and excellent selectivity, linearity, and sensitivity. The optimized chromatographic conditions significantly improved the ionization efficiency and, thus, the detection sensitivity for both pesticides. Persistence studies showed that fenpyroximate has higher initial residues and longer persistence than abamectin due to its greater lipophilicity and UV photostability, with half-lives of 2.3 and 2.45 days, respectively. The estimated pre-harvest intervals (PHI) of 2.6 days for abamectin and 6.9 days for fenpyroximate ensured residue levels below the MRL, supporting consumer safety. The study found that the risk to consumers from abamectin and fenpyroximate application in okra was negligible when used at approved and double approved rates. The risk assessment for both compounds at all intervals demonstrated an acceptable level of dietary risk. These findings provide important insights into the safe use of these pesticides on okra. However, this study is limited to a specific climatic region (Egypt), which may affect its applicability to other environments. It also focuses only on two pesticides without considering interactions with other agrochemicals. Additionally, long-term monitoring is needed to assess cumulative exposure and environmental persistence. Future research should explore the effects of environmental factors on pesticide degradation, compare dissipation across different crops, and evaluate long-term dietary risks.Abbreviations
| QuEChERS | Quick, easy, cheap, effective, rugged, and safe |
| MRL | Maximum residue limit |
| ADI | Acceptable daily intake |
| NEDI | National estimated daily intake |
| LOQ | The limit of quantitation |
| ME% | Matrix effect percent |
| STMRi | The median final residue obtained from the supervised trials (mg kg−1) |
| Fi | The average daily per capita consumption (kg per day) |
| SRM | Selective reaction monitoring |
| EC | Emulsifiable concentrate |
| SC | Suspension concentrate |
| PHI | Pre-harvest interval |
| HQc | Chronic hazard quotient |
Data availability
The data associated with this article have been included in the manuscript.Author contributions
Farag Malhat and Osama I. Abdallah: conceptualization, methodology, investigation, formal analysis, data curation, writing– original draft, writing review & editing, visualization, supervision, project administration. El-Sayed Saber and Nevein S. Ahmed: investigation, resources, data curation. Shokr Abel Salam Shokr: editing, funding acquisition, project administration.Conflicts of interest
The authors have no conflicts of interest to declare.References
- A. E. O. Elkhalifa, E. Alshammari, M. Adnan, J. C. Alcantara, A. M. Awadelkareem, N. E. Eltoum, K. Mehmood, B. P. Panda and S. A. Ashraf, Molecules, 2021, 26, 696 CrossRef CAS PubMed.
- O. E. Adelakun, O. J. Oyelade, B. I. O. Ade-Omowaye, I. A. Adeyemi and M. Van de Venter, Food Chem. Toxicol., 2009, 47, 1123–1126 CrossRef CAS PubMed.
- S. Fan, L. Guo, Y. Zhang, Q. Sun, B. Yang and C. Huang, Mol. Nutr. Food Res., 2013, 57, 2075–2078 CrossRef CAS.
- Z. Liao, J. Zhang, B. Liu, T. Yan, F. Xu, F. Xiao, B. Wu, K. Bi and Y. Jia, Molecules, 2019, 24, 1906 CrossRef CAS.
- S. Saha, M. R. Jadhav, T. P. A. Shabeer, K. Banerjee, B. K. Sharma, M. Loganathan and A. B. Rai, Proc. Natl. Acad. Sci., India, Sect. B, 2016, 86, 359–366 CrossRef CAS.
- T. F. Mekonnen, L. Byrne, U. Panne and M. Koch, Food Anal. Methods, 2018, 11, 2657–2665 CrossRef.
- National Center for Biotechnology Information (NCBI), PubChem Compound Summary for CID 9576412, Fenpyroximate, 2025, retrieved February 18, 2025, from https://pubchem.ncbi.nlm.nih.gov/compound/9576412 Search PubMed.
- European Food Safety Authority (EFSA), EFSA J., 2016, 14, 4382 Search PubMed.
- F. Malhat, O. Abdallah, C. Anagnostopoulos, M. Hussien, I. Purnama, R. Helmy, H. Soliman and D. El-Hefny, Front. Nutr., 2022, 9, 939012 CrossRef.
- R. P. Pohanish, Sittig's Handbook of Pesticides and Agricultural Chemicals, William Andrew, 2014 Search PubMed.
- C. Costas-Ferreira and L. R. F. Faro, Int. J. Mol. Sci., 2021, 22, 8413 CrossRef CAS.
- T. Pihlström, A. R. Fernández-Alba, C. F. Amate, M. E. Poulsen, B. Hardebusch, M. Anastassiades, R. Lippold, L. C. Cabrera, A. de Kok and F. O'Regan, SANTE, 2021, 11312, v2 Search PubMed.
- C. Ferrer, A. Lozano, A. Agüera, A. J. Girón and A. R. Fernández-Alba, J. Chromatogr. A, 2011, 1218, 7634–7639 CrossRef CAS PubMed.
- European Food Safety Authority (EFSA), A. Brancato, D. Brocca, L. Ferreira, L. Greco, S. Jarrah, R. Leuschner, P. Medina, I. Miron and A. Nougadere, EFSA J., 2018, 16, e05147 Search PubMed.
- F. Malhat and O. Abdallah, Environ. Monit. Assess., 2019, 191, 584 CrossRef CAS PubMed.
- IEDI, IEDI Calculations for FAO/WHO Acute Dietary Intake Assessment, 2014 Search PubMed.
- Á. Ambrus, Submission and Evaluation of Pesticide Residues Data for the Estimation of Maximum Residue Levels in Food and Feed, FAO, 2009 Search PubMed.
- European Food Safety Authority (EFSA), EFSA J., 2008, 6, 197 Search PubMed.
- European Food Safety Authority (EFSA), M. Anastassiadou, M. Arena, D. Auteri, A. Brancato, L. Bura, L. C. Cabrera, E. Chaideftou, A. Chiusolo and F. Crivellente, EFSA J., 2020, 18, e06009 Search PubMed.
- J. S. Algethami, M. A. M. Alhamami, M. F. Ramadan and O. I. Abdallah, Agriculture, 2022, 13, 116 CrossRef.
- M. M. Rahman, T. W. Na, A. M. Abd El-Aty, J.-H. Park, M. N. U. Al Mahmud, A. Yang, K. H. Park and J.-H. Shim, Environ. Monit. Assess., 2013, 185, 9461–9469 CrossRef CAS.
- F. Malhat, A. El-Mesallamy, M. Assy, W. Madian, N. M. Loutfy and M. T. Ahmed, Toxicol. Environ. Chem., 2013, 95, 1309–1317 CrossRef CAS.
- H. Diserens and M. Henzelin, J. Chromatogr. A, 1999, 833, 13–18 CrossRef CAS.
- S. H. Abd Al-Rahman, M. M. Almaz and I. A. Osama, Food Anal. Methods, 2012, 5, 306–311 CrossRef.
- K. A. Lewis, J. Tzilivakis, D. J. Warner and A. Green, Hum. Ecol. Risk Assess.: Int. J., 2016, 22, 1050–1064 CrossRef CAS.
- W. Farha, A. M. Abd El-Aty, M. M. Rahman, H.-C. Shin and J.-H. Shim, Environ. Monit. Assess., 2016, 188, 1–21 CrossRef CAS.
- D. Rugg, S. D. Buckingham, D. B. Sattelle and R. K. Jansson, Compr. Mol. Insect Sci., 2005, 1, 25–52 Search PubMed.
- M. S. Khalil and D. M. Darwesh, J. Plant Sci. Phytopathol., 2019, 3, 81–85 CrossRef.
- M. Swanson, W. Ivancic, J. Doe and G. K. O’Brien, J. Agric. Food Chem., 1995, 43, 337–342 CrossRef.
- A. Martijn, CIPAC, Encyclopedia of Agrochemicals, 2003 Search PubMed.
- A. El-Latif, A. A. Sallam and D. S. El-Hefny, J. Sohag Agrisci., 2022, 7, 127–137 Search PubMed.
- M. E. I. Badawy, M. S. Mahmoud and M. M. Khattab, J. Environ. Sci. Health, Part B, 2020, 55, 517–524 CrossRef CAS.
- S. H. Abd Al-Rahman, M. M. Almaz and N. S. Ahmed, Food Anal. Methods, 2012, 5, 564–570 CrossRef.
- H. A. Mahmoud, M. M. H. Arief, I. N. Nasr and I. H. Mohammed, J. Appl. Sci. Res., 2010, 6, 932–936 CAS.
- S. H. Abd-Alrahman, I. A. Osama and A. A. Mostafa, J. Anim. Vet. Adv., 2013, 12(14), 1228–1231 Search PubMed.
- P. Fantke, B. W. Gillespie, R. Juraske and O. Jolliet, Environ. Sci. Technol., 2014, 48, 8588–8602 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |