Open Access Article
Open Access ArticleA highly stretchable, self-adhesive, antimicrobial conductive hydrogel with guar gum/acrylic acid/MXene@AgNPs for multifunctional wearable sensors and electromagnetic interference shielding†
Tongle Pua,
Changgeng Lib,
Lin Yanga,
Xiu-zhi Tang c,
Yunjun Ruan
c,
Yunjun Ruan a and
Tong Guo
a and
Tong Guo *a
*a
aCollege of Big Data and Information Engineering, Guizhou University, Guiyang 550025, China. E-mail: tguo3@gzu.edu.cn
bSchool of Electronic Information, Central South University, Changsha 410083, China
cSchool of Aeronautics and Astronautics, Central South University, Changsha 410083, China
First published on 27th March 2025
Abstract
Multifunctional conductive hydrogels have attracted extensive attention in the fields of biomedicine and health monitoring. However, integrating excellent stretchability, self-adhesion, sensitive sensing, electromagnetic interference (EMI) shielding, and antibacterial properties into conductive hydrogels for wearable sensor applications remains a significant challenge. In this study, a multifunctional conductive hydrogel (GAMA) was prepared by incorporating MXene@Ag nanoparticles (AgNPs) as conductive fillers, which were uniformly dispersed within a dual-network structure of guar gum/acrylic acid. Serving as conductive agents, reinforcing fillers, and antibacterial components, MXene@AgNPs enable the GAMA hydrogel to achieve multifunctional integration and balanced performance. The GAMA hydrogel exhibits outstanding mechanical performance with a tensile strength of 97 kPa and elongation at a break of 850%, self-adhesion (21.5 kPa), and high conductivity (14.04 mS cm−1). Additionally, we employed this hydrogel as a flexible strain sensor to monitor human motion, achieving high sensitivity (gauge factor (GF) of 6.48 at 300% strain). The in situ synthesis of AgNPs on MXene nanosheets enhances polarization and interfacial losses of electromagnetic waves, endowing the hydrogel with an EMI shielding effectiveness of 34.5 dB. Furthermore, comprehensive biocompatibility evaluations confirm its excellent antibacterial performance, hemocompatibility, and cytocompatibility. Therefore, these properties endow the multifunctional GAMA hydrogel with great potential for applications in wearable sensors for human motion monitoring and EMI shielding.
1 Introduction
In recent years, flexible wearable electronic devices have attracted increasing attention in applications such as electronic skin,1,2 human motion monitoring,3–5 and soft robotics.6,7 In addition, as the demand for enhanced functionalities in these devices continues to grow across various sectors, the development of multifunctional integration has become crucial for advancing high-performance flexible wearable electronics.8,9 For instance, owing to superior biocompatibility, structural similarity to natural soft tissues, and excellent electrical conductivity, conductive hydrogels are promising candidates for flexible wearable sensors.3,10 However, as application environments become more complex, conductive hydrogels must combine excellent electrical conductivity and stress-sensing performance with protective features such as EMI shielding and antibacterial properties. Therefore, the multifunctional integration and balance of conductive hydrogels have become crucial research directions for the development of wearable sensors.Typically, incorporating conductive fillers such as nanocarbon materials (e.g., graphene, carbon nanotubes) and metal nanoparticles can effectively endow hydrogels with excellent electrical conductivity. However, the mechanical and electrical properties of hydrogels are influenced by the uniform dispersion of conductive fillers.11,12 MXene, a two-dimensional material similar to reduced graphene oxide (RGO), boasts outstanding electrical conductivity and hydrophilicity. It can disperse uniformly within hydrogels, significantly enhancing their electrical conductivity. Moreover, through the van der Waals interactions between its surface functional groups and polymer chains, MXene improves the mechanical properties of hydrogels. For example, Zhang et al.13 added MXene@CNFs to a polyacrylamide/gelatin double-network hydrogel, increasing the tensile strength from the original 80 kPa to 110 kPa. Additionally, due to its good electrical conductivity, this composite hydrogel exhibits a total electromagnetic shielding efficiency (EMI SE) of 33.8 dB at a thickness of 4 mm. More importantly, MXene, as a carrier, can be combined with other functional materials and uniformly dispersed in hydrogels, facilitating multifunctional integration and performance balance.
Considering the safety factors of wearable sensors, the components of conductive hydrogels should be derived from non-toxic or low-toxicity polymeric materials. Natural polymers such as chitosan,14 alginate,15 guar gum,16 gelatin,17 and nanocellulose18,19 are particularly suitable for applications in the field of wearable conductive hydrogels due to their excellent biocompatibility and safety. In addition, since hydrogel sensors are directly attached to human skin, there are gaps between the skin and the hydrogel that are similar to the living environment of cells. This situation easily leads to bacterial growth, posing a threat to human health. At the same time, it also shortens the service life of the hydrogel and affects its sensing performance.20–22 Silver nanoparticles (AgNPs) are widely used in antibacterial composite materials due to their advantages such as broad-spectrum antibacterial properties, durability, and good thermal stability. However, the synthesis of traditional AgNPs relies on chemical reduction methods, such as the reduction of silver nitrate by sodium borohydride. Although this method can rapidly prepare AgNPs, issues such as toxic byproducts, complex synthesis processes, and particle aggregation limit its applications in the fields of biomedicine and flexible electronics. Interestingly, the abundant functional groups on the surface of MXene nanosheets can directly reduce AgNO3, enabling the in situ formation of AgNPs. This strategy not only ensures the uniform distribution of AgNPs on the MXene surface but also promotes a synergistic effect that enhances both electrical conductivity and EMI performance. Furthermore, the introduction of AgNPs can significantly improve the antibacterial performance. The released Ag+ can achieve broad-spectrum and efficient inhibition of various pathogenic bacteria (such as Staphylococcus aureus) by mechanisms such as disrupting the bacterial cell membrane, inhibiting enzyme activity, and interfering with DNA replication. Moreover, its long-term effectiveness is superior to that of traditional antibacterial agents.
Herein, we designed a multifunctional hydrogel with electrical conductivity, high stretchability, self-adhesion, and antibacterial properties for applications in wearable sensors and electromagnetic interference (EMI) shielding fields. In detail, MXene loaded with in situ generated AgNPs was utilized as a multifunctional filler and incorporated into a mixed solution of natural guar gum and acrylic acid. Subsequently, through in situ polymerization, a conductive hydrogel based on a double-network structure of guar gum (GG)/acrylic acid (AA)/MXene@AgNPs (GAMA) was obtained. The GG/AA double-network hydrogel, rich in functional groups, exhibits excellent mechanical properties and self-adhesive properties. Additionally, owing to the van der Waals interactions between MXene and polymer chains, the mechanical properties of the composite hydrogel are further enhanced. Benefiting from the synergistic effect of MXene and AgNPs, the addition of MXene@AgNPs endows GAMA with excellent electrical conductivity, EMI shielding performance, antibacterial properties, and biocompatibility. In addition, when assembled into wearable sensors, the GAMA hydrogel effectively monitored body movements and vocal cord vibrations. Therefore, this study provides new insights into the design and fabrication of multifunctional hydrogels, demonstrating their potential applications in electromagnetic shielding and wearable electronic devices.
2 Materials and methods
2.1 Materials
The GG was purchased from Hefei Bomei Biotechnology Co., Ltd. Ti3C2 (MXene) was obtained from Foshan Xinxi Technology Co., Ltd. AA (AR, purity ≥99%), N,N′-methylene-bis-acrylamide (MBA, AR) and ammonium persulfate (APS, AR, purity ≥98%) were purchased from Aladdin Chemistry Co., Ltd. Silver nitrate was purchased from Sinopharm Chemical Reagent Co., Ltd. Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) were obtained from Guangdong Huankai Microbial Technology Co., Ltd. Human fibroblasts were from the cell bank (Chinese Academy of Sciences). Rabbit serum was obtained from Meryer Biochemical Technology Co., Ltd. All reagents were used directly without further purification.2.2 Preparation of GAMA hydrogel
First, 0.2 g of MXene powder was added to 10 mL of deionized water and sonicated for 30 min to obtain a uniformly dispersed suspension. Second, 0.1 g of AgNO3 powder was weighed and dissolved in 10 mL of deionized water under magnetic stirring for 10 min. Subsequently, the AgNO3 solution was added drop by drop to the MXene solution, followed by magnetic stirring for 10 min. The mixture was then sonicated for 1 h to yield a homogeneous MXene@AgNPs solution. Next, 0.5 g of GG was added to 20 mL of deionized water and stirred at 60 °C for 30 min until completely dissolved. After stirring for 30 min, 6 mL of AA was added. The mixture was then stirred at 60 °C for another 30 min until it became homogeneous. After that, MXene@AgNPs solution was added to the mixed solution of GG/AA with vigorous stirring at 60 °C for 1 h. Then, 0.014 M of APS initiator and 0.06 M of MBA crosslinker were added to make the mixture homogeneous, and it was quickly transferred to a glass plate mold, and in situ polymerization was carried out at 60 °C for 3 h to obtain GAMA hydrogel. The obtained samples obtained were denoted as GAM, GAMA1, and GAMA2 according to the mass of AgNO3 added (0, 0.1 g, 0.2 g). The control samples without MXene nanosheets and AgNPs are denoted as GA.2.3 Characterization
X-ray diffraction (XRD) patterns of the samples were measured using an X-ray diffractometer (Empyrean PANalytical B. V.). The morphology and elemental profiles of the samples were characterized by field emission scanning electron microscopy (FESEM, SU8010) and Oxford X-Max energy-dispersive X-ray spectrometer (EDS). Before the characterization, the hydrogel samples were freeze-dried and then fractured in liquid nitrogen to produce fresh cross-sections. The transmission electron microscopy (TEM) were acquired on JEOL JEM-F200. XPS spectra were recorded using an X-ray photoelectron spectrometer (Thermo Scientific K-Alpha+). UV-vis spectra were recorded using a UV-vis spectrophotometer (Shimadzu UV-1900). Fourier transform infrared spectra (FTIR) were recorded using an infrared spectrometer (Nicolet 470, Thermo Scientific) in the wavenumber range of 600 to 4000 cm−1. The tensile properties of the hydrogels were tested by using a universal tensile testing machine (CMT6103, MTS system), the sample size of the tensile specimens was 20 mm × 10 mm × 4 mm, and the loading rate was set at 50 mm min−1. For testing the adhesion strength of the hydrogels, a tensile–shear experiment was constructed using the universal tensile testing machine. The hydrogels were adhered to the surfaces of different substrates, such as wood, polytetrafluoroethylene (PTFE), copper, and glass, with a bonding area of 20 mm × 20 mm. The test was carried out at a tensile rate of 10 mm min−1. The adhesion strength of the hydrogel was calculated by dividing the maximum adhesion force by the initial bonding area. (The structural characterization of MXene@AgNPs, along with the silver release behavior and degradation behavior of the GAMA2 hydrogel, are provided in the ESI†).2.4 Conductivity tests
The conductivity of hydrogels was measured using an electrochemical workstation (CHI760E, Chenhua). The resistance of hydrogels with dimensions of 30 mm × 20 mm × 4 mm was tested using electrochemical AC impedance spectroscopy in the frequency range of 0.1–100 kHz and an amplitude of 0.1 V. The hydrogels were sandwiched between two copper foils during testing, and the ionic conductivity was calculated using eqn (1):
 | (1) |
2.5 Strain sensing tests
The sensing performance of the hydrogel sensor was evaluated using a Chenhua CHI760E electrochemical workstation. The evaluation was carried out with the assistance of a volunteer, and informed consent was obtained for the testing. The changes in real-time resistance of hydrogels with dimensions of 30 mm × 20 mm × 4 mm at different strains were tested by recording the i–t curves via the electrochemical workstation at a voltage of 2 V. Subsequently, the changes in relative resistance were calculated according to eqn (2):
 | (2) |
The gauge factor (GF) under tensile strain is calculated from eqn (3):
 | (3) |
2.6 Electromagnetic interference shielding measurements
The EMI shielding performance of the samples was tested in the frequency range of 8.2–12.4 GHz using a vector network analyzer (E5071C, AGILENT). The hydrogel samples were cut into rectangles of 22.86 mm × 10.16 mm × 4 mm. The EMI shielding effectiveness was defined as the logarithmic ratio of incident power to transmitted power. The total SE (SET) is the sum of reflection loss (SER), absorption loss (SEA), and multiple reflection loss (SEM). SEM can be neglected when SE is greater than 15 dB. The scattering parameters (S11, S12, S21, and S22) are measured, and SET, SEA, and SER were calculated according to the following equations:
 | (4) |
 | (5) |
 | (6) |
The absorption (A), reflection (R), and transmission (T) coefficients were calculated as:
| R = |S12|2 = |S21|2 | (7) |
| T = |S11|2 = |S22|2 | (8) |
| A = 1 − R − T | (9) |
2.7 Antimicrobial tests
All materials were sterilized in an autoclave for 30 min at 121 °C. The antimicrobial properties of the hydrogels were evaluated using Escherichia coli (Gram-negative bacteria) and Staphylococcus aureus (Gram-positive bacteria). First, the hydrogels were sterilized by UV light for 30 min. Subsequently, the hydrogels were placed in test tubes containing 10 mL of bacterial solution (1.0 × 104 CFU mL−1) and the tubes were placed in a shaking incubator for 4 h (37 °C, 200 rpm). After that, the bacterial solution was processed by gradient dilution method, and 100 μL of diluted bacterial solution was added to LB solid medium, coated well, and incubated in a sterile incubator for 24 h. The antibacterial rate was calculated as follows:
 | (10) |
2.8 Cell viability test
The cytotoxicity of the hydrogel on human fibroblasts was evaluated using the CCK-8 assay. The samples were sterilized by ultraviolet irradiation for 30 min. Subsequently, the samples were immersed in DMEM solution at 37 °C for 48 h. Then, the extracts were diluted to different concentrations (0, 0.01, 0.025, 0.05, 0.1, 0.25, 0.5, and 1.0 mg mL−1). Human fibroblasts were seeded into a 96-well plate at a density of 1 × 104 cells per well (100 μL per well). After pre-culturing for 24 h, the medium was replaced with an equal volume of fresh medium containing the extracts. Meanwhile, a blank control (pure DMEM medium) and a negative control (untreated cells) were set up. After 24 h of exposure, 10 μL of 10% CCK-8 reagent was added to each well and incubated in the dark for 1 h. The optical density (OD) value was measured at a wavelength of 450 nm using a microplate reader. The cell viability was calculated using eqn (11):
 | (11) |
2.9 Hemocompatibility of the GAMA2 hydrogels
The hemocompatibility of the hydrogel was detected by measuring the absorbance of hemoglobin released after the red blood cell (RBC) lysis. First, 5 mL of rabbit blood was collected into an anticoagulant tube. Centrifuge the blood at 1000 rpm for 10 min, then discard the supernatant. Wash the blood cells with physiological saline, and then centrifuge at 1500 rpm for 5 min. Repeat this washing and centrifugation process twice and retain the blood cell pellet. Next, dilute the blood cell pellet to a concentration of 2% (v/v) with PBS. Take 500 μL of the diluted blood cells and mix them at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio with 500 μL of hydrogel extracts at different concentrations (10, 50, 100, 200, 500 μg mL−1), PBS (negative control), and distilled water (positive control) respectively. Then, place the mixtures in an environment at 37 °C and let them stand for 1 h. After that, centrifuge at 3500 rpm for 5 min. Transfer the supernatant to a 96-well plate and measure the OD value at 542 nm. The calculation of the hemolysis rate is shown in eqn (12):
1 volume ratio with 500 μL of hydrogel extracts at different concentrations (10, 50, 100, 200, 500 μg mL−1), PBS (negative control), and distilled water (positive control) respectively. Then, place the mixtures in an environment at 37 °C and let them stand for 1 h. After that, centrifuge at 3500 rpm for 5 min. Transfer the supernatant to a 96-well plate and measure the OD value at 542 nm. The calculation of the hemolysis rate is shown in eqn (12):
 | (12) |
3 Results and discussion
3.1 Synthesis and characterization of hydrogels
The preparation process of GAMA hydrogels is shown in Fig. 1. The MXene nanosheets loaded with AgNPs were incorporated into a mixed solution of GG and AA, and then the dual-network structure of the GAMA conductive hydrogel was fabricated via in situ free-radical polymerization. The MXene@AgNPs were uniformly dispersed in the hydrogel and linked to the polymer chains via abundant hydrogen bonds.The microstructure and elemental distribution of the lyophilized GAMA hydrogels were characterized by SEM. As shown in Fig. 2a–c, the hydrogels exhibited a three-dimensional porous structure, with pore diameters of approximately 30 μm in the GA hydrogel and around 10 μm in the GAM and GAMA2 hydrogels. Fig. 2d shows the elemental distribution image of the GAMA2 hydrogel. Ti and Ag are distributed relatively uniformly, indicating the homogeneous dispersion of MXene and AgNPs within the hydrogel. Fig. 2e shows the XRD patterns of MXene@AgNPs powder, pure GA, GAM, and GAMA2 hydrogels. The pure GA hydrogel displayed a low-intensity broad peak at about 27°, indicating an amorphous crystal structure.23 After the addition of MXene nanosheets to the pure GA gel, a characteristic peak of MXene (002) appeared at 5.5° and the (002) peak of MXene nanosheets shifted to a smaller angle, indicating that the layer spacing of the MXene nanosheets became larger. With the introduction of MXene@AgNPs, three distinct diffraction peaks appeared in the GAMA2 hydrogel at 5.4°, 38.3°, and 44.5°. These peaks corresponded to the (002) facet of the MXene nanosheets and the (111) and (200) facets of the face-centered cubic structure of silver, respectively, suggesting the successful introduction of the AgNPs.
To further elucidate the intermolecular interactions in the GAMA2 hydrogels, the FTIR spectra of the pure GA, GAM, and GAMA2 are presented in Fig. 2f. All three hydrogels exhibited broad peaks at 3450 cm−1, which can be attributed to the absorption of water and the stretching vibration of the –OH group, indicating the formation of hydrogen bonds.24 The peak associated with C–O stretching vibration was observed at 1699 cm−1, where carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, hydrogen bond acceptor) and hydroxyl (–OH, hydrogen bond donor) groups can produce a high density of hydrogen bonding.25 This is in agreement with the broad peak at 3450 cm−1, which suggests the formation of hydrogen bonding between the polymer chains and MXene nanosheets. The XPS spectra of MXene powder, GA, GAM, and GAMA2 hydrogels are demonstrated in Fig. 2g. From these spectra, it can be observed that the peaks corresponding to Ti 2p and F 1s appeared in the XPS curves of the GAM and GAMA2 hydrogels. Additionally, the Ag 3d peaks are observed in the GAMA2 hydrogel, indicating the successful incorporation of MXene and AgNPs, which was further confirmed by the EDS image. The high-resolution spectra of C 1s and O 1s of GAMA2 hydrogels were further analyzed, as shown in Fig. 2h and i. The core energy level spectrum of C 1s had four peak components, C–C/C
O, hydrogen bond acceptor) and hydroxyl (–OH, hydrogen bond donor) groups can produce a high density of hydrogen bonding.25 This is in agreement with the broad peak at 3450 cm−1, which suggests the formation of hydrogen bonding between the polymer chains and MXene nanosheets. The XPS spectra of MXene powder, GA, GAM, and GAMA2 hydrogels are demonstrated in Fig. 2g. From these spectra, it can be observed that the peaks corresponding to Ti 2p and F 1s appeared in the XPS curves of the GAM and GAMA2 hydrogels. Additionally, the Ag 3d peaks are observed in the GAMA2 hydrogel, indicating the successful incorporation of MXene and AgNPs, which was further confirmed by the EDS image. The high-resolution spectra of C 1s and O 1s of GAMA2 hydrogels were further analyzed, as shown in Fig. 2h and i. The core energy level spectrum of C 1s had four peak components, C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (284.8 eV), C–O (285.4 eV), C–Ti (284 eV), and C
C (284.8 eV), C–O (285.4 eV), C–Ti (284 eV), and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (289.4 eV), and the core energy level spectrum of O 1s had two peak components, C–O (532 eV) and C
O (289.4 eV), and the core energy level spectrum of O 1s had two peak components, C–O (532 eV) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (533.3 eV). The strong C signal indicates the presence of abundant hydrophilic groups on the guar and acrylic chains, which provided abundant cross-linking sites in the hydrogel.
O (533.3 eV). The strong C signal indicates the presence of abundant hydrophilic groups on the guar and acrylic chains, which provided abundant cross-linking sites in the hydrogel.
3.2 Mechanical properties of hydrogels
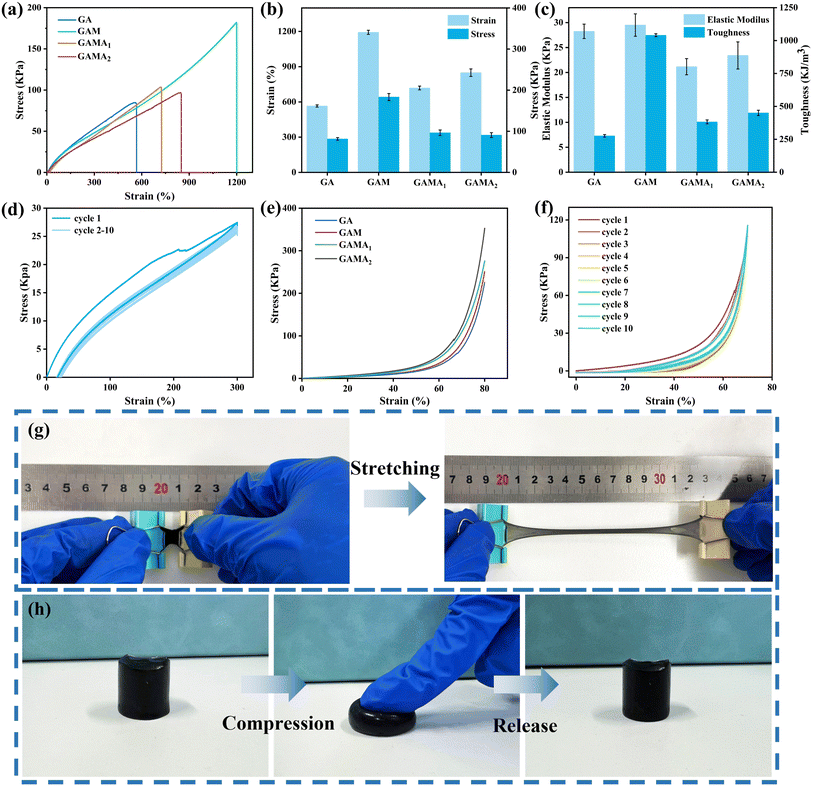
Excellent mechanical properties, good elasticity, and high stretchability are essential for hydrogels to govern the applicability of hydrogel sensors. Fig. 3a–c demonstrate the tensile stress–strain curves, strength and elongation, as well as the toughness and elastic modulus of GA, GAM, GAMA1, and GAMA2. The GA hydrogel exhibits poor mechanical properties, with a fracture strain of 567%, a tensile strength of 84.84 kPa, and a relatively low toughness of only 277 kJ m−3. After incorporating MXene nanosheets, the GAM hydrogel demonstrates significantly improved mechanical properties, with a tensile strength of 182 kPa, a fracture strain of 1198%, and a maximum toughness of 1041 kJ m−3, far surpassing those of the GA hydrogel. This enhancement primarily results from the large surface area and abundant polarized functional groups of MXene nanosheets, which facilitate stress transfer to more PAA and GG chains, effectively preventing stress concentration.13 However, after introducing MXene@AgNPs, the mechanical properties of the GAMA1 hydrogel declined compared to those of the GAM hydrogel, with a fracture strain of 724% and a tensile strength of 103.6 kPa. Moreover, as the AgNPs content increases, the tensile strength of the GAMA2 hydrogel further decreases. This is mainly because AgNPs adhere to the surface of MXene nanosheets, reducing the number of available polarized functional groups. Consequently, the interactions between MXene and both PAA and GG chains weaken, leading to a decline in tensile strength.Fig. 3d shows the loading–unloading curves of GAMA2 hydrogel under 300% tensile strain for 10 consecutive cycles. The significant hysteresis observed in the first cycle is likely attributed to the extensive breaking of hydrogen bonds, effectively dissipating energy and preserving the integrity of the gel network. After the first cycle of loading, the subsequent hysteresis loops nearly overlapped and the dissipated energy was almost constant, indicating a good cyclic stability. In addition, the hydrogels were also subjected to compression tests. Fig. 3e shows the compression stress–strain curves of the hydrogels with different conductive fillers added. It can be seen that the GAMA2 hydrogel exhibits the highest compression strength of 350 kPa. Fig. 3f shows the compression of GAMA2 hydrogel at 70% strain for 10 cycles at a rate of 10 mm min−1. The hysteresis curve and energy in the first cycle are higher than in subsequent cycles but stabilize with increasing cycle numbers, demonstrating the GAMA2 hydrogel's excellent fatigue resistance and rapid deformation recovery. As illustrated in Fig. 3g and h, the GAMA2 hydrogel exhibits exceptional stretchability, extending over 10 times its original length without rupture. Moreover, under the finger pressure, the hydrogel can be restored to its original shape without any damage after it is released from the finger, indicating that the hydrogel exhibits excellent shape recovery.
3.3 Adhesion properties of hydrogels
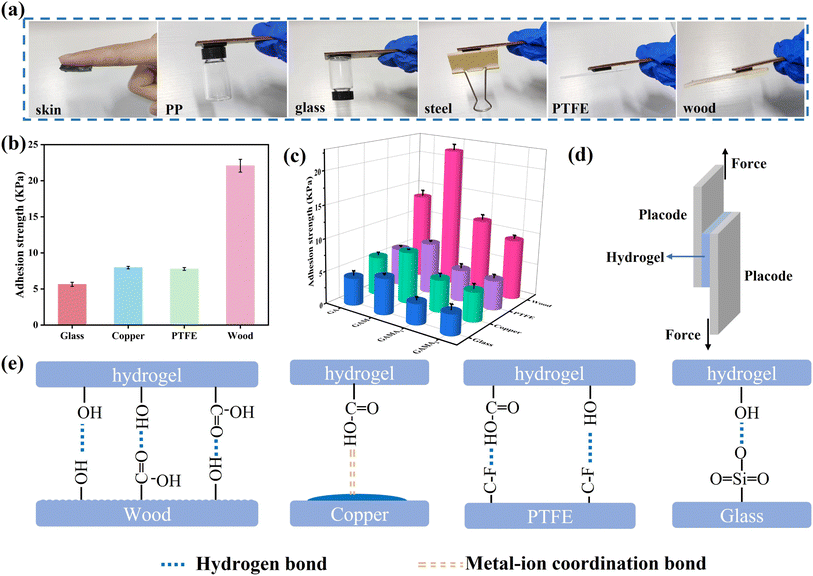
Self-adhesion ensures a tighter interface between the conductive hydrogel and the target surface, thereby maintaining the sensitivity of the sensing performance. As shown in Fig. 4a, the GAMA2 hydrogel exhibits excellent adhesion properties to various substrates, including skin, polypropylene (PP), glass, steel, polytetrafluoroethylene (PTFE), and wood. The good adhesion of hydrogels can be ascribed to hydrogen bonding and coordination interactions between the polymer network and the substrate. Multiple binding sites create strong interfacial connections, making the gel adhesive.To quantitatively evaluate the adhesion strength of the hydrogels, a tensile–shear testing system was used to evaluate the adhesion strength of hydrogels with different conductive fillers added to different substrates. As shown in Fig. 4d, all hydrogel specimens were cut into 20 mm × 20 mm × 4 mm pieces and bonded between two identical substrates. Fig. S7† shows the adhesion–displacement curves of different hydrogels on wood, glass, copper, and PTFE, indicating that the hydrogel exhibits the highest adhesion to wood and the lowest adhesion to glass. As illustrated in Fig. 4b–e, the hydrogel exhibits the highest bonding strength to wood among the four selected substrates. This is primarily attributable to the abundance of hydroxyl and carboxyl groups present on the surface of wood, which facilitate the formation of robust hydrogen bonds and hydrophobic interactions with the hydrogel. Additionally, the dimples on the wood surface enhance the contact area with the hydrogel, thereby intensifying the friction between the hydrogel and the wood.
The adhesion strength of the hydrogel to copper, PTFE, and glass decreased in that order, and the difference in adhesion strength between the different substrates may be due to different interfacial interactions. For copper, the carboxyl groups in the hydrogel formed coordination sites with copper atoms, which promoted the interfacial interactions between the hydrogel and the copper sheet. For PTFE, the adhesion mainly originated from the hydrogen bonding between the hydrogel and PTFE, so the adhesion strength was slightly weaker. For the glass plate, the adhesion strength is mainly determined by the hydrogen bonding between the interfaces and the electrostatic force, but the smooth surface of the glass plate makes the adhesion strength of the hydrogel to the glass the lowest among the four substrates. Among the four different hydrogels, the adhesion strength of GAM hydrogel to different substrates was the highest. Taking wood as an example, the adhesion strength of GAM hydrogel to wood was 21.5 kPa, which was significantly greater than that of GA hydrogel (13.3 kPa), GAMA1 hydrogel (11.3 kPa) and GAMA2 hydrogel (9.3 kPa). In comparison to pure GA hydrogels, the functional groups on the surface of a significant number of MXene nanosheets in GAM hydrogels are capable of forming hydrogen bonds with the substrate, thereby enhancing the adhesion strength. However, the introduction of MXene@AgNPs into GA hydrogels has resulted in the number of hydrogen bonds formed between the substrate and the hydrogel being reduced due to the presence of a large number of AgNPs on the surface of the MXene. Moreover, AgNPs also form metal–ligand bonds with the hydroxyl groups on the hydrogel surface, which further diminishes adhesion strength.
3.4 Conductivity and electromagnetic shielding properties of hydrogels
To investigate the electromagnetic interference shielding properties of hydrogels, the conductivity was initially measured.26 The electrical conductivity of GAM and GAMA is derived from the conductive network formed by the uniformly dispersed MXene nanosheets and MXene@AgNPs, respectively. Fig. 5a shows the relationship between the electrical conductivity of the hydrogel and the contents of MXene and AgNPs. With the addition of MXene nanosheets, the conductivity of the hydrogel increased from the insulating state of the pure GA hydrogel to 5.07 mS cm−1. Subsequently, due to the combined effect of MXene nanosheets and AgNPs, the conductivity further increased to 14.04 mS cm−1. The moderate conductivity of the hydrogel is associated with a higher electrical impedance and maintains a considerable attenuation capacity, leading to excellent EMI shielding properties of the hydrogel.27In daily applications, a material with an EMI SE of 20 dB can block about 99% of the incident electromagnetic wave energy.28 The EMI shielding performance of the hydrogel with a thickness of 4 mm was evaluated by measuring the scattering parameters (S11, S12, S21, and S22) using a vector network analyzer in the frequency range of 8.2–12.4 GHz. Fig. 5b compares the EMI SE values of GA hydrogels with different conductive fillers. The average EMI SE of pure GA hydrogels without any conductive fillers is 21.47 dB, which has reached the commercial 20 dB standard. Notably, the EMI shielding performance of GA hydrogel is mainly attributed to the polarization of water molecules under the influence of electromagnetic fields, as well as the changes in the extensive hydrogen bonding network.28 With the introduction of MXene nanosheets and MXene@AgNPs composite nanosheets, the average EMI SE of GAM and GAMA2 increased to 24.1 dB and 31 dB, respectively. More importantly, the trend of SET variations corresponds to that of conductivity, suggesting that the synergistic interaction between MXene and AgNPs significantly enhances EMI shielding performance.
To evaluate the EMI shielding mechanism of hydrogels, the contributions of absorption loss (SEA) and reflection loss (SER) of hydrogels were analyzed. As illustrated in Fig. 5c, the SET and SEA of GAMA2 hydrogel exhibit a significant growing trend, while the SER remains unchanged, demonstrating efficient electromagnetic wave attenuation with composite hydrogels. However, the attenuation of the EMI wave involves reflection first and then absorption. The calculated SER is based on the total power of the EMI wave, while the SEA is based on the power of the incident wave.29 To further evaluate the actual shielding mechanism of the hydrogel, the transmitted (T), absorbed (A), and reflected (R) power coefficients of the hydrogel are calculated in Fig. 5d. It demonstrates that the R of the pure GA hydrogel and GAM hydrogel are greater than the corresponding A of the GAM hydrogel. The R of pure GA hydrogels and GAM hydrogels are larger than the corresponding A, indicating that reflection loss is the predominant shielding mechanism. Furthermore, the R of GAM hydrogels are slightly larger than those of pure GA hydrogels, which is mainly due to the inevitable increase in impedance matching and electromagnetic wave reflection resulting from the incorporation of MXene nanosheets. The incorporation of MXene@AgNPs into the hydrogel results in an A greater than the R, indicating that the synergistic effect of MXene nanosheets and AgNPs promotes absorption rather than reflection. Furthermore, the power coefficient A increases as the content of AgNPs rises, while the R shows an opposite trend, suggesting that AgNPs enhance the hydrogel's absorption capability. In general, reflection attenuation is dependent on impedance mismatch at the interface between air and the shielding material. In contrast, absorption attenuation is associated with electromagnetic energy conversion, which is facilitated by induced currents and polarization relaxation.30–32
Fig. 5e illustrates the EMI shielding mechanism of the GAMA2 hydrogel. The incident electromagnetic waves are partially reflected, and only a small portion of the waves can penetrate through the hydrogel. The remaining electromagnetic waves are absorbed inside the GAMA2 hydrogel and converted into heat energy and dissipation.33 The absorption effect arises from the conductive loss caused by the conductive network formed by MXene/AgNPs, the dipolar polarization loss due to abundant functional groups and defects, and the interfacial polarization loss caused by rich heterogeneous interfaces. Additionally, the porous structure increases multiple reflections of the electromagnetic waves, which prolongs the propagation path of the incident waves and enhances the absorption effect. In summary, the excellent EMI shielding performance of GAMA2 is the result of the synergistic effects of multiple reflections, conductive loss, and polarization loss.34,35
3.5 Sensing properties of GAMA2 hydrogel
Due to its excellent tensile properties and conductivity, the porous GAMA2 hydrogel experiences changes in resistance under stress, thereby enabling the monitoring of human motion. In order to evaluate the potential of GAMA2 hydrogel as a flexible strain sensor, the relationship between its relative resistance with tensile strain was investigated. As illustrated in Fig. S8,† during the slow stretching of the hydrogel, the brightness of the light bulb gradually dimmed, which indicates that the stretching strain leads to an increase in the resistance of the hydrogel. Therefore, the strain sensitivity of the GAMA2 hydrogel as a strain sensor can be determined by fitting the slope of the resistance–strain curve during stretching. As shown in Fig. 6a, the GAMA2 hydrogel exhibits a gauge factor (GF) of 2.06 in the 0–100% strain range and 3.42 in the 100–200% strain range. Furthermore, the hydrogel demonstrates a GF of 6.4 in the 200–300% strain range. Overall, the hydrogel exhibits a substantial linear operating range and notable sensitivity, which are superior to that of previously reported hydrogel-based strain sensors.36,37 The strain-sensing response of the GAMA2 hydrogel stems from the changes in the electron transport pathways within the MXene nanosheet network during stretching. Additionally, the change to the three-dimensional interconnected porous structure promotes ion migration within the hydrogel. Therefore, the hydrogel can accurately detect tensile deformations of varying magnitudes. Notably, the relative change in resistance increases with tensile strain over a wide range of 5–200%, indicating that the strain sensor has a broad operational range, along with excellent responsiveness and repeatability (Fig. 6b and c).Furthermore, owing to its remarkable adhesion properties, the hydrogel can be directly affixed to human skin for real-time monitoring of fingers, wrists, throat, and other areas. As illustrated in Fig. 6d–g, as the bending angle of a finger increases from 0° to 90°, the relative resistance signal gradually rises due to slight stretching of the hydrogel strain sensor. Conversely, when the finger is maintained at a fixed bending angle, the relative resistance remains stable, indicating that the resistance increases with strain on the hydrogel. The stability of the relative resistance further suggests that the hydrogel has high consistency and stability. Notably, when the bending angle is varied from 0° to 90° and then back to 0°, the relative resistance changes consistently, demonstrating that the hydrogel sensor is capable of transiently detecting finger movements. Additionally, due to its high sensitivity, the hydrogel sensor can monitor small body movements. When placed on a volunteer's throat, the sensor shows regular and reproducible changes in relative resistance as the volunteer “repeatedly says “ni hao”. These results suggest that hydrogel sensors can serve as multifunctional wearable flexible sensors, which can quickly and accurately monitor various body movements and speech recognition.
3.6 Antimicrobial properties of GAMA2 hydrogel
Wearable hydrogel sensors attached to human skin are susceptible to bacterial growth, which not only compromises the normal functionality of the hydrogel but also endangers human health.38 Accordingly, the antimicrobial properties of the GAMA2 hydrogel were evaluated against Escherichia coli (a Gram-negative bacterium) and Staphylococcus aureus (a Gram-positive bacterium) using a plate colony counting method. By introducing differently doped hydrogels into the bacterial solution, varying colony counts could be observed. As illustrated in Fig. 7a–c, the inhibition rate of GA hydrogel on E. coli and S. aureus was 56.91% and 74.97%, respectively. The GAM hydrogel containing MXene nanosheets exhibits stronger inhibition against E. coli and S. aureus, with inhibition rates increasing to 81.45% and 78.83%, respectively. Furthermore, thanks to the potent antibacterial effect of AgNPs, the GAMA2 hydrogel demonstrates exceptional antimicrobial activity, with an antibacterial rate of 100% against both E. coli and S. aureus.3.7 Biocompatibility of GAMA2 hydrogel
In addition to antibacterial activity, biocompatibility and biosafety are crucial for antibacterial hydrogels.39 Therefore, the biocompatibility and biosafety of GAMA2 hydrogel were systematically evaluated. First, the CCK-8 assay was used to assess the effect of GAMA2 hydrogel on the viability of human fibroblasts. As shown in Fig. 8a, within the concentration range of 0.01–1 mg mL−1, GAMA2 hydrogel did not exhibit any toxicity to the cells, and the cell viability in each concentration group was maintained above 98%. This indicates that the extracts of GAMA2 hydrogel have good cytocompatibility with human fibroblasts within the tested concentration range. Next, an in vitro hemolysis assay was conducted to explore the blood compatibility of GAMA2 hydrogel. As shown in Fig. 8b, even when the concentration of GAMA2 reached 500 μg mL−1, the hemolysis rate caused by GAMA2 hydrogel was 3.26%, indicating that GAMA2 hydrogel has good blood compatibility. | ||
| Fig. 8 Biocompatibility of GAMA2 hydrogel. (a) Cell viability detected by CCK-8 assay after 24 h of treatment. (b) Hemolysis ratio analysis. | ||
4 Conclusion
In summary, we developed a GAMA hydrogel. The multifunctional conductive filler MXene@AgNPs were prepared by in situ reduction of AgNO3 using MXene nanosheets, and then it was introduced into the GG/AA dual-network structure. This hydrogel exhibited excellent stretchability, self-adhesion, efficient EMI shielding, sensing, antibacterial capability and biocompatibility. The good interfacial interaction between MXene and GA hydrogels guarantees the structural stability of the GAM hydrogel, with a tensile strength of 181 kPa at 1200% strain. Although the incorporation of MXene@AgNPs occupies part of the polymer functional groups, the composite hydrogel still maintains excellent self-adhesion properties. Coupled with the conductive loss and polarization loss induced by MXene@AgNPs, as well as the multiple reflection losses generated by the porous structure, the composite hydrogel exhibits an efficient EMI SE of 34.5 dB in the X-band. Moreover, due to its outstanding strain and conductivity performance, the GAMA hydrogel functions as a highly sensitive and reliable wearable sensor for human motion monitoring. Meanwhile, comprehensive biocompatibility evaluations have confirmed its excellent antibacterial performance, hemocompatibility, and cytocompatibility. Overall, the well-designed GAMA hydrogel integrates and balances multiple functionalities. The GAMA hydrogel demonstrates vast potential in wearable healthcare, flexible electronics, and biomedical engineering. By its multifunctional design, the current GAMA hydrogel is poised to expand its applications to dynamic health monitoring systems, smart wound dressings, protective coatings for implantable devices, as well as flexible electronics and soft robotics.Data availability
The datasets generated and analyzed in this current study are available from the corresponding author upon reasonable request.Author contributions
Tongle Pu: writing–original draft, review & editing, visualization, formal analysis, data curation. Changgeng Li: investigation, methodology, resources. Lin Yang: visualization, validation, investigation. Xiu-zhi Tang: funding acquisition, resources, supervision. Yunjun Ruan: funding acquisition, resources, supervision. Tong Guo: funding acquisition, resources, supervision, project administration.Conflicts of interest
No conflict of interest exists in the submission of this manuscript, and the manuscript is approved by all authors for publication. I would like to declare on behalf of my co-authors that the work described was original research that has not been published previously and is not under consideration for publication elsewhere, in whole or in part. All the authors listed have approved the manuscript that is enclosed.Acknowledgements
The authors are grateful for the financial support from the Natural Science Special (Special-term Professor) Research Foundation of Guizhou University (Grant No. [2021]23), the cultivation project of Guizhou University (Grant No. [2020]16) and the Guizhou Provincial Science and Technology Projects (Grant No. ZK [2022]52). Moreover, the work was supported by the Industry and Education Combination Innovation Platform of Intelligent Manufacturing and Graduate Joint Training Base at Guizhou University (Grant No. 2020-520000-83-01-324061) and Construction of Science and Technology Platform of Guiyang (Grant No. [2023]7-3).References
- K. Chen, K. Liang, H. Liu, R. Liu, Y. Liu, S. Zeng and Y. Tian, Nano-Micro Lett., 2023, 15, 102 CAS.
- D. Lu, M. Zhu, X. Li, Z. Zhu, X. Lin, C. F. Guo and X. Xiang, J. Mater. Chem. A, 2023, 11, 18247–18261 CAS.
- X. Li, L. He, Y. Li, M. Chao, M. Li, P. Wan and L. Zhang, ACS Nano, 2021, 15, 7765–7773 CAS.
- J. Wu, Q. Ma, Q. Pang, S. Hu, Z. Wan, X. Peng, X. Cheng and L. Geng, Carbohydr. Polym., 2023, 321, 121282 CAS.
- Y. Xie, X. Shi, S. Gao, C. Lai, C. Lu, Y. Huang, D. Zhang, S. Nie, F. Xu and F. Chu, J. Mater. Chem. A, 2024, 12, 5124–5132 CAS.
- J. Qu, Q. Yuan, Z. Li, Z. Wang, F. Xu, Q. Fan, M. Zhang, X. Qian, X. Wang, X. Wang and M. Xu, Nano Energy, 2023, 111, 108387 CAS.
- J. Jiang, S. Xu, H. Ma, C. Li and Z. Huang, Mater. Today Bio, 2023, 20, 100657 Search PubMed.
- Q. Li, B. Tian, J. Liang and W. Wu, Mater. Chem. Front., 2023, 7, 2925–2957 RSC.
- Z. Qin, Y. Li, X. Wang, Y. Liu, N. Li, Q. Xu, L. Ye and T. Jiao, J. Mater. Chem. A, 2024, 12, 10808 RSC.
- Z. Nie, K. Peng, L. Lin, J. Yang, Z. Cheng, Q. Gan, Y. Chen and C. Feng, Chem. Eng. J., 2023, 454, 139843 CrossRef CAS.
- C. Chen, S. Qiu, M. Cui, S. Qin, G. Yan, H. Zhao, L. Wang and Q. Xue, Carbon, 2017, 114, 356–366 CrossRef CAS.
- W. Zhang, P.-L. Wang, L.-Z. Huang, W.-Y. Guo, J. Zhao and M.-G. Ma, Nano Energy, 2023, 117, 108875 CrossRef CAS.
- R. Zhang, D. Xie, C. Zhang, Z. Xu, Y. Fang, W. Wang, M. Xu and Y. Song, J. Mater. Chem. A, 2023, 11, 24608–24617 RSC.
- H. Zhong, Y. Fang, M. Luo, L. Wang, J. Huang, G. Dai, K. Liu, J. Wu and J. Du, ACS Appl. Mater. Interfaces, 2024, 16, 28209–28221 CrossRef CAS PubMed.
- L. T. Gao, Y. M. Chen, Y. Aziz, W. Wei, X. Y. Zhao, Y. He, J. Li, H. Li, H. Miyatake and Y. Ito, Carbohydr. Polym., 2024, 330, 121812 CAS.
- T. Chen, X. Li, Q. Wang, Y. Li, L. Xu, Y. Yang, Y. Qiao, Y. Dai, J. Ke, H. Wan, S. Zhou and Z. Gao, Int. J. Biol. Macromol., 2024, 270, 132035 CAS.
- X. Zhang, K. Liu, M. Qin, W. Lan, L. Wang, Z. Liang, X. Li, Y. Wei, Y. Hu, L. Zhao, X. Lian and D. Huang, Carbohydr. Polym., 2023, 309, 120702 CAS.
- X. Nie, Y. Xie, X. Ding, L. Dai, F. Gao, W. Song, X. Li, P. Liu, Z. Tan, H. Shi, C. Lai, D. Zhang and Y. Lai, Carbohydr. Polym., 2024, 334, 122068 CAS.
- F. Gao, Y. Pang, Y. Wang, X. Yang, W. Song, X. Nie, Z. Tan, J. Zhou, Y. Xin, D. Wang, H. Shi, C. Lai and D. Zhang, ACS Sustain. Chem. Eng., 2024, 12, 13622–13633 CAS.
- K. Elkhoury, M. Morsink, L. Sanchez-Gonzalez, C. Kahn, A. Tamayol and E. Arab-Tehrany, Bioact. Mater., 2021, 6, 3904–3923 CAS.
- Y. Huang, L. Mu, X. Zhao, Y. Han and B. Guo, ACS Nano, 2022, 16, 13022–13036 CAS.
- C. Liu, Z. Xu, S. Chandrasekaran, Y. Liu and M. Wu, Carbohydr. Polym., 2023, 303, 120468 CAS.
- W. Kong, Z. Wang, M. Wang, K. C. Manning, A. Uppal, M. D. Green, R. Y. Wang and K. Rykaczewski, Adv. Mater., 2019, 31, 1904309 CAS.
- Z. Deng, Y. Guo, X. Zhao, P. X. Ma and B. Guo, Chem. Mater., 2018, 30, 1729–1742 CAS.
- H. Fang, J. Wang, L. Li, L. Xu, Y. Wu, Y. Wang, X. Fei, J. Tian and Y. Li, Chem. Eng. J., 2019, 365, 153–164 CAS.
- R. Zhang, D. Xie, C. Zhang, Z. Xu, Y. Fang, W. Wang, M. Xu and Y. Song, J. Mater. Chem. A, 2023, 11, 24608–24617 CAS.
- Y. Yang, B. Li, N. Wu, W. Liu, S. Zhao, C. J. Zhang, J. Liu and Z. Zeng, ACS Mater. Lett., 2022, 4, 2352–2361 CAS.
- S. Gong, X. Sheng, X. Li, M. Sheng, H. Wu, X. Lu and J. Qu, Adv. Funct. Mater., 2022, 32, 2200570 CAS.
- M. H. Al-Saleh, W. H. Saadeh and U. Sundararaj, Carbon, 2013, 60, 146–156 CAS.
- Y. Yang, S. Chen, W. Li, P. Li, J. Ma, B. Li, X. Zhao, Z. Ju, H. Chang, L. Xiao, H. Xu and Y. Liu, ACS Nano, 2020, 14, 8754–8765 CAS.
- Z. Wu, K. Pei, L. Xing, X. Yu, W. You and R. Che, Adv. Funct. Mater., 2019, 29, 1901448 CrossRef.
- Q. Liu, X. Liu, H. Feng, H. Shui and R. Yu, Chem. Eng. J., 2017, 314, 320–327 CrossRef CAS.
- W. Yang, B. Shao, T. Liu, Y. Zhang, R. Huang, F. Chen and Q. Fu, ACS Appl. Mater. Interfaces, 2018, 10, 8245–8257 CrossRef CAS.
- A. Iqbal, F. Shahzad, K. Hantanasirisakul, M.-K. Kim, J. Kwon, J. Hong, H. Kim, D. Kim, Y. Gogotsi and C. M. Koo, Science, 2020, 369, 446–450 CrossRef CAS PubMed.
- Y. Zhang, Y. Huang, T. Zhang, H. Chang, P. Xiao, H. Chen, Z. Huang and Y. Chen, Adv. Mater., 2015, 27, 2049–2053 CAS.
- Q. Wang, Q. Zhang, G. Wang, Y. Wang, X. Ren and G. Gao, ACS Appl. Mater. Interfaces, 2022, 14, 1921–1928 CAS.
- G. Huang, P. Wang, Y. Cai, K. Jiang and H. Li, J. Polym. Sci., 2023, 61, 1675–1687 CAS.
- Q. Xu, M. Hou, L. Wang, X. Zhang and L. Liu, Chem. Eng. J., 2023, 477, 147065 CAS.
- P. Xin, S. Han, J. Huang, C. Zhou, J. Zhang, X. You and J. Wu, Chin. Chem. Lett., 2023, 34, 108125 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00159e |
| This journal is © The Royal Society of Chemistry 2025 |