 Open Access Article
Open Access ArticleFrontier advances in molecular dynamics simulations for the design and optimization of oil-displacement polymers
Wenchao Jiang†
 abc,
Zhaowei Hou†
abc,
Xiaolin Wu
acd,
Jian Gai
abc,
Zhaowei Hou†
abc,
Xiaolin Wu
acd,
Jian Gai
 bc,
Liqiang Zheng
bc,
Liqiang Zheng
 e,
Yunchao Wangbc,
Chunlin Niebc,
Shouliang Lubc,
Xu Subc,
Jiayu Zhoubc,
Shu Yanbc,
Erlong Yang*f,
Bin Huangg,
Chi Dongh,
Jichao Sune,
Long Sui,
Shichun Yaobc,
Yane Wangbc,
Xiaodan Yubc,
Di Liubc,
Jiang Jiangbc,
Jiaming Wubc,
Xuesong Fubc,
Shuanghua Wangbc,
Xueying Subc,
Houfei Shanbc,
Lei Zhangj,
Guangming Qik,
Xiaoru Hek,
Jingya Lil,
Yuxiao Mam and
Yongping Wangn
e,
Yunchao Wangbc,
Chunlin Niebc,
Shouliang Lubc,
Xu Subc,
Jiayu Zhoubc,
Shu Yanbc,
Erlong Yang*f,
Bin Huangg,
Chi Dongh,
Jichao Sune,
Long Sui,
Shichun Yaobc,
Yane Wangbc,
Xiaodan Yubc,
Di Liubc,
Jiang Jiangbc,
Jiaming Wubc,
Xuesong Fubc,
Shuanghua Wangbc,
Xueying Subc,
Houfei Shanbc,
Lei Zhangj,
Guangming Qik,
Xiaoru Hek,
Jingya Lil,
Yuxiao Mam and
Yongping Wangn
aNational Key Laboratory for Multi-resource Collaborated Green Development of Continental Shale Oil, Daqing 163712, China. E-mail: jiangwenchao@petrochina.com.cn; houzhw@petrochina.com.cn; wuxldq@petrochina.com.cn
bExploration and Development Research Institute, PetroChina Daqing Oilfield Company Limited, Daqing 163712, China. E-mail: gaijian@petrochina.com.cn; wangyunchao@petrochina.com.cn; niechunlin@petrochina.com.cn; lushouliang@petrochina.com.cn; suxu@petrochina.com.cn; zhoujiayu@petrochina.com.cn; yanshu6487@petrochina.com.cn; yaoshch@petrochina.com.cn; wangyane@petrochina.com.cn; yuxiaodan@petrochina.com.cn; liudi-a@petrochina.com.cn; jiangjiang@petrochina.com.cn; wujiaming1998@petrochina.com.cn; fuxuesong@petrochina.com.cn; wangshuanghua@petrochina.com.cn; suxueying@petrochina.com.cn; shanhoufei1987@petrochina.com.cn
cResearch and Development Center of Sustainable Development of Continental Sandstone Mature Oilfield, Daqing 163712, China
dDaqing Oilfield Company Limited, Daqing 163453, China
eSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, China. E-mail: lqzheng@sdu.edu.cn; jichaosun@sdu.edu.cn
fMinistry of Education Key Laboratory of Enhanced Oil and Gas Recovery, Northeast Petroleum University, Daqing 163318, China. E-mail: yangerlong@nepu.edu.cn
gChongqing Institute of Unconventional Oil and Gas Development, Chongqing University of Science and Technology, Chongqing 401331, China. E-mail: huangbin@cqust.edu.cn
hSANYA Offshore Oil & Gas Research Institute, Northeast Petroleum University, Sanya 572024, China. E-mail: dongchi@nepu.edu.cn
iKey Laboratory of Ministry of Education for Advanced Materials in Tropical Island Resources, School of Chemistry and Chemical Engineering, Hainan University, Haikou 570228, China. E-mail: longsu@hainanu.edu.cn
jDaqing Oilfield Natural Gas Company, Daqing 163000, China. E-mail: tzhanglei@petrochina.com.cn
kNo. 2 Oil Production Company, PetroChina Daqing Oilfield Company Limited, Daqing 163414, China. E-mail: qiguangming@petrochina.com.cn; hexiaoru@petrochina.com.cn
lNo. 1 Oil Production Company, PetroChina Daqing Oilfield Company Limited, Daqing 163111, China. E-mail: lijingya@petrochina.com.cn
mToutai Oilfield Development Company Limited, PetroChina Daqing Oilfield Company Limited, Daqing 163000, China. E-mail: mayuxiao@petrochina.com.cn
nCNOOC China Limited, Tianjin Branch, Tianjin 300452, China. E-mail: wangyp50@cnooc.com.cn
First published on 5th June 2025
Abstract
This comprehensive review paper delves into the application and recent advancements of molecular dynamics (MD) simulation techniques in the design and optimization of polymers for oil displacement. Initially, the paper outlines the pivotal role of polymers in oil displacement, particularly highlighting the challenges associated with their stability and rheological property optimization under high-temperature and high-salinity conditions. Subsequently, it meticulously introduces the theoretical foundation of MD simulations, the selection of simulation parameters, and the emerging trends in the integration of machine learning with MD simulations. The core viewpoint revolves around the utilization of MD simulation techniques in elucidating the mechanisms of polymer-enhanced oil recovery (PEOR), optimizing polymer properties, and designing novel polymers. This includes observing polymer–oil interactions at the atomic scale and predicting the impact of polymer wettability changes on recovery efficiency. The unique contribution of this paper lies in systematically summarizing the application achievements of MD simulation techniques in the field of oil displacement polymers and providing insights for future research directions, thereby advancing the application and development of MD simulations in EOR polymer design. Future research endeavors will concentrate on developing multiscale simulation methodologies, exploring high-performance computing technologies, integrating experimental and simulation data, and assessing the environmental impact of polymers to foster the development of environmentally friendly oil displacement technologies. This review offers fresh perspectives to both the academic and industrial communities, particularly emphasizing the practical application potential in oilfield development and environmental management. By presenting an integrated view, this review paper serves as a catalyst for fostering innovative approaches and strategies in the domain of oil recovery enhancement through polymer technology.
1. Introduction
1.1 The crucial role of oil-displacement polymers
Enhanced Oil Recovery (EOR) technologies have been widely employed in the global petroleum industry, particularly as conventional oil extraction methods gradually become less effective. These technologies extend the lifecycle of oil fields and significantly improve recovery rates.1 EOR projects are primarily concentrated in countries such as China, the United States, and Canada, where the application of EOR technologies has greatly increased the utilization rate of crude oil.2–5 In recent years, with the increasing difficulty of oil resource extraction, chemical flooding techniques within EOR, especially polymer flooding for oil displacement, have gradually emerged as one of the core technologies in oil production.6Polymer flooding technology for oil displacement enhances the viscosity of injected water, thereby reducing the oil–water mobility ratio and enabling more uniform water advancement. This, in turn, improves the efficiency of mobilizing remaining oil.7,8 Numerous experiments have demonstrated that polymer flooding can increase recovery rates by 10% to 12%.9,10 The industrial application of this technology in the Daqing Oilfield has provided valuable experience for other oilfields globally.2,11 However, high-temperature and high-salinity environments pose significant challenges to the performance of polymer flooding. Under these extreme conditions, the stability and rheological properties of polymers are significantly reduced, thereby affecting recovery rates.12
To address these challenges, the design of polymers tailored to specific environments has emerged as a crucial task, with the development of novel materials such as biopolymers and nanoparticle-enhanced polymers gradually becoming research hotspots. Biopolymers like xanthan gum have garnered significant attention due to their excellent salt and temperature tolerance.13 Meanwhile, the combined use of polymers and nanoparticles has been proven to effectively enhance their stability and oil displacement efficiency.14 Furthermore, research has shown that ASP (alkali–surfactant–polymer) flooding and high-concentration polymer flooding techniques can further improve recovery rates, with the former increasing recovery rates by 10.1% to 22.9%,15–17 and the latter demonstrating outstanding economic and technical benefits. Simulation studies have indicated that, under these conditions, adjusting the molecular structure and rheological properties of polymers can significantly enhance their oil displacement efficiency.2,15 Synthetic polymers and natural polymers exhibit different advantages in EOR applications. Synthetic polymers, such as partially hydrolyzed polyacrylamide (HPAM), are widely used in high-temperature and high-salinity environments, but their salt tolerance needs improvement.18 Biobased polymers, on the other hand, are considered potential candidates for future polymer flooding due to their good environmental compatibility and renewability.12,19 Additionally, experimental and simulation studies have shown that adjustments in chain length, degree of branching, and functional groups can further improve polymer performance under complex reservoir conditions.7,20 These advancements have laid a solid foundation for the widespread application of polymer flooding technology globally and have pointed the way forward for the development of future EOR technologies.6,16
In industrial applications, polymer flooding technology not only possesses significant technical advantages but also has the potential to reduce the environmental impact of oilfield development. For instance, the utilization of biopolymers derived from natural sources contributes to mitigating environmental pollution and alleviating issues associated with biodegradation.13,19 Additionally, the integration of nanotechnology and multi-component composite flooding techniques offers further options for enhancing recovery rates in the future.21
Polymer flooding, as the cornerstone technology of chemical flooding, has achieved remarkable results in enhancing oil recovery and will continue to play a pivotal role in future oilfield development.
1.2 Molecular dynamics simulation techniques and their potential in polymer optimization
In recent years, the emergence of Molecular Dynamics (MD) simulation technology in materials science has provided a powerful tool for polymer optimization (Fig. 1). MD simulations, by accurately describing intermolecular interactions, can predict the physical, chemical, and mechanical properties of polymer materials, significantly accelerating the design and optimization process of new materials.22 Furthermore, MD simulations offer a cost-effective method to optimize polymer properties at the molecular level, reducing the need for expensive experimental testing. This is particularly valuable in the oil industry, where cost reduction is a critical factor. Additionally, MD simulations help address scaling challenges by enabling the prediction of polymer behaviour under a variety of field conditions, ensuring more efficient, economical, and scalable polymer flooding processes. Compared to traditional experimental methods, MD simulations offer direct insights into the microstructure and dynamic behaviour of polymers at the molecular scale, particularly demonstrating unique advantages in tackling complex multi-scale problems.23In the field of polymer optimization, MD simulation technology has been extensively applied to the study of polymers' mechanical properties. Through MD simulations of polymer materials such as polystyrene under shear deformation and tensile conditions, researchers have discovered that polystyrene exhibits viscoelastic behaviour under small strains and viscoplastic behaviour under large deformations.24 This investigation of stress responses at the molecular level provides a deeper understanding of the application of polymer materials in engineering fields.
MD simulations have also demonstrated a significant role in exploring the potential of polymers in Enhanced Oil Recovery (EOR) processes. EOR techniques commonly employ methods such as water flooding or chemical flooding to enhance the recovery rate of oil reserves, where polymers function as viscosity enhancers, increasing the viscosity of water to optimize oil displacement efficiency. Research using molecular dynamics simulations to investigate nanocellulose as a viscosity enhancer for EOR has shown that nanocellulose can effectively increase the viscosity of water but has a limited effect on oil–water interfacial tension.25 These findings provide guidance for the development of more efficient EOR polymer materials.
In summary, the rapid development and application of molecular dynamics (MD) simulation technology have significantly transformed the approach to polymer optimization and material design. It has not only demonstrated immense potential in predicting material performance but also facilitated more complex multiscale material research by enhancing computational efficiency. In the future, with further advancements in computational capabilities, MD simulations are poised to play an even more pivotal role in a broader range of materials science fields, particularly in the development of EOR and high-performance polymer composites.22,26
1.3 Objectives of this review
This paper aims to systematically review the latest advancements in the application of molecular dynamics (MD) simulation technology for the design and optimization of polymers used in oil displacement. By integrating existing literature, this paper will explore the unique advantages of MD simulations in addressing complex physicochemical challenges in the oil displacement process, particularly focusing on optimizing polymer stability and rheological properties in high-temperature and high-salinity environments. Additionally, this paper will summarize how MD simulation technology aids scientists in designing novel polymer materials to enhance oil recovery (EOR) and analyze the potential for future integration of this technology with other advanced methods, such as machine learning. The ultimate goal is to provide guidance for future research directions and to promote further application and development of MD simulation technology in the design of EOR polymers.2. Theoretical foundations and methods of molecular dynamics simulation
2.1 Basic principles of molecular dynamics simulation
Molecular dynamics (MD) simulation is a computational tool grounded in physics and mathematics, utilized to investigate the dynamic behaviour of molecular systems under specific conditions. The core theories of MD simulation encompass potential energy functions, intermolecular interactions, and the selection of time steps, which collectively determine the accuracy and stability of the simulation results. The fundamental principles of MD are based on Newton's laws of motion, whereby the interactions between molecules and atoms are described to simulate the trajectories and dynamic evolution of the system.27–29In MD simulations, the potential energy function or force field is crucial as it accurately describes the intermolecular interactions. Common potential energy functions include the Lennard-Jones potential and the Coulomb potential, primarily used to model van der Waals forces and electrostatic interactions.30,31 Different force field models are optimized for various types of interactions during their construction, such as the specific interactions in protein–ligand binding and the dynamic behaviour of ions in solution.26,29 In recent years, the application of enhanced sampling techniques in MD simulations, such as meta dynamics and variationally enhanced sampling, has provided effective means for more precise potential energy calculations and simulations over longer timescales.27,32 These enhanced sampling techniques, by improving the accuracy of the potential energy function, have broadened the applicability of MD simulations in biological macromolecule systems and significantly enhanced their reliability in analyzing biological systems.33,34
The model of intermolecular interactions between atoms represents another pivotal component of MD simulations. MD determines the trajectory of each atom by computing the forces and accelerations among them, thereby generating the temporal evolution information of the system. Typical interaction models encompass bonding forces, repulsive forces, and electrostatic forces, and the dynamic interplay of these forces provides theoretical support for studying the behaviour of biomolecules within cellular environments.35,36 For instance, in protein folding simulations, precise calculations of intermolecular forces can uncover the dynamic relationships among different amino acid side chains.31,37
The choice of time step length plays a significant role in MD simulations. Excessively large time steps can lead to system instability or even simulation failure, while excessively small time steps significantly increase computational costs. Therefore, careful selection is required to balance computational accuracy and efficiency. Generally, typical MD simulation time steps range from 1 to 2 femtoseconds (fs), but shorter time steps may be chosen when more precise kinetic process simulations are needed.38,39 Some modern optimization techniques, such as acceleration models based on graph neural networks and adaptive step size methods, allow for the use of slightly larger time steps while maintaining accuracy, enabling long-timescale simulations in complex molecular systems.35,40
2.2 Selection of simulation parameters
To ensure the accuracy of simulation results, it is crucial to make reasonable choices for simulation parameters, such as temperature, pressure, and molecular models. This not only affects the consistency between simulations and experimental conditions but also relates to the predictive capability of system properties.41,422.3 Integration of machine learning with molecular dynamics simulation
In recent years, the integration of machine learning with molecular dynamics simulations has emerged as a significant direction in materials science research. This integration is primarily manifested in accelerating simulation processes, enhancing simulation accuracy, and expanding research areas that are beyond the reach of traditional molecular dynamics (Fig. 2). By applying machine learning models to molecular dynamics simulations, researchers have achieved remarkable technological breakthroughs in the analysis and prediction of complex systems.51,52 | ||
| Fig. 2 A workflow integrating machine learning-assisted force field modelling with molecular dynamics simulations. This figure illustrates the specific application of machine learning in constructing force field models and performing molecular dynamics simulations. The process involves sampling reference geometric configurations from molecular dynamics trajectories (a), computing high-level theoretical energies and forces for these configurations (b), and utilizing these data to train a machine learning model to predict potential energy surfaces (c). Ultimately, this model is employed to accelerate path-integral molecular dynamics simulations for studying the behaviour of polymers under extreme conditions (d). This process not only enhances the efficiency and accuracy of simulations but also expands the capability to investigate complex material systems.51 | ||
A notable example of enhancing thermal stability using machine learning-driven force fields can be found in fluoropolymer simulations, where first-principles-based machine learning molecular dynamics (ML-MD) models have been used to predict the melting point, thermal expansion coefficient, and Young's modulus of polytetrafluoroethylene (PTFE) with high accuracy. In a comparison with traditional empirical force fields, the ML-based model predicted the melting point of PTFE at 327.5 °C with an error margin of just 1.5%, compared to the experimental value of 329 °C. The thermal expansion coefficient predicted by the ML model was 3.2 × 10−5 K−1, which was in excellent agreement with the experimental value of 3.1 × 10−5 K−1, showing the ML-driven force field's potential to accurately simulate polymer thermal stability (Fig. 3).52
 | ||
| Fig. 3 Comparison of predicted and experimental thermal properties of polytetrafluoroethylene (PTFE) using machine learning-driven force fields. Calculated Young's modulus of 60C390F780 with helix 157 using ML-FF-based MD simulations at a temperature range of 300 °C to 350 °C under standard atmospheric pressure.52 | ||
Similarly, machine learning-driven force field optimization has been applied to salt tolerance enhancement, particularly in water-soluble polymers used in extreme salinity environments. By employing Bayesian inference-based parameter optimization, the force field parameters were systematically refined to accurately describe polymer–water-ion interactions, leading to more reliable predictions of polymer conformational changes in saline solutions. For example, the model predicted a shift in the polymer's radius of gyration when exposed to 3 M NaCl solution, showing a reduction of 12%, which closely matched experimental observations. This approach has been demonstrated to improve the modelling accuracy of ionic effects on polymer behaviour, making it particularly useful for polymer flooding in high-salinity oil reservoirs (Fig. 4).53
 | ||
| Fig. 4 Prediction of polymer conformational change in saline environments using machine learning-driven force fields. Distribution of the label distance used for refinement and its mean value (vertical lines) for the initial reference simulation (κ0 = 20, green), the synthetic experimental data (κexp = 10, black dashed lines), BioEn (blue), and BioFF(orange). The confidence in the reference ensemble decreases top to bottom (θ = 10, 1, 0.1).53 | ||
Data sparsity is a significant challenge in force field optimization for polymer systems. Accurate machine learning models require large amounts of experimental and high-quality simulation data. However, due to the complexity of polymer systems, obtaining sufficient data is often difficult. Particularly for novel polymers or systems that have not yet been experimentally validated, the lack of adequate real-world data presents a significant obstacle to model training. To address data sparsity, the following approaches can be employed.
(1) Data augmentation: synthetic data generation or the use of data from similar systems can expand the training set. Techniques such as Synthetic Minority Over-sampling Technique (SMOTE) can effectively enhance the diversity of the dataset.
(2) Transfer learning: transfer learning allows knowledge learned from one domain to be applied to another, which reduces the need for large amounts of new data, particularly in cases where data is limited.
(3) Active learning: active learning is a machine learning technique where the model selects the most informative samples to label during the training process. This approach helps extract more valuable information from a limited training sample.
When using machine learning for data-driven simulation acceleration, generalization is a critical issue. Polymer systems exhibit high diversity, and the molecular structures and physical–chemical properties of different systems mean that a model trained on a specific system may not directly apply to others. If a model is trained solely on data from one system, it may suffer from overfitting, which reduces its performance on other systems. To improve generalization across different polymer systems, the following strategies can be implemented.
(1) Domain adaptation: domain adaptation, a technique in transfer learning, helps adjust models trained in one system to be applied to another, improving the model's ability to generalize.
(2) Cross-validation: cross-validation is an effective technique for assessing a model's generalization ability. By training and validating the model on different polymer systems, we can ensure that the model performs well across diverse environments.
(3) Multi-task learning: multi-task learning enables the model to learn relationships between different tasks, enhancing its generalization ability across multiple polymer systems. This is particularly useful when dealing with a variety of polymer types.
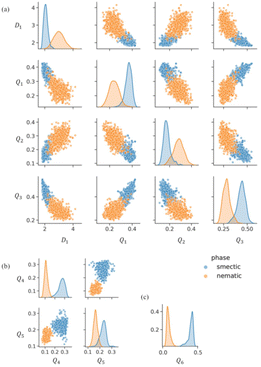 | ||
| Fig. 5 Utilization of machine learning-enhanced local structure analyzer (ML-LSA) for insights into liquid crystal polymers. This figure demonstrates the application of the Machine Learning-assisted Local Structure Analyzer (ML-LSA) in analyzing liquid crystal polymer (LCP) systems. By comparing the classification results using different combinations of features: (a) N = 4 with four features, (b) N = 8 with two features, and (c) N = 12 with one feature, ML-LSA is capable of automatically identifying and classifying the ordered structures within liquid crystal polymers. The blue and yellow points in the figure represent particles in smectic-like and nematic-like trajectories, respectively. The results indicate that as the number of neighbouring particles N increases, the classification accuracy of ML-LSA significantly improves. Specifically, the best classification performance is achieved with a single feature Q6 when N = 12, yielding a consistency index Xc of 0.92, which demonstrates that this feature can effectively distinguish between the smectic-like and nematic-like structures in liquid crystal polymers. These findings underscore the potential of ML-LSA in automatically identifying and classifying ordered structures in complex molecular systems.57 | ||
Computational cost is a significant challenge in inverse design and optimization. When integrating machine learning with molecular dynamics simulations, especially when simulating large or complex polymer systems, the computational resource demands can be extremely high. Inverse design typically requires simulating large numbers of candidate materials to identify the optimal design, and each simulation can be computationally intensive, leading to long processing times. To address computational cost, the following approaches can be employed.
(1) High-performance computing (HPC): leveraging high-performance computing resources can significantly accelerate simulations. Parallel computing and distributed computing techniques can share the computational load, making large-scale simulations more feasible.
(2) Simulation algorithm optimization: by optimizing existing molecular dynamics simulation algorithms, unnecessary computations and iterations can be reduced. For example, coarse-grained models can be used to reduce the number of particles in the simulation, thereby lowering the computational burden.
(3) Machine learning-assisted simulation acceleration: machine learning models can assist in accelerating molecular dynamics simulations by predicting potential energy, thus reducing the need for repetitive simulations. Additionally, machine learning can be used to predict trends in simulation results, helping to eliminate unfeasible designs early and reduce unnecessary calculations.
 | ||
| Fig. 6 Predictive capabilities of machine learning frameworks for shear strength at carbon nanotube-polymer interfaces. This figure illustrates a machine learning framework that predicts the shear strength of carbon nanotube-polymer interfaces based on molecular dynamics simulation data. This framework encompasses three primary steps: (1) functionalization of the molecular dynamics model, which involves creating covalent bonds on the surface of carbon nanotubes to simulate different chemical environments. (2) Execution of molecular dynamics simulations to quantify the critical pull-out force, i.e., the force required to pull a carbon nanotube out of the polymer matrix. (3) Extraction of feature representations from the carbon nanotube-epoxy interface and training of a machine learning model to rapidly predict the critical pull-out force. This model is capable of handling the large variability associated with pull-out forces, providing a powerful tool for the design and optimization of nanocomposites. Through this approach, researchers can quickly predict and optimize the performance of carbon nanotube-polymer interfaces without the need for time-consuming molecular dynamics simulations.61 | ||
Despite significant progress made in material science through the integration of machine learning with molecular dynamics simulations, challenges remain. For instance, effectively integrating data across different scales, enhancing the generalization capability of models, and maintaining efficient prediction performance in non-equilibrium systems are issues that still need to be addressed in the future.51,62 With the continuous optimization of deep learning algorithms and the enhancement of computational resources, this direction holds promise for playing an even more critical role in the study of complex material systems.
3. Molecular structure and performance requirements of oil-displacement polymers
3.1 Key performance requirements for oil-displacement polymers
The viscosity of polymer solutions is influenced by the conformation of the polymer chains, polymer concentration, solvent properties, and external conditions such as temperature and salinity. At low concentrations, polymer chains are more compact, resulting in lower viscosity. As concentration increases, the interactions between polymer chains strengthen, leading to a rise in viscosity. When the concentration exceeds the overlap concentration (C*), polymer chains begin to form a network structure, and viscosity increases significantly.
The addition of salt can alter the viscosity of polymer solutions because salt ions shield the electrostatic interactions between polymer chains, reducing the expansion of the polymer and consequently lowering the viscosity. The mechanisms by which different cations affect the polymer vary.68 Na+ and Ca2+ can directly enter the first hydration shell of acrylic acid anions and interact with them, forming stable dipole pairs. In contrast, Mg2+, due to its tightly bound hydration shell, can only interact with acrylic acid anions in the second hydration shell, resulting in an unstable dipole pair. These differences in direct contact and hydration state explain why Ca2+ and Na+ have a stronger effect on polymer solution properties, while Mg2+ has a weaker impact. Additionally, there are differences in the stability of the dipole pairs: Ca2+ forms stable “salt bridges” with multiple acrylic acid anions, significantly enhancing the coiling and aggregation of polymer chains, thereby reducing the viscosity of the solution. The dipole pairs formed by Na+ are relatively weak and have a smaller impact on polymer chain aggregation (Fig. 7). High temperatures affect the rheological properties of the solution by increasing the mobility of the polymer chains, which can lead to polymer degradation and a subsequent decrease in stability.
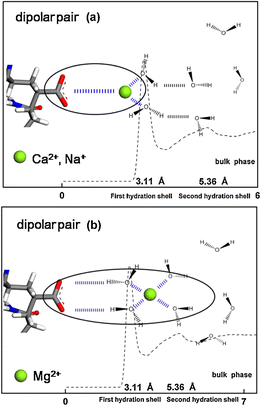 | ||
| Fig. 7 Schemes of dipolar pair formed by cations and acrylic anion: (a) Na+, Ca2+, and (b) Mg2+. This figure illustrates the dipole pair structures formed between different cations (Na+, Ca2+, and Mg2+) and acrylic acid anions, revealing the microscopic mechanism by which salts affect the properties of polymer solutions. This further supports the view that interactions between cations and polymers lead to conformational changes such as the coiling and aggregation of polymer chains. These microscopic structural changes directly influence the viscosity of polymer solutions.68 | ||
3.2 Design and application of different types of polymers
In Section 3.1, the key performance requirements of polymer flooding agents were reviewed, particularly their solubility, viscosity retention, interfacial activity, and stability in high-temperature and high-salinity environments. These performance requirements provide fundamental guidance for the design and application of polymers. In this section, the design strategies of different types of polymers were further explored, with a focus on the applications and developments of both conventional and novel polymers in complex environments.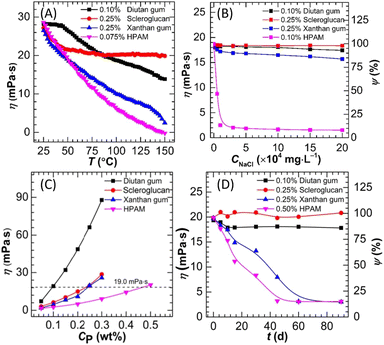 | ||
| Fig. 8 The viscosity changes of different polymers under different influencing factors. (A) Temperature. (B) Salinity. (C) Polymer concentration. (D) Time.71 | ||
To address this challenge, researchers have enhanced the performance of polymers through molecular structural modifications. Specifically, optimizing the molecular weight distribution of HPAM (hydrolytically polyacrylamide) has been found to improve its viscosity retention capacity. Utilizing field-flow fractionation techniques, studies have revealed that HPAM with a more concentrated molecular weight distribution can maintain 90% of its viscosity under a shear rate of 1 s−1, significantly enhancing its field applicability.64 Additionally, the introduction of hydrophobic groups to form hydrophobic associating polymers (HAPs) has proven to be an effective strategy. These polymers exhibit superior rheological properties and enhanced oil recovery efficiencies under high-salinity conditions.67
3.2.2.1 Hydrophobic associating polymers (HAPs). Hydrophobic associating polymers (HAPs) have improved their rheological properties and salt tolerance by introducing hydrophobic groups into their hydrophilic backbone (Fig. 9).67 Experimental results demonstrate that, under high-salinity conditions (200
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg per L NaCl), the viscosity of HAPs solutions is enhanced by 40% compared to traditional HPAM. Furthermore, they maintain good viscoelasticity at a shear rate of 1000 s−1.8,72 These characteristics make them an ideal choice for high-temperature, high-salinity reservoir environments. Quan et al.73 synthesized a hydrophobically associating polymer HPAAT containing sulfonate groups. This polymer is synthesized from acrylamide (AM), allyl polyethylene glycol (APEG), octadecyl dimethyl allyl ammonium chloride (DMAAC-18), and sodium styrene sulfonate (SSS) (Fig. 10). It exhibits excellent thickening and stability even under harsh reservoir conditions, making it particularly advantageous for its application in enhanced oil recovery (EOR). Hydrophobically associating polymers (HAPs) are created by incorporating hydrophobic monomers (such as alkyl or aromatic groups) into hydrophilic polymer chains (e.g., polyacrylamide). These hydrophobic monomers facilitate intermolecular hydrophobic interactions between polymer chains, which become significantly enhanced when the polymer concentration exceeds the critical aggregation concentration (CAC), leading to an increase in the viscosity of the polymer solution. The hydrophobic association strengthens the interactions between polymer chains, forming larger polymer aggregates, which in turn improve the flowability and microscopic oil displacement efficiency of the polymer in the reservoir.
000 mg per L NaCl), the viscosity of HAPs solutions is enhanced by 40% compared to traditional HPAM. Furthermore, they maintain good viscoelasticity at a shear rate of 1000 s−1.8,72 These characteristics make them an ideal choice for high-temperature, high-salinity reservoir environments. Quan et al.73 synthesized a hydrophobically associating polymer HPAAT containing sulfonate groups. This polymer is synthesized from acrylamide (AM), allyl polyethylene glycol (APEG), octadecyl dimethyl allyl ammonium chloride (DMAAC-18), and sodium styrene sulfonate (SSS) (Fig. 10). It exhibits excellent thickening and stability even under harsh reservoir conditions, making it particularly advantageous for its application in enhanced oil recovery (EOR). Hydrophobically associating polymers (HAPs) are created by incorporating hydrophobic monomers (such as alkyl or aromatic groups) into hydrophilic polymer chains (e.g., polyacrylamide). These hydrophobic monomers facilitate intermolecular hydrophobic interactions between polymer chains, which become significantly enhanced when the polymer concentration exceeds the critical aggregation concentration (CAC), leading to an increase in the viscosity of the polymer solution. The hydrophobic association strengthens the interactions between polymer chains, forming larger polymer aggregates, which in turn improve the flowability and microscopic oil displacement efficiency of the polymer in the reservoir.
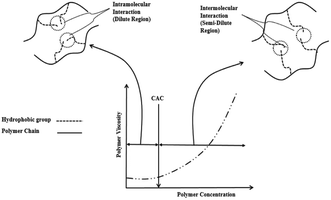 | ||
| Fig. 9 Evolution of viscosity in hydrophobically associating polymers across the critical aggregation concentration threshold. This figure demonstrates the viscosity changes of hydrophobically associating polymers before and after reaching the critical aggregation concentration (CAC).67 | ||
 | ||
| Fig. 10 (A) Molecular structure of HPAAT. (B) FTIR spectrum of HPAAT. (C) Nuclear magnetic resonance spectrum of HPAAT. The introduction of sulfonate groups further enhances the performance of HAPs. Sulfonate groups (–SO3H), as highly polar functional groups, significantly increase the hydrophilicity of the polymer, resulting in better solubility in water. These sulfonate groups can coordinate with sodium, calcium, magnesium, and other ions in the solution, reducing the shielding effect of salts on the polymer chains, thereby improving the stability and solubility of the polymer in high-salinity environments. This chemical interaction allows sulfonate-modified HAPs to maintain excellent rheological performance even under extreme conditions such as high salinity and temperature, making them a more reliable material for oil recovery.73 | ||
In addition, the negative charge of the sulfonate group enhances the compatibility of the polymer with the oil–water interface. By promoting the interaction between the hydrophilic part of the polymer and the water phase, as well as the association between the hydrophobic part and the oil phase, HAPs containing sulfonate groups can effectively promote adsorption at the oil–water interface, improving the distribution of oil and water. This structure enables the polymer to distribute more effectively in complex reservoirs, optimizing the reservoir.
3.2.2.2 Biobased polymers. Biobased polymers, represented by xanthan gum (XG), diutan gum (DG), and rhizobium-derived polymers, have garnered attention due to their environmental friendliness and exceptional performance. In simulated reservoir experiments, xanthan gum exhibited a viscosity retention rate of up to 85% under high-salinity conditions (200
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg per L NaCl) and high temperatures (80 °C), significantly higher than the 30% retention rate of traditional HPAM. Furthermore, it enhanced oil recovery by 16%, compared to the 10% increase achieved by HPAM.71,74 Additionally, rhizobium-derived polymers demonstrated superior viscosity retention capabilities, with an oil displacement efficiency of 25.7% (Fig. 11 and 12).74 This is partly attributed to the shear thinning (pseudoplasticity) properties exhibited by this biopolymer, similar to xanthan gum. When injected into the reservoir, the viscosity of the polymer solution decreases under high shear rates, which enhances injectability. As the solution moves further from the injection point and the shear rate decreases, the viscosity recovers, thereby improving the efficiency of oil displacement and contributing to a higher oil recovery rate.
000 mg per L NaCl) and high temperatures (80 °C), significantly higher than the 30% retention rate of traditional HPAM. Furthermore, it enhanced oil recovery by 16%, compared to the 10% increase achieved by HPAM.71,74 Additionally, rhizobium-derived polymers demonstrated superior viscosity retention capabilities, with an oil displacement efficiency of 25.7% (Fig. 11 and 12).74 This is partly attributed to the shear thinning (pseudoplasticity) properties exhibited by this biopolymer, similar to xanthan gum. When injected into the reservoir, the viscosity of the polymer solution decreases under high shear rates, which enhances injectability. As the solution moves further from the injection point and the shear rate decreases, the viscosity recovers, thereby improving the efficiency of oil displacement and contributing to a higher oil recovery rate.
 | ||
| Fig. 11 Oil recovery assay results using Rhizobium viscosum.74 | ||
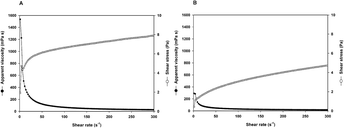 | ||
| Fig. 12 Comparative rheological analysis of rhizobium-derived polymers versus xanthan gum. This figure illustrates the viscosity performance of rhizobium-derived polymer (R. viscosum CECT 908) and xanthan gum (XG) at various shear rates under conditions of 40 °C. The rhizobium-derived polymer exhibits higher viscosity than xanthan gum under identical conditions, indicating its greater potential in enhancing the viscosity of injected water, particularly in high-temperature, high-salinity reservoir environments.74 (A) Rhizobium viscosum CECT 908 biopolymer (B) Xanthan gum. | ||
This enhanced viscosity retention suggests that rhizobium-derived polymers could be scaled up for field applications, where maintaining polymer performance under extreme reservoir conditions is crucial. These superior rheological properties make it feasible for large-scale synthesis, as polymers with stable viscosity under harsh conditions are essential for efficient and economical oil recovery at the industrial level. Additionally, the R. viscosum CECT 908 biopolymer has demonstrated stability at high shear rates (up to 300 s−1), high temperatures (up to 80 °C), and high salinities (up to 200 g per L of NaCl), making it particularly suitable for enhanced oil recovery (EOR) in mature, high-salinity reservoirs. Compared to xanthan gum, this biopolymer shows greater efficiency in oil displacement and is more resistant to mechanical degradation, which is crucial for scalable field applications. These findings underscore its potential for use in Microbial Enhanced Oil Recovery (MEOR), where the ability to maintain polymer stability and enhance oil recovery over long-term operations is essential for cost-effective and efficient field performance.74
Biobased polymers, particularly those synthesized from monoterpenes (e.g., limonene, pinene), offer significant advantages in terms of environmental sustainability. Monoterpene-based polymers have demonstrated good biodegradability, which makes them promising candidates for use in enhanced oil recovery (EOR) applications. These polymers degrade gradually in the reservoir environment, primarily through microbial activity, thereby reducing their long-term environmental impact. Specifically, under anaerobic conditions typical of oil reservoirs, monoterpene polymers exhibit slow degradation, which helps minimize the accumulation of residual polymer in the subsurface. Furthermore, the lifecycle analysis of biopolymer production shows that biopolymers consume fewer fossil resources compared to conventional petroleum-based polymers and can degrade into non-toxic substances after use, contributing to the sustainable development of oilfields.75
3.2.2.3 Nanoparticle-composite polymers. Nanoparticle-composite polymers exhibit significant performance advantages in enhanced oil recovery (EOR), particularly in improving the rheological properties, shear stability, temperature and salt tolerance, and oil displacement efficiency of polymers. Studies have shown that nanoparticles can effectively enhance the viscosity and stability of polymer solutions, thereby improving the efficiency of oil recovery.8,72
Specifically, nanoparticles (such as SiO2) strengthen the polymer network structure through hydrogen bonding and electrostatic interactions with polymer molecules (Fig. 13 and 14). This structure allows the polymer solution to maintain a high viscosity under high shear rates, thus improving the oil displacement efficiency (Fig. 15). For instance, the viscosity of the solution significantly increases when SiO2 nanoparticles are incorporated into polyacrylamide (PAM), especially at low shear rates, where the solution exhibits better shear-thinning behaviour.
 | ||
| Fig. 13 Mechanism of increasing fluid viscosity by combining silica nanoparticles with amphiphilic polymers.76 | ||
 | ||
| Fig. 14 Potential interactions between SiO2 and HPAM.77 | ||
 | ||
| Fig. 15 Macro–micro theoretical mechanism of SiO2 nanoparticles-polymer flooding system.78 | ||
Moreover, nanoparticle-composite polymers demonstrate excellent temperature and salt tolerance. Research has found that SiO2 nanoparticles can effectively enhance the stability of polymers in high-temperature and high-salinity environments. Under conditions of 60 °C, the addition of SiO2 significantly reduces the viscosity loss of HPAM solutions. In high-salinity environments, the addition of nanoparticles helps mitigate polymer degradation, increases polymer rigidity, and thus improves the rheological properties of the solution and oil recovery efficiency.
In terms of oil displacement performance, nanoparticle-composite polymers can alter the wettability of reservoir rocks, transforming them from oil-wet to water-wet, thereby promoting oil displacement. For example, the addition of SiO2 nanoparticles significantly reduces the interfacial tension between oil and water, further enhancing oil recovery rates.
3.2.2.4 Hyperbranched polymers. Hyperbranched polymers (HBPs) are a class of macromolecules with a three-dimensional dendritic structure. Due to their unique architecture, excellent properties, and easy synthesis methods, they have been widely applied in the field of enhanced oil recovery (EOR). Compared to traditional linear polymers, the molecular structure of hyperbranched polymers contains a greater number of terminal groups, which provide more functional groups. This characteristic makes them particularly attractive for applications in oilfield chemistry.
The synthesis of hyperbranched polymers typically involves the polycondensation reaction of ABx-type monomers, as discussed by Flory.79,80 By precisely controlling the monomer ratio, hyperbranched polymers of different generations can be synthesized. The branched structure of these polymers can be further improved by modifying their functional groups. For example, esterification reactions can be employed to react polyhydroxy compounds with monomers containing acidic groups, leading to the formation of the final hyperbranched polymer.
In the application of enhanced oil recovery, hyperbranched polymers exhibit excellent interfacial activity, low critical micelle concentration (CMC), and good emulsification properties. Qiao et al.81 synthesized a hyperbranched carboxylate-type polymer (m-HPAE), which had a CMC of 433.63 mg L−1, a Krafft point of 5 °C, and reduced surface tension to 28 mN m−1 (Fig. 16). These properties make this polymer outstanding in reducing oil–water interfacial tension and improving oil drop migration efficiency (Fig. 17). Through further modifications, m-HPAE can be functionalized via its abundant terminal groups, enhancing its performance in oilfield applications. For instance, when m-HPAE is used in combination with sodium dodecyl sulfate (LAS), the interfacial tension can be further reduced to 10−3 mN m−1. Experimental results have shown that oil recovery using m-HPAE alone or in combination with LAS increased by 10.4% to 32.21% compared to water flooding, with the combination system showing more significant effects, especially in low-permeability reservoirs.
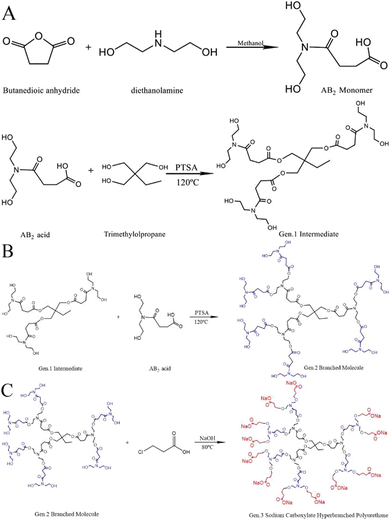 | ||
| Fig. 16 (A) Synthesis of the AB2 monomer and Gen.1 intermediate. (B) Synthesis of Gen.2 hyperbranched polyurethane (HPAE). (C) Synthesis of HPAE with sodium carboxylate at the end of Gen.2 (m-HPAE).81 | ||
 | ||
| Fig. 17 Contact angle of hydrophobic formation of the rock sample (A) and rock sample after m-HPAE treatment (B). This figure demonstrates the interface improvement capability of m-HPAE.81 | ||
In synthesizing the aforementioned discussions, it is evident that traditional polymers, such as partially hydrolyzed polyacrylamide (HPAM), continue to occupy a central position within the realm of chemical enhanced oil recovery (EOR) due to their cost-effectiveness and versatility across a wide range of applications. Nevertheless, the evolving landscape of reservoir conditions, characterized by increasing complexity, and the heightened emphasis on environmental stewardship, have necessitated the emergence of novel polymer classes. Notably, hydrophobic association polymers, bio-based polymers, and nanocomposite polymers have exhibited substantial promise and adaptability, marking a significant advancement in the field.
| Polymers | Advantages | Drawbacks |
|---|---|---|
| Natural polymers | (1) High biocompatibility inmost cases | (1) Relatively weak mechanical properties |
| (2) Suitable biodegradability | (2) Restricted sources | |
| (3) Non-toxic degradation products | (3) High production costs | |
| Synthetic polymers | (1) Low cost | (1) Lack of biological cues |
| (2) Easy to produce massively | ||
| (3) Adjustable mechanical properties | (2) Relatively poor biodegradability in most cases |
Natural polymers, such as xanthan gum and rhizobium-derived polymers, are generally more environmentally friendly due to their biodegradability. They can degrade gradually in the reservoir environment through microbial activity, which helps to minimize long-term environmental pollution. Furthermore, the degradation products of natural polymers are typically non-toxic and do not impose additional burdens on ecosystems, making them highly compatible with the environment. In addition to their biodegradability, natural polymers often exhibit high biocompatibility, which reduces negative impacts on the surrounding ecological system.
However, despite these advantages, natural polymers have certain limitations. Their mechanical strength is often weaker compared to synthetic polymers, and their performance can be severely compromised in extreme reservoir conditions, such as high temperature and high salinity. Furthermore, the cost of production for natural polymers tends to be higher, and their sources are often limited, making large-scale application challenging and economically burdensome in some cases.
On the other hand, synthetic polymers such as partially hydrolyzed polyacrylamide (HPAM) offer superior mechanical properties, thermal stability, and salt tolerance, making them particularly effective in harsh reservoir environments. These polymers are also cost-effective and easy to produce on a large scale, which makes them widely used in EOR applications. However, the production of synthetic polymers typically requires significant amounts of energy and chemical raw materials, which contribute to environmental impact. Additionally, many synthetic polymers have poor biodegradability, which raises concerns about their potential long-term environmental persistence and the risks of residual polymers in oil fields.
Thus, while synthetic polymers offer better performance under extreme conditions, their environmental trade-offs, particularly in terms of biodegradability and production impact, cannot be overlooked. The decision to use natural or synthetic polymers in EOR must therefore be based on a careful evaluation of the polymer's performance characteristics and its environmental footprint. Striking a balance between the polymer's ability to enhance oil recovery and its sustainability is crucial, especially as environmental concerns continue to gain prominence in the oil and gas industry.
3.2.4.1 Biobased and biodegradable polymers development. One of the most promising developments is the production of biobased and biodegradable polymers, such as polylactic acid (PLA). PLA, which is derived from renewable resources like corn starch, reduces reliance on fossil fuels.83 The synthesis of PLA is designed with green chemistry principles in mind, such as using enzyme-catalyzed polymerization techniques, which lower energy consumption and minimize by-product formation.84 Similarly, plant oil-based polycarbonates, synthesized through the copolymerization of epoxy plant oils (e.g., soybean oil, linseed oil) with CO2, not only utilize CO2 as a resource but also enhance the polymer's biodegradability. These materials can degrade naturally after use, reducing environmental residues and supporting the circular economy.85
3.2.4.2 Innovations in green synthesis technologies. In addition to biobased polymers, green synthesis technologies have seen innovations that improve the environmental sustainability of polymer production processes. Solvent-free polymerization is one such innovation, which eliminates volatile organic solvents like N,N-dimethylformamide (DMF), thereby reducing the emission of toxic substances during production. This method also decreases energy consumption, making it more eco-friendly.84,86 Another noteworthy approach is mechanochemical synthesis, where mechanical force, such as ball milling, is used to initiate polymerization reactions. This avoids the need for high temperature and pressure, significantly reducing energy consumption.87,88 Photopolymerization technologies also allow for polymerization at ambient temperatures, using light initiators to carry out reactions efficiently without solvents, thereby reducing VOC emissions.84
3.2.4.3 Circular economy and resource-efficient utilization. Furthermore, the resource-efficient utilization of CO2 has led to the synthesis of carbonates and polycarbonates from CO2, helping to mitigate greenhouse gas emissions and substitute petroleum-based raw materials. Zinc- or copper-catalyzed systems have been used to achieve high-efficiency copolymerization of CO2 with epoxides under mild conditions. This process aligns with the circular economy model, effectively turning waste CO2 into valuable products while reducing environmental impact.89
3.3 Causal analysis of polymer molecular structure and macroscopic properties
The intricate molecular architecture of polymers, characterized by variables like chain length and the presence of specific functional groups, profoundly impacts their macroscopic characteristics and the efficacy of oil displacement processes. Extensive and recent research endeavours have unequivocally shown that through meticulous adjustments to the polymer's molecular structure, remarkable improvements can be realized in terms of viscosity augmentation, salt resistance, and the injectability profile within the reservoir.3.3.1.1 Effect of molecular chain length on viscosity enhancement and injectivity. Viscosity enhancement is a key indicator for evaluating the efficiency of polymer flooding, as it directly affects the effectiveness and injectivity of the process. The molecular structure of the polymer, particularly its chain length and intermolecular interactions, determines its rheological properties. In polymer flooding, longer molecular chains generally provide stronger intermolecular forces, thereby enhancing the viscosity of the solution.
The study by Li et al.90 demonstrated that a polymer with 18 ethoxy (EO) units exhibited significant viscosity enhancement. At a concentration of 1500 mg L−1, the viscosity of this polymer was 113.1 mPa s, approximately 2.5 times higher than that of conventional polymers. This significant viscosity enhancement results from the longer molecular chains forming stronger aggregates through intermolecular hydrogen bonding and hydrophobic interactions, which lead to a denser three-dimensional network in the aqueous solution. The increase in EO chain length strengthens the interactions between molecular chains, promoting tighter molecular associations and enhancing the viscosity and stability of the solution. The growth of molecular chains significantly impacts the polymer's structure and dynamic properties. Additionally, Corredor et al.91 explored the combination of nanoparticles with HPAM and found that increasing molecular chain length and rigidity significantly enhanced both the viscosity and interfacial properties of the solution, particularly under high salinity conditions. However, although an increase in molecular chain length can enhance viscosity, it may also affect the injectivity of the polymer in the reservoir. During reservoir injection, longer molecular chains can reduce the polymer's permeability, thereby affecting its injection efficiency. The molecular chain length determines the permeability and distribution characteristics of the polymer during the injection process. The adaptive polymer (SAP) proposed by Zhang et al.92 demonstrated that shorter but more efficient molecular chains not only achieved good injectivity but also avoided the reservoir plugging issues caused by high molecular weight polymers.
3.3.1.2 Design of molecular chains in high-temperature and high-salinity environments. In high-salinity environments, the molecular chain length significantly affects the salt tolerance and degradation resistance of polymers. Polymers with longer molecular chains are generally more prone to degradation, especially under high-salinity conditions. Åsen's research93 found that high molecular weight HPAM polymers in high-salinity environments are prone to shear degradation, resulting in a loss of viscosity. However, molecular structure modifications, such as using ATBS copolymer monomers, can significantly improve their salt tolerance. In contrast, biopolymers like guar gum exhibit higher stability and degradation resistance in divalent salt (e.g., CaCl2) environments.94 Furthermore, in the study by Li et al.,90 the PAAP-18 polymer maintained a viscosity of 34.6 mPa s at a salinity of 20
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mg L−1 (at 50 °C and a polymer concentration of 1500 mg L−1), whereas the viscosity of the conventional polymer P1 decreased to 19.4 mPa s. This remarkable salt tolerance is primarily attributed to the unique amphiphilic molecular structure of PAAP-18 and the specific interactions among its components under high salinity conditions. The hydrophilic ethoxy (EO) units in the molecule enhance the polymer's dispersibility in high salinity solutions, while the hydrophobic segments promote the formation of a stable network structure. Under high salinity conditions, metal cations in the solution (such as Na+ and Ca2+) induce charge shielding effects, reducing the electrostatic repulsion between polymer chains and allowing them to aggregate more closely. This aggregation enhances the polymer's rheological properties and maintains its viscosity. Additionally, the enhanced hydrophobic interactions between alkyl or aromatic groups further stabilize the polymer's network structure and prevent chain dissociation.
000 mg L−1 (at 50 °C and a polymer concentration of 1500 mg L−1), whereas the viscosity of the conventional polymer P1 decreased to 19.4 mPa s. This remarkable salt tolerance is primarily attributed to the unique amphiphilic molecular structure of PAAP-18 and the specific interactions among its components under high salinity conditions. The hydrophilic ethoxy (EO) units in the molecule enhance the polymer's dispersibility in high salinity solutions, while the hydrophobic segments promote the formation of a stable network structure. Under high salinity conditions, metal cations in the solution (such as Na+ and Ca2+) induce charge shielding effects, reducing the electrostatic repulsion between polymer chains and allowing them to aggregate more closely. This aggregation enhances the polymer's rheological properties and maintains its viscosity. Additionally, the enhanced hydrophobic interactions between alkyl or aromatic groups further stabilize the polymer's network structure and prevent chain dissociation.Some reservoir conditions, where the environment is both highly saline and at very high temperatures, make the design of molecular chain length and structure particularly crucial. The acrylamide-based polymers (TAP-E-SB and TAP-S-B) synthesized by Tamsilian et al.95 exhibited excellent viscosity retention under high-temperature (up to 80 °C) and high-salinity conditions, with TAP-E-SB showing a significant viscosity increase at high temperatures, indicating good thermal stability and potential for enhanced oil recovery applications (Fig. 18). In contrast, traditional AM-based copolymers showed a tendency for viscosity decrease under high-temperature conditions, indicating poor thermal stability.
 | ||
| Fig. 18 Viscosity retention of acrylamide-based polymers under high temperature and high salinity conditions. This figure shows the temperature-dependent viscosity behaviour of different acrylamide-based polymers (including TAP-E-SB, TAP-S-B, and commercial AM-based copolymers) at a concentration of 2000 ppm in a 5% KCl aqueous solution.95 | ||
3.3.1.3 Optimization of molecular chain length and enhancement of interfacial properties. The effect of molecular chain length on interfacial properties is reflected in wettability and interfacial tension regulation. Qiannan et al.96 experimentally demonstrated that surfactant polymers, with larger molecular coil sizes and enhanced interfacial tension regulation capabilities, significantly improve reservoir wettability, causing the rock surface to transition from oil-wet to water-wet. This improvement in wettability aids in reducing oil–water interfacial tension and enhances the dispersion of oil droplets in the water phase, thereby increasing oil recovery efficiency (Fig. 19). Furthermore, the ability of surfactant polymers to significantly alter the wettability of reservoir rocks has important implications for their scalability in field applications. As shown in the experimental results, these polymers maintain their effectiveness at various concentrations, suggesting that they can be synthesized at large scales for field use without significant loss of performance. This characteristic makes surfactant polymers particularly promising for enhanced oil recovery (EOR) in mature oilfields, where maintaining stable wettability and improving oil recovery efficiency are critical for the success of large-scale operations. Additionally, Majeed et al.97 proposed sulfonated polymers that enhance solution viscosity and reduce liquid drainage, thereby improving foam stability and interfacial tension control capabilities. What's more, the PAAP-18 polymer synthesized by Li et al.90 with 18 ethoxy (EO) units can significantly reduce the interfacial tension (IFT) between oil and water, primarily due to its unique molecular structure and interfacial properties. PAAP-18 is an amphiphilic polymer, containing hydrophilic polyethylene glycol (PEG) segments and hydrophobic alkyl or aromatic groups in its molecular chain. This amphiphilic nature allows the polymer to align directionally at the oil–water interface, forming a stable adsorption layer that reduces interfacial tension. The presence of EO units enhances the polymer's hydrophilicity and flexibility, enabling PAAP-18 to adsorb more uniformly on the interface and adapt to interfacial deformations. Additionally, the intermolecular forces between PAAP-18 chains, such as hydrogen bonds and van der Waals forces, further strengthen its adsorption capacity and stability at the interface. Experimental results indicate that at a concentration of 1500 mg L−1, PAAP-18 can reduce the oil–water IFT from 44.4 mN m−1 to 13.0 mN m−1, whereas the conventional polymer P1 can only reduce it to 30.6 mN m−1. This demonstrates that PAAP-18 has superior interfacial activity and adsorption ability, which enable it to more effectively reduce interfacial tension and thereby significantly enhance heavy oil recovery efficiency.
 | ||
| Fig. 19 Change in rock contact angle before and after treatment with surfactant polymers.96 | ||
3.3.2.1 The role of hydrophilic functional groups. Hydrophilic functional groups, such as carboxyl and sulfonate groups, play a significant role in improving viscosity retention and enhancing oil recovery by enhancing polymer solubility and salt tolerance. For instance, Aguiar et al.98 demonstrated that polymers containing sulfonate groups can significantly enhance solution stability under high-salinity conditions while improving rheological properties. Additionally, biopolymers such as xanthan gum, due to the carboxyl and hydroxyl groups in their molecules, exhibit excellent salt tolerance and thermal stability, particularly under high-salinity and high-temperature environments (Fig. 20).70,99 These hydrophilic groups, through ionic interactions and intermolecular electrostatic repulsion, reduce the risk of molecular entanglement and degradation, providing effective solutions for complex reservoir conditions.
 | ||
| Fig. 20 TEM micrographs for diutan gum (a1 and a2), scleroglucan (b1 and b2), xanthan gum (c1 and c2), and HPAM (d1 and d2) at 25 and 85 °C (Cp = 0.05 wt%, no salt). Diutan gum, scleroglucan, and Xanthan gum were biopolymers produced through fermentation by the bacteria Sphingomonas, the plant pathogenic fungus Sclerotium, and the bacteria Xanthomonas, respectively.71 | ||
3.3.2.2 The contribution of hydrophobic functional groups. Hydrophobic functional groups within polymers contribute to enhancing both the viscoelasticity of solutions and the microscopic displacement efficiency by forming intermolecular hydrophobic association networks.8,67 For instance, oil-affinity-modified polymers, under high-temperature and high-salinity conditions, form viscous networks through the self-assembly effect of hydrophobic groups, significantly improving the fluid mobility control within reservoirs. Studies have shown that the synergistic action of nanoparticles with hydrophobic groups can further elevate the viscosity and shear stability of polymers, thereby reducing adsorption losses and enhancing oil recovery efficiency.8,72 Additionally, hydrophobic segments within thermosensitive polymers spontaneously form structured networks under high-temperature conditions, further augmenting their oil displacement performance.98
3.3.2.3 Enhancement of interfacial behaviour by surface-active functional groups. Surface-active functional groups, such as amide, long-chain alkyl, and sulfonate groups, play pivotal roles in reducing interfacial tension, altering rock wettability, and forming stable emulsions. For instance, experiments conducted by Qiannan et al.96 demonstrated that surface-active polymers can effectively drive residual oil out of pores by reducing interfacial tension to as low as 10−2 mN m−1 and simultaneously transforming the rock surface from oil-wet to water-wet. These surface-active groups also significantly enhance the emulsification capacity of oil under non-ultra-low interfacial tension conditions, further expanding the microscopic contact area for oil displacement (Fig. 21).
 | ||
| Fig. 21 Stability analysis of surfactant–polymer emulsions: insights into structural integrity. This figure illustrates the dehydration rate of emulsions generated by surface-active polymers over time, reflecting their emulsification capacity under non-ultra-low interfacial tension conditions. The higher the stability of the emulsions, the more effectively the surface-active polymers can stably disperse oil droplets in water, thereby enhancing oil washout efficiency. This emulsification effect is crucial for improving oil recovery in high-water-cut mature reservoirs.96 | ||
3.3.2.4 Adaptability of special functional groups to extreme environments. In high-temperature, high-salinity reservoirs, the incorporation of special functional groups is crucial for the optimal design of polymers. For instance, research by Liang et al.71 has shown that alginate and schizophyllan, which contain polysaccharide-based functional groups, maintain stable viscosity under high-temperature conditions, serving as effective alternatives to traditional polyacrylamide (HPAM). Furthermore, Couto et al.74 reported on a biopolymer produced by Rhizobium viscosum, whose natural functional group structure grants it high viscosity stability, maintaining efficient oil displacement performance even under conditions of 80 °C and 200 g per L NaCl. These natural functional groups not only reduce the risk of shear degradation of polymer molecules but also significantly enhance rheological properties and adsorption stability.
3.3.2.5 Environmental potential of functional groups in green polymers. Green polymers are increasingly gaining attention due to their natural functional group structures and environmentally friendly characteristics. For example, the hydroxyl and carboxyl groups in xanthan gum exhibit superior rheological properties under high-salinity conditions, far surpassing traditional synthetic polymers.99 Furthermore, the branched polymers prepared by combining chitosan and polyamidoamine (PAMAM) dendrimers exhibit significantly enhanced fluidity and oil displacement capability in porous media, thanks to their special functional groups. The optimization of these functional groups not only improves the oil displacement ability of the polymers but also demonstrates excellent biodegradability, providing a new direction for environmentally friendly Enhanced Oil Recovery (EOR) technologies.
As the challenges posed by extreme reservoir environments continue to escalate, the design of polymer functional groups will increasingly trend towards intelligence and multifunctionality. By integrating nanotechnology, smart responsive moieties (such as thermosensitive and pH-sensitive groups) along with green chemistry approaches, the performance and environmental adaptability of polymers will be further enhanced. These research endeavours provide novel insights and technical support for the efficient recovery of hydrocarbons from complex reservoirs in the future.
4. Application of molecular dynamics simulation in the design and optimization of oil-displacement polymers
4.1 Molecular-level analysis of polymer-driven oil recovery mechanisms
A profound understanding of the interactions between polymers and oil is crucial for optimizing polymer-flooding oil recovery techniques. The advancement of molecular dynamics simulation technology has provided a powerful tool for research in this field, enabling researchers to observe these interactions at the atomic scale and uncover how they influence oil droplet displacement and oil recovery efficiency.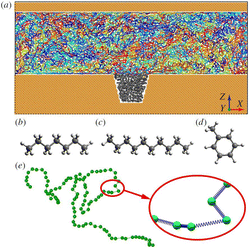 | ||
| Fig. 22 (a) Snapshot of the initial structure for MD simulations. The yellow part represents the wall of the pore; the grey part is the oil droplet trapped in the dead end; the colourful chains confined between the walls are polymer molecules. The molecular structures of (b) heptane, (c) decane and (d) toluene are also illustrated. (e) The detail of a representative polymer molecule. Inset illustrates the bead-spring model of the polymer chain.100 | ||
 | ||
| Fig. 23 Displacing behaviours of trapped oil by different flooding agents, namely (a) water and (b–d) polymers with varying chain lengths. This figure illustrates the displacement behaviour of oil droplets using different oil displacement agents. Specifically, (a) depicts the oil displacement effect of water flooding, while (b) to (d) show the displacement effects of polymers with varying chain lengths on oil droplets, respectively. The experimental results indicate that longer polymer chains (e.g., N = 250) are more effective than shorter chains (e.g., N = 100) in pulling oil droplets out of dead-end pores, with a significant reduction in the required injected pore volume (PV). To elaborate, polymers with N = 150 require 1.87 PV to fully displace oil droplets, whereas those with N = 250 only need 0.85 PV, demonstrating that longer polymer chains enhance oil displacement efficiency while reducing the amount of polymer required. These findings support the theory that viscoelastic polymers improve oil displacement efficiency in nanopores through their unique rheological properties, particularly in enhancing oil droplet displacement efficiency.100 | ||
4.2 Application of molecular dynamics simulation in polymer performance optimization
In Section 4.1, the molecular-level mechanisms underlying the interactions between polymers and oil were analyzed, highlighting the critical role of these interactions in enhancing oil displacement efficiency. The subsequent Section 4.2 will focus on how molecular dynamics simulations can be utilized to optimize the rheological properties of polymers, further improving their performance in enhanced oil recovery processes.Li et al. conducted molecular dynamics simulations to investigate the influence of hydrated montmorillonite on the viscosity of polyacrylamide (PAM) under confined shear conditions. Their research revealed that, as the temperature increased from 298 K to 343 K, the viscosity of PAM exhibited a decreasing trend. This may be attributed to the weakening of interactions between polymer chains due to the temperature rise, which subsequently affected the overall viscosity of the solution. Furthermore, the study also found that, at high shear rates, the viscosity reduction of PAM was more pronounced. This could be related to the adjustment of polymer chain conformations by shear forces, leading to a more ordered arrangement of polymer chains and thus reducing the viscosity of the solution.102
Chen et al. employed molecular dynamics simulations to explore the solution models of hydroxypropyl polyacrylamide (HPAM) and polyacrylamide (PAM) at varying NaCl concentrations, revealing the impact of salt concentration on the rheological properties of polymer solutions. The research findings indicated that, as the NaCl concentration increased, the viscosity of HPAM solutions decreased significantly. This may be attributed to the compression of the electrical double layer between carboxylate groups on HPAM molecules by the addition of NaCl, which reduced the repulsive interactions between molecules, leading to a greater tendency for polymer chains to aggregate and consequently lowering the solution's viscosity. In contrast, the viscosity changes of PAM solutions were not pronounced, possibly due to the lack of ionic groups on PAM molecules, rendering them insensitive to variations in salt concentration.103
These molecular-scale simulation results not only deepen our understanding of the rheological behaviour of polymer solutions but also provide crucial guidance for designing polymers with specific rheological properties. By adjusting the chemical structure of the polymers and external conditions (such as temperature, shear rate, and salt concentration), the rheological properties of the polymers can be optimized to meet specific requirements for oil displacement applications.
4.2.2.1 Molecular mechanisms underlying the alteration of polymer wettability. Ahsani et al. investigated the influence of hydrolyzed polyacrylamide (HPAM) on the wettability of carbonate surfaces. Through a combination of molecular dynamics simulations and experimental approaches, they discovered that the adsorption of HPAM significantly reduces the water contact angle, thereby transforming the oil reservoir rock surface from a strongly oil-wet state to a moderately oil-wet state. This transition is crucial for enhancing water mobility and oil displacement efficiency. Specifically, after HPAM adsorption, the water contact angle decreases to approximately 130° and 140° at ambient and reservoir temperatures, respectively, which aligns well with the experimental results, validating the accuracy of the simulation findings.104
Yuan et al. focused their research on the interaction between polyvinylpyrrolidone (PVP) and the surfactant AOT. Through dissipative particle dynamics (DPD) simulations, the researchers observed that the addition of AOT induced changes in the dihedral angle distribution of PVP chains, indicating the occurrence of interactions between the polymer and the surfactant. The impact of this interaction on wettability provides a theoretical basis for designing polymers with specific wettability alteration functionalities.105
4.2.2.2 Effectiveness of simulation applications in enhancing oil recovery. Molecular dynamics simulations not only aid in understanding the molecular mechanisms underlying wettability changes but also enable the prediction of oil recovery enhancements during polymer flooding processes. Ahsani et al. combined simulation and experimental methods to verify the effectiveness of HPAM in altering the wettability of carbonate surfaces at different temperatures. They found that as temperature increases, the adsorption tendency of HPAM on carbonate surfaces intensifies, potentially due to ion exchange and thermal decarboxylation phenomena. This discovery offers new directions for further molecular dynamics research, particularly in the application of different polymers as wettability modifiers through Enhanced Oil Recovery (EOR) processes.104
In summary, molecular dynamics simulation technology has played a pivotal role in research on polymer wettability alteration and oil recovery enhancement. By combining simulation and experimental methods, researchers can gain deeper insights into the interactions between polymers and reservoir rocks, as well as predict wettability changes and oil recovery enhancements during polymer flooding processes.
4.3 Application of molecular dynamics simulation in polymer design and solution characterization
4.3.1.1 Performance optimization of fluorocarbon-modified polyacrylamide. Fluorocarbon-modified polyacrylamide (FPAM), due to its unique hydrophobic properties, holds broad application prospects as an oil-displacement agent in oilfield chemistry. Research indicates that the introduction of fluorocarbon units into the polyacrylamide chain can significantly enhance the stability and efficiency of the polymer in high-salt and high-temperature environments. MD simulation technology is capable of simulating the self-assembly behaviour and intermolecular interactions of these polymers under different conditions, thereby providing theoretical guidance for experimental design.
For instance, Ni et al. investigated the hydrophobic association properties of aqueous solutions of fluorocarbon-modified polyacrylamide using Dissipative Particle Dynamics (DPD) simulations. The simulation results revealed that, as the length of the fluorocarbon chain increased, the hydrophobic interactions between polymer chains intensified, leading to enhanced solution association. Notably, P(AM-AANa-12F) exhibited lower hydrophobic association in pure water compared to saline solutions, and demonstrated a stronger positive salinity sensitivity. At a concentration of 0.05 mol/mol, the root mean square (RMS) end-to-end distance of P(AM-AANa-12F) was longer in pure water than in saline solutions, indicating that the stronger hydrophobic association was influenced by salinity. These findings provide important molecular-level insights for the design of salt-tolerant and high-temperature-resistant oil-displacement polymers (Fig. 24).106
 | ||
| Fig. 24 The RMS end-to-end distance of P(AM-AANa-12F) in salt solution at different concentrations under different temperature. This figure illustrates the impact of temperature on the root mean square (RMS) end-to-end distance of P(AM-AANa-12F) in saline solutions at various concentrations. The results indicate that, at 333 K, as the concentration increases, the RMS end-to-end distance of P(AM-AANa-12F) initially decreases and then increases. At higher temperatures, the thermal motion of the hydrophobic groups on the macromolecular chains intensifies, weakening the ice-like structure of surrounding water molecules and causing the macromolecular chains to contract. As the concentration rises, the polymer chains begin to form physical networks, which expand at higher temperatures, leading to an increase in the RMS end-to-end distance. When the concentration continues to increase, the associative behaviour of the polymers reaches a relative equilibrium, and the RMS end-to-end distance remains unchanged. These findings further corroborate the stability and efficiency of fluorocarbon-modified polyacrylamide in high-salinity, high-temperature environments, providing crucial molecular-level insights for the design of oil-displacement polymers suitable for high-temperature, high-salinity reservoirs.106 | ||
4.3.1.2 Enhancing the salt tolerance of polyacrylamide. Salt tolerance is a crucial performance indicator for polyacrylamide in oilfield applications. Yao et al. modified polyacrylamide by incorporating three functional monomers and employed molecular dynamics (MD) simulations to investigate the impact of these modifications on the polymer's salt tolerance. The simulation results revealed that the modified polyacrylamide (HM-HPAM) exhibited stronger salt tolerance compared to the unmodified polyacrylamide, with the salt tolerance increasing as the steric hindrance of the monomers increased. For instance, the intrinsic viscosity ([η]) of HM-HPAM1 was 5.2 dl g−1 at a NaCl concentration of 0 mol kg−1, decreasing to 4.1 dl g−1 at a NaCl concentration of 0.28 mol kg−1. This discovery holds significant implications for the design and optimization of polymers suitable for high-salinity environments.107
Furthermore, in this study, Yao et al. also investigated the dynamic properties of the modified polyacrylamide under different ionic strengths through MD simulations, encompassing the radius of gyration (Rg), hydrodynamic radius (RH), and intrinsic viscosity ([η]). The study found that as the NaCl concentration increased, the Rg of HM-HPAM decreased from 4.5 nm at 0 mol kg−1 to 3.2 nm at 0.28 mol kg−1, with corresponding decreases in RH and [η]. In contrast, the changes in HM-PAM were insignificant. These results indicate that the introduction of monomers with steric hindrance can reduce the coiling of molecular chains, thereby enhancing the salt tolerance of the polymer.107
4.3.2.1 Self-assembly of polyacrylamide and SDS. Wang et al. employed coarse-grained molecular dynamics simulations to investigate the self-assembly behaviour of polyacrylamide (PAM) and sodium dodecyl sulfate (SDS) in aqueous solutions. Their findings revealed that in the absence of SDS, PAM spontaneously curls to form aggregates. However, the addition of SDS causes PAM to stretch and ultimately forms the widely accepted “necklace” structure, where PAM is located at the interface between the hydrophobic and hydrophilic regions of the SDS micelles. Specifically, the radius of gyration (Rg) of PAM in the absence of SDS is 8.0 Å, while in the presence of SDS, Rg increases to 16.6 Å, indicating the stretching of PAM. This process primarily consists of three stages: firstly, PAM rapidly adsorbs SDS monomers, causing Rg to decrease rapidly from 8.0 Å to 11.7 Å. Secondly, as more SDS adsorbs, PAM gradually stretches, leading to an increase in Rg and finally, a stable “necklace” structure is formed. These discoveries highlight the primary role of hydrophobic interactions in the self-assembly of PAM and SDS, which is consistent with experimental results and other simulation studies.108
4.3.2.2 Interaction between neutral polymers and ionic surfactants. Yuan et al. utilized Dissipative Particle Dynamics (DPD) simulation methodology to investigate the interaction between neutral polymers and ionic surfactants in aqueous solutions. Their observations revealed a transition process in the polymers as the surfactant concentration increased: from a freely extended state to the formation of dispersed rod-like structures, subsequently evolving into a pearl-necklace-like micelles structure, and ultimately distributing within hexagonal and lamellar phases. Specifically, as the surfactant volume fraction increased from 0.01 to 0.05, the end-to-end distance of the polymers initially decreased and then increased, indicating a transformation from a freely extended state to a compressed rod-like structure, and ultimately the formation of the pearl-necklace micelles structure. This research provides intuitive molecular-level evidence for understanding the aggregation morphology of polymers in surfactant solutions.109
4.4 Application of molecular dynamics simulation in the study of polymer–surfactant interactions
4.4.1.1 Molecular simulation of CO2 foam stabilization by HPAM and ionic surfactants. Jia et al. conducted molecular dynamics simulations to investigate the interaction between hydrolyzed polyacrylamide (HPAM) and ionic surfactants (SDS and CTAB) at the interface between CO2 and the aqueous phase. Their findings revealed that CTAB exhibited a stronger ability to reduce the interfacial tension (IFT) between CO2 and water compared to SDS. Specifically, the IFT at the CTAB-treated interface decreased to approximately 10.766 mN m−1, while that at the SDS-treated interface was around 11.857 mN m−1, both significantly lower than the IFT between pure CO2 and the aqueous phase (32.457 mN m−1). Furthermore, the interfacial film formed by CTAB possessed a larger interfacial width (2.281 nm) and higher interfacial coverage, thereby more effectively reducing the energy per unit area at the interface. Competitive adsorption occurred between HPAM and the counterion Br on the CTAB interfacial film, whereas salt bridge structures formed between HPAM and Na+ near the SDS interface. These structural changes directly influenced the interfacial tension and foam stability.110
4.4.1.2 Simulation study on solution properties of polymer surfactant KL-6. Wu et al. conducted a study on the solution properties of the polymer surfactant KL-6 using dissipative particle dynamics (DPD) simulations. Their findings revealed that the critical association concentration (CAC) of KL-6 was 0.6 g L−1. Above this concentration, the viscosity of KL-6 solutions was significantly higher than that of HPAM solutions. Specifically, the viscosity of KL-6 solutions at 0.6 g L−1 was approximately twice that of HPAM solutions. Furthermore, at the same concentration, the surface tension of KL-6 solutions was 46% lower and the interfacial tension value was reduced by 37% compared to HPAM solutions, indicating that KL-6 polymer solutions exhibited higher surface activity. In saline solutions, KL-6 molecules formed a three-dimensional network structure, significantly enhancing its salt tolerance. This characteristic is particularly important in applications aimed at improving oil recovery.111
4.4.2.1 Aggregate structures of temperature- and salt-tolerant surfactants with polyacrylamide (PAM). The aggregate structures of temperature- and salt-tolerant surfactants with polyacrylamide (PAM) are crucial for enhancing oil displacement efficiency. Yuan et al. employed the dissipative particle dynamics (DPD) method to investigate the interactions between partially hydrolyzed polyacrylamide (HPAM) and ionic surfactants. Their findings revealed that as the surfactant concentration increases, the aggregate structures of the polymer and surfactants sequentially transition from a “bottle-brush” configuration to a “bead-on-string” structure, and further to a hexagonal phase and an inverse hexagonal phase at higher concentrations. These structural transformations directly influence the rheological properties and interfacial stability of the oil displacement system.112
Wu et al. have reviewed the applications of dissipative particle dynamics (DPD) and coarse-grained molecular dynamics (CG-MD) in studying the interactions between polymers and surfactants, highlighting that these simulation techniques can unveil the underlying patterns governing the structural morphologies of polymer/surfactant aggregates.113 As an illustrative example, Wang et al. employed the CG-MD method to investigate the self-assembly behaviour of polyacrylamide (PAM) and sodium dodecyl sulfate (SDS) in solution. Their findings revealed that the self-assembly process of PAM and SDS progresses through three distinct stages: the rapid coiling of PAM chains, the adsorption of SDS molecules onto the polymer backbone, and ultimately the formation of a “bead-on-string necklace” structure.108
4.4.2.2 Applications of multiscale simulation methods. Multiscale simulation methods have offered a comprehensive understanding of the aggregated structures formed by temperature- and salt-tolerant surfactants with polyacrylamide, spanning from individual molecules to multimolecular assemblies. For instance, Yuan et al. conducted research utilizing full-atom molecular dynamics (MD) to investigate the aggregated structures of surfactants and polymers, and subsequently developed coarse-grained (CG) model force fields. By comparing structural information obtained from both full-atom and CG models, they refined the parameters of the CG force fields. These research systems could reach dimensions of several tens of nanometers, offering a larger scope in terms of both temporal and spatial scales compared to full-atom MD studies. Furthermore, they enabled the investigation of aggregation kinetics over a broader range of time scales.112
4.5 Application of molecular dynamics simulation in the development of novel polymer-based oil recovery agents
4.5.1.1 Performance of modified papaya gum as a novel injectant. El-Hoshoudy et al. investigated the application of modified papaya gum (PGG) as a novel injectant in subsurface oil reservoirs through experimental and Monte Carlo simulation studies. They discovered that PGG exhibited significantly higher viscosity above the critical association concentration (0.6 g L−1). Specifically, the viscosity of PGG solutions at 0.6 g L−1 was more than 1.5 times that of natural guar gum (GG), indicating that PGG holds greater potential for enhanced oil recovery (EOR). In Monte Carlo simulations, PGG membranes exhibited higher stability and more negative electrostatic energies when interacting with SiO2− quartz crystals compared to GG membranes, further confirming the superior performance of PGG under reservoir conditions.114
4.5.1.2 Properties of silane polyacrylate composite materials. In a separate study, El-Hoshoudy et al. explored the synthesis, evaluation, and application of silane-polyacrylate composites as candidate materials for EOR. Through molecular dynamics simulations, they found that the introduction of silane coupling agents significantly enhanced the mechanical properties of the composites. Specifically, the bulk modulus of the silane-polyacrylate composites increased by approximately 40%, the elastic modulus by about 50%, Young's modulus by nearly 60%, and the shear modulus by roughly 70%. Meanwhile, the Poisson's ratio decreased by approximately 20%. These enhanced mechanical properties enabled the composites to exhibit better stability and effectiveness under high-shear, high-salt, and high-temperature reservoir conditions.115
The aforementioned studies clearly demonstrate the potential of novel injectants in enhancing oil recovery (EOR) and the application value of molecular dynamics simulations in assessing the performance of these novel materials.
4.5.2.1 Synthetic feasibility and viscosity reduction mechanism. The synthesis of amphiphilic low-molecular-weight polymers typically involves copolymerization reactions of multiple monomers. For instance, Ma et al. synthesized an amphiphilic low-molecular-weight polymeric viscosity reducer composed of acrylamide (AM), acrylic acid (AA), 2-acrylamido-2-methylpropanesulfonic acid (AMPS), and butylstyrene (PB) through an emulsion copolymerization method. The synthetic feasibility and viscosity reduction mechanism of this polymer were analyzed using molecular dynamics simulations via the Materials Studio software. Simulation results indicated that the addition of the polymer altered the potential energy, non-potential energy, density, and hydrogen bonding distribution of the crude oil, effectively reducing its viscosity. Upon adding the viscosity reducer, the potential energy of heavy oil significantly increased, potentially due to the adsorption of asphaltene and resin molecules onto the viscosity reducer, forming numerous branched structures and leading to a steric hindrance effect. Concurrently, the strong polar groups (–COO– and –SO3–) in AA and AMPS gradually dissociated the network structure of heavy oil from the inside out, promoting a reduction in its viscosity by approximately 86.14%. Structurally, the benzene rings in PB were able to intercalate into the layered structure of asphaltene, weakening the network structure of heavy oil. This structural design was inspired by previous research, which demonstrated that amphiphilic copolymers containing AM and n-butylstyrene could effectively adsorb emulsified oil droplets in heavy oil wastewater (Fig. 25).116
 | ||
| Fig. 25 Investigation of the effects of polymer viscosity reducers on the potential and non-potential energy profiles of heavy oil. This figure illustrates the impact of the viscosity reducer on the potential and non-potential energy of heavy oil after its addition. As depicted in the figure, compared to heavy oil without the viscosity reducer, the potential energy of heavy oil significantly increases upon the addition of the viscosity reducer. This phenomenon is primarily attributed to the adsorption of asphaltene and resin molecules onto the viscosity reducer, leading to the formation of numerous branched structures and, consequently, a steric hindrance effect. Simultaneously, the strong polar groups (–COO– and –SO3–) within the viscosity reducer gradually disintegrate the network structure of heavy oil from the inside out, facilitating a reduction in its viscosity. Experimental results indicate that with an increase in the concentration of the viscosity reducer, the viscosity of heavy oil decreases by approximately 86.14%, confirming the effectiveness of this amphiphilic low-molecular-weight polymeric viscosity reducer in lowering the viscosity of heavy oil.116 | ||
4.5.2.2 Application of molecular dynamics simulations in the advancement of amphiphilic low-molecular-weight polymer viscosity reducers. Molecular dynamics simulations have played a crucial role in the research and development of amphiphilic low-molecular-weight polymers as viscosity reducers. Through these simulations, researchers are able to predict the interactions between polymers and crude oil, thereby facilitating the optimization of polymer structural designs. For instance, Tesha conducted a study on the compatibility of polysulfone and polystyrene-co-maleic anhydride polymer blends, utilizing molecular dynamics simulations to provide simulated data on the performance of these blends. This data is indispensable for gaining insights into the interactions and compatibility between polymers. The favorable interactions between the two polymers were confirmed through Differential Scanning Calorimetry (DSC) analysis, evidenced by the observed shift in the glass transition temperature (Tg).117
In practical scenarios, molecular dynamics simulations offer researchers the unique opportunity to observe the interactions between polymer viscosity reducers and crude oil. This includes the adsorption of asphaltene and resin molecules onto the viscosity reducers, as well as the resultant steric hindrance effects. The combination of these simulation results with experimental data provides a scientific basis for the molecular design and performance optimization of viscosity reducers.
5. Future trends and prospects for oil-displacement polymer design
5.1 Design and potential applications of novel intelligent polymers
(1) Self-repairing polymers: the pursuit of polymers that possess the capability to self-heal and restore their performance within microfractures, thereby significantly prolonging their operational lifespan within oil reservoirs.
(2) Environmentally responsive polymers: the design and synthesis of polymers that are engineered to release active agents under precise environmental cues, ultimately enhancing oil recovery efficiency.
(3) Nanocomposite materials: the fusion of nanotechnology with intelligent polymers to bolster their robustness and stability under the challenging conditions prevalent in oil reservoirs.
Future endeavours in research should delve deeper into leveraging molecular dynamics simulations to fine-tune the performance characteristics of these intelligent polymers, ensuring they exhibit optimal responsiveness under the diverse conditions encountered within oil reservoirs.
(1) Biobased monomer polymerization: new biobased polymers are being developed by harnessing monomers derived from biomass, including lactic acid and polyhydroxyalkanoates (PHA), which serve as the foundational building blocks.
(2) Biodegradability evaluation: a rigorous assessment of the biodegradability of polymers within reservoir settings is conducted to ascertain their eco-friendliness once they have fulfilled their oil displacement roles.
(3) Minimizing ecological footprint: life cycle assessment (LCA) serves as a pivotal tool to refine polymer design, aiming to reduce their environmental impact throughout their entire existence from cradle to grave.
Future research endeavours should focus on pioneering novel biobased monomers and leveraging molecular dynamics simulations to foresee and tailor the degradation pathways and rates of these polymers, thereby guaranteeing their secure degradation post-oil displacement and mitigating their ecological footprint.
5.2 Future development of the integration of machine learning and molecular dynamics simulation
Federated learning is a distributed learning method that allows multiple participants to train models locally without sharing data. This approach is particularly valuable in polymer research, where data is often highly decentralized and involves multiple experimental and commercial institutions. Through federated learning, participants can share model updates instead of data, ensuring data privacy while improving the generalization ability of the models. This also enables the integration of diverse data from various sources, thus enhancing the adaptability of polymer design models across different experimental conditions.
Transfer learning allows models trained on one set of polymer systems to be applied to others, reducing the dependency on large amounts of new data. This technique is particularly useful for generalizing across diverse polymer types and systems, which is a common challenge in polymer design.
Active learning improves model efficiency by selecting the most informative data points for training, allowing models to perform well even with smaller datasets. This is particularly beneficial in polymer research, where data collection can be resource-intensive. By focusing on the most relevant data, active learning helps optimize the training process and reduces the need for large-scale data collection.
Additionally, meta-learning and self-supervised learning are emerging techniques that show great potential in polymer design. Meta-learning enables polymer design models to quickly adapt and make effective predictions when confronted with new types of polymers. Self-supervised learning, on the other hand, reduces the reliance on costly labelled data by training models using unlabelled data, which is especially useful in addressing the data scarcity issue in polymer design.
5.3 Industry applications and sustainability considerations
In addition to theoretical advancements, future research must focus on the practical application of these new polymers in real-world oilfield operations. Specifically, research should explore how biodegradable polymers can be used in microbial enhanced oil recovery (MEOR) systems to improve sustainability by reducing the environmental impact of chemical injection. Additionally, environmental life-cycle assessments (LCA) should be conducted to evaluate the long-term effects of polymer use in oil recovery processes, particularly in terms of biodegradation and toxicity. Future studies could explore the development of eco-friendly synthesis methods, such as green chemistry approaches for producing biodegradable polymers and the incorporation of renewable resources into polymer design to meet growing sustainability requirements.5.4 Unresolved challenges and future research directions
6. Conclusions
This comprehensive review paper delves into the pivotal advancements of molecular dynamics (MD) simulation technology in the realm of designing and optimizing polymers tailored for oil displacement applications. The paper starts by revisiting the crucial role that polymers play in oil displacement processes, highlighting the significant challenges posed by maintaining stability and optimizing rheological properties under harsh conditions, such as high temperatures and high salinity environments. Following this overview, the paper delves deeper into the theoretical backbone and methodologies of molecular dynamics simulations, meticulously explaining their fundamental principles, the intricacies involved in selecting appropriate simulation parameters, and the burgeoning trends in combining machine learning with molecular dynamics simulations that have emerged in recent times.The central thesis of this paper focuses on the application of molecular dynamics (MD) simulation technology in dissecting the molecular-level mechanisms underlying polymer-based oil displacement, refining polymer performance, and devising novel polymers. Leveraging MD simulations, researchers gain unprecedented insights into the atomic-scale interactions between polymers and oil, unveiling the displacement mechanisms of viscoelastic polymers within nanopores and elucidating the influence of interactions between polymer chains and oil droplets on displacement efficiency. Moreover, MD simulations have proven invaluable in probing the rheological properties and viscosity dynamics of polymer solutions, anticipating the ramifications of altered polymer wettability on recovery rates, and steering the design and performance optimization of innovative polymers. The distinctive contribution and novelty of this paper reside in its systematic compilation of the application outcomes of MD simulation technology within the realm of polymers for oil displacement, particularly emphasizing research on optimizing polymer stability and rheological properties under challenging reservoir conditions. By amalgamating existing literature, this paper not only addresses some of the research voids in MD simulation for enhanced oil recovery (EOR) polymer design but also offers a roadmap for future research endeavours, thereby fostering the advanced application and evolution of MD simulation technology in EOR polymer design.
Future research trajectories in this domain will prioritize the development of multiscale simulation methodologies, aiming to achieve a comprehensive understanding of the flow dynamics and interaction mechanisms of polymers within oil reservoir environments. A pivotal focus will be on advancing high-performance computing (HPC) technologies and refining simulation methodologies to address the complexity of these systems. Moreover, there is a pressing need to integrate experimental datasets with simulation results, thereby enhancing the predictive accuracy and reliability of the models employed. Simultaneously, rigorous assessments of the environmental footprint of polymers and the pursuit of environmentally friendly oil displacement technologies will be critical areas of emphasis. These research endeavours are poised to significantly propel the utilization of molecular dynamics (MD) simulations in practical oilfield development scenarios, particularly in the realms of enhancing oil recovery efficiencies and minimizing adverse environmental impacts.
The research outcomes presented herein contribute not only novel insights to the academic discourse but also present significant potential for practical applications within the industrial sphere, specifically in the areas of oilfield development and environmental stewardship. These findings enable us to leverage molecular dynamics (MD) simulation technology more effectively, thereby facilitating advancements in the design of oil-displacement polymers and addressing intricate reservoir challenges faced in practical scenarios.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Author contributions
Conceptualization: Z. H., W. J., X. W., B. H., L. Z.; methodology: W. J., J. G., C. N., E. Y., B. H., C. D.; investigation: W. J., L. S., J. S., X. S., S. W., J. Z., L. Z., J. W., Y. W., G. Q., X. H., J. L., Y. M., S. Y., Y. W., X. Y., D. L., J. J., X. F., X. S., H. S.; project administration: S. L., Z. H., X. W.; supervision: S. L., Z. H., X. W.; writing—original draft: W. J., J. G.; writing—review & editing: Z. H., X. W., E. Y., B. H.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by Youth Science and Technology Special Project of CNPC (grant number 2024DQ03244), Major Science and Technology Projects of CNPC (grant number 2023ZZ22), the Key Fund Joint Project of Chongqing (Project No. 2022NSCQ-LZX0205), the National Natural Science Foundation of China (NSFC) (No. 52274037) and the Hainan Provincial Joint Project of Sanya Yazhou Bay Science and Technology City (Grant No. 2021JJLH0059).References
- E. Lwisa, Chemical enhanced oil recovery, International Journal for Innovation Education and Research, 2021, 9, 3160, DOI:10.31686/IJIER.VOL9.ISS6.3160.
- A. Scott, L. Romero-Zerón and A. Penlidis, Evaluation of polymeric materials for chemical enhanced oil recovery, Processes, 2020, 8, 361, DOI:10.3390/pr8030361.
- A. O. Gbadamosi, S. Patil, M. Kamal, A. A. Adewunmi, A. Yusuff, A. Agi and J. Oseh, Application of polymers for chemical enhanced oil recovery: A review, Polymers, 2022, 14, 1433, DOI:10.3390/polym14071433.
- J. Dano, S. Abdelrahman and M. Ali, Simulation study on polymer flooding for enhanced oil recovery: A case study, Mater. Today: Proc., 2019, 11, 175, DOI:10.1016/j.matpr.2019.11.175.
- J. Wenchao, Prediction model for production well hydraulic fracturing effect of Block SN in Daqing Oilfield based on machine learning and model ensemble, Pet. Geol. Oilfield Dev. Daqing, 2023, 42, 64–72, DOI:10.19597/J.ISSN.1000-3754.202109045.
- P. Raffa, A. Broekhuis and F. Picchioni, Polymeric surfactants for enhanced oil recovery: A review, J. Petrol. Sci. Eng., 2016, 145, 723–733, DOI:10.1016/J.PETROL.2016.07.007.
- A. M. Firozjaii and H. Saghafi, Review on chemical enhanced oil recovery using polymer flooding: Fundamentals, experimental and numerical simulation, Petroleum, 2020, 9, 3, DOI:10.1016/j.petlm.2019.09.003.
- A. O. Gbadamosi, R. Junin, M. Manan, N. Yekeen and A. Agi, Hybrid suspension of polymer and nanoparticles for enhanced oil recovery, Polym. Bull., 2019, 1–38, DOI:10.1007/s00289-019-02713-2.
- W. Jiang, Z. Hou, X. Wu and K. Song, et al., A New Method for Calculating the Relative Permeability Curve of Polymer Flooding Based on the Viscosity Variation Law of Polymer Transporting in Porous Media, Molecules, 2022, 27, 3958, DOI:10.3390/molecules27123958.
- J. Jaffray, New Model Development for Polymer Chemical Enhanced Oil Recovery (EOR), Society of Petroleum Engineers, 2020, 204269-stu. DOI: DOI:10.2118/204269-stu.
- W. Jiang, Z. Hou and S. Yao, et al., Research on the timing for subsequent water flooding in alkali-surfactant-polymer flooding in Daqing oilfield based on Automated Machine Learning, Sci. Rep., 2024, 14(1), 27897, DOI:10.1038/s41598-024-79491-z.
- M. P. N. Matovanni, M. N. Ikhsanudin, R. I. Arvianto, J. Waluyo, S. Distantina, M. Kaavessina and S. H. Pranolo, The prospects and challenges of biopolymers for enhanced oil recovery (EOR), Equilibrium Journal of Chemical Engineering, 2023, 7(1), 78–86, DOI:10.20961/equilibrium.v7i1.73947.
- T. A. Musa, A. Ibrahim, H. Nasr-El-Din and A. Hassan, New insights into guar gum as environmentally friendly polymer for enhanced oil recovery in high-salinity and high-temperature sandstone reservoirs, J. Pet. Explor. Prod., 2021, 11, 1905–1913, DOI:10.1007/s13202-020-01080-3.
- P. Druetta and F. Picchioni, Polymer and nanoparticles flooding as a new method for enhanced oil recovery, J. Petrol. Sci. Eng., 2019, 2, 70, DOI:10.1016/j.petrol.2019.02.070.
- P. Druetta and F. Picchioni, Surfactant-polymer interactions in a combined enhanced oil recovery flooding, Energies, 2020, 6520, DOI:10.3390/en13246520.
- Y. An, X. Yao, J. Zhong, S. Pang and H. Xie, Enhancement of oil recovery by surfactant-polymer synergy flooding: A review, Polym. Polym. Compos., 2022, 30 DOI:10.1177/09673911221145834.
- T. N. Dantas, F. F. Viana, T. T. C. Souza, A. A. D. Neto and P. Aum, Study of single-phase polymer-alkaline-microemulsion flooding for enhancing oil recovery in sandstone reservoirs, Fuel, 2021, 302, 121176, DOI:10.1016/j.fuel.2021.121176.
- A. S. Santos, A. M. Marques, L. Palermo and C. Mansur, Influence of molar mass of partially hydrolyzed polyacrylamide on the treatment of produced water from enhanced oil recovery, Colloids Surf., A, 2020, 124042, DOI:10.1016/j.colsurfa.2019.124042.
- O. Olabode, T. Ojo, T. I. Oguntade and D. Oduwole, Recovery potential of biopolymer (B-P) formulation from Solanum tuberosum (waste) starch for enhancing recovery from oil reservoirs, Energy Rep., 2020, 6, 1448–1455, DOI:10.1016/j.egyr.2020.05.027.
- I. Arifin, G. Kadja and C. Radiman, Improving the viscosity of partially hydrolyzed polyacrylamide (PHPAM) solution for enhanced oil recovery (EOR) application, Key Eng. Mater., 2021, 874, 45–49, DOI:10.4028/www.scientific.net/KEM.874.45.
- Y. R. Sriwijaya, P. J. Ratri, T. R. Mayangsari, A. Adharis and S. S. Riswati, Synthesis of iron oxide (Fe2O3)-xanthan gum nanoparticle composites: Its potential as a chemical flooding media in enhanced oil recovery (EOR), J. Kim. Valensi, 2023, 53–58, DOI:10.15408/jkv.v9i1.29468.
- T. E. Gartner and A. Jayaraman, Modeling and simulations of polymers: A roadmap, Macromolecules, 2019, 755–786, DOI:10.1021/acs.macromol.8b01836.
- F. Grünewald, R. Alessandri, P. C. Kroon, L. Monticelli, P. C. T. Souza and S. Marrink, Polyply: A Python suite for facilitating simulations of macromolecules and nanomaterials, Nat. Commun., 2021, 13, 68, DOI:10.1038/s41467-021-27627-4.
- M. Ries, P. Steinmann, and S. Pfaller, Characterization of polystyrene under shear deformation using molecular dynamics, Developments and Novel Approaches in Nonlinear Solid Body Mechanics, 2021, vol. 14. DOI:10.1007/978-3-030-50460-1_14.
- M. Ledyastuti and J. Jason, Molecular dynamics simulation of nanocellulose-oil-water interaction in enhanced oil recovery application, IOP Conf. Ser.:Mater. Sci. Eng., 2021, 980, DOI:10.1088/1757-899X/980/1/012008.
- T. Nelson, A. White, J. Bjorgaard, A. E. Sifain, Y. Zhang, B. Nebgen and S. Tretiak, Non-adiabatic excited-state molecular dynamics: Theory and applications for modeling photophysics in extended molecular materials, Chem. Rev., 2020, 2215–2287, DOI:10.1021/acs.chemrev.9b00447.
- Y. I. Yang, Q. Shao, J. Zhang, L. Yang and Y. Gao, Enhanced sampling in molecular dynamics, J. Chem. Phys., 2019, 151(7), 070902, DOI:10.1063/1.5109531.
- Y. Wang, J. M. L. Ribeiro and P. Tiwary, Machine learning approaches for analyzing and enhancing molecular dynamics simulations, Curr. Opin. Struct. Biol., 2019, 61, 139–145, DOI:10.1016/j.sbi.2019.12.016.
- R. Lazim, S. Suh and S. Choi, Advances in Molecular Dynamics Simulations and Enhanced Sampling Methods for the Study of Protein Systems, Int. J. Mol. Sci., 2020, 21, 6339, DOI:10.3390/ijms21176339.
- L. Zheng, A. A. Alhossary, C. Kwoh and Y. Mu, Molecular Dynamics and Simulation, J. Chem. Phys., 2019, 550–566, DOI:10.1016/B978-0-12-809633-8.20284-7.
- H. Arya and T. Bhatt, Molecular dynamics simulations, The Design & Development of Novel Drugs and Vaccines, 2021. DOI:10.1016/B978-0-12-821471-8.00005-2.
- S. Wan, R. Sinclair and P. Coveney, Uncertainty quantification in classical molecular dynamics, Philos. Trans. R. Soc., A, 2020, 379, 20200082, DOI:10.1098/rsta.2020.0082.
- A. Semenov, Y. Bebikhov, M. Semenova and I. Yakushev, Development of a program for mathematical modeling of molecular dynamics processes, E3S Web Conf., 2023, 37103077, DOI:10.1051/e3sconf/202337103077.
- B. Narayan, Y. Yuan, A. Fathizadeh, R. Elber and N. Buchete, Long-time methods for molecular dynamics simulations: Markov State Models and Milestoning, Prog. Mol. Biol. Transl. Sci., 2020, 170, 215–237, DOI:10.1016/bs.pmbts.2020.01.002.
- Z. Li, K. Meidani, P. Yadav and A. Farimani, Graph Neural Networks Accelerated Molecular Dynamics, J. Chem. Phys., 2021, 156(14), 144103, DOI:10.1063/5.0083060.
- Z. F. Brotzakis, M. Vendruscolo and P. Bolhuis, A method of incorporating rate constants as kinetic constraints in molecular dynamics simulations, Proc. Natl. Acad. Sci. U. S. A., 2020, 118, e2012423118, DOI:10.1073/pnas.2012423118.
- X. Wu, L. Y. Xu, E. Li and G. Dong, Application of molecular dynamics simulation in biomedicine, Chem. Biol. Drug Des., 2022, 99, 789–800, DOI:10.1111/cbdd.14038.
- A. A. Laurent, Review of Algorithms Used in Molecular Dynamics Simulations, URNCST, 2019, 3(8) DOI:10.26685/urncst.157.
- R. Schneider, A. R. Sharma and A. Rai, Introduction to Molecular Dynamics, Molecular Dynamics Simulation of Nanocomposites Using BIOVIA Materials Studio, Lammps and Gromacs, 2019, ch. 1, DOI:10.1007/978-3-540-74686-7_1.
- P. R. Vlachas, J. Zavadlav, M. Praprotnik and P. Koumoutsakos, Accelerated Simulations of Molecular Systems through Learning of Effective Dynamics, J. Chem. Theory Comput., 2021, 17(8), 5269–5283, DOI:10.1021/acs.jctc.1c00809.
- C. Tian, K. Kasavajhala, K. A. A. Belfon, L. Raguette, H. Huang, A. N. Migues, B. Bickel, J. Wang, Y. Pincay, Q. Wu and C. Simmerling, ff19SB: Amino-acid specific protein backbone parameters trained against quantum mechanics energy surfaces in solution, J. Chem. Theory Comput., 2020, 16(1), 528–552, DOI:10.1021/acs.jctc.9b00591.
- H. Kim, B. Fábián and G. Hummer, Neighbor list artifacts in molecular dynamics simulations, J. Chem. Theory Comput., 2023, 19, 8919–8929, DOI:10.1021/acs.jctc.3c00777.
- J. Jung and Y. Sugita, Group-based evaluation of temperature and pressure for molecular dynamics simulation with a large time step, J. Chem. Phys., 2020, 153, 234115, DOI:10.1063/5.0027873.
- H. Hata, M. Nishiyama and A. Kitao, Molecular dynamics simulation of proteins under high pressure: Structure, function and thermodynamics, Biochim. Biophys. Acta, Gen. Subj., 2020, 1864(1) DOI:10.1016/j.bbagen.2019.07.004.
- M. Kim, E. Kim, S. Lee and J. S. Lee, New method for constant-NPT molecular dynamics, J. Phys. Chem. A, 2019, 123, 1689–1699, DOI:10.1021/acs.jpca.8b09082.
- S. Naserifar, J. J. Oppenheim, H. Yang, T. Zhou, S. Zybin, M. Rizk and W. Goddard, Accurate non-bonded potentials based on periodic quantum mechanics calculations for use in molecular simulations of materials and systems, J. Chem. Phys., 2019, 151, 154111, DOI:10.1063/1.5113811.
- A. Saeteaw, N. Sawang and T. Sutthibutpong, van der Waals parameters of inert, polar and non-polar gas molecules obtained from atomistic molecular dynamics simulations, J. Phys.: Conf. Ser., 2019, 1380, DOI:10.1088/1742-6596/1380/1/012094.
- L. Chen, S. Y. Wang and W. Tao, A study on thermodynamic and transport properties of carbon dioxide using molecular dynamics simulation, Energy, 2019, 179, 1094–1102, DOI:10.1016/J.ENERGY.2019.05.073.
- K. Li, B. Chen, Y. Song and M. Yang, Molecular dynamics simulation of the effects of different thermodynamic parameters on methane hydrate dissociation: An analysis of temperature, pressure and gas concentrations, Fluid Phase Equilib., 2020, 516, 112606, DOI:10.1016/j.fluid.2020.112606.
- Y. Du, H. Zhang, X. Li, Y. Zhang and S. Yuan, Molecular dynamics study on the suitable compatibility conditions of a CO2-cosolvent-light hydrocarbon system by calculating the solubility parameters, Energy Fuels, 2020, 34, 3483–3492, DOI:10.1021/acs.energyfuels.9b03883.
- F. Noé, A. Tkatchenko, K. R. Müller and C. Clementi, Machine learning for molecular simulation, Annu. Rev. Phys. Chem., 2019, 71, 361–390, DOI:10.1146/annurev-physchem-042018-052331.
- S. Hong, H. Chun, J. Lee, B. Kim, M. Seo, J. Kang and B. Han, First-principles-based machine-learning molecular dynamics for crystalline polymers with van der Waals interactions, J. Phys. Chem. Lett., 2021, 12, 6000–6006, DOI:10.1021/acs.jpclett.1c01140.
- J. Köfinger and G. Hummer, Empirical optimization of molecular simulation force fields by Bayesian inference, Eur. Phys. J. B, 2021, 94, 22, DOI:10.1140/epjb/s10051-021-00234-4.
- Z. Chen, D. Li, H. Wan, M. Liu and J. Liu, Unsupervised machine learning methods for polymer nanocomposites data via molecular dynamics simulation, Mol. Simul., 2020, 46(18), 1509–1521, DOI:10.1080/08927022.2020.1851028.
- J. Andrews, O. Gkountouna and E. Blaisten-Barojas, Forecasting molecular dynamics energetics of polymers in solution from supervised machine learning, Chem. Sci., 2022, 13, 7021–7033, 10.1039/d2sc01216b.
- H. Li, T. H. Tian, Y. Chen, S. Xiao, X. Zhao, Y. Gao and L. Zhang, Analyzing and predicting the viscosity of polymer nanocomposites in the conditions of temperature, shear rate, and nanoparticle loading with molecular dynamics simulations and machine learning, J. Phys. Chem. B, 2023, 127 DOI:10.1021/acs.jpcb.3c01697.
- H. Doi, K. Z. Takahashi, K. Tagashira, J. Fukuda and T. Aoyagi, Machine learning-aided analysis for complex local structure of liquid crystal polymers, Sci. Rep., 2019, 9, 16493, DOI:10.1038/s41598-019-51238-1.
- D. H. Nguyån, L. Tao and Y. Li, Integration of machine learning and coarse-grained molecular simulations for polymer materials: Physical understandings and molecular design, Front. Chem., 2022, 9, 820417, DOI:10.3389/fchem.2021.820417.
- J. Yang, L. Tao, J. He, J. McCutcheon and Y. Li, Machine learning enables interpretable discovery of innovative polymers for gas separation membranes, Sci. Adv., 2022, 8, abn9545, DOI:10.1126/sciadv.abn9545.
- J. Munshi, W. Chen, T. Chien and G. Balasubramanian, Machine learned metaheuristic optimization of the bulk heterojunction morphology in P3HT thin films, Comput. Mater. Sci., 2021, 187, 110119, DOI:10.1016/j.commatsci.2020.110119.
- A. Rahman, P. Deshpande, M. Radue, G. Odegard, S. Gowtham, S. Ghosh and A. Spear, A machine learning framework for predicting the shear strength of carbon nanotube-polymer interfaces based on molecular dynamics simulation data, Compos. Sci. Technol., 2021, 207, 108627, DOI:10.1016/j.compscitech.2020.108627.
- L. Tao, V. Varshney and Y. Li, Benchmarking machine learning models for polymer informatics: An example of glass transition temperature, J. Chem. Inf. Model., 2021, 61(11), 5395–5413, DOI:10.1021/acs.jcim.1c01031.
- J. Steindl, R. Hincapie, A. Borovina, C. Puls, J. Badstöber, G. Heinzmann and T. Clemens, Improved EOR polymer selection using field-flow fractionation, SPE Reservoir Eval. Eng., 2021, 25(1), 319–330, DOI:10.2118/207700-ms.
- J. Steindl, R. Hincapie, A. Borovina, C. Puls, J. Badstöber, G. Heinzmann, and T. Clemens, Improved Enhanced Oil Recovery Polymer Selection Using Field-Flow Fractionation, SPE Reservoir Evaluation & Engineering, 2022, DOI:10.2118/207700-pa.
- S. Jouenne, B. Levaché, M. Joly, C. Hourcq, M. Questel, and G. Heurteux, Universal viscosifying behavior of acrylamide-based polymers used in enhanced oil recovery, IOR 2019 – 20th European Symposium on Improved Oil Recovery, 2019, DOI:10.3997/2214-4609.201900140.
- S. Jouenne and B. Levaché, Universal viscosifying behavior of acrylamide-based polymers used in enhanced oil recovery, J. Rheol., 2020, 64(6), 1295–1313, DOI:10.1122/8.0000063.
- R. Afolabi, G. Oluyemi, S. Officer and J. Ugwu, Hydrophobically associating polymers for enhanced oil recovery – Part A: A review on the effects of some key reservoir conditions, J. Petrol. Sci. Eng., 2019, 180, 106162, DOI:10.1016/j.petrol.2019.06.016.
- Y. Rui, L. Ying and L. Chunxiu, Study about how the metal cationic ions affect the properties of partially hydrolyzed hydrophobically modified polyacrylamide (HMHPAM) in aqueous solution, Colloids Surf., A, 2013, 434, 16–24, DOI:10.1016/j.colsurfa.2013.05.036.
- M. H. Ar-Raihan, R. Salsabila, A. Adharis, P. J. Ratri and T. R. Mayangsari, Analysis of surfactant and polymer behavior on water/oil systems as additives in enhanced oil recovery (EOR) technology through molecular dynamics simulation: A preliminary study, J. Earth Energy Eng., 2023, 27–39, DOI:10.25299/jeee.2023.13958.
- A. Beteta, L. Nurmi, L. Rosati, S. Hanski, K. McIver, K. Sorbie, and S. Toivonen, Polymer chemical structure and its impact on EOR performance, SPE Asia Pacific Oil and Gas Conference and Exhibition, 2020, vol. 1. DOI:10.2118/200441-ms.
- K. Liang, P. Han, Q. Chen, X. Su and Y. Feng, Comparative study on enhancing oil recovery under high temperature and high salinity: Polysaccharides versus synthetic polymer, ACS Omega, 2019, 4(9), 10620–10628, DOI:10.1021/acsomega.9b00717.
- Y. Sun, W. Zhang, J. Li, R. S. Han and C. Lu, Mechanism and performance analysis of nanoparticle-polymer fluid for enhanced oil recovery: A review, Molecules, 2023, 28(11), 4331, DOI:10.3390/molecules28114331.
- H. Quan, Q. Lu, Z. Chen, Z. Huang and Q. Jiang, Adsorption-desorption behavior of the hydrophobically associating copolymer AM/APEG/C-18/SSS, RSC Adv., 2019, 9, 12300–12309, 10.1039/c9ra01932d.
- M. R. Couto, E. Gudiña, D. Ferreira, J. Teixeira and L. Rodrigues, The biopolymer produced by Rhizobium viscosum CECT 908 is a promising agent for application in microbial enhanced oil recovery, New Biotechnol., 2019, 49, 144–150, DOI:10.1016/j.nbt.2018.11.002.
- A. Li and G. Mao, Research progress of sustainable monoterpene polymers, Polym. Bull., 2024, 37, 616–629, DOI:10.14028/j.cnki.1003-3726.2024.23.351.
- W. Kang, C. Cao, S. Guo, X. Tang, Z. A. Lashari, Y. Gao, X. Zhang and M. W. Iqbal, Hongbin Yang Mechanism of silica nanoparticles' better-thickening effect on amphiphilic polymers in high salinity condition, J. Mol. Liq., 2019, 277, 254–260, DOI:10.1016/j.molliq.2018.12.092.
- Z. Hu, M. Haruna, H. Gao, E. Nourafkan and D. Wen, Rheological Properties of Partially Hydrolyzed Polyacrylamide Seeded by Nanoparticles, Ind. Eng. Chem. Res., 2017, 56, 3456–3463, DOI:10.1021/acs.iecr.6b05036.
- O. Santamaria, S. H. Lopera, M. Riazi, M. Minale, F. B. Cortés and C. A. Franco, Phenomenological study of the micro- and macroscopic mechanisms during polymer flooding with SiO2 nanoparticles, J. Pet. Sci. Eng., 2021, 198, 108135, DOI:10.1016/j.petrol.2020.108135.
- P. J. Flory, Molecular Size Distribution in Three Dimensional Polymers. II. Trifunctional Branching Units, J. Am. Chem. Soc., 1941, 63(11), 3091–3096, DOI:10.1021/ja01856a062.
- P. J. Flory, Molecular Size Distribution in Three Dimensional Polymers. VI. Branched Polymers Containing A R Bf-1 Type Units, J. Am. Chem. Soc., 1952, 74(11), 2718–2723, DOI:10.1021/ja01856a062.
- S. Qiao, Q. Liu, X. Zhang and H. Che, Syntheses of a hyperbranched polymer and its performance on enhanced oil recovery, Front. Chem., 2021, 9, 738717, DOI:10.3389/fchem.2021.738717.
- S. Zhu, W. Dou, X. Zeng, X. Chen, Y. Gao, H. Liu and S. Li, Recent Advances in the Degradability and Applications of Tissue Adhesives Based on Biodegradable Polymers, Int. J. Mol. Sci., 2024, 25, 5249, DOI:10.3390/ijms25105249.
- H. Yunzi, W. Walid, K. Kevin and C. Carol, Newly developed techniques on polycondensation, ring-opening polymerization and polymer modification: focus on poly(lactic acid), Materials, 2016, 9(3), 133, DOI:10.3390/ma9030133.
- A. Beena and T. Muringayil, Enhancing Polymer Sustainability: Eco-Conscious Strategies, Polymers, 2024, 16, 1769, DOI:10.3390/polym16131769.
- W. B. S. Farra, Synthesis and characterization of polycarbonates from epoxidized vegetable oils, propylene oxide and carbon dioxide, Doctoral thesis, Polytechnic University of Catalonia, Barcelona Tech, 2021, available, https://upcommons.upc.edu/bitstream/handle/2117/349572/TFWbS1de1.pdf?sequence=1.
- V. Aamod, L. Erlantz and A. Andrea, et al., Green Synthesis of Reticular Materials, Adv. Funct. Mater., 2023, 2304660, DOI:10.1002/adfm.202304660.
- J. Keogh, G. Deshmukh and H. Manyar, Green synthesis of glycerol carbonate via transesterification of glycerol using mechanochemically prepared sodium aluminate catalysts, Fuel, 2022, 310, 122484, DOI:10.1016/j.fuel.2021.12.039.
- G. Brahmachari and I. Karmakar, Green-inspired synthetic drives for organophosphorus compounds under solvent-free conditions, ARKIVOC, 2022, 3, 133–161, DOI:10.24820/ark.5550190.p011.807.
- K. Katsuhiko, Development of Carbon Dioxide-based Synthesis of Carbonate Derivatives, Carbamate Derivatives, and Silyl Formates, J. Jpn. Pet. Inst., 2024, 67(2), 45–51, DOI:10.1627/jpi.67.45.
- P. Li, F. Zhang, T. Zhu, C. Zhang, G. Liu and X. Li, Synthesis and properties of the active polymer for enhanced heavy oil recovery, Colloids Surf., A, 2021, 626, 127036, DOI:10.1016/j.colsurfa.2021.127036.
- L. Corredor, M. Husein and B. Maini, Impact of PAM-grafted nanoparticles on the performance of hydrolyzed polyacrylamide solutions for heavy oil recovery at different salinities, Ind. Eng. Chem. Res., 2019, 9888–9899, DOI:10.1021/acs.iecr.9b01290.
- Y. Zhang, Y. Feng, B. Li and P. Han, Enhancing oil recovery from low-permeability reservoirs with a self-adaptive polymer: A proof-of-concept study, Fuel, 2019, 136–146, DOI:10.1016/j.fuel.2019.04.034.
- S. M. Åsen, Synthetic Polymers for Enhanced Oil Recovery: Mechanical Degradation, and Alleviation Thereof, USPS Repository, 2021, DOI:10.31265/usps.89.
- I. Eiroboyi, S. Ikiensikimama, B. Oriji and I. Okoye, The effect of monovalent and divalent ions on biodegradable polymers in enhanced oil recovery, SPE J., 2019, 24 DOI:10.2118/198788-MS.
- Y. Tamsilian, A. Agirre, M. Fernández, J. Sheng and R. Tomovska, High-molar mass acrylamide-co-diacetoneacrylamide graft copolymers as viscosity enhancer for polymer flooding oil recovery, Polym. Test., 2020, 82, 106332, DOI:10.1016/j.polymertesting.2020.106332.
- Y. Qiannan, L. Yikun, L. Shuang, T. Shuai, S. Zhi and Y. Yang, Experimental study on surface-active polymer flooding for enhanced oil recovery: A case study of Daqing placanticline oilfield, NE China, Petrol. Explor. Dev., 2019, 46, 1206–1217, DOI:10.1016/s1876-3804(19)60274-0.
- T. Majeed, M. Kamal, T. Sølling, A. Mahboob and X. Zhou, Performance evaluation of novel polymers for CO2 foam enhanced oil recovery, SPE J., 2019, 24 DOI:10.2118/197839-MS.
- K. L. N. P. Aguiar, L. Palermo and C. Mansur, Polymer viscosifier systems with potential application for enhanced oil recovery: A review, Oil Gas Sci. Technol., 2021, 65, DOI:10.2516/ogst/2021044.
- V. H. Ferreira, K. Clinckspoor, A. B. Vermelho, V. Cardoso and R. Moreno, Mechanical degradation of biopolymers for enhanced oil recovery applications, SPE J., 2022, 2052–2072, DOI:10.2118/209579-pa.
- F. J. Cun, W. F. Chao, J. Jie, Y. B. Zhu, L. D. Tang and L. He, Molecular mechanism of viscoelastic polymer enhanced oil recovery in nanopores, R. Soc. Open Sci., 2018, 5(6), 180076, DOI:10.1098/rsos.180076.
- H. Wang, Exploration of the Interaction Mechanism between Active Polymers and Crude Oil, Pet. Geol. Recovery Effic., 2008, 15(5), 66–68 CAS.
- W. Li, J. Wang and D. Xu, Molecular simulations of the effect of hydrated montmorillonite on the viscosity of polyacrylamide under confined shear, J. Wuhan Univ. Technol., Mater. Sci. Ed., 2015, 6, 556–561, DOI:10.1007/s11595-015-1188-4.
- P. Chen, L. Yao and Y. Liu, et al., Experimental and theoretical study of dilute polyacrylamide solutions: effect of salt concentration, J. Mol. Model., 2012, 18(7), 3153–3160, DOI:10.1007/s00894-011-1332-9.
- T. Ahsani, Y. Tamsilian and A. Rezaei, Molecular dynamic simulation and experimental study of wettability alteration by hydrolyzed polyacrylamide for enhanced oil recovery: a new finding for polymer flooding process, J. Petrol. Sci. Eng., 2021, 196(3), 108029, DOI:10.1016/j.petrol.2020.108029.
- S. Yuan, C. Liu and G. Xu, et al., Mesoscopic Simulation on the Interaction between Polymer PVP and Surfactant AOT, Chem. J. Chin. Univ., 2003, 24(6), 1048–1051, DOI:10.3321/j.issn:0251-0790.2003.06.016.
- T. Ni, G. Huang, P. Gao, Y. Xu and M. Yang, Dissipative particle dynamics simulation on the association properties of fluorocarbon-modified polyacrylamide copolymers, Polym. J., 2011, 43, 635–641, DOI:10.1038/pj.2011.24.
- L. Yao, C. Panke, D. Bin, J. Jianhui, B. Bo and Z. Ge, Molecular design of modified polyacrylamide for the salt tolerance, J. Mol. Model., 2012, 18, 4529–4545, DOI:10.1007/s00894-012-1447-7.
- H. Wang, H. Zhang, C. Liu and S. Yuan, Coarse-grained molecular dynamics simulation of self-assembly of polyacrylamide and sodium dodecylsulfate in aqueous solution, J. Colloid Interface Sci., 2012, 386, 205–211, DOI:10.1016/j.jcis.2012.07.026.
- S. Yuan, Z. Cai and Y. Guo, Dynamic simulation on the interaction between polymer and surfactant in aqueous solution, Chem. J. Chin. Univ., 2002, 23, 585–589, DOI:10.1143/JPSJ.70.3739.
- J. Jia, H. Cai, Y. Liang, T. Tsuiji, M. Lin and B. Peng, Synergistic effect of hydrolyzed polyacrylamide and ionic surfactant to enhance the stability of CO2 foam: A molecular dynamics study, Pet. Sci. Bull., 2023, 1(01.005), 69–86, DOI:10.3969/j.issn.2096-1693.2023.
- K. Wu, X. Yao, B. Zhao, S. Han, J. Wei and H. Yang, Dissipative particle dynamics simulation and study on solution properties of polymer surfactants, Oil Drill. Prod. Technol., 2016, 38(6), 863–868, DOI:10.13639/j.odpt.2016.06.029.
- S. L. Yuan, G. Y. Xu, Z. T. Cai and Y. S. Jiang, Molecular simulation of dilute polyacrylamide solutions, Acta Phys.-Chim. Sin., 2002, 18(10), 833–839, DOI:10.1021/ie010349k.
- G. Wu, X. Ji, T. Zhang, S. Sun, C. Li and S. Hu, Molecular simulation studies on the interaction of polymer and surfactant: A review, Polymer, 2016, 8(1), 69–86, DOI:10.1016/j.molliq.2019.112104.
- A. N. El-hoshoudy, E. G. Zaki and S. M. Elsaeed, Experimental and Monte Carlo simulation of palmitate-guar gum derivative as a novel flooding agent in the underground reservoir, J. Mol. Liq., 2020, 112502, DOI:10.1016/j.molliq.2020.112502.
- A. N. El-hoshoudy, E. M. Mansour and S. M. Desouky, Experimental, computational and simulation oversight of silica-co-poly acrylates composite prepared by surfactant-stabilized emulsion for polymer flooding in unconsolidated sandstone reservoirs, J. Mol. Liq., 2020, 113082, DOI:10.1016/j.molliq.2020.113082.
- C. Ma, X. Liu, L. Xie, Y. Chen, W. Ren, W. Gu, M. Zhang and H. Zhou, Synthesis and Molecular Dynamics Simulation of Amphiphilic Low Molecular Weight Polymer Viscosity Reducer for Heavy Oil Cold Recovery, Energies, 2021, 14(14), 6856, DOI:10.3390/en14216856.
- J. M. Tesha, Molecular Dynamics Simulation of Polysulfone and Polystyrene-co-maleic Anhydride Blends Compatibility: A mesoscopic, Ewald Approach and Experimental Comparison, Research Square, preprint, 2021, rs-1109742, DOI:10.21203/rs.3.rs-1109742/v1.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |






