Open Access Article
Open Access ArticleSynthesis, characterization, and luminescence thermometry investigation of LiSrGd(WO4)3:Pr3+ phosphors
Refka Tahria,
Mouna Fhoulaa,
Fadwa Ayachia,
Tarak Koubaaa,
Mohamed Dammak *a,
Joan Josep Carvajal
*a,
Joan Josep Carvajal b and
Maria Cinta Pujolb
b and
Maria Cinta Pujolb
aLaboratoire de Physique Appliquée, Faculté des Sciences de Sfax, Département de Physique, Université de Sfax, BP 1171, Sfax, Tunisia. E-mail: madidammak@yahoo.fr; mohamed.dammak@fss.usf.tn
bUniversitat Rovira i Virgili, Departament de Química Física i Inorgànica, Materials in Green Chemistry (GreenMat), Marcel·li Domingo 1, Tarragona 43007, Spain
First published on 19th March 2025
Abstract
In this study, LiSrGd(WO4)3 (LSGW) phosphors doped with different concentrations of Pr3+ were synthesized via the traditional solid–solid method to explore their suitability for luminescence thermometry applications. Structural and morphological analyses using X-ray powder diffraction and scanning electron microscopy offered comprehensive insights. The energy gap value of LSGW, calculated at Eg = 3.69 eV for x = 5 at% Pr3+, suggested minimal alteration in the bandgap energy upon incorporation of Pr3+ ions into the LSGW host. The empirical energy levels for the free ion Pr3+ were derived from absorption and emission data are gathered from LSGW:Pr3+. The theoretical energy diagram and corresponding eigenstates for Pr3+ were calculated using block diagonalization of the Hamiltonian's matrix in the basis set of coupled states 〈4f2〉〈4f2τSLJ〉, considering intermediate coupling. Thermometric parameters were calculated using the luminescence intensity ratio strategy based on Pr3+ emission spectra for thermal coupled levels. Our findings revealed that the LIR1 (I531/I557) exhibited maximum absolute and relative sensitivities of 31.5 10−4 K−1 and 0.65% K−1, respectively, at 300 K, accompanied by remarkably low-temperature uncertainty (δT) values ranging from 0.031 to 0.037 K in the 300–440 K temperature range. These results show the importance of LiSrGd(WO4)3:Pr3+ phosphors for optical applications.
1. Introduction
Optical temperature sensors have recently received significant attention due to their potential applications in inaccessible, harsh, or hazardous environments.1–3 Recently, considerable focus has been given to contactless luminescence thermometry, which represents a new technique enabling temperature measurement with submicron spatial resolution and sub degree thermal precision.4–6 The luminescence (fluorescence) intensity ratio technique, known as LIR (FIR), is widely employed for optical temperature detection due to its quick measurement capability, high thermal resolution, and excellent precision.7,8 The LIR method exploits the temperature-induced variations in the intensity ratio of emission bands linked to the thermally-coupled levels (TCLs) of various lanthanide (Ln) ions. The energy separation (ΔE) between their TCLs ranges approximately from 200 to 2000 cm−1.9,10Trivalent praseodymium (Pr3+) stands out among activator Ln ions for its fluorescence spanning from the ultraviolet, to the visible and near-infrared wavelengths. Its emission characteristics include green-bluish and red light, attributed to transitions within the 3P0,1 and 1D2 multiplets.11–13 The incorporation of Pr3+ into phosphors has demonstrated notable sensing capabilities, enabled by cross relaxation (CR) processes between the 3P0 and 1D2 levels or between the thermally coupled levels (TCLs) 3P0 and 3P1. Additionally, researchers have investigated the interference of the metal–metal intervalence charge transfer (IVCT) state with Pr3+ luminescence as an innovative strategy for designing high-performance FIR-based thermometers. Previous studies have harnessed different Pr3+ transitions for FIR calculations based on their temperature-dependent intensity variations.14–22
For the design of highly efficient luminescent materials and sensors, the choice of a suitable host material is crucial. Specially, tungstate has a strong and broad charge transfer band in the near-ultraviolet region due to the (WO4)2 group. This allows it to enhance the luminescence of the fluorescent activation centre by absorbing near-ultraviolet energy and transferring it to the light emitting centre.23–25 Furthermore, tungstate compounds serve as excellent hosts for photoactive lanthanide ions, with potential applications in phosphors, lasers, photocatalysis, and negative thermal expansion.26,27 Numerous reports exist in the literature on tungstates, particularly those involving rare earth ions such as Sm, Ce, and Tm, there is a noticeable gap in research focused specifically on LiSrGd(WO4)3 hosts. This particular compound remains relatively understudied compared to its counterparts. The LiSrGd(WO4)3 structure is intriguing as it exhibits two polymorphic forms, transitioning between a monoclinic structure at low temperatures and an orthorhombic structure at higher temperatures, influenced primarily by the electronegativity of the rare earth ion.28 Gd2(WO4)3, a member of the rare earth tungstate family, holds particular significance due to its versatility and applications in various fields including optical fibers, sensors, scintillators, light-emitting diodes, and solid-state lasers.29 While Gd2(WO4)3 and its substitutions have been extensively investigated for their optical properties, including self-luminescence in the visible spectrum, there is a notable gap in research specifically addressing the LiSrGd(WO4) host.
This paper focuses on the synthesis and characterizations of LiSrGd(WO4)3:Pr3+ phosphors, with a notable emphasis on luminescent thermometry investigation. It begins with a detailed account of the synthesis process, followed by comprehensive characterizations including structural, morphological, and spectroscopic analyses. Results and discussions delve into various aspects such as structural and morphological characteristics, FTIR, UV-visible spectrum, photoluminescence, theoretical simulations of energy levels of free Pr3+ ion, and luminescence thermal stability with a specific focus on CIE changes in UC with temperature.
The study of luminescent thermometry explores the feasibility and accuracy of using luminophores for temperature sensing, highlighting their potential applications in advanced temperature measurement technologies.
2. Experimental section and characterizations
LiSrGd1−x(WO4)3:x% Pr3+ (x = 1, 3, 5, and 7 at%) were synthesized though the traditional solid-state reaction method. High-purity precursors (≥99%) including lithium carbonate (Li2CO3), strontium carbonate (SrCO3), praseodymium oxide (Pr6O11), gadolinium oxide (Gd2O3) and tungsten oxide (WO3) were mixed in stoichiometric proportions and ground into fine powders. The mixture was then placed in a crucible and heated up to 350 °C for 10 hours. After cooling, the resulting product was then weighed, manually crushed, and pressed into pellets. These pellets were sintered at 800 °C for 5 hours. The particle agglomeration was observed during the sintering process at 800 °C, where increased atomic diffusion promotes grain growth and particle coalescence. The synthesis of the LiSrGd1−xPrx(WO4)3 compound can be described by the following reaction:| 3Li2CO3 + 6SrCO3 + 3(1 − x)Gd2O3 + xPr6O11 + 9W2O3 + (18 − 4x)O2 → 6LiSrGd1−xPrx(WO4)3 + 9CO2 |
A Siemens-D8-θ/2θ diffractometer with a Cu Kα X-ray source (λ = 0.15418 nm) was employed for X-ray diffraction (XRD) measurements under grazing incidence. A Thermo Scientific Apreo 2 S LoVac field emission scanning electron microscope (FE-SEM) is used to study the morphology of the samples. The Fourier transform infrared (FT-IR) spectra of the material were obtained at room temperature within the 400–4000 cm−1 range using a PerkinElmer 1000 FTIR spectrometer. UV-vis-NIR absorption was measured using a PerkinElmer Lambda 365 UV-vis-NIR spectrometer. Photoluminescence spectra were measured using a Fluoromax 4P model Horiba spectrometer with a xenon arc lamp as the excitation source. Temperature-dependent DC emission was examined using a JOBIN YVON HR 320 spectrometer fitted with a temperature controller and a 450 nm laser excitation source.
3. Results and discussion
3.1. XRD and SEM
The XRD patterns of praseodymium doped LSGW synthesized in this work are shown in Fig. 1. All samples are crystalline. Most of the peaks can be assigned to the tetragonal scheelite crystalline phase, with the space group I41/a (no. 88).30 Small diffraction peaks can be observed at 2θ = 13.39° and 2θ = 14.4°, which should belong to the oxide precursors. In this crystal, Li, Sr, Gd and Pr3+ are distributed randomly in the S4 site that in CaWO4 occupies Ca2+; which is surrounded by 8 oxygens. It was possible to clearly observe the shift of the peak located at around 18°, corresponding to the (101) reflection and shown in the inset of Fig. 1, when the Pr3+ concentration increased. | ||
| Fig. 1 XRD patterns of the obtained products, LiSrGd(WO4)3:x at% Pr3+ (x = 1, 3, 5, and 7). Inset: angle shift of the (101) reflection related to the Pr concentration. | ||
Table 1 summarizes the unit cell parameters refined by using the Le Bail methodology using the TOPAS software. The introduction of Pr3+ in the crystalline structure affects the unit cell parameters, showing first a slight decrease in size for the two parameters a and c, that for Pr3+ concentrations of 5 at% or higher, increased again. This compression/stress should be expected when substituting Gd3+ or Sr2+ by Pr3+ because of the difference in ionic radii in a coordination environment of 8, that is Gd3+ = 1.05 Å, Pr3+ = 0.99 Å and Sr2+ = 1.18 Å. According to these data, a hypothesis that can be established is that Pr3+ tends to substitute first Sr2+ in the structure at low concentrations (below 5 at%), but then, as the concentration increases, it tends to substitute also Gd3+. However, no experiments have been done to confirm this.31,32
| % Pr | a (Å) | c (Å) | Volume (Å3) | Rexp/Rwp |
|---|---|---|---|---|
| 1 | 5.28683 | 11.53198 | 322.326 | 5.43/24.96 |
| 3 | 5.28671 | 11.52927 | 322.235 | 5.14/19.50 |
| 5 | 5.28710 | 11.53043 | 322.314 | 5.10/15.70 |
| 7 | 5.28845 | 11.53205 | 322.525 | 5.10/11.73 |
The observed shift of diffraction peaks toward higher 2θ angles compared to the standard JCPDS data can be attributed to the substitution of Gd3+ and/or Sr2+ by Pr3+ in the LiSrGd(WO4)3 lattice. This substitution induces a slight contraction of the unit cell, leading to peak shifts according to Bragg's law. Additionally, minor structural strain introduced during the solid-state synthesis process could further contribute to these shifts. Such peak displacements are commonly observed in doped phosphor systems and reflect changes in lattice parameters due to ionic substitution.
Fig. 2 shows the microstructure of LSGW particles doped with (1, 3, 5)% Pr3+ obtained at two different sizes (30 and 100 μm). Irregularly shaped particles, approximately 10 μm in size, are observed. Moreover, high-temperature heat treatments result in significant particle agglomeration.
3.2. FTIR spectroscopy
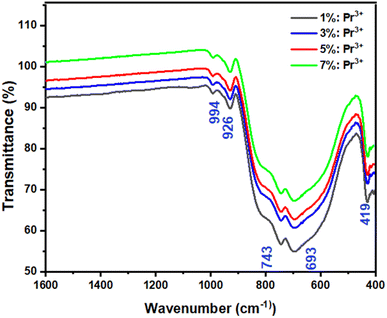
Fig. 3 shows the FTIR spectra of LSGW:3 at% Pr3+ and LSGW:5 at% Pr3+ phosphors. The set of vibrational bands below 1000 cm−1 belong to the characteristic absorption of WO42− group.33 The absorption peaks at 994 cm−1 and 926 cm−1 are ascribed to the stretching mode of the W![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and W–O bands, respectively.34,35 The peaks at 820 and 419 cm−1 is attributed to the stretching and bending modes of single WOW bridge, while the absorption peaks at 743 and 693 cm−1 are related to the stretching mode of the double WOOW bridge.34 Thus, the results of FTIR analysis further prove the formation of LSGW.
O and W–O bands, respectively.34,35 The peaks at 820 and 419 cm−1 is attributed to the stretching and bending modes of single WOW bridge, while the absorption peaks at 743 and 693 cm−1 are related to the stretching mode of the double WOOW bridge.34 Thus, the results of FTIR analysis further prove the formation of LSGW.
3.3. Optical characterization
The Kubelka–Munk function is used to calculate the band gap of LSGW phosphors from diffuse reflectance spectra. The remission function establishes a link between the sample's diffuse reflectance (R), the absorption coefficient (K), and the diffusion coefficient (S) through the well known equation:39
 | (1) |
Within the parabolic band structure, the energy band gap (Eg) can be obtained using the Tauc's relation given by the following equation:39,40
| (F(R)hν)2 = C(hν − Eg) | (2) |
The value of Eg, which is approximately 3.69 eV, is calculated by extrapolating the right portion of the curve (αhν)2 from the hν to zero curve. The allowable direct transition is characterized by nonlinear and linear sections in the plot of [F(R)hν]2 as a function of hν. The linear portion describes the fundamental absorption, while the nonlinear section refers to a residual absorption involving states of impurities.
The energy gap values of LSGW:x at% Pr3+, as illustrated in Fig. 5, were calculated to be: (a) Eg = 3.94 eV, (b) Eg = 3.93 eV, (c) Eg = 3.69 eV, and (d) Eg = 3.67 eV, for x = 1, 3, 5, and 7, respectively. The obtained results suggest that the incorporation of Pr3+ ions into the LSGW host induces a maximal variation of approximately 23 meV.
| EExp (cm−1) | EThe (cm−1) | EExp | EThe | ||
|---|---|---|---|---|---|
| 3H4 | 0 | 225.7 | 1D2 | — | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 207.4 207.4 |
| 3H5 | 2320 | 2310.8 | 3P0 | — | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 085.9 085.9 |
| 3H6 | 4492 | 4493.4 | 3P1 | — | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 629.1 629.1 |
| 3F2 | 5131 | 5120.6 | 1I6 | — | 21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 676.7 676.7 |
| 3F3 | 6494 | 6495.9 | 3P2 | — | 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 839.5 839.5 |
| 3F4 | 6966 | 6974.2 | Conduction band: 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842 842 |
||
| 1G4 | 9962 | 9956.3 | 0S0 | — | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124.5 124.5 |
To optimize the model parameters, a MATLAB program implementing the Nelder–Mead simplex method41 was used. The quality of the fit was assessed by the root mean square deviation (r.m.s.),42,43 calculated using eqn (3):
 | (3) |
| F2 | F4 | F6 | α | β | γ | ζ | |
|---|---|---|---|---|---|---|---|
| a F4 = 0.729248954 × F2 and F6 = 0.477047309 × F2. | |||||||
69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 361 361 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 581 581 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 088 088 |
14.65 | −423 | 1321 | 740.3 | |
The agreement between the theoretical and experimental energy levels for Pr3+ was good, with a root mean square deviation of approximately 20 cm−1. Table 3 shows the experimental and theoretical energy levels for Pr3+. These results confirm that the theoretical model is consistent with the known energy levels of the 4f2 configuration for free Pr3+ ions, as well as ions embedded in various crystal lattices.44
The wave functions or the eigenstates are represented in the form:
 | (4) |
These eigenvectors associated with the praseodymium multiplets along with their intermediate coupling coefficients are calculated and presented in Table 4.
| 3H | 3F | 1G | 1D | 3P | 1I | 0S | |
|---|---|---|---|---|---|---|---|
| 3H4 | 0.98618 | −0.03002 | 0.16295 | ||||
| 3H5 | 1 | ||||||
| 3H6 | 0.99857 | −0.05351 | |||||
| 3F2 | −0.98894 | −0.14769 | 0.01330 | ||||
| 3F3 | 1 | ||||||
| 3F4 | −0.11858 | −0.81478 | 0.56752 | ||||
| 1G4 | 0.11573 | −0.57900 | −0.80707 | ||||
| 1D2 | 0.14534 | −0.94750 | 0.28481 | ||||
| 3P0 | 0.99539 | 0.09586 | |||||
| 3P1 | 1 | ||||||
| 1I6 | 0.05351 | 0.99857 | |||||
| 3P2 | 0.02946 | −0.28360 | −0.95849 | ||||
| 0S0 | −0.09586 | 0.99539 |
The free ion energy multiplets of rare-earth ions like Pr3+ determine the luminescence properties and their dependence on temperature. The way these energy levels are populated at different temperatures affects the intensity ratios used for temperature measurement and hence the sensitivity and accuracy of the luminescent thermometry. Understanding this relationship allows for the design of more effective phosphors for specific thermometric applications.
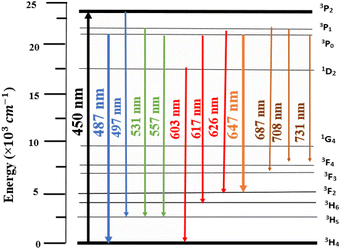
Luminescence of LSGW:Pr3+. The excitation spectrum (PLE) of LSGW:Pr3+ phosphors monitored at the 648 nm emission band shown in Fig. 6a is in accordance with the results in UV-vis diffuse reflectance spectra. It exhibits two distinct regions: a relatively wide charge transfer band (CTB) in the 200–400 nm range, originating from the (WO4)2− group,36 and the 4f–4f intra-configurational transition bands specific to the Pr3+ ion, occurring between 450 and 486 nm. These peaks are attributed to the internal transitions of the 4f2 configuration of the Pr3+ ion, with the most intense emission observed at 450 nm attributed to the 3H4 → 3P2 transition. Additionally, two other peaks are observed at 473 nm and 486 nm, corresponding to the 3H4 → 3P1 + 1I6 and 3H4 → 3P0 transitions, respectively.45 Fig. 6b displays the emission spectrum (PL) of LSGW:5 at% Pr3+ excited at 450 nm. The spectra exhibit several intense peaks resulting from intra-4f transitions. The most intense peak, appearing at 647 nm, is attributed to the 3P0 → 3F2 transition. Other less intense peaks are observed at 487 nm, 531 nm, 557 nm, 603 nm, and 617 nm, 687, 708, and 731 corresponding to the transitions 3P0 → 3H4, 3P1 → 3H5, 3P0 → 3H5, 1D2 → 3H4, 3P0 → 3H6, 3P1 → 3H5, 3P1 → 3F4 and 3P0 → 3F4 respectively.20,46,47 The energy level diagram and the possible optical transitions of Pr3+ ions in the LSGW host material are presented in Fig. 7.
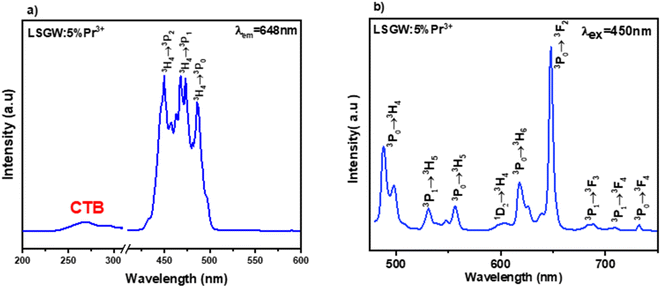 | ||
| Fig. 6 (a) Excitation spectrum measured by monitoring the 648 nm emission band, and (b) emission spectrum excited at 450 nm of LSGW:5% Pr3+. | ||
To investigate the impact of Pr3+ doping concentration on LSGW in optical properties, we prepared a series of samples with different doping concentrations (x = 1, 3, 5, and 7 at%) under consistent conditions. Emission spectra of these samples were then recorded within the visible range of 450–750 nm under blue excitation (450 nm), as depicted in Fig. 8a. All emission spectra present the same shape with a variation of their intensities when increasing the Pr3+ concentration. It is clearly seen that the emission intensity of 3P0 → 3F2 is more intense than that of 3P0 → 3H4 at x = 1 at%. At a concentration of 3 at%, both transitions, 3P0 → 3H4 and 3P0 → 3F2 exhibit nearly identical emission intensities. However, with an increase in concentration to 5 at%, the emission intensity of the 3P0 → 3H4 transition decreases while that of the 3P0 → 3F2 transition increases. This result can be explained by the radiative energy transfer between neighbouring luminescent centres of Pr3+ ions.18 The highest intensity emission spectrum was attained at a doping concentration of 5 at%. Beyond this concentration, the emission intensity decreases due to the phenomenon of luminescence quenching or self-extinction. Generally, this quenching process is due to the short average distance between the Pr3+ ions. To investigate the main cause of concentration quenching, we use the eqn (5) provided by Blasse to estimate the critical distance between Pr3+ activator ions.48
 | (5) |
 | (6) |
4. CIE chromaticity parameters
Fig. 9 shows the CIE chromaticity diagram for LSGW:x at% Pr3+ phosphors. It is clearly seen that the chromaticity coordinates of the compounds range from yellow to orange, with variation of the Pr3+ ions concentration. In order to find the practical utility of synthesized phosphors, the parameter Correlated Color Temperature (CCT) was calculated using the following formula proposed by McCamy:50| CCT = −449n3 + 3525n2 − 6823n + 5520.33 | (7) |
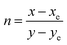 representing the convergence epicentre, which is located at point (xe = 0.3320 and ye = 0.1858) on the chromaticity diagram. The chromaticity coordinates as well as the CCT values are listed in Table 5. The obtained CCT values are all below 5000 K, indicating that the LSGW:Pr3+ phosphor can be used in white light-emitting diodes.
representing the convergence epicentre, which is located at point (xe = 0.3320 and ye = 0.1858) on the chromaticity diagram. The chromaticity coordinates as well as the CCT values are listed in Table 5. The obtained CCT values are all below 5000 K, indicating that the LSGW:Pr3+ phosphor can be used in white light-emitting diodes.
| Doping rate | x | y | CCT (K) |
|---|---|---|---|
| 1 at% | 0.41 | 0.39 | 3460 |
| 3 at% | 0.41 | 0.41 | 3498 |
| 5 at% | 0.43 | 0.41 | 3267 |
| 7 at% | 0.44 | 0.42 | 3010 |
5. Luminescent thermometry
In order to investigate the temperature effect, the temperature-dependent PL spectra of LSGW:5% Pr3+ were recorded from 300 to 440 K, under 450 nm excitation, as shown in Fig. 10. Notably, all emission spectra exhibit consistent spectral shapes, with no discernible shifts in peak position or full-width at half-maximum, indicating uniform distribution of crystal field. However, it is found that temperature has varying effects on emission-line intensity. Particular emphasis should be placed on transitions from thermally coupled 3P0(I1) and 3P1(I2) excited levels. In Fig. 11, the integrated intensities of 3P1 → 3H5 (531 nm), 3P0 → 3H5 (557 nm) and 3P0 → 3H6 (617 nm) are depicted against temperature variation. Notably, the emission intensity of the higher energy transition (3P1 → 3H5) increases, while the intensities of the lower-energy transitions (3P0 → 3H5 and 3P0 → 3H6) decrease as temperature increased, following a Boltzmann type distribution:21
 | (8) |
The temperature dependence of LIR between (I531 and I557) and (I531 and I617) relative to the temperature range 300–440 K are plotted in Fig. 12a and b, respectively. The experimental data are well fitted using eqn (1). The effective energy difference between the thermally coupled levels obtained from the fitting for LIR1 (I531/I557) is 403 cm−1, and for LIR2 (I531/I617), it is 324 cm−1. These values closely match the ΔE between the 3P1 and 3P0 states, which is approximately 500 cm−1.19 The most important parameters for temperature sensing applications are the absolute (Sa) and the relative thermal sensitivities (Sr) defined as follows:51
 | (9) |
 | (10) |
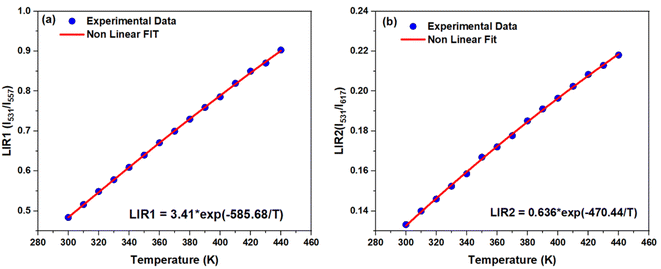 | ||
| Fig. 12 (a) The temperature dependent LIR1 (I531/I557) and (b) LIR2 (I531/I617) in 300–440 K temperature range. | ||
Fig. 13 shows the variation of Sa and Sr of the LSGW:Pr3+ phosphor as a function of temperature from 300 to 440 K. For LIR1 and LIR2 the Sa and Sr values decrease with temperature, and they take the maximum value at room temperature (300 K). The maximal absolute and relative thermal sensitivities were found to be 0.00315 K−1, and 0.65% K−1 for LIR1 and 0.0007 K−1 and 0.52% K−1 for LIR2. Table 6 shows the comparison of the Sr values for different materials doped with Pr3+ ions in different temperature ranges.
 | ||
| Fig. 13 Sa and Sr values in the 300–440 K temperature range corresponding to (a) LIR1 (I531/I557) and (b) LIR2 (I531/I617). | ||
| Phosphors | Sr (% K−1) | Temperature range (K) | References |
|---|---|---|---|
| SrMoO4:Pr3+ | 0.45 (I486/I601) | 298–498 | 17 |
| Na2La2Ti3O10:Pr3 | 1.96 (I611/I491) | 303–543 | 52 |
| Lu2GeO5:Pr3+ | 1.12 (I490/I608) | 150–550 | 53 |
| CaSc2O4:Pr3+ | 2.49 (I360/I630) | 275–490 | 55 |
| Gd2ZnTiO6:Pr3+ | 1.67 (I617/I492) | 293–433 | 56 |
| YVO4:Pr3+ | 0.78 (I595/I618) | 303–323 | 57 |
| CaMoO4:Pr3+ | 0.96 (I605/I490) | 303–573 | 58 |
| LiSrGd(WO4)3:Pr3+ | 0.65 (I531/I557) | 300–440 | This work |
| 0.52 (I531/I617) |
In the field of luminescent thermometry, LiSrGd(WO4)3:Pr3+ phosphors present a balanced performance in comparison to several other notable hosts. With a relative sensitivity (Sr) of 0.65% K−1 (I531/I557) and 0.52% K−1 (I531/I617), LiSrGd(WO4)3:Pr3+ exhibits moderate sensitivity values, outperforming SrMoO4:Pr3+ (Sr = 0.45% K−1)17 but falling behind more sensitive hosts like Na2La2Ti3O10:Pr3+ (Sr = 1.96% K−1).52
The temperature range of 300–440 K for LiSrGd(WO4)3:Pr3+ is narrower than that of Lu2GeO5:Pr3+ (150–550 K),53 yet it remains sufficient for various practical applications. These applications include biomedical diagnostics where the physiological temperature range is critical, microelectronics for real-time temperature monitoring of components, industrial processes requiring precise temperature control, environmental monitoring, household appliances for enhanced safety and efficiency, and energy systems such as battery management in electric vehicles and thermal management in solar panels.5,17,54 Additionally, the specific intensity ratios (I531/I557 and I531/I617) used by LiSrGd(WO4)3:Pr3+ offer unique advantages for precise temperature sensing, similar to the specialized ratios utilized by other phosphors. Although CaSc2O4:Pr3+ (Sr = 2.49% K−1)55 and Gd2ZnTiO6:Pr3+ (Sr = 1.67% K−1)56 demonstrate higher sensitivities, LiSrGd(WO4)3:Pr3+ provides a reliable alternative with its balanced sensitivity and adequate temperature range. This makes it a valuable candidate for luminescent thermometry applications, offering a practical solution with moderate sensitivity and a suitable operational temperature range.
In addition to having high sensing sensitivity, the performance of a thermometer must also be assessed based on its temperature resolution, which determines the precision of the temperature measurements. The temperature uncertainty (δT) value can be determined through various experimental methods: by measuring a series of emission spectra at the same temperature, using the temperature calibration curve, or monitoring the cooling of the sample.59 In this work, δT was obtained using the first method with the following expression:60
 | (11) |
 | ||
| Fig. 14 Value of ∂LIR/LIR obtained using 20 measurements at 298 K of (a) LIR1 and (b) LIR2 and (c and d) temperature uncertainty values δT. | ||
6. Conclusion
This study successfully synthesized LiSrGd(WO4)3 (LSGW) phosphors doped with Pr3+ using the solid-state method, confirming their potential for optical thermometry. Structural analyses verified Pr3+ incorporation with minimal bandgap alteration. Optimal doping (5 at%) yielded the highest emission intensity, while quenching effects at higher concentrations were attributed to dipole–dipole interactions. The LIR method demonstrated high sensitivity (Sa = 31.5 10−4 K−1, Sr = 0.65% K−1 at 300 K) and low temperature uncertainty (δT < 1 K) across 300–440 K, highlighting the competitive performance of LSGW:Pr3+ compared to similar materials. These findings establish LSGW:Pr3+ phosphors as promising candidates for precise and reliable optical temperature sensing.Data availability
All data underlying the results are available as part of the article and no additional source data are required.Conflicts of interest
There are no conflicts to declare.References
- V. Lojpur, M. G. Nikolić, D. Jovanović, M. Medić, Ž. Antić and M. D. Dramićanin, Appl. Phys. Lett., 2013, 103, 141912 CrossRef.
- K. Li, M. Shang, H. Lian and J. Lin, J. Mater. Chem. C, 2016, 4, 5507–5530 RSC.
- X. Wang, Q. Liu, Y. Bu, C. S. Liu, T. Liu and X. Yan, RSC Adv., 2015, 5, 86219–86236 RSC.
- I. E. Kolesnikov, A. A. Kalinichev, M. A. Kurochkin and E. Y. Kolesnikov, Mater. Des., 2019, 184, 108188 CrossRef CAS.
- M. Runowski, P. Wozny, I. R. Martín, V. Lavín and S. Lis, J. Lumin., 2019, 214, 116571 CrossRef CAS.
- B. del Rosal, D. Ruiz, I. Chaves-Coira, B. H. Juarez, L. Monge, G. Hong, N. Fernandez and D. Jaque, Adv. Funct. Mater., 2018, 28, 1806088 CrossRef.
- D. Jaque and F. Vetrone, Nanoscale, 2012, 4, 4301–4326 RSC.
- E. C. Ximendes, U. Rocha, T. O. Sales, N. Fernández, F. Sanz-Rodríguez, I. R. Martín, C. Jacinto and D. Jaque, Adv. Funct. Mater., 2017, 27, 1702249 CrossRef.
- M. Runowski, A. Shyichuk, A. Tymiński, T. Grzyb, V. Lavín and S. Lis, ACS Appl. Mater. Interfaces, 2018, 10, 172691–177279 CrossRef PubMed.
- S. F. León-Luis, U. R. Rodríguez-Mendoza, E. Lalla and V. Lavín, Sens. Actuators, B, 2011, 158, 208–213 CrossRef.
- Z. Liu and R. X. Wang, Chalcogenide Lett., 2022, 19, 471–481 CrossRef CAS.
- W. Ye, C. Ma, Y. Li, C. Zhao, Y. Wang, C. Zuo, Z. Wen, Y. Li, X. Yuan and Y. Cao, J. Mater. Chem. C, 2021, 9, 15201–15211 RSC.
- C. D. S. Brites, K. Fiaczyk, J. F. C. B. Ramalho, M. Sójka, L. D. Carlos and E. Zych, Adv. Opt. Mater., 2018, 6, 1701318 CrossRef.
- S. S. Zhou, G. C. Jiang, X. T. Wei, C. K. Duan, Y. H. Chen and M. Yin, J. Nanosci. Nanotechnol., 2014, 14, 3739–3742 CrossRef CAS PubMed.
- Y. Huang, L. Luo, J. Wang, Q. Zuo, Y. Yao and W. Li, J. Appl. Phys., 2015, 118, 044101 CrossRef.
- M. S. Pudovkin, S. L. Korableva, D. A. Koryakovtseva, E. V. Lukinova, A. V. Lovchev, O. A. Morozov and V. V. Semashko, J. Nanopart. Res., 2019, 21, 2–14 CrossRef.
- L. Li, P. Yang, W. Xia, Y. Wang, F. Ling, Z. Cao, S. Jiang, G. Xiang, X. Zhou and Y. Wang, Ceram. Int., 2021, 47, 769–775 CrossRef CAS.
- X. Tian, J. Li, H. Sheng, T. Li, L. Guo, C. Ji, Z. Huang, J. Wen, X. Liu, C. Li, J. Li and Y. Peng, Ceram. Int., 2022, 48, 3860–3868 CrossRef CAS.
- M. S. Pudovkin, O. A. Morozov, V. V. Pavlov, S. L. Korableva, E. V. Lukinova, Y. N. Osin, V. G. Evtugyn, R. A. Safiullin and V. V. Semashko, J. Nanomater., 2017, 2017, 3108586 Search PubMed.
- S. Jana, A. Mondal, J. Manam and S. Das, J. Alloys Compd., 2020, 821, 153342 CrossRef CAS.
- M. Runowski, P. Woźny, I. R. Martín, V. Lavín and S. Lis, J. Lumin., 2019, 214, 116571 CrossRef CAS.
- H. Zhang, Z. Gao, G. Li, Y. Zhu, S. Liu, K. Li and Y. Liang, Chem. Eng. J., 2020, 380, 122491 CrossRef CAS.
- B. Han, Y. Dai, J. Zhang, B. Liu and H. Sh, Ceram. Int., 2018, 44, 3734–3740 CrossRef CAS.
- K. Zhong, H. Ye, X. Wang, Y. Li and X. Yao, J. Mater. Sci.: Mater. Electron., 2021, 32, 17170–17181 CrossRef CAS.
- Q. Liu, X. Li, B. Zhang, L. Wang, Q. Zhang and L. Zhang, Ceram. Int., 2016, 42, 15294–15300 CrossRef CAS.
- L. Fan, Y. X. Fan, Y. H. Duan, Q. Wang, H. T. Wang, G. H. Jia and C. Y. Tu, Appl. Phys. B, 2009, 94, 553–557 Search PubMed.
- A. K. Parchur and R. S. Ningthoujam, Dalton Trans., 2011, 40, 7590–7594 Search PubMed.
- J. Bi, L. Wu, Y. Zhang, Z. Li, J. Li and X. Fu, Appl. Catal., B, 2009, 91, 135–143 CrossRef CAS.
- J. Yu, L. Qi, B. Cheng and X. Zhao, J. Hazard. Mater., 2008, 160, 621–628 CrossRef CAS PubMed.
- F. Ayachi, K. Saidi, M. Dammak, J. J. Carvajal and M. Cinta Pujol, RSC Adv., 2024, 14, 13494–13504 RSC.
- S. Ward, M. A. Isaacs, G. Gupta, M. Mamlouk and S. S. Pramana, Sustainable Energy Fuels, 2021, 5, 154–165 RSC.
- M. P. Selvam and K. J. Rao, Adv. Mater., 2000, 12, 1621–1624 CrossRef CAS.
- M. Song, W. Zhao, W. Ran, J. Xue, Y. Liu and J. H. Jeong, J. Alloys Compd., 2019, 803, 1063–1074 CrossRef CAS.
- L. Macalik, J. Hanuza and A. A. Kaminskii, J. Raman Spectrosc., 2002, 33, 92–103 CrossRef CAS.
- A. H. Ahmad and A. K. Arof, lonics, 2004, 10, 200–205 CAS.
- P. F. S. Pereira, I. C. Nogueira, E. Longo, E. J. Nassar, I. L. V. Rosa and L. S. Cavalcante, J. Rare Earths, 2015, 33, 113 CrossRef CAS.
- P. Nemec, B. Frumarová and M. Frumar, J. Non-Cryst. Solids, 2000, 270, 137 CrossRef CAS.
- M. Laroche, A. Braud, S. Girard, J. L. Doualan, R. Moncorge, M. Thuau and L. D. Merkle, J. Opt. Soc. Am. B, 1999, 16, 2269 CrossRef CAS.
- A. E. Morales, E. S. Mora and U. Pal, Rev. Mex. Fis. S, 2007, 53, 18 CAS.
- L. L. Noto, M. L. Chitambo, O. M. Ntwaeaborwa and H. C. Swart, Powder Technol., 2013, 247, 147–150 CrossRef CAS.
- J. A. Nelder and R. Mead, Comput. J., 1965, 7, 308 CrossRef.
- K. Rajnak, J. Chem. Phys., 1965, 43, 847 Search PubMed.
- K. M. Murdoch, N. M. Edelstein, L. A. Boatner and M. M. Abraham, J. Chem. Phys., 1996, 105, 2539 Search PubMed.
- M. L. Duan, X. F. Yang and J. H. Li, Adv. Mater. Res., 2011, 418–420, 665–669 Search PubMed.
- C. Basavapoornima, T. Maheswari, C. R. Kesavulu, W. Pecharapa, J. Kaewkhao and C. K. Jayasankar, AIP Conf. Proc., 2020, 2279, 060002 CrossRef.
- M. Runowskia, P. Woznya, I. R. Martínb, V. Lavínb and S. Lisa, J. Lumin., 2019, 214, 116571 Search PubMed.
- M. Fhoula, K. Saidi and M. Dammak, J. Alloys Compd., 2024, 979(5), 173537 CrossRef CAS.
- G. Blasse and B. C. Grabmaier, Luminescent Materials, Springer-Verlag, 1994 Search PubMed.
- D. L. Dexter and J. H. Schulman, J. Chem. Phys., 1954, 22, 1063–1070 Search PubMed.
- C. S. McCamy, Color Res. Appl., 1992, 17, 142–144 Search PubMed.
- I. E. Kolesnikov, A. A. Kalinichev, M. A. Kurochkin, D. V. Mamonova, E. Y. Kolesnikov and E. Lahderanta, J. Phys. Chem. C, 2019, 123, 5136–5143 CrossRef CAS.
- Y. Gao, F. Huang, H. Lin, J. Xu and Y. Wang, Sens. Actuators, B, 2017, 243, 137–143 CrossRef CAS.
- M. Sójka, C. D. S. Brites, L. D. Carlos and E. Zych, J. Mater. Chem. C, 2020, 8, 10086–10097 RSC.
- S. Kaur, H. Kaur, A. S. Rao and G. V. Prakash, Phys. B, 2024, 690, 416224 CrossRef CAS.
- S. Wang, S. Ma, G. Zhang, Z. Ye and X. Cheng, ACS Appl. Mater. Interfaces, 2019, 11, 42330–42338 CrossRef CAS PubMed.
- Y. Gao, Y. Cheng, T. Hu, Z. Ji, H. Lin, J. Xu and Y. Wang, J. Mater. Chem. C, 2018, 6, 11178–11183 RSC.
- H. Zhou, W. Gao, P. Cai, B. Zhang and S. Li, Solid State Sci., 2020, 104, 106283 Search PubMed.
- X. Tian, S. Xu, J. Wen, L. Zhu, C. Ji, Z. Huang, X. Wang, F. Luo, X. Liu, Y. Lu, J. Li, C. Li, Y. Peng, J. Cao and Z. He, Ceram. Int., 2023, 49, 27126–27137 CrossRef CAS.
- I. E. Kolesnikov, D. V. Mamonova, M. A. Kurochkin, E. Y. Kolesnikov and E. Lahderanta, ACS Appl. Nano Mater., 2021, 4, 1959–1966 CrossRef CAS.
- J. Stefanska and L. Marciniak, Adv. Photonics Res., 2021, 2, 2100070 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |