 Open Access Article
Open Access ArticleOnion peel derived carbon nanoparticles incorporated polysulfone membranes: enhanced dye removal from water†
Aman Sharma ab,
Soumi Datta
ab,
Soumi Datta c,
R. K. Sanjanab,
B. M. Poojab,
Suryasarathi Bose
c,
R. K. Sanjanab,
B. M. Poojab,
Suryasarathi Bose c and
Gurumurthy Hegde
c and
Gurumurthy Hegde *ab
*ab
aDepartment of Chemistry, School of Sciences, Christ University, Bengaluru, 560029, Karnataka, India. E-mail: murthyhegde@gmail.com
bCentre for Advanced Research and Development (CARD), Christ University, Bengaluru, 560029, Karnataka, India
cDepartment of Materials Engineering, Indian Institute of Science, Bengaluru, 560012, Karnataka, India
First published on 11th March 2025
Abstract
The ongoing discharge of hazardous dyes from industrial processes has intensified global water pollution, posing serious threats to aquatic ecosystems and human health. Addressing this challenge, our study explores the potential of bio-based carbon nanomaterials (CNM), synthesized from onion peel biowaste and designated as ON11, as effective agents in dye removal. These CNMs were incorporated into a mixed matrix membrane (MMM), using polysulfone (PSU) as the membrane substrate, to enhance dye adsorption. The CNM synthesis was achieved through a simple, eco-friendly process. We examined their impact on adsorption efficiency by introducing ON11 nanoparticles at varying concentrations into the PSU membrane (ON11@PSU). This CNM-embedded membrane structure offers a solution to challenges associated with the large-scale application of nanomaterials, particularly by minimizing leaching into water and improving durability. The ON11 and ON11@PSU membranes were characterized using various techniques, including SEM, Raman spectroscopy, XRD, optical profilometer, and FTIR, to confirm their behavior, morphology, and structural integrity. The surface area of ON11 was 423.26 m2 g−1, with BJH average pore diameter of 4.5 nm and BET pore volume of 0.26 cm3 g−1. ON11 nanoparticles were adsorptive in nature, and their utility in membrane adsorption is explored. The influence of parameters, including contact time, dye concentration, membrane thickness, pH, and adsorbent dosage, was systematically evaluated to optimize the dye adsorption efficiency of the ON11@PSU membrane pad. It was observed that the thickness of the 60 μm membrane (Sa = 2.170 μm and Sq = 2.75 μm) showed higher removal efficiency for all the selected dyes than the other thicknesses at the native pH itself. The MMM demonstrated its effectiveness as an adsorbent membrane, achieving maximum removal efficiencies of approximately 98% for MG dye, 92% for RhB dye, and 67% for MB dye. The negative zeta potential of adsorptive membranes enabled the electrostatic attraction of positively charged dyes, enhancing adsorption capacity. The findings contribute to developing sustainable and effective membrane utility as adsorbents, opening avenues for the effective use of agricultural waste products in environmental remediation applications.
1. Introduction
In recent years, the increasing global demand for textiles, pharmaceuticals, and various industrial processes has led to an alarming increase in the discharge of synthetic dyes into aquatic ecosystems. Water pollution caused by low utilization efficiency and uncontrollable discharge of organic dyes has greatly harmed the ecological environment and biological survival, resulting in a significant potable water deficit. In view of this, the global freshwater demand will be 40% higher than supply by 2030.1 The enduring presence of these dyes presents a major environmental hazard, underscoring the pressing need for sustainable and efficient wastewater treatment methods.2 Dyes like methylene blue (MB), malachite green (MG), and rhodamine B (RhB) pose a significant threat to the environment, humans, and aquatic organisms.3 Methylene blue is a cationic dye whose toxicity renders it hazardous to human health above a specific concentration.4,5 Long-term exposure to it may result in nausea, vomiting, anemia, and high blood pressure.6 Malachite green is a cationic, crystalline dye that is soluble in water and is highly utilized in the industrial and manufacturing sectors that produce silk, paint and pigment, food, textile dying, leather, paper, etc. It is carcinogenic, teratogenic, mutagenic, and persistent in nature. When ingested through water by humans, it damages the heart, kidneys, and organs.7,8 On the other hand, RhB is a red alkaline xanthene dye used extensively in food, paints, inks, fabrics, and pens because of its non-biodegradability, water solubility, and stability. Wastewater contaminates surface water, causing health and environmental problems such as respiratory, skin, and eye irritation, nervous system damage, and carcinogenic impacts.9,10Wastewater-containing dyes have been treated using a wide range of physical, chemical, and biological techniques, such as membrane filtration, adsorption, biosorption, coagulation/flocculation, advanced oxidation, photocatalytic degradation, phytoremediation, electrolytic treatment.6,11,12 Although these techniques have their own advantages and limitations. Adsorption, in contrast, has emerged as an alternative pollution detoxification method due to its low cost, effectiveness, sustainability, and convenience of use.7,9 Despite advances in particle adsorbents, their large-scale application for dye removal meets obstacles such as difficult separation and recovery, adsorbent loss, and issues with high-pressure drop and poor flow rate in packed-bed procedures.11 The separation process based on extraction requires a significant amount of solvents to remove organic contaminants. Membrane procedures provide an alternative to traditional separation approaches for overcoming this issue.13 Adsorptive membranes with adsorptive qualities have emerged, and research is being performed to increase dye rejection at low production and operational costs. Incorporating adsorbents into a polymer matrix of a substrate enhances dye rejection by capturing dye molecules through hydrogen bonding and electrostatic interactions, effectively preventing their passage through the membrane.14 Incorporating particulate adsorbents into separation membranes successfully solves the disadvantages of classical adsorbents. Classical adsorbents, such as powders or granules, often face limitations such as low reusability due to challenges in recovery and reuse, inefficient mass transfer caused by agglomeration or poor dispersibility in water, and high energy and cost requirements for post-adsorption separation processes like filtration or centrifugation. Incorporating particulate adsorbents, such as the Allium cepa peel-derived CNM in our study, into separation membranes successfully addresses these issues. The thin membrane walls (micron size) minimize packed bed height, increasing flow rates and minimizing pressure drops while removing the need for further adsorbent separation and recovery.11
Different materials, like Fe3O4/sulfonated graphene oxide (SGO),14 arginine arginine-modified montmorillonite,15 polyvinylpyrrolidone (PVP)–SiO2,16 etc., have been utilized to date in membranes for dye adsorption. Almost all parts of plant wastes could be used as adsorbents because they contain –COOH, –OH, or phenolic groups that could provide several types of bindings (e.g. charge interaction, hydrogen bonding, or hydrophobic interaction).17 Although there is no report to date on utilizing biomass-derived carbon nanospheres (CNS) into membranes for dye adsorption. Biomass-derived CNSs are mesoporous and have a spherical shape, making them a suitable candidate for dye degradation applications. CNSs have emerged as intriguing candidates for enhanced environmental remediation techniques due to their unique physicochemical features and abundance of surface functionalities.18 Also, the use of biowaste-derived nanomaterials helps to realize circular economy objectives as they allow the transformation of waste streams into high-added-value materials.19
The current study investigates a novel technique to address dye removal issues using carbon nanospheres generated from onion peels and embedding them in polysulfone (PSU) membranes. The PSU polymer was utilized for this study due to its excellent thermal and chemical stability, high mechanical strength, and ease of processing, which make it suitable for wastewater treatment applications. Additionally, PSU is cost-effective and widely available, offering an optimal balance of performance and scalability compared to higher-performance polymers. These factors align well with the objectives of our study and the practical feasibility of scaling up the developed membranes. Onion peels, an agricultural waste product, have received attention for their high carbon content and distinctive structural characteristics. Carbon nanospheres are derived from onion peels through a sustainable and cost-effective synthesis process, presenting a green, eco-friendly alternative for environmental applications.20 By integrating onion peel-derived carbon nanospheres into membranes, the adsorption parameter for the removal of synthetic dyes in wastewater is evaluated. Membrane-based technologies are flexible and can be synthesized on a large scale for water treatment purposes, and such treatment is efficient and has a low environmental impact.21 This work examines the combination of carbon nanospheres and membranes to increase dye adsorption, assist in the separation, and enable the breakdown of difficult dye molecules. It is envisaged that the present study will provide useful information on the advancement of wastewater treatment systems in an eco-friendly and sustainable manner to reduce the adverse impact of synthetic dyes on the environment.
2. Experimental section
2.1. Materials and reagents
The raw material precursor-Allium cepa (Onion) peels was collected from southern India (Karnataka) to synthesize CNM. The dried peels were ground to a fine powder using a Bajaj Rex mixer grinder. The tube furnace from NoPo Technologies was used for the pyrolysis process. Methylene blue (MB), malachite green (MG), and rhodamine b (RhB) dyes were procured from Sigma-Aldrich. Sodium hydroxide (NaOH) from CDH and hydrochloric acid (HCl) were procured from Thomas Baker. N-Methyl-2-pyrrolidone (NMP) was used as a solvent from Finar Pvt. Ltd. For the recyclability test, ethanol was used as a solvent. Polysulfone (PSU) was used as a membrane polymer procured from Sigma-Aldrich. Millipore water from Milli-Q, Progard® TS2 was used for all the studies.2.2. Synthesis of matrix mixed membrane pad (ON11@PSU-pad)
| CNS dosage in membrane (mg) | PSU (wt%) | NMP (wt%) | ON11 (wt%) | Refer as |
|---|---|---|---|---|
| — | 19.53 | 80.46 | — | PSU |
| 5 | 19.53 | 80.45 | 0.024 | ON11-5@PSU |
| 10 | 19.52 | 80.43 | 0.048 | ON11-10@PSU |
| 15 | 19.51 | 80.41 | 0.073 | ON11-15@PSU |
2.3. Materials characterization techniques
The morphology of ON11 and ON11@PSU membranes was analyzed using Scanning Electron Microscopy (Apreo 2S Thermoscientific and Zeiss). The surface functionalities were analyzed using the Fourier Transform Infrared Spectroscopy (FTIR) technique using Shimadzu IRSpirit. Raman spectra were analysed using a Renishaw inVia Raman microscope for information about the crystalline structure. Using a MiniFlex 600 from Rigaku Corporation, X-ray diffraction (XRD) patterns are used to assess the crystallinity of material. Brunauer–Emmett–Teller (BET) method was used to estimate surface area and pore size distribution using BELSORP MINI X, Japan at 77K N2 adsorption. An optical profilometer was used to calculate the surface roughness and topology of the ON11@PSU-pad membranes. The zeta potential of the ON11 was analyzed by Malvern Zetasiezer ZEN3600 Particle Analyzer. The absorbance of the dye concentration was noted using a UV-visible spectrophotometer (Ocean Optics).2.4. Dye adsorption experiments
The membranes were cut into standard-sized tokens to check the dye removal efficiency. One parameter was modified to assess the impact of other factors, including membrane thickness (30–120 μm), adsorbent dosage (0.024–0.073 wt%), contact period (10–70 min), and dye concentration (2.5–7.5 ppm). The rest of the parameters were considered constant. The effects of contact time, pH, membrane thickness, dye concentration, and nanoparticle dosage on dye adsorption were studied in detail. The studies were carried out in three trials, and the average error percentage is shown. Furthermore, the optimized parameters from the previous stage were used to study the effect of another parameter. The experiment was carried out in a beaker with a constant stirring rate, keeping the token membrane at the bottom. The physicochemical characteristics of the selected dyes is mentioned in Table 2. The absorbance before and after the contact time is checked using a UV-vis spectrophotometer, and the removal efficiency is calculated using eqn (1).
 | (1) |
Pseudo-first and pseudo-second order kinetic studies were performed and these models were fitted using linear square fitting in the Origin Pro 9.0 64-bit software.
3. Results and discussion
3.1. Physiochemical characterization of ON11 and ON11@PSU
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching and bending vibrations caused by carboxylic acid and alkene, respectively.
C stretching and bending vibrations caused by carboxylic acid and alkene, respectively.
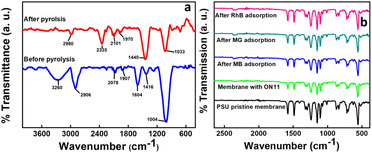 | ||
| Fig. 2 FTIR analysis of (a) ON11 CNS before and after pyrolysis, (b) ON11-10@PSU-pad before and after adsorption of respective dyes. | ||
After pyrolysis, the band at 2980 cm−1 corresponds to the hydroxyl group present. The stretching vibrations at 2335 cm−1 are caused by ambient CO2 adsorbed on the nanoparticles.26 Alkyne and allene (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) groups are represented by the 2101 and 1970 cm−1 bands, respectively. The band at 1440 cm−1 represents the methylene (C–H) group attached to the synthesized carbon nanospheres. The decrease in intensity is attributed to the degradation of the precursor during pyrolysis.27
C) groups are represented by the 2101 and 1970 cm−1 bands, respectively. The band at 1440 cm−1 represents the methylene (C–H) group attached to the synthesized carbon nanospheres. The decrease in intensity is attributed to the degradation of the precursor during pyrolysis.27
In the FTIR spectrum of the ON11@PSU membrane, as depicted in Fig. 2b, the peak at 1239 cm−1 is attributed to the stretching vibration of the C–O–C groups. The skeletal vibrations of aromatic hydrocarbons are associated with the peaks at 1484 and 1576 cm−1, while the symmetric and asymmetric stretching of sulfone groups are associated with the peaks at 1153 and 1325 cm−1. The peak at 560 cm−1 corresponds to a strong halogen bond (Cl−), and the peak at 1107 cm−1 to medium C–N stretching.28
 | ||
| Fig. 3 XRD analysis of (a) ON11, (b) ON11-10@PSU-pad before and after dye adsorption, and (c) Raman spectra of ON11. | ||
The XRD pattern of ON11-10@PSU membrane before and after dye adsorption is shown in Fig. 3b. The decrease in the peak intensity is observed after the dye adsorption, without any other significant changes in the pattern. The outcome implies that the dye-adsorbed carbon nanospheres might not induce the bulk phase shifts.29 It suggests that the membrane is amorphous. Additionally, the Raman spectrum (Fig. 3c) reveals the presence of a D-band, indicating structural defects and disorder within ON11, while the presence of a G-band confirms the sp2-hybridized carbon network characteristic of its graphitic structure. The intensity ratio of these bands was measured at 0.84, with the G-band observed at 1598 cm−1 and the D band at 1349 cm−1.30,31
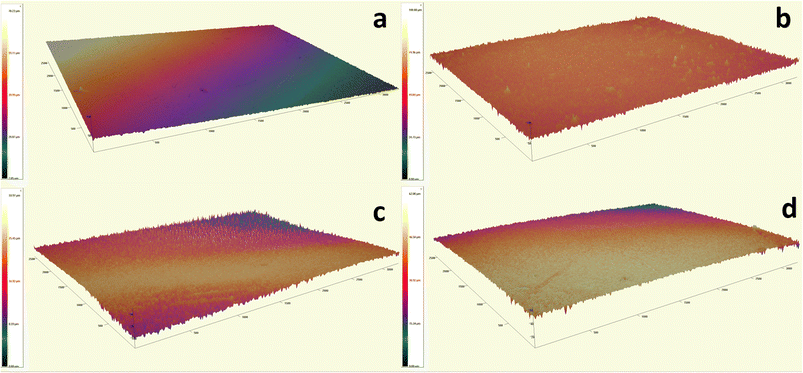 | ||
| Fig. 4 Optical Profilometry 3D-image of ON11-10@PSU pad at different thickness (a) 30 μm, (b) 60 μm, (c) 90 μm, and (d) 120 μm. | ||
Using an optical profilometer, surface roughness can be calculated, which is the average roughness (Sa) that is defined as the average height of all measured locations in the measurement.32 Eqn (2) contains the formula for calculating it.
 | (2) |
3.2. Application of ON11@PSU-pad in organic pollutant adsorption
 | ||
| Fig. 5 (a) Comparison of different dosages of ON11 as adsorbent itself and in membranes; (b) contact time studies of cationic dye removal with ON11-10@PSU-pad; (c) recyclability test using MG dye. | ||
| Dye | Polymer | Carbon type | Method | Removal efficiency (%) | Time (min) | Ref. |
|---|---|---|---|---|---|---|
| RhB | PSU + PVP (DMF solvent) | CNS synthesized hydrothermally | Ultrafiltration | 81.5 | — | 35 |
| CR | PSU (DMF solvent) | 30% activated carbon | Ultrafiltration | 99.5 | 360 | 36 |
| RhB | PSU | Aluminium and gallium hybrid | Ultrafiltration | 80 | — | 37 |
| MB | PSU | Activated carbon spheres (resorcinol and formaldehyde) | — | 99.9 | — | 38 |
| RhB | Polydopamine (hexane solvent) | Silk nanofibrils-MOF composite | Adsorption | 99.5 | — | 39 |
| MB | PSU (NMP solvent) | CNS from biowaste synthesized via pyrolysis | Adsorption | 67 | 60 | This work |
| MG | 98 | |||||
| RhB | 92 |
The membrane adsorption of dyes may be mediated by chemical bonding or interactions, such as electrostatic forces or hydrogen bonding, which make it less susceptible to the surface area offered by ON11 nanoparticles. As observed from the data above, the higher ON11 dosage shows a high removal efficiency of the dye as it provides more absorption sites.53–56 This trend is consistent with findings from adsorption experiments, where increasing adsorbent dosage results in a bigger surface area and more active sites for dye molecules to attach.57
3.3. Adsorption kinetic studies of ON11@PSU-pad
Adsorption kinetics assesses the rate at which an adsorbate is absorbed over time at a given concentration, providing insights into the diffusion of the adsorbate into the pores and suggesting a potential sorption mechanism.19 The pseudo-first-order (PFO) model, the linearized expression of the Lagergren pseudo-first-order rate equation,64 is represented as eqn (3):
 | (3) |
 | ||
| Fig. 7 (a) Pseudo-first-order and (b) pseudo-second-order kinetics for ON11-10@PSU-pad dye adsorption. | ||
| Kinetic models | MB | MG | RhB | |
|---|---|---|---|---|
| PFO | k1 (min−1) | 0.046 | 0.0467 | 0.0062 |
| qe (mg g−1) | 65.831 | 61.384 | 28.383 | |
| R2 | 0.78 | 0.77 | 0.59 | |
| PSO | k2 (g mg−1 min−1) | 2.8 × 10−4 | 1.18 × 10−4 | 4.05 × 10−5 |
| qe (mg g−1) | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063.6 063.6 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 889.53 889.53 |
3902.82 | |
| R2 | 0.99 | 0.99 | 0.99 | |
According to the pseudo-second-order (PSO) kinetic model, the adsorption occurs at two surface sites.65 The linear fit model of PSO kinetics can be given in eqn (4):
 | (4) |
This kinetics indicates a heterogeneous adsorbent surface, where different active sites have different affinities for the adsorbate. This is based on the assumption that the rate-limiting step could involve chemisorption, where valency forces come into play by sharing or exchanging electrons between the sorbent and sorbate.66
3.4. Reusability of ON11@PSU-pad
The reusability of ON11@PSU-pad was evaluated using MG dye over three cycles, as seen in Fig. 5c. For the recyclability test, membranes were submerged in ethanol and stirred for 10–15 minutes or until the dye desorption occurred. This method desorbed MG dye in ethanol, enabling the membrane to be regenerated and recycled.67 The removal efficiency remained consistent (∼90%) across the cycles. However, the structural integrity of the membrane diminished with subsequent reuse, primarily due to disintegration caused by repeated washing (refer to ESI Fig. S8† for recyclability test effect on structural integrity of membrane pad).The membrane pads can be utilized in modular treatment systems, such as layered filter systems, allowing for easy replacement and reusability. Our study demonstrated the recyclability of ON11@PSU-pad across multiple cycles, highlighting its cost-effectiveness and sustainability for repeated use. While this study is focused on cationic dyes, future functionalization of the membrane or testing with mixed dye systems could enable selective adsorption capabilities, enhancing its use in diverse wastewater streams. Beyond dye removal, the membranes could be tailored for selective adsorption of other pollutants, including heavy metals, pharmaceuticals, or pesticides, by modifying the carbon nanospheres or polymer matrix.
The PSU matrix used in ON11@PSU membranes is a non-biodegradable polymer; however, the integration of bio-based ON11 enhances the sustainability of the composite by utilizing biowaste, thereby aligning with circular economy principles. Using green solvents, natural additives, or bio-sourced materials during membrane fabrication could improve their eco-friendliness and biodegradability.68,69 While PSU was selected for its superior mechanical strength, thermal stability, and compatibility with ON11, future research will focus on incorporating ON11 into biodegradable polymer matrices. This approach aims to enhance the environmental sustainability of the membranes without compromising their performance. Additionally, strategies for recycling or repurposing PSU-based membranes after their functional lifetime could further mitigate environmental impact.
3.5. Dye adsorption mechanism on ON11@PSU-pad
The dye removal mechanism using the ON11@PSU-pad membrane pad is mainly based on the adsorption phenomenon. The surface charge of the membrane and its pore surface greatly depends on the adsorption performance.70,71 The zeta potential of carbon nanomaterial (ON11) is around −19.6 mV (refer to ESI Fig. S9†), and the PSU membrane72 surface is about −18 mV. The combined impact of these two significantly improves the total ON11@PSU-pad membrane surface and pore charge. SEM examination also shows that the nanomaterial is effectively dispersed across the membrane and pore surfaces (refer to ESI Fig. S10† for N2 adsorption–desorption curve and pore size distribution of ON11). The surface area, average pore diameter, and pore volume calculated from BET were 423.26 m2 g−1, 1.26 nm, and 0.26 cm3 g−1, respectively. BJH pore diameter was around 4.5 nm aligning with the mesoporous nature of the ON11. Adsorption occurs when the dye comes into contact with the negatively charged membrane surface, as the dye pollutant used in this investigation is cationic (MB, MG, RhB). This oppositely charged dye and membrane strengthened the electrostatic attraction between these two, primarily facilitating the adsorption procedure.73,74 For MB, MG, and RhB, the dye removal efficiency using the membrane pad is 67 ± 5%, 98 ± 3%, and 92 ± 3%, respectively, at 60 min contact time using 60 μm ON11-10@PSU-pad in native pH for 2.5 ppm concentration.A graphical representation is described in Fig. 8 to better understand the possible dye adsorption mechanism using the ON11@PSU membrane pad. In addition to electrostatic interactions, the dye adsorption mechanism involves hydrogen bonding, van der Waals forces, and π–π stacking interactions19,61 between the dye molecules and membrane surface. The selectivity of the prepared ON11@PSU pads towards different dyes depends on factors like pore size distribution and molecular sieving effect, polymer filler interaction and transport pathways, adsorption-dominated separation, and molecular interactions. The high surface area of the ON11 with oxygen-containing functional groups causes π–π interactions, enabling strong adsorption with dye molecules. This, in turn, enhances the retention of cationic dyes due to the electrostatic attraction with negatively charged ON11 and ON11@PSU membranes. The interfacial voids are created in the PSU membrane due to the dispersion of porous fillers, which visualizes improved permeability with controlled pore connectivity. The pore structure of the ON11 CNS incorporated into the PSU membrane plays a crucial role in dye adsorption. BET analysis revealed a mesoporous framework ranging from 2 to 10 nm (refer to ESI Fig. S10b†), which is well-suited for the molecular dimensions of MG (∼1.8 nm), RhB (∼1.5–1.7 nm), and MB (∼1.43 nm). These mesopores facilitate efficient dye diffusion and adsorption, while micropores may enhance surface interactions. The high removal efficiency of MG and RhB indicates stronger π–π stacking and electrostatic attractions, whereas MB likely exhibits weaker adsorption and faster diffusion, possibly due to its lower hydrophobic affinity. All of the mechanisms involved in the adsorption process confirmed the efficient adsorption of dye using ON11@PSU-pad from water as soon as it became immersed in the polluted water. Additionally, this membrane-based adsorption process has several advantages over solely using nanomaterials for adsorption. Nanomaterials have certain drawbacks, such as the difficulty of removing spent nanomaterials after water treatment, despite the fact that it is effective. However, the incorporation of nanomaterials into the membrane to create a MMM helps to hold the nanomaterial without compromising its adsorption effectiveness by providing a large surface area for contact with dye, and removal is also simple and feasible for reusability after treatment (see reusability study). These benefits prompted us to create a membrane pad that would more effectively and hassle-free adsorb the water contaminant.
4. Conclusion and future perspectives
In this study, we have developed a bio-based, onion peel-derived nanomaterial ON11-incorporated mixed matrix membrane (ON11@PSU) that demonstrates high efficacy in adsorbing cationic dyes like MB, MG, and RhB. This composite membrane proves to be a promising candidate for wastewater treatment. Incorporating Allium cepa-derived carbon nanospheres significantly enhanced the adsorption efficiency, with optimal dye removal achieved at a membrane thickness of 60 μm. The dye removal efficiency was evaluated by varying membrane thickness, layer of membrane pad, dye concentration, ON11 dosage, contact time, and solution pH. The 60 μm membranes exhibited enhanced adsorption, with maximum removal efficiencies of 67 ± 5% for MB, 98 ± 3% for MG, and 92 ± 3% for RhB at 2.5 ppm dye concentrations. By increasing the number of membrane pads, the removal efficiency also increases as surface area increases. Kinetic studies suggest that the adsorption process follows a pseudo-second-order model, indicating a chemisorption-driven mechanism. The membrane maintained strong performance through several reuse cycles, though further material refinement is needed to improve its structural stability for long-term applications.The limitations of this study include the diminished structural stability of ON11@PSU after repeated use as the physical wear resistance of the membrane needs improvement to enhance durability for long-term applications. Challenges in scaling up production using eco-friendly methods and the use of a non-biodegradable polymer matrix is required for future material optimization. Future work can focus on stability enhancement and expanding its application in mixed system of dyes and strengthening the membranes for long term applications. Furthermore, our future research will lead to the scaling up of membrane production utilizing environmentally friendly, cost-effective processes for real-world applications. This work aligns with circular economy concepts and provides a promising direction for sustainable water treatment technologies using bio-waste.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Aman Sharma: conceptualization, investigation, methodology, data curation, formal analysis, writing—draft manuscript, visualization, writing—original draft, revision of manuscript and editing, writing—review and editing. Soumi Datta: revision of manuscript and editing, visualization, formal analysis, reviewing. R. K. Sanjana: data curation, investigation. B. M. Pooja: data curation, investigation. Suryasarathi Bose: reviewing, validation, and final approval of the manuscript to be published. Gurumurthy Hegde: revision of manuscript and editing, supervision, validation, formal analysis, reviewing, and final approval of the manuscript to be published.Conflicts of interest
There are no conflicts to declare.References
- S. S. Vedula and G. D. Yadav, J. Indian Chem. Soc., 2022, 99, 100263 CrossRef CAS.
- J. Fang, Y. Chen, C. Fang and L. Zhu, Sep. Purif. Technol., 2022, 281, 119876 CrossRef CAS.
- A. Sharma, S. Sunny, J. Arulraj and G. Hegde, Nano Express, 2024, 5, 022002 CrossRef CAS.
- J. Cheng, C. Zhan, J. Wu, Z. Cui, J. Si, Q. Wang, X. Peng and L.-S. Turng, ACS Omega, 2020, 5, 5389–5400 CrossRef CAS PubMed.
- I. Khan, K. Saeed, I. Zekker, B. Zhang, A. H. Hendi, A. Ahmad, S. Ahmad, N. Zada, H. Ahmad, L. A. Shah, T. Shah and I. Khan, Water, 2022, 14, 242 CrossRef CAS.
- D. Pathania, S. Sharma and P. Singh, Arabian J. Chem., 2017, 10, S1445–S1451 CrossRef CAS.
- M. Abewaa, A. Mengistu, T. Takele, J. Fito and T. Nkambule, Sci. Rep., 2023, 13, 14701 CrossRef CAS PubMed.
- S. Srivastava, R. Sinha and D. Roy, Aquat. Toxicol., 2004, 66, 319–329 CrossRef CAS PubMed.
- A. Bazan-Wozniak, A. Jędrzejczak, R. Wolski, S. Kaczmarek, A. Nosal-Wiercińska, J. Cielecka-Piontek, S. Yagmur-Kabas and R. Pietrzak, Molecules, 2024, 29, 1412 CrossRef CAS PubMed.
- J. Sharma, S. Sharma, U. Bhatt and V. Soni, J. Hazard. Mater. Lett., 2022, 3, 100069 CrossRef CAS.
- H. Li, T. Li, W. Shi, Y. Tian, J. Liu and X. Qin, React. Funct. Polym., 2022, 170, 105135 CrossRef CAS.
- D. Teng, Y. Xu, T. Zhao, X. Zhang, Y. Li and Y. Zeng, J. Hazard. Mater., 2022, 425, 128004 CrossRef CAS PubMed.
- N. Benosmane, B. Boutemeur, S. M. Hamdi and M. Hamdi, Appl. Water Sci., 2022, 12, 104 CrossRef CAS.
- Y. Kang, J. Jang, Y. Lee and I. S. Kim, Desalination, 2022, 524, 115462 CrossRef CAS.
- E. Shokri, R. Yegani and A. Akbarzadeh, Appl. Clay Sci., 2017, 144, 141–149 CrossRef CAS.
- W. Zhu, Z. Wu, S. Zhao, F. Lv, Y. Zhang and S. Guo, Chem. Eng. Sci., 2023, 280, 119009 CrossRef CAS.
- C.-H. Lin, C.-H. Gung, J.-Y. Wu and S.-Y. Suen, J. Taiwan Inst. Chem. Eng., 2015, 51, 119–126 CrossRef CAS.
- P. M. Thabede, Appl. Sci., 2023, 13, 10511 CrossRef CAS.
- A. Sharma, J. M. Shivanna, A. N. Alodhayb and G. Hegde, Nanoscale Adv., 2024, 6, 3199–3210 RSC.
- B. Krishnappa, S. Saravu, J. M. Shivanna, M. Naik and G. Hegde, Environ. Sci. Pollut. Res. Int., 2022, 29, 79067–79081 CrossRef CAS PubMed.
- V. Molahalli, A. Shetty, A. Sharma, K. Bijapur, G. Soman and G. Hegde, in Nanoparticles and Plant-Microbe Interactions, Elsevier, 2023, pp. 35–68 Search PubMed.
- S. Supriya, G. Sriram, Z. Ngaini, C. Kavitha, M. Kurkuri, I. P. De Padova and G. Hegde, Waste Biomass Valorization, 2019, 11, 3821–3831 CrossRef.
- H. Yang, R. Yan, H. Chen, D. H. Lee and C. Zheng, Fuel, 2007, 86, 1781–1788 CrossRef CAS.
- M. S. Jyothi, M. Padaki, R. G. Balakrishna and R. K. Pai, J. Mater. Res., 2014, 29, 1537–1545 CrossRef CAS.
- H. L. Richards, P. G. L. Baker and E. Iwuoha, J. Surf. Eng. Mater. Adv. Technol., 2012, 02, 183–193 CAS.
- S. Modi, V. K. Yadav, N. Choudhary, A. M. Alswieleh, A. K. Sharma, A. K. Bhardwaj, S. H. Khan, K. K. Yadav, J.-K. Cheon and B.-H. Jeon, Materials, 2022, 15, 2393 CrossRef CAS PubMed.
- R.-F. Segundo, M. De La Cruz-Noriega, N. Milly Otiniano, S. M. Benites, M. Esparza and R. Nazario-Naveda, Molecules, 2022, 27, 625 CrossRef CAS PubMed.
- A. M. Alosaimi, Polymers, 2021, 13, 2792 CrossRef CAS PubMed.
- M. Arulkumar, P. Sathishkumar and T. Palvannan, J. Hazard. Mater., 2011, 186, 827–834 Search PubMed.
- V. S. Bhat, S. G. Krishnan, T. J. Jayeoye, T. Rujiralai, U. Sirimahachai, R. Viswanatha, M. Khalid and G. Hegde, J. Mater. Sci., 2021, 56, 13271–13290 CrossRef CAS.
- V. S. Bhat, S. Supriya and G. Hegde, J. Electrochem. Soc., 2019, 167, 037526 CrossRef.
- L. Mei and G. Guan, Nano TransMed, 2023, 2, e9130017 CrossRef.
- F.-G. Tseng, D. Bhalothia, K.-H. Lo, C.-H. Syu, Y.-C. Chen, A. Sihag, C.-W. Wang, H.-Y. T. Chen and T.-Y. Chen, Energy Adv., 2024, 3, 1283–1292 RSC.
- X. Zhang, X. Wang, T. Cheng and Q. Lin, J. Mol. Liq., 2023, 390, 123091 CrossRef CAS.
- H. M. Abbas, R. M. Kamel, M. E. A. Ali, A. H. Atta, H. M. A. Hassan and A. Shahat, Desalin. Water Treat., 2020, 193, 57–63 CrossRef CAS.
- P. G. Ingole, S. Y. Sawant, N. P. Ingole, R. R. Pawar, H. C. Bajaj, K. Singh, M. H. Cho and H. K. Lee, Membr. Water Treat., 2016, 7, 477–493 CrossRef.
- K. Parveen, U. Rafique and M. J. Akhtar, J. Environ. Health Sci. Eng., 2022, 20, 757–774 CrossRef CAS PubMed.
- M. Manoukian, H. Fashandi and H. Tavakol, Mater. Res. Express, 2019, 6, 055313 CrossRef CAS.
- X. Zhao, C. Wu, D. Dai, J. Ren, T. Li and S. Ling, iScience, 2023, 26, 107290 CrossRef CAS PubMed.
- E. S. Mansor, H. Ali and A. Abdel-Karim, Colloid Interface Sci. Commun., 2020, 39, 100314 CrossRef CAS.
- L. Zhang, H. Zhang, W. Guo and Y. Tian, Appl. Clay Sci., 2014, 94, 85–93 CrossRef.
- M. Farooq, S. Shujah, K. Tahir, S. Nazir, A. Ullah Khan, Z. M. Almarhoon, V. Jevtovic, H. S. Al-Shehri, S. Tasleem Hussain and A. Ullah, Inorg. Chem. Commun., 2022, 136, 109189 Search PubMed.
- J. Hou, Y. Chen, W. Shi, C. Bao and X. Hu, Appl. Surf. Sci., 2020, 505, 144145 CrossRef CAS.
- G. Yang, H. Gao, Q. Li and S. Ren, RSC Adv., 2021, 11, 15921–15926 RSC.
- G. L. Kyriakopoulos, K. Tsimnadis, I. Sebos and Y. Charabi, Crystals, 2024, 14, 742 CrossRef CAS.
- S. Dutta, B. Gupta, S. K. Srivastava and A. K. Gupta, Mater. Adv., 2021, 2, 4497–4531 Search PubMed.
- Z. Eren and F. N. Acar, Desalination, 2006, 194, 1–10 Search PubMed.
- E. Rápó and S. Tonk, Molecules, 2021, 26, 5419 CrossRef PubMed.
- C. Tejada-Tovar, Á. Villabona-Ortíz and Á. D. Gonzalez-Delgado, Water, 2021, 13, 1382 CrossRef CAS.
- N. K. Mondal and S. Kar, Appl. Water Sci., 2018, 8, 157 CrossRef.
- A. Khodabandehloo, A. Rahbar-Kelishami and H. Shayesteh, J. Mol. Liq., 2017, 244, 540–548 CrossRef CAS.
- A. E. Ofomaja, Biochem. Eng. J., 2008, 40, 8–18 Search PubMed.
- M. Naghizade Asl, N. M. Mahmodi, P. Teymouri, B. Shahmoradi, R. Rezaee and A. Maleki, Desalin. Water Treat., 2016, 57, 25278–25287 CrossRef CAS.
- C. Osagie, A. Othmani, S. Ghosh, A. Malloum, Z. Kashitarash Esfahani and S. Ahmadi, J. Mater. Res. Technol., 2021, 14, 2195–2218 Search PubMed.
- B. P. Chang, A. Gupta and T. H. Mekonnen, Chemosphere, 2021, 282, 131062 CrossRef CAS PubMed.
- L. Ceroni, S. Benazzato, S. Pressi, L. Calvillo, E. Marotta and E. Menna, Nanomaterials, 2024, 14, 522 Search PubMed.
- M. Saghanejhad Tehrani and R. Zare-Dorabei, RSC Adv., 2016, 6, 27416–27425 RSC.
- S. Lu, Y. Yang, X. Su, K. Yu and X. Wang, Sustainability: Sci. Pract. Policy, 2022, 14, 10935 CrossRef CAS.
- Y. Zhou, J. Lu, Y. Zhou and Y. Liu, Environ. Pollut., 2019, 252, 352–365 CrossRef CAS PubMed.
- S. N. Taqui, C. S. Mohan, B. A. Khatoon, M. E. M. Soudagar, T. M. Y. Khan, M. A. Mujtaba, W. Ahmed, A. Elfasakhany, R. Kumar and C. I. Pruncu, Biomass Convers. Biorefin., 2023, 13, 9119–9130 CrossRef CAS.
- A. A. E. A. Elfiky, M. F. Mubarak, M. Keshawy, I. E. T. E. Sayed and T. A. Moghny, Environ. Sci. Pollut. Res. Int., 2023, 30, 79091–79105 CrossRef CAS PubMed.
- S. T. Kadhum, G. Y. Alkindi and T. M. Albayati, Desalin. Water Treat., 2021, 231, 44–53 CrossRef CAS.
- A. R. Fischer, P. Werner and K.-U. Goss, Chemosphere, 2011, 82, 210–214 CrossRef CAS PubMed.
- M. I. Khan, S. Akhtar, S. Zafar, A. Shaheen, M. A. Khan, R. Luque and A. U. Rehman, Materials, 2015, 8, 4147–4161 CrossRef CAS PubMed.
- B. Baheri, R. Ghahremani, M. Peydayesh, M. Shahverdi and T. Mohammadi, Res. Chem. Intermed., 2016, 42, 5309–5328 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- J. Saleem, Z. K. B. Moghal, S. Pradhan, A. Hafeez, M. Shoaib, J. Alahmad and G. McKay, Polymers, 2024, 16, 1459 CrossRef CAS PubMed.
- Y. X. Foong, L. H. Yew and P. V. Chai, Mater. Today, 2021, 46, 2092–2097 Search PubMed.
- M. Ehsani, D. Kalugin, H. Doan, A. Lohi and A. Abdelrasoul, Appl. Sci., 2022, 12, 12837 CrossRef CAS.
- S. Dutta, A. Misra and S. Bose, Nanoscale, 2024, 16, 5188–5205 RSC.
- M. Elimelech, W. H. Chen and J. J. Waypa, Desalination, 1994, 95, 269–286 CrossRef CAS.
- P. Van der Meeren, H. Saveyn, S. Bogale Kassa, W. Doyen and R. Leysen, Phys. Chem. Chem. Phys., 2004, 6, 1408–1412 RSC.
- S. Dutta, R. Sen Gupta, K. Manna, S. Safikul Islam and S. Bose, Chem. Eng. J., 2023, 472, 145008 CrossRef CAS.
- S. Dutta, B. G. M. Patel, Y. Singh, G. Hegde and S. Bose, J. Membr. Sci., 2024, 694, 122422 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra00025d |
| This journal is © The Royal Society of Chemistry 2025 |







