DOI:
10.1039/D4RA09048A
(Review Article)
RSC Adv., 2025,
15, 12594-12608
Structural diversity and biological activities of terpenoids derived from Tripterygium wilfordii Hook. f.
Received
28th December 2024
, Accepted 5th April 2025
First published on 22nd April 2025
Abstract
Terpenoids, a heterogeneous group of natural products, have garnered considerable attention in the field of drug discovery. This is attributed to their vast diversity, intricate structural features, and extensive biological activities. Tripterygium wilfordii Hook. f., a traditional medicinal plant with widespread application in East Asia, is particularly enriched in terpenoids, which can be classified into sesquiterpenoids, diterpenoids, and triterpenoids. The present review provides a comprehensive elaboration of the chemical structures and biological activities of 217 terpenoids isolated from T. wilfordii. The purpose is to shed light on their potential in pharmacological research and to stimulate innovative drug discovery as well as clinical applications. These terpenoids display a broad spectrum of biological activities, such as antitumor, anti – inflammatory, immunosuppressive, and other therapeutic effects. Nevertheless, their clinical application is impeded by issues related to toxicity and poor bioavailability. Future research efforts should be concentrated on exploring effective strategies to alleviate toxicity and enhance drug delivery systems. In addition, in – depth investigation into the structure–activity relationships and the identification of new active constituents are crucial for the development of more potent and safer drugs. This review serves as an exhaustive reference for the discovery and development of novel drugs based on the natural active products of T. wilfordii, providing valuable insights and guidance for researchers in the relevant field.
Introduction
Tripterygium wilfordii Hook. f. (T. wilfordii), a liana plant belonging to the family Celastraceae, is predominantly distributed throughout East Asia, particularly in China.1 The roots, leaves, flowers, and fruits of T. wilfordii have been extensively utilized in the treatment of autoimmune and inflammatory diseases in China for decades, attributed to their traditional Chinese medicinal properties of being cold in nature, bitter and acrid in taste, distributed in the liver and kidney channels, and also employed as an insecticide.2 In recent years, terpenoids derived from T. wilfordii have garnered significant attention due to their diverse biological activities, including antitumor, anti-inflammatory, and immunosuppressive effects. It is noteworthy that the biological activities of terpenoids are closely related to their structural characteristics. The structural diversity of terpenoids is primarily characterized by the number of isoprene units and the presence or absence of nitrogen atoms. Sesquiterpenoids, diterpenoids, and triterpenoids are the main types of terpenoids found in T. wilfordii, each with distinct structural features and biological activities. The α,β-unsaturated lactone ring of diterpenoids, such as triptolide, is considered a key pharmacophore for their antitumor activity.3 The quinone structure of triterpenoids, such as celastrol, is directly associated with antioxidant and anti-inflammatory effects.4 Understanding the relationship between the structure and biological activity of these terpenoids is crucial for the development of new drugs and therapeutic agents. The structural diversity of terpenoids from T. wilfordii has been extensively studied to date.5 These compounds include sesquiterpenoids, diterpenoids, triterpenoids, flavonoids, lignans, steroids, and others, each contributing to the plant's medicinal properties.4 In this review article, we concentrate on the chemical structure and bioactivity of 217 terpenoids derived from T.wilfordii. We hope this article can provide a comprehensive overview and reference for drug development (Scheme 1).
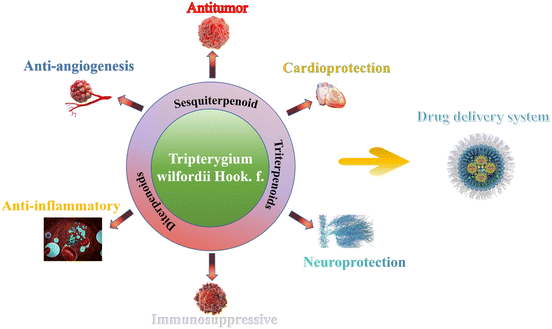 |
| | Scheme 1 Graphic illustration of biological activities of terpenoids derived from T. wilfordii. | |
The chemical constituents of T. wilfordii
Extensive research has been conducted on the root, leaves, flowers, and fruits of T. wilfordii. These compounds can be categorized into various types, including sesquiterpenoids, diterpenoids, triterpenoids, flavonoids, lignans, steroids, and others.5 In this section, we provide a comprehensive summary of the names, chemical structures, and subtypes of terpenoids that serve as the major constituents of T. wilfordii, highlighting their potential roles in the plant's medicinal properties.
Sesquiterpenoids isolated from T. wilfordii are primarily categorized into two groups: nitrogen-containing sesquiterpenoids (compounds 1–50, 51–79) and nitrogen-free sesquiterpenoids (compounds 80–102).6 The structural types of diterpenoids encompass abietane-type, kaurane-type, and other subtypes (compounds 103–150, 151–185).7 Triterpenoids, based on their carbon skeleton and substituents, predominantly belong to the friedelane type, oleanane type, ursane type, and related groups (compounds 186–217).8 The relevant information and chemical structures of terpenoids derived from T. wilfordii are depicted in Fig. 1 and Table 1.
 |
| | Fig. 1 The chemical structures of diterpenoids derived from T. wilfordii. | |
Table 1 Terpenoids isolated from T. wilfordii
| No |
Compounds |
Type |
Subtype |
Ref. |
| 1 |
Tripfordine A |
Sesquiterpenoid |
Nitrogen-containing |
9 |
| 2 |
Tripfordine B |
Sesquiterpenoid |
Nitrogen-containing |
9 |
| 3 |
Wilforine |
Sesquiterpenoid |
Nitrogen-containing |
9 and 10 |
| 4 |
Wilforgine |
Sesquiterpenoid |
Nitrogen-containing |
9 and 10 |
| 5 |
Wilfordine |
Sesquiterpenoid |
Nitrogen-containing |
11 and 12 |
| 6 |
Wilfortrine |
Sesquiterpenoid |
Nitrogen-containing |
11 and 12 |
| 7 |
Tripfordine C |
Sesquiterpenoid |
Nitrogen-containing |
9 |
| 8 |
Hypoglaunine A |
Sesquiterpenoid |
Nitrogen-containing |
13 and 14 |
| 9 |
Hypoglaunine B |
Sesquiterpenoid |
Nitrogen-containing |
9 and 13 |
| 10 |
Hypoglaunine D |
Sesquiterpenoid |
Nitrogen-containing |
9 and 13 |
| 11 |
Euonymine |
Sesquiterpenoid |
Nitrogen-containing |
9 |
| 12 |
Tripterdine C |
Sesquiterpenoid |
Nitrogen-containing |
15 |
| 13 |
Tripterdine D |
Sesquiterpenoid |
Nitrogen-containing |
15 |
| 14 |
Tripterdine E |
Sesquiterpenoid |
Nitrogen-containing |
15 |
| 15 |
Tripterdine J |
Sesquiterpenoid |
Nitrogen-containing |
15 |
| 16 |
Tripterygiumine A |
Sesquiterpenoid |
Nitrogen-containing |
5 and 16 |
| 17 |
Tripterygiumine B |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 18 |
Tripterygiumine C |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 19 |
Tripterygiumine D |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 20 |
Tripterygiumine E |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 21 |
Tripterygiumine F |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 22 |
Tripterygiumine G |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 23 |
Tripterygiumine H |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 24 |
Tripterygiumine I |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 25 |
Tripterygiumine J |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 26 |
Tripterygiumine K |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 27 |
Tripterygiumine L |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 28 |
Tripterygiumine M |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 29 |
Tripterygiumine N |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 30 |
Tripterygiumine O |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 31 |
Tripterygiumine P |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 32 |
Tripterygiumine Q |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 33 |
Tripterygiumine S |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 34 |
Tripterygiumine T |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 35 |
Tripterygiumine U |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 36 |
Tripterygiumine V |
Sesquiterpenoid |
Nitrogen-containing |
5 |
| 37 |
1-Desacetylwilforgine |
Sesquiterpenoid |
Nitrogen-containing |
17 |
| 38 |
1-Desacetylwilforine |
Sesquiterpenoid |
Nitrogen-containing |
17 |
| 39 |
9′-Hydroxy-2-nicotinoylwilforine |
Sesquiterpenoid |
Nitrogen-containing |
17 |
| 40 |
Tripterygiumine W |
Sesquiterpenoid |
Nitrogen-containing |
18 |
| 41 |
Wilfornine H |
Sesquiterpenoid |
Nitrogen-containing |
18 |
| 42 |
Tripterygiumine R |
Sesquiterpenoid |
Nitrogen-containing |
18 |
| 43 |
Triptersinine A |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 44 |
Triptersinine B |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 45 |
Triptersinine C |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 46 |
Triptersinine D |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 47 |
Triptersinine E |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 48 |
Triptersinine F |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 49 |
Triptersinine G |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 50 |
Triptersinine H |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 51 |
Triptersinine L |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 52 |
Triptersinine M |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 53 |
Triptersinine N |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 54 |
Triptersinine O |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 55 |
Triptersinine P |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 56 |
Triptersinine Q |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 57 |
Triptersinine R |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 58 |
Triptersinine S |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 59 |
Triptersinine T |
Sesquiterpenoid |
Nitrogen-containing |
19 |
| 60 |
Triptersinine Z4 |
Sesquiterpenoid |
Nitrogen-containing |
5 and 19 |
| 61 |
Triptersinine Z5 |
Sesquiterpenoid |
Nitrogen-containing |
5 and 19 |
| 62 |
Triptersinine Z6 |
Sesquiterpenoid |
Nitrogen-containing |
5 and 19 |
| 63 |
Triptersinine Z7 |
Sesquiterpenoid |
Nitrogen-containing |
5 and 19 |
| 64 |
Triptersinine Z8 |
Sesquiterpenoid |
Nitrogen-containing |
5 and 19 |
| 65 |
Wilforsinine C |
Sesquiterpenoid |
Nitrogen-containing |
5 and 20 |
| 66 |
Wilforsinine D |
Sesquiterpenoid |
Nitrogen-containing |
5 and 20 |
| 67 |
Wilforsinine E |
Sesquiterpenoid |
Nitrogen-containing |
5 and 20 |
| 68 |
Wilforsinine G |
Sesquiterpenoid |
Nitrogen-containing |
5 and 20 |
| 69 |
Wilforsinine H |
Sesquiterpenoid |
Nitrogen-containing |
5 and 20 |
| 70 |
Wilforsinine A |
Sesquiterpenoid |
Nitrogen-containing |
21 |
| 71 |
Wilforsinine B |
Sesquiterpenoid |
Nitrogen-containing |
21 |
| 72 |
Hypoglaunine E |
Sesquiterpenoid |
Nitrogen-containing |
13 |
| 73 |
Hypoglaunine F |
Sesquiterpenoid |
Nitrogen-containing |
13 |
| 74 |
Peritassine A |
Sesquiterpenoid |
Nitrogen-containing |
13 and 22 |
| 75 |
Wilfordinine A |
Sesquiterpenoid |
Nitrogen-containing |
13 |
| 76 |
Cangorin K |
Sesquiterpenoid |
Nitrogen-containing |
14 |
| 77 |
Dimacroregeline C |
Sesquiterpenoid |
Nitrogen-containing |
14 |
| 78 |
Dimacroregeline D |
Sesquiterpenoid |
Nitrogen-containing |
14 |
| 79 |
Euonine |
Sesquiterpenoid |
Nitrogen-containing |
13 and 22 |
| 80 |
Tripterdine A |
Sesquiterpenoids |
Nitrogen-free |
15 |
| 81 |
Tripterdine B |
Sesquiterpenoids |
Nitrogen-free |
15 |
| 82 |
Tripterdine F |
Sesquiterpenoids |
Nitrogen-free |
15 |
| 83 |
Tripterdine G |
Sesquiterpenoids |
Nitrogen-free |
15 |
| 84 |
Tripterdine H |
Sesquiterpenoids |
Nitrogen-free |
15 |
| 85 |
Tripterdine I |
Sesquiterpenoids |
Nitrogen-free |
15 |
| 86 |
Triptersinine I |
Sesquiterpenoids |
Nitrogen-free |
22 and 23 |
| 87 |
Triptersinine J |
Sesquiterpenoids |
Nitrogen-free |
22 and 23 |
| 88 |
Triptersinine K |
Sesquiterpenoids |
Nitrogen-free |
22 and 23 |
| 89 |
Wilforsinine F |
Sesquiterpenoids |
Nitrogen-free |
5 and 20 |
| 90 |
Triptregelol A |
Sesquiterpenoids |
Nitrogen-free |
22 and 23 |
| 91 |
Triptregelol B |
Sesquiterpenoids |
Nitrogen-free |
22 and 23 |
| 92 |
Triptersinine V |
Sesquiterpenoids |
Nitrogen-free |
5 |
| 93 |
Triptersinine W |
Sesquiterpenoids |
Nitrogen-free |
5 |
| 94 |
Triptersinine X |
Sesquiterpenoids |
Nitrogen-free |
5 |
| 95 |
Triptersinine Y |
Sesquiterpenoids |
Nitrogen-free |
5 |
| 96 |
Triptersinine Z1 |
Sesquiterpenoids |
Nitrogen-free |
5 |
| 97 |
Triptersinine Z2 |
Sesquiterpenoids |
Nitrogen-free |
5 |
| 98 |
Triptersinine Z3 |
Sesquiterpenoids |
Nitrogen-free |
5 |
| 99 |
Triptergosidol A |
Sesquiterpenoids |
Nitrogen-free |
6 |
| 100 |
Triptergosidol B |
Sesquiterpenoids |
Nitrogen-free |
6 |
| 101 |
Triptergosidol C |
Sesquiterpenoids |
Nitrogen-free |
6 |
| 102 |
Triptergosidol D |
Sesquiterpenoids |
Nitrogen-free |
6 |
| 103 |
Tripterycoside A |
Diterpenoids |
Abietane |
24 |
| 104 |
Tripterycoside B |
Diterpenoids |
Abietane |
24 |
| 105 |
Tripterycoside C |
Diterpenoids |
Abietane |
24 |
| 106 |
2α-Hydroxytriptonide |
Diterpenoids |
Abietane |
24 |
| 107 |
15-Hydroxytriptonide |
Diterpenoids |
Abietane |
24 |
| 108 |
Triptergulide A |
Diterpenoids |
Abietane |
25 and 26 |
| 109 |
Triptergulide B |
Diterpenoids |
Abietane |
25 and 26 |
| 110 |
Triptergulide C |
Diterpenoids |
Abietane |
25 and 26 |
| 111 |
Triptergulide D |
Diterpenoids |
Abietane |
25 and 26 |
| 112 |
Triptergulide E |
Diterpenoids |
Abietane |
25 and 26 |
| 113 |
Triptergulide F |
Diterpenoids |
Abietane |
25 and 26 |
| 114 |
Triptergulide G |
Diterpenoids |
Abietane |
25 and 26 |
| 115 |
Triptergulide H |
Diterpenoids |
Abietane |
25 and 26 |
| 116 |
Triptergulide I |
Diterpenoids |
Abietane |
25 and 26 |
| 117 |
Triptergulide J |
Diterpenoids |
Abietane |
25 and 26 |
| 118 |
Triptergulide K |
Diterpenoids |
Abietane |
25 and 26 |
| 119 |
Tripterlide A |
Diterpenoids |
Abietane |
27 |
| 120 |
Tripterlide B |
Diterpenoids |
Abietane |
27 |
| 121 |
Tripterlide C |
Diterpenoids |
Abietane |
27 |
| 122 |
Tripterlide D |
Diterpenoids |
Abietane |
27 |
| 123 |
Tripterlide E |
Diterpenoids |
Abietane |
27 |
| 124 |
Tripterlide F |
Diterpenoids |
Abietane |
27 |
| 125 |
16-Hydroxytriptolide |
Diterpenoids |
Abietane |
28 |
| 126 |
Triptolide |
Diterpenoids |
Abietane |
2 |
| 127 |
Triptonide |
Diterpenoids |
Abietane |
2 |
| 128 |
Hinokione |
Diterpenoids |
Abietane |
21 |
| 129 |
Triptonoterpene |
Diterpenoids |
Abietane |
28 |
| 130 |
Triptobenzene A |
Diterpenoids |
Abietane |
20 |
| 131 |
Wilforol F |
Diterpenoids |
Abietane |
7 |
| 132 |
Tripdiolide |
Diterpenoids |
Abietane |
28 |
| 133 |
Tripterolide |
Diterpenoids |
Abietane |
28 |
| 134 |
Triptolidenol |
Diterpenoids |
Abietane |
28 |
| 135 |
Tripchlorolide |
Diterpenoids |
Abietane |
28 |
| 136 |
Isotriptetraolide |
Diterpenoids |
Abietane |
28 |
| 137 |
Triptriolide |
Diterpenoids |
Abietane |
28 |
| 138 |
Tripdioltonide |
Diterpenoids |
Abietane |
28 |
| 139 |
Triptonolide |
Diterpenoids |
Abietane |
28 |
| 140 |
Triptophenolide |
Diterpenoids |
Abietane |
2 |
| 141 |
Triptophenolide methyl ether |
Diterpenoids |
Abietane |
28 |
| 142 |
Neotriptophenolide |
Diterpenoids |
Abietane |
28 |
| 143 |
Isoneotriptophenolide |
Diterpenoids |
Abietane |
28 |
| 144 |
Triptonoterpene methyl ether |
Diterpenoids |
Abietane |
28 |
| 145 |
Neotriptonoterpene |
Diterpenoids |
Abietane |
7 |
| 146 |
Triptonoterpenol |
Diterpenoids |
Abietane |
28 |
| 147 |
Triptoquinone A |
Diterpenoids |
Abietane |
7 and 21 |
| 148 |
Triptoquinone B |
Diterpenoids |
Abietane |
21 |
| 149 |
Triptoquinone C |
Diterpenoids |
Abietane |
28 |
| 150 |
Triptoquinone D |
Diterpenoids |
Abietane |
7 and 29 |
| 151 |
Triptoquinone E |
Diterpenoids |
Abietane |
29 |
| 152 |
Triptoquinone F |
Diterpenoids |
Abietane |
7 and 21 |
| 153 |
Triptoquinone G |
Diterpenoids |
Abietane |
28 |
| 154 |
Triptoquinone H |
Diterpenoids |
Abietane |
21 |
| 155 |
Triptinin A |
Diterpenoids |
Abietane |
20 |
| 156 |
Triptinin B |
Diterpenoids |
Abietane |
20 |
| 157 |
Triptobenzene B |
Diterpenoids |
Abietane |
7 |
| 158 |
Triptobenzene J |
Diterpenoids |
Abietane |
29 |
| 159 |
Triptobenzene M |
Diterpenoids |
Abietane |
7 |
| 160 |
Triptobenzene H |
Diterpenoids |
Abietane |
20 |
| 161 |
Triptobenzene I |
Diterpenoids |
Abietane |
30 |
| 162 |
Triptobenzene Q |
Diterpenoids |
Abietane |
31 |
| 163 |
Triptobenzene R |
Diterpenoids |
Abietane |
31 |
| 164 |
Triptobenzene S |
Diterpenoids |
Abietane |
31 |
| 165 |
Triptobenzene Y |
Diterpenoids |
Abietane |
31 |
| 166 |
Triregelin A |
Diterpenoids |
Abietane |
7 |
| 167 |
Triregelin B |
Diterpenoids |
Abietane |
7 |
| 168 |
Triregelin C |
Diterpenoids |
Abietane |
7 |
| 169 |
Triregelin D |
Diterpenoids |
Abietane |
7 |
| 170 |
Triregelin E |
Diterpenoids |
Abietane |
7 |
| 171 |
Triregelin F |
Diterpenoids |
Abietane |
7 |
| 172 |
Triregelin G |
Diterpenoids |
Abietane |
7 |
| 173 |
Triregelin H |
Diterpenoids |
Abietane |
7 |
| 174 |
Triregelin I |
Diterpenoids |
Abietane |
7 |
| 175 |
Triregelin J |
Diterpenoids |
Abietane |
7 |
| 176 |
Triregelin K |
Diterpenoids |
Abietane |
7 |
| 177 |
Doianoterpene A |
Diterpenoids |
Kaurane |
32 |
| 178 |
Doianoterpene B |
Diterpenoids |
Kaurane |
32 |
| 179 |
Doianoterpene C |
Diterpenoids |
Kaurane |
32 |
| 180 |
Neotripterifordin |
Diterpenoids |
Kaurane |
32 |
| 181 |
Doianoterpene D |
Diterpenoids |
Kaurane |
32 |
| 182 |
Ent-kauranol |
Diterpenoids |
Kaurane |
32 |
| 183 |
Tripterifordin |
Diterpenoids |
Kaurane |
28 |
| 184 |
Tripterinin |
Diterpenoids |
Kaurane |
28 |
| 185 |
Triregelin L |
Diterpenoids |
Kaurane |
7 |
| 186 |
3,4,6-Trihydroxy-2-oxo-1(10),3,5,7-tetraen-23,24-nor-D: A-friedooleana-29-oicacid |
Triterpenoids |
Friedelane |
5 |
| 187 |
2α,3α,23-Trihydroxyurs-12-en-28-oicacid |
Triterpenoids |
Friedelane |
5 |
| 188 |
2α,3α,23-Trihydroxyurs-12,20(30)-dien-28-oicacid |
Triterpenoids |
Friedelane |
5 |
| 189 |
2α, 3α, 24-Trihydroxyurs-12-en-28-oicacid |
Triterpenoids |
Friedelane |
5 |
| 190 |
Demethylzeylasteral |
Triterpenoids |
Friedelane |
8 |
| 191 |
Orthophenic acid |
Triterpenoids |
Friedelane |
8 |
| 192 |
Polpunonic acid |
Triterpenoids |
Friedelane |
8 |
| 193 |
Celastrol |
Triterpenoids |
Friedelane |
8 |
| 194 |
Triptocalline A |
Triterpenoids |
Friedelane |
8 |
| 195 |
Wilforol A |
Triterpenoids |
Friedelane |
8 |
| 196 |
Salaspermic acid |
Triterpenoids |
Friedelane |
16 |
| 197 |
Wilforic acid A |
Triterpenoids |
Friedelane |
28 |
| 198 |
Wilforic acid B |
Triterpenoids |
Friedelane |
28 |
| 199 |
Wilforic acid C |
Triterpenoids |
Oleanane |
28 |
| 200 |
28-Hydroxy-3-oxo-olean-12-en-29-oicacid |
Triterpenoids |
Oleanane |
13 |
| 201 |
3-acetoxy oleanolic acid |
Triterpenoids |
Oleanane |
13 |
| 202 |
3-Epikatonic acid |
Triterpenoids |
Oleanane |
8 |
| 203 |
3β-Acetyl-oleanolic acid |
Triterpenoids |
Oleanane |
33 |
| 204 |
Triptotriterpenic acid A |
Triterpenoids |
Oleanane |
16 |
| 205 |
Triptotriterpenic acid B |
Triterpenoids |
Oleanane |
8 |
| 206 |
Wilforlide A |
Triterpenoids |
Oleanane |
8 |
| 207 |
Wilforlide B |
Triterpenoids |
Oleanane |
8 |
| 208 |
22β-Hydroxytingenone |
Triterpenoids |
Ursane |
8 |
| 209 |
Demethylregelin |
Triterpenoids |
Ursane |
8 |
| 210 |
Regelin |
Triterpenoids |
Ursane |
8 |
| 211 |
Regelin C |
Triterpenoids |
Ursane |
8 and 13 |
| 212 |
Regelin D |
Triterpenoids |
Ursane |
8 and 13 |
| 213 |
Triptocallic acid A |
Triterpenoids |
Ursane |
8 |
| 214 |
Dulcioic acid |
Triterpenoids |
Ursane |
34 and 12 |
| 215 |
Regelindiol A |
Triterpenoids |
Ursane |
10 and 13 |
| 216 |
Regelindiol B |
Triterpenoids |
Ursane |
10 and 13 |
| 217 |
Triptotriterpenic acid C |
Triterpenoids |
Ursane |
10 |
Biological activities of terpenoids
The bioactive constituents extracted from T. wilfordii, characterized by diverse skeletal types and structural features, exhibit a broad range of biological activities with potential therapeutic applications. These effects encompass antitumor, anti-inflammatory, immunosuppressive, and neuroprotective properties, among others. In this section, we provide a comprehensive summary of the biological activities of the principal active ingredients derived from T. wilfordii, highlighting their significance in the realm of pharmacology.
Antitumor activity
A multitude of studies have demonstrated the potent antitumor activity of terpenoids derived from T. wilfordii. Triptolide (126), a quintessential abietane-type diterpenoid first isolated from T. wilfordii in 1972, has been shown to possess significant, broad-spectrum antitumor effects and diverse sensitizing properties.35 In recent years, an array of investigations have confirmed triptolide's substantial antitumor activity and therapeutic potential in various cancers, including breast cancer,3 lung cancer,35 liver cancer,35 colon cancer,36 thyroid cancer, and pancreatic cancer.3,36 As a predominant bioactive constituent in T. wilfordii, triptolide has been shown to exhibit significant antitumor properties by inducing apoptosis, autophagy, and cell senescence in a wide range of cancer cells, including conventional tumor cells, multidrug-resistant cancer cells, and certain cancer stem cells.35,36 In vitro experiments demonstrated that exposure of prostate cancer cell lines (PC-3, LNCaP, and C4-2) to 50 nM triptolide (126) for 24 h induced autophagy through the CaMKKβ-AMPK signaling pathway, which subsequently inhibited mTOR activity while activating ULK1 and Beclin 1. In vivo validation using a PC-3 xenograft nude mouse model showed that administration of triptolide (0.15 mg per kg per 2 days) for 18 days in combination with the autophagy inhibitor chloroquine resulted in significant tumor growth suppression compared to monotherapy groups.37 In subsequent in vivo studies, administration of triptolide at 1 mg kg−1 significantly reduced metastatic nodules of SKOV3 xenograft tumors in mice, without causing remarkable systemic toxicity during treatment.3 The molecular mechanisms of triptolide involved multiple signaling pathways, such as the RPL23-MDM2-p53 signaling pathway, transcription factor 3 activated signaling pathway, Akt/mTOR signaling pathway, NF-κB, vascular endothelial growth factor, and programmed cell death ligand 1.36,38,39 New dihydroagarofuran sesquiterpene polyol esters, including tripterdine A (80), tripterdine C (12), and tripterdine G (83), have exhibited inhibitory effects on the growth of human tumor cell lines (Huh7, MCF-7, and HCT-116).15 Cangoring K (76), dimacroregeline C (77), dimacroregeline D (78), and hypoglaunine A (8) have demonstrated potent cytotoxicity against SMMC7721 cells, with 20 h IC50 values ranging from 0.26 to 9.67 μM. This effect was achieved by disrupting the mitochondrial membrane potential.40 These four constituents, along with hypoterpene D, also showed similar inhibitory effects on LN-229 cells, with IC50 values ranging from 0.50 to 7.38 μM.14 Triptoquinone E (151) and triptoquinone F (152) have been reported to exhibit significant inhibition on human non-small cell lung cancer cell lines A549, human osteosarcoma cell lines HOS, and human breast cancer cells MCF-7.29 Celastrol (193), a friedelane-type triterpene derived from T. wilfordii, is another promising natural bioactive compound with a broad spectrum of activities against multiple complex diseases. It has been found to have positive anti-cancer effects on various cancer types, such as cervical cancer, hepatocellular cancer, prostate cancer, blood cancer, lung cancer, and colon cancer.4 The anticancer effects of celastrol are primarily achieved through multiple pathways, including inducing apoptosis,41 causing cell cycle arrest,41 inhibiting cell proliferation,42 targeting tumor-promoting inflammation,43 suppressing angiogenesis,44 and restraining cell invasion and metastasis.4,45 The antitumor effects of celastrol are associated with the up-regulation of caspase-3/7/9, E-cadherin, VEGF, bax, IκB, and STAT3, and down-regulation of HER2, p-AKT, p-ERK, MMP-9, ki-67, TNF-α, IL-6, and other genes involved in signaling pathways.4,41,42,45 In vitro studies showed celastrol (193) significantly suppressed proliferation of ovarian cancer A2780 and SKOV3 cells with an 72 h IC50 of around 2 μM, triggering G2/M phase arrest and apoptosis via ROS elevation.42 In vitro studies demonstrated that triptonide (127) effectively suppressed proliferation of PaTu8988 and Panc-1 pancreatic cancer cells with an IC50 of approximately 10.2 nM, inducing G2/M phase cell cycle arrest through activation of the MEKK4-MKK4-p38 signaling axis, accompanied by upregulated p21 expression and reduced CDK3 levels. In vivo experiments revealed that administration of 0.15 mg kg−1 triptonide significantly inhibited tumor growth in PaTu8988 xenograft models without inducing observable systemic toxicity.37 In addition, tripterdine C (12) inhibited the proliferation of HCT-116 colon cancer cells, with an IC50 value of 5.8 μM. The underlying mechanism was the suppression of the JAK/STAT3 pathway.15 Triptolidenol (134), an epoxy diterpene lactone diterpenoid obtained from T. wilfordii, has been shown to significantly suppress cell proliferation, cell migration, and induce cell cycle arrest at the S phase in human renal cell carcinoma through the mechanism of disrupting the NF-κB/COX-2 pathway by targeting ATP-binding sites of IKKβ.46 Wilforine (3) has been detected to re-sensitize MDR cancer cells with 72 h IC50 values exceeding 40 μM to chemotherapeutic drugs by binding to residues of P-gp such as LEU884, LYS887, THR176, and ASN172, thereby exerting an antitumor effect![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 Tripchlorolide (135) was demonstrated to effectively inhibit proliferation of A549 and cisplatin-resistant A549/DDP lung cancer cells at an optimal concentration of 200 nM, mechanistically attributed to suppression of AEG-1 expression, subsequent downregulation of MDR-1, and enhanced cisplatin chemosensitivity.48 Additionally, five triterpenes, including 28-hydroxy-3-oxo-olean-12-en-29-oic acid (200), regelin D (212), regelindiol B (216), 3-acetoxy oleanolic acid (201), and regelindiol A (215), have exhibited potent activity against HepG2 cells, A549 cells, and Hep3B cell lines, respectively, indicating the potential of these bioactive triterpenes for further investigation in advanced cancers.13 Table 2 and Fig. 2 provide a concise review of the antitumor effects of TwHF-based therapy, with the anticancer mechanisms of these triterpenoids detailed further in the current literature.
47 Tripchlorolide (135) was demonstrated to effectively inhibit proliferation of A549 and cisplatin-resistant A549/DDP lung cancer cells at an optimal concentration of 200 nM, mechanistically attributed to suppression of AEG-1 expression, subsequent downregulation of MDR-1, and enhanced cisplatin chemosensitivity.48 Additionally, five triterpenes, including 28-hydroxy-3-oxo-olean-12-en-29-oic acid (200), regelin D (212), regelindiol B (216), 3-acetoxy oleanolic acid (201), and regelindiol A (215), have exhibited potent activity against HepG2 cells, A549 cells, and Hep3B cell lines, respectively, indicating the potential of these bioactive triterpenes for further investigation in advanced cancers.13 Table 2 and Fig. 2 provide a concise review of the antitumor effects of TwHF-based therapy, with the anticancer mechanisms of these triterpenoids detailed further in the current literature.
Table 2 Antitumor activity of key terpenoids from T. wilfordii
| Compound |
Type |
Model |
Mechanism |
Dose/IC50 |
Ref |
| Triptolide (126) |
Diterpenoid |
PC-3 (prostate cancer) |
Modulates autophagy, LC3B activation |
50 nM in vitro |
37 |
| Nude mouse with PC-3 cell tumor |
0.15 mg kg−1 intraperitoneal injection |
| Celastrol (193) |
Triterpenoid |
A2780 (ovarian cancer cells) |
G2/M phase arrest and apoptosis ROS elevation |
2 μM in vitro |
42 |
| Nude mouse with A2780 cell tumor |
2 mg kg−1 intraperitoneal injection |
| Triptonide (127) |
Diterpenoid |
PaTu8988 (pancreatic cancer) |
MAPKP pathway suppression |
10 nM in vitro |
49 |
| Female NOD/SCID mouse with PaTu8988 cell tumor |
5 mg kg−1 intraperitoneal injection |
| Triptoquinone E (151) |
Diterpenoid |
A549 (lung cancer) |
Cytotoxicity |
35 μM in vitro |
29 |
| Wilforine (3) |
Sesquiterpenoid |
MDR cancer cells |
P-gp inhibition |
40 μM in vitro |
47 |
| Hypoglaunine A (8) |
Sesquiterpenoid |
SMMC7721 (liver cancer) |
Mitochondrial disruption |
0.29 μM in vitro |
40 |
| Tripterdine C (12) |
Sesquiterpenoid |
HCT-116 (colon cancer) |
Cytotoxicity |
5.8 μM in vitro |
15 |
| Triptolidenol (134) |
Diterpenoid |
768- O (renal cancer) |
IKKβ/COX-2 blockade |
87 nM in vitro |
46 |
| Cangorin K (76) |
Sesquiterpenoid |
LN-229 (glioblastoma) |
Cytotoxicity |
0.5 μM in vitro |
40 |
| Triptoquinone F (152) |
Diterpenoid |
MCF-7 (breast cancer) |
Cytotoxicity |
4.5 μM in vitro |
29 |
| Tripchlorolide (135) |
Diterpenoid |
A549 and A549/DDP (lung cancer) |
AEG-1 inhibition |
200 nM in vitro |
48 |
 |
| | Fig. 2 Schematic of the molecular targets of T. wilfordii in the treatment of tumor. | |
Anti-inflammatory activity
T.wilfordii has demonstrated significant anti-inflammatory effects, which are synergistically produced by its active constituents, including sesquiterpenoids, diterpenoids, and triterpenoids. Particularly in China, T. wilfordii is widely recognized for its therapeutic potential in treating rheumatoid arthritis, a common autoimmune disease.50 Network pharmacology studies have identified key genes such as AKT1, TNF-α, IL-6, CXCL8, MMP9, PTGS2, CASP3, and JUN, which are involved in the inflammatory response and are modulated by Triptolide (126) with a concentration of 50 nM for 24 h during renal injury. This diterpenoid has been reported to inhibit proliferation, induce apoptosis, and regulate the cell cycle in immunoglobulin A nephropathy mesangial cells, highlighting its molecular mechanisms.51 Furthermore, triptolide has been shown to act on rheumatoid arthritis-related pathways through eight targets, including MCL1, JUN, TNF, STAT1, RELA, IL23A, CASP3, and CDKN1A.52 A triptolide-phospholipid complex (TPCX) was engineered to enable transdermal rheumatoid arthritis therapy. Compared with free triptolide, TPCX demonstrated significantly improved aqueous solubility and in vitro skin permeation capacity. In arthritis models, TPCX topical formulation improved pharmacokinetics, reduced paw edema, and downregulated TNF-α, IL-1β, and IL-6 while protecting articular tissue. Transdermal delivery minimized systemic exposure, potentially reducing hepatorenal toxicity.53 Intraperitoneal celastrol (193) (1 mg per kg per day), administered as a 7-day pretreatment in endotoxemia or 1 day post-treatment in CLP-induced sepsis models, covalently targeted PKM2 and HMGB1, validated by ABPP, SPR, and molecular docking. This dual-binding inhibited Warburg metabolism and proinflammatory cytokine secretion (TNF-α, IL-1β, IL-6), improving survival (P < 0.05) and alleviating organ injury. Despite multi-target anti-inflammatory efficacy, low oral bioavailability and dose-dependent ALT/AST elevations with potential hepatorenal toxicity necessitate cautious translational optimization.54 Intra-articular celastrol-loaded chitosan-capped hollow mesoporous silica nanoparticles (CSL@HMSNs-Cs) enhanced the solubility of celastrol, while surface conjugation with chitosan conferred pH-responsive drug release, thereby improving CSL bioavailability. At 200 μg mL−1, they suppressed IL-1β/TNF-α/IL-6 and MMPs via NF-κB inhibition in vitro. In MIA-induced OA rats, treatment improved cartilage integrity, reduced effusion, and alleviated pain.55 Additional studies have confirmed celastrol's potent effects in treating rheumatoid arthritis, dementia, diabetic nephropathy, angiogenesis, and ulcerative colitis, with biological activity against various cell types including fibroblast-like synoviocytes, astrocytes, podocytes, human umbilical vein endothelial cells, and colonic epithelial cells.56 The anti-inflammatory activity of celastrol is associated with the expression of heme oxygenase-1, PI3K/Akt/mTOR, ROS/Akt/p70S6K, and FcεRI signaling pathways.5,56 The results of a growing number of studies suggested that inflammation was involved in regulating different stages of the tumorigenesis process. The role of inflammation in tumorigenesis was well-established, with Cyclooxygenase-2 (COX-2) playing a pivotal role in the inflammatory process and cancer development. The initiation of COX-2 transcription on account of binding of activated NF-κB and COX-2 promoter region.46,57 Triptolidenol (134) was demonstrated to suppressed NF-κB/COX-2 signaling by targeting IKKβ′s ATP-binding domainin ccRCC, reducing IL-1β, TNF-α, and COX-2 while inducing caspase-dependent apoptosis under 48-h treatment. This dual anti-inflammatory/antitumor profile suggests Triptolidenol's potential for COX-2-mediated ccRCC therapy with optimized dosing.46 Tripterdine C (12), tripterdine E (14), and tripterdine J (15) have exhibited potent anti-inflammatory effects by inhibiting the secretion levels of TNF-α and IL-6.15 Wilforine (3), wilfortrine (6), wilforgine (4), and wilfordine (5) have also shown inhibitory effects on cancer cells, with wilforine demonstrating anti-inflammatory effects in adjuvant arthritis rat models 11,12 Triptonide (127) administration significantly alleviated CFA-induced inflammatory pain in murine models by inhibiting AKT phosphorylation and suppressing TNF-α, IL-1β, and IL-6 expression, while in vitro experiments demonstrated its inhibitory effects on pro-inflammatory cytokine production in ND7/23 cells. Intravenous delivery (0.1, 0.5, 2.0 mg kg−1) attenuated paw edema and nociceptive hypersensitivity in arthritic mice through AKT pathway modulation and cytokine downregulation, indicating its potential as a therapeutic agent for chronic inflammatory disorders.58,59 Nine nitrogen-containing sesquiterpenoids, triptersinines A–L (43–51), have exhibited moderate inhibitory effects on NO nitric oxide (NO) production in lipopolysaccharide (LPS)-induced macrophages, along with neotripterifordin.12 These findings underscore the complex molecular interaction effects and reveal the potential pharmacological and molecular mechanisms of T. wilfordii in the treatment of inflammatory diseases. The studies summarized in Table 3 and Fig. 3, along with the current investigations and researches, highlight the diverse anti-inflammatory effects of T. wilfordii's constituents.34
Table 3 Summary of the anti-inflammatory activities of terpenoids
| Compound |
Type |
Model |
Dose |
Key targets |
Ref. |
| Triptolide (126) |
Diterpenoid |
IgAN mesangial cells |
50 nM in vitro |
p-JUN signaling |
35 and 51 |
| Wistar rats model of RA |
0.5 mg kg−1 transdermal administration |
TNF-a, IL-1b, IL-6 |
53 |
| Celastrol (193) |
Triterpenoid |
Chondrocytes in vitro |
200 μg mL−1 |
IL-1β, TNF-α, IL-6, MMP-3 and MMP-13 and NF-κB signaling pathway |
55 |
| Male BALB/c mice sepsis induced by cecal ligation puncture |
1 mg kg−1 intraperitoneal injection |
PKM2, HMGB1,TNF-α, IL-1β, IL-6 |
54 |
| Male BALB/c mice LPS-induced endotoxemia |
| Triptolidenol (134) |
Diterpenoid |
HK-2 ccRCC cells |
>1000 nM in vitro |
IKKβ/NF-κB, COX-2 |
46 and 57 |
| Caki-1 |
245 nM in vitro |
| ACHN |
140 nM in vitro |
| 786-O |
87 nM in vitro |
| Wilforine (3) |
Sesquiterpenoid |
Adjuvant arthritis rat (in vivo) |
10 mg kg−1 intragastric administration |
IL-6, IL-8 |
12 and 11 |
| Wilforgine (4) |
Sesquiterpenoid |
Adjuvant arthritis rat (in vivo) |
10 mg kg−1 intragastric administration |
IL-6, IL-8 |
12 and 11 |
| Triptonide (127) |
Diterpenoid |
Rat DRG neuron hybrid ND7/23 cells |
1 μg mL−1 in vitro |
AKT, TNF-α IL-1β, IL-6 |
58 and 59 |
| Mouse injection of CFA |
0.1, 0.5, 2.0 mg kg−1 Intravenous Administration |
 |
| | Fig. 3 Mechanisms of T. wilfordii and active compounds on anti-inflammatory activity. | |
Immunosuppressive activity
The immunosuppressive effects of terpenoids from T. wilfordii are related to their ability to modulate the immune response by suppressing the activation and proliferation of immune cells.60 Celastrol (193) has been shown to reduce the rate of IL-17 producing CD4+ and CD8+ T cells to Treg, demonstrating its potential effects to inhibit the expansion of antigen-reactive T cells and the migration of inflammatory cells into an arthritic joint.61 Celastrol also markedly suppresses the expression of TRAP, osteoclastic genes (Trap, MMP-9, CTR, Ctsk), and transcriptional factors (c-Jun, c-Fos, and NFATc1).5,62 Celastrol at concentrations from 0.03–30 μM showed significant immunomodulatory effects. It inhibited cytoplasmic DNA- and RNA-mediated IFN production in vitro and in vivo by targeting IRF3 and NF-κB pathways. In Trex1-/- mice, celastrol (0.2–5 mg kg−1) for 3 days reduced autoantibody production, and suppresses T cell activation. It also downregulated IFN-stimulated genes and protects against interferonopathy-related autoimmune diseases. These findings highlight celastrol's potential as an effective immunomodulatory agent for treating autoimmune disorders.58,63 Triptolide (126) has been detected in the treatment of rheumatoid arthritis, osteoarthritis, and skin allograft recipients, with findings indicating that its role in these diseases may contribute to the inhibition of the migration and invasion of rheumatoid fibroblast-like synoviocytes and suppression of IL-2, NLRP3, JNK, caspase-1, and other pro-inflammatory cytokines.34 Preconditioning with 0.1 μM triptolide enhanced umbilical cord mesenchymal stem cells' immunomodulatory capacity, significantly suppressed CD4+/CD8+ T-cell proliferation, elevated IL-10 secretion, and upregulated PD-L1/PD-L2 expression. This optimized protocol demonstrated improved therapeutic potential for T-cell-mediated immune dysregulation.64 Triptolide at 100 μg kg−1 suppressed JAK/STAT signaling, reduced IL-6, TNF-α, IFN-γ, and IL-10 expression, and increased CD4+CD25+Foxp3+ T cells in systemic lupus erythematosus mice, suggesting its therapeutic potential for SLE treatment.58 In experimental autoimmune encephalomyelitis models, Tripchlorolide (135), administered intraperitoneally at a dose of 40 μg kg−1, was found to reduce IL – 17 and IFN – γ levels by inhibiting the ERK1/2-NF-κB signaling pathway and did not induce side effects on serum biochemical and blood counts.65 Meanwhile, demethylzeylasteral (190) alleviated vitiligo progression in C57BL/6 mice by suppressing the JAK2-STAT1 pathway and downregulating CXCL9/10 expression and mitigated lupus nephritis by suppressing CD8+T-cell migration through the IFN-γ-JAK-STAT1-CXCL10 axis, with an in vitro concentration of 4.54 μM.66 Tripfordine exhibited anti-HIV activity at 1 μg mL−1 in H9 lymphocytes, while triptoquinone reduced IL-1α/β levels at 10 μg mL−1 in PBMCs, and euonine suppressed DTH and hemolysin responses at 80 mg kg−1 in DNCB-induced mice.28 A new study showed the potential of T. wilfordii as a treatment for paraquat-induced lung injury and fibrosis, as indicated by the regulation of ferroptosis through promoting the release of antioxidant enzymes superoxide dismutase (SOD) and reducing the expression of MDA and GSH via the activation of Nrf2/HO-1 signaling pathway.67 Ten sesquiterpenoids, including tripfordine A (1), tripfordine B (2), tripfordine C (7), wilforine (3), wilforgine (4), wilfordine (5), wilfortrine (6), hypoglaunine A (8), hypoglaunine B (9), and euonymine (11), were tested for cytotoxicities against H9 lymphocytes, with wilfordine, wilfortrine, hypoglaunine A, and hypoglaunine B showing anti-HIV activity with EC50 values under 2.54 μg mL−1.9 The role of these compounds in the treatment of autoimmune diseases remains a research focus, and these studies are summarized in Table 4 and Fig. 4.
Table 4 Immunosuppressive activities of terpenoids from T. wilfordii
| Compound |
Type |
Model |
Dose/IC50 |
Key targets |
Ref. |
| Celastrol (193) |
Triterpenoid |
RAW264.7 HEK293T BJ cells |
0.03–30 μM in vitro |
Cytoplasmic DNA- and RNA-mediated IFN production |
58 |
| Trex1-/- mice on C57BL/6 |
0.2–5 mg kg−1 intraperitoneal injection |
T Cell activation IRF3 activation |
63 |
| Triptolide (126) |
Diterpenoid |
UCMSCs |
0.01 μM in vitro |
IL-10 PD-L1 PD-L2 |
64 |
| Systemic lupus erythematosus mice |
100 μg/kg |
IL-6, TNF-α, IFN-γ, IL-10,CD4+CD25+Foxp3+T cell positivity |
58 |
| Tripchlorolide (135) |
Diterpenoid |
Female C57BL/6 mice |
40 μg kg−1 intraperitoneal injection |
ERK1/2-NF-κB, JAK/STAT |
65 |
| Demethylzeylasteral (190) |
Triterpenoid |
CDT8+ cell |
4.54 μM in vitro |
STAT1-CXCL9/10 signaling, JAK2 |
66 |
| C57Bl/6 mice with B16F10 cells |
5 mg kg−1 2.5 mg kg−1 intraperitoneal injection |
| Tripchlorolide (135) |
Diterpenoids |
EAE C57BL/6 mice model |
40 μg kg−1 intraperitoneal injection |
ERK1/2-NF-κB, JAK/STAT |
65 |
| Wilfordine (5) |
Sesquiterpenoid |
H9 lymphocytes |
20 μg mL−1 |
Anti-HIV activity |
9 |
| Wilfortrine (6) |
Sesquiterpenoid |
H9 lymphocytes |
>100 μg mL−1 |
Anti-HIV activity |
9 |
| Hypoglaunine B (9) |
Sesquiterpenoid |
H9 lymphocytes |
>100 μg mL−1 |
Anti-HIV activity |
9 |
| Euonymine (11) |
Sesquiterpenoid |
H9 lymphocytes |
>100 μg mL−1 |
Anti-HIV activity |
9 |
| Hypoglaunine A (8) |
Sesquiterpenoid |
H9 lymphocytes |
>100 μg mL−1 |
Anti-HIV activity |
9 |
| Tripfordine |
Sesquiterpenoid |
H9 lymphocytes |
1 μg mL−1 in vitro |
Anti-HIV activity |
28 |
| Triptoquinone |
Diterpenoid |
Peripheral blood mononuclear cells |
10 μg mL−1 in vitro |
IL-1α IL-1β |
28 |
| Euonine (79) |
Sesquiterpenoid |
DNCB-induced DTH reaction on mouse skin |
80 mg kg−1 intraperitoneal injection |
Hemolysin reaction |
28 |
 |
| | Fig. 4 The therapeutic mechanism of T. wilfordii in immunosuppressive activity. | |
Other activities
Various studies have indicated that triptolide (126) and celastrol (193) possess potential as anti-angiogenesis drugs due to their ability to downregulate the expression of angiogenic activators, including the canonical gene VEGF and VEGFR, as well as TNF-α, IL-17, Ang-1, Ang-2, ERK, p38, and other cytokines.68 These compounds have also demonstrated potent pharmacological cardioprotective effects through the nuclear accumulation of Nrf2 and the upregulation of its downstream target HO-1 in ischemic myocardial tissues. A network pharmacology study predicted the mechanism of T. wilfordii in treating myocardial fibrosis, suggesting that triptolide (126) is likely one of the principal active components, exerting its effects through the regulation of AGE-RAGE, PI3K-Akt, and MAPK pathways.69 Tripchlorolide (135) has emerged as a promising agent for modulating Aβ-related pathology in Alzheimer's disease (AD) at a concentration of 1 μM, potentially improving learning and memory function by crossing the blood–brain barrier, with its molecular mechanism possibly involving the regulation of NMDAR1, PSD-95, CaMKII, and BDNF.5 Triptonide (127) has been shown to lead to reversible male contraceptive effects in both mice and monkeys, targeting junction plakoglobin and disrupting its interactions with SPEM1 during spermiogenesis, indicating its potential as a promising male contraceptive agent.69
Structure and activity relationship
The structure–activity relationship of terpenoids from T. wilfordii reveals the interaction between specific functional groups in these compounds and biological targets, thereby producing therapeutic effects. For example, triptolide (126), an abietane-type diterpenoid, has a lactone ring and a ketone group at C-3. The lactone ring is vital for its antitumor activity as it can bind to and inhibit the NF-κB pathway, which is often overactivated in cancer cells. This inhibition suppresses the expression of pro-inflammatory and pro-survival genes, thus inducing apoptosis in tumor cells. The ketone group at C-3 also significantly contributes by interacting with the Akt/mTOR signaling pathway, further strengthening its anticancer effects.70–72 In liver cancer cells, the presence of a hydroxyl group at C-16 in triptolide has been shown to enhance its cytotoxicity by improving its ability to bind to and inhibit key cellular targets related to cell proliferation and survival.71 In the case of tripterifordin (183), a kaurane-type diterpenoid, the hydroxyl groups at C-3 and C-20 are critical for its anti-inflammatory activity. These hydroxyl groups allow the compound to interact with the NF-κB pathway, inhibiting the production of pro-inflammatory cytokines such as TNF-α and IL-6. This interaction reduces inflammation and tissue damage in diseases like rheumatoid arthritis.73 Celastrol (193) contains a ketone group at C-3 and a hydroxyl group at C-28. The ketone group at C-3 is essential for its anticancer activity as it can bind to and inhibit the PI3K/Akt/mTOR signaling pathway, which is frequently dysregulated in cancer cells. This inhibition suppresses cell proliferation and triggers apoptosis. The hydroxyl group at C-28 further boosts its capacity to interact with cellular targets involved in cancer cell survival and growth.74 Wilforine (3) has a pyridine ring and a hydroxyl group. The pyridine ring is crucial for its anti-inflammatory activity as it can bind to and inhibit the NF-κB pathway, decreasing the production of pro-inflammatory cytokines. The hydroxyl group enhances its ability to interact with cellular targets associated with inflammation and immune response.11,47 Consequently, the presence and arrangement of these functional groups greatly influence the compounds' ability to bind to and modulate key cellular pathways, thus determining their biological activities and therapeutic potential.
Toxicity of T. wilfordii
T. wilfordii, a traditional Chinese herb used in treating rheumatoid arthritis (RA), has gained widespread clinical applications due to the significant efficacy of its preparations, including Tripterygium Glycosides Tablets and Tripterygium Tablets. Over 30 clinical practice guidelines and consensus documents endorsed its use, with authoritative guidelines from American College of Rheumatology (ACR), European League Against Rheumatism (EULAR), and Chinese Society of Rheumatology (CSR) being widely adopted.12 Studies indicated that terpenoids in T. wilfordii exerted therapeutic effects through anti-inflammatory and immunomodulatory activities. However, they posed multi-organ toxicity risks, including significant harm to the digestive, urinary, reproductive, cardiovascular, hematopoietic and bone marrow systems.60,75,76
Hepatotoxicity
Meta-analyses revealed an overall adverse reaction rate of 11.7% for T. wilfordii preparations. Specific manifestations included alanine aminotransferase (ALT) elevation (8.6%), menstrual disorders in females (12.7%), cardiovascular events (4.9%), hematological abnormalities or mucosal damage (6.5–7.8%), as well as renal injury, alopecia, and weight loss.77,78 The hepatotoxic mechanisms involved lipid peroxidation and oxidative stress triggered by metabolites, characterized by elevated ALT/aspartate aminotransferase (AST), abnormal bile acid/bilirubin levels, and hepatocyte necrosis.60 Experimental studies demonstrated that Tripterygium Glycosides inhibited CYP27A1, CYP8B1, and SOD-1/GPX1 expression, reduced SOD and GPX activity, increased lipid peroxidation (LPO) levels, and disrupted mitochondrial function and antioxidant systems.79,80 Triptolide induced hepatocyte apoptosis via ROS generation, while celastrol exacerbated hepatocyte damage by suppressing CYP450 activity.81 These findings underscored that T. wilfordii-induced hepatotoxicity was closely linked to lipid peroxidation, oxidative stress, and inhibition of hepatic metabolic enzymes. Thus, clinical applications required vigilant monitoring of hepatotoxicity risks and exploration of interventions to mitigate adverse effects.
Nephrotoxicity
T. wilfordii nephrotoxicity was primarily attributed to diterpenoids, triterpenoids, and alkaloid metabolites, which directly damaged renal tubular epithelial cells, leading to oliguria, edema, hematuria, hypotension, and hyperkalemia.82–84 Animal studies confirmed that T. wilfordii extracts markedly increased serum creatinine, urea nitrogen, and uric acid levels in rats, accompanied by pathological changes such as glomerular atrophy and renal tubular liquefactive necrosis.85 The mechanisms involved triptolide-mediated upregulation of the Fas/FasL death receptor pathway and angiotensin II receptor synthesis impairment, resulting in ROS accumulation and apoptosis in glomerular cells.86
Cardiotoxicity
Blockade of the human ether-à-go-go-related gene (hERG) potassium channel was identified as a critical mechanism underlying drug-induced cardiotoxicity.87 Studies showed that T. wilfordii aqueous extracts at concentrations of 0.05 mg mL−1 and 0.1 mg mL−1 inhibited hERG current amplitudes by 21.4 ± 1.6% and 86.7 ± 5.7%, respectively. Celastrol directly bound to the hERG channel pore region, altering ion transport kinetics and reducing membrane potassium channel density without affecting activation/inactivation kinetics. This specific channel blockade was proposed as the key molecular mechanism of T. wilfordii cardiotoxicity.88
Reproductive toxicity
T. wilfordii disrupted reproductive function in both male and female animals through multiple pathways12 In males, active components (e.g., triptolide, celastrol) induced caspase-3-dependent spermatocyte apoptosis via Bax/Bcl-2 and Fas/FasL pathways activation, impaired Tnp1/Tnp2 expression leading to sperm malformation, and dysregulated cholesterol synthesis and testosterone-producing enzymes, causing testicular injury and spermatogenic dysfunction in a concentration- and time-dependent manner.89 In females, T. wilfordii inhibited granulosa cell proliferation, reduced antioxidant enzyme activity, and modulated Bax/Bcl-2 ratios, disrupting folliculogenesis and accelerating ovarian senescence.90 Additionally, its reproductive toxicity involved germ cell apoptosis, autophagic damage, hormonal synthesis dysregulation, and endocrine dysfunction.91,92
Drug delivery system
Despite T. wilfordii's therapeutic potential in cancer and autoimmune diseases, its clinical utility has been limited by poor aqueous solubility, inadequate targeting, and systemic toxicity. Novel drug delivery systems (DDSs), such as nanoparticles, polymeric micelles, and functionalized bioconjugates, enhanced the bioavailability and safety of T. wilfordii terpenoids by optimizing distribution and delivery efficiency. To mitigate triptolide toxicity, researchers developed water-soluble derivatives, including PG490-88, which entered Phase I clinical trials in the U.S. for prostate cancer treatment.93 Celastrol conjugated with polyethylene glycol (PEG) and EpCAM aptamer-modified dendrimers demonstrated improved solubility, potent antitumor activity in SW620 colon cancer cells, and reduced toxicity in AD293 cells, mice, and zebrafish.94,95 Celastrol-loaded PEG-polycaprolactone (PCL) nanoparticles retained metabolic regulatory effects in obesity models while avoiding gastrointestinal injury.96 A polydopamine@CEL-NS (PDA@CEL-NS) carrier system exhibited pH-responsive drug release and photothermal synergy, suppressing HepG2 cell proliferation, migration, and inducing apoptosis.97 Celastrol self-assembled nanoparticles displayed enhanced cytotoxicity in breast and lung cancer cells with tumor-targeting capability and minimal histological alterations.98,99 Celastrol-BSA-NPs prepared via high-pressure homogenization showed superior bioavailability and efficacy in diet-induced obesity.100 Triptolide-ADIBO conjugates modified with hyaluronic acid (HA) demonstrated potent antitumor effects in breast and liver cancers.101 These advancements highlight the role of modern DDSs in advancing T. wilfordii terpenoid applications. Therefore, developing targeted delivery systems for terpenoids remains a pivotal direction for future clinical translation.
Conclusions
In summary, terpenoids derived from Tripterygium wilfordii Hook. f. exhibit a diverse array of biological activities, including antitumor, anti-inflammatory, immunosuppressive, and other therapeutic effects. These compounds, characterized by their structural diversity and significant bioactivities, hold substantial promise for drug development. This review comprehensively summarizes the chemical structures and biological activities of 217 terpenoids from T. wilfordii, emphasizing their potential in pharmacological research and clinical applications. However, the clinical application of these terpenoids is constrained by their toxicity and poor bioavailability. Future research should prioritize the exploration of effective strategies to mitigate toxicity and enhance the drug delivery systems of these compounds. Furthermore, in-depth investigation into the structure–activity relationships and the identification of novel active constituents will facilitate the development of more efficacious and safer drugs. This review serves as a comprehensive reference for the discovery and development of novel drugs based on the natural active products of T. wilfordii, providing a solid foundation for future research and clinical translation.
Data availability
The present review article does not contain any new experimental data. Therefore, there are no new data sets generated or analyzed during the study to be made available. The article is based on a comprehensive literature review and analysis of previously published studies, which are properly cited within the manuscript.
Author contributions
Jiping li carried out the literature search and prepared a draft of the manuscript. Hong Liang, Likun Liu, Xiuli Gao, Yang Liu, Meng Zhang, Xiaoan Yuan, Shan Ren participated in a manuscript preparation and checked the literature. All authors have read and agreed to the published version of the manuscript. Wei Zhang conceptualized, organized, corrected and revised the manuscript.
Conflicts of interest
The authors declare no competing interests.
Acknowledgements
This study acknowledged the contributions of all participants. The work was financially supported by Qiqihar Science and Technology Program Joint Guidance Project (LSFGG-2023049), Scientific research project of Heilongjiang Provincial Health Commission (20230202040023), Scientific Research Project of Heilongjiang Provincial Education Department (2023-KYYWF-0865), Qiqihar Science and Technology Program Joint Guidance Project (LSFGG-2024103).
References
- J. Zhou, T. Hu, Y. Liu, L. Tu, Y. Song, Y. Lu, Y. Zhang, Y. Tong, Y. Zhao, P. Su, X. Wu, L. Huang and W. Gao, Phytochemistry, 2021, 190, 112868 CrossRef CAS PubMed.
- H. Guo, Z. Wang, L. Xu, H. Zhang, R. Chang and A. Chen, Electrophoresis, 2019, 40, 547–554 CrossRef CAS PubMed.
- P. Noel, D. D. Von Hoff, A. K. Saluja, M. Velagapudi, E. Borazanci and H. Han, Trends Pharmacol. Sci., 2019, 40, 327–341 CrossRef CAS PubMed.
- H. Y. Lim, P. S. Ong, L. Wang, A. Goel, L. Ding, A. Li-Ann Wong, P. C. Ho, G. Sethi, X. Xiang and B. C. Goh, Cancer Lett., 2021, 521, 252–267 CrossRef CAS PubMed.
- H. Lv, L. Jiang, M. Zhu, Y. Li, M. Luo, P. Jiang, S. Tong, H. Zhang and J. Yan, Fitoterapia, 2019, 137, 104190 CrossRef CAS PubMed.
- L. Ni, L. Li, Y. Qiu, F.-Y. Chen, C.-J. Li, J. Ma and D. Zhang, Fitoterapia, 2018, 128, 187–191 CrossRef CAS PubMed.
- D. Fan, S. Zhou, Z. Zheng, G.-Y. Zhu, X. Yao, M.-R. Yang, Z.-H. Jiang and L.-P. Bai, Int. J. Mater. Sci., 2017, 18, 147 Search PubMed.
- D. Fan, S. Parhira, G.-Y. Zhu, Z.-H. Jiang and L.-P. Bai, Fitoterapia, 2016, 113, 69–73 CrossRef CAS PubMed.
- M. Horiuch, C. Murakami, N. Fukamiya, D. Yu, T.-H. Chen, K. F. Bastow, D.-C. Zhang, Y. Takaishi, Y. Imakura and K.-H. Lee, J. Nat. Prod., 2006, 69, 1271–1274 CrossRef CAS PubMed.
- L. Guo, L. Duan, K. Liu, E.-H. Liu and P. Li, J. Pharm. Biomed. Anal., 2014, 95, 220–228 CrossRef CAS PubMed.
- X. Gao, X. Du, L. An, Y. Wang, L. Wang, Z. Wu, C. Huang and X. He, Phytomedicine, 2019, 54, 357–364 CrossRef CAS PubMed.
- Y. Zhang, X. Mao, W. Li, W. Chen, X. Wang, Z. Ma and N. Lin, Med. Res. Rev., 2021, 41, 1337–1374 CrossRef PubMed.
- C. Gao, L.-L. Lou, D. Wang, Y. Zhang, X.-X. Huang and S.-J. Song, J. Asian Nat. Prod. Res., 2017, 19, 725–731 CrossRef CAS PubMed.
- H. L. Ye, Y. Liu, J. Pan, W. Guan, Y. Liu, X. M. Li, S. Y. Wang, A. M. Algradi, B. Y. Yang and H. X. Kuang, Nat. Prod. Res., 2022, 36, 3979–3987 CrossRef CAS PubMed.
- Y.-L. Hu, T.-Q. Xu, W.-J. Yin, H.-Y. Cheng, X. Zhang, Y. Liu, Y.-B. Zhang and G.-X. Zhou, Fitoterapia, 2022, 160, 105205 CrossRef CAS PubMed.
- C. Zhang, Z. Yan, Y. Chen, Y. Zhang and X. Lü, Acta Acad. Med. Sin., 1994, 16, 466–468 CAS.
- C. M. Wu, L. M. Zhou, Y. F. Chai, Y. T. Wu and G. R. Fan, Chin. Chem. Lett., 2010, 21, 830–833 CrossRef CAS.
- C. Gao, X.-X. Huang, M. Bai, J. Wu, J.-Y. Li, Q.-B. Liu, L.-Z. Li and S.-J. Song, Fitoterapia, 2015, 105, 49–54 CrossRef CAS PubMed.
- D. Fan, G.-Y. Zhu, M. Chen, L.-M. Xie, Z.-H. Jiang, L. Xu and L.-P. Bai, Fitoterapia, 2016, 112, 1–8 CrossRef CAS PubMed.
- J. Xu, J. Lu, F. Sun, H. Zhu, L. Wang, X. Zhang and Z. Ma, Phytochemistry, 2011, 72, 1482–1487 CrossRef CAS PubMed.
- X.-D. Wang, W. Jia, W.-Y. Gao, R. Zhang, Y.-W. Zhang, J. Zhang, Y. Takaishi and H.-Q. Duan, J. Asian Nat. Prod. Res., 2005, 7, 755–759 CrossRef CAS PubMed.
- X.-X. Li, F.-Y. Du, H.-X. Liu, J.-B. Ji and J. Xing, J. Ethnopharmacol., 2015, 162, 238–243 CrossRef CAS PubMed.
- C. Wang, C.-J. Li, J.-Z. Yang, J. Ma, X.-G. Chen, Q. Hou and D.-M. Zhang, J. Nat. Prod., 2013, 76, 85–90 CrossRef CAS PubMed.
- J. Liu, Q. Wu, J. Shu, R. Zhang and L. Liu, Fitoterapia, 2017, 120, 126–130 CrossRef CAS PubMed.
- L. Ni, L. Li, Y. Zang, C. Li, J. Ma, T. Zhang and D. Zhang, Bioorg. Chem., 2019, 82, 68–73 CrossRef CAS PubMed.
- L. Ni, J. Ma, C. Li, L. Li, J. Guo, S. Yuan, Q. Hou, Y. Guo and D. Zhang, Tetrahedron Lett., 2015, 56, 1239–1243 CrossRef CAS.
- C. Wang, C.-J. Li, J. Ma, J.-Z. Yang, X.-G. Chen, L. Li and D.-M. Zhang, RSC Adv., 2015, 5, 30046–30052 RSC.
- R. Xu, J. M. Fidler and J. H. Musser, in Studies in Natural Products Chemistry, Elsevier, 2005, vol. 32, pp. 773–801 Search PubMed.
- M.-X. Xiu, X. Meng, N. Li, D. Wang, D.-X. Xiong, Y.-Y. Jiang, Y.-N. Sun, L. Chen and L. Cui, J. Asian Nat. Prod. Res., 2021, 23, 423–428 CrossRef CAS PubMed.
- X. Wang, W. Gao, Z. Yao, S. Zhang, Y. Zhang, Y. Takaishi and H. Duan, Chem. Pharm. Bull., 2005, 53, 607–610 CrossRef CAS PubMed.
- J.-Y. Li, Y. Peng, L.-Z. Li, P.-Y. Gao, C. Gao, S.-X. Xia and S.-J. Song, HCA, 2013, 96, 313–319 CrossRef CAS.
- N. Tanaka, N. Ooba, H. Duan, Y. Takaishi, Y. Nakanishi, K. Bastow and K.-H. Lee, Phytochemistry, 2004, 65, 2071–2076 CrossRef CAS PubMed.
- J. H. Shen, J. Integr. Plant Biol., 1992, 34, 475–479 CAS.
- X. Song, Y. Zhang and E. Dai, Mol. Med. Rep., 2020, 21, 2303–2310 CAS.
- J. Gao, Y. Zhang, X. Liu, X. Wu, L. Huang and W. Gao, Theranostics, 2021, 11, 7199–7221 CrossRef CAS PubMed.
- Y. Wei, Y. Wang, H. Xue, Z. Luan, B. Liu and J. Ren, Chin. J. Integr. Med., 2019, 25, 233–240 CrossRef CAS PubMed.
- F. Zhao, W. Huang, Z. Zhang, L. Mao, Y. Han, J. Yan and M. Lei, Oncotarget, 2016, 7, 5366–5382 CrossRef PubMed.
- Y.-Q. Zhang, Y. Shen, M.-M. Liao, X. Mao, G.-J. Mi, C. You, Q.-Y. Guo, W.-J. Li, X.-Y. Wang, N. Lin and T. J. Webster, Nanomed. Nanotechnol. Biol. Med., 2019, 15, 86–97 CrossRef CAS PubMed.
- A. M. Hamdi, Z.-Z. Jiang, M. Guerram, B. A. Yousef, H. M. Hassan, J.-W. Ling and L.-Y. Zhang, Biomed. Pharmacother., 2018, 103, 1557–1566 CrossRef CAS PubMed.
- H. L. Ye, Y. Liu, J. Pan, W. Guan, Y. Liu, X. M. Li, S. Y. Wang, A. M. Algradi, B. Y. Yang and H. X. Kuang, Nat. Prod. Res., 2022, 36, 3979–3987 CrossRef CAS PubMed.
- M. Chen, J. Yang, L. Li, Y. Hu, X. Lu, R. Sun, Y. Wang, X. Wang and X. Zhang, Sci. Rep., 2020, 10, 471 CrossRef CAS PubMed.
- L.-N. Xu, N. Zhao, J.-Y. Chen, P.-P. Ye, X.-W. Nan, H.-H. Zhou, Q.-W. Jiang, Y. Yang, J.-R. Huang, M.-L. Yuan, Z.-H. Xing, M.-N. Wei, Y. Li, Z. Shi and X.-J. Yan, Front. Oncol., 2019, 9, 2 CrossRef PubMed.
- L. Ni, L. Li, Y. Zang, C. Li, J. Ma, T. Zhang and D. Zhang, Bioorg. Chem., 2019, 82, 68–73 CrossRef CAS PubMed.
- Y. Zhu, X. Liu, P. Zhao, H. Zhao, W. Gao and L. Wang, Front. Pharmacol, 2020, 11, 25 CrossRef CAS PubMed.
- J. Shi, J. Li, Z. Xu, L. Chen, R. Luo, C. Zhang, F. Gao, J. Zhang and C. Fu, Front. Pharmacol, 2020, 11, 558741 CrossRef CAS PubMed.
- J. Jin, M. Zhou, X. Wang, M. Liu, H. Huang, F. Yan, Z. Yu, X. Shu, X. Huo, L. Feng, B. Zhang, S. Huang, S. Deng, C. Wang and X. Ma, Fitoterapia, 2021, 148, 104779 CrossRef CAS PubMed.
- Y.-T. Chang, Y.-C. Lin, L. Sun, W.-C. Liao, C. C. N. Wang, C.-Y. Chou, S. L. Morris-Natschke, K.-H. Lee and C.-C. Hung, Phytomedicine, 2020, 71, 153239 CrossRef CAS PubMed.
- T. Song, X. Lin, P. Huang, Y. Chen and L. Chen, Sci. Rep., 2022, 12, 11462 CrossRef CAS PubMed.
- B. Zhang, M. Meng, S. Xiang, Z. Cao, X. Xu, Z. Zhao, T. Zhang, B. Chen, P. Yang, Y. Li and Q. Zhou, Biochem. Pharmacol., 2019, 166, 70–81 CrossRef CAS PubMed.
- Y. Luo, X. Hou, A. Xi, M. Luo, K. Wang and Z. Xu, J. Ethnopharmacol., 2023, 307, 116211 CrossRef CAS PubMed.
- M. Xia, D. Liu, H. Liu, J. Zhao, C. Tang, G. Chen, Y. Liu and H. Liu, Front. Med., 2021, 8, 794962 CrossRef PubMed.
- W. Hu, W. Fu, X. Wei, Y. Yang, C. Lu and Z. Liu, J. Evidence-Based Complementary Altern. Med., 2019, 2019, 5276865 Search PubMed.
- X.-Y. Liu, W.-J. Pei, Y.-Z. Wu, F.-L. Ren, S.-Y. Yang and X. Wang, Drug Delivery, 2021, 28, 2127–2136 CrossRef CAS PubMed.
- P. Luo, Q. Zhang, T.-Y. Zhong, J.-Y. Chen, J.-Z. Zhang, Y. Tian, L.-H. Zheng, F. Yang, L.-Y. Dai, C. Zou, Z.-J. Li, J.-H. Liu and J.-G. Wang, Mil. Med. Res., 2022, 9, 22 CAS.
- T. Jin, D. Wu, X.-M. Liu, J.-T. Xu, B.-J. Ma, Y. Ji, Y.-Y. Jin, S.-Y. Wu, T. Wu and K. Ma, J. Nanobiotechnol., 2020, 18, 94 CrossRef CAS PubMed.
- W. Xin, Q. Wang, D. Zhang and C. Wang, Eur. J. Pharmacol., 2017, 814, 240–247 CrossRef CAS PubMed.
- J. Cui and J. Jia, CMC, 2021, 28, 3622–3646 CAS.
- J. Song, G.-N. He and L. Dai, Biomed. Pharmacother., 2023, 162, 114705 CrossRef CAS PubMed.
- Y.-J. Ling, T.-Y. Ding, F.-L. Dong, Y.-J. Gao and B.-C. Jiang, JPR, 2020, 13, 3195–3206 CrossRef CAS PubMed.
- Y. Shan, J. Zhao, K. Wei, P. Jiang, L. Xu, C. Chang, L. Xu, Y. Shi, Y. Zheng, Y. Bian, M. Zhou, S. J. Schrodi, S. Guo and D. He, Front. Pharmacol, 2023, 14, 1282610 CrossRef CAS PubMed.
- S. H. Venkatesha and K. D. Moudgil, in Anti-inflammatory Nutraceuticals and Chronic Diseases, eds. S. C. Gupta, S. Prasad and B. B. Aggarwal, Springer International Publishing, Cham, 2016, vol. 928, pp. 267–289 Search PubMed.
- B. Astry, S. H. Venkatesha, A. Laurence, A. Christensen-Quick, A. Garzino-demo, M. B. Frieman, J. J. O'Shea and K. D. Moudgil, Clin. Immunol., 2015, 157, 228–238 CrossRef CAS.
- Y. Liu, N. Xiao, H. Du, M. Kou, L. Lin, M. Huang, S. Zhang, S. Xu, D. Li and Q. Chen, Biochem. Pharmacol., 2020, 178, 114090 CrossRef CAS.
- H. He, A. Takahashi, T. Mukai, A. Hori, M. Narita, A. Tojo, T. Yang and T. Nagamura-Inoue, Front. Immunol., 2021, 12, 686356 CrossRef CAS PubMed.
- J. Zhang, Y. Zeng, J. Zhang, X. Pan, D. Kang, T. Huang and X. Chen, J. Neurochem., 2015, 133, 104–112 CrossRef CAS PubMed.
- Y. Chang, P. Kang, T. Cui, W. Guo, W. Zhang, P. Du, X. Yi, S. Guo, T. Gao, C. Li and S. Li, J. Transl. Med., 2023, 21, 434 CrossRef CAS PubMed.
- C.-Y. Song, M.-X. Feng, L. Li, P. Wang, X. Lu and Y.-Q. Lu, Ecotoxicol. Environ. Saf., 2023, 252, 114575 CrossRef CAS PubMed.
- W. Zhang, F. Li and W. Gao, J. Natl. Med. Assoc., 2017, 109, 142–148 Search PubMed.
- Y. Ming, L. Jiachen, G. Tao and W. Zhihui, CAD, 2023, 19, 68–79 CrossRef CAS PubMed.
- H. Wang, L. Chen and X. Ye, J. Peking Univ., Health Sci., 2018, 50, 607–612 CAS.
- Q. Tian, P. Zhang, Y. Wang, Y. Si, D. Yin, C. R. Weber, M. L. Fishel, K. E. Pollok, B. Qiu, F. Xiao and A. S. Chong, eLife, 2023, 12, e85862 CrossRef CAS PubMed.
- A. Glaviano, A. S. C. Foo, H. Y. Lam, K. C. H. Yap, W. Jacot, R. H. Jones, H. Eng, M. G. Nair, P. Makvandi, B. Geoerger, M. H. Kulke, R. D. Baird, J. S. Prabhu, D. Carbone, C. Pecoraro, D. B. L. Teh, G. Sethi, V. Cavalieri, K. H. Lin, N. R. Javidi-Sharifi, E. Toska, M. S. Davids, J. R. Brown, P. Diana, J. Stebbing, D. A. Fruman and A. P. Kumar, Mol. Cancer, 2023, 22, 138 CrossRef CAS PubMed.
- H. Luo, X. Wu, H. Huang, S. Chen, W. Yang, L. Zhang, H. Cui, J. Yang and A. Yang, J. Pharm. Biomed. Anal., 2016, 117, 195–204 CrossRef CAS PubMed.
- J. Yang, J. Liu, J. Li, M. Jing, L. Zhang, M. Sun, Q. Wang, H. Sun, G. Hou, C. Wang and W. Xin, Int. Immunopharmacol., 2022, 112, 109241 CrossRef CAS PubMed.
- Z. Ding, X. Wang, Y. Zhang, J. Liu, L. Wan, T. Li, L. Chen, N. Lin and Y. Zhang, Engineering, 2024, 39, 166–179 CrossRef CAS.
- N. Lin, Y.-Q. Zhang, Q. Jiang, W. Liu, J. Liu, Q.-C. Huang, K.-Y. Wu, S.-H. Tu, Z.-S. Zhou, W.-H. Chen, X.-X. Li, Y. Ding, Y.-F. Fang, J.-P. Liu, Z.-B. Li, D.-Y. He, Y.-L. Chen, Y.-Q. Lou, Q.-W. Tao, Q.-W. Wang, Y.-H. Jin, X. Liao, T.-X. Li and X.-Y. Wang, Front. Pharmacol, 2021, 11, 608703 CrossRef PubMed.
- C. Zhang, P.-P. Sun, H.-T. Guo, Y. Liu, J. Li, X.-J. He and A.-P. Lu, Front. Pharmacol, 2016, 7, 402 Search PubMed.
- C. Xi, S. Peng, Z. Wu, Q. Zhou and J. Zhou, Biomed. Pharmacother., 2017, 90, 531–541 CrossRef CAS PubMed.
- Z. Feng, C. Zhou, S. Dong, Z. Liu, T. Liu, L. Zhou and X. Zhou, Toxicol. in Vitro, 2019, 56, 141–149 CrossRef CAS PubMed.
- Z. Cao, B. Liu, L. Li, P. Lu, L. Yan and C. Lu, Toxicology, 2022, 469, 153134 CrossRef CAS PubMed.
- C. Jin, Z. Wu, L. Wang, Y. Kanai and X. He, Molecules, 2019, 24, 2162 CrossRef CAS PubMed.
- Q. Zhang, Y. Li, M. Liu, J. Duan, X. Zhou and H. Zhu, Int. J. Mater. Sci., 2018, 19, 305 Search PubMed.
- X.-X. Li, F.-Y. Du, H.-X. Liu, J.-B. Ji and J. Xing, J. Ethnopharmacol., 2015, 162, 238–243 CrossRef CAS PubMed.
- X. Feng, S. Fang and W. Chen, Adv. Integr. Med, 2019, 6, S102 CrossRef.
- S. Qing-Qing, W. Jing-Jing, R. O. Y. Debmalya, S. U. N. Li-Xin, J. Zhen-Zhou, Z. Lu-Yong and H. Xin, Chin. J. Nat. Med., 2020, 18, 196–205 Search PubMed.
- C. Zhang, N. Wang, Y. Xu, H.-Y. Tan, S. Li and Y. Feng, Int. J. Mater. Sci., 2018, 19, 2745 Search PubMed.
- X. Li, P. Wang, C. Wang, T. Jin, R. Xu, L. Tong, X. Hu, L. Shen, J. Li, Y. Zhou and T. Liu, J. Med. Chem., 2023, 66, 11792–11814 CrossRef CAS PubMed.
- W. Zhao, L. Xiao, L. Pan, X. Ke, Y. Zhang, D. Zhong, J. Xu, F. Cao, L. Wu and Y. Chen, Heliyon, 2019, 5, e02527 CrossRef PubMed.
- S. Xiong, Y. Li, Y. Xiang, N. Peng, C. Shen, Y. Cai, D. Song, P. Zhang, X. Wang, X. Zeng and X. Zhang, CDM, 2019, 20, 665–673 CrossRef CAS PubMed.
- Y. Zhu, L. Yao, Y. Guo, J. Zhang, Y. Xia, Z. Wei and Y. Dai, Reprod. Toxicol., 2024, 126, 108608 CrossRef CAS PubMed.
- D. Deng, J. Yan, W. Li, Y. Wu and K. Wu, J. Evidence-Based Complementary Altern. Med., 2022, 2022, 1–18 Search PubMed.
- Y. Dai, L. Sun, S. Han, S. Xu, L. Wang and Y. Ding, Front. Pharmacol, 2022, 13, 888968 CrossRef CAS PubMed.
- T. M. Kiviharju, P. S. Lecane, R. G. Sellers and D. M. Peehl, Clin. Cancer Res., 2002, 8, 2666–2674 CAS.
- Z. Qu, B. Zhang, Y. Chen and X. Yan, China Pharm., 2019, 30, 1470–1476 Search PubMed.
- P.-J. Ge, Construction of the Functional Dendrimer-Based Celastrol Delivery System for the Cancer-Targeted Treatment, Master’s Thesis, Xiamen University, 2018.
- J. Zhao, D. Luo, Z. Zhang, N. Fan, Y. Wang, H. Nie and J. Rong, J. Controlled Release, 2019, 310, 188–197 CrossRef CAS PubMed.
- G. Chen, Y. Liu, G. Shi, Y. Luo, S. Fu, A. Yang, Y. Zhou, Y. Wu, L. Lin and H. Li, J. Drug Delivery Sci. Technol., 2022, 75, 103630 CrossRef CAS.
- Y. Liu and J. Li, J. Colloid Interface Sci., 2023, 636, 216–222 CrossRef CAS PubMed.
- J. Song, G.-N. He and L. Dai, Biomed. Pharmacother., 2023, 162, 114705 CrossRef CAS PubMed.
- N. Fan, J. Zhao, W. Zhao, Y. Shen, Q. Song, H. C. Shum, Y. Wang and J. Rong, Biomater. Sci., 2022, 10, 984–996 RSC.
- W. Zheng, C. Wang, R. Ding, Y. Huang, Y. Li and Y. Lu, Int. J. Pharm., 2019, 572, 118721 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article a,
Hong Lianga,
Likun Liub,
Xiuli Gaob,
Yang Liuc,
Meng Zhangc,
Xiaoan Yuanb,
Shan Renb and
Wei Zhang*c
a,
Hong Lianga,
Likun Liub,
Xiuli Gaob,
Yang Liuc,
Meng Zhangc,
Xiaoan Yuanb,
Shan Renb and
Wei Zhang*c
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47 Tripchlorolide (135) was demonstrated to effectively inhibit proliferation of A549 and cisplatin-resistant A549/DDP lung cancer cells at an optimal concentration of 200 nM, mechanistically attributed to suppression of AEG-1 expression, subsequent downregulation of MDR-1, and enhanced cisplatin chemosensitivity.48 Additionally, five triterpenes, including 28-hydroxy-3-oxo-olean-12-en-29-oic acid (200), regelin D (212), regelindiol B (216), 3-acetoxy oleanolic acid (201), and regelindiol A (215), have exhibited potent activity against HepG2 cells, A549 cells, and Hep3B cell lines, respectively, indicating the potential of these bioactive triterpenes for further investigation in advanced cancers.13 Table 2 and Fig. 2 provide a concise review of the antitumor effects of TwHF-based therapy, with the anticancer mechanisms of these triterpenoids detailed further in the current literature.
47 Tripchlorolide (135) was demonstrated to effectively inhibit proliferation of A549 and cisplatin-resistant A549/DDP lung cancer cells at an optimal concentration of 200 nM, mechanistically attributed to suppression of AEG-1 expression, subsequent downregulation of MDR-1, and enhanced cisplatin chemosensitivity.48 Additionally, five triterpenes, including 28-hydroxy-3-oxo-olean-12-en-29-oic acid (200), regelin D (212), regelindiol B (216), 3-acetoxy oleanolic acid (201), and regelindiol A (215), have exhibited potent activity against HepG2 cells, A549 cells, and Hep3B cell lines, respectively, indicating the potential of these bioactive triterpenes for further investigation in advanced cancers.13 Table 2 and Fig. 2 provide a concise review of the antitumor effects of TwHF-based therapy, with the anticancer mechanisms of these triterpenoids detailed further in the current literature.





