Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Utility of 6-aza-2-thiothymine in the synthesis of novel [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one derivatives: synthesis, structure elucidation, molecular docking and in vitro anti-lung cancer activity†
Monica G. Kamela,
Farid M. Sroor *b,
Khaled Mahmoudc,
Heba I. Shafeyd,
Hamdi M. Hassaneen*a and
Laure Vendiere
*b,
Khaled Mahmoudc,
Heba I. Shafeyd,
Hamdi M. Hassaneen*a and
Laure Vendiere
aDepartment of Chemistry, Faculty of Science, Cairo University, Giza, Egypt. E-mail: hamdi_251@yahoo.com; hhassaneen@sci.cu.edu.eg
bOrganometallic and Organometalloid Chemistry Department, National Research Centre, 12622 Cairo, Egypt. E-mail: faridsroor@gmx.de; fm.sroor@nrc.sci.eg
cPharmacognosy Department, Pharmaceutical and Drug Industry Institute, National Research Centre, 12622-Dokki, Egypt
dCell Biology Department, National Research Centre, 12622-Dokki, Egypt
eLCC-CNRS, Université de Toulouse, CNRS, UPS, Toulouse, France
First published on 26th February 2025
Abstract
Using 6-aza-2-thiothymine (ATT) as a suitable precursor, a novel series of [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one derivatives (7a–j) was prepared by refluxing 6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one (3) with hydrazonoyl halides (1a–j) in chloroform in the presence of triethylamine. The structures of the newly synthesized compounds 7a–j were confirmed using spectral data, elemental analyses, and single-crystal X-ray diffraction results. All the synthesized triazolotriazin-7-one derivatives (7a–j) were evaluated as in vitro anti-cancer agents against PC3 (prostate cell line), A549 (lung carcinoma), PACA2 (pancreatic cancer cell line) and BJ1 (normal skin fibroblast) cell lines using MTT assay. Compounds 7a and 7g showed greater efficacy and low IC50 values (36.6 and 40.1 μM, respectively) compared to the reference drug, which exhibited an IC50 value of 43.8 μM on the lung cell line, and demonstrated safe mortality effect on the normal cell line (BJ1) with cytotoxicity percentages of 3.5% and 2.8%, respectively. These compounds (7a and 7g) were the most active compounds of the synthesized triazolotriazin-7-one derivatives (7a–j). They were further investigated to ascertain their mechanism of action using DNA fragmentation, DNA damage and gene expression (BCL-2, BAX, and p53 genes). Results indicated a significant increase in the expression levels of BCL-2 and a reduction in the expression of p53 and BAX genes in negative lung cancer cell lines. However, the treatment of negative cell lines with 7g improved the expression of the tested genes to a greater extent than that with 7a. Additionally, the DNA damage and DNA fragmentation levels were significantly elevated in the lung cancer cell line samples treated with 7a much more than 7g. Molecular docking was employed to explore the potential interactions between the most active compounds (7a and 7g) and two key enzymes, human 3-phosphoglycerate dehydrogenase (PHGDH) and phosphoserine aminotransferase (PSAT1), which play vital roles in the progression of lung cancer.
Introduction
Triazolotriazine moiety has attracted significant interest in medicinal chemistry owing to its unusual structural and electrical characteristics, which improve the efficacy of therapeutic candidates.1–4 Its core is useful in the development of nucleoside analogs and other therapeutic medicines because it shows enhanced lipophilicity and improved binding interactions with biological targets.1,5 Its incorporation in potential drug candidates has been demonstrated to improve antiviral and anti-cancer properties, mainly by increasing the drug's potency and selectivity against particular enzymes and receptors.6,7 Furthermore, triazolotriazine moiety can improve the pharmacokinetic profiles of drugs and stabilize nucleic acid structures, enhancing bioavailability and lowering toxicity, respectively.7,8 The significance of triazolotriazine derivatives in the synthesis of novel therapeutic agents highlights their potential for treating a range of diseases, such as cancer and viral infections.Lung cancer continues to be one of the most widespread and lethal types of cancer worldwide, causing serious problems to public health.9,10 Often discovered at an advanced stage, this disease is characterized by the uncontrolled proliferation of aberrant cells in the lungs, which results in poor prognosis and limited therapeutic options.11 The majority of lung cancer cases are caused by smoking, which is one of the main causes of the disease; however, risk factors for non-smokers include genetic predisposition, radon exposure, and environmental pollution.12 Moreover, the heterogeneity of lung cancer, which results in different subtypes displaying unique molecular profiles, makes treatment plans more challenging to implement and calls for personalized approaches.13 Targeted therapy and immunotherapy have made significant advances in lung cancer. However, issues including drug resistance, treatment expenses, and the necessity for early diagnostic technologies make it difficult to manage patients effectively and increase survival rates.14,15 Addressing these challenges requires an integrated strategy that includes prevention, early diagnosis, and the development of innovative therapeutic strategies, including discovering new drugs to improve the outcomes for patients with lung cancer.
Given the above information, the purpose of this study was to design and synthesize novel triazolo[4,3-b][1,2,4]triazin-7-one derivatives (7a–j) as the triazolotriazine family using 6-aza-2-thiothymine (ATT) as a suitable precursor and evaluate their anti-cancer activity against various cancer cell lines, including the PC3 (Prostate cell line), A549 (Lung carcinoma), PACA2 (Pancreatic cancer cell line) and BJ1 (normal skin fibroblast). The synthesis of the triazolo[4,3-b][1,2,4]triazin-7-one derivatives was of special interest since these compounds were not investigated in previous studies. The most promising compounds (7a and 7g) were examined in further studies to determine their mechanism of action using DNA fragmentation, DNA damage and gene expression (BCL-2, BAX, and p53 genes) as well as molecular docking.
Results and discussion
Chemistry
The reaction of 6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one (3)16 with hydrazonoyl halides (1a–j) in refluxing chloroform in the presence of triethylamine yielded a single product in each case (7a–j), as shown in Scheme 1 and Fig. 1. In order to clarify the preceding results, Scheme 2 proposes two plausible mechanistic sequences. In the first sequence (route A in Scheme 2), nitrilimines (2), which are generated in situ by the base-catalyzed dehydrohalogenation of hydrazonoyl halides (1),17–21 are thought to undergo 1,3-dipolar cycloaddition with the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond of triazinethione (3), resulting in the spiro intermediate 4, which is then subjected to a base-catalyzed ring cleavage to yield thiohydrazide (5). Subsequent intramolecular cyclization of 5 may result in two distinct heterocyclic intermediates, 6 and 8, which quickly lose hydrogen sulfide to form triazolo[4,3-b][1,2,4]triazin-7-one (7) or triazolo[3,4-c][1,2,4]triazin-5-one (9), respectively. In the intermediate 5, the less nucleophilic nitrogen (N-4) could lead to a preferential ring closure to form [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one (7) owing to the electronic effect of the carbonyl group.22 As an alternative, the reaction could include primary 1,3-addition, which results in amidrazones (10) that cyclize to give intermediate 11 and then compound 7 by the loss of hydrogen sulfide, as shown in route B in Scheme 2. It is suggested that route A is more likely to occur considering the following factors: (1) a C
S double bond of triazinethione (3), resulting in the spiro intermediate 4, which is then subjected to a base-catalyzed ring cleavage to yield thiohydrazide (5). Subsequent intramolecular cyclization of 5 may result in two distinct heterocyclic intermediates, 6 and 8, which quickly lose hydrogen sulfide to form triazolo[4,3-b][1,2,4]triazin-7-one (7) or triazolo[3,4-c][1,2,4]triazin-5-one (9), respectively. In the intermediate 5, the less nucleophilic nitrogen (N-4) could lead to a preferential ring closure to form [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one (7) owing to the electronic effect of the carbonyl group.22 As an alternative, the reaction could include primary 1,3-addition, which results in amidrazones (10) that cyclize to give intermediate 11 and then compound 7 by the loss of hydrogen sulfide, as shown in route B in Scheme 2. It is suggested that route A is more likely to occur considering the following factors: (1) a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S double bond appears to be a more reactive dipolarophile toward different 1,3-dipoles, (2) amidrazones of type 10 are known to be stable, but despite this, every attempt to separate them from the reaction mixtures was unsuccessful; (3) triazine-3-thione derivatives are mainly found in thione structures.23
S double bond appears to be a more reactive dipolarophile toward different 1,3-dipoles, (2) amidrazones of type 10 are known to be stable, but despite this, every attempt to separate them from the reaction mixtures was unsuccessful; (3) triazine-3-thione derivatives are mainly found in thione structures.23
 | ||
| Fig. 1 Chemical structures of all the synthesized [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one derivatives (7a–j). | ||
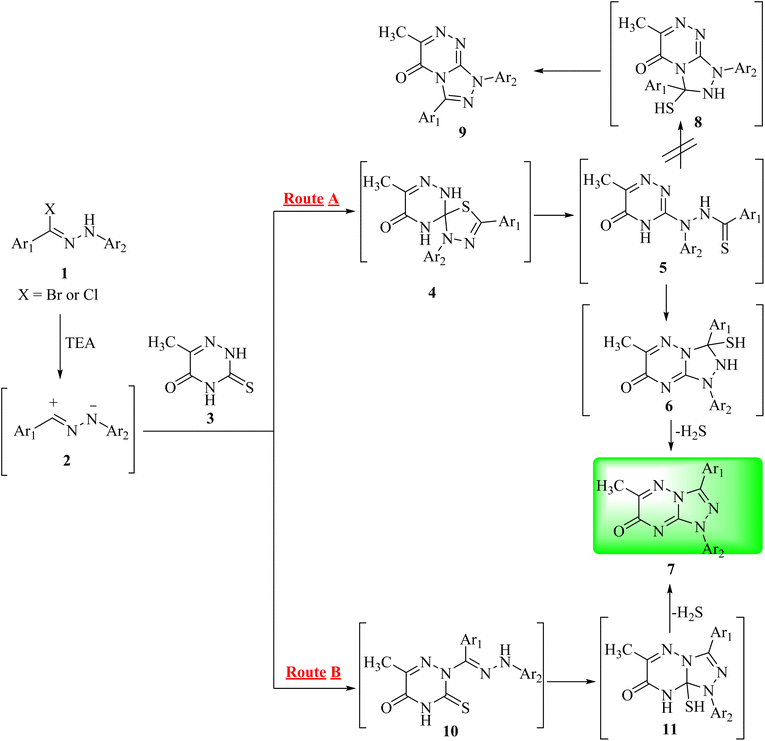 | ||
| Scheme 2 Plausible mechanism of the synthesis of [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one derivatives (7a–j). | ||
The structures of the isolated products were identified by their elemental analyses and spectroscopic data (1H NMR and 13C NMR). Their structures were assigned to be 7 rather than the isomeric structure 9 (Scheme 1 and Fig. 1). For example, the 1H NMR spectrum of compound 7b showed the following signals: triplet at δ 1.48 corresponding to the methyl protons in CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3, singlet at δ 2.45 corresponding to CH3 group protons, quartet at δ 3.03 corresponding to the methylene protons in C
3, singlet at δ 2.45 corresponding to CH3 group protons, quartet at δ 3.03 corresponding to the methylene protons in C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3 and pair of doublets at δ 8.30 and 8.45 corresponding to the protons of 4-NO2C6H4. Also, its 13C NMR spectrum showed 11 signals for asymmetric carbon atoms. Moreover, the single crystal X-ray structure of 7b confirmed the formation of a [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one scaffold (Table 1 and Fig. 2).
2CH3 and pair of doublets at δ 8.30 and 8.45 corresponding to the protons of 4-NO2C6H4. Also, its 13C NMR spectrum showed 11 signals for asymmetric carbon atoms. Moreover, the single crystal X-ray structure of 7b confirmed the formation of a [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one scaffold (Table 1 and Fig. 2).
| CCDC deposition number | 2390251 | μ (Cu Kα)/mm−1 | 0.95 |
| Chemical formula sum | C13H13N6O3 | Crystal size/mm | 0.19 × 0.18 × 0.14 |
| Formula weight/g mol−1 | 301.28 | T/K | 100 |
| Crystal color and shape | Orange, Bloc | θ rang/° | 4.2–79.4 |
| Crystal system | Monoclinic | Reflections collected | 2840 |
| Space group | P21/n | λ (Å) | 1.54184 |
| Unit cell parameters | |||
| a (Å) | 8.0047 (3) | F000 | 628 |
| b (Å) | 21.1874 (6) | Extinction correction | None |
| c (Å) | 8.0296 (3) | RInt | 0.042 |
| α (°) | 90 | Parameters/restraints | 203/4 |
| β (°) | 104.314 (4) | R[F2 > 2σ(F2)] | 0.043 |
| γ (°) | 90 | wR(F2) | 0.1294 |
| Unit cell volume/Å3 | 1319.53 (9) | Goodness-of-fit on F2 | 0.9825 |
| Molecules per cell Z | 4 | 1-Sigma level | 0.001 |
| Calcd density ρ/g cm−3 | 1.516 | Highest difference peak and hole/e Å−3 | 0.29/−0.64 |
As depicted in Table 1 and Fig. 2, single crystals containing 7b were found to be suitable for single-crystal X-ray diffraction. The crystal structure of compound 7b was deposited at Cambridge Crystallographic Data Centre under CCDC number 2390251. It was produced as orange crystals from a saturated DMF/acetonitrile solution at ambient temperature. Compound 7b forms a single molecule in the unit cell and crystallizes in the monoclinic cell with space group P21/n (Table 1). The presence of the fused [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one scaffold was clearly elucidated by the crystal structure determination of 3-ethyl-6-methyl-1-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7b) (Fig. 2). The selected examples of the bond lengths and angles of the [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one moiety, the distances of N7–N6 is 1.3721(14), N9–N1 is 1.3969(14), N7–C2 is 1.3648(16), C2–N1 is 1.3634 (15), N3–C2 is 1.3119(16), and C10–N1 is 1.4156(15), and the angles C8–N9–N1 is 106.15(10), O17–N16–O18 is 123.50(11), and N6–N7–C8 is 126.81(11), are in good agreement with those reported for 5-(4-bromophenyl)-4,6-dichloro-7-(2,4-dichlorophenyl)-7H-pyrrolo[2,3-d]pyrimidine, 4-amino-7-nitro-[1,2,4]triazolo[5,1-c][1,2,4]triazine-3-carbonitrile and 4,6-dichloro-7-(2,4-dichlorophenyl)-5-(3,4-dimethoxyphenyl)-7H-pyrrolo[2,3-d]pyrimidine.3,24,25 More selected bond lengths and angles are shown in Fig. 2 (see ESI†).
Anti-cancer activity
In the initial screening trial, a single dosage concentration of 100 μg ml−1 was applied for 48 hours. The cytotoxicity of the treated cells was determined by calculating the cell death percentage (% mortality) as a percentage of untreated cells (Table 2). Against the lung cancer cell lines (A549), the results demonstrated that four compounds, 7a, 7e, 7f and 7g, had considerable cytotoxic activity (80.4, 86.3, 69.1, and 82.3%, respectively), while compounds 7b, 7d and 7j showed limited cytotoxic effects of 35.2, 33.3 and 32.8%, respectively (Table 2). In the case of the prostate cell line (PC3), all compounds showed limited anti-cancer activity with mortality in the range of 15.9–35.1%, as depicted in Table 2. All the synthesized triazolotriazin-7-one derivatives (7a–j) demonstrated a low cytotoxic effect on the pancreatic cancer cell line (PACA2), as shown in Table 2. Additionally, to confirm the safety of the most effective compounds against cancer cells, their cytotoxicity was assessed against normal cells (BJ1). As visualized in Table 1, the most promising compounds, 7a, 7e, 7f and 7g, were found to be safe on the normal cells (BJ1) up to a concentration of 100 μg ml−1 with cytotoxicity percentages 3.5%, 6.4%, 3.2%, and 2.8%, respectively.
| Compound | PC3 (IC50, μM) | A549 (IC50, μM) | PACA2 (IC50, μM) | BJ1 |
|---|---|---|---|---|
| 7a | 28.2 ± 0.25 | 80.4 ± 0.66 (36.6 ± 0.33) | 20.4 ± 0.11 | 3.5 ± 1.16 |
| 7b | 24.5 ± 0.57 | 35.2 ± 1.22 | 3.6 ± 0.21 | 4.2 ± 0.98 |
| 7c | 27.3 ± 1.20 | 22.9 ± 0.81 | 10.5 ± 0.61 | 1.6 ± 0.38 |
| 7d | 17.9 ± 0.38 | 33.3 ± 0.22 | 11.5 ± 0.25 | 36.2 ± 1.11 |
| 7e | 35.1 ± 1.11 | 86.3 ± 0.74 (42.5 ± 0.27) | 8.6 ± 0.33 | 6.4 ± 0.85 |
| 7f | 33.4 ± 1.18 | 69.1 ± 0.77 | 5.6 ± 0.11 | 3.2 ± 0.63 |
| 7g | 14.6 ± 0.92 | 82.3 ± 0.82 (40.1 ± 0.84) | 13.4 ± 1.15 | 2.8 ± 0.71 |
| 7h | 24.2 ± 0.21 | 13.5 ± 0.33 | 21.3 ± 0.98 | 9.6 ± 0.93 |
| 7i | 18.4 ± 1.14 | 14.2 ± 0.24 | 9.6 ± 1.33 | 2.9 ± 1.22 |
| 7j | 15.9 ± 0.51 | 32.8 ± 0.54 | 7.2 ± 0.88 | 3.8 ± 0.77 |
| Dox | 100 (43.78 ± 0.54) | 100 (43.80 ± 0.17) | 100 (52.06 ± 0.42) | 1.10 ± 0.52 |
| Negative control | 0 | 0 | 0 | 0 |
Based on the results of Table 2, we continued our investigation into the molecular mechanisms underlying the potent lethal impact of the most active compounds on lung cancer cells (A549). Compounds 7a, 7e, 7f, and 7g showed limited mortality and high safety on the normal cells. Hence, secondary screening was performed on these compounds to ascertain their IC50 values and selectivity index. Table 2 illustrates that compounds 7a, 7e, and 7g had greater efficacy (IC50 values of 36.6, 42.5, and 40.1 μM, respectively) in comparison to the reference drug with an IC50 of 43.8 μM. Accordingly, compounds 7a and 7g with low IC50 values (36.6 and 40.1 μM, respectively) and safe mortality effect on the normal cell line (BJ1) with cytotoxicity percentages of 3.5, and 2.8%, respectively, are the most active compounds of the synthesized triazolotriazin-7-one derivatives (7a–j). Thus, in order to explore the potential mode of action of compounds 7a and 7g as powerful anti-lung cancer agents, we selected them for further studies. We used comet, RT-PCR, and DNA fragmentation tests to examine the effects of these compounds at the gene, protein, and DNA levels.
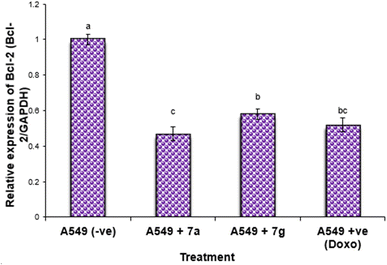
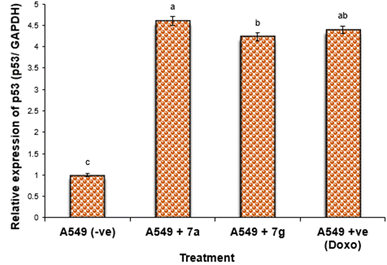
Nonetheless, the expression levels of the pro-apoptotic genes (p53 and BAX) were dramatically diminished in the negative samples of lung cancer cell lines compared to the treated lung cancer cell lines (Fig. 4 and 5). The expression levels of the anti-apoptotic gene BCL-2 were diminished in descending order in the cancer cell lines treated with 7g, Dox, and subsequently 7a in comparison to the negative control cancer cell lines. The expression levels of the pro-apoptotic genes (p53 and BAX) were elevated in a sequential manner in the cancer cell line treated with 7g, followed by Dox, and subsequently 7a, in comparison to the negative control cancer cell lines.
The results indicated that the anti-cancer action of the examined drugs was most prominently detected in 7g, followed by Dox, and subsequently 7a. Compound 7a showed much greater efficacy against the tumor cell line compared to 7g.
| Treatment | No. of samples | No. of cells | Classb | DNA damaged cells% (mean ± SEM) | ||||
|---|---|---|---|---|---|---|---|---|
| Analyzeda | Comets | 0 | 1 | 2 | 3 | |||
| a Number of cells examined per group.b Class 0 = no tail; 1 = tail length < diameter of the nucleus; 2 = tail length between 1× and 2× the diameter of the nucleus; and 3 = tail length > 2× the diameter of the nucleus. | ||||||||
| A549 (−ve) | 4 | 440 | 51 | 389 | 35 | 12 | 4 | 11.59 ± 0.65c |
| A549 + 7a | 4 | 440 | 124 | 316 | 37 | 45 | 42 | 28.18 ± 1.25a |
| A549 + 7g | 4 | 440 | 93 | 347 | 32 | 38 | 23 | 21.14 ± 0.85b |
| A549 + Dox | 4 | 440 | 105 | 335 | 34 | 41 | 30 | 23.86 ± 1.11b |
| Treatment | DNA fragmentation% (M ± SEM) | Change | Inhibition% |
|---|---|---|---|
| a Means with different superscripts (a, b, c) between groups in the same column are significantly different at P < 0.05. | |||
| A549 (−ve) | 12.54 ± 0.45c | 0.00 | 0.00 |
| A549 + 7a | 32.78 ± 1.35a | 20.24 | 27.78 |
| A549 + 7g | 25.57 ± 0.94b | 13.03 | 17.74 |
| A549 + Dox | 28.38 ± 1.06b | 15.84 | 19.69 |
| Comp. no. | Score kcal mol−1 | Moieties from compounds | Amino acid residues | Type of interaction, distance Å |
|---|---|---|---|---|
| 3-Phosphoglycerate dehydrogenase (PDB ID: 2G76) | ||||
| NAD | −7.7 | CO | Thr77, Arg235 | H-bonds 3.12, 2.99 |
O–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
His205, Ser102, Arg154, Ile155, Gly156 | H-bonds 3.12, 3.22, 3.25, 3.20, 2.86, 3.40 | ||
| NH2, N | Ser211, Asp174 | H-bonds 3.03, 3.37 | ||
| Phenyl ring | Arg235, Pro207 | Alkyl | ||
| CH2 | His205 | Carbon–H bond | ||
| 7a | −7.8 | NO2, N atoms of triazolo-triazine moiety | Ser211, His205, Asp174 | H-bonds, 3.09, 2.84, 3.13 |
| Phenyl and triazine rings | Pro175, Pro207, Thr206 | pi–alkyl, pi-sigma | ||
| Triazole ring | Asp174 | pi–anion | ||
| Ile176, Gly153, Arg154 | van der Walls | |||
| 7g | −8.6 | CO | Gly78 | H-bond 2.33 |
| Phenyl and triazolo-triazine rings | Thr77, Ile155, Ala234, Pro207, Arg235 | pi–donor H-bond, pi–alkyl | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| Phosphoserine aminotransferase (PDB ID: 7T7J) | ||||
| PLP | −6.5 | CO, OH, O–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N O, N |
Tr153, Lys198, Arg77, Gln197, Gly75, Asp174 | H-bond 3.00, 2.72, 3.01, 2.98, 2.26, 2.85, 3.59 |
P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
Gly75 | Carbon–hydrogen bond | ||
| Trp102 | van der Waals | |||
| 7a | −7.4 | NO2 | Ser177, Ser176, Thr153 | H-bonds 3.23, 3.18, 2.93, 3.49 |
| NO, phenyl and triazolo-triazine rings | Trp102, Gln197, Arg77 | Carbon hydrogen bond, pi–donor hydrogen bond, pi–pi stacked, pi–alkyl interactions | ||
| Cys149 | van der Waals | |||
| 7g | −8.5 | CO, N atom of triazine ring | Lys198, Trp102, Gly10 | H-bond 3.19, 3.01, 3.09 |
| Phenyl and 4-chloro phenyl rings | Pro11, Phe80, Trp102, Arg77 | pi–pi stacked, pi–alkyl | ||
| Triazole ring | Lys198 | pi–cation | ||
| Gln197 | van der Waals | |||
Autodock Vina within PyRx software version 8 was utilized to conduct the docking simulations, and the binding energy was used to report the results for each compound. A lower binding energy value signifies a stronger binding affinity. Hydrogen bonds, the strongest interactions, were examined in conjunction with hydrophobic bonds (carbon–hydrogen, van der Waals, Pi–anion, Pi–cation, Pi–sigma, alkyl, Pi–alkyl, etc.). The binding affinity and interaction characteristics of the selected compounds with the target enzymes are depicted in Table 5. The 2D and 3D docked poses, along with the interactions of the co-crystallized ligand, are illustrated in Fig. 9–14. The grid box with the XYZ dimensions 10.19 Å × 29.38 Å × −2.48 Å and center 25.05, 19.72, 23.93 (XYZ coordinates) was defined to cover the 3-phosphoglycerate dehydrogenase binding while for the phosphoserine aminotransferase enzyme (PDB ID: 7T7J), the grid box was identified to cover pyridoxal-5′-phosphate (PLP) with dimensions 16.00 Å × 14.48 Å × 18.09 Å and a center 14.85, 5.89, 11.21 (X, Y, Z).
 | ||
| Fig. 9 (A) 3D conformations of the native (orange), re-docked ligand (yellow), compound 7a (green), and compound 7g (blue) within the active site of human 3-phosphoglycerate dehydrogenase (PDB ID: 2G76) indicating that they are superimposed in the same position. (B) 2D conformations of the re-docked co-crystalline ligand NAD within the active site of human 3-phosphoglycerate dehydrogenase (PDB ID: 2G76). | ||
Assessment of the binding affinity of compounds 7a and 7g within the active site of human 3-phosphoglycerate dehydrogenase (PDB ID: 2G76)
Firstly, the docking setup was validated by the self-docking of the co-crystallized ligand NAD into the PHGDH active pocket (PDB ID: 2G76). The docking result indicates that the re-docked NAD was superimposed on the native ligand with a docking score of −7.7 kcal mol−1 and formed multiple interactions with the PHGDH active site (Fig. 9A and B). The NAD revealed intermolecular hydrogen bonds with Thr77, Arg235, His205, Ser102, Arg154, Ile155, Gly156, Ser211, and Asp174 and interacted via alkyl and carbon–hydrogen hydrophobic interactions with the Arg235, Pro207, and His205 amino acids of the binding site (Fig. 9B). Furthermore, compounds 7a and 7g were then docked into the PHGDH active site, which demonstrated better docking scores of −7.8 and −8.6 kcal mol−1 than NAD, respectively, and both of them were superimposed on the same position as the co-crystalline ligand (Table 5 and Fig. 9A). Compound 7a formed a stable complex with the enzyme active pocket by establishing three hydrogen bonds with Ser211, His205, Asp174, Ile176, Gly153, and Arg154 residues, along with various hydrophobic π–alkyl, π–sigma, π–anion, and van der Waals interactions similar to the co-crystalline ligand (Fig. 10). On the other hand, compound 7g exhibited one hydrogen bond with Gly78, in addition to the π-donor H-bond, π–alkyl with Thr77, Ile155, Ala234, Pro207, and Arg235 amino acids (Fig. 11). | ||
| Fig. 10 (A) 3D conformations of compound 7a within the active site of human 3-phosphoglycerate dehydrogenase (PDB ID: 2G76); (B) 2D conformations of compound 7a within the active site of human 3-phosphoglycerate dehydrogenase (PDB ID: 2G76). | ||
 | ||
| Fig. 11 (A) 3D conformations of compound 7g within the active site of human 3-phosphoglycerate dehydrogenase (PDB ID: 2G76). (B) 2D conformations of compound 7g within the active site of human 3-phosphoglycerate dehydrogenase (PDB ID: 2G76). | ||
Assessment of the binding affinity of compounds 7a and 7g within the active site of phosphoserine aminotransferase (PDB ID: 7T7J)
The co-crystallized ligand, pyridoxal-5′-phosphate (PLP), was re-docked into the active site of PSAT1, and the results showed that the re-docked PLP superimposed on the native ligand, achieving a docking score of −6.5 kcal mol−1 (Table 5 and Fig. 9A and B). Analysis of the binding site revealed that PLP formed hydrogen bonds, a carbon H-bond, and van der Waals interactions with the amino acids Thr153, Lys198, Arg77, Gln197, Gly75, Asp174, and Trp102 (Table 5 and Fig. 12B). | ||
| Fig. 12 (A) 3D conformations of the native (orange), re-docked ligand (yellow), compound 7a (green), and compound 7g (blue) within the active site of phosphoserine aminotransferase (PDB ID: 7T7J) indicating that they are superimposed in the same position. (B) 2D conformations of the re-docked co-crystalline ligand PLP within the active site of phosphoserine aminotransferase (PDB ID: 7T7J). | ||
The docking results for compounds 7a and 7g revealed docking scores of −7.4 and −8.5, respectively, outperforming the co-crystalline ligand PLP. Both compounds formed stable complexes with the PSAT1 active site and aligned with the same position as PLP (Table 5 and Fig. 12A). Compound 7a established four hydrogen bonds with Ser177, Ser176, and Thr153, along with hydrophobic interactions involving Trp102, Gln197, Arg77, and Cys149 residues (Fig. 13). On the other hand, compound 7g displayed three hydrogen bonds with Lys198, Trp102, and Gly10, in addition to the six pi–pi stacked, pi–alkyl, pi–cation, and van der Waals interactions with the amino acids Pro11, Phe80, Trp102, Arg77, Lys198, and Gln197 similar to the co-crystalline ligand (Fig. 14).
 | ||
| Fig. 13 (A) 3D conformations of compound 7a within the active site of phosphoserine aminotransferase (PDB ID: 7T7J). (B) 2D conformations of compound 7a within the active site of phosphoserine aminotransferase (PDB ID: 7T7J). | ||
 | ||
| Fig. 14 (A) 3D conformations of compound 7g within the active site of phosphoserine aminotransferase (PDB ID: 7T7J). (B) 2D conformations of compound 7g within the active site of phosphoserine aminotransferase (PDB ID: 7T7J). | ||
Materials and methods
Chemistry
Melting points were measured with an Electrothermal 9100 apparatus and were uncorrected. The IR spectra were recorded using a FTIR Bruker-vector 22 spectrophotometer and KBr pellets. The 1H and 13C NMR spectra were recorded in CDCl3 or DMSO-d6 as a solvent on the Varian Gemini NMR spectrometer at 300 MHz and 75 MHz, respectively. Chemical shifts were reported as δ values in ppm. The elemental analyses were performed at the Microanalytical Center, Cairo University. 6-Methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 16 and hydrazonoyl halides 1
16 and hydrazonoyl halides 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17–21 were prepared using the reported procedures.
17–21 were prepared using the reported procedures.
3,6-Dimethyl-1-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7a). Brown crystals (EtOH + DMF); mp 262–264 °C; yield (67%); 1H NMR (300 MHz, DMSO-d6) δ 2.32 (s, 3H, CH3), 2.58 (s, 3H, CH3), 8.38 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.47 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, DMSO-d6) δ 16.5, 18.4, 118.9, 124.5, 138.9, 145.7, 151.8, 153.0, 155.3, 161.7. Anal. calcd for C12H10N6O3 (286.25), C, 50.35; H, 3.52; N, 29.36. Found, C, 50.46; H, 3.39; N, 29.47.
3-Ethyl-6-methyl-1-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7b). Brown crystals (EtOH); mp 200–202 °C; yield (69%). 1H NMR (300 MHz, CDCl3) δ 1.48 (t, 3H, CH2C
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3, J ≈ 8 Hz), 2.45 (s, 3H, CH3), 3.03 (q, 2H, C
3, J ≈ 8 Hz), 2.45 (s, 3H, CH3), 3.03 (q, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3, J ≈ 8 Hz), 8.30 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.45 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 9.7, 17.5, 18.2, 119.5, 124.7, 141.0, 145.2, 147.9, 148.0, 155.7, 161.5. Anal. calcd for C13H12N6O3 (300.28), C, 52.00; H, 4.03; N, 27.99. Found, C, 52.11; H, 4.15; N, 27.86.
2CH3, J ≈ 8 Hz), 8.30 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.45 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 9.7, 17.5, 18.2, 119.5, 124.7, 141.0, 145.2, 147.9, 148.0, 155.7, 161.5. Anal. calcd for C13H12N6O3 (300.28), C, 52.00; H, 4.03; N, 27.99. Found, C, 52.11; H, 4.15; N, 27.86.
6-Methyl-1-(4-nitrophenyl)-3-propyl-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7c). Brown crystals (DMF); mp 202–204 °C; yield (64%). 1H NMR (300 MHz, CDCl3) δ 1.13 (t, 3H, CH2CH2C
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3, J ≈ 8 Hz), 1.91–1.99 (m, 2H, CH2C
3, J ≈ 8 Hz), 1.91–1.99 (m, 2H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH3), 2.48 (s, 3H, CH3), 3.00 (t, 2H, C
2CH3), 2.48 (s, 3H, CH3), 3.00 (t, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH2CH3, J ≈ 8 Hz), 8.34 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.49 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 13.6, 18.4, 19.2, 25.6, 119.7, 124.9, 141.2, 145.5, 147.0, 148.1, 156.0, 161.6. Anal. calcd for C14H14N6O3 (314.31), C, 53.50; H, 4.49; N, 26.74. Found, C, 53.61; H, 4.37; N, 26.87.
2CH2CH3, J ≈ 8 Hz), 8.34 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.49 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 13.6, 18.4, 19.2, 25.6, 119.7, 124.9, 141.2, 145.5, 147.0, 148.1, 156.0, 161.6. Anal. calcd for C14H14N6O3 (314.31), C, 53.50; H, 4.49; N, 26.74. Found, C, 53.61; H, 4.37; N, 26.87.
3-Isopropyl-6-methyl-1-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7d). Brown crystals (EtOH); mp 208–210 °C; yield (66%). 1H NMR (300 MHz, CDCl3) δ 1.52 (d, 6H, CH(C
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2, J ≈ 7 Hz), 2.47 (s, 3H, CH3), 3.43–3.52 (m, 1H, C
3)2, J ≈ 7 Hz), 2.47 (s, 3H, CH3), 3.43–3.52 (m, 1H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2), 8.32 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.48 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 18.3, 19.1, 24.9, 119.6, 124.7, 141.1, 145.2, 148.1, 150.9, 155.6, 161.5. Anal. calcd for C14H14N6O3 (314.31), C, 53.50; H, 4.49; N, 26.74. Found, C, 53.60; H, 4.38; N, 26.86.
(CH3)2), 8.32 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.48 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 18.3, 19.1, 24.9, 119.6, 124.7, 141.1, 145.2, 148.1, 150.9, 155.6, 161.5. Anal. calcd for C14H14N6O3 (314.31), C, 53.50; H, 4.49; N, 26.74. Found, C, 53.60; H, 4.38; N, 26.86.
3-Isobutyl-6-methyl-1-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7e). Brown crystals (EtOH); mp 202–204 °C; yield (63%). 1H NMR (300 MHz, CDCl3) δ 1.12 (d, 6H, CH2CH(C
![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 3)2, J ≈ 7 Hz), 2.30–2.39 (m, 1H, CH2C
3)2, J ≈ 7 Hz), 2.30–2.39 (m, 1H, CH2C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) (CH3)2), 2.48 (s, 3H, CH3), 2.93 (d, 2H, C
(CH3)2), 2.48 (s, 3H, CH3), 2.93 (d, 2H, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2CH(CH3)2, J ≈ 7 Hz), 8.36 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.50 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 18.4, 22.3, 26.4, 32.2, 94.7, 119.7, 124.9, 141.1, 145.5, 146.4, 156.0, 161.6. Anal. calcd for C15H16N6O3 (328.33), C, 54.87; H, 4.91; N, 25.60. Found, C, 54.99; H, 4.79; N, 25.72.
2CH(CH3)2, J ≈ 7 Hz), 8.36 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz), 8.50 (d, 2H, 4-NO2C6H4, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 18.4, 22.3, 26.4, 32.2, 94.7, 119.7, 124.9, 141.1, 145.5, 146.4, 156.0, 161.6. Anal. calcd for C15H16N6O3 (328.33), C, 54.87; H, 4.91; N, 25.60. Found, C, 54.99; H, 4.79; N, 25.72.
6-Methyl-1-phenyl-3-(p-tolyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7f). Beige crystals (DMF); mp 250–252 °C; yield (72%). 1H NMR (300 MHz, CDCl3) δ 2.47 (s, 3H, CH3), 2.53 (s, 3H, CH3), 7.33–7.38 (m, 3H, Ar-H), 7.51 (t, 2H, Ar-H, J ≈ 8 Hz), 8.18–8.24 (m, 4H, Ar-H); 13C NMR (75 MHz, CDCl3) δ 18.4, 20.9, 121.6, 125.1, 126.4, 128.7, 129.2, 131.5, 138.5, 140.9, 147.9, 151.4, 152.8, 161.7. Anal. calcd for C18H15N5O (317.35), C, 68.13; H, 4.76; N, 22.07. Found, C, 68.25; H, 4.63; N, 22.18.
3-(4-Methoxyphenyl)-6-methyl-1-phenyl-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7g). White crystals (DMF); mp 256–258 °C; yield (70%). 1H NMR (300 MHz, DMSO-d6) δ 2.34 (s, 3H, CH3), 3.87 (s, 3H, OCH3), 7.20 (d, 2H, Ar-H, J ≈ 9 Hz), 7.42 (t, 1H, Ar-H, J ≈ 8 Hz), 7.60 (t, 2H, Ar-H, J ≈ 8 Hz), 8.12 (d, 2H, Ar-H, J ≈ 9 Hz), 8.19 (d, 2H, Ar-H, J ≈ 9 Hz); 13C NMR (75 MHz, DMSO-d6) δ 18.2, 55.5, 113.8, 120.7, 122.8, 126.3, 128.9, 130.8, 138.4, 147.9, 151.4, 152.6, 160.4, 161.8. Anal. calcd for C18H15N5O2 (333.35), C, 64.86; H, 4.54; N, 21.01. Found, C, 64.98; H, 4.41; N, 21.14.
3-(4-Chlorophenyl)-6-methyl-1-phenyl-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7h). Yellow crystals (DMF); mp 245–247 °C; yield (74%). 1H NMR (300 MHz, CDCl3) δ 2.54 (s, 3H, CH3), 7.38 (t, 1H, Ar-H, J ≈ 8 Hz), 7.49–7.58 (m, 4H, Ar-H), 8.22 (d, 2H, Ar-H, J ≈ 8 Hz), 8.31 (d, 2H, Ar-H, J ≈ 9 Hz); 13C NMR (75 MHz, CDCl3) δ 18.6, 120.4, 122.0, 127.5, 128.0, 129.3, 131.9, 136.0, 138.1, 148.0, 151.9, 155.9, 161.9. Anal. calcd for C17H12ClN5O (337.77), C, 60.45; H, 3.58; N, 20.73. Found, C, 60.57; H, 3.72; N, 20.86.
3-(Furan-2-yl)-6-methyl-1-(4-nitrophenyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7i). Pale brown crystals (CH3CN); mp 269–271 °C; yield (71%). 1H NMR (300 MHz, DMSO-d6) δ 2.39 (s, 3H, CH3), 6.87–6.89 (m, 1H, furyl-H), 7.66 (d, 1H, furyl-H, J ≈ 3 Hz), 8.15 (d, 1H, furyl-H, J ≈ 1 Hz), 8.41–8.49 (m, 4H, 4-NO2C6H4); 13C NMR (75 MHz, DMSO-d6) δ 18.2, 112.6, 117.1, 119.8, 125.3, 136.5, 137.4, 141.2, 145.0, 147.1, 148.3, 154.9, 160.8. Anal. calcd for C15H10N6O4 (338.28), C, 53.26; H, 2.98; N, 24.84. Found, C, 53.38; H, 2.86; N, 24.95.
6-Methyl-1-(4-nitrophenyl)-3-(thiophen-2-yl)-[1,2,4]triazolo[4,3-b][1,2,4]triazin-7(1H)-one (7j). Pale brown crystals (DMF); mp 242–244 °C; yield (69%). 1H NMR (300 MHz, DMSO-d6) δ 2.38 (s, 3H, CH3), 7.36 (t, 1H, thieny-H, J ≈ 5 Hz), 8.05 (d, 1H, thieny-H, J ≈ 5 Hz), 8.22 (d, 1H, thieny-H, J ≈ 5 Hz), 8.38–8.47 (m, 4H, 4-NO2C6H4); 13C NMR (75 MHz, DMSO-d6) δ 18.3, 119.7, 120.4, 123.4, 125.3, 128.6, 131.7, 132.2, 139.9, 141.1, 144.9, 154.8, 160.8. Anal. calcd for C15H10N6O3S (354.34), C, 50.84; H, 2.84; N, 23.72. Found, C, 50.95; H, 2.71; N, 23.85.
Anti-cancer activity
Gene expression analysis
RNA isolation and reverse transcription (RT) reaction. The RNeasy Mini Kit (Qiagen, Hilden, Germany), augmented with a DNaseI (Qiagen) digestion step, was employed to extract total RNA from lung cancer cell lines following the manufacturer's procedure. The isolated total RNA was treated with one unit of RQ1 RNAse-free DNAse (Invitrogen, Germany) to eliminate DNA remnants, re-suspended in DEPC-treated water, and quantified photometrically at 260 nm. The purity of total RNA was evaluated using the 260/280 nm ratio, which ranged from 1.8 to 2.1.27
Furthermore, integrity was confirmed using ethidium bromide-stained examination of 28S and 18S bands via formaldehyde-containing agarose gel electrophoresis. Aliquots were utilized immediately for reverse transcription (RT); otherwise, they were preserved at −80 °C.
The poly(A) + RNA extracted from the lung cancer cell lines was reverse transcribed into the cDNA in a total amount of 20 μl utilizing the Revert Aid TM First Strand cDNA Synthesis Kit (Fermentas, Germany). A total of 5 μg of RNA was utilized with a master mix. The master mix comprised 50 mM MgCl2, 10× RT buffer (50 mM KCl; 10 mM Tris–HCl; pH 8.3), 10 mM of each dNTP, 50 μM oligo-dT primer, 20 IU ribonuclease inhibitor (50 kDa recombinant enzyme to suppress RNase activity), and 50 IU MuLV reverse transcriptase. Each sample mixture was centrifuged for 30 seconds at 1000 g and thereafter transferred to the thermocycler. The RT reaction was conducted at 25 °C for 10 minutes, subsequently at 42 °C for 1 hour, and concluded with a denaturation phase at 99 °C for 5 minutes. Subsequently, the reaction tubes containing RT-prepared samples were rapidly chilled in an ice chamber prior to cDNA amplification via the quantitative real-time polymerase chain reaction (qRT-PCR).
Quantitative real-time-PCR (qRT-PCR). The cDNA copy number of lung cancer cell lines was determined using the StepOne™ Real-Time PCR System from Applied Biosystems (Thermo Fisher Scientific, Waltham, MA, USA).
The PCR reactions were prepared in 25 μl mixes comprising 12.5 μl of 1× SYBR® Premix Ex Taq™ (TaKaRa, Biotech. Co. Ltd), 0.5 μl of 0.2 μM sense primer, 0.5 μl of 0.2 μM antisense primer, 6.5 μl of distilled water, and 5 μl of cDNA template.
The reaction protocol was divided into three stages. The initial step was conducted at 95 °C for a duration of 3 minutes. The second step comprised 40 cycles, each divided into three phases: (a) 95 °C for 15 seconds, (b) 55 °C for 30 seconds, and (c) 72 °C for 30 seconds. The third stage comprised 71 cycles, first at 60 °C and subsequently increasing by approximately 0.5 °C every 10 seconds until 95 °C. Every experiment incorporated a distilled water control. The particular primer sequences for the lung cancer cell lines (BCL-2, BAX, and p53 genes, Brito et al., 2012)28 were created and are presented in Table 6. A melting curve analysis was conducted at 95.0 °C after each qPCR to assess the quality of the utilized primers. The relative quantification of the target gene to the reference gene was assessed using the 2−ΔΔCT method.29–31
| Gene | Primer sequence | GenBank (accession no.) |
|---|---|---|
| a BCL-2: B-cell lymphoma-2 gene; BAX: BCL-2-associated X protein-encoding gene; p53: tumor suppressor gene; GAPDH: glyceraldehyde-3-phosphate dehydrogenase. | ||
| BCL-2 | F: TTCCGCGTGATTGAAGACAC | KY098818.1 |
| R: ACTTCATCACTATCTCCCGGT | ||
| p53 | F: GGAAATCTCACCCCATCCCA | AB082923.1 |
| R: CAGTAAGCCAAGATCACGCC | ||
| BAX | F: AACATGGAGCTGCAGAGGAT | L22474.1 |
| R: CCAATGTCCAGCCCATGATG | ||
| GAPDH | F: AGGTCGGAGTCAACGGATTT | NM_001357943.2 |
| R:ATCGCCCCACTTGATTTTGG | ||
DNA damage evaluation using the comet assay
The determination of DNA damage via the comet assay was conducted using lung cancer cell lines, following the methodology established by Olive et al. (1990).32 Following trypsin treatment to generate a single-cell suspension, approximately 1.5 × 104 cells were embedded in 0.75% low-gelling-temperature agarose and swiftly pipetted onto a pre-coated microscope slide.The samples underwent lysis for 4 hours at 50 °C in a solution containing 0.5% SDS and 30 mM EDTA at pH 8.0. Following an overnight rinse at room temperature in a Tris/borate/EDTA buffer, pH 8.0, samples underwent electrophoresis for 25 minutes at 0.6 V cm−1 and were subsequently stained with propidium iodide. The slides were examined with a fluorescence microscope equipped with a CCD camera, and 150 individual comet images were analyzed from each sample for a tail moment, DNA content, and percentage of DNA in the tail. Approximately 100 cells were analyzed per sample to assess the percentage of cells exhibiting comet-like DNA damage.
The non-overlapping cells were randomly selected and assigned a score on a scale of 0–3 based on the comet tail length migration and the relative proportion of DNA in the nucleus. Class 0 indicates no detectable DNA damage and no tail; class 1 indicates a tail length less than the diameter of the nucleus; class 2 indicates a tail length between 1× and 2× the nuclear diameter; and class 3 indicates a tail longer than 2× the diameter of the nucleus (Collins et al., 1997).33
DNA fragmentation assay
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g for 20 min. Fragmented DNA in the supernatant was extracted with an equal volume of a neutral phenol
000 g for 20 min. Fragmented DNA in the supernatant was extracted with an equal volume of a neutral phenol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) chloroform
chloroform![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isoamyl alcohol mixture (25
isoamyl alcohol mixture (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and analyzed electrophoretically on 2% agarose gels containing 0.1 μg ml−1 ethidium bromide.
1) and analyzed electrophoretically on 2% agarose gels containing 0.1 μg ml−1 ethidium bromide.Diphenylamine reaction procedure
The lung cancer cell lines (A549) were used to determine the quantitative profile of the DNA fragmentation. The cell lines were collected immediately after the culture and treatment with PT1 and PT7 as well as with Dox. The cancer cells were lysed in 0.5 ml of lysis buffer containing 10 mM Tris–HCl (pH 8), 1 mM EDTA, and 0.2% Triton X-100 and centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (Eppendorf) for 20 min at 4 °C. The pellets were re-suspended in 0.5 ml of lysis buffer. To the pellets (P) and the supernatants (S), 0.5 ml of 25% tri-chloroacetic acid (TCA) was added and incubated at 4 °C for 24 h. The cells were then centrifuged for 20 min at 10
000 rpm (Eppendorf) for 20 min at 4 °C. The pellets were re-suspended in 0.5 ml of lysis buffer. To the pellets (P) and the supernatants (S), 0.5 ml of 25% tri-chloroacetic acid (TCA) was added and incubated at 4 °C for 24 h. The cells were then centrifuged for 20 min at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm (Eppendorf) at 4 °C and the pellets were suspended in 80 ml of 5% TCA, followed by incubation at 83 °C for 20 min. Subsequently, to each cell sample, 160 ml of diphenylamine (DPA) solution [150 mg DPA in 10 ml glacial acetic acid, 150 ml of sulfuric acid and 50 ml acetaldehyde (16 mg ml−1)] was added and incubated at room temperature for 24 h (Gibb et al.,35 1997). The proportion of the fragmented DNA was calculated from the absorbance values at 600 nm wavelength using the formula:
000 rpm (Eppendorf) at 4 °C and the pellets were suspended in 80 ml of 5% TCA, followed by incubation at 83 °C for 20 min. Subsequently, to each cell sample, 160 ml of diphenylamine (DPA) solution [150 mg DPA in 10 ml glacial acetic acid, 150 ml of sulfuric acid and 50 ml acetaldehyde (16 mg ml−1)] was added and incubated at room temperature for 24 h (Gibb et al.,35 1997). The proportion of the fragmented DNA was calculated from the absorbance values at 600 nm wavelength using the formula:| % fragmented DNA = [OD(S)/[OD(S) + OD(P)]] × 100 |
Statistical analysis
All data were analyzed using the General Linear Model (GLM) procedure of the Statistical Analysis System (SAS) (1982),36 followed by the Scheffé-test to assess significant differences between groups. The values are expressed as mean ± SEM. All statements of significance were based on a probability of P < 0.05.Molecular docking simulation
The molecular docking of the most active compounds 7a and 7g with two enzymes that play an important role in lung cancer progression, namely, 3-phosphoglycerate dehydrogenase (PHGDH) and phosphoserine aminotransferase (PSAT1), was performed on Autodock 4 in PyRx software version 8.37,38 The 3D structures of the target enzymes were obtained from the RCSB protein data bank in the PDB format using codes 2G76 (3-phosphoglycerate dehydrogenase in complex with nicotinamide–adenine–dinucleotide (NAD)) and 7T7J (phosphoserine aminotransferase in complex with pyridoxal-5′-phosphate (PLP)).39,40Before the docking process, the enzymes underwent preparation, including removal of the co-crystallized ligands and water molecules, then optimization using the QuickPrep tool module in the MOE program. The resulting files were saved as pdb and then converted to the PDBQT format using Autodock Vina tools. Autodock tools were employed to set the size and the center of the grid box that covers the native ligands completely. The docking protocol was validated by re-docking the co-crystallized ligands into the enzyme-active sites and the RMSD was calculated using the DockRMSD server.41 The chemical structure of the selected compounds 7a and 7g were constructed with the ChemDraw ultra-10.0, saved as SDF files, then minimized by applying the MMFF94 force field and converted to pdbqt files using OpenBable tools involved in PyRx software. PyRx software presented the 8 most suitable docking poses of the ligand–protein complex after the docking was completed and subsequently ranked according to the binding energy. We selected the first docking pose, which is the most suitable pose where the ligands have the lowest binding energy, zero Å root-mean-square deviation (RMSD) and strongly interact with the protein's catalytic cavity, and visualized using BIOVIA Discovery Studio Visualizer 2021 for insights into the ligand binding position in the protein cavity.
Crystallography
The data for compound 7b was collected at a low temperature (100 K) on a Rigaku XtaLAB Synergy dual microfocus X-ray diffractometer, using a PhotonJet-S series of microfocus X-ray source, Cu-Kα radiation (λ = 1.54184 Å) and equipped with an Oxford Cryosystems Cooler Device. The cell parameters of the final unit were obtained by means of a least-squares refinement. The structure was solved by Direct Methods using SHELXT 2018/2 (ref. 42) and refined by means of least-squares procedures on a F2 using the program CRYSTALS.43 The Atomic Scattering Factors were taken from the International Tables for X-Ray Crystallography.44 All hydrogen atoms were geometrically placed and refined using a riding model.All non-hydrogen atoms were anisotropically refined, and in the last cycles of refinement, a weighting scheme was used, where weights are calculated from the following formula:
| w = 1/[2(Fo2) + (aP)2 + bP] |
Conclusions
In summary, we reported a novel series of [1,2,4]triazolo[4,3-b][1,2,4]triazin-7-one derivatives (7a–j) by the reaction of 6-methyl-3-thioxo-3,4-dihydro-1,2,4-triazin-5(2H)-one with the corresponding hydrazonoyl halides in chloroform. The chemical structures of these compounds were proven using spectral data, elemental analyses, and single-crystal X-ray diffraction. The anti-cancer activity of these compounds was determined against the PC3, A549, PACA2 and BJ1 using MTT assay. Two compounds, 7a and 7g, showed potent anti-cancer activities with low IC50 values (36.6 and 40.1 μM, respectively) compared to the reference drug with an IC50 value of 43.8 μM on lung cell lines and demonstrated safe mortality effect on the normal cell line (BJ1) with cytotoxicity percentages of 3.5% and 2.8%, respectively. These compounds (7a and 7g) were investigated further to determine their mechanism of action using DNA fragmentation, DNA damage and gene expression (BCL-2, BAX, and p53 genes). Molecular docking was employed to explore the binding affinity between the most active compounds (7a and 7g) and two key enzymes, PHGDH and PSAT1, which play vital roles in the progression of lung cancer.Data availability
The data supporting this article (copies of 1H, 13C NMR spectra and single-crystal X-ray details) have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- R. Aggarwal and G. Sumran, An insight on medicinal attributes of 1,2,4-triazoles, Eur. J. Med. Chem., 2020, 205, 112652 CrossRef CAS PubMed.
- P. Yang, X. Zheng, G. Zhang, C. Lei, G. Cheng and H. Yang, Construction of heterocycle-triazolotriazine framework energetic compounds: towards novel high-performance explosives, Chem. Commun., 2024, 60, 10588–10591 RSC.
- Q. Wang, Y. Shao and M. Lu, Amino-tetrazole functionalized fused triazolo-triazine and tetrazolo-triazine energetic materials, Chem. Commun., 2019, 55, 6062–6065 RSC.
- G. Zhang, W. Hu, J. Ma, H. Yang and G. Cheng, Combining 5,6-fused triazolo-triazine with pyrazole: A novel energetic framework for heat-resistant explosive, Chem. Eng. J., 2021, 426, 131297 CrossRef CAS.
- M. Mojzych, P. Tarasiuk, Z. Karczmarzyk, M. Juszczak, W. Rzeski, A. Fruzinski and A. Wozny, Synthesis, Structure and Antiproliferative Activity of New pyrazolo[4,3- e]triazolo[4,5-b][1,2,4]triazine Derivatives, Med. Chem., 2018, 14, 53–59 CAS.
- H. Peng, G. Kumaravel, G. Yao, L. Sha, J. Wang, H. Van Vlijmen, T. Bohnert, C. Huang, C. B. Vu, C. L. Ensinger, H. Chang, T. M. Engber, E. T. Whalley and R. C. Petter, Novel Bicyclic Piperazine Derivatives of Triazolotriazine and Triazolopyrimidines as Highly Potent and Selective Adenosine A2A Receptor Antagonists, J. Med. Chem., 2004, 47, 6218–6229 CrossRef CAS PubMed.
- Y. Guo, X. Peng, Y. Ji, Y. Zhang, J. Ding, Z. Zhan, J. Ai and W. Duan, Synthesis of triazolotriazine derivatives as c-Met inhibitors, Mol. Diversity, 2020, 25, 839–846 CrossRef PubMed.
- F. Akahoshi, S. Takeda, T. Okada, M. Kajii, H. Nishimura, M. Sugiura, Y. Inoue, C. Fukaya, Y. Naito, T. Imagawa and N. Nakamura, Synthesis and Pharmacological Activity of Triazolo[1,5-a]triazine Derivatives Inhibiting Eosinophilia, J. Med. Chem., 1998, 41, 2985–2993 CrossRef CAS PubMed.
- A. A. Thai, B. J. Solomon, L. V. Sequist, J. F. Gainor and R. S. Heist, Lung cancer, Lancet, 2021, 398, 535–554 CrossRef PubMed.
- B. Cheng, S. Xiong, C. Li, H. Liang, Y. Zhao, J. Li, J. Shi, L. Ou, Z. Chen, P. Liang, W. Liang and J. He, An annual review of the remarkable advances in lung cancer clinical research in 2019, J. Thorac. Dis., 2020, 12, 1056–1069 CrossRef PubMed.
- N. Samanci and E. Celik, The top 100 cited articles in lung cancer – a bibliometric analysis, Wspólcz. Onkol., 2020, 24, 17–28 CrossRef PubMed.
- M. F. Mridha, A. R. Prodeep, A. S. M. M. Hoque, M. R. Islam, A. A. Lima, M. M. Kabir, M. A. Hamid, Y. Watanobe and R. Morales, A Comprehensive Survey on the Progress, Process, and Challenges of Lung Cancer Detection and Classification, J. Healthc. Eng., 2022, 2022, 1–43 CrossRef PubMed.
- T. Barr, S. Ma, Z. Li and J. Yu, Recent advances and remaining challenges in lung cancer therapy, Chin. Med. J., 2024, 137, 533–546 CrossRef CAS PubMed.
- G. S. Jones and D. R. Baldwin, Recent advances in the management of lung cancer, Clin. Med., 2018, 18, s41–s46 CrossRef PubMed.
- L. Scharnetzki and J. H. Schiller, Lung Cancer, Chest, 2021, 159, 1721–1722 CrossRef PubMed.
- E. A. Falco, E. Pappas and G. H. Hitchings, 1,2,4-Triazine Analogs of the Natural Pyrimidines, J. Am. Chem. Soc., 2002, 78, 1938–1941 CrossRef.
- H. M. Hassaneen, H. A. H. Mousa, N. M. Abed and A. S. Shawali, Chemistry of C-Heteroarylhydrazidoyl Halides. Synthesis and Reactions of N-(p-Nitrophenyl)-C-(2-thienyl)-formohydrazidoyl Halides, Heterocycles, 1988, 27, 695 CrossRef CAS.
- T. Katada, S. Eguchi and T. Sasaki, Thermal cycloaddition reactions of thiocarbonyl compounds. Part 2. 1,3-dipolar cycloaddition reactions of adamantanethione with nitrile oxides, nitrilimines, and diazoalkanes, J. Chem. Soc., Perkin Trans. 1, 1984, 2641 RSC.
- N. M. Rateb, Synthesis of Some New Thiazole, Thiophene, and 2,3-Dihydro-1,3,4-thiadiazole and Pyrimidino[1,2-b]indazole Derivatives, Phosphorus, Sulfur, Silicon Relat. Elem., 2005, 180, 2361–2372 CrossRef CAS.
- D. L. Rector, S. D. Folz, R. D. Conklin, L. H. Nowakowski and G. Kaugars, Structure-activity relationships in a broad-spectrum anthelmintic series. Acid chloride phenylhydrazones. I. Aryl substitutions and chloride variations, J. Med. Chem., 2002, 24, 532–538 CrossRef PubMed.
- A. F. Hegarty, M. P. Cashman and F. L. Scott, Synthesis of aliphatic hydrazonyl bromides and kinetics of their conversion into hydrazides, J. Chem. Soc., Perkin Trans. 2, 1972, 1381 RSC.
- J. Daunis, H. Lopez and G. Maury, Heteroaromatic 10-.pi.-electron systems. New s-triazolo-as-triazines with a bridgehead nitrogen atom, J. Org. Chem., 2002, 42, 1018–1022 CrossRef.
- M. Tisler and Z. Vrbaski, Reaction of 4-Arylthiosemicarbazides with Some α-Keto Acids and Synthesis of Some Substituted 3-Thioxo-5-oxo-2,3,4,5-tetrahydro-1,2,4-triazines, J. Org. Chem., 2002, 25, 770–773 CrossRef.
- F. M. Sroor, A. F. El-Sayed and K. Mahmoud, Novel 5-Fluorouracil analogues versus perfluorophenyl ureas as potent anti-breast cancer agents: design, robust synthesis, in vitro, molecular docking, pharmacokinetics ADMET analysis and dynamic simulations, Bioorg. Chem., 2024, 153, 107944 CrossRef CAS PubMed.
- F. M. Sroor, W. M. Basyouni, W. M. Tohamy, T. H. Abdelhafez and M. K. El-awady, Novel pyrrolo[2,3-d]pyrimidine derivatives: Design, synthesis, structure elucidation and in vitro anti-BVDV activity, Tetrahedron, 2019, 75, 130749 CrossRef CAS.
- F. M. Sroor, A. M. Othman, K. Mahmoud and K. F. Mahrous, New 2,4-diaryl-3-azabicyclo[3.3.1]nonan-9-one derivatives as antimicrobial and anti-cancer agents: Synthesis, in-vitro and SAR studies, J. Mol. Struct., 2023, 1294, 136516 CrossRef CAS.
- F. M. Sroor, H. K. A. Elhakim, S. M. Abdelrehim, K. F. Mahrous, A. F. El-Sayed and I. A. Abdelhamid, New cyano-acrylamide derivatives incorporating the thiophene moiety: Synthesis, anti-cancer, gene expression, DNA fragmentation, DNA damage, and in silico studies, J. Mol. Struct., 2025, 1321, 140001 CrossRef CAS.
- A. F. Brito, A. M. Abrantes, C. Pinto-Costa, A. R. Gomes, A. C. Mamede, J. Casalta-Lopes, A. C. Gonçalves, A. B. Sarmento-Ribeiro, J. G. Tralhão and M. F. Botelho, Hepatocellular Carcinoma and Chemotherapy: The Role of p53, Chemotherapy, 2012, 58, 381–386 CrossRef CAS PubMed.
- N. Yasser, F. M. Sroor, H. M. El-Shorbagy, S. M. Eissa, H. M. Hassaneen and I. A. Abdelhamid, Synthesis, anticancer evaluation of novel hybrid pyrazole-based chalcones, molecular docking, DNA fragmentation, and gene expression: in vitro studies, RSC Adv., 2024, 14, 21859–21873 RSC.
- F. M. Sroor, A. A. F. Soliman, E. M. Youssef, M. Abdelraof and A. F. El-Sayed, Green, facile synthesis and evaluation of unsymmetrical carbamide derivatives as antimicrobial and anticancer agents with mechanistic insights, Sci. Rep., 2024, 14, 15441 CrossRef CAS PubMed.
- M. G. Kamel, F. M. Sroor, M. K. H. Hanafy, K. F. Mahrous and H. M. Hassaneen, Design, synthesis and potent anti-pancreatic cancer activity of new pyrazole derivatives bearing chalcone, thiazole and thiadiazole moieties: gene expression, DNA fragmentation, cell cycle arrest and SAR, RSC Adv., 2024, 14, 26954–26970 RSC.
- P. L. Olive, J. P. Banáth and R. E. Durand, Heterogeneity in Radiation-Induced DNA Damage and Repair in Tumor and Normal Cells Measured Using the “Comet” Assay, Radiat. Res., 2012, 178, AV35–AV42 CrossRef CAS PubMed.
- A. Collins, M. Dusinska, M. Franklin, M. Somorovska, H. Petrovska, S. Duthie, L. Fillion, M. Panayiotidis, K. Raslova and N. Vaughan, Comet assay in human biomonitoring studies: Reliability, validation, and applications, Environ. Mol. Mutagen., 1997, 30, 139–146 CrossRef CAS PubMed.
- A. Yawata, M. Adachi, H. Okuda, Y. Naishiro, T. Takamura, M. Hareyama, S. Takayama, J. C. Reed and K. Imai, Prolonged cell survival enhances peritoneal dissemination of gastric cancer cells, Oncogene, 1998, 16, 2681–2686 CrossRef CAS PubMed.
- R. K. Gibb, D. D. Taylor, T. Wan, D. M. O’Connor, D. L. Doering and Ç. Gerçel-Taylor, Apoptosis as a Measure of Chemosensitivity to Cisplatin and Taxol Therapy in Ovarian Cancer Cell Lines, Gynecol. Oncol., 1997, 65, 13–22 CrossRef CAS PubMed.
- SAS Institute, SAS User's Guide: Statistics, SAS Institute, Cary, N.C., 1982 Search PubMed.
- O. Trott and A. J. Olson, AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem., 2009, 31, 455–461 CrossRef PubMed.
- F. M. Sroor, A. F. El-Sayed and M. Abdelraof, Design, synthesis, structure elucidation, antimicrobial, molecular docking, and SAR studies of novel urea derivatives bearing tricyclic aromatic hydrocarbon rings, Arch. Pharm., 2024, 357, 2300738 CrossRef CAS PubMed.
- https://www.rcsb.org/structure/7T7J.
- https://www.rcsb.org/structure/2g76.
- E. W. Bell and Y. Zhang, DockRMSD: an open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism, J. Cheminf., 2019, 11, 40 Search PubMed.
- G. M. Sheldrick, A short history ofSHELX, Acta Crystallogr., Sect. A:Found. Adv., 2007, 64, 112–122 CrossRef PubMed.
- P. W. Betteridge, J. R. Carruthers, R. I. Cooper, K. Prout and D. J. Watkin, CRYSTALSversion 12: software for guided crystal structure analysis, J. Appl. Crystallogr., 2003, 36, 1487 CrossRef CAS.
- D. S. Moss, International tables for X-ray crystallography. Vol. IV edited by J. A. Ibers and W. C. Hamilton, Acta Crystallogr., 1975, 31, 2558 CrossRef.
- L. J. Farrugia, ORTEP-3 for Windows - a version ofORTEP-III with a Graphical User Interface (GUI), J. Appl. Crystallogr., 1997, 30, 565 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2390251. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ra08958h |
| This journal is © The Royal Society of Chemistry 2025 |