Open Access Article
Open Access ArticleSolvent extraction of boron from mildly alkaline salt lake brine in Tibet, China
Yujie Huangab,
Hailong Lub,
Shaolei Xieb,
Chenyu Zhaoab,
Jiguang Dongab,
Lejie Xub,
Yaru Qin*a,
Chenglong Shi*a and
Xiaowu Peng *b
*b
aSchool of Chemistry and Materials Science, Qinghai Minzu University, Xining 810007, China. E-mail: yaruqin@qhmu.edu.cn; shiclong@qhmu.edu.cn
bKey Laboratory of Green and High-end Utilization of Salt Lake Resources, Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining, Qinghai 810008, China. E-mail: pengxiaowu@isl.ac.cn
First published on 26th February 2025
Abstract
Since the level of resource depletion is maintained at a high level, the recovery of boron from salt lake brine has become an effective way to meet the increasing demand for boron. This study investigates the optimization of boron extraction from the weakly alkaline brine of the Laguocuo Salt Lake (LGCSL) in Tibet, China, a representative of Tibetan weakly alkaline salt lakes. We evaluated the efficacy of 2,2,4-trimethyl-1,3-pentanediol (TMPD) as an extractant within a solvent mixture of 2-butyl-1-octanol (C12–OH) and sulfonated kerosene. The extraction performance was systematically assessed through single-stage and multi-stage counter-current extraction experiments, examining variables such as extractant type, concentration, pH, temperature, and the presence of co-existing ions. Our results demonstrate that optimal boron extraction is achieved under conditions of pH 8.0, an organic-to-aqueous phase ratio (O/A) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5, and lower temperatures. Under these parameters, single-stage extraction efficiency surpassed 83%, while a three-stage process achieved an impressive 98.61% efficiency. Stripping experiments identified sodium hydroxide (NaOH) as an effective stripping agent, with a concentration of 0.3 mol L−1 and a phase ratio of 2
1.5, and lower temperatures. Under these parameters, single-stage extraction efficiency surpassed 83%, while a three-stage process achieved an impressive 98.61% efficiency. Stripping experiments identified sodium hydroxide (NaOH) as an effective stripping agent, with a concentration of 0.3 mol L−1 and a phase ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature yielding high stripping efficiency and significant boron concentration enrichment. To elucidate the extraction mechanism, Raman spectroscopy was employed to characterize the structural interactions between TMPD and boron complexes in the organic phase. Additionally, the influence of carbonate (CO32−) and bicarbonate (HCO3−) ions, prevalent in alkaline brines, on boron extraction was investigated. These ions were found to affect the extraction efficiency, likely through competitive interactions or complex formation, highlighting the necessity of their consideration in optimizing the extraction process. This study provides both theoretical insights and practical experimental data essential for the efficient recovery of boron from weakly alkaline salt lake brines.
1 at room temperature yielding high stripping efficiency and significant boron concentration enrichment. To elucidate the extraction mechanism, Raman spectroscopy was employed to characterize the structural interactions between TMPD and boron complexes in the organic phase. Additionally, the influence of carbonate (CO32−) and bicarbonate (HCO3−) ions, prevalent in alkaline brines, on boron extraction was investigated. These ions were found to affect the extraction efficiency, likely through competitive interactions or complex formation, highlighting the necessity of their consideration in optimizing the extraction process. This study provides both theoretical insights and practical experimental data essential for the efficient recovery of boron from weakly alkaline salt lake brines.
1. Introduction
With its unique chemical properties, boron plays a vital role in glass, ceramics, chemicals, materials, agriculture and other fields. China's boron reserves rank fourth in the world.1 It has the characteristic of concentrated distribution of boron resources. 97% of the identified resource reserves are distributed in Tibet Autonomous Region (37%), Liaoning Province (34%), Qinghai Province and Hubei Province. Among them, sedimentary reworked boron resources account for 38% of the total, salt lake-type boron ore resource reserves account for 52% of the total, and other types account for 10%.2 The majority of liquid brine boron resources are located on the Qinghai and Tibet Plateau. The solid boron ores are gradually being depleted with the exploitation of resources, while the boron dissolved in solution ore have not been effectively developed, especially the boron dissolved in solution resources in the salt lakes in Tibet. The salt lakes in Tibet are mainly divided into carbonate – type and sulfate – type salt lakes, among which ZhacangChaka, Mamico, and Zabuye are the most important, and the total storage of the three salt lakes is about 123.12 million ton.3 Due to environmental factors in Tibet, such as remote geographical location, inconvenient transportation, high altitude, and fragile ecological environment, they have not been effectively developed and utilized. For example, the Laguocuo Salt Lake (LGCSL) has only carried out a small experiment on brine evaporation, and compared with important salt lakes such as ZhacangChaka Salt Lake, no work has been carried out. Therefore, developing an efficient extraction technology for boron in the liquid salt lake boron resources in Tibet is an effective way to slow down imports from abroad.Regarding the research progress of the brine in the salt lakes on the Qinghai-Tibet Plateau, Lv et al.4 systematically summarized the characteristics of boron and lithium isotope fractionation in salt lake systems and the variation patterns of boron and lithium isotope compositions during the evolution of salt lakes. They also elaborated on the differences in boron isotope compositions from different sources, providing a theoretical basis for determining the origin of boron in Tibetan salt lakes. This research is based on the geological samples and environmental conditions of the Damxung Co Salt Lake. When the research results are extended to the entire Qinghai-Tibet Plateau and even other salt lakes in other regions, they may be limited by the unique local geological, hydrological, and climatic conditions. Peng et al.5 developed a new dilution method for extracting boron from the brine of sulfate-type salt lakes on the Qinghai-Tibet Plateau. The extraction efficiency can reach over 80%, which provides a new technical idea for the extraction of boron from Tibetan salt lakes. The research only focuses on sulfate-type salt lakes, and the applicability to other types of salt lakes, such as the carbonate-type salt lakes existing in the Tibet region, has not been explored, which limits the universal application scope of this method.
Traditional methods for extracting boron dissolved in solution, such as evaporation crystallization from brine or mining and processing of boron minerals, have limitations such as high energy consumption and equipment corrosion. In recent years, a large number of studies have been dedicated to developing advanced methods for extracting boron dissolved in solution. Currently, commonly used methods include: precipitation method,6–8 adsorption,9–11 ion exchange resin,12–14 membrane separation method,15–17 solvent extraction etc.18–21 Among them, the precipitation method has a mature process and simple equipment. Although it can achieve the precipitation separation of boron, due to the complex composition of the salt lake brine, the precipitation process may be interfered by other ions, resulting in poor precipitation effect or low purity. Moreover, the subsequent slag treatment is difficult to implement in the environment of scarce land resources in Tibet. Therefore, the overall applicability is limited. In the adsorption method, the adsorbent is inexpensive and has good selectivity. It can adsorb boron to a certain extent. However, for the salt lake brine with high boron concentration, a large amount of adsorbent and complex adsorption–desorption cycle operations may be required. Moreover, the impurities in the salt lake brine may affect the activity and life of the adsorbent. Therefore, the applicability is also limited to a certain extent. The ion exchange resin method has high selectivity and adsorption ability and can deeply remove boron. However, in practical application, it is necessary to overcome the influence of the complex ionic environment in the brine on the resin. Considering the cost and operation difficulty, there are a large number of sulfate and carbonate ions in the salt lake brine in Tibet. Therefore, the above-mentioned methods are not suitable for extracting boron from the brine of salt lakes in Tibet. Compared with other methods, the solvent extraction method shows many advantages, specifically including high extraction efficiency, wide application range, easy continuous operation, high product purity, strong process flexibility, and recyclability of the extractant. Among them, the recyclability of the extractant is mainly manifested in the single-stage boron extraction operation. Generally, the extractant does not reach the extraction saturation state. After the back-extraction operation of the boron-loaded organic phase using sodium hydroxide, the remaining organic phase can still retain a certain boron extraction capacity after corresponding purification treatment, thereby realizing the recycling of the organic phase. This recycling mechanism can significantly reduce the demand for fresh extractant in the boron extraction process. Therefore, this study will use the solvent extraction method to conduct research on the extraction of boron from the brine of salt lakes in Tibet. Therefore, this study will adopt the solvent extraction method to carry out research on the extraction of boron from the brine of the salt lakes in Tibet.
In this study, a solution of 2,2,4-trimethyl-1,3-pentanediol (TMPD) dissolved in 2-butyl-1-octanol (C12–OH) and sulfonated kerosene solvent was selected as the mixed alcohol extractant for the separation of boron from LGC brine, and an in-depth study on the factors affecting the extraction equilibrium was conducted. The process parameters of liquid–liquid extraction were studied and optimized. By changing the reaction conditions, such as the concentrations of TMPD and 2-butyl-1-octanol, pH, temperature, and phase ratio, the optimal extraction conditions were obtained. The mass transfer law, the structure of the extract complex, and the extraction reaction mechanism during the extraction process, as well as the reaction mechanism between boron ions and carbonate ions in carbonate-type salt lakes, were revealed. The effects of various factors on the extraction efficiency were determined, and through cascade cycle amplification experiments and process optimization, basic data for improving the cascade theory of complex salt lake systems and industrial design were provided. The results show that this process is a very promising method for deeply separating boron from weakly alkaline brine.
2. Material and methods
2.1. Materials
The extractant 2,2,4-trimethyl-1,3-pentanediol (TMPD, 97%) and the diluent 2-butyl-1-octanol (C12–OH, 97%) were purchased from TCI Co., Ltd. The molecular structures of TMPD and C12–OH are shown in Fig. 1 below. Boric acid (H3BO3, 99.5%) was purchased from Tianjin Kemio Chemical Reagent Co., Ltd and used as a boron source for the simulated feed solution. All experiments used deionized water. The boric acid was dissolved in deionized water, and the pH value of the experimental solution was adjusted by using HCl (wt%: 36–38%) or NaOH (wt%: 96%) to prepare the simulated brine. All reagents were of analytical purity and were used without further purification. The LGC brine used in this study was collected from the Laguo Cuo Salt Lake (LGCSL) in Tibet and provided by Tibet Ali Laguo Resources Co., Ltd. The main characteristics of the brine are shown in Table 1.| ρ (g cm−3) | pH | Ion concentrate solution (mol L−1) | ||||||
|---|---|---|---|---|---|---|---|---|
| H3BO3 | Li+ | Mg2+ | K+ | Na+ | CO32− | SO42− | ||
| 1.039 | 9.3 | 0.063 | 0.036 | 0.028 | 0.05 | 5.920 | 0.669 | 0.182 |
2.2. Single-stage extraction procedures
Single-stage extraction experiments were carried out in a separatory funnel at an ambient temperature of about (293 ± 2 K). A certain volume ratio of the aqueous phase and the organic phase was mixed and mechanically shaken at 300 rpm for 10 min (Strong Shaker, TAITEC, SR-2DW). After shaking, the mixture was allowed to stand until the two phases were separated, and the aqueous phase and the organic phase were collected for further analysis. Additionally, it should be noted that all the organic solvents used in the experiments were a mixed alcohol consisting of a 0.3 mol L−1 TMPD dissolved in 2-butyl-1-octanol and sulfonated kerosene as an extractant, with a phase ratio (O/A) of 1/1. To study the influence of ion concentration on boric acid extraction, eight kinds of simulated feed solutions were used, among which the concentration of boric acid is 0.0616 mol L−1, and the concentrations of different ions in each solution range from 0.01 to 0.09 mol L−1, including NaOH, Na2CO3, Na2SO4, NaCl, KCl, LiCl, MgCl2, and CaCl2. The remaining experiments used the real brine as the boron source. Single-factor experiments on TMPD concentration, temperature, phase ratio, and pH were carried out using the real brine. All single-factor experiments except the temperature experiment were carried out at room temperature.2.3. Multi-stage counter current extraction procedures
As shown in Fig. 2, a three-stage simulated counter-current extraction experiment was carried out using a separatory funnel. The experimental raw material was LGC brine which had been acidified to reach pH = 8. The extractant was selected as TMPD with a concentration of 0.3 mol L−1, 2-butyl-1-octanol was used as the cosolvent, and sulfonated kerosene 18A was used as the solvent. The phase ratio of the organic phase to the aqueous phase was set at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.
3.
The specific experimental steps were as follows: in the initial stage, 10 mL of the freshly prepared organic phase and 15 mL of the raw brine were accurately measured and injected into the second separatory funnel. Then, it was placed on an oscillator and oscillated for 10 minutes with specific oscillation parameters to promote the full progress of the extraction process and reach an equilibrium state. After the oscillation was completed, the separatory funnel was placed on a stable tabletop until the organic phase and the aqueous phase were clearly separated.
Subsequently, the organic phase (marked as O2) in the second separatory funnel was precisely transferred to the third separatory funnel using a separatory funnel, and at the same time, the aqueous phase (marked as A2) was transferred to the first separatory funnel. Next, freshly prepared acidified LGC brine was added to the third separatory funnel, and fresh extractant was added to the first separatory funnel. The two were again placed on the oscillator for oscillation operation to start a new round of the extraction process.
After the above operations were completed, the aqueous phase (A1) in the first separatory funnel and the organic phase (O3) in the third separatory funnel were simultaneously introduced into the second separatory funnel, thus obtaining the extract and the organic phase sample loaded with the target substance. After multiple cycles of operation, the boron content in the aqueous phase of the third separatory funnel gradually became stable, indicating that the entire system reached a stable state.
2.4. Analysis
The elemental ion concentrations in the aqueous phase were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES) (ICAP 6500 DUO; America Thermo Scientific). To ensure the accuracy of the results, strict calibration and quality control measures were taken during the experiment. Before each measurement, the ICP-AES instrument was calibrated with a standard solution of known concentration, and it was calibrated again after every 5 samples were measured, so that the measurement error was controlled within 5%. During data processing, only the data with a relative standard deviation (RSD%) less than 5% were retained for subsequent analysis to ensure the reliability and stability of the data. The pH values of all liquid samples in the experiment were measured by a pH meter (Mettler Toledo, SD 50). After extraction, the organic phases of complexes containing boron and without boron were measured by FTIR (Thermo Nicolet 670 spectrometer), and the aqueous phases of complexes containing boron were measured and analyzed by a spectroscopic Raman spectrometer at wavelengths from 0 to 4000 cm−1 (DXR; Thermo Fisher Scientific, Ltd, USA) and a gas-phase mass spectrometer.In this study, the formulas for extraction efficiency (E%), distribution ratio (D%), and stripping efficiency (SE%) are as follows. These formulas are derived from the mass balance principle in a simple two-phase extraction system, assuming that the extraction process reaches a quasi-equilibrium state within the experimental time frame, and that there are no significant side reactions or losses of the target analyte other than the extraction and stripping reactions considered.
 | (1) |
 | (2) |
 | (3) |
Among them, and respectively represent the concentrations of boron in the organic phase and the aqueous phase, with the unit of mol L−1.
3. Results and discussion
3.1. Effects of the extraction parameters on H3BO3 extraction
The extraction efficiencies of glycols with different alkyl chain lengths also vary. As shown in Fig. 3, the extraction efficiency of EHD is slightly higher than that of TMPD. Both EHD and TMPD are alkyl glycols, and the difference lies in the number of side chains and adjacent carbon atoms. In early research, EHD was often considered as a typical extractant for extraction. However, in terms of solvent loss rate (the solvent loss amounts of TMPD and EHD are shown in Table 2), the solvent loss of TMPD is lower than that of EHD, approximately 0.7 times. In terms of reaction activity, the reaction energy of TMPD is 1.3 times lower than that of EHD. Extraction agents with low reactivity typically exhibit greater stability and selectivity. The reason is that two new B–O–C bonds are produced during the reaction of TMPD with boric acid. The stability of this bond may be the key to the entire reaction activity. From the perspective of the electron distribution of B–O–C, the electron configuration of the final product of TMPD is also greater than that of the final product of EHD.23 The extraction performance of the extractant mainly depends on the number of adjacent carbon atoms at the center of the extractant and the number of electron configurations in the B–O–C region.24 Therefore, the extraction performance of TMPD is superior to that of EHD. Therefore, the extractant TMPD with relatively better extraction performance is selected and combined with eight common and easily accessible solvents (xylene, carbon tetrachloride, 1,2-dichloroethane, dichloromethane, butyl acetate, methyl tert-butyl ether, a mixture of isooctanol and sulfonated kerosene, and a mixture of 2-butyl-1-octanol and sulfonated kerosene) and applied to the extraction of boron from LGC brine to explore the relationship between different solvents and extraction efficiency.
| Organic solvent | Solvent loss (g L−1) | Experimental temperature (°C) |
|---|---|---|
| 2,2,4-Trimethyl-1,3-pentanediol | 12.86 | 20 |
| 2-Ethyl-1,3-hexanediol | 13.55 | 20 |
| 2-Butyl-1-octanol | 0.04 | 20 |
| TMPD/C12–OH | 12.90 | 20 |
| Isooctanol | 1.30 | 20 |
As shown in Fig. 4, dichloromethane has the highest extraction efficiency as a solvent, followed by 1,2-dichloroethane. However, for solvents, carbon tetrachloride, dichloromethane, and 1,2-dichloroethane are all volatile liquids with irritating odors. Since TMPD is inherently insoluble in kerosene, co-solvents (e.g., alcohols and ethers) need to be added to blend the two. Among them, a mixture of isooctanol and sulfonated kerosene and a mixture of C12–OH and sulfonated kerosene, when used as diluents, also achieve good extraction rates. Isooctanol and C12–OH are branched monohydric alcohols with different numbers of carbon chains, as the number of carbon atoms increases, the viscosity also increases. Since viscosity is not conducive to the separation of the two phases and affects the transfer between ions, there is an inverse relationship between viscosity and alcohol extraction rate. The extraction rate of isooctanol with 8 carbon atoms is slightly higher than that of 2-butyl-1-octanol with 12 carbons. In addition, as the number of carbon atoms increases, the solubility of monohydric alcohols gradually decreases. This is because the longer the carbon chain, the larger the hydrophobic group and the smaller the polarity of monohydric alcohols.25 C12–OH has low volatility and a high flash point, and its solvent loss rate is much lower than that of isooctanol (the solvent loss of isooctanol is 32.5 times that of C12–OH), as shown in Table 2. Under the same experimental conditions, C12–OH has low volatility, while the volatility of isooctanol is relatively high and its evaporation rate is faster. This makes isooctanol more likely to escape from the system into the surrounding environment during the experimental operation. This not only leads to solvent waste, but also the evaporated isooctanol causes a pungent smell to pervade the experimental area, posing a potential threat to the health of operators. The solvent loss rate of C12–OH is much lower than that of isooctanol. Solvent loss can bring about several negative impacts. For instance, it increases the organic matter content in the aqueous phase, thereby imposing more stringent requirements on subsequent treatment processes to reduce their environmental impact. Therefore, it is advisable to choose an organic solvent with minimal solvent loss to ensure the environmental friendliness, efficiency, and stability of the entire extraction process. In view of this, with a solvent loss rate of only 0.04 g L−1, C12–OH is more suitable as a cosolvent and diluent. Finally, in this experiment, TMPD is used as the extractant, 2-butyl-1-octanol is used as the cosolvent, and sulfonated kerosene is used as the diluent to extract boron from the brine of the LGC Salt Lake.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using a mixed organic solvent containing different concentrations of TMPD. At the same time, the extraction rate and viscosity of the mixed organic solvent containing different concentrations of 2-butyl-1-octanol TMPD under this experimental condition are compared to explore the concentration of the extractant and the concentration of the synergist suitable for the brine extraction in this experiment. The results are shown in Fig. 5. It is clear from Fig. 5 that the extraction rate of boron is positively correlated with the concentration of the extractant TMPD. When the content of the extractant TMPD in the organic phase is less than 0.3 mol L−1, the extraction efficiency of boron increases rapidly; when the content of the extractant TMPD is greater than 0.3 mol L−1, the extraction efficiency of boron gradually flattens out. At the same time, the distribution ratio shows the same trend as the extraction efficiency increases. As the concentration of the extractant TMPD in the organic phase increases, the distribution ratio also increases. It should be noted that when the concentration of 2-butyl-1-octanol in the extractant reaches a certain level, it has little impact on the extraction rate.
1 using a mixed organic solvent containing different concentrations of TMPD. At the same time, the extraction rate and viscosity of the mixed organic solvent containing different concentrations of 2-butyl-1-octanol TMPD under this experimental condition are compared to explore the concentration of the extractant and the concentration of the synergist suitable for the brine extraction in this experiment. The results are shown in Fig. 5. It is clear from Fig. 5 that the extraction rate of boron is positively correlated with the concentration of the extractant TMPD. When the content of the extractant TMPD in the organic phase is less than 0.3 mol L−1, the extraction efficiency of boron increases rapidly; when the content of the extractant TMPD is greater than 0.3 mol L−1, the extraction efficiency of boron gradually flattens out. At the same time, the distribution ratio shows the same trend as the extraction efficiency increases. As the concentration of the extractant TMPD in the organic phase increases, the distribution ratio also increases. It should be noted that when the concentration of 2-butyl-1-octanol in the extractant reaches a certain level, it has little impact on the extraction rate.
A mixed organic solvent of 0.1 mol L−1 and 0.4 mol L−1 TMPD with different concentrations of 2-butyl-1-octanol and kerosene is selected as the organic phase. The results are shown in Fig. 6(A). As can be seen from the figure, when the concentration of the cosolvent is greater than 0.4 mol L−1, the increase in the concentration of 2-butyl-1-octanol has little effect on the extraction rate and shows a relatively stable trend. A mixed alcohol extractant is formed by using 0.3 mol L−1 TMPD and different concentrations of 2-butyl-1-octanol and kerosene to explore the changes in its extraction rate and viscosity, as shown in Fig. 6(B). As can be seen from the figure, the concentration of 2-butyl-1-octanol is inversely proportional to the extraction rate and directly proportional to the viscosity. This is because as the concentration of 2-butyl-1-octanol increases, the viscosity of the organic phase also increases. We can draw such a conclusion that the viscosity of the extractant affects the extraction rate. In the extractant of this system, its viscosity depends on the additional amount of 2-butyl-1-octanol. The higher the viscosity, the lower the mass transfer rate and the longer the phase separation time, which is unfavorable for the extraction of boron.25
 | ||
| Fig. 6 Effect of different concentrations of 2-butyl-1-octanol on boron extraction (A); the relationship between different concentrations of 2-butyl-1-octanol and the viscosity of the extractant (B). | ||
The underlying mechanisms by which viscosity affects extraction efficiency lie in molecular diffusion, interfacial tension, and fluidity. Firstly, molecular diffusion affects the mass transfer efficiency. Since the molecules in low-viscosity solvents are more active and move more freely, they can collide with and interact with the target substances more frequently, thus promoting the diffusion of the target substances and improving the mass transfer efficiency and the extraction rate.26 Secondly, interfacial tension affects the two-phase separation. Viscosity will influence the properties of the liquid–liquid interface and then affect the interfacial tension and the phase separation efficiency.27 Low-viscosity solvents usually contribute to better phase separation, thus increasing the extraction rate.28 Finally, fluidity affects the mixing effect. Low-viscosity solvents have better fluidity and can provide a more thorough mixing effect, making the contact between the solvent and the raw materials more uniform and thus improving the extraction efficiency.29
Therefore, finally, 0.3 mol L−1 TMPD is selected as the extractant, and 0.3 mol L−1 2-butyl-1-octanol is selected as the cosolvent for further research on the brine.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The pH value of brine is adjusted by mixing HCl and NaOH as raw materials to explore the equilibrium pH suitable for the brine in this experiment. The results are shown in Fig. 7. As depicted in Fig. 7, when the equilibrium pH is within the range of 7 to 8, the extraction rate of boron by the extractant changes relatively little. However, when the pH exceeds 8, the trend of extraction efficiency for boron by the extractant significantly decreases. Specifically, the extraction efficiency of boron drops rapidly from 84.4% to 19.2%. Overall, there is a proportional relationship between the pH value of the original brine and the extraction efficiency, with the extraction efficiency gradually decreasing as the pH value of the brine increases. The distribution ratio also shows the same trend, decreasing from 7.6 to 0.24. This phenomenon can be attributed to the existence of boron in two forms at different pH levels: B(OH)3 and [B(OH)4]−.30,31 The chemical equilibrium between these two forms is highly sensitive to pH changes. When the pH is greater than 9, boron in the solution primarily exists as [B(OH)4]−, while when the pH is less than 9, boron mainly exists as B(OH)3. The extraction mechanism of the extractant for boron usually relies on specific chemical interactions with one of these forms. As the pH gradually increases, the molecules of boric acid (B(OH)3) decrease, while the ions of borate ([B(OH)4]−) increase. This transformation in the form of boron is not conducive to the formation of borate esters, which are crucial for the extraction process. Therefore, this reduces the extraction efficiency of the extractant for boron to some extent. In this study, the pH value of the original brine is 9.3. To reduce the amount of hydrochloric acid used in the experiment and considering the potential hazards of excessive acidity to humans and equipment, the equilibrium pH was ultimately set at 8 for further research.
1. The pH value of brine is adjusted by mixing HCl and NaOH as raw materials to explore the equilibrium pH suitable for the brine in this experiment. The results are shown in Fig. 7. As depicted in Fig. 7, when the equilibrium pH is within the range of 7 to 8, the extraction rate of boron by the extractant changes relatively little. However, when the pH exceeds 8, the trend of extraction efficiency for boron by the extractant significantly decreases. Specifically, the extraction efficiency of boron drops rapidly from 84.4% to 19.2%. Overall, there is a proportional relationship between the pH value of the original brine and the extraction efficiency, with the extraction efficiency gradually decreasing as the pH value of the brine increases. The distribution ratio also shows the same trend, decreasing from 7.6 to 0.24. This phenomenon can be attributed to the existence of boron in two forms at different pH levels: B(OH)3 and [B(OH)4]−.30,31 The chemical equilibrium between these two forms is highly sensitive to pH changes. When the pH is greater than 9, boron in the solution primarily exists as [B(OH)4]−, while when the pH is less than 9, boron mainly exists as B(OH)3. The extraction mechanism of the extractant for boron usually relies on specific chemical interactions with one of these forms. As the pH gradually increases, the molecules of boric acid (B(OH)3) decrease, while the ions of borate ([B(OH)4]−) increase. This transformation in the form of boron is not conducive to the formation of borate esters, which are crucial for the extraction process. Therefore, this reduces the extraction efficiency of the extractant for boron to some extent. In this study, the pH value of the original brine is 9.3. To reduce the amount of hydrochloric acid used in the experiment and considering the potential hazards of excessive acidity to humans and equipment, the equilibrium pH was ultimately set at 8 for further research.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
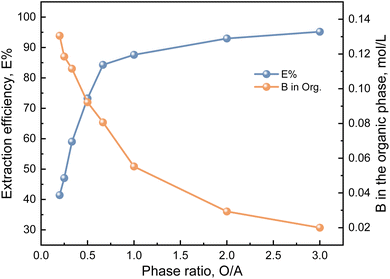
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4). Hydrochloric acid is used to adjust the equilibrium pH of the brine to 8.0. The experimental results are shown in Fig. 9. As can be seen from Fig. 9, the phase ratio in the TMPD and 2-butyl-1-octanol system has a greater influence on the extraction efficiency, and there is a positive proportional relationship between them. When the phase ratio is larger, the extraction efficiency of boron also increases. When the phase ratio (O/A) is in the range of 1
4). Hydrochloric acid is used to adjust the equilibrium pH of the brine to 8.0. The experimental results are shown in Fig. 9. As can be seen from Fig. 9, the phase ratio in the TMPD and 2-butyl-1-octanol system has a greater influence on the extraction efficiency, and there is a positive proportional relationship between them. When the phase ratio is larger, the extraction efficiency of boron also increases. When the phase ratio (O/A) is in the range of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 to 3
1.5 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the extraction efficiency increases from 32.35% to 87.58%. Continuing to increase the phase ratio will increase the ion concentration difference, which leads to an increase in mass transfer driving force, so the extraction efficiency also becomes higher. Therefore, the phase ratio is one of the primary factors affecting the efficiency of liquid–liquid extraction. Since the extraction efficiency difference is not obvious when the phase ratio (O/A) is 1
1, the extraction efficiency increases from 32.35% to 87.58%. Continuing to increase the phase ratio will increase the ion concentration difference, which leads to an increase in mass transfer driving force, so the extraction efficiency also becomes higher. Therefore, the phase ratio is one of the primary factors affecting the efficiency of liquid–liquid extraction. Since the extraction efficiency difference is not obvious when the phase ratio (O/A) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5. To increase the loading capacity of the extractant and reduce the amount of the extractant, finally, a phase ratio (O/A) of 1
1.5. To increase the loading capacity of the extractant and reduce the amount of the extractant, finally, a phase ratio (O/A) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 is selected for the research of cascade experiments.
1.5 is selected for the research of cascade experiments.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
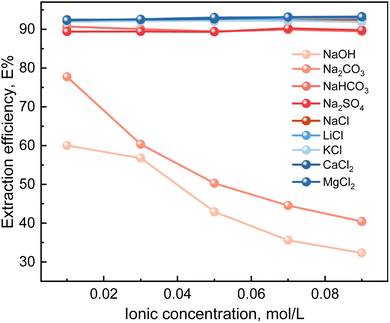
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results of the coexisting anion experiment are shown in Fig. 10. When no salting agent was added, the extraction of boric acid was 91.31%. As depicted in Fig. 10, only CO42− and OH− had a significant effect on the efficiency of boron extraction, while neither SO42− nor any of the other cations had much effect. Notably, the extraction efficiency of boron decreases progressively with increasing concentrations of CO32− and OH−, suggesting that the presence of both species can lead to inhibitory effects on the extraction process. This phenomenon may be because carbonic acid and hydroxides react with boric acid to form borates and borate ions, and the main compound containing borate ions among them is sodium tetraborate.
1. The results of the coexisting anion experiment are shown in Fig. 10. When no salting agent was added, the extraction of boric acid was 91.31%. As depicted in Fig. 10, only CO42− and OH− had a significant effect on the efficiency of boron extraction, while neither SO42− nor any of the other cations had much effect. Notably, the extraction efficiency of boron decreases progressively with increasing concentrations of CO32− and OH−, suggesting that the presence of both species can lead to inhibitory effects on the extraction process. This phenomenon may be because carbonic acid and hydroxides react with boric acid to form borates and borate ions, and the main compound containing borate ions among them is sodium tetraborate.
In terms of pH effects, sodium tetraborate and excess carbonate undergo hydrolysis in aqueous solutions, which increases the solution's pH. The speciation of boric acid and the extraction rate are closely pH-dependent. An increase in pH leads to a higher prevalence of borate ions, thereby reducing the extraction rate of boric acid. Regarding ionic strength, the dissolution of sodium tetraborate in water elevates the solution's ionic strength.32 The solvent extraction behavior of boric acid solution is significantly affected by ionic strength. The higher the ionic strength, the lower the extraction rate of boric acid. This effect is caused by the shielding effect of counter ions on boric acid complexes. The higher the ionic strength, the stronger the interaction between counter ions and boric acid complexes, leading to the transfer of boric acid complexes to the aqueous phase. This influence of ionic strength on the extraction behavior of boric acid reveals the importance of controlling ionic strength in the extraction process to optimize the extraction efficiency of boric acid. Thus, the control of the concentration of carbonate and hydroxide is very important for the effective extraction of boric acid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The shaking temperature is room temperature (293 K). By observing that the molar concentration of boron in the raffinate of single-stage extraction no longer changes, the result shows that the number of times of shaking required is 6. The experimental results are shown in Fig. 11. As can be seen from Fig. 11, after six single-stage extractions between the organic phase and fresh brine, after the fourth to sixth single-stage extractions, the boron in the raffinate hardly changes anymore, indicating that the saturated capacity has been reached. At this time, the saturated capacity of 0.3 mol L−1 TMPD is 0.1139 mol L−1. It can be found from this that single-stage extraction cannot completely extract boron from brine, and at the same time, the utilization rate of TMPD is relatively low. In practical applications, when the distribution coefficient of the target substance is low or a high-purity target substance is required, single-stage extraction is often difficult to meet the requirements. Currently, multistage extraction plays an important role. Therefore, multistage extraction is introduced in the subsequent stage to increase the total transfer rate of the target substance and improve the quality and yield of the product.
3. The shaking temperature is room temperature (293 K). By observing that the molar concentration of boron in the raffinate of single-stage extraction no longer changes, the result shows that the number of times of shaking required is 6. The experimental results are shown in Fig. 11. As can be seen from Fig. 11, after six single-stage extractions between the organic phase and fresh brine, after the fourth to sixth single-stage extractions, the boron in the raffinate hardly changes anymore, indicating that the saturated capacity has been reached. At this time, the saturated capacity of 0.3 mol L−1 TMPD is 0.1139 mol L−1. It can be found from this that single-stage extraction cannot completely extract boron from brine, and at the same time, the utilization rate of TMPD is relatively low. In practical applications, when the distribution coefficient of the target substance is low or a high-purity target substance is required, single-stage extraction is often difficult to meet the requirements. Currently, multistage extraction plays an important role. Therefore, multistage extraction is introduced in the subsequent stage to increase the total transfer rate of the target substance and improve the quality and yield of the product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and brine with a pH of 8.0 adjusted by HCl. This brine contains 0.063 mol L−1 of boric acid. Use a constant temperature shaker to extract boron from brine at different temperatures, which are 293 K, 303 K, 313 K, 323 K, and 333 K respectively. The results are shown in Fig. 12. It can be found from the figure that both the extraction efficiency and the distribution ratio are inversely proportional to the temperature. When the temperature increases, the extraction rate and the distribution ratio decrease accordingly. When the temperature rises from 293 K to 323 K, the extraction rate slowly decreases from 88.52% to 87.42%. This indicates that this process is an exothermic reaction. A lower temperature is conducive to boron extraction by the TMPD/2-butyl-1-octanol extraction system. Therefore, finally, the experiment is selected to be carried out at 293 K. Based on the influence of the TMPD/2-butyl-1-octanol system on the extraction of boric acid as shown in Fig. 12, the thermodynamic functions were analyzed and calculated. The enthalpy changes ΔH of boron extraction can be determined based on the slope of the curve of lg
3, and brine with a pH of 8.0 adjusted by HCl. This brine contains 0.063 mol L−1 of boric acid. Use a constant temperature shaker to extract boron from brine at different temperatures, which are 293 K, 303 K, 313 K, 323 K, and 333 K respectively. The results are shown in Fig. 12. It can be found from the figure that both the extraction efficiency and the distribution ratio are inversely proportional to the temperature. When the temperature increases, the extraction rate and the distribution ratio decrease accordingly. When the temperature rises from 293 K to 323 K, the extraction rate slowly decreases from 88.52% to 87.42%. This indicates that this process is an exothermic reaction. A lower temperature is conducive to boron extraction by the TMPD/2-butyl-1-octanol extraction system. Therefore, finally, the experiment is selected to be carried out at 293 K. Based on the influence of the TMPD/2-butyl-1-octanol system on the extraction of boric acid as shown in Fig. 12, the thermodynamic functions were analyzed and calculated. The enthalpy changes ΔH of boron extraction can be determined based on the slope of the curve of lg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D versus 1000/T (K) using the van't Hoff eqn (4). The result is shown in Fig. 12.
D versus 1000/T (K) using the van't Hoff eqn (4). The result is shown in Fig. 12.
 | (4) |
Among them, R is usually the gas constant, which is 8.314 J mol−1; C is the integration constant. Substituting the data in Fig. 12 into eqn (5), the following relationship is obtained:
 | (5) |
Therefore, the calculated enthalpy change is −2.757 kJ mol−1, which is less than 0. This result indicates that the reaction of extracting boron in this system is an exothermic reaction, and an increase in temperature is not conducive to the extraction of boric acid.
3.2. Striping extraction experiment
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, an equilibrium pH of 8.0, and a reaction temperature of 293 K. The loaded organic phase is collected, and the boron concentration in the loaded organic phase is 0.1124 mol L−1. Boronic acid is stripped by using different stripping agents (H2O, HCl, and NaOH) under the condition of a phase ratio (O/A) of 1
1, an equilibrium pH of 8.0, and a reaction temperature of 293 K. The loaded organic phase is collected, and the boron concentration in the loaded organic phase is 0.1124 mol L−1. Boronic acid is stripped by using different stripping agents (H2O, HCl, and NaOH) under the condition of a phase ratio (O/A) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The results are shown in Fig. 13. It can be found that the actual stripping effects of H2O and HCl are significantly worse than that of NaOH. The reason is that the stripping of the loaded organic phase is the hydrolysis process of borate. The borate generated under this system has a stable structure and is not easy to hydrolyze, so the stripping is relatively more difficult. Therefore, this experiment finally selects NaOH as the stripping agent.
1. The results are shown in Fig. 13. It can be found that the actual stripping effects of H2O and HCl are significantly worse than that of NaOH. The reason is that the stripping of the loaded organic phase is the hydrolysis process of borate. The borate generated under this system has a stable structure and is not easy to hydrolyze, so the stripping is relatively more difficult. Therefore, this experiment finally selects NaOH as the stripping agent.
To explore the influence of the concentration of the stripping agent on the stripping of boron in the TMPD/2-butyl-1-octanol system, different concentrations of NaOH solutions (0.1, 0.3, 0.5, 0.7, 0.9, and 1.2 mol L−1) were used for stripping under the condition that the phase ratio (O/A) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and the temperature is 293 K. The results are shown in Fig. 14. It can be seen from the experimental results that as the concentration of NaOH increases, the stripping rate gradually increases. This is because, during the stripping process, NaOH has a strong hydrolysis reaction with boron-containing ester complexes (detailed proof and elaboration will be provided below). The ester group in boron-containing ester complexes is easily attacked by hydroxide ions under alkaline conditions and the ester bond breaks. However, an important phenomenon was also observed in the experiment: when the concentration of NaOH exceeds 0.3 M, the emulsification phenomenon caused by NaOH becomes more and more serious. This phenomenon not only increases the time cost of the experiment but may also lead to the loss of the organic phase. The occurrence of the emulsification phenomenon may be because the addition of NaOH increases the polarity of the aqueous phase, thereby enhancing the interaction between the aqueous phase and the organic phase. This enhanced interaction is beneficial for the transfer of boron-containing ester complexes from the organic phase to the aqueous phase, but at the same time, it makes esters more prone to hydrolysis under alkaline conditions, that is saponification reaction. In view of this, the concentration of NaOH is finally determined to be 0.3 mol L−1.
1 and the temperature is 293 K. The results are shown in Fig. 14. It can be seen from the experimental results that as the concentration of NaOH increases, the stripping rate gradually increases. This is because, during the stripping process, NaOH has a strong hydrolysis reaction with boron-containing ester complexes (detailed proof and elaboration will be provided below). The ester group in boron-containing ester complexes is easily attacked by hydroxide ions under alkaline conditions and the ester bond breaks. However, an important phenomenon was also observed in the experiment: when the concentration of NaOH exceeds 0.3 M, the emulsification phenomenon caused by NaOH becomes more and more serious. This phenomenon not only increases the time cost of the experiment but may also lead to the loss of the organic phase. The occurrence of the emulsification phenomenon may be because the addition of NaOH increases the polarity of the aqueous phase, thereby enhancing the interaction between the aqueous phase and the organic phase. This enhanced interaction is beneficial for the transfer of boron-containing ester complexes from the organic phase to the aqueous phase, but at the same time, it makes esters more prone to hydrolysis under alkaline conditions, that is saponification reaction. In view of this, the concentration of NaOH is finally determined to be 0.3 mol L−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 2
4, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 4
1, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 5
1, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
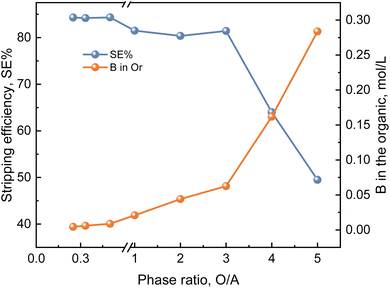
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) are selected to mix the organic phase and the aqueous phase. The experimental results are shown in Fig. 15. As can be seen from the figure, the stripping efficiency decreases with the increase of the phase ratio, and there is a negative correlation between the two. When R(O/A) < 1, the change in stripping efficiency is small. When R(O/A) > 3, the stripping efficiency rapidly decreases with the increase of the phase ratio. Increasing the phase ratio will reduce the overall stripping efficiency, and increase the stripping stages and stripping costs, which is not conducive to industrial production. It is obvious from the figure that when the phase ratio is 3, it is the node where the stripping efficiency rapidly decreases. Therefore, the stripping phase ratio (O/A) of 2
1) are selected to mix the organic phase and the aqueous phase. The experimental results are shown in Fig. 15. As can be seen from the figure, the stripping efficiency decreases with the increase of the phase ratio, and there is a negative correlation between the two. When R(O/A) < 1, the change in stripping efficiency is small. When R(O/A) > 3, the stripping efficiency rapidly decreases with the increase of the phase ratio. Increasing the phase ratio will reduce the overall stripping efficiency, and increase the stripping stages and stripping costs, which is not conducive to industrial production. It is obvious from the figure that when the phase ratio is 3, it is the node where the stripping efficiency rapidly decreases. Therefore, the stripping phase ratio (O/A) of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 is selected.
1 is selected.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to explore the influence of temperature on the stripping rate in the TMPD/2-butyl-1-octanol system, as shown in Fig. 16. As can be seen from figure, there is a negative correlation between temperature and stripping rate. When the temperature gradually rises, the stripping efficiency gradually decreases. When the temperature is >313 K, as the temperature increases, the stripping efficiency rapidly decreases. When the temperature is >313 K, the rate of decrease in stripping rate rapidly becomes smaller. The overall stripping efficiency is greater than 84%. When the temperature is 293 K, the stripping efficiency is greater than 88%. Therefore, finally, the stripping efficiency temperature is selected as room temperature (293 K). Additionally, the choice of room temperature for stripping is not only due to its high efficiency but also takes into account the convenience of operation and the economic nature of energy consumption, which is particularly important in industrial applications.
1 to explore the influence of temperature on the stripping rate in the TMPD/2-butyl-1-octanol system, as shown in Fig. 16. As can be seen from figure, there is a negative correlation between temperature and stripping rate. When the temperature gradually rises, the stripping efficiency gradually decreases. When the temperature is >313 K, as the temperature increases, the stripping efficiency rapidly decreases. When the temperature is >313 K, the rate of decrease in stripping rate rapidly becomes smaller. The overall stripping efficiency is greater than 84%. When the temperature is 293 K, the stripping efficiency is greater than 88%. Therefore, finally, the stripping efficiency temperature is selected as room temperature (293 K). Additionally, the choice of room temperature for stripping is not only due to its high efficiency but also takes into account the convenience of operation and the economic nature of energy consumption, which is particularly important in industrial applications.
3.3. Mechanism study
| Sample number | H3BO3/mol L−1 | NaHCO3/mol L−1 | pH |
|---|---|---|---|
| 3 | 0.06 | 0 | 5.43 |
| 3-1 | 0.06 | 0.001 | 7.19 |
| 3-2 | 0.06 | 0.01 | 7.51 |
| 3-3 | 0.06 | 0.1 | 7.95 |
| 3-4 | 0.06 | 0.2 | 7.99 |
| 3-5 | 0.06 | 0.5 | 8.04 |
| 3-6 | 0.06 | 1 | 7.99 |
 | ||
| Fig. 17 Raman spectra of boric acid with fixed concentration and sodium bicarbonate with different concentration. | ||
The ionic forms of boron in aqueous solution are relatively complex, and there are many interactions between borate anions existing simultaneously in aqueous solution. Nowadays, some researchers33,34 have proposed a model for the reaction of boric acid. In aqueous solutions containing carbonate and bicarbonate, the chemical equations for borate and four kinds of polyborates are as follows, eqn (6) to (8).
B(OH)3 + 2H2O ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) [B(OH)4]− + H3O+ [B(OH)4]− + H3O+
| (6) |
3B(OH)3 ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) [B3O3(OH)4]− + H2O + 2H2O+ [B3O3(OH)4]− + H2O + 2H2O+
| (7) |
4B(OH)3 ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) [B4O5(OH)4]2− + H2O + H2O+ [B4O5(OH)4]2− + H2O + H2O+
| (8) |
To confirm the attribution of the two peaks of sample 3-6 between 1000 and 1500 cm−1, and further confirm the complexes formed between CO32−, HCO3−, and H3BO3 as well as the main existing forms of borate ions, and explore the reasons why the existence of CO32− and HCO3− affects the boron extraction rate, the following two experiments were carried out respectively, as shown in Fig. 18(A) and (B). First, replicate samples 3 and 3-6 in the experiment in Fig. 17. Raman spectral analysis was performed on 0.06 mol L−1 H3BO3, 0.06 mol L−1 H3BO3, and 1 mol L−1 NaHCO3 and its raffinate after extraction, and this was classified as a set of analysis and control. The results are shown in Fig. 18(B). Zhu et al.35 gave the Raman spectra of potassium tetraborate and potassium metaborate solutions by using the Raman spectroscopy method and attributed each peak. Based on this, this study analyzes and controls each peak. We found that it is related to the vibration of the ion [B4O5(OH)4]2− at 687 cm−1. It can be seen that when NaHCO3 is added to H3BO3, the vibration peak intensity of the ion [B4O5(OH)4]2− at 687 cm−1 becomes stronger. This further indicates that NaHCO3 and H3BO3 will form borate Na2B4O5(OH)4. At 884 cm−1, it is related to the vibration peak of H3BO3 ions. It can be observed from the figure that the intensity of the boric acid vibration peak of the raffinate after extraction is weakened, indicating that H3BO3 in the solution forms a boric acid complex with TMPD, and the content of H3BO3 in the solution is reduced. At 1020 cm−1 and 1360 cm−1, it is related to the vibration peak of the ion HCO3−.36 Raman spectra of potassium carbonate and bicarbonate aqueous fluids at elevated temperatures and pressures: comparison with theoretical simulations. Combined with the two peaks with higher vibration amplitudes of sample 3-6 between 1000 and 1500 cm−1 in Fig. 17, the following conclusion can be drawn: due to the high concentration of sodium bicarbonate, after part of hydrochloric acid combines with boric acid to form borate, there is still excess bicarbonate. Therefore, the vibration peak intensities at 1020 cm−1 and 1360 cm−1 are stronger than those of the other six samples.
Secondly, the solid obtained by mixing 1 mol L−1 CO32− and 1 mol L−1 H3BO3 and performing evaporation crystallization, as well as analytically pure solid Na2B4O7 and solid K2B4O7·3H2O are analyzed by Raman spectroscopy, and the Raman spectra obtained from these three samples are classified as a set of reference controls. The absorption peak of the solid sample at 566 cm−1 is related to the vibration of the ion [B4O5(OH)4]2−, and the vibration amplitude of this absorption peak is the largest, indicating that the borate formed by sodium carbonate and boric acid is mainly Na2B4O5(OH)4. The absorption peak at 446 cm−1 is related to the vibration of the ion [B3O3(OH)4]−, and its vibration amplitude is relatively small. Thus, it can be inferred that there is a small amount of NaB3O3(OH)4 in the borate formed by sodium carbonate and boric acid. In addition, the absorption peak at 761 cm−1 is related to the ion [B(OH)4]−. This peak has a strong vibration, which means that more [B(OH)4]− is formed in this reaction process.
Combining Raman spectral analysis and pH changes, several reasons for the influence of carbonate and bicarbonate on boric acid extraction can be summarized as follows: firstly, carbonate and bicarbonate will react with boric acid, causing part of the boric acid in the solution to exist in the polymerized form – polyborate ions. The formation of its polymerized state reduces the amount of boric acid that can participate in complexation, and consequently leads to a decrease in the extraction rate. Polyborate ions are highly hydrated in aqueous solutions, forming stable hydrated ions. This hydration effect increases their solubility in the aqueous phase and makes it difficult for them to transfer to the organic phase, thus reducing the extraction rate.37 Polyborate ions form complex three-dimensional structures in aqueous solutions. In these structures, boron atoms are connected into rings by B–O–B bonds, forming stable polyborate ions. Such structures make polyborate ions more stable in aqueous solutions and difficult to form effective complexes with organic extractants, thereby resulting in a decrease in the extraction rate.38
Secondly, carbonate and bicarbonate will ionize to produce hydroxide ions in water, thus increasing the pH value of the solution. According to the existing forms of boric acid at different pH values, boric acid will be more inclined to exist in the ionic form of [B(OH)4]−. Since borate ions carry a negative charge, they have a relatively high solubility in water and a low affinity for the organic phase. Their interaction with organic extractants is weak, and it is difficult for them to form stable complexes.39 Therefore, borate ions have a relatively low solubility in the organic phase and a low extraction efficiency.
 | ||
| Fig. 19 FTIR infrared spectra before and after organic phase extraction, (A) is a partial enlargement of 600–2000 cm−1 in (B). | ||
From the perspective of molecular structure, the hydroxyl groups (–OH) in TMPD molecules play a crucial role. The boron atoms in boric acid molecules are sp2 hybridized, resulting in an empty p orbital, which exhibits electron-deficient characteristics.43 When TMPD comes into contact with boric acid, the empty p orbital of the boron atom can accept the lone pair of electrons from the hydroxyl oxygen atom, thereby initially forming a B–O–C coordinate bond.
3.4. Three-stage continuous counter-current extraction
To completely extract H3BO3 from brine, we conducted an experiment using the McCable–Thiele method to determine three-stage continuous countercurrent extraction (O/A = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3), as shown in Fig. 20. Taking LGC real brine as the raw material, its pH is adjusted to 8 with hydrochloric acid. Using 0.3 mol L−1 2-butyl-1-octanol as a cosolvent dissolved in 0.3 mol L−1 TMPD as the organic phase. The experimental results are listed in Table 4. Eventually, a raffinate containing 0.000877 mol L−1 of H3BO3 and an organic phase containing 0.061123 mol L−1 of boric acid were obtained, corresponding to an extraction efficiency of boric acid of 98.61%. The results indicate that after the fourth cycle, the extraction system reached a relatively stable state. The results show that as the number of extraction cycles increases, the extraction efficiency gradually improves and eventually stabilizes. The high extraction rate achieved in multi-stage countercurrent extraction is primarily attributed to the sustained high ion concentration gradient in the system. In the multi-stage countercurrent extraction process, the fresh organic phase is always in contact with the aqueous phase containing a relatively high concentration of boron. Starting from the first stage, boron in the aqueous phase continuously diffuses into the organic phase driven by the concentration gradient. When the organic phase moves to the next stage, it encounters a new aqueous phase with an even higher boron concentration, thereby maintaining the mass transfer driving force generated by the ion concentration gradient. This sustained concentration gradient provides a continuous “driving force” for boron transfer, enabling efficient migration of boron from the aqueous phase to the organic phase, significantly enhancing the extraction rate. As the number of extraction stages increases, the concentration gradient gradually diminishes, the mass transfer driving force weakens, and the amount of target substance transferred decreases accordingly, ultimately leading to stabilization of the extraction efficiency. Therefore, the three-stage countercurrent extraction experiment can significantly improve the extraction efficiency and distribution ratio of boric acid, achieving complete extraction of boric acid.
3), as shown in Fig. 20. Taking LGC real brine as the raw material, its pH is adjusted to 8 with hydrochloric acid. Using 0.3 mol L−1 2-butyl-1-octanol as a cosolvent dissolved in 0.3 mol L−1 TMPD as the organic phase. The experimental results are listed in Table 4. Eventually, a raffinate containing 0.000877 mol L−1 of H3BO3 and an organic phase containing 0.061123 mol L−1 of boric acid were obtained, corresponding to an extraction efficiency of boric acid of 98.61%. The results indicate that after the fourth cycle, the extraction system reached a relatively stable state. The results show that as the number of extraction cycles increases, the extraction efficiency gradually improves and eventually stabilizes. The high extraction rate achieved in multi-stage countercurrent extraction is primarily attributed to the sustained high ion concentration gradient in the system. In the multi-stage countercurrent extraction process, the fresh organic phase is always in contact with the aqueous phase containing a relatively high concentration of boron. Starting from the first stage, boron in the aqueous phase continuously diffuses into the organic phase driven by the concentration gradient. When the organic phase moves to the next stage, it encounters a new aqueous phase with an even higher boron concentration, thereby maintaining the mass transfer driving force generated by the ion concentration gradient. This sustained concentration gradient provides a continuous “driving force” for boron transfer, enabling efficient migration of boron from the aqueous phase to the organic phase, significantly enhancing the extraction rate. As the number of extraction stages increases, the concentration gradient gradually diminishes, the mass transfer driving force weakens, and the amount of target substance transferred decreases accordingly, ultimately leading to stabilization of the extraction efficiency. Therefore, the three-stage countercurrent extraction experiment can significantly improve the extraction efficiency and distribution ratio of boric acid, achieving complete extraction of boric acid.
| Raffinate | ICP test B/mg L−1 | H3BO3/mol L−1 | Extraction/% |
|---|---|---|---|
| R1 | 2.006 | 0.001237 | 98.04 |
| R2 | 1.823 | 0.001124 | 98.22 |
| R3 | 1.590 | 0.000981 | 98.44 |
| R4 | 1.485 | 0.000916 | 98.55 |
| R5 | 1.466 | 0.000904 | 98.56 |
| R6 | 1.461 | 0.000901 | 98.57 |
| R7 | 1.452 | 0.000895 | 98.58 |
| R8 | 1.422 | 0.000877 | 98.61 |
3.5. Two-stage continuous counter-current stripping extraction
To achieve the complete extraction of H3BO3 from the loaded organic phase, a series of experiments were conducted. The McCable–Thiele method was employed to determine a three-stage continuous countercurrent stripping process with an O/A ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, as depicted in Fig. 21. The loaded organic phase collected through three-stage countercurrent extraction served as the raw material, and 0.3 mol L−1 NaOH was utilized as the stripping agent. The experimental outcomes are presented in Table 5. Ultimately, a stripping raffinate containing 0.3371 mol L−1 H3BO3 was obtained, corresponding to a stripping efficiency of H3BO3 reaching 99.97%, and the boron was also enriched. After 5 cycles of the two-stage countercurrent extraction experiment, the reverse extraction system reached a relatively balanced state. The results demonstrate that the three-stage countercurrent stripping experiment can remarkably enhance the stripping efficiency of H3BO3. This indicates that boric acid is essentially completely stripped from the system. Through this approach, the extraction and separation of boric acid is optimized, utilizing advanced techniques and reagents to achieve efficient separation and purification. The use of countercurrent stripping allows for more thorough removal of the target compound, ensuring high purity and recovery rates. This method provides a reliable and effective solution for the extraction and purification of boric acid from complex mixtures.
1, as depicted in Fig. 21. The loaded organic phase collected through three-stage countercurrent extraction served as the raw material, and 0.3 mol L−1 NaOH was utilized as the stripping agent. The experimental outcomes are presented in Table 5. Ultimately, a stripping raffinate containing 0.3371 mol L−1 H3BO3 was obtained, corresponding to a stripping efficiency of H3BO3 reaching 99.97%, and the boron was also enriched. After 5 cycles of the two-stage countercurrent extraction experiment, the reverse extraction system reached a relatively balanced state. The results demonstrate that the three-stage countercurrent stripping experiment can remarkably enhance the stripping efficiency of H3BO3. This indicates that boric acid is essentially completely stripped from the system. Through this approach, the extraction and separation of boric acid is optimized, utilizing advanced techniques and reagents to achieve efficient separation and purification. The use of countercurrent stripping allows for more thorough removal of the target compound, ensuring high purity and recovery rates. This method provides a reliable and effective solution for the extraction and purification of boric acid from complex mixtures.
| Raffinate | ICP test B/mg L−1 | H3BO3/mol L−1 | Extraction/% |
|---|---|---|---|
| R1 | 3.495 | 0.3233 | 95.88 |
| R2 | 3.527 | 0.3263 | 96.76 |
| R3 | 3.586 | 0.3317 | 98.38 |
| R4 | 3.607 | 0.3337 | 98.95 |
| R5 | 3.627 | 0.3355 | 99.50 |
| R6 | 3.632 | 0.3360 | 99.64 |
| R7 | 3.642 | 0.3370 | 99.91 |
| R8 | 3.644 | 0.3371 | 99.97 |
4. Conclusion
Taking the C12–OH solution of 2,2,4-trimethyl-1,3-pentanediol (TMPD) and sulfonated kerosene as extractants and taking the Laguocuo Salt Lake, a typical representative of weakly alkaline salt lakes in Tibet, as the research object, the process of extracting boron by TMPD was systematically studied. In the extraction process, one molecule of TMPD reacts with one boric acid molecule to form a complex containing two C–O–B ester bonds. In the case of high concentrations of CO32− and OH− ions in brine, it has a greater impact on the boron extraction rate. When the concentrations of carbonate and bicarbonate ions are high, boric acid mainly exists in the forms of [B4O5(OH)4]2− and [B(OH)4]−, and a small part is [B3O3(OH)4]−; these forms of borate ions are difficult to combine with TMPD, so the extraction rate is low. The above was characterized by Fourier transform infrared spectroscopy and Raman spectroscopy, providing the mechanism of this reaction. In the extraction process research, through single-stage and multi-stage countercurrent extraction experiments, the effects of different extractants, extractant concentration, pH value, temperature, and coexisting ions on the extraction efficiency were investigated, and the appropriate extraction conditions were determined. The results show that under the conditions of pH = 8.0, O/A = 1/1.5, and lower temperature, the boron recovery rate is relatively ideal. The single-stage boron extraction rate is >83%, and the three-stage boron extraction rate is 98.61%. At the same time, the stripping experiment results show that NaOH is a suitable stripping agent. When the NaOH concentration is 0.3 mol L−1 and the phase ratio is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the stripping effect is good at room temperature, and the boron concentration is also enriched. The research results provide a theoretical and experimental basis for recovering boron from weakly alkaline salt lake brine and verify the feasibility of this method.
1, the stripping effect is good at room temperature, and the boron concentration is also enriched. The research results provide a theoretical and experimental basis for recovering boron from weakly alkaline salt lake brine and verify the feasibility of this method.
Data availability
All data supporting the findings of this study are included within the manuscript.Author contributions
Conceptualization, X. P.; methodology, Y. Q.; validation, H. L.; formal analysis, C. Z., L. X. and C. S.; investigation, Y. H. and J. D.; resources, H. L. and S. X.; data curation, Y. H. and C. Z.; writing original draft, Y. H.; writing—review and editing, Y. Q. and C. S.; visualization, J. D.; supervision, X. P. and S. X.; project administration, Y. Q. and X. P.; funding acquisition, Y. Q. and X. P. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by CAS Project for Young Scientists in Basic Research (Grant No. YSBR-039), the Tianjin University-Qinghai Minzu University Joint Innovation Fund (Grant No. 2023TQ002), the Natural Science Foundation of Qinghai Minzu University (Grant No. 2024XJMA02), and the Natural Science Foundation of Qinghai Province (Grant No. 2020-ZJ-953Q).References
- F. X. Zhang, S. Zhao, Z. Liu, H. P. Jing and W. Y. Zhang, Conservation and Utilization of Mineral Resources, 2019, 39(6), 142–151 Search PubMed.
- Y. A. Huo, Q. Y. Liu and Y. Wang, China Min. Mag., 2023, 45(2), 123–135 Search PubMed.
- Y. Y. Zhao, F. J. Nie, Z. Q. Hou, Z. Q. Li, X. T. Zhao and Z. B. Ma, Acta Petrol. Sin., 2008, 24(3), 519–530 CAS.
- Y. Y. Lv, J. Geomech., 2024, 30(1), 107–128 Search PubMed.
- J. Y. Peng, S. J. Bian, B. Zhang, Y. P. Dong and W. Li, Hydrometallurgy, 2017, 174, 47–54 CrossRef CAS.
- J. Y. Lin, Y. J. Shih, T. Y. Hsieh and Y. H. Huang, RSC Adv., 2016, 6, 63206–63213 RSC.
- C. Ma, J. F. Zhang, Y. T. Liu, L. Q. Sun and Q. F. Liu, Sep. Purif. Technol., 2024, 301, 141280 Search PubMed.
- A. Siciliano, C. Limonti, G. M. Curcio and F. Marchio, Journal of Water Process Engineering, 2022, 48, 102–110 Search PubMed.
- A. Melliti, W. Kheriji and I. Zidi, Water Sci. Technol., 2020, 81(2), 321–332 CrossRef CAS PubMed.
- M. B. Baskan and N. Atalay, Desalin. Water Treat., 2014, 52(6–7), 1191–1200 Search PubMed.
- T. Kameda, J. Oba and T. Yoshioka, J. Hazard. Mater., 2015, 293, 54–63 CrossRef CAS PubMed.
- A. Alharati, Y. Swesi, K. Fiaty and C. Charcosset, Desalin. Water Treat., 2018, 129, 34–41 CrossRef CAS.
- H. Kim, S. Kim and C. Kim, J. Environ. Chem. Eng., 2024, 12(4), 113159 CrossRef CAS.
- A. Alharati, Y. Swesi, K. Fiaty and C. Charcossect, Journal of Water Process Engineering, 2017, 17, 32–39 CrossRef.
- E. Çermikli, F. Şen, E. Altıok, J. Wolska, P. Cyganowski, N. Kabay, M. Arda and M. YuKsel, Desalination, 2020, 484, 114504 CrossRef.
- J. G. Neo, S. Japip, L. Luo, T. S. Chung, M. Weber and C. Maletzko, Sep. Purif. Technol., 2019, 222, 214–220 CrossRef CAS.
- A. Alkhudhiri, N. B. Darwish, M. W. Hakami, A. Abdullah, A. Alsadun and H. A. Homod, Membranes, 2020, 10(4), 1–16 Search PubMed.
- A. Balinski, V. Recksiek and N. Kelly, Processes, 2021, 9(2), 398 CrossRef CAS.
- K. C. Kim, N. I. Kim, T. Jiang, J. C. Kim and C. I. Kang, Hydrometallurgy, 2023, 210, 105705 Search PubMed.
- G. Almustafa, A. S. Darwish, T. Lemaoui, M. J. O'Connor, S. Amin and H. A. Arafat, Chem. Eng. J., 2021, 263, 131342 CrossRef.
- A. Yáñez-Fernández, M. J. Inestrosa-Izurieta, J. I. Urzúa and H. A. Arafat, Desalination, 2021, 517, 115269 CrossRef.
- A. E. Choukaife and L. Aljerf, J. Chromatogr. Sep. Tech., 2017, 1(4), 00024 Search PubMed.
- N. Matsuo, T. Ishihara, T. Oyanagi, K. Nakajima, M. Kunimoto, Y. Fukunaka and T. Homma, ECS Trans., 2020, 64(45), 91–97 CrossRef.
- K. Seval, C. Onac, A. Kaya and A. Akdogan, Membranes, 2021, 11(4), 291 CrossRef CAS PubMed.
- Z. Xu, H. Su, J. Zhang, W. Liu, Z. Zhu, J. G. Wang, J. Chen and T. Qi, RSC Adv., 2021, 11, 16096–16105 RSC.
- M. D. L. A. Fernández, J. Boiteux, M. Espino, F. J. V. Gomez and M. F. Silva, Anal. Chim. Acta, 2018, 1038, 1–10 CrossRef PubMed.
- L. Aljerf, K. Beasley, B. Smith and N. Ganeshan, International Journal of Biochemistry Advances, 2017, 1(1), 1–8 Search PubMed.
- F. Califano and N. James, J. Eng. Technol. Res., 2013, 5(4), 79–86 CAS.
- C. Bradtmöller and S. Scholl, Chem. Eng. Res. Des., 2015, 99, 75–86 CrossRef.
- N. Bachelier and J. Verchere, Polyhedron, 1995, 14(7), 949–954 Search PubMed.
- M. Badruk, N. Kabay, M. Demircioglu, H. Mordogan and U. Ipwkouglu, Sep. Sci. Technol., 1999, 34(13), 2521–2539 CrossRef.
- G. Xu, B. Feng and B. Jiang, J. Food Sci., 2010, 31(8), 75 Search PubMed.
- A. Anderko, P. Wang and M. Rafal, Fluid Phase Equilib., 2002, 194–197, 123–142 CrossRef CAS.
- A. Anderko, P. Wang and R. Young, Fluid Phase Equilib., 2002, 203, 141–176 CrossRef.
- F. Y. Zhu, C. H. Fang, Y. Fang, Y. Q. Zhou and L. D. Cao, J. Salt Lake Res., 2011, 1, 40–47 Search PubMed.
- J. Frantz, Chem. Geol., 1998, 152, 211–225 CrossRef CAS.
- Q. Z. Huang, J. S. Li, C. L. Ma, N. N. Wang, J. J. Pi, Z. X. Zhang, K. Chang, L. C. Kong, Y. Yang, X. Guo, Z. Y. Wang, J. H. Zhang, C. Pan, Y. F. Hu and Z. C. Liu, Sep. Purif. Technol., 2024, 358, 130252 CrossRef.
- F. Y. Zhu, L. G. Li, L. F. Jia, Y. F. Du, Y. Y. Wu, Y. H. Zhang, J. M. Pan, Y. Q. Zhou and J. S. Li, J. Mol. Liq., 2024, 408, 125390 CrossRef CAS.
- Z. J. Jiang, Z. D. Zhang, B. Z. Xia, S. Y. Xiong, L. R. Yang and Z. Li, Sep. Purif. Technol., 2024, 331, 125597 CrossRef CAS.
- X. W Peng, D. Shi, Y. Z. Zhang, L. C. Zhang, L. M. Ji and L. J. Li, J. Mol. Liq., 2021, 317, 115301 CrossRef.
- X. B. Fan, X. P. Yu, Y. F. Guo and T. Deng, J. Chem., 2018, 7530837 CAS.
- X. W. Peng, L. J. Li, D. Shi, L. C. Zhang, H. F. Li, F. Nie and F. G. Song, Hydrometallurgy, 2018, 177, 161–167 CrossRef CAS.
- X. D. Guan, P. Li, J. K. Zhang, Q. Q. Chang, J. C. Xiong, Y. W. Han, H. L. Zhang, Q. Li, L. L. Zhang, X. Y. Cao, H. J. Wang, Y. M. Yang, H. J. Xie and S. L. Zheng, Sep. Purif. Technol., 2024, 344, 127115 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |