Open Access Article
Open Access ArticleAtomic structures and electronic properties of different contact surfaces for CxFy–SiO2 triboelectric nanogenerator based on first-principles investigations
Baonan Jia *ab,
Jingming Gaob,
Jiaxiang Zhaob,
Jiahe Lianga,
Xinhui Zhangc,
Wendong Xiaoa,
Xiaoning Guan
*ab,
Jingming Gaob,
Jiaxiang Zhaob,
Jiahe Lianga,
Xinhui Zhangc,
Wendong Xiaoa,
Xiaoning Guan *b and
Pengfei Lu
*b and
Pengfei Lu b
b
aKey Laboratory of Knowledge Automation for Industrial Processes of Ministry of Education, School of Automation and Electrical Engineering, University of Science and Technology Beijing, Beijing 100083, China. E-mail: jiabaonan@163.com
bState Key Laboratory of Information Photonics and Optical Communications, Beijing University of Posts and Telecommunications, Beijing 100876, China. E-mail: guanxn@bupt.edu.cn
cSchool of Science, Xi'an University of Architecture and Technology, Xi'an 710055, China
First published on 17th January 2025
Abstract
Modification of the dielectric friction layer materials is an ideal way to enhance the output performance of a triboelectric nanogenerator (TENG), but current research mostly focuses on the metal–polymer or metal–SiO2 materials. In this work, we constructed different TENG models based on polymer CxFy–SiO2 electret materials, and the electronic properties of the different contact surfaces were investigated using first principles. We found that the charge transfer in CxFy–SiO2 materials occurred only at the contact interface, and it was partially affected by the terminal atoms near the SiO2 interface. The charge transfer of the polymer CxFy that was in contact with the O-terminated SiO2 achieved a more satisfactory effect. Among them, the II-C3F6–O model exhibited the highest amount of charge transfer because of the better hybridization of II-C3F6 with the O atoms of SiO2 layer. Our study showed that instead of adding different types of dielectric friction layers, varying the configurations of the same types of dielectric friction layers is an alternative way to regulate charge transfer. Furthermore, this strategy could provide new ideas for enhancing the performance of TENGs.
Introduction
With the continuous advancement of technology, microelectronic devices are increasingly being applied in the fields of self-powered flexible electronics and sensing. However, improving the energy efficiency of these devices to meet the demands of long-term use remains a pressing issue that needs to be addressed.1–7 The invention of triboelectric nanogenerators (TENG) marked a significant milestone in the field of self-powered systems, such as multimodal sensors, wearable electronic devices, and real-time human health monitoring, offering a novel approach to efficiently harvest mechanical energy.8–10 Current triboelectric nanogenerators still face issues such as insufficient output performance and low energy conversion efficiency,11–14 which limit their application in high-power energy generation because the dielectric friction layers cannot store charge for extended periods.15–25In recent years, the performance enhancement of TENG has been focused on the modification of its dielectric friction layers.26–31 Owing to their excellent ability to store charge for long periods, electret materials have become emerging materials for dielectric friction layers. Common electret materials mainly include inorganic electret materials, such as silicon dioxide, and organic electret materials, such as various polymers. In 2018, Wu et al.32 first investigated the mechanism of metal–polymer contact charging electrification via first-principles calculations and found that the stress on the contact region has a significant effect on the charge transfer. Further, Wu et al.33 investigated the charge transfer mechanism between metal and amorphous polymers and found that the modification of contact materials is an important method to improve the output power of TENG. Subsequently, Al–PE polymer,26 Cu–PVDF polymer,34 Au–PTFE polymer35 and Au–Nylon polymer35 have attracted widespread attention from researchers through first-principles investigations. In 2021, Antony et al.36 studied the electronic charge transfer of the hydroxylated metal/SiO2 interface using density functional theory and found that the separation distance between the contact surfaces must be small enough to produce electronic states within the apparent insulating bandgap. Owing to the unique physicochemical properties of metals, researchers have also paid significant attention to metal–SiO2 materials,37,38 such as Cu–SiO2, Au–SiO2, Pd–SiO2, Al–SiO2 and Ag–SiO2. In summary, current research on dielectric friction layers mainly focus on single electret materials, such as metal–polymer and metal–SiO2 materials; however, few studies are reported on polymer–SiO2 composite electret materials, indicating that there is still room for enhancing the output performance of TENG0073.
On this basis, we constructed polymer–SiO2 composite electret materials and modified the dielectric friction layers by changing the contact atoms and further altering the charge transfer. In this paper, interface models consisting of polymer CxFy (x = 2, y = 4; x = 3, y = 6) and SiO2 layer were considered, and the effect of different contact atoms on the electron transfer was investigated by first-principles calculations. The II-C3F6–O model, which consists of II-C3F6 and O-terminated SiO2, shows the best performance of electron transfer because II-C3F6 can hybridize more significantly with the O atoms of the SiO2 layer. Our study suggests that for the design of TENG, in addition to considering the different types of dielectric friction layers, different configurations of the same types can also be considered to enhance the performance of TENG.
Computational details
All the calculations in this paper are based on the Density Functional Theory (DFT) and were performed using the Vienna ab initio Simulation Package (VASP) software.39,40 Density Functional Theory is a first principles-based theoretical method that can achieve rigorous transformation from many-body problems into single-body problems. The Projector Augmented Wave (PAW) method41 was used to describe the interactions between the core electrons and the remaining valence electrons. The Perdew–Burke–Ernzerhof (PBE) functional within the generalized gradient approximation (GGA)42 was used to describe the exchange correlations of the electrons.VASP software was used to optimize the atomic structures and calculate the electronic properties of different contact models. The input energy was chosen to be 400 eV to ensure accuracy of calculations. For geometry optimization, all atomic positions and lattice structures were fully relaxed until convergence criteria were reached, with forces less than 0.05 eV per Å per atom and a total energy convergence criterion of 1.0 × 10−5 eV. For electronic properties, the same computational accuracy was used to obtain reliable results. According to previous studies,43,44 a vacuum layer of 20 Å was added to the z-axis to eliminate periodicity and avoid interaction between the two periodic units. In addition, considering the large contact models, we used a 10 × 10 × 1 K-point mesh around the gamma point in reciprocal space. The interlayer interaction of all interface models was carried out using Grimme's D3 correction45,46 to address the deficiencies in van der Waals forces.
Results and discussion
To better study the microscopic properties of triboelectric nanogenerators, we constructed interface models consisting of polymer CxFy (where x = 2, y = 4; x = 3, y = 6) and SiO2 materials.47,48 For the C3F6 polymer, according to the different atoms of the main and branched chains of the carbon chain, there are three conformations at the contact interface: CF2, CF3 and C2F2 atoms, respectively. We constructed three types of contact surface models, which were named I-C3F6, II-C3F6, and III-C3F6, as shown in Fig. 1(a–c), respectively. For the C2F4 polymer, on the other hand, the main and branched chains of the carbon chain are the same and there is only one conformation of CF2 atoms at the contact interface. Therefore, only one C2F4 contact surface model was constructed, as shown in Fig. 1(d). Similarly, based on the type of terminal atoms of SiO2 at the contact interface, we divided them into three configurations, and the atoms at the contact interface were O, Si, and SiO atoms, which were named as SiO2–O, SiO2–Si and SiO2–SiO, as shown in Fig. 1(e–g), respectively. Consequently, considering the different conformations of dielectric friction layers between the polymer CxFy and SiO2 materials, we considered a total of 12 different contact surface structures to investigate related microscopic properties for the nanogenerators.To determine the stability of the structure and the nature of interaction force between the two dielectric friction layers, the binding energies of interface models with different configurations were calculated as follows
| Eb = (ECxFy–SiO2 − ECxFy − ESiO2)/A | (1) |
| Material | Atomic number | a/Å | b/Å | Interlayer thickness/Å2 | Binding energy eV Å−2 |
|---|---|---|---|---|---|
| C2F4–SiO2–O | 45 | 5.46 | 4.87 | 2.719 | 0.034 |
| C2F4–SiO2–Si | 43 | 5.46 | 4.87 | 2.740 | 0.011 |
| C2F4–SiO2–SiO | 44 | 5.46 | 4.87 | 2.369 | 0.053 |
| I-C3F6–O | 54 | 6.37 | 4.89 | 2.024 | 0.038 |
| I-C3F6–Si | 52 | 6.37 | 4.89 | 2.093 | 0.032 |
| I-C3F6–SiO | 53 | 6.37 | 4.89 | 2.362 | 0.008 |
| II-C3F6–O | 57 | 6.37 | 4.89 | 1.568 | 0.046 |
| II-C3F6–Si | 55 | 6.37 | 4.89 | 1.378 | 0.024 |
| II-C3F6–SiO | 56 | 6.37 | 4.89 | 2.038 | 0.012 |
| III-C3F6–O | 56 | 6.37 | 4.89 | 1.118 | 0.019 |
| III-C3F6–Si | 54 | 6.37 | 4.89 | 1.426 | 0.002 |
| III-C3F6–SiO | 55 | 6.37 | 4.89 | 1.356 | 0.002 |
Analysis of the values in Table 1 indicates that when C2F4 and C3F6 are combined with SiO2, the contact area of C2F4 is smaller than that of C3F6 while the interlayer thickness is larger than that of C3F6. Consequently, it is easier for C3F6 to form a contact interface model with SiO2 than C2F4. Among the interface models consisting of SiO2 with different C3F6 configurations, the I-C3F6 model always has the largest interlayer thickness, whereas the II-C3F6 model has a suitable interlayer thickness. For the interface models consisting of C3F6 with different SiO2 configurations, the binding energies of the C3F6–O configurations are consistently larger than those of the C3F6–Si configurations and larger than those of the C3F6–SiO configurations. Based on previous studies,49,50 we screened the interface models with van der Waals interactions using 20–40 meV Å−1 as the range of binding energies. When the polymer C3F6 layer contacted with SiO-terminated SiO2, the binding energy is less than 0.02 eV Å−2. When the SiO2 layer contacted with II-C3F6, the formed configuration has the largest binding energy, of which the II-C3F6–O configuration has the most suitable binding energy of 0.046 eV Å−2.
In order to better understand the output performance, we analyzed the plane-averaged charge density difference of these 12 interface models and attempted a preliminary screening based on the amount and direction of charge transfer between the contact surfaces. It can be inferred from Fig. 2 that when the polymer CxFy is contacted with O-terminated SiO2 to form the CxFy–O interface model, the values of average charge density difference all reach up to 6 × 10−3 e Å−1. In contrast, when it is contacted with Si-terminated and SiO-terminated SiO2 to form the CxFy–Si and CxFy–SiO interface models, respectively, the values are much less likely to reach the same level in both dielectric friction layers.
For the CxFy–O, I-C3F6–SiO, and II-C3F6–SiO configurations, as shown in Fig. 2(a)–(d), (j) and (k), the six configurations have similar charge transfer modes. The difference values between the CxFy and SiO2 layers are almost equal, indicating a nearly unidirectional electron transfer. The configurations terminated with O atoms show a significantly larger charge transfer than those terminated with SiO atoms. In particular, the II-C3F6–O configuration has better charge transfer capacity with a larger amount of charge transfer, and the values of the average charge density difference reach up to 7.6 × 10−3 e Å−1, which represents an increase of 26.7% compared to the previously mentioned average value. For the CxFy–Si and C2F4–SiO configurations, as shown in Fig. 2(e)–(i), the frequent charge transfer at the interface implies that the electrons of these models do not simply transfer between the CxFy and SiO2 layers; some electrons accumulate near the interface instead of moving unidirectionally. For the III-C3F6–Si and III-C3F6–SiO configurations, as shown in Fig. 2(h) and (i), the charge transfers are too small and not considered in the subsequent calculations. In summary, among the above 12 interface conformations, the II-C3F6–O model has the most charge transfer and unidirectional electrons transfer.
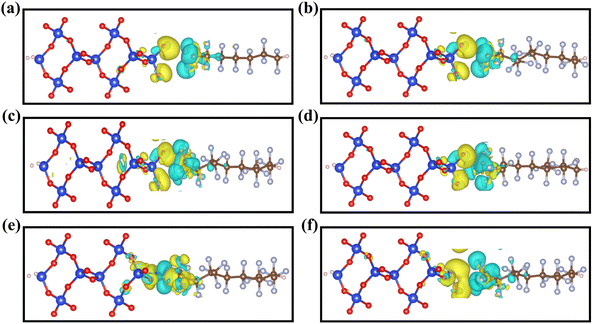
In order to understand the effect of different polymer friction layers on the interfacial properties, the charge density difference Δρ at the interfaces was computed to further investigate the charge transfer and charge redistribution between the CxFy and SiO2 interfaces.51
| Δρ = ρCxFy–SiO2 − ρCxFy − ρSiO2 | (2) |
It can be inferred from Fig. 3 that the yellow area indicates the charge depletion region and the blue area indicates the charge aggregation region. Charge transfer at the contact interface can be visualized more intuitively through the differential charge density map. We can judge the level of charge density based on the size of the area where the charge accumulates. The charge aggregation and depletion mainly occur at the contact interface between the polymer CxFy and SiO2 electret materials. There is more charge transfer occurring between the CxFy and O-terminated SiO2, no significant charge transfer between the CxFy and Si-terminated SiO2, and less charge transfer between the CxFy and SiO-terminated SiO2. For the polymer CxFy contacting the SiO2–O interface, as shown in Fig. 3(a–d), CxFy acquires electrons from the SiO2 interface, forming a charge depletion region at the SiO2 layer and a charge aggregation region at the CxFy layer. As shown in Fig. 3(c), II-C3F6 contact with the SiO2–O interface has a greater charge density. This indicates that the II-C3F6–O model has a strong charge transfer capability as the electret materials of the triboelectric nanogenerator. For the polymer II-C3F6 contacting different termination atoms of SiO2, as shown in Fig. 3(c, e and f), based on the different contact surfaces as well as the different sizes of the charge transfer region, it can be further judged that the II-C3F6–O structure has a better charge transfer ability and is more suitable to be the electret material for triboelectric nanogenerator.
The electrostatic potential difference between the two dielectric friction layers can be used to understand the amount of charge transfer and the time of storing charge. All configurations were analyzed by the value of differential charge density, and the variation of electrostatic potential along the z-direction was plotted, as shown in Fig. 4.52 The electrostatic potential differences between CxFy and SiO2–O, II-C3F6 and SiO2–Si, II-C3F6 and SiO2–SiO configurations were investigated. The electrostatic potential of the SiO2 layer is shown on the left side of the figure and that of the polymer layer is on the right side. By quantitatively analyzing the electrostatic potential, for the configurations of the CxFy contacting the O-terminated SiO2, as shown in Fig. 4(a–d), the difference in electrostatic potentials of the C3F6–O interface is significantly larger than that of the C2F4–O interface, which indicated that the charge transfer between the dielectric friction layers of the C3F6 and SiO2–O interfaces is closer, among which the II-C3F6 configuration performs optimally. For the configurations of II-C3F6 contacting different termination atoms of SiO2, as shown in Fig. 4(c, e and f), they possess almost the same trend of electrostatic potential change at the same distance. However, the difference in the electrostatic potential between the II-C3F6 and SiO2–O interfaces is the largest, as shown in Fig. 4(c), which indicates that the II-C3F6–O model possesses more charge transfer and has better power generation performance.
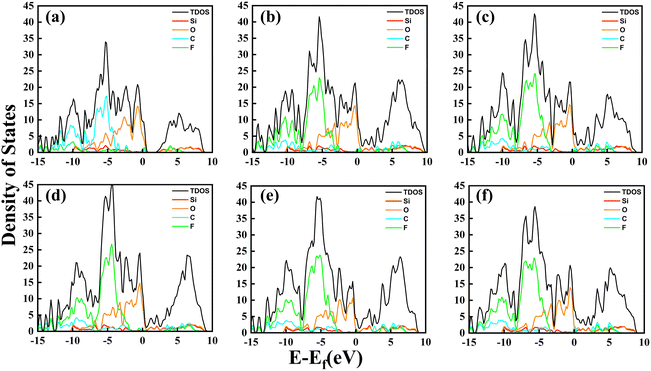
In order to gain insight into the nature of charge transfer between the dielectric friction layers composed of polymer CxFy and SiO2 electret materials, we calculated the total density of states (TDOS) and the projected density of states (PDOS) of the atoms for these interface models, as shown in Fig. 5. Due to the difference in the C2F4 and C3F6 extra-nuclear electronic structures, the TDOS peaks of the C2F4 structure in Fig. 5(a) are smaller than those of the other C3F6 structures. Also, there is a region with no density of states near 1 eV that is also different from the other C3F6 structures, a characteristic that is not conducive to electron transfer between dielectric friction layers.
In the interface models, as shown in Fig. 5(b)–(d), one layer of the dielectric friction layers is O-terminated SiO2 and the other layer contacts different C3F6 structures. It can be seen that above the Fermi energy level, for the I-C3F6 structure, the first peak of the O atom is close to the C and F atoms and shows hybridization in both contour and value. For the II-C3F6 structure, the hybridization of the O atom with C and F atom also changes obviously, and the change in the O atom is more obvious than that of the Si atom, which makes it reasonable to assume that the II-C3F6–O structure has better orbital hybridization and charge transfer. However, for the III-C3F6 structure, the first peak does not show significant hybridization with O atoms, which shows that the charge transfer of the III-structure is not as good as that of the I- and II-structures.
In the interface models, as shown in Fig. 5(c), (e) and (f), one layer of dielectric friction layers is the polymer II-C3F6 material, the other layer contacts different termination atoms of SiO2. For the SiO2–O model, the first peak on the O, C and F atoms shows obvious hybridization above the Fermi energy level. For the SiO2–Si model, the first peak also shows obvious hybridization but is numerically smaller than the O-terminated SiO2 model. For the SiO2–SiO model, the first peak has no obvious orbital hybridization compared with the other two models. Therefore, it is considered that the II-C3F6–O interface model, constituted by II-C3F6 in contact with the O-terminated SiO2, has better output performance, which also explains the charge transfer phenomenon between the dielectric friction layers.
Conclusions
In this study, considering the different contact surfaces between polymer CxFy and SiO2 electret materials, we constructed C3F6(C2F4)–SiO2 interface models as the dielectric friction layers. The charge transfer between the contact surfaces was investigated through first principles to study the intrinsic reasons of TENG modification. It was found that a better charge transfer effect was achieved when the polymer CxFy was in contact with the O-terminated SiO2. Among the interface models, the II-C3F6 configuration was likely to contact with the O atoms of the SiO2 layer, which not only had the largest amount of charge transfer but also possessed unidirectional charge transfer. Notably, the II-C3F6–O model possesses the largest electrostatic potential difference, indicating that this TENG structure has the best output performance. By calculating and analyzing the density of states, we found that the II-C3F6 configuration will hybridize more significantly with the O atoms of SiO2 compared to the other polymer configurations. This study reveals the intrinsic properties of TENG composed of polymer CxFy and SiO2 electret materials and provides theoretical guidance for modifying dielectric friction layers through the different configurations of the same materials. How to improve the charge storage capability of the dielectric friction layers through material composition design is currently a hot topic of concern. In future, dielectric friction layers composed of composite electret materials need to be expanded.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Baonan Jia: writing – original draft, investigation, methodology, formal analysis. Jingming Gao: investigation, data curation, software. Jiaxiang Zhao: investigation, data curation, software. Guoying Qin: investigation, data curation, software. Xiaoning Guan: writing – review & editing, formal analysis. Xinhui Zhang: investigation, formal analysis. Wendong Xiao: writing – review & editing, conceptualization. Pengfei Lu: investigation, conceptualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fundamental Research Funds for the Central Universities (No. 00007845), Foundation of Laboratory of Computational Physics (No. 6142A05QN22017) and project funded by China Postdoctoral Science Foundation (No. 2022M720516). We thank for the helpful discussion with Prof. Pengfei Guan and the computational support from the Beijing Computational Science Research Center (CSRC).Notes and references
- G. Ruhl, S. Wittmann, M. Koenig and D. Neumaier, Beilstein J. Nanotechnol., 2017, 8, 1056–1064 CrossRef CAS PubMed.
- J. Yang, K. Liu, X. Chen and D. Shen, Prog. Polym. Sci., 2022, 83, 100397 Search PubMed.
- A. I. Inamdar, S. Kamal, M. Usman, M.-H. Chiang and K.-L. Lu, Coord. Chem. Rev., 2024, 502, 215596 CrossRef CAS.
- X. Cao, Y. Xiong, J. Sun, X. Xie, Q. Sun and Z. L. Wang, Nano-Micro Lett., 2023, 15, 14 CrossRef CAS PubMed.
- X. Tao, X. Chen and Z. L. Wang, Energy Environ. Sci., 2023, 16, 3654–3678 RSC.
- H. Xiang, L. Peng, Q. Yang, Z. L. Wang and X. Cao, Sci. Adv., 2024, 10, eads2291 CrossRef CAS PubMed.
- Y. Jiang, X. Liang, T. Jiang and Z. L. Wang, Engineering, 2024, 33, 204–225 CrossRef CAS.
- F.-R. Fan, Z.-Q. Tian and Z. L. Wang, Nano Energy, 2012, 1, 328–334 CrossRef CAS.
- J. Luo and Z. L. Wang, EcoMat, 2020, 2, e12059 CrossRef CAS.
- J. Tao, L. Wang, K. Kong, M. Hu and Z. Dai, Biomimetics, 2022, 7, 216 CrossRef CAS PubMed.
- Y. Li, Y. Luo, H. Deng, S. Shi, S. Tian, H. Wu, J. Tang, C. Zhang, X. Zhang and J. W. Zha, Adv. Mater., 2024, 36, 2314380 CrossRef CAS PubMed.
- X. Zhu, Y. Hao, J. Yang, W. Su, H. Zhang, Y. Qin, C. Zhang and X. Li, Appl. Mater. Today, 2024, 41, 102492 CrossRef.
- K. Xiao, W. Wang, K. Wang, H. Zhang, S. Dong and J. Li, Adv. Funct. Mater., 2024, 34, 2404744 CrossRef CAS.
- W. Peng and S. Du, IEEE Trans. Circuits Syst. I Regul. Pap., 2023, 70, 3049–3062 Search PubMed.
- W. Sun, G. Ji, J. Chen, D. Sui, J. Zhou and J. Huber, Nano Energy, 2023, 108, 108248 CrossRef CAS.
- H. Zhang, P. Zhang, P. Li, L. Deng, W. Zhang, B. Liu and Z. Yang, Nano Res., 2022, 15, 7163–7171 CrossRef CAS.
- Q. M. Saqib, M. Y. Chougale, M. U. Khan, R. A. Shaukat, J. Kim, J. Bae, H. W. Lee, J.-I. Park, M. S. Kim and B. G. Lee, Nano Energy, 2021, 89, 106458 CrossRef CAS.
- Y. Zhu, Y. Zhao, L. Hou and P. Zhang, Micromachines, 2022, 13, 2053 CrossRef PubMed.
- P. Zhang, Y. Ma, H. Zhang and L. Deng, ACS Appl. Energy Mater., 2023, 6, 6598–6606 CrossRef CAS.
- L. Shooshtari, N. Rafiefard, M. Barzegar, S. Fardindoost, A. Irajizad and R. Mohammadpour, ACS Appl. Nano Mater., 2022, 5, 17123–17132 CrossRef CAS.
- X. Liu, Y. Wang, G. Wang, Y. Ma, Z. Zheng, K. Fan, J. Liu, B. Zhou, G. Wang and Z. You, Matter, 2022, 5, 4315–4331 CrossRef.
- W. Liu and J. Shi, Nano Energy, 2021, 89, 106479 CrossRef CAS.
- S.-N. Lai, C.-K. Chang, C.-S. Yang, C.-W. Su, C.-M. Leu, Y.-H. Chu, P.-W. Sha and J.-M. Wu, Nano Energy, 2019, 60, 715–723 CrossRef CAS.
- Y. Liu, G. Liu, T. Bu and C. Zhang, Mater. Today Energy, 2021, 20, 100686 CrossRef CAS.
- K. Xia and Z. Xu, Smart Mater. Struct., 2020, 29, 095016 CrossRef CAS.
- L. Li, X. Wang, P. Zhu, H. Li, F. Wang and J. Wu, Nano Energy, 2020, 70, 104476 CrossRef CAS.
- X. Chen, F. Wang, Y. Zhao, P. Wu, L. Gao, C. Ouyang, Y. Yang and X. Mu, Research, 2022, 2022, 9765634 CAS.
- G. Yinben, C. Zixi, W. Hongzhi and Z. Qinghong, J. Inorg. Mater., 2021, 36, 919–928 CrossRef.
- C. Xu, B. Zhang, A. C. Wang, W. Cai, Y. Zi, P. Feng and Z. L. Wang, Adv. Funct. Mater., 2019, 29, 1903142 CrossRef.
- G. Khandelwal, A. Chandrasekhar, N. P. Maria Joseph Raj and S.-J. Kim, Adv. Energy Mater., 2019, 9, 1803581 CrossRef.
- G. Khandelwal, M. K. Ediriweera, N. Kumari, N. P. Maria Joseph Raj, S. K. Cho and S.-J. Kim, ACS Appl. Mater. Interfaces, 2021, 13, 18887–18896 CrossRef CAS PubMed.
- J. Wu, X. Wang, H. Li, F. Wang, W. Yang and Y. Hu, Nano Energy, 2018, 48, 607–616 CrossRef CAS.
- J. Wu, X. Wang, H. Li, F. Wang and Y. Hu, Nano Energy, 2019, 63, 103864 CrossRef CAS.
- L. Li, X. Wang, Y. Hu, Z. Li, C. Wang and Z. Zhao, Adv. Funct. Mater., 2022, 32, 2109949 CrossRef CAS.
- D. Kang, J.-H. Hwang, Y.-J. Kim, P. Zhao, H. Y. Lee, J. Kim, M. S. Shin, S. Jeon, S. Kim and S.-W. Kim, Mater. Today, 2024, 72, 109–116 CrossRef CAS.
- A. C. Antony, D. Thelen, N. Zhelev, K. Adib and R. G. Manley, J. Appl. Phys., 2021, 129, 065304 CrossRef CAS.
- T.-R. Shan, B. D. Devine, S. R. Phillpot and S. B. Sinnott, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 83, 115327 CrossRef.
- J. Huang, E. Tea, G. Li and C. Hin, Appl. Surf. Sci., 2017, 406, 128–135 CrossRef CAS.
- V. Wang, N. Xu, J.-C. Liu, G. Tang and W.-T. Geng, Comput. Phys. Commun., 2021, 267, 108033 CrossRef CAS.
- Y. Y. Liang, D. M. Chen and J. F. Tong, Key Eng. Mater., 2012, 512, 490–493 Search PubMed.
- P. E. Blöchl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953 CrossRef PubMed.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- F. Withers, O. Del Pozo-Zamudio, A. Mishchenko, A. P. Rooney, A. Gholinia, K. Watanabe, T. Taniguchi, S. J. Haigh, A. Geim and A. Tartakovskii, Nat. Mater., 2015, 14, 301–306 CrossRef CAS PubMed.
- A. K. Geim and I. V. Grigorieva, Nature, 2013, 499, 419–425 CrossRef CAS PubMed.
- W. Liu, C.-L. Yang, Y.-T. Zhu and M.-S. Wang, J. Phys. Chem. C, 2008, 112, 1803–1811 CrossRef CAS.
- R. Chawla and S. Sharma, Compos. Sci. Technol., 2017, 144, 169–177 CrossRef CAS.
- K. Zhao, W. Sun, S. Li, Z. Song, M. Zhong, D. Zhang, B.-N. Gu, M.-J. Liu, H. Fu and H. Liu, Discover Nano, 2023, 18, 69 CrossRef CAS PubMed.
- D. L. Vu, C. D. Le and K. K. Ahn, Polymers, 2022, 14, 960 CrossRef CAS PubMed.
- T. Björkman, A. Gulans, A. V. Krasheninnikov and R. M. Nieminen, Phys. Rev. Lett., 2012, 108, 235502 CrossRef PubMed.
- Y. Chen, X. Guan, L. Yang, B. Jia, H. Zhao, L. Han, P. Guan and P. Lu, Appl. Surf. Sci., 2023, 611, 155679 CrossRef CAS.
- M. T. As-Samee, M. S. Hasan Khan, K. Kubra, M. R. Islam and M. T. Hasan, AIP Adv., 2023, 13, 065004 CrossRef CAS.
- Q. Campbell, J. Electrochem. Soc., 2023, 170, 031506 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |