Open Access Article
Open Access ArticlePhytochemical and antioxidant comparison of Quercus ilex and Quercus robur acorn extracts obtained by matrix solid-phase dispersion†
Diego Gonzalez-Iglesias *ab,
Laura Rubio
*ab,
Laura Rubio a,
Francisco Martinez-Vazquezb,
Aly Castillo
a,
Francisco Martinez-Vazquezb,
Aly Castillo abc,
Maria Celeiro
abc,
Maria Celeiro c,
Carmen Garcia-Jaresc and
Marta Lores
c,
Carmen Garcia-Jaresc and
Marta Lores b
b
ai-Grape, Via Isaac Peral, 32, 15890, Santiago de Compostela, Spain
bLIDSA, Department of Analytical Chemistry, Nutrition and Food Science, Faculty of Chemistry, Universidade de Santiago de Compostela, E-15782, Santiago de Compostela, Spain. E-mail: diegogonzalez.iglesias@usc.es
cCRETUS, Department of Analytical Chemistry, Nutrition and Food Science, Universidade de Santiago de Compostela, E-15782, Santiago de Compostela, Spain
First published on 22nd April 2025
Abstract
Oak (Quercus spp.) acorns are used in animal feed and in the treatment of specific diseases due to their nutritional value and high content of bioactive compounds. The aim of the present work is to investigate and compare polyphenolic compounds and the antioxidant activity of Quercus ilex and Quercus robur acorn extracts. This is performed using the matrix solid-phase dispersion (MSPD) extraction process, in an environmentally friendly way with different generally recognised as safe (GRAS) solvents. The GRAS solvents considered were an alcohol, a ketone, an ester and a glycol. Total polyphenolic content (TPC) and antioxidant activity (DPPH and ABTS scavenging test) were determined spectrophotometrically. The different antioxidant data obtained by two approaches are discussed. All Quercus robur extracts show better results than Quercus ilex in both total polyphenolic content and antioxidant activity, the highest results being obtained with ethyl lactate, 76 mgGAE g−1 DW and 2636 μmolTE g−1 DW, respectively. These results demonstrate the correlation between total polyphenolic content and antioxidant activity, and that free radical scavenging is concentration dependent. Individual quantification of the polyphenols was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS), with the major compounds being gallic acid, ellagic acid, catechin, quercetin and gallotannins in all extracts. MSPD, for the first time applied to acorns, has proven to be a good alternative to conventional processes for obtaining antioxidant extracts rich in bioactive compounds.
Introduction
Quercus is a genus of trees belonging to the family Fagaceae generally found in subtropical climate areas in various parts of the world, including Europe, North America and Asia.1 They are important members of most forests all over the world and have received great attention since ancient times due to their medicinal, ecological, and economical value.2 Usage of acorns, the fruit of Quercus trees, in nutrition has a long history. Oak acorns were especially used in Italy and Spain, providing up to 25% of the food consumed by the poorer classes.3 Quercus ilex L. (holm oak) is an evergreen tree distributed throughout the Mediterranean Basin while Quercus robur L. (pedunculate oak) is a deciduous tree and the most important natural forest community in northern Spain.4,5 Currently, acorns are mostly associated with animal feed, but they are receiving increasing attention for their potential as sources of essential nutrients for humans because of their content of carbohydrates, amino acids, proteins, lipids, and various sterols.6–9 Acorns, beside nutritional components, contain various bioactive compounds like polyphenols (tannins, gallic and ellagic acid, and different galloyl derivatives) which possess antioxidant activity.10Polyphenols are one of the most important classes of bioactive compounds and they are characterized by the ideal chemical structure for neutralization of oxygen radicals.11,12 Consuming polyphenol-rich foods has shown positive impacts in health increasing the protection against developing different types of cancers, cardiometabolic disorders, diabetes, and neurodegenerative diseases.13,14 Oak plants are able to synthesize a significant amount of phenols in vegetative and generative organs, which are essential for formation of systemic plant resistance. The antioxidant activity of polyphenols has long been known and research on its uses as natural antioxidants is of great importance in the pharmaceutical industry.15–17 Although free radicals are known to maintain homeostasis at the cellular level and work as signalling molecules, the excess of these are reported for oxidative stress and cause of various degenerative diseases. In this context, antioxidant capacity plays an important role in prevention, interception and repairing of the body through stopping the formation of ROS, radical scavenging, and repairing the enzymes involved in the process of cellular development.18 Quercus robur wood extracts are an important source of bioactive compounds possessing important antioxidants effects, and it has been demonstrated a high correlation between the content of total polyphenolic content and their antioxidant capacity.19 These results provide evidence that these plants could be potential sources of natural antioxidant agents and good candidates for future biomedical applications to promote human health with limited side effects.20 Many other studies indicated that acorn fruits are a potential source of various natural antioxidant compounds.21
The most common methodologies used to obtain bioactive extracts from Quercus consist of time-consuming multi-step processes such as dehulling, drying, maceration and concentration.6,22 The alternative technique proposed in this work, matrix solid-phase dispersion (MSPD), has never been previously used in the extraction of bioactive compounds from acorns. MSPD was first introduced by Barker et al. (1989) as a process for sample preparation, having the advantage of combining maceration, extraction, and filtration in a single process, eliminating centrifugation, drying, and separation steps.23 It has been widely applied in the extraction of polyphenolic compounds from algae and agro-industrial by-products.24–26 Due to the scaling versatility of the process and its combination with generally recognized as safe (GRAS) solvents, it is an attractive technique for practical application purposes in different areas.27
Therefore, the aim of the present work is to study the production of bioactive extracts from Quercus ilex and Quercus robur acorns by the MSPD extraction technique using GRAS solvents belonging to different chemical families. The solvents selected were hydro-organic mixtures of an alcohol, a ketone, an ester and a glycol. Antioxidant activity (AA) and total polyphenolic content (TPC) were evaluated as indicator parameters of the bioactivity of the extracts. The study includes the interspecific comparison of the antioxidant capacity between two of the main species of the genus Quercus, along with their target polyphenol profile determined by LC-MS/MS. For the first time, a comprehensive analysis of Quercus ilex and Quercus robur acorns incorporating the evaluation of these GRAS solvents according to a minimum requirements technique such as the MSPD is presented here.
Materials and methods
Standards and reagents
The standards employed for the quantification of the main polyphenols contained in the acorn extracts and the bioactivities, with their purity, suppliers, and CAS numbers are summarized in Table S1.† The solvents used for the extraction process were ethyl lactate from Lluch essence (Barcelona, Spain), acetone from Letslab (Barcelona, Spain), ethanol, propylene glycol, and ultrapure water MS-grade from Scharlab (Barcelona, Spain). Methanol MS-grade obtained by Sigma-Aldrich Chemie GmbH (Steinheim, Germany) and formic acid obtained by Merck (Darmstadt, Germany) were used for the mobile phase preparation in LC-MS/MS.Quercus acorns
Quercus ilex acorns were manually collected from the area of O Courel, Galicia (northwest of Spain) during the month of September 2022, and Quercus robur acorns from the area of Narón, Galicia also in September 2022. Acorns were properly identified and authenticated by Dr Francisco Javier Silva Pando, Department of Forest Ecosystems, Lourizán Forestry Research Centre, Xunta de Galicia, Galicia, Spain. Thereafter, the plant material was weighed and crushed without pre-treatment in an electric blender until an average particle diameter of about 5 mm was obtained. The milled acorns were placed in food-grade bags (20 cm × 20 cm) hermetically sealed for freezing (−18 °C) to avoid oxidation. Moisture content was measured by determining the loss of mass after drying in a moisture analyser ADAM PMB 202 using a temperature ramp up to 110 °C until stabilisation. Humidity values of 59.8% for Quercus ilex acorns and 65.3% for Quercus robur ones were found.Matrix solid-phase dispersion
1 g of crushed acorn was dispersed with 4 g SiO2 (particle size 0.707 mm) using a mortar and pestle for 5 min. Then, the mixture of disrupted acorns and SiO2 was transferred into a polypropylene cartridge containing a PTFE cellulose frit at the bottom, and 1 g of sand (to obtain a further degree of fractionation and sample clean-up). Finally, other cellulose frit was placed at the top to compress the mixture. Elution was made by gravity flow performed with 10 mL of the corresponding solvent, by maintaining a controlled extractive flow of 1 mL min−1. The extraction time was maintained constant (10 min) by regulating the flow rate using a discharge valve.The extractant GRAS solvents were isovolumetric mixtures of water with ethanol (E50), acetone (A50), ethyl lactate (L50) and propylene glycol (P50). While ethanol and acetone are two popular solvents for the characterisation of polyphenols and antioxidant properties, ethyl lactate and propylene glycol are unusual, however, they are also successful for the extraction of bioactive compounds in plants.28–30 Solvents and ratio were selected due to its high capacity for the extraction of various phenolic compounds from agro-industrial residues, resulting in extracts characterized by a high antioxidant activity and polyphenolic content.31–33
Total polyphenolic content
The Folin–Ciocalteu assay was used to determine the total polyphenolic content (TPC) of the Quercus ilex acorns extracts following Zhang's guidelines for microtitration in 96-well plates.34 Briefly, 20 μL of diluted extract (with a dilution factor of 125 in MilliQ water) was mixed with 100 μL of Folin–Ciocalteu reagent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v) and 80 μL of sodium carbonate solution (7.5 g L−1). The mixture was homogenized and kept in the dark for 30 min. Then, the absorbance was measured at 760 nm in a microplate reader (BMG LABTECH, Ortenberg, Germany). To express the TPC index, calibration curves of gallic acid covering a concentration range of 30–150 mg L−1 (0.200–0.800 absorbance unit [AU]) were employed. TPC was expressed as milligrams of gallic acid equivalent per gram of dry weight acorn (mgGAE g−1 DW).
10, v/v) and 80 μL of sodium carbonate solution (7.5 g L−1). The mixture was homogenized and kept in the dark for 30 min. Then, the absorbance was measured at 760 nm in a microplate reader (BMG LABTECH, Ortenberg, Germany). To express the TPC index, calibration curves of gallic acid covering a concentration range of 30–150 mg L−1 (0.200–0.800 absorbance unit [AU]) were employed. TPC was expressed as milligrams of gallic acid equivalent per gram of dry weight acorn (mgGAE g−1 DW).
Antioxidant activity
To evaluate the antioxidant activity (AA) of the extracts as well as their half inhibitory concentration (IC50) and 90% inhibitory concentration (IC90), 2,2-diphenylyl-1-picrylhydrazyl radical (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) assays were employed. To express the AA, a calibration curve of Trolox in the range of 3–31 mg L−1 (0.200–0.800 AU) was employed. The AA was represented as micromoles Trolox equivalent per gram of dry weight acorn (μmolTE g−1 DM). The half inhibitory concentration (IC50) of the samples was also measured and referred to the acorn content present in the extract (mg L−1).For the DPPH assay, the guidelines described by Symes were followed.35 Briefly, 100 μL of each extract at eight different concentration levels were placed in a 96-well plate and mixed with 100 μL of DPPH reagent prepared in methanol. The mixture was kept in the dark for 10 min and the measurement was performed at 515 nm.
The uptake of ABTS radical cations (ABTS+) by the extracts was determined as described by Xiao with minor modifications.36 Briefly, a 7 mM stock solution of ABTS was prepared in water by reacting it with 2.45 mM potassium persulphate aqueous. The mixture was stored in the dark at 25 °C for 16 h. The solution was diluted in water to obtain an absorbance of 0.700 (±0.004) at 748 nm. 50 μL of the extracts diluted at eight different concentration levels were placed in a 96-well plate and mixed with 200 μL of the stock solution. The mixture was kept in dark for 7 min and the measurement was performed at 748 nm.
LC-MS/MS analysis
The quantification of the polyphenols present in the extracts was performed by LC-MS/MS using a Thermo Scientific (San Jose, CA, USA) instrument based on a TSQ Quantum Ultra™ triple quadrupole mass spectrometer equipped with a heated electrospray ionization (HESI) source, and an Accela Open autosampler with a 20 μL loop. Optimal instrumental conditions were previously optimized by Celeiro.37 The chromatographic separation was performed employing a Kinetex C18 column (100 mm × 2.1 mm × 100 Å) obtained from Phenomenex (Torrance, CA, USA). The mobile phase was composed of water (A) and methanol (B), both with 0.1% formic acid. The eluted gradient started with 5% of B (held 5 min), it was increased to 90% of B in 11 min and kept constant for 3 min. Finally, initial conditions were reached in 9 min. Injection volume was 10 μL, with a flow rate of 0.2 mL min−1, and column temperature was set at 50 °C. Compound identification and detection were performed by selected reaction monitoring (SRM) working simultaneously in both negative and positive mode, monitoring two or three MS/MS transitions for each compound. The MS/MS parameters for all studied compounds were optimized by individual direct infusion and the most abundant collision-induced fragments were considered for quantification. Other HESI source parameters were the spray voltage: 3000 V, vaporizer temperature: 350 °C, sheath gas pressure: 35 au (arbitrary units), and ion sweep and auxiliar gas pressure: 0 and 10 au, respectively, and the capillary temperature: 320 °C. The system was operated by Xcalibur 2.2. and Trace Finder 3.1. software.Statistical analysis
All data were expressed as mean ± standard deviation. Extractions were carried out in triplicate and tests were run in triplicate (n = 9). After confirming the homoscedasticity of the data, an analysis of variance (ANOVA) was carried out and Fisher's test was also performed on the experimental data using Minitab, LLC. 20.3. All statistical operations were performed at a significance level of 5%.Results and discussion
Overall bioactive profile
The bioactivity of the extracts is highly dependent on the solvent used, due to the different antioxidant potential of compounds with different polarities, with polyphenols being the main cause of free radical inhibition.38 Therefore, the effect of ethanol, acetone, ethyl lactate and propylene glycol on the following bioactivity indices, TPC and AA are shown in Fig. 1. | ||
| Fig. 1 Response of the bioactive indicators total polyphenolic content (TPC) and antioxidant activity (AA) for each species and solvent used. | ||
The results shown in Fig. 1 highlight the superiority of Quercus robur acorn extracts over Quercus ilex, although both species show the same response profile for each solvent used, with L50 and A50 extracts outperforming E50 and P50 extracts. It should also be noted that in both species higher antioxidant activity results are obtained when the ABTS method is used. The exact values and their classification into groups are shown in Table 1.
| Acorn | TPC (mgGAE g−1) | AA DPPH (μmolTE g−1) | AA ABTS (μmolTE g−1) |
|---|---|---|---|
| a Mean value and standard deviation (x ± SD) (n = 9). The different letters in a same column by species denote a statistical difference with 95% confidence level. | |||
| Quercus ilex | |||
| E50 | 19 ± 1d | 244 ± 19d | 353 ± 32d |
| A50 | 24 ± 1c | 308 ± 20c | 434 ± 31c |
| L50 | 27 ± 1b | 347 ± 15b | 547 ± 44b |
| P50 | 19 ± 1d | 245 ± 18d | 368 ± 31d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Quercus robur | |||
| E50 | 42 ± 3d | 1328 ± 94c | 2576 ± 201c |
| A50 | 68 ± 3c | 1573 ± 80b | 2988 ± 89b |
| L50 | 76 ± 3b | 1811 ± 113b | 2636 ± 181c |
| P50 | 47 ± 2d | 1267 ± 102c | 2374 ± 151c |
The results shown in Fig. 1 and Table 1 reflect the importance of the solvent used to obtain bioactive extracts. The highest TPC values for both species are obtained using L50, being for Quercus robur 76 mgGAE g−1 and for Quercus ilex 27 mgGAE g−1, followed by A50 extracts. Regarding AA, the highest values using the DPPH method are obtained with L50 for both species, being for Quercus robur 1811 μmolTE g−1 and for Quercus ilex 347 μmolTE g−1, followed by the A50 extracts. However, the highest values of AA using the ABTS method for Quercus robur are obtained with A50 being 2988 μmolTE g−1 and for Quercus ilex with L50 being 547 μmolTE g−1.
Regarding the TPC of acorns, MSPD extraction has shown a yield 1.4 ± 0.1 times higher than solid–liquid extraction in water with magnetic stirring for 24 h, 11.9 ± 0.7 times higher than solid–liquid extraction in MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (80
H2O (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) with a subsequent concentrate step, and 9.2 ± 0.5 times higher than Soxhlet extraction in hexane with a subsequent concentrate step.39–41 Regarding the antioxidant activity according to the DPPH method, MSPD extraction offers a yield between 5.7 ± 0.1 and 11.0 ± 0.9 times higher than the above mentioned extraction procedures, and between 10.8 ± 0.9 and 14.5 ± 0.8 times higher according to the ABTS method. All these results showed higher bioactive compounds content than those obtained using other extraction techniques for Quercus acorns, wood and leaves, and other herbs, fruits and vegetables, some of them involving several steps including long maceration times, energy consumption for drying and/or the use of non-green solvents.19,42–48
20) with a subsequent concentrate step, and 9.2 ± 0.5 times higher than Soxhlet extraction in hexane with a subsequent concentrate step.39–41 Regarding the antioxidant activity according to the DPPH method, MSPD extraction offers a yield between 5.7 ± 0.1 and 11.0 ± 0.9 times higher than the above mentioned extraction procedures, and between 10.8 ± 0.9 and 14.5 ± 0.8 times higher according to the ABTS method. All these results showed higher bioactive compounds content than those obtained using other extraction techniques for Quercus acorns, wood and leaves, and other herbs, fruits and vegetables, some of them involving several steps including long maceration times, energy consumption for drying and/or the use of non-green solvents.19,42–48
The behaviour of the extracts obtained with E50 and P50 for both species is similar, while those of L50 and A50 are superior indicating that the higher the polyphenolic content of the extract, the higher its antioxidant activity, establishing a direct relationship that shows that polyphenols are the main cause.19 It is also observed that extracts obtained from Quercus robur acorns are more bioactive than those from Quercus ilex. On the other hand, all extracts perform better in inhibiting ABTS free radicals. This may be due to differences in the interaction of polyphenols with DPPH and ABTS radicals due to different inhibition mechanisms and could also explain the better performance of the Quercus robur extract with the ABTS method.45,49
Behaviour of free radical-inhibiting extracts
For a better understanding of the evolution of free radical inhibition as a function of extract concentration, curves were plotted for each extraction solvent, species and methodology. The results are shown in Fig. 2.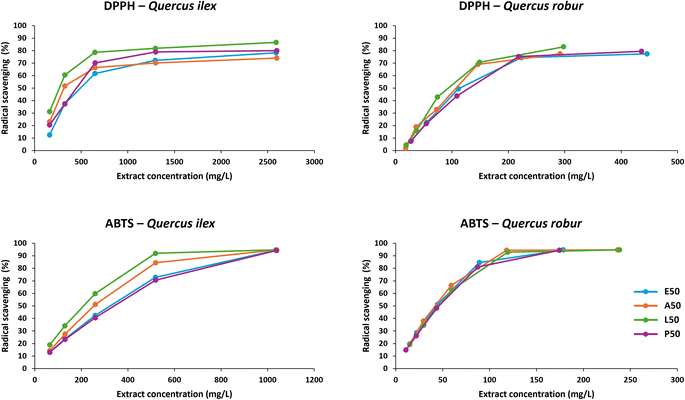 | ||
| Fig. 2 Evolution of free radical scavenging as a function of extract concentration for each species and solvent used. | ||
The inhibition curves for Quercus ilex acorn extracts show less overlap than those for Quercus robur, but with increasing concentration they all converge to similar values. The DPPH method does not allow to see the scavenging radical at high extract concentrations due to interferences for both species, regardless of the solvent used. The acorn extracts show a brownish colour due to the presence of carotenoids and other compounds that absorb at the same wavelength as DPPH.41,50 The interferences of these compounds with the DPPH method have already been demonstrated and, in this case, could be a limiting factor in the calculation of the IC90, which is a very interesting parameter when the extracts have a high antioxidant activity.51 The IC50 (DPPH & ABTS) and IC90 (ABTS) values are shown in Table 2 below.
| Acorn | IC50 DPPH (mg L−1) | IC50 ABTS (mg L−1) | IC90 ABTS (mg L−1) |
|---|---|---|---|
| a Mean value and standard deviation (x ± SD) (n = 9). The different letters in a same column by species denote a statistical difference with 95% confidence level. | |||
| Quercus ilex | |||
| E50 | 498 ± 39c | 348 ± 5b | 929 ± 31d |
| A50 | 315 ± 4b | 256 ± 17c | 735 ± 30c |
| L50 | 266 ± 4b | 210 ± 4b | 502 ± 8b |
| P50 | 448 ± 28c | 340 ± 17d | 943 ± 17d |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| Quercus robur | |||
| E50 | 117 ± 15bc | 47 ± 1c | 147 ± 7c |
| A50 | 108 ± 10bc | 42 ± 2b | 107 ± 1b |
| L50 | 92 ± 9b | 48 ± 1c | 118 ± 1b |
| P50 | 130 ± 10c | 49 ± 3c | 148 ± 9c |
The best IC50 values calculated by the DPPH method are obtained with L50, being for Quercus robur 92 mg L−1 and for Quercus ilex 266 mg L−1, (the lower, the better) followed by A50. In the case of ABTS, the best IC50 for Quercus robur is 42 mg L−1 and is obtained with A50, while for Quercus ilex it is 210 mg L−1 and is obtained with L50. The mean inhibitory concentration follows the same behaviour as IPT and AA, with Quercus robur extracts being more bioactive, and a higher bioactivity being observed when confronted with ABTS.
The IC50 calculated according to the DPPH method of acorn extracts from Quercus obtained by MSPD has been shown to be 1.9 ± 0.2 times higher than solid–liquid extraction in water with magnetic stirring for 24 h, and 4.5 ± 0.3 times higher according to the ABTS method. In addition, MSPD has also been shown to be 1.9 ± 0.2 times superior to ultrasound assisted extraction (UAE) in hexane for the calculation of the IC50 according to the ABTS method.39,52 On the other hand, this good antioxidant activity is also reflected with an inhibitory concentration (IC90) of 107 mg L−1 for Quercus robur acorns extracted with A50, which is a dilution factor 60 of the original extract. While the ABTS method has the advantage of no interference with the sample at high concentrations, the DPPH method has the practical advantage of not requiring reagent preparation 16 hours in advance.
Comparative profile of target polyphenols
To evaluate the presence of specific polyphenols, the acorn extracts were also directly analysed by LC-MS/MS. The resulting chromatograms were cross-checked against a spectral library of 70 different polyphenols. Thus, an unequivocal identification of the compounds was possible using commercially available standards of polyphenols and working in Selected Reaction Monitoring (SRM) mode, monitoring two or three MS/MS transitions per compound. A SRM reconstructed chromatogram for the Quercus ilex extract is depicted in Fig. 3. As can be seen, up to 12 target polyphenols were identified, including different phenolic acids, flavonoids and tannins. Gallic acid, ellagic acid, catechin, procyanidins, quercetin-3-O-glucoside, trigalloyl glucose and tetragalloyl glucose have been identified in Quercus acorns previously.40,53–55 Kaempferol glycosides, such as astragalin, are abundant in the genus Quercus, although this particular compound has not been identified previously in Quercus robur. Procyanidin A2 has also not been detected previously in reported studies on Quercus extracts.56 | ||
| Fig. 3 SRM reconstructed chromatogram obtained by LC-MS/MS analysis of L50 Quercus ilex acorn extract. | ||
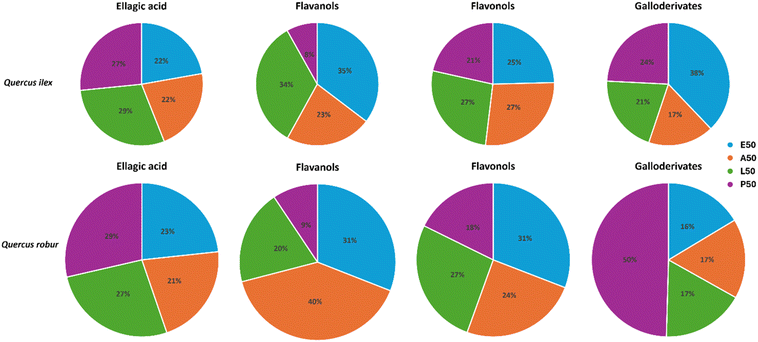
In the chromatogram corresponding to 1,3,6-trigalloylglucose, another peak with more intensity is observed, which could be an isomer due to sharing the same transitions, but its identification could not be carried out due to the lack of a standard. Another peak can also be observed in the chromatogram of procyanidin A2, which probably corresponds to procyanidin A1, but its identification cannot be assured due to the lack of a standard. In the chromatogram of quercetin-3-glucoside a peak corresponding to another quercetin-glycoside is also visible, most likely quercetin-3-galactoside, as they share molecular weight and transitions. Procyanidins B1, B2 and C1 were quantified as the sum of the three analytes. The quantification was performed using the corresponding pure standards of the target polyphenols analysed by LC-MS/MS to get the calibration data (R2 > 0.990 for them all) and results are shown in Table 3. In addition, Fig. 4 shows the distribution by polyphenolic families according to the extraction solvent used.
| Compound | RT (min) | Quercus ilex | Quercus robur | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| E50 | A50 | L50 | P50 | E50 | A50 | L50 | P50 | |||
| 1 | Gallic acid | 2.56 | 7.0 ± 0.5 | 13.2 ± 0.4 | 6.6 ± 0.3 | 18.5 ± 0.7 | 93 ± 10 | 129 ± 16 | 73 ± 5 | 354 ± 40 |
| 2 | ∑Procyanidins B1, B2, C1 | 5.01 | 36 ± 4 | 29.7 ± 0.8 | 32 ± 4 | 11 ± 1 | 19 ± 2 | 38 ± 4 | 15 ± 1 | 10.9 ± 0.8 |
| 3 | Catechin | 5.60 | 95 ± 13 | 54 ± 3 | 93 ± 9 | 20 ± 2 | 109 ± 10 | 128 ± 10 | 67 ± 8 | 28.±2 |
| 4 | 1,3,6-Trigalloylglucose | 5.87 | 15 ± 1 | 10.0 ± 0.7 | 10.2 ± 0.7 | 12.1 ± 0.7 | 14 ± 1 | 9.7 ± 0.7 | 44 ± 5 | 96 ± 8 |
| 5 | Procyanidin A2 | 7.98 | 4.6 ± 0.6 | 4.1 ± 0.4 | 4.6 ± 0.3 | 2.5 ± 0.2 | n.d | n.d | n.d | n.d |
| 6 | 1,2,3,6-Tetragalloylglucose | 7.95 | 72 ± 2 | 29 ± 4 | 37 ± 3 | 43 ± 4 | 79 ± 1 | 51 ± 1 | 78 ± 5 | 111 ± 13 |
| 7 | Quercetin-3-glucoside | 10.83 | 12.0 ± 0.4 | 12 ± 1 | 13.3 ± 0.6 | 9.6 ± 0.1 | 34 ± 3 | 20.9 ± 0.6 | 19 ± 1 | 9.8 ± 0.9 |
| 8 | Astragalin | 11.57 | n.d | n.d | n.d | n.d | < LOQ | < LOQ | 3.9 ± 0.4 | 2.1 ± 0.1 |
| 9 | Quercetin | 11.60 | 9 ± 1 | 11 ± 1 | 9.6 ± 0.9 | 8.9 ± 0.1 | 76 ± 7 | 66 ± 3 | 76 ± 4 | 53.0 ± 0.5 |
| 10 | Ellagic acid | 11.65 | 13.9 ± 0.1 | 14 ± 2 | 18.4 ± 0.8 | 17 ± 2 | 42 ± 3 | 39 ± 5 | 48 ± 4 | 51 ± 4 |
| ∑Polyphenols | 264.5 | 177.0 | 224.7 | 142.6 | 466.0 | 481.6 | 423.9 | 715.8 | ||
In Table 3, it should be noted that procyanidin A2 is only found in Quercus ilex acorns and that astragalin is only found in L50 and P50 extracts of Quercus robur, so these compounds can be used as specific markers. In conjunction with Fig. 4, the amount of ellagic acid extracted as a function of the solvent maintains the same profile for both species, where the maximum values were obtained with L50 and P50, being 18 and 16 mg kg−1, respectively for Quercus ilex, and 48 and 51 mg kg−1, respectively for Quercus robur. In the case of flavanols the extraction profile is slightly different, since for Quercus ilex the best solvents are E50 and L50, while for Quercus robur is A50, however for both species the worst is P50. For flavonols the same profile is observed where E50, A50 and L50 extract similar amounts and P50 is the worst. These results agree with those obtained in other studies showing that Quercus ilex acorns have less gallotannins than Quercus robur acorns, which is why they are sweeter and are often used for human food and livestock feed and a big difference is observed in the galloderivatives, as for Quercus robur a much higher amount is extracted with P50.57 On the other hand, the extract richest in target polyphenols for Quercus ilex acorns is E50 followed by L50, while for Quercus robur it is P50 followed by A50. Regardless of the lack of complete quantification, all extracts have a high concentration of target polyphenols ranging from 177–716 mg kg−1.
The high antioxidant activity of gallic acid, ellagic acid and gallotannins has been reported by other authors and it has been demonstrated that dietary supplementation with polyphenols improve growth performance and meat quality of broilers.58–61 These extracts are rich in different polyphenols which have diverse positive effects. Gallic acid increases n-3 long-chain polyunsaturated fatty acids; ellagic acid improves digestive enzyme activity, immune function, and intestinal functions; hydrolysable tannins increase antioxidant activity in ileal content and breast muscle; catechin and procyanidins B1 and B2 reduce Escherichia coli and lactic-acid bacteria ileal counts; and quercetin has potential as a complementary antimicrobial therapy for animal feed.62–66 The presence of these polyphenols at high concentrations gives the acorn extracts potential to be used as a new source of antioxidants and as a complementary animal feed.
Quercus robur has more polyphenols than Quercus ilex and its antioxidant activity is consequently better, but the difference between the results of the sum of the individual target polyphenols and the behaviour of the overall indices is evident. These targets do not illustrate all the polyphenols that either Quercus ilex or Quercus robur have, and that the difference between the sum and the TPC may be due to the presence of large hydrolysable tannins such as additional gallotannins or ellagitannins. These results show the need for further non-targeted in-depth analysis for the identification of other major polyphenols and their presence in the various extracts depending on the solvent used, to understand which are responsible for the observed differences in bioactivity.
In general, the best solvents for obtaining these extracts are L50 and A50. Although all solvents studied produce polyphenol-rich extracts with outstanding bioactivities. This opens up a wide range of application possibilities, selecting the right solvent according to the functional product to be developed. Thus, for example, ethyl lactate or propylene glycol are compatible with animal feed, so these extracts could be used directly as supplementary feed added to the drinking water; while extracts obtained with ethanol or acetone allow their easy elimination by volatilisation, facilitating their subsequent conversion into aqueous extracts with a wider range of applications, and solid extracts that can be used as functional ingredients in pelletised feed.
Conclusions
Bioactive extracts with high polyphenolic concentration and antioxidant activity were obtained from Quercus ilex and Quercus robur acorns using an extractive method with minimum solvent consumption and energy requirements, MSPD (matrix solid phase dispersion) system, prioritising solvents generally recognised as safe (GRAS) for subsequent characterization. Quercus robur acorn extracts showed higher bioactivity in each of the parameters evaluated than Quercus ilex. The best values for TPC, AA, IC50 and IC90 were obtained in both species using ethyl lactate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water and acetone
water and acetone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (50
water (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v) as extraction solvents. MSPD has proven to be a good alternative for obtaining bioactive extracts from acorns particularly rich in phenolic acids, flavonoids and tannins, which is in line with the reports in other Quercus species.
50, v/v) as extraction solvents. MSPD has proven to be a good alternative for obtaining bioactive extracts from acorns particularly rich in phenolic acids, flavonoids and tannins, which is in line with the reports in other Quercus species.
Data availability
All the data are available within the present manuscript and ESI.†Author contributions
Conceptualization, C. G.-J. and M. L.; methodology, L. R., A. C. and M. C.; formal analysis, C. G.-J. and M. L.; investigation, D. G.-I. and F. M.-V.; writing–original draft preparation, D. G.-I.; writing–review and editing, C. G.-J. and M. L.; project administration, M. L.; funding acquisition, M. L. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors thank Francisco Javier Silva Pando (Lourizán Forest Research Centre, Xunta de Galicia, Galicia, Spain) for performing the taxonomic identification. This research was supported by project 01_IN606D_2022_2665353 (Axencia Galega de Innovación). This research was supported by project PTQ2022-012516 from MCIN/AEI .Notes and references
- Y. Li, S. S. Zheng, T. R. Wang, M. H. Liu, G. Kozlowski, L. T. Yi and Y. G. Song, Evol. Ecol, 2024, 14, e70318 CrossRef PubMed.
- D. Şöhretoğlu and G. Renda, Phytochem. Rev., 2020, 19, 1379–1426 CrossRef.
- A. F. Hill, Economic Botany, McGraw-Hill Book Co. Inc, New York and London, 1937, pp. 374–375 Search PubMed.
- L. Toumi and R. Lumaret, Biochem. Syst. Ecol., 2001, 29, 799–817 CrossRef CAS PubMed.
- M. A. Balboa-Murias, A. Rojo, J. G. Álvarez and A. Merino, Ann. For. Sci., 2006, 63, 557–565 CrossRef CAS.
- S. Silva, E. M. Costa, A. Borges, A. P. Carvalho, M. J. Monteiro and M. M. E. Pintado, J. Food Meas. Charact., 2016, 10, 584–588 CrossRef.
- I. M. G. Lopes and M. G. Bernardo-Gil, Eur. J. Lipid Sci. Technol., 2005, 107, 12–19 CrossRef CAS.
- S. Rakić, S. Petrović, J. Kukić, M. Jadranin, V. Tešević, D. Povrenović and S. Šiler-Marinković, Food Chem., 2007, 104, 830–834 CrossRef.
- T. Akcan, R. Gökçe, M. Asensio, M. Estévez and D. Morcuende, J. Food Sci. Technol., 2017, 54, 3050–3057 CrossRef CAS PubMed.
- S. Rakić, D. Povrenović, V. Tešević, M. Simić and R. Maletić, J. Food Eng., 2006, 74, 416–423 CrossRef.
- H. K. Biesalski, L. O. Dragsted, I. Elmadfa, R. Grossklaus, M. Müller, D. Schrenk, P. Walter and P. Weber, Nutrition, 2009, 25, 1202–1205 CrossRef PubMed.
- C. G. Fraga, K. D. Croft, D. O. Kennedy and F. A. Tomás-Barberán, Food Funct., 2019, 10, 514–528 RSC.
- C. Manach, D. Milenkovic, T. Van de Wiele, A. Rodriguez-Mateos, B. de Roos, M. T. Garcia-Conesa, R. Landberg, E. R. Gibney, M. Heinonen, F. Tomás-Barberán and C. Morand, Mol. Nutr. Food Res., 2017, 61, 1600557 CrossRef PubMed.
- R. B. Martins, I. Gouvinhas, M. C. Nunes, J. A. Peres, A. Raymundo and A. I. R. N. A. Barros, Molecules, 2020, 25, 3568 CrossRef CAS PubMed.
- C. Garcia-Jares, A. Vazquez, J. P. Lamas, M. Pajaro, M. Alvarez-Casas and M. Lores, Antioxidants, 2015, 4, 737–749 CrossRef CAS PubMed.
- Y. Yao, W. Sang, M. Zhou and G. Ren, J. Agric. Food Chem., 2009, 58, 770–774 CrossRef PubMed.
- H. Wang, Y. J. Du and H. C. Song, Food Chem., 2010, 123, 6–13 CrossRef CAS.
- I. D. Bhatt, S. Rawat and R. S. Rawal, in Biotechnology for Medicinal Plants: Micropropagation and Improvement, ed. S. Chandra, H. Lata and A. Varma, Springer, Heidelberg, Berlin, 1st edition, 2013, vol. 13, pp. 295–326 Search PubMed.
- M. E. Alañón, L. Castro-Vázquez, M. C. Díaz-Maroto, M. H. Gordon and M. S. Pérez-Coello, Food Chem., 2011, 128, 997–1002 CrossRef.
- H. Meziti, H. Bouriche, S. Kada, I. Demirtas, M. Kizil and A. Senator, J. Pharm. Pharmacogn. Res., 2019, 7, 260–272 CrossRef CAS PubMed.
- A. F. Vinha, J. C. M. Barreira, A. S. G. Costa and M. B. P. P. Oliveira, Compr. Rev. Food Sci. Food Saf., 2016, 15, 947–981 CrossRef PubMed.
- S. T. B. Kazmi, M. Majid, S. Maryam, A. Rahat, M. Ahmed, M. R. Khan and I. ul Haq, Biomed. Pharmacother., 2018, 102, 728–738 CrossRef CAS PubMed.
- S. A. Barker, A. R. Long and C. R. Short, J. Chromatogr. A, 1989, 475, 353–361 CrossRef CAS PubMed.
- A. Castillo, M. Celeiro, M. Lores, K. Grgić, M. Banožić, I. Jerković and S. Jokić, Mar. Drugs, 2023, 21, 97 CrossRef CAS PubMed.
- A. Lončarić, M. Celeiro, A. Jozinović, J. Jelinić, T. Kovač, S. Jokić, J. Babić, T. Moslavac, S. Zavadlav and M. Lores, Foods, 2020, 9, 1521 CrossRef PubMed.
- E. Gómez-Mejía, D. Vicente-Zurdo, N. Rosales-Conrado, M. E. León-González and Y. Madrid, LWT–Food Sci. Technol., 2022, 169, 113988 CrossRef.
- E. Gato, A. Perez, A. Rosalowska, M. Celeiro, G. Bou and M. Lores, Plants, 2021, 10, 2801 CrossRef CAS PubMed.
- R. Teterovska, I. Sile, A. Paulausks, L. Kovalcuka, R. Koka, B. Mauriņa and D. Bandere, Plants, 2023, 12, 4108 CrossRef CAS PubMed.
- M. Lores, M. Pájaro, M. Álvarez-Casas, J. Domínguez and C. García-Jares, Talanta, 2015, 140, 134–142 CrossRef CAS PubMed.
- L. B. Garcia, G. A. Pires, D. A. J. Oliveira, L. A. O. Silva, A. F. Gomes, J. G. Amaral, G. R. Pereira and A. L. M. Ruela, Ind. Crops Prod., 2021, 161, 113181 CrossRef CAS.
- A. Castillo, M. Celeiro, L. Rubio, A. Bañobre, M. Otero-Otero, C. Garcia-Jares and M. Lores, Front. Nutr., 2022, 9, 1008457 CrossRef PubMed.
- I. M. Aroso, A. R. Araújo, J. P. Fernandes, T. Santos, M. T. Batista, R. A. Pires, J. F. Mano and R. L. Reis, Wood Sci. Technol., 2017, 51, 855–872 CrossRef CAS.
- L. G. Calvo, A. Castillo, R. A. Villarino, J. L. R. Rama, A. G. Abril and T. de Miguel, Antibiotics, 2023, 12, 1645 CrossRef CAS PubMed.
- Q. Zhang, J. Zhang, J. Shen, A. Silva, D. A. Dennis and C. J. Barrow, J. Appl. Phycol., 2006, 18, 445–450 CrossRef CAS.
- A. Symes, A. Shavandi, H. Zhang, I. A. M. Ahmed, F. Y. Al-Juhaimi and A. E. D. A. Bekhit, Antioxidants, 2018, 7, 52 CrossRef PubMed.
- F. Xiao, T. Xu, B. Lu and R. Liu, Food Front., 2020, 1, 60–69 CrossRef.
- M. Celeiro, J. P. Lamas, R. Arcas and M. Lores, Antioxidants, 2019, 8, 263 CrossRef CAS PubMed.
- W. Mikucka, M. Zielińska, K. Bułkowska and I. Witońska, J. Environ. Manage., 2022, 322, 116150 CrossRef CAS PubMed.
- S. Wahabi, K. Rtibi, C. Abidi, H. Tounsi, A. Ouerghui and H. Sebai, J. Med. Food, 2022, 25, 303–312 CrossRef CAS PubMed.
- E. Cantos, J. C. Espín, C. López-Bote, L. D. De la Hoz, J. A. Ordóñez and F. A. Tomás-Barberán, J. Agric. Food Chem., 2003, 51, 6248–6255 CrossRef CAS PubMed.
- F. Z. Makhlouf, G. Squeo, M. Barkat, A. Trani and F. Caponio, Food Res. Int., 2018, 114, 208–213 CrossRef CAS PubMed.
- M. R. Moreno-Jimenez, F. Trujillo-Esquivel, M. A. Gallegos-Corona, R. Reynoso-Camacho, R. F. González-Laredo, J. A. Gallegos-Infante, N. E. Rocha-Guzmán and M. Ramos-Gomez, Food Chem. Toxicol., 2015, 80, 144–153 CrossRef CAS PubMed.
- M. Celeiro, J. P. Lamas, R. Arcas and M. Lores, Energy Environ., 2020, 32, 981–1001 CrossRef.
- A. F. Likhanov, O. V. Sereda, V. M. Gryb, V. I. Melnyk, L. S. Osadchuk and T. Yuskevych, Biosyst. Divers, 2019, 27, 314–321 CrossRef.
- C. A. Rice-evans, N. J. Miller, P. G. Bolwell, P. M. Bramley and J. B. Pridham, Free Radical Res., 1995, 22, 375–383 CrossRef CAS PubMed.
- W. Zheng and S. Y. Wang, J. Agric. Food Chem., 2001, 49, 5165–5170 CrossRef CAS PubMed.
- Y. Cai, Q. Luo, M. Sun and H. Corke, Life Sci., 2004, 74, 2157–2184 CrossRef CAS PubMed.
- S. H. Zhou, Z. X. Fang, Y. Lü, J. C. Chen, D. H. Liu and X. Q. Ye, Food Chem., 2009, 112, 394–399 CrossRef CAS.
- W. Bors, W. Heller, C. Michel and M. Saran, Adv. Exp. Med. Biol., 1990, 264, 165–170 CrossRef CAS PubMed.
- S. Santoyo, M. Plaza, L. Jaime, E. Ibañez, G. Reglero and F. J. Señorans, J. Agric. Food Chem., 2010, 58, 8522–8527 CrossRef CAS PubMed.
- A. Castillo, S. Pereira, A. Otero, C. Garcia-Jares and M. Lores, J. Appl. Phycol., 2022, 34, 1537–1553 CrossRef CAS.
- Z. A. El-Agbar, R. R. Naik and A. K. Shakya, Orient. J. Chem., 2018, 34, 1368–1374 CrossRef CAS.
- E. Cadahía, L. Muñoz, B. F. De Simón and M. C. García-Vallejo, J. Agric. Food Chem., 2001, 49, 1790–1798 CrossRef PubMed.
- R. Brossa, I. Casals, M. Pintó-Marijuan and I. Fleck, Trees - Struct. Funct., 2009, 23, 401–408 CrossRef CAS.
- A. Karioti, A. R. Bilia and H. Skaltsa, Food Chem., 2010, 123, 131–142 CrossRef CAS.
- L. Molina-García, R. Martínez-Expósito, M. L. Fernández-De Córdova and E. J. Llorent-Martínez, J. Chem., 2018, 2018, 1–10 CrossRef.
- Ł. Łuczaj, A. Adamczak and M. Duda, Dendrobiology, 2014, 72, 103–111 CrossRef.
- S. Canas, V. Casanova and A. Pedro Belchior, J. Food Compos. Anal., 2008, 21, 626–633 CrossRef CAS.
- E. Bakkalbaşi, Ö. Menteş and N. Artik, Crit. Rev. Food Sci. Nutr., 2008, 49, 283–298 CrossRef PubMed.
- X. Chen, D. Zeng, X. Zeng and Q. Zeng, Animals, 2024, 14, 360 CrossRef PubMed.
- S. H. Abu Hafsa and S. A. Ibrahim, J. Anim. Physiol. Anim. Nutr., 2018, 102, 268–275 CrossRef CAS PubMed.
- K. Starčević, L. Krstulović, D. Brozić, M. Maurić, Z. Stojević, Ž. Mikulec, M. Bajić and T. Mašek, J. Sci. Food Agric., 2015, 95, 1172–1178 CrossRef PubMed.
- F. Wang, J. Chen, Y. Yin, M. Yang, Y. Xiao, Y. Cheng, L. Yin and C. Fu, J. Anim. Sci., 2022, 100, skac301 CrossRef PubMed.
- A. Brenes, A. Viveros, I. Goñi, C. Centeno, S. G. Sáyago-Ayerdy, I. Arija and F. Saura-Calixto, Poult. Sci., 2008, 87, 307–316 CrossRef CAS PubMed.
- S. Chamorro, C. Romero, A. Brenes, F. Sánchez-Patán, B. Bartolomé, A. Viveros and I. Arija, Food Funct., 2019, 10, 1444–1454 RSC.
- S. Wang, J. Yao, B. Zhou, J. Yang, M. T. Chaudry, M. Wang, F. Xiao, Y. Li and W. Yin, J. Food Prot., 2018, 81, 68–78 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08675a |
| This journal is © The Royal Society of Chemistry 2025 |