Open Access Article
Open Access ArticleLight-promoted borylation and lactonization of propargyl acetates using NHC-borane†
Hangcheng Ni *a,
Yu Lib,
Chaoming Lic and
Zhenxiang Liu*a
*a,
Yu Lib,
Chaoming Lic and
Zhenxiang Liu*a
aCollege of Pharmacy, Jinhua University of Vocational Technology, Jinhua, 321007, People's Republic of China. E-mail: nihc@hotmail.com; liuzhenxiang86@163.com
bJinhua Institute of Zhejiang University, Jinhua, 321000, People's Republic of China
cZhejiang CDMO Pharmaceutical Co., Ltd, Jinhua, 321000, People's Republic of China
First published on 11th February 2025
Abstract
In the absence of photoredox catalyst (PC), we have successfully achieved radical borylation and lactonization of propargyl acetates under light irradiation using N-heterocyclic carbene borane (NHC-borane), leading to the synthesis of various substituted 4-(NHC-boryl)-2(5H)-furanones. The reaction is triggered from the generation of an NHC-boryl radical, which is formed via hydrogen atom transfer (HAT) of NHC-borane with tert-butyl peroxide derived from the light-induced homolytic cleavage of di-tert-butyl peroxide (DTPB). During the lactonization process, carbon boron formation, carbon–carbon bond formation and cleavage are observed. This approach offers a facile and mild method for the construction of borylated furanones, opening a new possibility in radical borylation chemistry.
Introduction
Boranes and their derivatives serve as essential building blocks in synthetic chemistry, with a variety of methodologies developed for constructing boron-containing structures.1 Among these, cross-coupling reactions involving boryl radicals stand out as particularly effective for preparing such structures.2 Over the past decade, radical initiators such as azobis-isobutyronitrile have been employed to generate boryl radicals from NHC-borane under relatively harsh conditions.3 Curran's group and others has made significant contributions to this field, developing various methods through this process.3 Recently, with the advent of photocatalysis, this approach has emerged as a viable tool for the formation of boryl radicals, as reported by the groups of Yang, Wu, Wang, Leonori, Zhu and others.4 The generation of these radicals can occur through two distinct pathways: (a) direct oxidation of NHC-borane with a photocatalyst (PC); (b) HAT of NHC-borane facilitated by a photocatalyst (Fig. 1a). | ||
| Fig. 1 Radical borylation: (a) methods for boryl radical generation; (b) light-promoted borylation of propargyl acetates. | ||
Radical cyclization is a versatile strategy for constructing complex cyclic structures through careful substrate design.5 Among the various types of radical cyclization, those involving carbon–oxygen double bonds have been less extensively studied, likely due to the intricate reaction mechanisms that involve β-scission process of alkoxy radical.3c,6 Despite the challenges, these processes can lead to the formation of more intriguing products.
Lactones are prevalent in both natural and synthetic pharmaceutical compounds, such as 3-butylphthalide and (+)-bicuculline.7 Typically, photo-promoted radical lactonization utilizes carboxylic acids as precursors.8 The process involves the generation of a carboxylic acid radical through a photoredox process or ligand to metal charge transfer (LMCT) process, followed by the addition carbon-centered radical to carbon–oxygen bond to construct the lactone ring.
Herein we report the development of a light-promoted borylation of propargyl acetates using NHC-borane (Fig. 1b). The NHC ligand-stabilized boryl radical initiates a 5-exo cyclization with simple acetate esters, leading to the formation of stable borylated lactones, 4-(NHC-boryl)-2(5H)-furanones. This protocol involves boron–hydrogen bond activation, boron–carbon bond formation and carbon–carbon bond formation/cleavage during the lactonization process. These features collectively offer a facile approach for the synthesis of borylated furanones under mild conditions.
Results and discussion
As detailed in Table 1 (for more information, refer to the ESI†), the reaction of propargyl acetate (1a) with NHC-borane (2a, 2.0 equivalents) under 20 W LEDs (390–400 nm) and 2.0 equivalents of di-tert-butyl peroxide (DTBP) yielded the desired product (3a) with a 57% yield (Table 1, entry 1). The reaction did not proceed in the absence of light at 50 °C (entry 2). Upon screening various solvents, fluorobenzene (PhF) was found to be superior, resulting in a 65% yield of product 3a (entries 3–9). Other oxidants did not improve the yield (entries 10–14). The reaction was sensitive to air, yielding only 14% of the product under air (entry 15). Increasing the amount of NHC-borane to 5.0 equivalents was found to be optimal for this borylation reaction (entries 16–18), and the process was scalable to a larger scale (0.3 mmol of 1a), achieving an acceptable 78% yield (entry 19).| Entry | NHC-BH3 (equiv.) | Oxidant (equiv.) | Solvent (mL) | Yield |
|---|---|---|---|---|
| a All reactions were conducted at 0.1 mmol scale of 1a in a closed flask under nitrogen atmosphere with 20 W blue LED (390–400 nm) at ambient temperature.b Yield determined by HPLC using acetophenone as internal standard.c Reaction under dark at 50 °C.d Reaction conducted under air.e Reaction was conducted at 0.3 mmol scale of 1a in 3.0 mL of solvent. DTBP = tert-butylperoxide; TBHP = tert-butyl hydroperoxide. | ||||
| 1 | NHC-BH3 (2.0) | DTBP (2.0) | CH3CN (1.0) | 57% |
| 2c | NHC-BH3 (2.0) | DTBP (2.0) | CH3CN (1.0) | <5% |
| 3 | NHC-BH3 (2.0) | DTBP (2.0) | MeOH (1.0) | 60% |
| 4 | NHC-BH3 (2.0) | DTBP (2.0) | Acetone (1.0) | 57% |
| 5 | NHC-BH3 (2.0) | DTBP (2.0) | CH2Cl2 (1.0) | <5% |
| 6 | NHC-BH3 (2.0) | DTBP (2.0) | THF (1.0) | 28% |
| 7 | NHC-BH3 (2.0) | DTBP (2.0) | DMF (1.0) | 51% |
| 8 | NHC-BH3 (2.0) | DTBP (2.0) | DMSO (1.0) | 54% |
| 9 | NHC-BH3 (2.0) | DTBP (2.0) | PhF (1.0) | 65% |
| 10 | NHC-BH3 (2.0) | TBHP(2.0) | PhF (1.0) | <5% |
| 11 | NHC-BH3 (2.0) | (NH4)2S2O8 (2.0) | PhF (1.0) | <5% |
| 12 | NHC-BH3 (2.0) | K2S2O8 (2.0) | PhF (1.0) | <5% |
| 13 | NHC-BH3 (2.0) | di-tert-butyl disulfide (2.0) | PhF (1.0) | 23% |
| 14 | NHC-BH3 (2.0) | Dicumyl peroxide (2.0) | PhF (1.0) | 55% |
| 15d | NHC-BH3 (2.0) | DTBP (2.0) | PhF (1.0) | 14% |
| 16 | NHC-BH3 (3.0) | DTBP (2.0) | PhF (1.0) | 67% |
| 17 | NHC-BH3 (5.0) | DTBP (2.0) | PhF (1.0) | 74% |
| 18 | NHC-BH3 (7.0) | DTBP (2.0) | PhF (1.0) | 75% |
| 19e | NHC-BH3 (5.0) | DTBP (2.0) | PhF (3.0) | 78% |
Under the optimal reaction condition, we investigated the substrate scope of the borylation reaction with propargyl acetate (1), and the results were compiled in Table 2. A variety of aromatic alkynes, including those with functional groups such as alkyl, methoxy, halides, trifluoromethyl, and cyanide, were found to be compatible with our protocol, yielding the corresponding products in low to good yields (3a–3k). Aromatic rings with halides, which could be utilized as versatile coupling partners were obtained in 37–77% yield (3f–3i). Generally, aromatic alkynes with electron-donating groups provided higher yields than those with electron-withdrawing groups (3a–3i vs. 3j–3k). Propargyl acetates with pyridinyl or naphthalenyl groups yielded the corresponding lactones 3l and 3m in low yields. Additionally, we focused on phenyl propargyl acetates tethered with other geminal dialkyl groups. Typically, more sterically hindered dialkyl groups resulted in lower yields of lactones (3n–3q). Cyclization products were not obtained with propargyl acetates lacking dialkyl tethers or a tert-butyl group (3r–3t).
To gain some insight into the reaction mechanism, mechanistic studies were carried out. Addition of 1 equiv. of TEMPO or 1,1-diphenylethylene into the standard reaction almost inhibited the borylation reaction with the TEMPO or 1,1-diphenylethylene trapped adduct detected by HRMS, which indicated that a radical process related to acyl radical was likely to be involved in this reaction (Fig. 2a). Also, important vinyl radical intermediate was trapped by 1,1-diphenylethylene. Furthermore, the UV-visible spectra of DTPB fell in the 390–400 nm region, and no obvious interaction was observed between substrates and DTPB, demonstrating that DTPB should be the photo-active specie in the reaction (Fig. 2b). A light on/off experiment for the reaction of 1a with 2a under the standard reaction condition presented that the borylation reaction was almost ceased in the dark, which suggested that a chain propagation was not the main reaction pathway, and continuous irradiation of visible light was necessary for the reaction.
To elucidate the reaction mechanism, we conducted a series of mechanistic studies. The introduction of 1 equivalent of TEMPO or 1,1-diphenylethylene into the standard reaction nearly halted the borylation process, with the trapped adducts being detected by high-resolution mass spectrometry (HRMS), indicating that a radical process was likely involved in the reaction (Fig. 1a). Additionally, a crucial vinyl radical intermediate was successfully trapped by 1,1-diphenylethylene. The UV-vis spectrum of DTPB was found to fall within the 390–400 nm range, and no significant interaction was observed between the substrates and DTPB, confirming that DTPB is the photoactive species in the reaction (Fig. 2b). An on/off light experiment (Fig. 2c) with 1a and 2a under standard conditions revealed that the borylation reaction nearly ceased in the absence of light, suggesting that chain propagation is not the primary reaction pathway, and continuous irradiation with visible light is essential for the reaction to proceed.
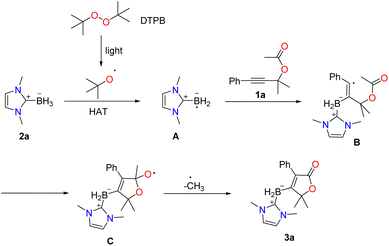
Based on the experimental results and existing literature,2–4,9,10 we propose a plausible mechanism (Scheme 1). Initially, DTPB undergoes homolytic cleavage under LED irradiation to generate an O-centered tert-butyl oxide radical. This tert-butyloxyl radical then abstracts a hydrogen atom from NHC-borane (2a), forming a boryl radical (A). The boryl radical (A) reacts with propargyl acetate (1a) to generate a vinyl radical (B). Radical (B) subsequently undergoes addition to the carbonyl oxygen double bond and a β-scission process of O- centered radical (C), leading to the formation of the final product (3a).
Conclusions
In summary, we have successfully accomplished a light-driven radical borylation/lactonization reaction using N-heterocyclic carbene borane stabilized borane and propargyl acetates, all without the need for a photocatalyst. This innovative approach has enabled the synthesis of a diverse array of substituted borylated lactones. Our mechanistic investigations have shed light on the role of peroxide, which is believed to participate in the HAT process for the generation of boryl radical. The reaction mechanism involves the activation of the boron–hydrogen bond, the formation of carbon–boron bonds, and the creation or cleavage of carbon–carbon bonds during the lactonization phase. This work not only simplifies the synthesis of borylated furanones under mild conditions but also opens new avenues for the synthesis of complex molecules with potential applications in various fields.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
H. Ni conceptualized the project. Y. Li and C. Li performed the experiments. H. Ni wrote the manuscript. Z. Liu provided funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge the financial support from Start-up Funding (Grant 1004) and development fund (Grant 1007) from Jinhua Branch of Sichuan Industrial Institute of Antibiotics and financial support from Jinhua Science and Technology Bureau (2020-1-003a).References
- (a) A. M. Barcellos, M. Sacramento, G. P. da Costa, G. Perin, E. J. Lenardão and D. Alves, Organoboron compounds as versatile reagents in the transition metal-catalyzed C–S, C–Se and C–Te bond formation, Coord. Chem. Rev., 2021, 442, 214012 Search PubMed; (b) A. B. Cuenca, R. Shishido, H. Ito and E. Fernández, Transition-metal-free B–B and B–interelement reactions with organic molecules, Chem. Soc. Rev., 2017, 46, 415–430 RSC; (c) M. Wang and Z. Shi, Methodologies and strategies for selective borylation of C–Het and C–C bonds, Chem. Rev., 2020, 120, 7348–7398 CrossRef CAS PubMed; (d) R. Bisht, C. Haldar, M. M. M. Hassan, M. E. Hoque, J. Chaturvedi and B. Chattopadhyay, Metal-catalysed C–H bond activation and borylation, Chem. Soc. Rev., 2022, 51, 5042–5100 RSC.
- (a) T. Taniguchi, Boryl radical addition to multiple bonds in organic synthesis, Eur. J. Org Chem., 2019, 6308–6319 CrossRef CAS; (b) T.-Y. Peng, F.-L. Zhang and Y.-F. Wang, Lewis base-boryl radicals enabled borylation reactions and selective activation of carbon–heteroatom bonds, Acc. Chem. Res., 2023, 56, 169–186 CrossRef CAS PubMed; (c) X. Chen, X. Zhou, J. He and X. Liu, Recent advances in photoinduced borylation via N-heterocyclic carbene boryl radicals, Synthesis, 2024, 56 DOI:10.1055/a-2405-2961.
- Selected examples: (a) T. Watanabe, D. Hirose, D. P. Curran and T. Taniguchi, Borylative radical cyclizations of benzo[3,4]cyclodec-3-ene-1,5-diynes and N-heterocyclic carbene-boranes, Chem.–Eur. J., 2017, 23, 5405–5409 CrossRef PubMed; (b) S.-C. Ren, F.-L. Zhang, J. Qi, Y.-S. Huang, A.-Q. Xu, H.-Y. Yan and Y.-F. Wang, Radical borylation/cyclization cascade of 1,6-enynes for the synthesis of boron-handled hetero- and carbocycles, J. Am. Chem. Soc., 2017, 139, 6050–6053 Search PubMed; (c) M. Shimoi, K. Maeda, S. J. Geib, D. P. Curran and T. Taniguchi, Esters as Radical Acceptors: β-NHC-Borylalkenyl Radicals Induce Lactonization by C–C Bond Formation/Cleavage on Esters, Angew. Chem., Int. Ed., 2019, 58, 6357–6361 Search PubMed.
- Selected examples: (a) W. Xu, H. Jiang, J. Leng, H.-W. Ong and J. Wu, Visible-light-induced selective defluoroborylation of polyfluoroarenes, gem-difluoroalkenes, and trifluoromethylalkenes, Angew. Chem., Int. Ed., 2020, 59, 4009–4016 CrossRef CAS PubMed; (b) P.-J. Xia, D. Song, Z.-P. Ye, Y.-Z. Hu, J.-A. Xiao, H.-Y. Xiang, X.-Q. Chen and H. Yang, Photoinduced single-electron transfer as an enabling principle in the radical borylation of alkenes with NHC-borane, Angew. Chem., Int. Ed., 2020, 59, 6706–6710 CrossRef CAS PubMed; (c) J. Qi, F.-L. Zhang, J.-K. Jin, Q. Zhao, B. Li, L.-X. Liu and Y.-F. Wang, New radical borylation pathways for organoboron synthesis enabled by photoredox catalysis, Angew. Chem., Int. Ed., 2020, 59, 12876–12884 CrossRef CAS PubMed; (d) N. Zhou, X.-A. Yuan, Y. Zhao, J. Xie and C. Zhu, Synergistic photoredox catalysis and organocatalysis for inverse hydroboration of imines, Angew. Chem., Int. Ed., 2018, 57, 3990–3994 Search PubMed; (e) J. H. Kim, T. Constantin, M. Simonetti, J. Llaveria, N. S. Sheikh and D. Leonori, A radical approach for the selective C–H borylation of azines, Nature, 2021, 595, 677–683 CrossRef CAS PubMed; (f) F. X. Li, X. Wang, J. Lin, X. Lou, J. Ouyang, G. Hu and Y. Quan, Selective multifunctionalization of N-heterocyclic carbene boranes via the intermediacy of boron-centered radicals, Chem. Sci., 2023, 14, 6341–6347 Search PubMed.
- (a) K. J. Romero, M. S. Galliher, D. A. Pratt and C. R. J. Stephenson, Radicals in natural product synthesis, Chem. Soc. Rev., 2018, 47, 7851–7866 Search PubMed; (b) M. Latrache and N. Hoffmann, Photochemical radical cyclization reactions with imines, hydrazones, oximes and related compounds, Chem. Soc. Rev., 2021, 50, 7418–7435 RSC.
- (a) X. Hu, G. X. Li, G. He and G. Chen, Minisci C–H alkylation of N-heteroarenes with aliphatic alcohols via β-scission of alkoxy radical intermediates, Org. Chem. Front., 2019, 6, 3205–3209 Search PubMed; (b) J. L. Shi, Z. Wang, Z. He, B. Dou and J. Wang, Visible light-promoted ring-opening alkenylation of cyclic hemiacetals through β-scission of alkoxy radicals, Chin. J. Chem., 2022, 41, 259–264 Search PubMed.
- (a) R. Karmakar, P. Pahari and D. Mal, Phthalides and Phthalans. Synthetic methodologies and their applications in the total synthesis, Chem. Rev., 2014, 114, 6213–6284 CrossRef CAS PubMed; (b) X. Diao, P. Deng, C. Xie, X. Li, D. Zhong, Y. Zhang and X. Chen, Drug Metab. Dispos., 2013, 41, 430–444 CrossRef CAS PubMed; (c) C. Zhao, K. P. Rakesh, S. Mumtaz, B. Moku, A. M. Asiri, H. M. Marwani, H. M. Manukumar and H.-L. Qin, Arylnaphthalene lactone analogues: synthesis and development as excellent biological candidates for future drug discovery, RSC Adv., 2018, 8, 9487–9502 RSC.
- (a) Q. Yang, Z. Jia, L. Li, L. Zhang and S. Luo, Visible-light promoted arene C–H/C–X lactonization via carboxylic radical aromatic substitution, Org. Chem. Front., 2018, 5, 237–241 RSC; (b) K. Wadekar, S. Aswale and V. R. Yatham, Cerium photocatalyzed dehydrogenative lactonization of 2-arylbenzoic acids, Org. Biomol. Chem., 2020, 18, 983–987 RSC.
- C. Wu, X. Hou, Y. Zheng, P. Li and D. Lu, Electrophilicity and nucleophilicity of boryl radicals, J. Org. Chem., 2017, 82, 2898–2905 CrossRef CAS PubMed.
- H. Jia, M. He, S. Yang, X. Yu and M. Bao, Visible-light-driven di-t-butyl peroxide-promoted the oxidative homo- and cross-coupling of phenols, Eur. J. Org Chem., 2022, 2022, e202101469 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08656b |
| This journal is © The Royal Society of Chemistry 2025 |