Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Nitrogen-rich metal–organic framework of nickel(II) as a highly efficient and reusable catalyst for the synthesis of cyclic carbonates at ambient pressure of CO2†
Reza Erfani-Ghorbani ,
Hossein Eshghi
,
Hossein Eshghi * and
Ali Shiri
* and
Ali Shiri
Department of Chemistry, Faculty of Science, Ferdowsi University of Mashhad, Mashhad, Iran. E-mail: heshghi@um.ac.ir
First published on 6th March 2025
Abstract
Nitrogen-rich metal organic frameworks (MOFs) structures have a great potential for the chemical fixation of CO2. In this direction, we have utilized the highly efficient nitrogen-rich dual linker MOF of nickel(II) as a heterogeneous catalyst in solvent-free chemical fixation of CO2 into cyclic carbonates at ambient pressure. In this present work, nitrogen-rich nickel-MOF, Ni-ImzAdn, was synthesized from imidazole and adenine as efficient nitrogen-rich linkers under hydrothermal conditions (Imz = Imidazole and Adn = Adenine). The Ni-ImzAdn was characterized thoroughly via various physicochemical analyses such as FT-IR, SEM, EDX, EDX-mapping, XRD, ICP-OES, BET, BJH, TG-DTA, CO2-TPD, and NH3-TPD. Ni-ImzAdn with adequate free nitrogen sites exhibit high catalytic activity in the cycloaddition of CO2 with styrene oxide (93% yield) at solvent-free and ambient pressure. The high activity of Ni–ImzAdn was attributed to the synergistic effect of strong Lewis acid and strong Lewis base sites on the catalyst, which were acquired by CO2 and NH3-TPD respectively. In addition, the MOF catalyst was presented as highly recyclable without significant loss of activity after six reaction cycles and low metal ion leaching (analyzed by (ICP-OES)). Thermogravimetry-differential thermal analysis (TG-DTA) showed the MOF catalyst had high thermal stability up to 318 °C.
1. Introduction
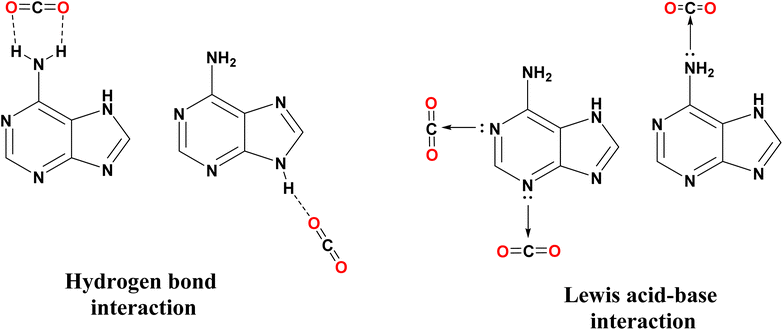
The increasing emission of carbon dioxide (CO2) into the atmosphere is a critical issue contributing to global warming. As a result, extensive research has been dedicated to utilizing CO2 as a feedstock for synthesizing valuable materials.1–5 The synthesis of cyclic carbonates is a 100% atom–economic reaction, a green process, and the most efficient artificial CO2 fixation.6,7 From the product viewpoint, cyclic carbonates have diverse applications, including their use as polar aprotic solvents, electrolytes in secondary batteries, and pharmaceutical intermediates.8–10 Due to the thermodynamic stability of CO2 (standard heat of formation of ΔHf = −394 kJ mol−1 and a standard Gibbs energy of ΔGf = −395 kJ mol−1) percentage conversion of cyclic carbonate is highly reliant on catalysts that can significantly lower the activation barrier for the reaction.11 Various heterogeneous catalysts with multiple active sites have been extensively studied for the cycloaddition of CO2 and epoxides to synthesize cyclic carbonates.12–18 Among them, MOFs are the class of materials that contain many inherently excellent properties, such as high surface area, structural diversity and rich functionalities.19,20 As a result, MOFs have gained significant attention as promising materials for the widespread utilization of CO2 in producing valuable chemicals.21–23 Extensive studies have been done on the modification of the nitrogen-rich metal–organic frameworks with Lewis acidic open metal sites and ligands containing electron-rich nitrogen atoms for CO2 fixation.24–28Bio-organic ligands are alternatives to toxic organic building blocks due to their biocompatibility, strong metal–ligand coordination, and CO2 capture capability.29–31 Adenine is a suitable ligand because of its multiple uncoordinated free nitrogen sites (consisting of one exocyclic amino-N atom and four heterocyclic N atoms) which could facilitate CO2 adsorption by Lewis acid-base and hydrogen bond interaction between CO2 and adenine ligands in chemical fixation of CO2 (Fig. 1).32–35
Imidazole is an N-containing heterocyclic ring that contains multi-binding sites for high CO2 adsorption capacity. This is due to strong hydrogen bonds –NH group and strong intramolecular dispersive π–π stacking bonding between imidazole and CO2 (the π–π stacking conformation is 15.1 kJ mol−1) (Fig. 2).36,37
Because of the aforesaid discussion, a suitable MOF-based catalysts for CO2 fixation under mild conditions should have a high density of CO2-philic sites such as nitrogen functionalities and Lewis acid sites (metal nodes), which combine high CO2 adsorption and epoxide activation. With this purpose, we have utilized Ni–ImzAdn MOF for CO2 cycloaddition with epoxide into cyclic carbonates under solvent-free conditions.
2. Experimental
2.1 Materials and methods
Epoxides (purity >98%), tetrabutylammonium bromide (TBAB, >98%), imidazole, adenine, and Ni(NO3)2·6H2O were procured from Sigma-Aldrich. Carbon dioxide (CO2 > 80%) was used for the synthesis of cyclic carbonates. Organic solvents such as methanol, chloroform, and ethanol were purchased from Merck Chemical Industries. The FT-IR spectrums were measured by the Thermo Nicolet Avatar 370. Leaching of metal was measured by the ICP-OES (inductively coupled plasma-optical emission spectrometry) method using the AMETEK (ARCOS FHE12) model Spectro Arcos-76004555 plasma. NMR spectra were recorded on a Bruker Avance III 300 MHz spectrometer. Field emission scanning electron microscopy (FE SEM; MIRA3 TESCAN) equipped with an energy dispersive X-ray spectroscopy (EDX) was used to analyze the morphology and dimensions of the samples. CO2/NH3 temperature programmed desorption (CO2/NH3-TPD) was performed on a NanoSROD (made by Sensiran Co., Iran) instrument. N2 adsorption–desorption isotherms were measured by using a GasSorb II (made by Toos Nano Co., Iran) instrument. XRD patterns were collected using a high-resolution diffractometer (Explorer, GNR Analytical Instruments, Italy). Thermogravimetry-differential thermal analysis (TG-DTA) was carried out using a Bahr STA-503 instrument. The samples (20.4 mg) were placed in aluminum pans and heated up to 800 °C at a rate of 5 °C min−1 under an air stream.2.2 Synthesis of the nickel-imidazole adenine MOF (Ni–ImzAdn)
The Ni–ImzAdn catalysis was prepared according to the literature with a slight modification.38 To be specific, 3 mmol of [Ni(NO3)2·6H2O] and 12 mmol of the organic ligands (imidazole (50%) + adenine (50%)) were dissolved in ethanol. The homogeneous solution mixture was transferred into a 100 mL Teflon-lined stainless steel autoclave and held at 180 °C for 10 h to achieve nucleation for the growth of the semi-crystalline MOF. The reaction mixture was cooled to room temperature and spring green-colored material was separated by filtration. The solid sample was further washed with methanol and chloroform for purification. The product was dried at 80 °C for 18 h to remove the residual solvent.2.3 General synthesis of cyclic carbonates
The CO2 and epoxide cycloaddition reactions were performed in a round bottom flask fitted with a Teflon stopcock flow control adaptor, a CO2 balloon, and a magnetic stirrer. The experiment was performed using CO2 cycloaddition with styrene oxide (SO) as a model reaction. In a typical experiment, the solvent-free condition was carried out by mixing epoxide (1.50 mmol), catalyst (0.020 g), and co-catalyst TBAB (0.15 mmol, 0.050 g) in a round-bottom flask, which was connected to a CO2 balloon. Then, the reaction was stirred at 100 °C for 8 h. After the completion of the chemical reaction (monitored by thin-layer chromatography (TLC)), the reactor was cooled to room temperature, and the catalyst was removed through centrifugation strategies. The resulting crude product was purified by column chromatography using ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n-hexane (4
n-hexane (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) to get the pure product (0.229 g, 1.39 mmol, 93% yield). The catalyst was washed with methanol (3 × 10 mL), chloroform (3 × 10 mL), and dried at 80 °C overnight for the next run.
10) to get the pure product (0.229 g, 1.39 mmol, 93% yield). The catalyst was washed with methanol (3 × 10 mL), chloroform (3 × 10 mL), and dried at 80 °C overnight for the next run.
3. Results and discussion
3.1 Catalyst characterizations
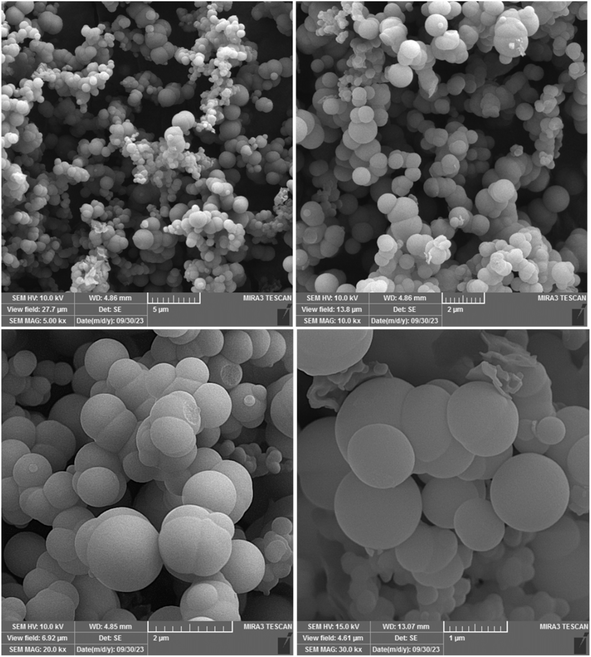
The FT-IR spectroscopy of Ni–ImzAdn MOF was measured, as illustrated in Fig. S1, ESI.† Absorption bands in the region 3201–3465 cm−1 are attributed to the NH and NH2 stretching vibrations of adenine and imidazole. The MOF catalyst shows a strong band for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond group that appears in the range of 1596–1643 cm−1 for adenine. The presence of peaks at 1205, 1335 and 1074 cm−1 can be attributed to the scissoring-wagging-stretching vibration of the imidazole molecule.38 Further, the O–Ni–O bond had a peak at 658 cm−1.38,39 The X-ray diffraction (XRD) shows the formation of the MOF materials and crystalline nature of the Ni–ImzAdn (Fig. S2, ESI†). The Ni–O peaks at 2θ = 10, 25, 16, and 33 are attributed to the formation of the nickel oxide clusters in Ni–ImzAdn MOF.38 The peak detected around 2θ = 13.1 is characteristic of adenine.38 Furthermore, the XRD pattern of the Ni–ImzAdn MOF shows that the peaks at 2θ = 8, 23, 26, and 30 correspond to the MOF formation.38 Moreover, due to factors including the nucleation of MOF materials, specific synthetic methods, solvothermal synthesis, temperature, solvents, reagent concentration of the Ni–ImzAdn MOF, the catalyst resulted into a semi-amorphous MOF.40,41 The synthesized framework in this study exhibits significant broadening in the region above 10 due to its amorphous structure or fine crystalline particles, making single-crystal analysis impossible. However, the main characteristic of the synthesized framework shows two sharp peaks at 2θ = 8 and 23. The surface morphology of the Ni–ImzAdn was performed using scanning electron microscopy (SEM), energy dispersive X-ray (EDX) analysis, and element mapping technique. The SEM revealed a spherical shape and smooth structure of Ni–ImzAdn (Fig. 3). The chemical constituents and the purity of the Ni–ImzAdn were done by energy dispersive X-ray (EDX) analysis and elemental mapping, where peaks for nickel, oxygen, nitrogen and carbon, were observed with ratios of 17.49, 13.55, 40.27, and 28.69, respectively, as shown in Fig. S3(b and c), ESI.† The nickel and other elements were dispersed uniformly on the catalyst surface.
N double bond group that appears in the range of 1596–1643 cm−1 for adenine. The presence of peaks at 1205, 1335 and 1074 cm−1 can be attributed to the scissoring-wagging-stretching vibration of the imidazole molecule.38 Further, the O–Ni–O bond had a peak at 658 cm−1.38,39 The X-ray diffraction (XRD) shows the formation of the MOF materials and crystalline nature of the Ni–ImzAdn (Fig. S2, ESI†). The Ni–O peaks at 2θ = 10, 25, 16, and 33 are attributed to the formation of the nickel oxide clusters in Ni–ImzAdn MOF.38 The peak detected around 2θ = 13.1 is characteristic of adenine.38 Furthermore, the XRD pattern of the Ni–ImzAdn MOF shows that the peaks at 2θ = 8, 23, 26, and 30 correspond to the MOF formation.38 Moreover, due to factors including the nucleation of MOF materials, specific synthetic methods, solvothermal synthesis, temperature, solvents, reagent concentration of the Ni–ImzAdn MOF, the catalyst resulted into a semi-amorphous MOF.40,41 The synthesized framework in this study exhibits significant broadening in the region above 10 due to its amorphous structure or fine crystalline particles, making single-crystal analysis impossible. However, the main characteristic of the synthesized framework shows two sharp peaks at 2θ = 8 and 23. The surface morphology of the Ni–ImzAdn was performed using scanning electron microscopy (SEM), energy dispersive X-ray (EDX) analysis, and element mapping technique. The SEM revealed a spherical shape and smooth structure of Ni–ImzAdn (Fig. 3). The chemical constituents and the purity of the Ni–ImzAdn were done by energy dispersive X-ray (EDX) analysis and elemental mapping, where peaks for nickel, oxygen, nitrogen and carbon, were observed with ratios of 17.49, 13.55, 40.27, and 28.69, respectively, as shown in Fig. S3(b and c), ESI.† The nickel and other elements were dispersed uniformly on the catalyst surface.
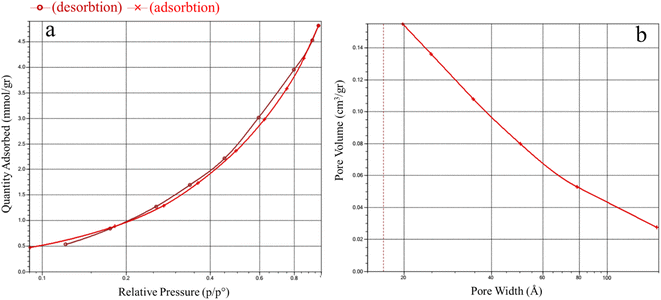
Brunauer–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) are the most important factors affecting the catalytic activity.40,42 The BET analysis results for Ni–ImzAdn exhibited the type II adsorption–desorption isotherm as per the International Union of Pure and Applied Chemistry (IUPAC) classification (Fig. 4(a)).38 In the range of P/Po (0.1–1), Ni–ImzAdn showed a slight increase in the volume of nitrogen adsorption, which indicates the presence of micropores in the structure. Moreover, the specific surface area and pore volume are approximately 161.8486 m2 g−1 and 0.146227 cm3 g−1, respectively. Additionally, the BJH isotherm in Fig. 4(b) clearly shows pores at approximately 1.9 nm that correspond to the micropores MOF. The pore size distribution and the surface functionality of MOF are the key issues for the CO2 adsorption capacities.43,44 Therefore, the microporous Ni–ImzAdn MOF with polar uncoordinated N-rich sites can enhance the adsorption of CO2.
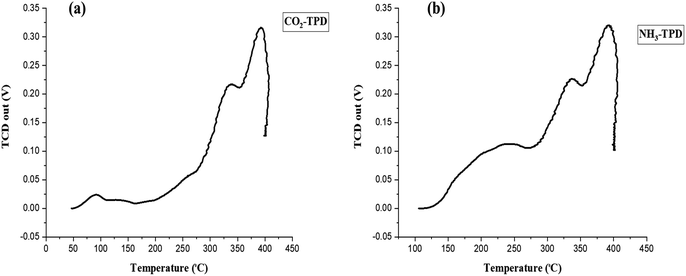
The MOF-catalyzed epoxide-CO2 cycloaddition reaction is closely related to the synergistic effect of Lewis acid–base site distributions.45,46 The acid–base nature of the MOF material was examined using NH3- and CO2-temperature programmed desorption (TPD). The basic strength and basicity of the catalysts were classified into two groups: weak (50–176 °C) and medium to strong (176–500 °C).47 According to Fig. 5(a) and Table 1, the CO2-TPD profile of Ni–ImzAdn showed one weak peak and two strong desorption peaks at 80, 320, and 380 °C, respectively. The weak peak of CO2 adsorbed on Ni–ImzAdn may be related to its π–π stacking bonding between imidazole and CO2. In contrast, strong Lewis basic sites were probably by the binding interaction between nitrogen sites of adenine and CO2. The surface acidity of the Ni–ImzAdn catalyst was evaluated by the NH3-TPD as shown in Fig. 5(b). Surface acidity can be classified into three main groups: weak (49.85–99.85 °C), medium (149.85–199.85 °C), and strong (319.85–499.85 °C).48 The NH3-TPD profile of Ni–ImzAdn showed three strong desorption peaks at 240, 340, and 380 °C, respectively. As illustrated in Table 1, the high-temperature desorption peaks could be ascribed to hydrogen bonding interactions of amino groups in adenine and imidazole with CO2, and NH3 adsorbed on metal nodes as Brønsted acid sites. Table 2 summarizes and compares the acidity and basicity of Ni–ImzAdn with other MOF catalysts that were previously used in the synthesis of cyclic carbonates.49–53 Among them, it was observed that the Ni–ImzAdn catalyst exhibited the highest desorption temperature of NH3 and CO2 during temperature-programmed desorption analysis.
| Entry | Temperature (°C) | NH3 desorption (mmol per g catalyst) | CO2 desorption (mmol per g catalyst) |
|---|---|---|---|
| 1 | 100–270 | 621.0 | — |
| 2 | 270–355 | 855.3 | — |
| 3 | 355–400 | 2481.6 | — |
| 4 | 50–165 | — | 48.4 |
| 5 | 165–355 | — | 567.2 |
| 6 | 355–400 | — | 1256.1 |
| Entry | CO2-TPD (°C) | NH3-TPD (°C) | MOF | Reference |
|---|---|---|---|---|
| a {(Me2NH2)2[Pb5(PTTPA)2(H2O)3]·2DMF·3H2O}n.b (Carboxymethylcellulose (CMC); 1-aminoethyl-3-methylimidazolium bromide (IL)).c Zn(tp)(bpy)0.5·(DMF)(H2O)0.5.d Zinc-1,3,5-benzenetricarboxylate.e [(CH3)2NH2][Co3(BTC)(HCOO)4(H2O)]·H2O. | ||||
| 1 | 142 | 122 | NUC-111aa | 49 |
| 2 | 142 | 148 | IL-Au@UiO-66-NH2/CMCb | 50 |
| 3 | 128 and 325 | 154, 251, and 457 | MOF-508ac | 51 |
| 4 | 145 and 280 | 270 | [Zn3(BTC)2]d | 52 |
| 5 | 320 | 115 | Co-BTCe | 53 |
| 6 | 80, 320, and 380 | 240, 340, and 380 | Ni–ImzAdn | This work |
Therefore, among them, Ni–ImzAdn had the most excellent catalytic performance and high synergistic effect of Lewis acid–base to facilitate the capture and conversion of CO2.
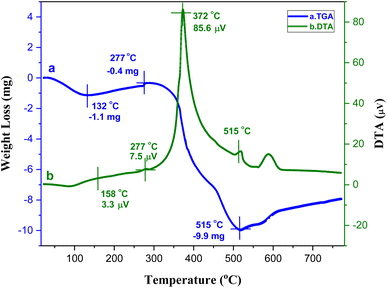
The thermal stability of the catalyst was examined by TG-DTA (Fig. 6). The initial weight loss (5.34%) occurred below 132 °C, which is attributed to the removal of moisture from the catalyst. Subsequently, another weight loss (1.94%) at 277 °C observed due to the evaporation of guest molecules, such as solvent molecules from the cavities of the MOF. Upon further continuation of heating above 318 °C, the sample started to disintegrate until it was completely decomposed at 515 °C due to the breaking of the network, indicating the high thermal stability of the Ni–ImzAdn MOF. In DTA curve, it is observed the significant exothermic peak at 372 °C belong to the decompositions of the linkers in the framework.
3.2 Catalytic activity for CO2 cycloaddition reaction
The heterogeneous nitrogen-rich MOF catalyst was examined for CO2 cycloaddition reaction at ambient pressure. The catalytic performance of the Ni–ImzAdn was investigated under different operating conditions. For the present purpose, the chemical fixation of CO2 with SO was considered as the model reaction in Table 3. As indicated in entry 1–5 in Table 3, no yield was observed in the absence of a catalyst, Ni(NO3)2·6H2O, imidazole, adenine, and in the presence catalyst without co-catalyst (TBAB). According to the data presented in entry 6, the fixation proceeds to around 24% yield using a catalytic amount of TBAB (0.15 mmol) alone; however, it can be increased to 93% yield with the addition of Ni–ImzAdn as catalyst (entry 12, Table 3). This may be because of the synergistic effects between acid–base bifunctional catalyst and TBAB. Furthermore, Ni–ImzAdn with rich accessible nitrogen sites can be incorporated to effectively capture and adsorption capacity of CO2 through chemical fixation. The results of the chemical fixation of CO2 with SO at various catalyst loadings (ranging from 10.0 mg to 60.0 mg) are shown in Table 3; entries 10–14. The low amount of catalyst (10 mg) led to a lower product yield (Table 3, entry 10). The yield to cyclic carbonate was excellent (in the range 93–95%) when the amount of catalyst increased from 10 mg to 60 mg (Table 3, entry 12–14). Nevertheless, due to the lower consumption of the catalyst and the proximity of yield in entries 12–14, moderate loading of catalyst (20 mg) was chosen as the optimal catalyst loading. The effect of the temperature and time in the CO2 cycloaddition reaction was investigated. The optimal cyclic carbonate content was 93% at 8 h and 100 °C (Table 3, entry 12). The results show that the yield decreases with increasing temperature to 120 °C (Table 3, entry 19). Considering that the reaction takes place under ambient pressure, the ability of the catalyst to capture and conversion CO2 gas at 120 °C is lower than at 100 °C. The effect of various solvents such as H2O, ethanol, toluene, n-hexane, and much more was investigated. The highest yield between various solvents was achieved with DMF (73%) at a temperature of 100 °C and ambient pressure of CO2 for 8 h (Table 3, entry 24). The heterogeneous catalytic performance under ambient air (approximately 0.04% CO2) showed low yield due to the low concentration of carbon dioxide (Table 3, entry 30).| Entry | Catalyst | Amount of catalyst (mg) | TBAB (mmol) | Temperature (°C) | Solvent | Time (h) | Yieldb (%) |
|---|---|---|---|---|---|---|---|
| a The CO2 cycloaddition reaction condition: styrene oxide (1.5 mmol), TBAB (0.15 mmol), and ambient pressure of CO2.b Determined by column chromatography.c Heterogeneous catalytic performance for fixation of CO2 from the ambient air (approximately 0.04 percent CO2 in air). | |||||||
| 1 | No catalyst | 0 | 0 | 100 | Solvent-free | 8 | 0 |
| 2 | Ni(NO3)2·6H2O | 8 | 0 | 100 | Solvent-free | 8 | 0 |
| 3 | Imidazole | 4 | 0 | 100 | Solvent-free | 8 | 0 |
| 4 | Adenine | 8 | 0 | 100 | Solvent-free | 8 | 0 |
| 5 | Ni–ImzAdn | 20 | — | 100 | Solvent-free | 8 | 0 |
| 6 | — | — | 0.15 | 100 | Solvent-free | 8 | 24 |
| 7 | Ni(NO3)2·6H2O | 8 | 0.15 | 100 | Solvent-free | 8 | 27 |
| 8 | Imidazole | 4 | 0.15 | 100 | Solvent-free | 8 | 38 |
| 9 | Adenine | 8 | 0.15 | 100 | Solvent-free | 8 | 41 |
| 10 | Ni–ImzAdn | 10 | 0.15 | 100 | Solvent-free | 8 | 80 |
| 11 | Ni–ImzAdn | 15 | 0.15 | 100 | Solvent-free | 8 | 86 |
| 12 | Ni–ImzAdn | 20 | 0.15 | 100 | Solvent-free | 8 | 93 |
| 13 | Ni–ImzAdn | 40 | 0.15 | 100 | Solvent-free | 8 | 95 |
| 14 | Ni–ImzAdn | 60 | 0.15 | 100 | Solvent-free | 8 | 95 |
| 15 | Ni–ImzAdn | 20 | 0.15 | r.t | Solvent-free | 8 | 7 |
| 16 | Ni–ImzAdn | 20 | 0.15 | 40 | Solvent-free | 8 | 11 |
| 17 | Ni–ImzAdn | 20 | 0.15 | 60 | Solvent-free | 8 | 33 |
| 18 | Ni–ImzAdn | 20 | 0.15 | 80 | Solvent-free | 8 | 54 |
| 19 | Ni–ImzAdn | 20 | 0.15 | 120 | Solvent-free | 8 | 86 |
| 20 | Ni–ImzAdn | 20 | 0.15 | Reflux | H2O | 8 | 10 |
| 21 | Ni–ImzAdn | 20 | 0.15 | Reflux | Ethanol | 8 | 30 |
| 22 | Ni–ImzAdn | 20 | 0.15 | Reflux | Toluene | 8 | 55 |
| 23 | Ni–ImzAdn | 20 | 0.15 | Reflux | n-Hexane | 8 | 40 |
| 24 | Ni–ImzAdn | 20 | 0.15 | 100 | DMF | 8 | 73 |
| 25 | Ni–ImzAdn | 20 | 0.15 | 100 | DMSO | 8 | 61 |
| 26 | Ni–ImzAdn | 20 | 0.15 | 100 | Solvent-free | 2 | 31 |
| 27 | Ni–ImzAdn | 20 | 0.15 | 100 | Solvent-free | 4 | 83 |
| 28 | Ni–ImzAdn | 20 | 0.15 | 100 | Solvent-free | 6 | 87 |
| 29 | Ni–ImzAdn | 20 | 0.15 | 100 | Solvent-free | 12 | 93 |
| 30 | Ni–ImzAdn | 20 | 0.15 | 100 | Solvent-free | 8 | 11c |
3.3 Catalytic activity towards different epoxides
Under the optimal reaction conditions, the coupling reaction of CO2 with various epoxides were investigated in the presence of Ni–ImzAdn (Table 4). It is worth mentioning that, all epoxides containing electron-donating or electron-withdrawing groups can be efficiently converted into the corresponding cyclic carbonates with good to high yield. A yield of 79% was achieved for 1,2-epoxycyclohexane, which can probably be attributed to the higher steric hindrance of the cyclohexene ring (Table 4, entry 5). 1H and 13C NMR spectra have been given in ESI.†3.4 Reusability study of the MOF catalyst
The recyclability of the catalyst is a significant aspect of the research on catalytic materials. As shown in Fig. S4 (ESI†), the initial performance of the catalyst can be remained after 6 runs of cycloaddition reaction, while the yield of product decreased only slightly. FT-IR and XRD analyses were used to investigate structural changes and MOF crystallinity. Furthermore, the ICP-OES technique was used to determine nickel leaching from the MOF catalyst after six catalytic cycles. The ICP-OES technique was successfully applied to detect the metal leaching in the fresh and 6th reused Ni–ImzAdn catalyst. According to the report in Table 5, nickel decreased from 0.06731 mmol (fresh sample) to 0.06692 mmol (after the 6th run). The catalyst was reused for six reaction runs, and no significant loss of activity was observed in the cycloaddition of CO2 with styrene oxide. However, no significant loss of catalytic activity and low metal leaching (3.9 × 10−4 mmol) at the end of six reaction runs, indicating that the metal nodes were stabilized by the free nitrogen sites of the Ni–ImzAdn.| Entry | Sample | Mass (g) | Volume (mL) | Dilution factor | Element | Instrument data (mg L−1) | Conversion content (mg kg−1) |
|---|---|---|---|---|---|---|---|
| 1 | Fresh catalyst | 0.02 | 25 | 100 | Ni | 158.085 | 197![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535 535 |
| 2 | Catalyst (6th run) | 0.02 | 25 | 100 | Ni | 157.177 | 196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
As shown in Fig. S5 (ESI†), after six reaction cycles, no chemical change in the heterogeneous catalyst structure was observed by FT-IR spectroscopy, that frequencies and intensities of all absorption bands are well-preserved. Additionally, as shown by the XRD patterns in Fig. S6 (ESI†), the XRD analysis of the 6th run reused Ni–ImzAdn catalyst shows the same positions of the XRD pattern in the fresh catalyst and a very slight decrease in intensity of the Ni–O peaks, which can be due to the trace loosening the metal nodes of MOF catalyst. Table 6, shows the comparative performance of Ni–ImzAdn MOF catalyst with other MOF-based catalysts reported in the literature for the conversion of styrene oxide to cyclic carbonate using CO2 under solvent-free conditions.54–58 As shown in Table 6, entry 2–6, for all catalysts much higher CO2 pressures are usually needed to achieve high conversion (from 8 to 20 bar in one case) at the closest temperature conditions. For example, similar conditions were employed by Verpoort and coworkers, with a ZIF-67 MOF,54 and Park and coworkers,57 with Cu-MOF in both the catalytic system, high pressure is required for CO2 fixation. On the opposite side, higher cyclic carbonate conversion (95.0%) was found to be at the ambient pressure by Ni–ImzAdn MOF catalyst. These results showed that the Ni–ImzAdn MOF has a high affinity for absorbing CO2 to carry out the fixation process.
3.5 Possible reaction mechanism
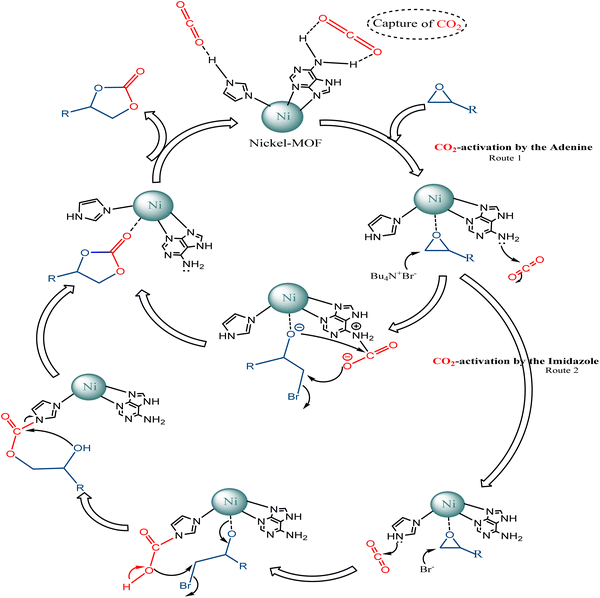
According to previous literature,59–61 a plausible reaction mechanism of the CO2/epoxide coupling by Ni–ImzAdn is shown in Fig. 7. As expected, the microporous Ni–ImzAdn MOF with polar uncoordinated N-rich sites have good CO2 uptake capacity due to the presence of adenine and imidazole inside the channels. On route number 1, the activation of CO2 occurs through a nucleophilic attack by adenine on the carbon atom of CO2 and the metal nodes provide acid sites for epoxide activation to promote ring-opening of the epoxide. In the second step of route 1, ring-opening of the epoxide takes place by a nucleophilic attack of the (Br−) anion from TBAB on the least-hindered carbon atom of Ni-epoxide to form metal-coordinated bromo-alkoxide. For the next step, the resulting alkoxide-ion reacts with the polarized CO2 molecule, which can cause the intramolecular cyclization to give the corresponding carbonate. On route number 2, the activation of CO2 occurs through a nucleophilic attack by –NH groups in the axial imidazole rings on the carbon atom of CO2 and the metal nodes provide acid sites for epoxide activation to promote ring-opening of the epoxide. Ring-opening of the epoxide takes place by a nucleophilic attack of the (Br−) anion from TBAB on the least-hindered carbon atom of Ni-epoxide to form metal-coordinated bromo-alkoxide. Finally, alkoxide-ion reacts with carbamic acid, then intramolecular cyclization occurs to give carbonate.244. Conclusion
In summary, Ni-based MOF, having strong Lewis acid-base dual-functional sites, high porosity, and amino-functional channels for high capture of CO2 gas molecules, was investigated towards catalytic cycloaddition of CO2 to epoxides to afford versatile and useful cyclic carbonate compounds under solvent-free condition. Also, due to the presence of N-containing ligands (imidazole and adenine) and open Ni(II) metal sites as high-density basic and acidic centers respectively, in comparison with many conventional MOFs that perform the CO2 fixation at high pressure, this MOF carried out the reaction at ambient pressure. Moreover, the catalyst exhibited high thermal and structural stability, along with excellent reusability, showing no significant decline in catalytic activity even after six cycles. These findings highlight the potential of this MOF as an efficient and durable heterogeneous catalyst.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Reza Erfani-Ghorbani: writing – original draft, methodology, investigation, formal analysis, data curation. Hossein Eshghi: supervision, conceptualization, writing – review & editing. Ali Shiri: supervision, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Ferdowsi University of Mashhad, under the Project no. 3/61310.References
- C. Kim, C.-J. Yoo, H.-S. Oh, B. K. Min and U. Lee, Review of carbon dioxide utilization technologies and their potential for industrial application, J. CO2 Util., 2022, 65, 102239 CrossRef CAS.
- L. Zhao, H.-Y. Hu, An-G. Wu, A. O. Terent’ev, L.-N. He and H.-R. Li, CO2 capture and in situ conversion to organic molecules, J. CO2 Util., 2024, 82, 102753 CrossRef CAS.
- G. Fiorani, A. Perosa and M. Selva, Sustainable valorisation of renewables through dialkyl carbonates and isopropenyl esters, Green Chem., 2023, 25(13), 4878–4911 RSC.
- Q.-W. Song, R. Ma, P. Liu, K. Zhang and L.-N. He, Recent progress in CO2 conversion into organic chemicals by molecular catalysis, Green Chem., 2023, 25, 6538–6560 RSC.
- E. Aytar, E. Yasar and A. Kilic, The efficient and reusable imidazolium-organoboron catalysts for green CO2 insertion reactions in solvent-free under atmospheric and high-pressure conditions, Fuel, 2024, 378, 132867 CrossRef CAS.
- A. Rehman, F. Saleem, F. Javed, A. Ikhlaq, S. W. Ahmad and A. Harvey, Recent advances in the synthesis of cyclic carbonates via CO2 cycloaddition to epoxides, J. Environ. Chem. Eng., 2021, 9(2), 105113 CrossRef CAS.
- T. Yan, H. Liu, Z. X. Zeng and W. G. Pan, Recent progress of catalysts for synthesis of cyclic carbonates from CO2 and epoxides, J. CO2 Util., 2023, 68, 102355 CrossRef CAS.
- S. Ravi, J. Kim, Y. Choi, H. H. Han, S. Wu, R. Xiao and Y.-S. Bae, Metal-free amine-anchored triazine-based covalent organic polymers for selective CO2 adsorption and conversion to cyclic carbonates under mild conditions, ACS Sustainable Chem. Eng., 2023, 11(3), 1190–1199 CrossRef CAS.
- E. Saha, H. Jungi, S. Dabas, A. Mathew, R. Kuniyil, S. Subramanian and J. Mitra, Amine-rich nickel(II)-xerogel as a highly active bifunctional metallo-organo catalyst for aqueous knoevenagel condensation and solvent-free CO2 cycloaddition, Inorg. Chem., 2023, 62(37), 14959–14970 CrossRef CAS PubMed.
- F. Pourhassan, R. Khalifeh and H. Eshghi, Well dispersed gold nanoparticles into the multi amine functionalized SBA-15 for green chemical fixation of carbon dioxide to cyclic carbonates under solvent free conditions, Fuel, 2021, 287, 119567 CrossRef CAS.
- M. Usman, A. Rehman, F. Saleem, A. Abbas, V. C. Eze and A. Harvey, Synthesis of cyclic carbonates from CO2 cycloaddition to bio-based epoxides and glycerol: an overview of recent development, RSC Adv., 2023, 13(33), 22717–22743 RSC.
- J.-Y. Chuang, K.-T. Liu, M. M. Lin, W.-Y. Yu, Ru-J. Jeng and M.-K. Leung, Two novel types of heterogeneous catalysts apply to synthesize cyclic carbonates through CO2 fixation on epoxides under mild condition, J. CO2 Util., 2023, 76, 102592 CrossRef CAS.
- P. Tyagi, D. Singh, N. Malik, S. Kumar and R. Singh Malik, Metal catalyst for CO2 capture and conversion into cyclic carbonate: progress and challenges, Mater. Today, 2023, 65, 133–165 CrossRef CAS.
- K. Wang, H. Li, Y. Lin, Yu-Z. Luo and Zi-J. Yao, Heterogeneous catalytic conversion of carbon dioxide and epoxides to cyclic carbonates, Surf. Interfaces, 2024, 103845 CrossRef CAS.
- Y. Liu, S. Li, Y. Chen, M. Li, Z. Chen, T. Hu, L. Shi, M. Pudukudy, S. Shan and Y. Zhi, Urea/amide-functionalized melamine-based organic polymers as efficient heterogeneous catalysts for CO2 cycloaddition, Chem. Eng. J., 2023, 474, 145918 CrossRef CAS.
- Y.-L. Wan, L. Wang and L. Wen, Amide-functionalized organic cationic polymers toward enhanced catalytic performance for conversion of CO2 into cyclic carbonates, J. CO2 Util., 2022, 64, 102174 CrossRef CAS.
- D. Kim and Na. Kyungsu, Organic–inorganic multifunctional hybrid catalyst giving catalytic synergies in cooperative coupling between CO2 and propylene oxide to propylene carbonate, J. CO2 Util., 2018, 27, 129–136 CrossRef CAS.
- B. Wang, L. Wang, J. Lin, C. Xia and W. Sun, Multifunctional Zn-N4 catalysts for the coupling of CO2 with epoxides into cyclic carbonates, ACS Catal., 2023, 13(15), 10386–10393 CrossRef CAS.
- C. C. Obi, J. T. Nwabanne, P. Kanuria Igbokwe, C. I. Idumah, V. U. Okpechi and H. C. Oyeoka, Novel advances in synthesis and catalytic applications of metal–organic frameworks-based nanocatalysts for CO2 capture and transformation, J. Environ. Chem. Eng., 2024, 112835 CrossRef CAS.
- S. Gulati, S. Vijayan, S. Kumar, B. Harikumar, M. Trivedi and R. S. Varma, Recent advances in the application of metal–organic frameworks (MOFs)-based nanocatalysts for direct conversion of carbon dioxide (CO2) to value-added chemicals, Coord. Chem. Rev., 2023, 474, 214853 CrossRef CAS.
- L. Hu, W. Xu, Q. Jiang, R. Ji, Z. Yan and G. Wu, Recent progress on CO2 cycloaddition with epoxide catalyzed by ZIFs and ZIFs-based materials, J. CO2 Util., 2024, 81, 102726 CrossRef CAS.
- Z. A. K. Khattak, N. Ahmad, H. A. Younus, H. Ullah, B. Yu, K. S. Munawar and M. Ashfaq, et al., Ambient conversion of CO2 and epoxides to cyclic carbonates using 3D amide-functionalized MOFs, Catal. Sci. Technol., 2024, 14(7), 1888–1901 RSC.
- A. Eskemech, H. Chand, A. Karmakar, V. Krishnan and R. R. Koner, Zn-MOF as a single catalyst with dual lewis acidic and basic reaction sites for CO2 fixation, Inorg. Chem., 2024, 63(8), 3757–3768 CrossRef CAS PubMed.
- R. R. Kuruppathparambil, T. M. Robert, R. S. Pillai, S. K. B. Pillai, S. K. K. Shankaranarayanan, D. Kim and D. Mathew, Nitrogen-rich dual linker MOF catalyst for room temperature fixation of CO2 via cyclic carbonate synthesis: DFT assisted mechanistic study, J. CO2 Util., 2022, 59, 101951 CrossRef CAS.
- Y. Hu, R. Abazari, S. Sanati, M. Nadafan, C. L. Carpenter-Warren, A. M. Z. Slawin, Y. Zhou and A. M. Kirillov, A dual-purpose Ce(III)–organic framework with amine groups and open metal sites: third-order nonlinear optical activity and catalytic CO2 fixation, ACS Appl. Mater. Interfaces, 2023, 15(31), 37300–37311 CrossRef CAS PubMed.
- X.-L. Yang, Y.-T. Yan, W.-J. Wang, H. Ze-Ze, W.-Y. Zhang, W. Huang and Y.-Y. Wang, A 2-fold interpenetrated nitrogen-rich metal–organic framework: dye adsorption and CO2 capture and conversion, Inorg. Chem., 2021, 60(5), 3156–3164 CrossRef CAS PubMed.
- Q. T. Nguyen, J. Na, Y.-R. Lee and K.-Y. Baek, Boosting catalytic performance for CO2 cycloaddition under mild condition via amine grafting on MIL-101-SO3H catalyst, J. Environ. Chem. Eng., 2024, 12(1), 111852 CrossRef.
- M. Saghian, S. Dehghanpour and M. Sharbatdaran, Amine-functionalized frameworks as highly actives catalysts for chemical fixation of CO2 under solvent and co-catalyst free conditions, J. CO2 Util., 2020, 41, 101253 CrossRef CAS.
- A. Zulys, F. Yulia, N. Muhadzib and N. Nasruddin, Biological metal–organic frameworks (Bio-MOFs) for CO2 capture, Ind. Eng. Chem. Res., 2020, 60(1), 37–51 CrossRef.
- H. Mohamed, N. Syahirah, K. A. Mohamed, F. Hussin and L. Ti Gew, A systematic review of amino acid-based adsorbents for CO2 capture, Energies, 2022, 15(10), 3753 CrossRef.
- M. Erzina, O. Guselnikova, R. Elashnikov, A. Trelin, D. Zabelin, P. Postnikov and J. Siegel, et al., BioMOF coupled with plasmonic CuNPs for sustainable CO2 fixation in cyclic carbonates at ambient conditions, J. CO2 Util., 2023, 69, 102416 CrossRef CAS.
- R. K. Gupta, M. Riaz, M. Ashafaq, Z.-Y. Gao, R. S. Varma, D.-C. Li, P. Cui, C.-H. Tung and D. Sun, Adenine-incorporated metal–organic frameworks, Coord. Chem. Rev., 2022, 464, 214558 CrossRef CAS.
- C. Pettinari and A. Tombesi, Metal–organic frameworks for carbon dioxide capture, MRS Energy Sustainability, 2020, 7, E35 CrossRef.
- F. Shen, J. Wu, G. Chen, Z. Wei, J. Wang, Z. Lin and K. Chai, Efficient capture of CO2 from flue gas and biogas by moisture-stable adenine-based ultramicroporous metal–organic framework, J. Environ. Chem. Eng., 2024, 113796 CrossRef CAS.
- Y. Rachuri, J. F. Kurisingal, R. Kumar Chitumalla, S. Vuppala, Y. Gu, J. Jang, Y. Choe, E. Suresh and D.-W. Park, Adenine-based Zn(II)/Cd(II) metal–organic frameworks as efficient heterogeneous catalysts for facile CO2 fixation into cyclic carbonates: a DFT-supported study of the reaction mechanism, Inorg. Chem., 2019, 58(17), 11389–11403 CrossRef CAS PubMed.
- H. M. Lee, Il S. Youn, M. Saleh, J. W. Lee and K. S. Kim, Interactions of CO2 with various functional molecules, Phys. Chem. Chem. Phys., 2015, 17(16), 10925–10933 RSC.
- R. A. Agarwal and N. K. Gupta, CO2 sorption behavior of imidazole, benzimidazole and benzoic acid-based coordination polymers, Coord. Chem. Rev., 2017, 332, 100–121 CrossRef CAS.
- R. Vanaraj, R. Vinodh, T. Periyasamy, S. Madhappan, C. Mohan Babu, S. P. Asrafali and R. Haldhar, et al., Capacitance enhancement of metal–organic framework (MOF) materials by their morphology and structural formation, Energy Fuels, 2022, 36(9), 4978–4991 CrossRef CAS.
- M. Zeraati, V. Alizadeh, P. Kazemzadeh, M. Safinejad, H. Kazemian and G. Sargazi, A new nickel metal organic framework (Ni-MOF) porous nanostructure as a potential novel electrochemical sensor for detecting glucose, J. Porous Mater., 2022, 1–11 Search PubMed.
- A. M. Spokoyny, D. Kim, A. Sumrein and C. A. Mirkin, Infinite coordination polymer nano-and microparticle structures, Chem. Soc. Rev., 2009, 38(5), 1218–1227 RSC.
- E. V. Shaw, A. M. Chester, G. P. Robertson, C. Castillo-Blas and T. D. Bennett, Synthetic and analytical considerations for the preparation of amorphous metal–organic frameworks, Chem. Sci., 2024, 15(28), 10689–10712 RSC.
- S. Das, P. Heasman, T. Ben and S. Qiu, Porous organic materials: strategic design and structure–function correlation, Chem. Rev., 2017, 117(3), 1515–1563 CrossRef CAS PubMed.
- H.-M. Wen, C. Liao, L. Li, A. Ali, Z. Alothman, R. Krishna, H. Wu, W. Zhou, J. Hu and B. Chen, A metal–organic framework with suitable pore size and dual functionalities for highly efficient post-combustion CO2 capture, J. Mater. Chem. A, 2019, 7(7), 3128–3134 RSC.
- M. Essalhi, M. Mohan, N. Dissem, N. Ferhi, A. Abidi, T. Maris and A. Duong, Two different pore architectures of cyamelurate-based metal–organic frameworks for highly selective CO2 capture under ambient conditions, Inorg. Chem. Front., 2023, 10(3), 1037–1048 RSC.
- J. Gu, X. Sun, X. Liu, Y. Yang, H. Shan and Y. Liu, Highly efficient synergistic CO2 conversion with epoxide using copper polyhedron-based MOFs with Lewis acid and base sites, Inorg. Chem. Front., 2020, 7(22), 4517–4526 RSC.
- S. Natarajan and K. Manna, Bifunctional MOFs in heterogeneous catalysis, ACS Org. Inorg. Au, 2023, 4(1), 59–90 CrossRef PubMed.
- G. Jiang, L. Zhang, Z. Zhao, X. Zhou, A. Duan, C. Xu and J. Gao, Highly effective P-modified HZSM-5 catalyst for the cracking of C4 alkanes to produce light olefins, Appl. Catal., A, 2008, 340(2), 176–182 CrossRef CAS.
- N.-Y. Topsøe, K. Pedersen and E. G. Derouane, Infrared and temperature-programmed desorption study of the acidic properties of ZSM-5-type zeolites, J. Catal., 1981, 70(1), 41–52 CrossRef.
- B. Zhao, C. Li, T. Hu, Y. Gao, L. Fan and X. Zhang, Robust {Pb10}-cluster-based metal–organic framework for capturing and converting CO2 into cyclic carbonates under mild conditions, Inorg. Chem., 2024, 63(30), 14183–14192 CrossRef CAS PubMed.
- X. Zhao, G. Chang, H. Xu, Y. Yao, D. Dong, S. Yang, G. Tian and X. Yang, A hierarchical metal–organic framework composite aerogel catalyst containing integrated acid, base, and metal sites for the one-pot catalytic synthesis of cyclic carbonates, ACS Appl. Mater. Interfaces, 2024, 16(6), 7364–7373 CrossRef CAS PubMed.
- X. Song, Y. Wu, D. Pan, J. Zhang, S. Xu, L. Gao, R. Wei, J. Zhang and G. Xiao, Dual-linker metal–organic frameworks as efficient carbon dioxide conversion catalysts, Appl. Catal., A, 2018, 566, 44–51 CrossRef CAS.
- C. Feng, X. Cao, L. Zhang, C. Guo, N. Akram and J. Wang, Zn 1, 3, 5-benzenetricarboxylate as an efficient catalyst for the synthesis of cyclic carbonates from CO2, RSC Adv., 2018, 8(17), 9192–9201 RSC.
- Y. Wu, X. Song, S. Xu, J. Zhang, Y. Zhu, L. Gao and G. Xiao, 2-Methylimidazole modified Co-BTC MOF as an efficient catalyst for chemical fixation of carbon dioxide, Catal. Lett., 2019, 149, 2575–2585 CrossRef CAS.
- B. Mousavi, S. Chaemchuen, B. Moosavi, Z. Luo, N. Gholampour and F. Verpoort, Zeolitic imidazole framework-67 as an efficient heterogeneous catalyst for the conversion of CO2 to cyclic carbonates, New J. Chem., 2016, 40(6), 5170–5176 RSC.
- T. Lescouet, C. Chizallet and D. Farrusseng, The origin of the activity of amine-functionalized metal–organic frameworks in the catalytic synthesis of cyclic carbonates from epoxide and CO2, ChemCatChem, 2012, 4(11), 1725–1728 CrossRef CAS.
- C.-Y. Gao, H.-R. Tian, J. Ai, L. Lei-Jiao, D. Song, Y.-Q. Lan and Z.-M. Sun, A microporous Cu-MOF with optimized open metal sites and pore spaces for high gas storage and active chemical fixation of CO2, Chem. Commun., 2016, 52(74), 11147–11150 RSC.
- A. C. Kathalikkattil, D.-W. Kim, J. Tharun, H.-G. Soek, R. Roshan and D.-W. Park, Aqueous-microwave synthesized carboxyl functional molecular ribbon coordination framework catalyst for the synthesis of cyclic carbonates from epoxides and CO2, Green Chem., 2014, 16(3), 1607–1616 RSC.
- M. H. Beyzavi, C. J. Stephenson, Y. Liu, O. Karagiaridi, J. T. Hupp and O. K. Farha, Metal–organic framework-based catalysts: chemical fixation of CO2 with epoxides leading to cyclic organic carbonates, Front. Energy Res., 2015, 2, 63 Search PubMed.
- S. Senthilkumar, M. S. Maru, R. S. Somani, H. C. Bajaj and S. Neogi, Unprecedented NH2-MIL-101 (Al)/n-Bu4NBr system as solvent-free heterogeneous catalyst for efficient synthesis of cyclic carbonates via CO2 cycloaddition, Dalton Trans., 2018, 47(2), 418–428 RSC.
- R. Das, S. Singh Dhankhar and C. M. Nagaraja, Construction of a bifunctional Zn(II)–organic framework containing a basic amine functionality for selective capture and room temperature fixation of CO2, Inorg. Chem. Front., 2020, 7(1), 72–81 RSC.
- J. F. Kurisingal, Y. Rachuri, Y. Gu, R. Kumar Chitumalla, S. Vuppala, J. Jang, K. Kumar Bisht, E. Suresh and D.-W. Park, Facile green synthesis of new copper-based metal–organic frameworks: experimental and theoretical study of the CO2 fixation reaction, ACS Sustainable Chem. Eng., 2020, 8(29), 10822–10832 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08614g |
| This journal is © The Royal Society of Chemistry 2025 |