Open Access Article
Open Access ArticleEco-friendly methods for the synthesis of N-acyl pyrazole derivatives with luminescent properties†
Eliza Świętczak ab,
Michał Rachwalskib and
Adam Marek Pieczonka
ab,
Michał Rachwalskib and
Adam Marek Pieczonka *b
*b
aUniversity of Lodz, Doctoral School of Exact and Natural Sciences, Matejki 21/23, 90-237 Lodz, Poland
bUniversity of Lodz, Faculty of Chemistry, Department of Organic and Applied Chemistry, Tamka 12, PL-91-403, Lodz, Poland. E-mail: adam.pieczonka@chemia.uni.lodz.pl
First published on 29th April 2025
Abstract
A comparison of green methods for the synthesis of N-acyl pyrazoles starting from aromatic carbohydrazide derivatives and 1,3-diketones is presented. Special attention was focused on reactions utilizing a ball mill enabling the synthesis of novel pyrazole derivatives with optimal yield and reproducibility. The use of this approach facilitated the synthesis of target compounds exhibiting solid-state luminescent properties attributable to the phenomenon of aggregation-induced emission (AIE). Additionally, efforts were made to prepare thin, luminescent solid-state films, allowing for the assessment of which synthesized compounds may have potential applications in organic electronics.
Introduction
Pyrazole derivatives represent a crucial class of heterocyclic organic molecules possessing substantial utilization potential, especially owing to their biological characteristics.1–3 However, the literature also reports pyrazole derivatives exhibiting luminescent properties in the solid state associated with the phenomenon of Aggregation-Induced Emission (AIE).4 N-Acyl pyrazole derivatives are less explored; however, their significance as biologically active compounds is equally important due to the presence of an additional carbonyl group.4–6 Compounds of this type can be obtained through direct acylation of the pyrazole ring utilizing, for example, acid chlorides,7,8 or through oxidative amidation reactions.9,10 Another approach involves cyclocondensation reactions utilizing carbohydrazide derivatives and carbonyl compounds.11 The most commonly described example in the literature is the reaction between hydrazides and 1,3-diketones, which requires additional activation to obtain the pyrazole ring. These reactions are most commonly catalysed by Lewis acids12 or Brønsted acids and can be conducted under solvent-free conditions.13 Reactions catalysed by microwaves14 or performed using grinding15 are also conducted without the use of a solvent, while the utilization of ultrasonication typically requires the use of a solvent such as ethanol.16 The synthesis of N-acyl pyrazole derivatives can even be carried out using solvents such as a mixture of glycerol–water17 or employing lemon juice as the reaction medium.18 Most of the presented synthetic methods can be considered as eco-friendly, however, they are typically described for a narrow scope of substrates.Taking this into account, as well as our interest in synthesizing compounds with luminescent properties,19 we have decided to conduct the synthesis of a series of N-acyl pyrazole derivatives using environmentally friendly methods, starting from aromatic hydrazide derivatives (Scheme 1).
Results and discussion
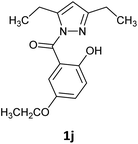
The initial reaction attempts were conducted utilizing furan-2-carboxylic acid hydrazide and 2,5-pentanedione, employing ultrasonic catalysis, microwave irradiation, grinding using a ball mill, as well as a reaction conducted in lemon juice. After optimizing the reaction conditions for each method, we expanded our investigations to include the hydrazides of biphenyl carboxylic acid and 2-naphthoic acid (Table 1). In all cases, the influence of the catalyst (piperidine, acetic acid, or sulfuric acid(VI)) and reaction time was examined. For reactions catalyzed by ultrasound and those conducted in a microwave reactor, the effect of temperature was also studied. In the case of ball mill utilization, the impact of milling frequency and the number of grinding balls on the efficiency of the process was investigated. In summary, the reactions conducted in the ultrasonic bath lasted for 75 minutes and required ethanol as a solvent, along with a catalytic amount of acetic acid as a catalyst. Synthesis in the vibratory ball mill required a catalytic amount of sulfuric acid but was conducted without a solvent for 60 minutes with the frequency 22 Hz. The reaction in the microwave reactor lasted for 20 minutes at 120 °C and was conducted without a solvent or catalyst. The synthesis conducted using lemon juice was carried out at room temperature for 24 hours using freshly squeezed lemon juice. In all cases, the products were obtained as solids, which were purified through the crystallization process.Based on the presented results, we have decided that the most versatile method for synthesizing N-acyl pyrazoles is mixing in a ball mill with the addition of a catalytic amount of H2SO4. In the next approach, we use a broader range of carbohydrazides and different diketones to determine the limitations of this method (Table 2). Because our research aimed to synthesize compounds with luminescent properties, we decided to use carbohydrazide derivatives with large aromatic substituents such as biphenyl, naphthyl, or a coumarin derivative. The presented method is suitable for the synthesis of derivatives containing bulky aromatic substituents (naphthyl, biphenyl, coumarin) and also tolerates the presence of an additional hydroxyl group but the best reactivity was observed for carbohydrazides with electron-donating substituents. Unfortunately, it is limited to derivatives with alkyl groups on the pyrazole ring (all attempts to synthesize 3,5-diphenyl-substituted pyrazole failed due to the lower electrophilicity of the carbonyl atoms in 1,3-diphenyl-1,3-propanedione compared to aliphatic diketones). Similarly, the attempt to use nitro-substituted hydrazide did not result in obtaining the expected product (see ESI info†). The reaction with an unsymmetrical diketone (6-methyl-2,4-heptadienone) resulted in a mixture of regioisomers 1k and 1l, from which only the major product 1k, could be isolated in pure form (Table 3).
| Product | Selected method | ||
|---|---|---|---|
| Ball mill | Ultrasonic catalysis | Microwave irradiation | |
| a —* Reaction not carried out. | |||
 |
50% | 40% | Trace amount |
 |
37% | —* | —* |
 |
42% | 18% | 37% |
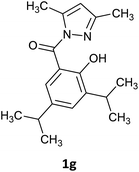 |
84% | —* | Trace amount |
 |
55% | 0% | 16% |
 |
34% | 0% | 23% |
 |
61% | 28% | Trace amount |
The structure of the compounds 1k and 1m was confirmed based on 2D NMR spectra. This compound is formed through the initial attack of the hydrazide on the less sterically hindered carbonyl atom, followed by cyclization involving the second carbonyl group. The same result was obtained for both tested carbohydrazides, and regardless of the method used, compound 1k and 1m predominantly formed (the regioisomer ratio was determined based on 1H NMR spectra).
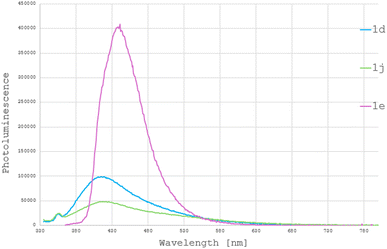
Among the obtained compounds, the most promising in terms of luminescent properties were the pyrazole derivatives of 4-ethoxy salicylic acid 1d and 1j, as well as the coumarin derivative 1e, for which we decided to conduct further investigations. To determine the application potential of the obtained compounds, absorption and emission spectra were recorded and their photoluminescence quantum yield (PLQY) was measured. Pyrazole derivatives with 4-ethoxy-2-hydroxyphenyl substituent (1d, 1j) exhibited similar absorption maxima (355–364 nm, see ESI info†) and emission maxima (420–440 nm, Fig. 1), characterized by low luminescence quantum yield in DCM solution (<1%), which increased to 5.2% for a thin solid-state layer. The benzocoumarin derivative 1e distinctly stood out, with a PLQY of 7.2% in DCM solution, increasing to 20% in a thin solid-state layer. The higher PLQY in the solid state is likely attributed to the aggregation-induced emission (AIE) effect, which has been described for both benzocoumarin derivatives20 and pyrazole derivatives.4
Thin solid-state layers were prepared to determine the film-forming properties of the obtained compounds and to measure their PLQY in the solid state. The thin layers were fabricated using the drop-casting method from toluene solutions onto quartz substrates. The layer prepared for the coumarin derivative was continuous without crystalline elements and emitted light uniformly (Fig. 2), indicating its potential utility as an emissive layer in OLEDs, for example. The luminescent properties and ability to form thin solid films are crucial for the potential application of the studied compounds in organic electronics. The presented preliminary studies confirm that compound 1e may be considered for further research, particularly regarding its use as an emissive layer in OLED devices. In the next stages of the study, alternative methods for fabricating thin solid films (such as spin-coating, dip-coating, and inkjet printing) will be employed. These techniques will facilitate the production of an OLED prototype incorporating AIE compounds as the emissive layer positioned between other functional layers, as presented in our previous studies.19
Conclusions
Our objective was to compare modern methods for the synthesis of N-acyl pyrazole derivatives, and we demonstrated that the use of a ball mill is the most versatile among the eco-friendly methods for synthesizing this group of derivatives. However, for the reaction of simple aromatic hydrazides with 2,4-pentadienone, the sonochemical method also enabled the synthesis of the expected products with high yields. Additionally, some of the obtained compounds exhibited luminescent properties. The most promising of these was the coumarin derivative 1e, for which a high-quality thin solid layer was also obtained. This, combined with its luminescent properties, suggests potential future applications of this compound, such as in the emissive layer of OLEDs. Typically, the synthesis of emitters used in OLEDs is complex and expensive, whereas N-acyl pyrazole derivatives can be synthesized using green, eco-friendly methods.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization and methodology, A. M. P. and M. R.; software, E. Ś., A. M. P. and M. R.; investigation, E. Ś.; writing—original draft preparation, E. Ś. and A. M. P.; writing—review and editing, M. R. and A. M. P.; supervision, M. R. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Notes and references
- J. V. Faria, P. F. Vegi, A. G. C. Miguita, M. S. Santos, N. Boechat and A. M. R. Bernardino, Recently reported biological activities of pyrazole compounds, Bioorg. Med. Chem., 2017, 25, 5891 CrossRef CAS PubMed.
- R. A. Menezes and K. S. Bhat, Synthetic aspects, structural insights and pharmacological potential of pyrazole derivatives: an overview, Discover Appl. Sci., 2025, 7, 137 CrossRef CAS.
- Z. Zeng, Ch. Liao and L. Yu, Molecules for COVID-19 treatment, Chin. Chem. Lett., 2024, 35, 109349 CrossRef CAS.
- S. Mukherjee, P. S. Salini, A. Srinivasan and S. Peruncheralathan, AIEE phenomenon: tetraaryl vs. triaryl pyrazoles, Chem. Commun., 2015, 51, 17148 RSC.
- P. Liao and C. He, Azole reagents enabled ligation of peptide acyl pyrazoles for chemical protein synthesis, Chem. Sci., 2024, 15, 7965 RSC.
- K. M. Short, M. A. Estiarte, S. M. Pham, D. C. Williams, L. Igoudin, S. Dash, N. Sandoval, A. Datta, N. Pozzi, E. Di Cera and D. B. Kita, Discovery of novel N-acylpyrazoles as potent and selective thrombin inhibitors, Eur. J. Med. Chem., 2023, 246, 114855 CrossRef CAS PubMed.
- K. Otrubova, S. Chatterjee, S. Ghimire, B. F. Cravatt and D. L. Boger, N-Acyl pyrazoles: Effective and tunable inhibitors of serine hydrolases, Bioorg. Med. Chem., 2019, 27, 1693 CrossRef CAS PubMed.
- Ch. Volpe, S. Meninno, G. Mirra, J. Overgaard, A. Capobianco and A. Lattanzi, Direct α-Imination of N-Acyl Pyrazoles with Nitrosoarenes, Org. Lett., 2019, 21, 5305 CrossRef CAS PubMed.
- J. M. Ovian, Ch. B. Kelly, V. A. Pistritto and N. E. Leadbeater, Accessing N-Acyl Azoles via Oxoammonium Salt-Mediated Oxidative Amidation, Org. Lett., 2017, 19, 1286 CrossRef CAS PubMed.
- G. P. Wadey, K. E. Doherty, A. L. Sandoval and N. E. Leadbeater, Preparation of novel acyl pyrazoles and triazoles by means of oxidative functionalization reactions, Heterocycl. Commun., 2023, 29, 20220158 CrossRef CAS.
- B. Á. Pereira, A. V. de Bastos, W. K. O. Teixeira, S. M. Silva, A. F. C. Flores and D. C. Flores, Synthesis of diverse N-acyl-pyrazoles via cyclocondensation of hydrazides with α-oxeketene dithioacetal, Mol. Diversity, 2017, 21, 1021 CrossRef CAS PubMed.
- G. Yang, X. He, B. Yu and Ch. Hu, Cu1.5PMo12O40-catalyzed condensation cyclization for the synthesis of substituted pyrazoles, Appl. Organomet. Chem., 2018, 32, 4532 CrossRef.
- Z. Wang and H. Qin, Solventless syntheses of pyrazole derivatives, Green Chem., 2004, 6, 90 RSC.
- B. R. Vaddula, R. S. Varma and J. Leazer, Mixing with microwaves: solvent-free and catalyst-free synthesis of pyrazoles and diazepines, Tetrahedron Lett., 2013, 54, 1538 CrossRef CAS.
- P. A. Channar, S. Afzal, S. A. Ejaz, A. Saeed, F. A. Larik, P. A. Mahesar, J. Lecka, J. Sevigny, M. F. Erben and J. Iqbal, Exploration of carboxy pyrazole derivatives: Synthesis, alkaline phosphatase, nucleotide pyrophosphatase/phosphodiesterase and nucleoside triphosphate diphosphohydrolase inhibition studies with potential anticancer profile, Eur. J. Med. Chem., 2018, 156, 461 CrossRef CAS PubMed.
- N. Ghareb, H. A. Elshihawy, M. M. Abdel-Daim and M. A. Helal, Novel pyrazoles and pyrazolo[1,2-a]pyridazines as selective COX-2 inhibitors; Ultrasound-assisted synthesis, biological evaluation, and DFT calculations, Bioorg. Med. Chem. Lett., 2017, 27, 2377 CrossRef CAS PubMed.
- Z. Min, Q. Zhang, X. Hong, X. Cao and X. Hu, A Green Protocol for Catalyst-Free Syntheses of Pyrazole in Glycerol-Water Solution, Asian J. Chem., 2015, 27, 3205 CrossRef CAS.
- V. Milovanovic, Z. D. Petrovic, S. Novakovic, G. A. Bogdanovic, D. Simijonovic and V. P. Petrovic, Structural characterization of benzoyl-1H-pyrazole derivatives obtained in lemon juice medium: Experimental and theoretical approach, J. Mol. Struct., 2019, 85, 1195 Search PubMed.
- J. A. Adamczyk, K. Zielonka, S. Kotarba, J. Saramak, I. Glowacki, M. Rachwalski and A. M. Pieczonka, Photophysical properties of novel fluorescent thin solid layers based on the Aggregation Induced Emission of alkoxy-substituted salicylaldehyde azines, J. Lumin., 2021, 229, 117668 CrossRef CAS.
- Y.-F. Sun, H.-P. Wang, Z.-Y. Chen and W.-Z. Duan, Solid-state Fluorescence Emission and Second-Order NonlinearOptical Properties of Coumarin-based Fluorophores, J. Fluoresc., 2013, 23, 123 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ra08527b |
| This journal is © The Royal Society of Chemistry 2025 |